Published online Jun 28, 2025. doi: 10.4329/wjr.v17.i6.107776
Revised: April 20, 2025
Accepted: June 7, 2025
Published online: June 28, 2025
Processing time: 90 Days and 2.4 Hours
Calvarial lesions are usually incidental and asymptomatic, rarely detected. However, these lesions can also present with pain, a palpable mass or a bone de
Core Tip: Calvarial lesions are usually incidental and asymptomatic, rarely detected, and mostly benign. In calvarial lesions, the patient's age, history of trauma or underlying systemic disease, and radiological features of the lesions [location (skull, diploic space), extent (focal, diffuse), multiplicity (solitary, multiple), attenuation (lytic, sclerotic, mixed), bone expansion, periosteal reaction, relationship to adjacent dura and diploic veins, suture crossing, transition zone, internal matrix and mineralization, and presence of soft tissue component] should be carefully evaluated. In calvarial lesions, diagnostic accuracy is increased when both computed tomography and magnetic resonance imaging findings are evaluated in conjunction with other modalities.
- Citation: Gökçe E, Beyhan M. Review of imaging modalities and radiological findings of calvarial lesions. World J Radiol 2025; 17(6): 107776
- URL: https://www.wjgnet.com/1949-8470/full/v17/i6/107776.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i6.107776
The skull begins to develop during the embryonic period, between the 23rd and 26th days of gestation, and is divided into the neurocranium and viscerocranium. The neurocranium surrounds the brain, while the viscerocranium forms the facial skeleton. The neurocranium develops from mesenchyme, while the viscerocranium develops from the first 3 branchial arches. The neurocranium is divided into the chondrocranium (skull base), which derives from paraxial mesoderm and forms by endochondral ossification, and the calvarium (skull vault), which forms by intramembranous ossification. The term "calvaria", of Latin origin, is used to describe the skull bones at the top of the head that surround the brain and are separate from the facial and mandibular bones[1]. The skull is composed of flat bones, including the frontal, parietal, and occipital bones; the zygomatic and squamous parts of the temporal bones; and the tip of the greater wing of the sphenoid bone[2]. During the calvarial bones' fetal and early postnatal development, the calvarium is unilaminar because there is only a single cortical table. However, in the sixth year of life, the three-layered calvarium emerges with the development of the middle diploic plate[1]. The calvarial bones consist of three layers: the middle intradiploic plate (diploic space), which consists of spongy bone and bone marrow; and the inner and outer cortical layers[3]. As the accumulation of the calvarium's inner and outer periosteal edges is slightly greater than the resorption of the endosteal surfaces, the cortical plates thicken proportionally. The inner plate of the calvarium is thinner in adults. This makes inner plate fractures more common in head traumas compared to the outer plate[1]. The scalp consists of skin, subcutaneous connective tissue, the galea aponeurotica, and subgaleal loose connective tissue[4]. The skull has outer and inner periosteal layers. The outer periosteal layer is called pericranium and the inner periosteal layer is called endosteum. The endosteum is the outer layer of the dura mater, which consists of a dense fibrous structure, while the inner layer of the dura mater consists of less dense fibrous connective tissue[1,3].
Calvarial bone tumors are rare. Primary neoplasms of the calvarium account for 0.8%-1% of all bone tumors[5]. Benign calvarial tumors are more common than their primary malignancies[4]. If the lesion arises directly from the calvarial bone, it is called a primary lesion; if it arises from adjacent structures and later affects the calvarial bone or metastasis, it is called a secondary lesion[6]. The patient's clinical history, age, symptoms and laboratory findings are important factors in the diagnosis of calvarial lesions. These lesions are usually asymptomatic and are detected incidentally on radiological images obtained for other reasons[4]. The most common symptoms of calvarial lesions in the literature are a palpable mass and headache, followed by exophthalmos, visual disturbances and loss of consciousness[5].
Plain radiography is usually the first imaging modality used to evaluate calvarial lesions. Computed tomography (CT) and/or magnetic resonance imaging (MRI) scans are then usually required. CT is an examination with higher diagnostic accuracy in detecting sclerotic or lytic lesions in bone and in determining the involvement of the calvarial tables and diploe when compared with plain radiographs. CT can also show calcification and sclerotic margins within a lesion. When soft tissue or intraaxial involvement is detected on plain radiography, MRI is preferred because of its ability to detect extraosseous involvement, especially when paramagnetic contrast is used. MRI can also detect intradiploic lesions before they erode the cortical tables. Cortical trabeculae appear hypointense on both T1-weighted images and T2-weighted images on MRI. Due to its high-fat content, the marrow is hyperintense in adults on T1-weighted images. In adolescents, the bone marrow signal is more heterogeneous because the marrow has not yet been completely replaced by fat[7]. If uniform low signal intensity is detected on T1-weighted images in the calvarium after the age of 7, bone marrow disease should be considered[3]. In addition, diffusion weighted imaging, MR spectroscopy and perfusion MRI techni
The various radiological features of calvarial lesions should be evaluated as a whole. Radiological findings of these lesions include the location (skull, diploic space), extent (focal, diffuse), multiplicity (solitary, multiple), attenuation (lytic, sclerotic, and mixed), bone expansion, periosteal reaction (solid, thin, lamellated, spiculated, hair-on-end, sunburst, and Codman’s triangle) relationship to adjacent dura and diploic veins, suture crossing, transition zone, internal matrix and mineralization, and the presence of a soft tissue component[3]. Accordingly, calvarial lesions may be solitary (single), multiple or diffuse, and lytic, sclerotic or mixed (Table 1). Calvarial lesions can often be osteogenic, chondrogenic, fibrogenic and vascular origin[9]. In addition, dural-based transdiploic lesions may be present, which can invade and cross the calvarium. The most classic transdiploic lesion is meningioma, but other neoplasms such as a solitary fibrous tumor, lymphoma, metastasis and plasmacytoma may also have transdiploic extension[3,8]. Malignant cancers of the scalp may occur with calvarial invasion, dural extension, and rarely intraparenchymal involvement. Most of these are squamous and basal cell carcinomas that arise as primary neoplasms from the skin. Metastases, melanoma, and various variants of sarcoma may also involve these locations[10].
| Appearances of calvarial lesions | ||
| Solitary lesions | Multiple lesions | Diffuse lesions |
| Metastasis1,2,3 | Metastasis1,2,3 | Metastasis1,2,3 |
| Fibrous dysplasia1,2,3 | Fibrous dysplasia1,2,3 | Fibrous dysplasia1,2,3 |
| Lymphoma1,2,3 | Lymphoma1,2,3 | Paget’s disease1,2,3 |
| Langerhans cell histiocytosis1 | Langerhans cell histiocytosis1 | Bone marrow hyperplasia (e.g., thalassemia1, myeloma1) |
| Dermoid and epidermoid cysts1 | Myeloma1 | Renal osteodystrophy1 |
| Primary bone tumors (e.g., aneurysmal bone cyst1, lipoma1) | Leukemia1 | Bone turnover abnormalities (e.g., hyperparathyroidism1) |
| Intraosseous meningioma1,2,3 | ||
| Intraosseous venous malformation1,3 | ||
| Osteoma2 | ||
| Osteomyelitis1 | ||
| Osteosarcoma2 | ||
Recognition of benign and malignant imaging findings is important for radiological diagnosis. A specific diagnosis can be made when a characteristic imaging feature is present[3]. Benign tumors usually have well-defined margins with sharp bone destruction and a narrow transition zone, and often have sclerotic margins. Malignant tumors have ill-defined margins, a wide transition zone, moth-eaten or infiltrated bone destruction, and usually have a soft tissue component. In generally slow-growing lesions, an unilamellar continuous pattern is found in the periosteum, whereas an interrupted periosteal reaction defines a more aggressive tumor[4,9]. Aggressive lesions may additionally extend from the destroyed bone into the adjacent scalp, dura mater, and/or brain parenchyma. On MRI, the adjacent dura mater is usually thickened, because of invasion or inflammatory effects of the malignant lesion, and shows contrast enhancement after paramagnetic contrast administration. Diploic veins and meninges adjacent to arachnoid granulations are contrast-enhancement with paramagnetic contrast, whereas the diploe is usually not contrast-enhancement. Therefore, dural contrast enhancement may be seen in normal meninges and benign calvarial lesions. Nodular, discontinuous contrast enhancement is associated with malignant tumor invasion, whereas continuous linear contrast enhancement usually indicates a reactive change. On post-gadolinium T1-weighted series, a thin hypointense line between the lesion and the dura mater with contrast enhancement indicates the presence of a potentially normal epidural space and indicates that the dura mater is not invaded[7].
Among all imaging features, the extent, multiplicity, and attenuation of the calvarial lesion and the patient's age may be sufficient to narrow the differential diagnosis[3]. Radiological diagnosis and differential diagnosis of calvarial lesions should be performed to decide the need for biopsy, surgical intervention, or conservative treatment[9]. For detailed evaluation, calvarial lesions can be classified into congenital and anatomical variants, traumatic and iatrogenic, idiopathic, infectious and inflammatory, metabolic, benign and malignant neoplastic causes[3]. Calvarial lesions in this classification are listed in Table 2. This article aims to review the imaging modalities and radiological findings of normal variants that may be confused with calvarial pathologies, systemic diseases affecting the calvarium, and benign and malignant calvarial lesions.
| Classification of calvarial lesions | |
| Anatomic and congenital variants | Idiopathic lesions |
| Arachnoid granulations | Hyperostosis frontalis interna |
| Venous lakes (Lacunae) | Bilateral parietal thinning |
| Parietal/biparietal foramina | Gorham’s disease |
| Lacunar skull | Parry-romberg syndrome |
| Dermoid cyst | Infectious and inflammatory lesions |
| Epidermoid cyst | Osteomyelitis |
| Skeletal dysplasia | Sarcoidosis |
| Convolutional markings and beaten copper skull | Langerhans cell histiocytosis |
| Tuberous sclerosis | Benign calvarial lesions |
| Encephalocele and atretic encephalocele | Fibrous dysplasia |
| Sinus pericranii | Osteoma |
| Aplasia cutis congenita | Primary intraosseous meningioma |
| External occipital protuberance | Intraosseous venous malformation |
| Iatrogenic and traumatic lesions | Aneurysmal bone cyst |
| Burr holes | Giant cell tumor |
| Flap osteonecrosis | Intraosseous lipoma |
| Congenital skull depression | Bone Desmoplastic Fibroma |
| Leptomeningeal cyst | Malignant calvarial lesions |
| Calcified cephal hematoma | Metastasis |
| Metabolic calvarial lesions | Multiple myeloma and plasmacytoma |
| Paget’s disease | Lymphoma |
| Chronic anemia | Leukemia |
| Hyperparathyroidism | Osteosarcoma |
| Renal osteodystrophy | Neuroblastoma |
| Acromegaly | Solitary fibrous tumor |
| Rickets and osteomalacia | Chordoma |
| Phenytoin-induced hyperostosis | Chondrosarcoma |
| Scurvy | Ewing’s sarcoma |
| Rhabdomyosarcoma | |
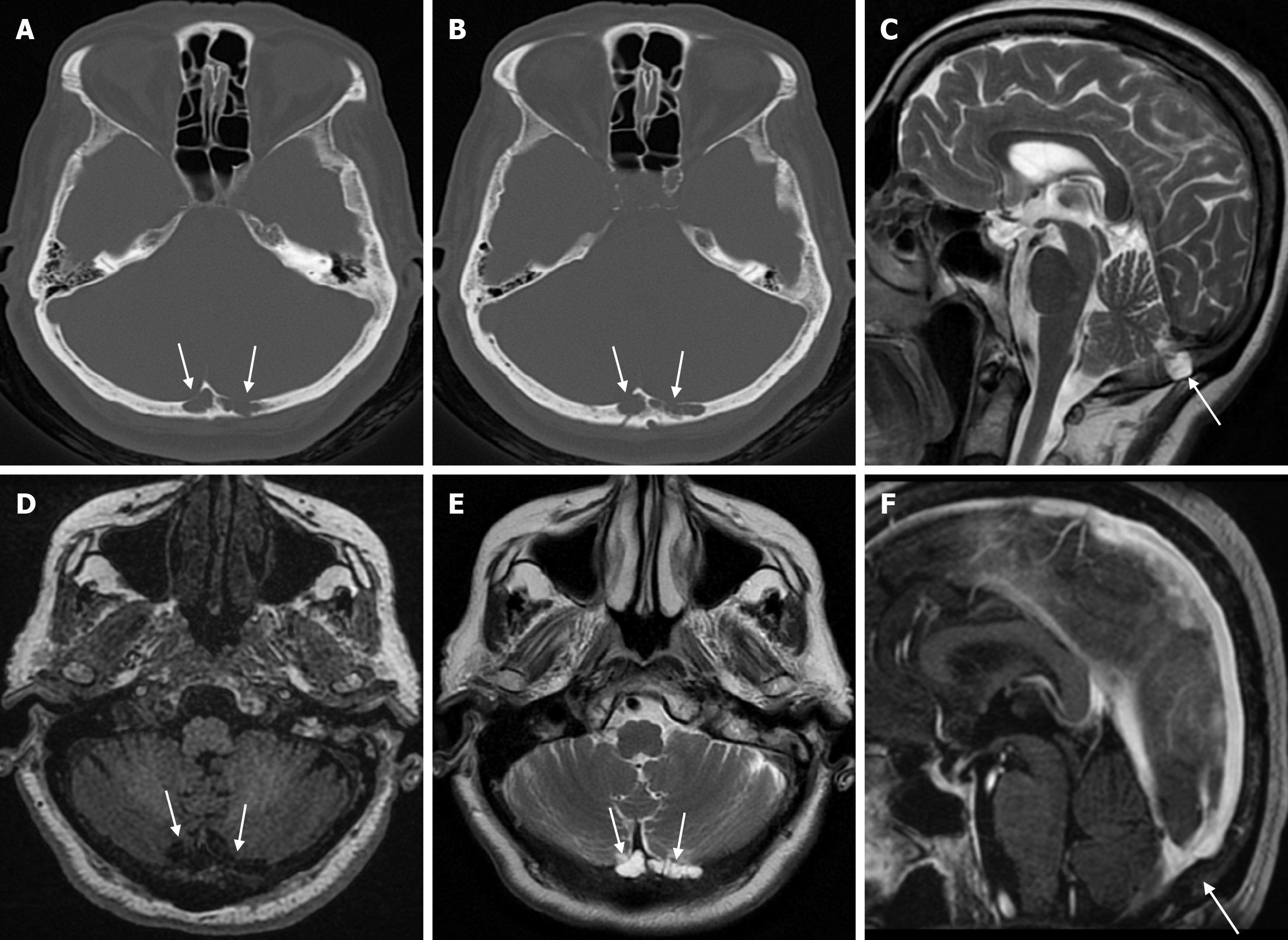
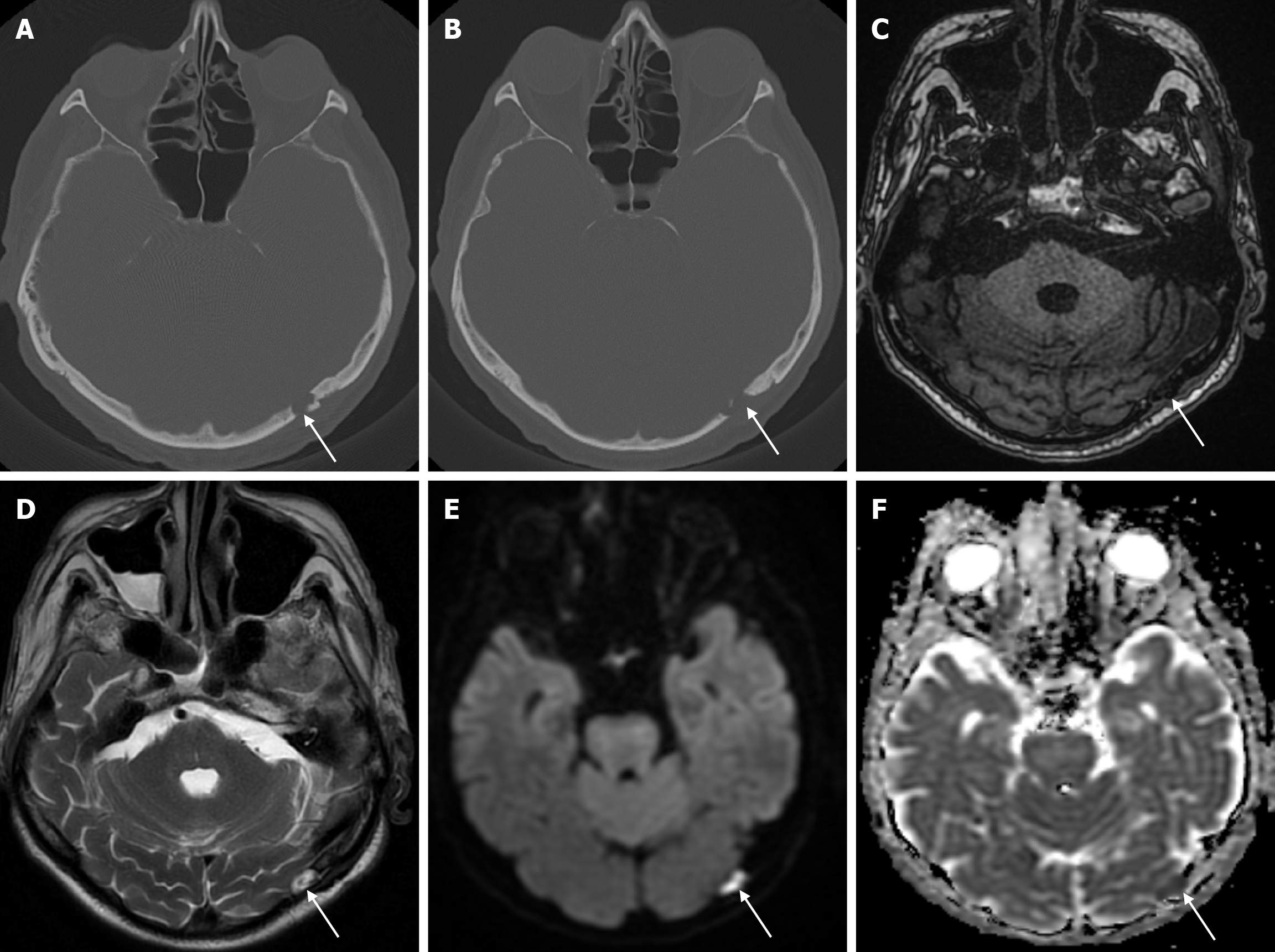
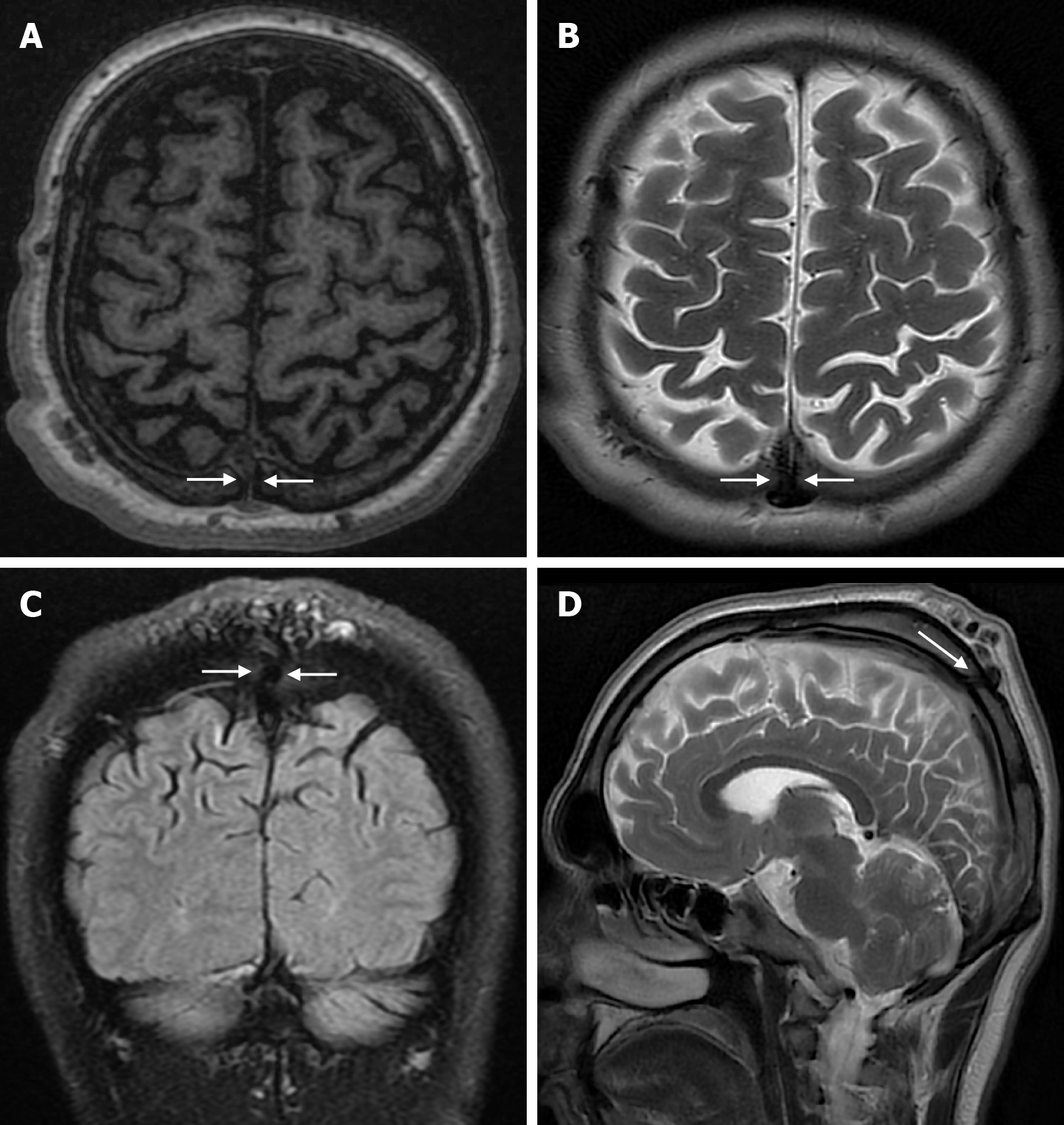
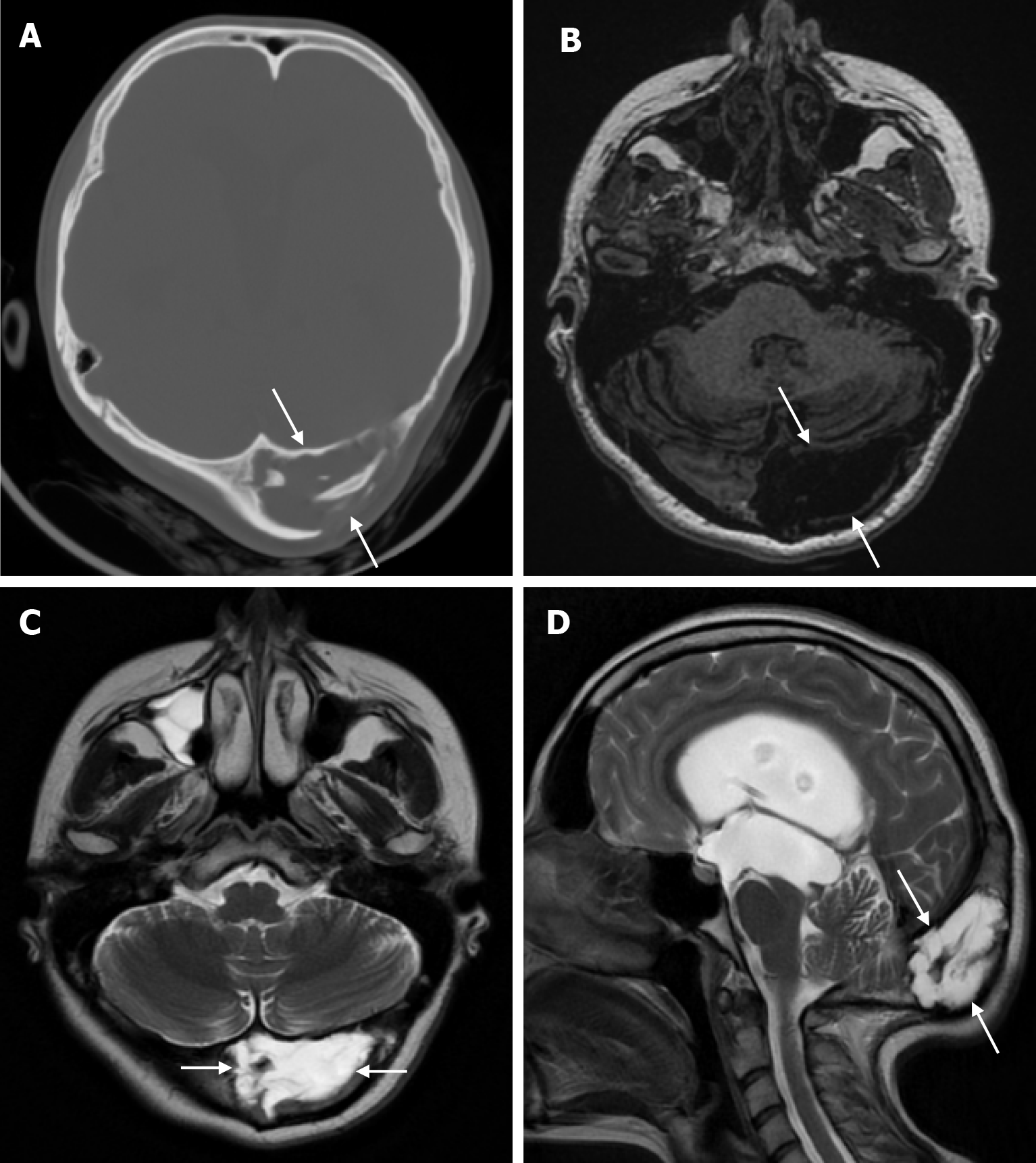
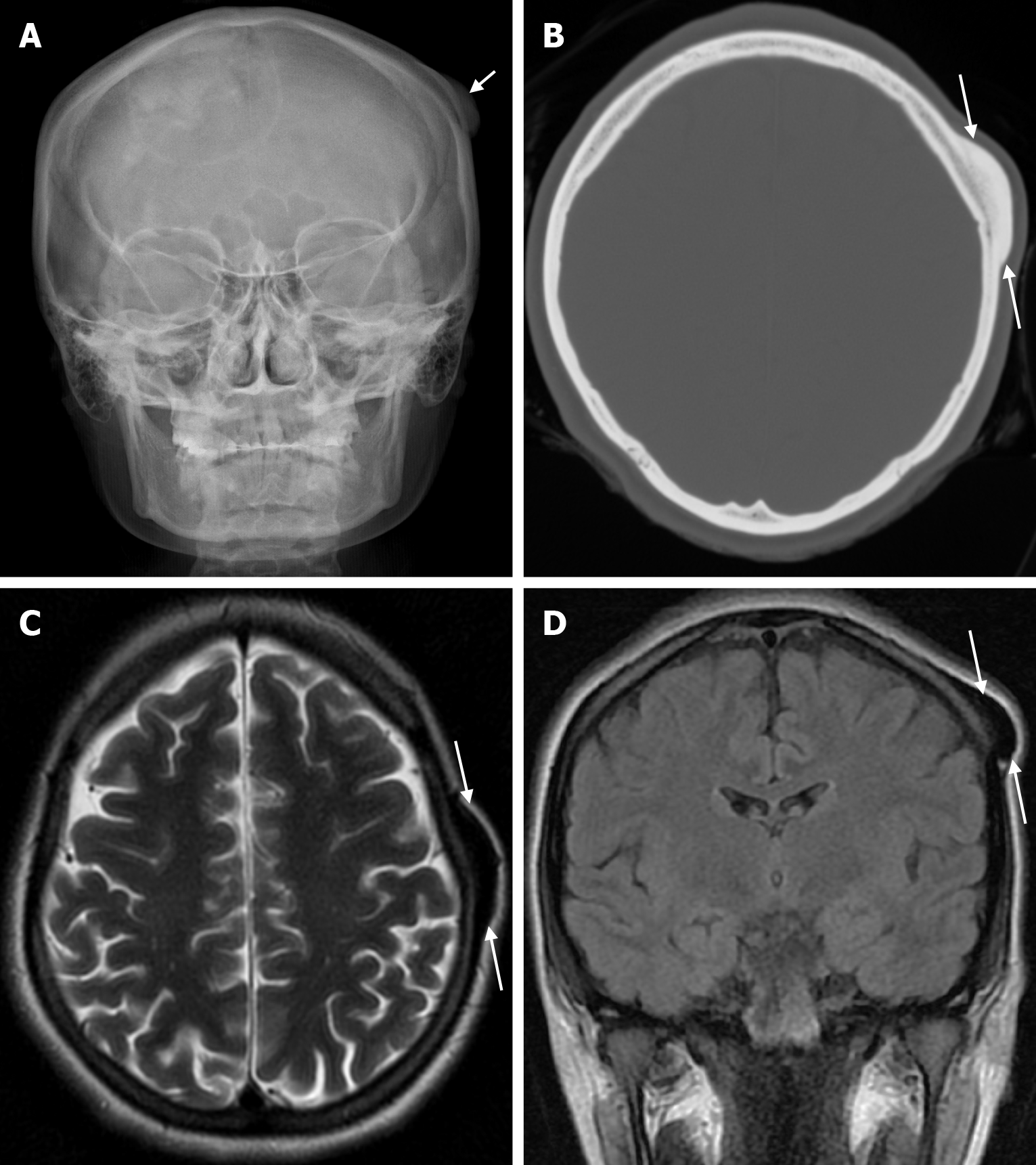
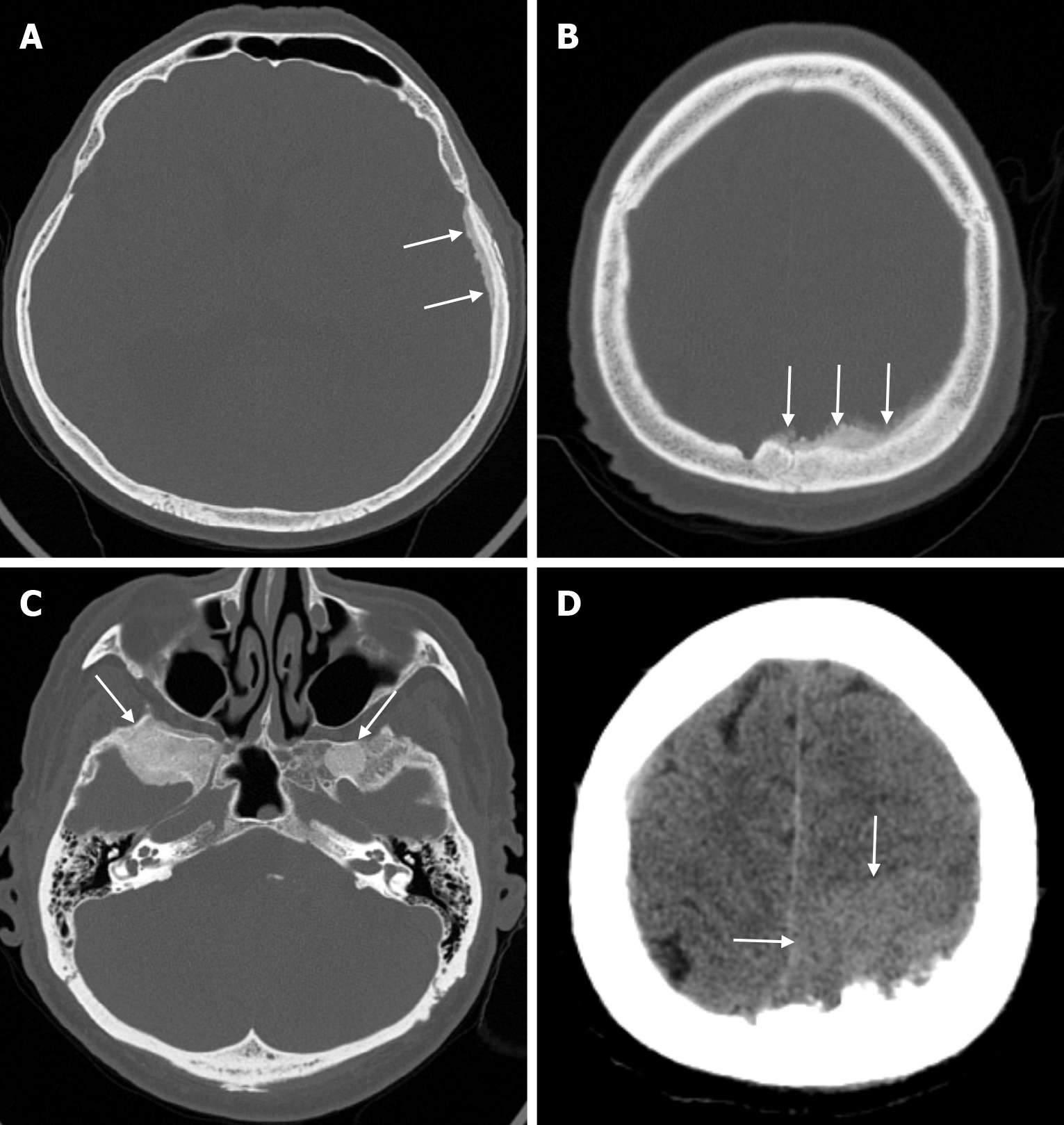
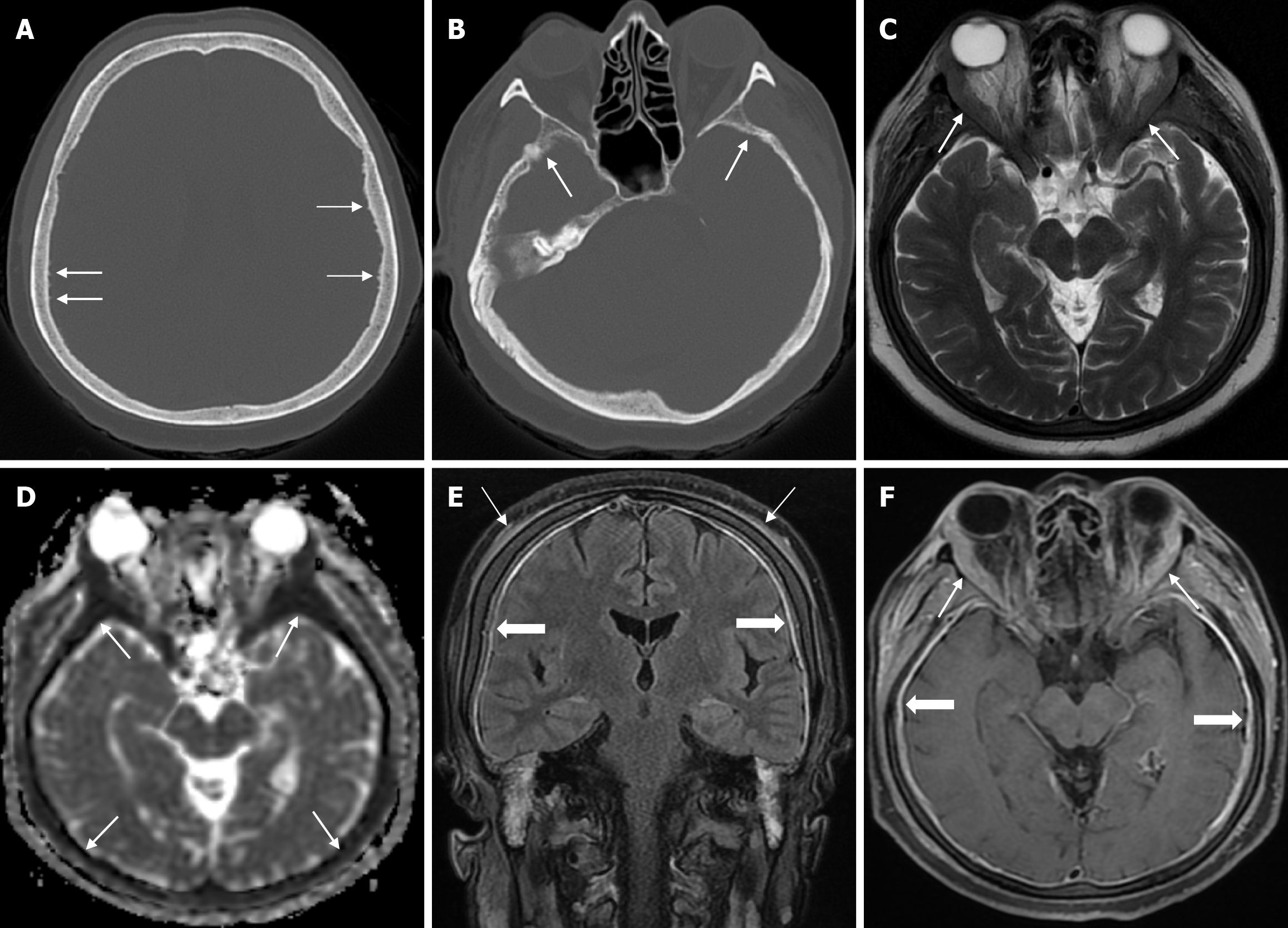
Arachnoid granulations (Pacchionian granulations) are cerebrospinal fluid-filled projections that extend from the subarachnoid space through openings in the dura into the venous sinuses and can be seen macroscopically. Their incidence varies from 0.3% to 55%. Arachnoid granulations are usually seen in millimeter dimensions, but may grow to fill the dural sinuses or arch the inner table[11]. In addition, intraosseous arachnoid granulations can sometimes extend to the inner table of the bone, causing scalloping, remodeling or bone erosion of the inner table[12]. The prevalence of arachnoid granulations increases with age, but there is no difference in gender distribution. Arachnoid granulations with a slight left hemispheric predominance are found in the dural venous sinuses, most frequently in the transverse sinuses and especially in the middle or lateral parts. The second most common location is the superior sagittal sinus, but they can be found anywhere in the dural venous sinuses. Arachnoid granulations are usually detected incidentally. If they fill and expand the dural sinuses, causing partial sinus obstruction, they may cause symptoms of increased intracranial pressure due to venous hypertension. They are usually associated with headaches. Arachnoid granulations may be seen as radiolucent areas on plain radiography of the skull or may cause compression on the inner table of the skull. On CT imaging, arachnoid granulations can be seen as sharply demarcated, hypodense structures in close relation to the dural venous sinus. On MRI, they usually show hyperintense signal on T2-weighted images and hypointense or isointense signal on T1-weighted images compared to brain parenchyma (Figure 1). On CT angiography, MR angiography or catheter angiography imaging, arachnoid granulations appear as oval or round filling defects in the dural venous sinuses during the venous phase[11]. Recent advances in radiology have made it possible to identify brain herniations into the arachnoid granulation by improving image quality with higher resolution and thinner slice three-dimensional T1-weighted and T2-weighted MRI sequences (Figure 2). Brain herniation into the arachnoid granulation is rare in the literature, and the incidence of brain herniation into the calvarial or dural sinuses has been reported to be 0.32%[13]. Arachnoid granulations may be confused with pathological processes in the dural venous sinuses[11]. In the differential diagnosis of arachnoid granulations, venous sinus thrombosis, dural-based tumors, or structures such as septa and fat that may cause filling defects in the dural venous sinuses should be considered[12]. Dural sinus thrombosis usually fills an entire sinus segment or several sinuses and may extend to the cortical veins, whereas arachnoid granulations appear as focal and well-defined defects. Fresh thrombus in the dural sinuses are hyperdense on CT and hyperintense on T1-weighted MRI. Differential tumor diagnosis can be made based on its shape, lack of contrast enhancement and diffusion restriction[11].
The transcalvarial venous channels are the openings in the calvarium that connect the dural venous sinuses to the extracranial veins via emissary veins. They are usually seen as serpentine or linear lucencies with sclerotic margins along the skull, and when these vessels are dilated they are known as venous lakes. Venous lakes are sometimes confused with fractures or sutures[14]. Venous lakes are present in about 15% of patients and are not associated with age or gender[15]. Venous lakes appear on CT as round or oval lucencies at the level of the skull base (Figure 3). On MRI, they show medium or low signal intensity on T1-weighted images, high signal on T2-weighted images, and significant contrast enhancement is seen in contrast-enhanced series[14]. It is important to distinguish venous structures from metastases. Metastases often show restricted diffusion within the lesion due to high cellularity. In addition, metastases may show increased blood flow within the lesion. Metastases often invade adjacent tissues[15].
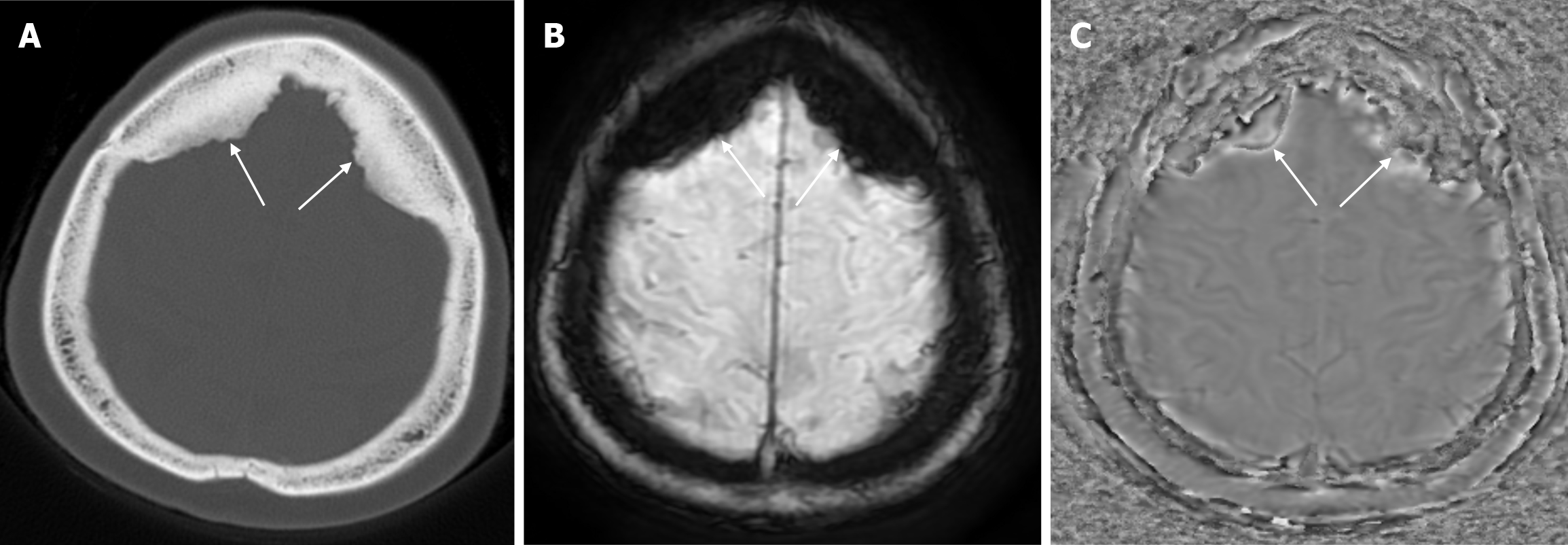
Small parietal foramina (approximately 1-2 mm in diameter) are intramembranous bone development defects that occur in 60% to 70% of the population. The parietal foramina close during the fifth month of normal embryonic development and give rise to emissary veins that anastomose with the superior sagittal sinuses. Enlarged parietal foramina (> 5 mm) occur at a rate of 1:15000 to 1:50000. These parietal/biparietal foramina arise as a result of abnormal calvarial ossification around the parietal notch during fetal development. At birth there is a large single midline or bilateral calvarial defect with the brain covered by the dura, pericranium and overlying scalp. Individuals with enlarged parietal foramina present with symptoms of severe headache, vomiting and intense pain when mild pressure is applied to the unprotected cerebral cortex. Parietal foramina may rarely be seen together with craniofacial and skeletal anomalies, myelomeningocele, and encephalocele. Genetic studies have shown a familial occurrence with autosomal dominant inheritance. Mutations in the MSX2 and ALX4 genes have been reported in almost 80% of parietal foramina cases[16]. CT scan shows single or double rounded defects in the parietal bone adjacent to the intersection of the sagittal and lambdoid sutures. The defects may be large and confluent along the midline. MRI is the preferred method to detect venous, cortical or meningeal abnormalities associated with the parietal foramina[14].
Lacunar skull, also known as Lückenschädel, is a fetal ossification disorder described in the early 19th century and rarely seen as an isolated defect. In this ossification defect, shallow or deep cavities form in the flat bones of the inner table of the skull due to mesenchymal dysplasia during intramembranous ossification. Lesions can be seen in any part of the skull but are more prominent in the frontal, parietal, and upper occipital bones. Lacunar skull development begins in utero but disappears by six months. Although it is not usually associated with any medical problem, it is painful when applied to the skin with local pressure, and these patients are at greater risk of brain damage from local trauma. Lacunar skull is often associated with neural tube defects, including spina bifida, meningocele, myelocele or encephalocele. Other congenital developmental anomalies such as rib and limb deformities, microcephaly, cleft palate and craniosynostosis may be present. A direct radiograph reveals that the craniolacuna has a distinctive "honeycomb" or "soap bubble" appearance. Large, rounded areas of reduced bone density are bordered by a pattern of thick bone that resembles a spiderweb. However, three-dimensional CT clearly shows numerous oval and round lacunae[17].
Dermoid cysts are benign congenital tumors consisting mainly of keratinized squamous epithelium, smooth muscle cells, hair follicles, and apocrine and sebaceous glands. They account for less than 1% of all intracranial tumors[18]. Dermoid cysts usually appear in the midline of the skull, near the anterior fontanelle. However, they can also appear in the posterior and middle cranial fossae. Dermoid cysts usually present in the first 3 decades of life[9]. The etiology of these tumors is related to primitive ectodermal cells and mesenchymal elements originating from neuroectodermal folds that close between the 3rd and 5th week of gestation and can also be caused by trauma. Patients are usually asymptomatic or present with mild symptoms such as headaches. However, if dermoid cysts rupture incidentally or as a result of trauma, the contents of the cyst may spread into the intracranial space, leading to chemical meningitis, arachnoiditis, aqueductal stenosis, ventriculitis, or seizures[18]. Dermoid cysts appear on plain radiography as an oval lytic lesion with sclerotic margins. On CT, dermoids appear as expansile, lytic, fat-dense, midline lesions with adjacent soft tissue and a soft tissue component that may extend intracranially. Intralesional calcifications may be present. Dermoid cysts have variable signal intensity on T2-weighted images on MRI but are usually hyperintense on T1-weighted images. Contrast enhancement of dermoid cysts may be slightly peripheral in about 25% of cases[9].
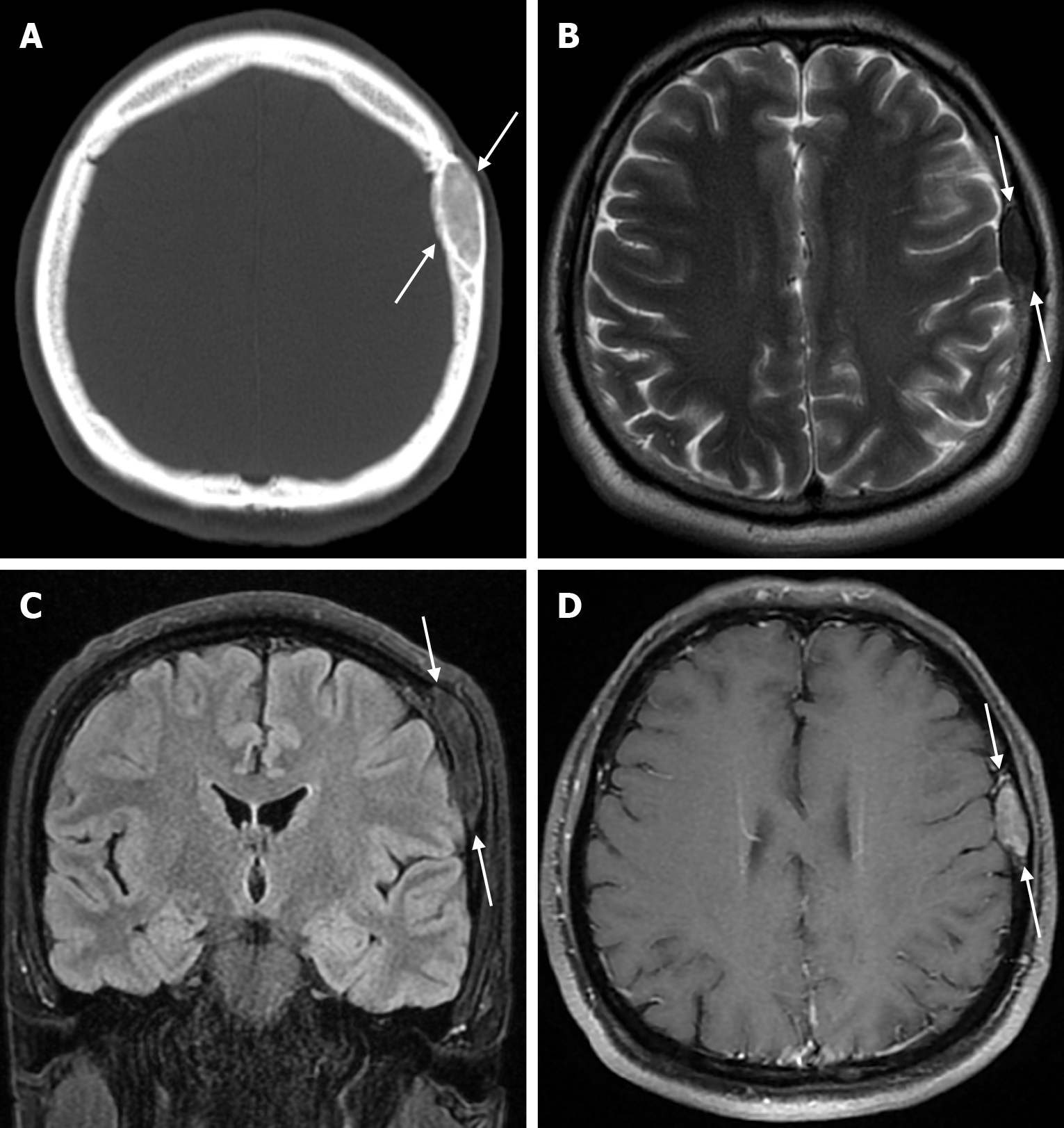
Epidermoid cysts are small or medium-sized, benign, and slow-growing tumors that arise from the remnants of ectodermal cells in the bones of the skull. These cysts can also develop after trauma or surgery. Epidermoid cysts are lined with squamous epithelium. They contain deposits of cholesterol and keratin. These cysts constitute less than 1% of primary intracranial tumours, while intradiploic epidermoid cysts account for less than 0.25% of intracranial tumours. These tumors can occur at any age from the first decade of life to the seventh decade, with a mean age of 32-38 years, and there is no gender predilection. Epidermoid cysts are most commonly found in the frontal, parietal and occipital regions and often involve more than one bone. Epidermoid cysts appear on plain radiography as round or lobulated lytic areas with smooth and sclerotic margins. On CT they appear as non-contrast enhanced intradiploic lytic hypodense lesions with smooth sclerotic borders. Epidermoid cysts often cause remodeling and widening of the inner and outer tables. Epidermoid cysts that appear hyperdense on CT due to bleeding, calcification or high protein content are called white epidermoids and are observed quite rarely. On MRI, epidermoid cysts appear isointense/hyperintense on T2-weighted and fluid-attenuated inversion recovery (FLAIR) images and slightly hyperintense on T1-weighted images (Figure 4). These lesions restrict diffusion on diffusion-weighted imaging but show no contrast enhancement on contrast enhanced sequences[19].
Skeletal dysplasias are heterogeneous group of genetic disorders of bone and cartilage. Approximately 450 different disorders, divided into 42 groups, are recognized in skeletal dysplasias, and more than 350 genes have been reported to be involved. Even the most common skeletal dysplasias, such as achondroplasia, are rare on their own, affecting about 1/30000 births, but skeletal dysplasias as a group are quite common, with an incidence of about 1/4000. Many skeletal dysplasias affect the skull, leading to significant complications such as neural compression and facial dysmorphism. Many skeletal dysplasias have unique and even pathognomonic findings[20].
The most common signs of cranial dysplasia are thickening of the skull bones and wormian bones. Wormian bones are small extra bones located between the cranial sutures, most commonly the lambdoid sutures. Dysplasias associated with wormian bones include cleidocranial dysplasia, pycnodysostosis, osteogenesis imperfecta and Hadju-Cheney syndrome. Skull thickening is usually seen in the spectrum of osteopetrosis and craniotubular dysplasia. Achondroplasia usually presents with narrowing of the skull base and compensatory cranial vault expansion[21]. Cleidocranial dysostosis is a rare autosomal dominant skeletal dysplasia resulting from heterozygous mutations in the runt-related gene, formerly called polyomavirus enhancer binding protein 2A or core binding factor A1. It is a polyostotic disorder characterized by incomplete intramembranous ossification of midline bony structures. Multiple wormian bones are characteristic of the skull due to intrasutural bone islands in the sagittal and lambdoid sutures. Premature fusion of the coronal suture or brachycephaly and frontal and parietal bossing have also been described[22].
Osteogenesis imperfecta is a genetic disorder caused by mutations in the type 1 procollagen genes, inherited as an autosomal dominant or recessive trait, characterized by decreased bone mass and increased bone fragility. Osteogenesis imperfecta is characterized by the triad of diffuse osteopenia, pencil-thin cortices and multiple fractures. In the skull, there are multiple wormian bones, a lucent calvarium, enlarged sinuses and platybasia[21].
Osteopetrosis is characterized by increased density in the medullary portions of bones where the cortices are preserved as a result of inadequate osteoclastic resorption of the bone within a bone. In osteopetrosis, diffuse sclerosis is present in the skull and progressive narrowing of the foramina causes cranial nerve compression. In addition, prognathism may occur with a predisposition to mandibular osteomyelitis[21].
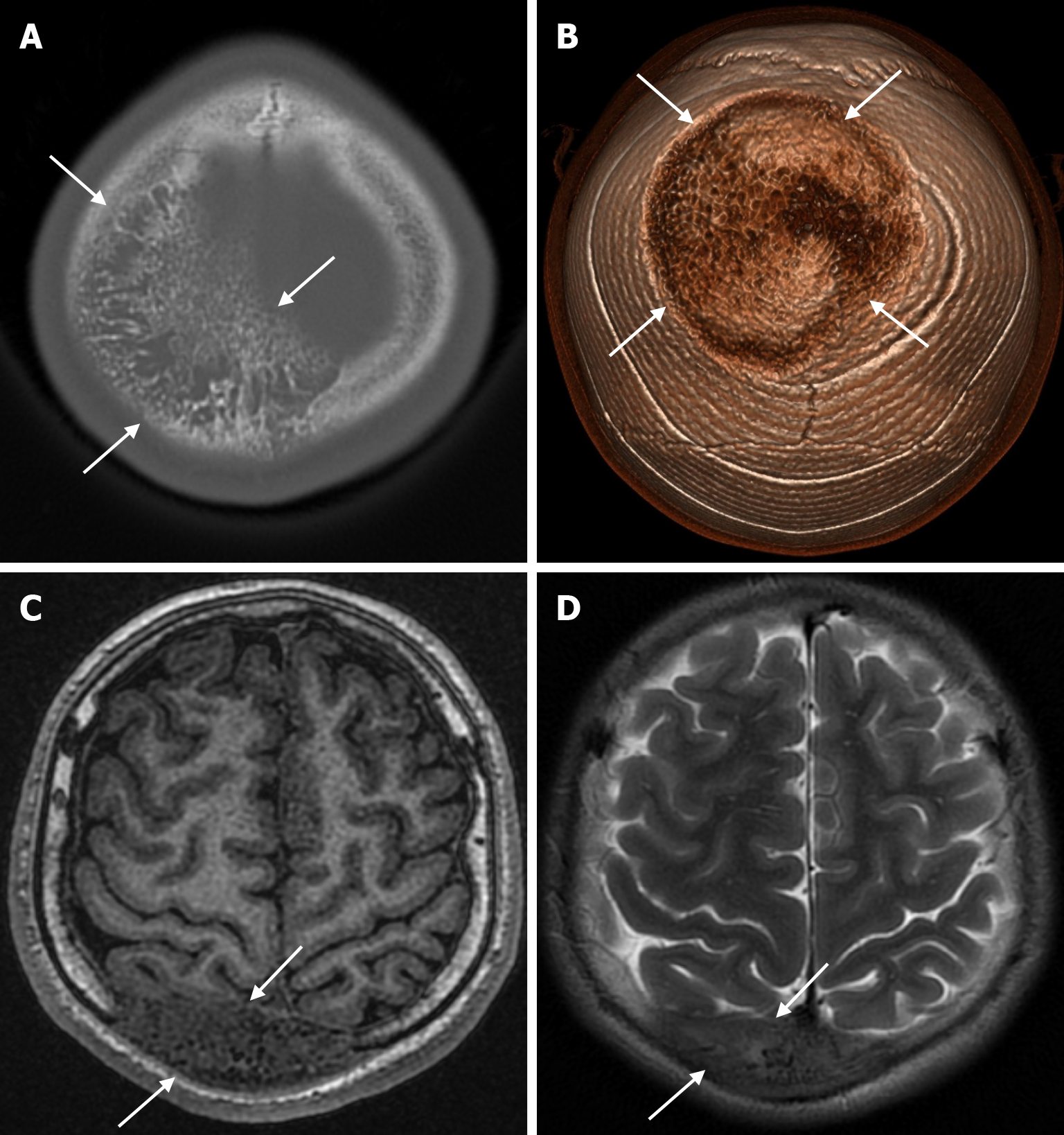
The beaten copper skull shows prominent convolutional markings on several bones of the skull. These convolutional markings normally appear between the ages of 2-3 and 5-7 years, which are periods of rapid brain growth. The occurrence of convolutional markings in children younger than 18 months should suggest a cause leading to increased intracranial pressure due to processes such as obstructive hydrocephalus, craniosynostosis or intracranial masses. Beaten copper skull pattern develops because of pressure applied to the soft skull by the growing brain. The beaten copper skull is usually confined to the posterior part of the inner table of the skull, but this appearance can affect the entire skull. Plain radiography of the skull can be an important diagnostic tool to detect increased intracranial pressure. In children, CT and three-dimensional reconstructions are best used to evaluate the convolutional markings (Figure 5) and cerebral ridges for surgical planning[23].
Tuberous sclerosis is a neurocutaneous syndrome with an autosomal dominant inheritance and an incidence of between 1/10000 and 1/200000 live births. Tuberous sclerosis may present with the classical clinical triad of skin lesions, mental retardation, and epileptic seizures. Three CT findings have been reported that can be seen in the skull associated with tuberous sclerosis: (1) High-density bone patchy areas that appear after puberty and are more common in the parietal regions can be seen in the calvarium. These changes develop due to hyperostosis of the inner table trabeculae and diploic spaces; (2) Both skull tables may show increased attenuation and thickening; and (3) Increased intracranial pressure findings (suture diastesis, sellar changes, and increased convolutional markings) that occur when subependymal hamartoma causes obstructive hydrocephalus are a rare finding[24].
Cephaloceles represent a spectrum of congenital malformations characterized by herniation of the brain and/or meninges through a defect in the skull. They can be subclassified according to the herniated intracranial contents. In encephalocele, the herniated sac contains brain tissue, meninges and cerebrospinal fluid. Encephalocele is rare, reported to occur in 1/4000 live births. The most common location of encephalocele is occipital (75%), followed by frontoethmoidal (15%) and basal (10%), while parietal encephalocele is rare. Most patients present with swelling of the head, depending on the location and size of the encephalocele. Most frontoethmoidal encephalocele present with a clinically visible lump at the root of the nose. These patients may have some degree of facial deformity, such as hypertelorism and proptosis. Basal encephalocele is internal and presents with nasal or nasopharyngeal swelling with varying degrees of obstruction, epistaxis and cerebrospinal fluid (CSF) rhinorrhoea[25].
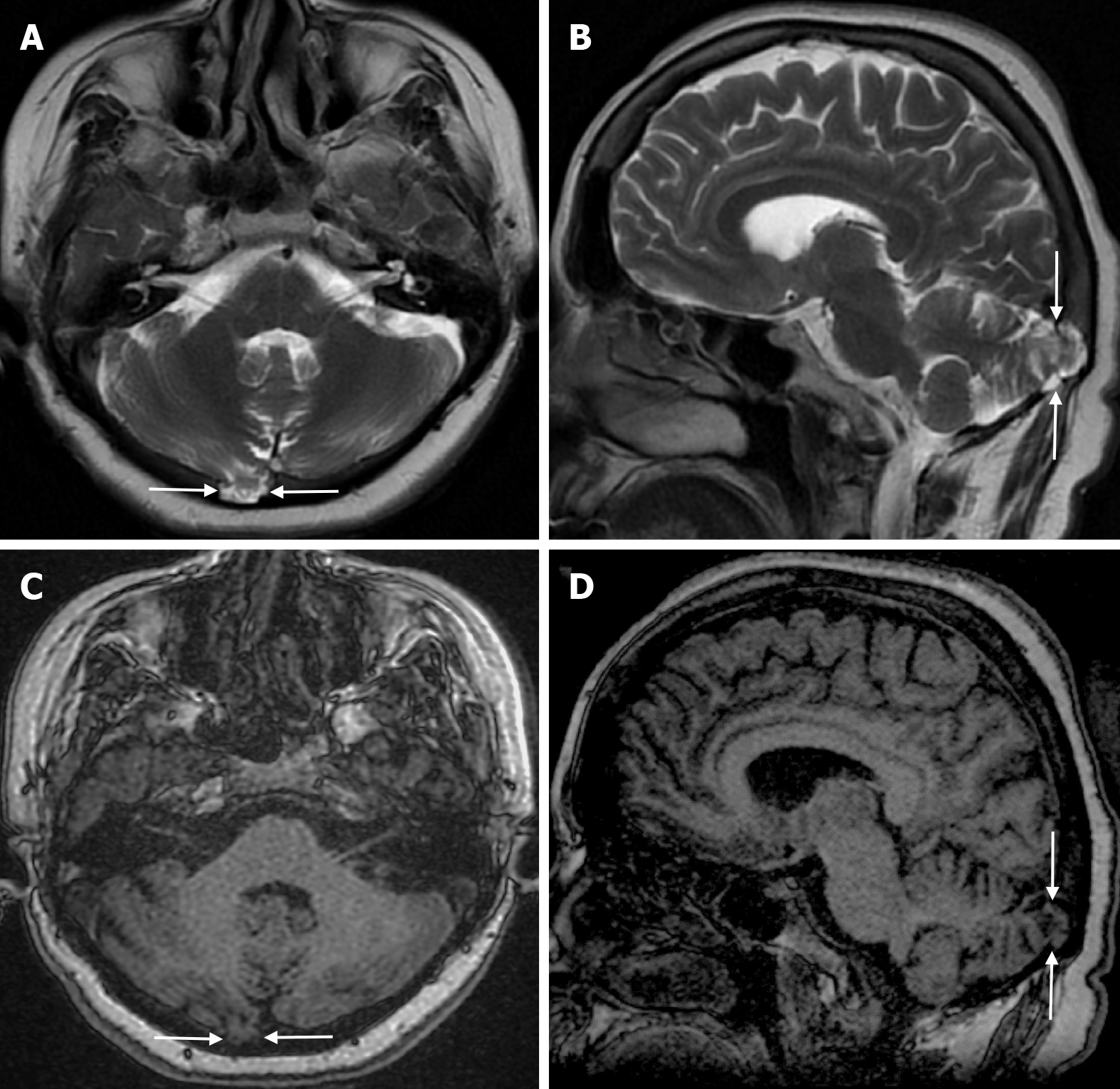
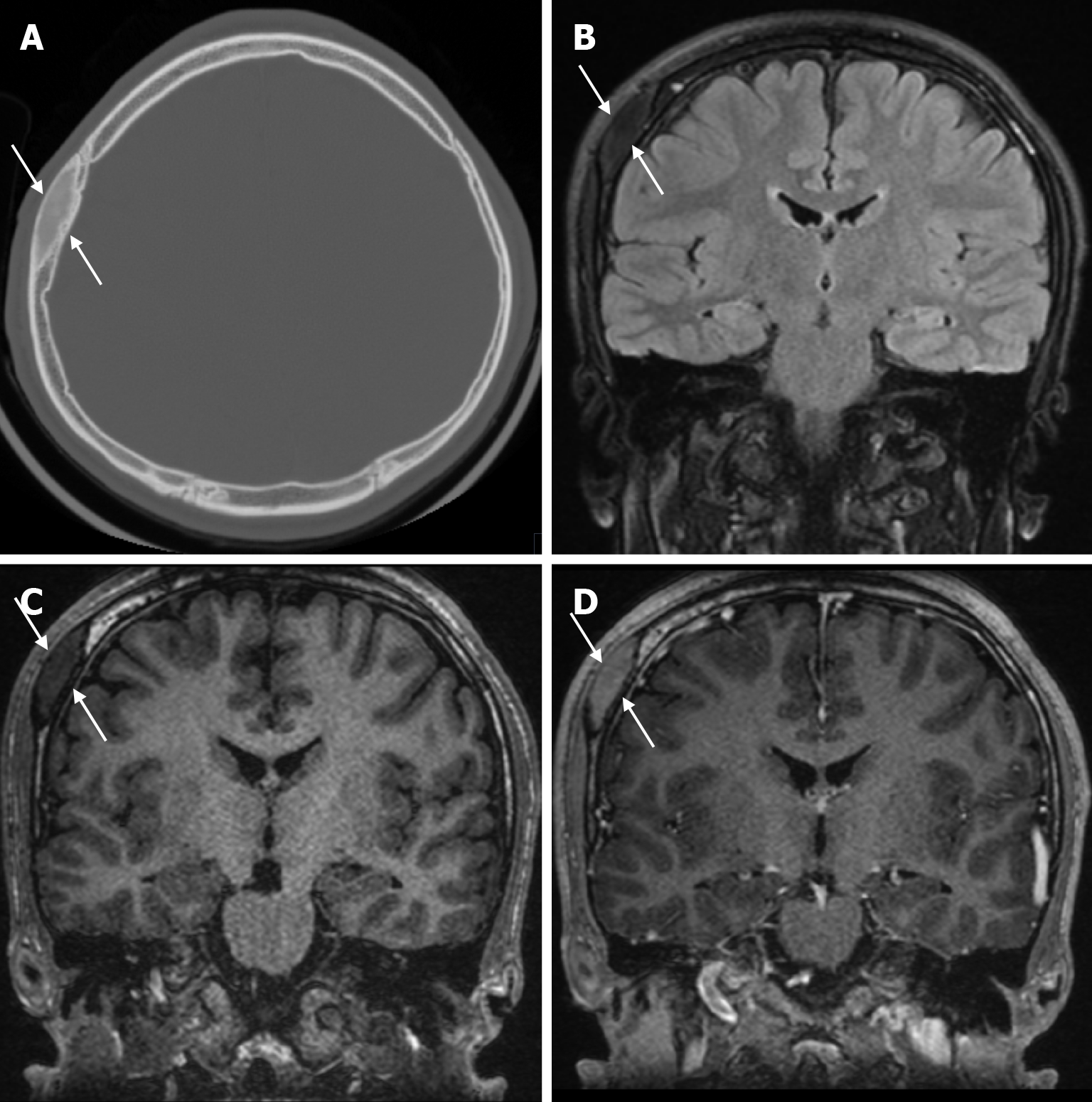
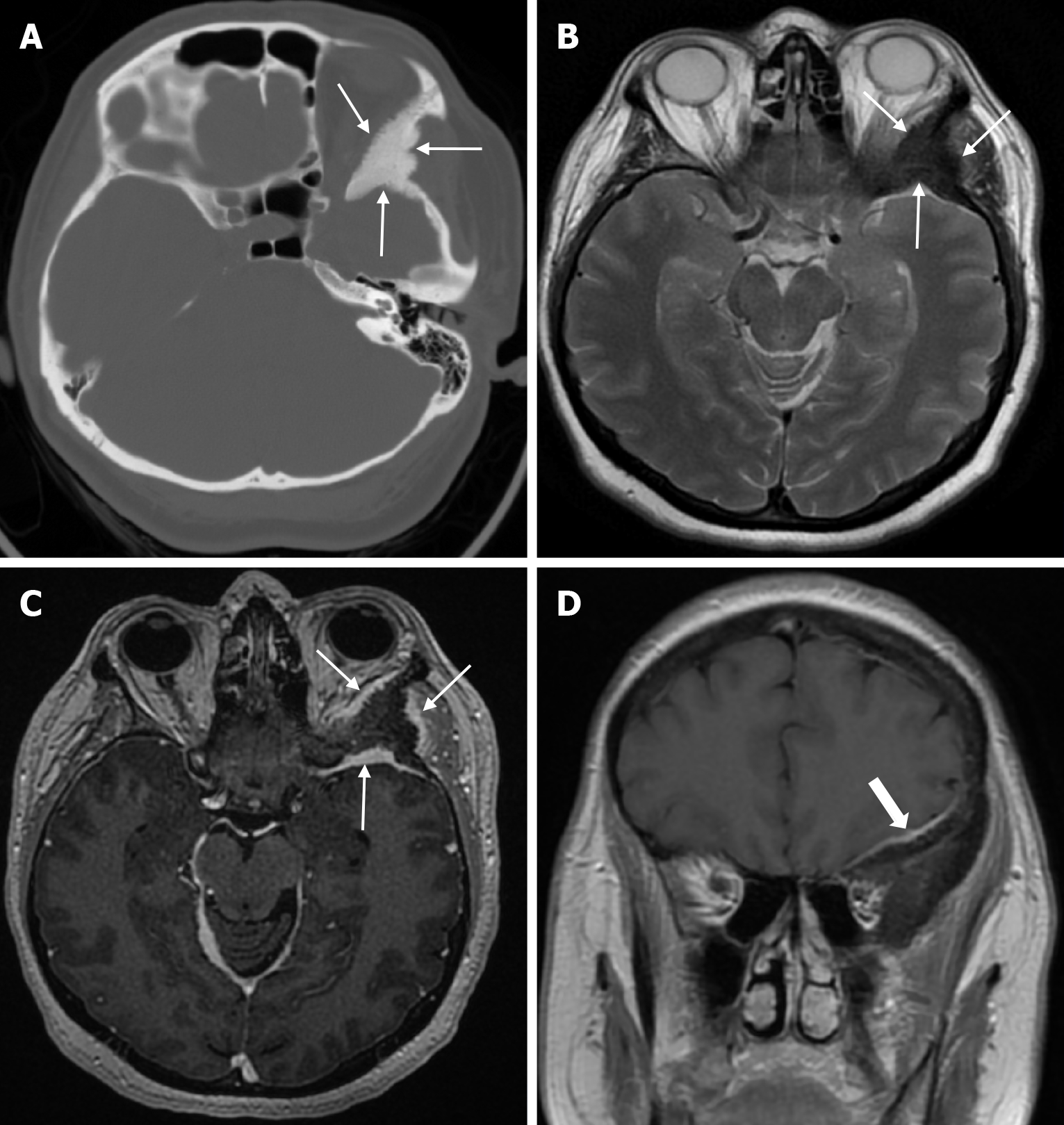
Atretic encephalocele is a midline scalp mass covered by skin containing meninges and neural remnants and/or degenerated brain tissue. In atretic encephaloceles, the brain tissue in the scalp degenerates and may remain attached to the dura mater by a fibrous stalk intracranially. Atretic cephaloceles may be associated with a persistent falcine vein characterized by a vertical embryonic position of the straight sinüs (Figure 6). Most atretic encephaloceles are parietal in location. On CT imaging, a subgaleal soft tissue mass may be seen in a “spinning top” configuration. CT imaging helps visualize bone defects, while MRI helps determine the contents of the encephalocele[3]. The lesions most commonly confused with atretic encephaloceles are sinus pericranii and dermoid cysts[25].
Sinus pericranii is a rare vascular anomaly with transosseous venous channels connecting the intracranial venous system to the extracranial veins[26]. It is usually congenital, but trauma is thought to be a predisposing factor. The congenital lesion has an endothelial lining, while the acquired lesion has a connective tissue lining. The male to female ratio is 2/1 and the condition can be seen in all age groups, but is usually diagnosed before the age of 30. Sinus pericranii is most commonly located near the midline. The frontal region is most commonly involved, followed by the parietal and occipital regions, while lateral localization is rare[27]. Sinus pericranii is often associated with other intracranial venous anomalies, such as developmental venous anomalies or venous vascular malformations. Affected patients typically present with a soft cutaneous mass that expands in the supine position or during the Valsalva manoeuvre and contracts on standing. Patients are usually asymptomatic, but headache, dizziness or nausea may occasionally occur. Sinus pericranii is classified as dominant (the majority of venous flow communicates with the sinus pericranii) or accessory (only a portion of intracranial venous flow communicates with the sinus pericranii). CT venography shows a calvarial defect and anomalous communication between the dural venous sinus and the extracranial veins (Figure 7). Thrombosis of the dominant sinus pericranii, where most intracranial venous drainage occurs, is associated with life-threatening complications such as venous congestion and/or infarction. Treatment is contraindicated in the dominant type. For accessory sinus pericranii, interventional (i.e. surgical or endovascular) treatment may be indicated to improve symptoms, prevent future traumatic haemorrhage and air embolism, and for cosmetic reasons[26]. Sinus pericranii should be suspected when a soft, fluctuant mass is detected near the intracranial sinus and can vary in size. MRI shows the relationship of the lesion to the underlying sinus, while direct injection of contrast medium through the wall of the mass shows rapid passage of contrast into the sinus[27]. Also, MRI shows variable signal intensity due to flow artefacts[26].
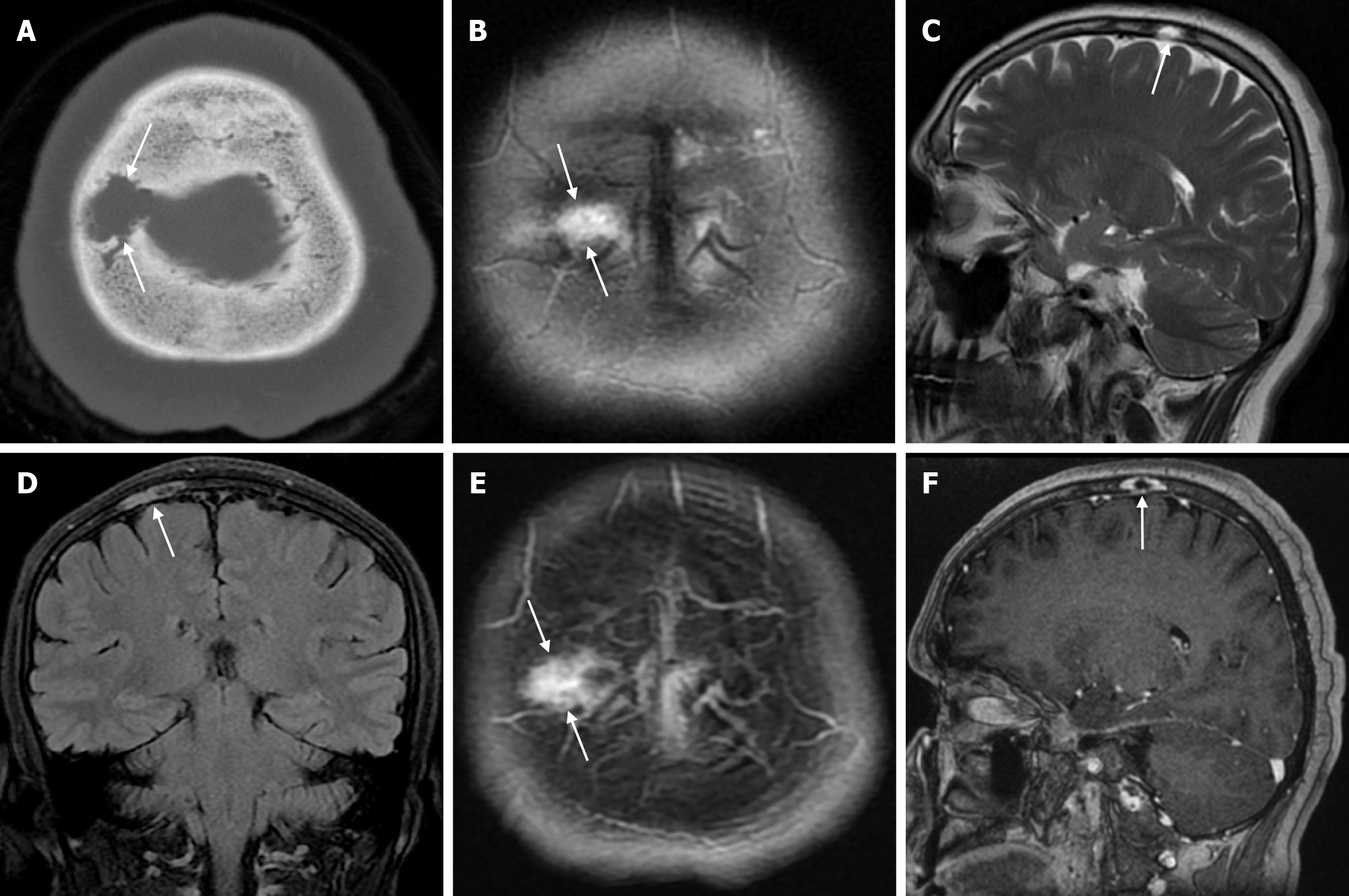
Aplasia cutis congenita is a rare condition characterized by localized congenital absence of skin. Aplasia cutis congenita rarely affects the trunk and limbs and may occur in isolation or as part of a heterogeneous group of syndromes. It is a severe cutaneous tissue deficiency that varies from absence of skin to full thickness defects extending deeper into bone and dura. Scalp lesions may be associated with complications such as haemorrhage, infection, thrombosis and seizures. The incidence of aplasia cutis in newborns is 0.5-1/10000; the female/male neonatal ratio is approximately 7/5. Most cases are sporadic, but genetic transmission (autosomal dominant and recessive inheritance) has been reported. In most cases, congenital aplasia cutis presents as a single lesion on the vertex lateral to the median line in the first days of life. The typical lesion is small (0.5-10 cm), well circumscribed, circular, oval, linear or stellate in appearance. When the skin lesion in aplasia cutis is larger than 10 cm, it may cause ulcerations that extend through the dermis, subcutaneous tissue, periosteum, and even the skull and dura. Large ulcerated lesions are usually inherited as an autosomal dominant trait. The hair collar sign (distorted hair growth around the scalp lesion) is a significant finding. The location of aplasia cutis congenita on the vertex may be partly explained by the fact that this is the area of maximum traction during rapid brain growth at 10 to 15 weeks of gestation. In the vast majority of cases, no complications are observed and simple wound care is sufficient. Complications that may worsen the prognosis such as haemorrhage, local infection, meningitis, sagittal sinus thrombosis, seizures and psychomotor retardation have been rarely reported[28]. A mortality rate of 20%-50% has been reported for aplasia cutis congenita. Pre-operative assessment includes history, physical examination, three-dimensional maxillofacial CT and brain MRI. Radiological examination includes evaluation of structures such as the brain and sagittal sinus, as well as the skull or dura defect in aplasia cutis congenita[29].
The external occipital protuberance is a normal anatomical structure that is the insertion site of the nuchal ligament on the posterior surface of the occipital bone at the level of the superior nuchal line. There are 3 types of external occipital protuberance depending on its shape: Type I, smooth shape; type II, crest-like shape; and type III, spine-like shape (Figure 8). Previous studies have shown that type I is more common in women and type III in men. Type III may occasionally present as a subcutaneous pseudotumor of the scalp. An external occipital protuberance (occipital spur) is defined as a bony prominence and is generally considered to be an anatomical variant. Although this variant is often asymptomatic, it can sometimes be painful enough to require surgical excision or, rarely, fracture after trauma. Recent studies have reported that the prevalence of external occipital protuberance is rapidly increasing in the young adult population, with a possible role for mechanical factors such as prolonged forward head extension. CT is more sensitive than plain radiography in detecting bone changes, and CT also offers the possibility of three-dimensional imaging[30].
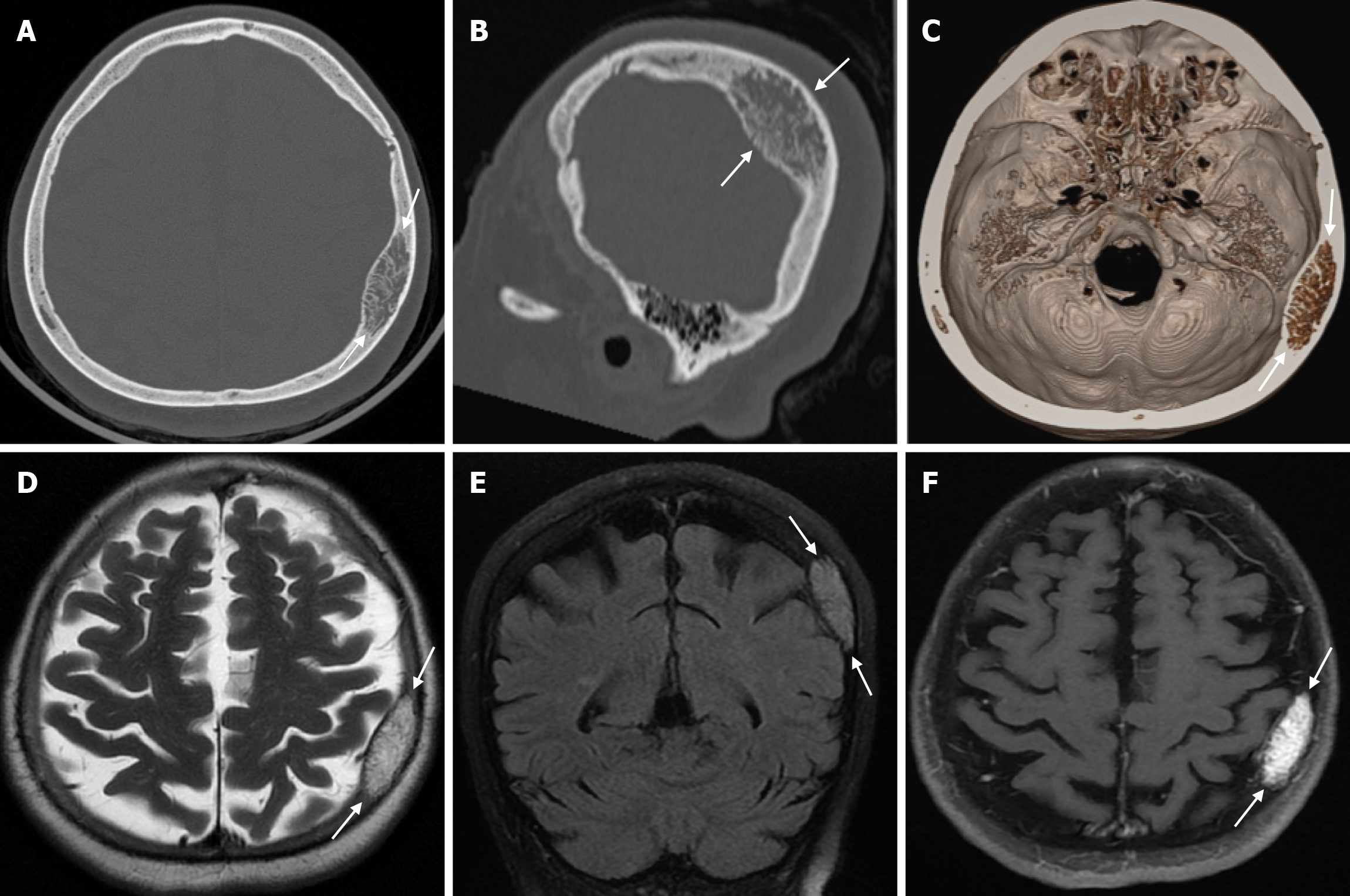
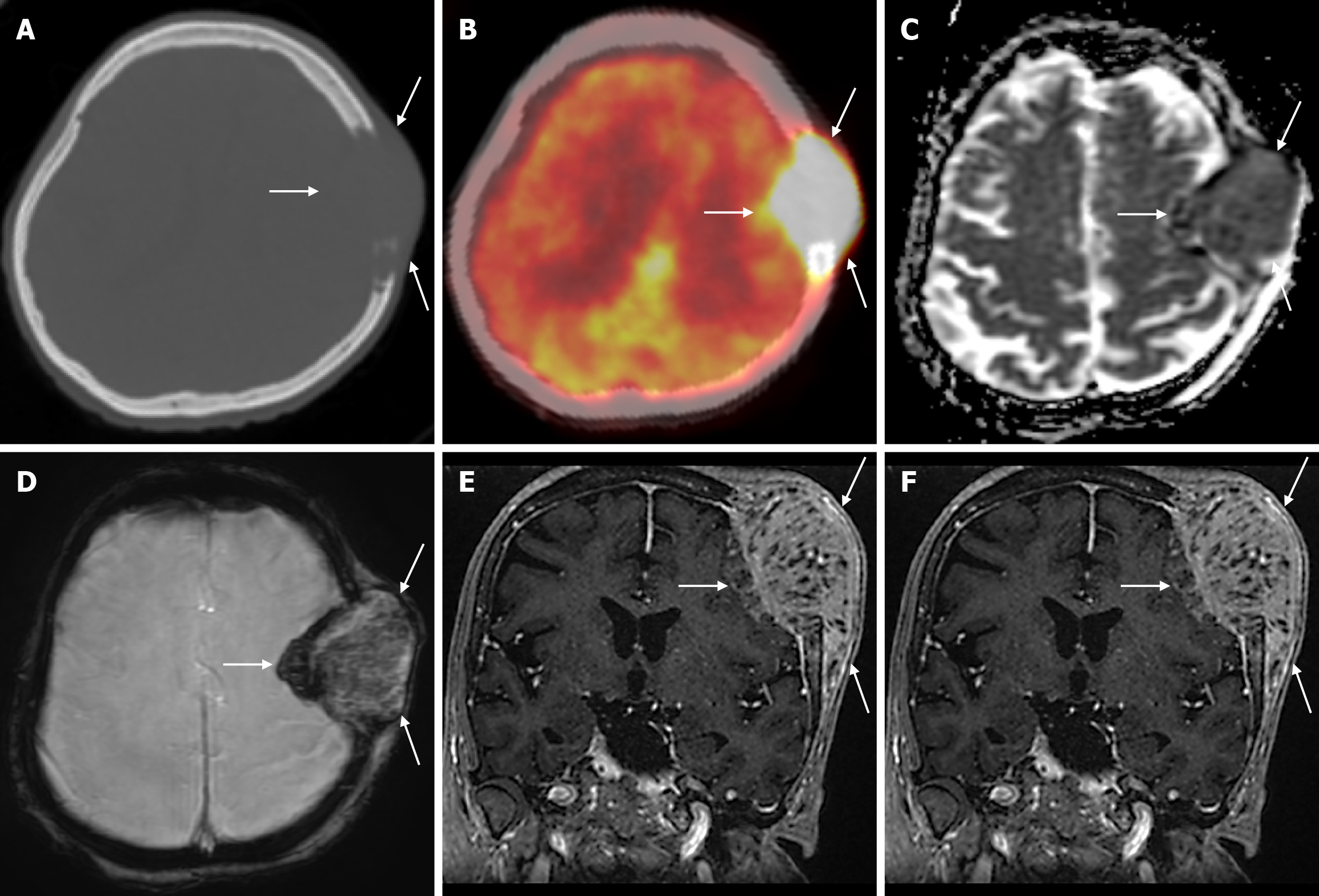
Burr holes appear on CT or plain radiography as round calvarial defects that cross the inner and outer table (Figure 9). They are usually small holes made in the calvarium with a surgical drill to insert a device (ventricular drainage or shunt catheter, intracranial pressure monitor, deep brain stimulator electrode or endoscope), to drain a chronic subdural hematoma, to provide access for a stereotactic brain biopsy or to prepare for the creation of a craniotomy flap. CT is the main imaging modality used to assess for potential complications due to its advantages of being fast, easily accessible in most hospitals, and compatible with monitoring equipment and implanted devices. In the early postoperative period, a normal internal fluid level may be seen in the burr holes, and occasionally a fluid collection or hematoma may be seen in the hole or in the adjacent subgaleal or subdural spaces. On contrast enhanced MRI, only the edges of the burr holes may be enhanced, or the enhancement may be more diffuse and fill the defect. This pattern of enhancement is normal, but burr holes can be mistaken for a bony lesion. The creation of a burr hole usually results in a small indentation of the overlying soft tissues, although not a problem in itself, which can have an undesirable cosmetic effect. Burr holes can be filled with bone allograft dust or fibrin glue and sometimes covered with a titanium plate. During the burr hole procedure, the burr may penetrate the skull, puncturing the dura mater and causing intracranial bleeding. The defect created by this penetration can be seen as an area of high signal intensity edema beneath the burr holes on T2-weighted MRI, known as the mushroom sign[31].
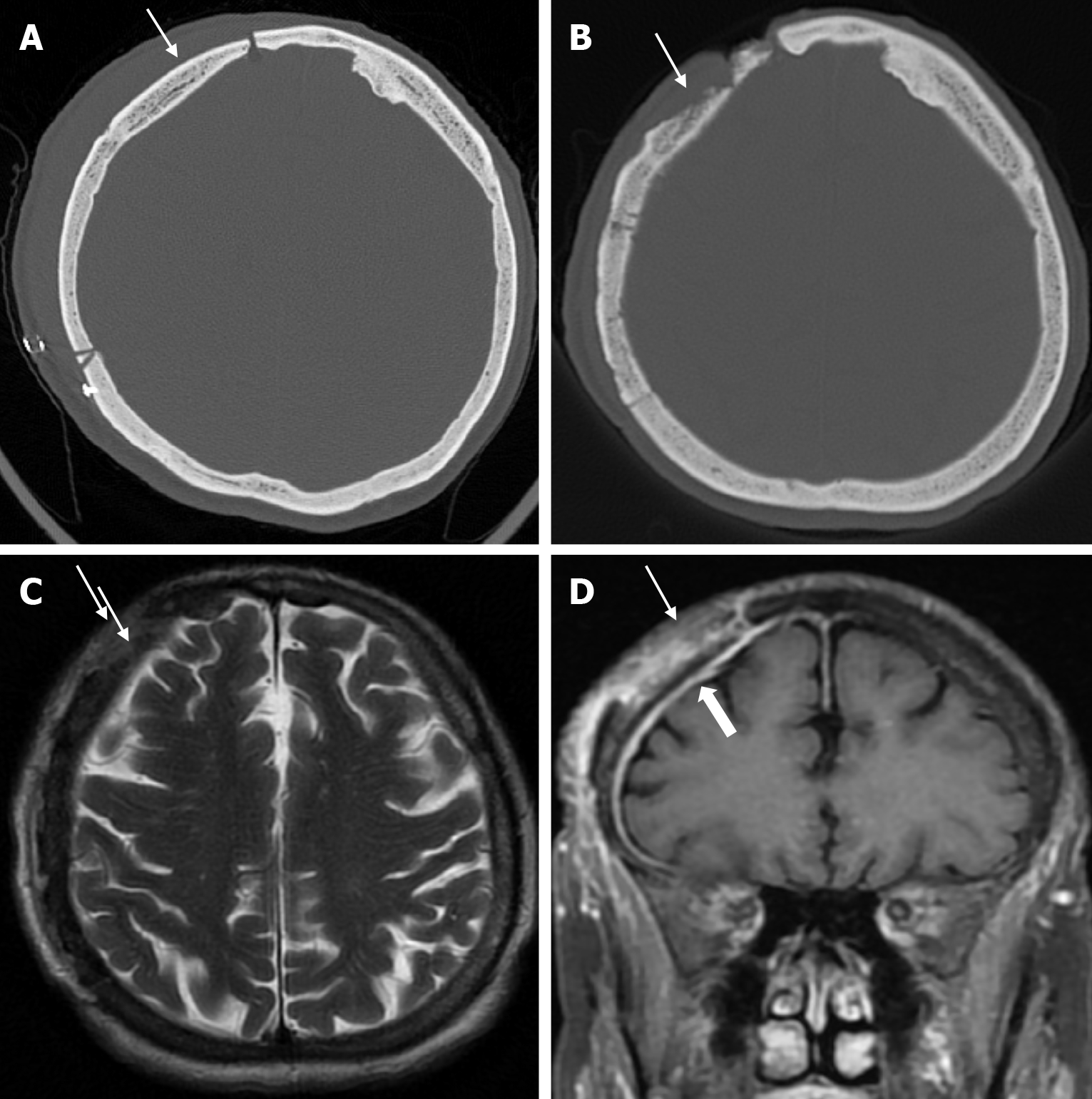
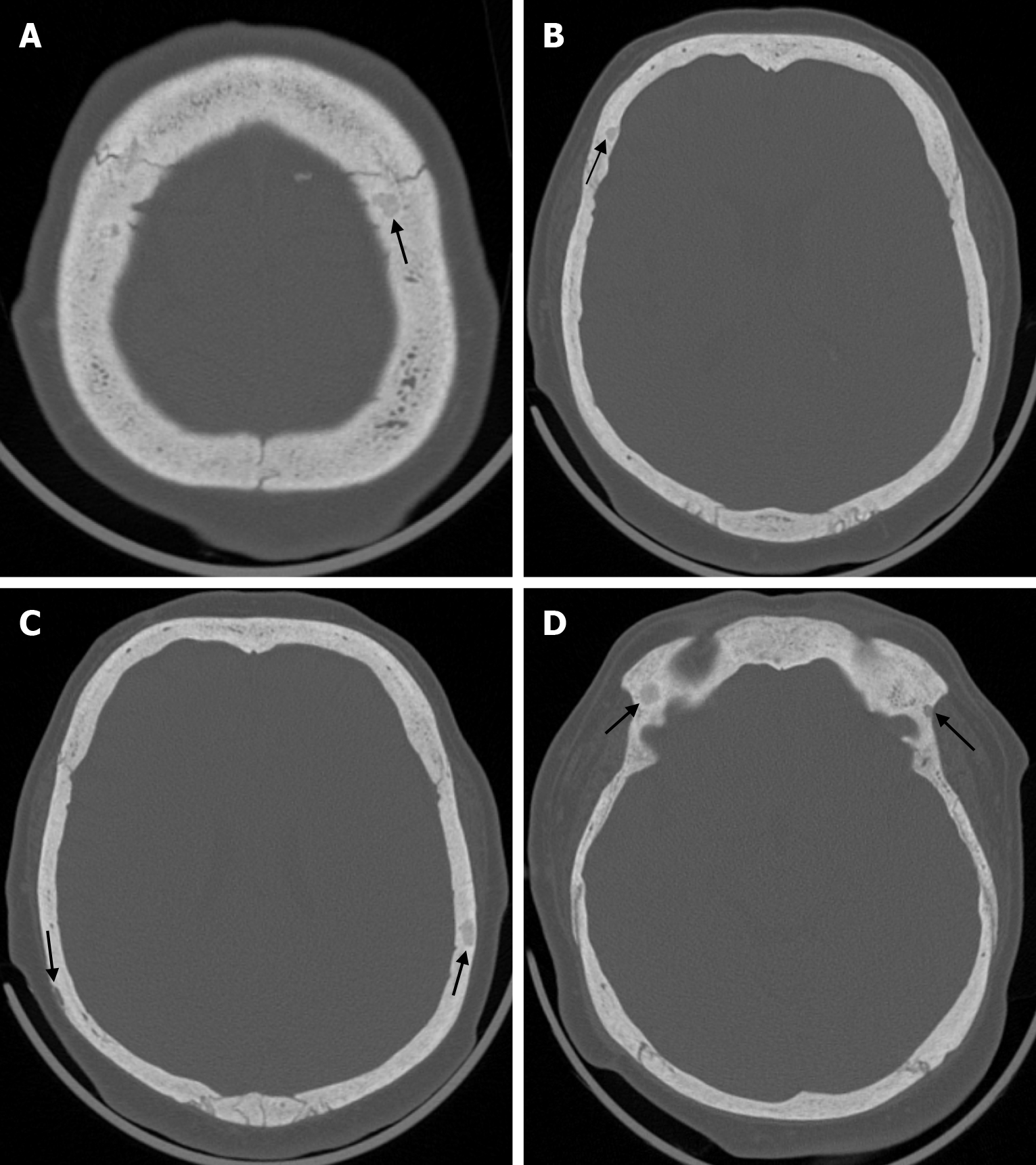
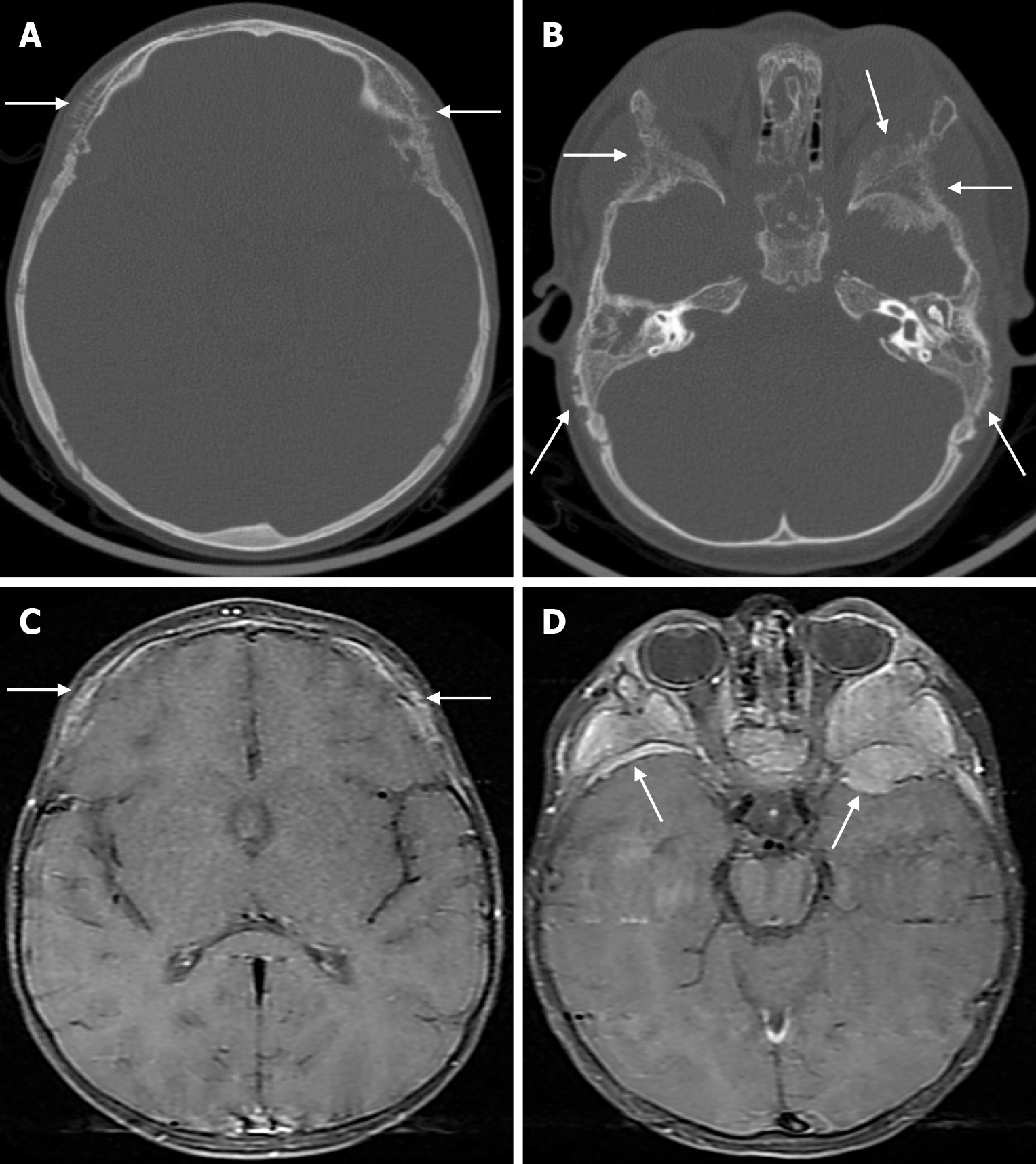
Osteonecrosis of the bone flap after autologous cranioplasty following decompressive craniectomy is a pathology seen in up to 35.1% of cases in adults and is aseptic in most cases. The incidence of bone flap resorption is much higher in the paediatric population, with rates of up to 81.1%. Several potential risk factors have been identified, including large bone flap size, the presence of a shunt system, and a long interval between craniectomy and cranioplasty[32]. In addition, several factors have been implicated in the development of flap osteonecrosis, including young age, multiple fractures, incompatibility between the replanted flap and the adjacent bone, and incorrect cryopreservation storage. Flap osteonecrosis is divided into two distinct categories based on CT characteristics. The more common type 1 flap os
Congenital depression of the fetal skull is a rare condition, occurring in 1-2.5/10000 live births. This depression can be associated with a fracture. However, depression without a fracture is more common. It can range from a small calvarial depression to a significant depression. Causes of congenital skull deformity include compression of the soft fetal skull in utero by a bony prominence of the maternal pelvis or spine, an uterine fibroid, a fetal limb, or a body part of a twin. Focal cortical atrophy, scarring and cerebro-meningeal adhesions may occur as a result of skull deformation. Although brain damage is rare, the risk increases if the depth of bony compression exceeds 5 mm. CT helps to identify fractures, underlying hematomas and cortical compression. Spontaneous recovery may occur because the skull of the newborn and infant is thin and flexible and therefore susceptible to remodelling. Treatment options range from conservative management to non-surgical intervention and surgical correction. In general, larger and deeper depressions are treated more aggressively[34].
Leptomeningeal cysts can develop as a rare complication of linear skull fractures in children. The incidence is reported in the literature to be between 0.05% and 0.1%. They are usually seen in children under the age of 3. These cysts most commonly occur in the cranial convexity but can also be seen in the posterior fossa and orbital roof. This complication develops as a result of dural tear associated with skull fracture. Laceration of the dura mater leads to herniation of the leptomeninges and filling with CSF. The continuous pulsatile pressure of the CSF and the expansion of the cyst cause resorption of the adjacent bone, erosion of the bone edges and expansion of the skull fracture. The fracture site, together with the thickness of the soft tissues, can be seen on plain radiography. While bone fractures can be better demonstrated with CT imaging, MRI is more useful in distinguishing leptomeningeal cysts from other pathologies. Leptomeningeal cysts are isointense with cerebrospinal fluid on both T1-weighted and T2-weighted images (Figure 10). Associated pathologies such as subdural fluid collections, hematomas, encephalomalacia, and ventricular dilatation may also be seen on CT imaging and MRI. Early diagnosis is important because of the risk of neurological deterioration and seizures, and treatment includes dural repair and cranioplasty[35].
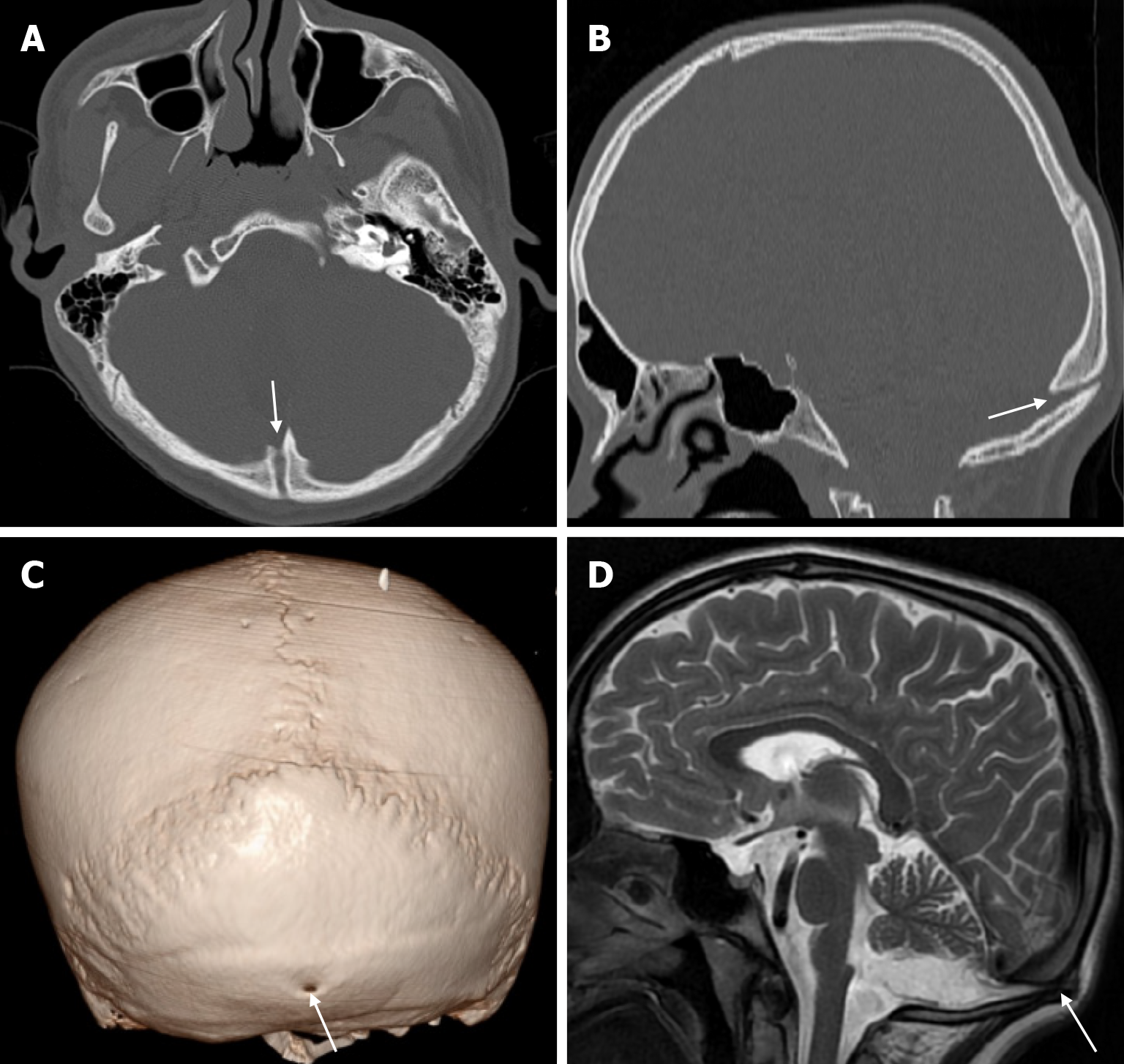
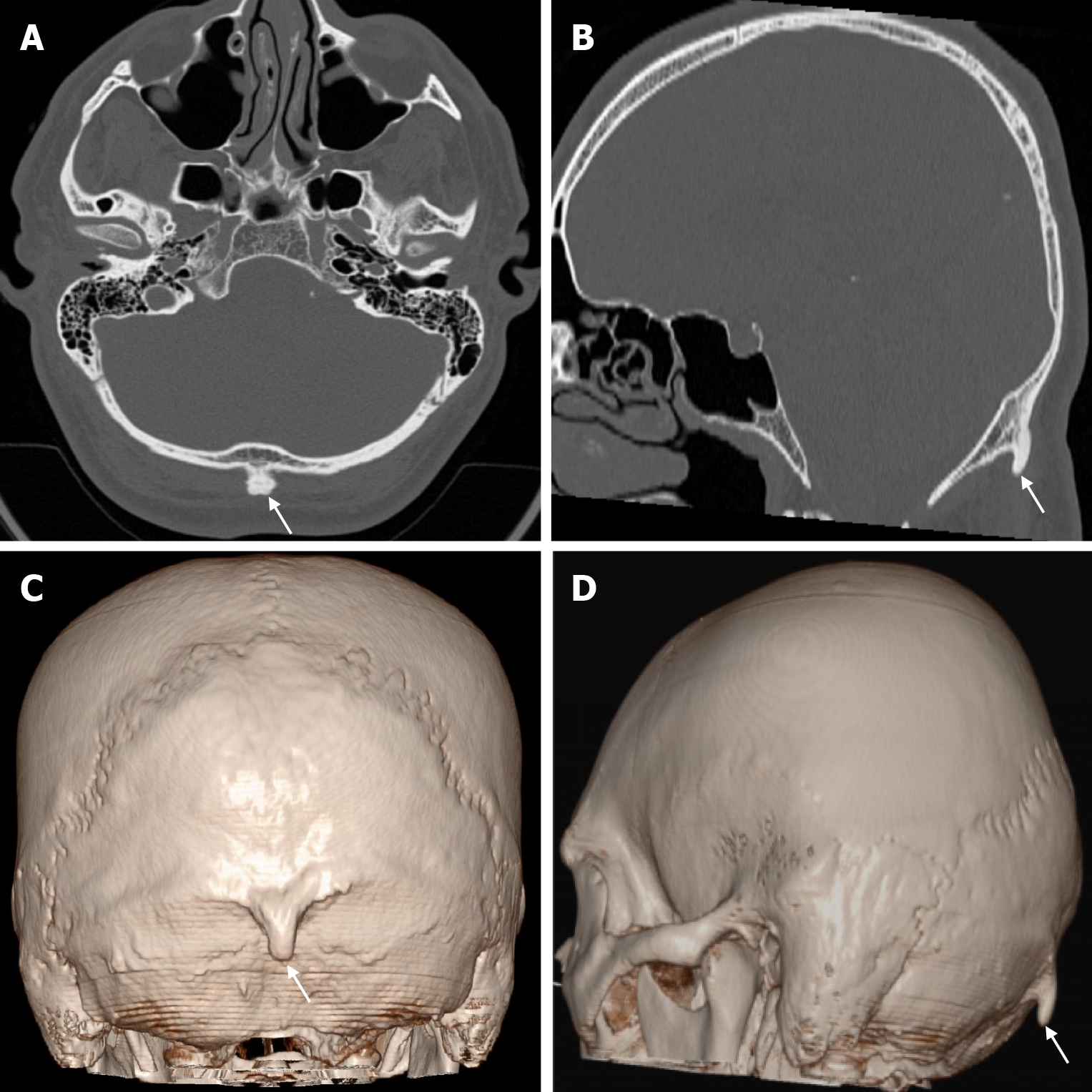
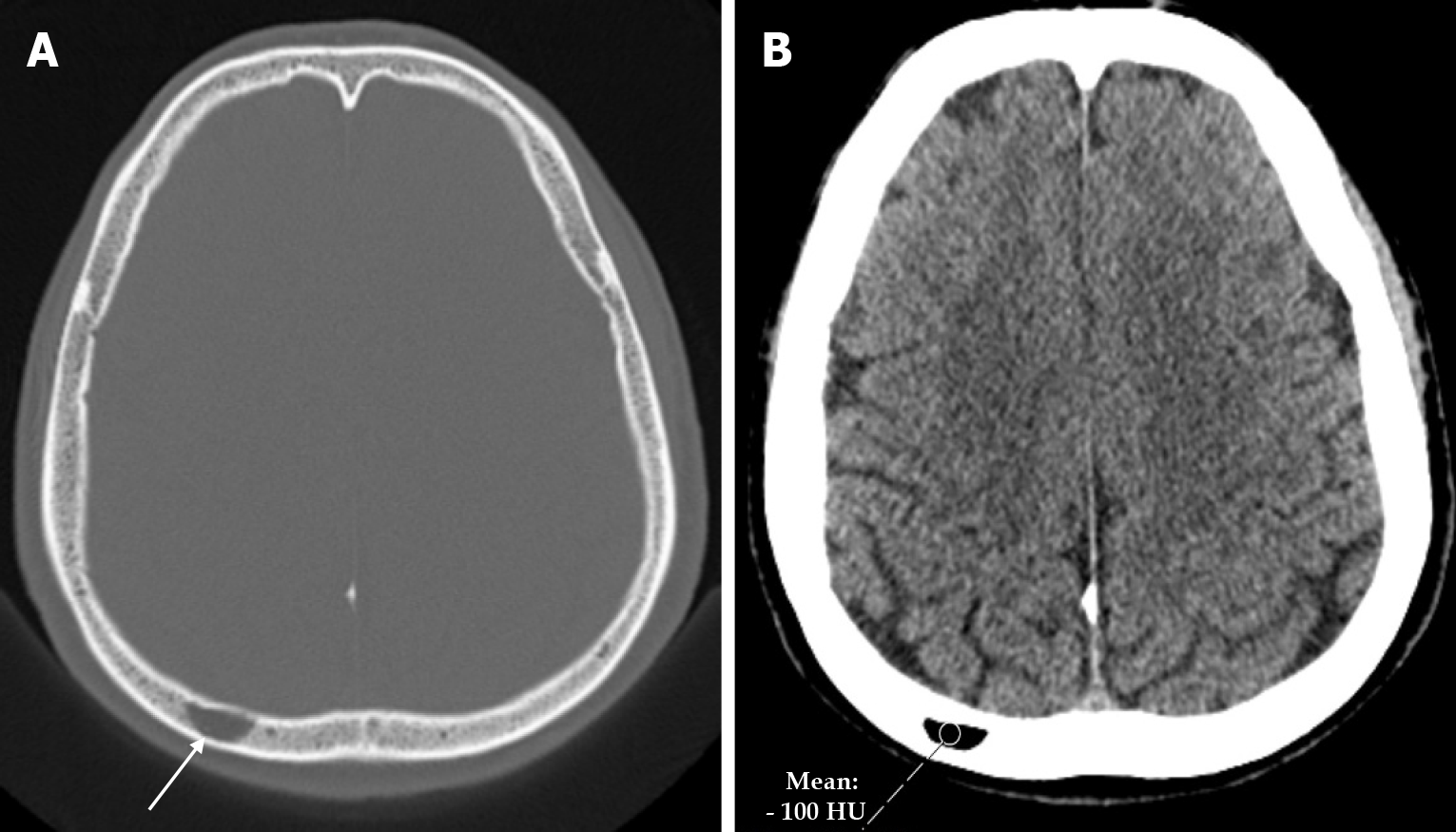
Cephalohematoma results from trauma during birth that lifts the scalp from the skull bone, including the pericranium, and ruptures the delicate vessels that run from the bone to the scalp. The most common location of a cephalohematoma is over the parietal and occipital bones and does not cross suture lines. Most cephalohematomas resolve spontaneously within the first month. In cases where the hematoma is not resorbed, a calcified cephalohematoma occurs as a result of progressive subpericranial osteogenesis. The incidence of cephalohematoma is 0.2%-3% of all births and calcification of the hematoma is seen in 3%-5% of all cephalohematomas. If the hematoma becomes large, it may collapse the flexible newborn skull. A calcified hematoma is seen on plain radiography as an expanding lesion surrounded by a radiopaque rim and variable thinning of the underlying calvarium, whereas on CT it is smooth, homogeneous, hypodense, surrounded by bone and no contrast enhancement[36]. Approximately 15% of hematomas are bilateral. It is important to distinguish a cephalohematoma from a subgaleal hematoma. A subgaleal hematoma may cross suture lines and cause hypovolemia, jaundice and coagulopathy. Skull fractures may be associated with a cephalohematoma. Linear skull fractures occur unilaterally in 5% of patients with cephalohematoma and bilaterally in 18%[37]. Calcified cephalohematoma is classified as type 1 or 2 depending on the contour of the inner table. A normally contoured inner table is type 1, whereas a depressed inner table is a type 2 calcified cephalohematoma[36].
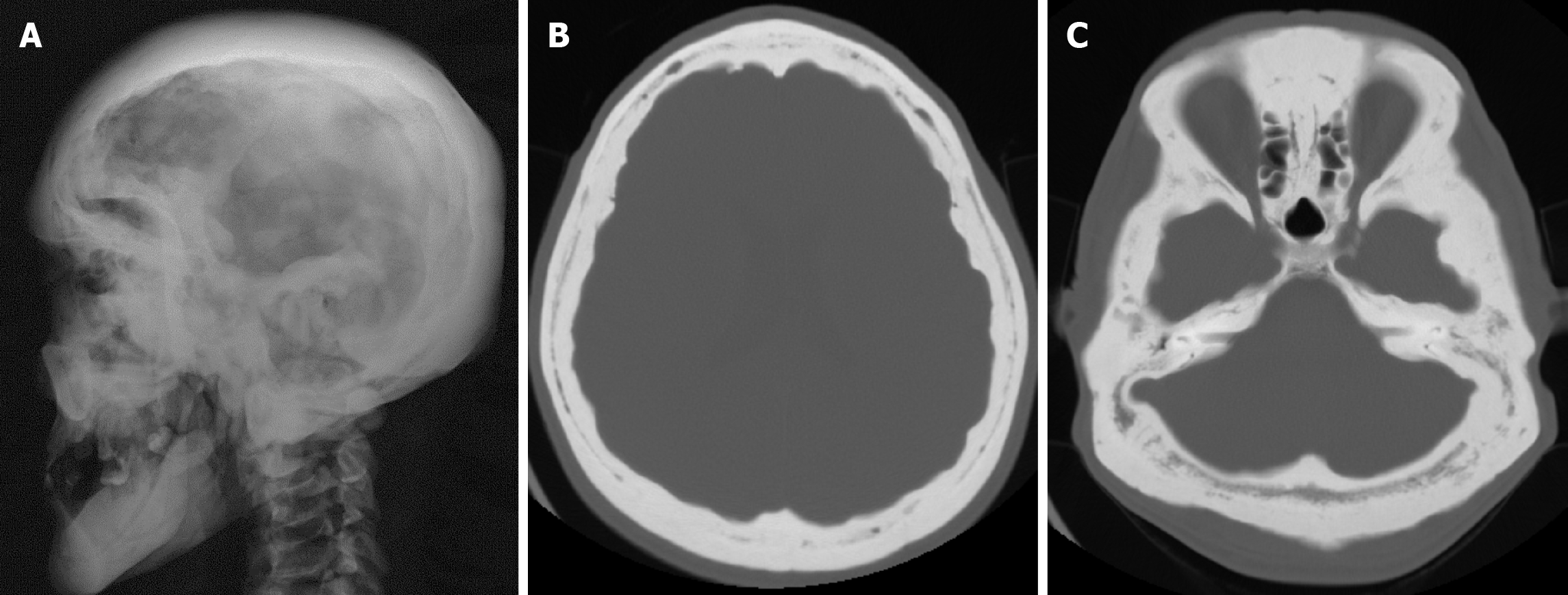
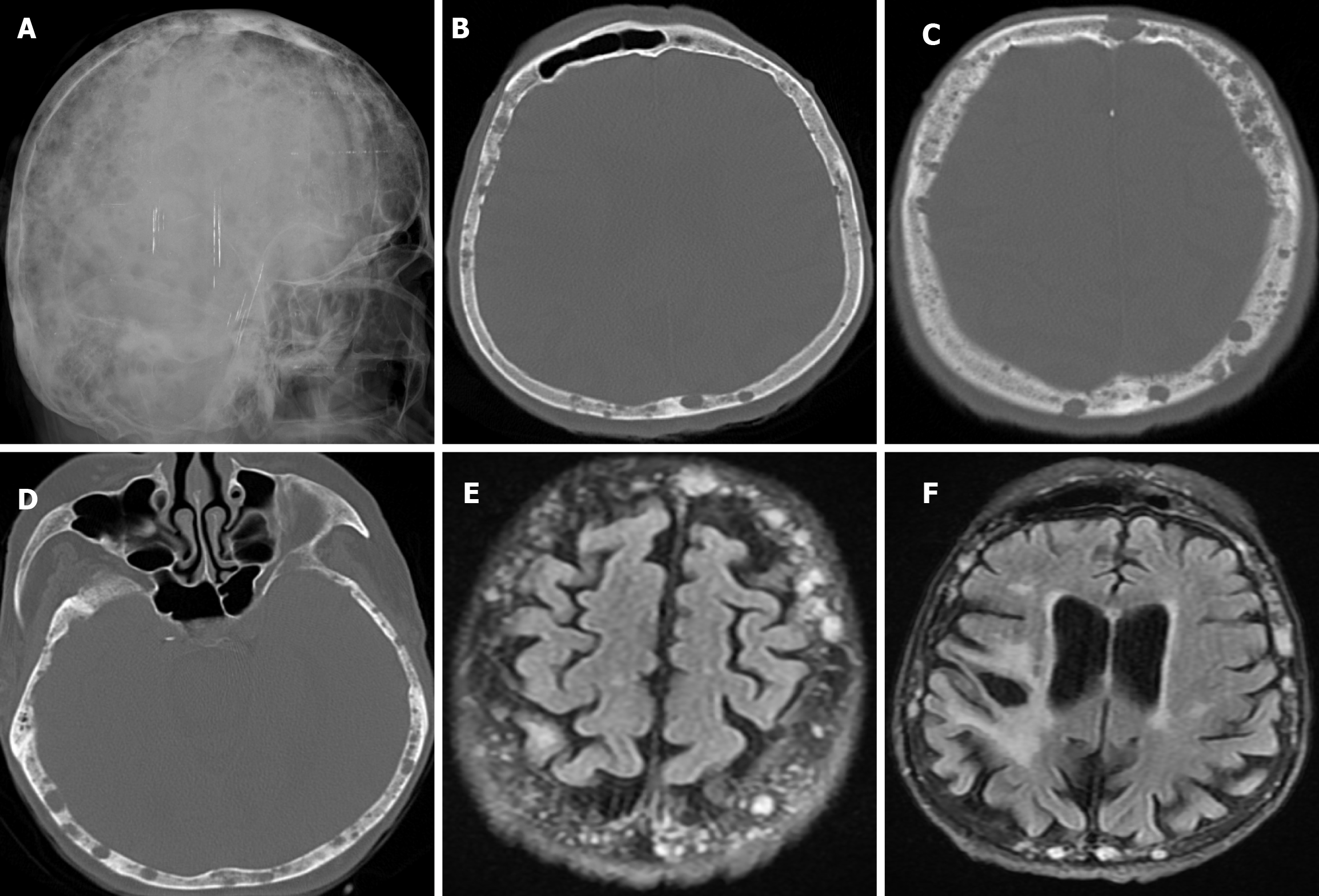
Hyperostosis frontalis interna is an overgrowth of the endocranial surface of the inner table of the skull's frontal bone and has been reported in 5%-12% of the general population[38]. Hyperostosis frontalis interna may be associated with many other syndromes or diseases such as Stewart-Morel, Klippel-Trenaunay-Weber, Troell-Junet, Frölich and Paget’s disease. However, it is recognized that it may also occur as an independent entity[39]. Hyperostosis frontalis interna is often found incidentally on plain radiography, CT or MRI. Hyperostosis frontalis interna is most commonly seen in postmenopausal women. It is also associated with hormonal imbalance, obesity, a history of headaches, and neurocognitive degenerative diseases. Advanced age, female sex, long-term estrogen stimulation and high leptin levels may also play a role. Irregular bone thickening is usually confined to the frontal bone, but may extend to the anterior parietal and temporal bones[40]. Radiographic findings include bilateral symmetrical calvarial thickening of the frontal bone (Figure 11), which may extend to the parietal bones. Calvarial thickening may be nodular or sessile, and focal or diffuse[39]. Contrast enhancement may be present in areas of hyperostosis frontalis interna and its long-term stability indicates a benign process[41]. In order to accurately determine the severity of hyperostosis frontalis interna, four different classifications have been made according to the morphological and histopathological features of the endocranium. Type A: single or multiple isolated bony prominences measuring less than 10 mm in diameter, located on the endocranial surface of the frontal bone. Type B: nodular bone formations covering less than 25% of the frontal bone. Type C: nodular bone formations covering up to 50% of the frontal bone. Type D: continuous nodular bone growth covering more than 50% of the frontal endocranium[37]. Differential diagnoses include osseous metastatic disease, Paget's disease and osteomatosis (Leontiasis Ossea or Virchow's disease)[40,41].
Bilateral parietal thinning has a prevalence of 0.25%-0.8% and is a rare, slowly progressive condition in adults. It is thought to be caused by a non-progressive congenital dysplasia of the diploe. There are opinions that the thinning may be due to an acquired and progressive disease, but it is not an anatomical variant or congenital diploe dysplasia. Parietal thinning is more common in females than males. The area between the sagittal suture and the parietal ridge is the characteristic region of bilateral parietal thinning. The radiological features are symmetrical thinning of the bilateral parietal bones, including the outer table and diploe, and a comb-like appearance. The inner table is usually intact. Bone scintigraphy shows symmetrical, well-defined photon defects in both parietal regions. Cranioplasty may be required in cases of progressive bone thinning to prevent the brain from being exposed to atmospheric pressure[42].
Gorham’s disease (Gorham-Stout syndrome, massive osteolysis of Gorham, phantom bone disease, disappearing bone disease, and vanishing bone disease) is a rare monostotic or polyostotic disorder of unknown cause characterized by osteoclast activation and bone resorption as a result of non-malignant intraosseous proliferation of hemangiomatous or lymphangiomatous tissue. Gorham’s disease is an inherited disorder with no gender bias and most patients are younger than 40 years of age. The calvarium is one of the least common sites of involvement. The initial radiological findings of Gorham's disease are radiolucent foci in intramedullary or subcortical regions resembling osteoporosis. This is followed by progressive bony deformity, cortical destruction and eventual loss of part of the skull. Bone destruction may continue for years and eventually stabilise. Tc-99m methylene diphosphate (Tc-99m MDP) bone scintigraphy may show increased uptake of radiopharmaceuticals on initial images and then an area of decreased uptake corresponding to an area of decreased bone. CT allows determination of the extent of osteolysis and three-dimensional imaging is useful in surgical planning for skull reconstruction. MRI allows visualisation of the vascular components of the lesion with a reticular enhancement pattern, along with edema-like signal abnormalities in the bone defect or involved bones. Differential diagnoses include metastasis, osteomyelitis, hereditary multicentric osteolysis, essential osteolysis with nephropathy and rheumatoid arthritis[43].
Parry-Romberg syndrome is a sporadic and rare, more common in females, insidious and self-limited progressive facial hemiatrophy with a slight left-sided predilection. It typically affects the skin and subcutaneous connective tissue. It may affect the underlying muscle, cartilage and bone structures at a later stage, with or without neurological symptoms developing. Parry-Romberg syndrome typically occurs first in children and young adults and progresses slowly with a highly variable course between 2 and 20 years, eventually reaching a "burn-out" phase and stabilising for no apparent reason, causing hemiatrophy. The exact etiology of this syndrome is unknown, but possible causes include infection, sympathetic cervical ganglion dysfunction, trauma, abnormal embryogenesis, and vasculopathy or autoimmune and inflammatory mechanisms. Affected areas typically begin in the maxillary or periorbital region and may extend to varying degrees to the forehead, perioral region, teeth, jaw, and neck. Neurological symptoms occur in 15%-20% of patients. The most common symptoms are facial pain, ipsilateral headaches, and seizures, which may be difficult to treat. Other neurological symptoms include trigeminal neuritis, facial paresthesia, cranial nerve dysfunction, hemiparesis, and cognitive impairment. Ophthalmological symptoms occur in 10% to 35% of patients and usually affect the ipsilateral orbit. Enophthalmos due to retrobulbar fat atrophy is common and other orbital abnormalities include uveitis, retinal or optic nerve changes. Common facial imaging findings include varying degrees of hemiatrophy with obliteration of fat planes, ipsilateral deviation of the aerodigestive system, and enophthalmos due to loss of retrobulbar fat. Intracranially, ipsilateral linear or discrete subcortical calcifications in the frontal lobe, white matter hypoattenuation on CT corresponding to hyperintense T2 signal intensity on MRI, and ipsilateral focal or hemispheric brain atrophy are the most common findings. These findings typically occur ipsilaterally on the affected side of the face and may worsen with disease progression. Differential diagnoses include Rasmussen encephalitis, hemifacial microsomia (first and second branchial arch syndrome), Goldenhar syndrome, partial lipodystrophy (Barraque-Simon syndrome) and silent sinus syndrome[44].
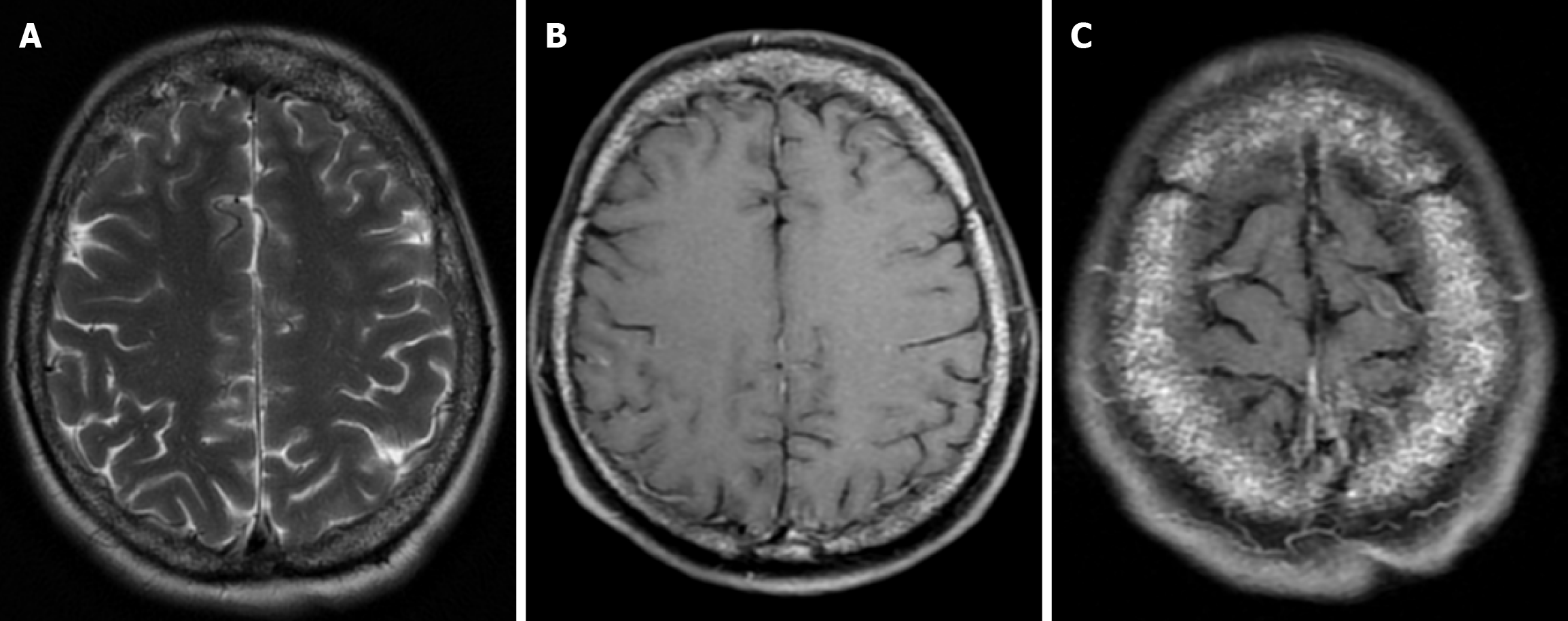
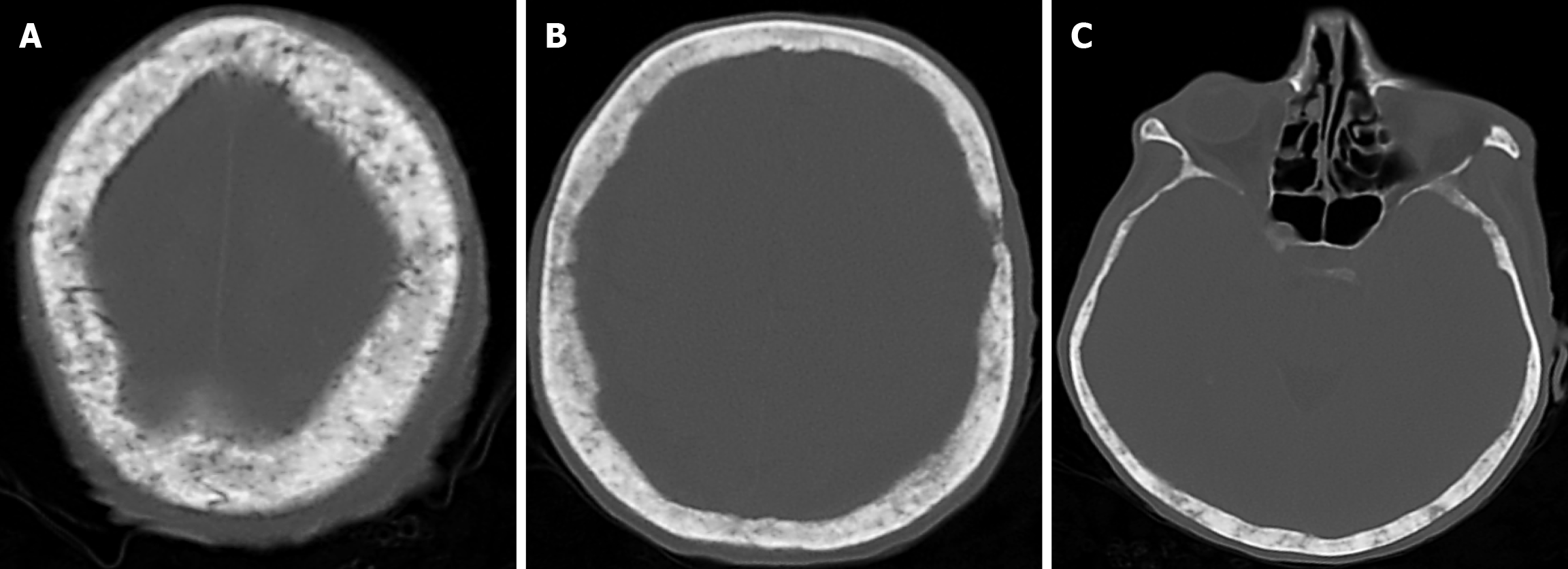
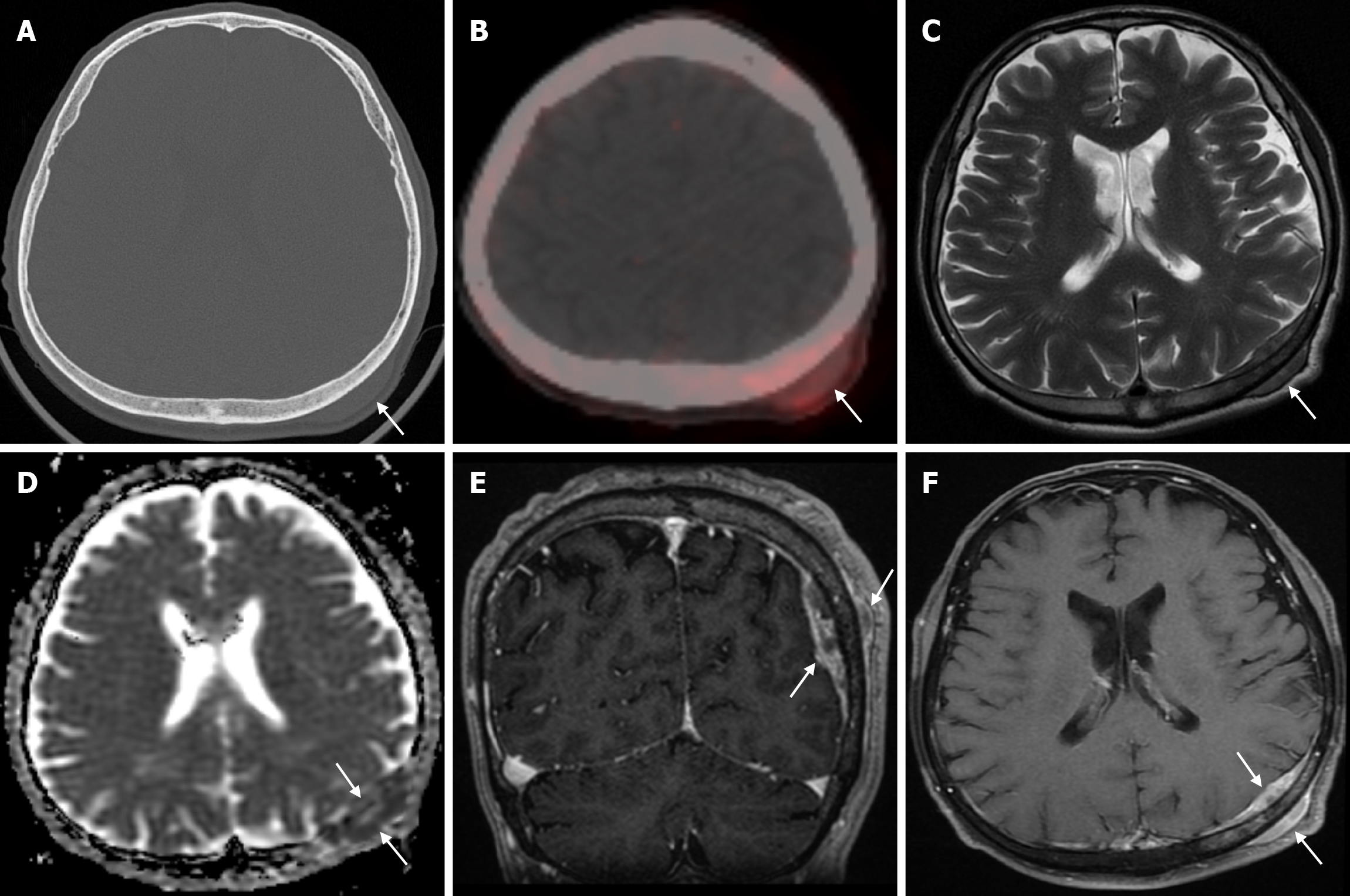
The prevalence of cranial osteomyelitis is 1.5% of all osteomyelitis cases, with a mortality rate of 20%-40%[45]. In developed countries, postoperative craniotomy infections are the most common cause of osteomyelitis, whereas in developing countries, sinusitis and scalp infections are the most common causes. It may occur as a complication of hematogenous spread or trauma. CT findings in the acute phase of osteomyelitis include areas of rarefaction and loss of diploic bone trabeculae; demineralization, erosion or thinning of the cortical bone table; and extracranial and subperiosteal abscesses. Diploic bone sclerosis and cortical bone thickening, radiolucent areas, and cortical disruption may occur in the chronic phase. Sequestration occurs as a result of the destruction of the skull base. On MRI may reveal an intracranial extension, particularly an epidural abscess and/or a subdural empyema. MRI findings in the acute phase of osteomyelitis include inflammatory replacement of diploic bone fat, widening of the diploic space, thinning of the skull base, increased signal intensity on T2-weighted images and contrast enhancement on T1-weighted images (Figure 12). In the chronic phase, sequestration, contrast enhancement in soft tissues and dura, and destruction of cortical bone fragments are seen. Bone scans using technetium-99m, gallium-67 citrate, single-photon emission computed tomography (SPECT), PET or PET-CT scans with 18F-fluorodeoxyglucose are highly effective in detecting cranial bone infections[46].
Sarcoidosis is a systemic inflammatory disease affecting multiple organs, most commonly the lungs, skin, and eyes. This disease is histopathologically characterized by the presence of non-caseating granulomas in the tissues[9]. Bone invol
Langerhans cell histiocytosis is a multisystem disorder of unknown cause characterized by abnormal proliferation of Langerhans cells. Langerhans cell histiocytosis most commonly affects children and young adults, especially males. Lesions are predominantly in the axial skeleton and often involve the calvarium.
The parietal bone is most commonly involved in the calvarium, followed by the frontal bone. Clinical diagnosis is usually made after palpation of the calvarial mass, and sometimes there may be pain or headache over the mass. The typical finding of Langerhans cell histiocytosis is a round and punched-out lytic lesion. A double-contoured appearance due to irregular destruction of the two tables on plain radiography is highly suggestive of Langerhans cell histiocytosis. On CT, sharply demarcated punched-out lytic lesions involving all three layers of the calvarium are seen in the intradiploic space, with no sclerotic margins or periosteal reaction. The lesion may also appear as beveled edges on CT due to asymmetric involvement of the inner and outer tables. As the lesion regresses, inflammatory cell infiltrates replace the normal bone, and a sclerotic rim can be seen. Granulomas appear slightly dense compared to the grey matter on CT. Residual bone density (button sequestrum) may be seen in the center of the lytic lesion. Lytic lesions may coalesce and a geographic pattern may be seen. MRI is a highly sensitive modality for detecting bone marrow involvement and soft tissue components that may show subgaleal or epidural extension. On MRI, they are hypointense compared to grey matter on T1-weighted images, heterogeneous, hyperintense on T2-weighted images, and show significant contrast enhancement. Dural and galleal reactive contrast enhancement is often seen. Langerhans cell histiocytosis has low apparent diffusion coefficient (ADC) values due to its high cellularity. Healing of lesions, whether spontaneous or with treatment, is associated with centripetal bone formation leading to the progressive disappearance of lesions[7,49-51]. Furthermore, although bone lesions of Langerhans cell histiocytosis usually have typical radiographic features, 18F-FDG PET-CT shows clinically and radiographically more indolent bone lesions[52].
Paget's disease, also known as osteitis deformans, is a chronic disease that causes abnormal and excessive remodeling of bones. It is estimated that Paget’s disease affects about 3% to 4% of people over the age of 40. The incidence of Paget’s disease increases markedly with age in both sexes, although there is a slight male predominance of 3/2. The disease has three main phases that explain the variable radiographic appearance. These are the lytic phase, in which osteoclastic resorption is initially active; the mixed phase, in which both osteoclastic and osteoblastic activity are seen, in which osteoblastic activity is an active process; and finally the blastic phase, in which osteoblastic activity gradually decreases, which is a late inactive process. Bone involvement in Paget’s disease is usually asymmetric, and cranial bones are involved in 25-65% of affected patients. Polyostotic Paget’s disease (65% to 90%) is more common than monostotic disease, and monostotic disease predominates in the axial skeleton. If the skull is affected, hearing loss and compression of cranial nerves in the foramina may be seen with increasing head size. In particular, platybasia, basilar invagination and hydrocephalus may be caused by the skull base.
The main techniques used to visualize Paget’s disease are plain radiography, bone scintigraphy, CT and MRI. On radiologic imaging of progressive osteolysis usually appears as well-defined wide areas of radiolucency affecting the frontal and occipital skull bones and is called circumscriptive osteoporosis. No sclerosis surrounds the skull lesions, and the inner table is more involved than the outer table, which usually crosses the suture lines. In the mixed phase, progressive osteolysis is characterized by coarsening the bony trabeculae along stress lines, cortical thickening and bony expansion.
Foci of sclerosis may be circular in the early stages of the blastic phase and may present a so-called cotton-wool appearance in previously areas of circumscribed osteoporosis. Later, sclerosis may progress to cross the sutures and cause marked thickening and widening of the diploic space, especially the inner table; this condition is called a tam-o'-shanter (wide and flattened) skull. Focal osteosclerosis of the skull has a cotton-wool appearance on plain radiography.
Bone scintigraphy is a sensitive method that can detect increased blood flow and osteoblastic activity accompanying osteoclastic activity in the disease. Bone scans show markedly increased radionuclide uptake throughout the abnormal bone in all three stages of Paget’s disease, particularly during the active phase. In osteoporosis circumscripta, however, the intense radionuclide uptake is confined to the margins of the lesion. In addition, non-specific tracer uptake scintigraphy has become a useful technique for determining the presence and distribution of polyostotic disease rather than for making a specific diagnosis or determining the extent of the pagetic lesion.
CT facilitates the diagnosis of pagetic abnormalities in bone that resemble findings seen on plain radiography. Because CT provides superior cortical and trabecular detail in a cross-sectional view, it better demonstrates osteolysis, trabecular coarsening, cortical thickening, and osseous expansion (Figure 13). In addition, CT often helps to assess complications such as fractures, spinal stenosis and secondary neoplasms.
MRI signals that the characteristics of Paget’s disease are variable, with three main patterns of involvement reflecting the natural history of the disease process at different stages. The most common pattern is a dominant fatty signal intensity in pagetic bone, which is seen in most patients and represents a long-standing disease. The second most common pattern corresponds to the early mixed active stage, when the affected bone shows heterogeneous, relatively low T1 and high T2 signal intensity. This pattern is also known as patchy appearance and is associated with the presence of granulation tissue, hypervascularity and edema, which is seen during active disease when there is abnormal and irregular bone mineralization. In the late blastic stage, low signal intensities are seen in pagetic bone on both T1-weighted and T2-weighted images due to changes in compact bone and fibrous tissue. In pagetic bones, preservation of the marrow signal may help exclude the diagnosis of a superimposed sarcoma. Increased contrast enhancement may be seen in pagetic bone as a complication indicator of hyperemia or secondary disease processes in active disease[53].
In the evaluation of Paget’s disease, standardized uptake values (SUVmax) of 18F-fluorodeoxyglucose PET-CT (18F-FDG PET-CT) in affected bones have been reported to range from 2.4 to 5.6. This variability is thought to be due to the presence of lytic or blastic phases of the disease. However, some manuscripts did not report 18F-FDG uptake in the localization of Paget’s disease or did not emphasize a correlation between osteoblastic and osteoclastic activity and 18F-FDG uptake. It has also been reported that 13% of Paget’s disease lesions have high 18F-FDG uptake, which may mimic the presence of metastatic disease. In addition, 18F-FDG PET-CT may be useful in evaluating sarcomatous malignant transformation of Paget’s disease. Malignant transformation to sarcoma has been estimated to occur in approximately 1% of patients with a long-standing Paget’s disease, but this phenomenon increases the risk of developing osteosarcoma in Pagetic patients by a factor of 30 compared to the general patient population over the age of 40. 18F-sodium fluoride (18F-NaF) is a PET radiopharmaceutical that specifically reflects bone blood flow and osteoblastic activity and is therefore used to assess skeletal metabolism. 18F-NaF PET-CT is able to assess both bone metastases and benign changes, and the increased osteoblastic activity specific to Paget’s disease can be clearly evaluated by this imaging modality[54].
Skeletal changes in chronic anemia are due to ineffective erythropoiesis as a result of impaired haemoglobin synthesis, peripheral destruction of erythrocytes and consequently reduced erythrocyte lifespan. Radiography show massive hyperplasia of the bone marrow associated with hyperactivity of the bone marrow in response to anemia. The hair-on-end sign is seen on plain radiography of the skull, CT and MRI as long, thin vertical lines of calcified spicules perpendicular to the bone surface, appearing as upright hairs. The hair-on-end appearance of the skull is a characteristic feature of chronic haemolysis, usually seen in patients with thalassaemia (Figure 14) and sickle cell disease. The incidence of hair-on-end appearance in patients with thalassaemia is 8.3%. Although rare, the hair-on-end sign has also been described in iron deficiency anemia, sickle cell disease, cyanotic congenital heart disease, hematological malignancies and nutritional deficiencies. The appearance of these projections indicates excessive medullary erythropoiesis in patients and is rare before the age of 5 years. As the bone lesions are explained by the relationship between proliferating bone marrow and bone cortex, only hypertransfusion initiated early in life will prevent the development of the abnormality[55].
Primary hyperparathyroidism is a condition in which there is no autonomous parathyroid hormone secretion, usually from a parathyroid adenoma, and no feedback inhibition by serum calcium. Secondary hyperparathyroidism is more frequently than primary hyperparathyroidism and occurs in response to low serum calcium levels. The most common cause of secondary hyperparathyroidism is chronic renal failure. This condition can also result from vitamin D deficiency or inadequate calcium intake. In 95% of patients with hyperparathyroidism, the skeletal findings are most easily recognized in the bones of the hands. The pathognomonic subperiosteal bone resorption of hyperparathyroidism begins as lacy irregularity and acro-osteolysis. However, intracortical, endosteal, trabecular, subligamentous, subchondral and subtendinous bone resorption may also occur. In the skull, bone resorption is described as a salt-and-pepper appearance and may result in loss of the distinction between the inner and outer skull tables. Trabecular resorption results in the trabeculae appearing smudgy. Intracortical resorption, also known as cortical tunnelling, is a common feature of hyperparathyroidism. Cortical thinning and the obscuring of the corticomedullary junction can be caused by endosteal resorption[56].
Brown tumors (osteoclastomas) result from activation of osteoclasts by parathyroid hormone. Brown tumors cause focal bone resorption, fibrous tissue deposition, necrosis and liquefaction. Radiologically, brown tumors are usually solitary but may be multifocal, with a risk of pathological fracture. They also present as sharply demarcated, centrally or eccentrically expanding lytic lesions[3]. Brown tumors may have a large soft tissue component, usually involving the facial bones. Although brown tumours were first described in primary hyperparathyroidism, they are now more commonly found in patients with chronic renal failure and secondary hyperparathyroidism. Treatment of hyperparathyroidism may also lead to regress of Brown tumors[56].
Renal osteodystrophy refers to abnormalities of bone morphology in chronic renal failure. Phosphate retention and reduced vitamin D conversion lead to hypocalcemia, which stimulates parathyroid hormone production and causes bone resorption. Therefore, factors such as secondary hyperparathyroidism and abnormal vitamin D metabolism contribute to renal osteodystrophy. Some imaging features of renal osteodystrophy overlap with those of hyperparathyroidism and rickets or osteomalacia. Skeletal findings in renal osteodystrophy include generalised demineralization, trabecular thickening, subperiosteal resorption, cortical thinning, bone cysts and pathological fractures. A diffuse increase in bone density is seen on imaging in patients with chronic renal failure. This finding is more common in the axial skeleton, which has more trabecular bone than cortical bone. Despite the increased radiodensity, the bone is structurally weak and more susceptible to stress fractures. This diffuse osteosclerosis is thought to be due to the anabolic effect of parathyroid hormone. The salt-and-pepper appearance in renal osteodystrophy (Figure 15) results from diffuse bone thickening with loss of distinction between the inner and outer tables of the skull and granular deossification due to low-density lytic foci scattered within the normal bone[9,56].
Acromegaly is a rare metabolic disorder that develops insidiously. It is characterised by chronically elevated levels of growth hormone and insulin-like growth factor 1 in the blood, which can lead to high morbidity and mortality. The average delay from onset of symptoms to diagnosis is estimated to be 7-10 years. The effects of growth hormone on the skeleton vary. This depends on the degree of skeletal maturity. Excessive secretion of growth hormone causes gigantism when the growth plates are still open and periosteal reactions with bone growth when the growth plates are closed. Radiographic enlargement of the sella turcica is due to the direct effect of the pituitary tumor (Figure 16). Other skull abnormalities result from the effects of excessive periosteal bone formation and include prominence of the occipital protuberance, cortical thickening of the calvarium, widening of the maxillary and frontal sinuses, enlargement and elongation of the mandible, increased mandibular angle and formation of a supraorbital crest. These changes result in a clinically significant prominence of the forehead[57].
Rickets and osteomalacia are two distinct clinical conditions in which bone mineralization is impaired. Rickets is a diffuse bone disease associated with calcium and phosphate homeostasis disorders that can result in joint deformities and stunted growth. Osteomalacia is a metabolic bone disease resulting from a chronic and severe deficiency of vitamin D or phosphate after growth has ceased, for any reason. Vitamin D or phosphate deficiency leads to defective bone mineralization and proximal muscle weakness with generalised or localized vague bone pain in different regions of the skeleton. Rickets affects the growing skeleton of infants and children, whereas osteomalacia affects adults after their growth plates have fused. Vitamin D deficiency is the most common etiological cause of both rickets and osteomalacia. Early skeletal deformities such as soft, thin skull bones known as craniotabes, which are the first signs of rickets, can occur in infants over three months of age. Frontal bumps and large fontanelles due to delayed closure of the fontanelles may be seen in the skull. In adults, demineralization leads to less hard bones (soft bones) with pathological fractures[58].
Long-term use of the anticonvulsant drug phenytoin (diphenylhydantoin) in patients with epilepsy has been associated with various side effects, including thickening of the skull bones and coarsening of facial features. It has been demon
Scurvy is a chronic vitamin C deficiency, which is necessary for the hydroxylation of many proteins, including collagen. A lack of vitamin C leads to abnormal collagen production, resulting in vascular fragility and an abnormal bone matrix. Both adults and adolescents may present with non-diagnostic diffuse osteopenia and cortical thinning due to cessation of osteoid formation. Porous subperiosteal new bone formation is observed on the ectocranial surface of the parietal and/or squamous temporal bones. Bilateral and subperiosteal new bone is diagnostic and plain radiography should be used for confirmation. Scurvy hemorrhages may be seen from temporal vessels arising from the lateral aspect of the parietal bones. Chronic or acute hemorrhage from meningeal vessels may also cause blood clots to form in the subdural space (subdural hematoma). On MRI, the bone marrow is heterogeneous on T1-weighted and T2-weighted images, and subperiosteal hemorrhage can be seen as periosteal contrast enhancement with increased subperiosteal T1 and T2 signal intensity[56,60].
Osteomas are the most common benign tumors of the calvarium. They are most frequently in people aged 40 to 50 and are more common in males. These lesions are composed of well-differentiated compact and cancellous bone and arise from the outer table in a juxtacortical location. Osteomas rarely arise from the inner table or diploe. The most common complaints are pain, tenderness and a slowly growing mass[3,61]. Most osteomas range in size from 2 to 30 mm, but exceptionally they can be much larger. Calvarial osteomas most commonly arise from the frontal bone near the frontal sinuses and can be seen anywhere in the cranial vault[62]. On CT, the osteoma is a juxtacortical, well-defined, sclerotic and homogeneous lesion (Figure 17). Unlike meningiomas, osteomas have homogeneous low signal intensity on all MRI sequences, are not contrast enhanced and do not contain soft tissue components[3,61].
Fibrous dysplasia, resulting from abnormal differentiation of immature osteoblasts, accounts for 7% of all benign bone tumors. It is most common in young adults and adolescents. Fibrous dysplasia may be monostotic (70%) or polyostotic (30%), and the skull is affected in both forms. It can also be associated with McCune-Albright syndrome and Mazabraud syndrome. Fibrous dysplasia usually affecting the frontal and temporal bones and can cross sutures. It is usually detected incidentally on radiological examinations, but may cause symptoms with mass effect, foraminal narrowing, and cranial nerve compression. Malignant transformation of fibrous dysplasia is extremely rare and has been reported in 0.4%-1% of cases. Fibrous dysplasia may be complicated by aneurysmal bone cysts or pathological fractures.
Fibrous dysplasia appears on CT as an intradiploic, expansile lesion with a characteristic ground glass matrix over most of the lesion (Figure 18). The outer table is more involved than the inner table (Figure 19). Three types have been defined according to their appearance on CT: Lytic, sclerotic and mixed (Figure 20). The more characteristic homogeneous sclerotic (ground-glass density) type is the most common. The signal and contrast enhancement properties of fibrous dysplasia lesions on MRI vary depending on the ratio of fibrous tissue and mineralized matrix within them. The homogeneous sclerotic type is the most common and the lesion typically has a low signal on T1-weighted and T2-weighted images. Signal intensity on T2-weighted images is heterogeneous and depends on the density of fibrous tissue, intralesional cellularity, and hemorrhagic or cystic components. Lesions of fibrous dysplasia with highly mineralized stroma tend to show low signal intensity on both T1-weighted and T2-weighted images, whereas lesions containing dense fibrous tissue tend to show intermediate signal intensity on T1-weighted images and high signal intensity on T2-weighted images. Contrast enhancement after gadolinium administration is also variable and not useful for diagnosis[9,49].
Fibrous dysplasia usually shows increased uptake on scintigraphy, but less commonly, no uptake may be seen in fibrous dysplasia. Although fibrous dysplasia is known not to show 18F-FDG hypermetabolism, a few cases have been reported where it mimics malignant bone involvement by showing increased FDG uptake on 18F-FDG PET-CT[63].
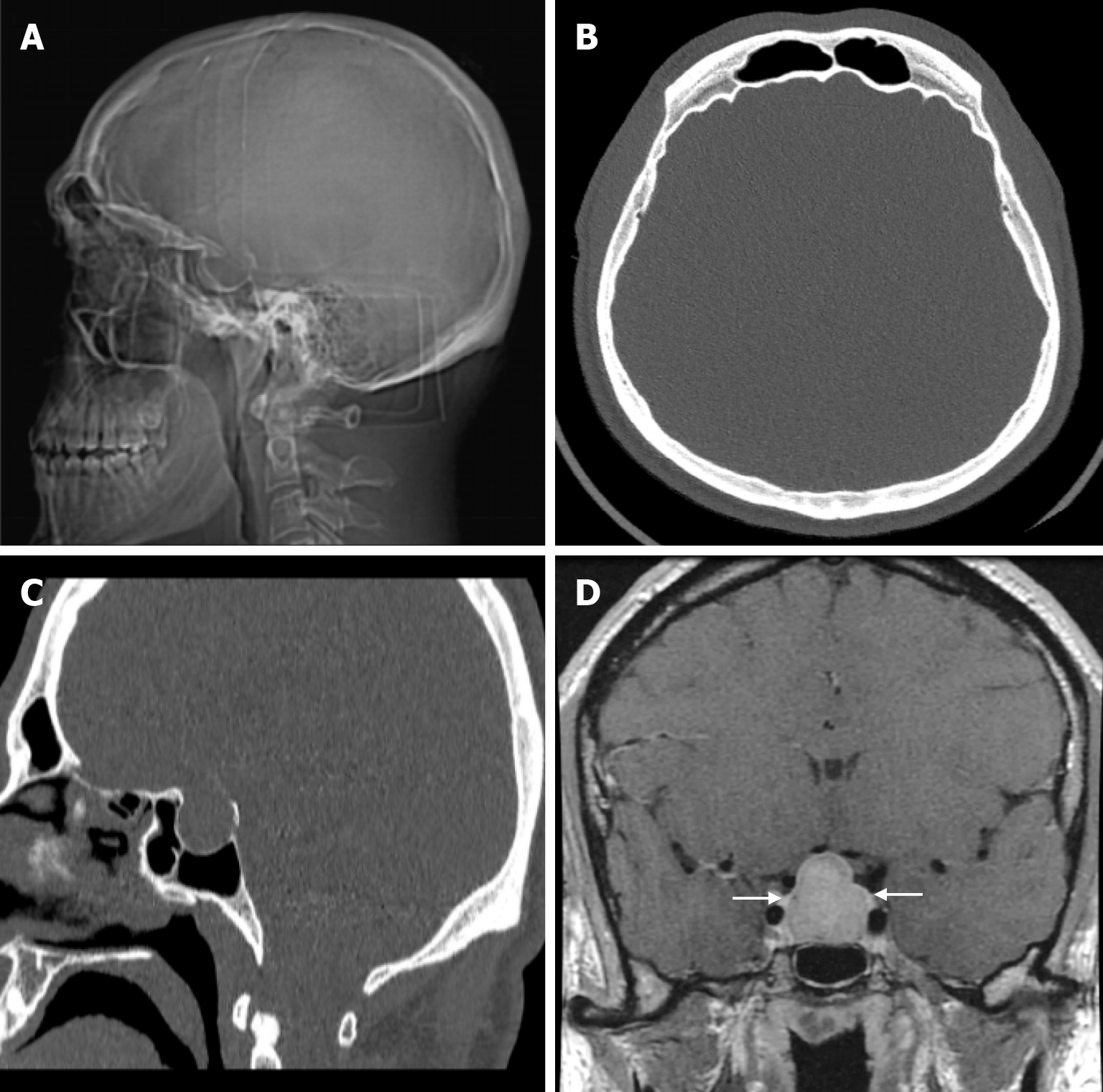
Meningiomas account for about 20% of all primary intracranial tumors and are the most common benign tumors. Primary extradural meningiomas are a rare subtype of meningioma, accounting for less than 2% of all meningiomas. Primary intraosseous meningiomas occur within the bone, particularly in the calvarium, and account for two-thirds of all primary extradural meningiomas. The frontoparietal and orbital regions are the most common sites for primary intraosseous meningiomas. The incidence is slightly higher in women and has a bimodal distribution, peaking between the second decade of life and the ages of 50 and 70. The most common presentation of primary intraosseous meningioma of calvarial origin is a slowly growing asymptomatic mass. Headache is the second most common symptom reported. The patho
Primary intraosseous meningioma is typically seen on CT as a sclerotic lesion with bone expansion, hyperostosis, and contour irregularities in the inner and/or outer table (Figure 21). On MRI, the tumor has a low signal on T1-weighted images and variable signal intensity on T2-weighted images, and contrast enhancement is not expected. The dural tail, which can be seen in intradural meningiomas, is not expected in intraosseous meningiomas. However, if it has caused a defect in the dura or an invasion, contrast enhancement may be seen in the dura[49,64]. Proton magnetic resonance spectroscopy (MRS) of the soft tissue component may characteristically show alanine (Ala, 1.47 ppm doublet inversion at long echo times). T2* dynamic susceptibility contrast-perfusion-weighted imaging (DSC-PWI) time-intensity curves (TIC) of the soft tissue component of primary intraosseous meningioma typically show a curve with little or no return from the baseline. These tumors characteristically have very high relative cerebral blood volume (rCBV) values[8].
Primary intraosseous meningiomas can be classified as osteoblastic, lytic or mixed types. Lytic and mixed types are less common in intraosseous meningiomas, with reporting frequencies ranging from 5% to 35%. Low-grade meningiomas are more prone to hyperostosis. In contrast, mixed hyperostotic and lytic types are more frequently associated with high-grade meningiomas. The differential diagnoses of intraosseous meningiomas include osteoma, fibrous dysplasia, enplaque meningioma, giant cell tumor, intraosseous venous malformation, epidermoid cyst, aneurysmal bone cyst, infection, plasmacytoma, osteogenic sarcoma, Langerhans cell histiocytosis, Paget disease, and osteoblastic skull metastasis[64].
Intraosseous lytic meningiomas have more malignant potential and usually show moderate homogeneous enhan
Recurrence rates for meningiomas arising from the calvarium range from 12.6% to 22%. Therefore, imaging and close follow-up are required to assess the extent of resection, detect early recurrence or malignant progression, and intervene timely[64,65].
Intraosseous venous malformations are a subtype of venous vascular malformations that occur primarily in bone. In the head and neck, they are most commonly found in the skull, skull base and facial skeleton. Further complicating the diagnosis of intraosseous venous malformations on imaging is the fact that these lesions are often mislabelled as haemangiomas in the radiological and clinical literature and in daily practice[66].
Intraosseous venous malformations are rare, benign, slow-growing lesions that account for 2%-10% of calvarial tumors and 0.2% of all bone neoplasms. The skull is the second most common site after the spine. In the calvarium, these pathologies tend to be confined to a single bone, most commonly the frontal and parietal bones. These lesions are more common in the 2nd to 4th decades of life and in women. Most of these lesions are small and present as palpable swellings on the scalp or as lesions found incidentally on radiological examination. They may be associated with headaches, rarely neurological symptoms, and may initially be extremely large. Calvarial venous malformations are thought to result from aberrant differentiation of intradiploic veins between the inner and outer table of the bone[67].
On plain radiography, calvarial intraosseous venous malformations appear as well-defined round or oval lytic lesions. As the lesion consists of malformed venous channels within the bone trabeculae, calvarial venous malformations occur primarily in the diploic space, with an expansile appearance and thinning of the overlying cortex. Although not entirely specific, thick trabeculations extending from the center of the lesion to the periphery give a classic mottled, spiculated, honeycomb, spoke or sunburst appearance. On CT, it appears as a well-circumscribed, intradiploic, expansile lytic lesion with trabeculations and spicules (Figure 22). There is a sharp demarcation between the lesion and normal bone, and peripheral sclerosis is seen in 30% of cases. In most cases, the outer table of the skull is widened with the preservation of the inner table. Variable bone density is thought to reflect osteoblastic activity due to chronic and recurrent hemorrhage. MRI findings are variable, and on T1-weighted imaging some lesions have high signals from thrombus or fat, which a fat-suppressed technique can distinguish. On T2-weighted images, lesions have a markedly hyperintense signal, reflecting slow-flowing blood or subacute thrombus. A larger lesion may show more hypointense foci due to thickened trabeculae. After paramagnetic contrast administration, focal areas of enhancement in the early phase and a diffuse enhancement pattern in the late phase are shown. Dural enhancement, similar to meningioma, is rarely seen[7,49,66].
The differential diagnosis of calvarial venous malformations should be made with intraosseous meningioma, fibrous dysplasia, dermoid and epidermoid cysts. Intraosseous meningioma, usually seen in older age groups and with associated bony sclerosis, also shows avid contrast enhancement and T2 hyperintensity signal. Fibrous dysplasia classically has a ground-glass or heterogeneous cystic appearance on CT and moderate to low T2 signal intensity reflecting fibrous content. Dermoid and epidermoid cysts typically show low CT density without an internal matrix, lack enhancement on MRI and characteristic diffusion restriction. If a calvarial lesion is large or growing at an alarming rate, prompt excision and/or tissue sampling should be performed, considering malignant lesions such as metastasis or myeloma, and lesions such as Langerhans cell histiocytosis. As with most lesions of the calvarium or skull base, diagnostic accuracy is increased when CT and MRI findings are interpreted together as complementary studies[66].
Aneurysmal bone cysts are well-circumscribed, expansile, and lucent lesions most commonly seen in children and adolescents. Only 3% to 6% of aneurysmal bone cysts occur in the skull. They are the primary lesion in two-thirds of cases, but may be secondary to an underlying lesion (e.g. fibrous dysplasia, chondroblastoma, giant cell tumor, and osteosarcoma). They can grow rapidly and cause symptoms by compressing intracranial structures. They appear as a sharply circumscribed, expansile lytic lesion with fine sclerotic margins, but when expansion is evident, the skull table appear distorted. This multiloculated lesion contains blood, which explains the frequent occurrence of fluid-fluid levels. Fluid-fluid levels within the locules and small cysts protruding into larger cysts give the characteristic soap bubble appearance. However, fluid-fluid levels have also been reported in other conditions, including Langerhans cell histiocytosis and metastatic neuroblastoma. The presence of a solid component suggests that the aneurysmal bone cyst is due to an underlying lesion (benign or malignant)[2,49].
Giant cell tumor (also known as osteoclastoma) accounts for 5% of all primary bone tumors and 20% of benign skeletal tumors. This tumor results from an overgrowth of osteoclasts and affects young adults between the ages of 20 and 45. It may present with pain or pathological fractures in the epiphyses of long bones. Giant cell tumors are extremely rare in the skull. In adults, the sphenoid and temporal bones are more commonly involved. This tumor is considered benign but can be aggressive and recurrent. On radiographs, it appears as a solid or mixed (solid-cystic) mass with well-defined but non-sclerotic edges and marked cortical thinning that may also lead to partial fracture. The tumor is hypointense on T2-weighted images due to hypercellularity, fibrous matrix, or hemorrhagic content. Giant cell tumors have high vascularity and therefore show intense contrast enhancement in contrast-enhanced series on MRI. If cystic areas are found, an associated secondary aneurysmal bone cyst should be suspected[8,51,68].
Intraosseous lipoma is a rare benign tumor that accounts for 0.1%-2.5% of all primary bone tumors. Only 4% of intraosseous lipomas are found in the skull. Intraosseous lipomas of the skull are divided into three groups according to their location: Calvarium, skull base, and facial skeleton. It is seen as a radiolucent mass in the skull on plain radiography. On CT it is seen as a spindle-shaped low density mass in continuity with the surrounding diploe (Figure 23), while on MRI it is seen as a homogeneous high signal intensity on T1-weighted and T2-weighted images. Common radiological features include calcification (57%), marginal sclerosis (74%) and cyst formation (67%). Intraosseous lipoma can show various histological changes such as atrophic bone trabeculae, fat necrosis, myxoid stroma and calcification. The differential diagnosis is often intraosseous venous malformation[69].
Desmoplastic fibroma of bone is a rare, locally aggressive intraosseous neoplasm that accounts for less than 0.1% of all primary bone tumors. Pathologically, this tumor creates an appearance consisting predominantly of spindle cells with abundant collagenous components and dilated vascular channels. This tumor can spread into surrounding tissues while maintaining its benign characteristics without necrosis, mitotic activity, or cellular atypia. Bone desmoplastic fibromas most commonly affect the long bones and mandible, and skull involvement is extremely rare. They usually present as slowly growing masses and may cause headaches, hearing changes, otorrhoea and head asymmetry. Bone desmoplastic fibromas range in size from 2 to 10 cm. On plain radiography they appear as lytic lesions with irregular margins without sclerosis. Desmoplastic fibroma of bone is seen on CT as lytic lesions with widening of the diploic space and typical thinning of the inner and outer tables of the skull. On MRI of these tumors, heterogeneous signal intensity is seen in T2-weighted series, while low contrast enhancement is seen in contrast-enhanced series due to dense connective tissue and hypocellularity. These imaging findings are not specific for desmoplastic fibroma of bone and may be seen in other conditions such as fibrous dysplasia, Langerhans cell histiocytosis, intraosseous venous malformation, metastasis, and low-grade osteosarcoma. However, the T2 shortening of non-sclerotic fibro-osseous lesions in T2-weighted series requires consideration of desmoplastic bone fibroma in the differential diagnosis of lytic skull lesions[50,70].
The most common malignant bone tumors in adults are metastases. Metastases are often seen after the fifth decade. They usually develop secondary to breast, prostate, lung, kidney or thyroid cancers in adults, while they are more common in children due to neuroblastoma or sarcoma. Breast cancer in adults and neuroblastoma in children are the most common causes of skull metastases. Skull metastases are known to occur in 15% to 25% of patients with advanced systemic cancer. Calvarial metastases usually involve all three layers of the skull. Spread is usually hematogenous and may extend from the adjacent dura and cranial nerves. The most likely mechanism of calvarial metastasis is the retrograde spread of metastases through the valveless venous plexus of Batson. Approximately half of patients with calvarial metastases are asymptomatic, and those with periosteal involvement or outer table present as a palpable mass that may be associated with localized pain. Involvement of the inner table can cause neurological symptoms such as headaches, neurological deficits, meningeal irritation and seizures. Increased intracranial pressure due to dural sinus involvement may be observed in patients with disseminated metastatic disease[4,71].
Depending on the primary tumor, metastases may have a lytic (Figure 24), sclerotic (Figure 25) or mixed pattern. Metastases usually appear as multiple expansile and lytic areas and lesions with accompanying soft tissue components extending into adjacent tissues. When solitary expansile lytic lesions are seen, metastases from thyroid or renal neoplasms should be suspected. Lung metastases (Figure 26) in the calvarium are both lytic and sclerotic. Sclerotic metastases are seen in prostate cancer (Figure 27) and osteosarcoma. Rapidly growing hypervascular lesions may present as blow-out expanding lesions[7,50].
On plain radiography, metastases appear as multiple lesions of varying size with poorly defined margins. CT usually shows the extent of bony destruction with irregular margins and permeative erosion. Radionuclide imaging may be more sensitive than CT in detecting calvarial metastases. The soft tissue component has variable density and contrast enhancement. On MRI, soft tissue is hyperintense on T2-weighted images and hypointense on T1-weighted images (replacing the normal hyperintense marrow). Sclerotic metastases are hypointense on T1-weighted and T2-weighted images. Calvarial metastases usually enhance homogeneously after paramagnetic contrast medium administration, but heterogeneous contrast enhancement, peripheral rim-like enhancement, or lack of enhancement in sclerotic lesions may be observed. MRI is more sensitive than CT in detecting dural invasion and associated intraparenchymal metastases. Dural tail sign due to dural invasion may be detected on MRI. There is a wide range of ADC values in ADC mapping in calvarial metastases depending on the primary histology, and restricted diffusion is detected in the solid components of cell-rich metastases[9,59,66]. Hypervascular tumors such as renal cell carcinoma, melanoma, and thyroid cancer may present with metastases that show intense contrast enhancement on contrast-enhanced series and color coding consistent with high perfusion on perfusion MRI series. MR DSC-PWI shows a TIC curve below baseline in metastases[8]. Bone metastases usually show increased vascularity compared to normal bone tissue due to the incorporation of new blood vessels to promote tumor growth. Therefore, metastases may show more intense contrast-enhancement and higher perfusion values than most benign lesions[72]. 11C-methionine PET-CT is more effective than 18F-FDG PET-CT in characterising tumors at the base of the skull[73].
Multiple myeloma is a malignant bone marrow neoplasm characterized by the monoclonal proliferation of plasma cells. It usually spreads multifocally or diffusely throughout the axial skeleton and rarely presents as a focal solitary lesion called a plasmacytoma. Multiple myeloma accounts for 10% of all hematological malignancies. This tumor is the most common primary malignant neoplasm of the skeleton in the adult population over 40 years of age, with a higher prevalence in males between the fifth and eighth decades of life. Plasmacytoma patients are generally younger than multiple myeloma patients. The most common symptom of these tumors is bone pain[7,9,74].
Obvious bone lesions are found on radiological examination in about 80% of multiple myeloma patients. The skull is involved in 40% of cases, with the parietal bone most commonly affected. Multiple myeloma can present in four main types in terms of bone involvement patterns. These are the diffuse form with multiple round lytic lesions, the diffuse form with diffuse osteopenia, solitary plasmacytoma, and osteosclerosing fibroma. Radiography shows multiple, small, round, lytic, punch-hole-shaped, and sharply demarcated lesions with non-sclerotic margins causing endosteal scalloping. These findings give a characteristic punched-out raindrop appearance on skull radiography (Figure 28). Lesions may coalesce to form larger lytic areas. The diffuse form with diffuse osteopenia is less common in the skull. CT is better at showing the presence and extent of lesions not seen on radiography and soft tissue involvement. MRI may show low signal intensity on T1-weighted images and intermediate signal intensity on T2-weighted images, or foci of pure and diffuse signal abnormality. Avid contrast enhancement is seen when the lesions are active and diffusion restricted, showing cortical infiltration into adjacent soft tissues with periosteal reactions[4,7,9,51]. Because myeloma lesions are only lytic, they are usually not detected by Tc-99m-based scintigraphy[49]. PET can provide prognostic information in these tumors and can be used to assess response to treatment[75].
Cranial plasmacytomas are usually completely lytic, cross the calvarium with a soft tissue component and show diffusion restriction. They are usually hypervascular with avid contrast enhancement. Flow voids can be present in plasmacytomas, which can lead to confusion with other hypervascular lesions such as hemangiopericytomas, although flow voids are usually more common in solitary fibrous tumors[3,51]. Plasmacytomas show early and intense contrast enhancement on T1-based dynamic contrast enhanced (DCE) MRI after gradual wash-out of the contrast agent[72].
Although bone involvement is relatively common in lymphoma, primary bone lymphoma is rare, accounting for less than 1% of all malignant lymphomas. Calvarial involvement is extremely rare[76]. Bone lymphoma usually arises from non-Hodgkin's lymphoma, a diffuse large cell-lymphoma. The most common symptom in the early stages of the disease is a gradually enlarging scalp mass. Neurological deficits may be the main clinical manifestation of the disease if intraparenchymal infiltration is present. The radiographic appearance of primary bone lymphoma can vary widely, from nearly normal-appearing bone to extensive permeative/destructive processes with cortical and soft tissue involvement. Skull involvement may be sclerotic, lytic or mixed. The most common radiographic finding of primary lymphoma is the appearance of a lytic destructive lesion with a permeative or moth-eaten appearance. The absence of lytic changes does not exclude the diagnosis of calvarial lymphoma. In the early stages of the disease, sclerotic changes may occur before significant bone destruction is seen. This can be explained by the tumor cells invading the diploic space before infiltrating the pericranial structures by progressing along the emissary veins, during which time the overall bone structure remains intact. On CT, the soft tissue component is homogeneously isodense to hyperdense. On MRI, the soft tissue is hypo
Leukemia is the most common cancer in children, and acute lymphoblastic leukemia is the most commonly diagnosed type and its incidence is increasing. Therefore, skull involvement by leukemic infiltrate is most commonly seen in children. Imaging findings depend on the stage of the disease and include diffuse bone marrow infiltration and contrast enhancement or more focal lytic lesions. Focal lesions are usually lytic and permeative with transdiploic extension, periosteal reaction and extraosseous soft tissue infiltration. Signal changes of bone marrow infiltration on MRI are typically hypointense on T1-weighted images and hyperintense on T2-weighted images, and lesions show diffusion restriction. As signal changes on MRI are non-specific, they cannot be used alone to distinguish leukemic bone marrow involvement from other bone marrow infiltrating pathologies such as hematological diseases, metastases and multiple myeloma[3,77]. The main finding that 18F-FDG PET-CT imaging can reveal in patients with leukemia is bone marrow infiltration in both acute and chronic forms. PET-CT in leukemia makes a significant contribution to the diagnosis and management of various types of leukemia, particularly in the evaluation of extramedullary infiltration, monitoring of leukemia relapse, detection of Richter's transformation (Richter’s transformation refers to the development of chronic lymphoblastic leukemia to another more aggressive lymphoma or lymphoid malignancy) in chronic forms, and evaluation of inflammatory activity associated with acute graft-versus-host disease[78].
Osteosarcoma is a malignant mesenchymal neoplasm in which tumor cells produce osteoid or immature bone. Although it is the most common primary bone tumor at all ages, osteosarcoma accounts for only 3%-5% of all childhood malignancies. Osteosarcomas primarily arise de novo, secondary to Paget’s disease, fibrous dysplasia, chronic osteomyelitis or previous radiotherapy, which may explain the rarity of paediatric cases. Primary osteosarcoma occurs in the young population, peak in the first and second decades of life, whereas secondary osteosarcoma affects the elderly. Craniofacial osteosarcomas are rare and usually present in the third or fourth decade of life. The most common craniofacial sites are the mandible and maxilla, followed by the calvarium and finally the base of the skull. In the skull, osteosarcoma usually has a lytic appearance with variable amounts of osteoid matrix. On CT, there is a poorly defined, aggressive periosteal reaction and new bone formation within the soft tissue component, and the transition zone is usually wide. On MRI, soft tissue involvement is seen with low signal intensity on T1-weighted images and heterogeneous signal intensity on T2-weighted images depending on the matrix of the lesion, and they are heterogeneously contrast enhanced after gadolinium application. Approximately 5%-10% of osteosarcomas initially present with lung metastases and therefore chest imaging should be performed[4,79].
Neuroblastoma arises from neural crest cells that have attacked the sympathetic system. Neuroblastoma is the most common extracranial solid tumor in childhood, accounting for 10% of childhood malignancies and 15% of malignancy-related deaths. In most cases, the disease is diagnosed before the age of 4. Metastases are present in 70% of patients at the time of diagnosis and are most commonly found in the bones. Calvarial/dural metastases occur in 25% of patients and may occasionally occur before the diagnosis of the primary tumor[80]. Neuroblastoma is the most common metastatic disease involving the skull in children. Neuroblastoma has radiographic features such as multiple lytic bone lesions, calvarial thickening, suture dehiscence and hair-on-end periosteal reaction (Figure 30). The hair-on-end appearance is due to the resistance of the inner periosteum, which prevents penetration of tumor cells, and removal of the periosteum results in plaque-like epidural deposits of tumor[3]. Dural metastases are most commonly seen on the extradural surface, usually with increased enhancement, thickening or plaque[80].
Meta-[123I]iodobenzylguanidine ([123I]mIBG) imaging (scintigraphy and SPECT-CT) used in nuclear medicine has high sensitivity and specificity for neuroblastoma (both around 90%). [124I]mIBG, [18F]mFBG, [68Ga]Ga-DOTA peptides and [18F]F-DOPA appear to be more sensitive and specific tracers than [123I]mIBG imaging, especially for detecting smaller lesions below the spatial resolution of SPECT. Only 18F-FDG PET-CT imaging is less sensitive and specific than [123I]mIBG imaging[81].
Solitary fibrous tumors, formerly known as hemangiopericytomas, are highly vascularized extraaxial tumors of mesenchymal origin. Solitary fibrous tumors are the most common nonmeningothelial mesenchymal neoplasms and share the common molecular feature of NAB2-STAT6 gene fusions. These tumors have been shown to arise from Zimmermann's pericytes, contractile spindle cells that line capillaries and post-capillary venules throughout the body. Nearly 25% of solitary fibrous tumors occur in the head and neck. They account for less than 1% of intracranial tumors. Primary osseous solitary fibrous tumors of the skull are extremely rare low-grade malignancies[82].
Solitary fibrous tumors are dural-based tumors that affect adults with a median age of 40-60 years with a slight male predominance. On imaging, it appears as an extra-axial, avidly contrast enhancement mass with flow voids and a possible dural tail, making it difficult to distinguish from a meningioma. However, a solitary fibrous tumor does not calcify and does not show hyperostosis because the bone involvement is completely lytic. If the dural base is narrow, it has the classic mushroom appearance. A solitary fibrous tumor shows a very specific high level of myoinositol (Myo, 3.55 ppm) on MRS[8]. A solitary fibrous tumor may cause lytic destruction of the skull base on CT and is poorly defined. On MRI, soft tissue T1-weighted and T2-weighted images show isointensity-mild hyperintensity, heterogeneous avid contrast enhancement and central foci of necrosis. Prominent internal flow voids are a typical feature on T2-weighted images, indicating diffusion restriction due to hypercellularity[51]. A solitary fibrous tumor should be considered in hypervascular and highly destructive bone lesions. Lesions should be followed for a long time due to the risk of distant metastasis and local recurrence[82].
Chordomas account for 1% of intracranial tumors and 4% of all primary bone tumors. Intracranial chordomas are a locally aggressive and relatively rare tumor of the skull base, thought to arise from embryonic remnants of the primitive notochord. Intracranial chordomas account for one-third of all chordomas and most commonly arise from spheno-occipital synchondrosis of the clivus. Chordomas are classified as classical, chondroid or dedifferentiated, depending on their composition Classical chordomas are the most common type, characterized by the absence of cartilage or additional mesenchymal components. In chondroid chordomas, both chordomatous and chondromatous features are present and they are detected more frequently in the spheno-occipital region of the skull base. Dedifferentiation and sarcomatous transformation are seen in 2%-8% of chordomas. Chordomas can occur at any age, but are most common in adults, with a peak prevalence in the fourth decade of life. Chordomas are more common in males by a ratio of 2/1. The most common initial complaints are diplopia associated with cranial nerve palsy due to the location of the tumor and headache. The most common cranial nerve involved is the sixth nerve, and headache is usually in the occipital or retroorbital region[83].
Most lesions are lytic, with frequent intratumoral calcifications or sequestered bone fragments on plain radiography. The four common radiological findings of the lesions are expansion, trabeculation, rarefaction and intratumoral calcifications. Because of the bone involvement and soft tissue components of these tumors, both CT and MRI are usually used to evaluate intracranial chordomas. On CT, intracranial chordomas typically appear as a centrally located (midline), well-circumscribed, expansile soft tissue mass originate from the clivus with associated widespread lytic bone destruction. In addition, intratumoral calcifications appear irregular on CT and are generally thought to represent sequestra from bone destruction rather than dystrophic calcifications within the tumor itself. MRI is the best imaging modality for both pre- and post-treatment evaluation of intracranial chordomas. Intracranial chordomas show moderate to low signal intensity on T1-weighted images, with occasional small hyperintense foci associated with intratumoral haemorrhage or mucus pools. They characteristically show very high signal intensity on T2-weighted images, probably due to the high fluid content of vacuolated cellular components. However, low signal fibrous septa can sometimes be seen. The contrast enhancement pattern of the tumor is usually heterogeneous, with moderate to marked enhancement, but sometimes has a honeycomb appearance due to intratumoral areas of low signal intensity. Radionuclide bone scans in chordoma may be normal or show decreased uptake in cold lesions. Diffusion-weighted imaging with T2 signals of lesions may be useful in distinguishing chordoma from chondrosarcoma. T2 signals appear brightest (hyperintense) in classic chordoma, isointense to the brain in chondrosarcoma (less bright) and poorly differentiated chordoma (even less bright). Chon
When clival chordomas are large, the mass may indent the pons with the characteristic thumb sign and elevate the pituitary gland, causing symptoms related to compression. It may also invade or displace other structures, including the cavernous sinus, jugular foramen, sphenoid sinus and posterior ethmoid sinus[9]. Intracranial chordomas are usually slow growing, but their close association with critical structures and extremely high local recurrence rates have often led to high mortality in the past. However, good results have been achieved with radical surgical resection, proton beam radiotherapy and combination therapy[83].
Chondrosarcomas are rare malignant tumors composed of embryonic cartilage, endochondral bone or remnants of primitive mesenchymal cells. Only 2% of all chondrosarcomas occur at the base of the skull and account for 0.1%-0.2% of all intracranial tumors. Despite the well-established association with Ollier and Maffucci diseases, skull base chondrosarcomas are most commonly de novo. These tumors are most common between the ages of 40 and 70 years, and there may be a slight female predominance (female/male ratio 1.1/1). As the majority of tumors are extraaxial at the clivus and temporo-occipital junction, their pathogenesis appears to be related to endochondral ossification of the skull base synchondroses. These extraaxial tumors occur mainly at the base of the skull in the petrous part of the temporal bone and in the petro-occipital, spheno-occipital and sphenopetrosal synchondroses. Cranial nerve deficits, particularly of the sixth cranial nerve, are common at the onset. Symptoms usually include headaches, facial paresthesias, hearing loss and/or tinnitus, double vision and gait abnormalities[84,85].
CT imaging of chondrosarcomas shows characteristic chondroid matrix calcifications consisting of arcs and rings, a sharp and non-sclerotic transition zone, and a lytic soft tissue mass in the petro-occipital fissure. These tumors appear to have low or intermediate signal on T1-weighted MR images. On T2-weighted images, it has a very high signal and may have interspersed low signal foci due to chondroid matrix calcification. On contrast enhanced imaging, it shows heterogeneous enhancement with classic annular enhancement lines within the tumor matrix[9].
They usually grow very slowly and erode the bones of the skull base; however, higher grade tumors characterized by rapid growth and early metastasis may be seen. The radiological features of chordomas and chondrosarcomas may overlap with similar localization and bone-destroying patterns, so histological confirmation is necessary for accurate diagnosis and treatment planning[85].
Ewing’s sarcoma is a rare neoplasm that accounts for 6%-9% of primary malignant bone tumors. It is the second most common cause of primary bone tumors in children. Primary calvarial involvement occurs in less than 1% of cases. Male patients are more commonly affected than female patients, with a reported rate of 1.6/1. The most common age group is 5-13 years, with 90% of patients under the age of 20. The temporal, parietal and frontal bones are commonly involved in this tumor, although occasionally the occipital and sphenoid bones may be involved. The most common symptoms associated with calvarial Ewing’s sarcoma are due to increased intracranial pressure. Localized headaches, swelling of the calvarium, papilledema and vomiting are the most commonly reported symptoms. Ewing’s sarcoma typically grows extradurally and often reaches a large size before invading the dura, causing clinical symptoms, or both. Plain radio
Rhabdomyosarcoma is a high-grade malignant neoplasm that morphologically resembles developing skeletal muscle and accounts for approximately 3% of all paediatric cancers. It is the most common soft tissue sarcoma in childhood and is rarely seen in adults. Rhabdomyosarcomas of the head and neck account for one-third of all rhabdomyosarcomas in children and adolescents. Rhabdomyosarcomas of the skull are extremely rare, with most cases arising from the skull base or temporal bone[89,90]. The median age of onset of rhabdomyosarcoma is 5 years, with 72%-81% of patients under 10 years of age. The incidence is higher in boys than girls, with a ratio of 1.4/1. CT of the head and neck can be used to assess bone involvement, including the skull base, anatomically. In general, soft tissue sarcomas do not have a specific MRI pattern due to the heterogeneity of tumor morphology and necrosis or haemorrhage. In general, they are isointense to muscle on T1-weighted images, hyperintense on T2 images and show avid contrast enhancement[91].
As rhabdomyosarcoma is rare, osteoma, osteosarcoma, Ewing’s sarcoma, Langerhans cell histiocytosis, plasmacytoma or chondrosarcoma should be considered first in primary skull tumors[90].
Calvarial lesions are rare and usually occur incidentally for a variety of reasons, ranging from malignant pathologies to congenital conditions, benign pathologies and anatomical variants. When the patient's age, clinical history, and symptoms are evaluated together, specific imaging findings, if any, help diagnose calvarial lesions. Defects in bone structure and lytic and sclerotic changes in patients are assessed by radiography and, ideally, CT, and the soft tissue components of the lesions and their relationship to the surrounding soft tissues are assessed by MRI in particular. As with most lesions of the calvarium or skull base, diagnostic accuracy is increased when CT and MRI are interpreted together as complementary studies and other imaging findings.
| 1. | Tubbs RS, Bosmia AN, Cohen-Gadol AA. The human calvaria: a review of embryology, anatomy, pathology, and molecular development. Childs Nerv Syst. 2012;28:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Mitra I, Duraiswamy M, Benning J, Joy HM. Imaging of focal calvarial lesions. Clin Radiol. 2016;71:389-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Khodarahmi I, Alizai H, Chalian M, Alaia EF, Burke CJ, Slasky SE, Wenokor C. Imaging Spectrum of Calvarial Abnormalities. Radiographics. 2021;41:1144-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Lloret I, Server A, Taksdal I. Calvarial lesions: a radiological approach to diagnosis. Acta Radiol. 2009;50:531-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Karatas D, Uygur S, Karabag H, Sayar H, Barut IT, Dagtekin A, Avci E. Calvarial Tumors: A Retrospective Analysis and Clinical Experience. Turk Neurosurg. 2023;33:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Yanmaz R, Bayaroğulları H. Radiological Approach to Osteolytic Benign Calvarial Lesions. Kafkas J Med Sci. 2017;7:144-152. [DOI] [Full Text] |
| 7. | Garfinkle J, Melançon D, Cortes M, Tampieri D. Imaging pattern of calvarial lesions in adults. Skeletal Radiol. 2011;40:1261-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Pons Escoda A, Naval Baudin P, Mora P, Cos M, Hernandez Gañan J, Narváez JA, Aguilera C, Majós C. Imaging of skull vault tumors in adults. Insights Imaging. 2020;11:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Gomez CK, Schiffman SR, Bhatt AA. Radiological review of skull lesions. Insights Imaging. 2018;9:857-882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 10. | Huntoon KM, Mayer RR, Fahim DK, Kumar S, Adelman DM, McCutcheon IE. Malignant primary tumors of scalp with cranial extension: multidisciplinary surgical strategies and outcomes. J Neurosurg. 2024;140:979-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | De Keyzer B, Bamps S, Van Calenbergh F, Demaerel P, Wilms G. Giant arachnoid granulations mimicking pathology. A report of three cases. Neuroradiol J. 2014;27:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Malekzadehlashkariani S, Wanke I, Rüfenacht DA, San Millán D. Brain herniations into arachnoid granulations: about 68 cases in 38 patients and review of the literature. Neuroradiology. 2016;58:443-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Battal B, Hamcan S, Akgun V, Sari S, Oz O, Tasar M, Castillo M. Brain herniations into the dural venous sinus or calvarium: MRI findings, possible causes and clinical significance. Eur Radiol. 2016;26:1723-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Choudhary G, Udayasankar U, Saade C, Winegar B, Maroun G, Chokr J. A systematic approach in the diagnosis of paediatric skull lesions: what radiologists need to know. Pol J Radiol. 2019;84:e92-e111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Benson JC, Hamilton-Cave M, Carr CM, Lane JI. Prevalence of intra-osseous veins and venous lakes in the posterior skull base on 3T MRI. Neuroradiol J. 2023;36:702-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Griessenauer CJ, Veith P, Mortazavi MM, Stewart C, Grochowsky A, Loukas M, Tubbs RS. Enlarged parietal foramina: a review of genetics, prognosis, radiology, and treatment. Childs Nerv Syst. 2013;29:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Kavurt AS, Demirel N, Cuni B, Tasci Yildiz Y, Baş AY. Isolated Lacunar Skull Defect in a Newborn. Türk Kadın Sağlığı ve Neonatoloji Dergisi. 2022;4:159-163. [DOI] [Full Text] |
| 18. | Kim SJ, Baek HJ, Ryu KH, Choi BH, Moon JI, Cho SB, Park SE, Bae K, Jeon KN, Cho EB, An HJ. Skull Base Dermoid Cyst in the Right Infratemporal Fossa Diagnosed Using the Dixon Technique: a Case Report and Review of Literature. Investig Magn Reson Imaging. 2017;21:114. [DOI] [Full Text] |
| 19. | Bikmaz K, Cosar M, Bek S, Gokduman CA, Arslan M, Iplikcioglu AC. Intradiploic epidermoid cysts of the skull: a report of four cases. Clin Neurol Neurosurg. 2005;107:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Calder AD, Gaunt T, Hickson M, Mankad K, Wilson LC. Major skull manifestations of skeletal dysplasias - pictorial essay. Pediatr Radiol. 2020;50:1658-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Panda A, Gamanagatti S, Jana M, Gupta AK. Skeletal dysplasias: A radiographic approach and review of common non-lethal skeletal dysplasias. World J Radiol. 2014;6:808-825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 70] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (5)] |
| 22. | Shen Z, Zou CC, Yang RW, Zhao ZY. Cleidocranial dysplasia: report of 3 cases and literature review. Clin Pediatr (Phila). 2009;48:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Desai V, Priyadarshini SR, Sharma R. Copper Beaten Skull! Can It be a Usual Appearance? Int J Clin Pediatr Dent. 2014;7:47-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Gasparetto EL, de Carvalho Neto A, Bruck I, Antoniuk S. Tuberous sclerosis and fibrous dysplasia. AJNR Am J Neuroradiol. 2003;24:835-837. [PubMed] |
| 25. | Pal NL, Juwarkar AS, Viswamitra S. Encephalocele: know it to deal with it. Egypt J Radiol Nucl Med. 2021;52:105. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Tamura G, Ogiwara H, Morota N. Characteristics of Recurrent Congenital Sinus Pericranii: Case Report and Review of the Literature. Pediatr Neurosurg. 2019;54:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Jung S, Lee JK, Kim SH, Kim JH, Kang SS, Lee JH. Parietal sinus pericranii: case report and technical note. Surg Neurol. 2000;54:270-2; discussion 273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Brzezinski P, Pinteala T, Chiriac AE, Foia L, Chiriac A. Aplasia cutis congenita of the scalp--what are the steps to be followed? Case report and review of the literature. An Bras Dermatol. 2015;90:100-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Lee YJ, Kim SA, Moon SH. An Aplasia Cutis Congenita: Suggestion of Management Algorithm. J Craniofac Surg. 2019;30:2493-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Jacques T, Jaouen A, Kuchcinski G, Badr S, Demondion X, Cotten A. Enlarged External Occipital Protuberance in young French individuals' head CT: stability in prevalence, size and type between 2011 and 2019. Sci Rep. 2020;10:6518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Sinclair AG, Scoffings DJ. Imaging of the post-operative cranium. Radiographics. 2010;30:461-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Ottenhausen M, Kalasauskas D, Kramer A, Neuhoff J, Serrano L, Schwandt E, Ringel F. Bone Flap Necrosis due to Low Grade Infection with Propionibacterium Acnes. World Neurosurg. 2019;124:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Ewald C, Duenisch P, Walter J, Götz T, Witte OW, Kalff R, Günther A. Bone flap necrosis after decompressive hemicraniectomy for malignant middle cerebral artery infarction. Neurocrit Care. 2014;20:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Agrawal SK, Kumar P, Sundaram V. Congenital depression of the skull in neonate: a case of successful conservative management. J Child Neurol. 2010;25:387-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Guler I, Buyukterzi M, Oner O, Tolu I. Post-traumatic leptomeningeal cyst in a child: computed tomography and magnetic resonance imaging findings. J Emerg Med. 2015;48:e121-e122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Wong CH, Foo CL, Seow WT. Calcified cephalohematoma: classification, indications for surgery and techniques. J Craniofac Surg. 2006;17:970-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Paul SP, Edate S, Taylor TM. Cephalhaematoma – a benign condition with serious complications: case report and literature review. Infant. 2009;5:146-148. |
| 38. | She R, Szakacs J. Hyperostosis frontalis interna: case report and review of literature. Ann Clin Lab Sci. 2004;34:206-208. [PubMed] |
| 39. | Raikos A, Paraskevas GK, Yusuf F, Kordali P, Meditskou S, Al-Haj A, Brand-Saberi B. Etiopathogenesis of hyperostosis frontalis interna: a mystery still. Ann Anat. 2011;193:453-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Stiene JM, Frank PW. Hyperostosis Frontalis Interna and a Question on Its Pathology: A Case Report. Am J Case Rep. 2022;23:e937450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 41. | Bhandari A, Greenhill M, Anthony M, Sharma S, Mushtaq R. MRI Imaging Appearance of Hyperostosis Frontalis Interna (HFI): A Case Report of Focal Benign Enhancement. Curr Med Imaging. 2024;20:e200723218926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 42. | Yiu Luk S, Fai Shum JS, Wai Chan JK, San Khoo JL. Bilateral thinning of the parietal bones: a case report and review of radiological features. Pan Afr Med J. 2010;4:7. [PubMed] |
| 43. | Lo CP, Chen CY, Chin SC, Juan CJ, Hsueh CJ, Chen A. Disappearing calvarium in Gorham disease: MR imaging characteristics with pathologic correlation. AJNR Am J Neuroradiol. 2004;25:415-418. [PubMed] |
| 44. | Wong M, Phillips CD, Hagiwara M, Shatzkes DR. Parry Romberg Syndrome: 7 Cases and Literature Review. AJNR Am J Neuroradiol. 2015;36:1355-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 45. | Osei-Yeboah C, Neequaye J, Bulley H, Darkwa A. Osteomyelitis of the frontal bone. Ghana Med J. 2007;41:88-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 46. | Akhaddar A. Cranial Osteomyelitis. In: Atlas of Infections in Neurosurgery and Spinal Surgery. Cham: Springer, 2017. [DOI] [Full Text] |
| 47. | Suri V, Singh A, Das R, Das A, Malhotra P, Jain S, Kumari S, Khandelwal N, Varma S. Osseous sarcoid with lytic lesions in skull. Rheumatol Int. 2014;34:579-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Thirunavukarasu V, Thirunavukarasu C, Willis T, Tsahtsarlis A. Osseous Sarcoidosis with a Solitary Lytic Skull Lesion: A Case Report. J Neurol Surg Rep. 2019;80:e27-e29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Colas L, Caron S, Cotten A. Skull Vault Lesions: A Review. AJR Am J Roentgenol. 2015;205:840-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 50. | Ugga L, Cuocolo R, Cocozza S, Ponsiglione A, Stanzione A, Chianca V, D'Amico A, Brunetti A, Imbriaco M. Spectrum of lytic lesions of the skull: a pictorial essay. Insights Imaging. 2018;9:845-856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 51. | Kee TP, Liauw L, Sathiyamoorthy S, Lee HY, Tan GSL, Yu WY. Large solitary lytic skull vault lesions in adults: radiological review with pathological correlation. Clin Imaging. 2020;59:129-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Luo ZH, Lu PX, Qi WL, Liao FX, Jin AF, Zen QY. Role of (18)F-FDG PET/CT in the diagnosis and management of patients with Langerhans cell histiocytosis. Quant Imaging Med Surg. 2022;12:3351-3363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 53. | Theodorou DJ, Theodorou SJ, Kakitsubata Y. Imaging of Paget disease of bone and its musculoskeletal complications: review. AJR Am J Roentgenol. 2011;196:S64-S75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 54. | Dondi F, Albano D, Treglia G, Bertagna F. Paget Disease as Common Pitfall on PET with Different Radiopharmaceuticals in Oncology: Not All That Glitters Is Gold! J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 55. | Amadi OP, Patel C, Tangella K, Khaliq W. 'Hair-on-end' sign: an important finding in clinical practice. QJM. 2012;105:905-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 56. | Chang CY, Rosenthal DI, Mitchell DM, Handa A, Kattapuram SV, Huang AJ. Imaging Findings of Metabolic Bone Disease. Radiographics. 2016;36:1871-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 57. | Baptista LC, Martins MM, Marcos VN. Radiographic findings in acromegaly: pictorial essay. Radiol Bras. 2023;56:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 58. | Alqahtani AS, Alshehri HM, Ahmed NF, Alatawi MH, Alshammari WMD, Saeedalshahrani A. Bone Deformity due to Rickets and Osteomalacia in Children and Adolescents. JPRI. 2021;33:59-65. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 59. | Chow KM, Szeto CC. Cerebral atrophy and skull thickening due to chronic phenytoin therapy. CMAJ. 2007;176:321-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 60. | Snoddy AME, Buckley HR, Elliott GE, Standen VG, Arriaza BT, Halcrow SE. Macroscopic features of scurvy in human skeletal remains: A literature synthesis and diagnostic guide. Am J Phys Anthropol. 2018;167:876-895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 61. | Arana E, Martí-Bonmatí L. CT and MR imaging of focal calvarial lesions. AJR Am J Roentgenol. 1999;172:1683-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 63. | Kim M, Kim HS, Kim JH, Jang JH, Chung KJ, Shin MK, Hwang HS, Kim BC, Jung SY. F-18 FDG PET-positive fibrous dysplasia in a patient with intestinal non-Hodgkin's lymphoma. Cancer Res Treat. 2009;41:171-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 64. | Poursina O, Qiu J. Primary intraosseous meningioma: a case of early symptomatic calvarial origin meningioma. J Surg Case Rep. 2024;2024:rjae676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 65. | Omofoye OA, Huynh T, Jhun R, Ashfaque H, Cronk K. Primary intraosseous meningioma of the calvarium: A systematic review. Clin Neurol Neurosurg. 2020;199:106283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 66. | Strauss SB, Steinklein JM, Phillips CD, Shatzkes DR. Intraosseous Venous Malformations of the Head and Neck. AJNR Am J Neuroradiol. 2022;43:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 67. | Krishnan P, Bhosle R, Patel S, Raju D, Cincu R, Moscote-Salazar LR, Gupta A, Agrawal A. Calvarial hemangiomas: Series of 6 cases and review of literature. World Neurosurg X. 2024;23:100297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 68. | Harris AE, Beckner ME, Barnes L, Kassam A, Horowitz M. Giant cell tumor of the skull: a case report and review of the literature. Surg Neurol. 2004;61:274-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 69. | Nagase S, Ogura K, Ashizawa K, Sakaguchi A, Hotchi S, Hishii M, Fukunaga M, Matsumoto T. Intraosseous Lipoma of the Calvaria in the Early Stage Resembling Normal Fatty Marrow. J Neurol Surg Rep. 2022;83:e29-e32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 70. | Deniz K, Ceylan D. Desmoplastic fibroma of the skull. Acta Neurochir (Wien). 2008;150:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 71. | Gupta S, Sharma G, Sajeevan S, Raut SN, Ahuja R, Joseph D, Gupta A, Gupta M. Varied Clinical Presentation and Management of Calvarial Metastases. Asian J Neurosurg. 2022;17:631-634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 72. | Eissa L, Bastawi RA. Skull base “intrinsic” bony mass lesions: conventional, diffusion and perfusion imaging with a proposed imaging approach. Egypt J Radiol Nucl Med. 2024;55:188. [DOI] [Full Text] |
| 73. | Tomura N, Mizuno Y, Saginoya T. PET/CT findings for tumors in the base of the skull: comparison of 18 F-FDG with 11 C-methionine. Acta Radiol. 2016;57:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 74. | Ludwig H, Novis Durie S, Meckl A, Hinke A, Durie B. Multiple Myeloma Incidence and Mortality Around the Globe; Interrelations Between Health Access and Quality, Economic Resources, and Patient Empowerment. Oncologist. 2020;25:e1406-e1413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 75. | Touzeau C, Moreau P. Multiple myeloma imaging. Diagn Interv Imaging. 2013;94:190-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 76. | Abdoh MG, Ajlan B, Basurrah AA, Al-Saiari S, Mujtaba SS, Rawah E, Brinji Z, Atteiah A, Farag AA. Primary Calvarial Lymphoma: A Case Report. Cureus. 2024;16:e55210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 77. | Cao W, Liang C, Gen Y, Wang C, Zhao C, Sun L. Role of diffusion-weighted imaging for detecting bone marrow infiltration in skull in children with acute lymphoblastic leukemia. Diagn Interv Radiol. 2016;22:580-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 78. | Zhao Z, Hu Y, Li J, Zhou Y, Zhang B, Deng S. Applications of PET in Diagnosis and Prognosis of Leukemia. Technol Cancer Res Treat. 2020;19:1533033820956993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 79. | Hadley C, Gressot LV, Patel AJ, Wang LL, Flores RJ, Whitehead WE, Luerssen TG, Jea A, Bollo RJ. Osteosarcoma of the cranial vault and skull base in pediatric patients. J Neurosurg Pediatr. 2014;13:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 80. | Khoei S, Boustani MR, Pak N. An atypical imaging characteristic of calvarial metastasis of neuroblastoma as multiple multi-loculated cystic masses with internal blood-fluid levels: a case report. BMC Neurol. 2022;22:471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 81. | Samim A, Tytgat GAM, Bleeker G, Wenker STM, Chatalic KLS, Poot AJ, Tolboom N, van Noesel MM, Lam MGEH, de Keizer B. Nuclear Medicine Imaging in Neuroblastoma: Current Status and New Developments. J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 82. | Idriceanu T, Lahiani W, Kalsoum E, Loganadane G, Palfi S. A Rare Case of Calvaria Solitary Fibrous Tumor: Case Report and Review of Literature. SN Compr Clin Med. 2023;5:150. [DOI] [Full Text] |
| 83. | Erdem E, Angtuaco EC, Van Hemert R, Park JS, Al-Mefty O. Comprehensive review of intracranial chordoma. Radiographics. 2003;23:995-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 183] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 84. | Awad M, Gogos AJ, Kaye AH. Skull base chondrosarcoma. J Clin Neurosci. 2016;24:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 85. | Palmisciano P, Haider AS, Sabahi M, Nwagwu CD, Bin Alamer O, Scalia G, Umana GE, Cohen-Gadol AA, El Ahmadieh TY, Yu K, Pathmanaban ON. Primary Skull Base Chondrosarcomas: A Systematic Review. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 86. | Mohindra S, Tripathi M, Batish A, Kapoor A, Patil NR, Mahendru S, Ahuja C, Chatterjee D. Primary Calvarial Ewing Sarcoma: A Case Series. J Neurol Surg B Skull Base. 2022;83:e181-e190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 87. | Sarigul B, Uysal E, Avci İ, Peker H, Celik S. Giant Calvarial Ewing's Sarcoma: A Case Report. J Neurol Surg Rep. 2018;79:e79-e82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 88. | Salunke PS, Gupta K, Malik V, Kumar N, Henke LE, Cai C, Chen WS, Pfeifer JD. Primary Ewing's sarcoma of cranial bones: analysis of ten patients. Acta Neurochir (Wien). 2011;153:1477-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 89. | Strah D, Stanley K, Oatmen K, Kylat RI, Dishop M, de la Maza M. An unusual presentation of neonatal rhabdomyosarcoma: a case report. Front Pediatr. 2023;11:1233334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 90. | An HR, Cho KJ, Song SW, Park JE, Song JS. Rhabdomyosarcoma of the skull with EWSR1 fusion and ALK and cytokeratin expression: a case report. J Pathol Transl Med. 2024;58:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 91. | de Vries ISA, van Ewijk R, Adriaansen LME, Bohte AE, Braat AJAT, Fajardo RD, Hiemcke-Jiwa LS, Hol MLF, Ter Horst SAJ, de Keizer B, Knops RRG, Meister MT, Schoot RA, Smeele LE, van Scheltinga ST, Vaarwerk B, Merks JHM, van Rijn RR. Imaging in rhabdomyosarcoma: a patient journey. Pediatr Radiol. 2023;53:788-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |