Published online Aug 19, 2025. doi: 10.5498/wjp.v15.i8.108933
Revised: June 3, 2025
Accepted: June 24, 2025
Published online: August 19, 2025
Processing time: 104 Days and 14.4 Hours
To illustrate the mechanisms of exosomal microRNAs (miRNAs) in common men
Core Tip: This synthesis highlights exosomal microRNAs as dual-functional tools bridging mechanistic insights into mental disorders and paving the way for personalized diagnostics/therapeutics. Focus on shared pathways (e.g., toll-like receptor 4, phosphatidylinositol 3-kinase/protein kinase B) may unify treatment strategies across diverse psychiatric conditions.
- Citation: He YN, Zhu HH, Zhou ZH, Qu KK. Exosomal microRNAs in common mental disorders: Mechanisms, biomarker potential and therapeutic implications. World J Psychiatry 2025; 15(8): 108933
- URL: https://www.wjgnet.com/2220-3206/full/v15/i8/108933.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i8.108933
Exosomes are nanoscale lipid structures ranging in diameter from 40 nm to 100 nm, which contain microRNAs (miRNAs), circular RNAs long non-coding RNAs, messenger RNAs (mRNAs), and other substances within their encapsulation[1-3]. These secreted membrane-bound particles originate from the inward budding of endosomal mem
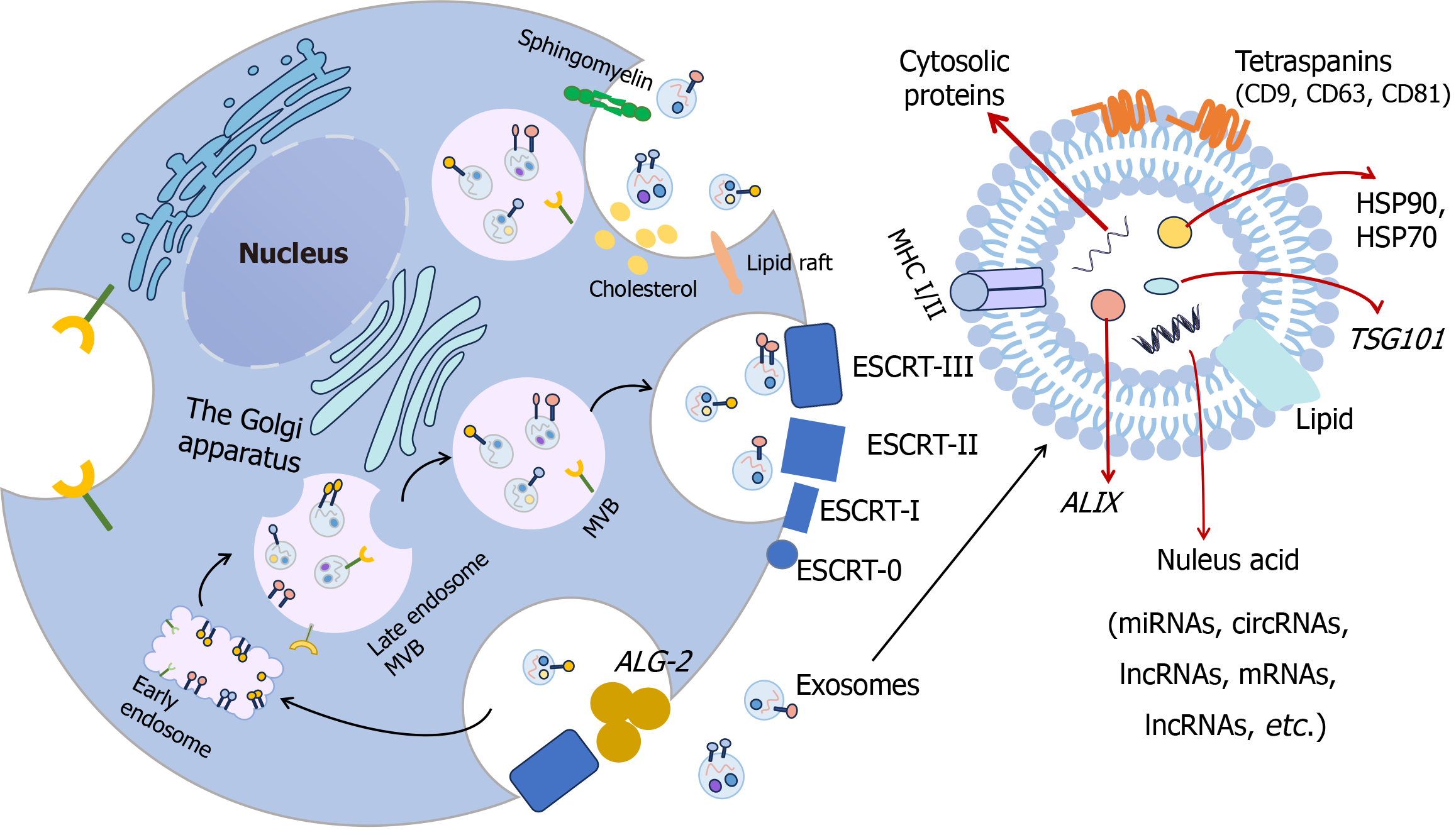
Recently, the mechanism of exosomes has attracted more and more interest from researchers. The mechanism of endosome sorting complex required for transport (ESCRT) is the most representative classical mechanism. The ESCRT machinery plays a pivotal role in orchestrating the biogenesis of exosomes through sequential activation of its core components ESCRT-I, ESCRT-II, and ESCRT-III. Mechanistically, ESCRT-III subunits assemble into membrane-associated polymeric structures that drive vesicle scission. This ESCRT-mediated process necessitates the calcium-binding apoptosis-linked gene-2-interacting protein X to facilitate the recruitment of ESCRT complexes to ubiquitinated cargo domains within late endosomal compartments (Figure 1). The coordinated action of these molecular components enables the precise intraluminal vesicle formation and subsequent exosomal secretion via membrane abscission mediated by the AAA+ + ATPase Vps4 complex. The mechanism of exosomes plays a vital role in diagnosing and treating mental disorders[6,7].
Mature miRNAs are naturally occurring small non-coding RNA molecules, with a canonical length of 22 ± 1 nucleotides[8]. These miRNAs exhibit partial complementarity with one or several mRNA molecules. The primary role of this process is to inhibit gene expression through various mechanisms, such as suppressing translation, cleaving mRNA, and removing poly (A) tails[9,10].
Several miRNAs can regulate a gene or a molecular pathway, thus forming a complex and powerful regulatory network[11]. This mechanism solves most pathological mechanism problems and broadens the understanding of miRNAs in mental disorders. In addition, miRNAs can be targeted to molecular regulation, bringing new hope for the precise treatment of mental disorders[10,11].
Recently, miRNAs have become a central focus in precision and molecular medicine research. Exosomes are regarded as highly suitable markers for detecting mental disorders, principally due to their unimpeded ability to traverse the blood-brain barrier (BBB). The overarching aim is to identify risk factors for diseases during the incipient stages of their onset, thereby enabling the implementation of preventive measures to curtail further disease progression. Notably, miRNAs have not been observed to cross the BBB freely. Significantly, exosomes conjugated with miRNAs possess the potential not only to predict the development of mental disorders but also to provide targeted therapeutic sites. This characteristic of exosomal miRNA associations could open up novel avenues for both the early diagnosis and targeted treatment of mental disorders, integrating the detection advantages of exosomes and the regulatory functions of miRNAs.
In this review, we summarize the pivotal roles and biomedical potential of exosomal miRNAs as follows: Firstly, exosomal miRNAs actively regulate diverse biological processes, including cell proliferation, differentiation, migration, and disease pathogenesis, leveraging the transport capacity of exosomes[12]. Secondly, these miRNAs exhibit broad detectability across multiple biofluids, such as saliva[13], urine[14], breast milk[15], peripheral blood[14], and cerebro
The application of exosomal miRNAs as promising biomarkers for disease diagnosis and therapeutic monitoring has driven extensive research into their isolation and detection methodologies. Despite significant advancements, the field currently lacks a standardized protocol for exosomal miRNAs isolation. Established isolation techniques primarily include ultra-speed centrifugation (differential ultracentrifugation and density gradient ultracentrifugation)[20], immu
For subsequent detection and quantification of exosomal miRNAs derived from human biofluids, researchers employ multiple analytical strategies such as quantitative real-time polymerase chain reaction (qRT-PCR), which is the gold standard for miRNA profiling due to its high sensitivity[27], while enzyme-linked immunosorbent assay enables protein-coupled miRNA detection[28]. Complementary approaches include flow cytometry[29] and immunoblotting (Western blot)[28]. While qRT-PCR dominates clinical applications due to its practicality, each method exhibits distinct advantages in specificity, throughput, and compatibility with downstream workflows. Consequently, method selection should be guided by experimental objectives and sample-specific considerations.
For subsequent detection and quantification of exosomal miRNAs derived from human biofluids, researchers employ multiple analytical strategies, qRT-PCR remains the gold standard for miRNA profiling due to its high sensitivity[27], while enzyme-linked immunosorbent assay enables protein-coupled miRNA detection[28]. Complementary approaches include flow cytometry[29] and immunoblotting (Western blot)[28]. While qRT-PCR dominates clinical applications due to its practicality, each method exhibits distinct advantages in specificity, throughput, and compatibility with down
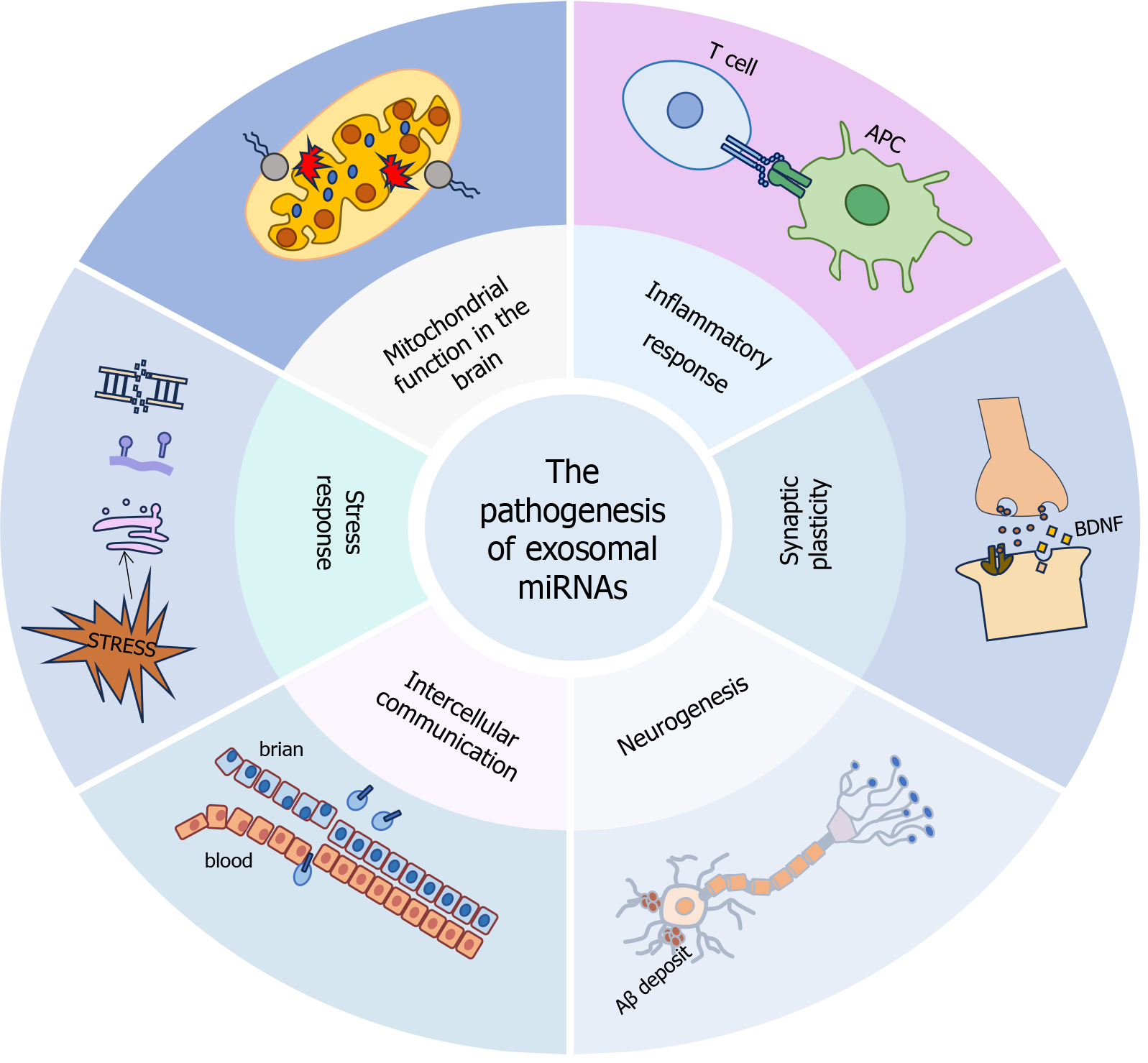
The complex biological specificity of exosome-mediated cell communication stems from two fundamental features: (1) Different internalization mechanisms, including clathrin-dependent endocytosis, macrophage action, and receptor-ligand docking systems; and (2) Intrinsic target cell selectivity is determined by surface tetradin topography and integrin-mediated tissue tropism (Figure 2). These increase the complexity of exosome functions in intercellular communication[30,31].
As there are no multivesicular bodies on neurons, many multivesicular bodies exist in their cell bodies and dendrites[31]. Due to this distribution, exosomes are crucial to signal transmission in the CNS. As a link carrier between the same or different types of cells, especially between neurons and glial cells, exosomal miRNAs play an essential role in the physiological and pathological processes of neuropsychiatric diseases, and have become an effective target for diagnosis and treatment[32]. For instance, in their research on Alzheimer’s disease (AD), Liu et al[33] discovered that miR-135a can traverse the BBB from CSF to peripheral blood serum. This finding presents novel targets for the diagnosis of AD[33]. Mesenchymal stem cell-derived exosomes improved ethanol-induced prefrontal cortex inflammation in adolescent mice, restored ethanol-treatment-induced myelin and synaptic disorders, as well as memory and learning impairments through intercellular communication[3].
Exosomes influence neurodegenerative disorders through two main mechanisms: (1) Regulating neural differentiation and development; and (2) Controlling synaptic vesicle activity in neurons. These dual functions emphasize exosomes as key players in neurogenesis and synaptic balance, connecting their activity to the progression of neurodegenerative disorders. Exosomes may promote or limit the aggregation of unfolded and abnormally folded proteins in the brain. In addition, exosomes can also take part in the clearance of misfolded proteins to play detoxification and neuroprotective functions[34-36]. For example, differentially expressed exosomes and their associated miRNAs in the denervated hip
Exosomal miRNAs exhibit intricate functions in neurogenesis. MiR-146a-5p regulates depression-related neurogenesis through the KLF4/CDKL5 pathway in major depressive disorder (MDD) model mice[38]. In another study of MDD model mice, miR-139-5p modulated neurogenesis and depressive behavior in mice[39]. Exosomal miRNAs are implicated in the pathophysiological processes of neurogenesis in both psychiatric and neurodevelopmental disorders. The injection of human urine-derived exosomal miR-21-5p into Rett syndrome (RTT) mouse models enhanced early neurogenesis via the EPha4/TEK signaling axis[40].
Synaptic plasticity plays a vital role in the mechanisms underlying cognition. This complex process allows for the up-regulation or down-regulation of synapses based on activity levels, which is essential for forming and consolidating memories. When this plasticity is disrupted due to genetic, environmental, or physiological factors, it can result in signi
Exosomes and exosomal miRNAs play a significant role in synaptic plasticity. Notably, miR-132-3p is crucial for this process. Exosomes derived from mesenchymal stem cells, rich in miR-132-3p, enhanced neuronal and synaptic function by activating the Ras/Akt/GSK3β pathway, thereby facilitating the recovery of cognitive function[43]. Due to its high abundance in brain tissue, miR-132-3p has attracted extensive research attention[44]. The Ras/Akt/GSK3β pathway is not only implicated in the pathogenesis of depression, but also plays a crucial role in other diseases, such as cerebrovascular disorders[43]. The interplay between miR-132-3p and the Ras/Akt/GSK3β pathway offers innovative perspectives on the applications of exosomes and exosomal miRNAs.
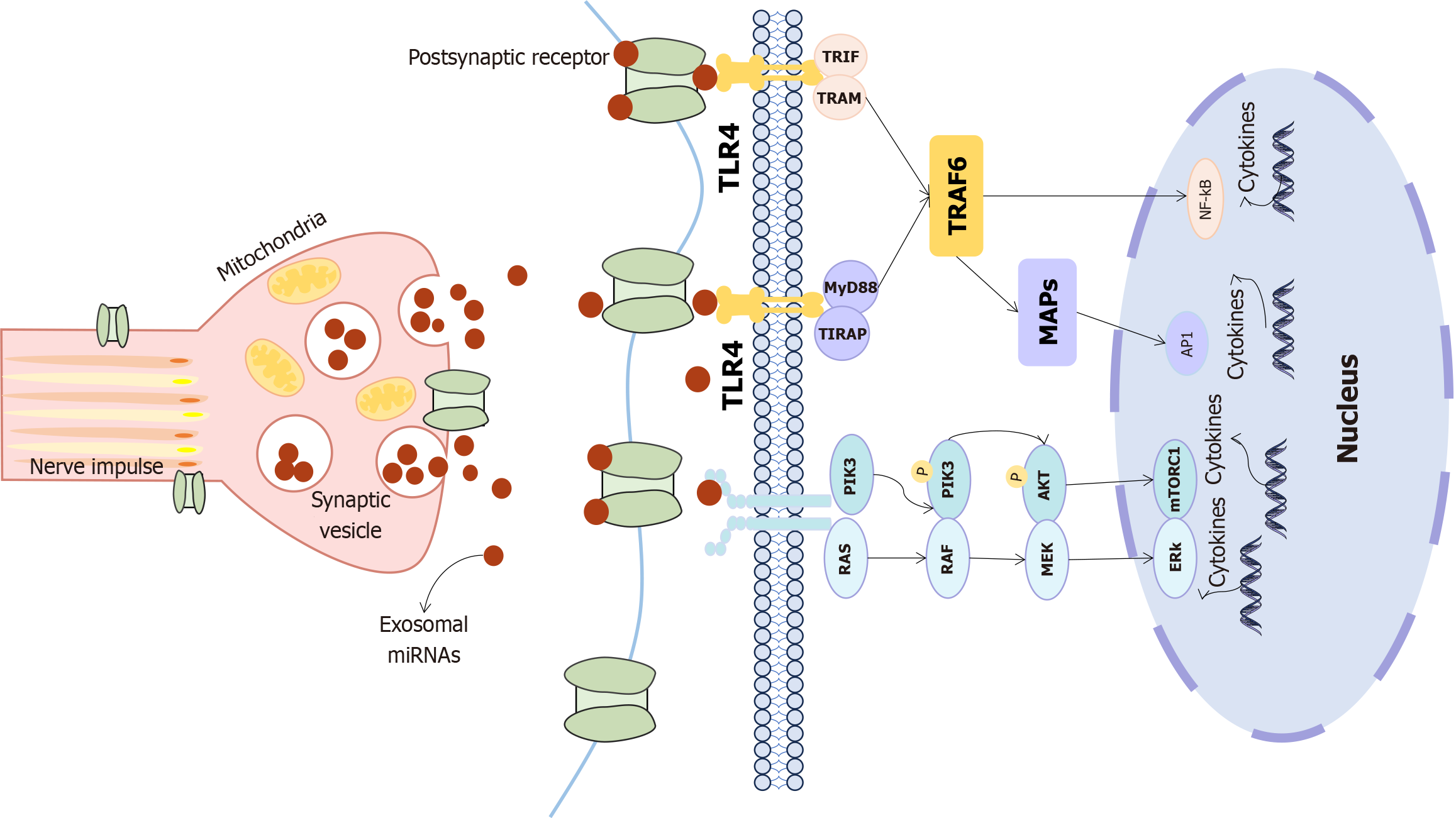
Exosome receptor-mediated pinocytosis crosses the BBB, establishing two-way communication channels and promoting molecular crosstalk between the CNS and the peripheral system. This inherent BBB penetration makes these vesicles key transporters of neuroactive biomolecules, making intercellular signaling essential for neurohomeostasis and pathological transmission. They can transport a wide range of biochemical molecules, including pro-inflammatory factors from the periphery to the CNS, where they target glial and neuronal cells. Conversely, during CNS inflammation, exosomes can deliver anti-inflammatory molecules to CNS cells, offering potential for targeted therapy[45]. The toll-like receptor 4 (TLR4) pathway is a well-established immune response axis that has been extensively researched in the context of immune regulation and mental disorders. Yang et al[46] reported that lipopolysaccharide-induced exosomal miR-146a modulates the expression of AD risk genes by suppressing the TLR4 signaling pathway[46].
Exosomes, characterized by their bidirectional transport capabilities, can transport substances that contribute to the amelioration of mental disorder symptoms. Wei et al[47] showed that anti-inflammatory microglia could modulate the AD cell model by transferring exosomal miR-223. Zhai et al[48] experimented by injecting exosomes and miR-22-loaded exosomes derived from adipose-derived mesenchymal stem cells into AD model mice. By comparing the two groups, it was found that the expression level of inflammatory factors in the group receiving regular exosomes was higher than that in the group administered miR-22-loaded exosomes. This finding suggests that the intervention with miR-22-loaded exosomes significantly downregulates inflammatory factors in the CSF, effectively preventing further nerve cell damage[48]. In particular, this study subjected exosomes and exosomal miRNAs to independent investigation. The findings suggest that exosomal miRNAs may mediate biological effects distinct from their parent exosomes in certain pathological contexts. This functional dichotomy may enable the development of precision therapeutic strategies for diseases such as AD. Furthermore, these results underscore the critical need to delineate the differential contributions of exosomal miRNAs vs whole exosomes in disease pathogenesis, which is essential for advancing their translation into clinical appli
Mitochondria are the primary energy producers within cells and participate in many vital cellular processes, encom
The dual effects of the increase in exosomal miR-137 and the deletion of COX6A2 lead to the stacking of damaged mitochondria, intensifying mitochondrial oxidative stress and damage to cortical parvalbumin interneurons (PVI), resulting in schizophrenia symptoms. It is worth noting that the mitochondrial antioxidant mitoquinone mesylate can reverse the overexpression of miR-137 induced by oxidative stress, the decrease in COX6A2, mitochondrial autophagy, and PVI damage. This reverse verifies that the upregulation of miR-137 and the subsequent defect in mitochondrial autophagy are one of the molecular mechanisms of oxidative stress-induced PVI damage[49].
Electroacupuncture in combination with neural stem cell-derived exosomes has been demonstrated to ameliorate depression-like behavior in rat models of MDD through the reversal of structural and functional impairments observed in synapses and mitochondria within the hippocampus[50]. However, a limitation of this study is the lack of separate investigations into electroacupuncture and exosomes, thereby leaving the specific role of exosomes somewhat ambi
Stress responses in the context of mental disorders encompass both biological factors and life events, which interact synergistically throughout the progression of mental disorders, with stressors such as oxidative stress, exposure to light, and various life events being included.
The injection of miR-139-5p antagonists treated depressive behavior in mildly stressed mice, suggesting that elevated levels of exosome miR-139-5p are likely to mediate depressive behavior induced by oxidative stress in mice[39]. Microglia-derived exosomes treated with 1070 nm light reduce endoplasmic reticulum stress that is induced by targeting the activating transcription factor-6 in AD model mice, thereby reducing AD symptoms[52]. In a study concentrating on post-traumatic stress disorder (PTSD) among veterans engaged in military operations within the United States, a correlation was observed between the severity of PTSD symptoms and the levels of exosomal proteins and exosomal miRNAs in the peripheral blood[53]. This finding suggests a possible association between the severity of PTSD symptoms and the presence of these molecular entities in the bloodstream. Essentially, exosomal proteins and exosomal miRNAs could serve as potential markers for assessing the severity of PTSD.
AD is a common neurodegenerative disease. Its pathological changes often manifest as senile plaques, neuronal tangles, hippocampal cellular granules, vacuolar degeneration, neuron loss, and so on (Figure 3). These pathological changes are related to genetic factors, abnormal β-amyloid metabolism, and neurotransmitter disorders[54]. Neurotransmitter disorders include exosomes and other transmitters, which are involved in intercellular communication, neurogenesis, and immune response, as well as impacting the occurrence and development of AD (Tables 1 and 2).
| miRNAs | Alteration | Model | Type tissue | Significance | Ref. | |
| Intercellular communication | miR-135a | Increase | AD mouse models, human patients | CSF, serum, plasma | ATP-binding cassette transporter-A1 reduces the false negatives of exosomal miRNAs. Possible biomarker for AD | Liu et al[33] |
| miR-193b | Increase | Cell models, AD mouse models, human patients | CSF, serum, cell | Possible biomarker for AD | Liu et al[55] | |
| miR-770-3p | AD mouse models | Brain tissue | As the downstream target of circ-Epc1, overexpression of circ-Epc1 affects M1 microglia and forms AD | Liu et al[56] | ||
| miR-29 | Decrease | AD rice models | Hippocampus | Exosomal miR-29 has protective effects on amyloid pathogenesis | Jahangard et al[57] | |
| miR-185-5p | Decrease | AD mouse models, AD cell models, human patients | Brain tissue, cell, serum and CSF | The expression of exosomal miR-185-5p was decreased by binding of APP 3’UTRs to miR-185-5p | Ding et al[58] | |
| miR-532-5p | AD mouse models | Brain tissue | The exosomal miR-532-5p targets EPHA4, and reduces EPHA4 its expression. Possible biomarker of AD | Liang et al[59] | ||
| miR1385p | AD mouse models, cell models | Brain tissue, hippocampus, cell | By targeting Tau as a protective factor for AD. Possible therapeutic targets | Meng et al[60] | ||
| miR-451a, miR-19a-3p | AD mouse models | Hippocampus | Possible biomarker for AD | Yan et al[61] | ||
| miR-29b-2 | Cell models | Cell | Reduces PSEN1 levels and inhibits secretory enzymes. Possible therapeutic targets | Lin et al[62] | ||
| miR-196b-5p, miR-339-3p, miR-34a-5p, miR-376b-3p, miR-677-5p, miR-721 | AD mouse models | Urine | Urinary exosomal miRNAs are promising to supplement or replace invasive cerebrospinal fluid. Possible biomarker for AD | Song et al[63] | ||
| Inflammatory response | miR-146a | Increase | AD mouse models | Hippocampus | Macrophage tolerance to TLR4 was induced | Yang et al[46] |
| miR-124-3p | AD mouse models | Brain tissue | Neurodegeneration is mitigated by targeting Rela/ApoE signaling pathways to transfer into hippocampal neurons | Ge et al[64] | ||
| miR-124 | Increase | AD cell models | Cell | Regulation by the secretome miR-124-3p released through the AD cell models. A promising therapy in AD | Garcia et al[65] | |
| miR-21 | Increase | AD mouse models | Brain tissue, hippocampus | Take part in immune processes. Possible biomarker for AD | Garcia et al[66] | |
| miR-146a-5p | AD mouse models, AD cell models | Cell, brain tissue, hippocampus, plasma | Targeted regulation of the HIF1α/mtROS pathway and improvement of NLRP3 inflammasome and inflammatory factors can alleviate cognitive impairment in IH mice | Zhang et al[67] | ||
| miR-223 | Cell models | Cell | Yb-1-mediated microglial exosomal sorting of miR-223 improves nerve cell damage repair and is a promising therapeutic target for AD | Wei et al[68] | ||
| miR-223 | Decrease | AD cell models | Cell | It acts as a protective factor for AD through the PI3K/Akt signaling pathway | Wei et al[47] | |
| miR-146a | Increase | AD mouse models | Hippocampus, CSF | Exosomal transfer of miR-146a is involved in the correction of cognitive dysfunction in AD | Nakano et al[69] | |
| miR-22 | AD mouse models | CSF and peripheral blood | MiR-22-loaded ADMSC-derived exosomes can reduce the release of inflammatory cytokines by inhibiting pyroptosis | Zhai et al[48] | ||
| miR-7670-3p | AD mouse models | Brain tissue | MiR-7700-3p reduces ATF6 expression, protects the integrity of dendritic spines in cortical and hippocampal neurons, and ultimately improves cognitive function | Chen et al[52] | ||
| Others | miR-124 | Increase | AD cell models | Cell | A novel diagnostic biomarker in circulating exosomes is a potential therapeutic target for AD whenever its deregulation is determined | Garcia et al[70] |
| miR-1306-5p | Decrease | Cell models, human patients | Cell, serum | CircAXL promotes the inhibition of PDE4A by targeting miR-1306-5p. Possible biomarker for AD | Meng et al[71] | |
| miR-211-5p | AD cell models | Cell | Inhibition of miR-211-5p may improve the efficacy of HUCMSC-derived exosomes in the treatment of AD by increasing the expression of NEP | Chen et al[72] |
| miRNA | Alteration | Model | Type tissue | Significance | Ref. |
| miR-320a, miR-328-3p, miR-204-5p | Decrease | Human patients | CSF | Potential targets for miR-328-3p involve AMPK signaling pathways associated with amyloid and tau metabolism in AD. Reliable biomarkers of AD | Tan et al[73] |
| miR-455-3p | Increase | Human patients | CSF | A potential biomarker for AD | Kumar and Reddy[74] |
| miR-9, miR-21, miR29-b, miR-122, miR-132 | Decrease | Human patients | Plasma | MiR-122 is related to alpha-tocopherol and may be a new targeted therapy | Boccardi et al[75] |
| miR-125b, miR-451a | Increase | Human patients | Peripheral blood | It plays a role through the PI3K/Akt signaling pathway. Possible biomarker for AD | Duan et al[76] |
| miR-342-5p | Increase | Human patients | Peripheral blood | Degradation of BACE1 mRNA in cells by targeting the 3’UTR sequence of BACE1 ameliorates β-Amyloid formation | Dong et al[77] |
| miR-16-5p, miR-25-3p, miR-92a-3p, miR-451a | Decrease | Human patients | Plasma | May have been involved in the early development of AD | Visconte et al[78] |
| miR-373, miR-204 | Decrease | Human patients | Peripheral blood | Potential biomarkers for AD | Taşdelen et al[79] |
| miR-30b-5p, miR-22-3p, miR-378a-3p | Decrease | Human patients | Peripheral blood | Potential biomarkers for the diagnosis of AD | Dong et al[80] |
| miR-483-5p, miR-502-5p | Decrease | Human patients | Plasma | Possible biomarker for AD | Liu et al[81] |
| miR-29c-3p | Increase | Human patients | Plasma | Possible biomarker for AD | Li et al[82] |
| miR-23a | Increase | Human patients | Plasma | Possible biomarker for AD | Barbagallo et al[83] |
| miR-126-3p, miR-142-3p, miR-146a-5p, miR-223-3p | Decrease | Human patients | CSF and peripheral blood | As a biomarker reflecting AD severity | Aharon et al[84] |
| miR-384 | Increase | Human patients | CSF and plasma | MiR-384 downregulates the expression and activity of ACE-1 to alleviate AD symptoms | Li et al[85] |
| miR-485-3p | Increase | Human patients | Saliva | Possible biomarker and treatment for AD | Ryu et al[86] |
| miR-486-3P | Increase | Human patients | Brain tissue and peripheral blood | Possible biomarker for AD | Cheng et al[87] |
| miR-185-5p | Human patients | Blood sample | Education decreased AD expression, depression increased AD expression, and participated in the production and accumulation of Aβ in patients, and enhanced depression, which participated in the production and accumulation of Aβ | Wang et al[88] |
In animal experiments related to AD, further understanding of the disease can be obtained by studying the patho
Liu et al[55] conducted a systematic investigation and demonstrated that the utilization of ABCA1 significantly enhances the detection efficiency of exosomes. Their findings revealed a consistent up-regulation of exosomal miR-135a and miR-193b across multiple experimental paradigms. The study employed a comprehensive approach, incorporating AD mouse models, in vitro cell cultures, and clinical samples, including CSF and serum obtained from AD patients. These results underscore the pivotal role of ABCA1 in exosome detection and suggest that miR-135a and miR-193b may serve as promising biomarkers for AD. It is necessary to conduct further research to elucidate the functional implications of these exosomal miRNAs in the pathogenesis of AD[33,55].
Studies on the brain tissue of AD model mice demonstrated that hypoxia-pretreated adipose-derived stem cell-derived exosomes can significantly enhance cognitive function. This improvement is mediated through the delivery of circEpc1, which regulates the M1/M2 polarization of microglia. Notably, circEpc1 functions as an upstream regulator of miR-770-3p. Intriguingly, miR-770-3p and circEpc1 do not exhibit synergistic changes during this process, suggesting a complex regulatory mechanism underlying their roles in microglial polarization and cognitive enhancement. These findings highlight the therapeutic potential of hypoxia-preconditioned adipose-derived stem cell exosomes in AD and provide insights into the molecular interactions between circEpc1 and miR-770-3p[56].
In a study focused on the hippocampus of a rat AD model, Jahangard et al[57] conclusively demonstrated the down-regulation of miR-29 expression. Subsequent experiments revealed that the administration of miR-29-enriched exosomes into the mouse hippocampus markedly reduced the extent of Aβ aggregation. These compelling findings suggest that miR-29 might serve as a significantly hopeful therapeutic miRNA target for the treatment of AD. This study revealed the prospects of exosome-based delivery systems for modulating miRNA expression and illustrated miR-29 as a critical player in mitigating Aβ pathology in AD. Ding et al[58] revealed that dysregulation of the amyloid precursor protein led to the binding and anchoring of miR-185-5p to the 3’untranslated region (3’UTR) of amyloid precursor protein. This interaction resulted in a significant reduction in exosome levels in both brain tissue and serum samples from AD patients and AD mouse models. In particular, this study was the first to identify a direct regulatory relationship between miR-185-5p and the 3’UTR region of amyloid precursor protein, providing new insights into the molecular mechanisms underlying exosome dysregulation in AD. These results point out the potential role of miR-185-5p in modulating amyloid precursor protein expression and its implications for exosome biogenesis in AD pathogenesis[58].
Liang et al[59] reported that in AD model mice, exercise led to an improvement in AD symptoms. This was achieved by exosomal miR-532-5p, which could cross the BBB, and targeted erythropoietin-producing hepatocellular carcinoma A4. In addition, Meng et al[60] discovered that miR-138-5p within neural stem cell-derived exosomes functioned as a protective factor against AD by targeting the Tau protein. Also, by a thorough study of the mechanism of action of fasudil on AD, Yan et al[61] identified that miR-451a and miR-19a-3p could serve as effective targets for diagnosing and treating AD. In cell culture investigations on AD, it was demonstrated that miR-29 b-2 decreased presenilin 1 and inhibited gamma-secretase activity. This indicates that miR-29 b-2 could be a promising target for developing AD therapies[62].
Notably, miR-196 b-5p, miR-339-3p, miR-34a-5p, miR-376 b-3p, miR-677-5p, and miR-721 were identified in the urine of AD model mice. This significant discovery not only highlights the potential of urinary exosomes as a non-invasive source of biomarkers for AD but also broadens the scope of exosome applications in disease diagnosis and monitoring. These findings demonstrate the utility of exosomes as valuable tools for identifying miRNA signatures in biofluids, offering new avenues for early detection and therapeutic intervention in AD[63].
Yang et al[46] injected lipopolysaccharide into the lateral ventricles of AD model mice. Their findings revealed that the expression of miR-146a in hippocampal exosomes was upregulated, and this upregulation was mediated through inhibition of the TLR4 signaling pathway. Research on brain tissues of cognitively impaired mice demonstrated that miR-124-3p transfers to hippocampal neurons and alleviates neurodegeneration by targeting the Rela/ApoE signaling pathway[64]. However, in another study, miR-124-3p was used as a target of miR-124 in AD cell models to regulate microglia inflammation and alleviate neurodegenerative changes[65]. These results suggest distinct yet potentially complementary roles of miR-124-3p and miR-146a in modulating neuroinflammation and neurodegeneration in AD, showing the complexity of miRNA-mediated regulatory mechanisms in the disease.
By studying the brain tissue and hippocampus of AD model mice, it was reported that miR-21 plays a role in repro
In an experiment employing AD cell models, exosomal miR-223 derived from microglia was demonstrated to alleviate neuronal damage and neuroinflammation[68]. Research on exosomes sourced from mesenchymal stem cells revealed that exosomal miR-223 can activate the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, suppress inflammation, and inhibit neuronal apoptosis in vitro by targeting phosphatase and tensin homolog deleted on chromosome ten. These observations present a novel potential avenue for treating AD[47]. These findings suggest a promising therapeutic strategy for AD, stressing the promise of exosomal miR-223 as a novel intervention to modulate neuroinflammation and neuronal survival.
Nakano et al[69] injected exosomes from bone marrow mesenchymal stem cells into the lateral ventricles of an AD mouse model and up-regulated miR-146a levels in the hippocampus. Also, alleviation of AD-related cognitive dys
In an AD model, mice treated with 1070-nm light modulation demonstrated significant alleviation of microglial inflammation. Specifically, exosomal miR-7670-3p was found to downregulate the expression of the target gene, activating transcription factor 6, thereby reducing endoplasmic reticulum estrogen receptor stress and subsequently attenuating the inflammatory response. These findings provide a novel therapeutic strategy for the treatment of AD, highlighting the potential of light-modulated exosomal miRNAs in regulating estrogen receptor stress and neuroinflammation[52]. This discovery paves the way for developing targeted interventions to address the underlying pathological mechanisms of AD.
MiR-124 showed different effects in two different AD model cell types. In SH-SWE neurons, the exosome miR-124 caused neurite growth, mitochondrial activation, and a decrease in the level of small Aβ oligomers. In contrast, in iNEU-PSEN cells, it reduced Tau phosphorylation. In addition, the use of miR-124 inhibitors resulted in a decrease in dendrite spine density. These findings suggest that the effects of miR-124 may be highly dependent on specific types of neuronal cells[70]. This cell type-specific function highlights the complexity of the regulatory mechanisms of miR-124 in AD and underscores the need for further study of its environmentally dependent effects.
When the circular AXL receptor tyrosine kinase was silenced in AD model cells, it was observed that this action inhibited phosphodiesterase 4A by liberating miR-1306-5p. Consequently, the pathological neurotoxicity of AD triggered by Aβ1-42 was eased[71]. Studies have demonstrated that purified exosomes derived from human umbilical cord mesenchymal stem cells exhibit overexpression of miR-211-5p inhibitors. This was significantly more effective than control exosomes in alleviating damage caused by Aβ1-40 treatment. These researchers reported that exosomal miR-211-5p inhibition enhanced the protective effects of human umbilical cord mesenchymal stem cells-derived exosomes against Aβ-induced neurotoxicity, highlighting their potential as a therapeutic intervention for AD[72]. This investigation under
In the study of clinical exosomes and AD, although there are still some gaps in the in-depth mechanism exploration compared with animal experiments, there have been many interesting discoveries with clinical application potential.
Clinical evidence reveals significant down-regulation of specific exosomal miRNAs particularly miR-320a, miR-328-3p, and miR-204-5p in CSF samples from AD patients. Notably, miR-328-3p has been mechanistically linked to the adenosine 5’-monophosphate-activated protein kinase signaling pathway, where it modulates critical pathways governing amyloid precursor protein processing and tau phosphorylation dynamics, suggesting its potential role in AD-related proteostasis dysregulation[73]. However, the exosomal miR-455-3p was up-regulated in CSF[74]. Although these exosomal miRNAs exhibit different trends in CSF, they can all be used as probable diagnostic markers for AD.
Boccardi et al[75] demonstrated that the plasma levels of exosomal miR-9, miR-21, miR-29b, miR-122, and miR-132 in patients were decreased. Specifically, miR-122 is associated with vitamin E’s participation in oxidative stress and might play a role in the onset of AD. Of particular interest, miR-122 has been functionally linked to vitamin E-mediated antio
Comprehensive profiling of peripheral blood-derived exosomes revealed significant down-regulation of multiple miRNA species in AD patients, including miR-16-5p, miR-25-3p, miR-92a-3p, miR-451a, miR-373, miR-204, miR-126-3p, miR-30 b-5p, miR-22-3p, miR-378a-3p, miR-483-5p, and miR-502-5p[78-81]. In peripheral blood, miR-29c-3p and miR-23a were up-regulated[82,83]. These studies provide compelling evidence that peripheral blood exosome miRNAs hold important promise as effective biomarkers for early AD diagnosis and prognostic assessment.
Some exosomal miRNAs are detectable in CSF and peripheral blood. For example, miR-126-3p, miR-142-3p, miR-146a-5p, and miR-223-3p exhibit down-regulation[84]. In addition, miR-384 shows an up-regulated expression pattern. Up-regulation of miR-384 exerts a regulatory effect on angiotensin-converting enzyme 1. Specifically, it can suppress the expression and activity of angiotensin-converting enzyme 1. By doing so, miR-384 can potentially mitigate the symptoms associated with AD[85].
Interestingly, patient saliva also contains exosome components, and the content of exosomes and exosomal miRNA can also predict AD, and the expression of miR-485-3p is up-regulated[86]. Studies on patients’ tissue and plasma exosomes showed that Has-miR-486-3P was up-regulated[87]. Moreover, Wang et al[88] discovered that miR-185-5p is associated with both education level and depression status. A higher level of education is linked to a reduced likelihood of AD manifestations, while depression exacerbates AD-related symptoms. Mechanistically, Has-miR-185-5p is involved in the production and accumulation of amyloid-β-β peptides.
Exosomes and exosomal miRNAs represent an emerging field of research in schizophrenia, offering exciting possibilities for improving diagnosis, treatment, and patient outcomes. As research progresses, they are poised to become integral to the precision medicine approach for schizophrenia, ultimately transforming how we understand and manage this complex disorder (Tables 3 and 4).
| miRNAs | Alteration | Model | Type tissue | Significance | Ref. |
| miR-137 | Increase | Gclm-KO mice, human patients | Plasma, prefrontal cortex | The changes in the level of miR-137/COX6A2 in plasma exosomes may be a marker of schizophrenia caused by PVI injury resulting from mitochondrial oxidative stress | Khadimallah et al[49] |
| miR-146a-5p | Increase | Schizophrenia mouse models, cell models | Plasma, cell | It may target NOTCH1, inhibit synaptic activity mediated by the Notch signaling pathway, and ultimately promote the occurrence and progression of schizophrenia in mice | Wang et al[89] |
| miR-223-3p | Increase | Mouse models, human postmortem brain, cell models | Cortical neuron, postmortem brain, astrocyte | miR-223 is a miRNA secreted by exosomes targeting glutamate receptors | Amoah et al[90] |
| miRNAs | Alteration | Model | Type tissue | Significance | Ref. |
| miR-203a-3p | Increase | Human patients, cells | Blood sample, cell | Targeted regulation of the 3’UTRs of DJ-1. Possible therapeutic target for schizophrenia | Tsoporis et al[91] |
| miR-675-3p | Human patients, cell models | Peripheral blood, cell | Associated with clozapine therapy. Understanding new pathogenesis | Funahashi et al[92] | |
| miR-486-5p, miR-199a-3p, miR-144-5p, miR-451a, miR-143-3p, miR-142- 3p | Decrease | Human patients | Urine | Urinary exosomal miRNA is a biomarker for predicting schizophrenia | Tomita et al[93] |
In the prefrontal cortex and blood of mice with disrupted redox homeostasis (Gclm-KO ± GBR), oxidative stress induces the up-regulation of exosomal miR-137. This increase in exosomal miR-137 expression results in the down-regulation of cytochrome c oxidase subunit 6A2, a critical component of mitochondrial complex IV, as well as a reduction in markers of mitochondrial phagocytosis. The consequent accumulation of damaged mitochondria further exacerbates oxidative stress, leading to selective impairment of parvalbumin-expressing interneurons, which are particularly vulnerable to redox imbalance[49]. These results demonstrate the role of exosomal miR-137 as a critical mediator in the interplay between oxidative stress, mitochondrial dysfunction, and neuronal pathology. Following its up-regulation, exosomal miR-146a-5p may suppress the synaptic activity of cortical pyramidal neurons in mice by targeting NOTCH1, a key component of the Notch signaling pathway. This inhibition of NOTCH1-mediated signaling disrupts synaptic function and neuronal communication, thereby contributing to the pathogenesis and progression of schizophrenia-like phenotypes in mice[89].
Preclinical studies (mouse models and cell cultures) demonstrated that elevated miR-223 expression in the orbitofrontal cortex is strongly associated with neuroinflammatory activation. Specifically, the expression of exosomal miR-223 in astrocytes was significantly elevated under inflammatory conditions. Antipsychotic treatment resulted in a significant down-regulation of exosomal miR-223 in astrocytes[90]. Clinical investigations and experimental models (encompassing murine studies and in vitro cell culture systems) have consistently demonstrated a strong association between miR-223 overexpression in the orbitofrontal cortex and neuroinflammatory activation. Detailed mechanistic analyses revealed that astrocyte-derived exosomal miR-223 exhibits marked up-regulation in response to pro-inflammatory stimuli. Notably, pharmacological intervention with antipsychotic agents was shown to effectively suppress this miR-223 elevation in astrocytic exosomes, suggesting a potential therapeutic modulation of neuroinflammatory pathways.
In a study of patients treated with olanzapine, miR-203a-3p was identified as a crucial mediator that targets the 3’UTR of DJ-1, a protein associated with schizophrenia. Antipsychotic monotherapy restores the antioxidant capacity of DJ-1 by modulating the expression of miR-203a-3p, thereby exerting a therapeutic effect[91]. In a study focusing on patients undergoing clozapine treatment, researchers discovered that compared with the control group, miR-675-3p expression was up-regulated following clozapine administration. This alteration in miR-675-3p expression holds potential as a biomarker for evaluating the efficacy of clozapine treatment[92].
The investigation of exosomes and their associated miRNAs extends beyond traditional sources such as nerve tissue and common body fluids like blood. For example, urinary exosomal miRNAs have emerged as promising predictive biomarkers for persistent psychotic experiences. In a comparative analysis involving 15 patients, miR-486-5p was identified as a potential biomarker. Additionally, six exosomal miRNAs, specifically miR-199a-3p, miR-144-5p, miR-451a, miR-143-3p, and miR-142-3p, exhibited significant up-regulation in this cohort[93].
The etiology of MDD remains a subject of diverse perspectives. A growing body of empirical data indicates that inflammation originating from multiple systems, including peripheral blood and the CNS, contributes to the development of MDD. Inflammation impacts the pathogenesis of MDD by regulating neurogenesis, dopaminergic and serotonergic metabolism, and activation of the hypothalamic-pituitary-adrenal axis. Conversely, MDD can influence cellular immune function via the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis, leading to alterations in the levels of central and peripheral inflammatory mediators[94,95] (Table 5).
| miRNAs | Alteration | Model | Type tissue | Significance | Ref. |
| miR-146a, miR-155 | Increase | Human patients | Blood sample | Interference by negative regulation of the TLR4 signaling pathway. It provides a potential diagnostic and therapeutic approach for MDD | Figueroa-Hall et al[96] |
| miR-21-5p, miR-145, miR-146a, miR-155 | Decrease | Human patients | Serum | Exosomal miRNA may play an important role in predicting the response to antidepressant drugs | Hung et al[97] |
| miR-9-5p | Increase | MDD mouse models, MDD cell models | Serum, cell | It polarizes M1 of microglia, leading to further neuronal damage. Possible be a new therapeutic target for MDD | Hung et al[98] |
| miR-144-5p | Decrease | MDD mouse models | Hippocampus | Mediated by the PI3K/Akt/FoxO1 signaling pathway. A new potential therapeutic target | Xian et al[99] |
| miR-139-5p | Increase | MDD mouse models | Serum, hippocampus | It is activated during stress and mediates depression-like behavior in mice. A potential new approach to the diagnosis and treatment of MDD | Wei et al[39] |
| miR-139-5p | Decrease | Human patients | Serum | A promising biomarker for diagnosing MDD | Wu et al[100] |
| miR-186-5p | Decrease | MDD mouse models | Hippocampus | The exosome SERPINF1 in peripheral blood uses miR-186-5p as a potential therapeutic target for this disease | Liang et al[101] |
The recognition, activation, and induction of pro-inflammatory mediators by TLRs may be a crucial connection between inflammation and MDD[96]. Serum analysis of patients indicated that the down-regulation of exosomal miR-146a and miR-155 Led to MDD through negative modulation of the TLR4 signaling cascade[97]. Further research demonstrated that, in serum exosomes, the expression profiles of miRNAs modulating the TLR4 signaling pathway, including miR-21-5p, miR-145, miR-146a, and miR-155, were down-regulated both before and after antidepressant treatment. After treatment, miR-145 and miR-146a displayed distinct expression patterns between the remission and non-remission groups, suggesting their potential as biomarkers for treatment response. Conversely, miR-21-5p and miR-155 could not distinguish between these two groups[98].
In other investigations exploring the relationship between inflammation and depression, various signaling pathways are implicated in MDD and inflammation. Analysis of serum and cells from MDD mouse models and cell-based models revealed that the up-regulation of miR-9-5p promoted the polarization of microglia towards the M1 phenotype. This polarized state triggered a pathological cascade characterized by the dysregulated secretion of pro-inflammatory mediators, including interleukin (IL)-1β, IL-6, and tumor necrosis factor-α, thereby amplifying neuronal injury through sustained neuroinflammatory responses. Mechanistically, this process occurs through suppression of the suppressor of cytokine signaling 2 expression, which in turn reactivates the Janus tyrosine kinase/signal transducer and activator of transcription 3 pathway that is usually inhibited by suppression of the suppressor of cytokine signaling 2[99]. In MDD model mice, miR-144-5p expression in the hippocampus is significantly down-regulated. This down-regulation gives rise to depression-like behaviors, including abnormal neurogenesis, neuronal apoptosis, altered synaptic plasticity, and neuroinflammation. The neuronal damage mediated by miR-144-5p is regulated by the PI3K/Akt/FoxO1 signaling pathway[100].
In the serum and hippocampus of MDD mice, miR-139-5p is up-regulated. This up-regulation causes neural damage in the adult hippocampus, leading to the development of depression-like behaviors in the mice. miR-139-5p is a negative regulator of neural stem cell proliferation and neuronal differentiation[39]. After validating the association between miR-139-5p and MDD in mice, the research team obtained consistent results by analyzing patient serum. Specifically, compared with healthy controls, miR-139-5p levels were elevated in the serum of patients. Thus, miR-139-5p holds potential as a biomarker for diagnosing and treating MDD[101]. In the hippocampus of MDD mice, miR-186-5p is down-regulated. This down-regulation inhibits serpin family F member 1 in the hippocampus, contributing to the manifestation of depression-like behaviors[102].
In an analysis of blood samples drawn from patients, researchers observed significant alterations in the expression levels of various miRNAs. Specifically, exosomal miR-484, miR-652-3p, and miR-142-3p exhibited marked down-regulation, indicating decreased presence. Conversely, exosomal miR-185-5p demonstrated a significant increase in expression, highlighting an upregulation of this miRNA. These modifications in the levels of exosomal miRNAs are not merely coincidental; they are intricately connected to multiple biological pathways. For instance, they have implications for key signaling pathways, including the PI3K/Akt signaling pathway, which plays a crucial role in cellular growth and survival. Additionally, these miRNAs participate in the pathways of fatty acid synthesis and metabolic processes, as well as the extracellular matrix and adhesion pathways, which are essential for maintaining tissue structure and facilitating cellular communication. Thus, the alterations in these specific miRNAs may have significant biological repercussions that warrant further investigation[103] (Table 6).
| miRNAs | Alteration | Model | Type tissue | Significance | Ref. |
| miR-484, miR-652-3p, miR-142-3p | Decrease | BD human patients | Plasma | Abnormally regulated miRNAs enrich multiple target pathways, including the PI3K/Akt signaling pathway, fatty acid biosynthesis/metabolism, and extracellular matrix | Ceylan et al[103] |
| miR-185-5p | Increase | BD human patients | Plasma | Regulate the adhesion pathway | Ceylan et al[103] |
Autism spectrum disorder: Exosomes rich in miR-29 b-3p cross the BBB to reach the medial prefrontal cortex and subsequently induce inhibition of insulin-like growth factor 1 expression in neurons. Excitatory neuron activation in the medial prefrontal cortex improved behavioral abnormalities in autism spectrum disorder (ASD) mice. miR-29 b-3p was up-regulated in the medial prefrontal cortex of mice[104]. Exosomes derived from human adipose-mesenchymal stem cells can improve neurodevelopmental abnormalities and inhibit the inflammatory microenvironment in the brain of ASD mice. This change is mediated through the miR-21a-3p/PI3K/AKT axis[105] (Table 7).
| Disease | miRNAs | Alteration | Model | Type tissue | Significance | Ref. |
| ASD | miR-29b-3p | Increase | Mouse | Medial prefrontal cortex | Exosome-derived miR-29 b-3p negatively regulates IGF-1 in the mPFC | Chen et al[104] |
| miR-21a-3p | ASD mouse models | Brian tissue | MiR-21a-3p/PI3K/Akt, as a signaling pathway, promotes neurogenesis and thus plays a regulatory role | Fu et al[105] | ||
| RTT | miR-21-5p | RTT mouse models | Brian tissue | Modulating the Epha4/TEK axis promotes early neurogenesis possible biomarker for RTT | Pan et al[40] |
RTT: RTT is a rare neurodevelopmental disorder. Pan et al[40] found that exosomal miR-21-5p derived from human uro-derived stem cells can promote early neurogenesis by modulating the EPha4/TEK axis, which may represent a promising therapeutic approach for this debilitating disease.
PTSD: In the PTSD mouse model, exosomal miR-15a-5p, miR-497a-5p, and miR-511-5p were down-regulated in the hippocampus and medial prefrontal cortex, and stress behaviors were expressed through brain-derived neurotrophic factor and FKBP5[106]. Plasma analysis of patients after explosive traumatic brain injury showed that exosomal miR-326 was found. Changes in miR-361 and miR-767-5p may be related to pathological mechanisms of dysregulation of cellular pathways associated with neurodegeneration, inflammation, and central hormone regulation[107]. Exosomal miR-139-5p was down-regulated in blood samples of veterans with mild traumatic brain injury, mainly because the target molecules of the glucocorticoid receptor signaling pathway were involved in this process[53] (Table 8).
| miRNAs | Alteration | Model | Type tissue | Significance | Ref. |
| miR-15a-5p, miR-497a-5p, miR-511-5p | Decrease | PTSD mouse models | Hippocampus, medial prefrontal cortex | Brain region-dependent regulation of miRNAs targeting FKBP5, BDNF, and other stress-related genes | Maurel et al[106] |
| miR-326, miR-361, miR-767-5p | PTSD human patients | Plasma | Possible biomarker of PTSD | Devoto et al[107] | |
| miR-139-5p | Decrease | PTSD human patients | Blood sample | MiR-139-5p was associated with the severity of PTSD symptoms in remote mTBI participants | Guedes et al[53] |
Exosomal miRNAs can mediate post-transcriptional gene silencing by binding to the 3'UTR or the open reading frame region of target miRNA, and play a role in disease neurogenesis, synaptic plasticity, inflammatory responses, mitochon
| 1. | Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6920] [Cited by in RCA: 6489] [Article Influence: 1297.8] [Reference Citation Analysis (0)] |
| 2. | Liao K, Niu F, Hu G, Yang L, Dallon B, Villarreal D, Buch S. Morphine-mediated release of miR-138 in astrocyte-derived extracellular vesicles promotes microglial activation. J Extracell Vesicles. 2020;10:e12027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 3. | Mellado S, Cuesta CM, Montagud S, Rodríguez-Arias M, Moreno-Manzano V, Guerri C, Pascual M. Therapeutic role of mesenchymal stem cell-derived extracellular vesicles in neuroinflammation and cognitive dysfunctions induced by binge-like ethanol treatment in adolescent mice. CNS Neurosci Ther. 2023;29:4018-4031. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Théry C. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1880] [Cited by in RCA: 2044] [Article Influence: 185.8] [Reference Citation Analysis (0)] |
| 5. | EL Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1956] [Cited by in RCA: 2568] [Article Influence: 214.0] [Reference Citation Analysis (0)] |
| 6. | Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D'Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanović MM, Kovács ÁF, Krämer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-'t Hoen EN, Noren Hooten N, O'Driscoll L, O'Grady T, O'Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6453] [Cited by in RCA: 7645] [Article Influence: 1092.1] [Reference Citation Analysis (1)] |
| 7. | Larios J, Mercier V, Roux A, Gruenberg J. ALIX- and ESCRT-III-dependent sorting of tetraspanins to exosomes. J Cell Biol. 2020;219:e201904113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 278] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 8. | Bartel DP. Metazoan MicroRNAs. Cell. 2018;173:20-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2329] [Cited by in RCA: 2642] [Article Influence: 377.4] [Reference Citation Analysis (0)] |
| 9. | Alles J, Fehlmann T, Fischer U, Backes C, Galata V, Minet M, Hart M, Abu-Halima M, Grässer FA, Lenhof HP, Keller A, Meese E. An estimate of the total number of true human miRNAs. Nucleic Acids Res. 2019;47:3353-3364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 406] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 10. | Wang SH, Zhang C, Wang Y. microRNA regulation of pluripotent state transition. Essays Biochem. 2020;64:947-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Hart M, Walch-Rückheim B, Krammes L, Kehl T, Rheinheimer S, Tänzer T, Glombitza B, Sester M, Lenhof HP, Keller A, Meese E. miR-34a as hub of T cell regulation networks. J Immunother Cancer. 2019;7:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Png KJ, Halberg N, Yoshida M, Tavazoie SF. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature. 2011;481:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 427] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 13. | Gallo A, Tandon M, Alevizos I, Illei GG. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One. 2012;7:e30679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 712] [Cited by in RCA: 861] [Article Influence: 66.2] [Reference Citation Analysis (2)] |
| 14. | Lv LL, Cao Y, Liu D, Xu M, Liu H, Tang RN, Ma KL, Liu BC. Isolation and quantification of microRNAs from urinary exosomes/microvesicles for biomarker discovery. Int J Biol Sci. 2013;9:1021-1031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 15. | Zhou Q, Li M, Wang X, Li Q, Wang T, Zhu Q, Zhou X, Wang X, Gao X, Li X. Immune-related microRNAs are abundant in breast milk exosomes. Int J Biol Sci. 2012;8:118-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 357] [Cited by in RCA: 437] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 16. | Sandau US, Magaña SM, Costa J, Nolan JP, Ikezu T, Vella LJ, Jackson HK, Moreira LR, Palacio PL, Hill AF, Quinn JF, Van Keuren-Jensen KR, McFarland TJ, Palade J, Sribnick EA, Su H, Vekrellis K, Coyle B, Yang Y, Falcón-Perez JM, Nieuwland R, Saugstad JA; International Society for Extracellular Vesicles Cerebrospinal Fluid Task Force. Recommendations for reproducibility of cerebrospinal fluid extracellular vesicle studies. J Extracell Vesicles. 2024;13:e12397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 17. | Mo Y, Leung LL, Mak CSL, Wang X, Chan WS, Hui LMN, Tang HWM, Siu MKY, Sharma R, Xu D, Tsui SKW, Ngan HYS, Yung MMH, Chan KKL, Chan DW. Tumor-secreted exosomal miR-141 activates tumor-stroma interactions and controls premetastatic niche formation in ovarian cancer metastasis. Mol Cancer. 2023;22:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 64] [Reference Citation Analysis (0)] |
| 18. | Li X, Liao J, Su X, Li W, Bi Z, Wang J, Su Q, Huang H, Wei Y, Gao Y, Li J, Liu L, Wang C. Human urine-derived stem cells protect against renal ischemia/reperfusion injury in a rat model via exosomal miR-146a-5p which targets IRAK1. Theranostics. 2020;10:9561-9578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 19. | Scheiber C, Klein HC, Schneider JM, Schulz T, Bechter K, Tumani H, Kapapa T, Flinkman D, Coffey E, Ross D, Čistjakovs M, Nora-Krūkle Z, Bortolotti D, Rizzo R, Murovska M, Schneider EM. HSV-1 and Cellular miRNAs in CSF-Derived Exosomes as Diagnostically Relevant Biomarkers for Neuroinflammation. Cells. 2024;13:1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 20. | Hernandez BJ, Strain M, Suarez MF, Stamer WD, Ashley-Koch A, Liu Y, Klingeborn M, Bowes Rickman C. Small Extracellular Vesicle-Associated miRNAs in Polarized Retinal Pigmented Epithelium. Invest Ophthalmol Vis Sci. 2024;65:57. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Zhang L, Wang H, Zhao G, Li N, Wang X, Li Y, Jia Y, Qiao X. Anti-Tim4 Grafting Strongly Hydrophilic Metal-Organic Frameworks Immunoaffinity Flake for High-Efficiency Capture and Separation of Exosomes. Anal Chem. 2021;93:6534-6543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 22. | Risha Y, Minic Z, Ghobadloo SM, Berezovski MV. The proteomic analysis of breast cell line exosomes reveals disease patterns and potential biomarkers. Sci Rep. 2020;10:13572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 23. | Kapoor KS, Harris K, Arian KA, Ma L, Schueng Zancanela B, Church KA, McAndrews KM, Kalluri R. High throughput and rapid isolation of extracellular vesicles and exosomes with purity using size exclusion liquid chromatography. Bioact Mater. 2024;40:683-695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 24. | Martínez-Greene JA, Hernández-Ortega K, Quiroz-Baez R, Resendis-Antonio O, Pichardo-Casas I, Sinclair DA, Budnik B, Hidalgo-Miranda A, Uribe-Querol E, Ramos-Godínez MDP, Martínez-Martínez E. Quantitative proteomic analysis of extracellular vesicle subgroups isolated by an optimized method combining polymer-based precipitation and size exclusion chromatography. J Extracell Vesicles. 2021;10:e12087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 25. | Zhao L, Wang H, Fu J, Wu X, Liang XY, Liu XY, Wu X, Cao LL, Xu ZY, Dong M. Microfluidic-based exosome isolation and highly sensitive aptamer exosome membrane protein detection for lung cancer diagnosis. Biosens Bioelectron. 2022;214:114487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 26. | Royo F, Théry C, Falcón-Pérez JM, Nieuwland R, Witwer KW. Methods for Separation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey Performed by the ISEV Rigor and Standardization Subcommittee. Cells. 2020;9:1955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 265] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 27. | Su L, Li R, Zhang Z, Liu J, Du J, Wei H. Identification of altered exosomal microRNAs and mRNAs in Alzheimer's disease. Ageing Res Rev. 2022;73:101497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 28. | Yao C, Ren J, Huang R, Tang C, Cheng Y, Lv Z, Kong L, Fang S, Tao J, Fu Y, Zhu Q, Fang M. Transcriptome profiling of microRNAs reveals potential mechanisms of manual therapy alleviating neuropathic pain through microRNA-547-3p-mediated Map4k4/NF-κb signaling pathway. J Neuroinflammation. 2022;19:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Tavasolian F, Lively S, Pastrello C, Tang M, Lim M, Pacheco A, Qaiyum Z, Yau E, Baskurt Z, Jurisica I, Kapoor M, Inman RD. Proteomic and genomic profiling of plasma exosomes from patients with ankylosing spondylitis. Ann Rheum Dis. 2023;82:1429-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 30. | Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1483] [Cited by in RCA: 2611] [Article Influence: 435.2] [Reference Citation Analysis (0)] |
| 31. | Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1298] [Cited by in RCA: 2000] [Article Influence: 181.8] [Reference Citation Analysis (0)] |
| 32. | Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G, Blot B, Haase G, Goldberg Y, Sadoul R. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci. 2011;46:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 453] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 33. | Liu CG, Meng S, Li Y, Lu Y, Zhao Y, Wang PC. MicroRNA-135a in ABCA1-labeled Exosome is a Serum Biomarker Candidate for Alzheimer's Disease. Biomed Environ Sci. 2021;34:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 34. | Budnik V, Ruiz-Cañada C, Wendler F. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci. 2016;17:160-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 544] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 35. | Howitt J, Hill AF. Exosomes in the Pathology of Neurodegenerative Diseases. J Biol Chem. 2016;291:26589-26597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 205] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 36. | Soria FN, Pampliega O, Bourdenx M, Meissner WG, Bezard E, Dehay B. Exosomes, an Unmasked Culprit in Neurodegenerative Diseases. Front Neurosci. 2017;11:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 37. | Cheng X, Li W, Zhao R, Li H, Qin J, Tian M, Zhang X, Jin G. The role of hippocampal niche exosomes in rat hippocampal neurogenesis after fimbria-fornix transection. J Biol Chem. 2021;296:100188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Fan C, Li Y, Lan T, Wang W, Long Y, Yu SY. Microglia secrete miR-146a-5p-containing exosomes to regulate neurogenesis in depression. Mol Ther. 2022;30:1300-1314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 135] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 39. | Wei ZX, Xie GJ, Mao X, Zou XP, Liao YJ, Liu QS, Wang H, Cheng Y. Exosomes from patients with major depression cause depressive-like behaviors in mice with involvement of miR-139-5p-regulated neurogenesis. Neuropsychopharmacology. 2020;45:1050-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 165] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 40. | Pan W, Xu X, Zhang M, Song X. Human urine-derived stem cell-derived exosomal miR-21-5p promotes neurogenesis to attenuate Rett syndrome via the EPha4/TEK axis. Lab Invest. 2021;101:824-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 41. | Lafourcade C, Ramírez JP, Luarte A, Fernández A, Wyneken U. miRNAs in Astrocyte-Derived Exosomes as Possible Mediators of Neuronal Plasticity. J Exp Neurosci. 2016;10:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 42. | Zhang H, Xie XH, Xu SX, Wang C, Sun S, Song X, Li R, Li N, Feng Y, Duan H, Li D, Liu Z. Oligodendrocyte-derived exosomes-containing SIRT2 ameliorates depressive-like behaviors and restores hippocampal neurogenesis and synaptic plasticity via the AKT/GSK-3β pathway in depressed mice. CNS Neurosci Ther. 2024;30:e14661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 43. | Ma X, Wang Y, Shi Y, Li S, Liu J, Li X, Zhong W, Pan Q. Exosomal miR-132-3p from mesenchymal stromal cells improves synaptic dysfunction and cognitive decline in vascular dementia. Stem Cell Res Ther. 2022;13:315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 44. | Wayman GA, Davare M, Ando H, Fortin D, Varlamova O, Cheng HY, Marks D, Obrietan K, Soderling TR, Goodman RH, Impey S. An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proc Natl Acad Sci U S A. 2008;105:9093-9098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 464] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 45. | Pape K, Tamouza R, Leboyer M, Zipp F. Immunoneuropsychiatry - novel perspectives on brain disorders. Nat Rev Neurol. 2019;15:317-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 314] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 46. | Yang J, Malone F, Go M, Kou J, Lim JE, Caughey RC, Fukuchi KI. Lipopolysaccharide-Induced Exosomal miR-146a Is Involved in Altered Expression of Alzheimer's Risk Genes Via Suppression of TLR4 Signaling. J Mol Neurosci. 2021;71:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 47. | Wei H, Zhu Z, Xu Y, Lin L, Chen Q, Liu Y, Li Y, Zhu X. Microglia-derived exosomes selective sorted by YB-1 alleviate nerve damage and cognitive outcome in Alzheimer's disease. J Transl Med. 2024;22:466. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 48. | Zhai L, Shen H, Sheng Y, Guan Q. ADMSC Exo-MicroRNA-22 improve neurological function and neuroinflammation in mice with Alzheimer's disease. J Cell Mol Med. 2021;25:7513-7523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 49. | Khadimallah I, Jenni R, Cabungcal JH, Cleusix M, Fournier M, Beard E, Klauser P, Knebel JF, Murray MM, Retsa C, Siciliano M, Spencer KM, Steullet P, Cuenod M, Conus P, Do KQ. Mitochondrial, exosomal miR137-COX6A2 and gamma synchrony as biomarkers of parvalbumin interneurons, psychopathology, and neurocognition in schizophrenia. Mol Psychiatry. 2022;27:1192-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 50. | Lyu Q, Shi LQ, Chen HY, Lu M, Liang XC, Ma XD, Zhou X, Ren L. Electroacupuncture combined with NSCs-Exo alters the response of hippocampal neurons in a chronic unpredictable mild stress paradigm in ovx rats. Life Sci. 2024;359:123235. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 51. | Li B, Chen Y, Zhou Y, Feng X, Gu G, Han S, Cheng N, Sun Y, Zhang Y, Cheng J, Zhang Q, Zhang W, Liu J. Neural stem cell-derived exosomes promote mitochondrial biogenesis and restore abnormal protein distribution in a mouse model of Alzheimer's disease. Neural Regen Res. 2024;19:1593-1601. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 52. | Chen C, Bao Y, Xing L, Jiang C, Guo Y, Tong S, Zhang J, Chen L, Mao Y. Exosomes Derived from M2 Microglial Cells Modulated by 1070-nm Light Improve Cognition in an Alzheimer's Disease Mouse Model. Adv Sci (Weinh). 2023;10:e2304025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 53. | Guedes VA, Lai C, Devoto C, Edwards KA, Mithani S, Sass D, Vorn R, Qu BX, Rusch HL, Martin CA, Walker WC, Wilde EA, Diaz-Arrastia R, Gill JM, Kenney K. Extracellular Vesicle Proteins and MicroRNAs Are Linked to Chronic Post-Traumatic Stress Disorder Symptoms in Service Members and Veterans With Mild Traumatic Brain Injury. Front Pharmacol. 2021;12:745348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 54. | Bassil F, Brown HJ, Pattabhiraman S, Iwasyk JE, Maghames CM, Meymand ES, Cox TO, Riddle DM, Zhang B, Trojanowski JQ, Lee VM. Amyloid-Beta (Aβ) Plaques Promote Seeding and Spreading of Alpha-Synuclein and Tau in a Mouse Model of Lewy Body Disorders with Aβ Pathology. Neuron. 2020;105:260-275.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 167] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 55. | Liu CG, Zhao Y, Lu Y, Wang PC. ABCA1-Labeled Exosomes in Serum Contain Higher MicroRNA-193b Levels in Alzheimer's Disease. Biomed Res Int. 2021;2021:5450397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 56. | Liu H, Jin M, Ji M, Zhang W, Liu A, Wang T. Hypoxic pretreatment of adipose-derived stem cell exosomes improved cognition by delivery of circ-Epc1 and shifting microglial M1/M2 polarization in an Alzheimer's disease mice model. Aging (Albany NY). 2022;14:3070-3083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 57. | Jahangard Y, Monfared H, Moradi A, Zare M, Mirnajafi-Zadeh J, Mowla SJ. Therapeutic Effects of Transplanted Exosomes Containing miR-29b to a Rat Model of Alzheimer's Disease. Front Neurosci. 2020;14:564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 58. | Ding L, Yang X, Xia X, Li Y, Wang Y, Li C, Sun Y, Gao G, Zhao S, Sheng S, Liu J, Zheng JC. Exosomes Mediate APP Dysregulation via APP-miR-185-5p Axis. Front Cell Dev Biol. 2022;10:793388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 59. | Liang X, Fa W, Wang N, Peng Y, Liu C, Zhu M, Tian N, Wang Y, Han X, Qiu C, Hou T, Du Y. Exosomal miR-532-5p induced by long-term exercise rescues blood-brain barrier function in 5XFAD mice via downregulation of EPHA4. Aging Cell. 2023;22:e13748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 60. | Meng S, Chen H, Deng C, Meng Z. Catalpol Mitigates Alzheimer's Disease Progression by Promoting the Expression of Neural Stem Cell Exosomes Released miR-138-5p. Neurotox Res. 2023;41:41-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 61. | Yan Y, Gao Y, Kumar G, Fang Q, Yan H, Zhang N, Zhang Y, Song L, Li J, Zheng Y, Zhang N, Zhang P, Ma C. Exosomal MicroRNAs modulate the cognitive function in fasudil treated APPswe/PSEN1dE9 transgenic (APP/PS1) mice model of Alzheimer's disease. Metab Brain Dis. 2024;39:1335-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 62. | Lin EY, Hsu SX, Wu BH, Deng YC, Wuli W, Li YS, Lee JH, Lin SZ, Harn HJ, Chiou TW. Engineered Exosomes Containing microRNA-29b-2 and Targeting the Somatostatin Receptor Reduce Presenilin 1 Expression and Decrease the β-Amyloid Accumulation in the Brains of Mice with Alzheimer's Disease. Int J Nanomedicine. 2024;19:4977-4994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 63. | Song Z, Qu Y, Xu Y, Zhang L, Zhou L, Han Y, Zhao W, Yu P, Zhang Y, Li X, Qin C. Microarray microRNA profiling of urinary exosomes in a 5XFAD mouse model of Alzheimer's disease. Animal Model Exp Med. 2021;4:233-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 64. | Ge X, Guo M, Hu T, Li W, Huang S, Yin Z, Li Y, Chen F, Zhu L, Kang C, Jiang R, Lei P, Zhang J. Increased Microglial Exosomal miR-124-3p Alleviates Neurodegeneration and Improves Cognitive Outcome after rmTBI. Mol Ther. 2020;28:503-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 65. | Garcia G, Fernandes A, Stein F, Brites D. Protective Signature of IFNγ-Stimulated Microglia Relies on miR-124-3p Regulation From the Secretome Released by Mutant APP Swedish Neuronal Cells. Front Pharmacol. 2022;13:833066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 66. | Garcia G, Pinto S, Ferreira S, Lopes D, Serrador MJ, Fernandes A, Vaz AR, Mendonça A, Edenhofer F, Malm T, Koistinaho J, Brites D. Emerging Role of miR-21-5p in Neuron-Glia Dysregulation and Exosome Transfer Using Multiple Models of Alzheimer's Disease. Cells. 2022;11:3377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 67. | Zhang Y, Miao Y, Xiong X, Tan J, Han Z, Chen F, Lei P, Zhang Q. Microglial exosomes alleviate intermittent hypoxia-induced cognitive deficits by suppressing NLRP3 inflammasome. Biol Direct. 2023;18:29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 68. | Wei H, Xu Y, Chen Q, Chen H, Zhu X, Li Y. Mesenchymal stem cell-derived exosomal miR-223 regulates neuronal cell apoptosis. Cell Death Dis. 2020;11:290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 69. | Nakano M, Kubota K, Kobayashi E, Chikenji TS, Saito Y, Konari N, Fujimiya M. Bone marrow-derived mesenchymal stem cells improve cognitive impairment in an Alzheimer's disease model by increasing the expression of microRNA-146a in hippocampus. Sci Rep. 2020;10:10772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 70. | Garcia G, Pinto S, Cunha M, Fernandes A, Koistinaho J, Brites D. Neuronal Dynamics and miRNA Signaling Differ between SH-SY5Y APPSwe and PSEN1 Mutant iPSC-Derived AD Models upon Modulation with miR-124 Mimic and Inhibitor. Cells. 2021;10:2424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 71. | Meng S, Wang B, Li W. CircAXL Knockdown Alleviates Aβ(1-42)-Induced Neurotoxicity in Alzheimer's Disease via Repressing PDE4A by Releasing miR-1306-5p. Neurochem Res. 2022;47:1707-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 72. | Chen H, Huang Z, Lei A, Yu X, Shen M, Wu D. miRNA-211-5p inhibition enhances the protective effect of hucMSC-derived exosome in Aβ(1-40) -induced SH-SY5Y cells by increasing NEP expression. J Biochem Mol Toxicol. 2024;38:e23624. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 73. | Tan YJ, Wong BYX, Vaidyanathan R, Sreejith S, Chia SY, Kandiah N, Ng ASL, Zeng L. Altered Cerebrospinal Fluid Exosomal microRNA Levels in Young-Onset Alzheimer's Disease and Frontotemporal Dementia. J Alzheimers Dis Rep. 2021;5:805-813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 74. | Kumar S, Reddy PH. Elevated levels of MicroRNA-455-3p in the cerebrospinal fluid of Alzheimer's patients: A potential biomarker for Alzheimer's disease. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 75. | Boccardi V, Poli G, Cecchetti R, Bastiani P, Scamosci M, Febo M, Mazzon E, Bruscoli S, Brancorsini S, Mecocci P. miRNAs and Alzheimer's Disease: Exploring the Role of Inflammation and Vitamin E in an Old-Age Population. Nutrients. 2023;15:634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 76. | Duan X, Zheng Q, Liang L, Zhou L. Serum Exosomal miRNA-125b and miRNA-451a are Potential Diagnostic Biomarker for Alzheimer's Diseases. Degener Neurol Neuromuscul Dis. 2024;14:21-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 77. | Dong Z, Gu H, Guo Q, Liu X, Li F, Liu H, Sun L, Ma H, Zhao K. Circulating Small Extracellular Vesicle-Derived miR-342-5p Ameliorates Beta-Amyloid Formation via Targeting Beta-site APP Cleaving Enzyme 1 in Alzheimer's Disease. Cells. 2022;11:3830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 78. | Visconte C, Fenoglio C, Serpente M, Muti P, Sacconi A, Rigoni M, Arighi A, Borracci V, Arcaro M, Arosio B, Ferri E, Golia MT, Scarpini E, Galimberti D. Altered Extracellular Vesicle miRNA Profile in Prodromal Alzheimer's Disease. Int J Mol Sci. 2023;24:14749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 79. | Taşdelen E, Özel Kızıl ET, Tezcan S, Yalap E, Bingöl AP, Kutlay NY. Determination of miR-373 and miR-204 levels in neuronal exosomes in Alzheimer's disease. Turk J Med Sci. 2022;52:1458-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 80. | Dong Z, Gu H, Guo Q, Liang S, Xue J, Yao F, Liu X, Li F, Liu H, Sun L, Zhao K. Profiling of Serum Exosome MiRNA Reveals the Potential of a MiRNA Panel as Diagnostic Biomarker for Alzheimer's Disease. Mol Neurobiol. 2021;58:3084-3094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 81. | Liu T, Li N, Pu J, Zhang C, Xu K, Wang W, Liu L, Gao L, Xu X, Tan J. The plasma derived exosomal miRNA-483-5p/502-5p serve as potential MCI biomarkers in aging. Exp Gerontol. 2024;186:112355. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 82. | Li Y, Xia M, Meng S, Wu D, Ling S, Chen X, Liu C. MicroRNA-29c-3p in dual-labeled exosome is a potential diagnostic marker of subjective cognitive decline. Neurobiol Dis. 2022;171:105800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 83. | Barbagallo C, Mostile G, Baglieri G, Giunta F, Luca A, Raciti L, Zappia M, Purrello M, Ragusa M, Nicoletti A. Specific Signatures of Serum miRNAs as Potential Biomarkers to Discriminate Clinically Similar Neurodegenerative and Vascular-Related Diseases. Cell Mol Neurobiol. 2020;40:531-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 84. | Aharon A, Spector P, Ahmad RS, Horrany N, Sabbach A, Brenner B, Aharon-Peretz J. Extracellular Vesicles of Alzheimer's Disease Patients as a Biomarker for Disease Progression. Mol Neurobiol. 2020;57:4156-4169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 85. | Li Y, Meng S, Di W, Xia M, Dong L, Zhao Y, Ling S, He J, Xue X, Chen X, Liu C. Amyloid-β protein and MicroRNA-384 in NCAM-Labeled exosomes from peripheral blood are potential diagnostic markers for Alzheimer's disease. CNS Neurosci Ther. 2022;28:1093-1107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 86. | Ryu IS, Kim DH, Ro JY, Park BG, Kim SH, Im JY, Lee JY, Yoon SJ, Kang H, Iwatsubo T, Teunissen CE, Cho HJ, Ryu JH. The microRNA-485-3p concentration in salivary exosome-enriched extracellular vesicles is related to amyloid β deposition in the brain of patients with Alzheimer's disease. Clin Biochem. 2023;118:110603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 87. | Cheng L, Vella LJ, Barnham KJ, McLean C, Masters CL, Hill AF. Small RNA fingerprinting of Alzheimer's disease frontal cortex extracellular vesicles and their comparison with peripheral extracellular vesicles. J Extracell Vesicles. 2020;9:1766822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 88. | Wang L, Zhen H, Sun Y, Rong S, Li B, Song Z, Liu Z, Li Z, Ding J, Yang H, Zhang X, Sun H, Nie C. Plasma Exo-miRNAs Correlated with AD-Related Factors of Chinese Individuals Involved in Aβ Accumulation and Cognition Decline. Mol Neurobiol. 2022;59:6790-6804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 89. | Wang Z, Wu T, Hu H, Alabed AAA, Cui G, Sun L, Sun Z, Wang Y, Li P. Plasma exosomes carrying mmu-miR-146a-5p and Notch signalling pathway-mediated synaptic activity in schizophrenia. J Psychiatry Neurosci. 2024;49:E265-E281. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 90. | Amoah SK, Rodriguez BA, Logothetis CN, Chander P, Sellgren CM, Weick JP, Sheridan SD, Jantzie LL, Webster MJ, Mellios N. Exosomal secretion of a psychosis-altered miRNA that regulates glutamate receptor expression is affected by antipsychotics. Neuropsychopharmacology. 2020;45:656-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 91. | Tsoporis JN, Ektesabi AM, Gupta S, Izhar S, Salpeas V, Rizos IK, Kympouropoulos SP, Dos Santos CC, Parker TG, Rizos E. A longitudinal study of alterations of circulating DJ-1 and miR203a-3p in association to olanzapine medication in a sample of first episode patients with schizophrenia. J Psychiatr Res. 2022;146:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 92. | Funahashi Y, Yoshino Y, Iga JI, Ueno SI. Impact of clozapine on the expression of miR-675-3p in plasma exosomes derived from patients with schizophrenia. World J Biol Psychiatry. 2023;24:303-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 93. | Tomita Y, Suzuki K, Yamasaki S, Toriumi K, Miyashita M, Ando S, Endo K, Yoshikawa A, Tabata K, Usami S, Hiraiwa-Hasegawa M, Itokawa M, Kawaji H, Kasai K, Nishida A, Arai M. Urinary exosomal microRNAs as predictive biomarkers for persistent psychotic-like experiences. Schizophrenia (Heidelb). 2023;9:14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 94. | Dantzer R. Depression and inflammation: an intricate relationship. Biol Psychiatry. 2012;71:4-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 95. | Dantzer R. Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa. Physiol Rev. 2018;98:477-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 619] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 96. | Figueroa-Hall LK, Paulus MP, Savitz J. Toll-Like Receptor Signaling in Depression. Psychoneuroendocrinology. 2020;121:104843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 97. | Hung YY, Wu MK, Tsai MC, Huang YL, Kang HY. Aberrant Expression of Intracellular let-7e, miR-146a, and miR-155 Correlates with Severity of Depression in Patients with Major Depressive Disorder and Is Ameliorated after Antidepressant Treatment. Cells. 2019;8:647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 98. | Hung YY, Chou CK, Yang YC, Fu HC, Loh EW, Kang HY. Exosomal let-7e, miR-21-5p, miR-145, miR-146a and miR-155 in Predicting Antidepressants Response in Patients with Major Depressive Disorder. Biomedicines. 2021;9:1428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 99. | Xian X, Cai LL, Li Y, Wang RC, Xu YH, Chen YJ, Xie YH, Zhu XL, Li YF. Neuron secrete exosomes containing miR-9-5p to promote polarization of M1 microglia in depression. J Nanobiotechnology. 2022;20:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 91] [Reference Citation Analysis (0)] |
| 100. | Wu X, Zhang Y, Wang P, Li X, Song Z, Wei C, Zhang Q, Luo B, Liu Z, Yang Y, Ren Z, Liu H. Clinical and preclinical evaluation of miR-144-5p as a key target for major depressive disorder. CNS Neurosci Ther. 2023;29:3598-3611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 101. | Liang JQ, Liao HR, Xu CX, Li XL, Wei ZX, Xie GJ, Cheng Y. Serum Exosome-Derived miR-139-5p as a Potential Biomarker for Major Depressive Disorder. Neuropsychiatr Dis Treat. 2020;16:2689-2693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 102. | Jiang M, Gu YF, Cai JF, Wang A, He Y, Feng YL. MiR-186-5p Dysregulation Leads to Depression-like Behavior by De-repressing SERPINF1 in Hippocampus. Neuroscience. 2021;479:48-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 103. | Ceylan D, Tufekci KU, Keskinoglu P, Genc S, Özerdem A. Circulating exosomal microRNAs in bipolar disorder. J Affect Disord. 2020;262:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 104. | Chen L, Xiong XY, Yao TT, Gui LN, Luo F, Du Y, Cheng Y. Blood exosome sensing via neuronal insulin-like growth factor-1 regulates autism-related phenotypes. Pharmacol Res. 2023;197:106965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 105. | Fu Y, Zhang YL, Liu RQ, Xu MM, Xie JL, Zhang XL, Xie GM, Han YT, Zhang XM, Zhang WT, Zhang J, Zhang J. Exosome lncRNA IFNG-AS1 derived from mesenchymal stem cells of human adipose ameliorates neurogenesis and ASD-like behavior in BTBR mice. J Nanobiotechnology. 2024;22:66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 106. | Maurel OM, Torrisi SA, Barbagallo C, Purrello M, Salomone S, Drago F, Ragusa M, Leggio GM. Dysregulation of miR-15a-5p, miR-497a-5p and miR-511-5p Is Associated with Modulation of BDNF and FKBP5 in Brain Areas of PTSD-Related Susceptible and Resilient Mice. Int J Mol Sci. 2021;22:5157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 107. | Devoto C, Guedes VA, Lai C, Leete JJ, Mithani S, Edwards K, Vorn R, Qu BX, Wilde EA, Walker WC, Diaz-Arrastia R, Werner JK, Kenney K, Gill JM. Remote blast-related mild traumatic brain injury is associated with differential expression of exosomal microRNAs identified in neurodegenerative and immunological processes. Brain Inj. 2022;36:652-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |