Published online Jun 26, 2025. doi: 10.4330/wjc.v17.i6.104832
Revised: February 26, 2025
Accepted: May 13, 2025
Published online: June 26, 2025
Processing time: 163 Days and 16.6 Hours
Right ventricular hypertrophy (RVH) occurs because of volume or pressure overload within the right ventricular (RV) system. RVH is associated with complex pathological changes, including myocardial cell injury, apoptosis, myocardial fibrosis, neuroendocrine disturbances, and abnormal water and liquid metabolism. Ferroptosis, a novel type of iron-dependent cell death characterized by lipid peroxide accumulation, is an important mechanism of cardiomyocyte death. However, the role of ferroptosis in RVH has rarely been studied. We hypothesize that hydrogen (H2), an experimental medical gas with superior distribution characteristics, inhibits ferroptosis.
To explore the protective effect of H2 on RVH and the mechanism by which H2 regulates ferroptosis.
An in vivo RVH rat model was induced by monocrotaline (MCT) in 30 male Sprague-Dawley rats. An H9C2 cell model was treated with angiotensin II to simulate pressure overload in the RV system in vitro. H2 was administered to rats by inhalation (2% for 3 hours daily for 21 days) and added to the cell culture medium. The Nrf2 inhibitor ML385 (1 μM) was used to investigate anti-ferroptotic mechanisms.
In MCT-treated rats, H2 inhalation decreased RVH; the RV wall thickness decreased from 3.5 ± 0.3 mm to 2.8 ± 0.2 mm (P < 0.05) and the RV ejection fraction increased from 45 ± 3% to 52 ± 4% (P < 0.05). In H9C2 cells, H2 alleviated hypertrophy. H2 inhibited ferroptosis by modulating the iron content, oxidative stress, and ferroptosis-related proteins, thereby restoring the Nrf2/HO-1 signaling pathway.
H2 retards RVH by inhibiting ferroptosis via Nrf2/HO-1 restoration, suggesting a new treatment strategy.
Core Tip: This study explored the protective effects of hydrogen (H2) on right ventricular hypertrophy (RVH) both in vivo and in vitro. The results revealed that H₂ inhibited ferroptosis, an iron-dependent cell death, by restoring the Nrf2/HO-1 pathway, offering new insights for treating RVH.
- Citation: Bai JC, Yang HX, Zhan CC, Zhao LQ, Liu JR, Yang W. Hydrogen alleviates right ventricular hypertrophy by inhibiting ferroptosis via restoration of the Nrf2/HO-1 signaling pathway. World J Cardiol 2025; 17(6): 104832
- URL: https://www.wjgnet.com/1949-8462/full/v17/i6/104832.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i6.104832
Right ventricular (RV) hypertrophy (RVH) is caused by volume or pressure overload and ultimately leads to RV dysfunction[1,2]. The right ventricle is more likely than the left ventricle to experience decreased cardiac function and even death under the same afterload[1]. Thus, improving RVH induced by pressure overload in the right cardiac system has great clinical significance. Although the clinical importance of RV function has been recognized, no effective therapies exist for RV dysfunction. Therefore, interventions should be developed to prevent RVH.
Several studies have suggested ferroptosis as a potential preventive target, particularly in diseases characterized by cardiomyocyte death[3]. Ferroptosis is a non-apoptotic form of cell death driven by the iron-dependent accumulation of lipid-based oxidative stress molecules[4]. Ferroptosis can induce cardiomyocyte injury, leading to cardiac remodeling. As ferroptosis contributes to heart damage, inhibiting ferroptosis can significantly improve the prognosis of myocardial damage[5-8]. System Xc- is the core antioxidant defense mechanism in ferroptosis[9]. The system Xc-/glutathione peroxidase 4 (GPX4) axis plays a central role in limiting lipid peroxidation. Disruption of system Xc-function can induce ferroptosis[10]. System Xc- is a heterodimer of member 11 of the light-chain solute carrier family 7 (SLC7A11) and member 2 of the heavy-chain solute carrier family 3 (SLC3A2), which are connected via disulfide bonds. SLC7A11 is an active subunit. Inhibition of SLC7A11, the light chain of system Xc-, attenuates glutathione (GSH) levels and GPX4 activity, leading to the accumulation of lethal lipid peroxides and the induction of ferroptosis[11,12]. Nrf2 has an anti-ferroptosis role and provides cardioprotection via an antioxidant effect[13]. Two critical targets associated with ferroptosis, system Xc and GPX4, are regulated by Nrf2. Nrf2 regulates the expression of various signaling proteins and enzymes by targeting downstream proteins such as SLC7A11, GPX4, and HO-1, thereby suppressing ferroptosis. Excess iron leads to ferroptosis through the regulation of iron metabolism[14]. TFR1 and FTH1 are important markers of ferroptosis[5,8]. TFR1 is considered a marker protein for ferroptosis. In addition, recent studies have confirmed that FTH1 is crucial for the initiation and promotion of cardiomyocyte ferroptosis[15]. Nrf2 induces SLC7A11 expression by directly binding to its promoter region, thereby regulating lipid peroxidation levels and morphological characteristics[16-18]. It is extremely important to elucidate the signaling pathways involved in cardiac hypertrophy and identify appropriate treatments to prevent or reverse this condition.
Hydrogen (H2) is a safe, economical, and convenient antioxidant. Ohsawa et al[19] first reported that H2 reduces the levels of cytotoxic oxygen free radicals. Our previous studies demonstrated that H2 attenuates doxorubicin-induced inflammation and cell apoptosis and reduces pyroptosis and fibrosis in diabetic cardiomyopathy. H2 also attenuates the production of reactive oxygen species (ROS) during thyroid hormone-induced cardiac hypertrophy[20-22]. However, whether H2 inhibits ferroptosis and attenuates RV cardiomyocyte damage caused by pressure overload in the right cardiac system remains unclear. In this study, we investigated the potential preventive effects of H2 against RVH in a monocrotaline (MCT) rat model simulating pressure overload of the right cardiac system and revealed that H2 inhibits ferroptosis and restores the Nrf2/HO-1 signaling pathway.
Considering the potential differences in cardiac function between male and female rats, and their different sensitivities to the model, only male rats were selected for this study. All procedures were performed in compliance with the guidelines of ARRIVE. The experimental protocol was performed in accordance with the Guide for the Care and Use of Laboratory Animals. This study was approved by the Animal Ethics Committee of the Fourth Affiliated Hospital of Harbin Medical University. Forty male Sprague-Dawley rats were housed in a controlled environment at the Animal Center of Harbin Medical University (Harbin, China) at a temperature of 22 ± 2 °C and a relative humidity of 55 ± 15% on a 12-hour light/dark cycle and were given ad libitum access to food and tap water.
In this study, a pressure overload model of the right cardiac system was established using a one-time subcutaneous injection of 60 mg/kg MCT. Subcutaneous injection of MCT causes minimal damage to animals during administration, is relatively simple to perform, has a high success rate, and is highly practical. Previous studies have reported 60 mg/kg as the most appropriate dose to induce pressure overload in the right heart system (MCT rat model). A dose that is too great leads to a significant increase in mortality; a dose that is too small does not effectively induce right heart failure and results in an experimental period that is too long[23,24]. The MCT rat model is a well-accepted model for studying the pathophysiology of pulmonary vascular remodeling and RV injury caused by pressure overload[25]. Forty male Sprague-Dawley rats (6-7 weeks old) were purchased from Liaoning Changsheng Biotechnology Co., Ltd. (Liaoning, China). Forty rats received a single subcutaneous injection of 60 mg/kg[26] of MCT (Absin Bioscience Inc, Shanghai, China) (n = 20) or saline (n = 20). The rats were divided into four groups: Control (n = 10), H2 (n = 10), MCT (n = 10), and MCT + H2 (n = 10) groups. The rats were weighed daily at 10:00 daily. Rats in the MCT and MCT + H2 groups received MCT injections, whereas rats in the control and H2 groups were injected with the same volume of saline. H2 (2%) was administered to rats in the H2 and MCT + H2 groups by inhalation for 3 hours twice daily for 28 days after the injection. H2 gas (2%) was deemed safe and effective and was produced and administered as reported previously[19-22,27]. Briefly, H2 gas was produced using an H2 generator (HA-300, SCDEALL, China). The H2 concentration was monitored in real time and maintained at 2% using an H2 detector (SDH-B101, Honeywell, United States). Rats and H2 treatments were prepared as previously described[22]. In brief, high-purity H2 was prepared using an H2 generator (output pressure: 0.4 MPa, flow rate: 80 mL/min) and injected into a rat ventilator (44 cm × 74 cm × 23 cm). The concentration of H2 was monitored using an H2 sensor and maintained at 2% ± 0.02%. The other bellows are filled with air. A fan was installed in the ventilation compartment to facilitate the flow of air or H2. The ventilation compartment contained food, water, and bedding so that the rats could live there for a long time. The rats were kept in the bellows for 6 hours per day for 28 days after MCT injection.
After 28 days, all rats were treated with 1% pentobarbital sodium (40 mg/kg). Transthoracic echocardiography was performed after the rats were subjected to abdominal anesthesia and placed in a supine position. Transthoracic echocardiography was used to assess RV structure and function. The probe was placed on the left side of the chest, and indices were measured using an ultrasound system (Philips CX50, WA, United States). The measured indices were the left ventricular ejection fraction (LVEF), RV end-diastolic dimension (RVEDD), RV end-systolic volume (RV-ESV), RV end-diastolic volume (RV-EDV), RV ejection fraction (RVEF) and RV free-wall (RVFW) thickness[26]. The RVEDD assesses RV diastolic function. The RVEF is the ratio of the RV end-diastolic and end-systolic volume differences to the end-diastolic volume, which is a good quantitative measure of RV systolic function. RVEF can reflect RV systolic function[28]. All measurements were performed by three experienced technicians who were unaware of the identities of the groups for five consecutive cardiac cycles then averaged[27].
The rats were administered an intraperitoneal injection of 0.1% 40 mg/kg sodium pentobarbital and fixed in the supine position. The abdominal cavity was opened layer-by-layer in the middle of the abdomen by incision, and the abdominal organs were moved to the right side with sterile cotton swabs to expose the abdominal aorta, which was separated with tweezers; blood samples were then collected through the abdominal aorta. Blood samples were centrifuged at 3000 × g and 4 °C for 15 minutes. The serum was collected and stored at −80 °C for further analysis. The brain natriuretic peptide (BNP) level is also a biomarker of cardiac function. Serum BNP, GSH, and GSH-Px levels were assessed using commercial kits (Nanjing Jiancheng Bioengineering Institute, China). The total superoxide dismutase (T-SOD) was assessed using a commercial kit (Wanlei, China) according to the manufacturer's instructions.
Malonaldehyde (MDA) is an indicator of lipid peroxidation. The MDA content in the RV tissue was detected using a commercial kit according to the manufacturer's instructions (Nanjing Jiancheng Bioengineering Institute, China). Frozen RV samples were thawed on ice. The ratio of heart tissue weight (g) to normal saline volume (mL) ratio was 1:9. After mechanical homogenization on ice, the samples were centrifuged at 3000 × g and 4 °C for 15 minutes. Similarly, Fe2+ in the RV tissue was assessed using a commercial kit according to the manufacturer's instructions (Nanjing Jiancheng Bioengineering Institute, China).
Heart tissue was placed in a 4% paraformaldehyde fixative solution for light microscopy, and conventional paraffin embedding was used for morphological analysis. Tissue samples collected from the same area of the heart rapidly after sacrifice were cut into 4-μm-thick paraffin sections for histological analysis[29]. Paraffin sections were stained with hematoxylin-eosin (HE, Beyotime, China) according to the manufacturer's instructions. Antinatriuretic peptide A (ANP) and BNP levels are the classic diagnostic and prognostic indicators of cardiac hypertrophy[3]. Immunohistochemistry (IHC) was performed using ANP (ab225844, 1:2000, Abcam), rabbit anti-BNP (ab243440, 1:4000, Abcam), anti-Nrf2 (WL02135, 1:200, Wanlei), and anti-HO-1 (WL02400, 1:100, Wanlei) antibodies. To quantify HE staining, we randomly selected multiple fields under a light microscope at 100 × or 200 × magnification. The cardiomyocyte size was quantified by measuring the diameter of the cardiomyocytes in each field, with at least 10 cells measured per field, and averaged for each section. Interstitial fibrosis was assessed by calculating the percentage of fibrotic area relative to the total tissue area in the selected fields.
For IHC staining, staining intensity was scored from 0 to 3 by two observers who were blinded to the groups. At least five fields per sample were evaluated and the average intensity score was calculated. The percentage of positively stained cells was determined by counting the cells in each field, dividing by the total cell number, and averaging across fields. To compare antigen expression among the samples, the immunoreactivity score was obtained by multiplying the average intensity score by the average percentage of positively stained cells. For Nrf2 and HO-1, subcellular localization was noted as cytoplasmic, nuclear, or both by observation at higher magnification, and the proportion of cells with each pattern was calculated for further analysis of protein function.
HE staining was employed to visualize general tissue morphology, including cardiomyocyte size and interstitial fibrosis, whereas IHC staining was employed to precisely detect and quantify the expression levels and subcellular localization of specific proteins (ANP, BNP, Nrf2, HO-1) relevant to cardiac hypertrophy and its regulatory mechanisms.
Histological sections of heart tissue were stained with wheat germ agglutinin (WGA) according to the manufacturer's instructions (4 μg/mL, Thermo Fisher Scientific Inc., United States) for morphometric measurement[29]. The tissues were observed using a fluorescence microscope, and images were collected in a dark room. Semi-quantitative analysis of three randomly selected fields per section was performed using ImageJ software.
WGA staining can clearly show the contours of cardiomyocytes, which is helpful for accurately measuring the area of cardiomyocytes, thus providing intuitive and reliable data for evaluating the degree of myocardial hypertrophy.
Paraffin sections of heart tissue were dewaxed for 1 hour and hydrated with tap and distilled water three times. Equal proportions of hydrochloric acid and potassium ferrocyanide were mixed to generate the Prussian blue staining solution. The tissues in the working solution were incubated for 3 minutes, then the samples were examined under an OLYMPUS (CX23) microscope, with images captured at 200 × magnification. The region of interest (total tissue area) was outlined using ImageJ software. The color threshold was adjusted to select areas with blue-stained iron deposits. The ratio of the blue-stained area to total area was calculated for each image. Multiple (5-10) random fields were analyzed per sample, and the average percentage value was computed to represent iron accumulation in the heart tissue.
Prussian blue staining enables the specific visualization and quantification of iron deposits in the heart tissue, which is crucial for assessing the level of iron accumulation related to ferroptosis and its potential impact on cardiac function.
Morphological changes in the mitochondria at the same position as the free wall of the right ventricle were observed by electron microscopy. The tissue was fixed at 4 °C with 2% glutaraldehyde in sodium bicarbonate buffer for 1 hour on ice with 1% osmium tetroxide. The sections were stained with uranyl acetate and observed under an H7650 transmission electron microscope (Hitachi, Japan).
H9C2 cells were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in DMEM containing 10% fetal bovine serum and 1% penicillin/streptomycin in a humidified 5% CO2 atmosphere at 37 °C. An angiotensin II (Ang II)-induced H9C2 cell model was constructed in vitro to study myocardial hypertrophy induced by pressure overload in the right cardiac system. The concentration gradients of Ang II were 0.01 μmol/L, 0.1 μmol/L, 1 μmol/L, 10 μmol/L, and 100 μmol/L. The degree of cell hypertrophy was most obvious at 1 mol/L after 24 h, which is consistent with previous research[30]. H9C2 cells were divided into different groups: Control, H2, Ang II (1 μmol/L), and Ang II+H2 groups. In addition, the Nrf2 inhibitor ML385 (1 μmol/L, HY-100523, MCE) was used in this study[24,31]. H9C2 cells were pretreated with ML385 for 2 hours then treated with Ang II plus H2 (Ang II+ML385+H2) for 24 hours. H2 treatment was performed as previously described[21]. In brief, H2 was dissolved in DMEM (H2 medium) at 0.4 MPa, O2 was dissolved in DMEM (O2 medium) at 42.5 mg/L saturation, and CO2 was dissolved in DMEM (CO2 medium) at atmospheric pressure. The three media (H2, O2, and CO2) were mixed proportionally with fetal bovine serum in a 75:20:5 vol.% ratio. The culture flask was then filled with a mixture of 75% H2, 20% O2, and 5% CO2, and the cells were cultured in a closed flask at constant pressure.
Cell viability was measured using the cell counting kit-8 (CCK-8, Beyotime, China) following the manufacturer’s instructions. Briefly, 5 × 103 cells/well from the different groups were cultured in 96-well plates. After treatment, 10 µL CCK-8 solution was added to each well at 37 °C for 2 hours before analysis. In addition, a lactate dehydrogenase (LDH, Nanjing Jiancheng Bioengineering Institute, China) release test was used to assess cell damage according to the manufacturer’s instructions.
H9C2 cardiomyocytes were collected from each group, homogenized, and centrifuged for 10 minutes, and the supernatant was collected. The MDA content, T-SOD activity, GSH content, and GSH-Px activity were determined. The MDA content of H9C2 cells in each group was assessed using a commercial kit (Nanjing Jiancheng Bioengineering Institute, China). Similarly, the activities of antioxidant enzymes such as T-SOD, GSH, and GSH-Px were determined in H9C2 cells from each group (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s instructions. In addition, Fe2+ levels were detected using a commercial kit (Elabscience, China) according to the manufacturer’s instructions.
The surface area of H9C2 cells was measured using a phalloidin staining kit (Beyotime, China) according to the manufacturer’s instructions. Briefly, H9C2 cells were fixed, permeabilized, and stained with phalloidin and 4,6-diamino-2-phenyl indole (DAPI, Beyotime, China) in a dark room. At least three images were captured using a fluorescence microscope. Subsequently, a sample size of no less than 30 cells was measured and analyzed using ImageJ software.
Phalloidin staining was used to specifically label actin filaments in H9C2 cells, enabling accurate measurement of the cell surface area, which is a key parameter for evaluating cell hypertrophy.
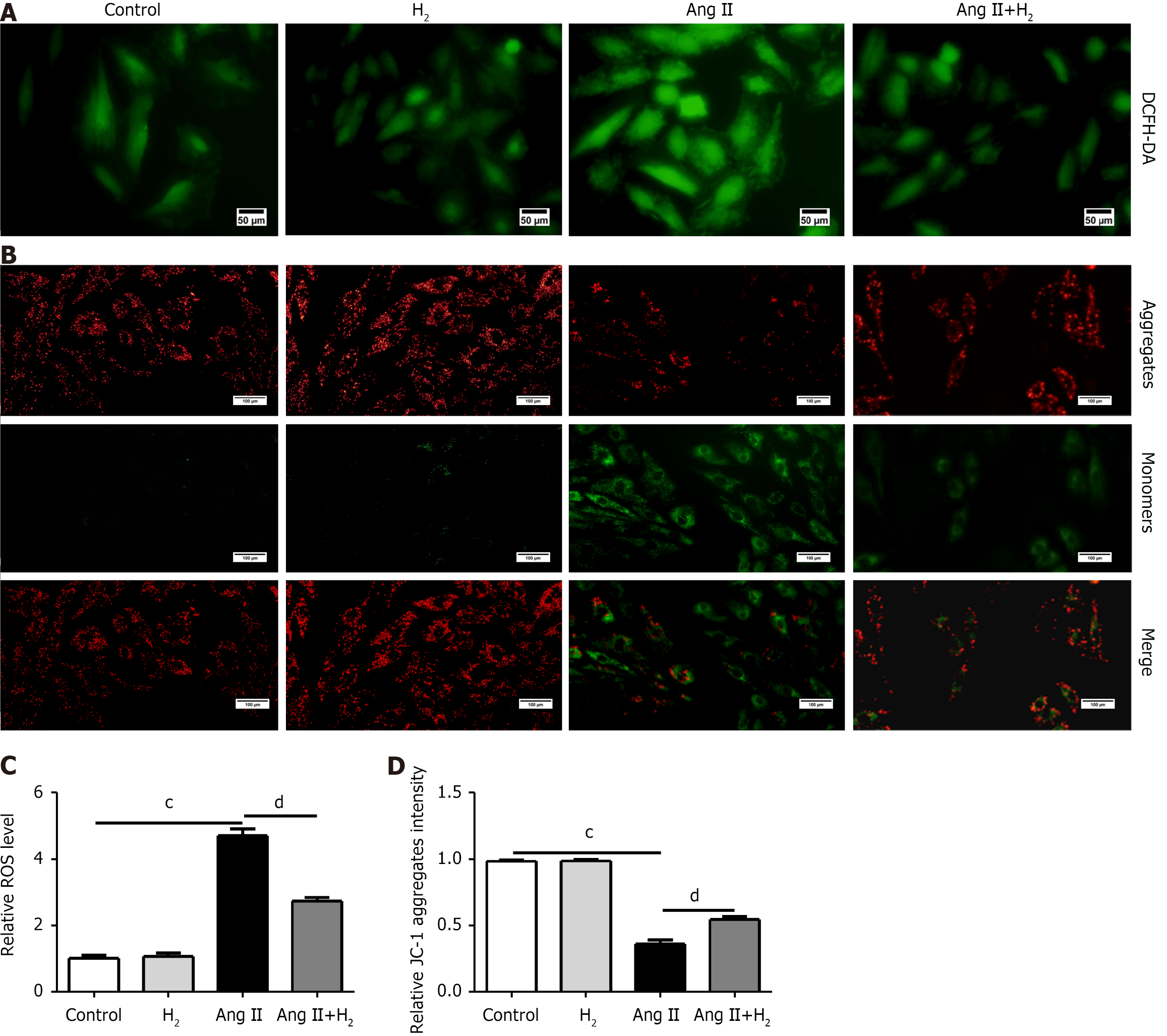
Cellular ROS production and mitochondrial membrane potential (MMP) in H9C2 cells were detected using an ROS assay kit (Solarbio, China) and a JC-1 assay kit (Beyotime, China) in accordance with the experimental protocol. In brief, H9C2 cells of different groups cultured in six-well plates were washed with phosphate-buffered saline (PBS) and incubated with DCFH-DA in a darkroom at 37 °C for 30 minutes. The DCFH-DA fluorescent probe concentration was 1:2000 according to the manufacturer’s instructions. Subsequently, the cells were washed three times with PBS, and fluorescence intensity was detected using a fluorescence microscope. In addition, H9C2 cells were washed three times then stained with JC-1 in a darkroom at 37 °C for 20 minutes. Red fluorescence of JC-1 aggregates and green fluorescence of JC-1 monomers were captured under a fluorescence microscope and analyzed using ImageJ software.
Quantitative PCR (qPCR) was performed according to the manufacturer’s instructions. For each group, total RNA was extracted from H9C2 cells in a six-well plate using TRIzol reagent (Sigma-Aldrich, T9424). cDNA was synthesized using a ReverTra Ace qPCR RT kit (TOYOBO, No. FSQ-101, Japan) according to the manufacturer’s instructions. The expression of ANP mRNA and BNP mRNA was normalized to β-actin. All samples were analyzed in triplicate. The primer sequences were as follows: ANP, forward, 5′-TCCGATAGATCTGCCCTCTT-3′; ANP, reverse, 5′-CTCCAATCCTGTCAATCCTACC-3′; BNP, forward, 5′-ATTCTGCTCCTGCTTTTCCT-3′; BNP, reverse, 5′-CCTTGGTCCTTTGAGAGCTGT-3′; β-actin, forward, 5′-GAGGTATCCTGACCCTGAAGTA-3′; β-actin, reverse, 5′-CACACGCAGCTCATTGTAGA-3.’
Protein from RV tissues and H9C2 cells was stored at −80 °C for western blotting[27]. Protein concentration was determined using a bicinchoninic acid assay kit (Beyotime, China). Each experiment was performed at least three times. Anti-ANP antibody (ab225844, 1:2000, Abcam), anti-BNP antibody (ab243440, 1:4000, Abcam), anti-Nrf2 antibody (A0674, 1:1000, ABclonal), anti-TFR1 antibody (sc-65882, 1:1000, Santa Cruz), anti-SLC7A11 antibody (A15604, 1:1000, ABclonal), anti-HO-1 antibody (A1346, 1:1000, ABclonal), anti-FTH1 antibody (WL05360, 1:500, Wanlei), anti-GPX4 antibody (A13309, 1:1000, ABclonal) and horseradish peroxidase-conjugated secondary antibodies (ZB-2301, ZB-2305, 1:1000, ZSGB) were used. Anti-β-actin (AC026, 1:50000, ABclonal) antibody was used as the internal control.
The normality of the data was first examined using the Shapiro-Wilk normality test with SPSS software (version 27.0; IBM, United States). If the data did not conform to a normal distribution, non-parametric statistical tests were used. To compare multiple groups, we used the Kruskal-Wallis test, which is a non-parametric alternative to one-way analysis of variance. Subsequently, Dunn’s test was applied for post-hoc analysis to determine which specific groups differed from each other. After confirming that all data conformed to a normal distribution, further data analyses were performed.
Data are presented as the mean ± SD. One-way analysis of variance with GraphPad Prism (GraphPad Software, CA, United States) was used for groups, and Tukey’s post-hoc test was used for statistical analysis. All experiments were repeated at least three times. The level of statistical significance was set to P < 0.05.
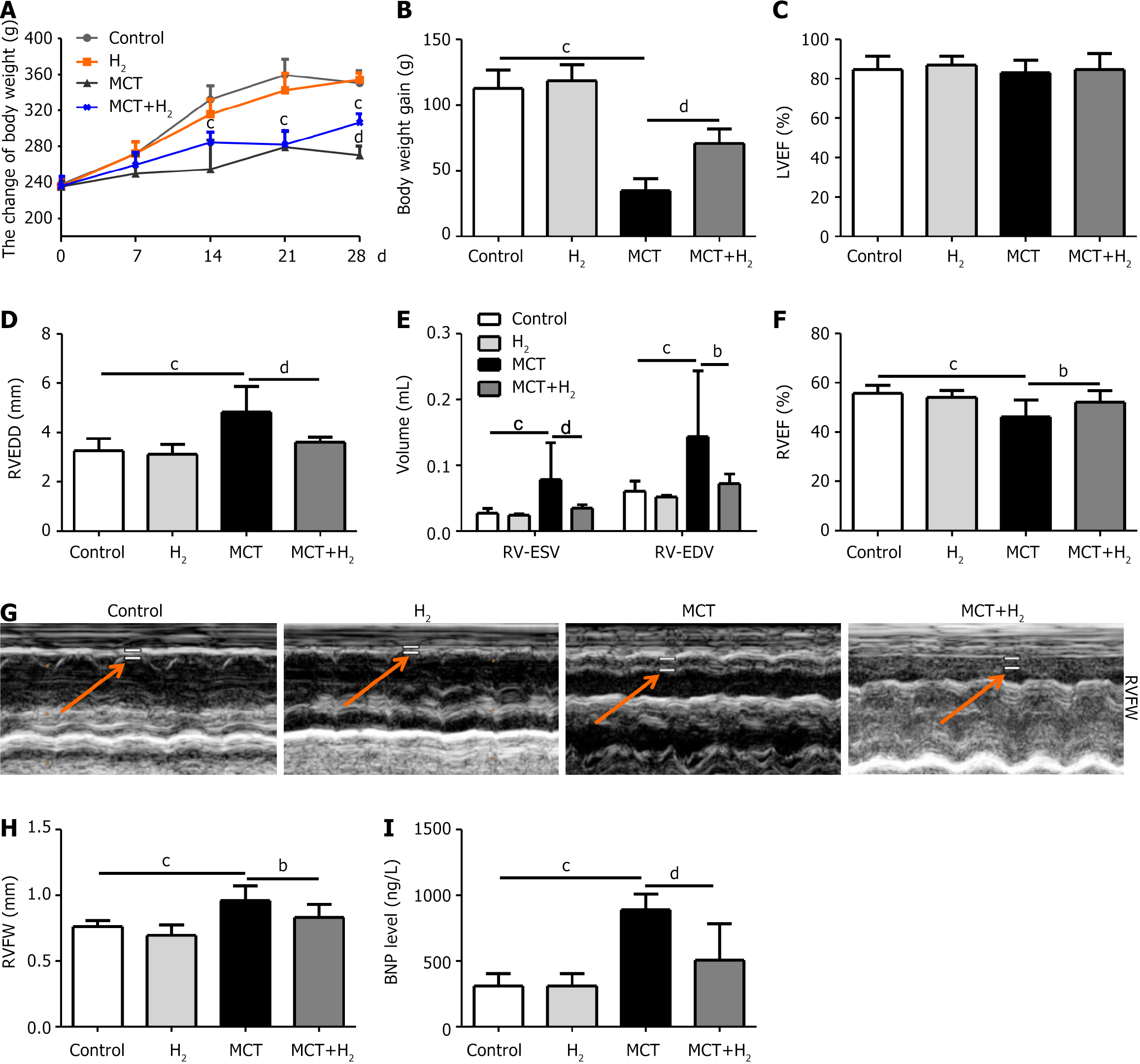
The weight of the rats was measured weekly, their general condition was observed, and no spontaneous death occurred within 28 days after MCT administration. The condition of the MCT rats was worse than that of the control group (Figure 1A), and body weight (BW) gain (Figure 1B) was significantly decreased. Conversely, the condition of the MCT rats in the H2 inhalation group was better than that of the MCT rats, with significantly increased BW and BW gains. The LVEF of the MCT group was lower than that of the other groups, but the difference was not significant, suggesting that the LVEF of MCT rats was not significantly decreased (Figure 1C), which was consistent with the model of RVH. We also evaluated the RV function in rats. RVEDD was significantly increased in the MCT group compared to that in the control group and was notably reduced by H2 treatment (Figure 1D). RV-ESV and RV-EDV were notably increased in the MCT group compared to those in the control group, but were decreased by H2 treatment (Figure 1E). RVEF was significantly decreased in the MCT group compared to that in the control group and was markedly increased by H2 treatment (Figure 1F). H2 inhalation alleviated abnormal morphological hypertrophy in MCT-treated rats. We assessed the RV structure in rats. RVFW thickness was increased in the MCT group compared to that in the control group, and this change was significantly alleviated by H2 treatment (Figure 1G and H).
Serum BNP levels in the MCT group were notably increased compared to those in the control group, but were significantly decreased after H2 treatment (Figure 1I). Thus, 28 days of H2 treatment alleviated abnormal morphological hypertrophy and improved cardiac function in MCT-treated rats.
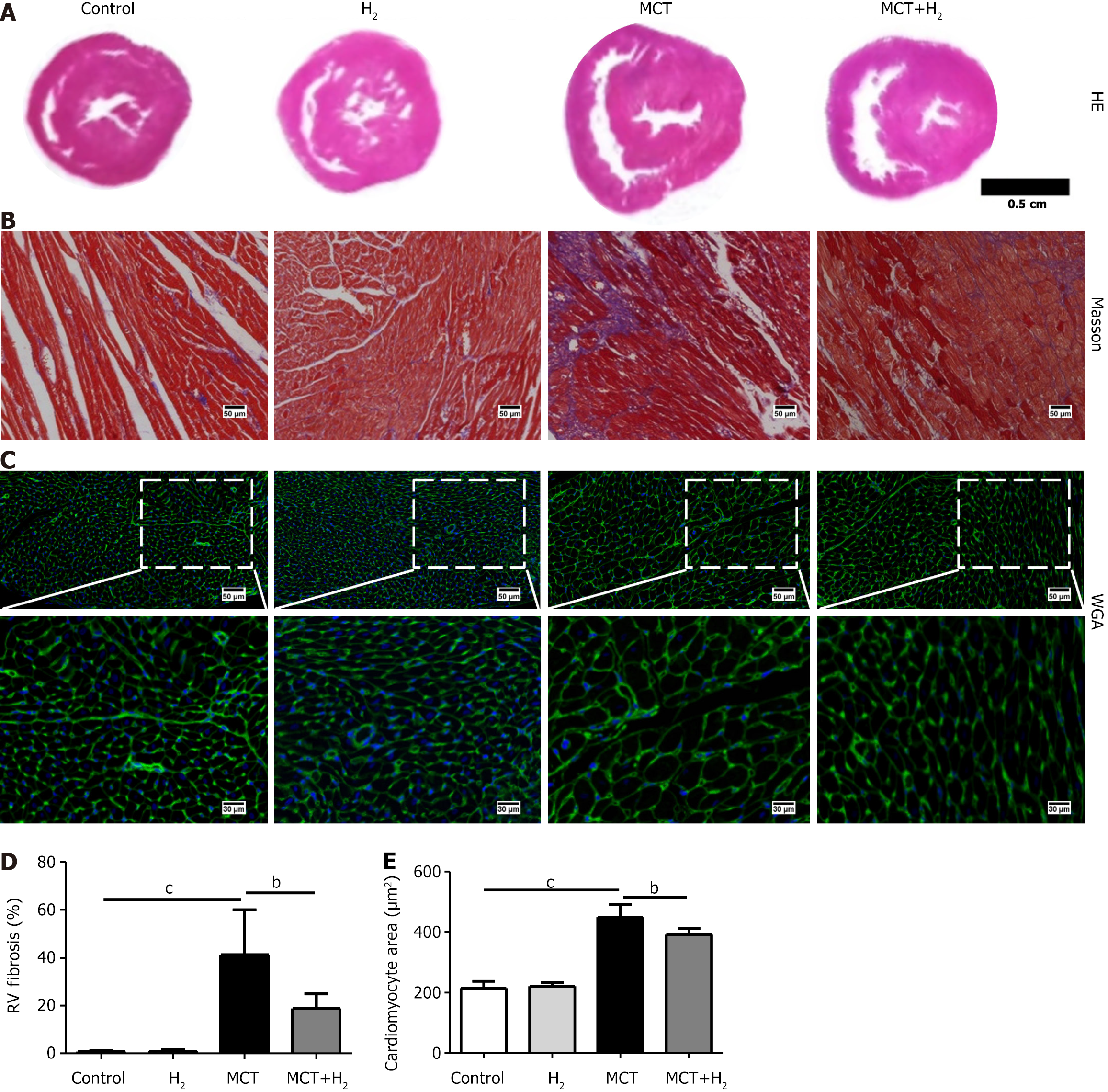
Based on the results of HE staining (Figure 2A), Masson’s trichrome staining (Figure 2B), and WGA staining (Figure 2C) of heart tissues, RV hypertrophy and fibrosis were significantly reduced in the MCT+H2 group compared with the MCT group. Cardiomyocyte fibrosis (Figure 2D) and area (Figure 2E) were increased in the MCT group, but these changes were reversed by H2 treatment.
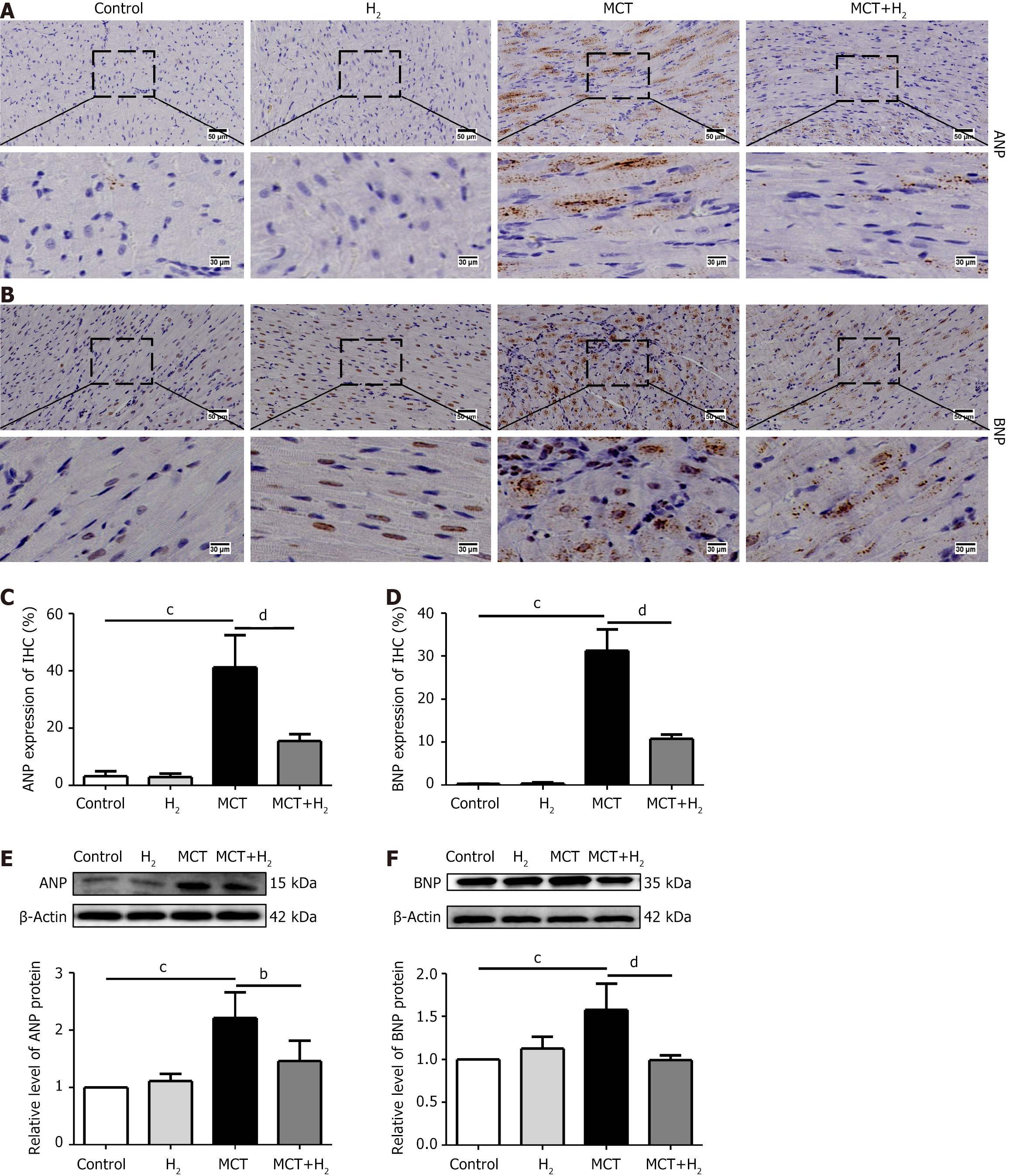
IHC staining showed that ANP and BNP levels in the MCT group were higher than those in the control group, and H2 treatment prevented this increase (Figure 3A-D). Similarly, the western blotting results showed that the expression of ANP and BNP was increased in the MCT group compared to that in the control group but decreased after H2 treatment (Figure 3E and F). These data confirmed that H2 has a protective effect against hypertrophy in MCT-treated rats.
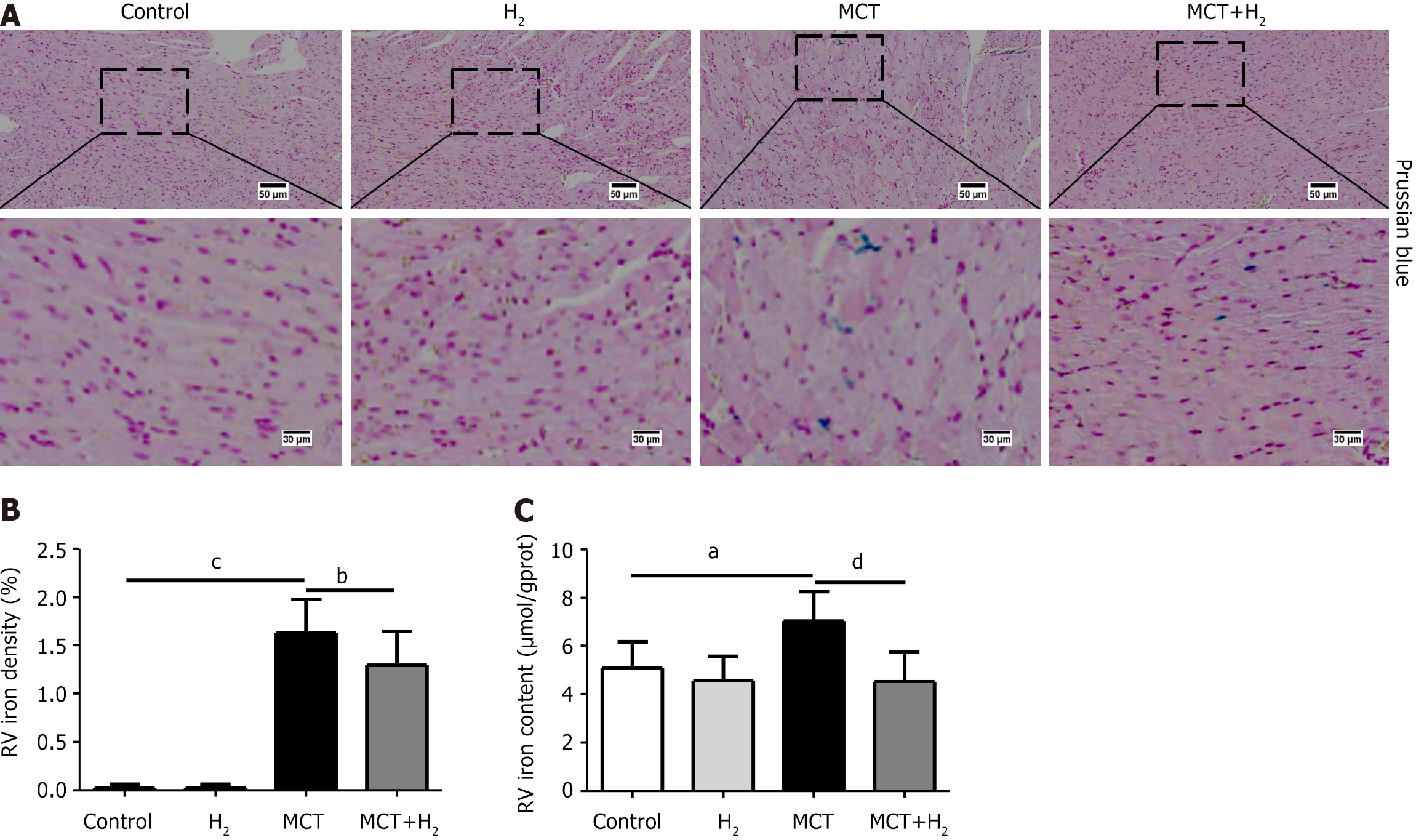
We assessed iron levels in the heart tissue, as iron is an important trigger of ferroptosis. Prussian blue staining showed that the number of iron-stained cells increased in MCT-treated rats. The iron content of RVH myocardial tissue was higher than that in normal rats. H2 reduced the iron content and proportion of Prussian blue iron-stained cells in the myocardium of MCT-treated rats (Figure 4A and B). The iron content of the RV tissue in the MCT group was notably elevated compared to that in the control group, but was reduced significantly after H2 treatment (Figure 4C).
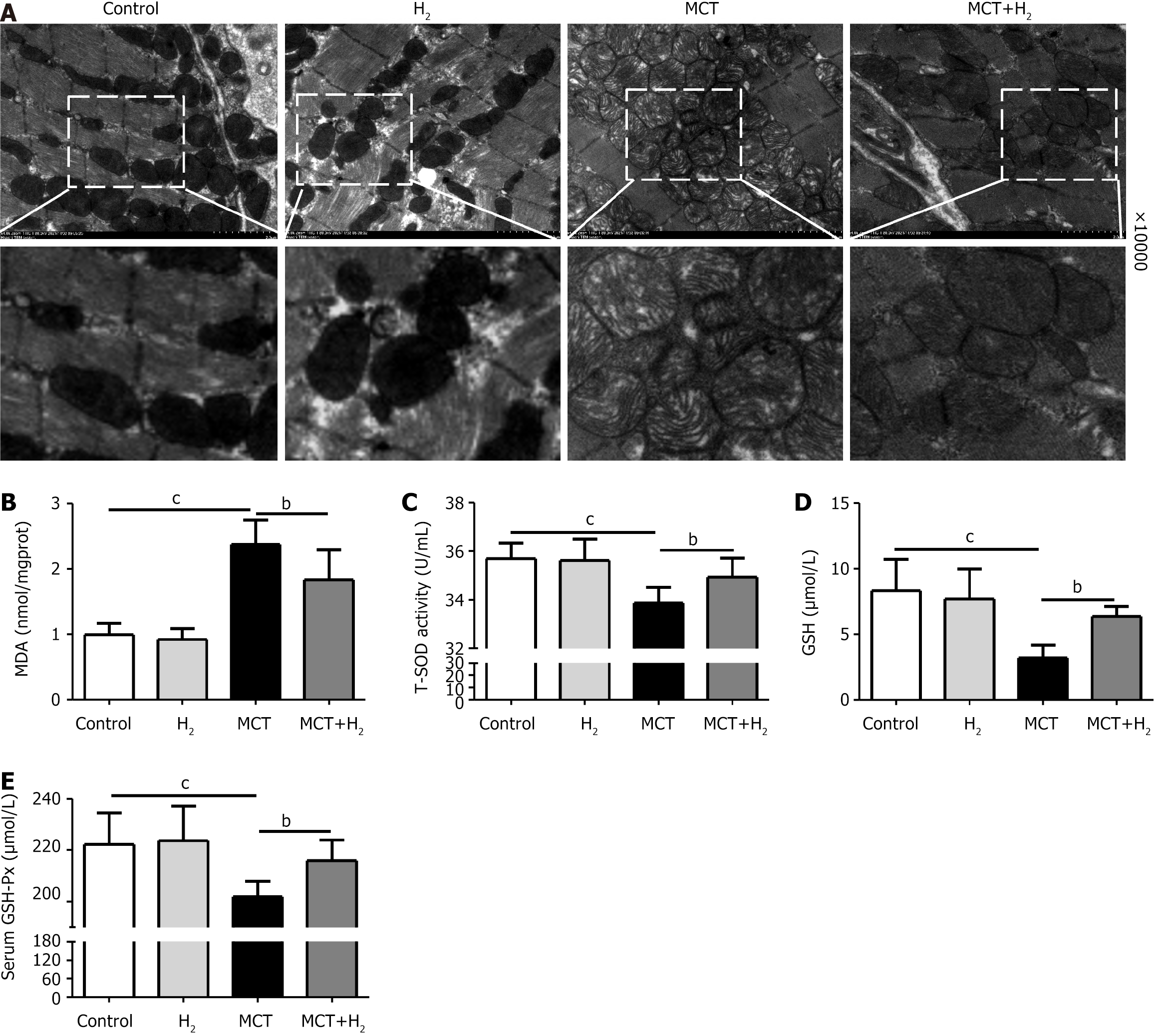
Next, we measured the mitochondrial morphology of the RV in each group using transmission electron microscopy (TEM) images. We observed that mitochondrial membrane density increased and mitochondrial cristae decreased or disappeared in MCT-treated rats compared to those in the control group. However, H2 inhalation significantly inhibited this effect (Figure 5A). To investigate the effect of H2 on MCT-induced oxidative stress, we assessed the levels of the oxidative stress-related indicators MDA, T-SOD, GSH, and GSH-Px. MDA is the primary marker of damage due to oxidative stress[32]. According to our results, MDA levels were significantly higher in the MCT group than in the control group and significantly lower in the MCT+H2 group than in the MCT group (Figure 5B). In addition, compared with the control group, serum T-SOD, GSH, and GSH-Px activities were downregulated in the MCT group and upregulated after H2 treatment (Figure 5C-E). In this regard, our results revealed that severe injury due to oxidative stress is an important mechanism underlying the occurrence and development of RVH, and that H2 inhalation suppressed oxidative stress in MCT-treated rats.
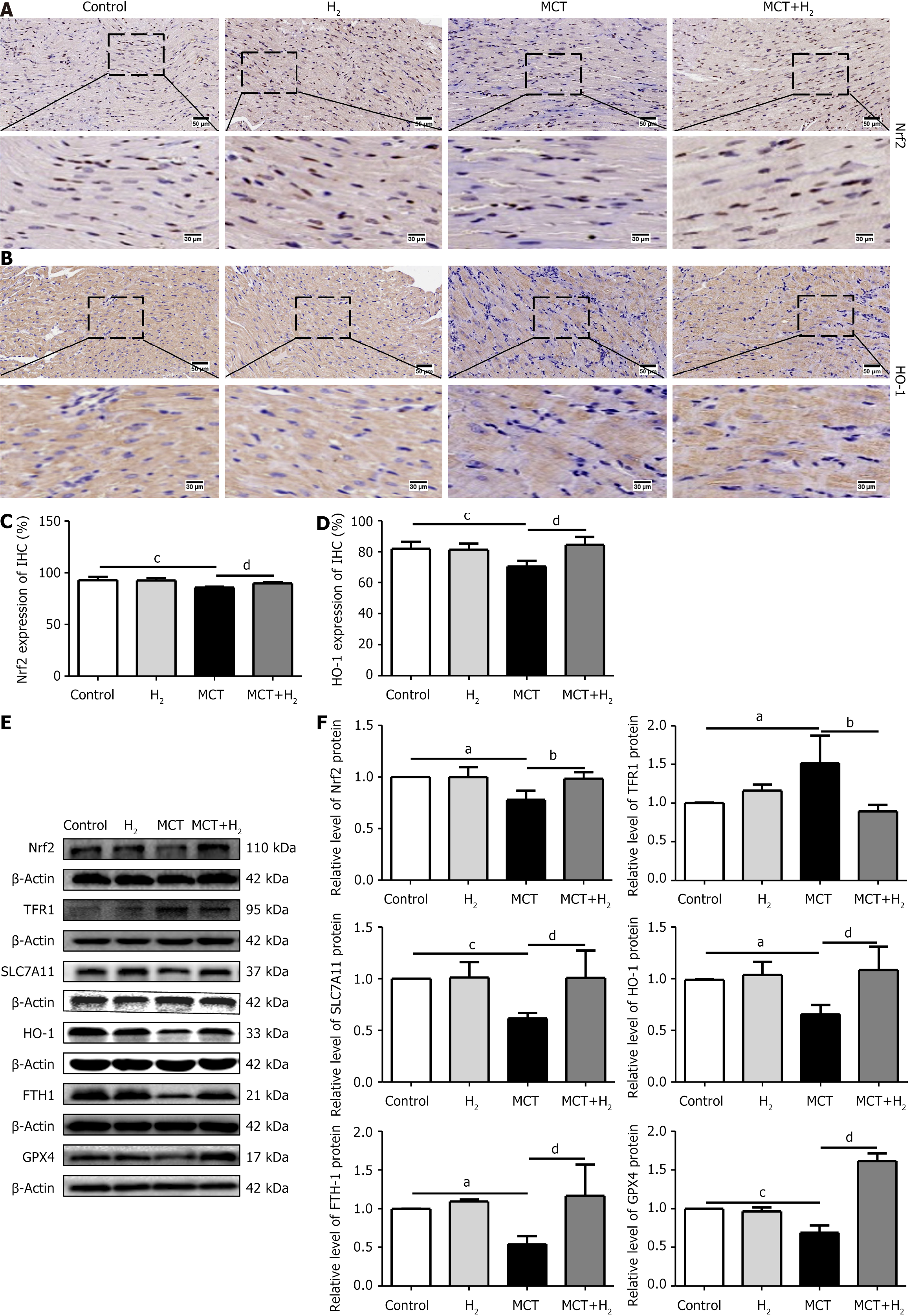
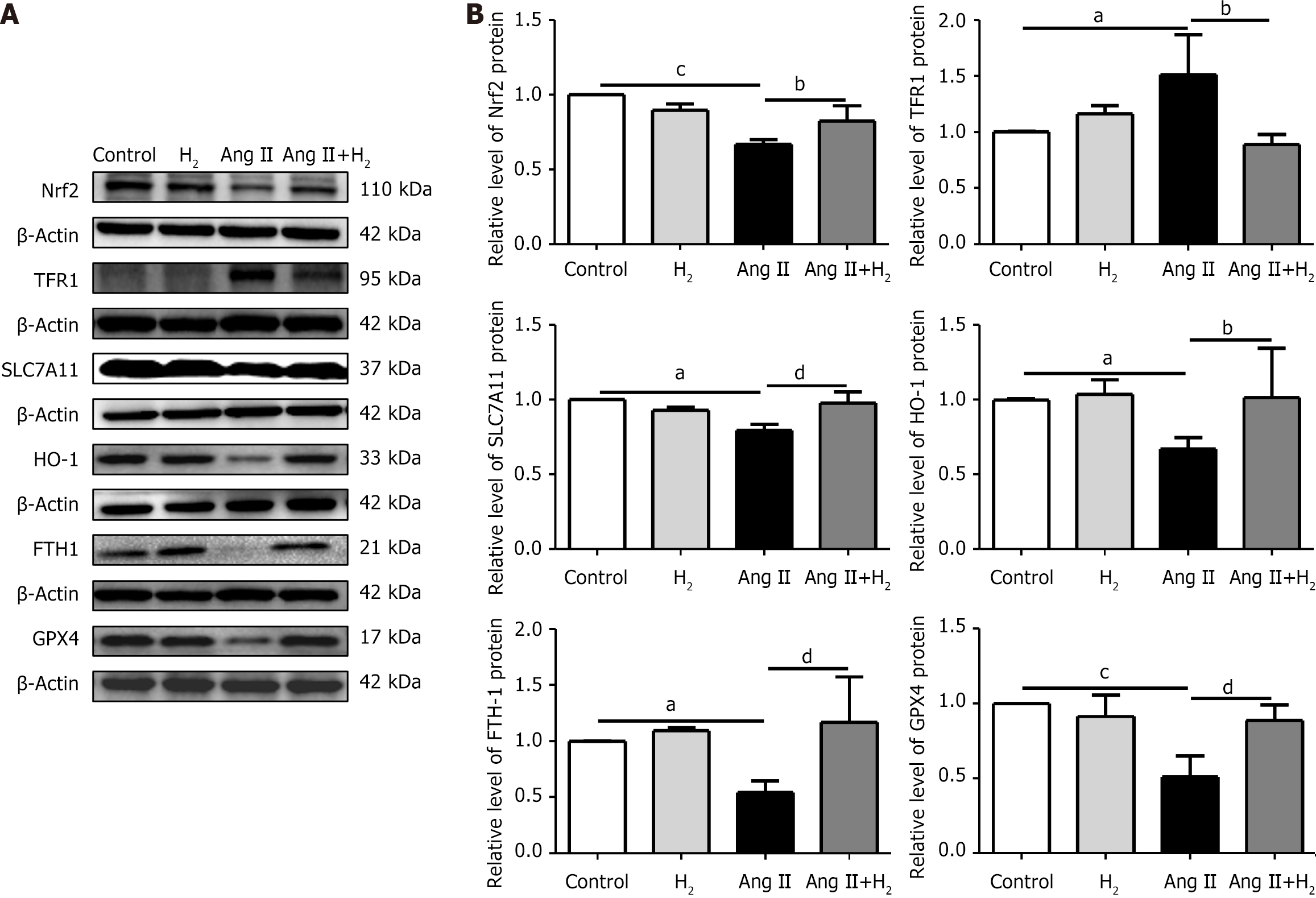
To elucidate the mechanisms involved in the prevention of RVH by H2 inhalation, we examined the effects of H2 on the core regulators of ferroptosis in the presence of RVH. IHC staining revealed the expression of Nrf2 (Figure 6A), and HO-1 (Figure 6B) in the MCT group was downregulated compared to that in the control group, and H2 treatment prevented this downregulation. Figure 6C and D also confirm this result. The expression levels of the ferroptosis-associated proteins Nrf2, HO-1, SLC7A11, FTH1, and GPX4 decreased after MCT administration, whereas the expression level of TFR1 increased. The levels of Nrf2, HO-1, SLC7A11, FTH1, and GPX4 were upregulated, whereas those of TFR1 were downregulated after H2 inhalation (Figure 6E and F). Ferroptosis occurred in the MCT rat model with pressure overload in the right cardiac system. H2 inhalation reduced right cardiac hypertrophy in MCT rats and improved cardiac function.
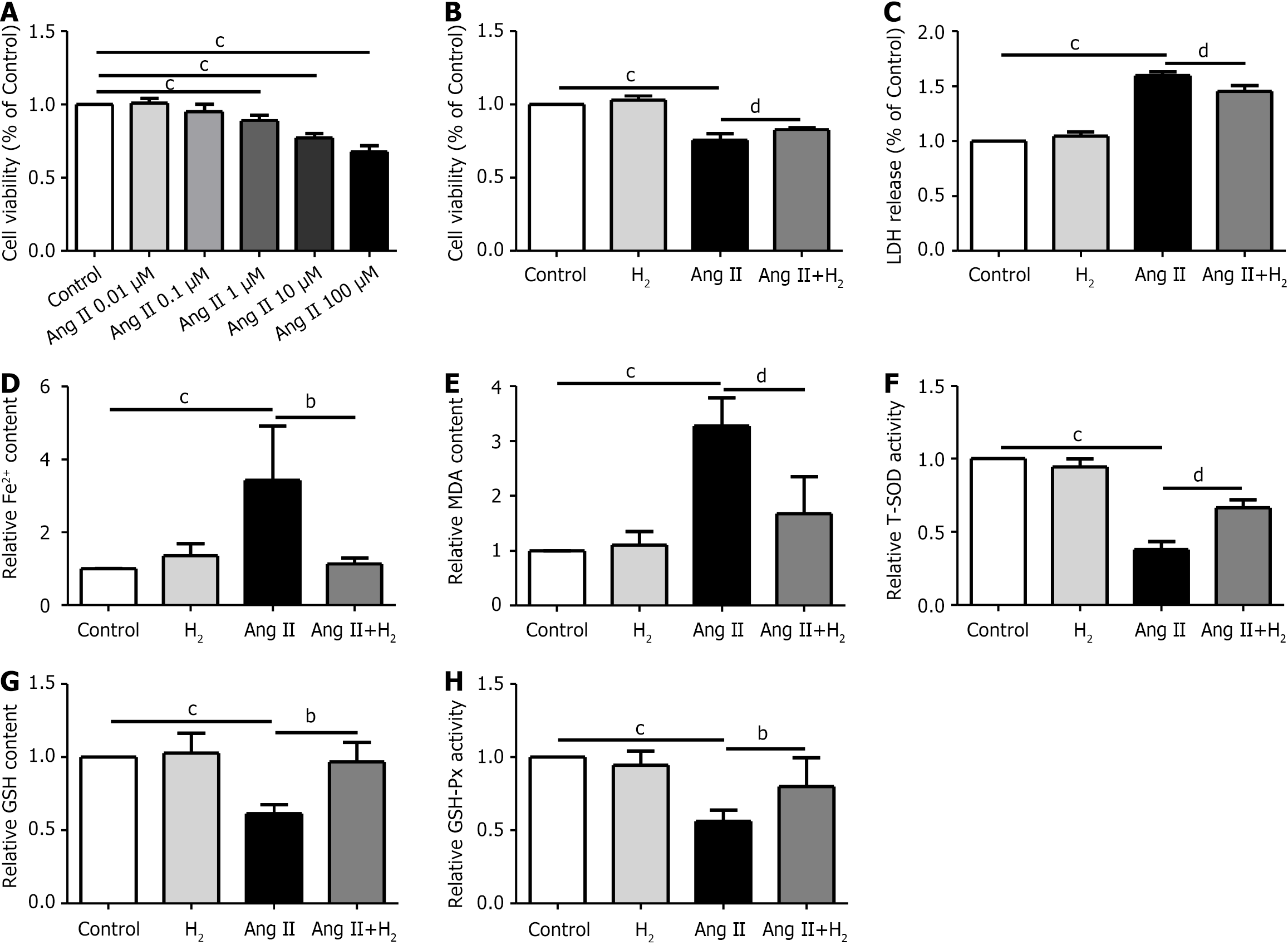
Hypertrophy was induced in H9C2 cells by administering different concentrations of Ang II (0, 0.01, 0.1, 1, 10, and 100 μmol/L) after 24 hours of serum starvation. Ang II-induced hypertrophy was obvious at a concentration of 1 μmol/L. A CCK-8 assay was performed to confirm the activity of H9C2 cells, and the degree of H9C2 cell damage was determined using an LDH release assay. The Fe2+ content in the Ang II group was higher than that in the control group, but was reduced after H2 treatment (Figure 7A-D). We then detected the oxidation-related indicators MDA, T-SOD, GSH, and GSH-Px, and the Fe2+ content in H9C2 cells. Our results suggest that MDA levels were higher in the Ang II group than in the control group and were lower after H2 treatment (Figure 7E). In addition, compared to the control group, T-SOD, GSH, and GSH-Px activities were downregulated in the Ang II group and upregulated after H2 treatment (Figure 7F-H). In this study, we confirmed that H2 alleviates iron accumulation and oxidative stress in Ang II-treated H9C2 cells.
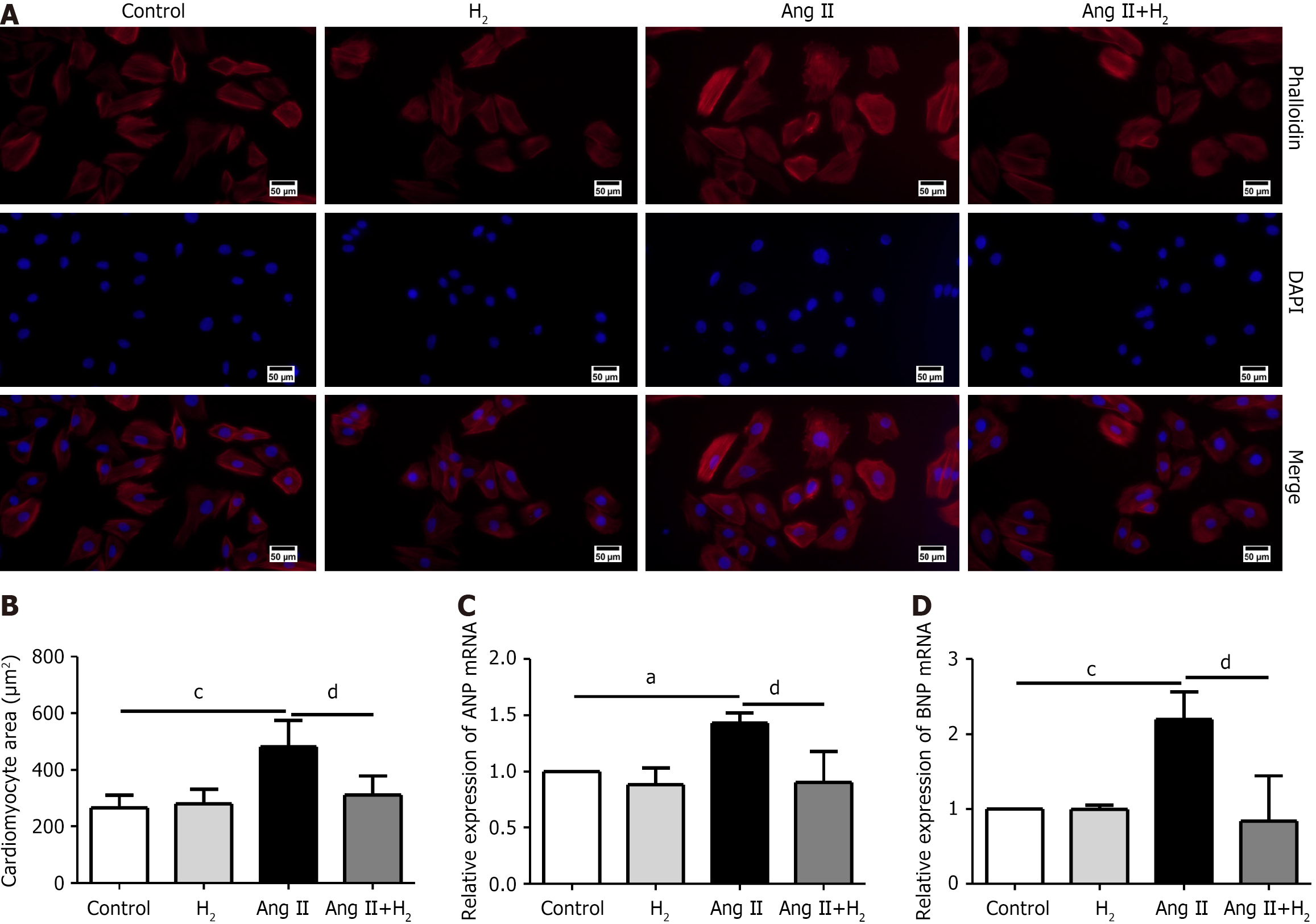
The effects of H2 on cardiomyocyte hypertrophy were assessed using phalloidin staining. Phalloidin and DAPI staining were combined (Figure 8A). The surface area of H9C2 cells in the different groups was measured. The cell surface areas were increased after Ang II induction, but decreased after H2 treatment (Figure 8B). We investigated the mRNA levels of hypertrophy-related indicators in H9C2 cells using qPCR. The qPCR results showed that the levels of ANP mRNA in the Ang II group were higher than those in the control group, but H2 treatment prevented this increase (Figure 8C). Furthermore, the mRNA expression of BNP increased in the Ang II group compared to that in the control group but decreased after H2 treatment (Figure 8D). We confirmed the ameliorative effect of H2 on the excessive growth of Ang II-treated H9C2 cells.
The levels of ROS (green fluorescent signal) in the H9C2 cells of each group were detected by DCFH-DA staining (Figure 9A). ROS levels were higher in the Ang II group than in the control group. H2 treatment significantly reversed this trend (Figure 9A). MMP levels were measured by JC-1 staining. An increased red/green fluorescence ratio indicates an increase in MMP. We analyzed the ratio of red-to-green fluorescence intensity (aggregates/monomers). The MMP in H9C2 cells was significantly decreased after Ang II treatment, but recovered by H2 treatment (Figure 9B). The same result can be found in Figure 9C and D.
To determine the mechanism by which H2 prevents cardiomyocyte hypertrophy in H9C2 cells, we examined ferroptosis-related indicators. The expression levels of proteins associated with ferroptosis, Nrf2, TFR1, SLC7A11, HO-1, FTH1, and GPX4, decreased after Ang II administration, whereas the expression of TFR1 protein was upregulated. After H2 treatment, the levels of Nrf2, TFR1, SLC7A11, HO-1, FTH1, and GPX4 proteins were upregulated, whereas the expression of TFR1 protein was downregulated (Figure 10). Overall, H2 reduced ferroptosis in H9C2 cells and increased the expression of components of the Nrf2/HO-1 pathway. Ferroptosis occurred in an Ang II cell model of right heart system pressure overload, and H2 reduced Ang II-induced cell hypertrophy. H2 inhibits ferroptosis by reducing the iron content, inhibiting oxidative stress, enhancing antioxidant activity, regulating the expression of various ferroptosis-related proteins, and alleviating myocardial hypertrophy caused by pressure overload.
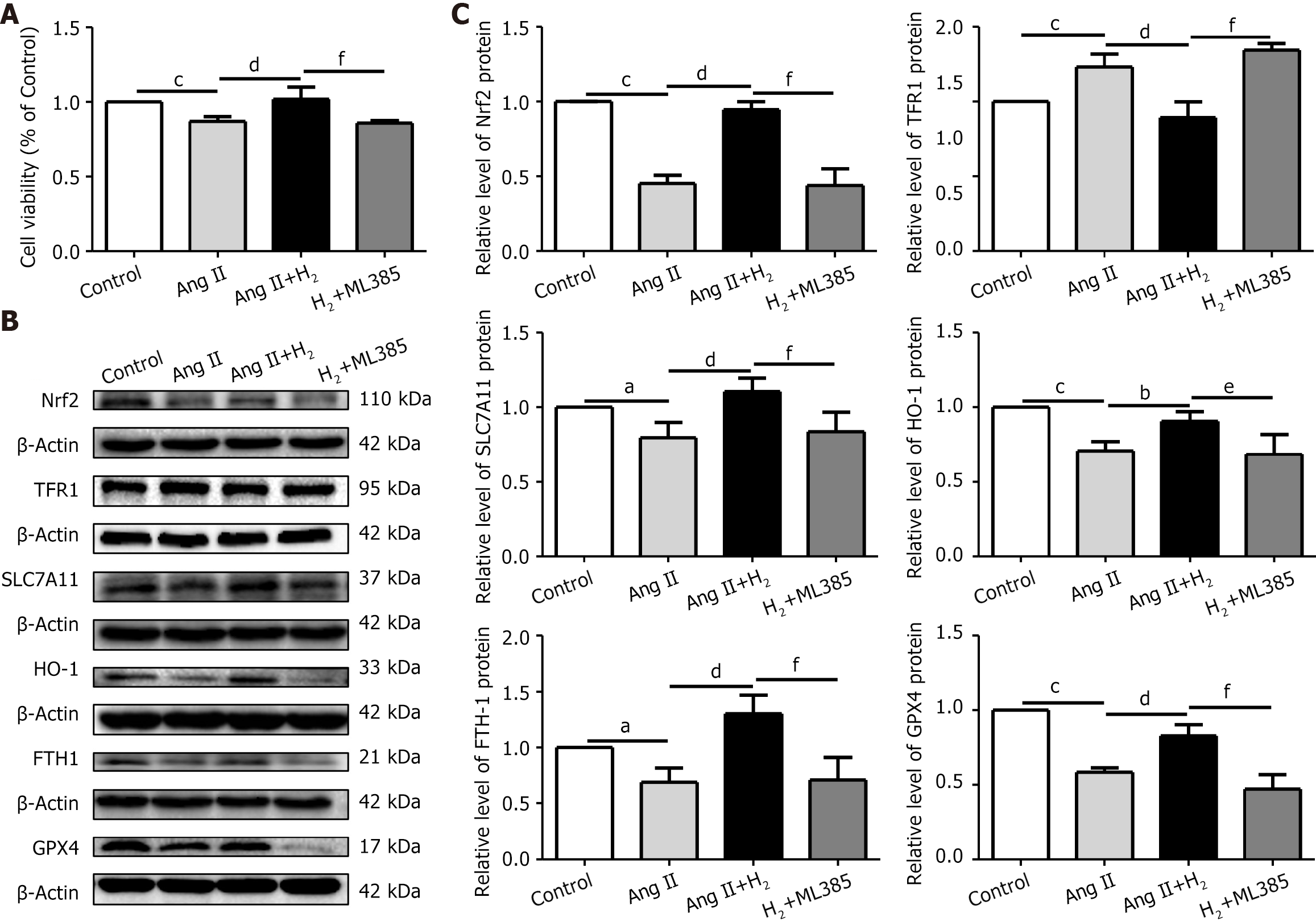
Mechanistically, the anti-ferroptotic effect of H2 is achieved by restoring the Nrf2/HO-1 signaling pathway. In particular, H2 treatment increased the expression of Nrf2 in Ang II-treated H9C2 cells. The specific Nrf2 inhibitor ML385 (1 μmol/L) was used in our study. Consistently, the Ang II+H2 group showed decreased ferroptosis compared to the control group, whereas ML385 treatment inhibited restoration of the Nrf2/HO-1 signaling pathway and negated the protective effects of H2 (Figure 11). In the pressure overload model of the right cardiac system, the inhibitory effect of H2 on ferroptosis was related to restoration of the Nrf2/HO-1 signaling pathway.
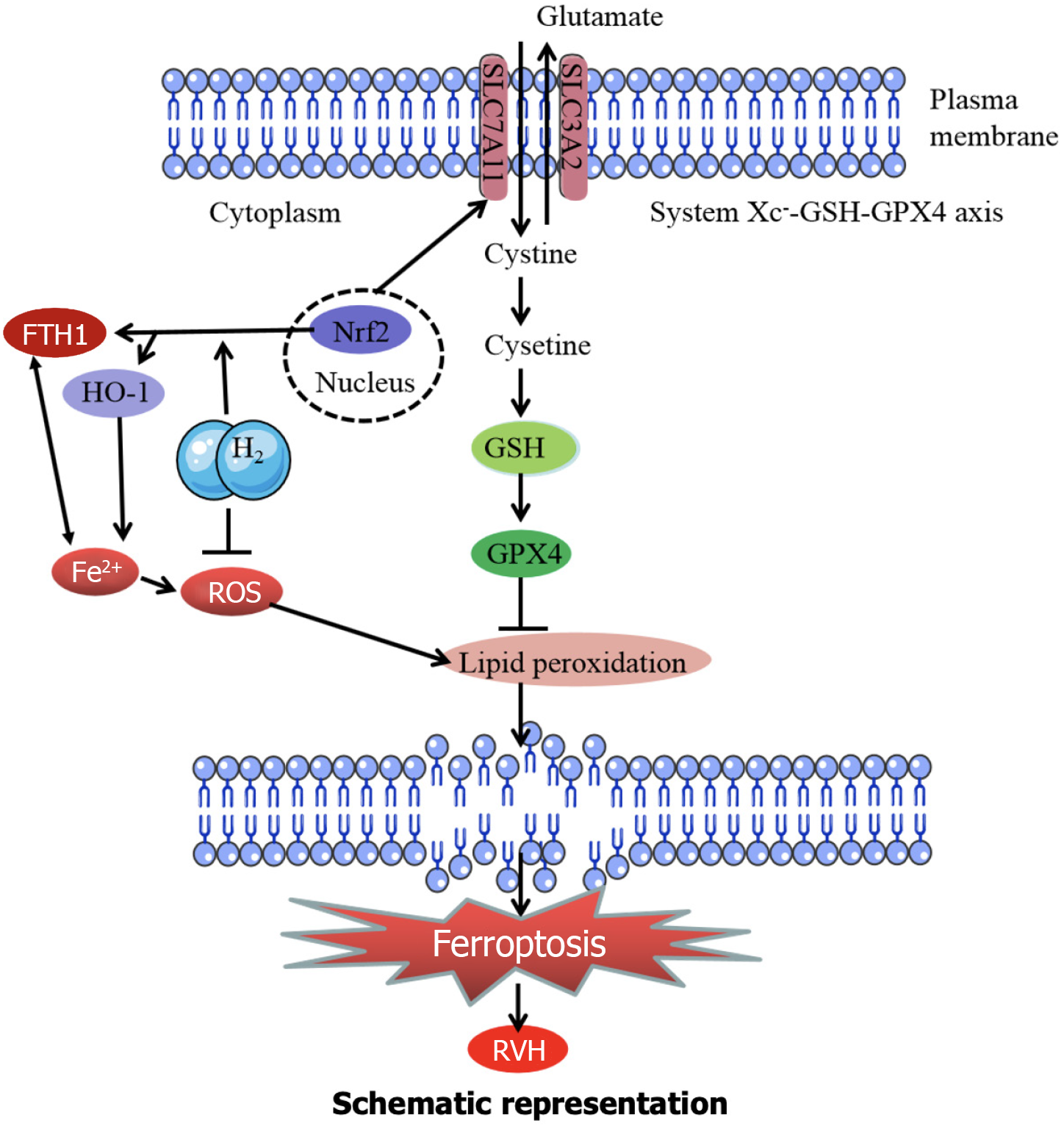
RVH is caused by long-term overload, which mainly refers to hypertrophy and enlargement of the RV, ultimately leading to right heart failure or even death[33]. RHV is easily overlooked in the early stages and associated with poor clinical prognosis and high mortality. RV function is the main determinant of survival in patients with pulmonary arterial hypertension[34]. The right ventricle is more susceptible to oxidative stress-induced damage than the left ventricle[35-37]. Effective treatment is important to prevent RVH; however, clinical options are limited. H2 is a biological antioxidant and can significantly inhibit injury in multiple organs by alleviating oxidative stress[19,38-40]. Based on previous studies, we investigated the protective effects of H2 against cardiac hypertrophy by inhibiting ferroptosis. Here, we present the first evidence that H2 alleviates RV hypertrophy and dysfunction by inhibiting ferroptosis in vivo. Furthermore, we confirmed that H2 inhibits ferroptosis by restoring the Nrf2/HO-1 signaling pathway in vitro in MCT-treated rats. H2 suppresses RVH through several key mechanisms. First, H2 inhibited ROS production. As depicted in Figure 12, ROS contributes to lipid peroxidation, which is a crucial step in ferroptosis. Thus, H2 prevents ferroptosis initiation by reducing ROS levels. Second, H2 likely interacts with Fe2+ ions. As Fe2+ contributes to the generation of ROS, interference with Fe2+ activity by H2 can decrease the formation of ROS and subsequent lipid peroxidation. Third, H2 restores the Nrf2 pathway. Once activated, Nrf2 translocates to the nucleus and induces the expression of HO-1, which has antioxidant properties that counteract oxidative stress and reduce ferroptosis. Finally, H2 helps maintain the GSH–GPX4 axis, which is essential for preventing lipid peroxidation because GSH and GPX4 work together to reduce lipid peroxidation. By preserving this axis, H2 prevents the progression of ferroptosis associated with RVH (Figure 12). It has been established that reducing agents like H2 can act on the redox-sensitive cysteine residues within KEAP1. The structure and function of KEAP1 are altered by specific chemical modifications of these residues. This alteration disrupts the normal inhibitory interaction between KEAP1 and NRF2 in the cytoplasm. Once the ability of KEAP1 to bind and sequester NRF2 is compromised, NRF2 is free to translocate to the nucleus. Inside the nucleus, NRF2 can bind to specific antioxidant response elements on DNA, initiating the transcription of a series of downstream target genes, including HO-1, which plays a crucial role in counteracting oxidative stress and regulating various physiological and pathological processes related to our observed outcomes.
The weights of the rats were measured weekly and their BW increased gradually every week. The rats in all groups were weighed and the average weekly weight and weight gain were calculated. Compared with the control group, BW was reduced at 14, 21, and 28 days after MCT injection, and weight increased after H2 inhalation. Comparing weight gain among rats in each group, weight gain 28 days after H2 administration was significantly lower than that in the MCT group. We suggest that a decrease in the heart function of rats led to impaired appetite and digestive system function, which resulted in a decrease in BW and weight gain.
Studies have investigated specific cardiac morphometric changes in MCT-induced RVH[26,31]. Echocardiography results showed that the LVEF in MCT rats was not significantly decreased, which was consistent with the RVH model. In addition, RVEDD, RV-ESV, RV-EDV, RVEF, and RVFW are indicators of RV structure and function[26,31]. We evaluated the RVEDD, RV-ESV, RV-EDV, RVEF, and RVFW using echocardiography. Consistent with prior studies, H2 inhalation improved RV function in MCT-treated rats, as determined by the decrease in RVEDD, RV-ESV, RV-EDV, RVFW, and BNP, and the increase in RVEF.
RVFW is an indicator of RV structural abnormalities[26,41]. To investigate the effect of H2 in RVH rats, RVFW was compared between groups and found to be increased in MCT-treated rats and decreased after H2 treatment. H2 inhalation alleviated the cardiomyocyte area and fibrosis in MCT-treated rats. HE staining showed that RVH was increased in MCT-treated rats and decreased by H2 inhalation. Masson staining showed that fibrosis in the right ventricle was increased in MCT-treated rats and decreased by H2 inhalation. In addition, WGA staining showed that H2 treatment significantly suppressed enlargement of the cardiomyocyte area caused by MCT. The cardiomyocyte area was severely increased in RVH rats and was significantly suppressed by H2 treatment. Furthermore, the hypertrophy-related markers ANP and BNP increased in the model group but decreased after H2. These results supported our hypothesis that the H2 strategy has a preventive effect against hypertrophy caused by pressure overload in the right cardiac system.
H2 has been explored in various diseases. In the central nervous system, H2 shows potential for treating conditions such as Parkinson's and Alzheimer’s diseases, as well as neonatal hypoxic-ischemic encephalopathy. The cardiovascular system improves heart function in myocardial infarction and reduces oxidative stress]. It also shows promise for the treatment of acute pancreatitis, sepsis, respiratory diseases, and other diseases. In China, the "H2 therapy instrument" has been approved as a class III medical device by the National Medical Products Administration for the auxiliary treatment of several diseases including cerebral ischemia, pulmonary ischemia, and others. Although the regulatory authorities in most countries have not achieved a widespread global consensus, existing research and partial approval indicate the potential of H2 for future clinical use.
Ferroptosis is typically characterized by iron accumulation, lipid peroxidation and mitochondrial dysfunction[3,42,43]. Iron is an essential element for many biological processes, including oxygen transport and storage, oxidative phosphorylation, and redox reactions. Excessive iron accumulation causes abnormal redox reactions leading to organ dysfunction. Excess iron produces ROS via the Fenton reaction, leading to ferroptosis. We found that the accumulation of iron and MDA led to ferroptosis and subsequent myocardial damage and deterioration. H2 regulates iron metabolism in MCT-treated rats. In our study, the regulation of iron metabolism and maintenance of iron homeostasis were decreased in MCT-treated rats. Ferroptosis mainly occurs because of inactivation of the cellular antioxidant system[44]. The activities of T-SOD, GSH, and GSH-Px antioxidant systems were significantly decreased in vivo and in vitro (Figure 7). Similarly, mitochondria in the RV tissue and H9C2 cells were identified using TEM and JC-1 assays. The results showed that the ameliorative effect of H2 on cardiohypertrophy was related to its ability to suppress oxidative stress.
Disruption of system Xc-function can induce ferroptosis[10]. The downregulation of SLC7A11 is believed to contribute to ferroptosis[3,45]. Our data suggest that SLC7A11 downregulation promotes ferroptosis in cardiomyocytes, which is consistent with previous studies[9,46]. In addition, GPX4 protein expression was significantly decreased in the model group, but significantly increased after H2 treatment. In our study, the iron content in the model group was higher than that in the control group and reduced after H2 administration. TFR1 is a ferroptosis marker. Recent studies confirmed that FTH1 is crucial for initiating and promoting cardiomyocyte ferroptosis[3]. Decreased FTH1 Levels indicate increased iron uptake and decreased iron storage. Thus, decreased FTH1 Levels may contribute to iron overload during ferroptosis. The model group showed decreased expression of FTH1 compared to the control group, which significantly increased after H2 treatment. In addition, the model group showed increased TFR1 expression compared to the control group, which was decreased after H2 treatment.
We clarified that the cardioprotective effects of H2 depend on ferroptosis and Nrf2/HO-1 restoration during cardiomyocyte hypertrophy. Nrf2 is an important transcription factor activated during regulation of the antioxidant response[30,47]. Similar to previous studies, we found that Nrf2 promotes the expression of HO-1 after H2 treatment. In our study, the protein expression levels of Nrf2 and HO-1 in the model group were downregulated compared to those in the control group, whereas H2 treatment prevented this downregulation.
In RVH, ferroptosis is caused by a pressure overload in the right cardiac system. H2 can inhibit ferroptosis by upregulating the Nrf2/HO-1 signaling pathway, reducing the iron content, inhibiting oxidative stress, enhancing antioxidant activity, and regulating a variety of ferroptosis-related proteins. The inhibitory effect of H2 on ferroptosis may be related to restoration of the Nrf2/HO-1 signaling pathway. H2 restores the Nrf2/HO-1 signaling pathway and inhibits the ferroptosis of cardiomyocytes caused by pressure overload in the right cardiac system, thereby reducing RVH.
This study has several limitations. The exact proportion of cardiomyocytes affected by ferroptosis or cell death in vivo and in vitro remains unclear because we did not perform TUNEL assays or blotting of cleaved caspase-3. Moreover, the role of Ang II in RVH is debatable in the MCT model, with incomplete exploration of the related physiological responses. Experiments on Nrf2 overexpression and knockout in mice are also lacking. In addition, the effects of H2 inhalation on ventricular wall thickness and Nrf2 activation, along with its relationship with ferroptosis and the Nrf2/HO-1 pathway, require further in-depth studies. For further research, we suggest applying a TUNEL assay and caspase-3 blotting, refining the model to better understand the role of Ang II, establishing Nrf2 gene-manipulated mouse models, and conducting more detailed studies on H2-related effects.
In conclusion, our study provides evidence that H2 retards the progression of cardiac hypertrophy caused by pressure overload in the right cardiac system by inhibiting ferroptosis and restoring the Nrf2/HO-1 signaling pathway. Our findings suggest that H2 inhalation represents a simple and convenient candidate strategy for protection against RVH. This approach shows potential for addressing RVH and warrants further exploration.
| 1. | Reddy S, Bernstein D. Molecular Mechanisms of Right Ventricular Failure. Circulation. 2015;132:1734-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 2. | Huang Y, Lei C, Xie W, Yan L, Wang Y, Yuan S, Wang J, Zhao Y, Wang Z, Yang X, Qin X, Fang Q, Fang L, Guo X. Oxidation of Ryanodine Receptors Promotes Ca(2+) Leakage and Contributes to Right Ventricular Dysfunction in Pulmonary Hypertension. Hypertension. 2021;77:59-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Fang X, Wang H, Han D, Xie E, Yang X, Wei J, Gu S, Gao F, Zhu N, Yin X, Cheng Q, Zhang P, Dai W, Chen J, Yang F, Yang HT, Linkermann A, Gu W, Min J, Wang F. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A. 2019;116:2672-2680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 595] [Cited by in RCA: 1490] [Article Influence: 248.3] [Reference Citation Analysis (0)] |
| 4. | Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, Noel K, Jiang X, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran Q, Rosenfeld CS, Salnikow K, Tang D, Torti FM, Torti SV, Toyokuni S, Woerpel KA, Zhang DD. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171:273-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4608] [Cited by in RCA: 4922] [Article Influence: 615.3] [Reference Citation Analysis (0)] |
| 5. | Menon AV, Liu J, Tsai HP, Zeng L, Yang S, Asnani A, Kim J. Excess heme upregulates heme oxygenase 1 and promotes cardiac ferroptosis in mice with sickle cell disease. Blood. 2022;139:936-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 116] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 6. | Ouyang S, You J, Zhi C, Li P, Lin X, Tan X, Ma W, Li L, Xie W. Ferroptosis: the potential value target in atherosclerosis. Cell Death Dis. 2021;12:782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 7. | Leng Y, Luo X, Yu J, Jia H, Yu B. Ferroptosis: A Potential Target in Cardiovascular Disease. Front Cell Dev Biol. 2021;9:813668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Hu H, Chen Y, Jing L, Zhai C, Shen L. The Link Between Ferroptosis and Cardiovascular Diseases: A Novel Target for Treatment. Front Cardiovasc Med. 2021;8:710963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 9. | Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2021;17:2054-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 1252] [Article Influence: 250.4] [Reference Citation Analysis (0)] |
| 10. | Ananth S, Miyauchi S, Thangaraju M, Jadeja RN, Bartoli M, Ganapathy V, Martin PM. Selenomethionine (Se-Met) Induces the Cystine/Glutamate Exchanger SLC7A11 in Cultured Human Retinal Pigment Epithelial (RPE) Cells: Implications for Antioxidant Therapy in Aging Retina. Antioxidants (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Yuan Y, Zhai Y, Chen J, Xu X, Wang H. Kaempferol Ameliorates Oxygen-Glucose Deprivation/Reoxygenation-Induced Neuronal Ferroptosis by Activating Nrf2/SLC7A11/GPX4 Axis. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 284] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 12. | Yu P, Zhang J, Ding Y, Chen D, Sun H, Yuan F, Li S, Li X, Yang P, Fu L, Yu S, Zhang J. Dexmedetomidine post-conditioning alleviates myocardial ischemia-reperfusion injury in rats by ferroptosis inhibition via SLC7A11/GPX4 axis activation. Hum Cell. 2022;35:836-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 13. | Liu XJ, Lv YF, Cui WZ, Li Y, Liu Y, Xue YT, Dong F. Icariin inhibits hypoxia/reoxygenation-induced ferroptosis of cardiomyocytes via regulation of the Nrf2/HO-1 signaling pathway. FEBS Open Bio. 2021;11:2966-2976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 14. | Baba Y, Higa JK, Shimada BK, Horiuchi KM, Suhara T, Kobayashi M, Woo JD, Aoyagi H, Marh KS, Kitaoka H, Matsui T. Protective effects of the mechanistic target of rapamycin against excess iron and ferroptosis in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2018;314:H659-H668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 272] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 15. | Fang X, Cai Z, Wang H, Han D, Cheng Q, Zhang P, Gao F, Yu Y, Song Z, Wu Q, An P, Huang S, Pan J, Chen HZ, Chen J, Linkermann A, Min J, Wang F. Loss of Cardiac Ferritin H Facilitates Cardiomyopathy via Slc7a11-Mediated Ferroptosis. Circ Res. 2020;127:486-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 519] [Article Influence: 103.8] [Reference Citation Analysis (0)] |
| 16. | Sanghvi VR, Leibold J, Mina M, Mohan P, Berishaj M, Li Z, Miele MM, Lailler N, Zhao C, de Stanchina E, Viale A, Akkari L, Lowe SW, Ciriello G, Hendrickson RC, Wendel HG. The Oncogenic Action of NRF2 Depends on De-glycation by Fructosamine-3-Kinase. Cell. 2019;178:807-819.e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 17. | Menegon S, Columbano A, Giordano S. The Dual Roles of NRF2 in Cancer. Trends Mol Med. 2016;22:578-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 494] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 18. | Kim KH, Jeong JY, Surh YJ, Kim KW. Expression of stress-response ATF3 is mediated by Nrf2 in astrocytes. Nucleic Acids Res. 2010;38:48-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1346] [Cited by in RCA: 1685] [Article Influence: 93.6] [Reference Citation Analysis (1)] |
| 20. | Gao Y, Yang H, Fan Y, Li L, Fang J, Yang W. Hydrogen-Rich Saline Attenuates Cardiac and Hepatic Injury in Doxorubicin Rat Model by Inhibiting Inflammation and Apoptosis. Mediators Inflamm. 2016;2016:1320365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Zou R, Nie C, Pan S, Wang B, Hong X, Xi S, Bai J, Yu M, Liu J, Yang W. Co-administration of hydrogen and metformin exerts cardioprotective effects by inhibiting pyroptosis and fibrosis in diabetic cardiomyopathy. Free Radic Biol Med. 2022;183:35-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 22. | Okabe K, Matsushima S, Ikeda S, Ikeda M, Ishikita A, Tadokoro T, Enzan N, Yamamoto T, Sada M, Deguchi H, Shinohara K, Ide T, Tsutsui H. DPP (Dipeptidyl Peptidase)-4 Inhibitor Attenuates Ang II (Angiotensin II)-Induced Cardiac Hypertrophy via GLP (Glucagon-Like Peptide)-1-Dependent Suppression of Nox (Nicotinamide Adenine Dinucleotide Phosphate Oxidase) 4-HDAC (Histone Deacetylase) 4 Pathway. Hypertension. 2020;75:991-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | McMurtry MS, Bonnet S, Wu X, Dyck JR, Haromy A, Hashimoto K, Michelakis ED. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ Res. 2004;95:830-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 362] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 24. | Zhou P, Gao G, Zhao CC, Li JY, Peng JF, Wang SS, Song R, Shi H, Wang L. In vivo and in vitro protective effects of shengmai injection against doxorubicin-induced cardiotoxicity. Pharm Biol. 2022;60:638-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Dignam JP, Scott TE, Kemp-Harper BK, Hobbs AJ. Animal models of pulmonary hypertension: Getting to the heart of the problem. Br J Pharmacol. 2022;179:811-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 26. | Tian L, Wu D, Dasgupta A, Chen KH, Mewburn J, Potus F, Lima PDA, Hong Z, Zhao YY, Hindmarch CCT, Kutty S, Provencher S, Bonnet S, Sutendra G, Archer SL. Epigenetic Metabolic Reprogramming of Right Ventricular Fibroblasts in Pulmonary Arterial Hypertension: A Pyruvate Dehydrogenase Kinase-Dependent Shift in Mitochondrial Metabolism Promotes Right Ventricular Fibrosis. Circ Res. 2020;126:1723-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 27. | Gao L, Jiang D, Geng J, Dong R, Dai H. Hydrogen inhalation attenuated bleomycin-induced pulmonary fibrosis by inhibiting transforming growth factor-β1 and relevant oxidative stress and epithelial-to-mesenchymal transition. Exp Physiol. 2019;104:1942-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Kanzaki Y, Fujita H, Sato K, Hosokawa M, Matsumae H, Morizane Y, Ohuchi H. Protrusion of KCNJ13 Gene Knockout Retinal Pigment Epithelium Due to Oxidative Stress-Induced Cell Death. Invest Ophthalmol Vis Sci. 2022;63:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Lyu L, Chen J, Wang W, Yan T, Lin J, Gao H, Li H, Lv R, Xu F, Fang L, Chen Y. Scoparone alleviates Ang II-induced pathological myocardial hypertrophy in mice by inhibiting oxidative stress. J Cell Mol Med. 2021;25:3136-3148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Salman M, Tabassum H, Parvez S. Tannic Acid Provides Neuroprotective Effects Against Traumatic Brain Injury Through the PGC-1α/Nrf2/HO-1 Pathway. Mol Neurobiol. 2020;57:2870-2885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 31. | Han JC, Guild SJ, Pham T, Nisbet L, Tran K, Taberner AJ, Loiselle DS. Left-Ventricular Energetics in Pulmonary Arterial Hypertension-Induced Right-Ventricular Hypertrophic Failure. Front Physiol. 2017;8:1115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Haro Girón S, Monserrat Sanz J, Ortega MA, Garcia-Montero C, Fraile-Martínez O, Gómez-Lahoz AM, Boaru DL, de Leon-Oliva D, Guijarro LG, Atienza-Perez M, Diaz D, Lopez-Dolado E, Álvarez-Mon M. Prognostic Value of Malondialdehyde (MDA) in the Temporal Progression of Chronic Spinal Cord Injury. J Pers Med. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 33. | Oknińska M, Zajda K, Zambrowska Z, Grzanka M, Paterek A, Mackiewicz U, Szczylik C, Kurzyna M, Piekiełko-Witkowska A, Torbicki A, Kieda C, Mączewski M. Role of Oxygen Starvation in Right Ventricular Decompensation and Failure in Pulmonary Arterial Hypertension. JACC Heart Fail. 2024;12:235-247. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Korf-Klingebiel M, Reboll MR, Polten F, Weber N, Jäckle F, Wu X, Kallikourdis M, Kunderfranco P, Condorelli G, Giannitsis E, Kustikova OS, Schambach A, Pich A, Widder JD, Bauersachs J, van den Heuvel J, Kraft T, Wang Y, Wollert KC. Myeloid-Derived Growth Factor Protects Against Pressure Overload-Induced Heart Failure by Preserving Sarco/Endoplasmic Reticulum Ca(2+)-ATPase Expression in Cardiomyocytes. Circulation. 2021;144:1227-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 35. | Schreckenberg R, Rebelo M, Deten A, Weber M, Rohrbach S, Pipicz M, Csonka C, Ferdinandy P, Schulz R, Schlüter KD. Specific Mechanisms Underlying Right Heart Failure: The Missing Upregulation of Superoxide Dismutase-2 and Its Decisive Role in Antioxidative Defense. Antioxid Redox Signal. 2015;23:1220-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Zungu-Edmondson M, Suzuki YJ. Differential stress response mechanisms in right and left ventricles. J Rare Dis Res Treat. 2016;1:39-45. [PubMed] |
| 37. | Türck P, Fraga S, Salvador I, Campos-Carraro C, Lacerda D, Bahr A, Ortiz V, Hickmann A, Koetz M, Belló-Klein A, Henriques A, Agostini F, da Rosa Araujo AS. Blueberry extract decreases oxidative stress and improves functional parameters in lungs from rats with pulmonary arterial hypertension. Nutrition. 2020;70:110579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | Hayashida K, Sano M, Ohsawa I, Shinmura K, Tamaki K, Kimura K, Endo J, Katayama T, Kawamura A, Kohsaka S, Makino S, Ohta S, Ogawa S, Fukuda K. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun. 2008;373:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 378] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 39. | Lee JW, Kim JI, Lee YA, Lee DH, Song CS, Cho YJ, Han JS. Inhaled hydrogen gas therapy for prevention of testicular ischemia/reperfusion injury in rats. J Pediatr Surg. 2012;47:736-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Qiu Z, He Y, Ming H, Lei S, Leng Y, Xia ZY. Lipopolysaccharide (LPS) Aggravates High Glucose- and Hypoxia/Reoxygenation-Induced Injury through Activating ROS-Dependent NLRP3 Inflammasome-Mediated Pyroptosis in H9C2 Cardiomyocytes. J Diabetes Res. 2019;2019:8151836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 244] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 41. | Luitel H, Sydykov A, Schymura Y, Mamazhakypov A, Janssen W, Pradhan K, Wietelmann A, Kosanovic D, Dahal BK, Weissmann N, Seeger W, Grimminger F, Ghofrani HA, Schermuly RT. Pressure overload leads to an increased accumulation and activity of mast cells in the right ventricle. Physiol Rep. 2017;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 42. | Cai W, Liu L, Shi X, Liu Y, Wang J, Fang X, Chen Z, Ai D, Zhu Y, Zhang X. Alox15/15-HpETE Aggravates Myocardial Ischemia-Reperfusion Injury by Promoting Cardiomyocyte Ferroptosis. Circulation. 2023;147:1444-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 181] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 43. | Liang Z, Miao Y, Teng X, Xiao L, Guo Q, Xue H, Tian D, Jin S, Wu Y. Hydrogen Sulfide Inhibits Ferroptosis in Cardiomyocytes to Protect Cardiac Function in Aging Rats. Front Mol Biosci. 2022;9:947778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 44. | Wu J, Ye J, Xie Q, Liu B, Liu M. Targeting Regulated Cell Death with Pharmacological Small Molecules: An Update on Autophagy-Dependent Cell Death, Ferroptosis, and Necroptosis in Cancer. J Med Chem. 2022;65:2989-3001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 45. | Jang S, Chapa-Dubocq XR, Tyurina YY, St Croix CM, Kapralov AA, Tyurin VA, Bayır H, Kagan VE, Javadov S. Elucidating the contribution of mitochondrial glutathione to ferroptosis in cardiomyocytes. Redox Biol. 2021;45:102021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 140] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 46. | Park TJ, Park JH, Lee GS, Lee JY, Shin JH, Kim MW, Kim YS, Kim JY, Oh KJ, Han BS, Kim WK, Ahn Y, Moon JH, Song J, Bae KH, Kim DH, Lee EW, Lee SC. Quantitative proteomic analyses reveal that GPX4 downregulation during myocardial infarction contributes to ferroptosis in cardiomyocytes. Cell Death Dis. 2019;10:835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 261] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 47. | Bhakkiyalakshmi E, Dineshkumar K, Karthik S, Sireesh D, Hopper W, Paulmurugan R, Ramkumar KM. Pterostilbene-mediated Nrf2 activation: Mechanistic insights on Keap1:Nrf2 interface. Bioorg Med Chem. 2016;24:3378-3386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |