Published online Jun 14, 2017. doi: 10.3748/wjg.v23.i22.4016
- This article has been corrected.
- See: World J Gastroenterol. Nov 28, 2024; 30(44): 4768-4770
Peer-review started: January 9, 2017
First decision: March 3, 2017
Revised: March 20, 2017
Accepted: May 4, 2017
Article in press: May 4, 2017
Published online: June 14, 2017
Processing time: 159 Days and 14.5 Hours
To investigate whether bone marrow mesenchymal stem cells (BMMSCs) modified with the HO-1 and CXCR3 genes can augment the inhibitory effect of BMMSCs on small bowel transplant rejection.
Lewis rat BMMSCs were cultured in vitro. Third-passage BMMSCs were transduced with the CXCR3/HO-1 genes or the HO-1 gene alone. The rats were divided into six groups and rats in the experimental group were pretreated with BMMSCs 7 d prior to small bowel transplant. Six time points (instant, 1 d, 3 d, 7 d, 10 d, and 14 d) (n = 6) were chosen for each group. Hematoxylin-eosin staining was used to observe pathologic rejection, while immunohistochemistry and Western blot were used to detect protein expression. Flow cytometry was used to detect T lymphocytes and enzyme linked immunosorbent assay was used to detect cytokines.
The median survival time of BMMSCs from the CXCR3/HO-1 modified group (53 d) was significantly longer than that of the HO-1 modified BMMSCs group (39 d), the BMMSCs group (26 d), and the NS group (control group) (16 d) (P < 0.05). Compared with BMMSCs from the HO-1 modified BMMSCs, BMMSCs, and NS groups, rejection of the small bowel in the CXCR3/HO-1 modified group was significantly reduced, while the weight of transplant recipients was also significantly decreased (P < 0.05). Furthermore, IL-2, IL-6, IL-17, IFN-γ, and TNF-α levels were significantly decreased and the levels of IL-10 and TGF-β were significantly increased (P < 0.05).
BMMSCs modified with the CXCR3 and HO-1 genes can abrogate the rejection of transplanted small bowel more effectively and significantly increase the survival time of rats that receive a small bowel transplant.
Core tip: In this paper, transplant recipient rats were pretreated with bone marrow mesenchymal stem cells (BMMSCs) in advance, and these rats were in a compromised immune state. CXCR3/HO-1 gene modified BMMSCs were injected into recipient rats after small bowel transplantation. The survival time of these rats was significantly prolonged; the number of cells that underwent apoptosis was significantly lower in the CXCR3/HO-1 modified BMMSCs group compared with rats from the HO-1 gene modified BMMSCs group, the native BMMSCs group, and the NS group. Furthermore, the percentage of regulatory T cells was significantly increased. Proinflammatory cytokines (IL-2, IL-6, IL-17, IFN-γ, and TNF-α) were significantly reduced, while anti-inflammatory cytokines (IL-10 and TGF-β) were significantly increased. Our data suggest that BMMSCs modified with the CXCR3 and HO-1 gene can reduce rejection of the small intestine more effectively than HO-1 modified BMMSCs and native BMMSCs.
- Citation: Yin ML, Song HL, Yang Y, Zheng WP, Liu T, Shen ZY. Effect of CXCR3/HO-1 genes modified bone marrow mesenchymal stem cells on small bowel transplant rejection. World J Gastroenterol 2017; 23(22): 4016-4038
- URL: https://www.wjgnet.com/1007-9327/full/v23/i22/4016.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i22.4016
In recent years, with improvements in surgical transplantation, immunosuppressive regimens, prevention of infection, and other key technological advances, small bowel transplantation has become an effective treatment for intestinal failure[1-4]. Currently, small bowel transplantation poses more clinical problems compared with transplantation of other organs such as the liver and kidney[5]. Specific problems include high rejection rates and the lack of effective preventive and treatment methods for such rejection. Clinical studies demonstrate that 30%-40% of recipients experience chronic rejection 5 years after transplantation, which requires removal of the transplanted small bowel, restoration of parenteral nutrition, or re-transplantation[6]. Therefore, the prevention and treatment of rejection after small bowel transplantation are a significant clinical problem that remains to be solved.
Bone marrow mesenchymal stem cells (BMMSCs) have the ability to proliferate in vitro, differentiate into cells with massive potential for immune regulation, and promote tissue repair in injured tissue[7,8]. They can secrete soluble cytokines through paracrine mechanisms, regulate inflammation and the immune response[9,10], and participate in complex regulation of immune cell function[11], mainly by inhibiting the most influential immune cells such as NK cells, T cells, and B cells[12]. A number of studies have shown that the immunomodulatory capacity of BMMSCs is capable of curing diseases, suggesting that they might be a novel approach for the treatment of disorders of the immune system[13,14]. BMMSCs are ideal candidates for cell transplantation and for the treatment of autoimmune diseases and prevention of rejection after solid organ transplantation[15]. However, a possible limitation to these cells is their ability to survive and to specifically target lesions[16]. Indeed, if BMMSCs are transplanted directly into a lesion, most tend to die within a few hours[17]. Therefore, the ability of BMMSCs to survive, proliferate, and migrate is an important problem that must be solved, if they can ever be used clinically. By modifying BMMSCs and interfering with or enhancing the expression of a certain gene may be an effective way to improve the survival rate of these cells in stem cell transplantation. In this study, the chemokine receptor (CXCR3) gene for chemotaxis[18] and the heme oxygenase-1 (HO-1) gene, which can enhance stem cell activity and prolong stem cell function[19], were transduced into BMMSCs to assess whether such modified BMMSCs could prevent or reduce rejection of transplanted small bowel in a rat model.
DMEM-F12 (Gibco, Grand Island, CA, United States), RPMI-1640 (Gibco), fetal bovine serum (FBS) (Biowest, Loire Valley, France), gel preparation kit (SDS-PAGE) (Boster, Suzhou, China), reverse transcription kit (Thermo Scientific, Massachusetts, MN, United States), SYBR Green kit (TaKaRa, Japan), antibodies against CD34 (FITC) (Santa Cruz Biotechnology, Santa Cruz, CA, United States), CD29 (PE), CD45 (PE), CD90 (FITC), RT1A (PE), RT1B (FITC) (Biolegend, San Diego, CA, USA), CD4 (FITC), CD25 (PE), FoxP3 (PE-Cyanine5) (eBioscience Inc, San Diego, CA, United States), Heme Oxygenase 1 (HO-1) (Abcam, Cambridge, United Kingdom), and CXCR3 (Affinity Bioreagent, United States), YAC-1 (Chinese Academy of Medical Sciences, Beijing, China), AD-Hmox1 (Shanghai Genechem Co. Ltd, Shanghai, China), AD-fusion gene (CXCR3/HO-1) (Shanghai Genechem Co. Ltd), and interleukin (IL)-2, IL-6, IL-10, IL-17, IL-23, tumor necrosis factor (TNF)-β, interferon (IFN)-γ, transforming growth factor (TGF)-β, and diamine oxidase (DAO) enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, United States) were employed in this study, as was HRP polymer (Jinmai Gene, Tianjin, China).
We surgically removed femurs and tibias from 2-3-wk-old Lewis rats that were sacrificed by cervical dislocation. The weight of the rats ranged from 40-60 g. After the bones were immersed in 75% alcohol for 5-10 min, the medulla canals of the bones were rinsed with DMEM-F12 and 10% FBS (complete medium) in order to generate single cell suspensions. Cells were incubated at 37 °C in an atmosphere containing 50 mL/L CO2. Approximately 10 d later, cells filled the bottom of the culture bottles, and these were the first cell culture passage. The cells were then subpassaged at a 1:1 ratio, amplified, purified, and expanded to third-passage cells. Finally, the third-passage BMMSCs were induced to differentiate as steatoblasts and osteoblasts after the phenotype test[20].
We prepared six 75-mm culture bottles for culturing 5 × 106 third-passage BMMSCs with 20 mL complete medium for 24 h. Then we discarded the medium. Two of the bottles received 5 mL of complete medium, two received 5 mL of HO-1 modified cells in complete medium, and the last two received 5 mL of CXCR3/HO-1 modified cells with complete medium, ensuring that all steps were done in dark conditions. The ratio of BMMSCs to genetically modified cells was 1:10. After incubation for 6 h, we discarded the medium and then added 20 mL of complete medium to culture for a further 48 h. Observation of expressed fluorescent protein was performed by fluorescence microscopy in the dark. Two types of BMMSCs were used to extract RNA and protein.
Experimental animals: The donors for small bowel transplantation were healthy male Brown Norway (BN) rats aged 6-8 wk and weighing 180-200 g. The recipients for small bowel transplantation were healthy male Lewis rats aged 6-8 wk and weighing 180-200 g. All animals were provided by the Experimental Animal Center of the Academy of Military Medical Sciences and standard rat food was provided ad libitum. The experimental animals were kept at 23 °C with 50% humidity and a 12 h light/dark cycle for 2 wk, with free access to water and food, and regular replacement of cage and clean bedding before the experiments. All experiments on animals followed the experimental animal ethical regulations, and were approved by the Ethics Committee of Tianjin First Central Hospital.
Donor surgery: After the BN rats were weighed and appraised, we performed abdominal anesthesia with 5% chloral hydrate (0.5 mL/100 g) and fully exposed the abdominal cavity by making an incision from the xiphoid to the pubic symphysis. We isolated a section of the superior mesenteric artery and portal vein for vascular anastomosis, and ligated the surrounding arteriovenous vein. Then, we separated a section of the small bowel from the Treitz Ligament to the cecum. We preserved the tissue in lactated Ringer’s solution at 4 °C after low-pressure enema with 5 mL of normal saline (NS) and then performed vascular perfusion with heparinized lactated Ringer’s solution (25 U/mL) at 4 °C.
Recipient surgery: Previously detailed steps for Lewis recipient rats were similar to those for donors. We isolated the inferior vena cava and abdominal aorta 2 cm under the left renal artery for vascular anastomosis, then occluded the proximal and distal branches of the abdominal aorta and inferior vena cava. Recipient abdominal aorta and inferior vena cava stomas, and the superior mesenteric artery and portal vein of the transplanted small bowel were subjected to arterial-arterial and venous-venous anastomosis. We then opened the blood vessels and straightened the intestine, before generating fistula on both sides of the abdominal wall. Using warm NS, we repeatedly washed the abdominal cavity, before finally closing the abdomen. According to different groups included in the experimental design, we injected the penis dorsal vein with the corresponding liquid.
Postsurgical management: Lewis rats were placed under 60 W heated lamps to awaken the animals, before they were all marked and fed. They were then isolated for 1-2 d. After the health of the rats had improved, they were placed in their respective groups. We observed the health of the rats and tended the stomas daily.
Experimental groups: The rats were divided into six groups. These was a “no small bowel transplantation” (NSBT) group and an IsoT group where transplantation occurred between the Lewis rats. According to different postoperative injections in the BN and Lewis rats, we divided another four groups into a normal saline group (NS), a BMMSCs (BM) group, an Adv-HO-1/BMMSCs (HB) group, and an Adv-(CXCR3+HO-1)/BMMSCs (HCB) group. Six time points (instant, 1 d, 3 d, 7 d, 10 d, and 14 d) (n = 6) were chosen for each group. Lewis rats in the IsoT and NS groups were injected with 1 mL of sterile NS via the dorsal penile vein, 7 d before surgery. Rats in the B, HB, and HCB groups were injected with a single-cell suspension that included 5 × 106 BMMSCs and 1 mL of sterile NS via the dorsal penile vein, 7 d before the operation. After transplantation, the IsoT group and NS group recipient rats were injected intravenously with 1 mL of sterile NS via the dorsal penile vein, while the BM group recipient rats were injected with a single-cell suspension including 5 × 106 BMMSCs and 1 mL of sterile NS. The HB group recipient rats were injected with a single-cell suspension including 5 × 106 Adv-HO-1 /BMMSCs and 1 mL of sterile NS, and the HCB group recipient rats were injected with a single-cell suspension that included 5 × 106 Adv-(CXCR3+HO-1)/BMMSCs and 1 mL of sterile NS. In addition, we prepared five more rats for each group and used them for survival analysis.
Small bowel transplantation and consequent effects: The survival of the rats in each group was observed, including the weight of the rats, changes in the mucosa, and secretions from the fistulas. The survival time and cause of death were recorded, and the survival rate was calculated.
The abdominal cavity was opened under anesthesia to observe the appearance of the transplanted intestine, including color, lumen diameter, and presence or absence of inflammatory masses and surrounding tissue adhesions. We then resected 2 cm of the intestine 12 cm distant from the stoma. After the resected intestine was cleaned and excised, it was soaked in 40 g/L of formaldehyde solution for detection of aforementioned immunological markers. We used paraffin to embed the sections, then made tissue sections for hematoxylin-eosin staining (HE staining), before observing the sections by optical microscopy. A pathologist who was blinded to the procedures examined the tissue sections under a light microscope. Six high power fields (× 200) were randomly selected from each slice to observe intestinal mucosal injury. Finally, we established the index of intestinal injury by referring to the literature[21].
Fluorescent double staining: The tissue was embedded in paraffin and 5 μm continuous sections were made. The sections were dewaxed and endogenous peroxidase was blocked by addition of 3% H2O2. After hydration, antigen repair and closure, we incubated the mixed primary antibodies (HO-1 at 1:250, CXCR3 at 1:400) with the sections and kept them in a 4 °C wet box overnight. On the next day, we re-warmed the sections and incubated them with the corresponding secondary antibody at 37 °C for 1 h. We then photographed the sections under a microscope. A negative control was run by using PBS instead of primary antibody.
Terminal deoxynucleotidyl transferase dUTP nick-end labeling: We strictly followed the kit instructions after baking at 70 °C. We used glycerol for sealing and selected multiple fields under a fluorescence microscope for analysis.
Western blot detection of cellular proteins: We collected protein samples from supernatant after adding the cells to 500 μL of RIPA lysis buffer for 30 min and centrifuging the contents in a 1.5 mL centrifuge tube (13000 × g for 5 min). The expressed proteins were detected by Western blot. After calculating the amount of sample and measuring the protein concentration, we performed electrophoresis and transfer, added primary antibodies against HO-1 (1:800) CXCR3 (1:800), and GAPDH (1:5000), and incubated them in a 4 °C wet box overnight. The secondary antibody was incubated at room temperature, and the color was then developed. The relative expression of HO-1 and CXCR3 was calculated by quantitative analysis.
In addition, we also prepared small intestinal tissue that was soaked in RIPA lysis buffer reagent (100 mg per 1 mL lysis buffer), macerated and centrifuged in 1.5 mL tubes (13000 × g for 5 min). After calculating the amount of sample and measuring the protein concentration, we performed electrophoresis and transfer, treated the samples with primary antibodies against HO-1 (1:400), CXCR3 (1:800), and GAPDH (1:5000), and incubated them at 4 °C overnight. The secondary antibody was incubated at room temperature, and the color was then developed. The relative expression of HO-1 and CXCR3 was calculated by quantitative analysis.
Trizol (1 mL) was added to each of the culture bottles and mixed for 10 min. After we measured the concentration, degree, and purity of RNA, cDNA was synthesized by reverse transcription and PCR was performed. With β-actin as a reference, the expression of HO-1 and CXCR3 mRNA was calculated by relative quantitative analysis. We used the Ct value as the statistical parameter; the relative ratio of the target gene and the β-actin gene was used as the evaluation standard, then relative quantification of the target gene was performed. The primer sequences are shown in Table 1.
| Target gene | Primer sequence |
| HO-1 | Forward: 5'-CTGGCTCTTTTCTTGG -3' |
| Reverse: 5'-ATGGTCAGAACATGGAC-3' | |
| CXCR3 | Forward: 5'-TCATGGCCTACTGCTATGC-3' |
| Reverse: 5'-CGACTTGGCCACGTCTAC-3' | |
| β-actin | Forward: 5'-GCGTGACATTAAAGAGAAGCTG-3' |
| Reverse: 5'-AGAAGCATTTGCGGTGCAC-3' |
Flow cytometry of BMMSCs: Third-passage BMMSCs were digested and prepared as single cell suspensions in 100 μL of PBS away from light. The number of cells was 5 × 105. We then added 0.625 μL of CD29 (PE), 2.5 μL of CD34 (FITC), 0.625 μL of CD45 (PE), 0.25 μL of CD90 (FITC), 0.625 μL of RT1A (PE), and 0.25 μL of RT1B (FITC), respectively. The blank group did not include antibody. All samples were incubated at 4 °C, washed and centrifuged (300 × g for 5 min), and resuspended in 200 μL of PBS. Flow cytometry was used to analyze the data.
Flow cytometry of T lymphocytes: We used 5% chloral hydrate while performing abdominal anesthesia. Rat spleens were harvested and macerated into a single cell suspension using a 200-μm nylon mesh. The cells were washed in RPMI 1640 medium and added to the separated lymphocytes at a 1:1 ratio. After centrifugation at 500 × g for 20 min, we collected buffy coat cells. Lymphocytes were precipitated after washing and centrifugation. The lymphocyte concentration was adjusted to 2 × 106/100 μL and then divided into a positive group and a control group. The control group did not have antibody. The positive group contained regulatory T cells (Tregs) to which we added 0.5 μL of CD4 antibody (FITC) and 0.625 μL of CD25 antibody (PE). After incubation at 4 °C, the cells were washed and centrifuged at 300 × g for 5 min, and perforated at 4 °C overnight, before being ruptured and resuspended in 100 μL PBS. Then, we added 5 μL of FoxP3 antibody (PE-Cyanine 5) and incubated the cells at 4 °C. The cells were then washed, centrifuged, and resuspended in 100 μL of PBS. CD4+CD25+FoxP3+ T cells were then detected.
Detection of NK cell activity: The target cells (YAC-1) were subcultured 24 h prior to the experiment, and cell viability was confirmed when live cells accounted for > 95% of the total population. Whole blood was taken from the inferior vena cava of the rats, and the lymphocyte separation fluid was added to obtain a cell ratio of 1:1. The buffy coat was collected after centrifugation. Then, we washed, centrifuged, and precipitated the cells to obtain effector cells (NK cells). Effector cells were resuspended. The ratio of effector cells to target cells was 100:1. The lactate dehydrogenase method[22] was used to detect NK cell activity.
Blood from normal and postoperative Lewis rats was collected via the inferior vena cava and stored at -80 °C. The ELISA kit was used to detect the cytokines IL-2, IL-6, IL-10, IL-17, IL-23, IFN-γ, TNF-α, and TGF-β, and DAO. We strictly followed the kit instructions.
SPSS statistical software, version 17.0 was used for statistical analyses. The measurement data, expressed as mean ± SD, were analyzed using single factor analysis of variance. Count data, expressed as percentages (%), were analyzed using the χ2 test. P-values < 0.05 were considered statistically significant.
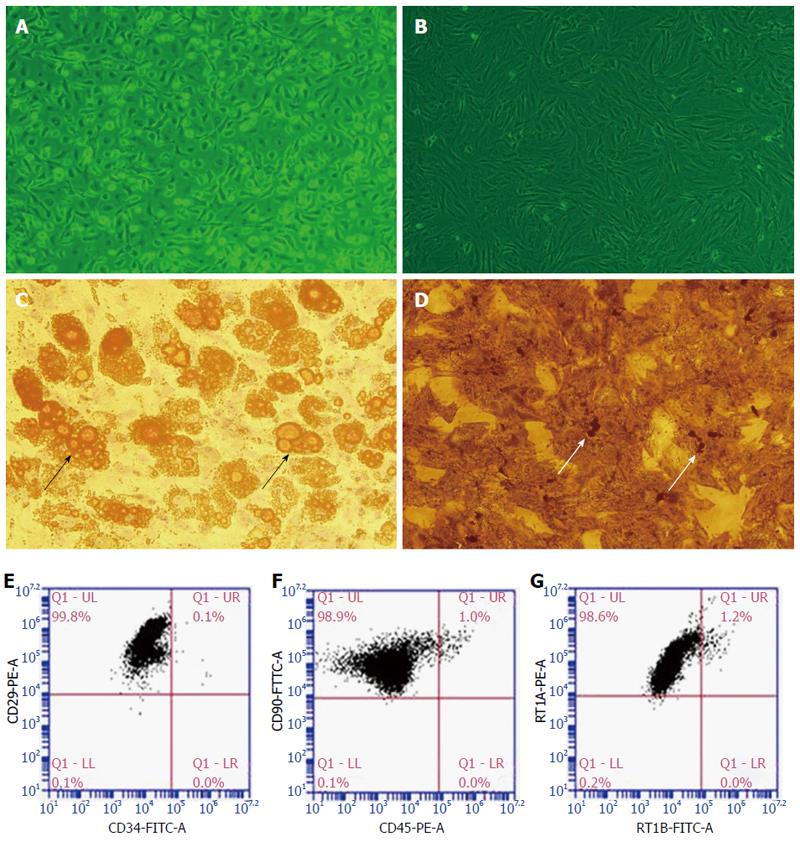
Identification of BMMSCs: Primary BMMSCs (Figure 1A) were cultured ex vivo and third-passage BMMSCs (Figure 1B) were typically spindle-like in appearance. BMMSCs were induced to differentiate into adipocytes (Figure 1C, with lipid droplets in the cytoplasm) and osteoblasts (Figure 1D, with calcium deposits in the cytoplasm) using different media. Flow cytometry analysis showed that the positive rates of CD29, CD90, and RT1A in the third-passage Lewis rat BMMSCs exceeded 98%; the negative rates of CD34, CD45, and RT1B also exceeded 95% (Figure 1E-G).
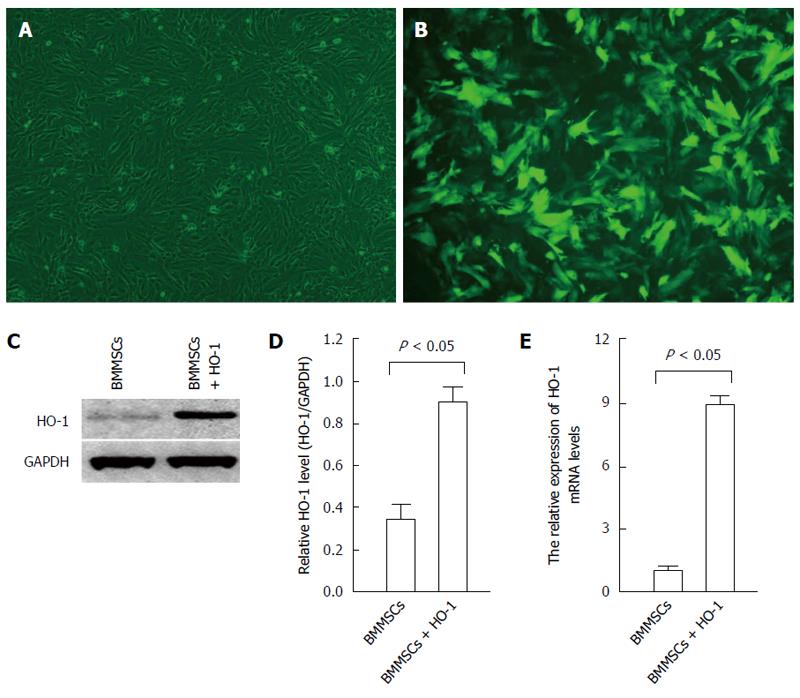
Characteristics of HO-1 modified BMMSCs: After infection with recombinant adenovirus expressing the HO-1 gene for 48 h, the BMMSCs were observed under a fluorescence microscope. The positive expression rate of green fluorescent protein (GFP) exceeded 85% (Figure 2A and B). The expression of HO-1 in HO-1 modified BMMSCs detected by Western blot was significantly higher than that of native BMMSCs (P < 0.05; Figure 2C and D). RT-PCR results showed that the expression of HO-1 mRNA in HO-1 modified BMMSCs was up-regulated, by a factor of 7.89 times compared with native BMMSCs (F = 39.49, P < 0.05; Figure 2E). These results showed that the HO-1 gene was successfully overexpressed in BMMSCs.
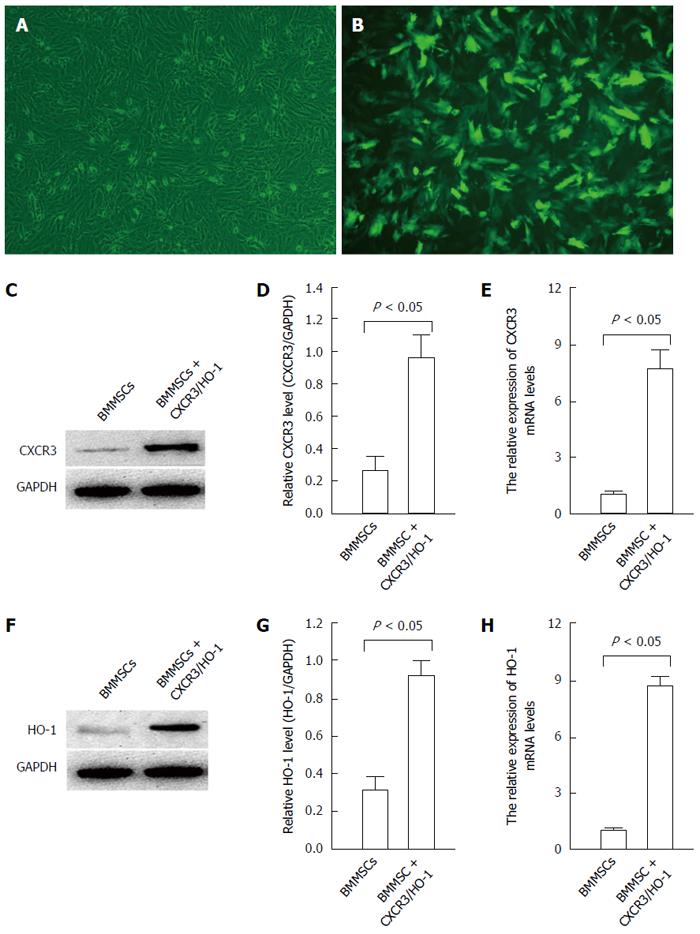
Increased expression of CXCR3 and HO-1 in BMMSCs after transduction with CXCR3/HO-1 genes: After infection with the GFP-labeled CXCR3/HO-1 gene for 48 h, the BMMSCs were observed under a fluorescence microscope. The positive expression rate of GFP exceeded 85% (Figure 3A and B). The expression of CXCR3 protein in CXCR3/HO-1 modified BMMSCs detected by Western blot was significantly higher than that of native BMMSCs (Figure 3C and D). RT-PCR results showed that the expression of CXCR3 mRNA in CXCR3/HO-1 modified BMMSCs was up-regulated, by a factor of 6.69 times higher than that of native BMMSCs (F = 33.28, P < 0.05; Figure 3E). The expression of HO-1 protein in CXCR3/HO-1 modified BMMSCs detected by Western blot was significantly higher than that of native BMMSCs (Figure 3F and G). RT-PCR results showed that the expression of HO-1 mRNA in CXCR3/HO-1 modified BMMSCs was up-regulated, by a factor of 7.71 times compared with native BMMSCs (F = 35.81, P < 0.05; Figure 3H). These results showed that the combination of CXCR3/HO-1 genes was successfully transduced into BMMSCs.
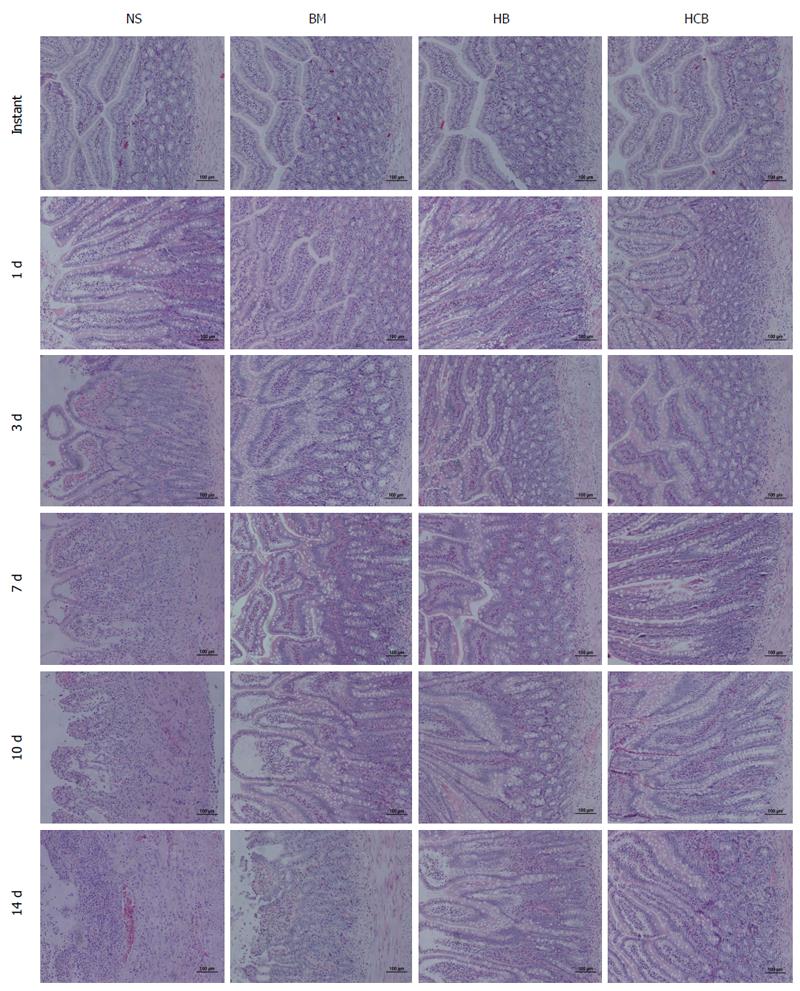
Pathological rejection of transplanted small bowel: Pathological results showed that there was no rejection in the NSBT group, while the IsoT group showed obvious rejection, and the injuries caused by ischemia-reperfusion fully recovered 7 d after transplantation. The rejection was similar between the NS group, BM group, HB group, and HCB group at instant and 1 d after transplantation; at the other time points, the rejection in the NS group was the most profound, and the injury was significantly higher than that of the BM group, HB group, and HCB group at the same time point (Figure 4). At the same time, HCB group had the least rejection, and the damage was significantly lower than that in the NS group, BM group, and HB group (Figure 4). At day 3, the BM group and HB group had only lymphocyte infiltration; at day 7, the BM group and HB group experienced mild rejection; at day 14, the BM group showed moderate rejection, while the HB group did not achieve moderate rejection (Figure 4).
General condition of the rats after small bowel transplantation: All of the rats recovered normally after transplantation. Rats in the NSBT group were normal in health. The mucosal color and secretion from the intestinal stoma were normal in the rats of the IsoT group. Rats in the NS group showed mild rejection 3 d after transplantation, with mucosal congestion and edema, and increased volume of thin secretions. The rejection was moderate 7 d after transplantation, with mucosal congestion turning dark, and the secretion becoming thick and in large volumes. The rejection was severe 14 d after transplantation, with severe mucosal necrosis and decreased volume of secretion. Rats in the BM group and the HB group showed mild rejection at day 7 and moderate rejection at day 14 after transplantation, and the rats in the HB group experienced less severe rejection compared with those in the BM group. Rats in the HCB group exhibited mild rejection at day 10 after transplantation, and showed no significant aggravation at day 14 after transplantation.
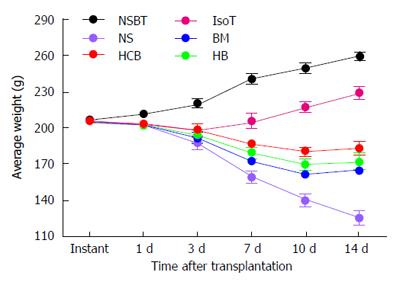
The average body weight (BW) of rats in the NSBT group increased by 3.82 g/d. The BW of rats in the IsoT group decreased slightly after transplantation, recovered to the preoperative BW at day 7 after transplantation, and later increased by 3.32 g/d. The average BW of rats at day 3 in the HCB group was higher than those in the NS group, BM group, and HB group (P < 0.05). There was no significant difference between the BM group and HB group (P > 0.05) but both groups had higher BW than the NS group (P < 0.05). Any two groups of the NS group, BM group, HB group, and HCB group showed a significant difference in the average BW of rats at 7 d, 10 d, and 14 d after transplantation, respectively (P < 0.05), among which BW in the HCB group was the highest, and BW in the NS group was the lowest (Figure 5). These results suggested that CXCR3/HO-1 modified BMMSCs significantly improved the survival quality of rats after transplantation than HO-1 modified BMMSCs and native BMMSCs.
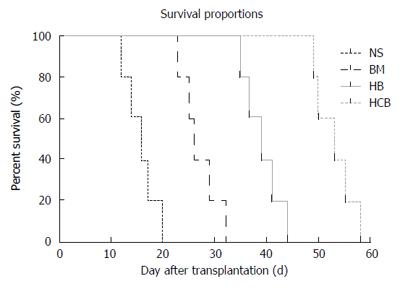
Improved post-transplant survival after treatment by CXCR3/HO-1 modified BMMSCs: The normal recipient (Lewis) rats in the NSBT group and IsoT group survived for more than 200 d after small bowel transplantation. The survival time of rats in the HCB group was the longest, followed by the HB group and BM group, while it was shortest in the NS group (P < 0.05; Figure 6). These results suggested that CXCR3/HO-1 modified BMMSCs could prolong the survival time of rats after transplantation more significantly than HO-1 modified BMMSCs and BMMSCs.
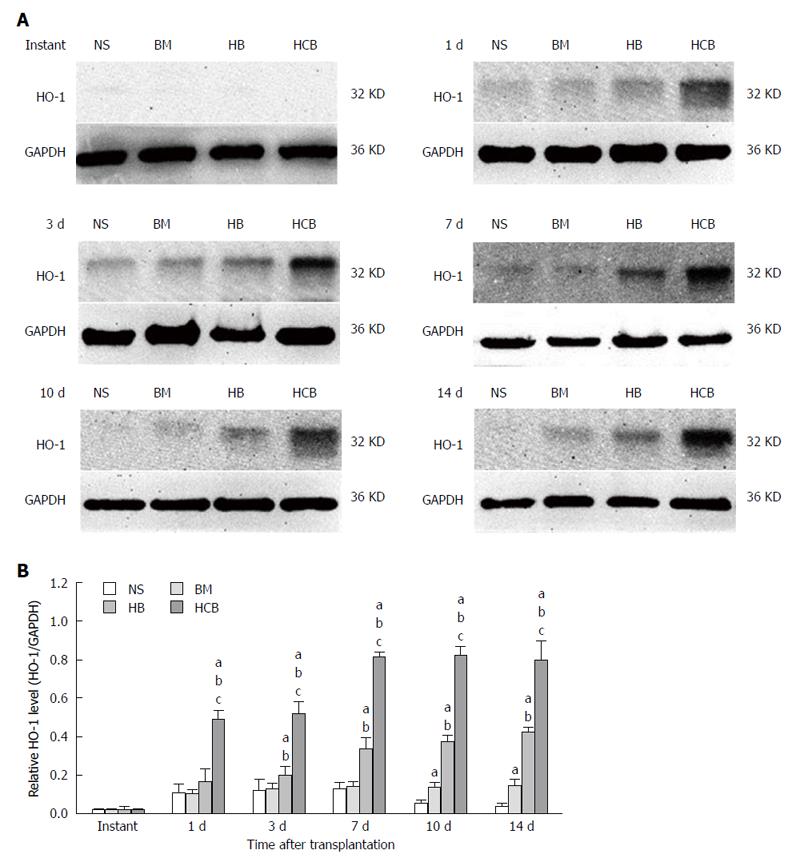
Increased expression of HO-1 in the transplanted small bowel after treatment with CXCR3/HO-1 modified BMMSCs: The expression of HO-1 protein in the transplanted small bowel was low at instant, and there was no significant difference between the four aforementioned groups (P > 0.05). The relative concentration of HO-1 protein in the HCB group was higher than those in the NS group, BM group, and HB group (P < 0.05) at every time point except immediately after transplantation. The relative concentration of HO-1 protein in the HCB group reached its maximum at day 7 after transplantation, and remained relatively stable thereafter (Figure 7). The relative concentration of HO-1 protein in the HB group was higher than that in the NS group and BM group (P < 0.05; Figure 7) at every time point except immediately after transplantation (instant). The relative concentration of HO-1 protein in the BM group was comparable to that in the NS group at 1 d, 3 d, and 7 d (P > 0.05), and was higher than that in the NS group at 10 d and 14 d (P < 0.05; Figure 7). These results suggested that transfused CXCR3/HO-1 modified BMMSCs could reach the transplanted small bowel more rapidly than transfused HO-1 modified BMMSCs and transfused BMMSCs, leading to early expression of large quantities of HO-1 protein.
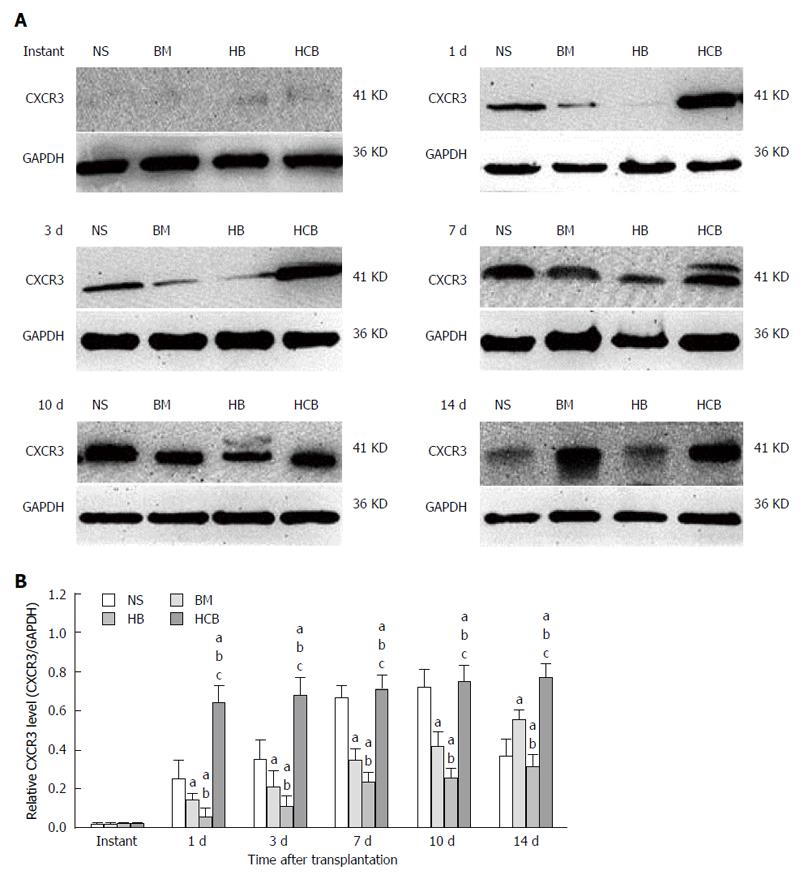
Increased expression of CXCR3 in the transplanted small bowel after treatment with CXCR3/HO-1 modified BMMSCs: The expression of CXCR3 protein in the transplanted small bowel was low at instant, and there was no significant difference between the four aforementioned groups (P > 0.05). The relative concentration of CXCR3 protein in the HCB group was higher than those in the NS group, BM group, and HB group (P < 0.05) at every time point except immediately after transplantation (at instant). The relative concentration of CXCR3 protein in the HB group was significantly lower than those in the NS group and BM group (P < 0.05) at every time point except immediately after transplantation (Figure 8). This was especially demonstrable at 3 d, 7 d, 10 d, and 14 d after transplantation. The relative concentration of CXCR3 protein in the BM group was significantly lower than that in the NS group at 1 d, 3 d, 7 d, and 10 d (P < 0.05; Figure 8), and was higher than that in the NS group at day 14 (P < 0.05). These results suggested that transfused CXCR3/HO-1 modified BMMSCs could reach the transplanted small bowel more rapidly than transfused HO-1 modified BMMSCs and transfused BMMSCs, leading to early expression of large quantities of CXCR3 protein.
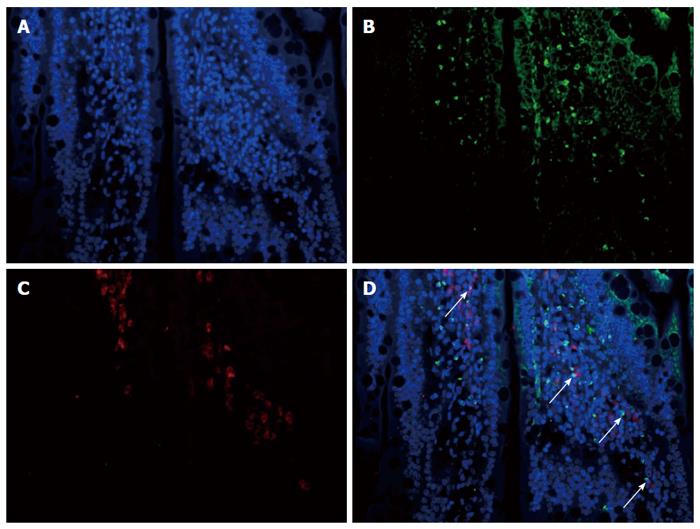
Increased co-expression of CXCR3 and HO-1 in the transplanted small bowel after treatment with CXCR3/HO-1 modified BMMSCs: CXCR3 and HO-1 proteins were found to be co-expressed in the same cells and localized in the transplanted small bowel (shown by the arrow in Figure 9) 1 d after double fluorescent histochemical staining.
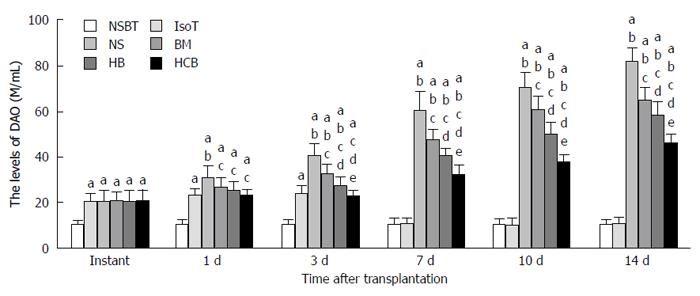
Effects of CXCR3/HO-1 modified BMMSCs on the function of transplanted small bowel: Serum concentrations of DAO in the IsoT group, NS group, BM group, HB group, and HCB group were comparable immediately after transplantation, and were all higher than that in the NSBT group (P < 0.05; Figure 10). Serum concentrations of DAO in the IsoT group nearly dropped to the level of the NSBT group (P > 0.05) at day 7 after transplantation. Serum concentration of DAO in the NS group was the highest, followed by those in the BM group and HB group, and the HCB group had the lowest level of DAO at 3 d, 7 d, 10 d, and 14 d after transplantation (P < 0.05). These results suggested that CXCR3/HO-1 modified BMMSCs could improve intestinal permeability much earlier and more significantly than HO-1 modified BMMSCs and BMMSCs.
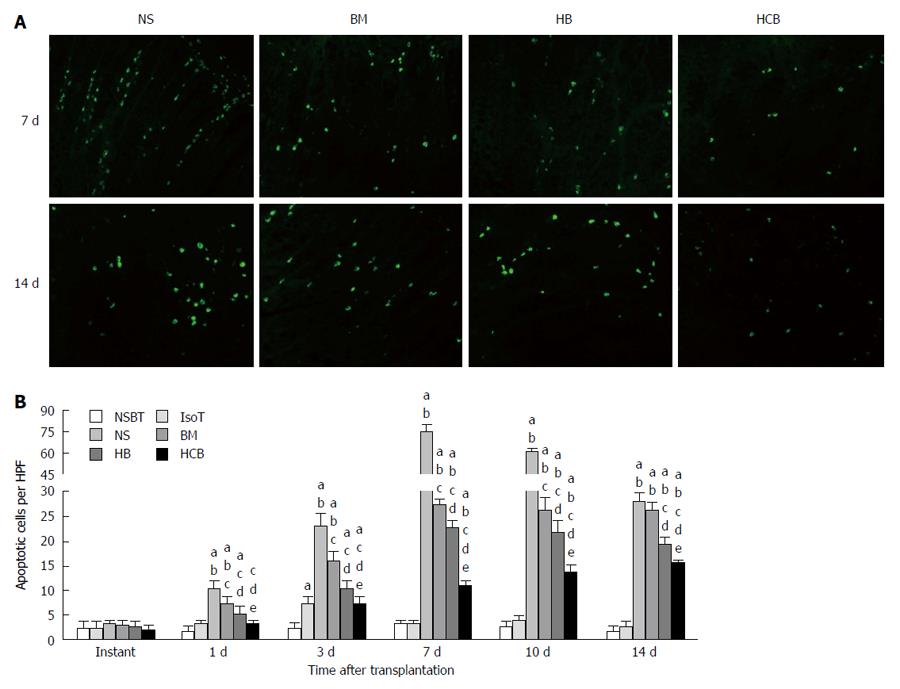
Decreased apoptosis in the transplanted small bowel after treatment with CXCR3/HO-1 modified BMMSCs: There was no significant difference in apoptosis between the six groups immediately after transplantation (P > 0.05; Figure 11). Apoptosis in the IsoT group increased at day 3 but nearly returned to the level of NSBT group by day 7. Apoptosis was the highest in the NS group, followed by the BM group and HB group, and was the lowest in the HCB group at 1 d, 3 d, 7 d, and 10 d (P < 0.05). At day 14 after transplantation, apoptosis was comparable between the NS group and BM group (P > 0.05), was higher in the BM group when compared with the HB group (P < 0.05), and was higher in the HB group when compared with the HCB group (P < 0.05; Figure 11). These results suggested that CXCR3/HO-1 modified BMMSCs could reduce the level of apoptosis in the transplanted small bowel more significantly than HO-1 modified BMMSCs and BMMSCs.
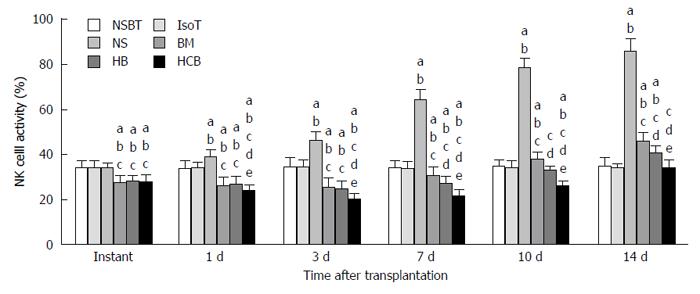
Inhibitory effects of CXCR3/HO-1 modified BMMSCs on NK cells: The activity of NK cells in the BM group, HB group, and HCB group was significantly lower than that in the NSBT group, IsoT group, and NS group immediately after transplantation (P < 0.05; Figure 12). The NSBT group and IsoT group showed no significant change at any of the time points. The activity of NK cells in the NS group increased significantly after transplantation, which was significantly higher than that in the NSBT group, IsoT group, BM group, HB group, and HCB group (P < 0.05). The activity of NK cells in the BM group, HB group, and HCB group initially increased but then decreased after transplantation. The activity of NK cells in the BM group was the highest, followed by the HB group, and was the lowest in the HCB group at every time point (P < 0.05; Figure 12). These results suggested that CXCR3/HO-1 modified BMMSCs could significantly reduce the activity of NK cells in the rats after small bowel transplantation, and were able to exert their effects much earlier than HO-1 modified BMMSCs and BMMSCs.
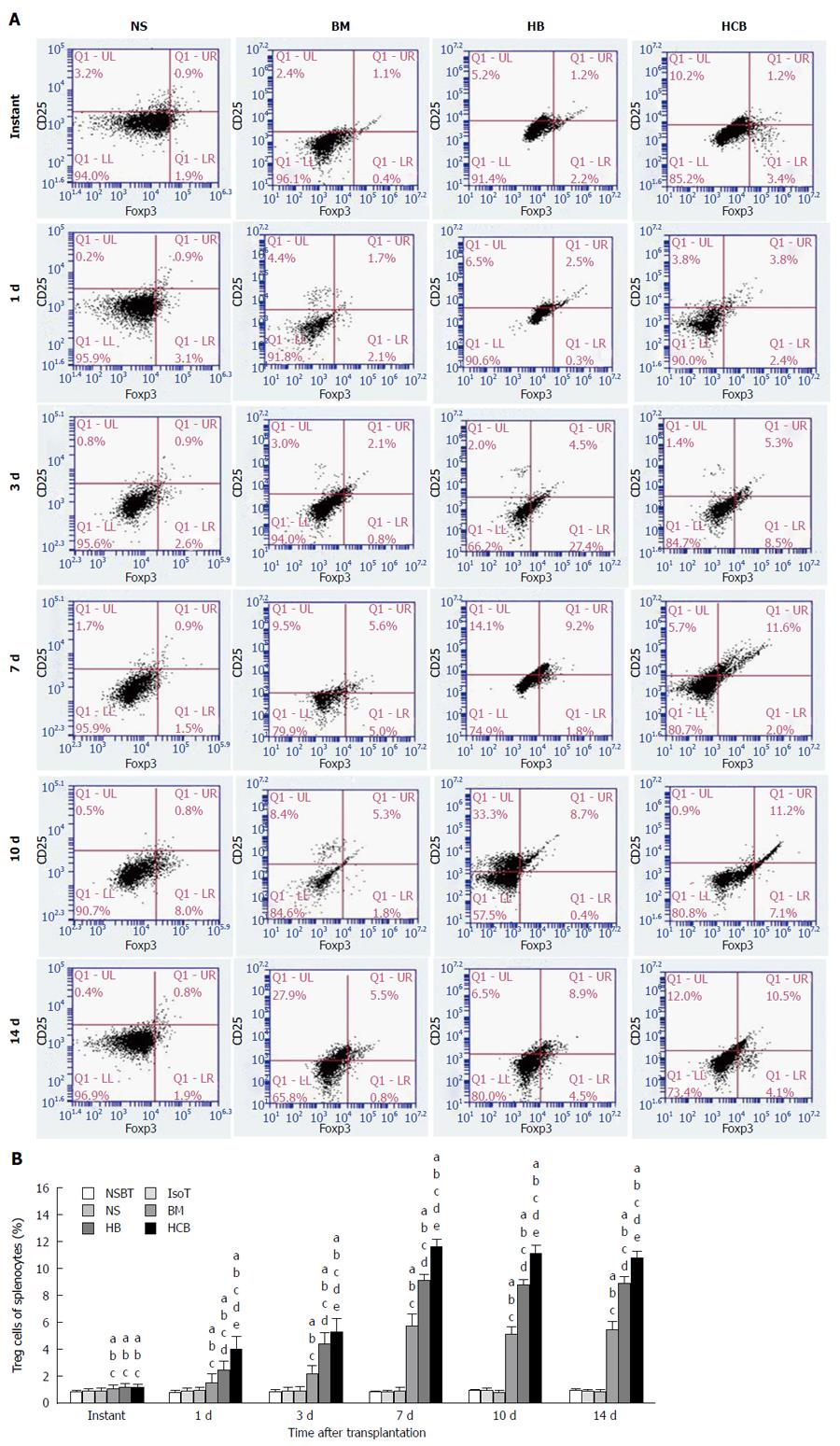
Increased ratio of Tregs after treatment with CXCR3/HO-1 modified BMMSCs: The ratio of Tregs in the BM group, HB group, and HCB group was comparable but were all significantly higher than that in the NBST group, IsoT group, and NS group at instant (P < 0.05). The ratio of Tregs in the NBST group, IsoT group, and NS group showed no significant change over time (P > 0.05; Figure 13), while the ratio of Tregs in the BM group, HB group, and HCB group increased significantly over time (P > 0.05; Figure 13). The ratio of Tregs in the HCB group was higher than that in the HB group and BM group, and the ratio in the HB group was higher than the BM group at the same time points. These results suggested that CXCR3/HO-1 modified BMMSCs could increase the ratio of Tregs in the rats after small bowel transplantation, much more significantly than HO-1 modified BMMSCs and BMMSCs.
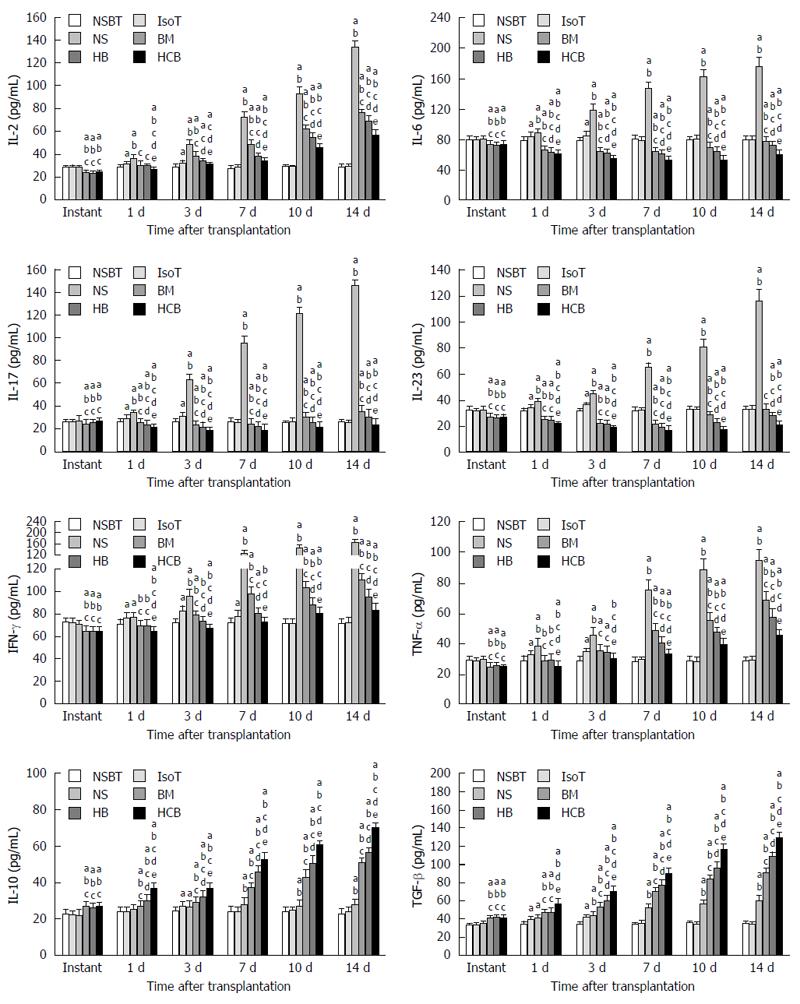
Effects of CXCR3/HO-1 modified BMMSCs on serum cytokines: Serum concentrations of Th1/Th2-related and Th17/Treg-related cytokines were detected. Cytokines in the BM group, HB group, and HCB group showed significant differences when compared with those in the NBST group, IsoT group, and NS group immediately after transplantation, with a reduction in proinflammatory cytokines and an increase in anti-inflammatory cytokines (P < 0.05; Figure 14). The concentrations of proinflammatory cytokines in the IsoT group were significantly higher than those in the NSBT group at 1 d and 3 d (P < 0.05), and were comparable to those in the NSBT group at day 7 (P > 0.05). The concentrations of proinflammatory cytokines in the NS group were significantly higher than those in the BM group, HB group, and HCB group at 1 d, 3 d, 7 d, 10 d, and 14 d (P < 0.05). Proinflammatory cytokine levels in the BM group were higher than those in the HB group, and those in the HCB group were the lowest at 1 d, 3 d, 7 d, 10 d, and 14 d (P < 0.05). The concentrations of anti-inflammatory cytokines in the HCB group were significantly higher than those in the NS group, BM group, and HB group (P < 0.05). The concentrations of anti-inflammatory cytokines in the HB group were higher than in the BM group, and those in the NS group were the lowest (P < 0.05). These results suggested that CXCR3/HO-1 modified BMMSCs could induce immunological changes in rat lymphocytes after small bowel transplantation more significantly than HO-1 modified BMMSCs and BMMSCs, resulting in increased secretion of anti-inflammatory cytokines and decreased secretion of proinflammatory cytokines.
With the progress in surgical technology, new immunosuppressive regimens, effective infection prevention, and perioperative management, the early survival rate of patients undergoing small bowel transplantation has been greatly improved[23]. However, the long-term outcome for small bowel transplantation is unsatisfactory[24]. The small bowel is an immune organ, with the small bowel mucosa being a perfect congenital immune system due to it being constantly exposed to exogenous antigens and microbial populations. As there is continual exchange of cells and antigens during congenital and acquired immune function, T cell-mediated acute cellular rejection[25] and antibody-mediated humoral rejection[26] are very likely to occur after small bowel transplantation. Studies have demonstrated that after small bowel transplantation, even under the application of a conventional immunization regimen, the incidence of rejection remains high[27,28]. The need to find a better method for induction of immune tolerance or transplantation rejection, has led to studies exploring whether BMMSCs can inhibit rejection after small bowel transplantation[29]. However, the effect of BMMSCs alone on acute immune rejection is poor, probably due to the low homing efficiency of BMMSCs after systemic infusion[30,31]. Treatment with BMMSCs results in an insufficient number of cells reaching in vivo lesions, and the survival time is also short when they do reach the target site.
Studies have shown that HO-1 and its metabolite system have antioxidant, anti-inflammatory, antiproliferative and immunomodulatory effects[18]. HO-1 is the rate-limiting enzyme in the metabolism of hemoglobin. This molecule can protect cells by exerting antioxidant activity and potent antiapoptotic activity and maintaining microcirculation[32]. Modification of BMMSCs with HO-1 enhances the ability of BMMSCs to tolerate anoxia-reoxygenation injury, thereby enhancing the survivability and proliferative capacity of these BMMSCs[33,34]. However, prolonged survival in rats is not ideal[34], and therefore this study aimed to make further improvements to the delivery of these important cells. In order to reduce the immune status of rats, we employed BMMSCs pretreatment before transplantation, which offers several advantages: (1) BMMSCs have especially poor immunogenic properties as multifunctional stem cells[35], allowing them to escape the killing effects of toxic T cells and natural killer cells[36]; (2) BMMSCs can interfere with the function of cytotoxic T cells, IFN-γ-secreting T cells, dendritic cells (DCs) and NK cells, consequently inhibiting the release of some inflammatory factors. Furthermore, BMMSCs can secrete some anti-inflammatory factors such as IL-10 and TGF-β[37], introducing a low immune state in the recipients[38]; and (3) BMMSCs can switch the immune response from that of a Th1 response to a Th2-driven response, inducing immune tolerance and a delay in rejection[39]. On this basis, we hypothesized that the addition of a gene that could guide BMMSCs to a damaged site of tissue would further enhance the effect of HO-1 modified BMMSCs.
Given that chemokines can be expressed and secreted by different histiocytes and immune cells under certain conditions, they bind to the corresponding chemokine receptors and affect effector cells differently[40]. The interaction of chemokines with their receptors controls the direct migration of various immune cells in the circulatory system and tissues and organs[41]. After the chemokine receptor binds to a specific chemokine, this stimulates calcium influx and results in cell chemotaxis, guiding cells to a specific part of the organism[42]. The chemokine receptor CXCR3 is a seven-subunit transmembrane G protein that is expressed in many organ lesions. They are found in parenchymal and inflammatory cells such as vascular endothelial cells, activated lymphocytes, macrophages and DCs but are not in the stationary phase of T lymphocytes, B lymphocytes and granulocytes[19].
It has been reported that CXCR3 and its ligands Mig, IP-10, and I-TAC are highly expressed during transplant rejection[43]. Th1 cells can specifically express CXCR3, which is typically a sign of activated Th1 lymphocytes[44] since the ligands of CXCR3 (Mig, IP-10, and I-TAC) can guide the activated Th1 lymphocytes to the tissue lesion[45]. Therefore, we hypothesized that modification of BMMSCs using the CXCR3 gene with specific binding of the CXCR3 receptor to the ligand, could propel BMMSCs more quickly to the injury site and in larger numbers. At the same time, BMMSCs were modified with the HO-1 gene to enhance the role of immunoregulation and damage repair, thereby protecting the long-term survival after small bowel transplantation. Our study was based on the chemotaxis of CXCR3 and we attempted to explore whether CXCR3 modified BMMSCs could reach the site of inflammatory injury faster as a more pure collection of BMMSCs. Our study aimed to sacrifice the recipient rats simultaneously and observe the expression of the recipient rats at the same time point; the rejection response caused by the inflammatory response gradually augments over time, while specific rejection of the CXCR3 ligand will increase[46-48]. Therefore, we chose to sacrifice rats at multiple time points to describe the results in greater detail. The transduction of both the HO-1 gene alone and CXCR3/HO-1 genes into BMMSCs resulted in GFP expression exceeding 85%, and the protein levels of HO-1 and CXCR3 were significantly higher than those in native BMMSCs. Furthermore, the expression of HO-1 and CXCR3 mRNA was significantly up-regulated in modified BMMSCs compared with native BMMSCs, thus confirming that we successfully obtained HO-1 modified BMMSCs and CXCR3/HO-1 modified BMMSCs. After transplantation, we showed that the protein levels of HO-1 and CXCR3 in the HCB group were significantly higher than those of the other groups 1 d after transplantation. Furthermore, immunofluorescence double staining showed that the expression of HO-1 and CXCR3 proteins was both present 1 d after surgery, which indicated that CXCR3/HO-1 modified BMMSCs could rapidly migrate to the transplanted small bowel. In addition, the levels of HO-1 and CXCR3 proteins in the HCB group were significantly higher than those in the other groups at the same time point, which indicated that CXCR3/HO-1 modified BMMSCs reached the transplanted small bowel in large numbers.
In our study, the median survival time after transplant surgery was significantly higher in the recipient rats which were pretreated with BMMSCs and treated with CXCR3/HO-1 modified BMMSCs (HCB group) (53 d) compared with the HB group (39 d), BM group (26 d), and NS group (16 d). This is likely to be related to the reduction in graft rejection, improvement in small bowel function, and a decrease in apoptosis. Pathological examination is a “gold standard” that reflects tissue damage; it scientifically and accurately reflects the degree of small bowel injury attributable to the rejection reaction[49,50]. Histopathological analysis showed that the transplanted small bowel in the HCB group had mild lymphocytic infiltration and a series of inflammatory reactions 7 d after surgery, while these phenomena in the HB group and BM group appeared 3 d after surgery. Furthermore, the infiltration of lymphocytes in the NS group was significantly different 1 d after surgery. DAO (95%) is mainly present in the cytoplasm of human and mammalian small bowel villus epithelial cells, with little or no distribution in the villi of the endometrium, or other tissues or cells. Apart from a small level of distribution in the endometrial villi, DAO is virtually absent from other tissues or cells[51]. When the bowel epithelial cells are damaged, this can lead to bowel barrier structural damage, so that villus epithelial cells will release significant quantities of DAO into the blood, leading to a rapid increase in DAO content. Thus, the DAO content in plasma directly reflects the damage to bowel epithelial cells and intestinal barrier structure, which is why it is measured[52-54]. We showed that the DAO content in plasma in the HCB group was significantly lower than those in the HB group, BM group, and NS group at the same postoperative time points, which confirmed that the degree of bowel function injury in the HCB group was ameliorated when compared with the other groups. We found that apoptosis in the IsoT group was similar to that in normal rats 7 d after transplantation, which indicated that ischemic injury had basically recovered by the 7th d after surgery; the level of apoptosis in the HCB group was lower than those of the HB group, BM group, and NS group at the same postoperative time points. However, the level of apoptosis in the NS group had clearly decreased by 10 d and 14 d after transplantation. Through pathological analysis, we found that a large number of necrotic mucosal cells had appeared. Therefore, the decrease in the NS group was not a result of an improved lesion but a false reduction caused by a large number of mucosal cells that had become necrotic and were being shed. Changes in body weight can also further reflect the rejection of the organ, i.e., the less profound the rejection, the heavier the rats. We showed that HCB rats weighed the heaviest compared with other experimental groups. We also showed that CXCR3/HO-1 modified BMMSCs were able to better protect transplanted small bowel, but the mechanism remains to be further elucidated.
BMMSCs regulate lymphocytes and induce the generation of regulatory T lymphocytes, which play an important role in both preventing rejection and maintaining immune tolerance[55]. Zhou et al[56] also found that BMMSCs have the ability to inhibit T cell responses both in vitro and in vivo, and that intravenous infusion of BMMSCs can alter the balance of Thl/Th2 cells. Th17 cells are helper T cells that can secrete IL-17, and they are iconic cells that play a positive role in immune regulation during the inflammatory response[57]. BMMSCs can inhibit the differentiation of CD4+ T cells into Thl cells and Thl7 cells and upregulate the proportion of CD4+ CD25+ Foxp3+ T cells (Tregs)[58]. Our findings showed that IL-6, IL-17, IL-23, IFN-γ, and TNF-α in serum in the preconditioned BM group, HB group, and HCB group were significantly lower than those in normal rats. The levels of IL-10 and TGF-β were significantly increased, the proportion of Tregs was significantly higher, and the activity of NK cells was also significantly lower than those observed in normal rats. These findings suggested that pretreated rats were in a low immune state.
Tregs are a regulatory T cell population distinct from Th1 and Th2 cells. They are thought to be a subset of Th3 cells that secrete TGF-β[59] and IL-10, both key in the maintenance of autoimmune tolerance[60], and important in the induction of immune tolerance after solid organ transplantation[61]. In the present study, we found that the proportion of Tregs from rats which were pretreated with BMMSCs was significantly higher than that of normal rats, and the levels of IL-10 and TGF-β were significantly higher than those of normal rats. At the same postoperative time, the proportion of Tregs in the HCB group was significantly higher than that in the HB group and BM group, while the HB group level was significantly higher than that in the BM group. These findings are similar to the results of a previous study[34] where the levels of IL-10 and TGF-β were highest in the HCB group. Thus, CXCR3/HO-1 modified BMMSCs exerted their effects earlier than HO-1 modified BMMSCs and BMMSCs.
The major Th1 cytokines are IFN-γ and IL-2[62], with such Th1 cytokines mediating proinflammatory cell immunity and acute rejection. In contrast, the major Th2 cytokine is IL-10; Th2 cells can down-regulate Th1 cells and consequently inhibit the cytotoxic effect of cytotoxic T lymphocytes, which is likely to be related to immune tolerance after transplantation[63-65]. Relevant studies have shown that IL-2 and IL-10 mutually orchestrate the final immune state[66]. Serum concentrations of IL-2 increase during acute rejection and are highly correlated with severity of rejection[67]. In contrast, high concentrations of IL-10 are associated with immune tolerance in solid organ transplantation[68]. Our results demonstrated that serum level of IL-2 in the HCB group treated with CXCR3/HO-1 modified BMMSCs was the lowest of all groups and was significantly lower than those observed in the NS group, BM group, and HB group. Furthermore, the level of IL-10 was the highest in the HCB group and significantly higher than those in the other three groups. For analysis of IFN-γ, we obtained similar results where the level of IFN-γ was significantly lower in the HCB group than in the NS group, BM group, and HB group. The cytokine profiles and trends mirror those of the pathological results of the transplanted small bowel, which is reassuring.
In recent years, studies have identified a new CD4+ T cell type 17 (T helper cell 17, Th17), which is different from Th1 and Th2. This subset of T cells are independently differentiated and have different regulation mechanisms, while secreting IL-17, IL-22, IL-26, and TNF-α[69]. Therefore, IL-17 is the most characteristic cytokine that is produced by Th17 cells[70,71]. IL-23 is mainly produced by activated DCs and macrophages in vivo, and IL-23 plays an important role in maintaining Th17 stability and promoting Th17 proliferation[72]. IL-23 can also activate macrophages and DCs to promote the secretion of TNF-α[73]. It has been reported that IL-23 can induce activated CD4+ T cells to proliferate and secrete IL-17/IL-17F, and enhance IL-23/Th17-induced autoimmune and inflammatory diseases. The binding of IL-17/IL-17F to the receptor may promote the expression of IL-1, IL-6, IL-8, TNF-α, and chemokines, thereby promoting neutrophil aggregation to the chronic inflammatory location[74]. BMMSCs can interfere with the function of DCs and macrophages, resulting in decreased levels of IL-23. TGF-β can promote the differentiation of Tregs by inducing the expression of Treg transcription factor Foxp3[75]. We demonstrated that the levels of IL-23, IL-17, and IL-6 in the pretreated BM group, HB group, and HCB group were significantly lower than those in normal rats, and also found that the levels of IL-23, IL-17, and IL-6 in the HCB group were significantly lower than those in the NS group, BM group, and HB group at the same postoperative time points. Data have shown that the concentration of TNF-α and the rejection status were positively correlated[76]. We found that the concentration of TNF-α in the HCB group was the lowest compared with those in the other groups. At the same postoperative time, those animals in the HCB group had the highest level of TGF-β, while also having similarly high levels of Tregs compared with the other groups. We speculate that CXCR3/HO-1 co-transduced BMMSCs are better in modulating immunity by influencing the interaction of cytokines with a better concomitant regulatory effect.
In this study, we found that pretreatment with BMMSCs can render recipient rats into a low immune state, which is beneficial in reducing rejection after transplantation. Introduction of the HO-1 gene may be able to solve the activity problems of BMMSCs when injected into the body, and the CXCR3 gene can increase the homing ability of these BMMSCs. BMMSCs modified with the HO-1 and CXCR3 genes can quickly reach the injured site in large numbers, and thus appear to be able to reduce the rejection of small bowel transplants. We believe we have finally achieved the purpose of prolonging the survival period of rats after small bowel transplantation.
We thank Key Laboratory of Emergency and Care Medicine of Ministry of Health and Tianjin Key Laboratory of Organ Transplantation for allowing this work to be performed in their laboratories.
Small bowel transplantation is an ideal method for the treatment of end-stage intestinal failure. However, the development of small bowel transplantation has lagged behind that of other organ transplants because the incidence of rejection is high. Bone marrow mesenchymal stem cells (BMMSCs) play an important role in the regulation of immune function and are an ideal choice for cell transplantation in the treatment of autoimmune diseases and the prevention of rejection after solid organ transplantation. However, BMMSCs have a limited ability to survive and do not specifically migrate to the lesion in any meaningful capacity. Therefore, it is important to enhance the survival activity and quantity of BMMSCs at the injury site.
BMMSCs play an important role in mitigating rejection and maintaining immune tolerance. However, treatment with BMMSCs tends to result in an insufficient number of cells that can target in vivo lesions and they have a short survival time. It is important to find a way to modify the BMMSCs, thereby increasing their activity and quantity at the injury location.
They found that BMMSCs modified with the CXCR3 and HO-1 genes can significantly reduce the rejection of small bowel transplantation. Thus, chemokine receptor CXCR3 and HO-1 synergistically play an important role in this process.
BMMSCs are likely to be an ideal cell therapy for the treatment of organ transplant rejection in the future. By enhancing cell activity and quantity at the injury location, this can further enhance the effect of improving the rejection of transplanted small bowel. This is a step forward for clinical application of gene modified BMMSCs.
Diamine oxidase is a highly active intracellular enzyme that exists in human and mammalian small bowel villi. It plays a role in histamine production and a variety of polyamine metabolism processes, and its activity is closely related to the synthesis of nucleic acid and protein in mucosal cells. Its presence can reflect the intact nature of the intestinal barrier and the degree of injury.
The authors investigated the protective effects of BMMCCs modified with the CXCR3 and HO-1 genes on the rejection of small bowel transplantation. They found that CXCR3/HO-1 modified BMMSCs were able to improve the survival of the recipient rats. The whole project involved a large amount of work and has a certain degree of innovation in its execution.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Koepsell SA S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Roskott AM, Nieuwenhuijs VB, Dijkstra G, Koudstaal LG, Leuvenink HG, Ploeg RJ. Small bowel preservation for intestinal transplantation: a review. Transpl Int. 2011;24:107-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Trevizol AP, David AI, Yamashita ET, Pecora RA, D’Albuquerque LA. Intestinal and multivisceral retransplantation results: literature review. Transplant Proc. 2013;45:1133-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Yildiz BD. Where are we at with short bowel syndrome and small bowel transplant. World J Transplant. 2012;2:95-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 4. | Grant D, Abu-Elmagd K, Mazariegos G, Vianna R, Langnas A, Mangus R, Farmer DG, Lacaille F, Iyer K, Fishbein T. Intestinal transplant registry report: global activity and trends. Am J Transplant. 2015;15:210-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 297] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 5. | Abu-Elmagd KM, Costa G, Bond GJ, Wu T, Murase N, Zeevi A, Simmons R, Soltys K, Sindhi R, Stein W. Evolution of the immunosuppressive strategies for the intestinal and multivisceral recipients with special reference to allograft immunity and achievement of partial tolerance. Transpl Int. 2009;22:96-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Abu-Elmagd KM, Wu G, Costa G, Lunz J, Martin L, Koritsky DA, Murase N, Irish W, Zeevi A. Preformed and de novo donor specific antibodies in visceral transplantation: long-term outcome with special reference to the liver. Am J Transplant. 2012;12:3047-3060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 7. | Ho MS, Mei SH, Stewart DJ. The Immunomodulatory and Therapeutic Effects of Mesenchymal Stromal Cells for Acute Lung Injury and Sepsis. J Cell Physiol. 2015;230:2606-2617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Chen J, Li C, Chen L. The Role of Microvesicles Derived from Mesenchymal Stem Cells in Lung Diseases. Biomed Res Int. 2015;2015:985814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol. 2013;91:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 389] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 10. | Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 749] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 11. | Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42-48. [PubMed] |
| 12. | Shi M, Liu ZW, Wang FS. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol. 2011;164:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Ju S, Teng GJ, Lu H, Jin J, Zhang Y, Zhang A, Ni Y. In vivo differentiation of magnetically labeled mesenchymal stem cells into hepatocytes for cell therapy to repair damaged liver. Invest Radiol. 2010;45:625-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, Pascual CY, Aller MA, Arias J, Arnalich-Montiel F. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012;12:574-591. [PubMed] |
| 15. | Sensebé L, Krampera M, Schrezenmeier H, Bourin P, Giordano R. Mesenchymal stem cells for clinical application. Vox Sang. 2010;98:93-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 16. | Hodgkinson CP, Gomez JA, Mirotsou M, Dzau VJ. Genetic engineering of mesenchymal stem cells and its application in human disease therapy. Hum Gene Ther. 2010;21:1513-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 455] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 18. | Yamashita K, Ollinger R, McDaid J, Sakahama H, Wang H, Tyagi S, Csizmadia E, Smith NR, Soares MP, Bach FH. Heme oxygenase-1 is essential for and promotes tolerance to transplanted organs. FASEB J. 2006;20:776-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | García-López MA, Sánchez-Madrid F, Rodríguez-Frade JM, Mellado M, Acevedo A, García MI, Albar JP, Martínez C, Marazuela M. CXCR3 chemokine receptor distribution in normal and inflamed tissues: expression on activated lymphocytes, endothelial cells, and dendritic cells. Lab Invest. 2001;81:409-418. [PubMed] |
| 20. | Liu T, Fu NN, Song HL, Wang YL, Wu BJ, Shen ZY. Suppression of MicroRNA-203 improves survival of rat bone marrow mesenchymal stem cells through enhancing PI3K-induced cellular activation. IUBMB Life. 2014; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Wu T, Abu-Elmagd K, Bond G, Nalesnik MA, Randhawa P, Demetris AJ. A schema for histologic grading of small intestine allograft acute rejection. Transplantation. 2003;75:1241-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 22. | Xu CJ, Ding GQ, Fu JY, Meng J, Zhang RH, Lou XM. Immunoregulatory effects of ethyl-acetate fraction of extracts from Tetrastigma hemsleyanum Diels et. Gilg on immune functions of ICR mice. Biomed Environ Sci. 2008;21:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Pécora RA, David AI, Lee AD, Galvão FH, Cruz-Junior RJ, D’Albuquerque LA. Small bowel transplantation. Arq Bras Cir Dig. 2013;26:223-229. [PubMed] |
| 24. | Izamis ML, Tolboom H, Uygun B, Berthiaume F, Yarmush ML, Uygun K. Resuscitation of ischemic donor livers with normothermic machine perfusion: a metabolic flux analysis of treatment in rats. PLoS One. 2013;8:e69758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Fosby B, Karlsen TH, Melum E. Recurrence and rejection in liver transplantation for primary sclerosing cholangitis. World J Gastroenterol. 2012;18:1-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 103] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 26. | Cuadrado A, San Segundo D, López-Hoyos M, Crespo J, Fábrega E. Clinical significance of donor-specific human leukocyte antigen antibodies in liver transplantation. World J Gastroenterol. 2015;21:11016-11026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Selvaggi G, Gaynor JJ, Moon J, Kato T, Thompson J, Nishida S, Levi D, Ruiz P, Cantwell P, Tzakis AG. Analysis of acute cellular rejection episodes in recipients of primary intestinal transplantation: a single center, 11-year experience. Am J Transplant. 2007;7:1249-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Koo J, Dawson DW, Dry S, French SW, Naini BV, Wang HL. Allograft biopsy findings in patients with small bowel transplantation. Clin Transplant. 2016;30:1433-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Yang Y, Song HL, Zhang W, Wu BJ, Fu NN, Zheng WP, Dong C, Shen ZY. Reduction of acute rejection by bone marrow mesenchymal stem cells during rat small bowel transplantation. PLoS One. 2014;9:e114528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Takahashi T, Tibell A, Ljung K, Saito Y, Gronlund A, Osterholm C, Holgersson J, Lundgren T, Ericzon BG, Corbascio M. Multipotent mesenchymal stromal cells synergize with costimulation blockade in the inhibition of immune responses and the induction of Foxp3+ regulatory T cells. Stem Cells Transl Med. 2014;3:1484-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, Kyurkchiev S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells. 2014;6:552-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 468] [Article Influence: 42.5] [Reference Citation Analysis (4)] |
| 32. | Chen C, Zhang F, Zhang Z, Peng M, Wang Y, Chen Y. TLR4 signaling-induced heme oxygenase upregulation in the acute lung injury: role in hemorrhagic shock and two-hit induced lung inflammation. Mol Biol Rep. 2013;40:1167-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Wu B, Song HL, Yang Y, Yin ML, Zhang BY, Cao Y, Dong C, Shen ZY. Improvement of Liver Transplantation Outcome by Heme Oxygenase-1-Transduced Bone Marrow Mesenchymal Stem Cells in Rats. Stem Cells Int. 2016;2016:9235073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Yang Y, Song HL, Zhang W, Wu BJ, Fu NN, Dong C, Shen ZY. Heme oxygenase-1-transduced bone marrow mesenchymal stem cells in reducing acute rejection and improving small bowel transplantation outcomes in rats. Stem Cell Res Ther. 2016;7:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Pontikoglou C, Deschaseaux F, Sensebé L, Papadaki HA. Bone marrow mesenchymal stem cells: biological properties and their role in hematopoiesis and hematopoietic stem cell transplantation. Stem Cell Rev. 2011;7:569-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 36. | Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, Deans RJ, McIntosh KR. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12:47-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 433] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 37. | Valcz G, Krenács T, Sipos F, Leiszter K, Tóth K, Balogh Z, Csizmadia A, Muzes G, Molnár B, Tulassay Z. The role of the bone marrow derived mesenchymal stem cells in colonic epithelial regeneration. Pathol Oncol Res. 2011;17:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med. 1989;169:493-502. [PubMed] |
| 39. | Huang H, He J, Teng X, Yu Y, Ye W, Hu Y, Shen Z. Combined intrathymic and intravenous injection of mesenchymal stem cells can prolong the survival of rat cardiac allograft associated with decrease in miR-155 expression. J Surg Res. 2013;185:896-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Schulz O, Hammerschmidt SI, Moschovakis GL, Förster R. Chemokines and Chemokine Receptors in Lymphoid Tissue Dynamics. Annu Rev Immunol. 2016;34:203-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 41. | Frick VO, Rubie C, Keilholz U, Ghadjar P. Chemokine/chemokine receptor pair CCL20/CCR6 in human colorectal malignancy: An overview. World J Gastroenterol. 2016;22:833-841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 42. | Balkwill FR. The chemokine system and cancer. J Pathol. 2012;226:148-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 327] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 43. | Halloran PF, Fairchild RL. The puzzling role of CXCR3 and its ligands in organ allograft rejection. Am J Transplant. 2008;8:1578-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129-134. [PubMed] |
| 45. | Colvin RA, Campanella GS, Sun J, Luster AD. Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function. J Biol Chem. 2004;279:30219-30227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 209] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 46. | Shino MY, Weigt SS, Li N, Palchevskiy V, Derhovanessian A, Saggar R, Sayah DM, Gregson AL, Fishbein MC, Ardehali A. CXCR3 ligands are associated with the continuum of diffuse alveolar damage to chronic lung allograft dysfunction. Am J Respir Crit Care Med. 2013;188:1117-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Hricik DE, Nickerson P, Formica RN, Poggio ED, Rush D, Newell KA, Goebel J, Gibson IW, Fairchild RL, Riggs M. Multicenter validation of urinary CXCL9 as a risk-stratifying biomarker for kidney transplant injury. Am J Transplant. 2013;13:2634-2644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 201] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 48. | Pranzatelli MR, Tate ED, McGee NR, Travelstead AL, Verhulst SJ, Ransohoff RM. Expression of CXCR3 and its ligands CXCL9, -10 and -11 in paediatric opsoclonus-myoclonus syndrome. Clin Exp Immunol. 2013;172:427-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Jonecová Z, Tóth Š, Varga J, Staško P, Kovalčinová B, Maretta M, Veselá J. The immediate response of jejunal mucosa to small bowel heterotopic allotransplatation in rats. Tissue Cell. 2014;46:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 50. | Wong YL, Lautenschläger I, Dombrowsky H, Zitta K, Bein B, Krause T, Goldmann T, Frerichs I, Steinfath M, Weiler N. Correction: Hydroxyethyl Starch (HES 130/0.4) Impairs Intestinal Barrier Integrity and Metabolic Function: Findings from a Mouse Model of the Isolated Perfused Small Intestine. PLoS One. 2015;10:e0127136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Bounous G, Echavé V, Vobecky SJ, Navert H, Wollin A. Acute necrosis of the intestinal mucosa with high serum levels of diamine oxidase. Dig Dis Sci. 1984;29:872-874. [PubMed] |
| 52. | Fukudome I, Kobayashi M, Dabanaka K, Maeda H, Okamoto K, Okabayashi T, Baba R, Kumagai N, Oba K, Fujita M. Diamine oxidase as a marker of intestinal mucosal injury and the effect of soluble dietary fiber on gastrointestinal tract toxicity after intravenous 5-fluorouracil treatment in rats. Med Mol Morphol. 2014;47:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 53. | Mahmood MM, Watt J, Ahmed JM. Thrombus aspiration during primary percutaneous coronary intervention for acute myocardial infarction: A review of clinical evidence and guidelines. World J Cardiol. 2015;7:889-894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 54. | Zhou SS, Tian F, Chen YD, Wang J, Sun ZJ, Guo J, Jin QH. Combination therapy reduces the incidence of no-reflow after primary per-cutaneous coronary intervention in patients with ST-segment elevation acute myocardial infarction. J Geriatr Cardiol. 2015;12:135-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 55. | Ma OK, Chan KH. Immunomodulation by mesenchymal stem cells: Interplay between mesenchymal stem cells and regulatory lymphocytes. World J Stem Cells. 2016;8:268-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 56. | Zhou HP, Yi DH, Yu SQ, Sun GC, Cui Q, Zhu HL, Liu JC, Zhang JZ, Wu TJ. Administration of donor-derived mesenchymal stem cells can prolong the survival of rat cardiac allograft. Transplant Proc. 2006;38:3046-3051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 57. | Kang SK, Shin IS, Ko MS, Jo JY, Ra JC. Journey of mesenchymal stem cells for homing: strategies to enhance efficacy and safety of stem cell therapy. Stem Cells Int. 2012;2012:342968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 58. | Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010;16:203-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 503] [Cited by in RCA: 468] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 59. | Shevach EM. Certified professionals: CD4(+)CD25(+) suppressor T cells. J Exp Med. 2001;193:F41-F46. [PubMed] |
| 60. | Radić M. Role of Helicobacter pylori infection in autoimmune systemic rheumatic diseases. World J Gastroenterol. 2014;20:12839-12846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 61. | Germani G, Rodriguez-Castro K, Russo FP, Senzolo M, Zanetto A, Ferrarese A, Burra P. Markers of acute rejection and graft acceptance in liver transplantation. World J Gastroenterol. 2015;21:1061-1068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 62. | Chen G, Wu D, Wang Y, Cen J, Feng Y, Sun A, Tang X, Chang H, Zhu Z. Expanded donor natural killer cell and IL-2, IL-15 treatment efficacy in allogeneic hematopoietic stem cell transplantation. Eur J Haematol. 2008;81:226-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 63. | Zhang XG, Lü Y, Wang B, Li H, Yu L, Liu C, Wu Z, Liu XM. Cytokine production during the inhibition of acute vascular rejection in a concordant hamster-to-rat cardiac xenotransplantation model. Chin Med J (Engl). 2007;120:145-149. [PubMed] |
| 64. | Gleissner CA, Zastrow A, Klingenberg R, Kluger MS, Konstandin M, Celik S, Haemmerling S, Shankar V, Giese T, Katus HA. IL-10 inhibits endothelium-dependent T cell costimulation by up-regulation of ILT3/4 in human vascular endothelial cells. Eur J Immunol. 2007;37:177-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 65. | Zhang H, Stern JN, Strominger JL. T cell receptors in an IL-10-secreting amino acid copolymer-specific regulatory T cell line that mediates bystander immunosuppression. Proc Natl Acad Sci USA. 2009;106:3336-3341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 66. | Bharat A, Fields RC, Steward N, Trulock EP, Patterson GA, Mohanakumar T. CD4+25+ regulatory T cells limit Th1-autoimmunity by inducing IL-10 producing T cells following human lung transplantation. Am J Transplant. 2006;6:1799-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 67. | van Emmerik N, Baan C, Vaessen L, Jutte N, Quint W, Balk A, Bos E, Niesters H, Weimar W. Cytokine gene expression profiles in human endomyocardial biopsy (EMB) derived lymphocyte cultures and in EMB tissue. Transpl Int. 1994;7 Suppl 1:S623-S626. [PubMed] |
| 68. | Cottrez F, Hurst SD, Coffman RL, Groux H. T regulatory cells 1 inhibit a Th2-specific response in vivo. J Immunol. 2000;165:4848-4853. [PubMed] |
| 69. | Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 903] [Cited by in RCA: 862] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 70. | Pène J, Chevalier S, Preisser L, Vénéreau E, Guilleux MH, Ghannam S, Molès JP, Danger Y, Ravon E, Lesaux S. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol. 2008;180:7423-7430. [PubMed] |
| 71. | Geginat J, Paroni M, Maglie S, Alfen JS, Kastirr I, Gruarin P, De Simone M, Pagani M, Abrignani S. Plasticity of human CD4 T cell subsets. Front Immunol. 2014;5:630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 217] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 72. | Peng J, Yang XO, Chang SH, Yang J, Dong C. IL-23 signaling enhances Th2 polarization and regulates allergic airway inflammation. Cell Res. 2010;20:62-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 73. | Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 790] [Article Influence: 41.6] [Reference Citation Analysis (1)] |
| 74. | Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, Cua DJ, Powrie F, Cheever AW, Maloy KJ. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485-2494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 475] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 75. | Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1569] [Cited by in RCA: 1541] [Article Influence: 90.6] [Reference Citation Analysis (0)] |
| 76. | Wei XC, Yang DD, Han XR, Zhao YA, Li YC, Zhang LJ, Wang JJ. Bioactivity of umbilical cord blood dendritic cells and anti-leukemia effect. Int J Clin Exp Med. 2015;8:19725-19730. [PubMed] |