Published online Oct 16, 2025. doi: 10.12998/wjcc.v13.i29.111096
Revised: July 5, 2025
Accepted: August 13, 2025
Published online: October 16, 2025
Processing time: 66 Days and 19 Hours
Gestational diabetes mellitus (GDM) has emerged as a global public health cha
To synthesize current evidence on the pathophysiology, diagnosis, management, complications, and individualized treatment strategies of GDM.
We conducted a narrative review in accordance with PRISMA guidelines. Pub
GDM results from a complex interplay among progressive insulin resistance, β-cell dysfunction, immune dysregulation, and placental inflammation. Emerging evidence indicates that hyperglycemia before formal diagnosis can impair fetal programming via epigenetic mechanisms. GDM increases a mother’s risk of developing type 2 diabetes mellitus seven- to tenfold and raises the incidence of cardiovascular disease, preeclampsia, and cesarean delivery. Offspring are at higher risk of macrosomia, neonatal hypoglycemia, and future metabolic and cardiovascular disorders. Lifestyle modification remains the cornerstone of therapy and, when necessary, can be supplemented with pharmacologic agents such as metformin or insulin. Postpartum follow-up, breastfeeding support, and preconception counseling are vital to long-term metabolic health.
GDM requires precision-based, life-course care. Future priorities include early risk detection, biomarker validation, unified diagnosis, and culturally sensitive interventions to improve maternal-child outcomes.
Core Tip: This narrative review explores the latest evidence on gestational diabetes mellitus (GDM), emphasizing its pathophysiology, clinical management, and long-term cardiometabolic consequences. It highlights the role of early hyper
- Citation: Luo QJ, Ni Q. Life-course management of gestational diabetes mellitus: A narrative review. World J Clin Cases 2025; 13(29): 111096
- URL: https://www.wjgnet.com/2307-8960/full/v13/i29/111096.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i29.111096
Gestational diabetes mellitus (GDM) is currently the most common metabolic disorder of pregnancy. It affects approximately 14%-25% of pregnancies worldwide, amounting for more than 18 million births each year[1-3]. The rising incidence of GDM parallels global trends in maternal obesity, sedentary lifestyles, and delayed childbearing[4-6].
Clinically, GDM is associated with a broad spectrum of adverse perinatal outcomes, including preeclampsia, cesarean section, macrosomia, neonatal hypoglycemia, and respiratory distress syndrome[7-9]. In the long term, GDM contributes to increased risks of type 2 diabetes mellitus (T2DM) and cardiovascular disease in mothers, as well as obesity and metabolic syndrome in their offspring[10-12].
Over the past two decades, the prevalence of GDM has risen substantially—from under 5% in some high-income countries to over 25% in certain regions of Asia and the Middle East—driven by changing diagnostic criteria and shifting population risk profiles[13,14]. Adoption of the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria—endorsed by the World Health Organization (WHO) and the American Diabetes Association (ADA)—has increased reported GDM diagnoses by up to 75% in some regions[15]. However, this expansion has sparked debate over potential overdiagnosis, particularly when applied in early pregnancy without robust clinical trial validation[16,17].
Despite broad international endorsement of these criteria, substantial variability persists in screening practices, diagnostic thresholds, and timing across countries, hindering efforts toward standardized global management[18,19]. Moreover, GDM is increasingly recognized as a heterogeneous, temporally dynamic disorder rather than a single disease entity. Emerging classification models that consider variations in insulin sensitivity, β-cell function, genetic predisposition, and gestational timing may facilitate more precise, tailored interventions[20,21]. However, most current clinical guidelines continue to rely on uniform diagnostic cutoffs and standardized treatment protocols.
This narrative review synthesizes the latest advances across seven key domains: Pathophysiology, epidemiology, diagnostic methodologies, clinical management, maternal-fetal outcomes, molecular insights, and public health perspectives. By integrating recent scientific developments and addressing ongoing controversies, we aim to support a shift toward risk-stratified, individualized care for GDM.
This narrative review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines to ensure methodological transparency and rigor. A comprehensive literature search was performed across four major databases—PubMed, EMBASE, Web of Science, and Scopus—to identify peer-reviewed articles published between January 2017 and March 2025. The search strategy combined Medical Subject Headings and free-text terms, including: “gestational diabetes mellitus”, “GDM”, “pregnancy”, “hyperglycemia”, “insulin resistance”, “epigenetics”, and “maternal outcomes”.
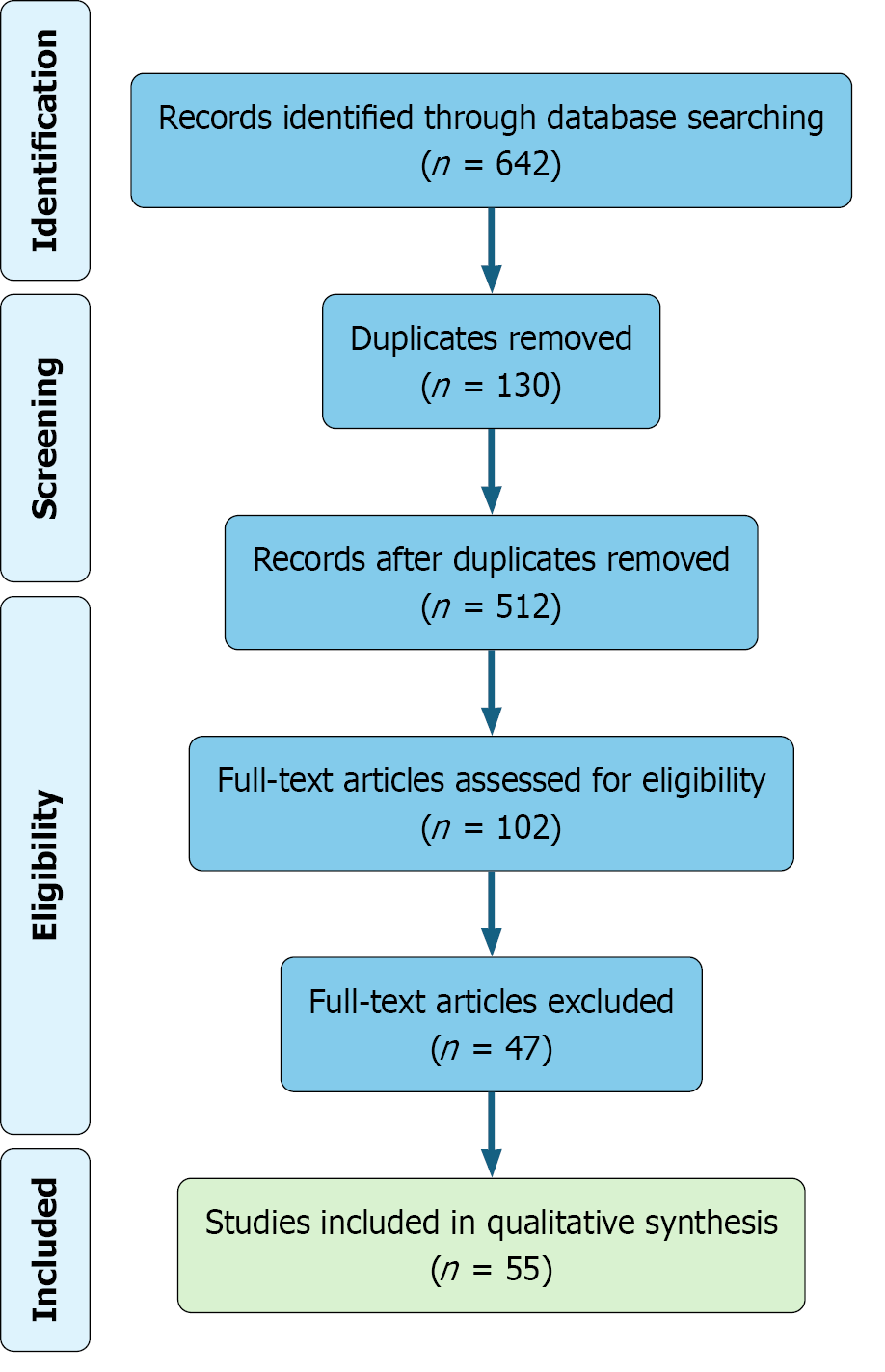
All references were imported into EndNote for deduplication and screening. After removing 130 duplicate records, 512 unique articles remained and were screened by title and abstract. Full-text review was conducted for 102 studies that met the preliminary inclusion criteria. Of these, 47 articles were excluded due to methodological limitations, insufficient relevance, or incomplete outcome data. A total of 55 studies were ultimately included in the qualitative synthesis based on scientific quality, clinical significance, and relevance to the review objectives.
Although this review was informed by PRISMA principles to improve methodological transparency and reporting quality, it does not constitute a systematic review. No meta-analysis was conducted, and the review protocol was not registered in PROSPERO or any other database. The primary objective was to provide a comprehensive narrative synthesis of current evidence, rather than a quantitative aggregation or statistical evaluation. The full study selection process is presented in Figure 1.
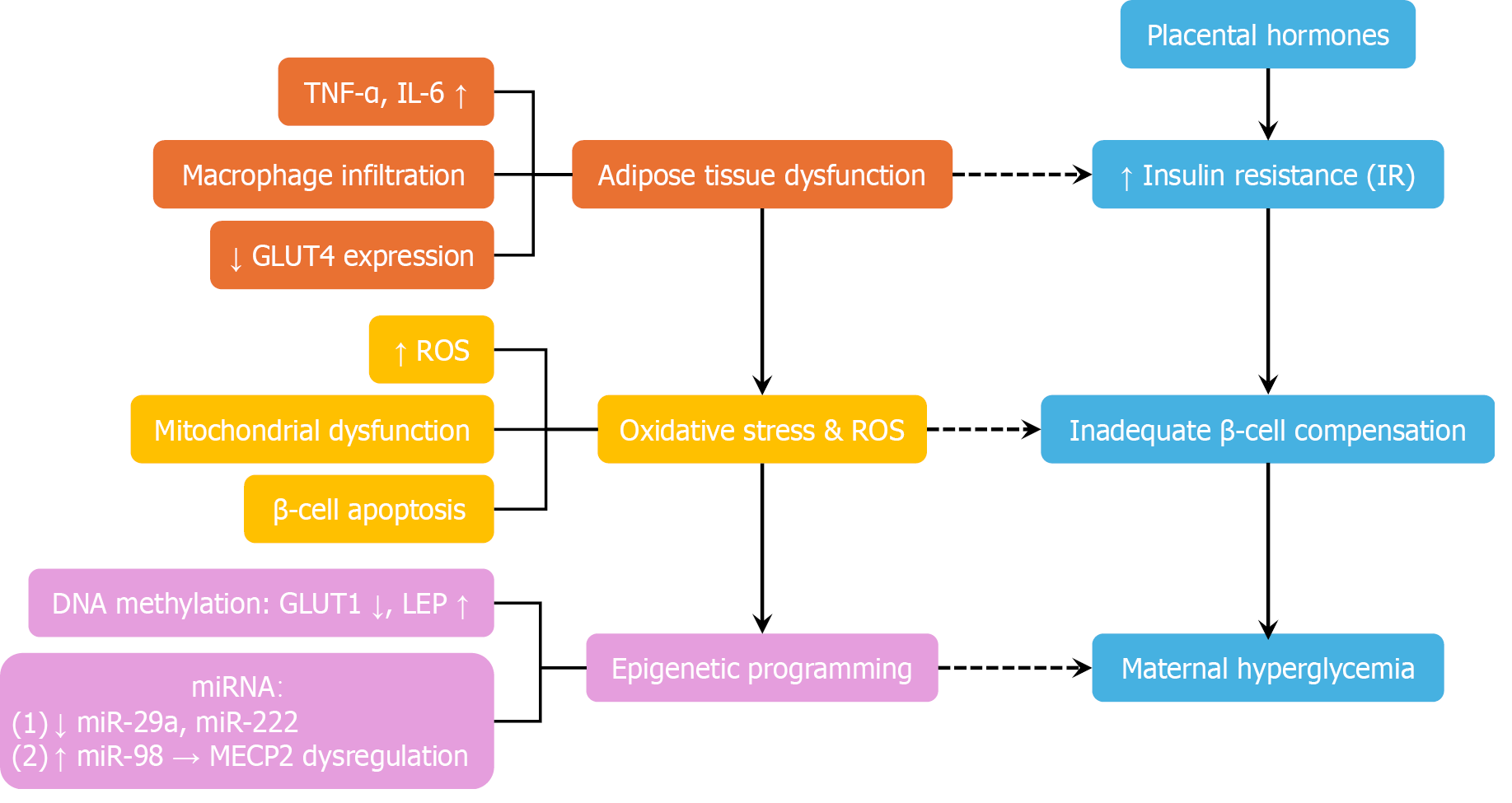
Hormone-induced insulin resistance: GDM develops when the physiological insulin resistance of pregnancy exceeds the compensatory capacity of maternal pancreatic β-cells, resulting in hyperglycemia[21,22]. Normally, placental hormones—including human placental lactogen, estrogen, progesterone, and placental growth hormone—induce insulin resistance to redirect maternal nutrients toward fetal growth[23,24]. In GDM, this adaptation becomes maladaptive. Insulin sensitivity can decline by up to 80%, particularly in overweight or obese women, whose preexisting insulin resistance and impaired β-cell function exacerbate metabolic dysregulation[1,4].
Adipose tissue dysfunction and inflammatory mediators: Dysfunctional adipose tissue plays a pivotal role in GDM pathogenesis. Adipocyte hypertrophy and immune cell infiltration foster chronic low-grade inflammation, characterized by elevated levels of TNF-α, interleukin-6 (IL-6), and C-reactive protein (CRP). These inflammatory cytokines impair insulin signaling through serine phosphorylation of insulin receptor substrate-1 (IRS-1) and suppression of GLUT4 expression[25-27]. At the placental level, inflammation disrupts trophoblast invasion, alters nutrient transport, and contributes to fetal overgrowth and macrosomia[9,28].
Adipokine imbalance: GDM is characterized by disrupted adipokine secretion, with elevated leptin concentrations and reduced adiponectin levels. This imbalance decreases insulin sensitivity, impairs β-cell function, and aggravates placental dysfunction, thereby exacerbating hyperglycemia[1,26].
β-cell dysfunction and failure of compensation: Central to GDM is β-cell dysfunction, including impaired insulin secre
Genetic and epigenetic susceptibility: Genetic studies have identified both T2DM-shared (e.g., GCK, CDKAL1, and HNF1A) and GDM-specific variants associated with placental function and neuroendocrine regulation[31]. Epigenetic modifications—including altered DNA methylation of leptin, SLC6A4, ADIPOQ, and GLUT1/3 in fetal tissues—suggest a mechanism for intergenerational transmission of metabolic risk[32].
Metabolomic and lipid dysregulation: Metabolomic profiling in GDM has revealed elevated levels of branched-chain amino acids and triglycerides, which contribute to insulin resistance and fetal overgrowth independently of maternal body mass index (BMI)[7,9]. These signatures may serve as biomarkers for GDM subtypes and predictors of adverse outcomes.
Oxidative stress and cellular injury: Oxidative stress is a hallmark of GDM pathophysiology. Hyperglycemia, elevated free fatty acids, and advanced glycation end products activate inflammatory pathways via Toll-like receptor signaling and NF-κB activation. This leads to reactive oxygen species generation, causing β-cell and endothelial damage[33]. GDM placentas exhibit diminished antioxidant defenses and increased vulnerability to oxidative injury, impairing angiogenesis and exacerbating fetal complications[26,34].
Immune dysregulation: Recent research implicates immune dysregulation in GDM, including shifts in regulatory T cell (Treg)/Th17 balance, polarization of macrophages toward a proinflammatory M1 phenotype, and abnormal galectin expression in maternal serum and placental tissue. These findings highlight a complex interplay among immune, endocrine, and metabolic systems in GDM pathogenesis[26,28].
Summary: The pathophysiology of GDM involves a multifaceted network of hormonal, inflammatory, metabolic, and immunologic disruptions. Figure 2 presents an integrative schematic summarizing these mechanisms, including placental hormone influence, adipose-derived inflammation, oxidative stress, and epigenetic modifications. This complexity underlines the necessity for personalized diagnostic and therapeutic approaches in GDM management.
Global burden and trends: The global prevalence of GDM is steadily increasing, representing a major public health challenge. According to the International Diabetes Federation, hyperglycemia affects approximately 14% of pregnancies worldwide, with over 80% attributed to GDM—equivalent to nearly one in six live births each year[1]. Based on standar
Regional disparities: Considerable interregional variation exists. The Middle East and North Africa region reports the highest prevalence at 27.6%, followed by Southeast Asia (20.8%), the Western Pacific (14.7%), and Africa (14.2%). In contrast, North America and Europe report relatively lower rates of 7.1% and 7.8%, respectively[13].
Within-region and country-level variability: Even within regions, GDM prevalence is highly heterogeneous. For example, among 24 European countries, the average prevalence is 10.9%, yet country-specific estimates range from 1.8% in Sweden to 66.1% in Moldova[6]. In Asia, prevalence ranges from 1.2% to nearly 50%, influenced by ethnic background, urbanization levels, and diagnostic protocols[18].
National trends—case examples: In mainland China, GDM prevalence increased 3.5-fold between 1999 and 2012, reaching 14.8% under pooled IADPSG data, with urban areas reporting higher rates than rural counterparts[12]. In Taiwan, prevalence rose from 7.6% in 2004 to 13.4% in 2015, with the highest rates observed among women aged ≥ 41 and in the eastern and southern regions[14]. In the United States, GDM prevalence increased by 78%—from 4.6% in 2006 to 8.2% in 2016—with steeper rises among Asian women and those of lower socioeconomic status[35].
Diagnostic criteria and detection: The rising burden of GDM is partially attributed to evolving diagnostic criteria. The adoption of the IADPSG thresholds has driven a marked increase in case detection—for instance, a 3.5-fold rise in Australia between 2010 and 2019[18]. While these criteria enhance sensitivity, they may also elevate false positive rates and increase clinical workload, prompting discussion on threshold calibration.
Recurrence and predictors: GDM recurrence remains a significant clinical concern. Meta-analyses estimate a pooled recurrence rate of 47.6%, with higher rates observed among African American, Latina, and Asian populations[10]. Risk factors for recurrence include advanced maternal age, elevated pre-pregnancy BMI, prior insulin therapy, interpregnancy weight gain, and elevated OGTT values in prior pregnancies.
Early-onset GDM and screening debates: Recent studies suggest that up to 70% of GDM cases may present in early pregnancy, particularly under more sensitive diagnostic criteria[16]. However, the accuracy of early screening remains contentious. For instance, the threshold of fasting plasma glucose (FPG) ≥ 5.1 mmol/L may yield excessive false positives in certain populations. As a result, some experts advocate for revised cut-offs (e.g., FPG ≥ 5.5 mmol/L) to avoid overtreatment.
Summary and public health relevance: The global rise, geographic heterogeneity, and high recurrence of GDM under
Lack of global consensus: The screening and diagnosis of GDM remain subjects of ongoing debate, largely due to the lack of a universally accepted standard. Prominent international organizations—including the IADPSG, the ADA, the WHO, and the American College of Obstetricians and Gynecologists (ACOG)—have each proposed differing diagnostic approaches, contributing to global variation in clinical practice.
Diagnostic approaches: The IADPSG and WHO recommend a one-step 75-g OGTT, typically performed between 24 and 28 weeks of gestation. A diagnosis of GDM is made if any of the following plasma glucose values are met or exceeded: FPG ≥ 5.1 mmol/L (92 mg/dL); 1-hour plasma glucose ≥ 10.0 mmol/L (180 mg/dL); 2-hour plasma glucose ≥ 8.5 mmol/L (153 mg/dL)[15,18,22].
In contrast, the ACOG continues to endorse the two-step approach. The initial screening involves a 50-g glucose challenge test (GCT), administered without fasting. A plasma glucose level ≥ 140 mg/dL one-hour post-load prompts a follow-up 100-g OGTT. GDM is diagnosed if two or more of the following values are abnormal: Fasting ≥ 95 mg/dL (5.3 mmol/L); 1-hour ≥ 180 mg/dL (10.0 mmol/L); 2-hour ≥ 155 mg/dL (8.6 mmol/L); 3-hour ≥ 140 mg/dL (7.8 mmol/L)[24,30].
These differing criteria have led to substantial variation in GDM prevalence across populations, complicating international efforts to standardize care. A comparative summary of these diagnostic strategies is presented in Table 1.
| Guideline | Test type | Timing | Fasting threshold (mg/dL) | 1-hour | 2-hour | Diagnosis criteria |
| IADPSG | 75-g OGTT | 24-28 weeks | ≥ 92 | ≥ 180 | ≥ 153 | Any one abnormal |
| ADA | 75-g OGTT | 24-28 weeks | ≥ 95 | ≥ 180 | ≥ 155 | Any two abnormal |
| ACOG | 50-g GCT + 100-g OGTT | 24-28 weeks | ≥ 95 | ≥ 180 | ≥ 155 | Two-step approach |
| WHO | 75-g OGTT | 24-28 weeks | ≥ 92 | - | ≥ 153 | Any one abnormal |
One-step vs two-step debate: The one-step diagnostic method has been widely adopted in countries such as China and Australia. However, its implementation has resulted in a 2-3-fold increase in GDM diagnoses, raising concerns about overdiagnosis, increased healthcare costs, and limited evidence of improved maternal or neonatal outcomes[14,36,37].
Randomized controlled trials—including the ScreenR2GDM and GEMS studies—reported no significant differences between the one-step and two-step approaches in terms of reducing large-for-gestational-age (LGA) births, cesarean delivery, or neonatal complications. However, the one-step method resulted in a greater proportion of women receiving treatment[1,21].
National and contextual variations: In response to the above-mentioned findings, several national guidelines—including those from Korea and Taiwan—allow for either approach based on resource availability, clinical infrastructure, and feasibility[14,36]. Tailoring screening strategies to local health system capacity may improve implementation and equity.
Timing and threshold considerations: Most international guidelines recommend GDM screening between 24 and 28 weeks of gestation. However, early screening in high-risk individuals—such as those with obesity, prior GDM, or a family history of diabetes—is increasingly being explored. Despite this, early-pregnancy FPG levels show limited sensitivity. Recent proposals suggest raising the diagnostic threshold (e.g., from 5.1 to 5.5 mmol/L) to minimize false positives and prevent unnecessary interventions[16,26].
Universal vs selective screening: Universal screening has been shown to identify more cases of GDM, especially in regions with a high prevalence. In contrast, selective screening based on risk factors may miss 35%-47% of cases[1,13]. In low-resource settings, the International Federation of Gynecology and Obstetrics (FIGO) recommends the use of hand
Emerging diagnostic tools: Advances in molecular diagnostics and omics-based approaches offer promising tools for early risk prediction and subtype identification.
Biomarkers such as β-muricholic acid, urinary metabolites, and inflammatory proteins [e.g., sex hormone-binding globulin (SHBG), CRP, and IL-6] have demonstrated high predictive performance (area under the curve > 0.95)[10,26].
Polygenic risk scores, immune gene panels (e.g., FABP4 and CXCL10), and metabolomic subtyping strategies may facilitate precision diagnosis and personalized interventions[20,28].
Controversies and future directions: Despite the broad international endorsement of IADPSG criteria by major organizations such as the WHO, ADA, and FIGO, ongoing controversies remain regarding their cost-effectiveness, psychological impact, and actual clinical benefit. Achieving global consensus will require harmonization of diagnostic thresholds, improved risk stratification models, and the development of adaptable, context-specific screening protocols.
Maternal age: Advanced maternal age is one of the most consistently recognized non-modifiable risk factors for GDM. Women aged ≥ 35 years have up to a 4-fold higher risk than those < 25 years[2,8,12]. In Asian populations, the risk increases by approximately 12.7% for each year beyond age 18[30]. A large-scale European study reported a 2.14-fold higher prevalence of GDM among women aged ≥ 30 years compared to younger counterparts[6].
Adiposity and gestational weight gain: Prepregnancy overweight and obesity are the most modifiable and impactful risk factors for GDM. Obesity (BMI ≥ 30 kg/m²) is associated with a 6.8-fold higher risk. Overweight status (BMI 25-29.9 kg/m²) confers a 2.3-fold increased risk[6,8]. Each unit increase in BMI is linked to a 9%-12% higher risk of LGA infants or macrosomia[7]. Excessive gestational weight gain, particularly during the second trimester, significantly elevates GDM risk—especially in older or overweight women[2].
Family and obstetric history: Personal or familial history of metabolic or obstetric complications markedly increases the risk of developing GDM. A family history of diabetes is associated with as much as a 3.6-fold increase in GDM risk. A prior diagnosis of GDM is the strongest predictor of recurrence, with an odds ratio of approximately 21.1 and a re
| Risk level | Key characteristics | Recommended screening approach |
| High | BMI ≥ 30 kg/m², prior GDM, strong family history, PCOS | Early OGTT (< 20 weeks), repeat at 24-28 weeks |
| Moderate | BMI 25-29.9 kg/m², age ≥ 30 years, non-White ethnicity | Standard OGTT at 24-28 weeks |
| Low | BMI < 25 kg/m², age < 25 years, no risk factors | Routine screening or selective as per policy |
Lifestyle and sociodemographic factors: Sociodemographic and behavioral factors play a substantial role in GDM risk. Low socioeconomic status, physical inactivity, and sedentary behavior of ≥ 4 hours per day are independently associated with increased GDM risk[12,35]. Seasonal patterns (e.g., winter conception), urban living, and residence in economically aged communities have also been linked to higher prevalence[14].
Pronounced ethnic disparities: South and East Asian, Hispanic, and African American women exhibit higher GDM prevalence. However, progression to T2DM following GDM may occur more frequently among White and Black women[39].
Dietary and environmental factors: Nutritional patterns, micronutrient status, and lifestyle behaviors significantly shape insulin sensitivity and GDM risk. Diets rich in processed foods, saturated animal fats, and added sugars contribute to GDM pathogenesis. Conversely, low-glycemic index (GI) and high-fiber diets have been shown to enhance insulin sensitivity and reduce GDM risk. Additional contributors include vitamin D deficiency, chronic psychological stress, and poor sleep quality, which are associated with impaired glucose regulation and heightened metabolic risk[25,30].
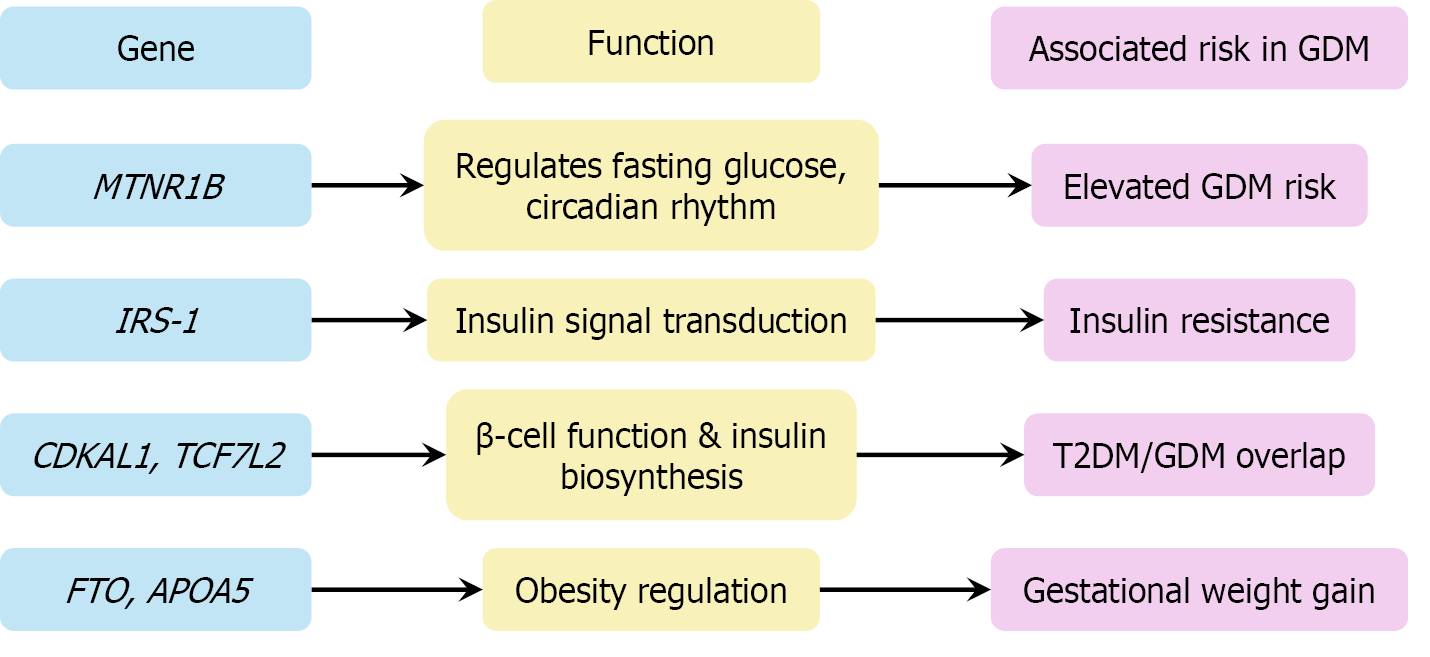
Genetic and epigenetic risk: Genetic predisposition and epigenetic changes contribute to GDM susceptibility and may drive intergenerational metabolic risk transmission. Genetic susceptibility loci such as TCF7 L2, MTNR1B, and CDKAL1 are strongly associated with GDM and T2DM. Polygenic risk scores have demonstrated predictive value for GDM, particularly when integrated with clinical or metabolic risk factors[9,20]. Epigenetic modifications—including placental DNA methylation, altered microRNA (miRNA) expression, and exosome-mediated signaling—may influence both maternal metabolic adaptations and fetal programming, contributing to intergenerational transmission of metabolic risk[29,32].
Summary: A comprehensive risk stratification model for GDM should incorporate three key domains: (1) Non-modifiable factors: Maternal age, genetic predisposition, ethnicity, and obstetric history; (2) Modifiable factors: Pre-pregnancy BMI, physical activity, dietary quality, and micronutrient status; and (3) Molecular and epigenetic markers: Genetic polymor
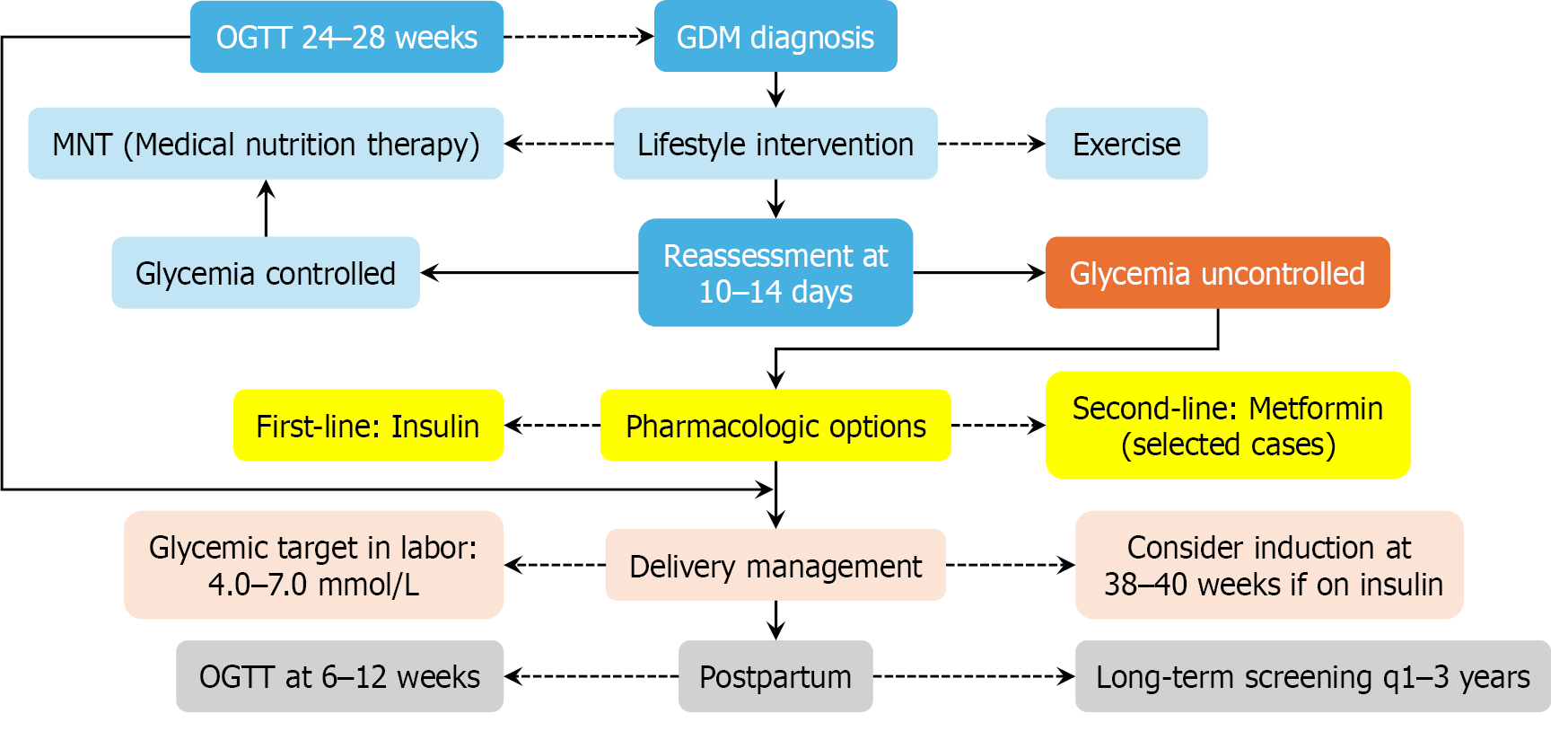
To support clinical implementation, a simplified algorithm depicting the diagnostic and therapeutic workflow for GDM is presented in Figure 3.
Lifestyle and nutritional therapy: Lifestyle intervention is the first-line treatment for GDM. Medical nutrition therapy, individualized to caloric and metabolic needs, generally includes: Carbohydrates: 40%-50% of daily intake (≥ 175 g/day); Fats: 20%-35%, favoring unsaturated sources; and Proteins: 10%-35%, derived from lean sources. Approximately 70%-85% of GDM cases are successfully managed through dietary modification and exercise[22,40]. Moderate physical activity—such as 30 minutes per day of walking or resistance training—improves insulin sensitivity and glycemic control. Post
Glucose monitoring and glycemic targets: Blood glucose monitoring guides therapy adjustments and predicts pregnancy outcomes. ADA 2024 recommended targets[22,24] are shown in Table 3. Monitoring modalities include: Self-monitoring of blood glucose; Clinic-based testing; Continuous glucose monitoring (promising for type 1 diabetes and those under evaluation for GDM[21]; and Pharmacologic therapies (if lifestyle measures fail to achieve target glycemia within 10-14 days, then pharmacologic treatment is initiated).
| Time point | Target glucose (mg/dL) |
| Fasting | < 95 |
| 1 hour postprandial | < 140 |
| 2 hours postprandial | < 120 |
First-line therapy: Insulin remains the gold standard for efficacy and fetal safety. Oral alternatives include metformin (preferred due to lower neonatal hypoglycemia rates compared to glyburide) and glyburide (crosses the placenta; associated with higher risk of neonatal hypoglycemia). Emerging concerns exist over the long-term effects of metformin, with evidence suggesting increased BMI in offspring at 7-9 years of age[32]. Experimental therapies (e.g., GLP-1 receptor agonists and DPP-4 inhibitors) are under investigation for postpartum T2DM prevention[1], but are not approved for patients during pregnancy.
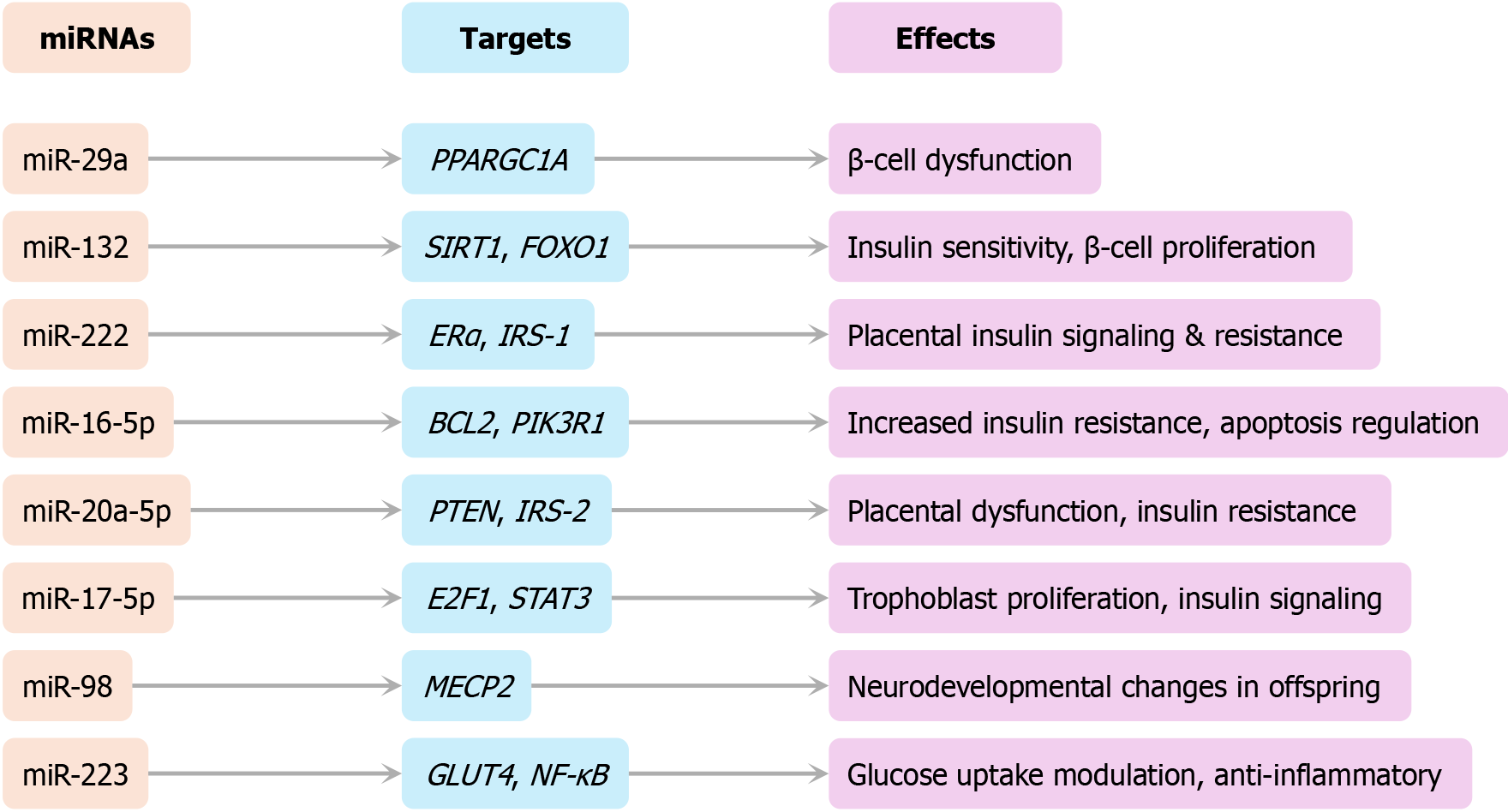
Recent advances and controversies: Key developments in GDM management include distinct strategies for early GDM (< 20 weeks) due to low reproducibility of early OGTT results[21]. IADPSG diagnostic controversy includes increased detection but no clear neonatal outcome benefit[15]. Biomarker-based prediction efforts include: (1) Proteins: SHBG, FABP4, GDF-15, and adiponectin; (2) MiRNAs: MiR-222 and miR-223; and (3) Insulin resistance indices: HOMA-IR and metabolomic subtypes[7,9]. Precision medicine models for GDM risk prediction and tailored care are actively being evaluated[21,28].
Intrapartum and postpartum management: Intrapartum care includes: (1) Hourly glucose monitoring; (2) Maintaining blood glucose between 4.0 and 7.0 mmol/L; and (3) Using insulin or glucose infusions as required[38]. Postpartum care includes: (1) Discontinuing insulin unless hyperglycemia persists; (2) Administering a 75-g OGTT at 6-12 weeks post
Patient education and health system considerations: Effective patient communication is essential for adherence and outcomes. Identified barriers include: (1) Cultural mismatches in dietary guidance; (2) Overmedicalization of pregnancy; and (3) Fragmented multidisciplinary care pathways[37]. Proposed solutions include: (1) Telehealth-based dietary and glycemic counseling; (2) One-day comprehensive GDM clinics; and (3) Multilingual education and structured behavior-change interventions[12,41].
GDM is increasingly recognized not merely as a transient metabolic adaptation of pregnancy but as a complex, polygenic disorder with epigenetic regulation. While it shares numerous susceptibility loci with T2DM, GDM also involves pregnancy-specific genetic and regulatory mechanisms.
Genetic susceptibility: Many genetic loci implicated in GDM are also linked to T2DM, underscoring a shared patho
These findings position GDM within a broader metabolic continuum that overlaps with T2DM, while also reflecting unique gestational influences, particularly involving placental signaling. The functional implications of key GDM-associated genetic loci are summarized in Figure 4, highlighting the overlap with type 2 diabetes and the mechanistic relevance of insulin signaling, β-cell function, and metabolic regulation.
Epigenetic reprogramming: Maternal hyperglycemia induces stable, tissue-specific DNA methylation changes in both maternal and fetal compartments. Placental targets include HOOK2, RDH12, COPS8, and PIK3R5—genes linked to nutrient sensing and metabolic signaling. Fetal genes include: MEST, NR3C1, and TNFRSF1B—associated with glucocorticoid response and inflammation. These epigenetic alterations persist into cord blood and have been associated with increased insulin resistance and dysregulated lipid metabolism in offspring[23,42]. Furthermore, oxidative stress and low-grade inflammation observed in GDM may amplify these epigenetic lesions, enhancing the risk of long-term meta
MiRNAs: MiRNAs serve as post-transcriptional regulators and show dysregulated expression profiles in GDM. Down
Metabolomics: Metabolomic profiling has identified several biomarkers elevated in GDM: (1) 2-Hydroxybutyrate, an indicator of insulin resistance and oxidative stress; and (2) Choline, which plays a role in hepatic lipid metabolism and fetal neurodevelopment. Animal and human studies suggest that maternal diet and GDM exposure modulate epigenetic marks in key regulators (e.g., adiponectin, leptin, and PPARG), predisposing offspring to long-term metabolic disorders[9].
These molecular and epigenetic alterations provide a mechanistic bridge between maternal GDM exposure and long-term offspring outcomes. A conceptual summary of this gene-to-phenotype axis is illustrated in Figure 6.
Translational barriers: Despite the promise of omics-based biomarkers in GDM risk stratification and prediction, major translational barriers remain: (1) Lack of population-specific standardization of cut-off thresholds; (2) Absence of clinical validation in prospective settings; and (3) Cost, accessibility, and technical feasibility in routine obstetric care. To bridge the gap from bench to bedside, future efforts must focus on multicenter prospective trials and the development of har
GDM is increasingly recognized not merely as a transient pregnancy complication but as a sentinel event that predicts long-term metabolic and cardiovascular morbidity. Suboptimal glycemic control during pregnancy poses serious risks to both maternal and neonatal outcomes, with sequelae that extend well beyond delivery.
Maternal complications: Inadequately managed GDM is associated with increased risks of preeclampsia, preterm labor, excessive gestational weight gain, cesarean delivery, and postpartum hemorrhage[24,30].
The most consequential long-term complication for mothers is T2DM: Women with prior GDM have an approximately 10-fold increased risk of developing T2DM. It was estimated that 30%-50% will convert to T2DM within 5-10 years postpartum, with conversion risk modulated by ethnicity, residual β-cell function, and lifestyle factors[24,30]. Beyond metabolic disease, GDM is also an independent risk factor for cardiovascular complications, including hypertension, coronary artery disease, stroke, and congestive heart failure[44].
Emerging evidence suggests that maternal diabetes may induce lasting alterations in fetal cardiometabolic pro
Fetal and neonatal complications: Intrauterine exposure to maternal hyperglycemia profoundly disrupts fetal metabolic homeostasis. Key perinatal complications include: Macrosomia (15%-45% prevalence); Neonatal hypoglycemia; Respira
Macrosomia—defined as birth weight > 4000 g—is among the most consistent neonatal consequences and is associated with an increased risk of labor complications and surgical delivery. Neonatal hypoglycemia typically presents within hours after birth due to persistent fetal hyperinsulinemia, a compensatory response to transplacental glucose overload. If prolonged, it may impair neurodevelopment.
Long-term offspring outcomes: Exposure to GDM in utero predisposes offspring to a spectrum of adverse long-term outcomes, including childhood and adolescent obesity, impaired glucose tolerance, early-onset T2DM, dyslipidemia and hypertension, and premature cardiovascular disease[46].
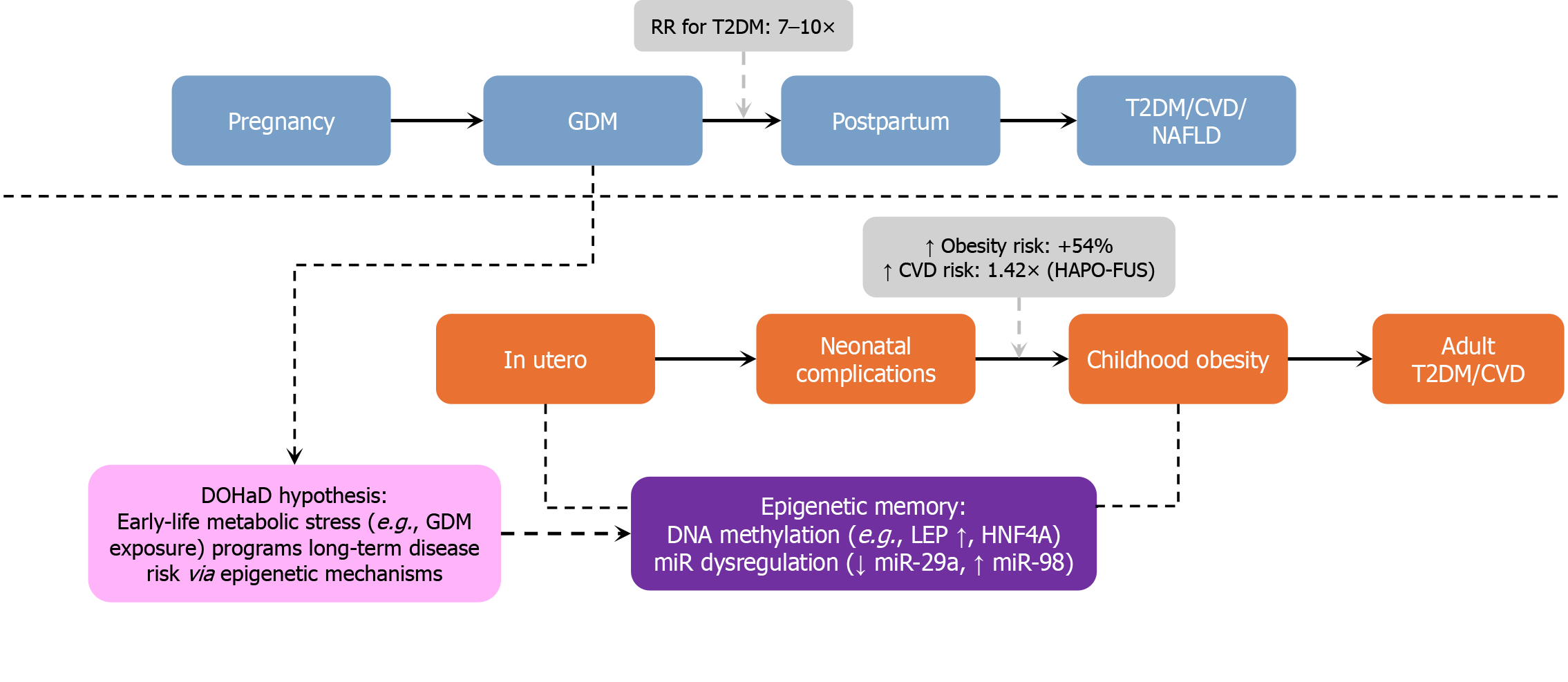
These observations support the Developmental Origins of Health and Disease (DOHaD) paradigm, in which intrau
Key supporting evidence includes: HAPO-FUS (10-14 years) and EPOCH (12-15 years) cohorts show increased central adiposity, impaired glucose tolerance, and insulin resistance among GDM-exposed offspring[46,47]. A meta-analysis of 32 studies (over 3.3 million offspring) confirmed that in utero exposure to GDM significantly increases the risk of obesity (odds ratio [OR] = 1.57), type 2 diabetes (OR = 4.5), and multiple components of metabolic syndrome, including elevated blood pressure, BMI, triglycerides, and LDL cholesterol levels[48]. Danish national registry data report an approximately 29% increase in early adulthood cardiovascular events in GDM-exposed individuals[49].
GDM has evolved from a clinical obstetric issue into a pressing global public health concern. Its far-reaching conse
Global burden and rising prevalence: The global prevalence of GDM continues to escalate, driven by rising maternal age, obesity, and sedentary lifestyles. In South and Southeast Asia, GDM is now a major contributor to maternal and perinatal morbidity, potentially reversing progress in reducing stillbirths and congenital anomalies. South Asia bears some of the world's highest stillbirth rates—up to 25.15 per 1000 Live births—a burden exacerbated by undiagnosed or poorly managed GDM[50]. In many low- and middle-income countries, GDM surveillance is inadequate, with limited population-based prevalence data, low screening coverage, and fragmented care delivery systems[14,51]. These realities emphasize the need to integrate GDM into national maternal health frameworks, particularly in resource-limited contexts where the potential for intervention is greatest.
Cardiometabolic sequelae and chronic disease prevention: GDM is a powerful predictor of future chronic disease in both mothers and offspring. It is associated with a 7-10-fold increased risk of developing T2DM within 5-10 years postpartum, and elevated lifetime risks of stroke, myocardial infarction, heart failure, and metabolic syndrome[22,44].
Importance of structured long-term follow-up: Guidelines have recommended a 75-g OGTT at 4-12 weeks postpartum, and regular metabolic screening every 1-3 years, stratified by individual risk level[6]. Furthermore, GDM exposure in utero is now firmly associated with intergenerational risk transmission. Offspring of mothers with GDM face increased likelihood of obesity, insulin resistance, and early-onset T2DM[14].
Policy recommendations and preventive strategies: To translate the evidence into action, Table 4 summarizes multilevel strategies recommended across the GDM continuum, from preconception to postpartum care.
| Level | Strategic action |
| Preconception | Promote weight optimization and screen high-risk women[2] |
| Antenatal | Implement universal OGTT screening in high-burden regions[14] |
| Postpartum | Ensure OGTT follow-up, breastfeeding support, and metabolic surveillance[44] |
| Health system | Harmonize diagnostic criteria and integrate GDM into chronic disease frameworks[6] |
Data gaps and health equity considerations: Gaps in diagnostic standardization and surveillance impede global policy efforts. Within Europe, national GDM prevalence ranges from 1.8% to 66.1%, reflecting variability in screening strategies and diagnostic thresholds[6]. Vulnerable populations—including women with low socioeconomic status, ethnic mino
Practical barriers to postpartum follow-up: Despite the well-established risks of T2DM and cardiovascular disease following GDM, postpartum follow-up and long-term preventive care remain grossly inadequate in many healthcare systems—especially in low-resource or underserved populations. Qualitative evidence from Nagraj et al[52] highlights key barriers at both the patient and provider levels. Women often lack awareness of the long-term consequences of GDM and do not perceive the need for continued monitoring unless symptomatic. Meanwhile, healthcare providers cite fragmented referral systems, insufficient integration between obstetric and primary care, and limited provider training as persistent obstacles to continuity of care.
Sinha et al[53] further identified logistical and psychosocial barriers, including lack of childcare, transportation difficulties, postpartum exhaustion, and ambiguous provider communication. Importantly, the study also recognized several facilitators that could be leveraged to improve adherence—such as strong provider recommendations, automated reminder systems, culturally tailored education, and pre-scheduled follow-up appointments.
These qualitative insights are corroborated by large-scale retrospective data. In a 2023 analysis by D’Amico et al[54], less than 20% of women with a history of GDM received diabetes-related care in primary care settings within six months postpartum, and only one-third completed the standard six-week postpartum visit. The study found that breakdowns in structured referral pathways contributed significantly to care gaps.
Collectively, these findings underscore the urgent need for integrated care models that bridge obstetric and primary care, particularly for at-risk populations. Strategies such as telehealth follow-up, community health worker engagement, electronic referral tracking, and patient-centered education may help close the implementation gap and mitigate the long-term cardiometabolic burden associated with GDM.
GDM exerts profound effects on fetal development, encompassing immediate perinatal complications as well as long-term cardiometabolic consequences. These outcomes are primarily mediated by maternal hyperglycemia and resultant fetal hyperinsulinemia, with growing evidence pointing to epigenetic mechanisms that program disease risk in later life.
Macrosomia and neonatal morbidity: Macrosomia is a hallmark feature of GDM, observed in approximately 15%-45% of affected pregnancies. A systematic review by Mistry et al[50] found that 6 out of 8 studies confirmed a strong association between GDM and macrosomia, with incidence rates as high as 28%. This overgrowth is primarily driven by fetal hyperinsulinemia and is associated with a higher risk of LGA infants and birth trauma, including shoulder dystocia. GDM also increases the likelihood of neonatal hypoglycemia, RDS, and admission to neonatal intensive care units (NICU). Even in treated pregnancies, these risks persist[16,26,30]. Interestingly, the Treatment of Booking Gestational Diabetes Mellitus pilot trial found that although early GDM treatment reduced the incidence of LGA births, it was paradoxically associated with an increased rate of NICU admissions, primarily due to a higher prevalence of small-for-gestational-age (SGA) neonates[16].
Congenital anomalies: Although GDM is typically diagnosed after the period of organogenesis, it is associated with an increased risk of congenital anomalies, likely due to undiagnosed preexisting insulin resistance and β-cell dysfunction.
A large United States national cohort (n > 29 million) found that GDM was linked to a 28% increase in overall congenital anomaly risk (relative risk [RR] = 1.28)[51]. Specific associations include: Cyanotic congenital heart disease (RR = 1.50); Hypospadias (RR = 1.29); Cleft lip/palate (RR = 1.28-1.40); Down syndrome (RR = 1.38); and Spina bifida (RR = 1.13).
These findings suggest that GDM, even when diagnosed later in pregnancy, may reflect a latent pathophysiological state with developmental consequences.
Stillbirth and growth restriction: GDM contributes not only to fetal overgrowth but also to intrauterine growth restriction and stillbirth. In one study, the stillbirth rate in the GDM group was 4.8% compared to 0% in the control group (P = 0.02)[50]. These adverse outcomes may result from placental vasculopathy, chronic fetal acidosis, and subclinical maternal inflammation. This dual risk of overgrowth and restriction reflects the heterogeneous and dynamic nature of GDM’s impact on fetal development.
Long-term cardiometabolic programming: In utero exposure to maternal hyperglycemia has enduring effects on the offspring’s metabolic health. Findings from the HAPO-FUS and EPOCH cohorts show that GDM-exposed children are at significantly increased risk for obesity (increased by 54%), insulin resistance (increased by 50%), and cardiovascular disease (1.42-fold increase in adulthood)[11]. In addition, studies by Plows et al[25] and Alejandro et al[30] reinforce these findings, reporting increased rates of childhood obesity, impaired glucose tolerance, and early-onset T2DM.
Epigenetic and predictive markers: GDM-associated fetal programming is mediated, in part, by epigenetic changes, including: DNA methylation in insulin-related genes; Altered expression of adipokines such as leptin and adiponectin; and Mitochondrial stress in placental and fetal hepatic tissue[11]. These molecular changes may underpin long-term disease susceptibility and highlight opportunities for early prediction and intervention. Emerging clinical tools, such as fetal abdominal circumference assessment at 24-28 weeks, may help personalize glycemic targets and reduce fetal overgrowth risk. However, standardized guidelines for their application are still lacking[36].
GDM is increasingly recognized as a sentinel marker of future cardiometabolic disease for both mother and child. Far from being a transient gestational complication, GDM signals heightened lifetime risk, necessitating proactive long-term surveillance and preventive care strategies. Figure 7 depicts the longitudinal trajectory of metabolic and cardiovascular risk associated with GDM, which affects both maternal and offspring health across the lifespan. This figure underscores the intergenerational consequences of in utero hyperglycemia and the urgency of early intervention.
Pregnancy-associated maternal complications: Short-term maternal complications of GDM include: (1) Preeclampsia, which occurs in over 20% of GDM pregnancies[50]; (2) Cesarean delivery: Data are mixed, with some studies showing higher rates[50]; and (3) Gestational hypertension and mental health: No significant differences between early vs deferred treatment groups in the TOBOGM trial[17].
Postpartum progression to T2DM: GDM is a strong predictor of future T2DM: (1) Incidence: 30%-60% conversion within 5-10 years[30,36]; (2) Relative risk: 7-10-fold increase[11,25]; (3) Pooled meta-analysis: RR = 8.92 (95%CI: 7.84-10.14)[39]; (4) Ethnic disparities: Asian women convert at higher rates, even with similar BMI[36]; and (5) Screening: OGTT remains more sensitive than FPG for detecting early β-cell dysfunction[36].
Long-term cardiometabolic morbidity: Even in the absence of T2DM, women with a history of GDM are at increased risk of: (1) Hypertension, dyslipidemia, and CVD; (2) Non-alcoholic fatty liver disease (NAFLD); and (3) Chronic kidney disease and diabetic retinopathy[11].
Postpartum obesity and intergenerational risk: Prepregnancy obesity and GDM have synergistic effects: Women with both conditions are more likely to retain excess postpartum weight and develop hypertension, T2DM, and CVD[4]. Their offspring face higher risks of central adiposity, insulin resistance, and early-onset metabolic disease[46,47,55].
Offspring outcomes and developmental programming: Offspring of GDM pregnancies exhibit: (1) +54% risk of childhood obesity; (2) A 1.42-fold increased risk of cardiovascular events by age 35-40[11]; and (3) Persistent risks inde
Neurodevelopmental and epigenetic sequelae: Beyond cardiometabolic risk, GDM-exposed offspring may be at increased risk of ADHD (RR = 2.00) and autism spectrum disorder (RR = 1.48), especially in cases involving maternal obesity or pharmacologic treatment[32]. Epigenetic changes in genes like HNF4A and RREB1 are observed in cord blood, supporting intergenerational transmission of metabolic dysfunction[36].
Protective role of lactation and missed preventive opportunities: Lactation offers significant metabolic benefits, yet postpartum care gaps limit its long-term preventive potential. Lactation lowers maternal T2DM risk by 30%-50% and supports β-cell function[36]. Breastfeeding mothers show 40% greater β-cell preservation four years postpartum. However, many women report a discontinuity of care after delivery[37], representing a critical missed opportunity for long-term prevention and education.
A consolidated overview of maternal and offspring risks is provided in Table 5. GDM is not merely a pregnancy-related diagnosis—it is a powerful early warning signal of lifelong cardiometabolic risk. Comprehensive postpartum care should incorporate structured long-term follow-up, patient education, lactation support, and early pediatric inter
| Risk domain | Maternal impact | Offspring impact |
| T2DM | 7- to 10-fold increased risk within 10 years | Increased insulin resistance |
| Cardiovascular disease | A 2-fold increased risk (even without T2DM) | Early-onset CVD, hypertension |
| Weight trajectory | Higher postpartum weight retention | Higher adiposity and central fat distribution |
| Epigenetic programming | Persistent inflammation, β-cell stress | Altered methylation of insulin signaling genes |
| Preventive leverage | Breastfeeding, early screening | Healthy lifestyle education, pediatric monitoring |
GDM has evolved from a narrowly defined obstetric condition into a major public health concern at the intersection of global epidemics of obesity, T2DM, and cardiovascular disease. Emerging evidence supports a paradigm shift toward longitudinal, individualized, and prevention-focused models of care.
Clinical gaps and implementation challenges: Several barriers impede optimal GDM management in routine clinical practice: (1) Heterogeneity of interventions: Variation in nutritional counseling, physical activity protocols, and lack of cultural tailoring limits the generalizability and scalability of effective interventions[5]; (2) Inconsistent screening: Diagnostic discrepancies between IADPSG, ADA, and WHO guidelines lead to variable case detection and inconsistent care planning[15]; and (3) Poor postpartum follow-up: Despite clear guidelines, the rate of postpartum OGTT completion remains unacceptably low—particularly among underserved populations and in low-resource settings[41].
Priority clinical insights: Evidence-based strategies targeting modifiable risks and surveillance can guide more effective, individualized GDM care: (1) Integrating regular physical activity into routine prenatal care: Enhancing insulin sen
System-level and policy implications: The rising global burden of GDM reflects broader shifts in demography and health behavior. Key drivers include delayed childbearing, rapid urbanization, and increasing maternal obesity preva
Summary of clinical recommendations: Based on the reviewed evidence, Table 6 consolidates key clinical recommendations across care domains, providing a framework for comprehensive GDM management from screening to postpartum follow-up.
| Domain | Clinical recommendation |
| Prenatal care | Integrate structured lifestyle guidance into routine visits |
| Screening | Employ early risk-based screening using BMI, age, ethnicity, and history |
| Postpartum | Ensure OGTT at 6-12 weeks and initiate annual T2DM risk surveillance |
| Education | Improve cultural and psychosocial sensitivity in patient communication |
| Health systems | Standardize GDM diagnosis globally; expand access and continuity of care |
GDM care must transcend glycemic control and embrace a life-course strategy targeting both maternal and intergene
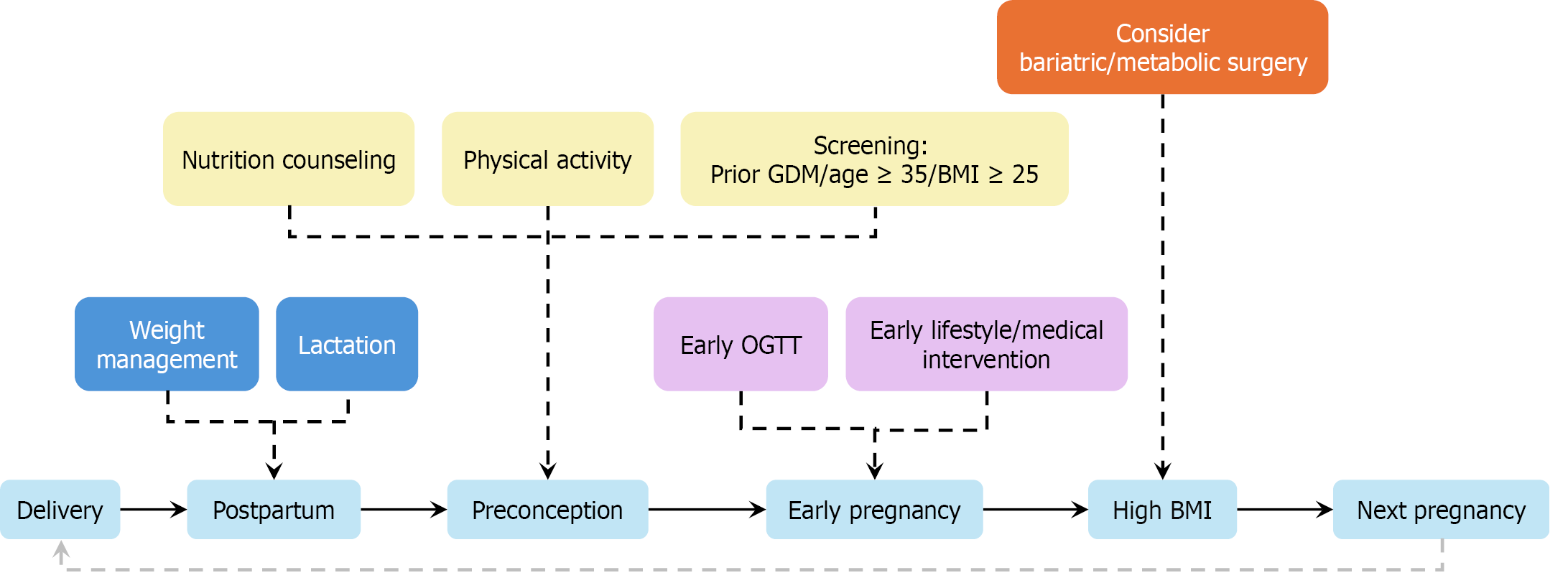
Recurrent GDM affects up to 84% of high-risk women, amplifying cumulative maternal-fetal complications and entrenching intergenerational metabolic vulnerability[10]. Preventive strategies must be stratified by risk phenotype and initiated early—ideally in the postpartum or preconception periods. To visualize the temporally stratified approach for GDM recurrence prevention, Figure 8 presents a comprehensive timeline incorporating postpartum, preconception, and early pregnancy strategies based on individual risk profiles.
Current limitations: Most trials have failed to prevent GDM recurrence due to: (1) Delayed intervention (initiated mid-pregnancy, after key metabolic windows); (2) Absence of phenotype-based stratification (e.g., insulin-resistant vs lean GDM); and (3) Lack of pharmacologic efficacy: Neither metformin nor probiotics have consistently reduced recurrence[10].
Proven preventive strategies: Timely lifestyle or surgical interventions can substantially reduce GDM recurrence, particularly in high-risk women: (1) Early lifestyle intervention: In the RADIEL trial (Finland), lifestyle intervention was initiated before 20 weeks in women with prior GDM and/or obesity. The outcome was a significant reduction in recurrence (adjusted P = 0.044)[10]; (2) Prepregnancy bariatric surgery: This was associated with a 77% reduction in GDM recurrence (OR = 0.23; 95%CI: 0.15-0.36); and (3) Considerations: Micronutrient deficiency and SGA risk should be considered[10].
Clinical implementation matrix: To inform evidence-based prevention strategies, Table 7 summarizes the effectiveness of current GDM recurrence interventions based on clinical evidence.
| Strategy | Effectiveness | Comments |
| Preconception lifestyle | ++ | Strongest evidence, cost-effective |
| Mid-late pregnancy lifestyle | - | Typically implemented too late for meaningful metabolic benefit |
| Metformin | - | No proven preventive effect |
| Probiotics | - | Promising but inconsistent |
| Bariatric surgery | +++ | Consider for BMI ≥ 35 kg/m² and failed lifestyle efforts |
Recommendations: Targeted, phenotype-informed strategies and early action are essential to prevent GDM recurrence and optimize maternal-fetal outcomes: (1) Identifying high-risk women early (prior GDM, age ≥ 35 years, and BMI ≥ 25 kg/m²); (2) Initiating lifestyle intervention preconceptionally; (3) Stratifying by GDM phenotype to distinguish insulin-resistant subtypes; (4) Considering surgery: For BMI ≥ 35 kg/m² with recurrent GDM; and (5) Promoting multidisciplinary care: Obstetrics, endocrinology, and primary care teams should be linked.
Controversies and evidence gaps: Unresolved issues in screening, pharmacotherapy, biomarkers, and global criteria hinder consensus and limit progress in GDM management.
Early screening uncertainty: Although early-onset GDM (< 20 weeks) comprises up to 70% of cases, current FPG thresholds (e.g., ≥ 5.1 mmol/L) have low specificity and predictive value[17]. Early treatment (e.g., in TOBOGM trial) reduced LGA but increased SGA births, raising safety concerns: (1) Long-term safety of pharmacologic therapy: Metformin is associated with a higher childhood BMI at 7-9 years[32,36]. Glyburide is Associated with increased neonatal hypoglycemia risk due to placental transfer; (2) Lack of standardized precision tools: Biomarkers such as SHBG, FABP4, GDF-15, and miRNAs (e.g., miR-222 and miR-223) are promising but remain unincorporated in clinical practice due to validation and standardization challenges[9,20,26]; (3) Ambiguous management of early-onset GDM: Clear protocols are currently lacking. Risk may be higher, but treatment thresholds are extrapolated from later gestation data[21]; and (4) Inconsistent global diagnostic criteria: Prevalence varies from < 2% to > 60% across regions due to differing criteria (e.g., IADPSG, WHO, and ADA), impeding unified care delivery[13,18].
Future directions: To address the above gaps, researchers should conduct stratified RCTs by GDM phenotype, follow metformin-exposed offspring for long term, validate predictive biomarkers across ethnicities and assays, develop region-specific, standardized diagnostic models, and integrate ethics and health equity into national policy reform.
Summary: To synthesize the core findings and ongoing debates in GDM research and management, we provide a concise tabular summary (Table 8) outlining current clinical standards, key controversies, and recommended directions for future investigation across three major domains: Screening timing, pharmacologic treatment, and epigenetics. GDM recurrence is both foreseeable and preventable—yet remains under-addressed in standard clinical care. Early, risk-adapted inter
| Dimension | Current standard | Controversies | Recommended research |
| Screening timing | 24-28 weeks OGTT | Early screening utility unclear | TOBOGM and similar RCTs |
| Pharmacologic treatment | Insulin; Metformin (optional) | Long-term safety of metformin | Childhood follow-up & mechanistic studies |
| Epigenetics | Not routinely applied | Inconsistent miRNA & methylation data | Multicenter validation & standardization |
GDM is an escalating global health concern with wide-reaching implications for maternal, fetal, and intergenerational metabolic health. While adoption of the IADPSG criteria has enhanced screening standardization and early detection, it has also raised important concerns regarding overdiagnosis, healthcare burden, and uncertain long-term benefit[15,21]. Clinical trials such as ACHOIS and TOBOGM demonstrate that timely intervention reduces neonatal complications like macrosomia and respiratory distress syndrome; however, improvements in maternal outcomes, particularly hypertensive disorders, remain modest[17]. The maternal burden of GDM extends far beyond pregnancy. Women with prior GDM face a 7-10-fold increased risk of progressing to T2DM, especially those of Asian descent or with overweight/obesity[30,36]. Simultaneously, fetal exposure to maternal hyperglycemia initiates epigenetic reprogramming and metabolic dysregulation, increasing long-term risk for obesity, insulin resistance, and cardiometabolic disease[11,32]. A paradigm shift is urgently needed from pregnancy-limited care to a life-course prevention framework. This entails: (1) Establishing inte
GDM exemplifies the critical link between reproductive and metabolic health, providing a unique opportunity to implement precision medicine strategies that interrupt the cycle of chronic disease transmission across generations. To fully harness this potential, clinical care models must evolve—driven by translational research, harmonized international guidelines, and a firm commitment to equity, prevention, and personalized care.
| 1. | Modzelewski R, Stefanowicz-Rutkowska MM, Matuszewski W, Bandurska-Stankiewicz EM. Gestational Diabetes Mellitus-Recent Literature Review. J Clin Med. 2022;11:5736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 78] [Reference Citation Analysis (0)] |
| 2. | Yong HY, Mohd Shariff Z, Mohd Yusof BN, Rejali Z, Tee YYS, Bindels J, van der Beek EM. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep. 2020;10:8486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 3. | Choudhury AA, Devi Rajeswari V. Gestational diabetes mellitus - A metabolic and reproductive disorder. Biomed Pharmacother. 2021;143:112183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 174] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 4. | Brunner K, Linder T, Klaritsch P, Tura A, Windsperger K, Göbl C. The Impact of Overweight and Obesity on Pregnancy: A Narrative Review of Physiological Consequences, Risks and Challenges in Prenatal Care, and Early Intervention Strategies. Curr Diab Rep. 2025;25:30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Laredo-Aguilera JA, Gallardo-Bravo M, Rabanales-Sotos JA, Cobo-Cuenca AI, Carmona-Torres JM. Physical Activity Programs during Pregnancy Are Effective for the Control of Gestational Diabetes Mellitus. Int J Environ Res Public Health. 2020;17:6151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 6. | Paulo MS, Abdo NM, Bettencourt-Silva R, Al-Rifai RH. Gestational Diabetes Mellitus in Europe: A Systematic Review and Meta-Analysis of Prevalence Studies. Front Endocrinol (Lausanne). 2021;12:691033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 7. | Francis EC, Powe CE, Lowe WL Jr, White SL, Scholtens DM, Yang J, Zhu Y, Zhang C, Hivert MF, Kwak SH, Sweeting A; ADA/EASD PMDI. Refining the diagnosis of gestational diabetes mellitus: a systematic review and meta-analysis. Commun Med (Lond). 2023;3:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 8. | Zhang Y, Xiao CM, Zhang Y, Chen Q, Zhang XQ, Li XF, Shao RY, Gao YM. Factors Associated with Gestational Diabetes Mellitus: A Meta-Analysis. J Diabetes Res. 2021;2021:6692695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 9. | Lu W, Hu C. Molecular biomarkers for gestational diabetes mellitus and postpartum diabetes. Chin Med J (Engl). 2022;135:1940-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Egan AM, Enninga EAL, Alrahmani L, Weaver AL, Sarras MP, Ruano R. Recurrent Gestational Diabetes Mellitus: A Narrative Review and Single-Center Experience. J Clin Med. 2021;10:569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Wicklow B, Retnakaran R. Gestational Diabetes Mellitus and Its Implications across the Life Span. Diabetes Metab J. 2023;47:333-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 12. | Juan J, Yang H. Prevalence, Prevention, and Lifestyle Intervention of Gestational Diabetes Mellitus in China. Int J Environ Res Public Health. 2020;17:9517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 13. | Wang H, Li N, Chivese T, Werfalli M, Sun H, Yuen L, Hoegfeldt CA, Elise Powe C, Immanuel J, Karuranga S, Divakar H, Levitt N, Li C, Simmons D, Yang X; IDF Diabetes Atlas Committee Hyperglycaemia in Pregnancy Special Interest Group. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group's Criteria. Diabetes Res Clin Pract. 2022;183:109050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 546] [Article Influence: 182.0] [Reference Citation Analysis (0)] |
| 14. | Su FL, Lu MC, Yu SC, Yang CP, Yang CC, Tseng ST, Yan YH. Increasing trend in the prevalence of gestational diabetes mellitus in Taiwan. J Diabetes Investig. 2021;12:2080-2088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Saeedi M, Cao Y, Fadl H, Gustafson H, Simmons D. Increasing prevalence of gestational diabetes mellitus when implementing the IADPSG criteria: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2021;172:108642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 125] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 16. | Raets L, Beunen K, Benhalima K. Screening for Gestational Diabetes Mellitus in Early Pregnancy: What Is the Evidence? J Clin Med. 2021;10:1257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Simmons D, Immanuel J, Hague WM, Teede H, Nolan CJ, Peek MJ, Flack JR, McLean M, Wong V, Hibbert E, Kautzky-Willer A, Harreiter J, Backman H, Gianatti E, Sweeting A, Mohan V, Enticott J, Cheung NW; TOBOGM Research Group. Treatment of Gestational Diabetes Mellitus Diagnosed Early in Pregnancy. N Engl J Med. 2023;388:2132-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 159] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 18. | Dłuski DF, Ruszała M, Rudziński G, Pożarowska K, Brzuszkiewicz K, Leszczyńska-Gorzelak B. Evolution of Gestational Diabetes Mellitus across Continents in 21st Century. Int J Environ Res Public Health. 2022;19:15804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Amiri FN, Faramarzi M, Bakhtiari A, Omidvar S. Risk Factors for Gestational Diabetes Mellitus: A Case-Control Study. Am J Lifestyle Med. 2021;15:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Powe CE, Hivert MF, Udler MS. Defining Heterogeneity Among Women With Gestational Diabetes Mellitus. Diabetes. 2020;69:2064-2074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Sweeting A, Wong J, Murphy HR, Ross GP. A Clinical Update on Gestational Diabetes Mellitus. Endocr Rev. 2022;43:763-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 397] [Article Influence: 132.3] [Reference Citation Analysis (1)] |
| 22. | American Diabetes Association Professional Practice Committee. 15. Management of Diabetes in Pregnancy: Standards of Care in Diabetes-2024. Diabetes Care. 2024;47:S282-S294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 98] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 23. | Dalfrà MG, Burlina S, Del Vescovo GG, Lapolla A. Genetics and Epigenetics: New Insight on Gestational Diabetes Mellitus. Front Endocrinol (Lausanne). 2020;11:602477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 24. | Lende M, Rijhsinghani A. Gestational Diabetes: Overview with Emphasis on Medical Management. Int J Environ Res Public Health. 2020;17:9573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 25. | Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The Pathophysiology of Gestational Diabetes Mellitus. Int J Mol Sci. 2018;19:3342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 817] [Cited by in RCA: 1020] [Article Influence: 145.7] [Reference Citation Analysis (0)] |
| 26. | Sharma AK, Singh S, Singh H, Mahajan D, Kolli P, Mandadapu G, Kumar B, Kumar D, Kumar S, Jena MK. Deep Insight of the Pathophysiology of Gestational Diabetes Mellitus. Cells. 2022;11:2672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 27. | Ruszała M, Pilszyk A, Niebrzydowska M, Kimber-Trojnar Ż, Trojnar M, Leszczyńska-Gorzelak B. Novel Biomolecules in the Pathogenesis of Gestational Diabetes Mellitus 2.0. Int J Mol Sci. 2022;23:4364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Pan X, Jin X, Wang J, Hu Q, Dai B. Placenta inflammation is closely associated with gestational diabetes mellitus. Am J Transl Res. 2021;13:4068-4079. [PubMed] |
| 29. | Dłuski DF, Wolińska E, Skrzypczak M. Epigenetic Changes in Gestational Diabetes Mellitus. Int J Mol Sci. 2021;22:7649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 30. | Alejandro EU, Mamerto TP, Chung G, Villavieja A, Gaus NL, Morgan E, Pineda-Cortel MRB. Gestational Diabetes Mellitus: A Harbinger of the Vicious Cycle of Diabetes. Int J Mol Sci. 2020;21:5003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 169] [Article Influence: 33.8] [Reference Citation Analysis (1)] |
| 31. | Elliott A, Walters RK, Pirinen M, Kurki M, Junna N, Goldstein JI, Reeve MP, Siirtola H, Lemmelä SM, Turley P, Lahtela E, Mehtonen J, Reis K, Elnahas AG, Reigo A, Palta P, Esko T, Mägi R; Estonian Biobank Research Team; FinnGen, Palotie A, Daly MJ, Widén E. Distinct and shared genetic architectures of gestational diabetes mellitus and type 2 diabetes. Nat Genet. 2024;56:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 40] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 32. | Chu AHY, Godfrey KM. Gestational Diabetes Mellitus and Developmental Programming. Ann Nutr Metab. 2020;76 Suppl 3:4-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 33. | Saucedo R, Ortega-Camarillo C, Ferreira-Hermosillo A, Díaz-Velázquez MF, Meixueiro-Calderón C, Valencia-Ortega J. Role of Oxidative Stress and Inflammation in Gestational Diabetes Mellitus. Antioxidants (Basel). 2023;12:1812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 63] [Reference Citation Analysis (0)] |
| 34. | Carrasco-Wong I, Moller A, Giachini FR, Lima VV, Toledo F, Stojanova J, Sobrevia L, San Martín S. Placental structure in gestational diabetes mellitus. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 35. | Zhou T, Du S, Sun D, Li X, Heianza Y, Hu G, Sun L, Pei X, Shang X, Qi L. Prevalence and Trends in Gestational Diabetes Mellitus Among Women in the United States, 2006-2017: A Population-Based Study. Front Endocrinol (Lausanne). 2022;13:868094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 36. | Moon JH, Jang HC. Gestational Diabetes Mellitus: Diagnostic Approaches and Maternal-Offspring Complications. Diabetes Metab J. 2022;46:3-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 152] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 37. | Craig L, Sims R, Glasziou P, Thomas R. Women's experiences of a diagnosis of gestational diabetes mellitus: a systematic review. BMC Pregnancy Childbirth. 2020;20:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 38. | Mensah GP, Ten Ham-Baloyi W, van Rooyen DRM, Jardien-Baboo S. Guidelines for the nursing management of gestational diabetes mellitus: An integrative literature review. Nurs Open. 2020;7:78-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | You H, Hu J, Liu Y, Luo B, Lei A. Risk of type 2 diabetes mellitus after gestational diabetes mellitus: A systematic review & meta-analysis. Indian J Med Res. 2021;154:62-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 40. | Rasmussen L, Poulsen CW, Kampmann U, Smedegaard SB, Ovesen PG, Fuglsang J. Diet and Healthy Lifestyle in the Management of Gestational Diabetes Mellitus. Nutrients. 2020;12:3050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 41. | Liu K, Clarke GS, Grieger JA. A low-intensity nutrition intervention targeting triglycerides in gestational diabetes: a feasibility RCT. J Clin Endocrinol Metab. 2025;dgaf291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 42. | Valencia-Ortega J, Saucedo R, Sánchez-Rodríguez MA, Cruz-Durán JG, Martínez EGR. Epigenetic Alterations Related to Gestational Diabetes Mellitus. Int J Mol Sci. 2021;22:9462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 43. | Chen Z, Gong L, Zhang P, Li Y, Liu B, Zhang L, Zhuang J, Xiao D. Epigenetic Down-Regulation of Sirt 1 via DNA Methylation and Oxidative Stress Signaling Contributes to the Gestational Diabetes Mellitus-Induced Fetal Programming of Heart Ischemia-Sensitive Phenotype in Late Life. Int J Biol Sci. 2019;15:1240-1251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 44. | Xie W, Wang Y, Xiao S, Qiu L, Yu Y, Zhang Z. Association of gestational diabetes mellitus with overall and type specific cardiovascular and cerebrovascular diseases: systematic review and meta-analysis. BMJ. 2022;378:e070244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 45. | Agarwal P, Morriseau TS, Kereliuk SM, Doucette CA, Wicklow BA, Dolinsky VW. Maternal obesity, diabetes during pregnancy and epigenetic mechanisms that influence the developmental origins of cardiometabolic disease in the offspring. Crit Rev Clin Lab Sci. 2018;55:71-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 140] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 46. | Scholtens DM, Kuang A, Lowe LP, Hamilton J, Lawrence JM, Lebenthal Y, Brickman WJ, Clayton P, Ma RC, McCance D, Tam WH, Catalano PM, Linder B, Dyer AR, Lowe WL Jr, Metzger BE; HAPO Follow-up Study Cooperative Research Group; HAPO Follow-Up Study Cooperative Research Group. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): Maternal Glycemia and Childhood Glucose Metabolism. Diabetes Care. 2019;42:381-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 193] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 47. | Hockett CW, Harrall KK, Moore BF, Starling AP, Bellatorre A, Sauder KA, Perng W, Scherzinger A, Garg K, Ringham BM, Glueck DH, Dabelea D. Persistent effects of in utero overnutrition on offspring adiposity: the Exploring Perinatal Outcomes among Children (EPOCH) study. Diabetologia. 2019;62:2017-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 48. | Guan J, Qiu J, Li L, Fu M, Zhang M, Wu Y, Xu Y, Ding H, Gao Q. A meta-analysis of adverse offspring health outcomes in patients with gestational diabetes mellitus. Diabetes Obes Metab. 2025;27:3555-3567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 49. | Yu Y, Arah OA, Liew Z, Cnattingius S, Olsen J, Sørensen HT, Qin G, Li J. Maternal diabetes during pregnancy and early onset of cardiovascular disease in offspring: population based cohort study with 40 years of follow-up. BMJ. 2019;367:l6398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 191] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 50. | Mistry SK, Das Gupta R, Alam S, Kaur K, Shamim AA, Puthussery S. Gestational diabetes mellitus (GDM) and adverse pregnancy outcome in South Asia: A systematic review. Endocrinol Diabetes Metab. 2021;4:e00285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 51. | Wu Y, Liu B, Sun Y, Du Y, Santillan MK, Santillan DA, Snetselaar LG, Bao W. Association of Maternal Prepregnancy Diabetes and Gestational Diabetes Mellitus With Congenital Anomalies of the Newborn. Diabetes Care. 2020;43:2983-2990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 52. | Nagraj S, Hinton L, Praveen D, Kennedy S, Norton R, Hirst J. Women's and healthcare providers' perceptions of long-term complications associated with hypertension and diabetes in pregnancy: a qualitative study. BJOG. 2019;126 Suppl 4:34-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Sinha DD, Williams RC, Hollar LN, Lucas HR, Johnson-Javois B, Miller HB, Stoermer A, Colditz GA, James AS, Herrick CJ. Barriers and facilitators to diabetes screening and prevention after a pregnancy complicated by gestational diabetes. PLoS One. 2022;17:e0277330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 54. | D'Amico R, Dalmacy D, Akinduro JA, Hyer M, Thung S, Mao S, Fareed N, Bose-Brill S. Patterns of Postpartum Primary Care Follow-up and Diabetes-Related Care After Diagnosis of Gestational Diabetes. JAMA Netw Open. 2023;6:e2254765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 55. | Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ. 2017;356:j1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 747] [Article Influence: 93.4] [Reference Citation Analysis (0)] |