Copyright
©The Author(s) 2025.
World J Clin Cases. Oct 16, 2025; 13(29): 111096
Published online Oct 16, 2025. doi: 10.12998/wjcc.v13.i29.111096
Published online Oct 16, 2025. doi: 10.12998/wjcc.v13.i29.111096
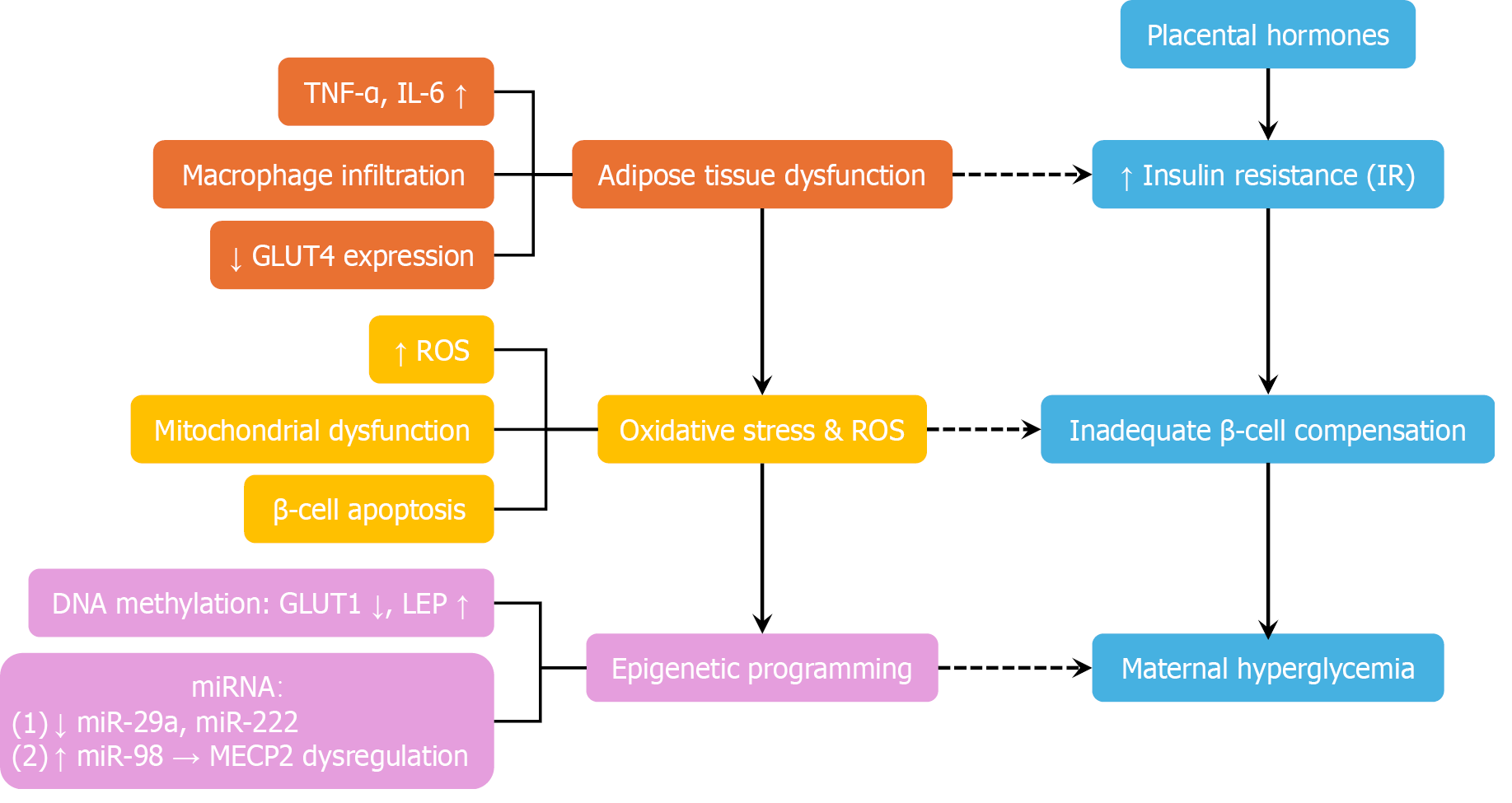
Figure 2 Pathophysiology of gestational diabetes mellitus: A multi-pathway model.
This diagram illustrates the multifactorial pathophysiology of gestational diabetes mellitus. Placental hormones—including human placental lactogen, estrogen, and placental growth hormone—progressively induce insulin resistance to support fetal nutrient supply. In susceptible women with insufficient β-cell compensation, this adaptive mechanism fails, resulting in maternal hyperglycemia. Simultaneously, adipose tissue dysfunction—characterized by macrophage infiltration, elevated proinflammatory cytokines [e.g., tumour necrosis factor alpha (TNF-α) and interleukin-6 (IL-6)], and decreased GLUT4 expression—further aggravates insulin resistance. Oxidative stress and mitochondrial dysfunction contribute to β-cell apoptosis and impaired insulin secretion. Epigenetic modifications, such as altered DNA methylation [e.g., decreased GLUT1 and increased leptin (LEP)] and dysregulated mRNAs (e.g., downregulated miR-29a and upregulated miR-98), are implicated in fetal metabolic programming and may perpetuate transgenerational cardiometabolic risk. Blue blocks - core insulin-glucose axis: Placental hormones, increased insulin resistance, inadequate β-cell compensation, and maternal hyperglycemia. Orange blocks - adipose inflammation and insulin resistance: Elevated TNF-α, elevated IL-6, reduced GLUT4 expression, and adipose tissue dysfunction. Yellow blocks - oxidative stress and mitochondrial dysfunction: Increased reactive oxygen species and β-cell apoptosis. Purple blocks - epigenetic mechanisms: Altered DNA methylation (e.g., decreased GLUT1 and increased LEP) and dysregulated miRNAs. TNF-α: Tumor necrosis factor alpha; IL-6: Interleukin-6; GLUT4: Glucose transporter type 4; ROS: Reactive oxygen species; LEP: Leptin; miR: MicroRNA.
- Citation: Luo QJ, Ni Q. Life-course management of gestational diabetes mellitus: A narrative review. World J Clin Cases 2025; 13(29): 111096
- URL: https://www.wjgnet.com/2307-8960/full/v13/i29/111096.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i29.111096