Published online Jun 19, 2025. doi: 10.5498/wjp.v15.i6.105751
Revised: April 2, 2025
Accepted: April 27, 2025
Published online: June 19, 2025
Processing time: 110 Days and 5.4 Hours
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder currently lacking effective therapeutic interventions. Pathological hallmarks of AD include intracellular neurofibrillary tangles (NFTs) and extracellular amyloid beta (Aβ) plaques. Neuroplastin 65 (NP65), highly expressed in the brain, has been previously shown to mitigate cognitive impairments and decrease Aβ plaques in the AD mouse model, suggesting that NP65 is involved in AD neuropathology. However, direct evidence linking NP65 expression to AD pathogenesis in human brain remains absent.
To quantify NP65 isoform expression gradients across distinct neuroanatomical regions in the healthy brain and investigate the alterations of NP65 expression in the AD brain.
Immunohistochemical, immunofluorescent and western blot analyses were used to investigate NP65 expression in 19 postmortem brains (AD = 10, controls = 9). Double immunostaining with 6E10 and or phosphorylated-micro
In controls, NP65 was highly expressed in a wide-range of brain areas. AD cases showed significantly increased NP65 immunoreactivity across multiple brain regions, including the frontal and temporal cortex, hippocampus, and cerebellum, compared to controls. Western blot analysis consistently confirmed significantly elevated NP65 expression in the hippocampus of AD patients relative to controls. Double immunostaining demonstrated partial colocalization of NP65 with NFTs and Aβ plaques in AD brain tissue.
Our findings demonstrate a significant increase of NP65 protein, which colocalizes with NFTs and Aβ plaques in AD brains, providing direct evidence supporting a critical role of NP65 expression in the neuropathological mechanisms of this disease.
Core Tip: Alzheimer’s disease (AD) is a progressive neurodegenerative disorder, with pathological hallmarks including intracellular neurofibrillary tangles and extracellular amyloid beta (Aβ) plaques. Synaptic membrane glycoprotein neuroplastin 65 (NP65) has been previously shown to mitigate cognitive impairments and decrease Aβ plaques in the AD mouse model. However, direct evidence linking NP65 expression to AD pathology in human brain remains absent. The present study shows that NP65 is highly expressed in a wide-range of brain areas and is significantly increased in AD cases. In addition, we found colocalization of NP65 with neurofibrillary tangles and Aβ plaques in AD brain. Thus, this study provides direct evidence of NP65 involvement in AD pathology.
- Citation: Zheng YN, Wang Y, Chen L, Xu LZ, Zhang L, Wang JL, Liu J, Zhang QL, Yuan QL. Increased expression of the neuroplastin 65 protein is involved in neurofibrillary tangles and amyloid beta plaques in Alzheimer’s disease. World J Psychiatry 2025; 15(6): 105751
- URL: https://www.wjgnet.com/2220-3206/full/v15/i6/105751.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i6.105751
Alzheimer’s disease (AD) is a neurodegenerative disease that is clinically characterized by progressive cognitive deficits. The number of AD patients will rise to 82 million by 2050, and become a global crisis[1]. Clinically, the principal manifestations of AD patients are the progressive decline of memory, language and mental disorders, and the progressive weakening of cognition, including temporal and spatial judgment, and finally dementia[2]. Whole brain atrophy occurs, which is characterized by a smaller volume and thinner cortex, all of which are accompanied by amyloid beta (Aβ) protein deposits throughout the brain neuropil. Other histopathological features of AD include neurofibrillary tangles (NFTs) caused by hyperphosphorylation of tau protein within neurons, diffuse inflammatory necrotic foci, and loss of neurons [3]. Available therapies, such as cholinesterase blockers and N-methyl-D-aspartate receptor agonists, can only relieve symptoms but cannot block the progress of AD. Recently, several amyloid-binding antibodies have been approved for patient treatment in the United States (e.g., lecanemab, donanemab, and aducanumab), and clinical trials are underway on other Aβ binding antibodies and immunization protocols[4]. Therefore, targeting the study of Aβ production, deposition and degradation is helpful in developing new therapeutic methods [5]. Interestingly, several studies have shown that certain cell adhesion molecules (CAMs), such as L1 and neuroplastin (NP) 65, are involved in the formation of Aβ plaques in APP/PS1 mice (a commonly used AD model of cerebral amyloidosis)[6-8], indicating a potential role of CAMs in the pathogenesis of AD.
The NP isoforms NP65 and NP55 are encoded by a single gene (NPTN in humans, Nptn in rodents) and result from alternative splicing of the mRNA [9]. Both isoforms are single-spanned transmembrane proteins belonging to the immunoglobulin superfamily with two (NP55) and three (NP65) Ig domains, respectively. NP65 and NP55 are solely distinguished by the absence of the Ig1 module in NP55 but the presence in NP65[9]. In rodents, NP55 is widely localized in various tissues, whereas NP65 is mainly expressed in the brain and retina[10-13]. In the human brain, NP65 is strongly expressed in the cerebral cortex, striatum, hippocampus, hypothalamus and cerebellum[10,14]. Notably, NP55 is the major isoform in the rodent cerebellum, but NP65 is the major isoform in the human cerebellum[10,15]. Functionally, NP65, as a synaptic glycoprotein, is related to synaptic plasticity, and involved in neuropsychiatric diseases[12,16]. Our serial reports have shown that NP65 is related to anxiety in mice, as NP65 deficient mice clearly display anxiety-like behaviors, in several paradigms including the hole-board test and marble burying test, open-field test and light-dark transition test[17-19]. Additionally, a single nucleotide polymorphism in the NP locus is reported to be associated with cortical thickness and intellectual ability in adolescents[20]. Furthermore, our previous studies have shown that NP65 deficiency improves learning and memory in wild-type mice[17,18]. Therefore, these reports suggest a role for NP65 in neuropsychiatric disease.
Our recent studies have shown that NP65 deficiency alleviates cognitive deficits and decreases Aβ plaque load and neuroinflammation in APP/PS1 mice[8]. Given that NP65 is highly expressed in the cerebral cortex, hippocampus and cerebellum in the human brain, it is necessary to explore whether NP65 is involved in the pathogenesis of AD. Previously, NP65 immunoreactivity was only investigated in the hippocampus of AD patients, showing an increase in NP65 immunoreactivity at the early stage but not the late stage of AD when compared to controls[21]. Therefore, it is necessary to further determine whether NP65 is involved in the pathogenesis of AD. Here, we thoroughly investigated the expression and distribution of NP65 in postmortem human brain and further investigated whether the expression of NP65 is altered in distinctive regions of the AD brain, such as the hippocampus, frontal and temporal cortex, and cerebellum. Interestingly, we found a significant increase in NP65 in a wide range of brain regions in AD and partial co-localization of NP65 with Aβ plaques and NFTs in the AD brain. These results provide direct evidence that NP65 is involved in the pathogenesis of AD, indicating a new potential therapeutic target for AD.
Human postmortem brains were obtained from the NeuroBioBank of Central South University, which receives willed body donations with consent[22]. This NeuroBioBank is supported by the Red Cross Society of China and provides cadavers for medical education and research. In total ten cases were included in the AD group, of which six cases were definitely diagnosed as AD according to their medical records, and the other four cases were recorded as dementia and clinically regarded as AD patients. Furthermore, using the specific 6E10 antibody against Aβ plaques and the phosphorylated-microtubule-associated protein tau (p-Tau) antibody against NFTs[3], all ten brains were confirmed as AD cases with NFTs and Aβ plaques (Supplementary Figures 1 and 2). Age-matched (controls: 72.33 ± 10.33 years, AD: 79 ± 7.85 years, t = -0.109, ns) cases of older patients who did not die of neurological diseases, including cancer and cardiovascular diseases, were included as controls and were also confirmed by the 6E10 and p-Tau antibodies to have negative results (Supplementary Table 1). The present study was approved by the Ethics Committee for Research and Education at Xiangya School of Medicine in 2020, approval No. 2020KT-37, in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
The brain was dissected within 15 hours after death. One half of the brain was freshly frozen at -80 °C for western blot or other molecular biology detection, while the other half was immersed in neutral formalin for 20 days for section staining. To prepare brain sections, the cerebral hemisphere and hippocampus were cut coronally into slices (1.0 cm thick); the cerebellum was sagittally cut into slices (1.0 cm thick); and the brainstem was cut horizontally into slices (0.5 cm thick). These brain slices were then placed in 15%, and 30% sucrose in 0.01 mol phosphate buffer (PBS) until they sank to the bottom at -4 °C. They were then cut into sections (20 μm thick) using a cryostat (CM 1950, Leica, Heidelberg, Germany). These sections were stored in a cryoprotectant at -20 °C before use.
The procedures for human brain sectioning were described previously and modified[23,24]. In brief, after being rinsed with PBS, the human brain sections were pretreated with 5.0% hydrogen peroxide in methanol for 30 minutes at room temperature and then washed with PBS to neutralize endogenous peroxidase activity. To expose the antigen, the sections were treated with 95% formic acid for 10 minutes (for the 6E10 antigen), or treated with 2 × saline sodium citrate buffer in a water bath at 65 °C for 60 minutes (for the NP65 antigen). The sections were then treated with 0.01M Tris-HCI for 10 minutes and immersed in a blocking solution (5% horse serum and 0.5% Triton X-100 in PBS) on a shaking rocker for 2 hours at room temperature. Finally, the sections were incubated with primary antibodies, including mouse anti-6E10 (1:1000, Abcam, United States), rabbit anti-p-Tau (1:1000, Abcam, United States) and goat anti-NP65 (1:200, R and D Systems, United States) at 4 °C overnight (Table 1). The sections were then incubated with biotinylated horse anti-goat secondary immunoglobulin G (IgG) or goat anti-mouse/rabbit IgG (1:200, Vector Laboratories, United States) for 1 hour and further with an avidin-biotin-horseradish peroxidase complex kit (1:200, Vector Laboratories, United States) for 2 hours at room temperature. Immunoreactivity was visualized using 0.03% hydrogen peroxide and 0.05% 3, 3’-diami
| Antibody | Manufacturer | Product code | Host species | Dilution |
| Htr3A | Abcam | ab13897 | Goat | 1:300 |
| Aβ | Novus | 2-62566 | Mouse | 1:500 |
| APP | Abcam | ab32136 | Rabbit | 1:300 |
| BACE-1 | Santa | AF10748 | Rabbit | 1:200 |
| p-Tau | Abcam | ab62639 | Mouse | 1:1000 |
| 594-conjugated affinipure anti-goat IgG | Jackson | 705-585-003 | Donkey | 1:500 |
| 488-conjugated affinipure donkey anti-rabbit IgG | Jackson | 711-545-152 | Donkey | 1:500 |
| 488-conjugated affinipure donkey anti-mouse IgG | Jackson | 715-545-150 | Donkey | 1:500 |
| Horse anti-mouse/rabbit/goat IgG antibody | Vector | BA-1300-2.2 | Horse | 1:500 |
| ABC-HRP kit | Vector | PK-6100 | - | 1:500 |
In addition, immunofluorescent staining of NP65 alone or with 6E10 or p-Tau (AT-8, a marker for NFTs) was performed according to a previous report[24]. For this staining, the sections were treated with a 95% sodium citrate solution (pH 6.0) in a water bath at 65 °C for 60 minutes and then cooled to room temperature. After being blocked with 7% donkey serum in 0.1% Triton X-100 for 3 hours at room temperature, the sections were incubated with primary antibodies, including goat-anti NP65 or antibody cocktails: NP65 with 6E10 or p-Tau overnight at 4 °C. After thoroughly washing with PBS, the sections were incubated in fluorescein isothiocyanate (FITC)-conjugated or Cy3-conjugated anti-goat or anti-mouse and anti-rabbit secondary IgG (1:1000, Jackson Immuno-Research Laboratories, United States) for 2 hours at room temperature, and counterstained with diamidino-phenyl-indole (Beyotime Biotechnology, Shanghai, China). Primary and secondary antibodies were diluted with 7% donkey serum in 0.1% Triton X-100 in 0.01 mol PBS. All sections were treated with 0.1% Sudan black to block autofluorescence before being covered with VECTASHIELD® antifading mounting media (Vector Laboratories, United States). A negative control was set up by omitting the primary antibody.
For semi-quantification of NP65 expression, two blind investigators performed the analysis of immunohistochemical and immunofluorescent staining, respectively. The images were captured under a 200 × or 400 × magnification using a microscope (Olympus FV1000, Tokyo, Japan) and semi-quantitatively analyzed with ImageJ software v1.51 (National Institutes of Health, Bethesda, MD, United States). The optical density (OD) of NP65 immunoreactivity was determined by subtracting the background of the designated region of interest (ROI) using ImageJ software v1.51 (National Institutes of Health, Bethesda, MD, United States). In immunohistochemical analysis, the upper 40 and lower 255 threshold were set to subtract the background of ROI; in immunofluorescence analysis, the upper 75 and lower 255 threshold were set to subtract the background of ROI. The averaged OD of 3 selected ROIs was used as the OD of each individual. Signal intensity per field was then calculated. The immunoreactivity data are derived from three cases with three slides of each individual.
For detecting levels of NP65 protein in the hippocampus from AD (n = 3) and controls (n = 3), the block of hippocampus (15-20 g) was taken out from the -80 °C refrigerator and rinsed with PBS. It was then cut into pieces. The cell membrane protein of the hippocampus was extracted using a membrane protein extraction kit (Beyotime, China), according to the manufacturer’s instructions. In brief, the tissues were lysed with reagent A with 1 mmol/L phenylmethylsulfonyl fluoride buffer in an ice bath for 10 minutes. They were then centrifuged at 14000 × g at 4 °C for 10 minutes and the supernatant was collected. The supernatant was further centrifuged at 700 × g at 4 °C for 30 minutes. The pellet was collected and dissolved with reagent B in an ice bath for 10 minutes. It was then centrifuged at 14000 × g at 4 °C for 5 minutes. The supernatant was the membrane protein and was collected for protein detection. After determining the protein concentration, 15 μg of protein samples were electrophoretically separated in 10% sodium dodecyl sulfate polyacrylamide gels and transferred to polyvinylidene fluoride membranes. These membranes were blocked with 5% bovine serum albumin in Tris-buffered saline containing 0.05% Tween-20 for 1 hour at room temperature. They were then incubated overnight at 4 °C with goat anti-NP65 (1:1000, R and D Systems, Minnesota, United States) and rabbit anti-glyceraldehyde-3-phosphate dehydrogenase (1:2000, Beyotime, Shanghai, China). The membranes were then washed and incubated with anti-rabbit IgG-horseradish peroxidase and anti-goat IgG-horseradish peroxidase (1:1000, Beyotime) for 2 hours at room temperature. Immunoreactive bands were visualized and images were analyzed quantitatively using ImageJ software (NIH, Bethesda, United States). Targeted protein levels were normalized to those of glyceraldehyde-3-phosphate dehydrogenase from two separate experiments.
Statistical analyses were carried out using SPSS 22.0 (IBM SPSS, Chicago, IL, United States) for Windows. All data were expressed as mean ± SEM. An unpaired Student’s t-test was used to evaluate the differences between two groups. Statistical significance was set at P < 0.05.
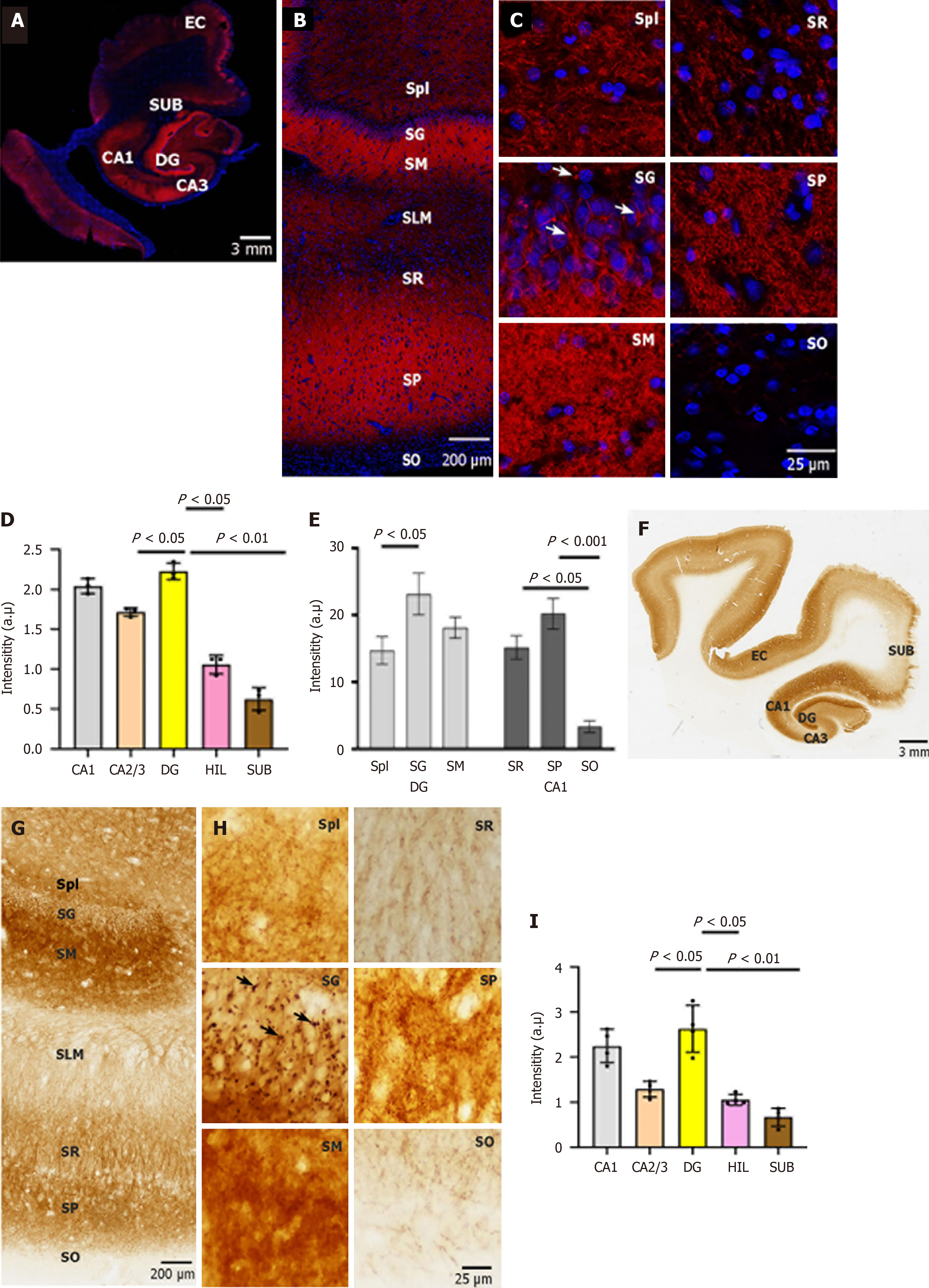
Although the distribution of NP65 in some regions of the human brain has been reported[10,14,21], detailed information on NP65 in the entire brain remains unclear. In the present study, we first investigated the distribution of NP65 in the aged human brain (from individuals who died of no neurological or psychiatric disorders), as controls for AD cases. In the hippocampal formation, using both immunohistochemistry and immunofluorescence staining, we found that NP65 expression was most abundant in the dentate gyrus (DG) and cornu ammonis (CA) 1 regions, while moderate in the CA2-3 regions of the hippocampus and the hilar part of the DG, as demonstrated by statistical analysis (Figure 1). This distribution is similar to that in the rodent hippocampus[12], whereas it is different to a previous report showing that CA1-CA4 exhibited a rather uniform NP65 immunostaining in the human hippocampus[10]. In the CA1, prominent NP65 staining was found in the stratum pyramidale, moderate staining in the stratum radiatum, and faint staining in the stratum oriens (Figure 1A-E). This distribution differs to that in the rodent brain, as it was reported that strong NP65 immunoreactivity was found in the stratum oriens and stratum radiatum of the rodent CA1[12]. In the DG, the most intense immunoreactivity was found in the stratum granulosum (SG) and stratum moleculare and less intense in the stratum plexiforme (Figure 1A-E), similar to previous reports in rodent and human brains[12,14]. At the cellular level, NP65 immunoreactivity was highly localized on the cell membranes of granular and pyramidal neurons and in the neuropil (Figure 1C and H). Strikingly, NP65 immunoreactive materials appeared as discrete knots in the cell membranes of granular neurons (Figure 1C and H indicated by arrow), strikingly similar to the NP55 immunoreactivity in Purkinje cell somata of the rat cerebellar cortex[15]. In the stratum pyramidale and the neuropil of the hippocampus, NP65 immunoreactivity showed punctate, plexus and laminar patterns in the CA1 region (Figure 1C and H).
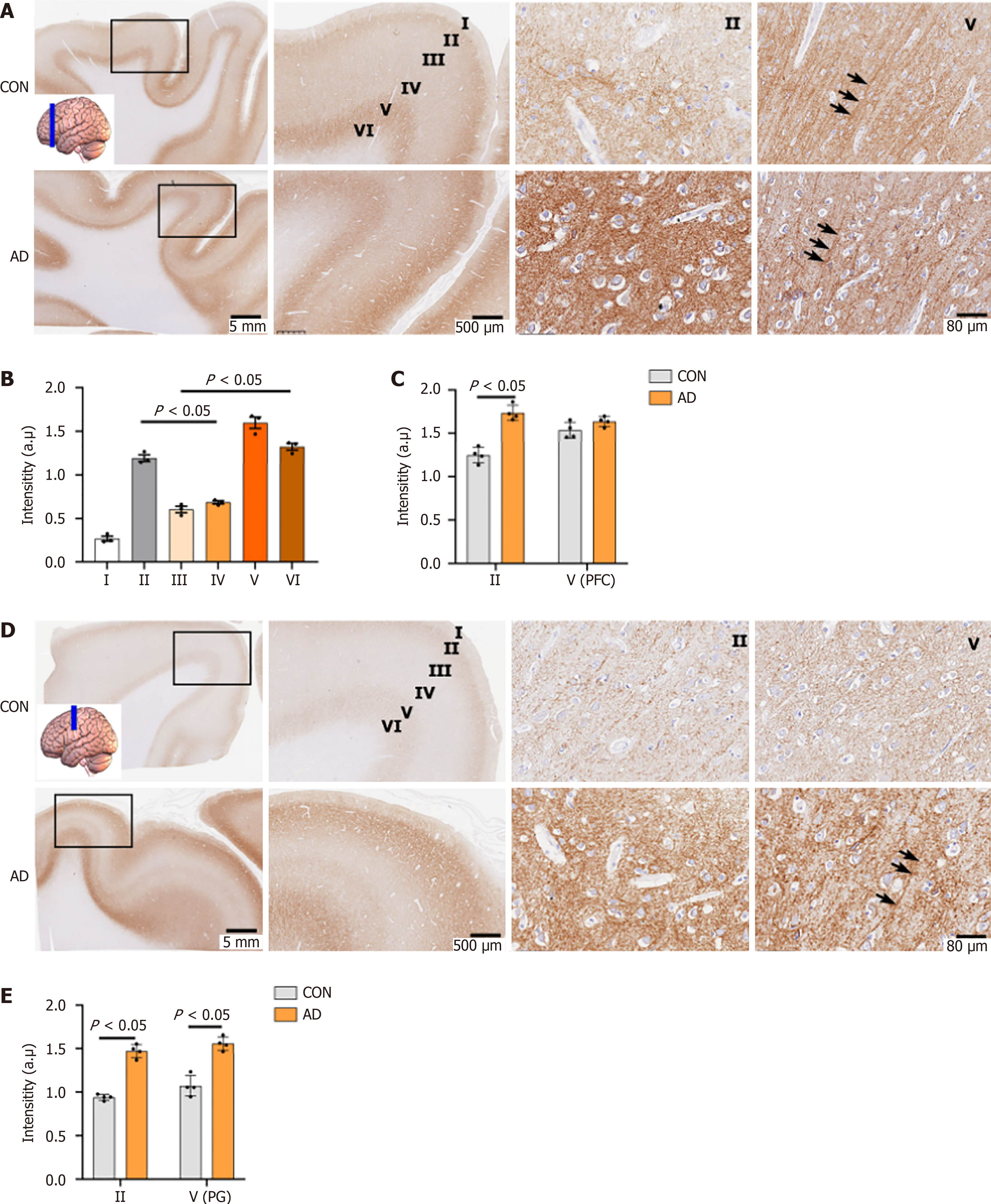
In the cerebral cortex, the overall distribution of NP65 was uneven and showed regional differences, inconsistent with one report showing no obvious regional differences[10]. With regard to the layers of the neocortex, the most intense immunoreactivity was found in layers II and V/VI (Figure 2), while other layers were moderately labelled (Figure 2A, B and D), consistent with the distribution pattern of NP65 in the rat neocortex[12]. In the dorsolateral frontal cortex, the prefrontal cortex (PFC) exhibited prominent immunostaining relative to the precentral gyrus (Figure 2A and D). Notably, prominent NP65 immunoreactivity was localized at the apical dendrites (indicated by an arrow in Figure 2A) and cell membranes of the pyramidal neurons in layer V of the PFC (Figure 2A). In addition, the white matter underlying the cerebral cortex showed no immunoreactivity (Figure 2A and D).
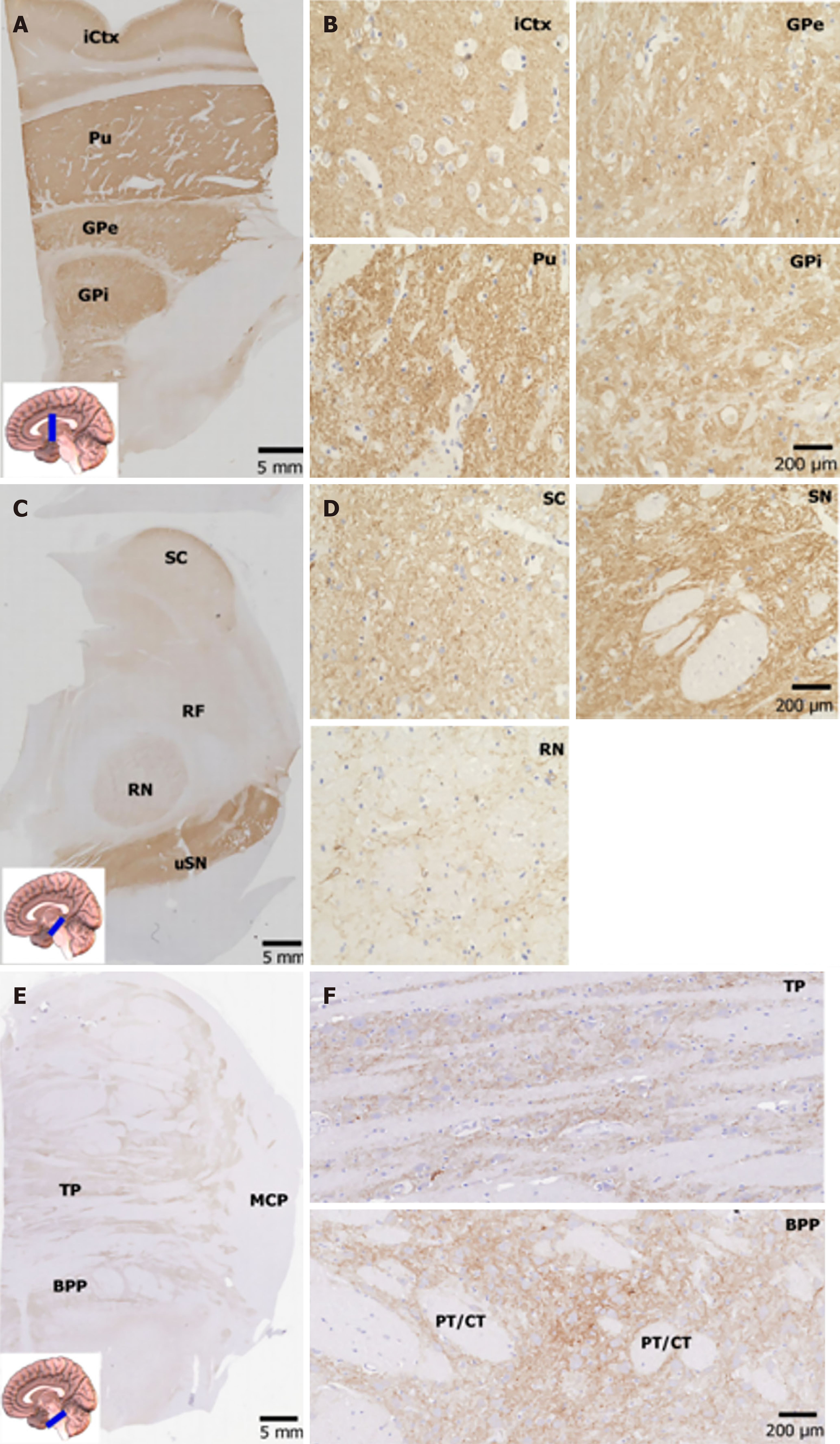
In the lentiform nucleus, the putamen and globus pallidus displayed intense NP65 immunoreactivity (Figure 3A and B), which differs greatly from the distribution pattern in the rat lentiform, showing strong staining in the putamen but faint in the globus pallidus[12]. The high-power view showed that NP65 immunoreactivity was strong and punctate in the putamen, while it formed more dense networks in the globus pallidus (Figure 3A and B). In the superior colliculus of the midbrain, prominent NP65 staining was present in the substantia nigra, moderate staining was present in the superior colliculus, and faint staining was present in the red nucleus and reticular formation. There was no staining in the crus cerebri (Figure 3C). The high-power view showed that NP65 immunostaining was strongly positive in punctate and fibers in the substantia nigra, while there were less intense positive fibers in the red nucleus (Figure 3D). The general NP65 immunoreactivity in the brain stem was obviously faint compared with that in the cerebral hemisphere (Figure 3A-F). In addition, in the basilar part of the pons, a few nerve fibers and the pontine nucleus were moderately stained, while the longitudinal pyramidal tract was devoid of any immunoreaction (Figure 3F).
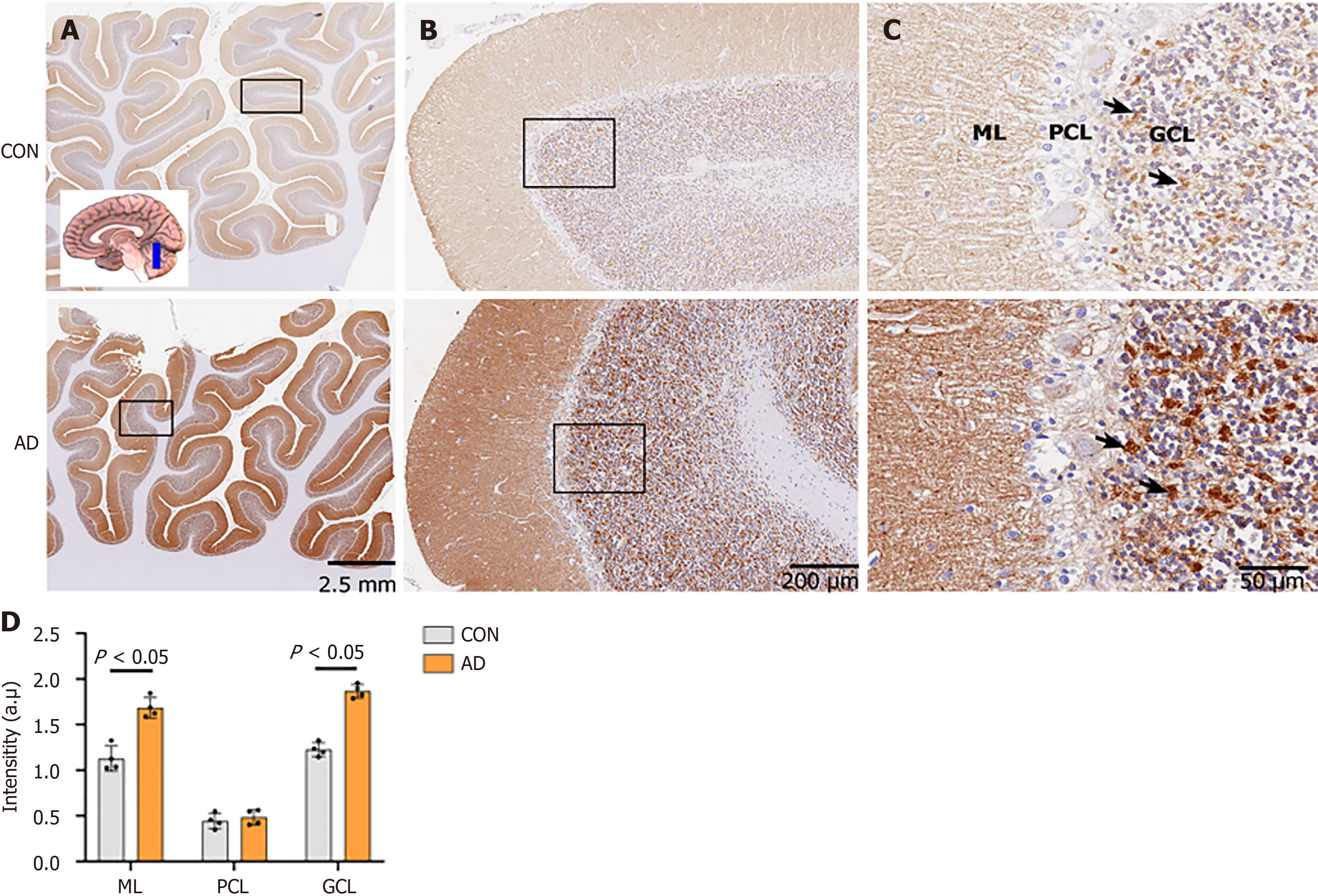
In the cerebellum, NP65 immunostaining was present in the cerebellar cortex, and absent in the medulla. Strikingly similar to a previous postmortem report[10], strong immunoreactivity was found in the granule cell layer (GCL) and the molecular layer (ML) but not in the Purkinje cell layer (PCL) (Figure 4A-C). This distribution is also similar to that of NP55 previously observed in the mouse cerebellum[15]. In the GCL, strong immunoreactivity appeared blocky or granular (indicated by an arrow in Figure 4C), seemingly representing synaptic glomeruli, as previously reported[15].
Exploring the immunohistochemical and immunofluorescent approaches, we observed that the expression levels of NP65 were significantly elevated in the hippocampus, temporal and frontal cortex, and cerebellum in the AD group compared to controls. However, the cellular localization of NP65 immunoreactive deposits in AD had not changed. Intracellular immunoreactivity, as previously reported[21], was not found.
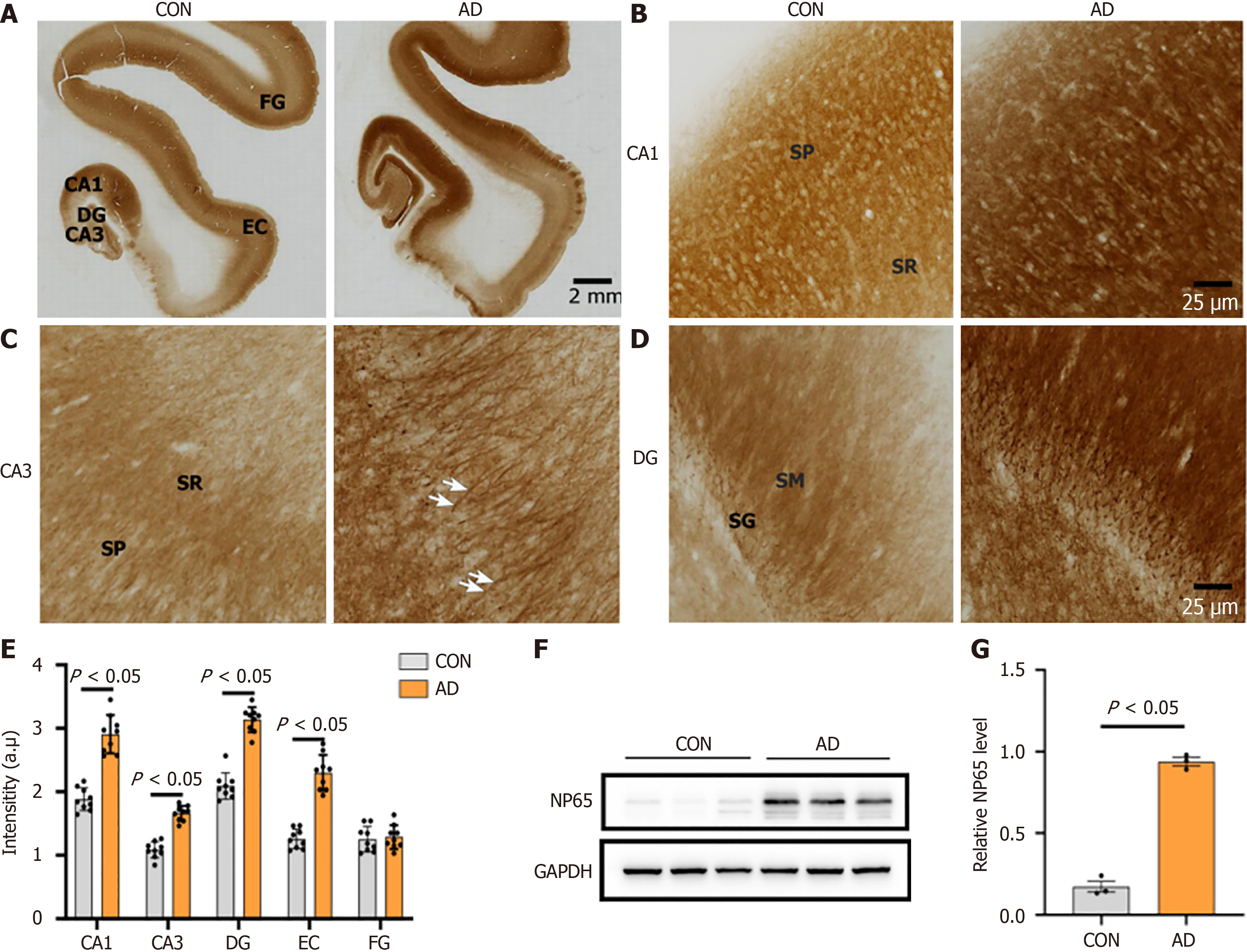
In the hippocampal formation, we found that NP65 immunoreactivity in the DG, CA1 and CA3 of the hippocampus in the AD group was significantly increased compared to the control group (Figure 5A-E), partially consistent with Ilic’s report showing increased NP65 expression at an early stage of AD[21]. Notably, the apical dendrites of the pyramidal cells in CA3 showed strongly positive immunoreactivity in AD cases (Figure 5C, indicated by an arrow). Moreover, western blot analysis was performed to validate the immunohistochemical staining results and showed a significant increase in NP65 protein in the AD hippocampus compared with the controls (Figure 5F and G).
In the inferior and medial temporal cortex, the entorhinal cortex (EC) of the para-hippocampal gyrus displayed higher immunoreactivity while the fusiform gyrus showed comparable immunoreactivity in the AD group compared with the controls (Figure 5A-E). In the dorsolateral-frontal cortex, NP65 immunoreactivity in layer II of the dorsolateral PFC and in layers II and V of the precentral gyrus was significantly increased in the AD group relative to the controls (Figure 2A and C-E). Note that strongly positive apical dendrites in layer V are more visible in AD than in controls (Figure 2A and D, indicated by an arrow).
In the cerebellar cortex, the ML and the GCL showed a significant increase in NP65 immunoreactivity, while the PCL showed unchanged immunoreactivity in the AD group when compared to the controls (Figure 4D). Strikingly, the GCL of the AD group showed more intense, and blocky or granular immunoreactivity compared to the controls (Figure 4C, indicated by an arrow). Taken together, the present results clearly show that NP65 expression is significantly increased in wide areas of the AD brain.
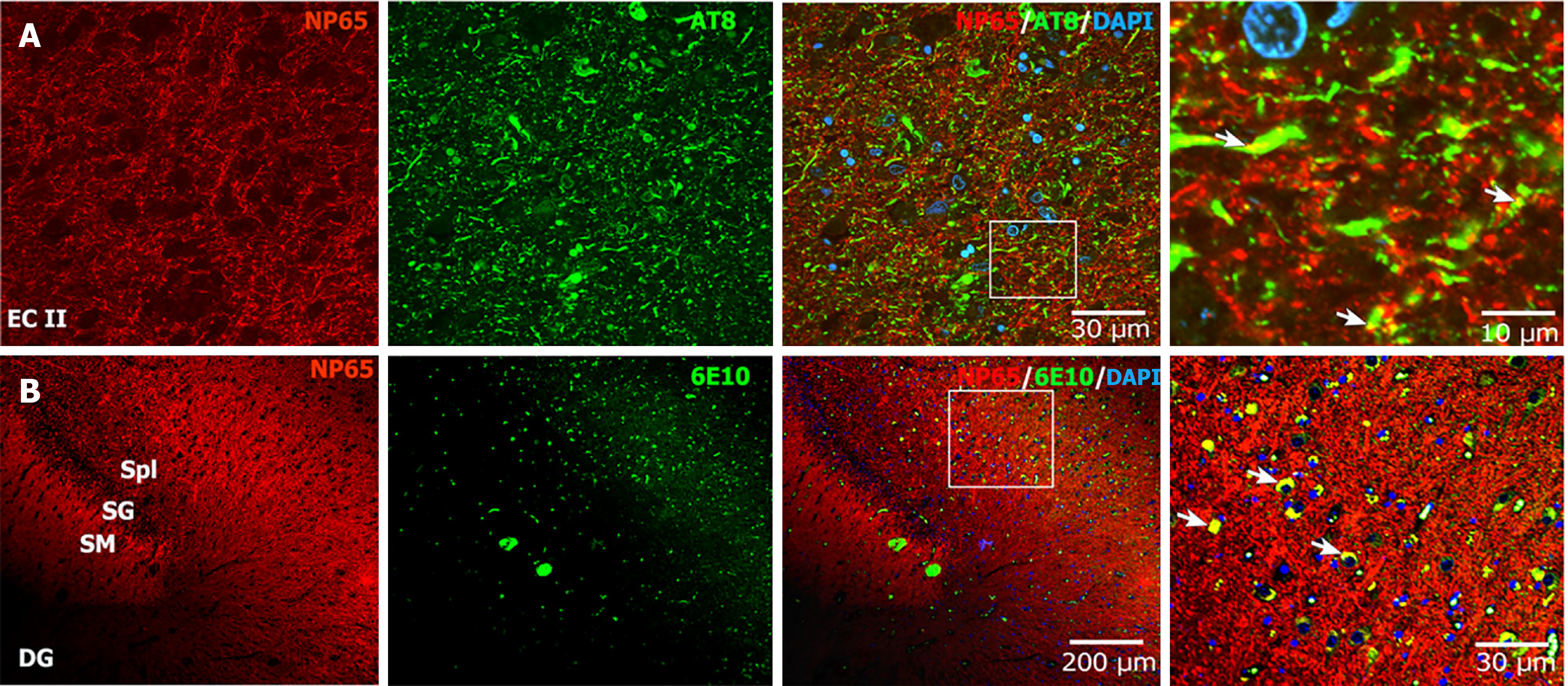
For Aβ and NFTs, which are the major neuropathological features of AD, we investigated whether NP65 is involved in Aβ and p-Tau pathology. Interestingly, double-immunofluorescent staining of NP65 with p-Tau (a marker for NFTs) showed that NP65 immunoreactive punctate structures partially colocalized with the NFTs in the EC of AD (Figure 6A). In addition, the double-immunofluorescent staining of NP65 with 6E10 (a marker for Aβ plaques) displayed that NP65 immunostaining also partially colocalized with diffuse and punctate Aβ plaques in the DG (Figure 6B, indicated by an arrow). Together, we show for the first time that NP65 partially colocalizes with NFTs and Aβ plaques in the AD human brain.
In this study, we showed that NP65 is significantly increased in a wide range of postmortem AD human brain regions, such as the frontal and temporal cortex, hippocampus, DG, and cerebellar cortex. Importantly, we found that NP65 was partly colocalized with NFTs and Aβ plaques in AD. Combined with our previous study showing a decreased Aβ plaques in APP/PS1 mice when NP65 is deficient[8], these results provide evidence that NP65 is involved in the pathology of AD.
Recently, some neural CAMs have been reported to be involved in the pathogenesis of AD in mice. For example, L1, as a membrane CAM, shows an increase in the cerebrospinal fluid of AD patients[25]. In addition, L1 is reported to be decreased in the hippocampus of APPswe mice, and upregulation of L1 or the L1-70 fragment results in a decrease in Aβ plaques in APP/PS1 or APPswe mice (mouse models of AD)[7,26]. These results suggest that L1 expression may be negatively associated with Aβ plaque formation. Interestingly, NP65, another member of the CAMs, has been reported to play crucial roles in neuronal survival, neurite outgrowth, synapse formation, hippocampal CA1 synaptic plasticity, and cognitive function as well as neuronal response following ischemic injury in mice[12,18,27]. Notably, our recent study showed that the deficiency of NP65 resulted in alleviated cognitive deficits, decreased Aβ plaques and reduced microglial activation and neuroinflammation in APP/PS1 mice[8]. Additionally, it was reported that the NP65 immunoreactive product was significantly increased in the hippocampus of AD patients with a short duration[21]. Therefore, these results suggest the NP65 might be involved in the pathogenesis of AD.
The distribution of NP65 in the brain shows species differences in rats, mice and humans[10,12,14,15]. Despite there being more reports on NP65 distribution in rodent brains[11,12,15], only two groups have reported on the NP65 distribution in some regions of the human brain[10,14]. To determine whether NP65 is involved in AD pathogenesis, it is necessary to clarify the detailed characteristics of its expression in sublayers of the cortex, its cellular and subcellular distribution, and its distribution in a wide range of regions in the human brain[10]. Interestingly, the present study showed that NP65 immunoreactivity is most prominent in the PFC. Moreover, strongly-positive apical dendrites were visible in layer V of the PFC. These results are inconsistent with a previous report that showed no regional differences in the cerebral cortex[10]. In the cerebellum, the most intense NP65 immunoreactivity was found in the ML and GCL. The blocky immunoreactive product may be synaptic glomeruli in the GCL, as previously reported in the human cerebellum or NP55 in the rat cerebellum[10,15]. In the hippocampal formation, the most prominent NP65 immunoreactivity was found in the SG and stratum molecular of the DG, consistent with a previous report[10,14]. Unexpectedly, we found prominent punctate structures in the SG, greatly different to the tiny punctate structures in other brain regions. As NP65 can be localized to both pre- and post-synaptic structures[13,28], this large punctate structure may be a synapse indicating the projection of the EC to the granular cells. Notably, we have identified for the first time that there is abundant NP65 immunolabelling in the substantia nigra in the midbrain, implying a potential role in Parkinson’s disease. At the cellular level, NP65 was localized on the cell membrane and dendrites of some principal neurons, such as pyramidal neurons in the hippocampus, pyramidal neurons in layer II and V of the cerebral cortex, and localized in the neuropil. It showed punctate, blocky, and continuous plexuses, positive nerve fibers and a dense network in appearance. The medulla and projection fibers in the brainstem were always devoid of any immunoreaction.
In the present study, intracellular NP65 immunoreactivity was not detected throughout all sections. Interestingly, a previous report showed NP65 immunoreactivity in the somata of neurons of the anterior hypothalamus in normal human brain[10]. This may reflect the complexity of NP65 expression in different subregions and different types of neurons, as there is no brain atlas of NP65 distribution in the human brain. Taken together, the present results show that NP65 is differentially expressed in divergent neurons and is most abundant in the PFC, hippocampal formation, cerebellar cortex, and substantia nigra in the midbrain throughout the human brain.
Next, NP65 expression was detected in the AD and control human brain. Interestingly, we found that the NP65 immunoreactive products were significantly increased in a wide range of brain regions in the AD group compared with the controls, such as the hippocampal formation, frontal and EC, and cerebellar cortex. Furthermore, western blot analysis further confirmed this increase of NP65 protein in the AD hippocampus compared to controls. At the subcellular level, there was no difference in the localization of NP65 immunoreactivity between the AD and control groups. In all AD cases, NP65 was not found to be ectopically expressed in the cytoplasm. Inconsistently, a previous report showed that intracellular NP65 immunoreactivity was found in neurons of the subicular pyramidal layer of the hippocampal formation[21]. This discrepancy may be due to the different methods, severity of AD and altered trafficking and degradation of NP65 protein in AD pathology. Remarkably, the NP65 immunolabelled apical dendrites in layer V in the PFC and apical dendrites of pyramidal cells in CA3 were more prominent and clearly visible in AD cases compared to controls. Additionally, the granular cell layer of the cerebellar cortex exhibited more intense immunoreactivity and appeared blocky in AD cases than in controls. Therefore, the present results clearly show a significant increase in the expression of NP65 in vast brain areas of AD cases.
Finally, we explored whether NP65 is related to Aβ plaques and NFTs, which are the neuropathological hallmarks of AD. More importantly, we found for the first time that NP65 was partly colocalized with NFTs and Aβ plaques in the AD human brain, confirmed by double-immunofluorescent staining. These results indicate that NP65 may affect the formation of Aβ plaques and NFTs in AD pathology.
Our recent studies have shown that NP65 knockout alleviates cognitive deficits and reduces Aβ plaques and neuroinflammation in APP/PS1 mice[8]. Notably, NP65 knockout reverses the increase in 5-hydroxytryptamine receptor 3A (Htr3A) levels in APP/PS1 AD model[8]. Combined with the previous study showing that Htr3A interneurons contribute to Aβ production and inhibiting Htr3A expression decreases Aβ plaques in APP/PSI mice[23], it is thus hypothesized that NP65 deficiency decreases Aβ plaques by downregulating Htr3a expression in APP/PS1 mice. Recent studies have proved that NP65/55 is required for plasma membrane Ca2+ adenosine triphosphatase expression and essential auxiliary subunits of plasma membrane Ca2+ adenosine triphosphatase and key regulators of Ca2+ clearance[29,30]. Ca2+ imbalance of homeostasis could promote brain function deficits and accumulation of Aβ in AD patients. Given that Htr3 is an ionotropic receptor with Ca2+ permeability[31], it is possibly that NP65 could affect Ca2+ imbalance of homeostasis in Htr3A interneurons, which contribute to Aβ generation. However, how NP65 likely affects the formation of NFTs and Aβ in AD pathology by regulating Htr3A interneurons remains unknown. Taken together, our present findings suggest that NP65 may be involved in the formation of Aβ plaques and NFTs in human AD pathology. In the near future, using 3xTg-AD mice that develop Aβ plaques and NFTs[32], we anticipate examining how the up- or down-regulation of NP65 level affects the formation of Aβ plaques and NFTs and the underlying mechanisms. In addition, using co-immunoprecipitation and protein mass spectrometry to determine the interacting proteins with NP65 may identify potential mechanisms of NP65 involved in AD pathology.
In conclusion, our results show that the NP65 protein is significantly increased and colocalized with NFTs and Aβ plaques in the brains of individuals with AD. Combined with our previous report on AD mice[8], our findings suggest that NP65 is involved in the formation of Aβ plaques in AD, making it a potential target for the treatment of this disease.
| 1. | Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, Cummings J, van der Flier WM. Alzheimer's disease. Lancet. 2021;397:1577-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1527] [Cited by in RCA: 2651] [Article Influence: 662.8] [Reference Citation Analysis (0)] |
| 2. | Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, Van der Flier WM. Alzheimer's disease. Lancet. 2016;388:505-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1969] [Cited by in RCA: 2277] [Article Influence: 253.0] [Reference Citation Analysis (0)] |
| 3. | Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol. 2009;68:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 491] [Cited by in RCA: 478] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 4. | Buccellato FR, D'Anca M, Tartaglia GM, Del Fabbro M, Scarpini E, Galimberti D. Treatment of Alzheimer's Disease: Beyond Symptomatic Therapies. Int J Mol Sci. 2023;24:13900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 5. | Karran E, De Strooper B. The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nat Rev Drug Discov. 2022;21:306-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 418] [Article Influence: 139.3] [Reference Citation Analysis (0)] |
| 6. | Djogo N, Jakovcevski I, Müller C, Lee HJ, Xu JC, Jakovcevski M, Kügler S, Loers G, Schachner M. Adhesion molecule L1 binds to amyloid beta and reduces Alzheimer's disease pathology in mice. Neurobiol Dis. 2013;56:104-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Aksic M, Jakovcevski I, Hamad MIK, Jakovljevic V, Stankovic S, Vulovic M. The Neuroprotective Effect of Neural Cell Adhesion Molecule L1 in the Hippocampus of Aged Alzheimer's Disease Model Mice. Biomedicines. 2024;12:1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Wu DD, Cheng J, Zheng YN, Liu YT, Hou SX, Liu LF, Huang L, Yuan QL. Neuroplastin 65 deficiency reduces amyloid plaque formation and cognitive deficits in an Alzheimer's disease mouse model. Front Cell Neurosci. 2023;17:1129773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Langnaese K, Beesley PW, Gundelfinger ED. Synaptic membrane glycoproteins gp65 and gp55 are new members of the immunoglobulin superfamily. J Biol Chem. 1997;272:821-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Bernstein HG, Smalla KH, Bogerts B, Gordon-Weeks PR, Beesley PW, Gundelfinger ED, Kreutz MR. The immunolocalization of the synaptic glycoprotein neuroplastin differs substantially between the human and the rodent brain. Brain Res. 2007;1134:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Langnaese K, Mummery R, Gundelfinger ED, Beesley PW. Immunoglobulin superfamily members gp65 and gp55: tissue distribution of glycoforms. FEBS Lett. 1998;429:284-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Smalla KH, Matthies H, Langnäse K, Shabir S, Böckers TM, Wyneken U, Staak S, Krug M, Beesley PW, Gundelfinger ED. The synaptic glycoprotein neuroplastin is involved in long-term potentiation at hippocampal CA1 synapses. Proc Natl Acad Sci U S A. 2000;97:4327-4332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Kreutz MR, Langnaese K, Dieterich DC, Seidenbecher CI, Zuschratter W, Beesley PW, Gundelfinger ED. Distribution of transcript and protein isoforms of the synaptic glycoprotein neuroplastin in rat retina. Invest Ophthalmol Vis Sci. 2001;42:1907-1914. [PubMed] |
| 14. | Herrera-Molina R, Mlinac-Jerkovic K, Ilic K, Stöber F, Vemula SK, Sandoval M, Milosevic NJ, Simic G, Smalla KH, Goldschmidt J, Bognar SK, Montag D. Neuroplastin deletion in glutamatergic neurons impairs selective brain functions and calcium regulation: implication for cognitive deterioration. Sci Rep. 2017;7:7273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Marzban H, Khanzada U, Shabir S, Hawkes R, Langnaese K, Smalla KH, Bockers TM, Gundelfinger ED, Gordon-Weeks PR, Beesley PW. Expression of the immunoglobulin superfamily neuroplastin adhesion molecules in adult and developing mouse cerebellum and their localisation to parasagittal stripes. J Comp Neurol. 2003;462:286-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Ilic K, Mlinac-Jerkovic K, Sedmak G, Rosenzweig I, Kalanj-Bognar S. Neuroplastin in human cognition: review of literature and future perspectives. Transl Psychiatry. 2021;11:394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Amuti S, Tang Y, Wu S, Liu L, Huang L, Zhang H, Li H, Jiang F, Wang G, Liu X, Yuan Q. Neuroplastin 65 mediates cognitive functions via excitatory/inhibitory synapse imbalance and ERK signal pathway. Neurobiol Learn Mem. 2016;127:72-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Li H, Liu Y, Gao X, Liu L, Amuti S, Wu D, Jiang F, Huang L, Wang G, Zeng J, Ma B, Yuan Q. Neuroplastin 65 modulates anxiety- and depression-like behavior likely through adult hippocampal neurogenesis and central 5-HT activity. FEBS J. 2019;286:3401-3415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Cheng J, Chen L, Zheng YN, Liu J, Zhang L, Zhang XM, Huang L, Yuan QL. Disfunction of dorsal raphe nucleus-hippocampus serotonergic-HTR3 transmission results in anxiety phenotype of Neuroplastin 65-deficient mice. Acta Pharmacol Sin. 2024;45:1393-1405. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Desrivières S, Lourdusamy A, Tao C, Toro R, Jia T, Loth E, Medina LM, Kepa A, Fernandes A, Ruggeri B, Carvalho FM, Cocks G, Banaschewski T, Barker GJ, Bokde AL, Büchel C, Conrod PJ, Flor H, Heinz A, Gallinat J, Garavan H, Gowland P, Brühl R, Lawrence C, Mann K, Martinot ML, Nees F, Lathrop M, Poline JB, Rietschel M, Thompson P, Fauth-Bühler M, Smolka MN, Pausova Z, Paus T, Feng J, Schumann G; IMAGEN Consortium. Single nucleotide polymorphism in the neuroplastin locus associates with cortical thickness and intellectual ability in adolescents. Mol Psychiatry. 2015;20:263-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Ilic K, Mlinac-Jerkovic K, Jovanov-Milosevic N, Simic G, Habek N, Bogdanovic N, Kalanj-Bognar S. Hippocampal expression of cell-adhesion glycoprotein neuroplastin is altered in Alzheimer's disease. J Cell Mol Med. 2019;23:1602-1607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Yan XX, Ma C, Bao AM, Wang XM, Gai WP. Brain banking as a cornerstone of neuroscience in China. Lancet Neurol. 2015;14:136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Liu LF, Liu YT, Wu DD, Cheng J, Li NN, Zheng YN, Huang L, Yuan QL. Inhibiting 5-hydroxytryptamine receptor 3 alleviates pathological changes of a mouse model of Alzheimer's disease. Neural Regen Res. 2023;18:2019-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 24. | Ai JQ, Luo R, Tu T, Yang C, Jiang J, Zhang B, Bi R, Tu E, Yao YG, Yan XX. Doublecortin-Expressing Neurons in Chinese Tree Shrew Forebrain Exhibit Mixed Rodent and Primate-Like Topographic Characteristics. Front Neuroanat. 2021;15:727883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | von Gunten A, Kövari E, Bussière T, Rivara CB, Gold G, Bouras C, Hof PR, Giannakopoulos P. Cognitive impact of neuronal pathology in the entorhinal cortex and CA1 field in Alzheimer's disease. Neurobiol Aging. 2006;27:270-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Ogbodo JO, Agbo CP, Njoku UO, Ogugofor MO, Egba SI, Ihim SA, Echezona AC, Brendan KC, Upaganlawar AB, Upasani CD. Alzheimer's Disease: Pathogenesis and Therapeutic Interventions. Curr Aging Sci. 2022;15:2-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 27. | Owczarek S, Soroka V, Kiryushko D, Larsen MH, Yuan Q, Sandi C, Berezin V, Bock E. Neuroplastin-65 and a mimetic peptide derived from its homophilic binding site modulate neuritogenesis and neuronal plasticity. J Neurochem. 2011;117:984-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Willmott T, Skitsa I, Hill I, Mummery R, Beesley PW. Molecular characterisation and structural relationship of the synapse-enriched glycoproteins gp65 and gp55. J Neurochem. 1992;58:2037-2043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Bhattacharya S, Herrera-Molina R, Sabanov V, Ahmed T, Iscru E, Stöber F, Richter K, Fischer KD, Angenstein F, Goldschmidt J, Beesley PW, Balschun D, Smalla KH, Gundelfinger ED, Montag D. Genetically Induced Retrograde Amnesia of Associative Memories After Neuroplastin Ablation. Biol Psychiatry. 2017;81:124-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Gong D, Chi X, Ren K, Huang G, Zhou G, Yan N, Lei J, Zhou Q. Structure of the human plasma membrane Ca(2+)-ATPase 1 in complex with its obligatory subunit neuroplastin. Nat Commun. 2018;9:3623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 31. | Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991;254:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 753] [Cited by in RCA: 740] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 32. | Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer's disease. Neurobiol Aging. 2003;24:1063-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 714] [Article Influence: 34.0] [Reference Citation Analysis (0)] |