Published online Aug 15, 2025. doi: 10.4251/wjgo.v17.i8.105006
Revised: April 3, 2025
Accepted: July 2, 2025
Published online: August 15, 2025
Processing time: 215 Days and 21.6 Hours
Gastric cancer (GC) is a type of cancer which causes high cancer-related mortality. Surgical operation and systematic chemical therapies are primary choices for the treatment of GC patients with advanced stages, however, the 5-year overall survival is only around 30%.
To investigate the role of mesenchymal stem cell (MSC)-derived long non-coding RNAs (lncRNA) NKILA in fatty acid oxidation and chemoresistance in GC cells, mediated through the miR-485-5p/STAT3 pathway.
GC cell lines (AGS and MKN45) were co-cultured with human bone marrow-derived MSCs were cultured. The MSC identity was confirmed by flow cytometry (CD73, CD90, CD105 > 95% positive, CD34, CD45 negative). Co-culture of GC cells and MSCs was performed in Transwell plates, where MSCs were placed in the upper chamber and GC cells in the lower chamber for 72 hours. For transfections, pcDNA-NKILA vectors, shSTAT3, and miR-485-5p mimics were utilized. Colony formation, apoptosis assays (Annexin V/PI staining), sphere formation, and flow cytometry were performed to evaluate cell proliferation, stemness, and chemoresistance. qPCR was used to analyze gene expression (Sox2, Oct4, CD133, LIN28, NKILA), and Western blotting assessed protein levels of stemness markers. Luciferase reporter assays were conducted to confirm miR-485-5p/STAT3 interactions, and biotin-labeled RNA pulldown was used to assess RNA-protein binding. Fatty acid oxidation was evaluated using a CPT1 activity assay and β-oxidation rate detection. ATP levels were measured to assess the energetic status of GC cells. Clinical GC tissue samples were collected from patients at our hospital for validation.
MSCs were found to enhance the stemness and chemoresistance of GC cells. Co-culturing MKN45 and AGS cells with MSCs significantly increased sphere-forming ability and the expression of key cancer stem cell markers (SOX2, Oct4, LIN28, CD133), indicating that MSCs promote stem-like properties. Flow cytometry confirmed an enrichment of CD44+ and CD133+ subpopulations in MSC-treated GC cells. Additionally, MSC co-culture reduced chemotherapy-induced apoptosis and enhanced cell proliferation, suggesting a protective role in chemotherapy resistance. MSC-derived lncRNA NKILA further promoted stemness and chemoresistance, enhancing expression of stem cell markers and protecting cells from oxaliplatin and 5-FU-induced apoptosis. MSC co-culture also induced fatty acid oxidation in GC cells, as shown by increased CPT1 activity, β-oxidation rates, and ATP levels. NKILA mediated these effects by upregulating STAT3, which was confirmed to regulate fatty acid oxidation and chemoresistance. NKILA’s interaction with miR-485-5p further promoted STAT3 expression and fatty acid oxidation, reinforcing its role in maintaining stemness and enhancing chemoresistance.
MSCs enhance the stemness and chemoresistance of GC cells by secreting lncRNA NKILA, which promotes fatty acid oxidation through STAT3 activation. NKILA modulates the miR-485-5p/STAT3 axis, thereby increasing energy metabolism and supporting cancer stem cell properties. Targeting NKILA or the miR-485-5p/STAT3 pathway offers potential therapeutic strategies to overcome chemoresistance in GC.
Core Tip: Mesenchymal stem cells enhance the stemness and chemoresistance of gastric cancer cells by secreting long non-coding RNAs NKILA, which promotes fatty acid oxidation through STAT3 activation. NKILA modulates the miR-485-5p/STAT3 axis, thereby increasing energy metabolism and supporting cancer stem cell properties. Targeting NKILA or the miR-485-5p/STAT3 pathway offers potential therapeutic strategies to overcome chemoresistance in gastric cancer.
- Citation: Lyu XJ, Zhou L, Jiang XM, Zheng D. Mesenchymal stem cell-derived lncRNAs NKILA contributes to stemness and chemoresistance by fatty acid oxidation in gastric cancer via miR-485-5p/STAT3. World J Gastrointest Oncol 2025; 17(8): 105006
- URL: https://www.wjgnet.com/1948-5204/full/v17/i8/105006.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i8.105006
Gastric cancer (GC) is a type of cancer which causes high cancer-related mortality[1,2]. Surgical operation and systematic chemical therapies are primary choices for the treatment of GC patients with advanced stages, however, the 5-year overall survival is only around 30%[2]. Chemical agents such as Oxaliplatin (Oxa), 5-fluorouracil (5-FU), and Cisplatin are widely applied in the clinical treatment of cancers including GC, however, the frequently emerged drug resistance largely limited the therapeutic efficacy and clinical outcome[3,4]. Hence, exploring the mechanisms underlying drug resistance and looking for novel therapeutic targets is imperative for GC treatment.
In recent years, increasing pieces of evidence have revealed the important role of the tumor microenvironment (TME) in the developing drug resistance of cancer cells[5]. Noteworthy, the TME consists of various cell types, including the mesenchymal stromal cells (MSCs)[6]. MSCs communicate with cancer cells through secreting various signaling chemicals such as chemokines and cytokines, thus participating in the initiation and progression of cancers[7]. Besides, MSCs also facilitate cancer cells to generate stemness and further promote their drug resistance ability[8,9].
A well-known characteristic of cancer is abnormal metabolism, which is also thought to contribute to the chemoresistance of cancer cells[10]. Fatty acid oxidation (FAO) is important signaling that modulates the degradation of fatty acids and the production of NADPH and ATP[11]. FAO-mediated aberrant lipid metabolism has been revealed to be correlated with cancer development[12]. Studies have reported that FAO facilitates cancer cell stemness and drug resistance in cancers patients, including GC[13]. Recent studies suggested that MSCs stimulate the FAO process of GC cells, which contributes to drug resistance. However, the mechanisms underlying this phenomenon are unclear.
Long non-coding RNAs (lncRNAs) represent a class of regulatory molecules that orchestrate cancer progression through miRNA sponging and epigenetic modulation[14-16]. LncRNA NKILA exhibits context-dependent roles across malignancies, promoting EMT in hepatocellular carcinoma while suppressing metastasis in osteosarcoma[17-20]. Intriguingly, its function in GC - particularly in MSC-mediated metabolic adaptation - remains unexplored.
Geographical disparities highlight GC's multifactorial etiology, with incidence rates ranging from 32.1/100000 males in East Asia to 4.7/100000 in North Africa[17,18]. This heterogeneity underscores the need for targeted therapies addressing both tumor-intrinsic and microenvironmental drivers[21,22].
Here, we elucidate a novel MSC-GC crosstalk axis: MSC-derived lncRNA NKILA enhances stemness and chemoresistance by upregulating STAT3 via miR-485-5p sponging, thereby activating FAO. Our findings position NKILA as a potential therapeutic target to disrupt microenvironment-fueled treatment resistance.
AGS and MKN45 GC cell lines were obtained from Procell (China). Primary human bone marrow-derived MSCs (HUXMA-01001, Procell, China) were cultured in α-MEM medium (Hyclone, United States) supplemented with 10% FBS and 1% penicillin/streptomycin. MSC identity was confirmed by flow cytometry for surface markers CD73/CD90/CD105 (> 95% positivity) and absence of hematopoietic markers CD34/CD45 (antibodies from BioLegend, United States). All cells were maintained at 37 °C in 5% CO2. For co-culture experiments, 1 × 105 MSCs were seeded in the upper chamber of 6-well Transwell plates with 0.4 μm polyester membranes (Corning, United States), while 5 × 104 GC cells were plated in the lower chamber. Co-cultures were maintained in complete RPMI 1640 medium for 72 hours prior to subsequent assays.
Oxa (61825-3, 5 μM), 5-FU, and the FAO inhibitor etomoxir (ETX) were purchased from Sigma. The oligonucleotides pcDNA-NKILA vectors and empty pcDN13.1 vectors, shSTAT3, miR-485-5p mimics, and negative control were synthesized by RiboBio (China). Transfections were performed using Lipofectamine 3000 (Thermo, United States) according to the manufacturer’s protocol.
Colony formation was conducted to assess cell proliferation. In short, GC cells were cultured in 6-well plates (1000 cells per well) for 2 weeks, then fixed and stained with 0.5% crystal violet (Beyotime, China) in methanol for 20 minutes. The images were taken, and colonies were counted. Cell apoptosis was determined by Annexin V/PI staining (KA3805) (Sigma, United States) and analyzed by flow cytometer (BD Biosciences, United States).
GC cells were seeded into a 96-well plate and incubated with DMEM/F12 (Thermo, United States) that was supplemented with B27 (1%; Sigma, United States), EGF (20 ng/mL, Peprotech, United States), bFGF (20 ng/mL, Peprotech, United States), and 1% penicillin/streptomycin for 10 days. Then the number of the spheres was calculated.
GC cells were collected after indicated treatment, fixed in BSA solution (15561-020) (Thermo, United States), then stained with FITC-labeled anti-CD44 and Cy5-labeled anti-CD133 antibodies (Biolegend, United States) in dark for 30 minutes. The positive staining was then measured by a FACS flow cytometer (BD Biosciences, United States).
The total RNA was extracted from GC cells and patient tissues using TRIzol solution (Thermo, United States). The cDNA was synthesized using High-Capacity cDNA reverse transcription kits (AB1453A, Thermo, United States). The expression of Sox2, Oct4, CD133, LIN28, and NKILA was analyzed by SYBR Green qPCR Mix (4402959, Thermo, United States) based on the 2-ΔΔCt method. GAPDH was used as the internal control for normalization.
Cells were homogenized by using RIPA lysis buffer (LA2670, SolarBio, China) to obtain total protein. Equal quantities of protein were divided by 18%-10% SDS-PAGE, transferred to NC membranes, blocked in 5% non-fat milk for 60 minutes, then incubated with primary antibodies overnight at 4 °C. Subsequently, the protein bands were hatched with secondary antibodies at room temperature and visualized after reacting with ECL reagent (Millipore, Germany). Primary antibodies (KD/KO) against Sox2, Oct4, CD133, LIN28, and GAPDH were purchased from Proteintech (China) and referred to the manufacturer's protocols.
Wild type or mutated NKILA or STAT3 was cloned into pmirGLO promoter gene vectors to obtain pmirGLO-NKILA-WT/MUT and pmirGLO-STAT3-WT/MUT. The vectors were transfected to GC cells with miR-485-5p mimics and hatched for 48 hours. The luciferase activity was measured using Promega dual-luciferase reporter gene assay kit (E1910) (United States).
The biotin-labeled wild-type or mutated miR-485-5p probes (485-5p) were designed and synthesized by RiboBio (China). RNA pulldown assay was performed using C-1 magnetic beads (Invitrogen, United States). Cells were transfected with NKILA and the biotin-labeled miR-485-5p, harvested and sonicated, then incubated with the magnetic beads for 6 hours at 4 °C. The enrichment of NKILA was assessed by qPCR assay.
The activity of CPT1 was measured using a CPT1 assay kit (MBS2602676) (FineTest, China) following the manufacturer's instructions.
The ATP level was measured by using an ATP detection kit (ab113849) (Abcam, China) as per the manufacturer's description.
Fatty Acid β-oxidation rate was measured using FAO Assay kit (ab217602) (Abcam, United States) followed by manufacturer's instructions.
Tumor samples and adjacent non-tumor tissues were collected from GC patients who received treatment at our hospital (June 2020 to October 2021).
All experiments were performed with ≥ 3 biological replicates. Data were shown as means ± SEM. Data normality was first verified by Shapiro-Wilk test. Between-group comparisons were analyzed using the following methods:
For single-variable comparisons between two groups, unpaired Student's t-test (normal distribution) or Mann-Whitney U test (non-normal distribution) was applied.
For multi-group comparisons, one-way ANOVA (normal distribution) or Kruskal-Wallis test (non-normal distribution) was used, with post-hoc corrections (Tukey's test for ANOVA, Dunn's test for Kruskal-Wallis).
Two-way ANOVA with Sidak's multiple comparisons was employed for drug treatment × MSC co-culture interaction analyses.
Statistical analyses were performed using SPSS (Version 19.0) and GraphPad Prism 9.0. Error bars represent SEM throughout the figures. P < 0.05 was considered statistically significant.
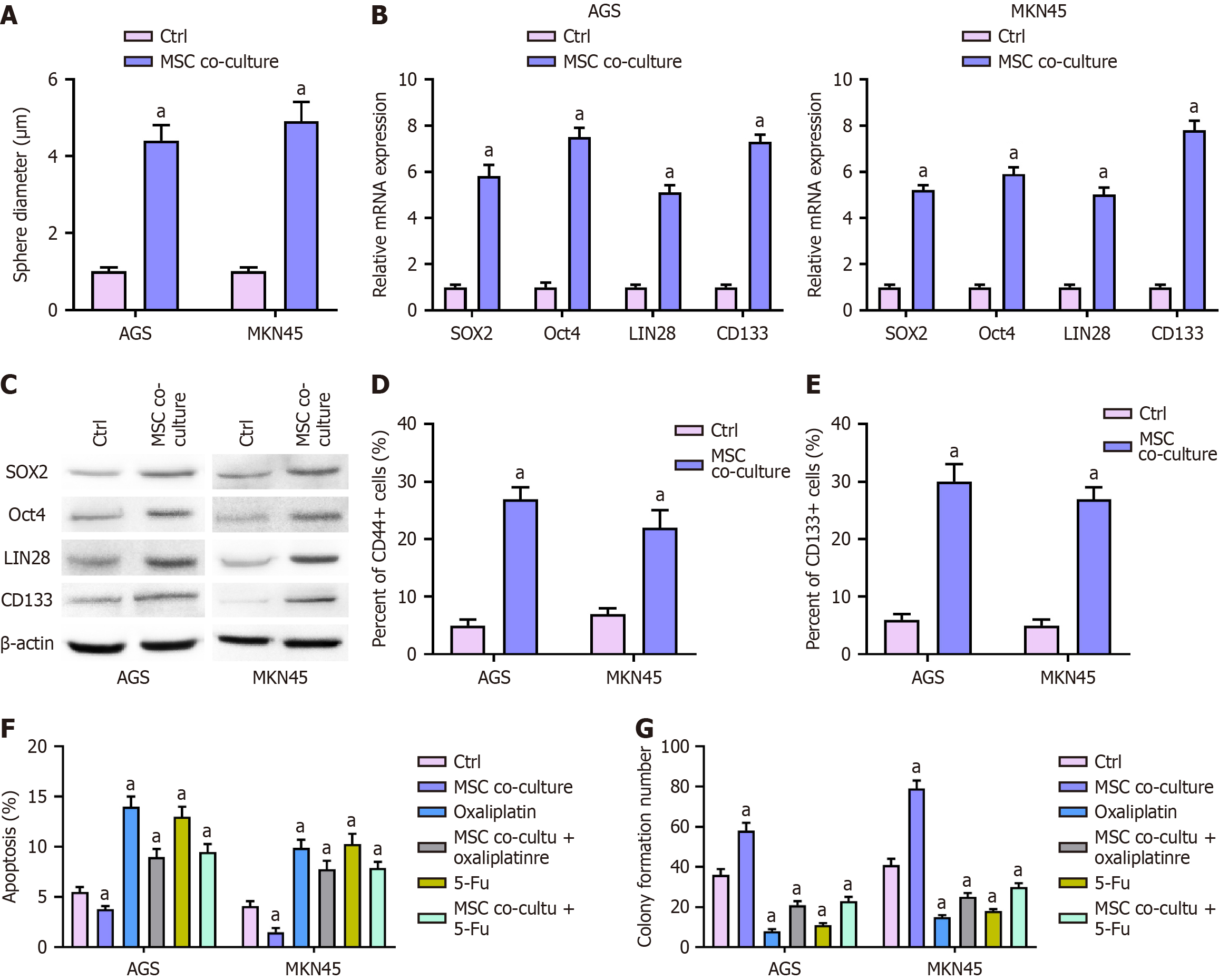
Sphere formation assay: To assess the effect of MSCs on stemness, MKN45 and AGS cells were co-cultured with MSCs and subjected to a sphere formation assay (Figure 1A). The results demonstrated a significant increase in sphere-forming ability in the MSC co-culture group compared to the control (P < 0.01), indicating that MSCs promote the stem-like properties of GC cells.
Expression of cancer stem cell biomarkers: MSC co-culture significantly upregulated the expression of key cancer stem cell markers, including SOX2, Oct4, LIN28, and CD133, at both the mRNA and protein levels. qPCR analysis confirmed a marked increase in the mRNA expression of these markers in MSC-treated MKN45 and AGS cells (P < 0.01; Figure 1B). Consistently, Western blot analysis revealed elevated protein levels of SOX2, Oct4, LIN28, and CD133 in the MSC co-culture group (Figure 1C), further supporting the induction of stemness by MSCs.
Flow cytometry analysis of CD44+ and CD133+ subpopulations: Flow cytometry analysis demonstrated a significant increase in the proportion of CD44+ and CD133+ subpopulations in MSC-treated MKN45 and AGS cells (P < 0.01; Figure 1D and E), indicating an enrichment of cancer stem-like cells upon MSC exposure.
Effects on apoptosis and proliferation: To examine the impact of MSCs on chemoresistance, MKN45 and AGS cells were co-cultured with MSCs and treated with Oxa or 5-Fu. Flow cytometry analysis revealed that MSCs significantly reduced chemotherapy-induced apoptosis (P < 0.01; Figure 1F). In colony formation assays, MSC co-culture significantly enhanced the proliferative capacity of GC cells and reversed the growth inhibition caused by Oxa and 5-Fu (P < 0.01; Figure 1G), suggesting a protective role of MSCs in chemotherapy resistance.
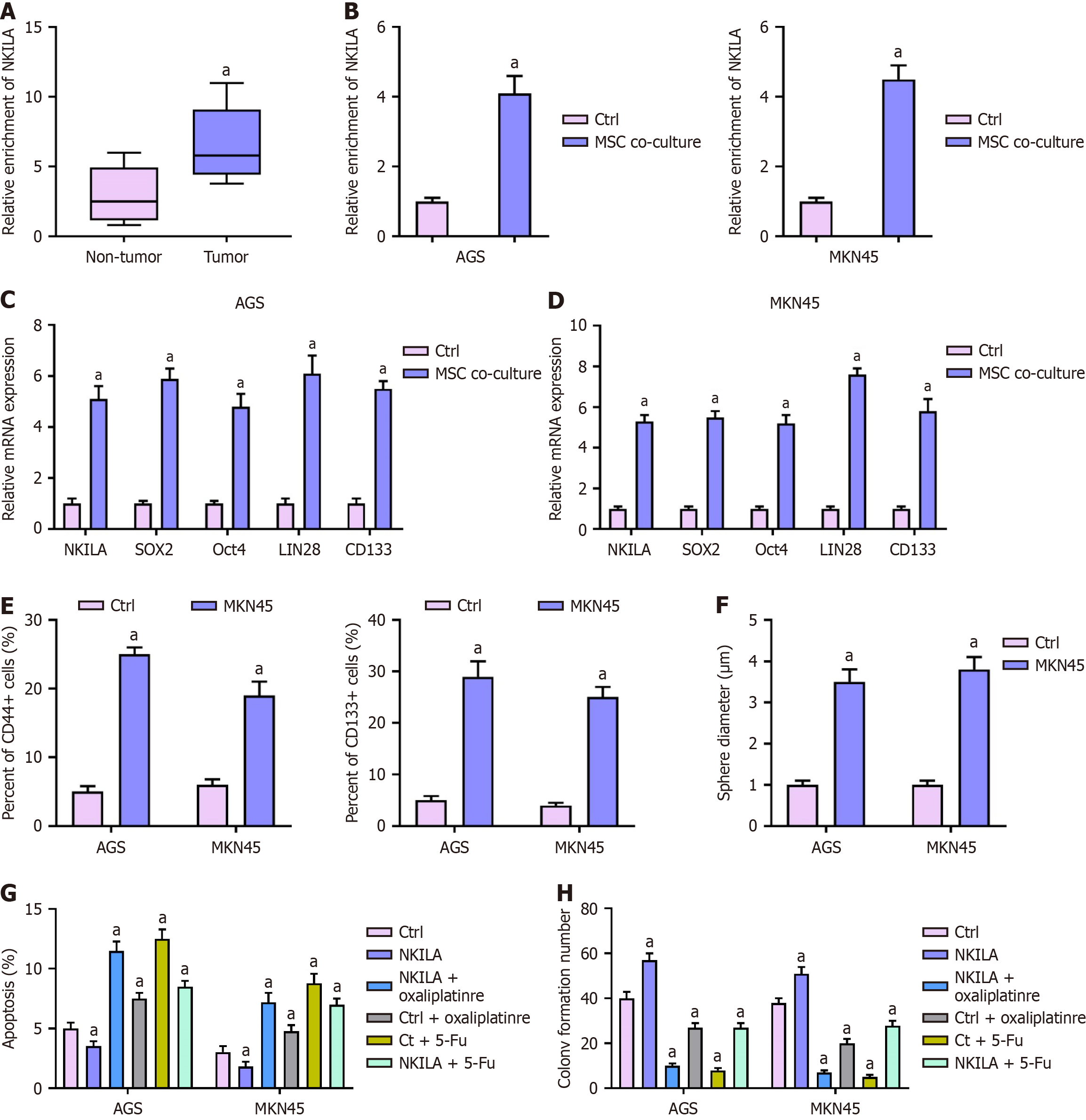
Expression of NKILA in clinical tissues and cells: We first evaluated the expression of NKILA in clinical GC tissues and adjacent non-tumor tissues by qPCR (Figure 2A). Statistical analysis revealed that NKILA expression was significantly higher in tumor tissues than in non-tumor tissues (P < 0.01). Subsequently, we examined NKILA expression in MKN45 and AGS cells co-cultured with MSCs (Figure 2B). In comparison to the Ctrl group, MSC co-culture significantly increased NKILA expression in both MKN45 and AGS cells (P < 0.01).
Effect of NKILA overexpression on cancer stem cell markers: MKN45 and AGS cells were transfected with an NKILA overexpression plasmid (Figure 2C-F). The mRNA levels of SOX2, Oct4, LIN28, and CD133 were assessed by qPCR (Figure 2C and D). One-way ANOVA showed a significant increase in the mRNA expression of these stem cell markers in the NKILA overexpression group compared to the Ctrl group (P < 0.01). Flow cytometry analysis also revealed a higher proportion ofCD44+ and CD133+ cells in the NKILA overexpression group (P < 0.01; Figure 2E). Furthermore, sphere formation assays demonstrated that the sphere diameter was significantly larger in NKILA-overexpressing cells than in Ctrl cells (P < 0.01; Figure 2F), indicating that NKILA overexpression enhanced cell stemness.
Effect of NKILA overexpression on cell apoptosis and proliferation: MKN45 and AGS cells were transfected with the NKILA overexpression plasmid and then treated with Oxa or 5-Fu (Figure 2G and H). Flow cytometry analysis showed that NKILA overexpression inhibited chemotherapy-induced apoptosis in both cell lines (P < 0.01; Figure 2G). Colony formation assays revealed that while Oxa or 5-Fu treatment decreased colony formation in the Ctrl group, NKILA overexpression significantly restored colony formation and cell proliferation (P < 0.01; Figure 2H).
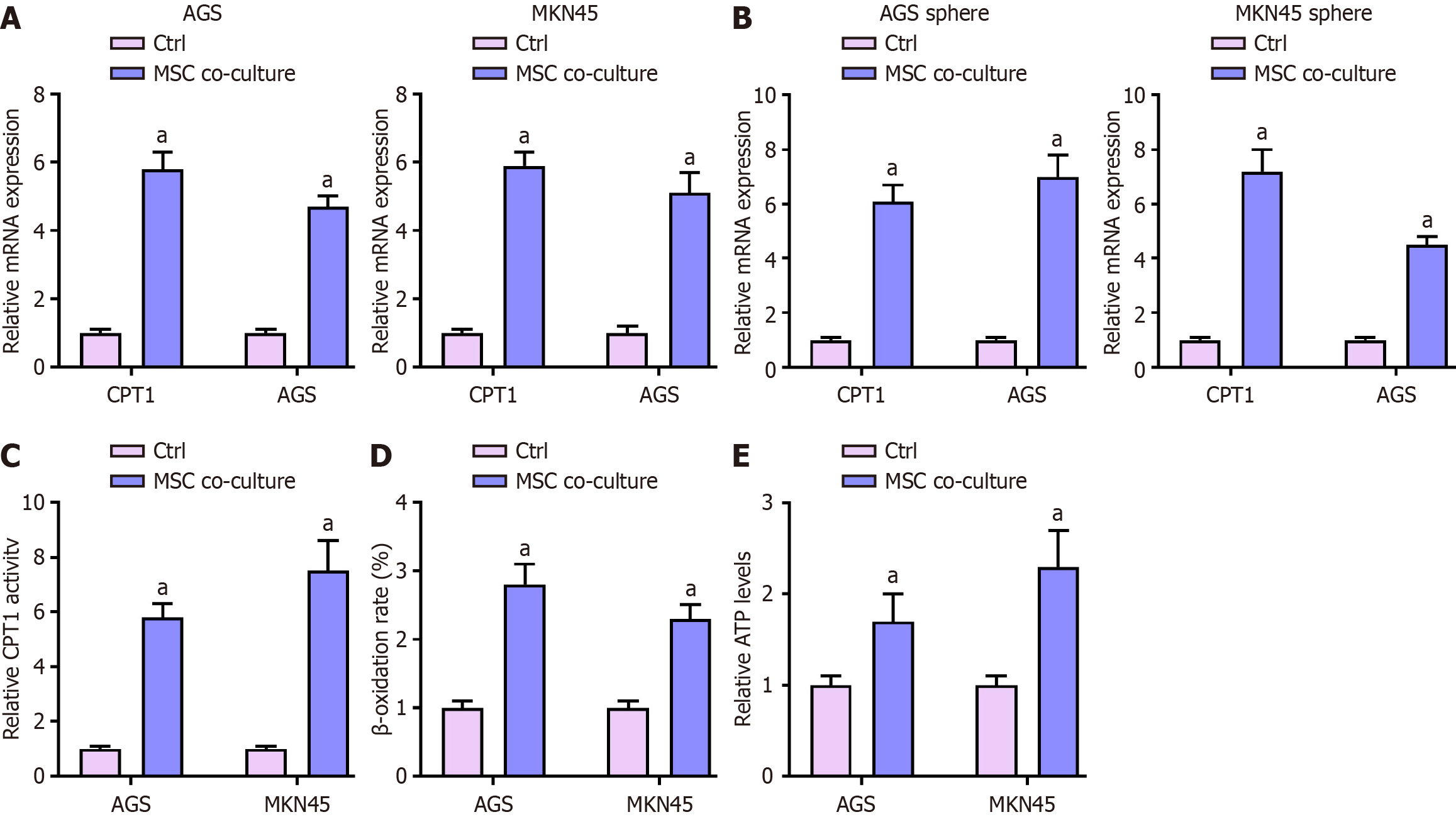
We investigated the effect of MSCs on FAO in GC cells by co-culturing MKN45 and AGS cells with MSCs.
First, we measured the mRNA expression levels of CPT1 and ACS in MKN45 and AGS cells by qPCR (Figure 3A). Statistical analysis showed that MSC co-culture significantly upregulated the mRNA levels of CPT1 and ACS in both MKN45 and AGS cells (P < 0.01). Similarly, the mRNA expression levels of CPT1 and ACS in MKN45 and AGS sphere cells were also significantly increased upon MSC co-culture (P < 0.01; Figure 3B).
Next, we assessed CPT1 activity in both cell lines (Figure 3C). One-way ANOVA revealed that MSC co-culture significantly increasedCPT1 activity in MKN45 and AGS cells (P < 0.01). We then analyzed β-oxidation rates (Figure 3D), finding that the MSC co-culture group exhibited significantly higher β-oxidation rates compared to the Ctrl group (P < 0.01). Lastly, we determined the relative ATP levels in these cells (Figure 3E). Statistical analysis showed that MSC co-culture significantly increased ATP levels in both MKN45 and AGS cells (P < 0.01).
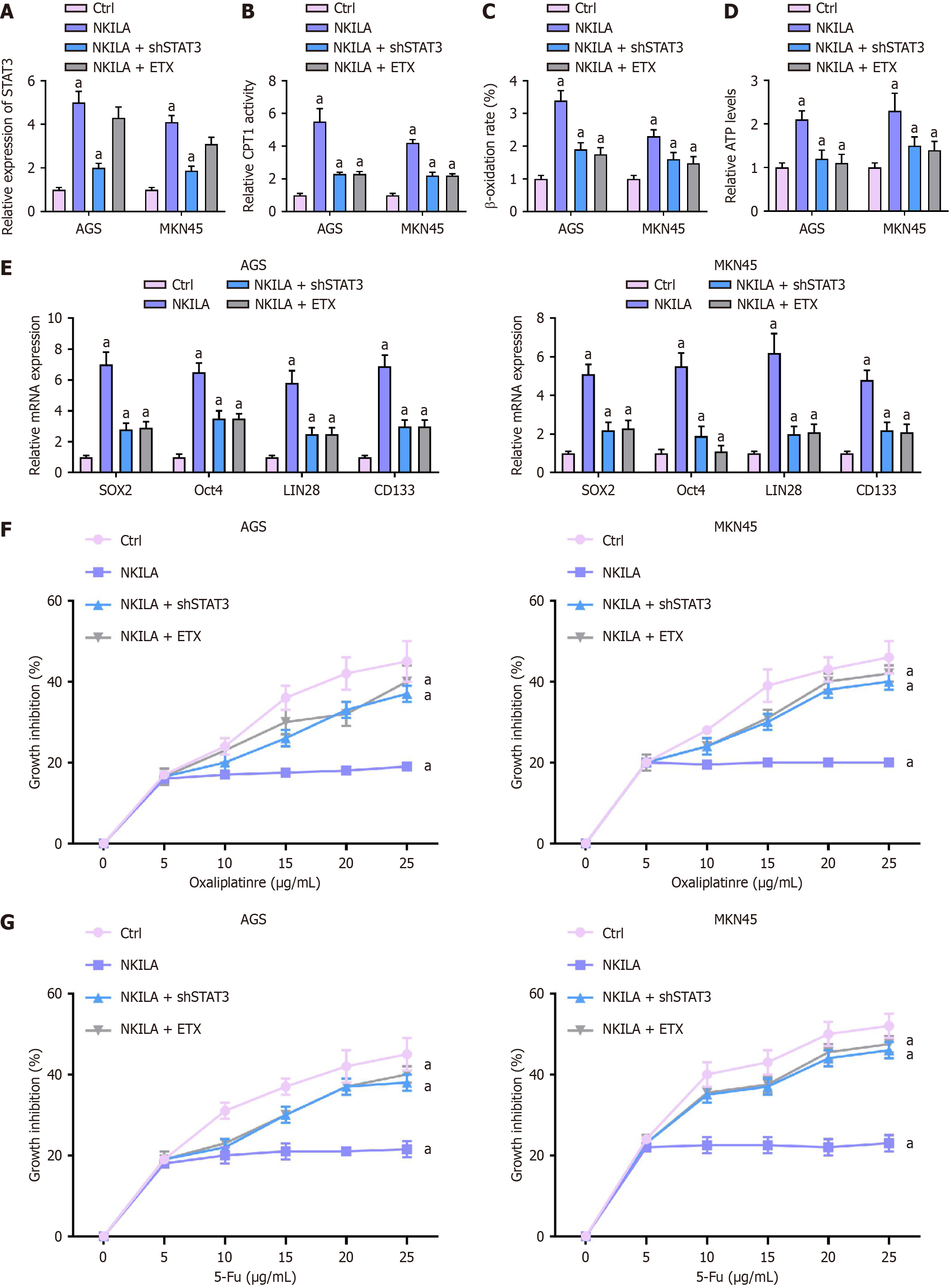
We investigated the effect of NKILA on FAO in GC cells by transfecting MKN45 and AGS cells with NKILA and co-treating them with STAT3 shRNA or ETX.
First, STAT3 expression was measured by qPCR (Figure 4A). Statistical analysis with one-way ANOVA revealed that, in both AGS and MKN45 cells, STAT3 expression was significantly lower in the NKILA + shSTAT3 and NKILA + ETX groups compared to the NKILA group (P < 0.01).
CPT1 activity, the CPT1 activity was assessed (Figure 4B). One-way ANOVA showed that the CPT1 activity in both AGS and MKN45 cells was significantly higher in the NKILA group compared to the Ctrl group. However, CPT1 activity was notably reduced in the NKILA + shSTAT3 and NKILA +ETX groups compared to the NKILA group (P < 0.01). β-oxidation: Β-oxidation was analyzed (Figure 4C). One-way ANOVA indicated that the β-oxidation rate in the NKILA group was higher than in the Ctrl group for both cell lines. In contrast, the β-oxidation rates in the NKILA + shSTAT3 and NKILA + ETX groups were significantly lower than in the NKILA group (P < 0.01).
Relative ATP levels were measured (Figure 4D). Statistical analysis showed that ATP levels were significantly higher in the NKILA group compared to the Ctrl group. In contrast, ATP levels were significantly reduced in the NKILA + shSTAT3 and NKILA + ETX groups compared to the NKILA group (P < 0.01).
The mRNA expression of SOX2, Oct4, LIN28, and CD133 was measured by qPCR (Figure 4E). One-way ANOVA indicated that the expression of these cancer stem cell markers was significantly higher in the NKILA group compared to the Ctrl group. However, in the NKILA + shSTAT3 and NKILA + ETX groups, the expression levels of SOX2, Oct4, LIN28, and CD133 were significantly reduced compared to the NKILA group (P < 0.01).
Chemoresistance to Oxa or 5-Fu was assessed by CCK-8 (Figure 4F and G). One-way ANOVA showed that cell viability was significantly higher in the NKILA group compared to the Ctrl group under both Oxa and 5-Fu treatments, indicating enhanced chemoresistance. However, in the NKILA + shSTAT3 and NKILA + ETX groups, cell viability was significantly reduced compared to the NKILA group when treated with Oxa or 5-Fu (P < 0.01).
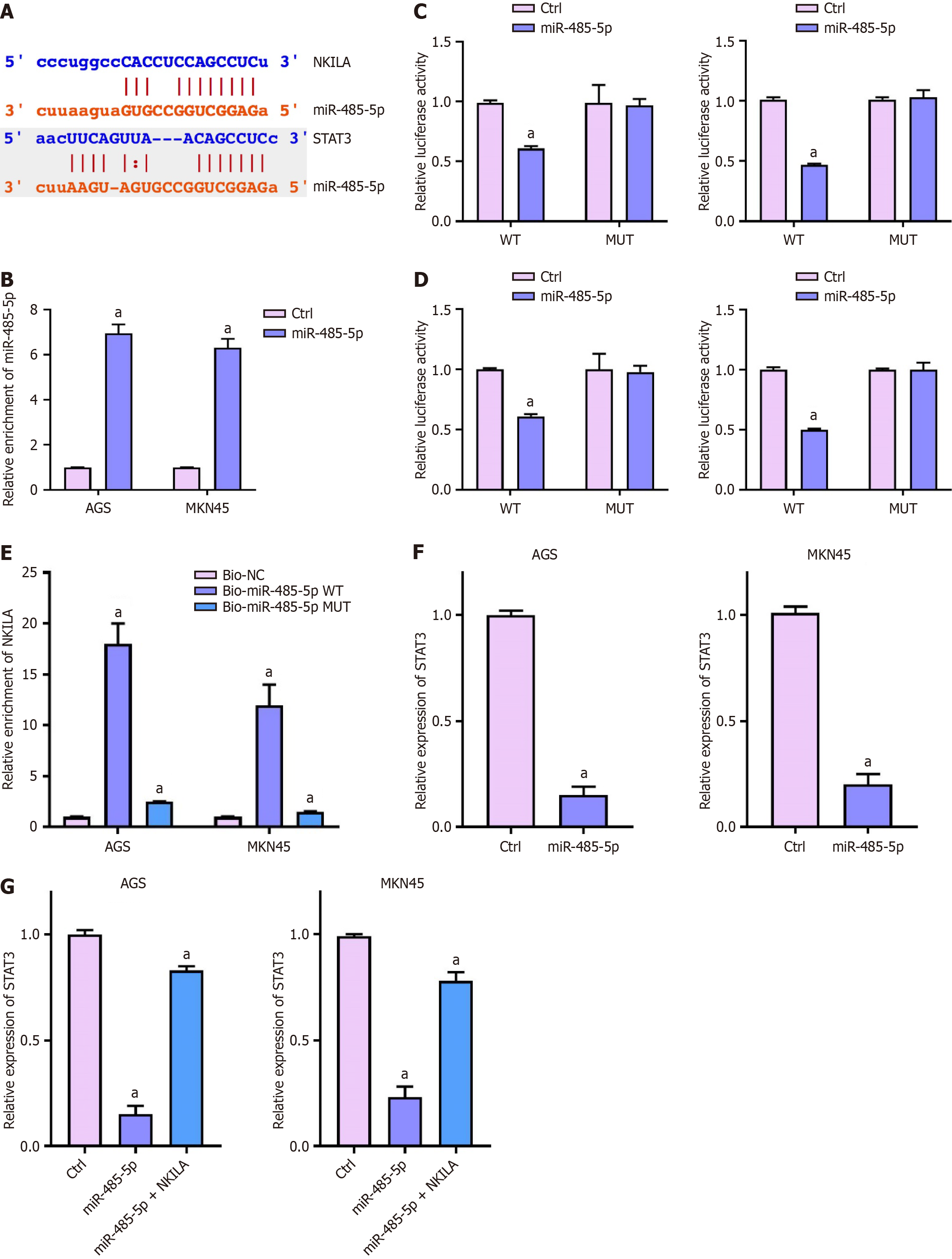
Next, we identified the binding sites of miR-485-5p between NKILA and STAT3 (Figure 5A). The miR-485-5p mimic was transfected into MKN45 and AGS cells, and the expression of miR-485-5p was measured by qPCR (Figure 5B). One-way ANOVA showed that miR-485-5p expression was significantly upregulated in both cell lines after transfection (P < 0.01), confirming successful mimic transfection.
The luciferase activity of NKILA was measured using a reporter gene assay (Figure 5C). One-way ANOVA revealed that miR-485-5p significantly inhibited the luciferase activity of NKILA in both MKN45 and AGS cells (P < 0.01), suggesting a direct interaction between miR-485-5p and NKILA.
Similarly, luciferase assays on the STAT3 3'UTR were performed (Figure 5D). One-way ANOVA indicated that miR-485-5p also inhibited luciferase activity in both cell lines (P < 0.01), suggesting miR-485-5p directly regulates STAT3.
The direct interaction between miR-485-5p and NKILA was confirmed by RNA pulldown (Figure 5E). One-way ANOVA showed that the enrichment of NKILA was significantly higher in the Bio-miR-485-5p WT group compared to the Bio-NC and Bio-miR-485-5pMUT groups in both AGS and MKN45 cells (P < 0.01), confirming the direct binding between miR-485-5p and NKILA.
We next measured STAT3 expression in MKN45 and AGS cells transfected with themiR-485-5p mimic (Figure 5F). One-way ANOVA indicated that miR-485-5psignificantly repressed STAT3 expression in both cell lines (P < 0.01).
We then transfected MKN45 and AGS cells with both the miR-485-5p mimic and the NKILA overexpression plasmid (Figure 5G). One-way ANOVA showed that NKILA overexpression could restore STAT3 expression that was suppressed by miR-485-5pin both cell lines (P < 0.01).
MKN45 and AGS cells were transfected with NKILA and co-treated with miR-485-5p mimic.
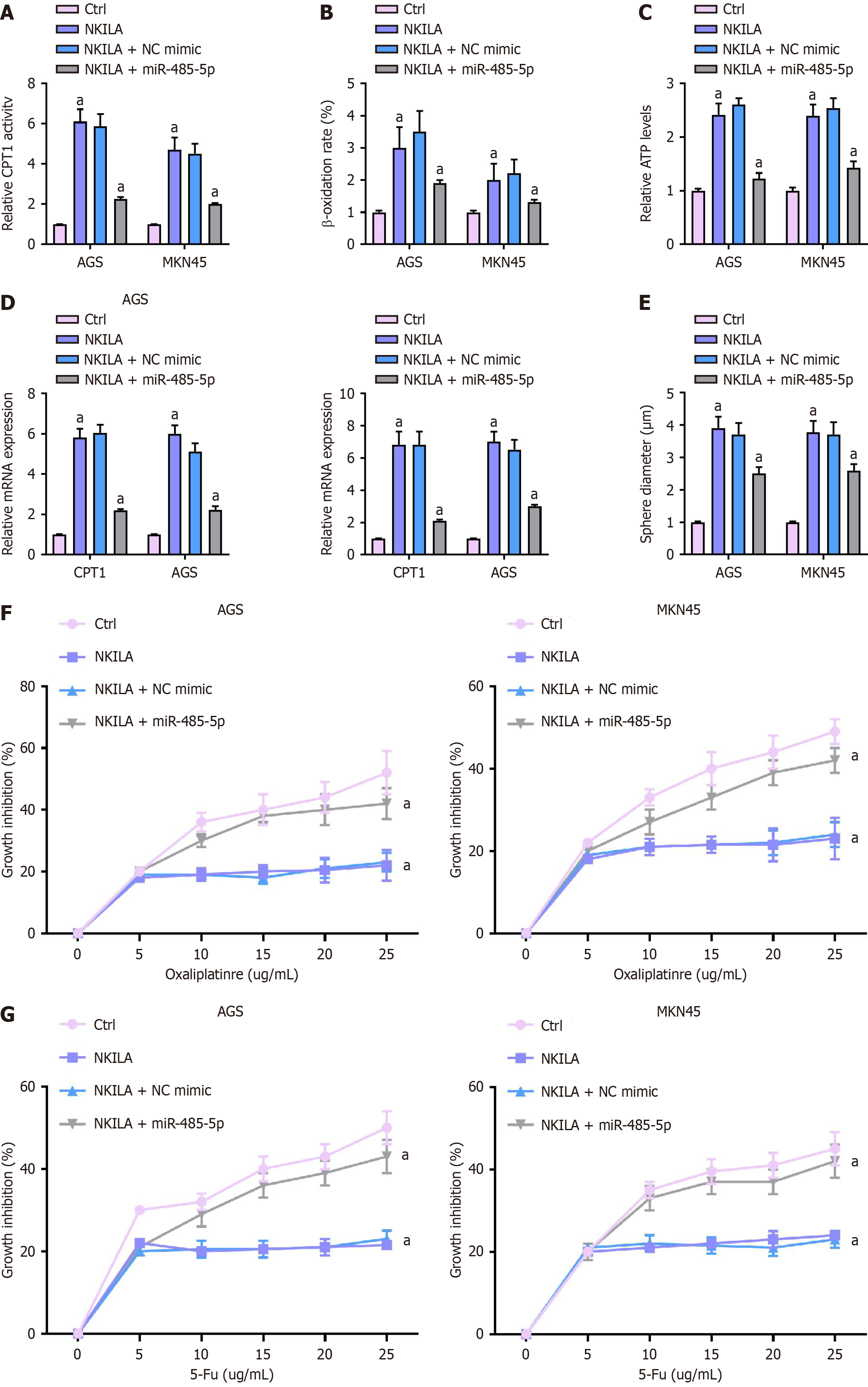
First, CPT1 activity in the cells was assessed (Figure 6A). One-way ANOVA was used to compare the Ctrl, NKILA, NKILA + NC mimic, and NKILA + miR-485-5p mimic groups. Results showed that in the NKILA group, CPT1 activity in AGS and MKN45 cells was significantly higher compared to the Ctrl group. However, in the NKILA + miR-485-5p mimic group, CPT1 activity was notably reduced compared to the NKILA group (P < 0.01).
The β-oxidation rate in the cells was analyzed (Figure 6B). One-way ANOVA showed that the β-oxidation rate was elevated in the NKILA group compared to the Ctrl group. However, the β-oxidation rate was significantly decreased in the NKILA + miR-485-5pmimic group compared to the NKILA group (P < 0.01).
The relative ATP levels in the cells were determined (Figure 6C). One-way ANOVA analysis revealed that ATP levels were elevated in the NKILA group compared to the Ctrl group. However, ATP levels were significantly lower in the NKILA + miR-485-5p mimic group compared to the NKILA group (P < 0.01).
The mRNA expression of cancer stem cell markers, including SOX2, Oct4, LIN28, and CD133, was measured by qPCR (Figure 6D). One-way ANOVA showed that the expression of these markers was upregulated in the NKILA group compared to the Ctrl group. However, miR-485-5p mimic reversed this upregulation, significantly decreasing the expression of these markers in the NKILA + miR-485-5p mimic group (P < 0.01).
Stemness was assessed using a sphere formation assay (Figure 6E). One-way ANOVA revealed that NKILA-induced sphere formation in MKN45 and AGS cells was reversed by miR-485-5p mimic. Sphere-forming ability was significantly reduced in the NKILA + miR-485-5p mimic group compared to the NKILA group (P < 0.01).
Chemoresistance to Oxa and 5-Fu was evaluated using CCK-8 (Figure 6F and G). One-way ANOVA analysis indicated that cell viability was higher in the NKILA group under Oxa or 5-Fu treatment, indicating enhanced chemoresistance. However, in the NKILA + miR-485-5p mimic group, cell viability was significantly reduced compared to the NKILA group under the same treatments (P < 0.01).
GC serves as the third leading cause of cancer-related mortality globally and is regarded as the most common malignancy of the digestive system. FAO is associated with the chemoresistance of GC cells and could be regulated by MSCs. Nevertheless, the function of MSCs-derived lncRNA NKILA in FAO-associated chemoresistance of GC cells is still obscure. In the current work, we discovered the effect of MSCs-derived lncRNA NKILA on FAO and chemoresistance in GC. Additionally, the obtained findings revealed that MSCs improved GC cells' stemness, chemoresistance, and FAO. In clinical GC tissue and GC cells co-cultured with MSCs, NKILA was elevated. NKILA improved the expression of SOX2, Oct4, LIN28, and CD133 in GC cells.
LncRNA NKILA plays a crucial role in cancer development. It has been reported that NKILA represses retinoblastoma by inhibiting XIST[23]. NKILA inhibits TGF-β-associated epithelial-mesenchymal transition in breast cancer by the NF-κB pathway[24,25]. NKILA/NF-κB signaling regulates laryngeal cancer progression[26]. The current study noticed that STAT3 expression was increased by NKILA but decreased by STAT3 shRNA in the case of MKN45 and AGS cells. Previously, a study reported that NKILA contributes to immune evasion by regulating activation-induced cell death in cancer[24]. LncRNA NKILA enhances EMTby miR-485-5p in liver cancer[19]. The findings in the current study revealed that MSCs improved GC cells' stemness, chemoresistance, and FAO. In clinical GC tissue and GC cells co-cultured with MSCs, NKILA was elevated. NKILA enhanced the expression of SOX2, Oct4, LIN28, and CD133 in GC cells. In the current study, it was observed that overexpression of NKILA increased CD44+ and CD133+ GC cells and the sphere formation of the cells. Moreover, the NKILA overexpression was able to reverse Oxa and 5-Fu-induced apoptosis and Oxa and 5-Fu-inhibited proliferation of GC cells. NKILA promoted the CPT activity, β-oxidation, and relative ATP levels, and STAT3 depletion of FAO inhibitor ETX could inhibit the phenotype. The depletion of STAT3 or ETX could reverse NKILA-upregulated SOX2, Oct4, LIN28, and CD133 in GC cells. Similarly, The Oxa and 5-Fu resistance of GC cells induced by NKILA was reversed by STAT3 silencing and ETX. The clinical significance of NKILA in GC should be confirmed in future studies.
A previous study reported that STAT3 promotes transcriptional activation of EZH2 to regulate the poor prognosis of GC[27]. Between NKILA and STAT3, we discovered the miR-485-5p binding sites. In MKN45 and AGS cells, the overexpression of miR-485-5p by its mimic was confirmed. MiR-485-5p reduced the luciferase activity of NKILA and STAT3 3'UTR in MKN45 and AGS cells. LncRNA PVT1 enhances the angiogenesis of GC by targeting the STAT3/VEGFA signaling[28]. miR-485-5p represses breast cancer progression by inhibiting MUC1[29]. miR-485-5p is a tumor suppressor and potential biomarker of colorectal cancer[30]. LINC01224 contributes to the progression of colorectal cancer by targeting miR-485-5p/MYO6 signaling[31]. Our mechanical investigation showed NKILA enhanced STAT3 expression by targeting miR-485-5p in GC cells. The NKILA-induced CPT activity, β-oxidation, and relative ATP levels were inhibited miR-485-5p in GC cells. The miR-485-5p could reverse NKILA-upregulated SOX2, Oct4, LIN28, and CD133 in GC cells. MiR-485-5p reversed the NKILA-induced sphere formation of GC cells. MiR-485-5p reversed the Oxa and 5-Fu resistance of GC cells induced by NKILA. Other targets of NKILA and miR-485-5p need to be explored in future research.
While this study provides novel insights into NKILA-mediated chemoresistance, several limitations should be acknowledged: (1) Human cell model limitations: Although our in vitro co-culture system partially mimics MSC-GC interactions, the reliance on immortalized cell lines and lack of TME components (e.g., immune cells, stromal matrix, hypoxia) may limit clinical translatability. Future studies should employ patient-derived organoids or patient-derived xenograft (PDX) models to better recapitulate human pathophysiology while addressing ethical constraints of direct human tissue research; (2) Unresolved NKILA delivery mechanisms: Although we demonstrated MSC-derived NKILA's functional role, the specific transport vehicles (exosomes vs tunneling nanotubes) mediating NKILA transfer remain uncharacterized. Live-cell imaging and exosome depletion experiments are needed to clarify this critical mechanistic step; (3) Pending in vivo validation: The therapeutic potential of NKILA targeting requires validation in orthotopic GC mouse models with NKILA knockdown/overexpression. These models would simultaneously assess tumor-specific efficacy and systemic toxicity profiles of STAT3/FAO inhibitors; and (4) Unexplored alternative pathways: While miR-485-5p/STAT3 is central to our findings (Figures 5 and 6), other NKILA-regulated pathways (e.g., Wnt/β-catenin or Hippo-YAP) may synergistically regulate stemness and warrant systematic exploration.
Finally, it was concluded that MSCs-derived lncRNA NKILA contributes to stemness and chemoresistance by FAO in GC via miR-485-5p/STAT3 MSCs-derived lncRNA NKILA contributed to stemness and chemoresistance by FAO in GC via miR-485-5p/STAT3.
Our work establishes MSC-derived NKILA as a master regulator of FAO-driven chemoresistance in GC via the miR-485-5p/STAT3 axis. These findings open avenues for NKILA-based therapeutic strategies and highlight the importance of metabolic reprogramming in cancer stem cells. Future studies should address the translational challenges outlined above to advance this paradigm toward clinical application.
| 1. | Biagioni A, Skalamera I, Peri S, Schiavone N, Cianchi F, Giommoni E, Magnelli L, Papucci L. Update on gastric cancer treatments and gene therapies. Cancer Metastasis Rev. 2019;38:537-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 127] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 2. | Yang W, Ma J, Zhou W, Cao B, Zhou X, Yang Z, Zhang H, Zhao Q, Fan D, Hong L. Molecular mechanisms and theranostic potential of miRNAs in drug resistance of gastric cancer. Expert Opin Ther Targets. 2017;21:1063-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 3. | Yuan L, Xu ZY, Ruan SM, Mo S, Qin JJ, Cheng XD. Long non-coding RNAs towards precision medicine in gastric cancer: early diagnosis, treatment, and drug resistance. Mol Cancer. 2020;19:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 4. | Chen Z, Li Y, Tan B, Zhao Q, Fan L, Li F, Zhao X. Progress and current status of molecule-targeted therapy and drug resistance in gastric cancer. Drugs Today (Barc). 2020;56:469-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2209] [Cited by in RCA: 2947] [Article Influence: 327.4] [Reference Citation Analysis (0)] |
| 6. | Bartosh TJ, Ullah M, Zeitouni S, Beaver J, Prockop DJ. Cancer cells enter dormancy after cannibalizing mesenchymal stem/stromal cells (MSCs). Proc Natl Acad Sci U S A. 2016;113:E6447-E6456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 135] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 7. | Timaner M, Tsai KK, Shaked Y. The multifaceted role of mesenchymal stem cells in cancer. Semin Cancer Biol. 2020;60:225-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 8. | Wu H, Mu X, Liu L, Wu H, Hu X, Chen L, Liu J, Mu Y, Yuan F, Liu W, Zhao Y. Bone marrow mesenchymal stem cells-derived exosomal microRNA-193a reduces cisplatin resistance of non-small cell lung cancer cells via targeting LRRC1. Cell Death Dis. 2020;11:801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 9. | Le Naour A, Prat M, Thibault B, Mével R, Lemaitre L, Leray H, Joubert MV, Coulson K, Golzio M, Lefevre L, Mery E, Martinez A, Ferron G, Delord JP, Coste A, Couderc B. Tumor cells educate mesenchymal stromal cells to release chemoprotective and immunomodulatory factors. J Mol Cell Biol. 2020;12:202-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer. 2013;13:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 1003] [Article Influence: 83.6] [Reference Citation Analysis (0)] |
| 11. | Corn KC, Windham MA, Rafat M. Lipids in the tumor microenvironment: From cancer progression to treatment. Prog Lipid Res. 2020;80:101055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 271] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 12. | Cheng S, Wang G, Wang Y, Cai L, Qian K, Ju L, Liu X, Xiao Y, Wang X. Fatty acid oxidation inhibitor etomoxir suppresses tumor progression and induces cell cycle arrest via PPARγ-mediated pathway in bladder cancer. Clin Sci (Lond). 2019;133:1745-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 13. | Chen T, Wu G, Hu H, Wu C. Enhanced fatty acid oxidation mediated by CPT1C promotes gastric cancer progression. J Gastrointest Oncol. 2020;11:695-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Chi Y, Wang D, Wang J, Yu W, Yang J. Long Non-Coding RNA in the Pathogenesis of Cancers. Cells. 2019;8:1015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 581] [Cited by in RCA: 597] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 15. | Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36:5661-5667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 757] [Cited by in RCA: 1267] [Article Influence: 158.4] [Reference Citation Analysis (0)] |
| 16. | Kumar MM, Goyal R. LncRNA as a Therapeutic Target for Angiogenesis. Curr Top Med Chem. 2017;17:1750-1757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 194] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 17. | Tan YT, Lin JF, Li T, Li JJ, Xu RH, Ju HQ. LncRNA-mediated posttranslational modifications and reprogramming of energy metabolism in cancer. Cancer Commun (Lond). 2021;41:109-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 456] [Article Influence: 91.2] [Reference Citation Analysis (0)] |
| 18. | Bhan A, Soleimani M, Mandal SS. Long Noncoding RNA and Cancer: A New Paradigm. Cancer Res. 2017;77:3965-3981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2088] [Cited by in RCA: 2163] [Article Influence: 270.4] [Reference Citation Analysis (0)] |
| 19. | Wang Y, Li C, Shi Y, Kuai J. LncRNA NKILA Promotes Epithelial-Mesenchymal Transition of Liver Cancer Cells by Targeting miR-485-5p. J Oncol. 2021;2021:1281031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Zhang GD, Li Y, Liao GJ, Qiu HW. LncRNA NKILA inhibits invasion and migration of osteosarcoma cells via NF-κB/Snail signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:4118-4125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 21. | Zhang W, Guo Q, Liu G, Zheng F, Chen J, Huang D, Ding L, Yang X, Song E, Xiang Y, Yao H. NKILA represses nasopharyngeal carcinoma carcinogenesis and metastasis by NF-κB pathway inhibition. PLoS Genet. 2019;15:e1008325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 22. | Zhu X, Du J, Yu J, Guo R, Feng Y, Qiao L, Xu Z, Yang F, Zhong G, Liu F, Cheng F, Chu M, Lin J. LncRNA NKILA regulates endothelium inflammation by controlling a NF-κB/KLF4 positive feedback loop. J Mol Cell Cardiol. 2019;126:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Lyu X, Ma Y, Wu F, Wang L, Wang L. LncRNA NKILA Inhibits Retinoblastoma by Downregulating lncRNA XIST. Curr Eye Res. 2019;44:975-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Huang D, Chen J, Yang L, Ouyang Q, Li J, Lao L, Zhao J, Liu J, Lu Y, Xing Y, Chen F, Su F, Yao H, Liu Q, Su S, Song E. NKILA lncRNA promotes tumor immune evasion by sensitizing T cells to activation-induced cell death. Nat Immunol. 2018;19:1112-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 335] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 25. | Wu W, Chen F, Cui X, Yang L, Chen J, Zhao J, Huang D, Liu J, Yang L, Zeng J, Zeng Z, Pan Y, Su F, Cai J, Ying Z, Zhao Q, Song E, Su S. LncRNA NKILA suppresses TGF-β-induced epithelial-mesenchymal transition by blocking NF-κB signaling in breast cancer. Int J Cancer. 2018;143:2213-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 26. | Yang T, Li S, Liu J, Yin D, Yang X, Tang Q. lncRNA-NKILA/NF-κB feedback loop modulates laryngeal cancer cell proliferation, invasion, and radioresistance. Cancer Med. 2018;7:2048-2063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 27. | Pan YM, Wang CG, Zhu M, Xing R, Cui JT, Li WM, Yu DD, Wang SB, Zhu W, Ye YJ, Wu Y, Wang S, Lu YY. STAT3 signaling drives EZH2 transcriptional activation and mediates poor prognosis in gastric cancer. Mol Cancer. 2016;15:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 28. | Zhao J, Du P, Cui P, Qin Y, Hu C, Wu J, Zhou Z, Zhang W, Qin L, Huang G. LncRNA PVT1 promotes angiogenesis via activating the STAT3/VEGFA axis in gastric cancer. Oncogene. 2018;37:4094-4109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 279] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 29. | Wang X, Zhou X, Zeng F, Wu X, Li H. miR-485-5p inhibits the progression of breast cancer cells by negatively regulating MUC1. Breast Cancer. 2020;27:765-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | Pan Y, Qin J, Sun H, Xu T, Wang S, He B. MiR-485-5p as a potential biomarker and tumor suppressor in human colorectal cancer. Biomark Med. 2020;14:239-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 31. | Gu J, Dong L, Wang Y, Nie W, Liu W, Zhao JA. LINC01224 promotes colorectal cancer progression through targeting miR-485-5p/MYO6 axis. World J Surg Oncol. 2021;19:281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |