Published online Aug 16, 2025. doi: 10.12998/wjcc.v13.i23.105011
Revised: March 24, 2025
Accepted: May 7, 2025
Published online: August 16, 2025
Processing time: 146 Days and 21 Hours
Macrophages play a crucial role in the tumor microenvironment, displaying remarkable plasticity that allows them to either suppress or promote tumor progression. Their polarization into M1 or M2 phenotypes could have significant prognostic implications, and manipulating this polarization may offer a novel approach to controlling colorectal neoplasms.
To evaluate the infiltration rates of M1 and M2 macrophages in colorectal neoplasia, specifically comparing cases with and without metalloproteinase mutations. Additionally, it sought to explore potential prognostic factors as
The study involved two cohorts of patients diagnosed with colorectal neoplasia: 33 patients with metalloproteinase mutations and 33 without. Macrophage quantity and polarization were assessed using markers indicative of M1 (iNOS) and M2 (CD163, CD206) macrophages. Prognostic factors and survival outcomes related to colorectal cancer (CRC) were also analyzed.
Among the 61 patients, 28 (45.9%) exhibited metalloproteinase mutations, while 33 (54.1%) did not. Tumor staging revealed that 16.9% were in stage I, 34.2% in stage II, 42.4% in stage III, and 8.5% in stage IV. The study recorded 12 patient deaths (19.7%), with 21.2% from the control group and 17.9% from the mutation group. M2 macrophages, identified by CD163 and CD206 markers, had mean counts of 23 and 17, respectively, with standard deviations of 21 and 17. In contrast, M1 macrophages, identified by iNOS, had a mean count of five per site, with a standard deviation of 11.
The study found no statistically significant differences in macrophage density between groups, irrespective of metalloproteinase mutation status, age, gender, tumor region, staging, TILS, tumor recurrence, or clinical outcomes. No association was observed between macrophage polarization and CRC prognosis or survival. However, patients with metalloproteinase mutations demonstrated a better survival rate, suggesting a potential protective role of this mutation in colorectal neoplasia.
Core Tip: This study investigates the prognostic significance of macrophage polarization in colorectal cancer (CRC), focusing on patients with and without metalloproteinase mutations. Using M1 (inducible nitric oxide synthase) and M2 (CD163, CD206) markers, macrophage densities were quantified and correlated with survival and clinical outcomes. While no significant differences in macrophage density or polarization were observed between groups, the study highlights a potential protective role of metalloproteinase mutations, as patients harboring these mutations demonstrated improved survival rates. This insight may guide future research into the tumor microenvironment and its implications for CRC prognosis and therapy.
- Citation: Brambilla E, Brambilla DJF, Tregnago AC, Riva F, Pasqualotto FF, Soldera J. Exploring macrophage polarization as a prognostic indicator for colorectal cancer: Unveiling the impact of metalloproteinase mutations. World J Clin Cases 2025; 13(23): 105011
- URL: https://www.wjgnet.com/2307-8960/full/v13/i23/105011.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i23.105011
Colorectal cancer (CRC) is the third most common type of malignant tumor worldwide. In 2020, global statistics revealed 1.9 million new CRC cases and 940000 deaths, marking a notable rise from previous years[1]. Similarly, in Brazil, estimates predict 45600 new cases annually from 2023 to 2025, equating to 21.10 cases per 100000 inhabitants. Women are expected to have slightly higher case numbers than men[2].
Risk factors for CRC can be classified as intrinsic or extrinsic. Intrinsic factors include genetic and epigenetic changes, such as oncogene activation and signals from the cellular microenvironment, which are pivotal in cancer development. Mutations in key genes like APC, KRAS, and TP53 are central to CRC pathogenesis. Extrinsic factors include lifestyle choices and environmental influences, such as obesity, lack of physical activity, smoking, alcohol use, and the consumption of processed foods. These extrinsic factors induce metabolic dysregulation and chronic inflammation, promoting carcinogenesis through cellular damage and alterations in pathways that regulate cell proliferation and programmed cell death[3].
CRC is a complex disease characterized by various molecular processes leading to the establishment of pathological features, from initiation to metastasis. These features, termed the "hallmarks of cancer," were outlined by Hanahan and Weinberg[4] in 2011 and include ten key characteristics: Genomic instability, sustained proliferative signaling, evasion of tumor suppressors, resistance to cell death, cellular immortality, angiogenesis promotion, metastasis capability, altered energy metabolism, immune evasion, and tumor-induced inflammation.
Significantly, many of these hallmarks are not solely dependent on the cancer cells but are also influenced by the tumor microenvironment (TME), which consists of the surrounding cellular and molecular milieu. The TME plays a pivotal role in cancer progression by fostering angiogenesis, immune evasion, and metastasis. Thus, the interactions between cancer cells and their microenvironment are critical for the progression and dissemination of CRC[4].
Cancer progression involves multiple steps, including genetic and epigenetic changes traditionally thought to affect only cancer cells. Recent understanding emphasizes the dynamic interactions between cancer cells and the TME. Paget's "seed and soil" analogy highlights this relationship, where the TME, consisting of fibroblasts, endothelial cells, immune cells, extracellular matrix components, and soluble factors, plays a crucial role[5,6].
The tumor stroma, composed of blood vessels, fibroblasts, and immune cells, supports cancer growth, promoting angiogenesis and suppressing immune responses. Cancer-associated fibroblasts (CAFs) are pivotal in this environment, releasing cancer cells from growth suppression and driving local inflammation and invasion. CAFs also promote angiogenesis and inhibit cytotoxic T lymphocytes, contributing to tumor progression[4].
Due to the complexity of cancer processes, efforts focus on targeting TME interactions for therapeutic advancements. Cancer cells often develop resistance to current treatments, making other TME components, such as more stable stromal cells, promising targets for intervention. Macrophages, abundantly present in tumors and associated with poor prognosis in some cancers, represent another potential therapeutic target[7].
The role of macrophages in the prognosis of CRC remains under-researched and requires further elucidation. Macrophages are immune cells present in nearly all tissues, and they express specific markers like CD68, CD163, and iNOS[7-9]. Originally identified by Metchnikoff in the 19th century, macrophages maintain tissue integrity through patrolling and clearing pathogens and debris[9]. Monocyte-derived macrophages originate from bone marrow, while embryonic macrophages are derived from the yolk sac and fetal liver. Both types are essential for tissue homeostasis and inflammation recovery, particularly through apoptotic cell removal via efferocytosis[9,10].
Carcinogens trigger inflammation, typically mediated by innate immunity. Macrophages, along with neutrophils, respond to harmful signals, engulfing pathogens and secreting molecules like nitric oxide to degrade them[10]. These cells are classified into M1 (pro-inflammatory) and M2 (anti-inflammatory) types[11]. M1 macrophages produce tumoricidal molecules and sustain inflammation by recruiting other immune cells like T cells[7,12]. CSF-1 has been shown to affect macrophage infiltration and tumor progression, with inhibition increasing CD8+ T-cell recruitment and reducing tumors[13].
When inflammation fails to control tumors, M2 macrophages resolve the inflammation, remove apoptotic cells, regulate tissue integrity, and are exploited by cancer cells to promote tumor growth and invasion[9,14,15]. Tumor-associated macrophages (TAMs) originate from monocytes recruited into the tumor tissue, differentiating in response to signaling through cytokines, chemokines, and growth factors produced by stromal cells and the TME[16]. Macrophages are key components of the TME, and M2 macrophages are predominant in most tumors, promoting invasive phenotypes[7]. High densities of M2 macrophages are linked to poor prognosis in various cancers, though in CRC, M2 macrophages correlate with a better prognosis. This discrepancy may arise from differences in macrophage marker identification and their phenotypic plasticity[11].
The TME in CRC is shaped by somatic and genetic mutations, alongside epigenetic changes. The immune system exerts selective pressure on tumor cells through the recognition of non-self antigens, mediated by immune checkpoints that regulate cell repair or destruction. Failures in this system may allow tumor cells to evade detection, leading to their persistence[17]. Immunosuppressive mechanisms in CRC include the recruitment of regulatory immune cells, such as Tregs and M1 macrophages. Additionally, the Warburg effect, where glycolysis is favored even under normoxia, supports neoplastic proliferation and creates an inhospitable environment for T cells[17].
Microsatellite instability (MSI) tumors, which comprise 15% of CRC cases, exhibit a high mutation rate and a significantly increased presentation of neoantigens compared to microsatellite-stable tumors, making them more su
Li et al's meta-analysis[19] revealed that patients with wild-type metalloproteinases had better survival compared to mutated forms, although differing methods for macrophage identification complicate conclusions. Recent advances show promising immunotherapy outcomes in MSI-positive CRC patients, but the absence of MSI in some tumors leaves a gap for alternative therapeutic exploration. Macrophage interactions within the TME remain a critical area for future research to improve CRC treatments[20].
This study aims to evaluate the infiltration rates of M1 and M2 macrophages in CRC tumors, both with and without mutations in metalloproteinases. Specifically, the study seeks to examine the correlation between macrophage infiltration rates and key prognostic factors of CRC, as well as to explore the impact of these infiltration rates on patient survival.
This retrospective study assessed 33 cases of colon and rectal adenocarcinoma with mutations in the metalloproteinases MLH1, PMS2, MSH6, and MSH2. A control group of 33 cases was selected, consisting of the next consecutive patients diagnosed with CRC, who exhibited a wild-type profile for the same metalloproteinases. The analysis focused on clinicopathological variables, including age at diagnosis, clinical staging (stages I-IV), treatment modalities (surgery or chemoradiotherapy), disease-free survival, and overall survival. The study followed the REMARK guidelines established by the NCI-EORTC Working Group on Cancer Diagnostics, ensuring adherence to the gold standard for biomarker research.
The medical records were obtained from the Pathology Unit database of the General Hospital of Caxias do Sul. The data collection encompassed records between January 1, 2018, and December 31, 2021. The General Hospital of Caxias do Sul is a reference center for CRC treatment, providing comprehensive data on CRC cases, including molecular and histopathological profiles relevant for biomarker analysis.
The patient cohort consisted of 33 individuals with colon and rectal adenocarcinoma exhibiting mutations in metalloproteinases, alongside 33 control patients with a wild-type metalloproteinase profile. Clinical data, including diagnosis date, staging, and survival outcomes, were collected from electronic medical records. All aspects of patient confidentiality and data protection were strictly maintained, with approvals from the hospital’s Scientific and Editorial Council and the Research Ethics Committee, as well as compliance with privacy protocols.
The variables under investigation included the presence of macrophage membrane markers CD68, iNOS, CD206, and CD163. CD68 served as a marker for total lymphocytes, while CD206 and CD163 were used to identify M2-polarized macrophages, with iNOS indicating M1-polarized macrophages. Original hematoxylin-eosin stained slides were reviewed for diagnostic confirmation. Areas with the highest concentration of intratumoral macrophages were identified and subsequently examined on the immunohistochemistry slides.
Immunohistochemistry slides were prepared using 3 μm thick cuts from tissue fixed in 10% buffered formalin and embedded in paraffin. The slides underwent deparaffinization and rehydration, followed by antigen retrieval using Tris-EDTA (Thermo Fisher, Waltham, MA) at 95 °C for 20 minutes. Primary antibodies against iNOS (dilution 1:100, polyclonal, Sigma-Aldrich®), CD206 (dilution 1:300, 5C11, Sigma-Aldrich®), CD163 (dilution 1:200, polyclonal, ArigoBio®), and CD68 (dilution 1:1500, K-p1, Cell Marque®) were incubated overnight and subsequently stained with DAB. Macrophages from the tissue itself served as internal controls. The immunohistochemistry slides were examined independently by two pathologists to evaluate the percentage of stained macrophages. The percentage was assessed visually at high magnification (400×). Discrepancies in interpretation were simultaneously reviewed by both pathologists, leading to a consensus.
The data analysis involved descriptive and inferential statistical methods for group comparisons. Quantitative variables were described using mean and standard deviation, while qualitative variables were presented as absolute and relative frequencies. Inferential statistical analyses employed both parametric and non-parametric methods, considering the independent design of the variables. For quantitative variables, one-way and two-way analysis of variance (ANOVA) tests were performed, with normality and homogeneity of variances checked to apply the appropriate statistical tests. Multivariate analyses, including MANOVA, were also conducted.
For qualitative variables, the χ2 test was used to assess independence. Fisher's exact test was applied for cases with two-way contingency tables, while Pearson's χ2 test of maximum likelihood ratio was used for larger tables. Additionally, cluster analysis was performed using the k-means algorithm, determining the optimal number of clusters via the elbow method. This involved plotting explained variance against the number of clusters to select the most appropriate grouping. To evaluate the internal quality of clusters, the stability of the ideal solution was calculated using Jaccard bootstrap values with 10000 iterations. A two-tailed P value of < 0.05 was deemed statistically significant. All analyses were conducted using R programming language (version 4.3.1, The R Foundation for Statistical Computing).
The sample consisted of 66 cases. Of this total, five patients did not meet all the required data criteria and were excluded, leaving 61 patients for statistical evaluation. Regarding the presence of mutations in metalloproteinases, 33 patients (54.1%) did not exhibit mutations, while 28 patients (45.9%) presented the mutation (Table 1).
| Total | No mutation, n = 33 | With mutation, n = 28 | P value | ||||
| n | % | n | % | n | % | ||
| Age (year) | 0.1351 | ||||||
| ≤ 60 | 15 | 24.6 | 11 | 33.3 | 4 | 14.3 | |
| > 60 | 46 | 75.4 | 22 | 66.7 | 24 | 85.7 | |
| Sex | 0.0931 | ||||||
| Female | 43 | 70.5 | 20 | 60.6 | 23 | 82.1 | |
| Male | 18 | 29.5 | 13 | 39.4 | 5 | 17.9 | |
| Location | 0.1541 | ||||||
| Right colon | 44 | 72.1 | 21 | 54.5 | 23 | 78.6 | |
| Left colon | 17 | 27.9 | 12 | 45.5 | 5 | 21.4 | |
| Staging | 0.051 | ||||||
| I | 10 | 16.9 | 3 | 9.7 | 7 | 25.0 | |
| II | 19 | 32.2 | 7 | 22.6 | 12 | 42.9 | |
| III | 25 | 42.4 | 18 | 58.0 | 7 | 25 | |
| IV | 5 | 8.5 | 3 | 9.7 | 2 | 7.1 | |
| Colectomy | 0.248 | ||||||
| Right side | 39 | 63.9 | 18 | 54.5 | 21 | 75.0 | |
| Left side | 15 | 24.6 | 10 | 30.3 | 5 | 17.9 | |
| Total | 7 | 11.5 | 5 | 15.2 | 2 | 7.1 | |
| Lymphocytes | 0.24 | ||||||
| Low | 30 | 50.0 | 18 | 54.6 | 12 | 44.4 | |
| Moderate | 18 | 30.0 | 7 | 21.2 | 11 | 40.7 | |
| Intense | 12 | 20.0 | 8 | 24.2 | 4 | 14.9 | |
| Reccurence | 0.4431 | ||||||
| No | 49 | 87.5 | 26 | 83.9 | 23 | 92.0 | |
| Yes | 7 | 12.5 | 5 | 16.1 | 2 | 8.0 | |
| Clinical outcome | 1.01 | ||||||
| Disease-free | 49 | 80.3 | 26 | 78.8 | 23 | 82.1 | |
| Death | 12 | 19.7 | 7 | 21.2 | 5 | 17.9 | |
The samples were categorized into two groups: Patients aged up to 60 years and patients older than 60 years. This division showed that 46 patients (75.4%) were over 60 years old, with no statistically significant difference between the groups with and without metalloproteinase mutations (P = 0.135). Concerning gender, 43 samples (70.5%) were from female patients, and again, no statistical difference was found between the groups with and without metalloproteinase mutations (P = 0.093).
When evaluating tumor location, 44 patients (65.6%) had lesions located in the right colon, while 17 patients (27.9%) had lesions located in the left colon. No statistically significant difference was found between the groups with and without metalloproteinase mutations (P = 0.062). Surgeries performed included right-sided colectomy in 39 cases (63.9%), left-sided colectomy in 15 cases (24.6%), and total colectomy in seven cases (11.5%). Among the total colectomies, four patients had neoplasms in the right colon and three in the left colon. There was no statistical difference regarding the presence or absence of mutations in these two groups (P = 0.248).
Using staging to group the samples, 10 patients were in stage I (16.9%), 19 were in stage II (34.2%), 25 were in stage III (42.4%), and five were in stage IV (8.5%). When taking metalloproteinase mutations into account, stages I and II combined had the highest number of samples with mutations (67.9%), while stages III and IV together had the highest number of samples without mutations (67.7%). A statistically significant difference was identified between the staging groups with and without metalloproteinase mutations (P = 0.05).
By the end of the data collection period, seven patients (12.5%) exhibited disease recurrence, of whom two had metalloproteinase mutations (8%) and five did not (16.1%), with no statistically significant difference between the groups (P = 0.443).
There were 12 deaths (19.7%) during the study: Seven (21.2%) in the control group and five in the mutation group (17.9%). No statistically significant difference was identified between clinical outcomes and the presence or absence of mutations (P = 1.0). When analyzing by stage, one death occurred in a patient in stage I, five in stage II, three in stage III, and three in stage IV. The highest mortality occurred in stage IV, accounting for 60% of the cases (Table 2). Of the total deaths, three were due to coronavirus disease 2019 (COVID-19) and were unrelated to tumor recurrence. Among the patients who died from COVID-19 complications, one was in stage I and two in stage II, all of whom had metalloproteinase mutations. Additionally, two patients in the mutation group died from adenocarcinoma, and both were in stage IV.
| Total | Stage I, n = 10 | Stage II, n = 19 | Stage III, n = 25 | Stage IV, n = 5 | P value | ||||||
| n | % | n | % | n | % | n | % | n | % | ||
| Instability | 0.05a | ||||||||||
| No mutation | 33 | 52.5 | 3 | 30.0 | 8 | 40.0 | 18 | 72.0 | 4 | 66.66 | |
| With mutation | 28 | 47.5 | 7 | 70.0 | 12 | 60.0 | 7 | 28.0 | 2 | 33.33 | |
| Age (year) | 0.61 | ||||||||||
| ≤ 60 | 15 | 24.6 | 1 | 10.0 | 6 | 30.0 | 7 | 28.0 | 1 | 16.7 | |
| > 60 | 46 | 75.4 | 9 | 90.0 | 14 | 70.0 | 18 | 72.0 | 5 | 83.3 | |
| Sex | 0.055 | ||||||||||
| Female | 43 | 70.5 | 9 | 90.0 | 17 | 85.0 | 14 | 56.0 | 3 | 50.0 | |
| Male | 18 | 29.5 | 1 | 10.0 | 3 | 15.0 | 11 | 44.0 | 3 | 50.0 | |
| Location | 0.722 | ||||||||||
| Right colon | 44 | 72.1 | 8 | 80.0 | 16 | 80.0 | 17 | 68.0 | 3 | 50.0 | |
| Left colon | 17 | 27.9 | 2 | 20.0 | 4 | 20.0 | 8 | 32.0 | 3 | 50.0 | |
| Colectomy | 0.342 | ||||||||||
| Right side | 39 | 63.9 | 7 | 70.0 | 15 | 75.0 | 14 | 56.0 | 3 | 50.0 | |
| Left side | 15 | 24.6 | 3 | 30.0 | 3 | 15.0 | 6 | 24.0 | 3 | 50.0 | |
| Total | 7 | 11.5 | 0 | 0.0 | 2 | 10.0 | 5 | 20.0 | 0 | 0.0 | |
| Lymphocytes | 0.874 | ||||||||||
| Low | 30 | 50.0 | 3 | 33.3 | 10 | 50.0 | 14 | 56.0 | 3 | 50.0 | |
| Moderate | 18 | 30.0 | 4 | 44.4 | 6 | 30.0 | 7 | 28.0 | 1 | 16.6 | |
| Intense | 12 | 20.0 | 2 | 22.2 | 4 | 20.0 | 4 | 16.0 | 2 | 33.3 | |
| Recurrence | 0.59 | ||||||||||
| No | 49 | 87.5 | 9 | 100.0 | 16 | 89.0 | 19 | 82.6 | 5 | 83.3 | |
| Yes | 7 | 12.5 | 0 | 0.0 | 2 | 11.0 | 4 | 17.4 | 1 | 16.7 | |
| Clinical outcome | 0.022a | ||||||||||
| Disease-free | 49 | 80.3 | 8 | 80.0 | 17 | 85.0 | 22 | 88.0 | 2 | 33.3 | |
| Death | 12 | 19.7 | 1 | 10.0 | 3 | 15.0 | 3 | 12.0 | 4 | 66.6 | |
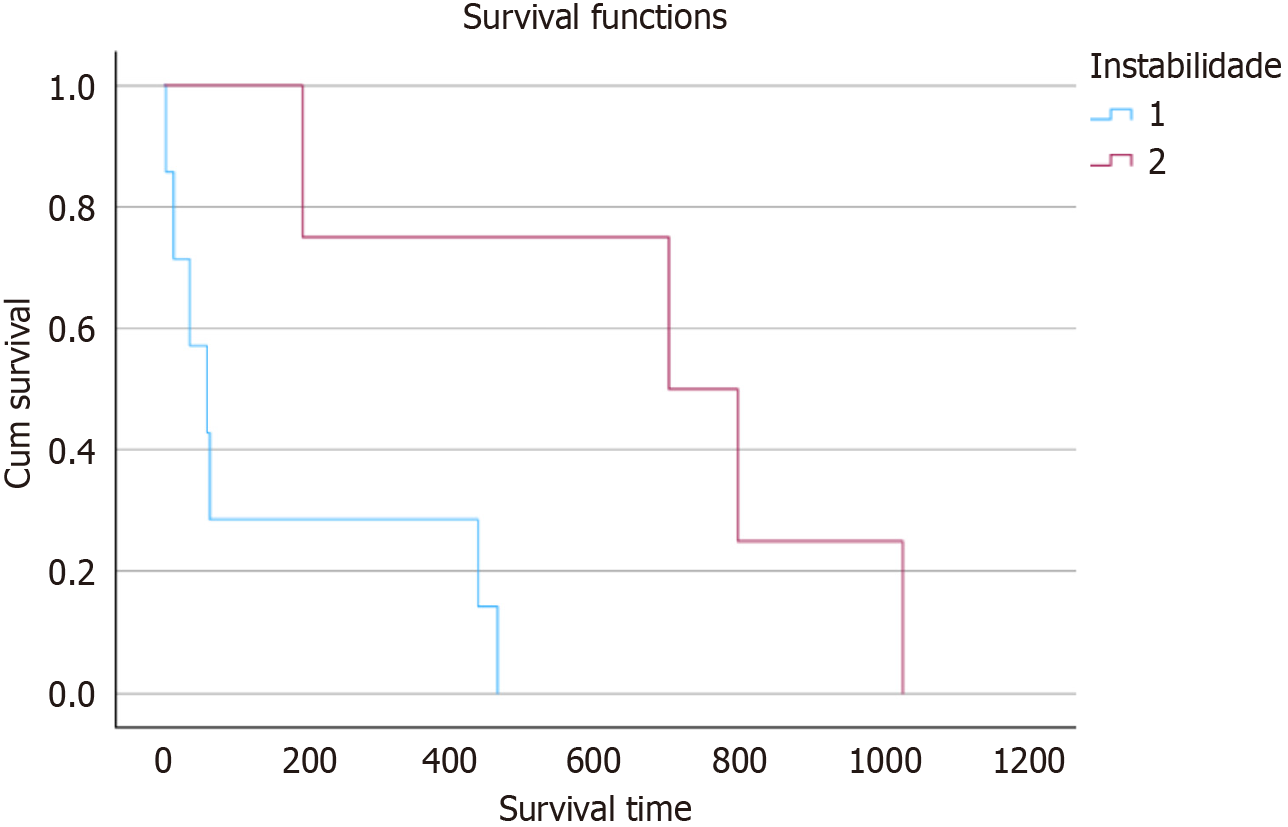
In terms of time to death among patients with and without metalloproteinase mutations (Figure 1), the survival curves showed a statistically significant difference (P = 0.016). The group of patients with mutations exhibited longer survival, while those without mutations tended to have an earlier death. The data also showed considerable heterogeneity regarding the presence of macrophages within the neoplasms. In general, the quantitative variables exhibited a very high standard deviation, making their evaluation difficult. The CD68 marker for total macrophages showed an average of 32 macrophages per field with a standard deviation of 26. The CD163 and CD206 markers, indicative of M2-polarized macrophages, had averages of 23 and 17 macrophages, with standard deviations of 21 and 17, respectively (Table 3). In contrast, the iNOS marker for M1-polarized macrophages showed an average of five macrophages per field with a standard deviation of 11.
| n | % | mean ± SD | P value | ||
| CD68 | 0.757 | ||||
| Right side | No mutation | 21 | 47.7 | 31.667 ± 25.997 | |
| With mutation | 23 | 52.3 | 30.0 ± 25.849 | ||
| Left side | No mutation | 12 | 70.6 | 33.75 ± 28.534 | |
| With mutation | 5 | 29.4 | 37.2 ± 32.042 | ||
| CD163 | 0.256 | ||||
| Right side | No mutation | 21 | 47.7 | 23.881 ± 20.987 | |
| With mutation | 23 | 52.3 | 20.109 ± 19.934 | ||
| Left side | No mutation | 12 | 70.6 | 21.750 ± 24.076 | |
| With mutation | 5 | 29.4 | 33.3 ± 28.504 | ||
| CD206 | 0.117 | ||||
| Right side | No mutation | 21 | 47.7 | 19.057 ± 19.358 | |
| With mutation | 23 | 52.3 | 14.226 ± 15.599 | ||
| Left side | No mutation | 12 | 70.6 | 15.458 ± 12.648 | |
| With mutation | 5 | 29.4 | 27.4 ± 23.954 | ||
| iNOS | 0.179 | ||||
| Right side | No mutation | 21 | 47.7 | 6.767 ± 13.487 | |
| With mutation | 23 | 52.3 | 3.57 ± 4.831 | ||
| Left side | No mutation | 12 | 70.6 | 3.383 ± 4.522 | |
| With mutation | 5 | 29.4 | 8.21 ± 15.199 |
When examining the effect of patient variables on macrophage density as assessed by the CD163, iNOS, and CD206 markers collectively, a statistically significant interaction effect was observed between instability and clinical outcome (Table 4, P = 0.019). Individually, the macrophage density obtained through these markers did not demonstrate a statistically significant difference between the groups when considering the studied variables, such as metalloproteinase mutation, age, gender, tumor location, staging, TME, recurrence, and clinical outcomes (Table 5).
| Degrees of freedom | F | Hotelling-Lawley test | P value | |
| Instability | 1 | 0.314 | 0.005 | 0.958 |
| Age | 1 | 0.537 | 0.028 | 0.659 |
| Location | 1 | 0.058 | 0.003 | 0.936 |
| Staging | 3 | 1.697 | 0.296 | 0.094 |
| Recurrence | 1 | 0.650 | 0.038 | 0.586 |
| Clinical outcome | 1 | 1.929 | 0.102 | 0.305 |
| Instability vs location | 1 | 1.061 | 0.006 | 0.322 |
| Instability vs clinical outcome | 1 | 2.592 | 0.141 | 0.019 |
| Total | CD68 | CD163 | iNOS | CD206 | ||||||
| n | % | mean ± SD | P value | mean ± SD | P value | mean ± SD | P value | mean ± SD | P value | |
| Instability | 0.753 | 0.868 | 0.524 | 0.690 | ||||||
| No mutation | 33 | 54.1 | 32.424 ± 26.52 | 23.106 ± 21.809 | 5.536 ± 11.11 | 17.748 ± 17.097 | ||||
| With mutation | 28 | 45.9 | 31.286 ± 26.541 | 22.464 ± 21.694 | 4.398 ± 7.518 | 16.579 ± 17.598 | ||||
| Age (year) | 0.483 | 0.706 | 0.993 | 0.251 | ||||||
| ≤ 60 | 15 | 24.6 | 33.0 ± 21.696 | 22.5 ± 17.687 | 5.377 ± 9.082 | 20.067 ± 15.374 | ||||
| > 60 | 46 | 75.4 | 31.543 ± 27.861 | 22.913 ± 22.877 | 4.896 ± 9.819 | 16.280 ± 17.804 | ||||
| Sex | 0.295 | 0.496 | 0.701 | 0.275 | ||||||
| Female | 43 | 70.5 | 33.953 ± 26.403 | 23.919 ± 21.043 | 4.526 ± 6.575 | 19.191 ± 18.764 | ||||
| Male | 18 | 29.5 | 27.0 ± 26.183 | 20.167 ± 23.208 | 6.181 ± 14.644 | 12.483 ± 11.825 | ||||
| Location | 0.77 | 0.923 | 0.332 | 0.551 | ||||||
| Right colon | 44 | 72.1 | 30.795 ± 25.63 | 21.909 ± 20.293 | 5.095 ± 4.803 | 16.532 ± 17.457 | ||||
| Left colon | 17 | 27.9 | 34.765 ± 28.619 | 25.147 ± 25.121 | 4.803 ± 8.772 | 18.971 ± 16.879 | ||||
| Staging | 0.371 | 0.454 | 0.402 | 0.973 | ||||||
| I | 10 | 16.9 | 24.5 ± 21.141 | 15.65 ± 15.431 | 5.06 ± 6.184 | 16.1 ± 15.911 | ||||
| II | 19 | 32.2 | 37.368 ± 25.732 | 24.079 ± 21.545 | 6.029 ± 8.282 | 18.684 ± 17.811 | ||||
| III | 25 | 42.4 | 28.84 ± 26.226 | 22.42 ± 22.71 | 2.868 ± 3.554 | 17.808 ± 19.248 | ||||
| IV | 5 | 8.5 | 43.0 ± 38.987 | 34.4 ± 28.369 | 12.8 ± 28.066 | 10.94 ± 8.029 | ||||
| Colectomy | 0.93 | 0.994 | 0.59 | 0.634 | ||||||
| Right side | 39 | 63.9 | 31.026 ± 26.462 | 22.474 ± 20.703 | 4.954 ± 10.326 | 18.069 ± 17.915 | ||||
| Left side | 15 | 24.6 | 33.4 ± 28.306 | 23.567 ± 24.243 | 3.777 ± 5.634 | 16.033 ± 14.502 | ||||
| Total | 7 | 11.5 | 33.571 ± 24.616 | 23.071 ± 23.816 | 8.0 ± 12.342 | 14.957 ± 20.454 | ||||
| Lymphocytes | 0.636 | 0.593 | 0.544 | 0.417 | ||||||
| Low | 30 | 50.0 | 33.867 ± 28.295 | 36.25 ± 27.89 | 6.555 ± 11.843 | 16.033 ± 16.511 | ||||
| Moderate | 18 | 30.0 | 26.944 ± 22.37 | 22.567 ± 23.103 | 2.339 ± 3.0 | 15.261 ± 15.029 | ||||
| Intense | 12 | 20.0 | 36.25 ± 27.89 | 21.306 ± 20.569 | 5.583 ± 9.758 | 24.1 ± 21.661 | ||||
| Recurrence | 0.499 | 0.719 | 0.388 | 0.941 | ||||||
| No | 49 | 87.5 | 33.98 ± 26.279 | 24.337 ± 22.125 | 5.637 ± 10.421 | 17.518 ± 16.553 | ||||
| Yes | 7 | 12.5 | 30.714 ± 31.282 | 22.929 ± 22.606 | 2.507 ± 3.446 | 22.286 ± 25.063 | ||||
| Clinical outcome | 0.252 | 0.429 | 0.208 | 0.167 | ||||||
| Disease-free | 49 | 80.3 | 30.261 ± 25.51 | 23.378 ± 21.317 | 4.578 ± 6.32 | 10.392 ± 9.601 | ||||
| Death | 12 | 19.7 | 27.917 ± 30.261 | 20.5 ± 23.434 | 6.796 ± 17.917 | 18.882 ± 18.273 | ||||
Due to the high degree of heterogeneity in the data, machine learning methods were employed to assess possible patterns in macrophage density. From these data, two distinct groups were identified: (1) Group 1 with higher macrophage density; and (2) Group 2 with lower macrophage density per field (Table 6). Additionally, a statistically significant difference was found between these groups concerning macrophage density as identified by the CD68 marker (P < 0.01). Group 1 had an average of 64 macrophages per field, while Group 2 had an average of 17 macrophages per field. Using the CD206 marker, a statistically significant difference was also found between the groups (P < 0.01), with Group 1 showing an average of 38 macrophages per field, and Group 2 showing an average of 7 macrophages per field. Lastly, the CD163 marker showed an average of 50 macrophages per field in Group 1 and 15 macrophages per field in Group 2 (P < 0.01).
| Group 1, n = 19 | Group 2, n = 42 | P value | |
| CD 68 | 64.737 ± 17.048 | 17.048 ± 12.61 | < 0.01 |
| CD163 | 50.053 ± 10.488 | 15.29 ± 8.897 | < 0.01 |
| iNOS | 9.474 ± 15.19 | 2.996 ± 4.389 | 0.064 |
| CD 206 | 38.211 ± 14.635 | 7.712 ± 6.462 | < 0.01 |
| Age (complete years) | 64.947 ± 11.038 | 66.405 ± 12.655 | 0.548 |
| Time to death (days) | 161.333 ± 238.123 | 421.875 ± 391.745 | 0.279 |
The clustering analysis results are supported by the correlations between the measurements obtained using the different markers to identify macrophages (Table 7). When evaluating these two clusters, none of the studied variables, such as the presence of metalloproteinase mutations, staging, tumor location, or survival, showed a statistically significant relationship (Table 8).
| Total | Group 1, n = 19 | Group 2, n = 42 | P value | ||||
| n | % | n | % | n | % | ||
| Instability | 0.785a | ||||||
| No mutation | 33 | 52.5 | 11 | 57.9 | 22 | 52.4 | |
| With mutation | 28 | 47.5 | 8 | 42.1 | 20 | 47.6 | |
| Age (year) | 1.0a | ||||||
| ≤ 60 | 15 | 24.6 | 5 | 26.3 | 10 | 23.8 | |
| > 60 | 46 | 75.4 | 14 | 73.7 | 32 | 76.2 | |
| Sex | 0.14a | ||||||
| Female | 43 | 70.5 | 16 | 84.2 | 27 | 64.3 | |
| Male | 18 | 29.5 | 3 | 15.8 | 15 | 35.71 | |
| Location | 0.76a | ||||||
| Right colon | 44 | 72.1 | 13 | 68.4 | 31 | 73.8 | |
| Left colon | 17 | 27.9 | 6 | 31.6 | 11 | 26.2 | |
| Staging | 0.96 | ||||||
| I | 10 | 16.9 | 3 | 16.7 | 7 | 17.1 | |
| II | 19 | 32.2 | 6 | 33.3 | 13 | 31.7 | |
| III | 25 | 42.4 | 7 | 38.9 | 18 | 43.9 | |
| IV | 5 | 8.5 | 2 | 11.1 | 3 | 7.3 | |
| Colectomy | 0.592 | ||||||
| Right side | 39 | 63.9 | 13 | 68.4 | 26 | 61.9 | |
| Left side | 15 | 24.6 | 5 | 26.3 | 10 | 23.8 | |
| Total | 7 | 11.5 | 1 | 5.3 | 6 | 14.3 | |
| Lymphocytes | 0.91 | ||||||
| Low | 30 | 50.0 | 18 | 54.6 | 12 | 44.4 | |
| Moderate | 18 | 30.0 | 7 | 21.2 | 11 | 40.7 | |
| Intense | 12 | 20.0 | 8 | 24.2 | 4 | 14.9 | |
| Recurrence | 0.679a | ||||||
| No | 49 | 87.5 | 16 | 84.2 | 33 | 78.5 | |
| Yes | 7 | 12.5 | 3 | 15.8 | 4 | 9.5 | |
| Clinical Outcome | 0.737a | ||||||
| Disease-free | 50 | 82.0 | 16 | 84.2 | 34 | 81.0 | |
| Death | 11 | 18.0 | 3 | 15.8 | 8 | 19.0 | |
Macrophages are essential for maintaining tissue integrity, hemostasis, and repair, performing specialized functions that vary by organ and gene expression[21]. They originate from fetal hematopoiesis, first in the yolk sac and then in the liver, and integrate into tissues. In the colon, embryonic macrophages are replaced by monocyte-derived macrophages formed in the bone marrow postnatally[22]. Macrophages play critical roles in hemostasis, inflammation resolution, angiogenesis, and the clearance of apoptotic cells, exhibiting diverse functions influenced by tissue type and pathological conditions. This diversity is regulated by epigenetic responses related to interferon and metabolism[10]. Macrophages secrete inflammatory mediators, such as TNF-α, which can either protect against pathogens (M1) or lead to tissue damage; thus, regulation towards an anti-inflammatory phenotype (M2) is vital for healing[23].
TME consists of various non-tumor cells, including fibroblasts, endothelial cells, and immune cells, along with extracellular components such as chemokines and cytokines. Investigating macrophages is crucial due to their polarized functions[24]. Generally categorized as M1 (pro-inflammatory) or M2 (anti-inflammatory), M2 macrophages include TAMs, which are often linked to poor prognoses[9]. TAMs promote inflammation, facilitate immune evasion, and enhance tumor progression[6]. Macrophage regulation is influenced by tumor cell properties and TME characteristics, prompting increased interest in targeting TAMs for immunotherapy[10].
In this study, CD68 was used to identify macrophages, with iNOS marking M1 and CD163 and CD206 marking M2 macrophages. M1 macrophages are identified by high TNF-α and iNOS expression, while M2 macrophages show elevated arginase 1, IL-10, CD163, CD204, and CD206[25]. The suitability of CD163 as an M2 marker is debated, leading to the decision to use both CD163 and CD206 in this evaluation[26-30].
Multiple methodologies exist for assessing macrophages in tumor tissues. This study utilized the PD-L1 IHC 22C3 pharmDx© protocol, originally designed for lung cancer, to evaluate macrophages in CRC samples[31]. Two pathologists identified the area of highest macrophage concentration using CD68 staining and calculated the percentage of macrophages marked by CD163, CD206, and iNOS through the Tumor Proportion Score.
Forssell et al[32] linked elevated macrophage levels at tumor margins to improved prognosis, although our study did not consider macrophage localization due to sample size constraints. Furthermore, we classified tumors based on metalloproteinase mutations, noting that these mutations are often linked to earlier disease stages, a relationship corroborated by previous studies[10,33,34]. Tumors with metalloproteinase mutations also displayed increased survival rates[14], as observed in our findings (P = 0.016). It is important to note that a correlation between overall survival and disease-free time was demonstrated previously, which aimed to identify a group that would benefit from adjuvant chemotherapy in stage II colon cancer patients[35].
In our sample, only two patients with repair protein mutations succumbed to CRC, while the remaining three deaths in this group involved patients in stages I and II of cancer, resulting from COVID-19. Other study variables, including age, sex, tumor location, type of surgery performed, TILs, and macrophage count, did not show significant associations with survival rates. It is important to note that a correlation between overall survival and disease-free time was demonstrated by Feng et al[35], which aimed to identify a group that would benefit from adjuvant chemotherapy in stage II colon cancer patients[35].
No significant differences in macrophage density were found between patients with and without metalloproteinase mutations, aligning with Edin et al[36], while Bauer et al[37] reported greater macrophage infiltration in MSI cases without examining prognostic factors.
Regarding the number of macrophages obtained from immunohistochemical analysis, our results depict a positive correlation between the quantity of macrophages identified using the markers CD68, CD163, CD206, and iNOS. These findings partially align with the study conducted by Koelzer et al[34] in 2016, which found a correlation between CD68 and CD163 but not between CD68 and iNOS[34].
Although TAMs are frequently presented as factors of poor prognosis[38,39], this relationship remains controversial in the context of CRC, as demonstrated by the meta-analysis conducted by Zhang et al[40], which compared the presence of macrophages with disease prognosis[40].
Such uncertainty persists in light of conflicting findings within the literature. For instance, Khorana et al[41] demonstrated that TAM infiltration and vascular endothelial growth factor are associated with a better prognosis[41]. Conversely, Shabo et al[27] observed higher recurrence rates and lower survival for rectal tumors with increased CD163 expression. Additionally, TAMs have potential as predictors of hepatic metastases, with a decrease in M1 macrophages and an increase in M2 macrophages observed in stage IIIB patients, who are more likely to develop metastases[42].
Another meta-analysis exploring 24 studies indicated that a high rate of TAM infiltration was associated with favorable five-year survival rates for CRC cases (hazard ratio = 0.69, 95% confidence interval = 0.55–0.87), albeit with high heterogeneity[19]. Recent work by Elomaa et al[43] further demonstrated that PD-L1+ macrophages—predominantly M1-polarized and closely associated with tumor cells—correlate with improved clinical outcomes. Moreover, patients without deficiencies in repair proteins exhibited better prognoses, suggesting that the molecular context, including potential protective effects conferred by metalloproteinase mutations, may influence macrophage behavior[19]. In our study, although overall macrophage quantity did not show significant differences across prognostic variables, this observation mirrors findings by Li et al[19], where 16 of 27 articles reported similar inconsistencies. In contrast, emerging evidence from single-cell analyses has identified distinct macrophage subpopulations, such as EEF1G+ macrophages, that are closely linked to metastatic potential and immunometabolic remodeling[44]. Together, these studies highlight that variations in macrophage subpopulation characterization and underlying molecular mechanisms may account for discrepancies in prognostic associations.
The cluster analysis in our study identified two well-defined groups based on macrophage quantity; however, cross-referencing these clusters with prognostic outcomes did not reveal significant differences. This contrasts with findings from Koelzer et al[34], where specific macrophage phenotypes were associated with patient prognosis. In support of this, Donadon et al[45] showed that macrophage morphology—delineating small and large subsets—correlates with distinct functional states and survival outcomes, emphasizing that quantitative assessment alone may be insufficient. Ad
Overall, discrepancies have been observed in the literature regarding the quantity of macrophages. The polarization of macrophages appears to be a crucial factor in tumor progression and, thus, holds potential for prognostic prediction. Furthermore, it is important to consider the site of infiltration, whether in the stroma or at the tumor front. Although the results of this study did not present statistically significant inferences, the findings pave the way for more in-depth investigations. Identifying patterns in macrophage quantity from a prognostic perspective, as well as the possibility of manipulating these cells through immunotherapy, represents key research directions aimed at advancing cancer medicine.
This study is subject to several limitations. The small sample size of patients with the identified mutation, while including all available cases at our service, remains relatively small for studies investigating complex tumor biology. This may limit the representativeness and statistical power of the results, potentially affecting the ability to fully elucidate the relationship between macrophage polarization and various prognostic factors. Additionally, the evaluation of ma
In this study, no differences were found in the presence of polarized macrophages, whether M1 or M2, between groups of patients with or without mutations in DNA repair genes. Additionally, no statistically significant differences were identified when evaluating predictive factors related to CRC and survival. These results may be attributed to the small sample size of patients, as well as the limited observation period.
The authors would like to extend our sincere gratitude to the Centro de Patologia Médica for their invaluable assistance with the immunohistochemistry analyses. We are also grateful to the Postgraduate Program at the Universidade de Caxias do Sul for the support provided through the PhD program, and to Hospital Geral de Caxias do Sul for their ongoing support in facilitating this research. Their contributions were instrumental in the completion of this study, which is Brambilla B PhD thesis.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64624] [Article Influence: 16156.0] [Reference Citation Analysis (176)] |
| 2. | Instituto Nacional de Câncer - Inca. Normas e Recomendações do Instituto Nacional de Câncer/MS. Rev Bras Cancerol. 2023;46:23-33. [RCA] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 3. | Thanikachalam K, Khan G. Colorectal Cancer and Nutrition. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 478] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
| 4. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 47129] [Article Influence: 3366.4] [Reference Citation Analysis (5)] |
| 5. | Wang H, Tian T, Zhang J. Tumor-Associated Macrophages (TAMs) in Colorectal Cancer (CRC): From Mechanism to Therapy and Prognosis. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 230] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 6. | Xiao Y, Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. 2021;221:107753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 1135] [Article Influence: 227.0] [Reference Citation Analysis (2)] |
| 7. | Kim J, Bae JS. Tumor-Associated Macrophages and Neutrophils in Tumor Microenvironment. Mediators Inflamm. 2016;2016:6058147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 547] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 8. | Scodeller P, Simón-Gracia L, Kopanchuk S, Tobi A, Kilk K, Säälik P, Kurm K, Squadrito ML, Kotamraju VR, Rinken A, De Palma M, Ruoslahti E, Teesalu T. Precision Targeting of Tumor Macrophages with a CD206 Binding Peptide. Sci Rep. 2017;7:14655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 9. | Viola A, Munari F, Sánchez-Rodríguez R, Scolaro T, Castegna A. The Metabolic Signature of Macrophage Responses. Front Immunol. 2019;10:1462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 553] [Cited by in RCA: 1318] [Article Influence: 219.7] [Reference Citation Analysis (0)] |
| 10. | DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19:369-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1310] [Cited by in RCA: 1633] [Article Influence: 272.2] [Reference Citation Analysis (0)] |
| 11. | Aras S, Zaidi MR. TAMeless traitors: macrophages in cancer progression and metastasis. Br J Cancer. 2017;117:1583-1591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 470] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 12. | Anderson NR, Minutolo NG, Gill S, Klichinsky M. Macrophage-Based Approaches for Cancer Immunotherapy. Cancer Res. 2021;81:1201-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 444] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 13. | Strachan DC, Ruffell B, Oei Y, Bissell MJ, Coussens LM, Pryer N, Daniel D. CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8(+) T cells. Oncoimmunology. 2013;2:e26968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 317] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 14. | IJsselsteijn ME, Sanz-Pamplona R, Hermitte F, de Miranda NFCC. Colorectal cancer: A paradigmatic model for cancer immunology and immunotherapy. Mol Aspects Med. 2019;69:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2416] [Cited by in RCA: 2566] [Article Influence: 122.2] [Reference Citation Analysis (0)] |
| 16. | Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4154] [Cited by in RCA: 4846] [Article Influence: 242.3] [Reference Citation Analysis (1)] |
| 17. | Sillo TO, Beggs AD, Morton DG, Middleton G. Mechanisms of immunogenicity in colorectal cancer. Br J Surg. 2019;106:1283-1297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Taieb J, Svrcek M, Cohen R, Basile D, Tougeron D, Phelip JM. Deficient mismatch repair/microsatellite unstable colorectal cancer: Diagnosis, prognosis and treatment. Eur J Cancer. 2022;175:136-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 146] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 19. | Li J, Li L, Li Y, Long Y, Zhao Q, Ouyang Y, Bao W, Gong K. Tumor-associated macrophage infiltration and prognosis in colorectal cancer: systematic review and meta-analysis. Int J Colorectal Dis. 2020;35:1203-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 20. | Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, El Dika IH, Segal N, Shcherba M, Sugarman R, Stadler Z, Yaeger R, Smith JJ, Rousseau B, Argiles G, Patel M, Desai A, Saltz LB, Widmar M, Iyer K, Zhang J, Gianino N, Crane C, Romesser PB, Pappou EP, Paty P, Garcia-Aguilar J, Gonen M, Gollub M, Weiser MR, Schalper KA, Diaz LA Jr. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N Engl J Med. 2022;386:2363-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 897] [Article Influence: 299.0] [Reference Citation Analysis (0)] |
| 21. | Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2920] [Article Influence: 194.7] [Reference Citation Analysis (0)] |
| 22. | Nielsen SR, Schmid MC. Macrophages as Key Drivers of Cancer Progression and Metastasis. Mediators Inflamm. 2017;2017:9624760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 226] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 23. | Vinnakota K, Zhang Y, Selvanesan BC, Topi G, Salim T, Sand-Dejmek J, Jönsson G, Sjölander A. M2-like macrophages induce colon cancer cell invasion via matrix metalloproteinases. J Cell Physiol. 2017;232:3468-3480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (3)] |
| 24. | Lavin Y, Merad M. Macrophages: gatekeepers of tissue integrity. Cancer Immunol Res. 2013;1:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 25. | Heng Y, Zhu X, Lin H, Jingyu M, Ding X, Tao L, Lu L. CD206(+) tumor-associated macrophages interact with CD4(+) tumor-infiltrating lymphocytes and predict adverse patient outcome in human laryngeal squamous cell carcinoma. J Transl Med. 2023;21:167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 26. | Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, Klapproth K, Schäkel K, Garbi N, Jäger D, Weitz J, Schmitz-Winnenthal H, Hämmerling GJ, Beckhove P. Low-dose irradiation programs macrophage differentiation to an iNOS⁺/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 661] [Cited by in RCA: 839] [Article Influence: 69.9] [Reference Citation Analysis (2)] |
| 27. | Shabo I, Olsson H, Sun XF, Svanvik J. Expression of the macrophage antigen CD163 in rectal cancer cells is associated with early local recurrence and reduced survival time. Int J Cancer. 2009;125:1826-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 28. | Barros MH, Hauck F, Dreyer JH, Kempkes B, Niedobitek G. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS One. 2013;8:e80908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 453] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 29. | Xu ZJ, Gu Y, Wang CZ, Jin Y, Wen XM, Ma JC, Tang LJ, Mao ZW, Qian J, Lin J. The M2 macrophage marker CD206: a novel prognostic indicator for acute myeloid leukemia. Oncoimmunology. 2020;9:1683347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 30. | Konstantinov AS, Kovaleva OV, Samoilova DV, Shelekhova KV. Role of macrophages in progression of colorectal cancer: a contrast with the traditional paradigm. Int J Clin Exp Pathol. 2022;15:403-411. [PubMed] |
| 31. | Agilent/Dako. PD-L1 IHC 22C3 pharmDx Interpretation Manual - NSCLC. Santa Clara: Agilent Technologies, 2018. Available from: https://www.agilent.com/cs/library/usermanuals/public/29158_pd-l1-ihc-22C3-pharmdx-nsclc-interpretation-manual.pdf. |
| 32. | Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. 2007;13:1472-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 434] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 33. | Varela GB, Santos FSD, Brambilla DJF, Brambilla E. Prevalência de Alterações nas Proteínas de Reparo do Adenocarcinoma Colorretal em Serviço de Referência no Sul do Brasil. J Coloproctol. 2021;. [DOI] [Full Text] |
| 34. | Koelzer VH, Canonica K, Dawson H, Sokol L, Karamitopoulou-Diamantis E, Lugli A, Zlobec I. Phenotyping of tumor-associated macrophages in colorectal cancer: Impact on single cell invasion (tumor budding) and clinicopathological outcome. Oncoimmunology. 2016;5:e1106677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 35. | Feng Q, Chang W, Mao Y, He G, Zheng P, Tang W, Wei Y, Ren L, Zhu D, Ji M, Tu Y, Qin X, Xu J. Tumor-associated Macrophages as Prognostic and Predictive Biomarkers for Postoperative Adjuvant Chemotherapy in Patients with Stage II Colon Cancer. Clin Cancer Res. 2019;25:3896-3907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 36. | Edin S, Wikberg ML, Dahlin AM, Rutegård J, Öberg Å, Oldenborg PA, Palmqvist R. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS One. 2012;7:e47045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 322] [Cited by in RCA: 387] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 37. | Bauer K, Michel S, Reuschenbach M, Nelius N, von Knebel Doeberitz M, Kloor M. Dendritic cell and macrophage infiltration in microsatellite-unstable and microsatellite-stable colorectal cancer. Fam Cancer. 2011;10:557-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Oosterling SJ, van der Bij GJ, Meijer GA, Tuk CW, van Garderen E, van Rooijen N, Meijer S, van der Sijp JR, Beelen RH, van Egmond M. Macrophages direct tumour histology and clinical outcome in a colon cancer model. J Pathol. 2005;207:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Jedinak A, Dudhgaonkar S, Sliva D. Activated macrophages induce metastatic behavior of colon cancer cells. Immunobiology. 2010;215:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 40. | Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7:e50946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 677] [Cited by in RCA: 803] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 41. | Khorana AA, Ryan CK, Cox C, Eberly S, Sahasrabudhe DM. Vascular endothelial growth factor, CD68, and epidermal growth factor receptor expression and survival in patients with Stage II and Stage III colon carcinoma: a role for the host response in prognosis. Cancer. 2003;97:960-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 42. | Cui YL, Li HK, Zhou HY, Zhang T, Li Q. Correlations of tumor-associated macrophage subtypes with liver metastases of colorectal cancer. Asian Pac J Cancer Prev. 2013;14:1003-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 43. | Elomaa H, Ahtiainen M, Väyrynen SA, Ogino S, Nowak JA, Lau MC, Helminen O, Wirta EV, Seppälä TT, Böhm J, Mecklin JP, Kuopio T, Väyrynen JP. Spatially resolved multimarker evaluation of CD274 (PD-L1)/PDCD1 (PD-1) immune checkpoint expression and macrophage polarisation in colorectal cancer. Br J Cancer. 2023;128:2104-2115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 44. | Hua Y, Ma X, Zhao X, Wei X, Mu X, Zhang X. Characterization of metastasis-specific macrophages in colorectal cancer for prognosis prediction and immunometabolic remodeling. Sci Rep. 2024;14:26361. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 45. | Donadon M, Torzilli G, Cortese N, Soldani C, Di Tommaso L, Franceschini B, Carriero R, Barbagallo M, Rigamonti A, Anselmo A, Colombo FS, Maggi G, Lleo A, Cibella J, Peano C, Kunderfranco P, Roncalli M, Mantovani A, Marchesi F. Macrophage morphology correlates with single-cell diversity and prognosis in colorectal liver metastasis. J Exp Med. 2020;217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |