Published online Feb 26, 2023. doi: 10.12998/wjcc.v11.i6.1393
Peer-review started: November 8, 2022
First decision: November 24, 2022
Revised: December 7, 2022
Accepted: February 2, 2023
Article in press: February 2, 2023
Published online: February 26, 2023
Processing time: 108 Days and 5.9 Hours
Patients with obstructive jaundice caused by intrahepatic bile duct stones can be effectively managed by surgery. However, some patients may develop postope
A 52-year-old male patient admitted to the hospital on October 23, 2021, with a progressive exacerbation of jaundice, was found to have multiple intrahepatic bile duct stones with the diagnoses of obstructive jaundice and acute cholecystitis. Subsequently, the patient underwent left hepatectomy with biliary exploration, stone extraction, T-tube drainage, and cholecystectomy without developing any intraoperative complications. The patient had a dark urine color with worsening jaundice postoperatively and did not respond well to plasma exchange and other symptomatic and supportive treatments. Since the progressive increase in postoperative bilirubin could not be clinically explained with any potential reason, including, if not at all, viral infection, cholangitis, autoimmune liver disease, and other causes, the patient underwent whole-exon screening for any genetic diseases, which surprisingly identified UGT1A1 and ABCB11 gene mutations related to glucuronidation of indirect bilirubin as well as bile acid transport in hepatocytes, respectively. Thus, we hypothesized that postoperative refractory cholestasis might result from UGT1A1 and ABCB11 gene mutations and further recommended liver transplantation to the patient, who eventually declined it and died from liver failure six months later.
Surgery may aggravate cholestasis in patients with multiple intrahepatic bile duct stones and cholestasis associated with UGT1A1 and ABCB11 gene mutations. A liver transplant may be the best option if active medical treatment fails.
Core Tip: We presented a case of multiple intrahepatic bile duct stones, cholestasis, and progressive jaundice after surgical treatment, which was diagnosed upon the finding of adenosine triphosphate-binding cassette subfamily B member 11 (ABCB11) and uridine 5’-diphospho-glucuronosyltransferase 1A1 (UGT1A1) gene mutation from the genetic study. Since the patient refused to undergo liver transplantation, his postoperative aggravating jaundice was medically managed, but he soon died due to liver failure. The UGT1A1 gene is related to the glucuronidation of indirect bilirubin. Its mutation leads to increased indirect bilirubin, while the ABCB11 gene is involved in bile transport, and its mutation may lead to disturbance of bile acid transport, changes in bile composition, cholestasis, and the formation of intrahepatic bile duct stones. Surgical treatment in such patients may induce exacerbation of cholestasis, and liver transplantation should be the preferred treatment if medical management fails.
- Citation: Jiang JL, Liu X, Pan ZQ, Jiang XL, Shi JH, Chen Y, Yi Y, Zhong WW, Liu KY, He YH. Postoperative jaundice related to UGT1A1 and ABCB11 gene mutations: A case report and literature review. World J Clin Cases 2023; 11(6): 1393-1402
- URL: https://www.wjgnet.com/2307-8960/full/v11/i6/1393.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i6.1393
Obstructive jaundice caused by intrahepatic bile duct stones is relatively common, and its chief symptoms entail progressive, painless jaundice, occasionally accompanied by right upper quadrant pain, anorexia and greasy skin, itchy skin, dark yellow urine, and clay-colored stool. Primary intrahepatic stone disease (PIS) tends to occur regionally with familial aggregation[1] and carries a higher incidence in Southeast Asia than in Western countries[2,3]. Recurrent episodes and continued progression to advanced stages of PIS may lead to biliary cirrhosis or intrahepatic cholangiocarcinoma[4], two of which account for the most important causes of death from benign biliary tract disease.
PIS requires complex management with multiple surgical interventions, and hepatectomy has been the primary choice. However, less than 1% of patients may experience increased postoperative jaundice, liver failure, and even life-threatening conditions, which are related to various factors, including the underlying liver disease, the number of liver segments removed, intraoperative liver ischemia time, intraoperative continuous blood transfusion, postoperative hematoma, parenteral nutrition, anesthetics and drugs (such as antibiotics, analgesics), and sepsis and oxidative stress[5-7]. Moreover, although the PIS pathogenesis remains undefined, the disease possesses distinct ethnic and regional characteristics. Therefore, genetic factors may also be involved in the pathogenesis of PIS. Currently, gene mutation-related multiple intrahepatic bile duct stones, cholestasis, and postoperative jaundice aggravation have not received much clinical attention. We report a case of uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) and adenosine triphosphate- binding cassette subfamily B member 11 (ABCB11) gene mutations with multiple intrahepatic bile duct stones, cholestasis, and progressive jaundice after surgical treatment. Furthermore, we present a literature review on the potential effect, mechanism, and prognosis of UGT1A1 and ABCB11 gene mutations on intrahepatic bile duct stones and postoperative jaundice.
A 52-year-old male was admitted to the hospital on October 23, 2021, with yellowish skin and sclera for three months and a progressive exacerbation of postoperative jaundice for one month.
Three months ago, the patient noticed yellowish skin/sclera and dark-brown urine without any apparent predisposition, which had worsened with abdominal pain a month later, so he was admitted to a local hospital for further management. The post-admission examination of liver function test revealed normal transaminases but elevated levels of direct bilirubin, indirect bilirubin, gamma-glutamyl transpeptidase (GGT), and total bile acid (Table 1). Upper abdominal computed tomography (CT) examination showed multiple intrahepatic and extrahepatic bile duct stones and obstructions, cholestasis, and gallbladder enlargement. The patient was diagnosed with obstructive jaundice having multiple intrahepatic bile duct stones with acute cholecystitis, so he underwent left external hepatectomy with bile duct exploration, stone extraction, T-tube drainage, and cholecystectomy on September 9, 2021. The abdominal pain was significantly relieved postoperatively without fever, nausea, vomiting, or any sign of abdominal distension, but the urine color had been persistently getting darker with aggravation of jaundice of skin and sclera. Liver function re-examination showed a progressive increase in the bilirubin level compared to before the operation. Thus, the patient was further managed with plasma exchange and symptomatic and supportive treatments. However, the effect was not good, and he was admitted to our service for further diagnosis and treatment.
| Date | ALT, U/L | AST, U/L | TBIL, μmol/L | DBIL, μmol/L | IBIL, μmol/L | GGT, U/L | ALP, U/L | TBA, U/L |
| Ref: 0-40 | Ref: 0-34 | Ref: 5.1-19.0 | Ref: 1.7-6.8 | Ref: 1.7-13.2 | Ref: 10-60 | Ref: 45-120 | Ref: 0-50 | |
| 2021-09-06 (Pre-operative) | 33.0 | 28.0 | 135.1↑ | 84.1↑ | 51.0↑ | 77.0↑ | 105.0 | 208.0↑ |
| 2021-09-10 (Post-operative) | 311↑ | 545↑ | 167.6↑ | 106.9↑ | 60.7↑ | 48.0 | 126.0↑ | 120.8↑ |
| 2021-09-22 | 39.0 | 23.0 | 187.1↑ | 109.4↑ | 77.7↑ | 32.0 | 78.0 | 53.0↑ |
| 2021-09-28 (Before discharge) | 47.0↑ | 32.0 | 286.2↑ | 166.2↑ | 120.0↑ | 34.0 | 97.0 | 80.9↑ |
| 2021-10-23 (On readmission) | 43.0↑ | 32.0 | 593.3↑ | 285.9↑ | 307.4↑ | 36.0 | 109.0 | 131.4↑ |
| 2021-10-30 (After drug treatment) | 30.0 | 27.0 | 602.2↑ | 283.2↑ | 319.0↑ | 23.0 | 97.0 | 213.6↑ |
| 2021-11-14 (After plasma exchange) | 22.0 | 33.0 | 230.6↑ | 141.1↑ | 89.5↑ | 28.0 | 76.0 | 151.9↑ |
| 2021-11-17 (Before discharge) | 29.0 | 44.0↑ | 336.7↑ | 193.5↑ | 143.2↑ | 34.0 | 88.0 | 183.0↑ |
He reported a history of hypertension for ten years and did not take medication regularly. However, he denied a history of long-term heavy drinking, exposure to poisons and traditional Chinese medicines, and trauma.
The patient denied any family history of malignancy and other hereditary disorders.
The patient had a Body Mass Index of 23.44, and his vital signs were stable, with clear consciousness. Moreover, the patient presented with the face of chronic liver disease and severe jaundice of the skin and sclera but did not have liver palm and spider nevus. Heart and lung examinations did not find any obvious abnormality, while the abdominal physical localized a T-tube drainage tube in place at the right abdomen with palpable tenderness at the right upper quadrant in the absence of rebound pain, muscle tension, and shifting dullness.
Dynamic changes in biochemical indicators of liver function tests were listed as follows (Table 1): On Sep 6, 2021, preoperative LFTs were triglyceride (TG) as 3.62 mmol/L (Ref: < 1.7 mmol/L), total cholesterol (TC) as 4.46 mmol/L (Ref: < 5.2 mmol/L), high density lipoprotein cholesterol (HDL-C) as 0.17 mmol/L (Ref:0.91-1.55 mmol/L), and low density lipoprotein cholesterol(LDL-C): 3.14 mmol/L(Ref: < 3.12 mmol/L).
On October 23, 2021, postoperative LFTs showed TG as 3.46 mmol/L, TC as 5.16 mmol/L, HDL-C as 0.64 mmol/L, and LDL-C as 3.23 mmol/L.
On October 23, 2021, C-reactive protein and coagulation function were checked with normal findings, hepatitis B and viral infection were ruled out with negative antigen in the serum antibody tests, and a negative autoimmune antibody panel ruled out autoimmune hepatitis. Other tests were in the normal ranges, including thyroid function tests, ceruloplasmin, total iron binding capacity, and unsaturated iron binding capacity.
On October 23, 2021, bile drainage fluid was sent for culture, which came back with positive Pseudomonas putida, being sensitive to antibiotics such as piperacillin, ceftriaxone, and ceftazidime.
On November 17, 2021, the culture came back with negative gram-negative bacilli lipopolysaccharide and fungal (1-3)-B-D glucan.
On November 19, 2021, metagenomics next-generation sequencing (mNGS) on peripheral blood was reported back without detections of bacteria, mycobacteria, fungi, viruses, mycoplasma, Chlamydia, rickettsia, and parasites.
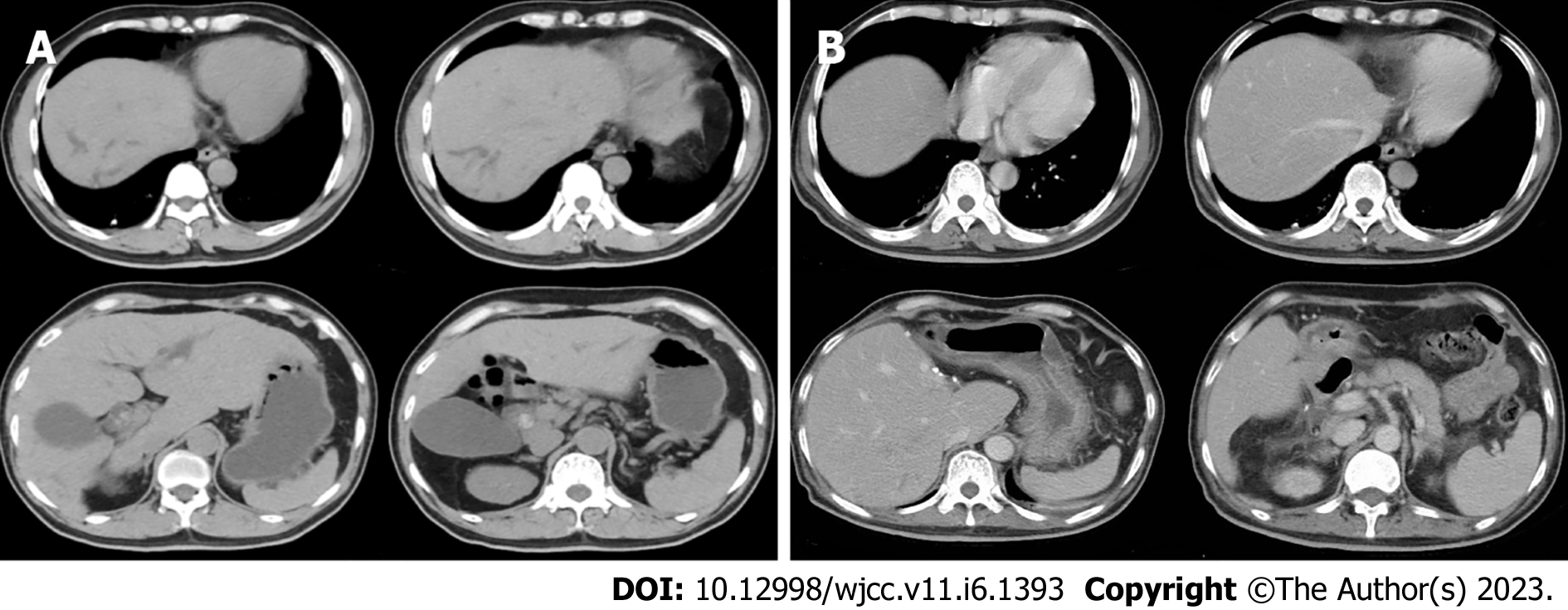
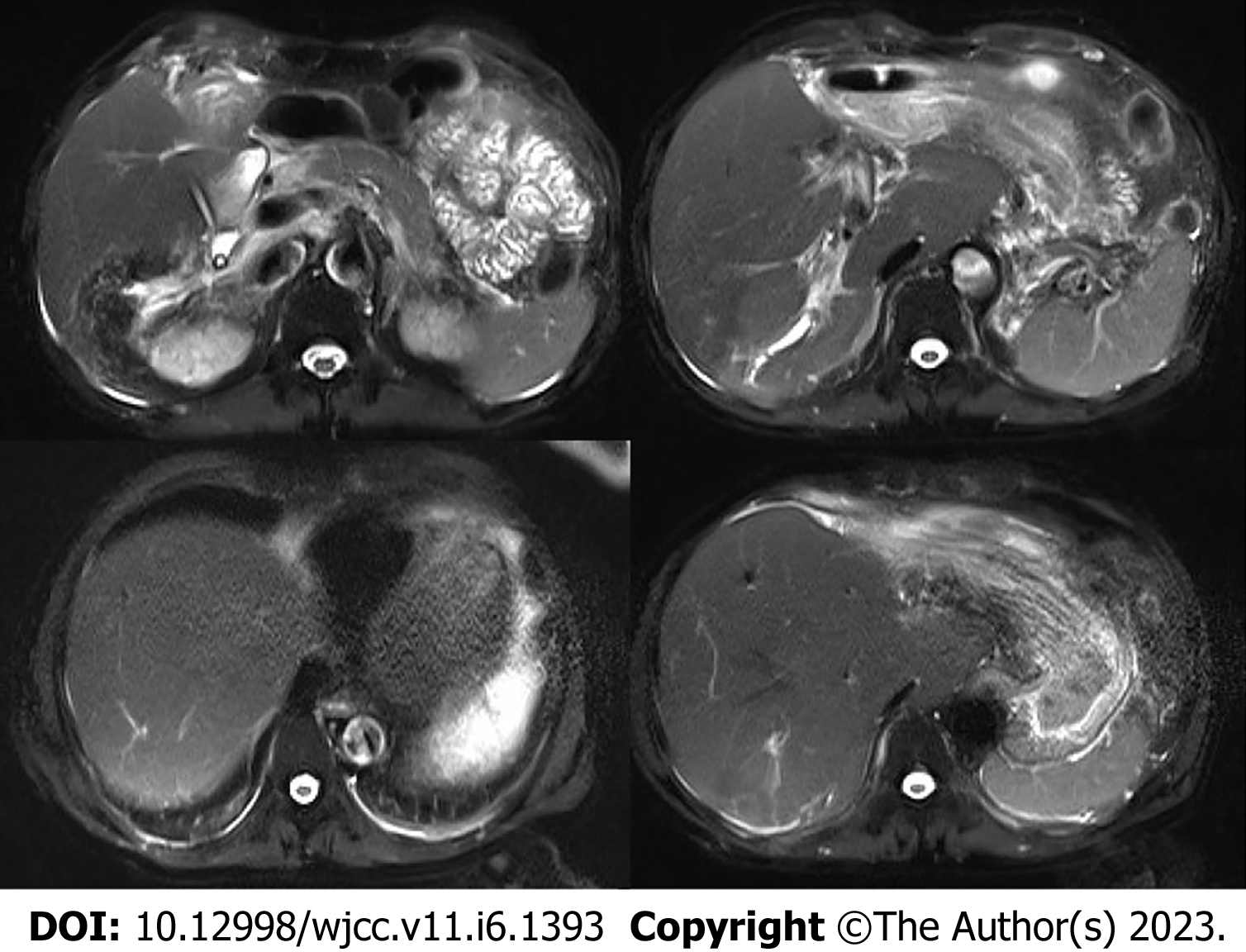
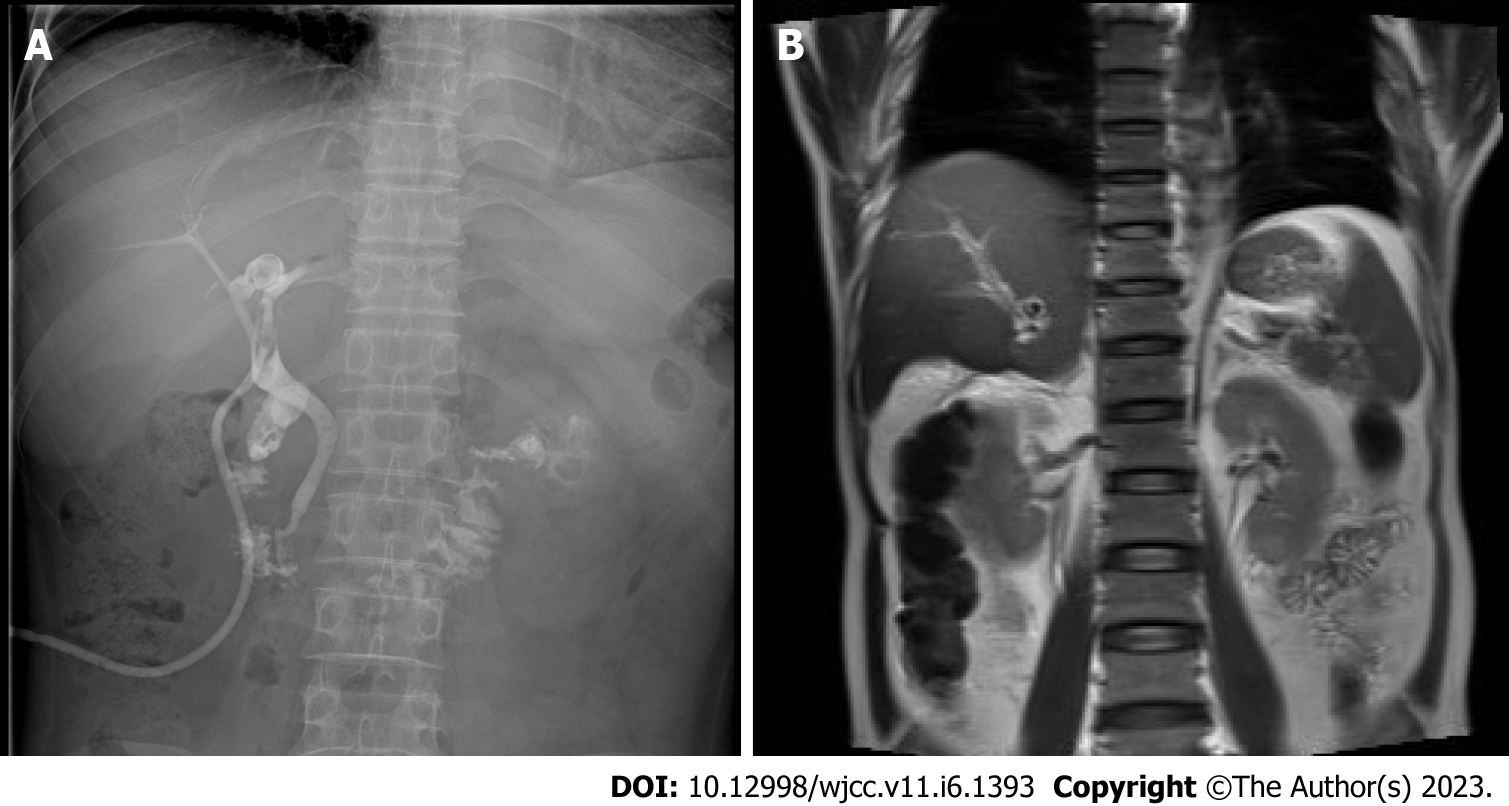
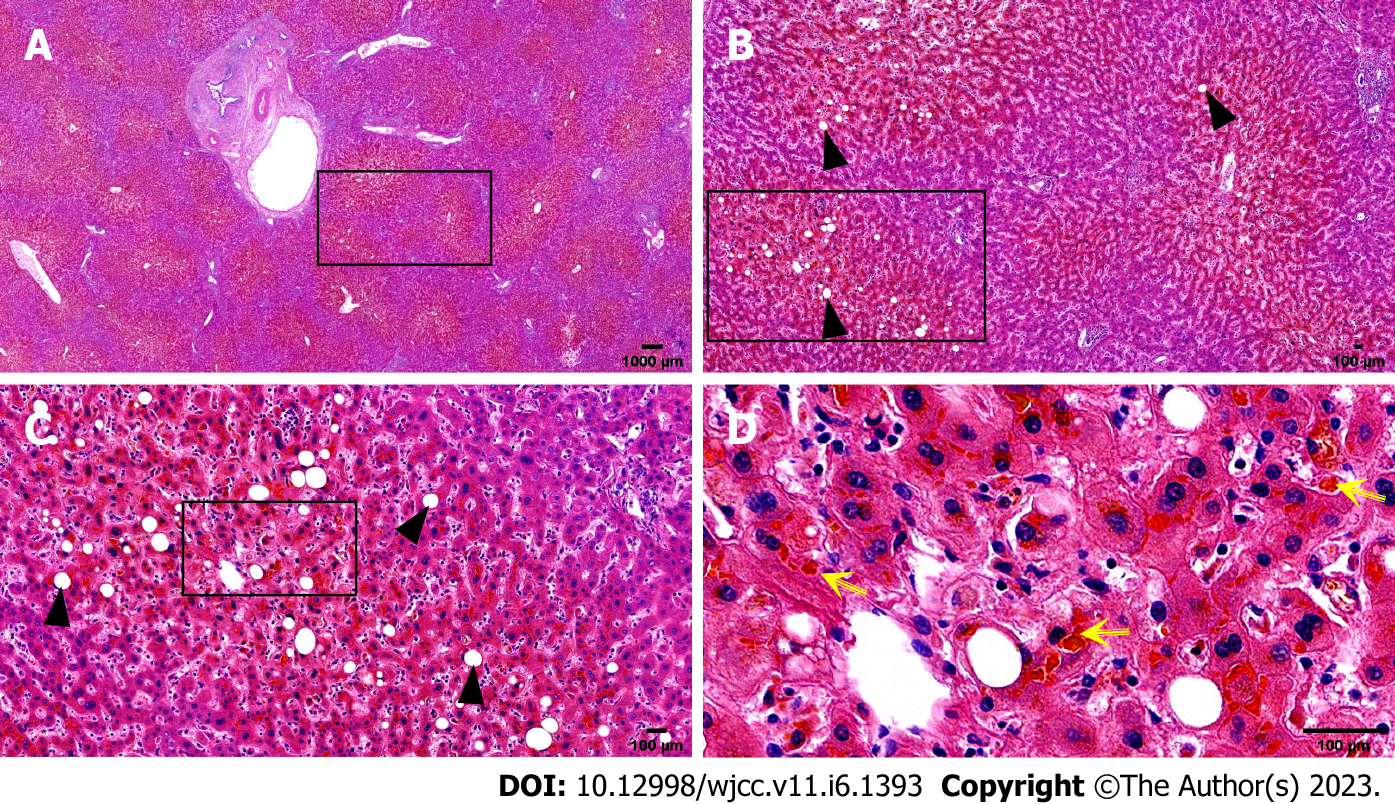
A preoperative non-contrast CT scan of the upper abdomen unveiled left intrahepatic and multiple bile duct stones with dilated intra-and extrahepatic bile ducts and an enlarged gallbladder (Figure 1A). Postoperative non-contrast CT scan and magnetic resonance imaging of the upper abdomen illustrated signs of liver cirrhosis with multiple intrahepatic bile duct stones in the upper right posterior lobe of the liver with cholangitis, peri-cholangitis, and portal hypertension (Figure 1B) and (Figure 2). Besides, postoperative magnetic resonance cholangiopancreatography further confirmed multiple intrahepatic bile duct stones with mild dilatation in the upper right posterior lobe of the liver (Figure 3A), and T-tube angiography could not visualize the right and left hepatic duct but the lower end of the common bile duct (Figure 3B). Postoperative histopathologic examination exhibited cholestatic liver disease and intrahepatic bile duct dilatation with chronic inflammation (Figure 4). Furthermore, sequential immunohistochemical staining unveiled mild and delicate hyperplasia along biliary ducts, then Alpha-Smooth Muscle Actin staining spotted a few activated hepatic stellate cells, Masson and Sirius red staining revealed fibrous tissue hyperplasia in the portal area with an interlobular short fibrous septum, Periodic Acid Schiff Diastase Stain displayed a little waxy substance in Kupffer cells, and Prussian blue signified a little iron granules deposition in hepatocytes and Kupffer cells. Besides, copper staining was negative.
During hospitalization, the patient was managed on the diagnoses of cholestatic hepatitis, biliary tract infection, and decompensated liver cirrhosis with piperacillin-tazobactam sodium, dexamethasone, and supportive medications for protecting the liver and eliminating jaundice. After the treatments, re-examining white blood cells and C-reactive protein did not confirm any ongoing infection. However, the bilirubin was significantly higher than before. Afterward, intermittent plasma exchange replacement therapy with double plasma molecular absorption system (DPMAS) was administered to the patient four times to control progressive jaundice but failed to stop the progression.
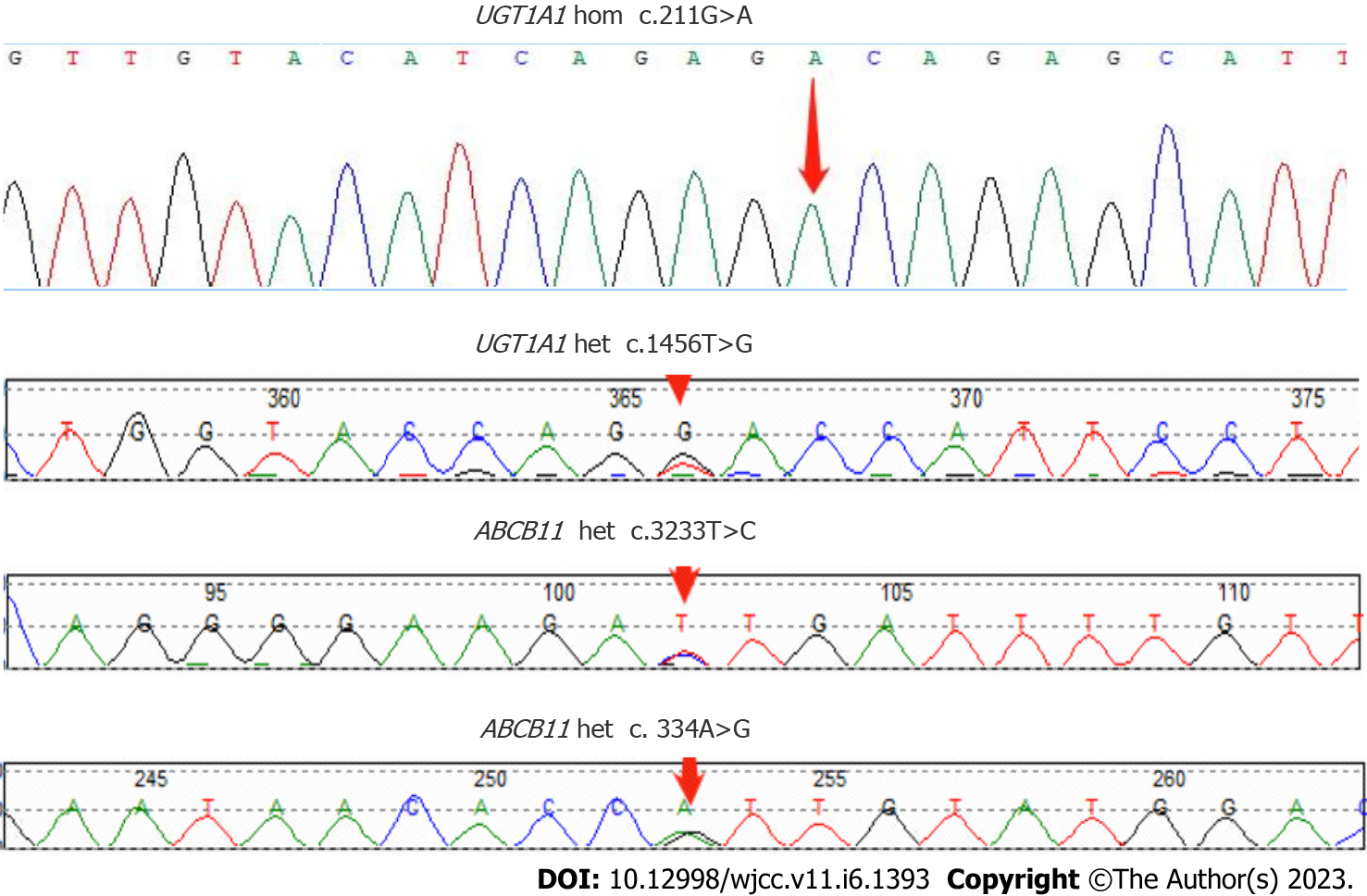
CT examination was repeated after this admission to unveil the signs of cirrhosis, which was paradoxical to the preoperative finding of normal liver. Further pathological examination of the resected liver tissue revealed fibrous tissue hyperplasia in the hepatic portal area, suggesting that the patient only possessed early hepatic fibrosis before surgery. Taken together, we advised the patient of whole-exon screening to fish out any genetic disorder to search for the cause of cirrhosis, which surprisingly revealed UGT1A1 and ABCB11 gene mutations (Table 2 and Figure 5).
| Gene | Chromosomal position | Basic variation information | Zygote type | Inheritance patterns |
| UGT1A1 | chr2:234669144 | NM_000463.3:c.211G>A (p.Gly71Arg) | Hom | AR |
| chr2:234681059 | NM_000463.3:c.1456T>G (p.Tyr486Asp) | Het | AR | |
| ABCB11 | chr2:169787353 | NM_003742.4:c.3233T>C (p.Ile1078Thr) | Het | AR |
| chr2:169869837 | NM_003742.4:c.334A>G (p.Ile112Val) | Het | AR |
Based on the clinical, pathological, and genetic findings, the patient was diagnosed with intrahepatic bile duct stones with cholestasis associated with UGT1A1 and ABCB11 gene mutations, biliary tract infection, and decompensated liver cirrhosis.
Piperacillin and tazobactam sodium were first given to prevent infection, dexamethasone to reduce inflammation, compound glycyrrhizic acid monoamine S, adenosine methionine and ursodeoxycholic acid to protect the liver and eliminate jaundice, and plasma exchange therapy DPMAS was given later, but the effect was poor. Liver transplantation was recommended, but the patient and his family refused for financial reasons.
Since the patient and his family refused liver transplantation, his condition quickly deteriorated after discharge, and he died of liver failure six months later.
The patient, diagnosed with obstructive jaundice from multiple intrahepatic bile duct stones, underwent left hepatectomy combined with biliary exploration, T-tube drainage, and cholecystectomy. However, he developed aggravation of postoperative jaundice, featured as the progressive increase of bilirubin, imaging signs of liver cirrhosis without blatant biliary obstruction, and ineffective medical therapy, including plasma exchange DPMAS. This postoperative jaundice with significant cellular injuries was pathologically characterized by cholestasis on the resected left liver, which was further examined with the whole-exon examination, identifying hereditary disease genes that showed UGT1A1 and ABCB11 gene mutations. The UGT1A1 gene encodes the enzyme to glucuronidate indirect bilirubin for its removal from the body. Hence, its mutation causes indirect bilirubin elevation as the main feature. ABCB11 gene encodes the bile salt export pump (BSEP), and its mutation leads to bile acid transport disorder, changes in bile composition, cholestasis, and the formation of cholesterol stones with the elevation of direct bilirubin. In this case, both direct and indirect bilirubin were significantly elevated. Therefore, by comprehensive analysis, multiple intrahepatic bile duct stones and cholestasis, in this case, may be related to UGT1A1 and ABCB11 gene mutations.
The UGT1A1 gene is located at 2q37 of the human genome and encodes uridine 5'-diphosphate-glucuronidase, the only enzyme to glucuronidate bilirubin in the liver[8,9]. UGT1A1 gene mutations cause unconjugated hyperbilirubinemia in both Crigler-Najjar syndrome (CN) and Gilbert syndrome (GS)[10]. Crigler-Najjar syndrome is classified as type I and type II variants, which reduce UGT1A1 enzymatic activity to 0% and 10%, respectively, while the enzyme in GS can only release 30% of its activity[10]. The ABCB11 gene is located at locus 2q24 in the human genome and contains 28 exons[11], which can be translated into BSEP with a molecular weight of approximately 150–170 kDa. The BSEP proteins, which can be found along the canalicular membrane of hepatocytes, consist of two tandem homologous moieties to harbor an intracellular nucleotide-binding domain (NBD) and six transmembrane domains, which can be energized for transporting bile acids out of the cells upon hydrolysis of ATP by NBD. Mutations in the ABCB11 gene result in the accumulation of primary bile acids in the intercellular space of the liver, thereby increasing the levels of bilirubin in serum and bile salts in the liver and blood. Studies have shown that mutations in the ABCB11 gene are closely associated with cholestasis, including benign recurrent intrahepatic cholestasis[12,13], progressive familial intrahepatic cholestasis[14,15] and intrahepatic cholestasis of pregnancy[16,17]. In addition, beyond its transporting capacity, BSEP can facilitate cholesterol solubilization and inhibit its supersaturated crystallization[18,19]. Taken together, decreased bile salt secretion due to the BSEP alteration from the mutation of the ABCB11 gene subsequently superimposes the formation of cholesterol gallstones along with abnormal bile metabolism and composition changes, which contribute to the pathogenesis of PIS[20]. Furthermore, cholestasis assists in depositing bile components to form intrahepatic bile duct stones and accumulates toxic bile acids, which lead to biliary wall injury and inflammation[21]. Long-term inflammation can lead to the thickening and stenosis of the biliary wall, thereby exacerbating cholestasis and promoting the formation of intrahepatic bile duct stones, further exacerbating cholestasis. This vicious cycle thus leads to disease progression[22,23]. In our patient, the point mutation of c.211G>A (p.Gly71Arg) and c.1456T>G (p.Tyr486Asp) was found in exon 1 and exon 5 of the UGT1A1 gene, respectively, which are most frequently seen in CN-II[24,25]. Along with the ABCB11 gene mutation, both genes cooperatively aggravate intrahepatic cholestasis and hepatocellular injury before and after the surgery.
Intrahepatic bile duct stones and cholestasis can be managed with hepatic lobectomy, cholangiojejunostomy, Roux-Y anastomosis, simple choledocholithotomy, T-tube drainage, or liver transplantation according to the different intra- and extrahepatic biliary stones and clinical manifestations of each patient. After the obstructive pathologies in most patients with obstructive jaundice are removed surgically, the bilirubin levels drop, and the liver function gradually improves. However, in a few patients, bilirubin levels do not decrease but increase after surgery with the aggravation of jaundice and persistent intrahepatic cholestasis[26]. Postoperative cholestasis has many predisposing factors, including ischemia-reperfusion injury, the basis of liver cirrhosis, biliary tract infection, and the down-regulation of the expression level of bile acid efflux transporter in liver tissue. Long-term intrahepatic and extrahepatic biliary stones complicated by repeated suppurative cholangitis along the intrahepatic bile duct cause hepatic parenchyma fibrosis, hyperplasia, and pseudolobular formation, subsequently leading to the development of cholestatic liver cirrhosis[27,28], which dysfunctions hepatic uptake, transport, esterification and absorption of bilirubin and further elevates bilirubin level to make the pathogenesis fall into a vicious circle. Although the factors of biliary obstruction can be relieved by the operation, the hepatic injury from the persistent obstruction was deteriorated by anesthesia, ischemia, and inflammatory reactions. In addition to liver cirrhosis, sepsis with endotoxemia can also contribute to postoperative intrahepatic cholestasis by breaching the bile excretion function of hepatocytes[29,30], impairing the ability of hepatocytes to uptake bile salts, affecting the permeability of the cell membrane, and de-functioning bile transport. Furthermore, post-hepatectomy hyperbilirubinemia may be associated with hepatic mitochondrial dysfunction or impaired expression of bile excretion pumps on hepatocyte canalicular membranes[31-33].
In this report, our patient did not exhibit any evidence of liver cirrhosis in the preoperative upper abdominal imaging studies but had the feature of liver cirrhosis in the upper abdominal CT and magnetic resonance imaging one month after the operation. Normally, cirrhosis cannot develop in such a short time unless we might preoperatively omit the existence of this disease entity so that the patient underwent the inappropriate liver resection without classifying the patient according to Child’s criteria. Otherwise, another hidden pathology was blinded until the postoperative stage. The postoperative pathological examination of the specimen from the surgery showed signs of early hepatic fibrosis, and the postoperative molecular pathology identified UGT1A1 and ABCB11 gene mutations, thereby giving us another perspective on the underlying causes of postoperative developments of cholestasis and cirrhosis. While managing the postoperative cholestasis of this patient, we not only performed T-tube angiography, which showed no obvious intra- and extrahepatic biliary obstruction but also treated the patient with antibiotics according to the drug sensitivity results to the infection and hormone therapy to reduce inflammation. However, since his bilirubin had progressively increased and was not controlled even with plasma exchange DPMAS replacement therapy, we sent the patient to receive genetic testing to identify the underlying causes, which showed the genetic defects in UGT1A1 and ABCB11, thus singling out liver transplantation as the only effective method to the patient.
Interestingly, during the postoperative management, the coagulation function of the patient was always in the normal range, suggesting that the synthesis function of his lever was never perturbed. Such clinical uniqueness could be explained by the fact that his UGT1A1 and ABCB11 genes affect bilirubin metabolism and bile transport rather than significantly damaging liver cells and obstructing intra- and extrahepatic bile ducts for postoperative liver decompensation and lack of efficacy in the given treatment. Another issue related to our patient was low serum GGT levels that were paradoxical to its elevation in patients with biliary obstruction. Low GGT could be considered a potential laboratory indicator for non-obstruction caused while working up the patients with cholestasis.
UGT1A1 and ABCB11 gene mutations associated with multiple intrahepatic bile duct stones and cholestasis are rare, so they are easy to ignore in clinical practice. Such patients have poor surgical treatment effects, which may induce aggravation of cholestasis, a progressive increase in bilirubin, and rapid progression of liver fibrosis. Therefore, liver transplantation may be the first choice for patients with multiple intrahepatic bile duct stones and cholestasis related to UGT1A1 and ABCB11 gene mutations after active medical treatments are ineffective. Patients with clinically found cholestasis should pay attention to whether the GGT level is elevated, which is more valuable for the preliminary judgment of hereditary metabolic intrahepatic cholestasis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Furugen M, Japan; Sanal MG, India; Yao G, China S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Meier Y, Pauli-Magnus C, Zanger UM, Klein K, Schaeffeler E, Nussler AK, Nussler N, Eichelbaum M, Meier PJ, Stieger B. Interindividual variability of canalicular ATP-binding-cassette (ABC)-transporter expression in human liver. Hepatology. 2006;44:62-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 163] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 2. | Lorio E, Patel P, Rosenkranz L, Patel S, Sayana H. Management of Hepatolithiasis: Review of the Literature. Curr Gastroenterol Rep. 2020;22:30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (2)] |
| 3. | Li C, Wen T. Surgical management of hepatolithiasis: A minireview. Intractable Rare Dis Res. 2017;6:102-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (37)] |
| 4. | Meng ZW, Han SH, Zhu JH, Zhou LY, Chen YL. Risk Factors for Cholangiocarcinoma After Initial Hepatectomy for Intrahepatic Stones. World J Surg. 2017;41:835-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Tandon A, Roorda AK, Legha P, Sangoi A, Triadafilopoulos G. Mellow Yellow: Diagnosis and Management of Multifactorial Postoperative Jaundice. Dig Dis Sci. 2016;61:2226-2230. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Chung C, Buchman AL. Postoperative jaundice and total parenteral nutrition-associated hepatic dysfunction. Clin Liver Dis. 2002;6:1067-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Pauli-Magnus C, Meier PJ. Hepatobiliary transporters and drug-induced cholestasis. Hepatology. 2006;44:778-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 191] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 8. | van Es HH, Bout A, Liu J, Anderson L, Duncan AM, Bosma P, Oude Elferink R, Jansen PL, Chowdhury JR, Schurr E. Assignment of the human UDP glucuronosyltransferase gene (UGT1A1) to chromosome region 2q37. Cytogenet Cell Genet. 1993;63:114-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 47] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Memon N, Weinberger BI, Hegyi T, Aleksunes LM. Inherited disorders of bilirubin clearance. Pediatr Res. 2016;79:378-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 10. | Aiso M, Yagi M, Tanaka A, Miura K, Miura R, Arizumi T, Takamori Y, Nakahara S, Maruo Y, Takikawa H. Gilbert Syndrome with Concomitant Hereditary Spherocytosis Presenting with Moderate Unconjugated Hyperbilirubinemia. Intern Med. 2017;56:661-664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annu Rev Biochem. 2002;71:537-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1164] [Cited by in RCA: 1122] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 12. | Hayashi H, Naoi S, Hirose Y, Matsuzaka Y, Tanikawa K, Igarashi K, Nagasaka H, Kage M, Inui A, Kusuhara H. Successful treatment with 4-phenylbutyrate in a patient with benign recurrent intrahepatic cholestasis type 2 refractory to biliary drainage and bilirubin absorption. Hepatol Res. 2016;46:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Thoeni C, Waldherr R, Scheuerer J, Schmitteckert S, Roeth R, Niesler B, Cutz E, Flechtenmacher C, Goeppert B, Schirmacher P, Lasitschka F. Expression Analysis of ATP-Binding Cassette Transporters ABCB11 and ABCB4 in Primary Sclerosing Cholangitis and Variety of Pediatric and Adult Cholestatic and Noncholestatic Liver Diseases. Can J Gastroenterol Hepatol. 2019;2019:1085717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Waisbourd-Zinman O, Surrey LF, Schwartz AE, Russo PA, Wen J. A Rare BSEP Mutation Associated with a Mild Form of Progressive Familial Intrahepatic Cholestasis Type 2. Ann Hepatol. 2017;16:465-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Keitel V, Burdelski M, Warskulat U, Kühlkamp T, Keppler D, Häussinger D, Kubitz R. Expression and localization of hepatobiliary transport proteins in progressive familial intrahepatic cholestasis. Hepatology. 2005;41:1160-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 190] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Anzivino C, Odoardi MR, Meschiari E, Baldelli E, Facchinetti F, Neri I, Ruggiero G, Zampino R, Bertolotti M, Loria P, Carulli L. ABCB4 and ABCB11 mutations in intrahepatic cholestasis of pregnancy in an Italian population. Dig Liver Dis. 2013;45:226-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Lim TY, Coltart I, Foskett P, Thompson R, Strautnieks S, Penna L, Williamson C, Miquel R, Heneghan MA. Donor transmitted mutation of the ABCB11 gene and ensuing intrahepatic cholestasis of pregnancy in a liver transplant recipient. Liver Transpl. 2017;23:1229-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Kubitz R, Dröge C, Kluge S, Stindt J, Häussinger D. Genetic variations of bile salt transporters. Drug Discov Today Technol. 2014;12:e55-e67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Chen R, Wang J, Tang S, Zhang Y, Lv X, Wu S, Yang Z, Xia Y, Chen D, Zhan S. Role of polymorphic bile salt export pump (BSEP, ABCB11) transporters in anti-tuberculosis drug-induced liver injury in a Chinese cohort. Sci Rep. 2016;6:27750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Caplan MS, Cohn RA, Langman CB, Conway JA, Shkolnik A, Brouillette RT. Favorable outcome of neonatal aortic thrombosis and renovascular hypertension. J Pediatr. 1989;115:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Li T, Apte U. Bile Acid Metabolism and Signaling in Cholestasis, Inflammation, and Cancer. Adv Pharmacol. 2015;74:263-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 224] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 22. | Ran X, Yin B, Ma B. Four Major Factors Contributing to Intrahepatic Stones. Gastroenterol Res Pract. 2017;2017:7213043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Zollner G, Trauner M. Mechanisms of cholestasis. Clin Liver Dis. 2008;12:1-26, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Aono S, Yamada Y, Keino H, Hanada N, Nakagawa T, Sasaoka Y, Yazawa T, Sato H, Koiwai O. Identification of defect in the genes for bilirubin UDP-glucuronosyl-transferase in a patient with Crigler-Najjar syndrome type II. Biochem Biophys Res Commun. 1993;197:1239-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 111] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Maruo Y, Nakahara S, Yanagi T, Nomura A, Mimura Y, Matsui K, Sato H, Takeuchi Y. Genotype of UGT1A1 and phenotype correlation between Crigler-Najjar syndrome type II and Gilbert syndrome. J Gastroenterol Hepatol. 2016;31:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Modha K. Clinical Approach to Patients With Obstructive Jaundice. Tech Vasc Interv Radiol. 2015;18:197-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 27. | Liu FB, Yu XJ, Wang GB, Zhao YJ, Xie K, Huang F, Cheng JM, Wu XR, Liang CJ, Geng XP. Preliminary study of a new pathological evolution-based clinical hepatolithiasis classification. World J Gastroenterol. 2015;21:2169-2177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Wijarnpreecha K, Thongprayoon C, Sanguankeo A, Upala S, Ungprasert P, Cheungpasitporn W. Hepatitis C infection and intrahepatic cholestasis of pregnancy: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2017;41:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 29. | Aslan AN, Sari C, Baştuğ S, Sari SÖ, Akçay M, Durmaz T, Bozkurt E. Severe jaundice due to intrahepatic cholestasis after initiating anticoagulation with rivaroxaban. Blood Coagul Fibrinolysis. 2016;27:226-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Corrêa TD, Cavalcanti AB, Assunção MS. Balanced crystalloids for septic shock resuscitation. Rev Bras Ter Intensiva. 2016;28:463-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Arai T, Nagino M, Nimura Y. [Hepatic failure following resection of cholestatic liver]. Nihon Geka Gakkai Zasshi. 2004;105:664-668. [PubMed] |
| 32. | Peralta C, Jiménez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J Hepatol. 2013;59:1094-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 476] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 33. | Bernhardt GA, Zollner G, Cerwenka H, Kornprat P, Fickert P, Bacher H, Werkgartner G, Müller G, Zatloukal K, Mischinger HJ, Trauner M. Hepatobiliary transporter expression and post-operative jaundice in patients undergoing partial hepatectomy. Liver Int. 2012;32:119-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |