Published online Jun 6, 2022. doi: 10.12998/wjcc.v10.i16.5165
Peer-review started: October 22, 2021
First decision: December 27, 2021
Revised: December 29, 2021
Accepted: March 15, 2022
Article in press: March 15, 2022
Published online: June 6, 2022
Processing time: 223 Days and 8 Hours
Early detection of colorectal neoplasms, including colorectal cancers (CRCs) and advanced colorectal adenomas (AAs), is crucial to improve patient survival. Circulating microRNAs (miRNAs) in peripheral blood are emerging as nonin
To identify candidate circulating cell-free miRNAs as diagnostic biomarkers in patients with colorectal neoplasms.
The study was divided into three phases: (1) Candidate miRNAs were selected from three public miRNA datasets using differential gene expression analysis methods; (2) an independent set of serum samples from 60 CRC patients, 60 AA patients and 30 healthy controls (HCs) was included and analyzed by quantitative real-time polymerase chain reaction for miRNAs, and their diagnostic power was detected by receiver operating characteristic (ROC) analysis; and (3) the origin and function of miRNAs in cancer patients were investigated in cancer cell lines and tumor tissues.
Based on bioinformatics analysis, miR-627-5p and miR-199a-5p were differentially expressed in both the serum and tissues of patients with colorectal neoplasms and HCs and were selected for further study. Further validation in an independent cohort revealed that both circulating miR-627-5p and miR-199a-5p were sequentially increased from HCs and AAs to CRCs. The diagnostic power of miR-672-5p yielded an area under the curve (AUC) value of 0.90, and miR-199a-5p had an AUC of 0.83 in discriminating colorectal neoplasms from HCs. A logistic integrated model combining miR-199a-5p and miR-627-5p exhibited a higher diagnostic performance than either miRNA. Additionally, the levels of serum miR-627-5p and miR-199a-5p in CRC patients were significantly lower after surgery than before surgery and the expression of both miRNAs was increased with culture time in the culture media of several CRC cell lines, suggesting that the upregulated serum expression of both miRNAs in CRC might be tumor derived. Furthermore, in vitro experiments revealed that miR-627-5p and miR-199a-5p acted as tumor suppressors in CRC cells.
Serum levels of miR-199a-5p and miR-627-5p were markedly increased in patients with colorectal neoplasms and showed strong potential as minimally invasive biomarkers for the early screening of colorectal neoplasms.
Core Tip: This report is the first on the diagnostic usefulness of circulating miR-627-5p and miR-199a-5p in patients with colorectal neoplasms. We identified that serum levels of miR-627-5p and miR-199a-5p were markedly elevated in patients with colorectal neoplasia and appeared to be novel biomarkers for the non-invasive screening of colorectal neoplasia. An integrated model combining miR-199a-5p and miR-627-5p obtained a better discriminative capacity than each miRNA alone. Additionally, miR-627-5p and miR-199a-5p had different expression levels in the serum of cancer patients before and after surgery and in vitro gain-of-function experiments demonstrated that both microRNAs played crucial roles in regulating the progression and invasion of colorectal cancer cells.
- Citation: Zhao DY, Zhou L, Yin TF, Zhou YC, Zhou GYJ, Wang QQ, Yao SK. Circulating miR-627-5p and miR-199a-5p are promising diagnostic biomarkers of colorectal neoplasia. World J Clin Cases 2022; 10(16): 5165-5184
- URL: https://www.wjgnet.com/2307-8960/full/v10/i16/5165.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i16.5165
Colorectal cancer (CRC) is one of the most common malignant digestive tumors, ranking fourth regarding incidence and mortality rates among all cancer types in 2018 and posing a tremendous threat to global population health[1]. The 5-year survival rate of CRC patients highly depends on the tumor stage at diagnosis, approximately 90% for stage I disease and less than 8% for stage IV disease, highlighting the crucial importance of early cancer detection and diagnosis to reduce the burden of this disease[2,3]. Most CRCs start with aberrant crypt foci, evolving into benign premalignant lesions (adenomas), and ultimately progressing to colorectal tumors (adenocarcinomas) over the span of years[4]. The classical process of colorectal tumorigenesis lends itself to screening. Notably, advanced colorectal adenoma (AA) is considered the protracted and treatable preclinical phase and is suitable for population screening due to its significant correlation with a high risk of progression to invasive cancer[5]. Thus, developing cost-effective screening tools for colorectal neoplasms, including CRCs and AAs, is crucial.
Although several advances in diagnostic techniques have been confirmed to effectively decrease the mortality of CRC patients, several challenges persist regarding current CRC screening tools, such as the lack of a universally accepted strategy, the lack of a high specificity and sensitivity strategy, and the high cost of screening advanced adenoma[6]. For example, colonoscopy is currently the most effective and straightforward examination to diagnose advanced lesions and prevent cancer progression by resecting precancerous polyps in an en bloc manner[4]. However, this modality comprises prior bowel cleansing, an invasive procedure, with a relatively high expense, leading to poor compliance of patients and exposing patients to potentially procedural risks[7]. Alternative, noninvasive stool-based screening tools, such as the fecal occult blood test, have been criticized for the poor detection of advanced adenomas and high false-positive rates due to other confounding illnesses, such as gingival bleeding[8]. Additionally, circulating tumor markers, such as carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA199), have been emerging as potential alternatives for the minimally invasive detection and prevention of CRC. Unfortunately, their less-than-desirable sensitivity and specificity have hampered their clinical use in the early detection of CRC and premalignant lesions[9]. Therefore, identifying a simple, inexpensive, noninvasive or minimally invasive screening approach with good sensitivity and specificity to improve the detection of early colorectal neoplasms is required.
CRC originates from a number of genomic instabilities, epigenetic alterations, and genetic aberrations, which offer the foundation for detecting serum or plasma to identify tumor-specific fingerprints[10]. MicroRNAs (miRNAs) are endogenously derived non-coding RNA sequences of 19-25 nucleotides that regulate the expression of target genes through either miRNA degradation or translational repression[11]. Since their discovery in 1993, every cancer type detected by miRNA sequencing has demonstrated that miRNA expression profiles differ between normal tissues and tumor tissues, including CRC[12]. Altered miRNA expression exerts a tumor-suppressive or oncogenic function in regulating signaling pathways involved in CRC progression from normal to premalignant lesions, thereby contributing to malignancy[13-15]. Furthermore, Chen et al[16] demonstrated that peripheral blood contains a number of stable miRNAs secreted from various tumor tissues, raising the possibility of their potential use as noninvasive diagnostic markers for cancer. In fact, miRNAs are superior to mRNAs due to their small sizes, easy detectability, and stability. As serum or plasma miRNAs are resistant to RNase digestion, they remain stable for extended storage even when subjected to 10 freeze-thaw cycles, boiling, and low or high pH[16]. An increasing number of studies have demonstrated that several circulating miRNAs have exhibited the potential to distinguish individuals with AA and CRC from healthy volunteers, such as upregulated miR-29a, miR-92a, and miR-21[17,18]. Thus, circulating cell-free miRNAs are likely robust and novel molecular markers in early colorectal neoplasm diagnosis. However, few miRNAs have been widely applied in clinical practice because of the failure to distinguish patients with AAs from healthy volunteers, the small sample sizes, and the lack of pathological features of subjects.
As the development of genome-sequencing technology has strongly catalyzed the understanding of cancer biology, an increasing number of publicly available genomic datasets, such as the Gene Expression Omnibus (GEO), has been utilized to identify potential biomarkers for cancer diagnosis. In the present study, the miRNA expression profiles of the GEO GSE25609, GSE115513, and GSE41655 datasets were used to mine differentially expressed miRNAs coexisting in serum and tissues between patients with colorectal neoplasms and healthy controls (HCs). Next, we screened circulating cell-free miR-199a-5p and miR-627 as potential diagnostic biomarkers of colorectal neoplasms, and explored their expression in serum, tissues and cancer cell lines.
All the preoperative peripheral blood samples and neoplasm tissues were obtained from patients who underwent endoscopy resulting in colorectal neoplasia, confirmed by histology as adenocarcinoma and AA at China-Japan Friendship Hospital from September 2019 to April 2021. The postoperative peripheral blood samples from 15 patients with CRC were collected 30 d after surgical resection. AA was defined as a large (≥ 1 cm) tubular adenoma, an adenoma with at least 25% villous elements, or a high-grade intraepithelial neoplasm. HC subjects were selected from those undergoing annual health checks without major abnormalities, and individuals undergoing screening colonoscopy. Individuals with a personal history of radiotherapy, chemotherapy, and bowel resection, malignant tumors in other organs, inflammatory bowel disease, hereditary CRC, or familial adenomatous polyposis, were excluded from both groups. Each blood sample was first centrifuged at 4000 × g for 10 min at 4°C. Next, the serum was aliquoted into cryovial tubes, labelled and stored at -80°C before use. All the colorectal samples were quick-frozen by liquid nitrogen and stored at -80°C. Each specimen was subjected to no more than 3 freeze/thaw cycles before analysis. All the participating subjects provided written informed consent to use their blood and tissue samples in the study, and the study protocol was approved by the ethics committee of China-Japan Friendship Hospital (No. 2018-116-K85-1). Details of the clinicopathological parameters of all the participants are listed in Table 1.
| Factors | Phase II | Phase III | |||||||
| CRC (n = 60) | AA (n = 60) | HC (n = 30) | P value | CRC (n = 30) | AA (n = 33) | HC (n = 20) | P value | ||
| Age (yr) | 66.95 ± 10.52 | 62.70 ± 10.58 | 63.60 ± 7.60 | 0.06 | 65.40 ± 10.10 | 60.27 ± 10.07 | 61.40 ± 8.15 | 0.10 | |
| Gender | Male | 41 (68.33%) | 42 (70.00%) | 20 (66.67%) | 0.95 | 21 (70%) | 25 (75.76%) | 17 (85.00%) | 0.48 |
| Female | 19 (31.67%) | 18 (30.00%) | 10 (33.33%) | 9 (30%) | 8 (24.24%) | 3 (15.00%) | |||
| Lymph node metastasis | Present | 21 (35.00%) | 9 (30%) | ||||||
| Absent | 39 (65.00%) | 21 (70%) | |||||||
| TNM stage | I-II | 37 (61.67%) | 20 (66.67%) | ||||||
| III-IV | 23 (38.33%) | 10 (33.33%) | |||||||
| Histology | SSA | 0 (0%) | 1 (1.67%) | 0 (0%) | 1 (3.03%) | ||||
| TA | 0 (0%) | 35 (58.33%) | 0 (0%) | 18 (54.55%) | |||||
| TVA | 0 (0%) | 24 (40.00%) | 0 (0%) | 14 (42.42%) | |||||
| Adenocarcinoma | 60 (100%) | 30 (100%) | |||||||
Our study was divided into three phases: Phase I, bioinformatics analysis; phase II, marker validation and phase III, exploration phase.
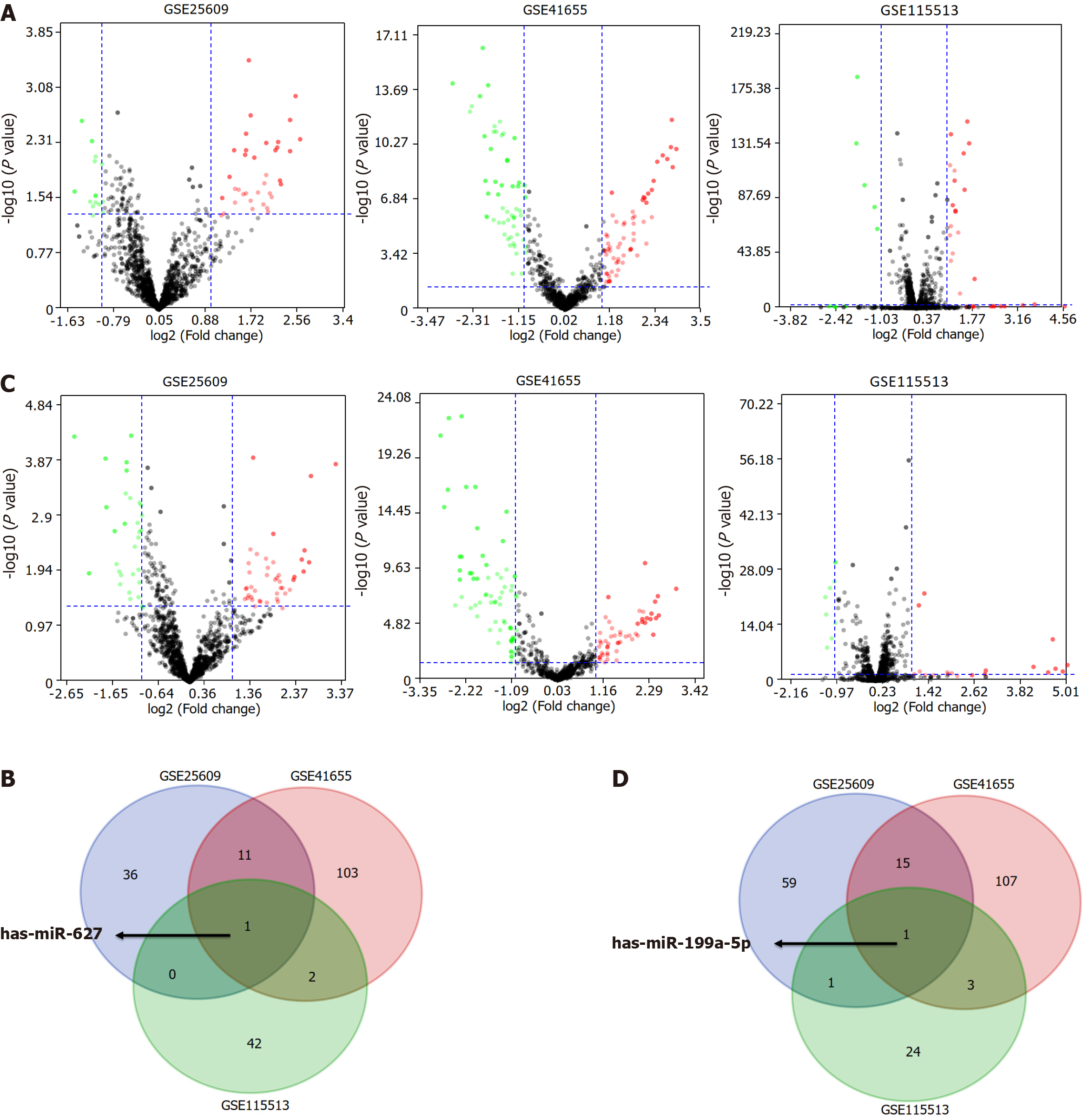
Phase I - Bioinformatics analysis: Three independent miRNA expression profiling datasets obtained from the GEO database were included in this study: GSE25609, GSE41655, and GSE115513. GSE25609 was submitted by Giraldez et al[19] and contained 61 plasma samples from 21 patients with CRC, 20 patients with AA, and 20 HCs. GSE41655 was submitted by Shi et al (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE41655) and comprised 33 CRC tissues, 59 adenoma tissues and 15 normal colorectal mucosae. GSE115513 was submitted by Slattery et al[20] and included 750 tumor tissues, 103 adenoma tissues and 649 normal mucosae. Differential gene expression analysis was employed to determine the differences in the miRNA expression levels between colorectal neoplasm samples and non-malignant samples using the “limma” package in R software. Differentially expressed genes (DEGs) in both plasma and tissues were then screened for further analysis.
Phase II – Marker validation: The levels of circulating miR-627-5p and miR-199a-5p identified in phase I were verified in serum samples from 60 patients with CRC, 60 patients with AA, and 30 HCs using quantitative real-time polymerase chain reaction (qRT-PCR).
Phase III - Exploration phase: The origin and function of circulating miR-627-5p and miR-199a-5p in cancer patients were investigated in tumor tissues and CRC cell lines. First, an independent group of 30 patients with CRC, 33 patients with AA and 20 HCs were enrolled to explore the levels of miR-199a-5p and miR-627-5p in neoplasm tissues. Next, fifteen pairs of serum samples obtained from cancer patients after and before surgical resection were used to identify the expression alterations of miR-199a-5p and miR-627-5p between preoperative and postoperative patients. Finally, we performed cell experiments to explore the roles of miR-199a-5p and miR-627-5p in colorectal cell viability, invasion, apoptosis and migration.
Experiments were performed using the normal colonic mucosal epithelial cell (FHC) and human colon carcinoma cell lines (HCT116, RKO, and SW480) obtained from the American Type Culture Collection. Cells were cultured in RPMI 1640 medium or Dulbecco’s Modified Eagle’s Medium (Invitrogen, Carlsbad, CA, United States) comprising 100 IU/mL of penicillin, 100 μg/mL of streptomycin, and 10% fetal bovine serum, and incubated in an atmosphere of 5% CO2 in air, a minimum relative humidity of 95%, and a temperature of 37°C.
In total, 1.5 × 106 SW480 cells/well (three replicates per group) were plated into 6-well culture plates. When the confluence reached 60%-70%, the miR-627-5p mimic, miR-199a-5p mimic, and mimic negative control (NC) oligos synthesized by GenePharma (Shanghai Province, China) were transfected into the cells using Lipofectamine™ RNAiMAX Transfection Reagent (Invitrogen, Carlsbad, CA, United States), respectively, based on the manufacturer’s protocol. The sequences used in our study are shown below: miR-627-5p mimic, forward 5’-CCCAGUGUUCAGACUACCUGUUC-3’ and reverse 5’-ACAGGUAGUCUGAACACUGGGUU-3’; miR-199a-5p mimic, forward 5’-GUGAGUCUCUA
Total RNA containing small RNAs was isolated from serum, tissues, and cells using an RNAprep Pure Cell Kit (Solarbio, Beijing, China) and then subjected to cDNA synthesis using a Hifair® Ⅱ1st Strand cDNA Synthesis Kit (YESEN, Shanghai, China). RT-PCR for miR-199a-5p, miR-627-5p and endogenous control U6 snRNA was performed using Hieff® qPCR SYBR® Green Master Mix (No Rox) (YESEN, Shanghai, China) and gene-specific stem-loop primers provided by Sangon Biotech in triplicate for each sample according to the manufacturer’s protocol. The sequences of primers used for qRT-PCR are listed in Supplementary Table 1. RT-PCR was performed using a LineGene 9600 Plus Real-Time PCR system (Bioer Technology) and default thermal cycling conditions. The reactions were initially incubated at 95°C for 5 min, followed by 40 cycles with denaturation for 10 s at 95°C, annealing for 20 s at 60°C, and extension for 20 s at 72°C. The relative quantities of miRNAs were calculated using the comparative threshold method and the average within cycle threshold (CT) values (2-∆∆Ct).
Cell Counting Kit-8 (CCK-8, Solarbio, Beijing, China) assays were used to investigate cell viability ability. SW480 cells were seeded in 96-well plates at a density of 1.5 × 103/well and transfected with mimics or mimic NC oligos before the CCK-8 assay as mentioned above. Following incubation at 0, 24, 48, and 72 h, the medium was removed, and 100 μL of serum-free medium supplemented with 10 µL of CCK-8 solution was placed in each well, followed by incubation for 30 min at 37°C in the dark. The absorbance at 450 nm was recorded using a spectrophotometric microtiter plate reader.
For the scratch wound assay, SW480 cell lines were seeded in 6-well culture plates at a density of 1.5 × 106 cells/well and cultured to reach confluence. CRC cells were subsequently treated with either mimics or mimic NC oligos according to a previously described protocol. The monolayer was scratched manually using a sterile 200 μL micropipette tip and washed with phosphate buffered saline solution to clear the exfoliated cells, and photographs were captured of all the long wounds at 0 and 24 h post-wounding. The migration capability of cells was calculated as follows: Migration rate = (G0-G24)/G0, where G0 represents the initial gap distance at 0 h and G24 represents the gap distance at 24 h post-wounding.
For the cell invasion assay, 6.5-mm Transwell chambers precoated with Matrigel (Corning, Bedford, MA, United States) were used following the manufacturer’s protocol. The transfected cells were first resuspended in 200 µL serum-free medium and then loaded into the top compartment containing 100 μL of serum-free medium at a density of 1 × 107/well. Additionally, 600 μL of RPMI 1640 supplemented with 10% fetal bovine serum was placed in the bottom compartment as a chemoattractant. After incubation for 72 h, the upper cells were gently removed with a cotton swab and the invaded cells on the lower surface of the bottom compartment were then fixed in 4% paraformaldehyde for 20 min, stained with 0.1% crystal violet solution for 15 min, and subsequently photographed at 400 × magnification by inverted fluorescence microscopy equipped with a digital camera. The number of invaded cells was counted in 5 random high-power fields.
An Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis kit (7seabiotech, Shanghai, China) was utilized to detect cell apoptosis following the manufacturer’s protocol. After transfection for 48 h, the detached and adherent cells were digested with 0.25% trypsin and harvested by transferring them with the medium into 1.5 mL centrifuge tubes. The transfected cells were subsequently resuspended in 400 µL of binding buffer at a density of at least 1 × 105 cells/mL, after which 10 μL of Annexin V-FITC and 5 μL of PI staining buffer were successively added to the cell suspension. Following incubation in the dark, the samples were immediately analyzed using an imaging flow cytometer to detect the cell apoptosis rate.
To investigate the underlying biological mechanisms of miR-199a-5p and miR-627-5p, target genes were first screened using three miRNA prediction databases miRTarBase, miRDB, and targetScan, and were subsequently subjected to a Venn diagram to identify the overlapping genes covered in 3 databases. Functional enrichment analysis including gene ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were conducted using the “org.Hs.eg.db” and “clusterProfiler” packages based on R language with the thresholds of a P value < 0.05 and a Q value < 0.05.
The data were expressed as median (interquartile range) or means ± SD as appropriate. The Wilcoxon rank-sum test was used to analyze differences in miRNA expression. Comparisons among the three groups were performed using χ2 test or one-way analysis of variance where appropriate. Receiver operating characteristic (ROC) curves were established to assess the ability of serum miR-199a-5p and miR-627-5p to serve as diagnostic markers of CRC and AA. The diagnostic performance of miRNA combinations was calculated using a binary regression model. Youden’s index (sensitivity + specificity-1) was employed to calculate the optimal cut-off point of the expression of serum miRNA expression. All calculations were performed using IBM SPSS (version 26.0) and the graphs were depicted using GraphPad Prism (version 9) and R software (version 3.6.1). P values less than 0.05 were deemed significant unless otherwise specified.
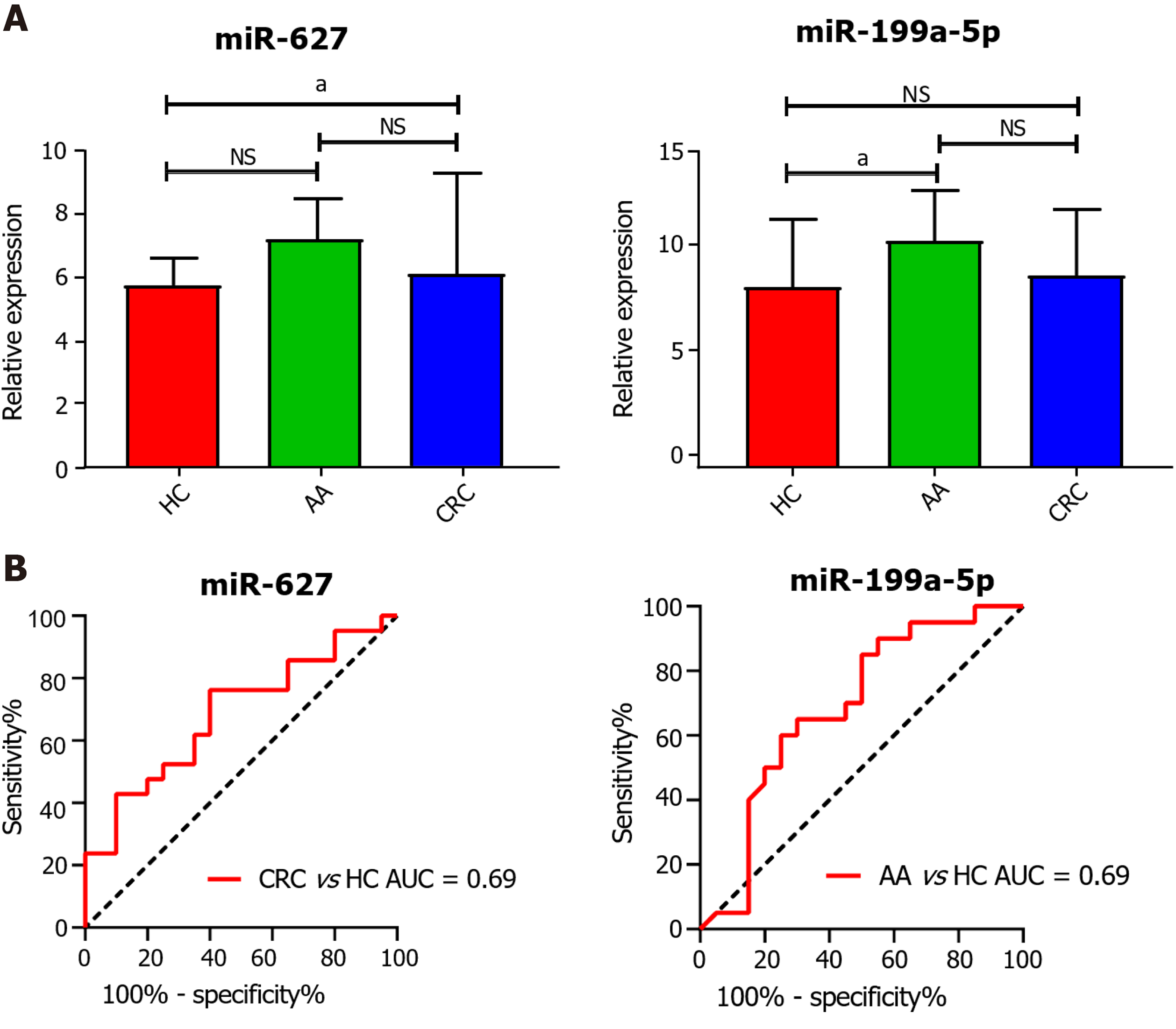
In the initial screening step aimed at identifying the candidate circulating miRNAs, we conducted differential expression analysis in the GSE25609, GSE41655, and GSE115513 datasets based on the cut-off criteria of a|log2 fold change| ≥ 1.00 and a false discovery rate < 0.05. In total, 48 differentially expressed genes (DEGs) in GSE25609, 117 DEGs in GSE41655, and 45 DEGs in GSE115513 were found to be differentially expressed between the CRC and control groups (Figure 1A). The DEGs in the three datasets intersected and miR-627 was screened as a candidate circulating miRNA for further analysis (Figure 1B). Additionally, 76 DEGs in GSE25609, 126 DEGs in GSE41655, and 29 DEGs in GSE115513 were identified to be differentially expressed between the adenoma and control groups, and miR-199a-5p was selected for further study (Figure 1C and D). Next, we analyzed the expression of plasma miR-627 and miR-199a-5p in the GSE25609 dataset and evaluated the usefulness of the miRNAs as diagnostic biomarkers of CRC and AA. The level of plasma miR-627 was markedly increased in the CRC group (P = 0.04, Figure 2A), while that of plasma miR-199a-5p was significantly upregulated in the AA group relative to the control group (P = 0.04, Figure 2A). ROC analysis demonstrated that plasma expression of miR-627 discriminated the CRC group from the normal controls with an area under the curve (AUC) = 0.69 (CI: 0.52-0.85, Figure 2B), while the plasma expression of miR-199a-5p discriminated the AA group from the control group with an AUC = 0.69 (CI: 0.52-0.86, Figure 2B). Thus, both miR-627 and miR-199a-5p were preliminarily selected for further study.
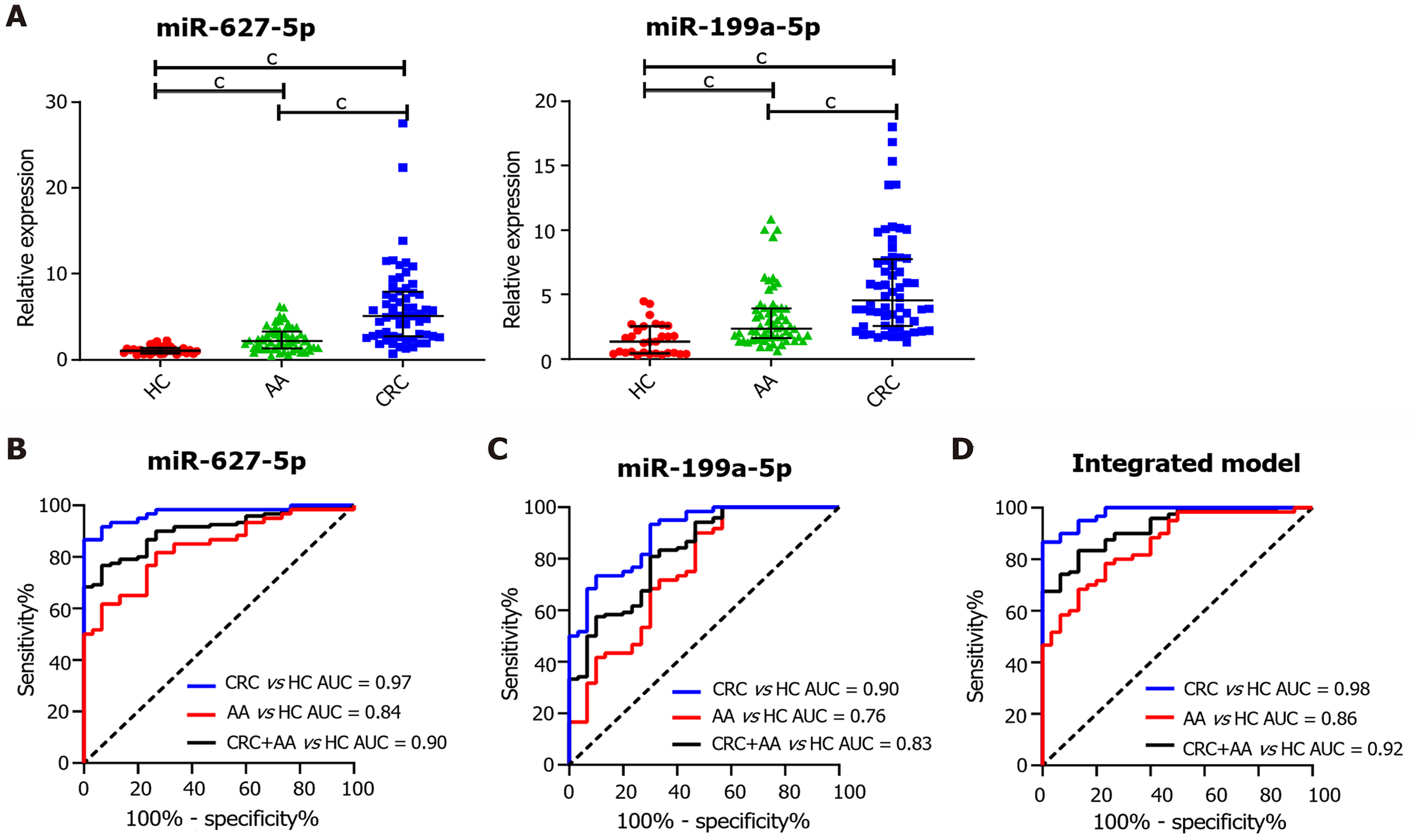
The levels of circulating cell-free miR-627-5p and miR-199a-5p in a validation cohort of serum specimens comprising 30 HCs, 60 AA patients and 60 CRC patients were examined by qRT-PCR. First, we observed that circulating miR-627-5p and miR-199a-5p were confirmed to be highly upregulated in patients with CRC and AA compared with HCs (Figure 3A, P < 0.001). Compared to patients with AA, serum miR-627-5p and miR-199a-5p were also highly increased in patients with CRC (Figure 3A, P < 0.001). Next, ROC analysis was employed to calculate the screening efficiency of miR-627-5p and miR-199a-5p in discriminating patients with colorectal neoplasms from HCs (Figure 3B-D). The AUC, sensitivity, specificity, and cut-off point were generated to describe the diagnostic value of tumor markers (Table 2). The AUC value for CEA was 0.70 to differentiate cancer patients from HCs with a sensitivity of 0.32 and a specificity of 1.00, while the AUC value for CA199 was 0.54 with the sensitivity of 0.12 and specificity of 1.00. By contrast, the performance of miR-627-5p yielded an AUC of 0.97 with an optimal sensitivity of 0.87 and a specificity of 1.00 at a cut-off of 2.21; miR-199a-5p showed an AUC of 0.90, a sensitivity of 0.93 and a specificity of 0.70 at a cut-off of 1.79, demonstrating the competitive power of both serum miRNAs to predict CRC patients.
| AUC | Sensitivity | Specificity | Cut-off value | P value | ||
| CRC vs HC | miR-627-5p | 0.97 | 0.87 | 1.00 | 2.21 | < 0.001 |
| miR-199a-5p | 0.90 | 0.93 | 0.70 | 1.79 | < 0.001 | |
| CEA | 0.70 | 0.32 | 1.00 | 7.83 | < 0.01 | |
| CA199 | 0.54 | 0.12 | 1.00 | 42.50 | 0.52 | |
| Integrated model | 0.98 | 0.87 | 1.00 | 0.84 | < 0.001 | |
| AA vs HC | miR-627-5p | 0.84 | 0.62 | 0.93 | 1.87 | < 0.001 |
| miR-199a-5p | 0.76 | 0.90 | 0.53 | 1.35 | < 0.001 | |
| CEA | 0.50 | 0.80 | 0.33 | 1.44 | 0.95 | |
| CA199 | 0.43 | 1.00 | 0.04 | 1.31 | 0.27 | |
| Integrated model | 0.86 | 0.78 | 0.77 | 0.59 | < 0.001 | |
| CRC+AA vs HC | miR-627-5p | 0.90 | 0.77 | 0.93 | 1.87 | < 0.001 |
| miR-199a-5p | 0.83 | 0.81 | 0.70 | 1.78 | < 0.001 | |
| CEA | 0.60 | 0.90 | 0.30 | 1.28 | 0.12 | |
| CA199 | 0.49 | 0.07 | 1.00 | 42.50 | 0.80 | |
| Integrated model | 0.92 | 0.83 | 0.87 | 0.74 | < 0.001 |
Additionally, the diagnostic power of these markers in distinguishing AA patients from HCs was also analyzed (Table 2). As the AUCs of CEA and CA199 reached 0.50 and 0.43, respectively, their predictive value seemed to be limited. MiR-627-5p was identified as a promising predictor, with an AUC of 0.84. The sensitivity and specificity were 0.62 and 0.93, respectively, and the cut-off value was 1.87. Circulating cell-free miR-199a-5p discriminated AA patients from controls with an AUC of 0.76. The optimal sensitivity and specificity with respect to a cut-off value of 1.35 were 0.90 and 0.53, respectively. Additionally, we examined the capacity of these markers to differentiate patients with CRC and AA from HCs. The diagnostic power of miR-627-5p yielded an AUC value of 0.90 with a sensitivity of 0.77 and a specificity of 0.93 at a cutoff value of 1.87; miR-199a-5p yielded an AUC of 0.83, a sensitivity of 0.81 and a specificity of 0.70 at a cut-off of 1.78. In comparison, CEA yielded an AUC value of 0.60 and CA199 yielded an AUC value of 0.49. These results revealed that circulating cell-free miR-627-5p and miR-199a-5p predicted colorectal neoplasms with high efficiency and could be used as new biomarkers.
We determined whether the combination of miR-627-5p and miR-199a-5p would demonstrate a synergistic effect in improving the diagnostic performance in predicting colorectal neoplasms compared with each miRNA alone. Hence, logistic analysis was employed to combine miR-627-5p with miR-199a-5p and construct an integrated model for diagnostic prediction (Table 2). ROC analysis demonstrated that the integrated model differentiated patients with CRC from HCs with an AUC value of 0.98, a sensitivity of 0.87 and a specificity of 1.00. The diagnostic capacity of this model to distinguish AA patients from controls yielded an AUC of 0.86 with a sensitivity of 0.78 and a specificity of 0.77. Additionally, the AUC was 0.92 to separate patients with CRC and AA from the control group with a sensitivity of 0.83 and a specificity of 0.87. These results revealed that the integrated model showed better discriminative capacity than each miRNA alone.
We further compared the differences in serum miR-627-5p and miR-199a-5p among different subgroups. The Wilcoxon rank-sum tests revealed that circulating miR-627-5p and miR-199a-5p expression was not correlated with age, TNM stage, lymph node metastasis, or histological grade in CRC patients (Table 3). A significant increase in serum miR-627-5p and miR-199a-5p was observed in CRC patients with tumor sizes ≥ 4 cm (miR-627-5p: P = 0.04; miR-199a-5p: P = 0.03) and male gender (miR-627-5p: P = 0.03; miR-199a-5p: P = 0.04). However, none of the clinical pathology characteristics of AA patients, such as age, gender, adenoma number, histology, and intraepithelial neoplasia, were highly correlated with either miRNA (Supplementary Table 2).
| Variable | n (%) | miR-627-5p | miR-199a-5p | |||
| Median (IQR) | P value | Median (IQR) | P value | |||
| Age (yr) | < 65 | 24 (40.00) | 4.15 (2.43-8.82) | 0.23 | 4.14 (2.36-6.52) | 0.38 |
| ≥ 65 | 36 (60.00) | 5.92 (3.36-8.41) | 3.86 (2.55-9.87) | |||
| Gender | Male | 41 (68.33) | 6.46 (3.24-7.60) | 0.03 | 7.80 (5.76-10.04) | 0.04 |
| Female | 19 (31.67) | 4.16 (2.22-6.05) | 5.82 (3.89-7.91) | |||
| Tumor size | < 4 cm | 20 (33.33) | 4.30 (2.22-6.05) | 0.04 | 6.15 (3.91-7.79) | 0.03 |
| ≥ 4 cm | 40 (66.67) | 6.12 (3.46-7.59) | 7.71 (5.57-9.99) | |||
| Lymph node metastasis | Absent | 39 (65.00) | 5.10 (2.59-7.19) | 0.93 | 5.87 (3.86-8.98) | 0.52 |
| Present | 21 (35.00) | 5.07 (2.63-8.20) | 3.97 (2.26-7.55) | |||
| TNM stage | I-II | 37 (61.67) | 5.10 (2.78-7.22) | 0.77 | 6.75 (3.87-10.04) | 0.17 |
| III-IV | 23 (38.33) | 5.07 (2.74-8.45) | 3.89 (1.91-7.22) | |||
| Histological grade | Well/moderately differentiated | 49 (81.67) | 4.16 (1.92-6.05) | 0.33 | 5.82 (3.97-9.26) | 0.36 |
| Poorly differentiated/mucinous | 11 (18.33) | 5.07 (2.78-7.16) | 3.85 (3.44-5.87) | |||
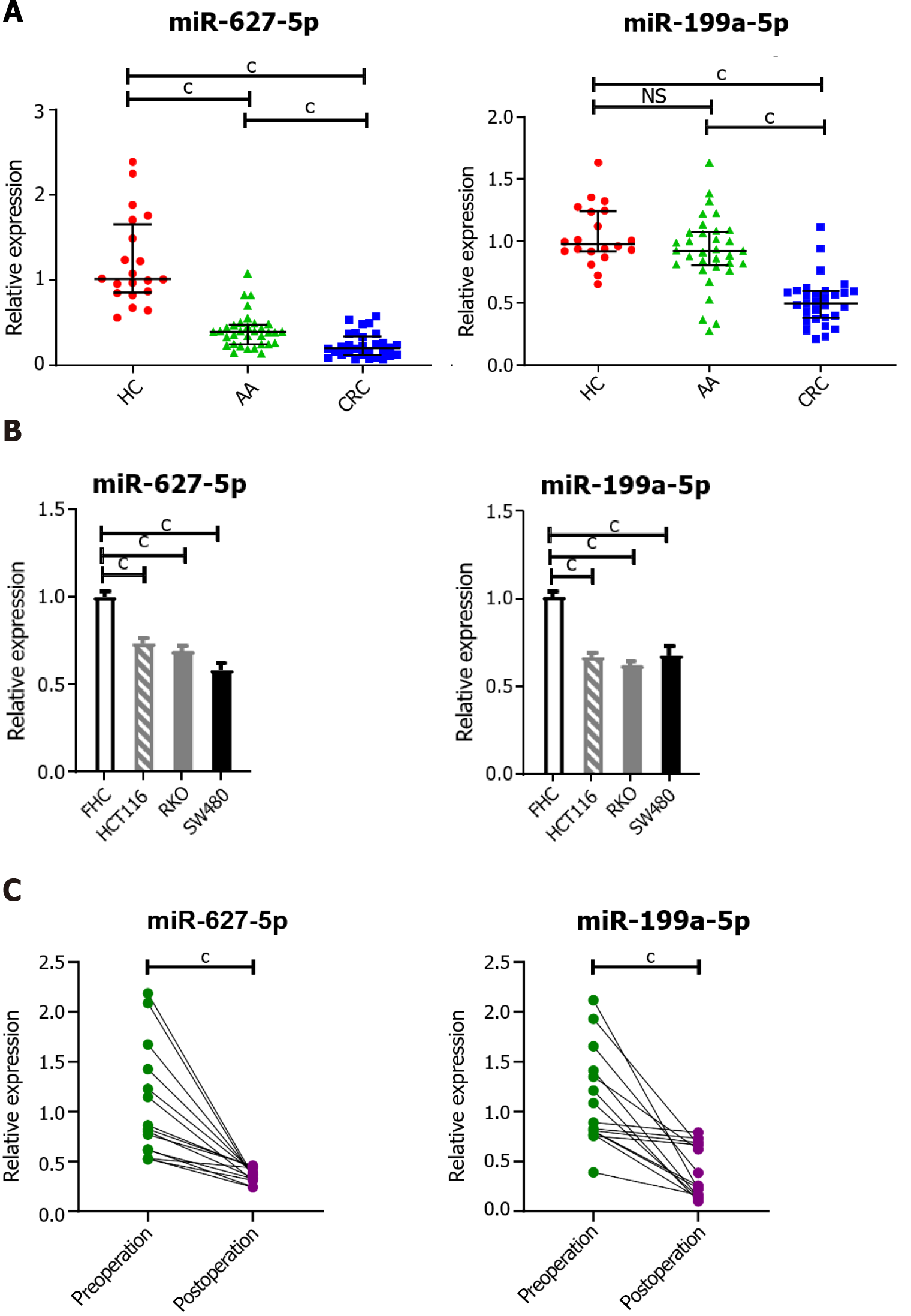
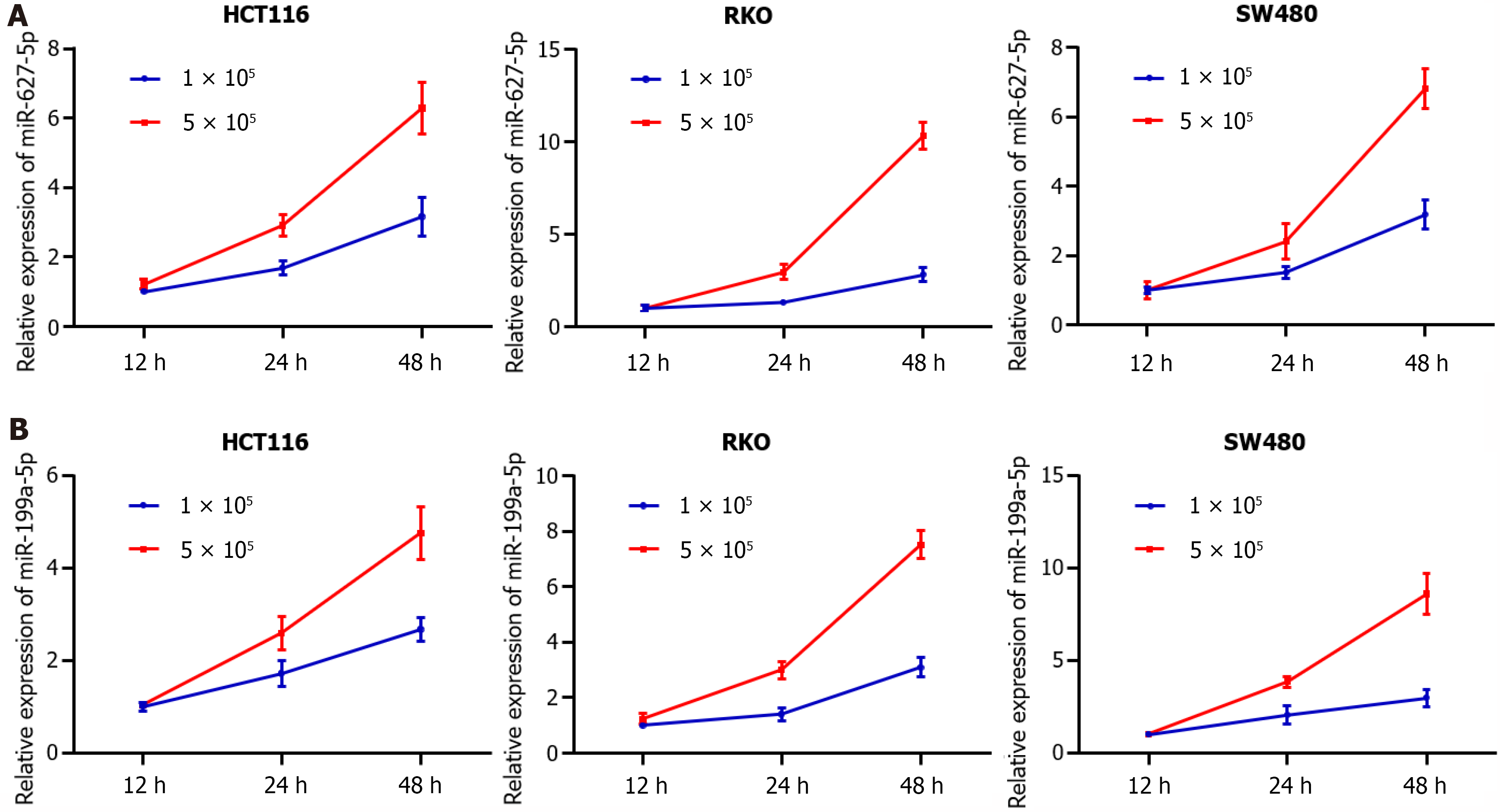
Mounting evidence has demonstrated that circulating miRNAs are secreted into blood by cancer cells or are released from apoptotic and necrotic cancer cells[21]. To prove that circulating miRNAs in serum are of tumor origin, an independent cohort of 30 CRC patients, 33 AA patients and 20 HCs was enrolled to examine the tissue expression levels of miR-627-5p and miR-199a-5p (Figure 4A). Unexpectedly, the levels of both miR-627-5p and miR-199a-5p were markedly decreased in CRC patients compared with those in HCs and patients with AA (P < 0.001, Figure 4A). Notably, miR-627-5p expression was significantly reduced in AA tissues relative to that in normal controls (P < 0.001, Figure 4A), but no significant difference was found in miR-199a-5p expression between AA tissues and controls (P = 0.12, Figure 4A). Consistent with this finding, the expression levels of miR-627-5p and miR-199a-5p were markedly decreased in human colon cancer cell lines compared to the FHC cell line (P < 0.001 vs FHC cell line, Figure 4B). Next, the serum levels of both miRNAs were measured in another independent cohort of 15 CRC patients before and 1 mo after surgical removal. Their expression levels were markedly decreased in serum postoperatively compared to those preoperatively (P < 0.001, respectively, Figure 4C). Additionally, the expression of both miRNAs increased with culture time and cell numbers in the culture media of colon cancer cell lines (HCT116, RKO, and SW480, Figure 5).
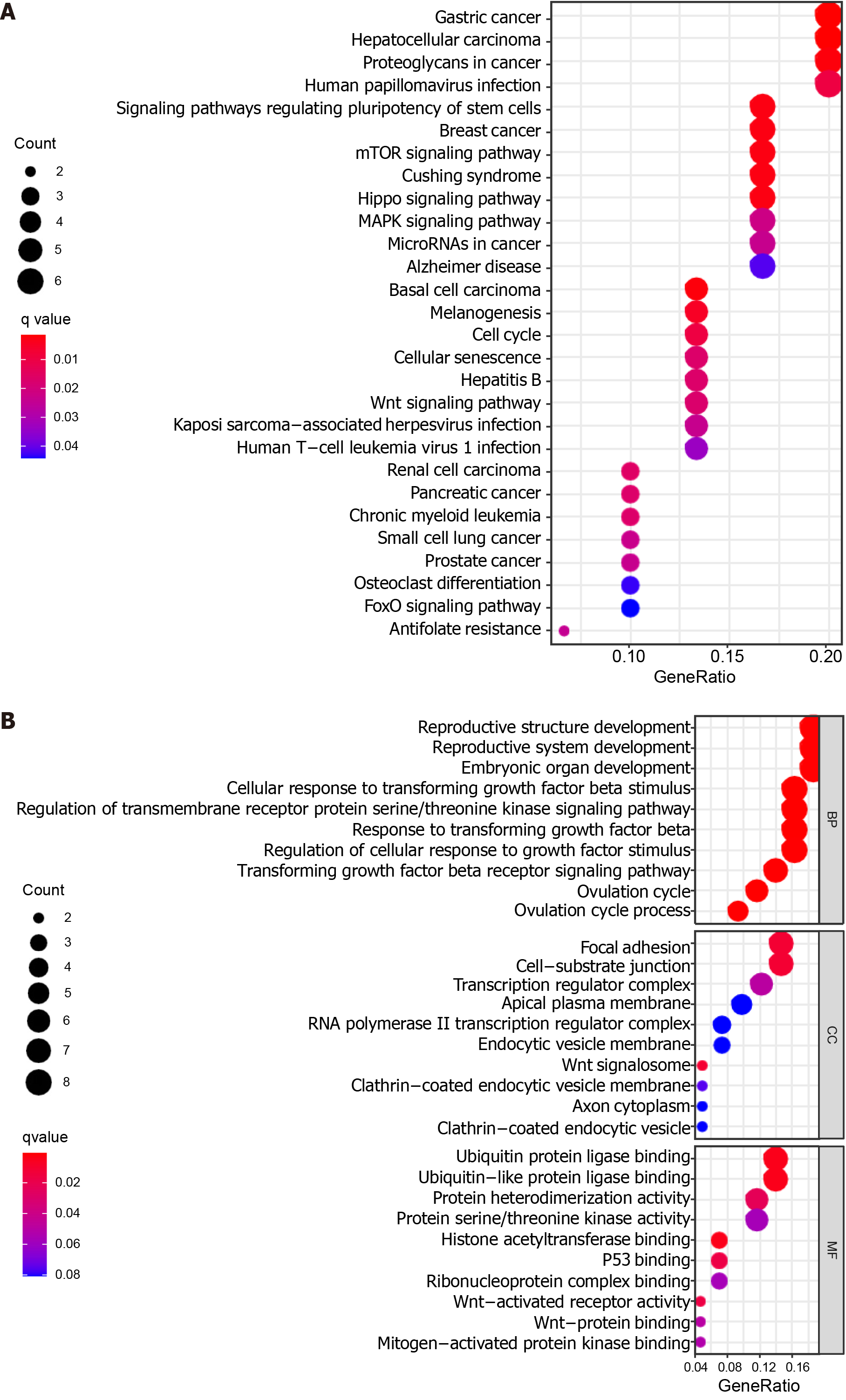
To elucidate the potential biological functions of miR-627-5p and miR-199a-5p, 8 overlapping target genes for miR-627-5p and 35 overlapping target genes for miR-199a-5p were first explored using the TargetScan, miRDB, and miRTarBase databases (Supplementary Figure 1). Subsequently, functional enrichment analysis revealed that 28 KEGG pathways and 462 GO terms were significantly enriched for the target genes (Supplementary Tables 3 and 4). The top ten terms in the biological process (BP), cellular component (CC), and molecular function (MF) groups from the GO results are depicted in Figure 6B. For the BP results, it was noted that the target genes were related to the response to the transforming growth factor beta receptor signaling pathway. CC results revealed that the target genes were primarily located at the Wnt signalosome and focal adhesion. For the MF results, the target genes were primarily involved in the biological functions of several oncogenes, such as p53 binding, Wnt-activated receptor activity, and Wnt-protein binding. Additionally, KEGG analysis demonstrated that the target genes were significantly associated with multiple cancer-related pathways, such as the Wnt signaling pathway, mammalian target of rapamycin (mTOR) signaling pathway, and hippo signaling pathway (Figure 6A). These target genes were also involved in regulating various cancers (e.g., gastric cancer, hepatocellular carcinoma, breast cancer, pancreatic cancer, and prostate cancer). These results revealed the potential roles of the miRNAs in affecting cancer proliferation, invasion and metastasis.
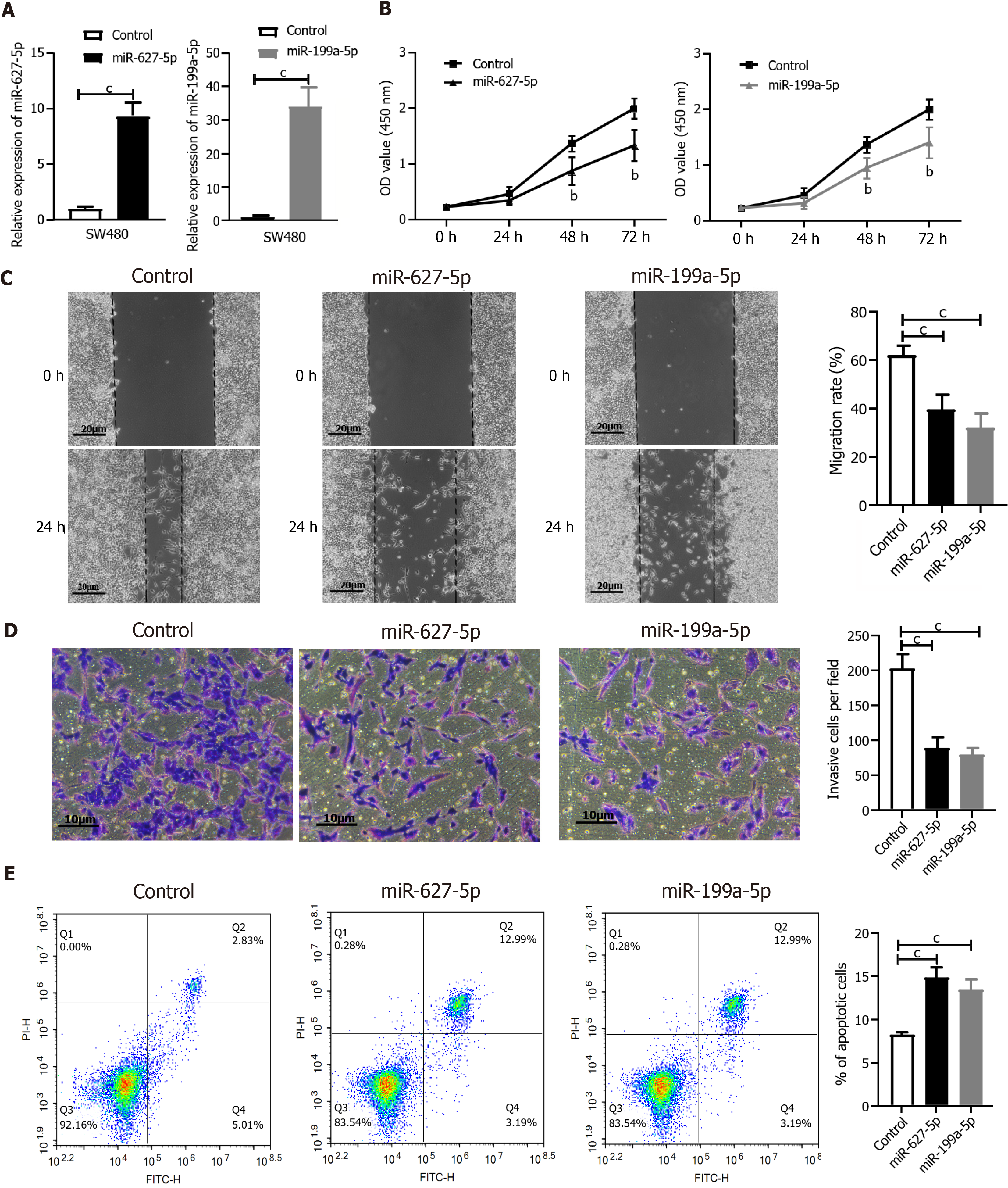
To further validate the function of miR-627-5p and miR-199a-5p, SW480 cells were treated with either a miR-627-5p mimic or a miR-199a-5p mimic for a series of in vitro functional assays. The efficiency of both miR-672-5p mimic and miR-199a-5p transfection was analyzed by qRT-PCR in SW480 cells (P < 0.001, respectively, Figure 7A). Next, the CCK-8 assay was applied to measure the effect of miRNAs on the viability of tumor cells, the wound healing assay was employed to investigate the role of miRNAs in the migration ability of CRC cells, the Transwell assay was applied to observe cell invasion features, and flow cytometry was conducted to test the function of miRNAs in cell apoptosis. Both miR-627-5p and miR-199a-5p mimics resulted in a marked downregulation of cell viability (Figure 7B), cell motility (Figure 7C), and invasive ability of SW480 cells (Figure 7D). Additionally, elevated levels of miR-627-5p and miR-199a-5p expression markedly increased the number of apoptotic cells compared to that in the control groups (Figure 7E).
Identifying non-invasive tumor markers for the early screening of colorectal tumors and precancerous lesions is critical to reduce cancer incidence and extend the long-term survival of patients through timely recognition and radical surgery of advanced colorectal neoplasia. With the development of genome-sequencing technology and comprehensive bioinformatic analysis, the potential abilities of circulating miRNAs released from cancer cells as non-invasive biomarkers have been recognized by an increasing number of scientists. Notably, using circulating miRNAs as tumor markers is a better strategy than using RNA or protein biomarkers due to their reproducible, highly stable, and consistent characteristics on individuals of the same species. In the present study, we first identified that circulating miR-627-5p and miR-199a-5p were markedly elevated in patients with colorectal neoplasia and appeared to be novel biomarkers in the non-invasive screening of colorectal neoplasia.
In the discovery stage, we screened candidate circulating cell-free miRNAs in serum as liquid biopsy biomarkers of colorectal neoplasm using bioinformatic analysis from three GEO datasets. Two overlapping miRNAs, miR-627 and miR-199a-5p, were identified and subjected to ROC analysis. As the mature sequences of miR-627 were designated as “-5p” and “-3p” and a growing number of studies have focused on the role of miR-627-5p in cancer development[22-24], we chose miR-627-5p for further validation. Thus, we speculated that circulating miR-199a-5p and miR-627-5p have potential as biomarkers of colorectal neoplasia. In the validation stage, we sought to determine their efficiencies in discriminating patients with CRC and AA from HCs by target quantification methods. However, previous studies have demonstrated that not all of the miRNAs detected by next-generation sequencing could be steadily achieved using the qRT-PCR method[25]. Several factors have influenced the successful detection of miRNAs, including the relatively low expression of specific miRNAs, sequence length variations of unique miRNAs, and bias of detection methods. We confirmed that the circulating expression levels of miR-199a-5p and miR-627-5p were markedly increased in patients with precancerous lesions and CRC compared with neoplasm-free controls by qRT-PCR, particularly those diagnosed with CRC, suggesting that the increase in both miRNAs occurred during colorectal carcinogenesis. Additionally, serum miR-199a-5p and miR-627-5p levels were significantly elevated in male CRC patients and in those with larger tumor sizes, but were relatively stable in patients with other clinicopathological characteristics, such as age, tumor location, histological grade, and TNM stage. Colorectal adenomas comprise several histological types, including sessile serrated adenoma, TA, and tubulovillous adenoma (TVA). Considering that the traditional adenoma-carcinoma sequence developed by the accumulation of multiple genomic events contributes to 70%-90% of CRC[4], we mainly included patients with TA and TVA in the present study. Although different subtypes of precursor colorectal lesions may involve different genetic alterations and distinct miRNA changes[26], our study demonstrated no difference in the serum miR-199a-5p and miR-627-5p levels between TA and TVA patients, further confirming the promise of both miRNAs as robust biomarkers for precancerous adenomas. However, considering the small sizes in the subgroups, these results should be further validated in an independent large cohort.
To date, a variety of studies have demonstrated that components of colorectal tumors (such as miRNAs, mRNAs, proteins and exosomes), which are released into biological fluids, can be detected by liquid biopsy and used as circulating biomarkers[21]. However, most studies only focused on the predictive power of circulating biomarkers in the identification of CRC and not in the diagnosis of precancerous lesions. As early as 2009, a study showed that miR-92 was markedly increased in CRC patients and had a high diagnostic accuracy with an AUC value of 0.885[27], but patients with adenoma were not included in the study. Aberrant expression of circulating exosomal miR-125a-3p has been detected in the serum of patients with early stage colon cancer, but its diagnostic accuracy was relatively low (AUC = 0.685)[25]. Overexpression of serum metadherin mRNA from CRC patients when compared to HCs was firstly described by Ghafar et al[28] in 2020 and its diagnostic accuracy (AUC = 0.976) was significantly higher than other routine tumor markers. However, the potential utility of circulating metadherin mRNA in the identification of AAs has not been studied. The most noteworthy results of the present study were that serum miR-627-5p and miR-199a-5p could act as novel minimally invasive biomarkers for colorectal neoplasm screening. By enrolling an independent cohort including CRC, AA and HCs, we conducted a powerful statistical analysis to draw conclusions. Circulating cell-free miR-199a-5p showed promising potential in differentiating CRC patients from HCs (AUC = 0.90), but its diagnostic efficiency in discriminating AAs from controls was relatively low (AUC = 0.76). MiR-627-5p exhibited better performance in differentiating cancer patients from controls (AUC = 0.97) and AA patients from HCs (AUC = 0.84), revealing that the marked upregulated expression of serum miR-627-5p was more likely to reflect the early stage during the progression from precancerous lesions to neoplasia. Additionally, circulating miR-627-5p could discriminate patients with colorectal neoplasms from the neoplasm-free group (AUC = 0.90). Concurrently, the AUCs for the conventional tumor markers CEA and CA199 were lower than those for both miRNAs. Considering that the optimal target lesion for CRC prevention would be AA, which has a higher risk of evolving into a tumor, miR-627-5p could be a better minimally invasive biomarker for the early screening of colorectal neoplasms.
Currently, a number of studies have demonstrated that combined genes can be used to establish integrated diagnostic models that help to improve the diagnostic accuracy. For example, Giraldez et al[19] established a combined signature consisting of miR-19a, miR19-b, and miR-15b, discriminating early colorectal tumors with a specificity of 79.25% and a sensitivity of 80.95%. Another study constructed a serum-based four-miRNA signature (miR-23a-3p, miR-27a-3p, miR-142-5p and miR-376c-3p) that could discriminate patients with colon cancer from control groups with an AUC of 0.92[29]. In the present study, a logistic integrated model combining miR-199a-5p and miR-627-5p exhibited a higher diagnostic performance than using either miRNA alone, and it diagnosed AA with both high sensitivity and specificity as robustly as CRC. Several advantages exist regarding the application of the integrated model as a practical and economic non-invasive screening strategy for colorectal neoplasms: (1) The integration of miR-199a-5p and miR-627-5p built a more reliable diagnostic model than conventional tumor markers and each miRNA alone, reflecting the complex molecular mechanisms of CRC initiation and development; (2) the use of blood samples may lead to better patient compliance than guiding patients to conduct bowel preparation and endoscopy; and (3) the diagnostic model comprising only two miRNAs would save the expense of laboratory testing and lower the training requirements for clinical doctors, thereby promoting its potential usefulness in clinical practice.
Growing evidence has demonstrated that dysregulated expression of miRNAs plays key roles in modulating multiple genes crucial in cancer initiation, progression and metastasis[13]. Hence, we retrieved the target genes of miR-627-5p and miR-199a-5p and functionally annotated them to further understand the biological role of both miRNAs. The target genes were mainly related to multiple critical signaling pathways involved in the multistep carcinogenesis, such as Wnt, mTOR, Hippo, transforming growth factor beta receptor signaling pathway and focal adhesion. Abundant research has documented that constitutive activation of Wnt signaling is the pathological basis of many malignancies, including CRC[30]. Transforming growth factor beta signaling pathways have been proved to be essential for the proliferation, angiogenesis, and invasion of CRC[31]. These pathways represented only a small subset of target genes participating in signaling pathways, supporting the function of miR-627-5p and miR-199a-5p in regulating cancer initiation and progression. In our study, miR-627-5p was demonstrated to be not only sequentially decreased from normal colorectal tissues and precancerous lesions to cancer tissues, but also significantly reduced in the human colon cancer cell lines compared with than in FHC cells. MiR-199a-5p was markedly reduced in CRC tissues and cell lines but not significantly decreased in AA tissues. Additionally, in vivo gain-of-function assays revealed that enhanced expression of both miRNAs suppressed the aggressive behavior of CRC cells, suggesting their suppressive role in CRC development. It is worth mentioning that the same miRNA may exert different functions depending on the circumstances, for example, promoting tumor growth in several cancer types, and acting as a tumor suppressor in other tumor types[32]. MiR-627-5p has been demonstrated to be reduced in several cancer tissues, including CRC[33], glioblastoma multiforme[24], oral squamous cell carcinoma[22], and hepatocellular carcinoma[34], supporting its suppressor role in cancer progression. However, Shin et al[35] mentioned that miR-627-5p was markedly increased in patients diagnosed with gastric cancer when compared with their non-tumor counterparts and was considered a potential oncogene in gastric cancer. The regulatory mechanism of miR-627-5p in CRC has not been documented to date, but a recent publication has revealed that decreased expression of miR-627-5p exerts inhibitory effects on hepatocellular carcinoma possibly by targeting the BCL3 transcription coactivator[34]. An in vitro study reported that miR-627-5p suppressed cell proliferation, retarded migration, and promoted apoptosis by increasing YBX2 expression in oral squamous cell carcinoma[22]. Further research is required to explore the underlying mechanism of miR-627-5p in the carcinogenesis of CRC. MiR-199a-5p, a member of the miR-199 family, has been demonstrated to be a proliferation inhibition gene in a variety of tumors, including non-small cell lung cancer[36], laryngeal cancer[37], breast cancer[38], and hepatocellular carcinoma[39]. Extensive studies have been performed to explore the suppressive function of miR-199a-5p in CRC. For example, in a clinical study with a small sample size, miR-199a-5p expression was markedly decreased in clinical CRC tissues than in the control group and inversely correlated with discoidin domain receptor 1 (DDR1) expression. Additionally, downregulated miR-199a-5p increased the level of DDR1, activating epithelial-to-mesenchymal transition related signaling, thus enhancing the migratory and invasive abilities of human CRC cells[40]. These findings were similar to those of another study with a large sample size, showing that the expression level of miR-199a-5p was sequentially reduced in T2, T3 and T4 colorectal tumors compared to that in normal tissues[39]. The study also demonstrated that miR-199a-5p suppressed cell proliferation, migration and invasion of CRC cells by negatively regulating integrin alpha-3[41]. Zhu et al[42] suggested that miR-199a-5p suppressed the growth of CRC cells potentially by inactivating the phosphoinositide 3-kinase/AKT signaling pathway. These previous studies supported the notion that miR-199a-5p plays a crucial role as a tumor suppressor by targeting various genes in CRC, providing a new therapeutic target to inhibit CRC growth and metastasis.
It is worth noting that the levels of miR-627-5p and miR-199a-5p were increased in CRC serum but decreased in tumor tissues. The inconsistency between the serum level of miRNAs and tissue expression validated in CRC and AA suggested that the release of miRNAs is complex during the course of cancers. In general, the circulating miRNA expression pattern should be closely correlated with the tissue expression pattern in the same cancer type. Hur et al[43] found that circulating miR-203 levels were markedly increased in CRC patients with liver metastasis compared with those in primary CRC patients, a finding that was concordant with the markedly high expression in liver metastasis specimens compared with matched primary CRC tissues. However, discrepancies between the circulating levels and tissue levels have been observed in many miRNAs. miR-145 showed the opposite trend, with upregulated expression in non-small cell lung cancer serum[44] and reduced expression in tissues[45]. Similarly, Lian et al[46] suggested that serum miR-199a-3p level was markedly higher in osteosarcoma patients than in HCs, whereas Duan et al[47] reported that miR-199a-3p was decreased in the same tumor tissues. Thus, the miRNA expression patterns between serum and tissues may be opposite. In our study, the levels of miR-627-5p and miR-199a-5p were significantly decreased in postoperative serum samples compared with those in preoperative samples and their expression levels increased with culture time and cell numbers in the culture media of colon cancer cell lines, which suggested that both miRNAs might be tumor-derived. The conflicting data between intracellular and extracellular profiles of miR-199a-5p and miR-627-5p questioned the existence of a specific secretion mechanism for circulating miRNAs. Accumulating evidence has demonstrated that different sources of miRNAs are present in the biological fluids of cancer patients[21,48]. miRNAs can be actively secreted by tumor cells into extracellular fluids by encapsulation with membrane-bound vesicles, including exosomes and shedding microvesicles, or by binding to Argonaute proteins. Some circulating miRNAs are regarded as merely byproducts of tumor cell apoptosis and necrosis. Additionally, circulating tumor cells and blood platelets are also packed with intracellular miRNAs. Therefore, we hypothesized that circulating miR-199a-5p and miR-627-5p originate from the active secretion of tumor cells and passive release from death cells, likely representing biological processes for miRNA clearance in tumor tissues.
Despite some strengths of the present study, it also has some weaknesses. First, a major issue is that we did not enroll patients with other diseases such as inflammatory bowel disease and other cancer types to confirm the diagnostic efficiency of both miRNAs. Second, the small sample size in the control group may reduce the validity and reliability of our diagnostic study. Indeed, recruiting truly healthy volunteers is practically difficult, requiring the collection of each individual’s personal health information and a thorough medical examination. Further research with a large sample size is anticipated to validate whether both miRNAs can be used in routine clinical diagnosis. Third, an optimal protocol for sample preparation is also a key issue in our study. Arroyo et al[49] suggested that the blood clotting procedure may induce the secretion of a number of miRNAs from platelets, thus influencing the abundance of tumor-specific miRNAs. Thus, the use of blood plasma is preferred in future studies. Finally, the origin of circulating extracellular miR-199a-5p and miR-627-5p in patients with colorectal neoplasms was unclear and a series of in vivo and in vitro experiments should be performed to answer this question and further clarify the underlying mechanism of both miRNAs in the initiation and progression of colorectal neoplasms.
To our best knowledge, our study is the first to validate the markedly elevated levels of serum miR-199a-5p and miR-627-5p in patients with CRC and AA, revealing the potential of both circulating cell-free miRNAs in serum as liquid biopsy biomarkers of colorectal neoplasms. An integrated model combining miR-199a-5p and miR-627-5p obtained an enhanced discriminative capacity and can serve as a practical and economic non-invasive screening strategy for colorectal neoplasms. Furthermore, in vitro gain-of-function experiments suggested that both miRNAs act as tumor suppressors in the tumorigenesis of CRC.
Identifying non-invasive tumor biomarkers for the early screening of colorectal cancer (CRC) and advanced adenomas (AAs), appears to be a key measure to reduce cancer incidence and extend the long-term survival of patients through timely recognition and radical surgery of early-onset CRC and precancerous lesions.
Due to the lack of high specificity and sensitivity screening strategies, a number of patients with CRC are diagnosed in advanced stages, which is a bottleneck in decreasing the mortality rate of cancer patients. With the development of genome-sequencing technology, circulating microRNAs (miRNAs) are considered potential non-invasive screening biomarkers of colorectal neoplasms.
To examine the efficiency of circulating miR-627-5p and miR-199a-5p in serum samples to delineate patients with CRC and AA from healthy controls (HCs).
Candidate miRNAs were first selected from three public miRNA datasets using bioinformatic analysis methods. An independent set of serum samples from 60 patients with CRC, 60 patients with AA, and 30 HCs was included to detect the diagnostic power of candidate miRNAs. The origin and function of candidate miRNAs were then explored in cancer cell lines and tumor tissues.
We first identified that circulating miR-627-5p and miR-199a-5p were markedly elevated in patients with colorectal neoplasia and both miRNAs could discriminate CRCs and AAs from the control group with high sensitivity and specificity. The combination of miR-199a-5p and miR-627-5p was a more reliable diagnostic model than conventional tumor markers and each miRNA alone. Additionally, the expression levels of both miRNAs were markedly reduced in postoperative serum samples compared to those in preoperative samples and their expression levels increased with culture time and cell numbers in the culture media of cancer cell lines, which suggested both miRNAs might be tumor-derived.
Serum miR-672-5p and miR-199a-5p have strong potential as practical and economic non-invasive biomarkers for early screening of colorectal neoplasms.
Circulating miRNAs in peripheral blood offer new opportunities for promising early detection of colorectal neoplasms.
The authors thank Gene Expression Omnibus (GEO) for providing invaluable datasets for statistical analyses.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abd-Elsalam S, Egypt; Batyrbekov K, Kazakhstan; Sato T, Japan S-Editor: Chen YL L-Editor: Webster JR P-Editor: Qi WW
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55701] [Article Influence: 7957.3] [Reference Citation Analysis (132)] |
| 2. | O'Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1063] [Cited by in RCA: 1161] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 3. | Gellad ZF, Provenzale D. Colorectal cancer: national and international perspective on the burden of disease and public health impact. Gastroenterology. 2010;138:2177-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 4. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 2979] [Article Influence: 496.5] [Reference Citation Analysis (3)] |
| 5. | Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE, Quinn VP, Ghai NR, Levin TR, Quesenberry CP. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1552] [Article Influence: 141.1] [Reference Citation Analysis (0)] |
| 6. | Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ, Etzioni R, McKenna MT, Oeffinger KC, Shih YT, Walter LC, Andrews KS, Brawley OW, Brooks D, Fedewa SA, Manassaram-Baptiste D, Siegel RL, Wender RC, Smith RA. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68:250-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 1304] [Article Influence: 186.3] [Reference Citation Analysis (0)] |
| 7. | Rutter MD, Nickerson C, Rees CJ, Patnick J, Blanks RG. Risk factors for adverse events related to polypectomy in the English Bowel Cancer Screening Programme. Endoscopy. 2014;46:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 8. | Lee MW, Pourmorady JS, Laine L. Use of Fecal Occult Blood Testing as a Diagnostic Tool for Clinical Indications: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2020;115:662-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF, Bast RC Jr; ASCO. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313-5327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1110] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 10. | Nguyen LH, Goel A, Chung DC. Pathways of Colorectal Carcinogenesis. Gastroenterology. 2020;158:291-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 305] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 11. | Lau NC, Lim LP, Weinstein EG, Bartel DP. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294:858-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2408] [Cited by in RCA: 2411] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 12. | Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5705] [Cited by in RCA: 6021] [Article Influence: 316.9] [Reference Citation Analysis (0)] |
| 13. | To KK, Tong CW, Wu M, Cho WC. MicroRNAs in the prognosis and therapy of colorectal cancer: From bench to bedside. World J Gastroenterol. 2018;24:2949-2973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 116] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (2)] |
| 14. | Hur K, Toiyama Y, Takahashi M, Balaguer F, Nagasaka T, Koike J, Hemmi H, Koi M, Boland CR, Goel A. MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut. 2013;62:1315-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 451] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 15. | Yu Y, Kanwar SS, Patel BB, Oh PS, Nautiyal J, Sarkar FH, Majumdar AP. MicroRNA-21 induces stemness by downregulating transforming growth factor beta receptor 2 (TGFβR2) in colon cancer cells. Carcinogenesis. 2012;33:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 220] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 16. | Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W, Wang J, Jiang X, Xiang Y, Xu C, Zheng P, Zhang J, Li R, Zhang H, Shang X, Gong T, Ning G, Zen K, Zhang CY. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3218] [Cited by in RCA: 3549] [Article Influence: 208.8] [Reference Citation Analysis (0)] |
| 17. | Huang Z, Huang D, Ni S, Peng Z, Sheng W, Du X. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int J Cancer. 2010;127:118-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 750] [Cited by in RCA: 749] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 18. | Sabry D, El-Deek SEM, Maher M, El-Baz MAH, El-Bader HM, Amer E, Hassan EA, Fathy W, El-Deek HEM. Role of miRNA-210, miRNA-21 and miRNA-126 as diagnostic biomarkers in colorectal carcinoma: impact of HIF-1α-VEGF signaling pathway. Mol Cell Biochem. 2019;454:177-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 19. | Giráldez MD, Lozano JJ, Ramírez G, Hijona E, Bujanda L, Castells A, Gironella M. Circulating microRNAs as biomarkers of colorectal cancer: results from a genome-wide profiling and validation study. Clin Gastroenterol Hepatol. 2013;11:681-8.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 20. | Slattery ML, Herrick JS, Pellatt DF, Stevens JR, Mullany LE, Wolff E, Hoffman MD, Samowitz WS, Wolff RK. MicroRNA profiles in colorectal carcinomas, adenomas and normal colonic mucosa: variations in miRNA expression and disease progression. Carcinogenesis. 2016;37:245-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 21. | Marcuello M, Vymetalkova V, Neves RPL, Duran-Sanchon S, Vedeld HM, Tham E, van Dalum G, Flügen G, Garcia-Barberan V, Fijneman RJ, Castells A, Vodicka P, Lind GE, Stoecklein NH, Heitzer E, Gironella M. Circulating biomarkers for early detection and clinical management of colorectal cancer. Mol Aspects Med. 2019;69:107-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 192] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 22. | Chen F, Liu M, Yu Y, Sun Y, Li J, Hu W, Wang X, Tong D. LINC00958 regulated miR-627-5p/YBX2 axis to facilitate cell proliferation and migration in oral squamous cell carcinoma. Cancer Biol Ther. 2019;20:1270-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Fan Z, Zheng J, Xue Y, Liu X, Wang D, Yang C, Ma J, Liu L, Ruan X, Wang Z, Liu Y. NR2C2-uORF targeting UCA1-miR-627-5p-NR2C2 feedback loop to regulate the malignant behaviors of glioma cells. Cell Death Dis. 2018;9:1165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Li Z, Zhang J, Zheng H, Li C, Xiong J, Wang W, Bao H, Jin H, Liang P. Modulating lncRNA SNHG15/CDK6/miR-627 circuit by palbociclib, overcomes temozolomide resistance and reduces M2-polarization of glioma associated microglia in glioblastoma multiforme. J Exp Clin Cancer Res. 2019;38:380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 25. | Wang J, Yan F, Zhao Q, Zhan F, Wang R, Wang L, Zhang Y, Huang X. Circulating exosomal miR-125a-3p as a novel biomarker for early-stage colon cancer. Sci Rep. 2017;7:4150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 26. | Siskova A, Cervena K, Kral J, Hucl T, Vodicka P, Vymetalkova V. Colorectal Adenomas-Genetics and Searching for New Molecular Screening Biomarkers. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Ng EK, Chong WW, Jin H, Lam EK, Shin VY, Yu J, Poon TC, Ng SS, Sung JJ. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009;58:1375-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 900] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 28. | Abdel Ghafar MT, Gharib F, Abdel-Salam S, Elkhouly RA, Elshora A, Shalaby KH, El-Guindy D, El-Rashidy MA, Soliman NA, Abu-Elenin MM, Allam AA. Role of serum Metadherin mRNA expression in the diagnosis and prediction of survival in patients with colorectal cancer. Mol Biol Rep. 2020;47:2509-2519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 29. | Vychytilova-Faltejskova P, Radova L, Sachlova M, Kosarova Z, Slaba K, Fabian P, Grolich T, Prochazka V, Kala Z, Svoboda M, Kiss I, Vyzula R, Slaby O. Serum-based microRNA signatures in early diagnosis and prognosis prediction of colon cancer. Carcinogenesis. 2016;37:941-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 30. | Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461-1473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1646] [Cited by in RCA: 1882] [Article Influence: 235.3] [Reference Citation Analysis (0)] |
| 31. | Itatani Y, Kawada K, Sakai Y. Transforming Growth Factor-β Signaling Pathway in Colorectal Cancer and Its Tumor Microenvironment. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 178] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 32. | Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5384] [Cited by in RCA: 5601] [Article Influence: 294.8] [Reference Citation Analysis (0)] |
| 33. | Padi SK, Zhang Q, Rustum YM, Morrison C, Guo B. MicroRNA-627 mediates the epigenetic mechanisms of vitamin D to suppress proliferation of human colorectal cancer cells and growth of xenograft tumors in mice. Gastroenterology. 2013;145:437-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 34. | Wang J, Chen T, Wang L, Yao B, Sun L, Chen S, Liu Q. MicroRNA-627-5p inhibits the proliferation of hepatocellular carcinoma cells by targeting BCL3 transcription coactivator. Clin Exp Pharmacol Physiol. 2020;47:485-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Shin VY, Ng EK, Chan VW, Kwong A, Chu KM. A three-miRNA signature as promising non-invasive diagnostic marker for gastric cancer. Mol Cancer. 2015;14:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 36. | Li Y, Wang D, Li X, Shao Y, He Y, Yu H, Ma Z. MiR-199a-5p suppresses non-small cell lung cancer via targeting MAP3K11. J Cancer. 2019;10:2472-2479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 37. | Li DJ, Wang X, Yin WH, Niu K, Zhu W, Fang N. MiR-199a-5p suppresses proliferation and invasion of human laryngeal cancer cells. Eur Rev Med Pharmacol Sci. 2020;24:12200-12207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 38. | Li W, Wang H, Zhang J, Zhai L, Chen W, Zhao C. miR-199a-5p regulates β1 integrin through Ets-1 to suppress invasion in breast cancer. Cancer Sci. 2016;107:916-923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Jiang J, Gusev Y, Aderca I, Mettler TA, Nagorney DM, Brackett DJ, Roberts LR, Schmittgen TD. Association of MicroRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis, and patient survival. Clin Cancer Res. 2008;14:419-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 422] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 40. | Hu Y, Liu J, Jiang B, Chen J, Fu Z, Bai F, Jiang J, Tang Z. MiR-199a-5p loss up-regulated DDR1 aggravated colorectal cancer by activating epithelial-to-mesenchymal transition related signaling. Dig Dis Sci. 2014;59:2163-2172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 41. | Tian L, Chen M, He Q, Yan Q, Zhai C. MicroRNA199a5p suppresses cell proliferation, migration and invasion by targeting ITGA3 in colorectal cancer. Mol Med Rep. 2020;22:2307-2317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 42. | Zhu QD, Zhou QQ, Dong L, Huang Z, Wu F, Deng X. MiR-199a-5p Inhibits the Growth and Metastasis of Colorectal Cancer Cells by Targeting ROCK1. Technol Cancer Res Treat. 2018;17:1533034618775509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 43. | Hur K, Toiyama Y, Okugawa Y, Ide S, Imaoka H, Boland CR, Goel A. Circulating microRNA-203 predicts prognosis and metastasis in human colorectal cancer. Gut. 2017;66:654-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 44. | Chen X, Hu Z, Wang W, Ba Y, Ma L, Zhang C, Wang C, Ren Z, Zhao Y, Wu S, Zhuang R, Zhang Y, Hu H, Liu C, Xu L, Wang J, Shen H, Zhang J, Zen K, Zhang CY. Identification of ten serum microRNAs from a genome-wide serum microRNA expression profile as novel noninvasive biomarkers for nonsmall cell lung cancer diagnosis. Int J Cancer. 2012;130:1620-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 231] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 45. | Liu K, Chen H, You Q, Ye Q, Wang F, Wang S, Zhang S, Yu K, Li W, Gu M. miR145 inhibits human nonsmall-cell lung cancer growth by dual-targeting RIOK2 and NOB1. Int J Oncol. 2018;53:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Lian F, Cui Y, Zhou C, Gao K, Wu L. Identification of a plasma four-microRNA panel as potential noninvasive biomarker for osteosarcoma. PLoS One. 2015;10:e0121499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 47. | Duan Z, Choy E, Harmon D, Liu X, Susa M, Mankin H, Hornicek F. MicroRNA-199a-3p is downregulated in human osteosarcoma and regulates cell proliferation and migration. Mol Cancer Ther. 2011;10:1337-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 48. | Turchinovich A, Weiz L, Burwinkel B. Extracellular miRNAs: the mystery of their origin and function. Trends Biochem Sci. 2012;37:460-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 415] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 49. | Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003-5008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2345] [Cited by in RCA: 2631] [Article Influence: 187.9] [Reference Citation Analysis (0)] |