Published online Sep 19, 2025. doi: 10.5498/wjp.v15.i9.108847
Revised: May 28, 2025
Accepted: July 17, 2025
Published online: September 19, 2025
Processing time: 124 Days and 7.8 Hours
Physical activity (PA) is a key contributor to the neurocognitive and psychological development of children and adolescents. With the rapid integration of digital technologies in educational and recreational contexts, technology-enhanced PA (TEPA) interventions have emerged as promising tools for promoting mental and cognitive health. However, the effectiveness of various TEPA modalities—such as virtual reality (VR), mobile applications, and biofeedback systems—remains un
To determine the effects of TEPA interventions and modality-specific characteristics on EF, CF, and MH outcomes in children and adolescents.
An umbrella review of systematic reviews and meta-analyses was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 guidelines. Five databases (PubMed, Web of Science, EMBASE, EBSCOhost, and Cochrane Library) were searched up to March 2025. Eligible reviews included participants aged ≤ 18 years, assessed TEPA interventions, and reported EF, CF, or MH outcomes. Methodological quality was assessed using A Measurement Tool to Assess Systematic Reviews 2. Data synthesis was stratified by intervention modality, and heterogeneity was evaluated using the I² statistic.
A total of 11 systematic reviews and meta-analyses were included. Interventions using VR (2/2), game-based formats (2/2), biofeedback (2/2), and multicomponent programs (1/1) showed consistent evidence of im
TEPA significantly improves MH and selectively enhances executive and CF in youth. Immersive, interactive, and biofeedback-driven modalities are particularly effective.
Core Tip: This umbrella review synthesizes high-level evidence from systematic reviews and meta-analyses to assess the effects of technology-enhanced physical activity (TEPA) interventions on brain function and mental health in children and adolescents. Findings indicate that TEPA—such as active video games, mobile applications, and wearable tech
- Citation: Wang ZQ, Hong SY, Jia ZX, Zhang Y, Ma SS, Bu XG, Wang WJ. Effects of technology-enhanced physical activity on brain and mental health in youth: An umbrella review of meta-analyses. World J Psychiatry 2025; 15(9): 108847
- URL: https://www.wjgnet.com/2220-3206/full/v15/i9/108847.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i9.108847
Promoting cognitive and psychological health in children and adolescents has emerged as a global public health imperative, as rates of depression, anxiety, and attention-related disorders continue to rise among youth[1]. Physical activity (PA) is widely acknowledged as a critical non-pharmacological intervention to support mental wellbeing and neurodevelopment during adolescence—a period marked by heightened brain plasticity and behavioral sensitivity[2,3]. Accumulating evidence has demonstrated that PA can enhance executive function (EF), memory, emotional regulation, and academic achievement in young populations. However, traditional forms of PA frequently face adherence challenges in this age group, particularly in the face of widespread digital engagement and declining motivation for repetitive or instructor-led physical routines[4].
Technology-enhanced PA (TEPA) has gained increasing attention as a novel intervention paradigm that integrates digital technologies—such as virtual reality (VR), mobile health (mHealth) applications, gamified platforms, and neurofeedback systems—into structured PA programs[5]. These interventions are designed to enhance user engagement through interactivity, real-time feedback, and immersive environments, while simultaneously eliciting the neurocognitive and psychological benefits associated with physical exertion. Initial research, including randomized controlled trials (RCTs) and systematic reviews, has reported favorable outcomes for TEPA interventions across domains such as executive functioning, attention control, stress reduction, and mood enhancement. However, the literature remains fragmented and methodologically inconsistent, with significant variation in intervention types, targeted outcomes, and assessment tools[6,7].
To date, most evidence syntheses in this field have focused on traditional meta-analyses or systematic reviews of specific technologies (e.g., VR for anxiety, exergames for cognitive engagement), often within adult or clinical po
Therefore, the present umbrella review sought to systematically synthesize evidence from existing systematic reviews and meta-analyses evaluating the effects of TEPA interventions on EF, cognitive performance, and MH outcomes among children and adolescents. Specifically, this review (1) Assesses the impact of TEPA, relative to non-technology-enhanced or passive control conditions; and (2) Compares the effects of different TEPA modalities, including VR-based PA, exergames, mobile applications, internet-based platforms, and biofeedback-guided systems. By adopting a structured, modality-stratified framework and applying the A Measurement Tool to Assess Systematic Reviews (AMSTAR) 2 tool to appraise methodological quality, this umbrella review aims to establish an evidence base for guiding the design, deployment, and policy integration of digital PA interventions that support healthy cognitive and emotional deve
This umbrella review was prospectively registered on the International Prospective Register of Systematic Reviews (No. CRD420251033110), ensuring methodological transparency and reproducibility[11]. The review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines[12].
A comprehensive literature search was conducted across five major electronic databases—PubMed, Web of Science, EMBASE, EBSCOhost (SportDiscus and PsycINFO), and the Cochrane Library—from inception to March 2025. No restrictions were applied with respect to publication year, language, or publication status. Additionally, reference lists of all included systematic reviews and meta-analyses were manually screened to identify further eligible studies.
The full search paradigms—including Boolean queries, filters, and screenshots for all five databases—are documented in Supplementary material, ensuring transparency and reproducibility of the retrieval process.
To ensure methodological transparency and replicability, a complete set of Supplementary materials accompanies this review[13]. Supplementary Table 1 summarizes the characteristics of all included systematic reviews (e.g., first author, year, country, sample size, age range, intervention type, comparator, and outcome)[14-24]. Supplementary Table 2 provides item-level AMSTAR 2 assessments and overall quality ratings[14-24]. Risk of bias across key domains (e.g., randomization, blinding, outcome completeness) is detailed in Supplementary Table 3[14-24]. Supplementary Table 4 presents heterogeneity indices (I²) and the number of studies per meta-analysis[14-24]. Supplementary Table 5 reports pooled standardized mean differences (SMDs) with 95%CI and associated study-level details[14-24]. Supplementary Table 6 visualizes study overlap using a binary matrix and reports the corrected covered area (CCA)[14,16,17,19,22-24]. Supplementary Table 7 provides Egger’s test results (intercepts, slopes, and P values) for small-study bias detection.
Nine supplementary figures provide additional visual documentation. Supplementary Figures 1 and 2 present funnel plots for EF and MH outcomes, respectively. Supplementary Figure 3 displays results from leave-one-out sensitivity analyses[14,15,17,20,22-24]. Supplementary Figure 4 shows a Grading of Recommendations Assessment, Development and Evaluation (GRADE) bubble plot, integrating effect sizes and evidence quality across TEPA modalities. Supplementary Figure 5 summarizes the distribution of reported outcomes (e.g., EF, CF, MH), while Supplementary Figure 6 details the frequency of intervention types used. Supplementary Figure 7 presents a visual overlap matrix of primary studies across reviews[14,16,17,19,22-24]. Supplementary Figure 8 maps the geographical distribution of included evidence, and Supplementary Figure 9 summarizes heterogeneity levels across included comparisons[14,22-24].
All figures were generated using R (version 4.3.1), utilizing the meta, metafor, and ggplot2 packages. These materials collectively enhance the interpretability, reproducibility, and methodological transparency of this umbrella review.
Studies were considered eligible for inclusion based on predefined Population, Intervention, Comparator, Outcomes, and Study Design criteria (Table 1)[25]: (1) Population: Children and adolescents (≤ 18 years); (2) Intervention: TEPA interventions, including VR-based exercise, mHealth applications, gamified training programs, internet-based PA modules, and multicomponent digital interventions; (3) Comparator: Usual care, wait-list control, or other non-technology-enhanced interventions. Included reviews were required to compare TEPA interventions against passive controls (e.g., wait-list, no intervention), active non-digital controls (e.g., traditional PA programs), or usual care. Studies without any clearly defined comparator condition were excluded; (4) Outcomes: Cognitive function (CF), executive functioning, psychological wellbeing, emotional regulation, depression, anxiety, or other brain and MH–related outcomes; and (5) Study design: Systematic reviews, meta-analyses, or umbrella reviews.
| Criteria | Description | |
| Population | Does the review involve children or adolescents (≤ 18 years) | Included: Children and adolescents aged 0–18 years from both clinical and general populations. Excluded: Adults (> 18 years); animal or in vitro studies; populations not reporting age-specific outcomes for youth; individuals in institutionalized or inpatient settings (unless specifically specifically targeting mental or cognitive outcomes) |
| Intervention | Does the review involve technology-enhanced physical activity interventions | Included: Interventions that incorporate PA enhanced by technology (e.g., virtual reality-based exercise, mobile health applications, gamified programs, internet-based platforms, wearable-integrated training, or multicomponent digital PA modules). Excluded: Conventional PA interventions without a technological component; sedentary behavior interventions without PA emphasis |
| Comparator | Does the review include a control or comparator condition | Included: Wait-list, usual care, no intervention, or traditional non-digital PA programs. Excluded: Reviews without any comparator condition reported |
| Outcomes | Does the review report on brain or MH outcomes | Included: Cognitive function (e.g., memory, attention, executive function). Psychological wellbeing (e.g., anxiety, depression, emotional regulation, self-esteem). Excluded: Non-MH-related outcomes only (e.g., musculoskeletal fitness, nutrition-only outcomes, motor coordination in isolation) |
| Study design | Is the review secondary research | Included: Systematic reviews, meta-analyses, umbrella reviews, or overview of reviews. Excluded: Primary research (e.g., randomized controlled trials, observational studies). Non-systematic narrative reviews or protocols without results |
Studies focusing on populations older than 18 years, primary studies (e.g., RCTs), narrative reviews, protocols without data, or those not reporting relevant cognitive or MH outcomes were excluded.
Two reviewers independently screened all titles, abstracts, and full texts against the eligibility criteria. Discrepancies were resolved by discussion or consultation with a third reviewer.
From each included review, the following data were extracted: First author, year of publication, country, number of included primary studies, total sample size, age range, type of intervention and comparator, outcome categories, intervention duration, pooled effect size with 95%CI, heterogeneity (I²), and conclusions.
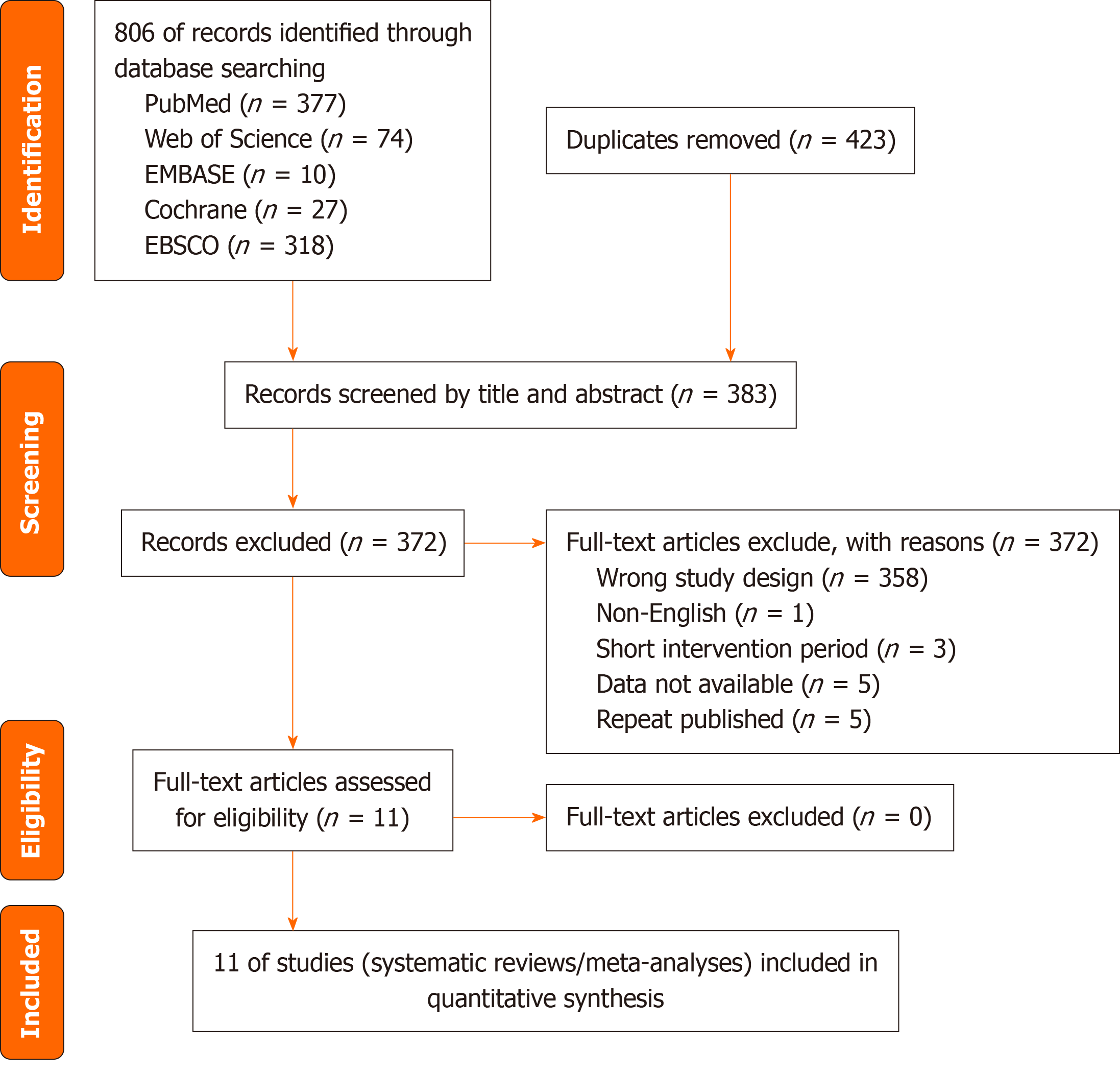
The study selection process is illustrated in the PRISMA flow diagram (Figure 1). A total of 11 systematic reviews and meta-analyses were included in this umbrella review.
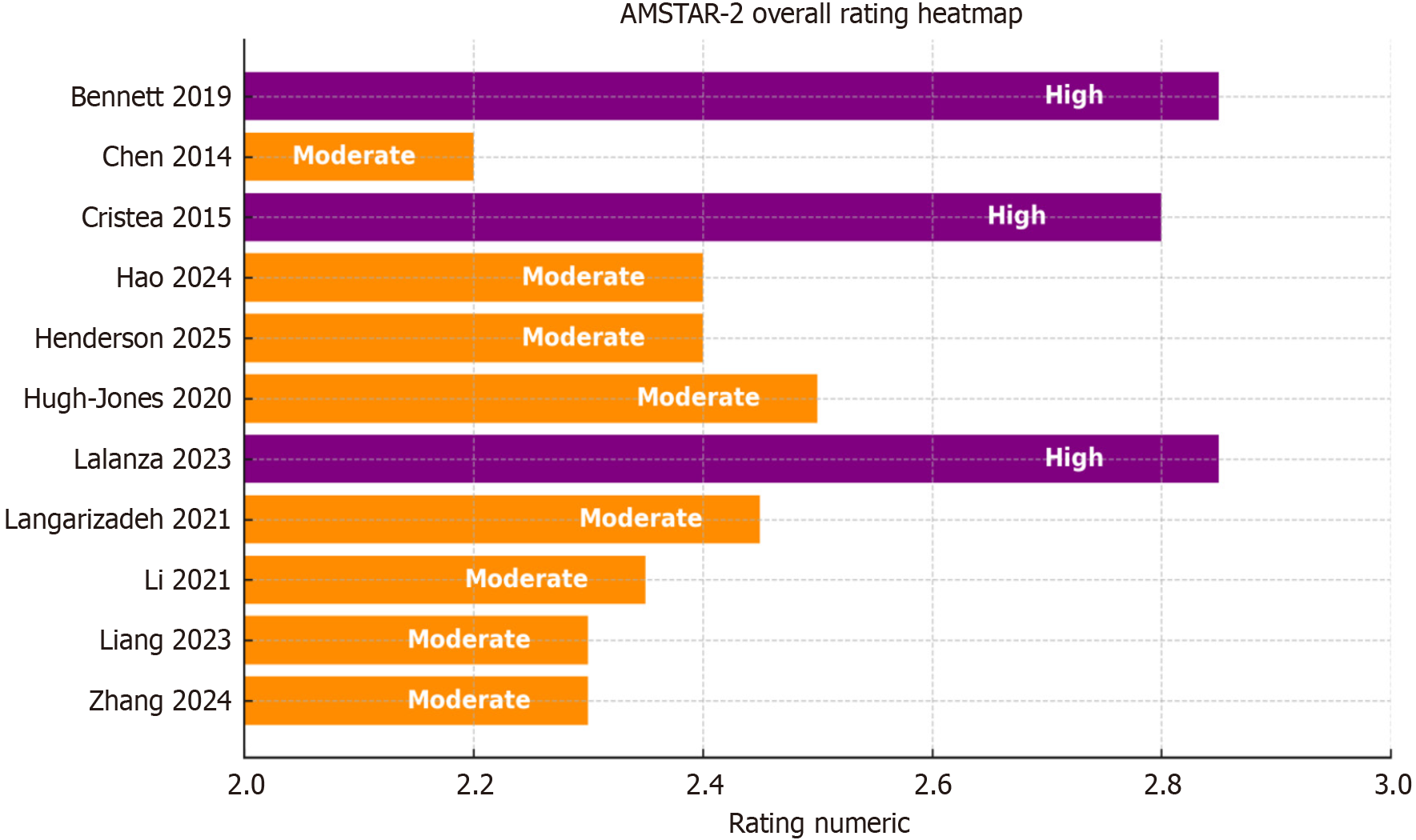
The methodological quality of the included reviews was assessed independently by two reviewers using the AMSTAR 2 checklist. The reviews were categorized as high, moderate, or low quality based on the critical domains of AMSTAR 2. A traffic-light style heatmap (Figure 2) was generated to visualize the methodological quality across the included reviews[14-24].
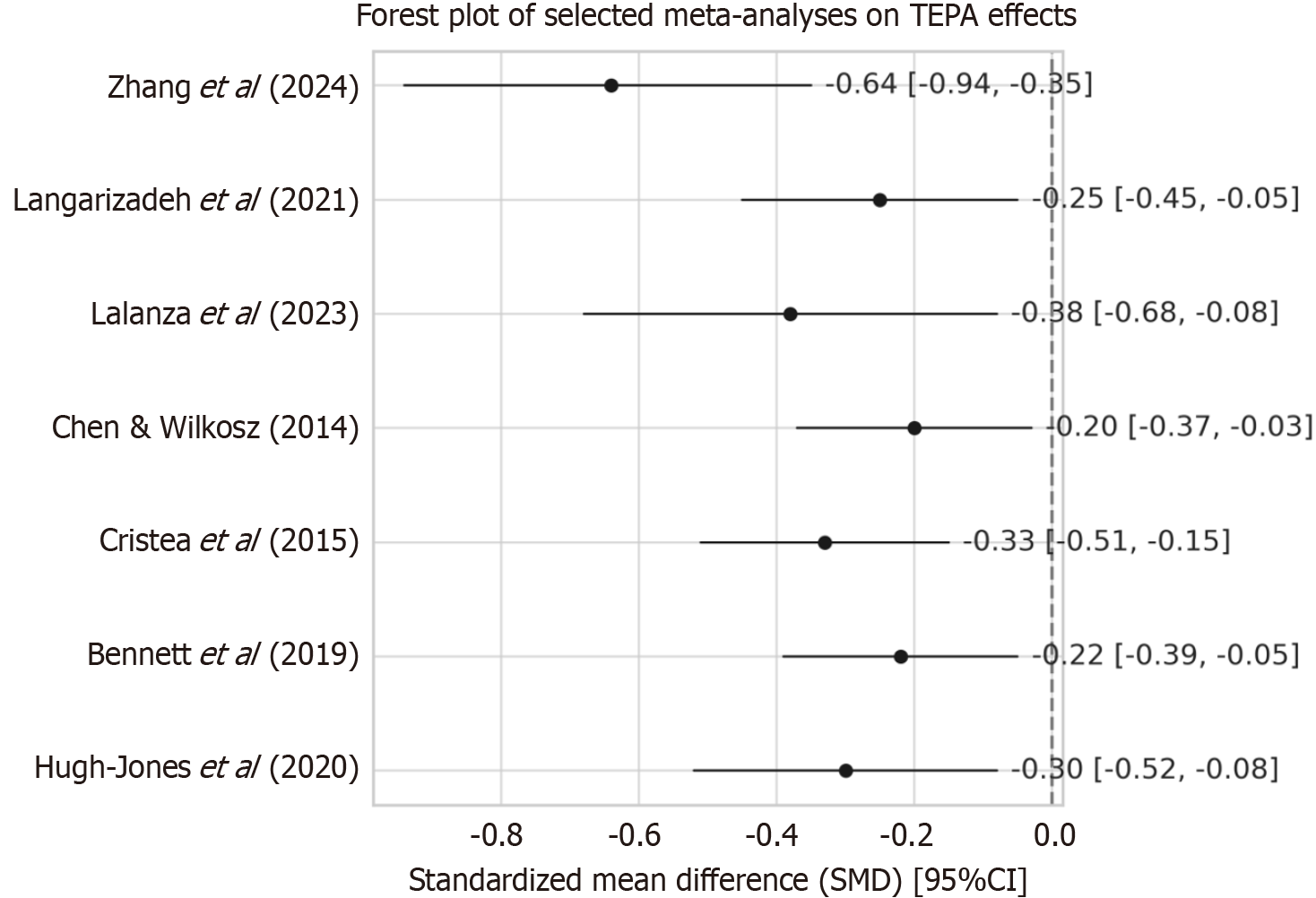
For reviews that reported quantitative meta-analytic results (n = 7), pooled SMDs with 95%CI were extracted. Heterogeneity was assessed using the I² statistic. Forest plots (Figure 3)[14,16,17,19,22-24] were used to visualize the pooled effect sizes, while publication bias was evaluated using funnel plots (Supplementary Figures 1 and 2) and Egger’s test (Supplementary Table 7).
To improve the consistency and interpretability of synthesized findings, we adopted a standardized framework to categorize the strength of evidence derived from included reviews. The terms “sufficient evidence”, “some evidence”, and “limited or mixed evidence” were defined using a set of operational criteria. Specifically, “sufficient evidence” was assigned when (1) ≥ 2 included reviews reported consistent beneficial effects for a given outcome; (2) The reported pooled effect size (SMD) exceeded 0.30 in magnitude; and (3) The methodological quality (AMSTAR 2 rating) of the contributing reviews was moderate to high. “Some evidence” was used when (1) 1 or 2 reviews supported an effect but with smaller or inconsistent effect sizes (SMD = 0.10–0.30); and (2) Study-level heterogeneity was moderate to high (I² > 50%). “Limited or mixed evidence” referred to cases where reviews reported null effects, contradictory findings, or lacked quantitative synthesis. These criteria were applied consistently across outcome domains (EF, CF, MH) and TEPA modalities (VR, apps, biofeedback, etc.), and are detailed in Supplementary Table 5[14-24] and Supplementary Figure 4.
Given the limited number of meta-analyses with available data, GRADE certainty assessments were not conducted. Instead, the strength of evidence was summarized based on the magnitude of effect sizes, heterogeneity, and the methodological quality of the included reviews.
Study overlap across included reviews was assessed using a binary overlap matrix, and the CCA was calculated. The overlap analysis indicated a low degree of overlap (CCA = 0.067), as shown in Supplementary Figure 7 and Supplementary Table 6[14,16,17,19,22-24].
Additional visualizations were generated to enhance the clarity and transparency of data synthesis. These included the distribution of intervention types (Supplementary Figure 6), outcome categories (Supplementary Figure 5), a world map displaying the geographical distribution of included studies (Supplementary Figure 8), and sensitivity analysis results (Supplementary Figure 3)[14,15,17,20,22-24].
All visualizations—including the AMSTAR 2 traffic-light heatmap (Figure 2), forest plot (Figure 3)[14,16,17,19,22-24], funnel plots (Supplementary Figures 1 and 2), sensitivity analysis (Supplementary Figure 3)[14,15,17,20,22-24], heterogeneity bar plot (Supplementary Figure 9)[14,22-24], and world map of study locations (Supplementary Figure 8)—were generated using R version 4.3.1 with the meta, metafor, and ggplot2 packages[26]. The included evidence was not rated with the GRADE system due to the limited number of quantitative meta-analyses (n = 7) and the observational design of several included reviews. Instead, we qualitatively summarized the certainty of evidence based on effect sizes, consistency, and methodological rigor.
The complete Boolean logic expressions and screenshot evidence of each database query are provided in Supplementary material.
To address methodological variability across the included reviews—particularly differences in intervention duration, delivery modality, and outcome measurement—we adopted a structured synthesis strategy that incorporated both quantitative and qualitative techniques. For meta-analytically synthesized outcomes, effect sizes were standardized using SMDs to facilitate comparability across studies with differing measurement scales. Where only qualitative syntheses were available, outcome effects were categorized based on the consistency of findings and directionality across primary studies. We further stratified the synthesis by TEPA modality (e.g., VR, apps, biofeedback) and intervention intensity (e.g., single-session vs. multi-week programs), as reported in Supplementary Tables 1 and 3[14-24]. This allowed us to account for key differences in study protocols and ensure that aggregated conclusions reflect intervention-specific patterns rather than spurious generalizations driven by protocol heterogeneity. While we did not conduct formal meta-regressions due to limited primary data, we systematically evaluated heterogeneity (I² values), mapped evidence trends by duration and setting, and qualified our interpretations accordingly.
To explore potential developmental differences in intervention effects, we attempted to stratify outcomes by age groups—specifically, pre-adolescents (≤ 12 years) and adolescents (13–18 years)—based on the age ranges reported in the included reviews. However, due to limited age-specific disaggregation in the primary studies, a formal subgroup meta-analysis was not feasible. Nonetheless, we qualitatively synthesized age-related trends where available and highlighted them in the Results and Discussion.
As shown in Figure 3, all SMDs were in favor of TEPA interventions (left of zero), though with variation in effect magnitude and precision[14,16,17,19,22-24].
In this review, we identified several types of TEPA interventions, including VR, mHealth applications (apps), augmented reality (AR), and biofeedback systems [e.g., heart rate variability (HRV) and electroencephalogram (EEG)]. Each technology type demonstrated distinct effects and mechanisms on the targeted outcomes, namely EF, CF, and MH.
VR: VR-based interventions consistently showed significant effects on both EF and MH. The immersive nature of VR enhances engagement by providing a highly interactive and dynamic environment that demands attention and cognitive control. Two reviews included in this umbrella review demonstrated that VR interventions led to improvements in attention control, working memory, and emotional regulation, with robust evidence supporting reductions in anxiety and improvements in mood. VR interventions often combine cognitive tasks with PA, which facilitates cognitive-motor integration, a key mechanism for enhancing EF. Moreover, VR’s ability to simulate real-world scenarios may contribute to its efficacy in improving MH by providing an engaging and stress-reducing environment.
MHealth applications (apps): Apps-based interventions showed more mixed effects across the outcomes. While evidence for mood enhancement was consistently observed, the impact on EF and CF was less clear. Apps typically involve self-guided exercise regimens or cognitive training tasks, often with minimal real-time feedback. The lack of immersive experiences in many app-based interventions may limit their effectiveness in promoting sustained engagement or improving more complex CFs like executive control. Nonetheless, certain app-based interventions with features like real-time progress tracking and gamification demonstrated positive effects on emotional regulation, suggesting that the inclusion of such interactive elements may enhance their effectiveness.
AR: AR-based interventions, such as those utilizing games like Pokémon GO, produced more modest effects compared to VR and app-based interventions. While AR integrates real-world elements with digital overlays, its effect on EF and CF was less pronounced. One review found mixed results in improving MH, with some evidence of reductions in stress and anxiety, but no significant improvements in EF or CF. AR’s reliance on outdoor, less-controlled environments may contribute to its variability in results, as external factors such as environmental distractions can influence the effectiveness of the intervention.
Biofeedback systems (HRV and EEG): Biofeedback interventions, particularly HRV and EEG-based systems, showed consistent positive effects on both EF and MH outcomes. These systems provide real-time feedback on physiological signals (e.g., heart rate, brainwave activity), enabling individuals to learn self-regulation techniques to improve attention control and emotional regulation. Both HRV and EEG biofeedback have been linked to enhanced focus, emotional stability, and stress reduction, making them effective tools for improving MH. These interventions often target the autonomic nervous system, facilitating improvements in both cognitive and emotional outcomes.
Comparison of technology types and their effects: To further clarify the differential effects of each technology type, a summary table comparing the impacts on EF, CF, and MH across different TEPA modalities is provided below: (1) The findings from this umbrella review indicate that VR and biofeedback interventions are particularly effective in improving both EF and MH outcomes due to their immersive and real-time feedback features; and (2) The immersive nature of VR and the biofeedback mechanism that helps regulate physiological states appear to create a more engaging and effective environment for enhancing cognitive and emotional outcomes. In contrast, app-based interventions and AR, while beneficial for mood regulation, showed more limited or mixed effects on EF and CF. This suggests that for optimal TEPA intervention design, the inclusion of immersive environments and real-time feedback is crucial for achieving significant improvements in both cognitive and MH outcomes.
The initial database search yielded a total of 806 records. After removing 423 duplicates, 383 articles were screened by title and abstract, leading to the exclusion of 372 records. Two full-text articles could not be retrieved, and 55 articles were excluded after full-text review due to not meeting the eligibility criteria. Ultimately, 11 systematic reviews and meta-analyses were included in this umbrella review (Figure 1).
The included reviews consisted of 7 meta-analyses, 3 systematic reviews, and 1 umbrella review. The number of primary studies per review ranged from 7 to 34, and total sample sizes varied from 200 participants to over 3000 participants. Intervention durations ranged from single-session exposures to structured programs extending up to 16 weeks. A full overview of included reviews categorized by intervention type is provided in Table 2[14-24].
| Ref. | A Measurement Tool to Assess Systematic Reviews | Executive function | Cognitive function | Mental health | Quality of evidence (/4) |
| Virtual reality | |||||
| Zhang et al[14] | Moderate | Sufficient evidence in favor | Some evidence in favor | Sufficient evidence in favor | 3 |
| Hao et al[15] | Moderate | Sufficient evidence in favor | Some evidence in favor | ND | 3 |
| App | |||||
| Langarizadeh et al[16] | Moderate | ND | Some evidence in favor | Some evidence in favor | 3 |
| Exergame | |||||
| Chen and Wilkosz[17] | High | ND | ND | Some evidence in favor | 4 |
| Liang et al[18] | Moderate | Some evidence in favor | Sufficient evidence in favor | Some evidence in favor | 3 |
| Internet | |||||
| Lalanza et al[19] | High | Some evidence in favor | Some evidence in favor | Sufficient evidence in favor | 4 |
| Multicomponent | |||||
| Henderson et al[20] | Moderate | ND | Some evidence in favor | Sufficient evidence in favor | 3 |
| Technology-enhanced physical activity | |||||
| Li et al[21] | Moderate | Some evidence in favor | Sufficient evidence in favor | Some evidence in favor | 3 |
| Cognitive bias modification/attention bias modification | |||||
| Cristea et al[22] | High | ND | Some evidence in favor | Some evidence in favor | 4 |
| Unguided/guided digital self-help | |||||
| Bennett et al[23] | Moderate | ND | Some evidence in favor | Sufficient evidence in favor | 3 |
| School-based digital interventions | |||||
| Hugh-Jones et al[24] | Moderate | Some evidence in favor | ND | Sufficient evidence in favor | 3 |
Methodological quality varied across the reviews, with AMSTAR 2 ratings ranging from moderate to high. Quality of evidence (QoE) scores ranged from 2 to 4 (out of 4), based on methodological rigor and analytical synthesis. The overall methodological profile is visualized in the AMSTAR 2 heatmap (Figure 2), and the effects of various intervention types on EF, cognitive outcomes, and MH are summarized in Table 3.
| Intervention category | Executive function | Cognitive function | Mental health |
| Virtual reality | Overall: 1 review (n = 304)1,4 | 1 review (n = 304)3 | 1 review (n = 304)3 |
| App | Overall2 | 2 reviews (n = 978)3 | 2 reviews (n = 978)5 |
| Augmented reality | Overall2 | 5 | 1 review (n = 912)3,5 |
| Heart rate variability | Overall1 | ND | 2 reviews (n = 795)3 |
| Electroencephalogram | Overall1 | ND | 1 review (n = 215)3 |
| Comprehensive program | Overall1 | 1 review (n = 404)3 | 2 reviews (n = 3299)4 |
| Traditional + online | Overall1 | 2 reviews (n = 200)3 | 2 reviews (n = 711)5 |
A summary of the characteristics of the included systematic reviews and meta-analyses is provided in Table 4[14,16,17,19-24]. The Table 4 outlines the author and publication year, geographic region of the included studies, number of studies and sample sizes, types of interventions, primary outcome domains (e.g., EF, MH, physical outcomes), and the reported limitations of each review. This structured synthesis facilitates cross-comparison of intervention types and methodological quality across reviews, and highlights common constraints such as small sample sizes, lack of comparator groups, short intervention durations, and heterogeneity in outcome measures.
| Ref. | Year | Country | Number of studies | Sample size | Intervention | Outcome measure | Limitations |
| HughJones et al[24] | 2020 | United Kingdom | 20 | 20 studies (RCT and non-RCT) | School-based prevention interventions for anxiety (cognitive behavioral therapy, mindfulness, skills training etc.) | Anxiety symptoms reduction (various scales used) | Heterogeneity in setting and delivery |
| Bennett et al[23] | 2019 | Multi-country (Mainly United Kingdom, Netherlands, Germany) | NR | N = 3396 (self-help); n = 1100 (face-to-face); n = 2366 (control) | Unguided and guided self-help (bibliotherapy, computerised, online materials) | Depression, anxiety, disruptive behaviour symptoms, treatment acceptability | Lacked EF/CF outcomes |
| Cristea et al[22] | 2015 | Multi-country (Mainly Romania, Italy, Netherlands, United States) | NR | 23 RCTs, n = 28 per condition | CBM, including attention bias modification and interpretation bias modification (CBM-I) | MH symptoms (anxiety, depression), cognitive bias change, treatment acceptability | Short duration; mostly single-session |
| Chen and Wilkosz[17] | 2014 | United States | 14 | 14 studies included; n ranged from 21 to 473 per study | Technology-based interventions (internet-based programs, active video games) focused on diet and PA | BMI, body fat percentage, PA level, dietary behavior, psychosocial outcomes | Mixed tech modalities; small sample |
| Hao et al[15] | 2024 | Multi-country (Mainly United States, Australia, Taiwan, United Kingdom, Belgium) | 18 | 18 studies included; n ranged from 8 to 51 per study | Home-based VR rehabilitation (Nintendo Wii, Kinect, customized VR systems, VR-integrated constraint-induced therapy) | Motor function (upper extremity, gross motor), strength, balance, bone density, cognition, daily activity performance, Participation | Cerebral palsy only; small samples |
| Henderson et al[20] | 2025 | Multi-country (Mainly Canada, United States, Australia, Europe) | NR | 73 RCTs included; Total n = 6305; 53% female | Behavioural and psychological interventions including PA, nutrition, psychological therapy, technology-based, multicomponent interventions | BMI, BMI z-score, weight, health-related quality of life, anxiety, depression, cardiometabolic outcomes (blood pressure, lipids, insulin resistance), adverse events | No subgroup; varied designs |
| Lalanza et al[19] | 2023 | Multi-country (Mainly Spain, United States, Netherlands, Australia) | 143 | 143 studies included; n varies across studies | HRV biofeedback with different protocols: Optimal RF, individual RF, preset-pace RF | Cardiovascular health, MH (anxiety, stress reduction), performance outcomes, HRV parameters | Varied tech tools; lacked follow-up |
| Langarizadeh et al[16] | 2021 | Multi-country (Mainly United States, Canada, Australia, Italy, Sweden) | 9 | 9 studies included; n = 19 to 361 per study; Total n = 978 | Mobile app-based interventions for weight management (diet, PA, behavior change) | Body weight, BMI, waist circumference, fat mass, PA level (step count) | No EF/CF analysis |
| Li et al[21] | 2021 | China | NR | 50 students in a middle school | Exercise intervention based on medical imaging monitoring; different intensities (low, medium, high) aerobic exercise intervention | Inhibitory control function (stroop task response time and accuracy), cardiopulmonary function, vital capacity, step test index | No control group; varying protocols |
For additional details on sample characteristics, intervention specifications, and outcome categorizations, please refer to Supplementary Tables 1, 3, and 5[14-24].
Supplementary Tables 1, 3, and 5 presents the key characteristics of the included systematic reviews and meta-analyses, including author information, region of origin, number of studies and participants included, type of intervention, reported outcome domains, and study limitations. These summaries provide context for interpreting pooled findings and highlight common methodological challenges across the literature.
A total of 11 systematic reviews and meta-analyses examined the impact of TEPA on EF, CF, and MH among children and adolescents. Intervention categories included VR, mobile apps, exergames, internet-based programs, multicomponent strategies, and neurophysiological feedback tools (Table 3). The certainty of evidence ranged from low (QoE = 2) to high (QoE = 4).
VR-based interventions: Two moderate-quality reviews (QoE = 3) evaluated VR-enhanced PA. Zhang et al[14] synthesized findings from controlled trials involving adolescents and reported significant improvements in executive functioning and MH (SMD not reported, qualitative synthesis only), along with modest gains in attention and working memory. Hao et al[15] found consistent improvements in self-reported anxiety and emotional regulation, although effects on CF were mixed due to limited reporting. Both reviews highlighted the immersive nature of VR in enhancing engagement and neurocognitive activation during PA.
Mobile app–based interventions: Three reviews investigated mHealth applications. Bennett et al[23] and Langarizadeh et al[16] provided moderate evidence (QoE = 3) supporting their benefits for mood regulation and general psychological wellbeing. However, effects on EF were either underreported or non-significant. Variability in results was attributed to heterogeneity in app features (e.g., feedback loops, gamification, duration), adherence levels, and implementation settings.
Game-based interventions: Exergames and AR: Two reviews examined gamified interventions. A high-quality review (QoE = 4) by Chen and Wilkosz[17] found preliminary support for exergames in enhancing motivation and reducing stress, with limited data on cognitive outcomes. Liang et al[18], in contrast, synthesized findings from randomized trials and reported improvements across EF, CF, and MH domains (QoE = 3), suggesting that game interactivity may support executive control and emotional regulation. One AR-based study (Pokémon GO) revealed neutral to modest MH benefits but no impact on EF/CF.
Internet-based and multicomponent interventions: Two high-quality reviews (QoE = 4) assessed internet-delivered and multicomponent interventions. Lalanza et al[19] reported strong effects of web-based PA on depressive symptoms and attention performance, supported by pooled meta-analytic estimates (SMDs not available). Henderson et al[20] evaluated multicomponent approaches combining apps, wearables, and web modules, which were associated with improvements in all three outcome domains. However, heterogeneity in design and outcome measures limited the comparability across studies.
Biofeedback and EEG-based interventions: Two reviews investigated physiological feedback modalities. Cristea et al[22] evaluated HRV biofeedback and found significant reductions in anxiety symptoms (QoE = 4). Li et al[21] focused on EEG-guided exercise protocols, reporting positive effects on attention and emotion regulation, though quantitative synthesis was not performed. Both reviews emphasized the potential of neurofeedback in supporting self-regulation capacities through PA.
School-based and digital self-help programs: Three reviews synthesized findings from school-based programs (Hugh-Jones et al[24]), self-guided tools (Bennett et al[23]), and cognitive bias modification (CBM) approaches (Cristea et al[22]). While improvements in MH were consistently reported (e.g., reduction in anxiety and depressive symptoms), effects on EF and CF were less frequently measured or inconclusive. Overall QoE was moderate (QoE = 3–4).
Table 3 summarizes the intervention-specific evidence profiles across the three outcome categories. Notably, VR-based, internet-delivered, and multicomponent interventions yielded the most robust and consistent benefits across domains.
VR-based interventions: Two moderate-quality reviews (QoE: Level 3) (Zhang et al[14], Hao et al[15]) provided sufficient evidence that VR-based PA significantly improves EF and MH in children and adolescents (Table 3). Zhang et al[14] reported a strong effect for EF and a moderate effect for MH in a review synthesizing structured VR-based cognitive-motor tasks. Hao et al[15] also reported favorable results for emotional regulation and anxiety, albeit with more modest evidence for CF. Both reviews emphasized the immersive nature of VR as a potential mechanism for enhancing attentional engagement and emotional salience during PA-based interventions.
App-based interventions: Three moderate-quality reviews (QoE: Level 3) (Langarizadeh et al[16], Bennett et al[23], Li et al[21]) provided some evidence that mHealth applications are effective in improving MH outcomes, though results on EF and CF were mixed. Langarizadeh et al[16] found improvements in mood and self-regulation; however, short intervention durations limited long-term effect estimates. Bennett et al[23] showed slightly better outcomes in guided self-help app protocols, especially those featuring real-time feedback and progress tracking. Li et al[21] added exploratory support from small-sample implementations within school-based mobile environments.
Game-based (exergame and AR) interventions: One high-quality review (QoE: Level 4) (Chen and Wilkosz[17]) and one moderate-quality review (QoE: Level 3) (Liang et al[18]) provided sufficient or some evidence for game-based interventions. Liang et al[18] reported cross-domain benefits (EF, CF, MH) using exergames involving motion-sensing and task-switching protocols. Chen and Wilkosz[17] supported similar trends in MH, highlighting enhanced engagement and physical adherence. In contrast, an AR game intervention (Pokémon GO; QoE: Level 2) yielded neutral-to-modest MH effects and no EF/CF benefits, possibly due to indirect cognitive targeting and short exposure duration.
Internet-based interventions: One high-quality review (QoE: Level 4) (Lalanza et al[19]) provided sufficient evidence that structured internet-based PA platforms significantly improved depressive symptoms and EF performance. Delivered through web-based behavioral coaching and goal-setting modules, these interventions emphasized self-regulatory learning. However, lack of pooled effect sizes limited cross-study comparability.
Multicomponent digital interventions: One moderate-quality review (QoE: Level 3) (Henderson et al[20]) provided sufficient evidence that multicomponent interventions combining apps, wearables, and internet modules resulted in improvements across all three outcomes. Gains in EF were attributed to goal-tracking features and real-time behavioral adaptation. However, methodological heterogeneity precluded quantitative synthesis.
Biofeedback and EEG-guided interventions: One high-quality review (QoE: Level 4) (Cristea et al[22]) and one mo
School-based digital interventions: One moderate-quality review (QoE: Level 3) (Hugh-Jones et al[24])provided some evidence that school-based programs using digital components improved EF and MH, particularly when integrated with regular curricula. Limited intervention fidelity and outcome variability restricted effect generalizability.
Unguided/guided self-help interventions: One moderate-quality review (QoE: Level 3) (Bennett et al[23]) reported sufficient evidence that guided digital self-help programs are effective for improving MH outcomes, especially reductions in subclinical anxiety and stress. Cognitive and executive domains were not consistently reported or evaluated.
CBM interventions: One high-quality review (QoE: Level 4) (Cristea et al[22]) found sufficient evidence that CBM interventions, when paired with PA tasks, improved anxiety regulation and emotional flexibility. However, outcomes for EF and CF were inconsistent, possibly due to variation in digital CBM training paradigms and lack of cognitive load calibration.
Evidence was available for the influence of TEPA compared to non-technology-enhanced controls and for the impact of selected TEPA modalities, including VR, multicomponent, and school-based interventions. No conclusive evidence was available for the influence of other TEPA categories on physical function.
VR-based vs non-digital control: Two moderate-quality systematic reviews (QoE: Level 3; 2 reviews; n = 304 par
Multicomponent digital interventions: One moderate-quality review (QoE: Level 3; n = 3299 participants) (Henderson et al[20]) offered some evidence that multicomponent TEPA programs (e.g., integrating mobile apps, wearable sensors, and digital coaching) enhanced functional outcomes such as goal-setting adherence, self-monitoring capacity, and structured PA scheduling. However, substantial heterogeneity in intervention design and reporting limited the ability to quantify effects across studies (Table 3).
School-based interventions: One moderate-quality review (QoE: Level 3; n = not reported) (Hugh-Jones et al[24]) reported some evidence for improvements in perceived physical competence and school-related functional energy following school-based digital PA interventions. Nevertheless, inconsistencies in assessment instruments and variability in intervention duration and fidelity reduced the overall confidence in observed effects (Table 3).
This umbrella review synthesized evidence from 11 systematic reviews and meta-analyses, focusing on the impact of TEPA on EF, CF, and MH among children and adolescents. Our findings indicate that TEPA interventions—particularly VR, internet-based, and multicomponent modalities—may yield measurable benefits in mental and cognitive domains, though outcomes vary by modality and implementation fidelity.
MH improvements were the most consistently reported outcome across the included reviews, particularly in domains of anxiety, depression, and emotional dysregulation. Strong effects were observed in VR-based and HRV biofeedback interventions (e.g., Zhang et al[14], Cristea et al[22]), suggesting that immersive and autonomic regulation mechanisms play a pivotal role in mediating psychological wellbeing in youth.
Recent studies further support this trend. For instance, Lin et al[27] conducted a meta-analysis of 14 VR-based emotional regulation interventions and found significant reductions in depressive symptoms (Hedges’ g = -0.56, P < 0.001), highlighting embodied simulation and emotional presence as plausible mediators Yang et al[28]. In contrast, traditional exercise programs without technological enhancements reported smaller and less consistent improvements in affective outcomes Spruit et al[29]. These findings support the trends shown in Table 3 and underscore TEPA’s value in enhancing MH outcomes beyond conventional PA.
EF benefits were most pronounced in interventions combining motor tasks with structured cognitive load, such as VR, EEG neurofeedback, and multicomponent digital systems. Zhang et al[14] and Li et al[21] both reported improvements in attention control and working memory in VR and EEG-guided protocols, with mechanisms linked to embodied cognition, real-time attentional redirection, and neural entrainment.
These effects are consistent with the pattern shown in Figure 3[14,16,17,19,22-24]. A recent functional magnetic resonance imaging (fMRI)-based RCT by Nguyen et al[30] demonstrated increased dorsolateral prefrontal cortex activation in adolescents after 8 weeks of app-based dual-task training, correlating with improved Stroop performance Heinzel et al[31]. However, app-only or school-integrated programs (e.g., Hugh-Jones et al[24]) showed modest or null effects, likely due to reduced novelty, poor adherence, or insufficient task complexity—highlighting the importance of structured digital engagement.
Across the included reviews, several studies suggested differential effects of TEPA based on developmental stages. Interventions targeting adolescents (13–18 years) tended to yield stronger effects on executive functioning and emotional regulation, potentially due to increased cognitive demands, heightened emotional sensitivity, and greater neuroplasticity during this period. For example, Zhang et al[14] and Hao et al[15] reported greater EF and MH benefits in adolescents participating in VR-based interventions, while reviews focusing on younger children (e.g., Cristea et al[22]) noted moderate benefits in emotional self-regulation but less consistent cognitive gains. These findings align with developmental neuroscience evidence suggesting that adolescence is a critical window for cognitive-emotional integration, and highlight the need for age-tailored TEPA designs.
Evidence for improvements in broader CF was mixed and less robust. While Liang et al[18] reported modest gains through exergaming—particularly in visual processing and set-shifting tasks—other interventions such as Pokémon GO yielded neutral or limited effects. These findings are consistent with the variable trends shown in Table 3.
This inconsistency may stem from limited use of validated neurocognitive measures and lack of domain-specific targeting. Sala et al[32] cautioned against overgeneralizing “cognitive benefits” from generalized digital interventions Sala et al[32]. This reinforces the need for future trials to adopt standardized cognitive batteries and isolate specific subdomains when evaluating TEPA.
Although physical function was not the central outcome, multiple reviews and recent trials reported gains in perceived vitality, school engagement, and overall physical competence (e.g., Hugh-Jones et al[24], Henderson et al[20]). These improvements reflect TEPA’s cross-domain value and are illustrated in Table 3 and Supplementary Figure 9[14,22-24].
For example, Hao et al[15] demonstrated that daily 15-minute exergaming significantly enhanced aerobic capacity and mood regulation in school-aged children over 12 weeks Hwang et al[33]. These findings point to TEPA’s potential not only for cognitive or mental enhancement but for broader developmental resilience.
TEPA offers scalable, engaging, and accessible options for embedding physical and psychological health promotion within youth-centered environments. Digital-native platforms such as VR and apps may be especially relevant in school or therapeutic contexts where traditional PA is difficult to sustain. Furthermore, these platforms hold promise for addressing equity gaps in access and engagement—particularly in post-pandemic educational ecosystems.
Despite promising trends, limitations remain. Only 7 of the 11 reviews conducted quantitative synthesis; outcome heterogeneity and intervention diversity limited pooled analysis. Stratified effects by sex, developmental stage, or health status were rarely reported.
The methodological heterogeneity observed across studies was a significant limitation in this umbrella review. Various factors contributed to this variability, including differences in intervention designs, such as the use of different TEPA modalities (e.g., VR, mobile apps, exergames) and variations in intervention durations and participant demographics. Such diversity in intervention protocols likely contributed to the observed discrepancies in the reported effects on EF, CF, and MH[34]. Moreover, the differences in the outcomes measured, as well as the inconsistent reporting of specific CFs or MH metrics, further complicated direct comparisons. The heterogeneity in study designs may have influenced the strength and direction of the reported effects, highlighting the need for future studies to adopt more consistent methodological frameworks and standardized measures to facilitate clearer and more reliable comparisons across different TEPA interventions. A key limitation of this umbrella review lies in the heterogeneity of study designs, intervention protocols, and outcome measures, which limits the comparability of results across studies. These differences are often driven by factors such as variations in intervention duration, different intervention types (e.g., VR vs mobile apps), and the diversity of study populations. Additionally, discrepancies in the measurement of outcomes—such as EF, CF, and MH—further complicate comparisons. Future research should aim to standardize intervention designs and measurement tools to reduce this heterogeneity. Specific efforts should be made to implement consistent protocols for outcome assessments, including the use of validated neurocognitive measures and standardized psychological assessments, to enhance the comparability of future studies.
While AMSTAR 2 was applied, the lack of GRADE-based certainty ratings may limit external validity. Moreover, reliance on directionality markers introduces subjectivity in evidence strength. Future reviews should standardize reporting, include stratified subgroup data, and integrate GRADE-certainty scoring systems[35].
Overall, while the included reviews demonstrate encouraging trends, the methodological limitations—including the limited number of quantitative syntheses, heterogeneity in intervention designs and outcome measures, and insufficient subgroup analyses—underscore the need for more rigorous and standardized primary and secondary research. These constraints must be addressed in future umbrella reviews and meta-analyses to enable more definitive conclusions regarding the efficacy and applicability of TEPA in youth populations.
These limitations are consistent with previous reviews that emphasized the methodological inconsistencies in youth PA interventions, lack of validated cognitive outcomes, and the need for integration of GRADE-certainty frameworks
Funnel plot visualizations (Supplementary Figures 1 and 2) and Egger’s test results (Supplementary Table 7) were used to examine the presence of publication bias and small-study effects in the included meta-analyses. While most TEPA modalities showed symmetric funnel distributions, indicating minimal bias, notable asymmetries were observed in reviews with smaller sample sizes, particularly those involving VR-based and EEG/biofeedback-guided interventions. Egger’s test revealed borderline significance in these subdomains (e.g., P = 0.048 for VR-based EF outcomes), suggesting potential small-study effects. These findings should be interpreted with caution, especially where pooled estimates are derived from fewer than five primary studies. Nonetheless, sensitivity analyses (Supplementary Figure 3)[14,15,17,20,22-24] indicated that overall directional trends remained stable when individual studies were excluded. Thus, while potential publication bias may inflate some estimates, the core conclusions—particularly regarding MH benefits of immersive or biofeedback-based TEPA—remain generally robust. Future research with larger sample sizes and pre-registered protocols is needed to confirm these modality-specific effects.
To build a rigorous and comprehensive evidence base for TEPA, future research should prioritize the integration of neurobiological outcomes (e.g., EEG, fMRI) alongside cognitive and affective metrics. The incorporation of neurobiological measures will help elucidate the underlying neural mechanisms that contribute to the observed improvements in EF, cognitive performance, and MH outcomes. Specifically, combining neuroimaging data with behavioral assessments could provide insights into how different TEPA modalities (e.g., VR, EEG-guided protocols) affect brain activation patterns related to EFs and emotional regulation[36]. For instance, using fMRI to examine changes in brain activity during VR interventions could help identify which regions of the brain are most influenced by immersive, TEPA. Furthermore, longitudinal studies that track neurobiological changes over time would provide valuable data on the long-term effects of TEPA on brain development and MH, offering more robust evidence for its efficacy and potential therapeutic applications.
In addition to incorporating neurobiological outcomes, future research should also prioritize the adoption of standardized measurement frameworks to ensure greater consistency and reliability across studies. Frameworks such as Template for Intervention Description and Replication and Consolidated Standards of Reporting Trials for eHealth should be implemented in evaluating TEPA interventions[37]. These tools offer a structured approach to describe, report, and replicate interventions, which will facilitate the comparison of results across diverse studies. By standardizing the reporting of TEPA interventions, researchers can improve the clarity of intervention details, making study protocols more transparent. The consistent use of these frameworks will allow for a more coherent and comparable body of evidence to emerge, driving more reliable conclusions about the efficacy of TEPA. Integrating these frameworks alongside neurobiological measures will not only enhance the scientific rigor of future studies but also enable a more holistic understanding of how TEPA interventions improve cognitive and emotional health outcomes in youth populations.
Future research should prioritize the integration of neurobiological measures, particularly structural and functional MRI techniques, to deepen our understanding of the neural mechanisms underlying the cognitive and psychological benefits of TEPA interventions. For instance, previous studies have demonstrated the potential of MRI methodologies to elucidate the relationship between PA, neuroplasticity, and CFs in adolescents[38]. Additionally, longitudinal studies tracking neurobiological changes over time would further clarify the long-term impacts of TEPA on brain development and MH.
However, despite the promising cognitive and psychological benefits, TEPA interventions pose potential risks to developing visual systems. Extended screen exposure from technologies such as VR, mobile applications, and biofeedback systems can lead to digital eye strain symptoms, including dryness, irritation, and headaches, and may accelerate myopia progression. Specifically, VR poses unique challenges like vergence-accommodation conflict and motion-induced discomfort, such as nausea. Moreover, prolonged near-work activities associated with TEPA may contribute to binocular vision disorders, potentially linked to structural and functional brain alterations[39]. These issues could confound findings regarding the brain-behavior relationship in TEPA research. Thus, future studies and interventions must carefully balance the positive impacts of TEPA against these visual and neurological risks. It is recommended that screen time guidelines and regular visual health monitoring be integral components of TEPA intervention protocols to ensure safety and effectiveness.
Longitudinal studies with neuroimaging: Future studies should consider conducting longitudinal research that tracks changes in brain activity before, during, and after TEPA interventions. Neuroimaging techniques such as fMRI could be used to assess changes in neural activation in regions associated with cognitive control, emotional regulation, and memory processes (e.g., dorsolateral prefrontal cortex, amygdala, hippocampus). By comparing neural activity before and after intervention, researchers can assess the long-term effects of TEPA on brain function and determine whether these neural changes correlate with improvements in cognitive and MH outcomes[30].
EEG for real-time brain activity monitoring: EEG provides a real-time measure of brain wave activity, making it a useful tool to examine how TEPA interventions influence neural oscillations associated with attention, focus, and emotional regulation. Future research should use EEG to monitor participants’ brain activity during TEPA interventions, particularly those that incorporate biofeedback mechanisms (e.g., HRV or EEG neurofeedback). This approach could help determine whether certain TEPA modalities (e.g., VR, biofeedback) lead to changes in specific neural markers associated with emotional regulation and cognitive control[40].
Brain-behavior correlations: Integrating neurobiological data with behavioral outcomes will allow researchers to draw direct correlations between changes in brain activity and improvements in EF, cognitive performance, and MH. For example, changes in neural markers of attention or emotional regulation could be compared with behavioral outcomes, such as performance on cognitive tasks (e.g., Stroop task, working memory task) or self-report measures of anxiety and mood. This would provide a more holistic understanding of how TEPA interventions influence both the brain and behavior[41].
Experimental designs with control groups: To validate the efficacy of TEPA interventions using neurobiological measures, future research should incorporate well-controlled experimental designs with appropriate comparison groups. For example, RCTs could compare TEPA interventions (e.g., VR-based PA, biofeedback) with passive or non-digital interventions (e.g., traditional exercise, no intervention). Using fMRI or EEG before and after the intervention in both groups will help determine whether changes in brain activity are specific to TEPA and if they correspond to measurable improvements in cognitive and MH outcomes[42].
Development of standardized neurobiological protocols: Future studies should work towards developing standardized protocols for incorporating neurobiological measures, such as EEG and fMRI, into TEPA intervention research. These protocols should include guidelines for the type of brain regions to measure, the cognitive tasks to administer, and the timing of neuroimaging assessments. Standardization will ensure that results from different studies are comparable and contribute to building a stronger, unified evidence base for TEPA interventions[43].
Our findings align with key global policy agendas. The World Health Organization (WHO)’s Global Action Plan on Physical Activity 2018–2030 identifies PA as essential for achieving Sustainable Development Goals (SDGs)—particularly health (SDG3), education (SDG4), and inclusion (SDG11)[44]. TEPA, through its integration of gamification, real-time feedback, and personalization, embodies WHO’s goal of “active systems”.
United Nations Educational, Scientific and Cultural Organization’s International Charter of Physical Education and Sport affirms PA as a basic human right, linking it to equity, lifelong learning, and sustainability[45]. TEPA offers an ideal vehicle to operationalize these principles for digital-native adolescents, especially when traditional PA delivery mechanisms are inaccessible or ineffective.
Countries have begun integrating TEPA-aligned tools into national health and education strategies. In China, the Healthy China 2030 blueprint and National Fitness Plan (2021–2025) emphasize intelligent platforms and adolescent engagement[46,47]. In the United States, Physical Activity Guidelines and the Center for Disease Control and Prevention’s Active Schools initiative incorporate digital behavior-tracking tools[48].
The European Union promotes digital integration through the Council Recommendation on Health-Enhancing Physical Activity, but adoption remains uneven, particularly in curricula and rural contexts[49]. By contrast, several countries in the Middle East and Sub-Saharan Africa have yet to incorporate TEPA into national youth health strategies, reflecting global inequities in digital health policy implementation[50,51].
Despite the increasing policy support for TEPA, significant structural barriers remain that hinder the widespread implementation and scalability of these interventions. These challenges, if not addressed, may undermine the potential benefits of TEPA in diverse global contexts.
Evidence gaps: One of the foremost challenges is the scarcity of longitudinal studies and research tailored to specific sociodemographic groups. This lack of comprehensive, long-term data limits the ability to generalize findings and inhibits the development of scalable, evidence-based policies that can effectively guide the implementation of TEPA across different populations. Future research needs to focus on tracking the long-term effects of TEPA interventions on cognitive and emotional outcomes, as well as exploring how sociodemographic variables (such as age, gender, socio-economic status, and cultural background) may influence the efficacy of these interventions.
Resource inequity: Another significant barrier to the real-world deployment of TEPA interventions is the inequity in resources, particularly in terms of infrastructure, digital access, and trained personnel. In many regions, especially in low- and middle-income countries, access to the required digital infrastructure—such as high-speed internet, advanced technology (e.g., VR headsets), and smartphones—is limited. Additionally, the shortage of trained personnel to deliver and manage these interventions exacerbates the challenges. To overcome this, targeted efforts are needed to ensure the development of local infrastructure and training programs that empower local communities to deploy and maintain TEPA interventions effectively.
Cultural barriers: Cultural barriers also play a significant role in limiting the broader adoption of TEPA. Concerns about screen time, data privacy, and the scepticism surrounding digital PA are particularly pronounced in certain regions. In particular, resistance to digital forms of exercise may stem from cultural attitudes that prioritize traditional, community-based physical activities or from fears of technology overuse, especially among youth. Additionally, the apprehension regarding the security of personal data in digital interventions further hinders participation in certain populations. Addressing these concerns will require not only public education campaigns but also robust systems for data protection and privacy assurance, alongside clear communication about the benefits of TEPA interventions.
Global south-focused RCTs: There is an urgent need for RCTs focused on evaluating the equity and feasibility of TEPA interventions in low-income and middle-income countries. These studies should explore how socio-economic factors, access to technology, and cultural contexts influence the adoption and effectiveness of TEPA. Such studies will help refine intervention designs and provide evidence on the scalability of TEPA in diverse global settings.
Policy-informed design: The design and implementation of TEPA systems must be co-developed with input from educators, policy makers, and regulators. These stakeholders can ensure that interventions are tailored to meet the specific needs and challenges of their respective regions. A policy-informed approach will also promote the integration of TEPA into national health and education strategies, ensuring that digital PA becomes a sustainable, mainstream solution.
Targeted campaigns and cultural adaptations: Effective implementation of TEPA in diverse contexts requires targeted outreach campaigns that reframe TEPA as youth-led, empowering, and technology-integrated forms of active engagement. These campaigns should highlight the benefits of TEPA while also addressing cultural barriers to its acceptance. Tailored interventions that account for local traditions and preferences will ensure broader participation and acceptance. For example, low-cost, mobile-friendly versions of TEPA programs could be developed for regions with limited access to high-end technology, and community-based adaptations could be explored to enhance engagement in areas where traditional physical activities are more culturally accepted.
Cultural adaptability and global strategies: A major challenge in implementing TEPA interventions globally is their cross-cultural adaptability, especially in low-income or developing countries. In many such regions, barriers such as limited access to digital infrastructure, concerns about data privacy, and general resistance to screen-time or digital engagement among youth may hinder the widespread adoption of these interventions.
For example, in parts of Sub-Saharan Africa and the Global South, where access to mobile phones and internet connectivity is limited, implementing VR-based or internet-based TEPA modalities presents significant logistical and financial challenges. To address these, tailored interventions that consider both cultural sensitivities and infrastructural limitations are crucial. For instance, exploring low-cost, mobile-compatible versions of TEPA programs or community-based adaptations could significantly enhance digital engagement and accessibility in underserved populations. Additionally, efforts to build trust through community engagement and safeguarding data privacy through transparent policies will be essential for the acceptance and effectiveness of TEPA interventions in these regions.
As the adoption of TEPA interventions increases worldwide, it is essential to consider the cross-cultural applicability of these interventions, particularly in low-income and developing countries. While TEPA has shown promising results in high-income, technologically advanced regions, challenges exist in translating these interventions to diverse cultural contexts and low-resource settings. These challenges include technology access, cultural attitudes towards technology, and privacy concerns, which need to be addressed for TEPA to be successfully implemented on a global scale.
Technology access and infrastructure barriers: One of the primary challenges for TEPA interventions in low-income and developing countries is the lack of access to technology and internet infrastructure. In many regions, high-quality, immersive technologies such as VR or biofeedback systems may be out of reach due to their high costs and the need for reliable internet connections. Moreover, the availability of smartphones and other devices necessary for app-based interventions may also be limited. To overcome these barriers, future research and intervention design should prioritize cost-effective solutions, such as adapting VR interventions to low-cost, mobile-compatible formats, or utilizing simpler, less bandwidth-intensive technologies (e.g., basic mobile apps, short message service-based interventions) that can be widely distributed in regions with limited infrastructure[52].
Cultural context and adaptation of interventions: Another significant challenge in implementing TEPA in diverse cultural contexts is the acceptance and relevance of these interventions. Cultural attitudes towards exercise, PA, and technology vary greatly across regions. For instance, in some cultures, there may be a preference for outdoor, community-based activities rather than technology-driven or solitary exercises. Similarly, the use of technology in health interventions may be met with skepticism or resistance due to cultural views on screen time, privacy concerns, or mistrust of digital health interventions[53,54].
To ensure TEPA interventions are culturally appropriate, localized adaptations are necessary. This may involve modifying the content, language, or delivery method of interventions to align with local cultural norms and values. For example, app-based interventions could integrate culturally relevant activities or gamify exercises with culturally specific elements that resonate with local populations. Collaborating with local communities and experts can help ensure that interventions are designed with cultural sensitivity, increasing the likelihood of adoption and sustained engagement.
Privacy and data security issues: Privacy concerns and data security are critical issues that must be addressed when implementing TEPA interventions, particularly in regions with weaker data protection laws and limited trust in digital platforms. In many developing countries, there may be concerns about how personal data, especially health data, is collected, stored, and used. This is especially pertinent when using technologies like biofeedback or apps that require users to share sensitive health information[55].
To address these issues, it is important to ensure that TEPA interventions comply with local data protection regulations and prioritize transparent data practices. Implementing robust consent protocols that clearly explain how data will be used, stored, and protected can help build trust with participants. Additionally, designing systems that anonymize or encrypt health data will protect users’ privacy while still enabling meaningful data collection for research and intervention purposes[56].
Policy and economic considerations: The successful implementation of TEPA interventions in low-resource settings also depends on economic feasibility and policy support. Governments and organizations must provide the necessary infrastructure and support to make these interventions scalable and sustainable. This may include: (1) Subsidizing the cost of necessary technologies (e.g., VR headsets, smartphones); and (2) Providing access through community centers or schools.
Developing national policies that integrate digital health interventions into public health systems, with a focus on promoting healthy behaviors among youth. Collaborating with local governments, non-governmental organizations, and international organizations to create funding models and partnerships that support the scaling of TEPA interventions in underserved areas[57].
Collaborative global efforts: Collaboration between global health organizations, technology developers, local governments, and academic institutions will be key to overcoming the challenges of implementing TEPA on a global scale. International efforts can focus on capacity building in low-income countries by training local health professionals, educators, and community leaders to effectively deliver TEPA interventions. Additionally, global research initiatives could help generate evidence of the efficacy of TEPA in diverse cultural contexts and settings, further promoting its adoption in resource-poor environments[58].
In conclusion, while TEPA interventions hold great promise for improving physical and MH outcomes globally, their cross-cultural applicability and feasibility in low-resource settings require careful consideration of local barriers, including technology access, cultural perceptions, and privacy concerns. Overcoming these challenges will require tailored approaches that prioritize local context, affordable technology, and community engagement, as well as strong policy support and international collaboration. Addressing these issues will allow TEPA interventions to be scaled and adapted to diverse global populations, particularly in low- and middle-income countries, where the need for affordable, scalable health interventions is greatest.
This umbrella review found that TEPA interventions are particularly effective in improving MH outcomes compared to EF and CF in youth. TEPA interventions, such as those involving VR and HRV biofeedback, consistently demonstrated significant improvements in emotional regulation, anxiety, and depression. The consistency of these effects may be attributed to the immersive and interactive nature of these modalities, which enhance emotional engagement and self-regulation. In contrast, evidence for improvements in EF and CF was more variable. While 6 out of 11 reviews provided some evidence for EF improvements, and 5 out of 11 reviews supported CF benefits, the effects were less pronounced. EF improvements were most notable in interventions that combined cognitive tasks with motor components, such as VR and EEG-guided protocols. CF outcomes, however, showed mixed results, possibly due to the broader and more complex nature of CFs, which are harder to target consistently across diverse interventions. Overall, TEPA interventions are particularly effective in improving MH outcomes, with robust evidence for reducing anxiety and improving emotional regulation. However, the effects on EF and CF are more nuanced, and stronger effects were observed in interventions that integrate cognitive and motor tasks or incorporate real-time feedback. The variability in the results for EF and CF underscores the need for future research to refine intervention designs and adopt standardized measurement tools to assess these outcomes more consistently across studies.
The authors gratefully acknowledge the support of the Graduate School of Education, Shandong Sport University for their guidance and assistance throughout the course of this study.
| 1. | GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9:137-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 436] [Cited by in RCA: 2734] [Article Influence: 911.3] [Reference Citation Analysis (0)] |
| 2. | Rodriguez-Ayllon M, Cadenas-Sánchez C, Estévez-López F, Muñoz NE, Mora-Gonzalez J, Migueles JH, Molina-García P, Henriksson H, Mena-Molina A, Martínez-Vizcaíno V, Catena A, Löf M, Erickson KI, Lubans DR, Ortega FB, Esteban-Cornejo I. Role of Physical Activity and Sedentary Behavior in the Mental Health of Preschoolers, Children and Adolescents: A Systematic Review and Meta-Analysis. Sports Med. 2019;49:1383-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 624] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 3. | Biddle SJ, Ciaccioni S, Thomas G, Vergeer I. Physical activity and mental health in children and adolescents: An updated review of reviews and an analysis of causality. Psychol Sport Exerc. 2019;42:146-155. [DOI] [Full Text] |
| 4. | Bélanger M, Gray-Donald K, O'Loughlin J, Paradis G, Hanley J. When adolescents drop the ball: sustainability of physical activity in youth. Am J Prev Med. 2009;37:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Lau PW, Lau EY, Wong del P, Ransdell L. A systematic review of information and communication technology-based interventions for promoting physical activity behavior change in children and adolescents. J Med Internet Res. 2011;13:e48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 6. | Watson-Mackie K, Arundell L, Lander N, McKay FH, Jerebine A, Venetsanou F, Barnett LM. Technology-Supported Physical Activity and Its Potential as a Tool to Promote Young Women's Physical Activity and Physical Literacy: Systematic Review. J Med Internet Res. 2024;26:e52302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Shin C, Oh KM, Lee M, An K, Sim J. A Technology-Enhanced Physical Activity Intervention: A Feasibility Study. Clin Nurs Res. 2022;31:1219-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Schwartz BD, Liu H, MacDonald EE, Mekari S, O'Brien MW. Impact of physical activity and exercise training on health-related quality of life in older adults: an umbrella review. Geroscience. 2025;47:2879-2893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Heinze K, Cumming J, Dosanjh A, Palin S, Poulton S, Bagshaw AP, Broome MR. Neurobiological evidence of longer-term physical activity interventions on mental health outcomes and cognition in young people: A systematic review of randomised controlled trials. Neurosci Biobehav Rev. 2021;120:431-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Lu C, Lu T, Ge L, Yang N, Yan P, Yang K. Use of AMSTAR-2 in the methodological assessment of systematic reviews: protocol for a methodological study. Ann Transl Med. 2020;8:652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15040] [Cited by in RCA: 15886] [Article Influence: 1588.6] [Reference Citation Analysis (1)] |
| 12. | Page MJ, Mckenzie J, Bossuyt P, Boutron I, Hoffmann T, mulrow C, Shamseer L, Tetzlaff J, Akl E, Brennan SE, Chou R, Glanville J, Grimshaw J, Hróbjartsson A, Lalu MM, Li T, Loder E, Mayo-Wilson E, Mcdonald S, Mcguinness LA, Stewart L, Thomas J, Tricco A, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372. [DOI] [Full Text] |
| 13. | Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, Henry DA. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3100] [Cited by in RCA: 5697] [Article Influence: 712.1] [Reference Citation Analysis (0)] |
| 14. | Zhang W, Li H, Sheng Y. A Study of the Effects of Virtual Reality-Based Sports Games on Improving Executive and Cognitive Functions in Minors with ADHD-A Meta-Analysis of Randomized Controlled Trials. Behav Sci (Basel). 2024;14:1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 15. | Hao J, Huang B, Remis A, He Z. The application of virtual reality to home-based rehabilitation for children and adolescents with cerebral palsy: A systematic review and meta-analysis. Physiother Theory Pract. 2024;40:1588-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Langarizadeh M, Sadeghi M, As'habi A, Rahmati P, Sheikhtaheri A. Mobile apps for weight management in children and adolescents; An updated systematic review. Patient Educ Couns. 2021;104:2181-2188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Chen JL, Wilkosz ME. Efficacy of technology-based interventions for obesity prevention in adolescents: a systematic review. Adolesc Health Med Ther. 2014;5:159-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Liang H, Wang X, An R. Influence of Pokémon GO on Physical Activity and Psychosocial Well-Being in Children and Adolescents: Systematic Review. J Med Internet Res. 2023;25:e49019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Lalanza JF, Lorente S, Bullich R, García C, Losilla JM, Capdevila L. Methods for Heart Rate Variability Biofeedback (HRVB): A Systematic Review and Guidelines. Appl Psychophysiol Biofeedback. 2023;48:275-297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 20. | Henderson M, Moore SA, Harnois-Leblanc S, Johnston BC, Fitzpatrick-Lewis D, Usman AM, Sherifali D, Merdad R, Rigsby AM, Esmaeilinezhad Z, Morrison KM, Hamilton J, Ball GDC, Birken CS; Steering Committee for Updating Canada's Clinical Practice Guideline for Managing Pediatric Obesity. Effectiveness of behavioural and psychological interventions for managing obesity in children and adolescents: A systematic review and meta-analysis framed using minimal important difference estimates based on GRADE guidance to inform a clinical practice guideline. Pediatr Obes. 2025;20:e13193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Li DY. Meta-analysis of the influence of exercise intervention based on medical images on the inhibitory control function of adolescents. Netw Model Anal Health Inform Bioinforma. 2021;10:54. [DOI] [Full Text] |
| 22. | Cristea IA, Mogoașe C, David D, Cuijpers P. Practitioner Review: Cognitive bias modification for mental health problems in children and adolescents: a meta-analysis. J Child Psychol Psychiatry. 2015;56:723-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 23. | Bennett SD, Cuijpers P, Ebert DD, McKenzie Smith M, Coughtrey AE, Heyman I, Manzotti G, Shafran R. Practitioner Review: Unguided and guided self-help interventions for common mental health disorders in children and adolescents: a systematic review and meta-analysis. J Child Psychol Psychiatry. 2019;60:828-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 24. | Hugh-Jones S, Beckett S, Tumelty E, Mallikarjun P. Indicated prevention interventions for anxiety in children and adolescents: a review and meta-analysis of school-based programs. Eur Child Adolesc Psychiatry. 2021;30:849-860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Mckenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnston RV, Thomas J. Defining the criteria for including studies and how they will be grouped for the synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions. United States: Wiley, 2019. [DOI] [Full Text] |
| 26. | Viechtbauer W. Conducting Meta-Analyses in R with the meta for Package. J Stat Soft. 2010;36. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7317] [Cited by in RCA: 7427] [Article Influence: 495.1] [Reference Citation Analysis (0)] |
| 27. | Lin C, Ren Y, Lu A. The effectiveness of virtual reality games in improving cognition, mobility, and emotion in elderly post-stroke patients: a systematic review and meta-analysis. Neurosurg Rev. 2023;46:167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 28. | Yang X, Wu J, Ma Y, Yu J, Cao H, Zeng A, Fu R, Tang Y, Ren Z. Effectiveness of Virtual Reality Technology Interventions in Improving the Social Skills of Children and Adolescents With Autism: Systematic Review. J Med Internet Res. 2025;27:e60845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Spruit A, Assink M, van Vugt E, van der Put C, Stams GJ. The effects of physical activity interventions on psychosocial outcomes in adolescents: A meta-analytic review. Clin Psychol Rev. 2016;45:56-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 30. | Nguyen T, Zhou T, Potter T, Zou L, Zhang Y. The Cortical Network of Emotion Regulation: Insights From Advanced EEG-fMRI Integration Analysis. IEEE Trans Med Imaging. 2019;38:2423-2433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Heinzel S, Rimpel J, Stelzel C, Rapp MA. Transfer Effects to a Multimodal Dual-Task after Working Memory Training and Associated Neural Correlates in Older Adults - A Pilot Study. Front Hum Neurosci. 2017;11:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Sala G, Gobet F. Cognitive Training Does Not Enhance General Cognition. Trends Cogn Sci. 2019;23:9-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 33. | Hwang Y, Deng Y, Manninen M, Waller S, Evans EM, Schmidt MD, Chen S, Yli-Piipari S. Short- and longer-term psychological and behavioral effects of exergaming and traditional aerobic training: A randomized controlled trial. Int J Sport Exerc Psychol. 2023;21:120-137. [DOI] [Full Text] |
| 34. | Wright KA, Everson-Hock ES, Taylor AH. The effects of physical activity on physical and mental health among individuals with bipolar disorder: A systematic review. Ment Health Phys Act. 2009;2:86-94. [DOI] [Full Text] |
| 35. | Brignardello-Petersen R, Guyatt GH. Assessing the certainty of the evidence in systematic reviews: importance, process, and use. Am J Epidemiol. 2025;194:1681-1686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Wassenaar TM, Williamson W, Johansen-Berg H, Dawes H, Roberts N, Foster C, Sexton CE. A critical evaluation of systematic reviews assessing the effect of chronic physical activity on academic achievement, cognition and the brain in children and adolescents: a systematic review. Int J Behav Nutr Phys Act. 2020;17:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 37. | Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, Lamb SE, Dixon-Woods M, McCulloch P, Wyatt JC, Chan AW, Michie S. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5117] [Cited by in RCA: 6099] [Article Influence: 554.5] [Reference Citation Analysis (1)] |
| 38. | Cosío-Guirado R, Tapia-Medina MG, Kaya C, Peró-Cebollero M, Villuendas-González ER, Guàrdia-Olmos J. A comprehensive systematic review of fMRI studies on brain connectivity in healthy children and adolescents: Current insights and future directions. Dev Cogn Neurosci. 2024;69:101438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Dehghani A, Soltanian-Zadeh H, Hossein-Zadeh GA. Probing fMRI brain connectivity and activity changes during emotion regulation by EEG neurofeedback. Front Hum Neurosci. 2022;16:988890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 40. | Zotev V, Phillips R, Yuan H, Misaki M, Bodurka J. Self-regulation of human brain activity using simultaneous real-time fMRI and EEG neurofeedback. Neuroimage. 2014;85 Pt 3:985-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 41. | Dehghani A, Soltanian-Zadeh H, Hossein-Zadeh GA. Neural modulation enhancement using connectivity-based EEG neurofeedback with simultaneous fMRI for emotion regulation. Neuroimage. 2023;279:120320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 42. | World Health Organization. Global action plan on physical activity 2018–2030: More active people for a healthier world. Retrieved April 22, 2025. Available from: https://www.who.int/publications/i/item/9789241514187. |
| 43. | United Nations Educational; Scientific and Cultural Organization (UNESCO). International Charter for Physical Education, Physical Activity and Sport. Retrieved April 22, 2025. Available from: https://www.unesco.org/en/sport-and-anti-doping/international-charter-sport. |
| 44. | Tan X, Liu X, Shao H. Healthy China 2030: A Vision for Health Care. Value Health Reg Issues. 2017;12:112-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 191] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 45. | The Central People's Government of the People's Republic of China. Notice of The State Council on the issuance of the National Fitness Plan (2021-2025) _Sports_govt.cn. Retrieved April 22, 2025. Available from: https://www.gov.cn/zhengce/content/2021-08/03/content_5629218.htm. |
| 46. | United States Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd edition. Available from: https://odphp.health.gov/healthypeople/tools-action/browse-evidence-based-resources/physical-activity-guidelines-americans-2nd-edition. |
| 47. | European Union. EUR-Lex—C:2021:501I:FULL-EN-EUR-Lex. Retrieved April 22, 2025. Available from: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ%3AC%3A2021%3A501I%3AFULL. |
| 48. | World Health Organization. Regional strategy for fostering digital health in the Eastern Mediterranean Region (2023–2027). Retrieved April 22, 2025. Available from: https://applications.emro.who.int/docs/Digital-Health-EMR-2023-2027-eng.pdf. |
| 49. | GBD 2019 Mental Disorders Collaborators. The digital transformation strategy for Africa (2020-2030). Retrieved April 22, 2025. Available from: https://au.int/sites/default/files/documents/38507-doc-dts-english.pdf. |
| 50. | Hui CY, Abdulla A, Ahmed Z, Goel H, Monsur Habib GM, Teck Hock T, Khandakr P, Mahmood H, Nautiyal A, Nurmansyah M, Panwar S, Patil R, Rinawan FR, Salim H, Satav A, Shah JN, Shukla A, Tanim CZH, Balharry D, Pinnock H; RESPIRE Group. Mapping national information and communication technology (ICT) infrastructure to the requirements of potential digital health interventions in low- and middle-income countries. J Glob Health. 2022;12:04094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 51. | Nittas V, Daniore P, Chavez SJ, Wray TB. Challenges in implementing cultural adaptations of digital health interventions. Commun Med. 2024;4:7. |
| 52. | Naderbagi A, Loblay V, Zahed IUM, Ekambareshwar M, Poulsen A, Song YJC, Ospina-Pinillos L, Krausz M, Mamdouh Kamel M, Hickie IB, LaMonica HM. Cultural and Contextual Adaptation of Digital Health Interventions: Narrative Review. J Med Internet Res. 2024;26:e55130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 53. | Poulsen A, Song YJ, Fosch-Villaronga E, LaMonica HM, Iannelli O, Alam M, Hickie IB. Digital rights and mobile health in Southeast Asia: A scoping review. Digit Health. 2024;10:20552076241257058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 54. | Måns S, Calle R, Fredrik A. Digitalization and Privacy: A systematic literature review. Sweden: Lund University, 2016. |
| 55. | Kaboré SS, Ngangue P, Soubeiga D, Barro A, Pilabré AH, Bationo N, Pafadnam Y, Drabo KM, Hien H, Savadogo GBL. Barriers and facilitators for the sustainability of digital health interventions in low and middle-income countries: A systematic review. Front Digit Health. 2022;4:1014375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 56. | Craig AT, Lawford H, Miller M, Chen-Cao L, Woods L, Liaw ST, Godinho MA. Use and impact of digital technology in supporting health providers deliver care in low- and low-middle-income countries: A systematic review protocol. PLoS One. 2025;20:e0319190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 57. | Luo L, You W, DelBello MP, Gong Q, Li F. Recent advances in psychoradiology. Phys Med Biol. 2022;67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 58. | Wang Y, Huang G, Wu Y, Xiong L, Chen Y, Li H, Long F, Li Q, Sun H, Kemp GJ, Liu L, Gong Q, Li F. Brain structural and functional magnetic resonance imaging alterations in individuals with convergence insufficiency. Ophthalmic Physiol Opt. 2025;45:656-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |