Published online Oct 15, 2016. doi: 10.4239/wjd.v7.i18.449
Peer-review started: March 28, 2016
First decision: May 26, 2016
Revised: August 1, 2016
Accepted: August 17, 2016
Article in press: August 18, 2016
Published online: October 15, 2016
Processing time: 197 Days and 2.7 Hours
To examine the epidemic of diabetes mellitus (DM) and its impact on mortality from all-cause and cardiovascular disease (CVD), and to test the effect of antidiabetic therapy on the mortality in United States adults.
The analysis included a randomized population sample of 272149 subjects ages ≥ 18 years who participated in the National Health Interview Surveys (NHIS) in 2000-2009. Chronic conditions (hypertension, DM and CVD) were classified by participants’ self-reports of physician diagnosis. NHIS-Mortality Linked Files, and NHIS-Medical Expenditure Panel Survey Linkage Files on prescribed medicines for patients with DM were used to test the research questions. χ2, Poisson and Cox’s regression models were applied in data analysis.
Of all participants, 22305 (8.2%) had DM. The prevalence of DM significantly increased from 2000 to 2009 in all age groups (P < 0.001). Within an average 7.39 (SD = 3) years of follow-up, male DM patients had 1.56 times higher risk of death from all-cause (HR = 1.56, 95%CI: 1.49-1.64), 1.72 times higher from heart disease [1.72 (1.53-1.93)], 1.48 times higher from cerebrovascular disease [1.48 (1.18-1.85)], and 1.67 times higher from CVD [1.67 (1.51-1.86)] than subjects without DM, respectively. Similar results were observed in females. In males, 10% of DM patients did not use any antidiabetic medications, 38.1% used antidiabetic monotherapy, and 51.9% used ≥ 2 antidiabetic medications. These corresponding values were 10.3%, 40.4% and 49.4% in females. A significant protective effect of metformin monotherapy or combination therapy (except for insulin) on all-cause mortality and a protective but non-significant effect on CVD mortality were observed.
This is the first study using data from multiple linkage files to confirm a significant increased prevalence of DM in the last decade in the United States. Patients with DM have significantly higher risk of death from all-cause and CVD than those without DM. Antidiabetic mediations, specifically for metformin use, show a protective effect against all-cause and CVD mortalities.
Core tip: The study is one of the first projects to use a 10-years nationally linked dataset. The results highlight a new epidemic of diabetes in the United States. It addresses the impact of diabetes on cardiovascular disease and all-cause mortality. The study is also one of the first studies to explore the association between glucose lowering drug use and health outcomes using health survey data from the real-world.
- Citation: Liu L, Simon B, Shi J, Mallhi AK, Eisen HJ. Impact of diabetes mellitus on risk of cardiovascular disease and all-cause mortality: Evidence on health outcomes and antidiabetic treatment in United States adults. World J Diabetes 2016; 7(18): 449-461
- URL: https://www.wjgnet.com/1948-9358/full/v7/i18/449.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i18.449
Diabetes mellitus (DM) is the seventh leading cause of death in the United States. Of 2543279 death certificates from all-causes in 2010, 2.9% of deaths (n = 73932) clinically listed DM as the main cause of death, and more than 9% of deaths (n = 234051) were attributable to DM as a comorbid cause of death in the United States. It is estimated that more than 1.4 million Americans are diagnosed with DM every year. In 2012, 29.1 million Americans, or 9.3% of the population, had DM. Of the 29.1 million, 21 million were diagnosed, and 8.1 million were undiagnosed[1]. A similar increased prevalence of DM has been estimated worldwide[2]. It is clear that DM has posed a serious public health problem in the United States and in the world[1-4], not only because DM is a leading cause of death, but also DM is a significant risk factor for cardiovascular disease (CVD). CVD is the number one killer of the Americans[5-10]. Although the overall trend in the prevalence of DM and its impact on risk of CVD have been examined by several studies, some were limited to their small sample sizes[11], some were limited to their study designs [such as findings from the Behavior Risk Factor Surveillance Systems that are conducted using a telephone survey with a very low response rate (< 40%)][12], and some were limited to a cross-sectional analysis design[7,13]. Furthermore, patterns of antidiabetic treatment and its impact on long-term health outcomes are less known. In the present study, we aimed to examine the trend of DM, and the impact of DM on CVD (hypertension, coronary heart disease and stroke), and risk of mortality from CVD and all-cause using a nationally representative sample in the United States. Findings from the study may add new evidence of the burdens of DM to the body of the literatures, the patterns of antidiabetic medications usage, and the magnitudes of DM and drug use on all-cause and CVD mortalities.
Participants ages 18 years and older in the 2000-2009 National Health Interview Surveys (NHIS) were included in the study. The NHIS has been conducted annually since 1960 by the United States National Center for Health Statistics (NCHS), which is a part of the Centers for Disease Control and Prevention (CDC)[14]. NHIS is a cross-sectional household interview survey that serves as the principal source of information on the health of the noninstitutionalized civilian population of the United States[15]. Uniform sampling and interviewing processes for core variables are continuous throughout each year’s survey. The sampling plan follows a multistage area probability design that permits the representative sampling of households and non-institutionalized groups. In NHIS, one adult per household is randomly chosen to participate in a completed interview from approximately 30000 households containing about 85500 persons, of them about 30000 adults ages 18 and older. Participants’ vital status (alive or deceased) are followed yearly and linked to death certificates in the National Death Index system (NHIS-Mortality Linked File). This Linked File provides an important opportunity for health professionals to estimate the risk of mortality prospectively on the basis of the NHIS participants’ baseline characteristics[14]. In the present study, we applied the most recently released NHIS-Mortality Linked File, which had follow-up information for subjects who participated in NHIS in and before 2009, and followed up through the end of 2011 (December 31, 2011). We examined the past one decade trend of DM between 2000 and 2009, and risk of mortality in patients with DM. Of total 287530 participants ages 18 and older, we excluded 15381 who had missing information on prevalent DM status at baseline (n = 237), and those who were lost to follow-up (n = 15144) during the course of follow-up between 2000 and 2011, yielding a final analytic sample of 272149 adults (94.7% = 272149/287530). To examine the patterns of medication use in patients with DM, we further linked the study sample at individual participant’s level with the Medical Expenditure Panel Surveys (MEPS)[16]. The NHIS, NHIS-Mortality Linked File and MEPS have been approved by the Institutional Review Board of the United States CDC NCHS and are available through the NCHS[14]. The present analysis has been approved by Drexel University Institutional Review Board (# 1605004544).
Two groups of health outcomes in patients with and without DM were examined: (1) all-cause mortality; and (2) CVD mortality. Mortality data were defined using ICD-10: Heart disease (ICD10: I00-I09, I11, I13, I20-I51), cerebrovascular disease (ICD10: I60-I69). CVD includes the two major forms of heart disease and cerebrovascular disease (ICD10: I00-I09, I11, I13, I20-I51 and ICD10: I60-I69). Predictors and covariates included: (1) demographic factors: Age, gender, race/ethnicity and education attainments (< high-school graduate, high-school graduate, and ≥ college); (2) lifestyle related factors: Body mass index [BMI, calculated by weight (kg)/height (m2)], cigarette smoking (never smoked, formerly smoked, or currently smoker), alcohol consumption (not a drinker: < 12 drinks in entire life, former drinker: No drinks in previous year, and current drinker), and physical activity. BMI was classified into four groups on the basis of the World Health Organization (WHO) definition (underweight: < 18.5, normal weight: 18.5-24.9, overweight: 25-29.9, and obese: ≥ 30 kg/m2). Physical activity status was grouped on the basis of current guidelines (active: ≥ 150 min per week of moderate-intensity equivalent leisure-time aerobic activity; insufficiently active: 10-149 min per week of moderate-intensity equivalent leisure-time aerobic activity); (3) CVD related chronic conditions: Hypertension, coronary heart disease (CHD) and stroke. Baseline CVD includes patients who had CHD and/or stroke. The baseline chronic conditions were classified by participants’ self-reports of diagnoses made by a doctor or health professional; and (4) DM, oral glucose-lowering medication and insulin use were classified according to DM patients’ prescription records.
A serial analysis was conducted to test the study hypotheses and fit the time-events prediction models. The first group analysis included the basic characteristics description of the study participants and tested gender differences using univariate analysis, including t test for continuous variables, and χ2 tests for categorical variables. Changes in the prevalence of DM from 2000 to 2009 by sex and ages (18-54, 55-64, 65-74 and ≥ 75) were tested using simple linear regression models. The second group analysis involved estimates of mortality rates (per 1000 person-year) from all-cause, heart disease, cerebrovascular disease, and total CVD. We used Poisson regression to calculate mortality per person years. The third group analysis estimated the hazard ratios of DM (yes/no) for the risk of mortality from all-cause, heart disease, cerebrovascular disease, and total CVD using Cox’s proportional hazard regression models. In the analysis, five multivariate adjusted Cox’s models were performed by gender. Model 1 adjusted for age (years) and race/ethnicity (NH-White, NH-Black, Hispanics, and other groups). Model 2 adjusted for age, race/ethnicity and education level (< higher school, high school, and ≥ college). Model 3 adjusted for the covariates used in Model 2 plus three behavioral factors (smoking, alcohol consumption and physical activity). Model 4 adjusted for the covariates used in Model 3 plus hypertension. Because patients with CVD at baseline may have an increased risk of mortality, we excluded those patients in Model 5 and adjusted the same covariates as used in Model 4. Interactions of gender and DM on risk of mortality were tested using SAS Proc GENMOD. The fourth group analysis involved in estimates of the prevalence of glucose lowering medication and insulin use. We examined hazard ratios of monotherapy and combinations of glucose lowering medication and insulin use for the risk of mortalities compared to those without antidiabetic medication use. In the last group analysis, we compared baseline differences in 5 preventable factors’ age-race-adjusted standardized rates (education level, as a marker for economic status, smoking, physical activity, BMI and hypertension) between males and females using logistic regression in order to explain a potential gender difference in the relative risk of all-cause and CVD mortality in patients with DM.
All data analyses were performed using SAS version 9.3, with complex sample modules that take the sample design of NHIS, including stratification, clustering and weight into consideration (SAS Institute, Cary, NC). Statistical significance was determined for a two-sided test at a P value < 0.05.
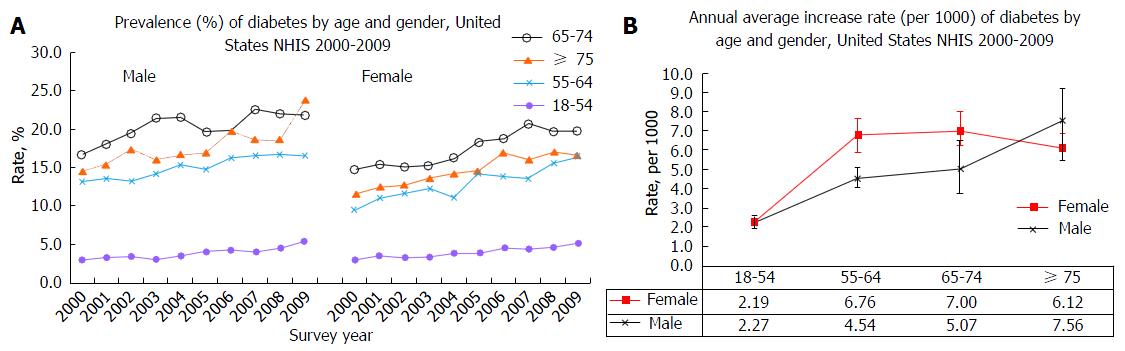
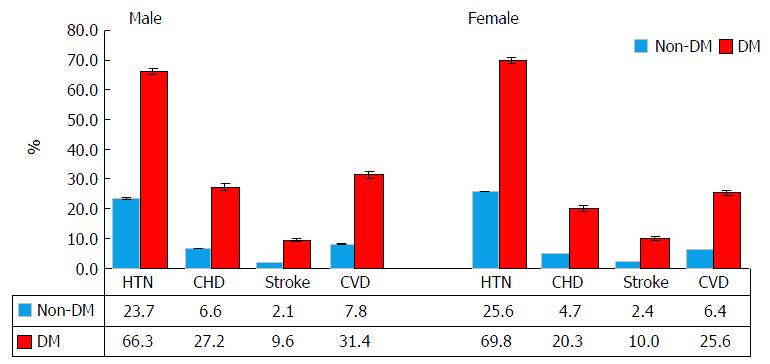
Of 272149 subjects participated in 2000-2009 NHIS, 22305 (8.2%) had diabetes (male: 9892, and female: 12413). The prevalence of DM significantly increased from 2000 to 2009 in all age groups for males and females (P < 0.001) (Figure 1A). The annual increase rates (per 1000) were 2.27, 4.54, 5.07, and 7.56 for male aged 18-54, 55-64, 65-74 and ≥ 75, respectively (test for linear trend, all P < 0.01). The corresponding values in females were 2.19, 6.76, 7.00 and 6.12, respectively (P < 0.01). Females had 1.49 times higher annual increase in those aged 55-64, and 1.38 higher in those aged 65-74 compared to males (Figure 1B). There were significant differences in demographic, behavior factors and medical conditions between those with and without DM in males and females (Table 1 and Figure 2).
| Male | Female | |||||||||||||
| Non-DM (n = 109507) | DM (n = 9892) | Non-DM (n = 140337) | DM (n = 12413) | |||||||||||
| No.a | Rateb (SEP) | No.a | Rateb (SEP) | P value | No.a | Rateb | (SEP) | No.a | Rateb | (SEP) | P valuec | |||
| Age, mean, yr | 44.9 | (0.10) | 60.3 | (0.14) | < 0.0001 | 46.6 | (0.1) | 60.7 | (0.16) | < 0.0001 | ||||
| Race/ethnicity | ||||||||||||||
| NH-White | 71683 | 74.5 | (0.25) | 6062 | 70.9 | (0.55) | < 0.0001 | 89088 | 73.8 | (0.25) | 6782 | 66.6 | (0.55) | < 0.0001 |
| NH-Black | 13345 | 10.4 | (0.18) | 1712 | 14.2 | (0.40) | 21517 | 12.5 | (0.20) | 2923 | 19.2 | (0.46) | ||
| Hispanics | 19251 | 10.7 | (0.17) | 1646 | 10.4 | (0.36) | 23779 | 9.8 | (0.15) | 2260 | 10.7 | (0.36) | ||
| Others | 5228 | 4.4 | (0.10) | 472 | 4.5 | (0.25) | 5953 | 3.9 | (0.08) | 448 | 3.4 | (0.21) | ||
| Education | ||||||||||||||
| Less than HS | 19941 | 15.3 | (0.18) | 2682 | 24.0 | (0.52) | < 0.0001 | 25377 | 15.0 | (0.16) | 4066 | 28.8 | (0.50) | < 0.0001 |
| HS Graduated | 30474 | 28.0 | (0.21) | 2887 | 30.6 | (0.58) | 39612 | 28.4 | (0.19) | 3873 | 33.1 | (0.54) | ||
| ≥ College | 58314 | 56.6 | (0.30) | 4231 | 45.3 | (0.58) | 74451 | 56.5 | (0.27) | 4370 | 38.1 | (0.53) | ||
| Smoking status | ||||||||||||||
| No smoker | 54430 | 49.6 | (0.24) | 3667 | 36.7 | (0.58) | < 0.0001 | 87384 | 60.8 | (0.21) | 7344 | 58.2 | (0.51) | < 0.0001 |
| Former smoker | 26644 | 24.9 | (0.19) | 4293 | 44.6 | (0.60) | 24800 | 19.1 | (0.14) | 3075 | 26.4 | (0.47) | ||
| Current smoker | 27507 | 25.5 | (0.19) | 1834 | 18.7 | (0.46) | 27211 | 20.1 | (0.16) | 1893 | 15.4 | (0.35) | ||
| Alcohol consumption | ||||||||||||||
| Never | 16415 | 14.3 | (0.19) | 1660 | 16.5 | (0.43) | < 0.0001 | 41116 | 27.0 | (0.23) | 5159 | 39.7 | (0.55) | < 0.0001 |
| Former | 15028 | 13.8 | (0.15) | 3348 | 34.0 | (0.61) | 19335 | 14.1 | (0.13) | 3389 | 28.2 | (0.51) | ||
| Current | 75204 | 71.8 | (0.22) | 4668 | 49.5 | (0.62) | 77255 | 58.9 | (0.25) | 3670 | 32.1 | (0.54) | ||
| Exercise | ||||||||||||||
| Inactive | 38394 | 34.5 | (0.32) | 4485 | 47.0 | (0.62) | < 0.0001 | 55965 | 38.9 | (0.29) | 6416 | 55.2 | (0.62) | < 0.0001 |
| Insufficiently active | 27605 | 27.2 | (0.18) | 2178 | 24.6 | (0.50) | 40073 | 31.0 | (0.18) | 2724 | 25.1 | (0.49) | ||
| Sufficiently active | 38609 | 38.3 | (0.26) | 2473 | 28.3 | (0.59) | 38637 | 30.0 | (0.22) | 2158 | 19.8 | (0.45) | ||
| BMI, kg/m2 | ||||||||||||||
| Overweight | 46884 | 43.5 | (0.18) | 3595 | 36.2 | (0.55) | < 0.0001 | 38254 | 28.1 | (0.15) | 3250 | 28.0 | (0.45) | < 0.0001 |
| Obesity | 24128 | 22.4 | (0.17) | 4372 | 46.1 | (0.59) | 31193 | 22.4 | (0.15) | 6168 | 52.8 | (0.53) | ||
| Medical condition | ||||||||||||||
| Hypertension | 25582 | 23.7 | (0.19) | 6501 | 66.3 | (0.56) | < 0.0001 | 35594 | 25.6 | (0.17) | 8662 | 69.8 | (0.48) | < 0.0001 |
| Coronary heart Dis | 7020 | 6.6 | (0.09) | 2598 | 27.2 | (0.48) | < 0.0001 | 6435 | 4.7 | (0.07) | 2450 | 20.3 | (0.41) | < 0.0001 |
| Stroke | 2243 | 2.1 | (0.05) | 956 | 9.6 | (0.30) | < 0.0001 | 3244 | 2.4 | (0.05) | 1233 | 10.0 | (0.32) | < 0.0001 |
| CVD | 8434 | 7.8 | (0.10) | 3019 | 31.4 | (0.51) | < 0.0001 | 8699 | 6.4 | (0.09) | 3109 | 25.6 | (0.43) | < 0.0001 |
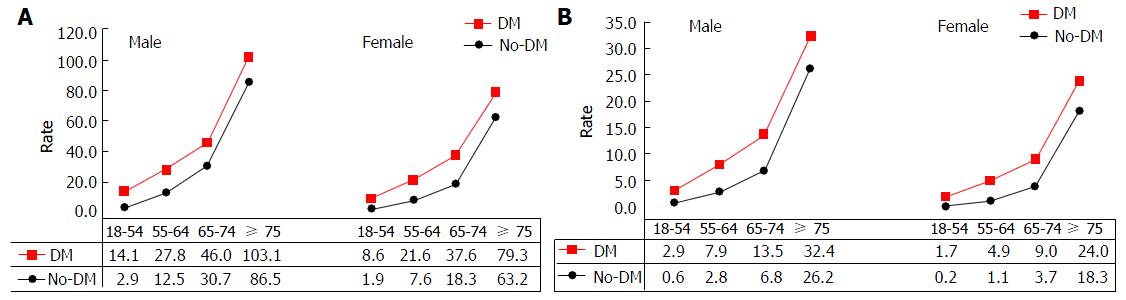
Table 2 shows that mortality from all-cause, heart disease, cerebrovascular disease (CBVD) and CVD increased with age in subjects with or without DM. However, patients with DM had significantly higher mortality than those without DM in both males and females, except for CBVD in males aged 18-54.9 (P = 0.063), and aged ≥ 75 (P = 0.694), and in females aged ≥ 75 (P = 0.371). Figure 3A depicts an overall increase in all-cause mortality with increased age in males and females, but a greater increased trend for those aged ≥ 65. Similar trend for CVD mortality is shown in Figure 3B. Subjects without DM had a much lower mortality rate from all-cause and CVD before the age of 65 as compared to those with DM and age of 65 and older.
| Male | Female | ||||||||||
| Non-DM | DM | Non-DM | DM | ||||||||
| Event | Rate | Event | Rate | P value | Event | Rate | Event | Rate | P value | ||
| All-cause | |||||||||||
| No. of death | 8363 | 2278 | 9485 | 2450 | |||||||
| Age | 18-54.9 | 1765 | 2.9 | 317 | 14.1 | < 0.0001 | 1384 | 1.9 | 249 | 8.6 | < 0.0001 |
| 55-64.9 | 1271 | 12.5 | 473 | 27.8 | < 0.0001 | 989 | 7.6 | 403 | 21.6 | < 0.0001 | |
| 65-74.9 | 1874 | 30.7 | 677 | 46.0 | < 0.0001 | 1681 | 18.3 | 693 | 37.6 | < 0.0001 | |
| ≥ 75 | 3453 | 86.5 | 811 | 103.1 | < 0.0001 | 5431 | 63.2 | 1105 | 79.3 | < 0.0001 | |
| Heart disease | |||||||||||
| No. of death | 1707 | 543 | 1603 | 506 | |||||||
| Age | 18-54.9 | 288 | 0.5 | 62 | 2.8 | < 0.0001 | 115 | 0.2 | 41 | 1.4 | < 0.0001 |
| 55-64.9 | 244 | 2.4 | 116 | 6.8 | < 0.0001 | 108 | 0.8 | 72 | 3.9 | < 0.0001 | |
| 65-74.9 | 350 | 5.7 | 164 | 11.1 | < 0.0001 | 241 | 2.6 | 132 | 7.2 | < 0.0001 | |
| ≥ 75 | 825 | 20.7 | 201 | 25.6 | 0.032 | 1139 | 13.2 | 261 | 18.7 | < 0.0001 | |
| CBVD | |||||||||||
| No. of death | 376 | 112 | 621 | 134 | |||||||
| Age | 18-54.9 | 50 | 0.1 | 4 | 0.2 | 0.063 | 58 | 0.1 | 8 | 0.3 | 0.002 |
| 55-64.9 | 38 | 0.4 | 19 | 1.1 | < 0.001 | 33 | 0.3 | 19 | 1.0 | < 0.0001 | |
| 65-74.9 | 67 | 1.1 | 35 | 2.4 | < 0.001 | 96 | 1.0 | 34 | 1.8 | 0.003 | |
| ≥ 75 | 221 | 5.5 | 54 | 6.9 | 0.694 | 434 | 5.0 | 73 | 5.2 | 0.371 | |
| CVD | |||||||||||
| No. of death | 2083 | 655 | 2224 | 640 | |||||||
| Age | 18-54.9 | 338 | 0.6 | 66 | 2.9 | < 0.0001 | 173 | 0.2 | 49 | 1.7 | < 0.0001 |
| 55-64.9 | 282 | 2.8 | 135 | 7.9 | < 0.0001 | 141 | 1.1 | 91 | 4.9 | < 0.0001 | |
| 65-74.9 | 417 | 6.8 | 199 | 13.5 | < 0.0001 | 337 | 3.7 | 166 | 9.0 | < 0.0001 | |
| ≥ 75 | 1046 | 26.2 | 255 | 32.4 | 0.038 | 1573 | 18.3 | 334 | 24.0 | < 0.0001 | |
Of the total study sample, within an average 7.39 (SD = 3) years of follow-up, the results show that after adjustment for age and race/ethnicity, male patients with DM vs non-DM had 1.56 times higher risk of death from all-cause (HR = 1.56, 95%CI: 1.49-1.64), 1.72 times higher from heart disease (HR = 1.72, 95%CI: 1.53-1.93), 1.48 times higher from CBVD (HR = 1.48, 95%CI: 1.18-1.85), and 1.67 times higher from CVD (HR = 1.67, 95%CI: 1.51-1.86), respectively (Model 1, Table 3). Similar results were observed in females. After further adjustment for the inclusion of education (Model 2), and behavior risk factors (cigarette smoking, alcohol consumption, and physical inactivity, Model 3), the corresponding HRs of DM for the risk of the mortalities remained statistically significant in patients with DM vs those without DM in males and in females. Model 4 shows that after a further control of the effect of hypertension, the HRs were attenuated compared to Model 3, specifically the impact of DM on the risk of death from CBVD became a borderline significance in males (P = 0.06). Finally, we excluded those who had heart disease and stroke at baseline (Model 5), the results show that HRs were further attenuated, except for a slight but non-significant increase in HR for death from CBVD in females.
| Male (n = 119399) | Female (n = 152750) | Excess HR1 | ||||||
| Mortality | HR | (95%CI) | P value | HR | (95%CI) | P value | Rate, % | P value |
| DM vs non-DM | ||||||||
| Model 1 | ||||||||
| All-cause | 1.56 | (1.49-1.64) | < 0.0001 | 1.69 | (1.61-1.78) | < 0.0001 | 8.3 | 0.02 |
| Heart Dis | 1.72 | (1.53-1.93) | < 0.0001 | 2.02 | (1.81-2.25) | < 0.0001 | 17.4 | 0.05 |
| CBVD | 1.48 | (1.18-1.85) | 0.001 | 1.43 | (1.15-1.77) | 0.001 | -3.5 | 0.35 |
| CVD | 1.67 | (1.51-1.86) | < 0.0001 | 1.85 | (1.69-2.03) | < 0.0001 | 10.5 | 0.16 |
| Model 2 | ||||||||
| All-cause | 1.54 | (1.47-1.62) | < 0.0001 | 1.62 | (1.55-1.71) | < 0.0001 | 5.5 | 0.13 |
| Heart Dis | 1.70 | (1.51-1.91) | < 0.0001 | 1.95 | (1.74-2.17) | < 0.0001 | 14.5 | 0.09 |
| CBVD | 1.48 | (1.18-1.86) | 0.001 | 1.39 | (1.12-1.72) | 0.003 | -6.2 | 0.70 |
| CVD | 1.66 | (1.49-1.84) | < 0.0001 | 1.79 | (1.63-1.96) | < 0.0001 | 7.8 | 0.29 |
| Model 3 | ||||||||
| All-cause | 1.47 | (1.39-1.55) | < 0.0001 | 1.55 | (1.47-1.63) | < 0.0001 | 5.5 | 0.15 |
| Heart Dis | 1.62 | (1.44-1.82) | < 0.0001 | 1.80 | (1.60-2.03) | < 0.0001 | 11.2 | 0.21 |
| CBVD | 1.35 | (1.04-1.75) | 0.023 | 1.40 | (1.12-1.75) | 0.003 | 3.4 | 0.85 |
| CVD | 1.57 | (1.40-1.75) | < 0.0001 | 1.68 | (1.52-1.86) | < 0.0001 | 7.4 | 0.35 |
| Model 4 | ||||||||
| All-cause | 1.42 | (1.35-1.49) | < 0.0001 | 1.50 | (1.42-1.58) | < 0.0001 | 5.6 | 0.15 |
| Heart Dis | 1.58 | (1.33-1.69) | < 0.0001 | 1.65 | (1.48-1.89) | < 0.0001 | 4.4 | 0.19 |
| CBVD | 1.28 | (0.99-1.66) | 0.06 | 1.33 | (1.06-1.66) | 0.013 | 3.9 | 0.83 |
| CVD | 1.46 | (1.31-1.63) | < 0.0001 | 1.58 | (1.42-1.75) | < 0.0001 | 8.2 | 0.32 |
| Model 5 - in those without baseline CVD | ||||||||
| All-cause | 1.32 | (1.23-1.41) | < 0.0001 | 1.40 | (1.32-1.50) | < 0.0001 | 6.1 | 0.19 |
| Heart Dis | 1.22 | (1.03-1.45) | 0.019 | 1.43 | (1.21-1.69) | < 0.0001 | 17.2 | 0.19 |
| CBVD | 1.24 | (0.93-1.67) | 0.137 | 1.41 | (1.10-1.82) | 0.008 | 13.7 | 0.53 |
| CVD | 1.22 | (1.04-1.43) | 0.017 | 1.45 | (1.26-1.67) | < 0.0001 | 18.9 | 0.08 |
Females appeared to have a higher HRs of DM for mortality from all-cause, heart disease and CVD than males. However, the increased HRs in females became non-significant after adjusting for age, race/ethnicity and education (Model 2), adjusting for behavior factors (Model 3), adjusting hypertension (Model 4), and excluding those who had heart disease and stroke at baseline (Model 5).
Table 4 shows that in males, 10% of patients with DM did not use any antidiabetic medications, and 38.1% of DM patients used antidiabetic monotherapy, and 51.9% used ≥ 2 antidiabetic medications. The corresponding values of the prevalence of those who did not use any antidiabetic medication, those who used 1 only (i.e., monotherapy), and those who used ≥ 2 were 10.3%, 40.4% and 49.4% in females respectively. Of those with monotherapy in males, 37.2% patients used insulin, followed by metformin (27.3%), sulfonylureas (25.9%) and others (9.6%). The most common three monotherapies in females were metformin (33.2%), insulin (33.0%), and sulfonylureas (21.9%), (gender differences: P = 0.008). Among patients with combined antidiabetic medication therapies, the most frequent combination was metformin and sulfonylureas (20.1% in males, and 21.9% in females). No significant differences in the proportions of combined therapies between males and females were observed (P = 0.42).
| Male | Female | ||||
| % | (SEP) | % | (SEP) | P value | |
| By groups | |||||
| Monotherapy | 38.11 | (1.19) | 40.36 | (1.06) | 0.291 |
| Combination | 51.89 | (1.27) | 49.38 | (1.10) | |
| No drug | 10.00 | (0.83) | 10.26 | (0.66) | |
| Monotherapy | |||||
| Biguanides (Metformin) | 27.32 | (1.89) | 33.24 | (1.57) | 0.008 |
| SU | 25.86 | (1.72) | 21.88 | (1.34) | |
| Insulin | 37.20 | (1.85) | 33.01 | (1.42) | |
| Others | 9.63 | (1.16) | 11.87 | (0.95) | |
| Combination | |||||
| Metformin + SU | 20.05 | (1.34) | 21.94 | (1.10) | 0.422 |
| TZD + Any (insulin excluded) | 13.56 | (1.25) | 13.11 | (0.87) | |
| Insulin + Any (TZD excluded) | 15.25 | (1.38) | 16.91 | (1.07) | |
| Metformin + SU + TZD | 7.72 | (0.86) | 6.75 | (0.69) | |
| Metformin + SU + Insulin | 6.89 | (0.87) | 4.97 | (0.61) | |
| Any other combinations | 36.52 | (1.85) | 36.32 | (1.44) | |
Table 5 shows that after adjustment for key covariates, patients with treatment of antidiabetic medication vs those without had 7% lower risk of mortality from all-cause (HR = 0.93, 95%CI: 0.73-1.18, P = 0.56, Model 2) and 4% lower risk from CVD (HR = 0.96, 95%CI: 0.60-1.54, P = 0.87, Model 2), although these associations did not reach statistical significance. However, DM patients with metformin monotherapy had a significantly decreased risk of all-cause mortality (HR = 0.55, 95%CI: 0.38-0.80, P = 0.002, Model 2), but those with insulin monotherapy showed an increased risk of all-cause mortality (HR = 1.71, 95%CI: 1.31-2.24, P < 0.0001, Model 2). A protective but non-significant effect of the treatment of antidiabetic medications (except for sulfonylureas and insulin use) on CVD mortality was observed.
| All-cause mortality | Mortality from CVD | ||||||||||||
| Model 1 | Model 2 | Model 1 | Model 2 | ||||||||||
| HR | (95%CI) | P value | HR | (95%CI) | P value | HR | (95%CI) | P value | HR | (95%CI) | P value | ||
| Medication use vs no-use | 0.87 | (0.68-1.11) | 0.251 | 0.93 | (0.73-1.18) | 0.556 | 0.88 | (0.54-1.44) | 0.613 | 0.96 | (0.60-1.54) | 0.873 | |
| Monotherapy (ref: Non-drug use) | |||||||||||||
| Biguanides (Metformin) | 0.53 | (0.36-0.77) | 0.001 | 0.55 | (0.38-0.80) | 0.002 | 0.82 | (0.42-1.61) | 0.564 | 0.87 | (0.45-1.68) | 0.681 | |
| SU | 0.89 | (0.66-1.21) | 0.456 | 0.91 | (0.67-1.23) | 0.529 | 1.10 | (0.66-1.83) | 0.716 | 1.11 | (0.67-1.84) | 0.696 | |
| Insulin | 1.65 | (1.26-2.16) | < 0.001 | 1.71 | (1.31-2.24) | < 0.0001 | 1.51 | (0.86-2.66) | 0.153 | 1.58 | (0.92-2.70) | 0.094 | |
| Combination | |||||||||||||
| Metformin + SU | 0.75 | (0.55-1.01) | 0.059 | 0.81 | (0.60-1.10) | 0.168 | 0.77 | (0.41-1.43) | 0.403 | 0.87 | (0.48-1.57) | 0.632 | |
| TZD + Any (insulin excluded) | 0.87 | (0.60-1.26) | 0.468 | 0.98 | (0.67-1.42) | 0.905 | 0.43 | (0.23-0.82) | 0.011 | 0.52 | (0.28-0.98) | 0.042 | |
| Insulin + Any (TZD excluded) | 1.27 | (0.90-1.78) | 0.171 | 1.33 | (0.94-1.86) | 0.103 | 1.37 | (0.69-2.72) | 0.365 | 1.45 | (0.75-2.81) | 0.264 | |
| Metformin + SU + TZD | 0.40 | (0.25-0.65) | < 0.001 | 0.43 | (0.27-0.70) | 0.001 | 0.54 | (0.24-1.18) | 0.120 | 0.58 | (0.27-1.25) | 0.164 | |
| Metformin + SU + Insulin | 0.64 | (0.41-1.01) | 0.053 | 0.67 | (0.43-1.05) | 0.080 | 0.75 | (0.44-1.29) | 0.293 | 0.80 | (0.47-1.37) | 0.418 | |
| Other combination | 0.63 | (0.46-0.86) | 0.004 | 0.68 | (0.50-0.93) | 0.016 | 0.59 | (0.31-1.09) | 0.091 | 0.64 | (0.35-1.19) | 0.157 | |
In patients with DM, a combination of metformin, sulfonylureas and thiazolidinedione showed a significantly reduced risk of all-cause mortality compared to those who did not use a combination therapy (HR = 0.43, 95%CI: 0.27-0.70, P = 0.001, Model 2 in Table 5). A significantly reduced risk of all-cause mortality was observed as well in patients with any other combined drug therapies (0.68, 0.50-0.93, P = 0.016, Model 2). No significant association between combination medication use and risk of CVD mortality was observed for DM patients with or without combination therapies, except for those with thiazolidinedione plus any other antidiabetic medications (excluding insulin) (0.52, 0.28-0.98, P = 0.042).
Table 6 shows that DM patients with insulin monotherapy showed an increased risk of heart disease mortality than those without insulin monotherapy (1.91, 1.12-3.26, P = 0.018, Model 2). A combination of metformin, sulfonylureas and insulin was significantly and negatively associated with heart disease mortality (0.35, 0.20-0.62, P < 0.0001, Model 2). No statistical significance in mortality from CBVD was observed in DM patients with or without medication (neither monotherapy nor combinations).
| Heart disease mortality | Cerebrovascular disease (CBVD) mortality | |||||||||||||
| Model 1 | Model 2 | Model 1 | Model 2 | |||||||||||
| HR | (95%CI) | P value | HR | (95%CI) | P value | HR | (95%CI) | P value | HR | (95%CI) | P value | |||
| Medication use vs no-use | 1.01 | (0.62-1.65) | 0.982 | 1.08 | (0.67-1.74) | 0.741 | 0.58 | (0.19-1.78) | 0.339 | 0.67 | (0.23-1.95) | 0.457 | ||
| Monotherapy | ||||||||||||||
| Biguanides (Metformin) | 0.80 | (0.38-1.67) | 0.550 | 0.83 | (0.40-1.70) | 0.608 | 0.94 | (0.22-4.01) | 0.938 | 1.20 | (0.30-4.82) | 0.792 | ||
| SU | 1.26 | (0.76-2.11) | 0.373 | 1.27 | (0.76-2.13) | 0.364 | 0.71 | (0.23-2.24) | 0.560 | 0.76 | (0.25-2.34) | 0.629 | ||
| Insulin | 1.85 | (1.06-3.24) | 0.031 | 1.91 | (1.12-3.26) | 0.018 | 0.60 | (0.16-2.23) | 0.445 | 0.71 | (0.23-2.21) | 0.547 | ||
| Combined | ||||||||||||||
| Metformin + SU | 0.82 | (0.41-1.67) | 0.586 | 0.91 | (0.47-1.80) | 0.794 | 0.59 | (0.14-2.47) | 0.472 | 0.71 | (0.17-2.94) | 0.632 | ||
| TZD + Any (insulin excluded) | 0.55 | (0.28-1.08) | 0.082 | 0.68 | (0.35-1.30) | 0.242 | 0.14 | (0.02-1.18) | 0.070 | 0.15 | (0.02-1.32) | 0.088 | ||
| Insulin + Any (TZD excluded) | 1.79 | (0.89-3.61) | 0.103 | 1.85 | (0.94-3.64) | 0.074 | 0.44 | (0.13-1.53) | 0.196 | 0.50 | (0.15-1.66) | 0.257 | ||
| Metformin + SU + TZD | 0.61 | (0.27-1.40) | 0.245 | 0.65 | (0.30-1.42) | 0.280 | 0.34 | (0.04-2.83) | 0.316 | 0.41 | (0.04-3.95) | 0.438 | ||
| Metformin + SU + Insulin | 0.33 | (0.19-0.59) | 0.000 | 0.35 | (0.20-0.62) | < 0.0001 | 1.66 | (0.55-5.02) | 0.368 | 1.91 | (0.64-5.73) | 0.245 | ||
| Other combinations | 0.71 | (0.37-1.37) | 0.307 | 0.78 | (0.42-1.47) | 0.444 | 0.28 | (0.06-1.31) | 0.106 | 0.31 | (0.07-1.41) | 0.129 | ||
The present study, using data from one of the largest national health survey systems and multiple linkage files, examined the burden of DM and its impact on CVD and all-cause mortality among adults in the United States. The study adds new evidence to the body of scientific literatures regarding antidiabetic medication profiles and health outcomes in patients with DM. The main findings show that: (1) the prevalence of DM significantly increased in all age groups for males and females in the last decade; (2) patients with DM had 1.47 to 1.62 times higher risk of death from all-cause and CVD in males, and 1.55 to 1.68 times higher in females compared to those without DM; (3) about 40% of patients with DM used antidiabetic monotherapy, and about 50% used combined antidiabetic therapy, however 10% of patient with DM did not use any medication in both males and females; and (4) in patients with DM, using metformin monotherapy or a combined therapy of metformin with other antidiabetic medications showed a significantly reduced risk of all-cause mortality. This protective association remained significant after adjustment for age, sex, race/ethnicity, survey year, antihypertensive drug, and anti-dyslipidemia medication use.
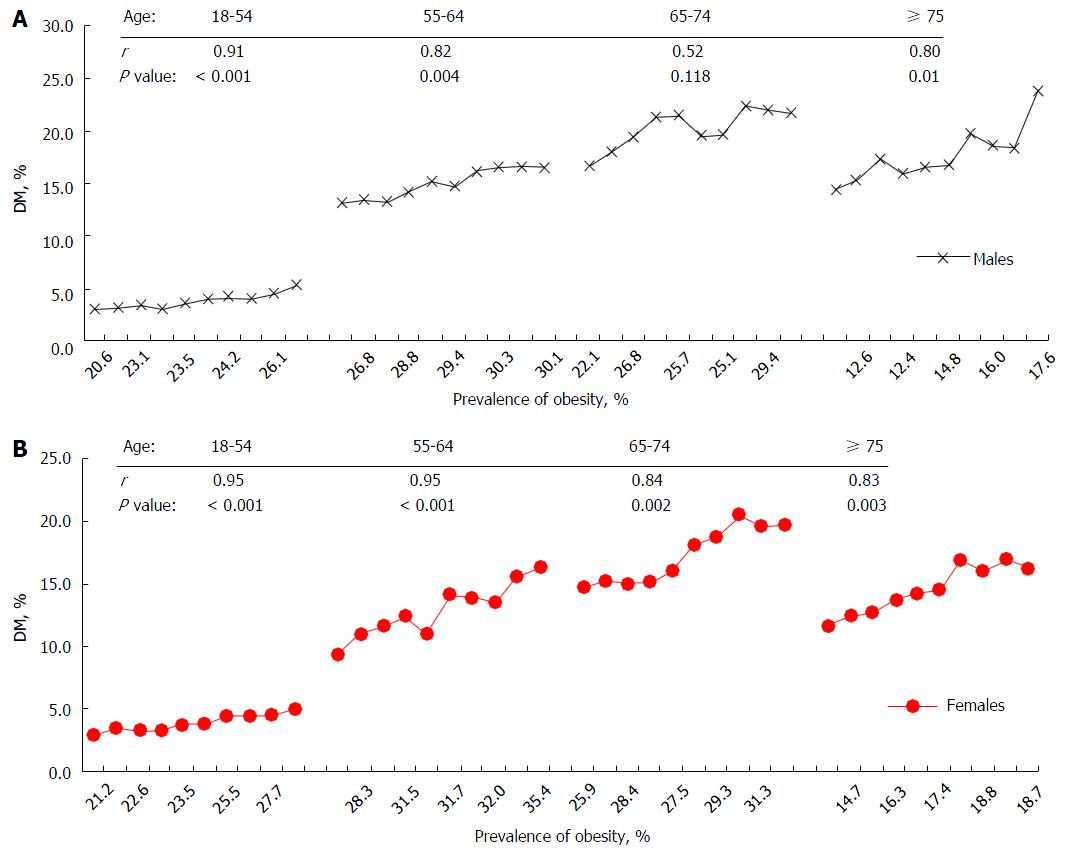
The present study confirmed an increased prevalence of DM in the last decade in the United States This finding is consistent with previous reports[1-4], and provides new evidence at the national level. Several factors may contribute to the increased rates. Of them, an increased prevalence of obesity across the nation may contribute to the increased trend of DM. We analyzed obesity rate using the same NHIS data. Figure 4 depicts a positive correlation trend between the prevalence of obesity and DM between 2000 and 2009 by four age groups in males (Figure 4A) and in females (Figure 4B) between 2000 and 2009. The highest correlation coefficient (r) was shown in ages 18-54 (r = 0.91, P < 0.001) in males, and ages 18-54 (r = 0.95, P < 0.001), and 55-64 (r = 0.95, P < 0.001) in females. Given the well-known pathophysiological mechanisms of obesity and risk of DM, this finding suggests that control of obesity would play a pivotal role in stopping the unwelcome trends of DM. In addition, females aged 55-74 have a greater increased trend of DM than males (Figure 1B). Although it is unclear why there is a notable increase in this age group for females, changes in female hormone at pre- and post-menopausal ages may partly explain this gender difference in the risk of DM and other chronic diseases[5,7,17-21]. Data from the Women’s Health Initiative Hormone Trial suggest that combined therapy with estrogen and progestin reduces the incidence of DM[21].
As demonstrated in several studies, we observed an excess relative risk (i.e., hazard ratio) of DM for all-cause and heart disease in females vs males. However, this excess risk became non-significant after adjustment for key covariates. Findings using data from the earlier Framingham Heart Study (FHS) surveys (1970s and 1980s) demonstrated a significant excess risk of recurrent myocardial infarction and fatal coronary heart disease for women with DM vs men with DM[22,23]. Our non-significant results are not consistent with the previous report. It may be attributable to the different datasets we used from the FHS. For example, the majority of participants in FHS were white middle class individuals who may have different risk profiles from minorities and people with lower social status. Furthermore, a decreased relative risk of DM for CVD in recent generations has been observed because of early diagnosis and disease prevention as well as more advanced treatment than two or three decades ago. Nevertheless, this relatively higher risk of DM for coronary heart disease in women vs men should be still taken into consideration in CVD risk assessment and disease prevention. In the study, among 5 preventable CVD risk factors that we examined, 4 (percent of individuals with lower socioeconomic status, assessed by education level, the proportion of individuals who were physically inactive, the proportion of individuals with obesity, and the proportion of individuals who had hypertension) were significantly higher in females than males, although males had a higher smoking rate than females (Table 7). These risk factors differences may partly explain the relative risk difference between genders. It is clear further studies are needed to assess the gender differences, including studies of the established and emerging risk predictors[15,24-28].
| Patients with DM at baseline | |||||
| Male (n = 9892) | Female (n = 12413) | ||||
| Rate | (SEP) | Rate | (SEP) | P value | |
| Education | |||||
| Less than HS | 21.53 | (0.50) | 24.82 | (0.47) | < 0.0001 |
| HS Graduated | 28.44 | (0.59) | 30.61 | (0.55) | 0.0100 |
| ≥ College | 50.03 | (0.60) | 44.57 | (0.56) | < 0.0001 |
| Smoking status | |||||
| No smoker | 40.92 | (0.58) | 61.60 | (0.53) | < 0.0001 |
| Former smoker | 36.03 | (0.58) | 17.81 | (0.49) | < 0.0001 |
| Current smoker | 23.05 | (0.46) | 20.59 | (0.35) | < 0.0001 |
| Exercise | |||||
| Inactive | 42.80 | (0.63) | 49.34 | (0.62) | < 0.0001 |
| Insufficiently active | 26.88 | (0.52) | 27.98 | (0.49) | 0.1000 |
| Sufficiently active | 30.32 | (0.61) | 22.68 | (0.47) | < 0.0001 |
| BMI, kg/m2 | |||||
| Overweight | 34.21 | (0.55) | 25.82 | (0.46) | < 0.0001 |
| Obesity | 47.13 | (0.60) | 53.88 | (0.51) | < 0.0001 |
| Medical condition | |||||
| Hypertension | 52.54 | (0.58) | 54.55 | (0.46) | 0.0100 |
The present study provides new evidence of the patterns of antidiabetic medicine usage and their impact on all-cause and CVD mortalities in patients with DM. Treatments with metformin, insulin, and sulfonylureas were the top three medications in the study population. More than one third of patients took insulin, which is commonly given to patients either for a short-term use because of significantly out of control serum glucose, or for long-term glucose control because their DM has progressed over many years (commonly between 10 and 20 years) and their pancreas can no longer make enough insulin to respond to other glucose-lowering medications[5,29]. Similar to previous studies, findings from the present study suggest a significant protective effect on all-cause mortality, and a protective effect on CVD mortality for those using metformin or metformin combined with other glucose-lowering medications. Metformin, a class of medications known as “biguanides” and a first-line agent for type 2 DM (T2DM) pharmacotherapy, is one of the most prescribed drugs worldwide[30]. It has been suggested that the potential mechanisms by which metformin reduces the risk of mortality is lowering blood glucose by reducing hepatic glucose output, decreasing intestinal glucose absorption, and controlling body weight by decreasing food intake[30-32], The mechanism of the cardiovascular effect of metformin was reported to improve lipoprotein profiles in diabetic patients by decreasing plasma concentrations of free fatty acid, triglycerides, total cholesterol and LDL cholesterol and increased HDL cholesterol[30]. Meanwhile, all-cause mortality includes deaths from cancer as well. Several studies have shown a significant risk reduction in cancer incidence and mortality among diabetic patients on metformin use relative to other antidiabetic drugs use[33]. Furthermore, in considering that biguanides demonstrate a better safety profile than most oncology drugs in current anticancer drug use, nonconventional routes for administering diabetobiguanides for cancer treatment has been suggested[34]. Findings from the present study support the current knowledge of metformin therapy in the reduction of CVD and total mortality, although further studies are needed in detail on its specific association with CVD and cancers.
In addition to the strength of using a large-scale sample size, the present study has several other advantages. First, using the NHIS-Mortality Linked Files, we were able to test the association between DM and risk of outcomes prospectively. Second, by using NHIS-MEPS linkage Files, we were able to test the patterns of medications which paves the way for us to further test more details on the association between pharmacotherapy and disease outcomes using a nationally representative dataset.
Similar to any study, however, the present study has several limitations. First, we were unable to classify whether a patient with DM was type 1 DM (T1DM) or T2DM because the NHIS data did not collect the information. Therefore, findings from the study cannot be applied to interpret risk differences between T1DM and T2DM. However, although T1DM can occur at any age, it is most often diagnosed in children, adolescents, or young adults. The NHIS’s participants were aged 18 and older. Furthermore, it is well-known that the majority of total DM are T2DM in general population, we may be able to assume the majority DM cases in the NHIS data were T2DM. Second, baseline predictors were measured once only, that any changes in the study variables after baseline may affect the prospective estimates of the associations between baseline predictors and health outcomes. Findings of the study should be on the basis of the hypothesis that these changes, if any, were randomized across all participants, so that a potential time-varying bias would be small when a study uses a large-scale sample size[35]. Third, participants’ medical conditions at baseline were self-reported physician-diagnosis of disease (hypertension, CHD, stroke and DM), therefore possible recall bias may occur. However, the recall bias might have a relatively small effect, because the use of self-reports of physician-diagnosis of disease have been confirmed as a valid approach in large-scale population health surveys in the United States[36,37]. Fourth, the NHIS did not have data on participants’ physical exams and laboratory tests (i.e., without exact blood pressure measures, and measures from serum lipids and metabolic biomarkers), which may not only lead to underestimate the prevalence of hypertension and DM, but also limit us to quantitatively estimate the association between antidiabetic drug use and changes in serum HbA1c (a biomarker of glycaemia control status in diabetic patients) and lipid profiles, and their impacts on the study outcomes. Therefore, the findings of the study provide a relatively conservative estimate of the burdens of disease. Fifth, we were unable to test subgroups of antidiabetic drugs’ effects on the study outcomes, such as the subgroups of sulfonylureas, because the detail data was not available from NHIS-MESP Linkage File. Sixth, in multivariate analysis, we cannot always be able to control adequately for confounding factors. We may not even know about them and chance cannot be discarded although it is highly unlikely.
Despite the limitations discussed the above, three clear and important conclusions follow the present study. First, the prevalence of DM significantly increased in all age groups in the past decade, with specific increase in females aged 55-74 compared to males. Second, DM is a significant predictor for mortality from all-cause and CVD in both genders, with a slightly higher excess relative risk in females vs males. Third, about 10% of patients with DM do not receive antidiabetic therapy. DM patients who received metformin monotherapy or combination of metformin with other antidiabetic medications (except insulin) showed a significant protective effect on all-cause mortality.
The study used data from the National Health Interview Surveys (NHIS), NHIS Mortality Linked Files and Medical Expenditure Panel Surveys (MEPS) from the United States Centers for Disease Control and Prevention - National Center for Health Statistics (CDC-NCHS). Points of view however, are those of the authors and do not necessarily represent the position of the CDC-NCHS. The authors gratefully acknowledge the assistance of Miss Gabrielle Pyronneau for her careful proofreading and editing of the manuscript.
Diabetes mellitus (DM) is a leading cause of death in the United States. The present study examined the trend of DM using data from nationally representative surveys from 2000 to 2009. It is one of the first studies that address the epidemic of DM in the nation, and its serious impact on population health. Furthermore, findings from the study add new evidence of glucose-lowering treatment and health outcomes in patients with DM using data from the real world, instead of using a sample from very selective participants that are commonly applied in clinical trials.
The amount of data in the real world has been exploding, and analyzing large-scale data sets, so called Big data is becoming a key basis of competition and productivity in epidemiological studies of DM. The present study using a large-scale dataset from multiple sources not only addresses the epidemic of DM in the United States, but also advances the research methods to build a national cohort sample by taking the advantages of national health survey data at baseline linking health medication prescription and vital statistics for a decade time period. Findings from this approach provide a unique opportunity to address drug effects on health outcomes using data from real world.
The innovations of the study are characterized by its research design linking data from multiple sources, and building up a representative sample of national cohort study.
The study design adds new research approach to the body of study designs using data from population based studies. Findings from the study are very informative for counties that are experiencing an increasing trend of obesity and diabetes in the world.
Cross-sectional study design; Prospective study design; Linked Files.
This is a timely, interesting and informative report.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fu JF, Joven J, Ozdemir S, Rabkin SW, Sicari R, Tan XR S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Association AD. Statistics About Diabetes. [accessed 2014 Jan 15]. Available from: http://www.diabetes.org/diabetes-basics/statistics/. |
| 2. | Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9344] [Cited by in RCA: 8965] [Article Influence: 426.9] [Reference Citation Analysis (1)] |
| 3. | Liu L, Yin X, Morrissey S. Global variability in diabetes mellitus and its association with body weight and primary healthcare support in 49 low- and middle-income developing countries. Diabet Med. 2012;29:995-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Meetoo D, McGovern P, Safadi R. An epidemiological overview of diabetes across the world. Br J Nurs. 2007;16:1002-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570-2581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1841] [Cited by in RCA: 1826] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 6. | Berry C, Tardif JC, Bourassa MG. Coronary heart disease in patients with diabetes: part I: recent advances in prevention and noninvasive management. J Am Coll Cardiol. 2007;49:631-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215-2222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3857] [Cited by in RCA: 3461] [Article Influence: 230.7] [Reference Citation Analysis (0)] |
| 8. | Rutter MK, Meigs JB, Sullivan LM, D’Agostino RB, Wilson PW. Insulin resistance, the metabolic syndrome, and incident cardiovascular events in the Framingham Offspring Study. Diabetes. 2005;54:3252-3257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 217] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Leon BM, Maddox TM. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6:1246-1258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 587] [Cited by in RCA: 722] [Article Influence: 72.2] [Reference Citation Analysis (13)] |
| 10. | NCHS. Leading Causes of Death. [updated 2014 Jun 8]. Available from: http://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm. |
| 11. | Wong ND, Glovaci D, Wong K, Malik S, Franklin SS, Wygant G, Iloeje U. Global cardiovascular disease risk assessment in United States adults with diabetes. Diab Vasc Dis Res. 2012;9:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Centers for Disease Control and Prevention (CDC). Increasing prevalence of diagnosed diabetes--United States and Puerto Rico, 1995-2010. MMWR Morb Mortal Wkly Rep. 2012;61:918-921. [PubMed] |
| 13. | Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988-2012. JAMA. 2015;314:1021-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1462] [Cited by in RCA: 1461] [Article Influence: 146.1] [Reference Citation Analysis (0)] |
| 14. | NCHS-NHIS. National Health Interview Survey. Available from: http://www.cdc.gov/nchs/nhis.htm. |
| 15. | Liu L, Núnez AE, An Y, Liu H, Chen M, Ma J, Chou EY, Chen Z, Eisen HJ. Burden of Cardiovascular Disease among Multi-Racial and Ethnic Populations in the United States: an Update from the National Health Interview Surveys. Front Cardiovasc Med. 2014;1:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | AHRQ-MEPS. Medical Expenditure Panel Survey. Available from: http://meps.ahrq.gov/mepsweb/. |
| 17. | Butler J, Rodondi N, Zhu Y, Figaro K, Fazio S, Vaughan DE, Satterfield S, Newman AB, Goodpaster B, Bauer DC. Metabolic syndrome and the risk of cardiovascular disease in older adults. J Am Coll Cardiol. 2006;47:1595-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 143] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 18. | Best LE, Hayward MD, Hidajat MM. Life course pathways to adult-onset diabetes. Soc Biol. 2005;52:94-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Yamori Y, Liu L, Ikeda K, Mizushima S, Nara Y, Simpson FO. Different associations of blood pressure with 24-hour urinary sodium excretion among pre- and post-menopausal women. WHO Cardiovascular Diseases and Alimentary Comparison (WHO-CARDIAC) Study. J Hypertens. 2001;19:535-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 914] [Cited by in RCA: 933] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 21. | Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, Bassford T, Burke G, Torrens J, Howard BV. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia. 2004;47:1175-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 423] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 22. | Abbott RD, Donahue RP, Kannel WB, Wilson PW. The impact of diabetes on survival following myocardial infarction in men vs women. The Framingham Study. JAMA. 1988;260:3456-3460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 255] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035-2038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2337] [Cited by in RCA: 2165] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 24. | Kabadi SM, Lee BK, Liu L. Joint effects of obesity and vitamin D insufficiency on insulin resistance and type 2 diabetes: results from the NHANES 2001-2006. Diabetes Care. 2012;35:2048-2054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Liu L, Chen M, Hankins SR, Nùñez AE, Watson RA, Weinstock PJ, Newschaffer CJ, Eisen HJ. Serum 25-hydroxyvitamin D concentration and mortality from heart failure and cardiovascular disease, and premature mortality from all-cause in United States adults. Am J Cardiol. 2012;110:834-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Liu L. Joint Effects of Serum 25 (OH) D and C-Reactive Protein Concentration on Coronary Heart Disease and All-cause Mortality in Patients with Diabetes Mellitus. J Heart Health. 2015;1. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Liu L, Nettleton JA, Bertoni AG, Bluemke DA, Lima JA, Szklo M. Dietary pattern, the metabolic syndrome, and left ventricular mass and systolic function: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2009;90:362-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Sun J, Rangan P, Bhat SS, Liu L. A Meta-Analysis of the Association between Helicobacter pylori Infection and Risk of Coronary Heart Disease from Published Prospective Studies. Helicobacter. 2016;21:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5314] [Cited by in RCA: 5282] [Article Influence: 310.7] [Reference Citation Analysis (0)] |
| 30. | Batchuluun B, Sonoda N, Takayanagi R, Inoguchi T. The Cardiovascular Effects of Metformin: Conventional and New Insights. J Endocrinol Diabetes Obes. 2014;2:1035. |
| 31. | Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:550-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 758] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 32. | Lee A, Morley JE. Metformin decreases food consumption and induces weight loss in subjects with obesity with type II non-insulin-dependent diabetes. Obes Res. 1998;6:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 214] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 33. | DeCensi A, Puntoni M, Guerrieri-Gonzaga A, Cazzaniga M, Serrano D, Lazzeroni M, Vingiani A, Gentilini O, Petrera M, Viale G. Effect of Metformin on Breast Ductal Carcinoma In Situ Proliferation in a Randomized Presurgical Trial. Cancer Prev Res (Phila). 2015;8:888-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Menendez JA, Quirantes-Piné R, Rodríguez-Gallego E, Cufí S, Corominas-Faja B, Cuyàs E, Bosch-Barrera J, Martin-Castillo B, Segura-Carretero A, Joven J. Oncobiguanides: Paracelsus’ law and nonconventional routes for administering diabetobiguanides for cancer treatment. Oncotarget. 2014;5:2344-2348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Szklo M, Nieto FJ. Epidemiology Beyond the Basics. Sudbury, MA: Jones and Bartlett 2007; . |
| 36. | Glymour MM, Avendano M. Can self-reported strokes be used to study stroke incidence and risk factors?: evidence from the health and retirement study. Stroke. 2009;40:873-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 37. | Bush TL, Miller SR, Golden AL, Hale WE. Self-report and medical record report agreement of selected medical conditions in the elderly. Am J Public Health. 1989;79:1554-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 355] [Article Influence: 9.9] [Reference Citation Analysis (0)] |