Published online Aug 15, 2025. doi: 10.4251/wjgo.v17.i8.108887
Revised: May 27, 2025
Accepted: July 1, 2025
Published online: August 15, 2025
Processing time: 108 Days and 22.5 Hours
Acute variceal bleeding (AVB) represents a life-threatening complication in hepatocellular carcinoma (HCC) patients undergoing systemic therapy, mainly including immune checkpoint inhibitors (ICIs) and antivascular drugs used alone or in combination. The pathogenesis of AVB in this population may involve tumor-related factors, treatment-induced effects, or progression of underlying portal hypertension. Identifying high-risk factors for AVB is crucial for the management of this patient population.
To develop and validate a risk prediction model for AVB occurrence in cirrhotic HCC patients receiving ICI-based systemic therapy.
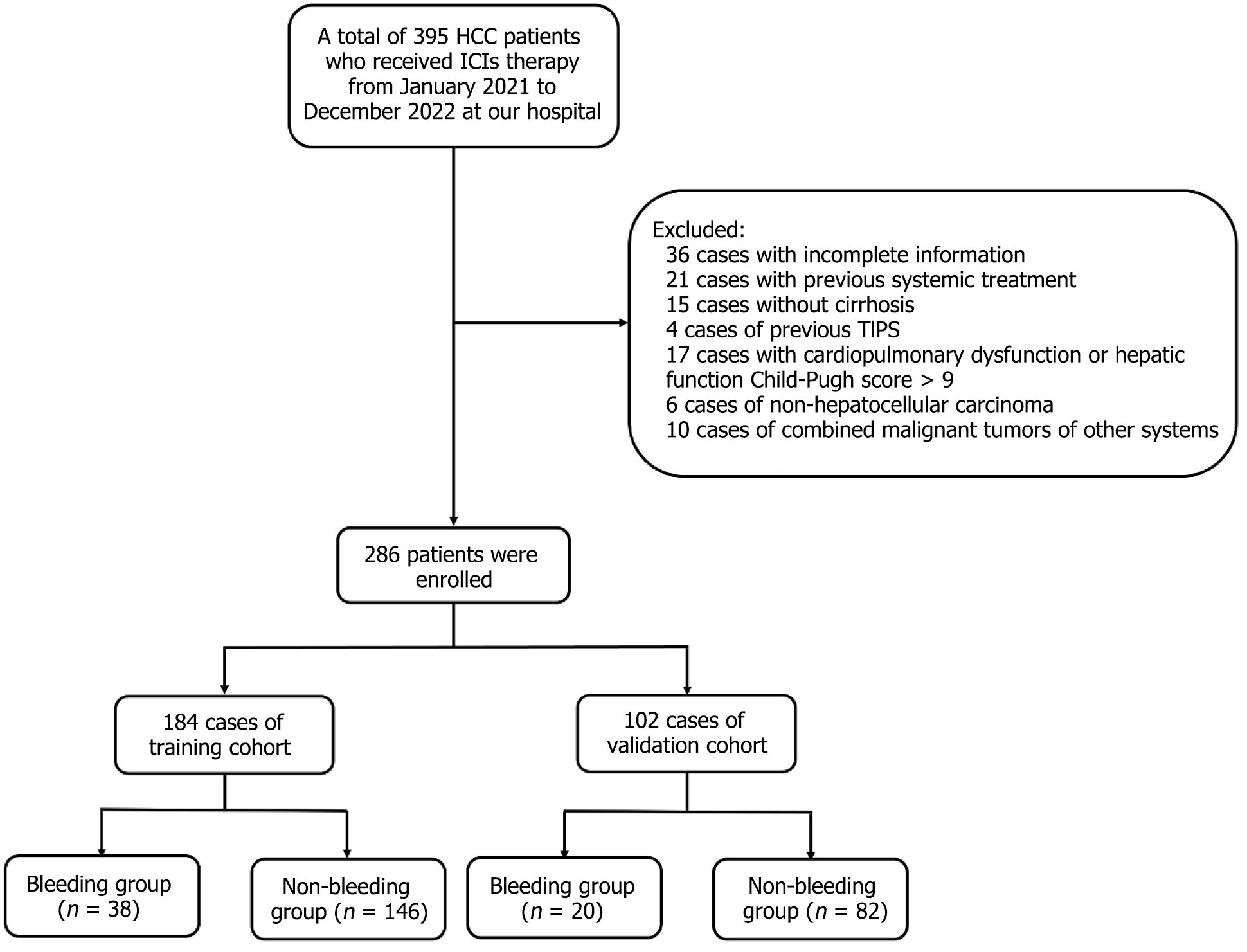
This retrospective study analyzed 286 HCC patients (2021-2022) receiving ICIs (mono-/combination therapy), randomly split into training (n = 184) and validation (n = 102) cohorts. In the training cohort, bleeding vs non-bleeding groups were compared for general information, etiological data, laboratory indicators, tumor staging, systemic treatment drugs, variceal bleeding history, and endoscopic treatment history. Risk factors for AVB were identified and used to establish a logistic regression model for predicting bleeding, which was further validated in the validation cohort.
The bleeding group had significantly higher proportions of patients with platelet count ≥ 100 × 109/L, alpha-fetoprotein ≥ 400 ng/mL, tumor diameter ≥ 5 cm, portal vein tumor thrombosis, ascites, bleeding history, prior endoscopic treatment, albumin-bilirubin grade level 2-3, fibrosis-4 index (FIB-4) ≥ 4.57, and prognostic nutritional index < 45 compared to the non-bleeding group. Multivariate analysis identified tumor diameter ≥ 5 cm, portal vein thrombosis, bleeding history, and elevated FIB-4 as independent risk factors for bleeding (P < 0.05). A predictive model based on these factors showed good discrimination, with area under the receiver operating characteristic curve values of 0.861 (training) and 0.816 (validation).
A history of pre-ICI bleeding significantly increases recurrent bleeding risk, necessitating close monitoring. The FIB-4 fibrosis model, combined with tumor features, can also serve as a predictive factor for bleeding.
Core Tip: Hepatocellular carcinoma patients with a history of variceal bleeding, tumor diameter ≥ 5 cm, portal vein tumor thrombosis, or elevated fibrosis-4 index (≥ 4.57) are at significantly increase the risk of recurrent variceal bleeding during immune checkpoint inhibitor (ICI)-based systemic therapy. Closely monitor these individuals for early signs of bleeding. Prior to initiating ICIs, consider endoscopic screening in high-risk patients.
- Citation: Zhang X, Song LM, Zheng YP, Qian BX, Liang J, Wang FM. Risk prediction of acute variceal bleeding in hepatocellular carcinoma patients undergoing systemic therapy based on immune checkpoint inhibitors. World J Gastrointest Oncol 2025; 17(8): 108887
- URL: https://www.wjgnet.com/1948-5204/full/v17/i8/108887.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i8.108887
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second leading cause of cancer-related mortality worldwide[1]. China bears the highest global burden of primary liver cancer, accounting for the greatest incidence and mortality rates worldwide. The majority of cases develop against a background of hepatic cirrhosis, with over two-thirds of HCC patients presenting at advanced stages when curative interventions are no longer feasible. Recent advances in immune checkpoint inhibitor (ICI)-based systemic therapies have significantly expanded the therapeutic landscape for HCC management. First-line agents such as atilizumab combined with bevacizumab, sorafenib, lenvatinib, and donafenib have shown significant benefits in objective remission rate, median survival time, and progression-free survival, marking a new therapeutic era in HCC[2]. However, it has been demonstrated that the use of systemic therapies increases the risk of acute variceal bleeding (AVB).
On one hand, ICIs may exacerbate underlying liver disease by inducing intrahepatic inflammatory responses, which could increase intrahepatic vascular resistance and portal pressure, thereby raising the risk of upper gastrointestinal hemorrhage. However, current clinical evidence remains limited, and the precise mechanistic pathways underlying this phenomenon require further elucidation. On the other hand, both bevacizumab and tyrosine kinase inhibitors (TKIs) target the vascular endothelial growth factor receptor, which may consequently modulate portal hemodynamics. The Imbrave150 phase II clinical trial showed that bevacizumab significantly increased the risk of bleeding, ranked as the second most common adverse reaction, following hypertension[3]. The impact of TKIs on bleeding risk remains controversial. A 2023 meta-analysis of randomized controlled trials demonstrated that sorafenib was associated with a 16.7% incidence of bleeding events (relative risk: 2.00, 95%CI: 1.14-3.29; P < 0.05) compared to control therapies[4]. In contrast, accumulating evidence from multiple clinical studies indicates that lenvatinib - whether as monotherapy or in combination with ICIs - does not significantly increase bleeding risk[5,6].
This study sought to: (1) Identify clinical predictors of AVB in patients undergoing ICIs-based systemic therapy; (2) Evaluate the differential bleeding risks associated with various therapeutic regimens; and (3) Establish an evidence-based framework to guide both treatment selection and clinical monitoring protocols.
This retrospective study included HCC patients hospitalized at the Third Central Hospital of Tianjin from January 2021 to December 2022 who received systemic treatment based on ICIs. All patients met the diagnostic criteria for HCC based on radiologic or histologic findings, according to the American Association for the Study of the Liver guidelines[7]. Tumor staging was based on the Barcelona Clinic Liver Cancer (BCLC) classification[8].
Inclusion criteria: (1) Diagnosis of HCC; and (2) Receiving at least one cycle of ICI therapy, with or without TKIs.
Exclusion criteria: (1) Non-HCC; (2) Concurrent malignant tumors of other systems; (3) Severe heart, lung, or liver disease (Child-Pugh C > 9) or kidney dysfunction; (4) Absence of cirrhosis; (5) History of transjugular intrahepatic portosystemic shunt; (6) History of previous systemic therapy; and (7) Incomplete data (36 cases): Number of tumours (missing in 15 cases, 3.8%), maximum tumor diameter (missing in 7 cases, 1.8%), lactate dehydrogenase (LDH) values (missing in 4 cases, 1.0%), antivascular combination therapy records (missing in 21 cases, 5.3%).
Data was collected from enrolled patients one week before the first ICI treatment. The following information was gathered: General information (gender, age, etiological factors, and presence of cirrhosis); laboratory data [blood count, liver function, kidney function, and alpha-fetoprotein (AFP)]; tumor characteristics (number of tumors, size, presence of vascular invasion, and distant metastases); portal hypertension (PHT) status (history of bleeding and endoscopic treatment).
Albumin (ALB)-bilirubin classification (ALBI) was calculated as: ALBI = [log10 bilirubin (mmol/L) × 0.66 + ALB (g/L) × -0.085].
Prognostic nutritional index (PNI) was calculated as: PNI = serum ALB (g/L) + 5 × lymphocyte count (× 109/L).
Patients were classified according to predetermined criteria. Due to a limited number of grade 3 patients (n = 8), grades 2 and 3 were combined for analysis.
Liver fibrosis-4 index (FIB-4) was calculated as: FIB-4 = [age × aspartate aminotransferase (AST)] ÷ [square root of platelets × alanine aminotransferase (ALT)].
We enrolled cirrhotic patients who characteristically exhibit elevated FIB-4 that may exceed conventional stratification thresholds, therefore, patients were categorized based on the median value.
The primary endpoint of this study was the development of variceal bleeding (presenting with hematemesis and/or melena, confirmed by endoscopy to be caused by esophageal or gastric variceal bleeding), and the secondary endpoint was patient death or last documented attendance at our institution. All enrolled patients were randomly assigned to a training cohort and a validation cohort.
ICIs include carilizumab 200 mg, tirilizumab 200 mg, atilizumab 1200 mg, and sindilizumab 200 mg, administered as a single fixed dose via intravenous injection every three weeks. Combination therapies included anti-angiogenic drugs such as bevacizumab 15 mg/kg (fixed dose, intravenous injection, every three weeks); lenvatinib mesylate 8 mg (body weight < 60 kg) or 12 mg (body weight ≥ 60 kg), taken orally once daily; sorafenib tosylate 400 mg, taken orally twice daily; and regorafenib 160 mg, taken orally once daily for the first 21 days of each treatment cycle (28 days per course).
Patients may also have received local therapies, including radiofrequency ablation, transarterial chemoembolization, or hepatic arterial infusion chemotherapy.
All statistical analyses were performed using SPSS 26.0 and R 4.1.2. Continuous variables were first assessed for normality using Shapiro-Wilk tests. Normally distributed data were expressed as mean ± SD and compared using independent samples t test. Non-normally distributed variables were summarized as median (interquartile range) and analyzed with Mann-Whitney U tests. Categorical variables were presented as frequencies (percentages), with between-group differences evaluated by χ² tests or Fisher's exact tests as appropriate. Restricted cubic splines (RCS) combined with logistic regression modeling was performed to investigate potential nonlinear associations between continuous variables and outcomes. Logistic regression was performed to identify factors affecting bleeding and to develop a predictive model. The final predictive model was visualized using a nomogram construction. The model’s performance was evaluated using receiver operating characteristic (ROC), calibration curves, and decision curve analysis (DCA). External validation was employed to assess the model’s prediction effectiveness. A P value of < 0.05 was considered statistically significant.
A total of 286 patients were included in this study. Of these, 223 were male and 63 were female, with ages ranging from 37 to 84 years, and a mean age of 62.34 ± 9.23 years. There were 54 cases in BCLC stage A, 81 cases in stage B, and 151 cases in stage C. ICIs were predominantly used with caririlizumab in 201 cases (70.3%), followed by tirilizumab in 60 cases (21.0%). A total of 167 patients (58.4%) received ICIs combined with TKIs, while 23 (8.0%) were treated with bevacizumab, and the remaining patients did not receive anti-angiogenic drugs. Variceal bleeding occurred in 58 patients (20.3%) during treatment. All patients were randomly assigned to a training cohort (184 patients) and a validation cohort (102 patients; Figure 1). No statistically significant differences were observed between the two groups (P > 0.05) in terms of gender, age, etiology, tumor BCLC stage, immunotherapeutic drugs, combination with anti-vascular drugs, or the number of bleeding events during treatment (Table 1).
| Clinical characteristics | Training cohort (n = 184) | Validation cohort (n = 102) | Z/χ2 | P value |
| Sex | 0.208 | 0.648 | ||
| Female | 39 (21.2) | 24 (23.5) | ||
| Male | 145 (78.8) | 78 (76.5) | ||
| Age (years) | 0.001 | 0.983 | ||
| < 60 | 76 (41.3) | 42 (41.2) | ||
| ≥ 60 | 108 (58.7) | 60 (58.8) | ||
| Etiology | 0.356 | 0.949 | ||
| Hepatitis B | 143 (77.7) | 78 (76.5) | ||
| Hepatitis C | 18 (9.8) | 9 (8.8) | ||
| Alcoholic liver disease | 13 (7.1) | 8 (7.8) | ||
| Other | 10 (5.4) | 7 (6.9) | ||
| BCLC staging | 1.526 | 0.466 | ||
| A | 32 (17.4) | 22 (21.6) | ||
| B | 50 (27.2) | 31 (30.4) | ||
| C | 102 (55.4) | 49 (48.0) | ||
| Immunotherapy drugs | 2.028 | 0.363 | ||
| Camrelizumab | 125 (67.9) | 76 (74.5) | ||
| Tislelizumab | 40 (21.7) | 20 (19.6) | ||
| Other | 19 (10.3) | 6 (5.9) | ||
| Combined antivascular drugs | 1.000 | 0.607 | ||
| TKIs | 106 (57.6) | 61 (59.8) | ||
| Bevacizumab | 17 (9.2) | 6 (5.9) | ||
| None | 61 (33.2) | 35 (34.3) | ||
| Bleeding events | 0.044 | 0.833 | ||
| Without | 146 (79.3) | 82 (80.4) | ||
| With | 38 (20.7) | 20 (19.6) | ||
| Bleeding time | 3.00 (1.00,10.00) | 4.00 (2.00,11.00) | -0.579 | 0.563 |
The training cohort consisted of 184 patients, among whom 38 experienced variceal bleeding events during treatment. A comparison of clinical characteristics between the bleeding and non-bleeding groups showed no statistically significant differences (P > 0.05) in terms of gender, age, etiology, immunotherapy drugs, use of anti-angiogenic drugs, combination with local therapy, TBIL, ALB, LDH, tumor number, presence of distant metastasis, BCLC stage, or Child-Pugh grade. However, The proportion of patients in the bleeding group with platelet count (PLT) ≥ 100 × 109, AFP ≥ 400 ng/mL, tumor diameter ≥ 5cm, combined portal vein tumor thrombus (PVTT), ascites, pre-treatment bleeding history, pre-treatment endoscopic treatment history, ALBI grades 2 and 3, and FIB-4 ≥ 4.57 was significantly higher than that in the non-bleeding group, while the proportion of patients with PNI ≥ 45 was significantly lower than that in the non-bleeding group. All differences were statistically significant (P < 0.05; Table 2). The RCS analysis for the relationship between the continuous variables ALBI, PLT, FIB-4, PNI, AFP and bleeding risk are detailed in Supplementary Figure 1.
| Clinical characteristics | Non-bleeding group (n = 146) | Bleeding group (n = 38) | t/Z/χ2 | P value |
| Sex | 0.001 | 0.981 | ||
| Female | 31 (21.2) | 8 (21.1) | ||
| Male | 115 (78.8) | 30 (78.9) | ||
| Age (years) | 0.013 | 0.910 | ||
| < 60 | 60 (41.1) | 16 (42.1) | ||
| ≥ 60 | 86 (58.9) | 22 (57.9) | ||
| Etiology | 4.141 | 0.233 | ||
| Hepatitis B | 110 (75.3) | 33 (86.6) | ||
| Hepatitis C | 15 (10.3) | 3 (7.9) | ||
| Alcoholic liver disease | 13 (8.9) | 0 (0.0) | ||
| Other | 8 (5.5) | 2 (5.3) | ||
| Immunotherapy drugs | 3.268 | 0.199 | ||
| Camrelizumab | 101 (69.2) | 24 (63.2) | ||
| Tislelizumab | 33 (22.6) | 7 (18.4) | ||
| Other | 12 (8.2) | 7 (18.4) | ||
| Combined antivascular drugs | 5.490 | 0.062 | ||
| TKIs | 90 (61.6) | 16 (42.1) | ||
| Bevacizumab | 11 (7.5) | 6 (15.8) | ||
| None | 45 (30.8) | 16 (42.1) | ||
| Combined local treatment | 0.917 | 0.338 | ||
| Without | 35 (24.0) | 12 (31.6) | ||
| With | 111 (76.0) | 26 (68.4) | ||
| TBIL (µmol/L) | 3.657 | 0.056 | ||
| < 20 | 83 (56.8) | 15 (39.5) | ||
| ≥ 20 | 63 (43.2) | 23 (60.5) | ||
| ALB (g/L) | 1.403 | 0.236 | ||
| ≥ 35 | 103 (70.5) | 23 (60.5) | ||
| < 35 | 43 (29.5) | 15 (39.5) | ||
| LDH (µ/L) | 3.344 | 0.067 | ||
| < 250 | 100 (68.5) | 20 (52.6) | ||
| ≥ 250 | 46 (31.5) | 18 (47.4) | ||
| PLT (× 109) | 3.889 | 0.049 | ||
| < 100 | 80 (54.8) | 14 (36.8) | ||
| ≥ 100 | 66 (45.2) | 24 (63.2) | ||
| AFP (ng/mL) | 4.748 | 0.029 | ||
| < 400 | 104 (71.2) | 20 (52.6) | ||
| ≥ 400 | 42 (28.8) | 18 (47.4) | ||
| Number of tumours, (%) | 0.020 | 0.887 | ||
| Solitary tumor | 44 (30.1) | 11 (28.9) | ||
| Multiple tumors | 102 (69.9) | 27 (71.1) | ||
| Maximum tumour diameter (cm) | 10.270 | 0.001 | ||
| < 5 | 99 (67.8) | 15 (39.5) | ||
| ≥ 5 | 47 (32.2) | 23 (60.5) | ||
| Combined portal vein tumor thrombosis | 46 (31.5) | 19 (50.0) | 4.514 | 0.034 |
| Combined distant metastases | 48 (32.9) | 9 (23.7) | 1.192 | 0.275 |
| BCLC staging | 2.080 | 0.353 | ||
| A | 27 (18.5) | 5 (13.2) | ||
| B | 42 (28.8) | 8 (21.1) | ||
| C | 77 (52.7) | 25 (65.8) | ||
| Combined ascites | 28 (19.2) | 15 (39.5) | 6.935 | 0.008 |
| History of pre-treatment bleeding | 10 (6.8) | 19 (50.0) | 42.286 | < 0.001 |
| History of endoscopy treatment | 21 (14.4) | 18 (47.4) | 19.640 | < 0.001 |
| Child-Pugh grade | 0.016 | 0.900 | ||
| A | 119 (81.5) | 30 (78.9) | ||
| B | 27 (18.5) | 8 (21.1) | ||
| ALBI grade | 8.218 | 0.004 | ||
| Grade 1 | 64 (43.8) | 7 (18.4) | ||
| Grades 2 and 3 | 82 (56.2) | 31 (81.6) | ||
| FIB-4 grade | 10.745 | 0.001 | ||
| < 4.57 | 82 (56.2) | 10 (26.3) | ||
| ≥ 4.57 | 64 (43.8) | 28 (73.7) | ||
| PNI grade | 13.804 | 0.001 | ||
| ≥ 45 | 62 (42.5) | 4 (10.5) | ||
| 40-44.9 | 37 (25.3) | 17 (44.7) | ||
| < 40 | 47 (32.2) | 17 (44.7) |
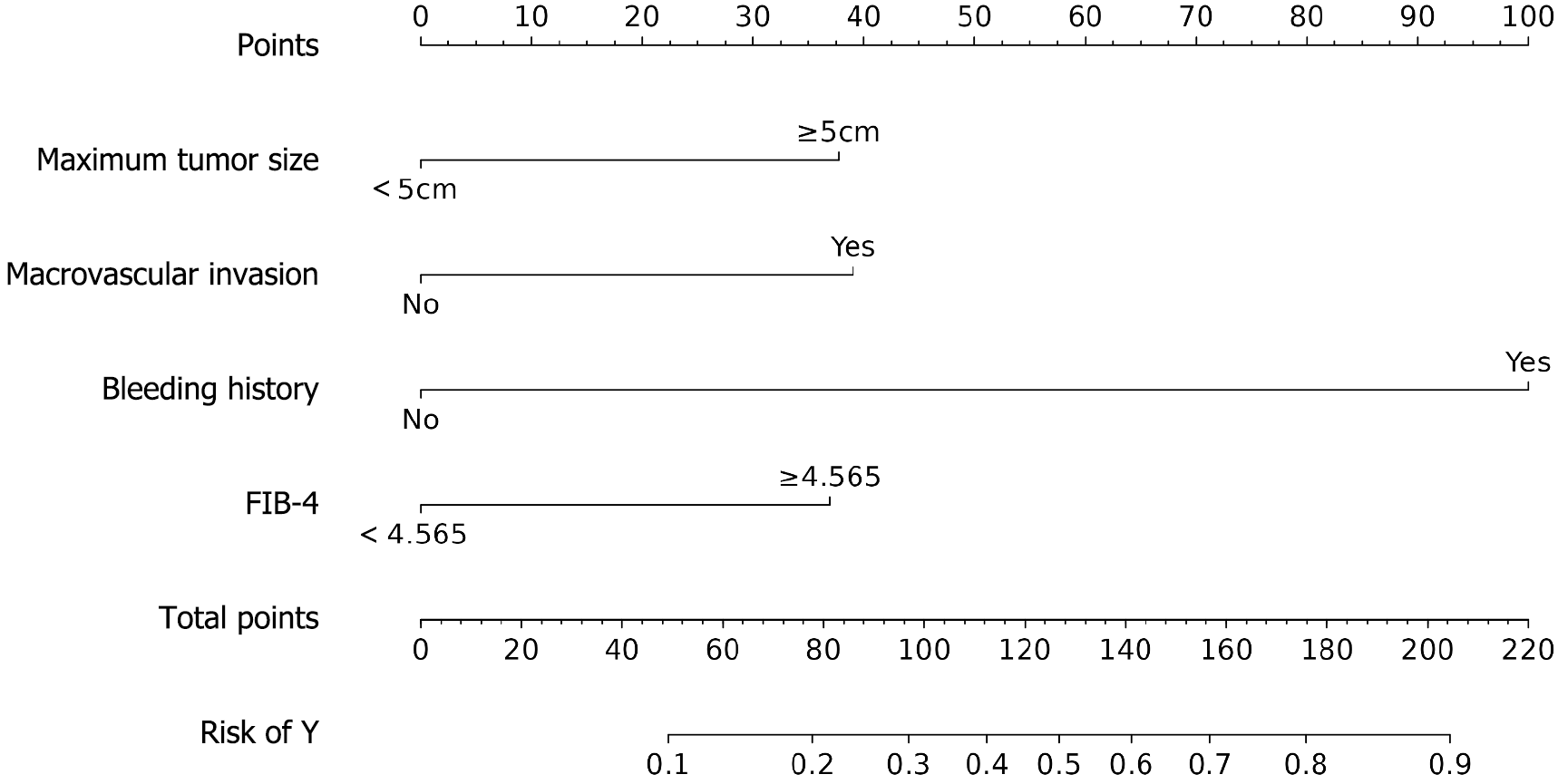
A binary logistic regression model was constructed, with bleeding status as the dependent variable and the following covariates: PLT, AFP, maximum tumor diameter, PVTT, ascites, pretreatment bleeding history, pretreatment endoscopic therapy, ALBI grade, FIB-4 grade, and PNI grade. The analysis revealed that a maximum tumor diameter ≥ 5 cm, PVTT, pretreatment bleeding history, and high FIB-4 grade were independent risk factors for bleeding (all P < 0.05). Patients with a maximum tumor diameter ≥ 5 cm had a 2.908-fold increased risk of bleeding compared to those with smaller tumors. Similarly, the presence of PVTT was associated with a 3.017-fold higher bleeding risk. Notably, a history of pretreatment bleeding conferred the strongest risk, with an odds ratio of 16.923 (95%CI: 5.951–48.123). Additionally, an elevated FIB-4 grade (≥ 4.57) was linked to a 2.841-fold increased bleeding risk (Table 3; Figure 2).
| Factor | B | SE | Wald χ2 | P value | OR (95%CI) |
| Maximum tumor diameter ≥ 5 cm | 1.067 | 0.466 | 5.248 | 0.022 | 2.908 (1.167-7.246) |
| Combined portal vein tumor thrombosis | 1.104 | 0.472 | 5.465 | 0.019 | 3.017 (1.195-7.616) |
| History of pre-treatment bleeding | 2.829 | 0.533 | 28.143 | 0.000 | 16.923 (5.951-48.123) |
| FIB-4 ≥ 4.57 | 1.044 | 0.468 | 4.973 | 0.026 | 2.841 (1.135-7.115) |
| Constant | -3.587 | 0.530 | 45.817 | 0.000 | - |
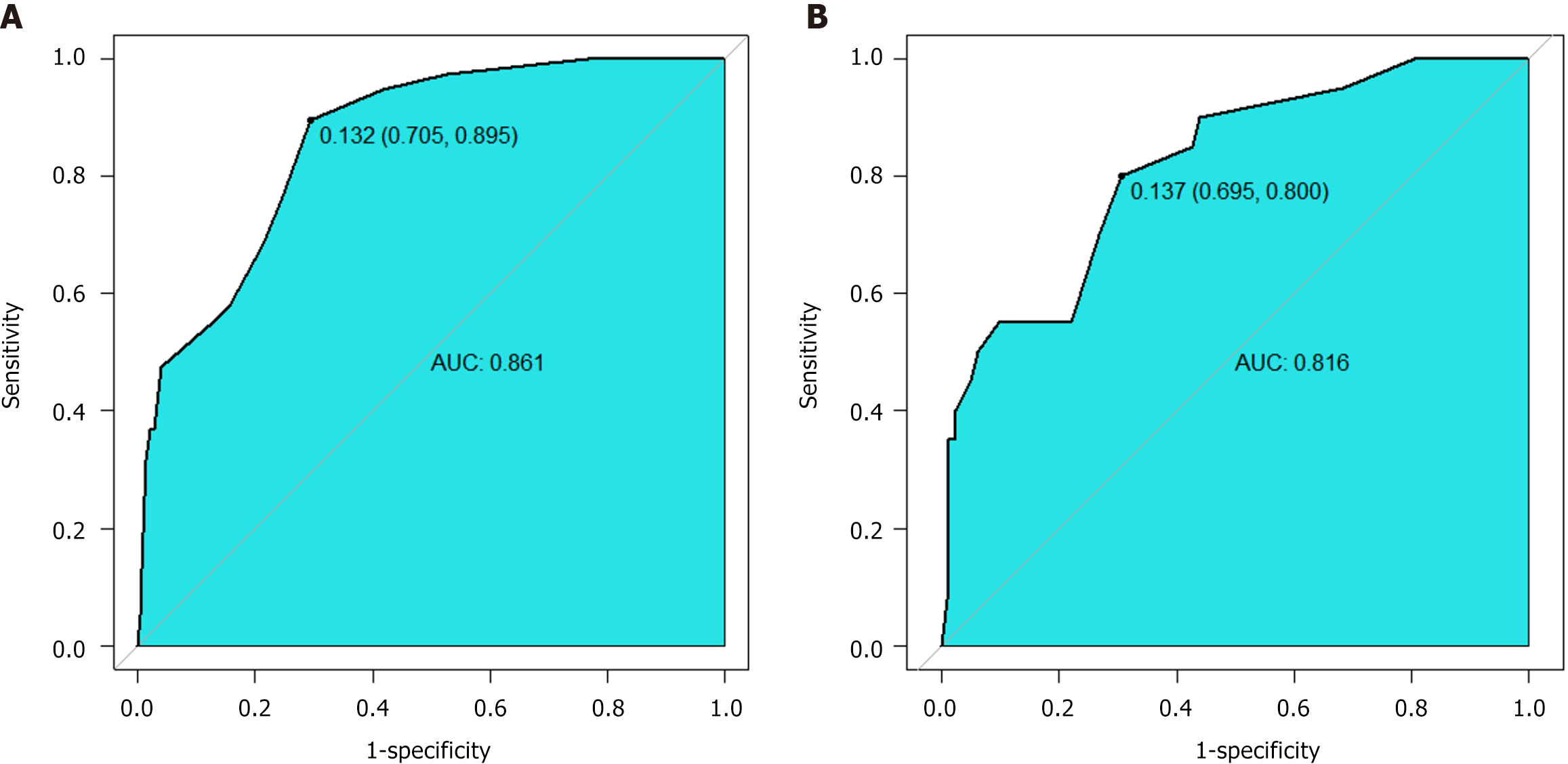
The joint probability was calculated using the established logistic regression model, and the ROC curve was plotted. The area under the curve (AUC) for the training cohort was 0.861 (95%CI: 0.802-0.920). At the optimal cutoff value of 0.132, the model demonstrated 70.5% sensitivity and 89.5% specificity, indicating that the model has good predictive value for bleeding. The AUC for the validation cohort was 0.816 (95%CI: 0.714-0.919), suggesting that the model also discriminated well in the validation population (Figure 3).
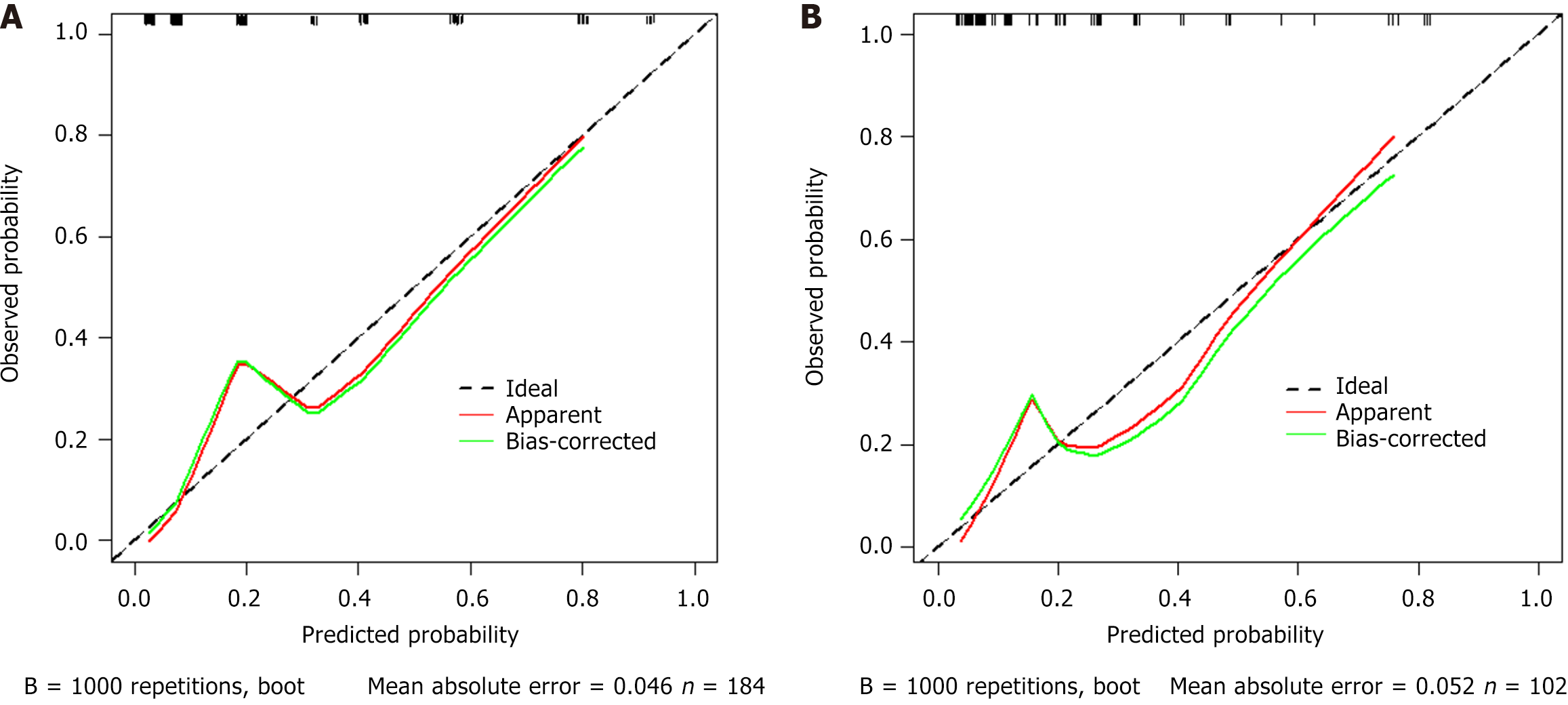
Internal validation with 1000 bootstrap replicates showed good model fit (Hosmer-Lemeshow P = 0.067) and discrimination (C-statistic 0.861). External validation maintained calibration stability (P = 0.161), indicating excellent calibration performance and readiness for clinical implementation (Figure 4).
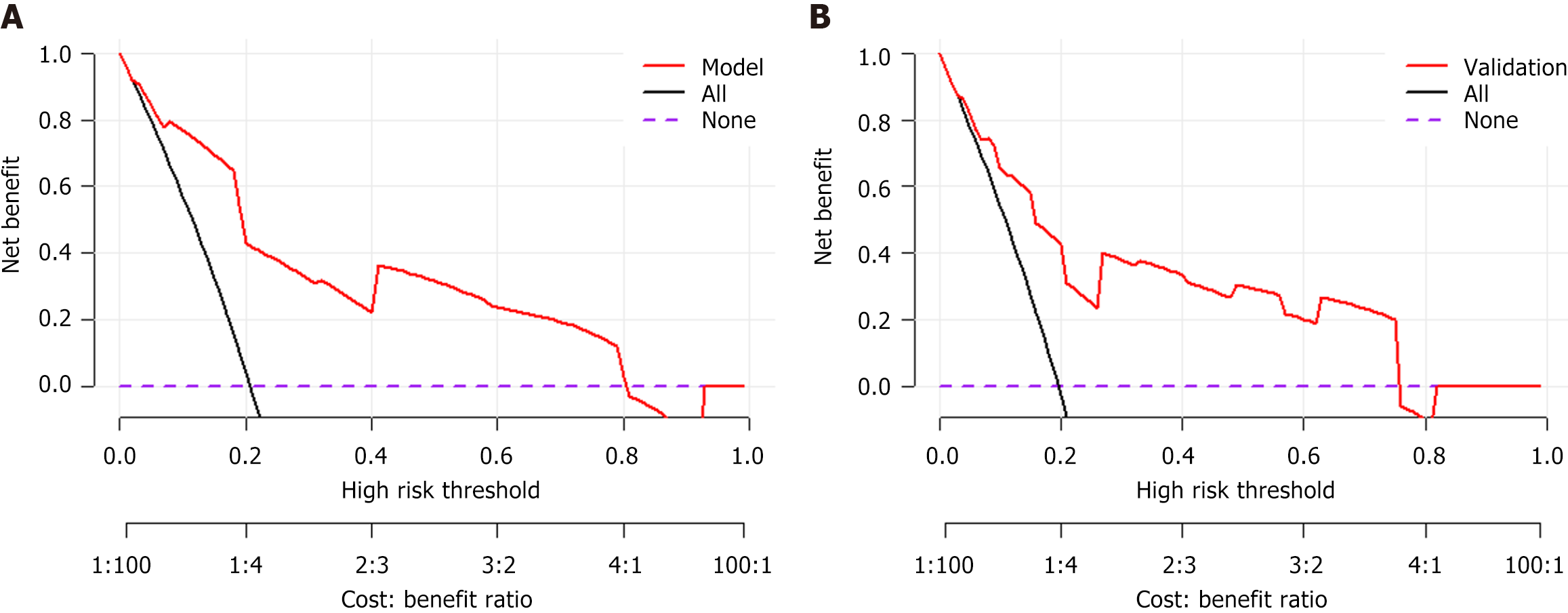
DCA was performed to evaluate clinical utility by quantifying net benefit across threshold probabilities (0%-100%). The 'None' strategy (all patients are classified as negative) and 'All' strategy (all patients are classified as positive) served as references. The DCA demonstrated clinical utility across threshold probabilities 0%-80%, with net benefit superiority over 'treat none' strategy (reference line at y = 0; Figure 5). The DCA results confirm the model's clinical utility, with superior net benefit compared to alternative strategies.
PHT and HCC represent two major complications of cirrhosis that profoundly impact patient survival and prognosis. These conditions are pathophysiologically interrelated: HCC elevates portal pressure through both arteriovenous shunt formation and progressive architectural distortion of the liver parenchyma. Furthermore, tumor invasion into the portal vein and its tributaries directly exacerbates PHT. Clinical studies have consistently identified HCC as an independent predictor of adverse outcomes in PHT-related upper gastrointestinal bleeding[9]. In HCC patients with PHT-related variceal rupture and bleeding, the necessity to interrupt or discontinue anticancer therapy may adversely impact overall survival. With the expanding application of systemic therapies in clinical practice, the incidence of treatment-associated bleeding events is anticipated to rise[10]. Consequently, a thorough pretherapeutic evaluation of upper gastrointestinal bleeding risk is essential to optimize clinical management and therapeutic interventions in this patient population. This prospective study systematically evaluated patients receiving systemic therapy for HCC to identify predictive factors for variceal hemorrhage. Our comprehensive analysis incorporated multiple clinical dimensions including: Hepatic functional reserve, degree of liver fibrosis, nutritional parameters, systemic inflammatory markers, tumor characteristics, therapeutic regimens, and prior PHT management. These findings provide clinically actionable insights to guide risk stratification and therapeutic decision-making in this high-risk population.
Multivariable analysis revealed four independent predictors of variceal hemorrhage in our cohort: Large tumor burden (maximum diameter ≥ 5 cm), pretreatment bleeding history, and advanced hepatic fibrosis (FIB-4 ≥ 4.57). Most notably, pretreatment bleeding history emerged as the most prominent independent risk factor, conferring a 16.923-fold increased risk (95%CI: 5.951-48.123) of variceal hemorrhage during systemic therapy compared to patients without prior bleeding episodes.
Larrey et al[11] investigated risk factors for AVB during atezolizumab-bevacizumab combination therapy. Their analysis similarly identified prior AVB history as a significant predictor of bleeding events, reinforcing that previous AVB episodes substantially increase hemorrhagic risk during anti-VEGF therapy. Our cohort included a limited subset of patients receiving bevacizumab (n = 23, 8.0%), yet the findings robustly demonstrate that prior AVB history serves as not only a specific risk enhancer for bevacizumab-associated hemorrhage, but also a general prognostic marker for bleeding risk across all systemic therapies.
Furthermore, HCC lesions exceeding 5 cm in maximal diameter and those with PVTT constitute significant high-risk features for AVB development. While the association between tumor burden and AVB risk is clinically recognized, the quantitative relationship between specific tumor size thresholds and bleeding risk remains under characterized in existing literature. The current findings demonstrate that HCC patients with tumor diameters ≥ 5 cm exhibit significantly elevated risks of AVB. This association may be mechanistically explained by mass effect-induced vascular compression, where larger tumor volumes potentially distort intrahepatic vasculature architecture, thereby exacerbating PHT and predisposing to AVB. Nevertheless, this pathophysiological hypothesis requires validation through dedicated mechanistic studies.
HCC patients with PVTT demonstrate significantly higher incidence rates of AVB compared to those without vascular invasion, primarily attributable to hemodynamic alterations secondary to portal venous obstruction. This clinical observation was corroborated by Lim et al[12] in a propensity-matched cohort analysis of 1709 HCC patients (with vs without PVTT), with findings that align precisely with our current results.
The FIB-4 index serves as a clinically validated, non-invasive tool for evaluating hepatic fibrosis in chronic liver disease. This composite score integrates routinely measured parameters, including age, ALT, AST, and platelet count, providing both practical accessibility and superior diagnostic accuracy compared to alternative non-invasive fibrosis assessment models[13]. Extensive clinical evidence demonstrates that the FIB-4 index exhibits superior predictive performance for both variceal presence and bleeding risk in cirrhosis compared to conventional non-invasive fibrosis models, including: Simple biochemical ratios (AST/ALT), serum index-based models (AST-to-platelet ratio index, FIB-4, King's score), imaging-incorporated parameters (PC/SD ratio)[14]. Using the median FIB-4 index of 4.57 as the cutoff, we stratified patients into high- and low-risk cohorts. The high FIB-4 group demonstrated a significantly elevated bleeding risk (OR: 2.841, 95%CI: 1.135-7.115; P < 0.05) compared to the low-risk group. These findings validate FIB-4 as a reliable noninvasive indicator of PHT severity. The FIB-4 serves as a clinically valuable predictor of AVB risk in HCC patients receiving systemic therapy, offering an efficient screening tool for identifying high-risk individuals in routine practice.
This study further evaluated the impact of combining bevacizumab or TKIs with ICIs on AVB risk. Existing evidence indicates that bevacizumab potentiates bleeding risk by impairing mucosal repair and aggravating PHT[15-17]. In our training cohort (n = 184), 17 patients received ICI-bevacizumab combination therapy, with a higher-though not statistically significant-proportion of bevacizumab use observed in the bleeding group (6/38, 15.8%) vs the non-bleeding group (11/146, 7.5%; P = 0.12). While this trend aligns with the drug’s known bleeding risk profile, the limited sample size and preemptive exclusion of high-risk patients (e.g., those with moderate-severe varices) may have attenuated the observed effect. Notably, TKI-ICI combinations demonstrated no significant association with AVB risk, corroborating prior reports 5-6. These findings support the judicious use of targeted therapies in HCC patients with PHT, particularly favoring TKIs in those with significant variceal risk.
This investigation systematically evaluated HCC patients with cirrhosis undergoing ICI therapy to identify predictors of AVB. While existing evidence remains limited and inconclusive, our findings offer clinically actionable insights that may enhance risk stratification and therapeutic decision-making for this high-risk population. Nevertheless, several study limitations warrant consideration. First, the limited cohort size receiving bevacizumab combination therapy (n = 23, 8.0%) may constrain accurate risk quantification for bevacizumab-associated AVB. Second, as a single-center retrospective analysis with 77.3% HBV-positive patients, our findings may have limited generalizability to populations where HCV infection (predominant in Western countries) or alcohol-related liver disease drive HCC pathogenesis. External validation in multicenter, ethnically diverse cohorts is needed to verify these observations.
This study definitively established that four key clinical factors: Prior variceal hemorrhage, PVTT, tumor diameter ≥ 5 cm, and elevated FIB-4 are significantly associated with AVB risk during ICI therapy for HCC. These parameters enable robust risk stratification to guide both therapeutic decision-making and proactive surveillance during systemic treatment.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55853] [Article Influence: 7979.0] [Reference Citation Analysis (132)] |
| 2. | Dong Y, Wong JSL, Sugimura R, Lam KO, Li B, Kwok GGW, Leung R, Chiu JWY, Cheung TT, Yau T. Recent Advances and Future Prospects in Immune Checkpoint (ICI)-Based Combination Therapy for Advanced HCC. Cancers (Basel). 2021;13:1949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Ma N, Nicholas A, Wang Y, Li L, Zhu AX, Finn RS. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 963] [Article Influence: 321.0] [Reference Citation Analysis (0)] |
| 4. | Chen Y, Ma X, Zhang X, Luo J, An L, Zhang Y, Chang X, Dong Z, Zhang W, Kong H, Zhao J, Ding H, Liu F, Yang Y. Prevention of variceal rebleeding in cirrhotic patients with advanced hepatocellular carcinoma receiving molecularly targeted therapy: a randomized pilot study of transjugular intrahepatic portosystemic shunt versus endoscopic plus β-blocker. Hepatol Int. 2022;16:1379-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Harding JJ, Merle P, Rosmorduc O, Wyrwicz L, Schott E, Choo SP, Kelley RK, Sieghart W, Assenat E, Zaucha R, Furuse J, Abou-Alfa GK, El-Khoueiry AB, Melero I, Begic D, Chen G, Neely J, Wisniewski T, Tschaika M, Sangro B. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 746] [Article Influence: 186.5] [Reference Citation Analysis (0)] |
| 6. | Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Mody K, Llovet JM. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol. 2020;38:2960-2970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 661] [Cited by in RCA: 877] [Article Influence: 175.4] [Reference Citation Analysis (0)] |
| 7. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3031] [Article Influence: 433.0] [Reference Citation Analysis (3)] |
| 8. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2876] [Article Influence: 110.6] [Reference Citation Analysis (1)] |
| 9. | Lee YR, Park SY, Tak WY. Treatment Outcomes and Prognostic Factors of Acute Variceal Bleeding in Patients with Hepatocellular Carcinoma. Gut Liver. 2020;14:500-508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Thabut D, Kudo M. Treatment of portal hypertension in patients with HCC in the era of Baveno VII. J Hepatol. 2023;78:658-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 11. | Larrey E, Campion B, Evain M, Sultanik P, Blaise L, Giudicelli H, Wagner M, Cluzel P, Rudler M, Ganne-Carrié N, Thabut D, Allaire M. A history of variceal bleeding is associated with further bleeding under atezolizumab-bevacizumab in patients with HCC. Liver Int. 2022;42:2843-2854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Lim J, Kim HI, Kim E, Kim J, An J, Chang S, Kim SO, Lee HC, Lee YS, Shim JH. Variceal bleeding is aggravated by portal venous invasion of hepatocellular carcinoma: a matched nested case-control study. BMC Cancer. 2021;21:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Dong XQ, Wu Z, Zhao H, Wang GQ; China HepB-Related Fibrosis Assessment Research Group. Evaluation and comparison of thirty noninvasive models for diagnosing liver fibrosis in chinese hepatitis B patients. J Viral Hepat. 2019;26:297-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Kraja B, Mone I, Akshija I, Koçollari A, Prifti S, Burazeri G. Predictors of esophageal varices and first variceal bleeding in liver cirrhosis patients. World J Gastroenterol. 2017;23:4806-4814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4708] [Article Influence: 941.6] [Reference Citation Analysis (2)] |
| 16. | Boige V, Malka D, Bourredjem A, Dromain C, Baey C, Jacques N, Pignon JP, Vimond N, Bouvet-Forteau N, De Baere T, Ducreux M, Farace F. Efficacy, safety, and biomarkers of single-agent bevacizumab therapy in patients with advanced hepatocellular carcinoma. Oncologist. 2012;17:1063-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 17. | Siegel AB, Cohen EI, Ocean A, Lehrer D, Goldenberg A, Knox JJ, Chen H, Clark-Garvey S, Weinberg A, Mandeli J, Christos P, Mazumdar M, Popa E, Brown RS Jr, Rafii S, Schwartz JD. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26:2992-2998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 396] [Article Influence: 23.3] [Reference Citation Analysis (0)] |