Published online Aug 15, 2025. doi: 10.4251/wjgo.v17.i8.106781
Revised: May 8, 2025
Accepted: June 20, 2025
Published online: August 15, 2025
Processing time: 127 Days and 20.8 Hours
Gastric cancer (GC) has a high prevalence and mortality overall. GEN1 is asso
To explore the cellular processes associated with GC will help to elucidate the mechanism of the occurrence and development of GC and discover potential therapeutic targets.
The detection of GEN1 expression at mRNA and protein levels was done by real-time quantitative polymerase chain reaction and western blotting. The function of GEN1 was verified by loss-of-function experiments in AGS cells. The genes co-expressed with GEN1 were searched from the stomach adenocarcinomas (STAD) data in The Cancer Genome Atlas database. Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of the genes co-expressed with GEN1 to further identify the pathways involved in GEN1. Rescue experiments using ferroptosis inhibitor ferrostatin-1 and chemotherapeutic sensitivity assays with cisplatin were also performed.
Significant up-regulation of GEN1 was observed in GC cell lines AGS and MGC-803. Inhibition of GEN1 induced cell apoptosis and decreased cell proliferation, cycle progression, migration in AGS cells. There were 264 genes co-expressed with GEN1 in STAD cohort (r > 0.4, P < 0.001). KEGG enrichment analysis showed that GEN1 might be associated with the cell cycle, Fanconi anemia pathway, homologous recombination, oocyte meiosis and cellular senescence in GC. Furthermore, CCNA2, CCNB1, CCNB2, cyclin-dependent kinase (CDK) 1, CDK2 and polo-like kinase 1 protein levels were lower in GEN1-knockdown AGS cells, manifesting that GEN1 was associated with the cell cycle pathway in AGS cells. Downregulation of GEN1 decreased adenosine triphosphate content and elevated reactive oxygen species in AGS cells, suggesting that GEN1 silencing led to mitochondrial dysfunction in AGS cells. In addition, GEN1 silencing caused an overt decrease in FTH1 and GPX4 protein levels and a significant elevation in ACSL4 protein levels, implying that GEN1 silencing promoted AGS cell ferroptosis. Treatment with ferrostatin-1 rescued cell viability loss induced by GEN1 knockdown, confirming ferroptosis as a key death mechanism. Additionally, GEN1-deficient AGS cells showed enhanced sensitivity to cisplatin, with a significantly reduced half-maximal inhibitory concentration compared to control cells.
GEN1 promotes GC cell proliferation and migration while suppressing apoptosis and ferroptosis. Targeting GEN1 not only disrupts mitochondrial function and cell cycle progression but also sensitizes GC cells to ferroptosis and chemotherapy. These findings highlight GEN1 as a potential therapeutic target for enhancing treatment efficacy in gastric cancer.
Core Tip: GEN1 promotes gastric cancer (GC) cell proliferation and migration while suppressing apoptosis and ferroptosis. Targeting GEN1 not only disrupts mitochondrial function and cell cycle progression but also sensitizes GC cells to ferroptosis and chemotherapy. These findings highlight GEN1 as a potential therapeutic target for enhancing treatment efficacy in GC.
- Citation: Zhang Q, Yuan ZG, Zheng KF, Chen K. GEN1 regulates cell proliferation, migration, apoptosis and ferroptosis in gastric cancer. World J Gastrointest Oncol 2025; 17(8): 106781
- URL: https://www.wjgnet.com/1948-5204/full/v17/i8/106781.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i8.106781
Gastric cancer (GC) is an aggressive gastrointestinal tumor and the fourth leading cause of global cancer mortality[1]. The main treatments for GC include gastrectomy, targeted immunotherapy, radiotherapy and chemotherapy, and the prognosis of the tumor is closely related to the stage at which it is diagnosed[2,3]. The morphological, genetic and molecular heterogeneity of GC can influence treatment decisions, and 5-year survival rates in early-stage patients exceed 90%, but the prognosis in late-stage patients is poor[4]. This cancer is caused by specific changes in genes that affect the ability of cells to grow and divide[5]. Therefore, exploring the cellular processes associated with GC will help to elucidate the mechanism of the occurrence and development of GC and discover potential therapeutic targets.
Intermediates called Holliday junctions (HJs) form during meiosis and homologous DNA repair. The prerequisite for proper separation and replication of chromosomes is that HJs are disassembled[6,7]. In 1991, Escherichia coli ruvC was found to be the bacterial “HJs” dissociating enzyme[8,9]. The enzyme in yeast is called Yen 1. GEN1, the direct homologue of Yen 1, is this enzyme in humans[10,11]. Both Yen 1 and GEN1 belong to the Rad2/Xeroderma pigmentosum group G (XPG) family of structure-specific nucleases. Notably, GEN1 collaborates with key DNA repair proteins such as BRCA1/BRCA2 and RAD51 to ensure faithful homologous recombination repair, and its dysfunction may synergize with defects in these pathways to exacerbate genomic instability[12,13]. GEN1 is essential for maintaining centrosome integrity; its loss triggers abnormal centrosome amplification, DNA damage, and apoptosis[14]. Pharmacological inhibition of GEN1 enhances the susceptibility of SKBR3 breast cancer cells to 5-fluorouracil, underscoring its therapeutic potential[15].
Emerging evidence implicates GEN1 overexpression in multiple cancers, including colorectal and breast malignancies, where it correlates with aggressive phenotypes and chemoresistance[16,17]. However, its role in GC remains unexplored. Given the reliance of cancer cells on DNA repair pathways for survival, targeting GEN1 may exploit a critical vulnerability in GC.
Here, we investigated the functional role of GEN1 in GC progression. We validated its overexpression in GC cell lines and demonstrated that GEN1 silencing suppresses proliferation, migration, and cell cycle progression while inducing apoptosis and ferroptosis in AGS cells. Bioinformatics analysis of The Cancer Genome Atlas (TCGA)-stomach adenocarcinomas (STAD) data revealed that GEN1-associated genes are enriched in cell cycle regulation, Fanconi anemia, and homologous recombination pathways. Mechanistically, GEN1 knockdown downregulated cyclins (CCNA2, CCNB1/CCNB2), cyclin-dependent kinase (CDK) (CDK1/2), and polo-like kinase 1 (PLK1), implicating GEN1 in cell cycle control. Our findings establish GEN1 as a pro-tumorigenic factor in GC and highlight its dual role in DNA repair and redox homeostasis.
Gene expression profiling interactive analysis 2.0 (http://gepia2.cancer-pku.cn/#index), including TCGA and GTEx databases, was used to detect GEN1 expression in STAD.
Three GC cell lines BGC-823 (CBP60477), AGS (CBP60476) and MGC-803 (CBP60485) were cultured in Roswell Park Memorial Institute-1640 medium (Thermo Fisher, United States) supplemented with 10% fetal bovine serum (FBS) (Thermo Fisher). The human gastric epithelial cell line-1 (GES-1) (CBP60512) was cultured in Dulbecco’s Modified Eagle’s Medium supplemented with 10% FBS (Ausbian, United States). All cell lines were bought from Cobioer biosciences co., ltd (Nanjing, Jiangsu Province, China) and cultured under air containing 5% carbon dioxide and in an incubator at a temperature set at 37 °C.
According to the RNA interference sequence design principle, the GEN1 gene was used as a template to design and select three 19-21nt RNA interference target sequences [short hairpin (sh) GEN1-1: 5’-TGCGTAATCTTGGTGGGAAA-3’; shGEN1-2: 5’-AACGTATTAAGCCTAAAGAAA-3’; shGEN1-3: 5’-TGGTAAAGACCTGCAATGTTA-3’] to knock down GEN1. ShRNA interference sequences were designed according to the selected target sequences and cloned into the BR-V108 lentivirus vector following the instructions for fermentas T4 DNA ligase. After the lentivirus was packaged, AGS cells were transfected with 400 μL of lentivirus (1 × 107 TU/mL) using lipofectamine 2000 transfection reagent (Thermo Fisher). After 72 hours of culture, the cell infection efficiency was observed by fluorescence microscope.
Total RNA extraction from collected cells was performed according to the operating instructions for TRIzol reagents (Sigma, United States). The concentration and quality of the extracted RNA were determined by Nanodrop 100 spectrophotometer (2000/2000C, Thermo Fisher). RNA reverse transcription was performed to obtain complementary DNA according to the operating instructions that come with Hiscript QRT supermix Hiscript QRT supermix for quantitative polymerase chain reaction (qPCR) (+ gDNA WIPER) (R123-01, Vazyme). The PCR reactions were carried out by utilizing an AceQ qPCR SYBR Green master mix (Q111-02, Vazyme) and run on the real-time PCR system (ViiA™ 7) made by Applied Biosystems (United States). The comparative cycle threshold 2-ΔΔCT approach was exploited to calculate the relative quantitative, and the results were normalized to the values of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Primers for GAPDH (forward primer: 5’-TGACTTCAACAGCGACACCCA-3’; reverse primer: 5’-CACCCTGTTGCTGTAGCCAAA-3’) and GEN1 (forward primer: 5’-CATTGTTCCGTATGTTCC-3’; reverse primer: 5’-TTCACTGAGTTGCCTATC-3’) were used.
The collected cells were rinsed with phosphate-buffered saline (PBS) solution. Total proteins were extracted by radioimmunoprecipitation assay lysis buffer (Sigma), followed by the determination of protein concentrations by the bicinchoninic acid assay protein assay kit (23225, HyClone-Pierce, United States). Equal amounts of the proteins (20 μg) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrophoretically transferred to a polyvinylidene difluoride membrane. The membranes were blocked with 5% skim milk at room temperature and incubated overnight at 4 °C with primary antibodies against GEN1 (ab198989, 1:3000, Abcam, United States), CCNA2 (ab181591, 1:2000, Abcam), CCNB1 (ab32053, 1:5000, Abcam), CCNB2 (ab185622, 1:1000, Abcam), CDK1 (ab133327, 1:5000, Abcam), CDK2 (ab32147, 1:2000, Abcam), PLK1 (ab17056, 1:2000, Abcam), FTH1 (ab75972, 1:2000, Abcam), GPX4 (ab125066, 1:2000, Abcam), ACSL4 (ab155282, 1:2000, Abcam), and GAPDH (AP0063, 1:3000, Bioworld, United States). After incubation at room temperature with rabbit anti-IgG secondary antibody (A0208, 1:3000, Affinity, United States), western blots were developed using an enhanced chemiluminescence-plus chemiluminescence test kit as per the manufacturer’s protocol.
Cells in the logarithmic growth phase were digested by trypsin and made into cell suspension using the complete medium. The cells were inoculated in 96-well plates (Corning, United States) at a density of 2000 cells per well and 10 μL of cell counting kit-8 (CCK-8) reagent (Sigma) was added within 2 hours before the end of culture. 4 hours later, 96-well plates were placed on the oscillator and oscillated for 2-5 minutes. A microplate reader (Tecan infinite, Switzerland) was used to detect the optical density value at 450 nm.
To confirm the involvement of ferroptosis in GEN1 knockdown-induced cell death, AGS cells transfected with shCtrl or shGEN1 were pretreated with 1 μM ferrostatin-1 (Selleckchem, United States) for 2 hours before cell viability measurement. Three experimental groups were included: ShCtrl: Negative control with scrambled shRNA, shGEN1: AGS cells transfected with shGEN1, shGEN1 + ferrostatin-1: ShGEN1-transfected cells treated with ferrostatin-1. The CCK-8 assay was performed as described previously, with absorbance measured at 450 nm after 48 hours of treatment.
To evaluate the chemosensitization effect of GEN1 knockdown, AGS cells transfected with shCtrl or shGEN1 were treated with cisplatin (Sigma-Aldrich, United States) at concentrations of 0, 2, 5, and 10 μM for 48 hours. Cell viability was determined by CCK-8 assay, and the half-maximal inhibitory concentration (IC50) of cisplatin was calculated using GraphPad Prism 8.0 [nonlinear regression analysis, log (inhibitor) vs normalized response model].
When the cells grew to about 80% coverage, the cells were digested with trypsin and collected. Cells were washed with PBS (potential of hydrogen = 7.2-7.4) precooled at 4 °C and then fixed with precooled 70% ethanol. One hour later, the cells were washed and they were stained with the cell stain staining containing RNase. Flow cytometry was used to detect the cells, with a cell passing rate of 200-350 cell/second.
Cell apoptosis was evaluated by using the apoptosis detection kit (eBioscience, United States), In short, the collected AGS cells were washed with PBS and 1 × binding buffer. Subsequently, 1 × cell stain buffer was used so that the cells were eventually resuspended at a density of 1 × 106. The cell suspension (100 μL) was added with 5 μL annexin V-APC. After staining for 5 minutes, the supernatant was removed by centrifugation and stained with 5 μL of propidium iodide. Cells were detected after supplementation with 1 × cell stain buffer to 300 μL.
Cells in the logarithmic phase were collected and planked at a cell density of 5 × 104 cells /well. When the cells reached more than 90% confluence, the scratch meter was used to align the central part of the 96-well plate and nudge upward to create scratches. The plate was gently rinsed and then added to a low-concentration serum medium. Photographs were taken using a fluorescence microscope (Olympus) at 0 hour and 24 hours.
Transwell inserts (Corning, United States) were used to detect the migrating ability of AGS cells. Cells (5 × 104 cells/well) were inoculated in the upper chamber with a medium free of FBS (200 μL) and in the lower chamber with a complete medium containing 10% FBS (600 μL). After incubation for 48 hours, cells migrated to the lower side of the polycarbonate membrane were fixed in 4% paraformaldehyde and then stained with crystal violet. Finally, the cells were captured using an inverted microscope (Olympus, Japan) and counted.
The gene co-expression relationship with GEN1 was identified by analyzing STAD data in the TCGA database (https://www.cancer.gov/about-nci/organization/ccg/research/structural-genomics/tcga). The Kyoto Encyclopedia of Genes and Genomes (KEGG) database was also used to identify biological pathways and diseases involved in genes co-expressed with GEN1[18]. The potential correlation between these genes was analyzed using Cytoscape.
The analysis of adenosine triphosphate (ATP) concentration in AGS cells was carried out following the instructions provided by the ATP chemiluminescence assay kit (E-BC-F002, Elabscience, Wuhan, Hubei Province, China).
In brief, AGS cells were plated in 24-well plates for more than 12 hours, followed by stabilization with paraformaldehyde (Beyotime, Shanghai, China) for 30 minutes. Dihydroethidium staining (30 μM, DHE, Invitrogen) was performed for 5 minutes. The intensity of DHE fluorescence was measured using an inverted fluorescence microscope (Leica, Solms, Germany).
All data were presented as mean ± SD of three independent experiments. Statistical analysis was performed using GraphPad Prism 8.0 version (GraphPad Software, United States) and SPSS 18.0 software (SPSS Inc., United States). Statistical evaluation was performed by using the unpaired t-test and analysis of variance. The difference was statistically significant when P < 0.05.
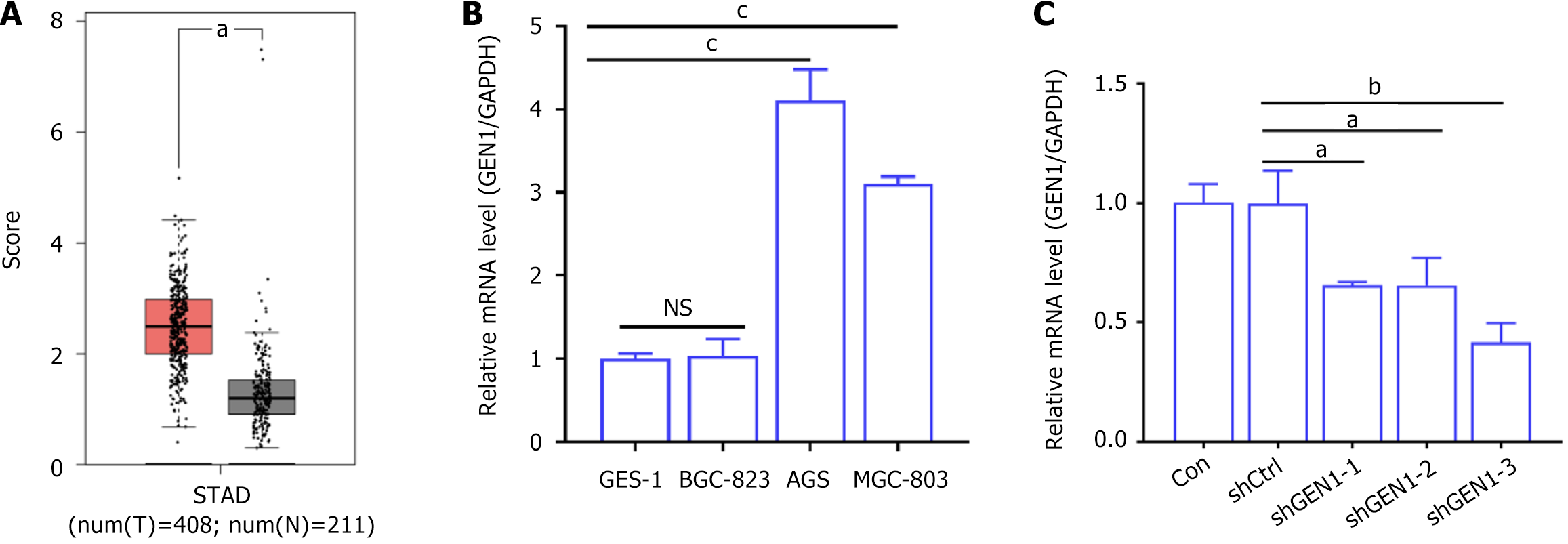
GEN1 was retrieved from the GEPIA2 database to analyze and compare whether there were differences in the expression levels of GEN1 in tumor tissues and normal tissues of GC patients. As exhibited in Figure 1A, GEN1 had higher levels in STAD samples than that in normal tissues. To investigate the function of GEN1 in GC, GEN1 mRNA expression levels were detected in multiple GC cell lines, and real time-qPCR showed higher expression levels of GEN1 mRNA in AGS and MGC-803 cell lines compared to the GES-1 cell line, while there was no significant change in the BGC-823 cell line (Figure 1B). Because GEN1 mRNA expression levels were highest in AGS cells, we investigated the function of GEN1 in GC by transfecting GEN1-specific shRNAs to down-regulate GEN1 in AGS cells. Transfection with shGEN1-3 had the highest knockdown efficiency, so shGEN1-3 was selected for subsequent investigation (Figure 1C). These results implied the association of GEN1 with GC.
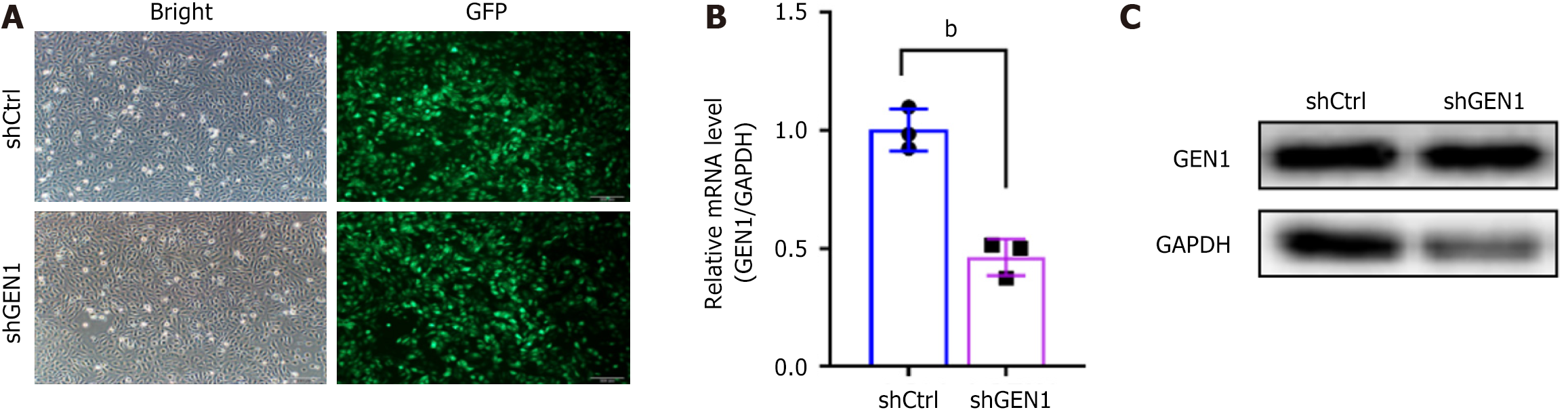
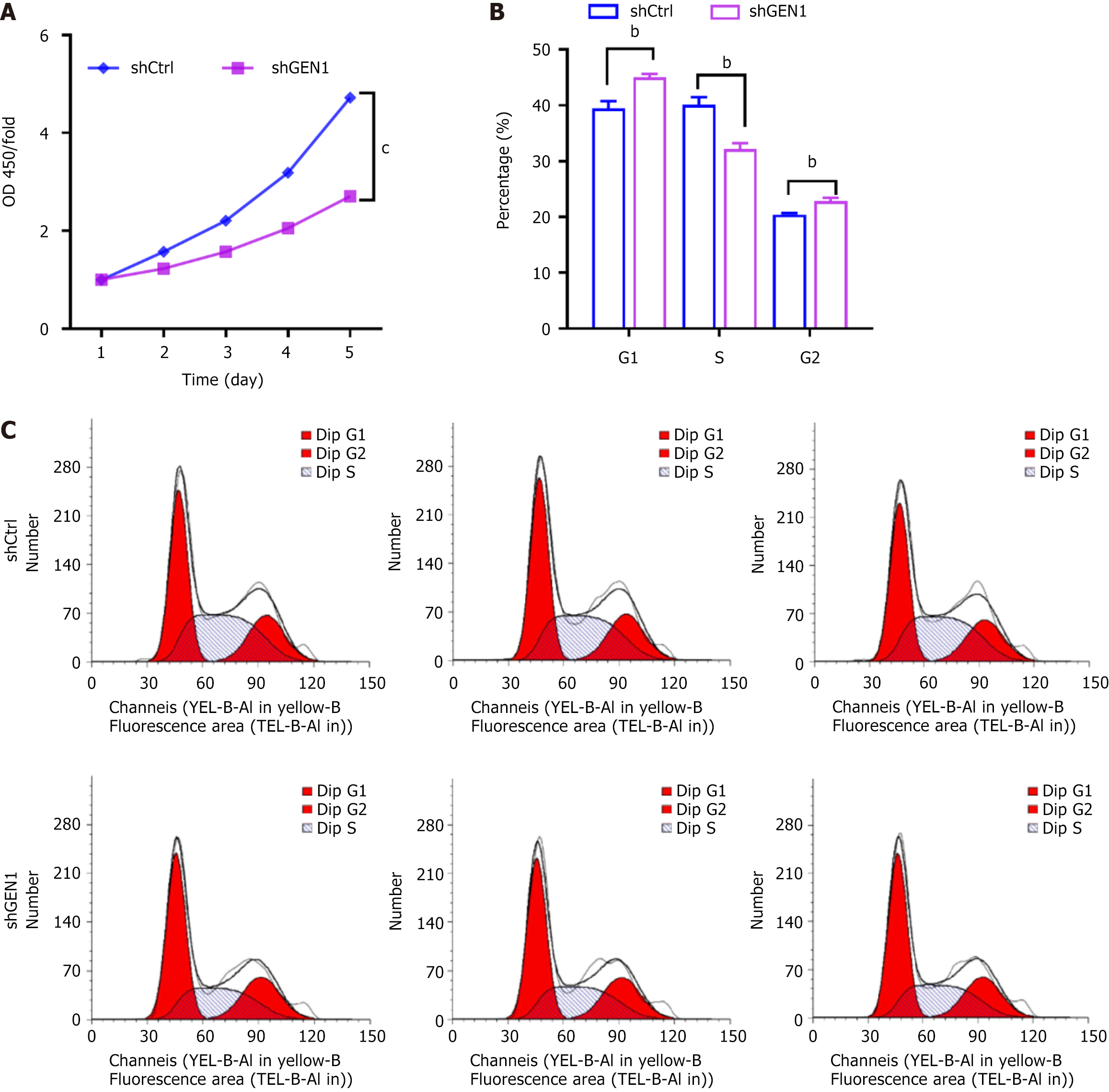
The specific shGEN1-3 targeting GEN1 was named shGEN1. Seventy-two hours after infection, fluorescence microscopy showed that the infection efficiency of cells in both groups exceeded 80%. Moreover, GEN1 mRNA expression levels were down-regulated in AGS cells after transfection with shGEN1. Similar GEN1 protein results were observed in AGS cells (Figure 2). Our data showed that GEN1 silencing lessened AGS cell proliferation, as demonstrated by CCK-8 assays (Figure 3A). Flow cytometry analysis showed that the number of AGS cells had a significant decrease in the S phase, with a concurrent increase of cells in the G0/G1 phase upon of GEN1 knockdown (Figure 3B and C). All results showed that GEN1 knockdown inhibited cell proliferation in AGS cells.
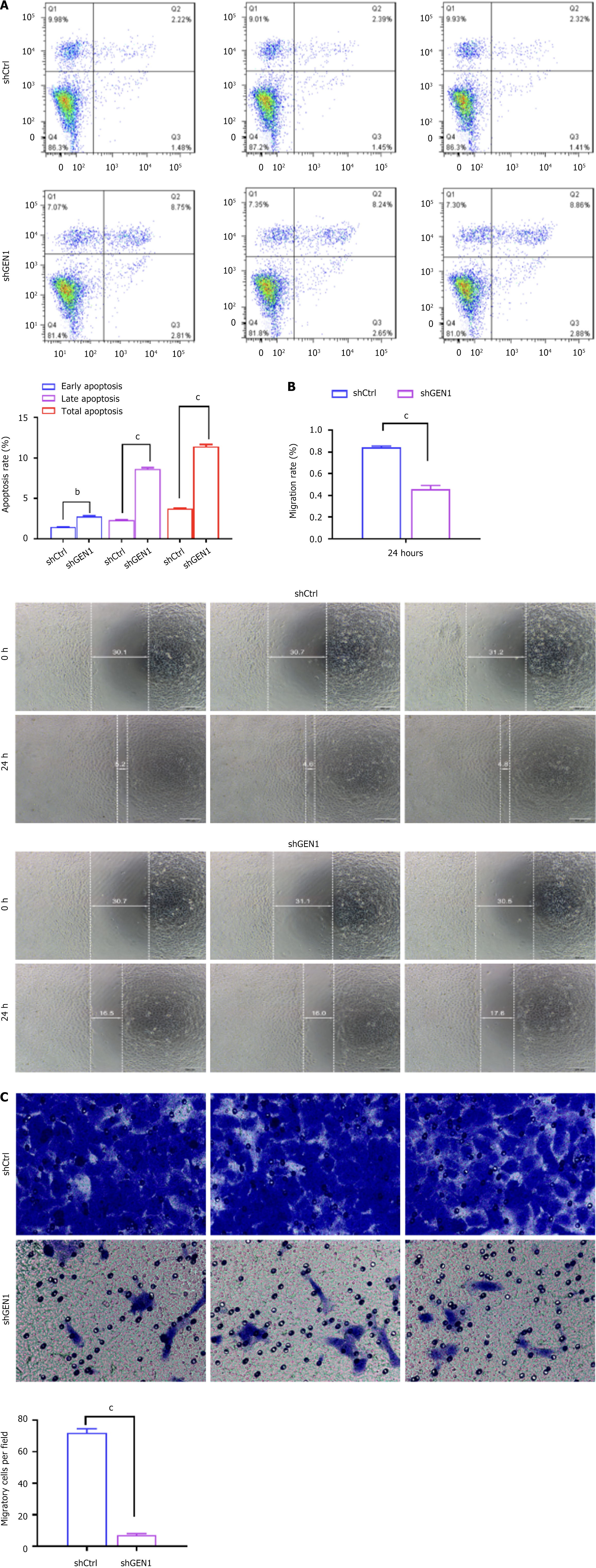
We further investigated the effect of GEN1 knockdown on AGS cell apoptosis and migration. Apoptosis analysis showed that both early apoptosis and late apoptosis were enhanced depending on the interference of GEN1 (Figure 4A). Wound-healing assays showed that interference with GEN1 caused a significant decrease in the migration rate of AGS cells (Figure 4B). Further transwell assays confirmed that down-regulation of GEN1 significantly reduced the migrating ability of AGS cells (Figure 4C). Collectively, interference with GEN1 induced AGS cell apoptosis and lowered AGS cell migration.
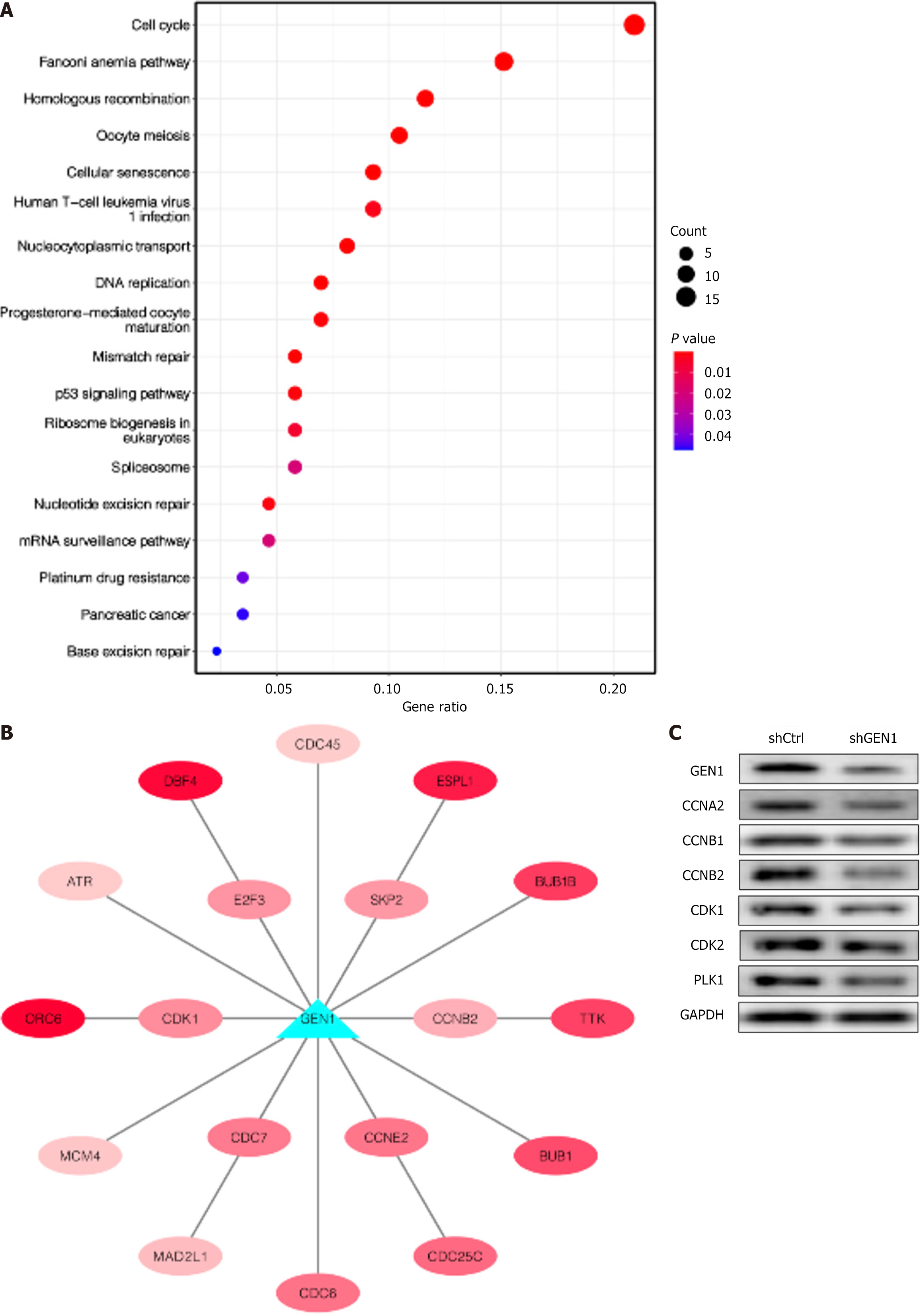
Further identification of genes co-expressed with GEN1 (r > 0.4, P < 0.001) was performed by analyzing the STAD data of the TCGA database. A total of 264 genes were found, all of which were positively correlated with GEN1. Subsequently, we conducted KEGG enrichment analysis for these positively related genes, and results were shown in Figure 4A, which suggested that these genes were associated with the cell cycle, Fanconi anemia pathway, homologous recombination, oocyte meiosis and cellular senescence, and GEN1 is most likely to participate in the cell cycle pathway (Figure 5A). The genes of the cell cycle pathway were visualized by Cytoscape, and red represented a positive correlation, with the darker the color, the stronger the correlation (Figure 5B). To further understand the relationship between GEN1 and the cell cycle pathway, multiple cell cycle-associated proteins in GEN1-knockdown cells were conducted. The results of western blotting showed that GEN1 down-regulation lessened CCNA2, CCNB1, CCNB2, CDK1, CDK2 and PLK1 protein levels in AGS cells (Figure 5C), indicating that GEN1 was involved in cell cycle pathways.
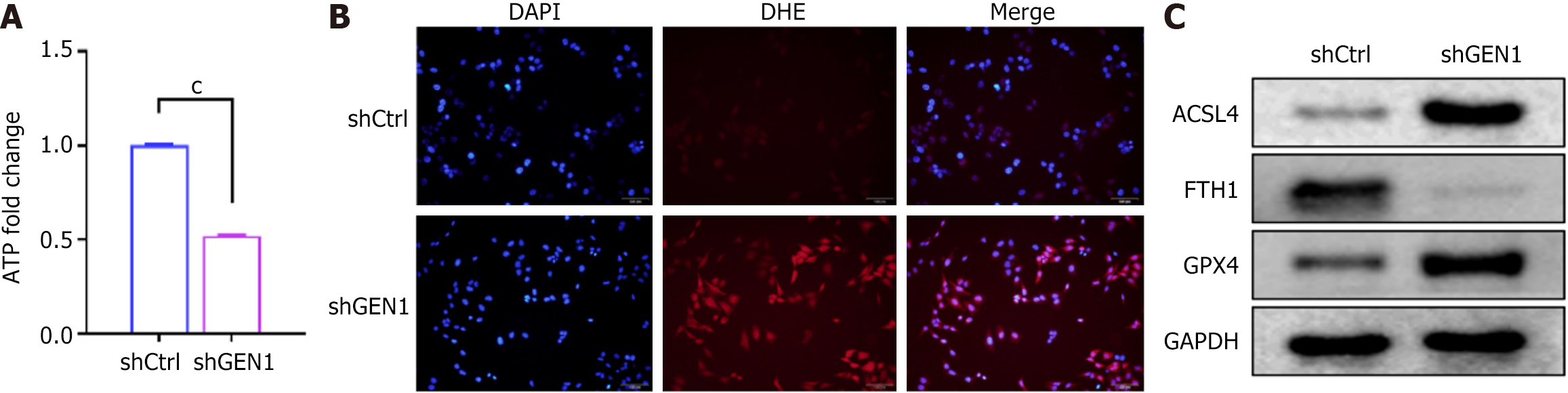
Based on the close relationship between abnormal lipid metabolism, accumulation of reactive oxygen species (ROS), and abnormal iron metabolism with the development of GC, we investigated the effect of GEN1 silencing on ferroptosis in AGS cells. Our results exhibited that GEN1 inhibition caused an overt reduction in intracellular ATP content in AGS cells (Figure 6A). DHE staining showed that GEN1 knockdown elevated ROS production in AGS cells (Figure 6B). Importantly, FTH1 and GPX4 protein levels were markedly reduced in GEN1-knockdown cells, but ACSL4 protein levels were overtly upregulated (Figure 6C). These results manifested that GEN1 silencing promoted AGS cell ferroptosis.
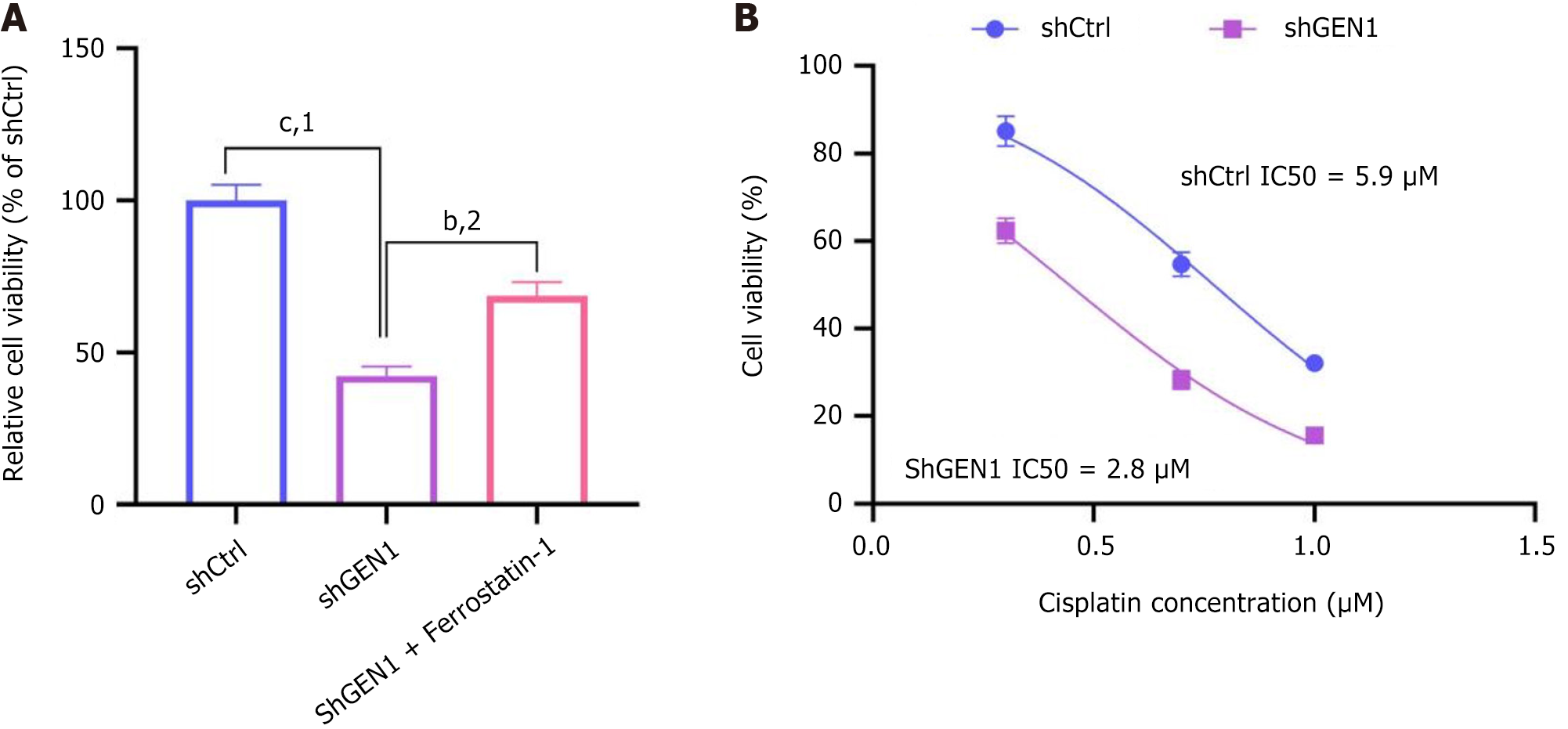
To validate the role of GEN1 in ferroptosis, we treated shGEN1-transfected AGS cells with the ferroptosis inhibitor ferrostatin-1. As shown in Figure 7A, ferrostatin-1 significantly rescued the cell viability reduction caused by GEN1 silencing (shGEN1: 42.3% ± 3.1% vs shGEN1 + ferrostatin-1: 68.7% ± 4.5%, P < 0.01), confirming that GEN1 deficiency triggers ferroptosis-dependent cell death. Furthermore, GEN1 knockdown enhanced the sensitivity of AGS cells to cisplatin. The IC50 of cisplatin in shGEN1 cells (2.8 ± 0.3 μM) was 2.3-fold lower than that in shCtrl cells (5.9 ± 0.6 μM, P < 0.001) (Figure 7B). These results suggest that targeting GEN1 may enhance the efficacy of conventional chemotherapy in GC.
GC is an important global healthcare problem due to its overall high prevalence and mortality[19]. The search for key biomarkers for the diagnosis and treatment of GC is a hot spot in today’s research, with the rapid development of molecular biology[4,20]. At present, some biomarkers closely related to GC progression have been identified, but few have been used in clinical practice. Therefore, the search for biomarkers closely related to GC progression is indispensable for the development of accurate GC treatment strategies.
GEN1 belongs to the Rad2/XPG nuclease family. A series of studies have verified that GEN1 specifically binds to and breaks down HJs[21,22]. Many studies hypothesize that proper resolution of HJs is key to correcting DNA repair. Many tumor cells are damaged by DNA damage drugs probably by damaging their DNA. GEN1 interference has been shown to increase drug sensitivity in fruit flies, either alone or in combination with other genes[23]. Therefore, this study aims to investigate the effect of GEN1 on the malignant phenotypes of GC cells and its involvement in the pathway in this tumor.
GEN1 has been shown to play a key role in protecting the genome to ensure proper development and proliferation of B lymphocytes. Previous studies have shown that GEN1 does not make a significant contribution to breast cancer susceptibility as a susceptibility gene[24]. However, the GEN1 response to DNA damage suggests that changes in GEN1 may contribute to the development of breast cancer[25]. Furthermore, down-regulation of GEN1 enhanced the susceptibility of SKBR3 cells to 5-fluorouracil but had no effect on MCF-7 cells[16]. Based on the GEPIA2 database, it was found that GEN1 was over-expressed in GC samples. We explored the expression of GEN1 in GC cells and observed that GC cells had higher GEN1 Levels than GES-1 cells, which was consistent with the trend of GEN1 expression in GC tumors in GEPIA2. Loss-of-function experiments in the study showed that silencing of GEN1 inhibited AGS cell proliferation, induced AGS cell apoptosis and decreased AGS cell migration, implying that GEN1 exerted an oncogenic property in GC. Unfortunately, we did not investigate the effect of GEN1 silencing on subcutaneous tumorigenesis of AGS cells in xenograft mice, which could be explored in the future.
Bioinformatics analysis (TCGA database) of genes co-expressed with GEN1 to explore the pathways involved in GEN1. There were 264 genes positively correlated with GEN1, and KEGG enrichment analysis showed that these genes were associated with the cell cycle, Fanconi anemia pathway, homologous recombination, oocyte meiosis and cellular senescence. It has been reported that GEN1 plays an important role in the regulation of centrosome integrity[26]. Moreover, cell cycle kinases and phosphatases control GEN1 to inhibit deleterious joint molecular-processing[27,28]. Therefore, we analyzed whether GEN1 mediates GC progression by affecting cell cycle pathway, and the results showed that GEN1 down-regulation decreased the levels of cell cycle-related proteins (CCNA2, CCNB1, CCNB2, CDK1, CDK2 and PLK1) in AGS cells. Upregulation of CCNA2 in GC patients had been revealed to possess a poor prognosis[29]. High levels of CCNA2, CCNB1, and CCNB2 were identified to be related with poor overall survival in Kaplan-Meier Plotter dataset[30]. ESRRA accelerated tumor growth by regulating the CDC25C/CDK1/CCNB1 pathway in GC[31]. CDK2 repressed SIRT5 in GC cells, thus elevating aerobic glycolysis[32]. PLK1 inhibition could enhance cell sensitivity to DDP in SGC-7901/DDP cells[33]. Mechanistically, GEN1 knockdown downregulated key cell cycle regulators (CCNA2, CCNB1/CCNB2, CDK1/2, PLK1) (Figure 5C). While the precise regulatory mechanism remains to be elucidated, we propose two hypotheses: (1) GEN1 may transcriptionally activate cyclins/CDKs by resolving replication-associated DNA damage, thereby preventing replication stress-induced degradation of these proteins[34]; and (2) GEN1 deficiency could activate the ATM/ATR-Chk1/2-p21 axis, leading to CDK inhibition and cell cycle arrest[35].
Iron overload and lipid peroxidation pathways are central links in the process of ferroptosis[36]. Excessive ferrous ion (Fe2+) generates ROS through Fenton reaction, causing lipid peroxidation and triggering cell ferroptosis[37]. In addition, ROS can also produce large amounts of lipid peroxides by attacking polyunsaturated fatty acids in the lipid membrane, resulting in membrane damage and cell death. As an important part of ferroptosis execution, ACSL4 is enriched with special oxidation-sensitive fatty acids in the membrane, making cells sensitive to ferroptosis and is a marker of ferroptosis[38]. GPX4, a negative regulatory factor of ferroptosis, can convert lipid hydroperoxides into lipid alcohols, which is a process that prevents the formation of Fe2+-dependent toxic lipid ROS[39]. FTH1 with iron oxidase activity is a major intracellular iron storage protein localized to the cytoplasm, nucleus, and mitochondria and reduces intracellular Fe2+ concentrations, playing a major role in iron metabolism[40]. Our data demonstrate that GEN1 silencing elevates ROS, depletes ATP, upregulates ACSL4, and downregulates GPX4/FTH1 (Figure 6), hallmarks of ferroptosis. Critically, rescue experiments with the ferroptosis inhibitor ferrostatin-1 partially restored cell viability in GEN1-knockdown cells (Figure 7A), directly linking GEN1 loss to ferroptosis activation. While the interplay between GEN1’s DNA repair function and ferroptosis remains unclear, we hypothesize that unresolved DNA damage due to GEN1 deficiency may exacerbate mitochondrial dysfunction and lipid peroxidation. Notably, GEN1’s potential crosstalk with ferroptosis regulators like p53 (which suppresses SLC7A11)[41] or NRF2 (which activates antioxidant genes)[42] warrants future investigation. A key translational finding is that GEN1 knockdown sensitizes GC cells to cisplatin, reducing its IC50 by 2.3-fold (Figure 7B). This aligns with studies showing that GEN1 inhibition enhances 5-fluorouracil efficacy in breast cancer[43]. We propose that GEN1-deficient GC cells accumulate DNA damage and ferroptotic stress, rendering them vulnerable to chemotherapeutics. This dual vulnerability could be exploited in combinatorial regimens, for example, pairing GEN1 inhibitors with platinum drugs or ferroptosis inducers (e.g., erastin).
Our study has limitations: (1) The lack of in vivo xenograft data to validate GEN1’s tumorigenic role; (2) Unexplored correlations between GEN1 expression and GC patient prognosis; and (3) Incomplete mechanistic insights into GEN1’s regulation of cell cycle proteins and ferroptosis pathways. Future work should address these gaps by: (1) Profiling GEN1 in GC clinical cohorts; (2) Testing GEN1 inhibitors in preclinical models; and (3) Exploring interactions between GEN1 and ferroptosis regulators (e.g., p53, NRF2) via co-immunoprecipitation or clustered regularly interspaced short palindromic repeats screens.
GEN1 drives GC progression by promoting cell cycle progression and suppressing ferroptosis. Its knockdown inhibits proliferation, induces apoptosis/ferroptosis, and enhances cisplatin sensitivity, positioning GEN1 as a dual therapeutic target for GC. Our findings bridge DNA repair, redox biology, and chemotherapy response, offering a roadmap for translating GEN1 inhibition into clinical strategies.
| 1. | Norwood DA, Montalvan-Sanchez E, Dominguez RL, Morgan DR. Gastric Cancer: Emerging Trends in Prevention, Diagnosis, and Treatment. Gastroenterol Clin North Am. 2022;51:501-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 2. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2847] [Article Influence: 569.4] [Reference Citation Analysis (5)] |
| 3. | Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71:264-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 1082] [Article Influence: 270.5] [Reference Citation Analysis (0)] |
| 4. | Lei ZN, Teng QX, Tian Q, Chen W, Xie Y, Wu K, Zeng Q, Zeng L, Pan Y, Chen ZS, He Y. Signaling pathways and therapeutic interventions in gastric cancer. Signal Transduct Target Ther. 2022;7:358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 163] [Article Influence: 54.3] [Reference Citation Analysis (1)] |
| 5. | Gwee YX, Chia DKA, So J, Ceelen W, Yong WP, Tan P, Ong CJ, Sundar R. Integration of Genomic Biology Into Therapeutic Strategies of Gastric Cancer Peritoneal Metastasis. J Clin Oncol. 2022;40:2830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 59] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 6. | Kaczmarczyk AP, Déclais AC, Newton MD, Boulton SJ, Lilley DMJ, Rueda DS. Search and processing of Holliday junctions within long DNA by junction-resolving enzymes. Nat Commun. 2022;13:5921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Song Q, Hu Y, Yin A, Wang H, Yin Q. DNA Holliday Junction: History, Regulation and Bioactivity. Int J Mol Sci. 2022;23:9730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Dunderdale HJ, Benson FE, Parsons CA, Sharples GJ, Lloyd RG, West SC. Formation and resolution of recombination intermediates by E. coli RecA and RuvC proteins. Nature. 1991;354:506-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 190] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Xia J, Mei Q, Rosenberg SM. Tools To Live By: Bacterial DNA Structures Illuminate Cancer. Trends Genet. 2019;35:383-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Rass U, Compton SA, Matos J, Singleton MR, Ip SC, Blanco MG, Griffith JD, West SC. Mechanism of Holliday junction resolution by the human GEN1 protein. Genes Dev. 2010;24:1559-1569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Bailly AP, Freeman A, Hall J, Déclais AC, Alpi A, Lilley DM, Ahmed S, Gartner A. The Caenorhabditis elegans homolog of Gen1/Yen1 resolvases links DNA damage signaling to DNA double-strand break repair. PLoS Genet. 2010;6:e1001025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Carreira R, Aguado FJ, Hurtado-Nieves V, Blanco MG. Canonical and novel non-canonical activities of the Holliday junction resolvase Yen1. Nucleic Acids Res. 2022;50:259-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Chan YW, West SC. Spatial control of the GEN1 Holliday junction resolvase ensures genome stability. Nat Commun. 2014;5:4844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Li H, Wu J, Xu Q, Pang Y, Gu Y, Wang M, Cheng X. Functional genetic variants of GEN1 predict overall survival of Chinese epithelial ovarian cancer patients. J Transl Med. 2024;22:577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Wechsler T, Newman S, West SC. Aberrant chromosome morphology in human cells defective for Holliday junction resolution. Nature. 2011;471:642-646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 16. | Wu Y, Qian Y, Zhou G, Lv J, Yan Q, Dong X. Effect of GEN1 interference on the chemosensitivity of the breast cancer MCF-7 and SKBR3 cell lines. Oncol Lett. 2016;11:3597-3604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Xia J, Chen LT, Mei Q, Ma CH, Halliday JA, Lin HY, Magnan D, Pribis JP, Fitzgerald DM, Hamilton HM, Richters M, Nehring RB, Shen X, Li L, Bates D, Hastings PJ, Herman C, Jayaram M, Rosenberg SM. Holliday junction trap shows how cells use recombination and a junction-guardian role of RecQ helicase. Sci Adv. 2016;2:e1601605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Altermann E, Klaenhammer TR. PathwayVoyager: pathway mapping using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. BMC Genomics. 2005;6:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 255] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 19. | Petryszyn P, Chapelle N, Matysiak-Budnik T. Gastric Cancer: Where Are We Heading? Dig Dis. 2020;38:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (1)] |
| 20. | Yu C, Chen J, Ma J, Zang L, Dong F, Sun J, Zheng M. Identification of Key Genes and Signaling Pathways Associated with the Progression of Gastric Cancer. Pathol Oncol Res. 2020;26:1903-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Chan YW, West S. GEN1 promotes Holliday junction resolution by a coordinated nick and counter-nick mechanism. Nucleic Acids Res. 2015;43:10882-10892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Sobhy MA, Bralić A, Raducanu VS, Takahashi M, Tehseen M, Rashid F, Zaher MS, Hamdan SM. Resolution of the Holliday junction recombination intermediate by human GEN1 at the single-molecule level. Nucleic Acids Res. 2019;47:1935-1949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Carvajal-Garcia J, Crown KN, Ramsden DA, Sekelsky J. DNA polymerase theta suppresses mitotic crossing over. PLoS Genet. 2021;17:e1009267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Turnbull C, Hines S, Renwick A, Hughes D, Pernet D, Elliott A, Seal S, Warren-Perry M, Gareth Evans D, Eccles D; Breast Cancer Susceptibility Collaboration UK, Stratton MR, Rahman N. Mutation and association analysis of GEN1 in breast cancer susceptibility. Breast Cancer Res Treat. 2010;124:283-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Sun L, Zhang Y, Pan Z, Li B, Sun M, Zhang X. Expression and localization of GEN1 in mouse mammary epithelial cells. J Biochem Mol Toxicol. 2014;28:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Gao M, Rendtlew Danielsen J, Wei LZ, Zhou DP, Xu Q, Li MM, Wang ZQ, Tong WM, Yang YG. A novel role of human holliday junction resolvase GEN1 in the maintenance of centrosome integrity. PLoS One. 2012;7:e49687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Wild P, Matos J. Cell cycle control of DNA joint molecule resolution. Curr Opin Cell Biol. 2016;40:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Matos J, West SC. Holliday junction resolution: regulation in space and time. DNA Repair (Amst). 2014;19:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 29. | Lu XQ, Zhang JQ, Zhang SX, Qiao J, Qiu MT, Liu XR, Chen XX, Gao C, Zhang HH. Identification of novel hub genes associated with gastric cancer using integrated bioinformatics analysis. BMC Cancer. 2021;21:697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Zhang HP, Li SY, Wang JP, Lin J. Clinical significance and biological roles of cyclins in gastric cancer. Onco Targets Ther. 2018;11:6673-6685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Li FN, Zhang QY, Li O, Liu SL, Yang ZY, Pan LJ, Zhao C, Gong W, Shu YJ, Dong P. ESRRA promotes gastric cancer development by regulating the CDC25C/CDK1/CyclinB1 pathway via DSN1. Int J Biol Sci. 2021;17:1909-1924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 32. | Tang Z, Li L, Tang Y, Xie D, Wu K, Wei W, Xiao Q. CDK2 positively regulates aerobic glycolysis by suppressing SIRT5 in gastric cancer. Cancer Sci. 2018;109:2590-2598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 33. | Chen Z, Chai Y, Zhao T, Li P, Zhao L, He F, Lang Y, Qin J, Ju H. Effect of PLK1 inhibition on cisplatin-resistant gastric cancer cells. J Cell Physiol. 2019;234:5904-5914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Song G, Liu J, Tang X, Zhong J, Zeng Y, Zhang X, Zhou J, Zhou J, Cao L, Zhang Q, Li Y. Cell cycle checkpoint revolution: targeted therapies in the fight against malignant tumors. Front Pharmacol. 2024;15:1459057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 35. | Soni A, Duan X, Stuschke M, Iliakis G. ATR Contributes More Than ATM in Intra-S-Phase Checkpoint Activation after IR, and DNA-PKcs Facilitates Recovery: Evidence for Modular Integration of ATM/ATR/DNA-PKcs Functions. Int J Mol Sci. 2022;23:7506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1891] [Cited by in RCA: 2423] [Article Influence: 605.8] [Reference Citation Analysis (0)] |
| 37. | He YJ, Liu XY, Xing L, Wan X, Chang X, Jiang HL. Fenton reaction-independent ferroptosis therapy via glutathione and iron redox couple sequentially triggered lipid peroxide generator. Biomaterials. 2020;241:119911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 202] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 38. | Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A, Prokisch H, Trümbach D, Mao G, Qu F, Bayir H, Füllekrug J, Scheel CH, Wurst W, Schick JA, Kagan VE, Angeli JP, Conrad M. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1074] [Cited by in RCA: 2702] [Article Influence: 300.2] [Reference Citation Analysis (0)] |
| 39. | Forcina GC, Dixon SJ. GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis. Proteomics. 2019;19:e1800311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 653] [Article Influence: 108.8] [Reference Citation Analysis (0)] |
| 40. | Di Sanzo M, Quaresima B, Biamonte F, Palmieri C, Faniello MC. FTH1 Pseudogenes in Cancer and Cell Metabolism. Cells. 2020;9:2554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 41. | Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H, Baer R, Gu W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1173] [Cited by in RCA: 2397] [Article Influence: 239.7] [Reference Citation Analysis (0)] |
| 42. | Sun X, Ou Z, Xie M, Kang R, Fan Y, Niu X, Wang H, Cao L, Tang D. HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene. 2015;34:5617-5625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 501] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 43. | Du X, Yu M, Ju H, Xue S, Li Y, Wu X, Xu H, Shen Q. Inhibition of MAPK/ERK pathway activation rescues congenital anomalies of the kidney and urinary tract (CAKUT) in Robo2(PB/+) Gen1(PB/+) mice. Biochem Biophys Res Commun. 2023;653:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |