Published online May 15, 2025. doi: 10.4251/wjgo.v17.i5.104522
Revised: February 27, 2025
Accepted: April 21, 2025
Published online: May 15, 2025
Processing time: 143 Days and 5.4 Hours
Colorectal cancer (CRC) is a major cause of cancer-related mortality, with limited therapeutic options for advanced stages. Simvastatin, primarily used to lower cholesterol, has shown potential as an anticancer agent. It may exert its effects by inhibiting SERPINE1, a protein implicated in CRC progression, and activating the cyclic guanosine monophosphate-protein kinase G (cGMP/PKG) signaling pa
To study the effects of simvastatin on the function of colon cancer cells and to uncover the underlying mechanisms.
NCM460, HCT-116, and SW620 cell lines were used for in vitro experiments with simvastatin at doses of 20 μM, 40 μM, and 80 μM. The Stitch database was used to analyze the target genes of simvastatin, whereas STRING was used to investigate SERPINE1 and its related pathways. HCT-116 and SW620 cells were transfected with single-cell RNA sequencing reveals SERPINE1 with or without Rp-8-Br-cGMP (a PKG inhibitor). Cell toxicity, proliferation, and migration were evaluated using the cell counting kit-8, colony formation, and Transwell assays, respectively. Apoptosis was analyzed via flow cytometry, and levels of reactive oxygen species (ROS), malondialdehyde (MDA), glutathione (GSH), and ferrous ion (Fe2+) were detected using commercial kits. Real-time polymerase chain reaction and western blotting were used to analyze gene expression.
Simvastatin dose-dependently inhibited the proliferation and migration of HCT-116 and SW620 cells while promoting apoptosis. It downregulated Ki-67, proliferating cell nuclear antigen, MMP2, and MMP9, and upregulated Bax, particularly at higher doses. Simvastatin increased the ROS, MDA, and Fe2+ levels while decreasing the GSH levels. It downregulated SLC7A11 and ferroportin and upregulated TRF1. SERPINE1 was identified as a core target, with related genes enriched in the cGMP/PKG pathway. SERPINE1 knockdown increased GUCY1B1 and PRKG1 levels, decreased cell viability, and altered oxidative stress markers, with the effects being reversed by Rp-8-Br-cGMP.
Simvastatin effectively inhibited the proliferation and migration of colon cancer cells and promoted apoptosis through the modulation of key targets, such as SERPINE1 and the cGMP/PKG signaling pathway.
Core Tip: Simvastatin significantly reduces the proliferation and migration of HCT-116 and SW620 colon cancer cell lines in a dose-dependent manner, while promoting apoptosis. Treatment with simvastatin enhances oxidative stress markers (reactive oxygen species, malondialdehyde, ferrous ion) and decreases glutathione levels, indicating its potential to induce ferroptosis in colon cancer cells through the modulation of the cyclic guanosine monophosphate-protein kinase G signaling pathway. SERPINE1 was identified as a core target of simvastatin, with its downregulation linked to increased GUCY1B1 and PRKG1 levels, suggesting a crucial role in the drug’s mechanism of action.
- Citation: Liu Y, Ge H, Fan ZM, Lu T, He L, Li M, Zhao HR, Leng Q. Simvastatin inhibits proliferation and migration, promotes oxidative stress and ferroptosis in colon cancer. World J Gastrointest Oncol 2025; 17(5): 104522
- URL: https://www.wjgnet.com/1948-5204/full/v17/i5/104522.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i5.104522
Colorectal cancer (CRC) is the third most prevalent type of cancer worldwide and the second most common cause of cancer-related fatalities[1]. Epidemiological studies indicate rising incidence of CRC, especially in low- and middle-income countries where lifestyle changes and increased life expectancy are significant contributing factors[2]. The clinical significance of CRC is accentuated by its asymptomatic manifestation in the early stages, which frequently results in delayed diagnosis and a less favorable prognosis[3]. Effective screening initiatives have demonstrated a remarkable reduction in mortality rates, underscoring the importance of early detection and intervention. Understanding the epidemiology of CRC is of paramount importance in formulating targeted prevention and treatment strategies to address this formidable public health concern. CRC develops over a protracted period, typically spanning 10-15 years, during which genetic mutations accumulate, leading to the formation of adenomatous polyps that can progress to invasive cancer[4]. Adenocarcinoma accounts for approximately 90% of all colorectal malignancies[5]. CRC commonly metastasizes to the liver and lungs, with dissemination to the peritoneum and distant lymph nodes also possible[6]. The incidence of CRC is increasing, and recent estimates suggest that it will rank as the third most frequently diagnosed cancer in Canada, representing approximately 11% of all new cancer cases[2].
The prognosis for patients with CRC varies significantly, depending on the stage at which the disease is diagnosed[7]. For instance, patients with localized CRC have a remarkable five-year relative survival rate of 90%, whereas those diagnosed with distant metastases have a dismal survival rate of 14%[8]. Recent advancements in treatment methods, such as traditional chemotherapy, radiotherapy, and targeted therapies, have significantly enhanced the outcomes for patients with CRC[9]. However, the prognosis of patients with advanced disease remains unfavorable due to issues, such as drug resistance and low response rates to treatment. The chemotherapy landscape for CRC is limited to a handful of approved agents, including 5-fluorouracil, oxaliplatin, irinotecan, capecitabine, and targeted therapies, such as cetuximab, panitumumab, and bevacizumab[10,11]. Despite these advances, the challenges of recurrence and metastasis in late-stage CRC persist, necessitating the exploration of novel therapeutic approaches.
Among the compounds under investigation, statins, which are commonly known for their cholesterol-lowering effects, have garnered attention for their potential antitumor properties[12]. Simvastatin, a widely used lipophilic statin for cholesterol management, exhibits various pharmacological effects beyond lipid lowering. It exhibits potential antitumor properties by inducing apoptosis, inhibiting tumor cell proliferation, and reducing inflammation in various cancers. Recent studies have indicated that simvastatin may enhance the efficacy of conventional cancer therapies and improve patient outcomes, making it a promising candidate for adjunctive treatment in oncology. Further studies are necessary to fully understand the mechanisms of action and therapeutic applications of this compound in cancer care. Simvastatin has demonstrated significant anticancer activity in various studies, particularly in preclinical models of colon cancer[13,14]. The proposed mechanisms of action of simvastatin in cancer include its ability to induce apoptosis, inhibit tumor angiogenesis, and suppress inflammatory pathways. Notably, recent research has highlighted that simvastatin may exert its anticancer effects through the modulation of key signaling pathways, such as the cyclic guanosine monophosphate (cGMP)-dependent protein kinase G (PKG) pathway[15].
SERPINE1 levels are abnormally elevated in CRC[16]. SERPINE1, also known as plasminogen activator inhibitor-1, has emerged as a critical factor in cancer progression, particularly in CRC[17]. This serine protease inhibitor facilitates tumor metastasis by promoting the invasion of cancer cells into surrounding tissues[18]. Elevated levels of SERPINE1 have been associated with poor prognosis in various cancers, including CRC[19]. Recent studies have indicated that SERPINE1 may influence cancer progression through the p38-mitogen-activated protein kinase signaling pathway[16], further emphasizing its role as a potential therapeutic target.
The cGMP/PKG signaling pathway is essential for controlling several cellular functions, such as the relaxation of vascular smooth muscle, cell proliferation, and apoptosis. In rectal cancer, alterations in this pathway have been linked to tumor growth and metastasis. Increased levels of cGMP may promote the survival and proliferation of tumor cells, indicating that targeting the cGMP/PKG pathway could offer innovative therapeutic approaches for treating rectal cancer and enhancing patient outcomes. The cGMP/PKG signaling pathway has been increasingly recognized for its anticancer properties in various malignancies[20]. PKG activation suppresses cell growth, migration, and metastasis[21]. This signaling cascade is crucial for various physiological processes, such as cell apoptosis, neuronal signaling, and bone growth, underscoring its broad biological significance. Recent studies have indicated that activating the cGMP/PKG pathway can considerably reduce the formation of colorectal adenomas[22], underscoring its potential as a therapeutic target in CRC management.
Based on the abovementioned findings, we hypothesized that simvastatin exerts its anticancer effects by inhibiting SERPINE1, which leads to the activation of the cGMP/PKG signaling pathway, ultimately resulting in reduced proliferation and migration of CRC cells. This study was aimed at elucidating the mechanisms by which simvastatin influences SERPINE1 expression and the subsequent activation of the cGMP/PKG pathway to obtain insights that could inform novel therapeutic strategies for CRC management. The objectives of this study were to: (1) Assess the impact of simvastatin on SERPINE1 expression in CRC cell lines; (2) Investigate the effects of simvastatin on the cGMP/PKG signaling pathway; and (3) Evaluate the resultant changes in the proliferation and migration of CRC cells.
Primary antibodies specific to Ki-67 (ab92742, 1:5000), proliferating cell nuclear antigen (PCNA) (ab92552, 1:2000), MMP2 (ab92536, 1:2000), MMP9 (ab76003, 1:2000), Bax (ab32503, 1:2000), Bcl-2 (ab182858, 1:2000), SLC7A11 (ab307601, 1:1000), ferroportin (FPN) (ab239511, 1:1000), TRF1 (ab192629, 1:1000), SERPINE1 (ab317604, 1:1000), GUCY1B1 (ab154841, 1:2000), PRKG1 (ab110124, 1:1000), and β-actin (ab6276, 1:5000) were purchased from Abcam (Cambridge, United Kingdom). Cell counting kit-8 (CCK-8) (C0037), Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (C1062M), reactive oxygen species (ROS) assay kit (S0035M), enzyme-linked immunosorbent assay kits for malondialdehyde (MDA) (S0131M) and glutathione (GSH) (S0053) were sourced from Beyotime (Beijing, China). A ferrous ion (Fe2+) assay kit was obtained from Dojingo (Ferro-orange kit, Shanghai, China). Rp-8-Br-cGMP, a specific inhibitor of cyclic GMP-dependent protein kinase (PKG), was purchased from Cayman Chemicals (18441, United States).
NCM460, HCT116, and SW620 cell lines were obtained from the Shanghai branch of the Chinese Academy of Sciences’ Type Culture Collection. These cells were maintained in RPMI 1640 medium (Gibco, United States), supplemented with 10% fetal bovine serum (Gibco, United States). Additionally, the culture medium was fortified with 100 units per milliliter of penicillin/streptomycin (HyClone, Shanghai, China). The cells were kept under standard culturing conditions, which included an incubation at 37 °C in an environment of 5% carbon dioxide (CO2). Experiments were conducted during the logarithmic phase of cell growth.
HCT116 and SW620 cells were treated with simvastatin at doses of 20, 40, and 80 μM for 24, 48, or 72 hours. In another in vitro experiment, these two cell lines were transfected with single-cell RNA sequencing reveals SERPINE1 (si-SERPINE1), with or without Rp-8-Br-cGMP, for 24 hours.
Si-SERPINE1 and single-cell RNA sequencing reveals-negative control (si-NC) were procured from RiboBio (Guangzhou, China). The sequences of si-SERPINE1 and si-NC are listed below: Si-NC: 5’-CAGCTTGCTTTGAATAAAACATA-3’; Si-SERPINE1 (si-SERPINE1-1): 5’-ATGGAAATTGACCATACAATTTC-3’; Si-SERPINE1-2: 5’-CGACATGTTCAGACAGTTTCA-3’; Si-SERPINE1-3: 5’-CAGCAGATTCAAGCAGCTATG-3’.
HCT116 and SW620 cell lines were transfected with the constructs using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, United States) for 24 hours, adhering to the guidelines outlined in the instruction manual.
The cytotoxic effect was assessed using the CCK-8 assay. Cells were seeded in 96-well plates at a density of 3 × 103 cells/well and cultured for 24, 48, or 72 hours. They were then treated with simvastatin at concentrations of 20, 40, and 80 μM, or with si-SERPINE1, with or without Rp-8-Br-cGMP. Following incubation, the cells were exposed to 1 mg/mL CCK-8 solution (C0037, Beyotime, China). After 3 hours, the absorbance was measured at 450 nm using an ELX-800 spectrometer (Bio-Tek Instruments).
Cells were plated in 6-well plates at a density of 1 × 105 cells/well and incubated for 24 hours at 37 °C in a 5% CO2 atmosphere to facilitate attachment. Thereafter, the cells were subjected to specific treatments, including drug administration, or were used as controls. After 48 hours, the cells were fixed with 4% paraformaldehyde for 10 minutes at room temperature and washed twice with phosphate-buffered saline (PBS) to eliminate excess fixative. Subsequently, the cells were stained with 0.1% crystal violet solution for 15 minutes at room temperature. The cells were then gently rinsed with PBS to remove any unbound dye. For quantifying cell viability, 30% acetic acid was added to dissolve crystal violet for 15 minutes at room temperature, ensuring thorough mixing. The optical density (OD) of the resulting solution was measured at 590 nm using a spectrophotometer (Agilent Technologies, Santa Clara, CA, United States). Each experiment was conducted in triplicate to ensure the reproducibility and reliability of results. The OD values were used to assess the relative cell viability, and the results were expressed as a percentage of the control group.
The Transwell migration assay was performed to compare the migratory abilities of HCT116 and SW620 cells. Cell suspensions were introduced into the upper chamber of the Transwell, whereas the lower chamber was filled with medium supplemented with 20% fetal bovine serum. After incubating for 24 hours, non-migrated cells were carefully removed using a cotton swab. The migrated cells were then fixed with 4% paraformaldehyde for 15 minutes and subsequently stained with 0.1% crystal violet for 30 minutes. Excess stain was gently rinsed off with PBS. The cells that migrated to the underside of the membrane were quantified under a light microscope. To ensure consistency and reliability of the results, the experiment was repeated three times.
To assess apoptosis, approximately 1 × 106 cells/well were seeded into 6-well culture plates. Following exposure to different experimental conditions, apoptosis was evaluated using the Annexin V-FITC kit (C1062M, Beyotime, China), according to the protocol provided by the manufacturer. For quantification, 100 μL of the cell suspension was combined with 5 μL of Annexin V-FITC and 5 μL of propidium iodide (PI). This mixture was incubated in the dark at room temperature for 15 minutes. After incubation, 400 μL of binding solution was added, and the samples were subjected to flow cytometry analysis using a BD FACSCalibur (BD Biosciences). The percentage of apoptotic cells, encompassing early and late stages, was determined by examining the fluorescence emitted from the Annexin V and PI stains, following the manufacturer’s instructions.
Intracellular ROS levels were assessed using the ROS assay kit (S0035M, Beyotime, China). After the treatment, the cells in each group were rinsed with PBS. A solution of dihydroethidium diluted in Dulbecco’s modified eagle medium was then added to each well, and the cells were incubated at 37 °C in the dark for 20 minutes. Following incubation, the cells were washed three times with Dulbecco’s modified eagle medium. The resulting red fluorescence was examined using a fluorescent inverted microscope, and the intensity was quantified using the ImageJ software (version 1.41; Bethesda, MD).
Following different treatments, the levels of GSH and MDA in the cells were assessed using specific activity assay kits (S0053, S0131M, Beyotime Biotechnology, China). Measurements were performed according to the instructions provided with the assay kits. A microplate reader (MS-8496 F, Sumitomo Bakelite Co., Ltd., Tokyo, Japan) was used to determine the levels of MDA and GSH in the cells. Absorbance was recorded at 450 nm for MDA and 405 nm for GSH. These readings allowed quantification of the respective MDA and GSH content within the cells.
Cells were seeded at a density of 1 × 105 cells/mL in 12-well culture plates. Intracellular Fe2+ concentrations were measured using a Ferro-orange kit (Dojindo, Molecular Technologies Inc., Shanghai, China), following the manufacturer’s instructions. Briefly, after various treatments, the cells were incubated in serum-free medium for 4 hours. They were then stained with 1 μM Ferro-orange in Hank’s balanced salt solution for 30 minutes at 37 °C. The fluorescence intensity was measured using a Synergy H1 automatic microplate spectrophotometer (BioTek Instruments, Vermont, United States) at an excitation wavelength of 543 nm and an emission wavelength of 580 nm.
The molecular structure and simplified molecular input line entry specification (SMILES) representation of simvastatin were obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov). Target prediction for simvastatin was conducted using the STITCH database (http://stitch.embl.de/), limiting the search to “Homo sapiens” species.
The protein-protein interaction (PPI) network for SERPINE1 genes was obtained from the STRING database (version 11.0; https://string-db.org/), applying a minimum interaction score of ≥0.4. Subsequently, the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis for SERPINE1 and its associated genes within the modules was performed using the KEGG database.
Total RNA was extracted using TRIzol reagent (R0011; Beyotime, Beijing, China). Following this, complementary DNA was synthesized using a complementary DNA synthesis kit (D7168S; Beyotime, Beijing, China). Polymerase chain reaction (PCR) was performed using an ABI 7900 fluorescence quantitative PCR system (ABI, United States). The primers used in this study are listed in Table 1. The process began with an initial denaturation at 95 °C for 10 minutes. This was followed by 40 cycles, each comprising denaturation at 95 °C for 10 seconds, annealing at either 52.8 or 56.2 °C for 15 seconds, and extension at 72 °C for 20 seconds. A temperature gradient ranging from 72 °C to 95 °C was implemented, increasing by 1 °C at each step, with automatic gain calibration conducted prior to the initial run. Glyceraldehyde-3-phosphate dehydrogenase expression served as a reference for normalizing the messenger RNA (mRNA) levels, and relative expression was determined using the 2-ΔΔCT method.
| Gene name | Species | Primers (5’-3’) |
| SERPINE1 | Human | Forward primer: TTCAAGATTGATGACAAGGGC |
| Reverse primer: CTCATCCTTGTTCCATGGC | ||
| GUCY1B1 | Human | Forward primer: GAGGTGTGGGAAGACATCAAG |
| Reverse primer: GCCTCTTTTATAAAGCACATAAGCA | ||
| PRKG1 | Human | Forward primer: GGACAGGACTCATCAAGCATAC |
| Reverse primer: CTTCACGAGTGACATTTACCGTT | ||
| GAPDH | Human | Forward primer: GGAGCGAGATCCCTCCAAAAT |
| Reverse primer: GGCTGTTGTCATACTTCTCATGG |
Cells were lysed using radio immunoprecipitation assay buffer (Beyotime, Beijing, China), and total protein concentration was determined using a commercial kit (Beyotime, Beijing, China). A total of 60 μg of protein was separated via 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis and subsequently transferred onto a polyvinylidene fluoride membrane (ISEQ00010, Sigma-Aldrich, Beijing, China). To minimize nonspecific binding, the membranes were treated with a blocking solution of 5% milk powder in Tris-buffered saline (TBS) containing 0.05% Tween 20 (37573, TBST, Thermo Scientific, Beijing, China).
The membranes were incubated overnight at 4 °C with primary antibodies, including human polyclonal antibodies against Ki-67, PCNA, MMP2, MMP9, Bax, Bcl-2, SLC7A11, FPN, TRF1, SERPINE1, GUCY1B1, PKG, and β-actin, all sourced from Abcam (United Kingdom). After three washes with TBST, the membranes were incubated with a goat anti-human secondary antibody conjugated to horseradish peroxidase (A0201, diluted 1:1000; Beyotime, Beijing, China). The protein blots were analyzed using enhanced chemiluminescence, with β-actin serving as the internal control. Protein levels were quantified densitometrically using the ImageJ software (version 1.41).
Data analysis was performed using the SPSS Statistics software (version 20, Chicago, IL, United States). Continuous variables were averaged, and standard deviations were calculated. A t-test was performed to evaluate differences between two groups. For comparisons involving three or more groups, one-way analysis of variance was conducted, followed by an LSD test for post-hoc analysis. The findings indicated a significant difference between the two groups (P < 0.05).
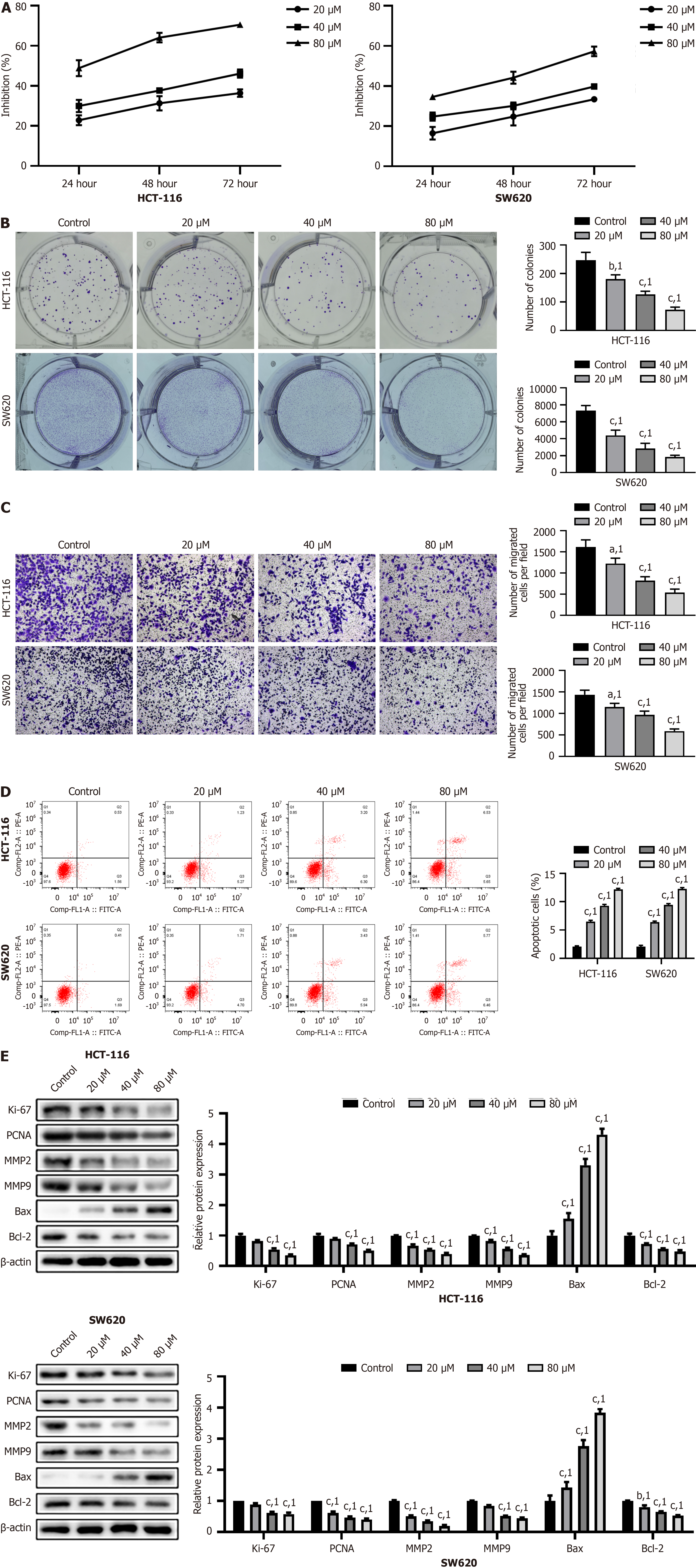
HCT-116 and SW620 cells were used as in vitro models of colon cancer. Simvastatin inhibited cell viability and proliferation in a time- and dose-dependent manner, as evident from the results of the CCK-8 and colony formation assays (P < 0.001, Figure 1A and B). Results of the Transwell assay showed that cell migration was significantly reduced following simvastatin treatment, particularly at a dose of 80 μM (P < 0.001, Figure 1C). Furthermore, flow cytometry revealed a significant increase in cell apoptosis after simvastatin treatment, especially at the 80 μM dose (P < 0.001, Figure 1D). Western blotting was performed to assess the protein levels of markers related to proliferation, migration, and apoptosis. After simvastatin treatment, we observed downregulation of Ki-67, PCNA, MMP2, MMP9, and Bcl-2 and upregulation of Bax (P < 0.001, Figure 1E). Overall, simvastatin effectively inhibited cell viability, proliferation, and migration, while promoting apoptosis in HCT-116 and SW620 colon cancer cells, as evidenced by significant changes in protein markers associated with these processes.
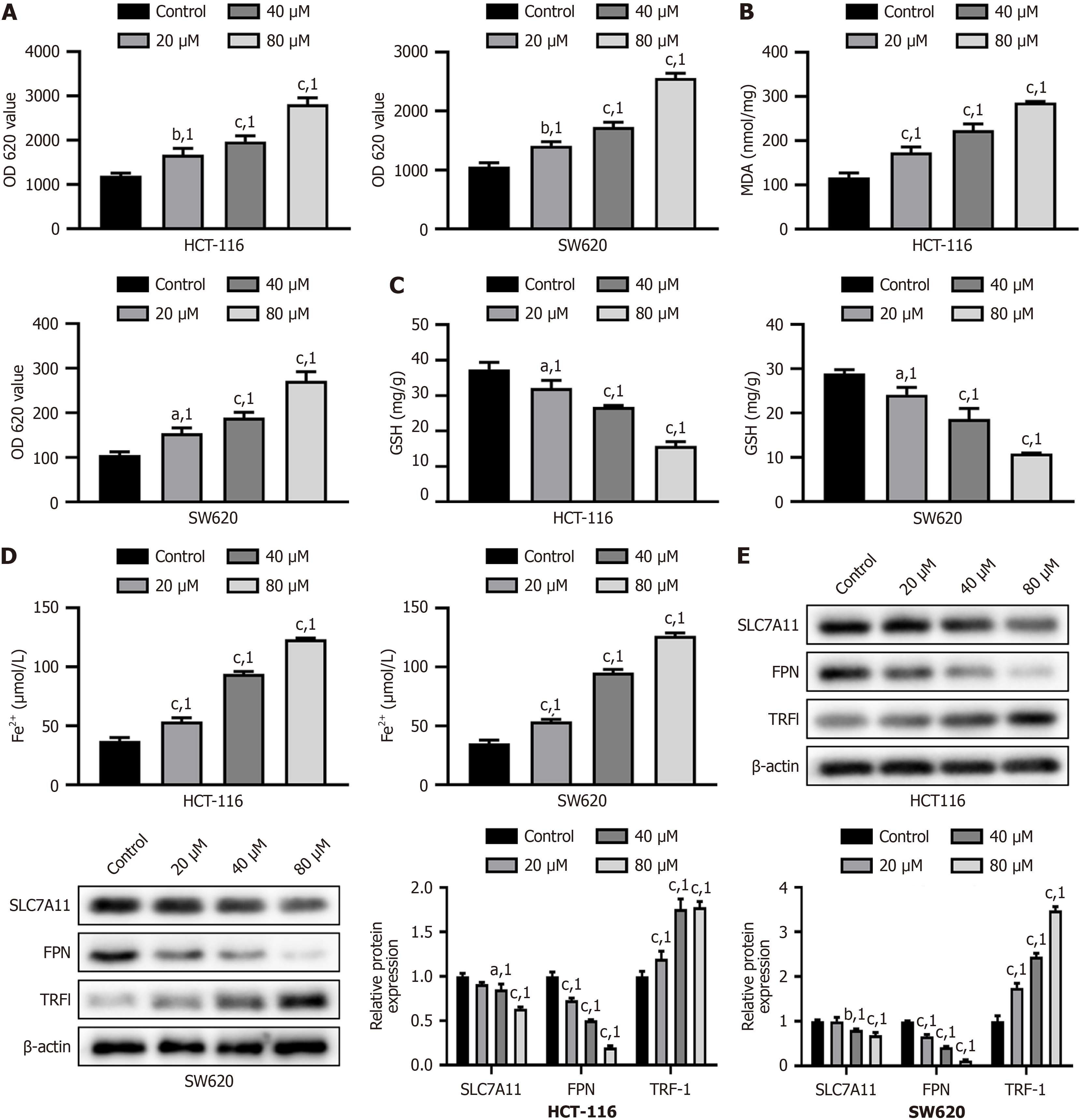
Next, we assessed the oxidative stress in cells by measuring ROS, MDA, and GSH levels. Simvastatin increased ROS and MDA levels in a dose-dependent manner and decreased GSH levels (P < 0.001, Figure 2A-C) in HCT-116 and SW620 cells. Additionally, after simvastatin treatment, we observed a dose-dependent increase in Fe2+ levels, along with downregulation of SLC7A11 and FPN, and upregulation of TRF1 (P < 0.001, Figure 2D and E). Overall, simvastatin induced oxidative stress in HCT-116 and SW620 cells by increasing ROS and MDA levels, while decreasing GSH levels, and caused a dose-dependent increase in Fe2+ content and changes in the expression of related proteins.
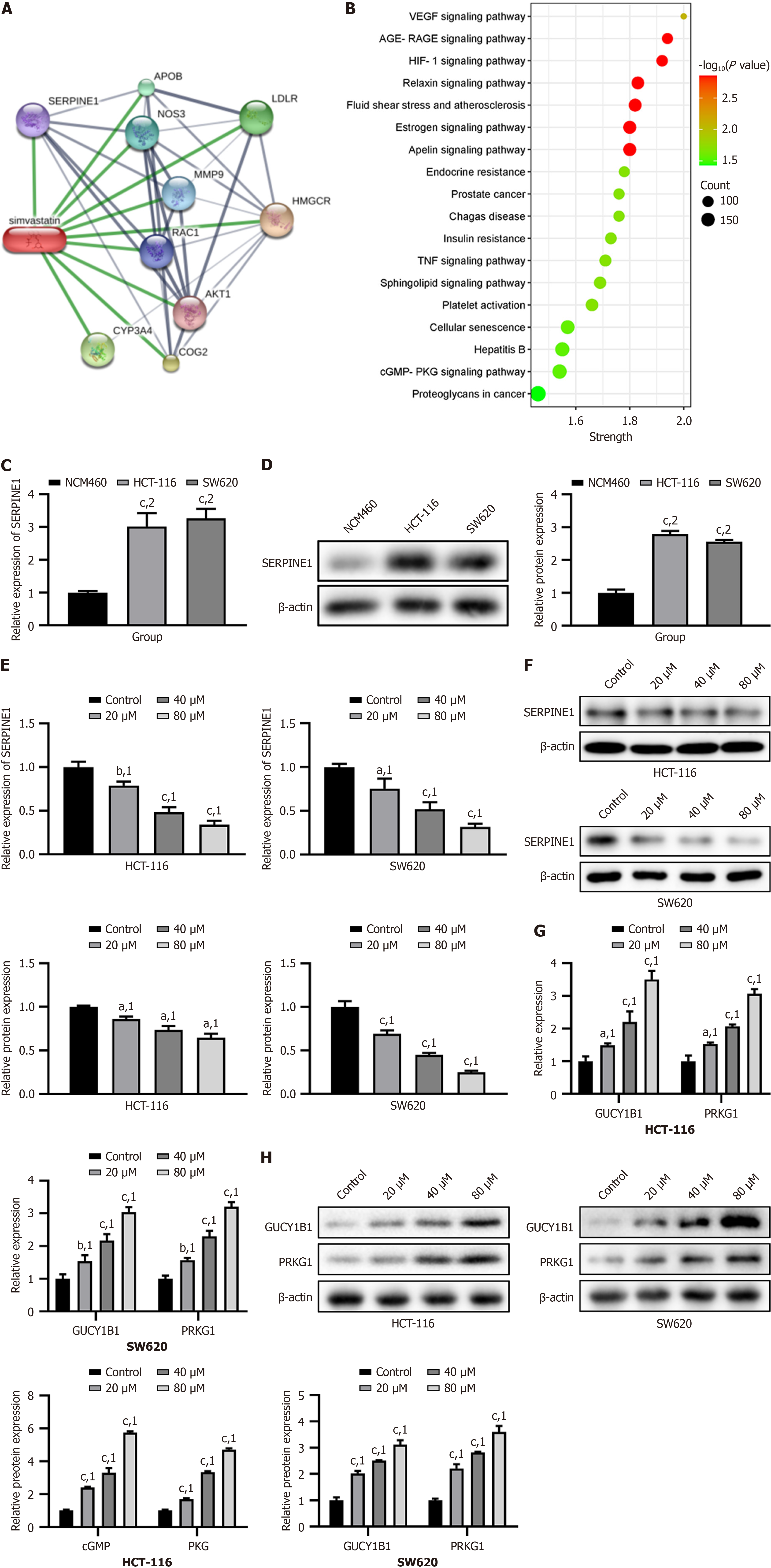
Next, we used the STITCH and STRING databases to identify the core target of simvastatin. The PPI network revealed that SERPINE1, with the highest degree, was the core target of simvastatin (Figure 3A). The KEGG pathway enrichment analysis revealed that SERPINE1 and related genes were primarily enriched in the cGMP-PKG signaling pathway (Figure 3B). We then assessed the mRNA and protein levels of SERPINE1 in NCM460, HCT-116, and SW620 cells and found significant increases in HCT-116 and SW620 cells (P < 0.001, Figure 3C and D). After simvastatin treatment, the mRNA and protein levels of SERPINE1 decreased in a dose-dependent manner in HCT-116 and SW620 cells (P < 0.05, Figure 3E and F). GUCY1B1 is a guanylate cyclase. Its main function is to catalyze the conversion of guanosine triphosphate to cGMP. We observed an increase in GUCY1B1 and PRKG1 at both the mRNA and protein levels in HCT-116 and SW620 cells following simvastatin treatment (P < 0.001, Figure 3G and H). Simvastatin could reduce SERPINE1 expression in HCT-116 and SW620 cells in a dose-dependent manner. Simultaneously, simvastatin also increased the levels of GUCY1B1 and PRKG1 proteins related to the cGMP-PKG signaling pathway.
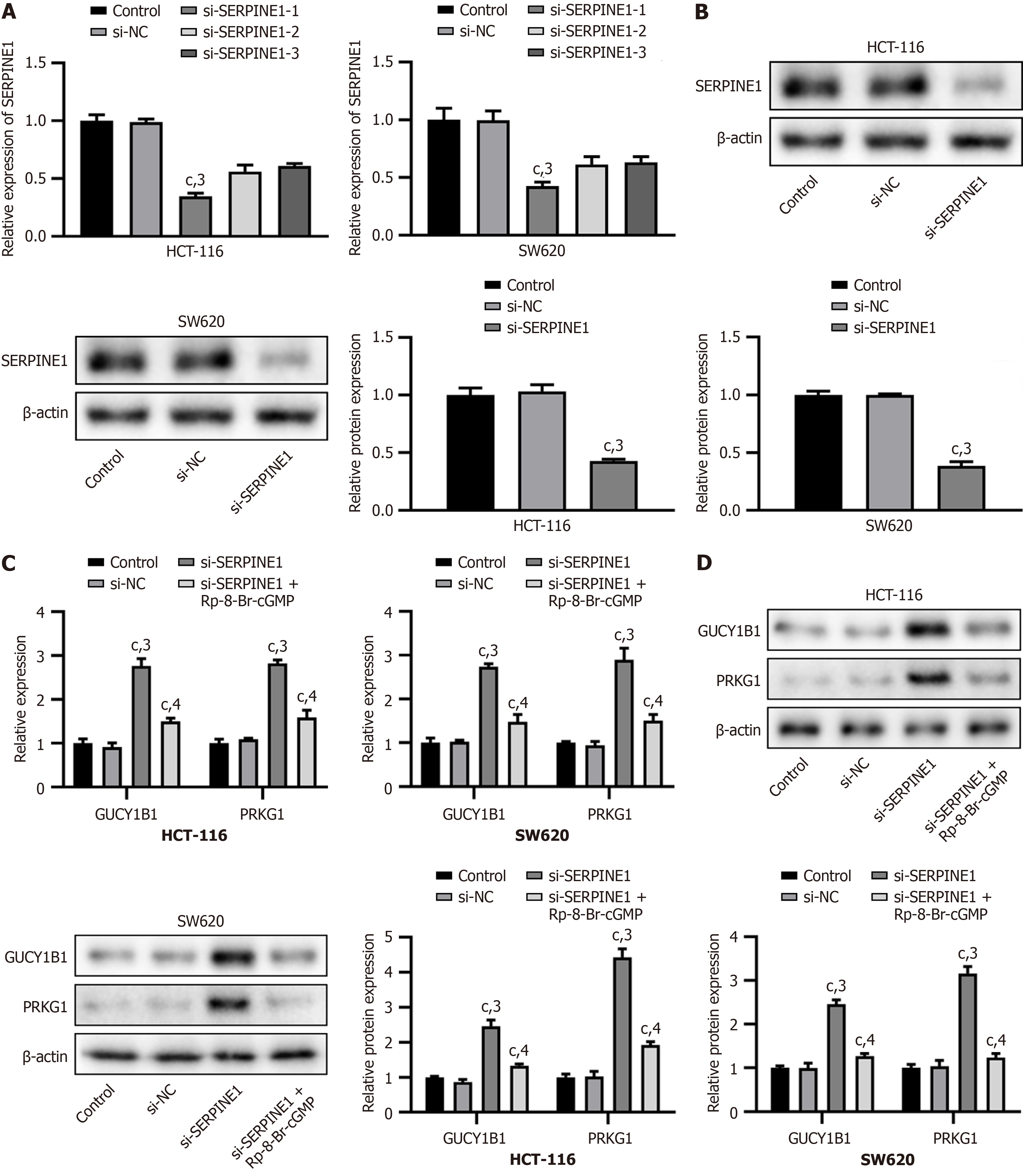
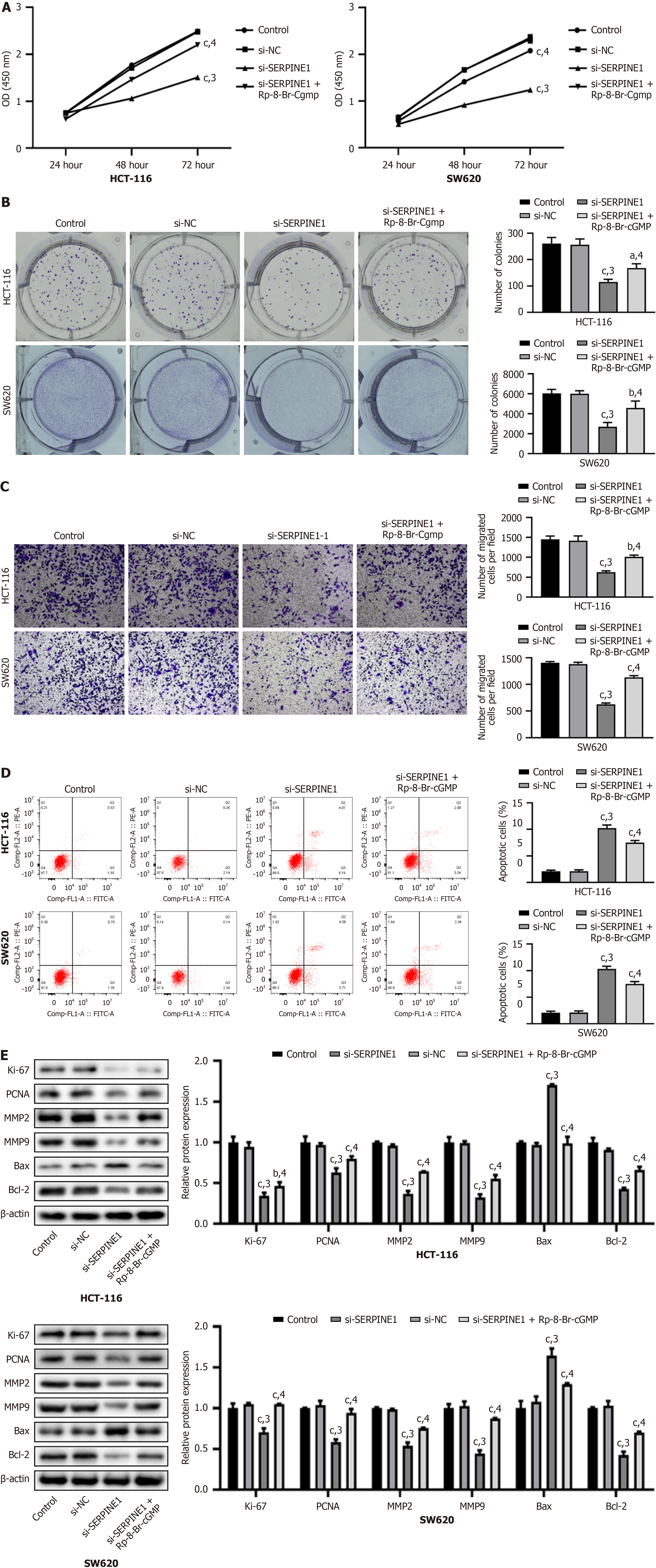
We used real-time PCR and western blot analyses to analyze the transfection efficiency of si-SERPINE1. After transfection with si-SERPINE1, we observed a significant decrease in the mRNA and protein levels of SERPINE1 (si-SERPINE1-1) in HCT-116 and SW620 cells (P < 0.001, Figure 4A and B). Additionally, the mRNA and protein levels of GUCY1B1 and PRKG1 were significantly increased following si-SERPINE1 transfection (P < 0.001); however, these levels were reversed by Rp-8-Br-cGMP, a specific inhibitor of cyclic GMP-PKG (P < 0.001, Figure 4C and D). Transfection with si-SERPINE1 significantly reduced SERPINE1 levels in HCT-116 and SW620 cells, while increasing GUCY1B1 and PRKG1 levels, which were subsequently reversed by the PKG inhibitor Rp-8-Br-cGMP.
Transfection with si-SERPINE1 significantly reduced cell viability and proliferation, as evident from the results of the CCK-8 and colony formation assays (P < 0.001); however, these effects were counteracted by Rp-8-Br-cGMP (P < 0.05, Figure 5A and B). The results of the Transwell assay indicated a notable decrease in cell migration following si-SERPINE1 transfection (P < 0.001), which was restored by Rp-8-Br-cGMP (P < 0.001, Figure 5C). Additionally, flow cytometry showed a significant increase in apoptosis after si-SERPINE1 transfection (P < 0.001), which was reversed by Rp-8-Br-cGMP (P < 0.001, Figure 5D). Following si-SERPINE1 transfection, we observed a downregulation of Ki-67, PCNA,
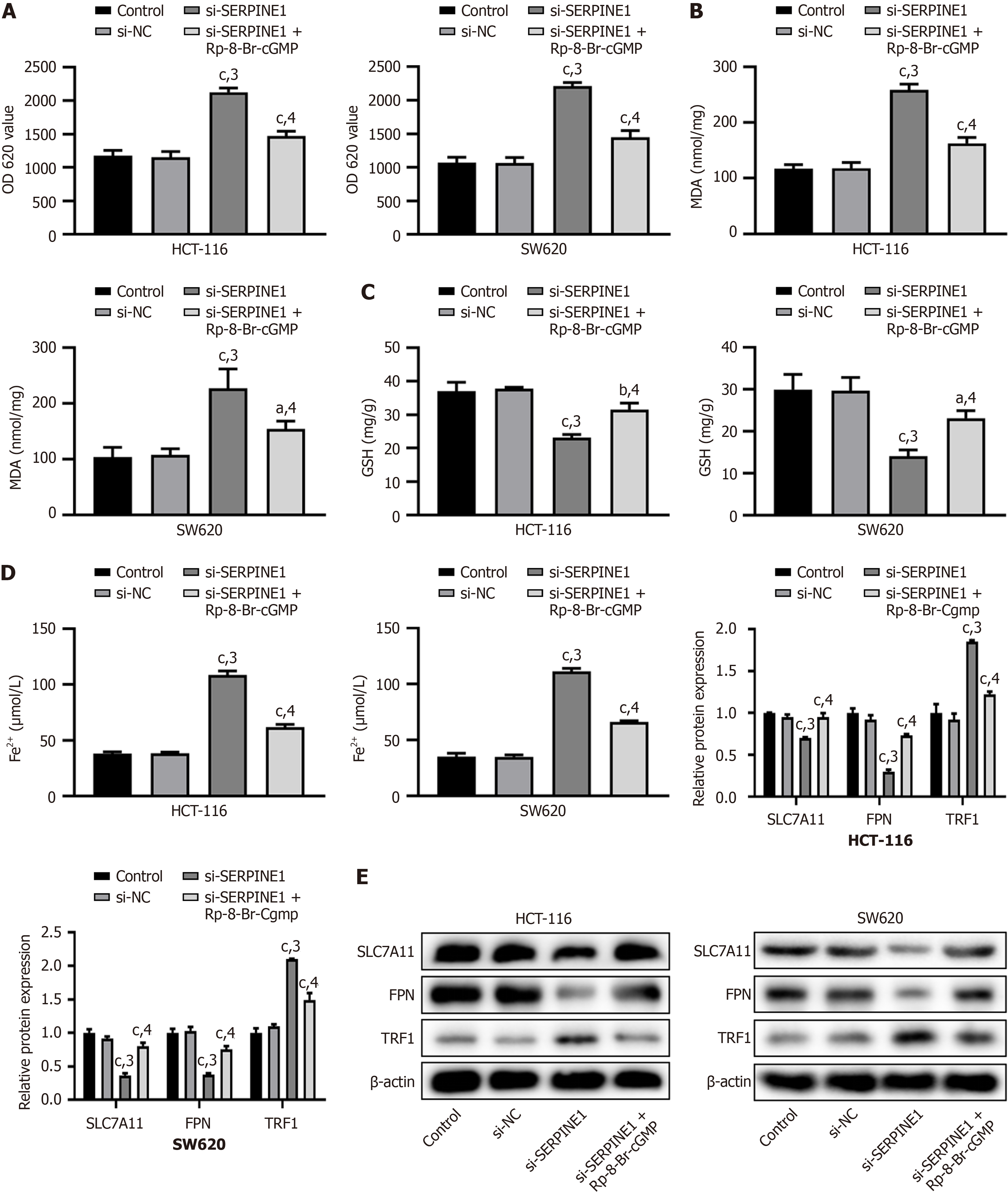
Transfection with si-SERPINE1 led to increased ROS and MDA levels and decreased GSH levels (P < 0.001); however, these changes were reversed by Rp-8-Br-cGMP (P < 0.001, Figure 6A-C) in HCT-116 and SW620 cells. Additionally, following si-SERPINE1 transfection, we observed an increase in Fe2+ levels (P < 0.001), along with downregulation of SLC7A11 and FPN, and upregulation of TRF1 (P < 0.001), but these effects were also reversed by Rp-8-Br-cGMP (P < 0.001, Figure 6D and E). Transfection with si-SERPINE1 increased ROS and MDA levels, decreased GSH levels, elevated Fe2+ levels, and altered the expression of key proteins, and all these changes were reversed by the PKG inhibitor Rp-8-Br-cGMP.
Colon cancer remains the leading cause of cancer-related morbidity and mortality worldwide[23,24]. Despite advances in treatment, understanding the mechanisms underlying colon cancer progression and searching for effective therapeutic agents are crucial. Simvastatin has been studied for its ability to influence cancer cell proliferation, migration, and apoptosis[25,26]. In this study, we investigated the effects of simvastatin on colon cancer cell lines (HCT-116 and SW620) and elucidated the underlying mechanisms involving the SERPINE1 and cGMP/PKG signaling pathways.
Our findings indicated that simvastatin effectively inhibited cell viability and proliferation, reduced migration, and promoted apoptosis in colon cancer cells in a dose-dependent manner. This was accompanied by an increase in oxidative stress markers and a significant downregulation of SERPINE1, revealing its role as a core target of simvastatin. The activation of the cGMP/PKG signaling pathway further supports these effects. SERPINE1 knockdown enhanced oxidative stress and ferroptosis, highlighting its pivotal role in modulating the response to simvastatin treatment.
Simvastatin treatment led to a significant reduction in the viability and proliferation of colon cancer cells, corroborating previous studies showing that simvastatin can inhibit cell growth in various cancer types[27,28]. The mechanisms underlying this effect may include the downregulation of proliferation markers, such as Ki-67 and PCNA, which is consistent with findings in a previous study, wherein similar trends were observed in lung cancer cells treated with simvastatin[29]. The ability of simvastatin to induce apoptosis in HCT-116 and SW620 cells is particularly noteworthy as it emphasizes the potential of statins to promote programmed cell death in malignancies. This effect may be attributed to multiple mechanisms, including inhibition of the mevalonate pathway, which disrupts essential cellular functions in cancer cells. Additionally, the role of simvastatin in modulating the inflammatory pathways and in enhancing the immune response could further contribute to its anticancer effects. Exploring the synergistic potential of simvastatin and conventional therapies may lead to improved treatment strategies, highlighting its potential as an adjunct in cancer management.
The ability of simvastatin to decrease cell migration, as shown by the Transwell assay results, aligns with previous studies suggesting that simvastatin can impede cancer cell metastasis[30]. Downregulation of MMP2 and MMP9, which are critical players in the metastatic process, highlights the therapeutic potential of simvastatin in preventing the spread of colon cancer. The increase in apoptotic markers, particularly Bax, reinforces the notion that simvastatin induces both growth inhibition and antimetastatic effects[31].
Our findings that simvastatin increases ROS and MDA levels while decreasing GSH levels suggest a shift toward oxidative stress, consistent with previous reports highlighting the role of oxidative stress in cancer therapy[32]. This phenomenon may promote apoptosis and ferroptosis in colon cancer cells. The observed increase in Fe2+ levels post-simvastatin treatment is consistent with findings from studies suggesting that iron dysregulation can enhance oxidative stress and promote ferroptosis[33].
Identification of SERPINE1 as a core target of simvastatin reinforces its significance in cancer biology. The downregulation of SERPINE1 in response to simvastatin is a critical finding as SERPINE1 has been implicated in cancer progression and metastasis[34]. The activation of the cGMP/PKG signaling pathway following SERPINE1 knockdown suggests a novel regulatory mechanism through which simvastatin exerts its anticancer effects. Previous research has indicated that cGMP signaling can mediate various cellular processes, including apoptosis and migration[35], thus supporting our findings.
Knockdown experiments using si-SERPINE1 provided compelling evidence that SERPINE1 plays a critical role in modulating the effects of simvastatin. The increased levels of GUCY1B1 and PRKG1 following SERPINE1 knockdown, and the reversal of these effects by the PKG inhibitor Rp-8-Br-cGMP, highlight the intricate interplay between these signaling pathways. This finding is particularly important as it suggests that targeting SERPINE1 may enhance the therapeutic efficacy of simvastatin in colon cancer treatment.
One of the strengths of this study is the comprehensive approach used to elucidate the mechanisms by which simvastatin affects colon cancer cells, including a range of assays that assess viability, proliferation, migration, apoptosis, and oxidative stress. In addition, the use of multiple cell lines enhanced the robustness of our findings. The limitations of the study include the lack of in vivo experiments, which are necessary to confirm the translational potential of these results. Furthermore, although this study identified SERPINE1 as a core target, further exploration of other potential targets and pathways affected by simvastatin would provide a more holistic view of its anticancer mechanisms.
In conclusion, our study demonstrates that simvastatin effectively inhibits the proliferation and migration of colon cancer cells while promoting apoptosis through modulation of SERPINE1 and activation of the cGMP/PKG signaling pathway. These findings not only support the potential use of simvastatin as an adjunct therapeutic agent in colon cancer but also underscore the need for further research to explore its clinical applicability. Delving deeper into the mechanisms of action of simvastatin may ultimately help identify novel strategies for the treatment and management of colon cancer.
In conclusion, our study demonstrates that simvastatin effectively inhibits the proliferation and migration of colon cancer cells while promoting apoptosis through the modulation of SERPINE1 and activation of the cGMP/PKG signaling pathway. These findings not only support the potential use of simvastatin as an adjunct therapeutic agent in colon cancer but also underscore the need for further research to explore its clinical applicability. By delving deeper into the mechanisms of simvastatin’s action, we may ultimately identify novel strategies for the treatment and management of colon cancer.
| 1. | Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14:101174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 687] [Cited by in RCA: 1339] [Article Influence: 334.8] [Reference Citation Analysis (5)] |
| 2. | Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 1574] [Article Influence: 262.3] [Reference Citation Analysis (2)] |
| 3. | Marzouk O, Schofield J. Review of histopathological and molecular prognostic features in colorectal cancer. Cancers (Basel). 2011;3:2767-2810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Testa U, Pelosi E, Castelli G. Colorectal cancer: genetic abnormalities, tumor progression, tumor heterogeneity, clonal evolution and tumor-initiating cells. Med Sci (Basel). 2018;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 177] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 5. | Luo C, Cen S, Ding G, Wu W. Mucinous colorectal adenocarcinoma: clinical pathology and treatment options. Cancer Commun (Lond). 2019;39:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 189] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 6. | Pretzsch E, Bösch F, Neumann J, Ganschow P, Bazhin A, Guba M, Werner J, Angele M. Mechanisms of Metastasis in Colorectal Cancer and Metastatic Organotropism: Hematogenous versus Peritoneal Spread. J Oncol. 2019;2019:7407190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 189] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 7. | Ratto C, Sofo L, Ippoliti M, Merico M, Doglietto GB, Crucitti F. Prognostic factors in colorectal cancer. Literature review for clinical application. Dis Colon Rectum. 1998;41:1033-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 103] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Ranasinghe R, Mathai M, Zulli A. A synopsis of modern - day colorectal cancer: Where we stand. Biochim Biophys Acta Rev Cancer. 2022;1877:188699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Saad AM, Abdel-Rahman O. Initial systemic chemotherapeutic and targeted therapy strategies for the treatment of colorectal cancer patients with liver metastases. Expert Opin Pharmacother. 2019;20:1767-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Andrei P, Battuello P, Grasso G, Rovera E, Tesio N, Bardelli A. Integrated approaches for precision oncology in colorectal cancer: The more you know, the better. Semin Cancer Biol. 2022;84:199-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 11. | Lam M, Lum C, Latham S, Tipping Smith S, Prenen H, Segelov E. Refractory Metastatic Colorectal Cancer: Current Challenges and Future Prospects. Cancer Manag Res. 2020;12:5819-5830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Sassano A, Platanias LC. Statins in tumor suppression. Cancer Lett. 2008;260:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Li G, Zheng J, Xu B, Ling J, Qiu W, Wang Y. Simvastatin inhibits tumor angiogenesis in HER2-overexpressing human colorectal cancer. Biomed Pharmacother. 2017;85:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Relja B, Meder F, Wilhelm K, Henrich D, Marzi I, Lehnert M. Simvastatin inhibits cell growth and induces apoptosis and G0/G1 cell cycle arrest in hepatic cancer cells. Int J Mol Med. 2010;26:735-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | MacDougall DA, Pugh SD, Bassi HS, Lotteau S, Porter KE, Calaghan S. Simvastatin Promotes Cardiac Myocyte Relaxation in Association with Phosphorylation of Troponin I. Front Pharmacol. 2017;8:203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Wang Y, Wang J, Gao J, Ding M, Li H. The expression of SERPINE1 in colon cancer and its regulatory network and prognostic value. BMC Gastroenterol. 2023;23:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 17. | Li S, Wei X, He J, Tian X, Yuan S, Sun L. Plasminogen activator inhibitor-1 in cancer research. Biomed Pharmacother. 2018;105:83-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 18. | Pavón MA, Arroyo-Solera I, Céspedes MV, Casanova I, León X, Mangues R. uPA/uPAR and SERPINE1 in head and neck cancer: role in tumor resistance, metastasis, prognosis and therapy. Oncotarget. 2016;7:57351-57366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 19. | Wang S, Pang L, Liu Z, Meng X. SERPINE1 associated with remodeling of the tumor microenvironment in colon cancer progression: a novel therapeutic target. BMC Cancer. 2021;21:767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 20. | Ren Y, Zheng J, Yao X, Weng G, Wu L. Essential role of the cGMP/PKG signaling pathway in regulating the proliferation and survival of human renal carcinoma cells. Int J Mol Med. 2014;34:1430-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Jiang L, Lan T, Chen Y, Sang J, Li Y, Wu M, Tao Y, Wang Y, Qian H, Gu L. PKG II inhibits EGF/EGFR-induced migration of gastric cancer cells. PLoS One. 2013;8:e61674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Li N, Xi Y, Tinsley HN, Gurpinar E, Gary BD, Zhu B, Li Y, Chen X, Keeton AB, Abadi AH, Moyer MP, Grizzle WE, Chang WC, Clapper ML, Piazza GA. Sulindac selectively inhibits colon tumor cell growth by activating the cGMP/PKG pathway to suppress Wnt/β-catenin signaling. Mol Cancer Ther. 2013;12:1848-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 23. | Aihaiti Y, Song Cai Y, Tuerhong X, Ni Yang Y, Ma Y, Shi Zheng H, Xu K, Xu P. Therapeutic Effects of Naringin in Rheumatoid Arthritis: Network Pharmacology and Experimental Validation. Front Pharmacol. 2021;12:672054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 24. | Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3058] [Cited by in RCA: 3298] [Article Influence: 412.3] [Reference Citation Analysis (3)] |
| 25. | Wang F, Liu W, Ning J, Wang J, Lang Y, Jin X, Zhu K, Wang X, Li X, Yang F, Ma J, Xu S. Simvastatin Suppresses Proliferation and Migration in Non-small Cell Lung Cancer via Pyroptosis. Int J Biol Sci. 2018;14:406-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 26. | Chen MC, Tsai YC, Tseng JH, Liou JJ, Horng S, Wen HC, Fan YC, Zhong WB, Hsu SP. Simvastatin Inhibits Cell Proliferation and Migration in Human Anaplastic Thyroid Cancer. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Spampanato C, De Maria S, Sarnataro M, Giordano E, Zanfardino M, Baiano S, Cartenì M, Morelli F. Simvastatin inhibits cancer cell growth by inducing apoptosis correlated to activation of Bax and down-regulation of BCL-2 gene expression. Int J Oncol. 2012;40:935-941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 28. | Wang G, Cao R, Wang Y, Qian G, Dan HC, Jiang W, Ju L, Wu M, Xiao Y, Wang X. Simvastatin induces cell cycle arrest and inhibits proliferation of bladder cancer cells via PPARγ signalling pathway. Sci Rep. 2016;6:35783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 29. | Ma W, Wei S, Li Q, Zeng J, Xiao W, Zhou C, Yoneda KY, Zeki AA, Li T. Simvastatin Overcomes Resistance to Tyrosine Kinase Inhibitors in Patient-derived, Oncogene-driven Lung Adenocarcinoma Models. Mol Cancer Ther. 2024;23:700-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 30. | Mandal CC, Ghosh-Choudhury N, Yoneda T, Choudhury GG, Ghosh-Choudhury N. Simvastatin prevents skeletal metastasis of breast cancer by an antagonistic interplay between p53 and CD44. J Biol Chem. 2011;286:11314-11327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 31. | Schointuch MN, Gilliam TP, Stine JE, Han X, Zhou C, Gehrig PA, Kim K, Bae-Jump VL. Simvastatin, an HMG-CoA reductase inhibitor, exhibits anti-metastatic and anti-tumorigenic effects in endometrial cancer. Gynecol Oncol. 2014;134:346-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Hecht F, Pessoa CF, Gentile LB, Rosenthal D, Carvalho DP, Fortunato RS. The role of oxidative stress on breast cancer development and therapy. Tumour Biol. 2016;37:4281-4291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 179] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 33. | Mancardi D, Mezzanotte M, Arrigo E, Barinotti A, Roetto A. Iron Overload, Oxidative Stress, and Ferroptosis in the Failing Heart and Liver. Antioxidants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 91] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 34. | Chen S, Li Y, Zhu Y, Fei J, Song L, Sun G, Guo L, Li X. SERPINE1 Overexpression Promotes Malignant Progression and Poor Prognosis of Gastric Cancer. J Oncol. 2022;2022:2647825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 35. | Guo D, Tan YC, Wang D, Madhusoodanan KS, Zheng Y, Maack T, Zhang JJ, Huang XY. A Rac-cGMP signaling pathway. Cell. 2007;128:341-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |