Copyright
©2014 Baishideng Publishing Group Inc.
World J Hepatol. Aug 27, 2014; 6(8): 580-595
Published online Aug 27, 2014. doi: 10.4254/wjh.v6.i8.580
Published online Aug 27, 2014. doi: 10.4254/wjh.v6.i8.580
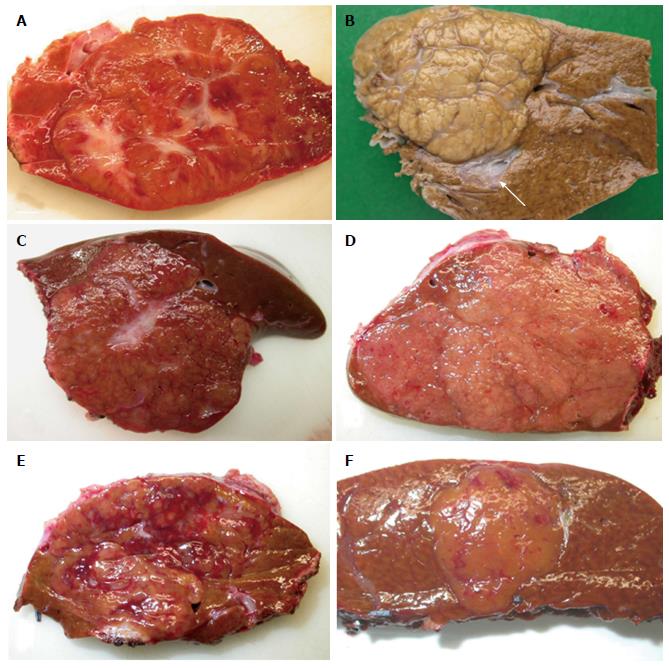
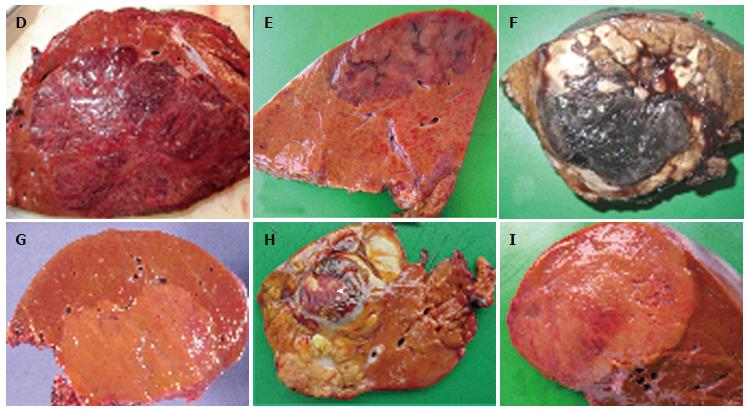
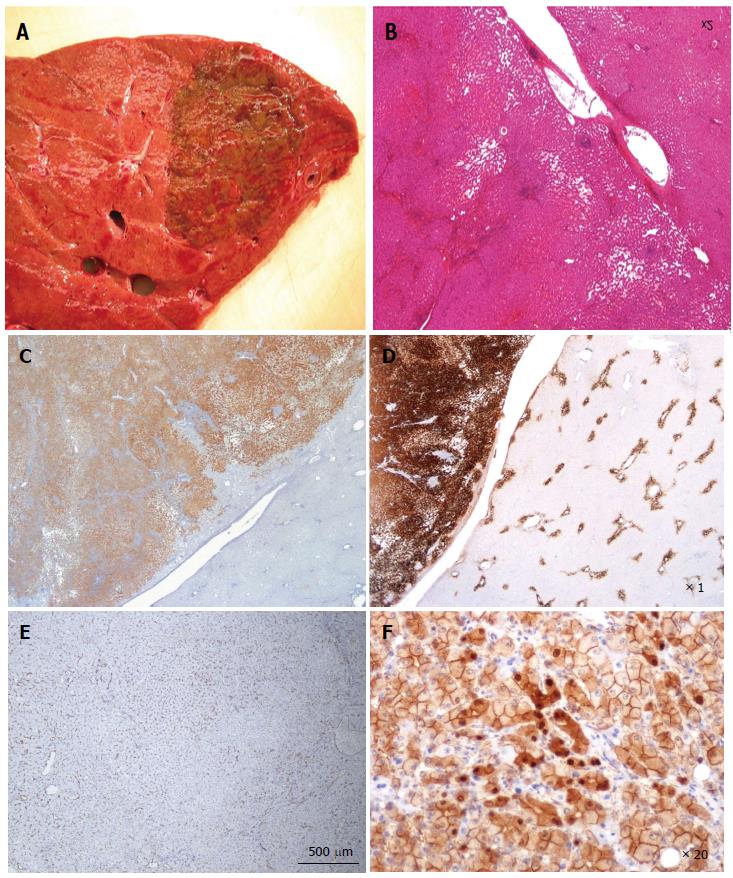
Figure 1 Focal nodular hyperplasia the macroscopic aspects.
A: Man born in 1988; 100 kg/1.95 m; abdominal pain; abnormal liver function tests; magnetic resonance imaging (MRI): liver mass 9.5 cm: Focal nodular hyperplasia (FNH)/hepatocellular adenoma (HCA) (bisegmentectomy in 2009). Fresh specimen: typical aspect of FNH: Tan, vaguely plurinodular tumor, non encapsulated, with central stellate scar. This macroscopic aspect is typical and the diagnosis of FNH is evident. The diagnosis was confirmed on HE and CK7. B: Woman born in 1951; acute abdominal pain followed by discomfort and pain on abdominal palpation; ultrasound (US): 2 nodules, largest one 3.5 cm. Magnetic resonance imaging (MRI): FNH (left hepatectomy in 2008). Fixed specimen: plurinodular tumor with thin fibrous bands, non encapsulated but well demarcated from surrounding liver parenchyma, with large portal tract at the interface (arrow). The diagnosis of FNH is evident. The diagnosis was confirmed on HE and CK7. C: Woman born in 1986; abdominal pain; MRI 2007: FNH 6.4 cm close to the biliary convergence; US in 2000: hemangioma 15 mm; 2004: 4.5 cm (tumorectomy in 2007). Fresh specimen: pedunculated irregular nodule with eccentric fibrous scar, well demarcated from the surrounding liver. The diagnosis of FNH is likely. D: Woman born in 1965; oral contraceptives for 18 years; abdominal pain; imaging (6 cm): FNH [surgery in 2005 (tumorectomy)]. Fresh specimen: clear-tan, vaguely plurinodular tumor, without clear-cut fibrous scar. The diagnosis of FNH is not self-evident. The diagnosis was confirmed on HE, CK7 and glutamine synthase (GS). E: Woman born in 1961; abnormal liver function tests; liver US: nodule 5.5 cm, MRI and US favors HCA over FNH (segmentectomy VII in 2009). Fresh specimen: irregular cut surface with tan nodules separated by congestive/reddish, atrophic areas. The diagnosis of FNH is unlikely. F: Woman born in 1956; check-up for arterial hypertension; liver imaging (2 cm nodule): probable HCA (tumorectomy in 2003) (other nodules were found). Fresh specimen: well-limited, non encapsulated, smooth nodule, with small reddish areas, without any fibrous bands or scar visible. The diagnosis of FNH is unlikely. The diagnosis was confirmed by HE, CK7 and GS.
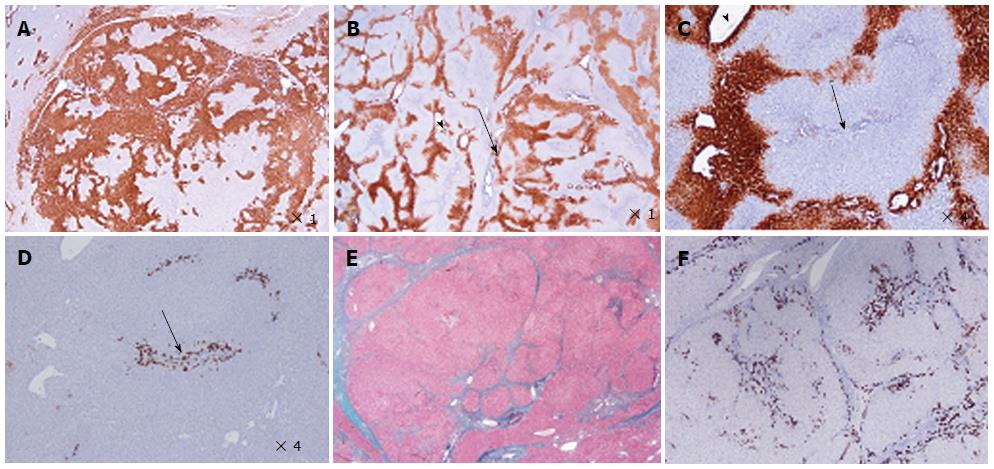
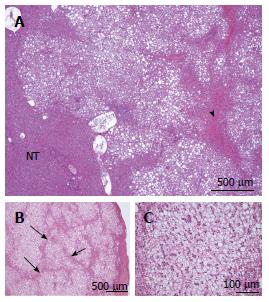
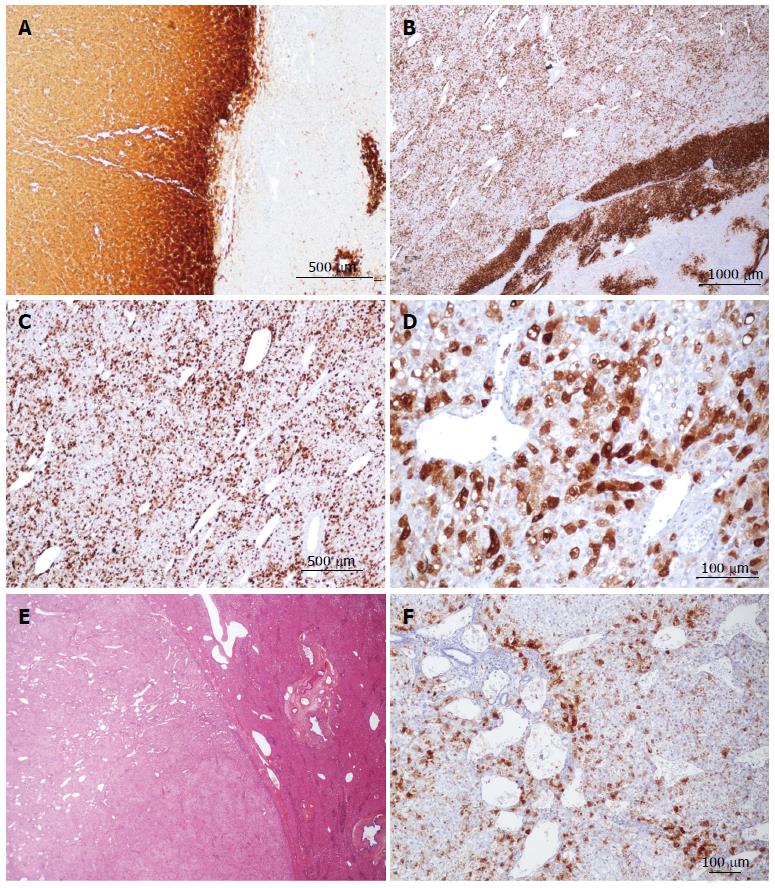
Figure 2 Focal nodular hyperplasia microscopic typical features on Masson’s trichrome, glutamine synthase and CK7 immunostainings.
A: Same patient as Figure 1C. Glutamine synthase (GS) immunostaining: typical aspect of focal nodular hyperplasia (FNH) with large anastomosed positive areas in a “map-like” pattern[11]. B-D: Woman born in 1969; abnormal liver function tests (GGT); ultrasound (US): nodule interpreted as a hemangioma, computed tomography scan and magnetic resonance imaging: FNH 8 cm, segment VIII with minor dilatation of the right biliary tree (compression by the tumor). Left lobectomy + segmentectomy VIII in 2002 (other nodule segment II: 0.5 cm). B, C: GS immunostaining: typical aspect of FNH with anastomosed positive areas often centered by veins (arrowhead) and at distance of fibrous bands (arrows). C and D are from 2 serial sections. D: CK7 immunostaining: ductular reaction at the periphery of fibrous bands. E, F: Same patient as Figure 1C. E: Masson’s trichrome - fibrous bands surrounding benign hepatocytic nodules of different sizes. F: CK7 immunostaining - prominent ductular reaction at the junction between parenchymal nodules and fibrous bands. Typical aspect of FNH.
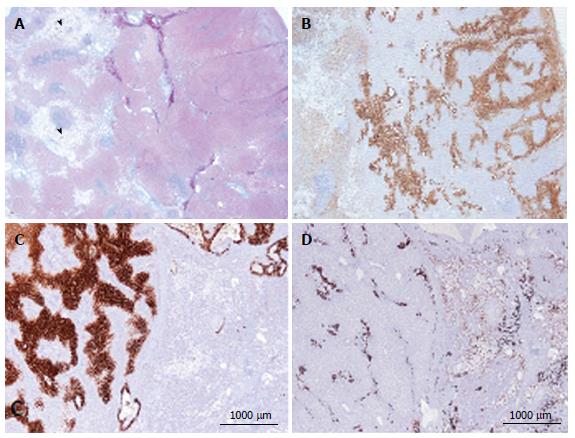
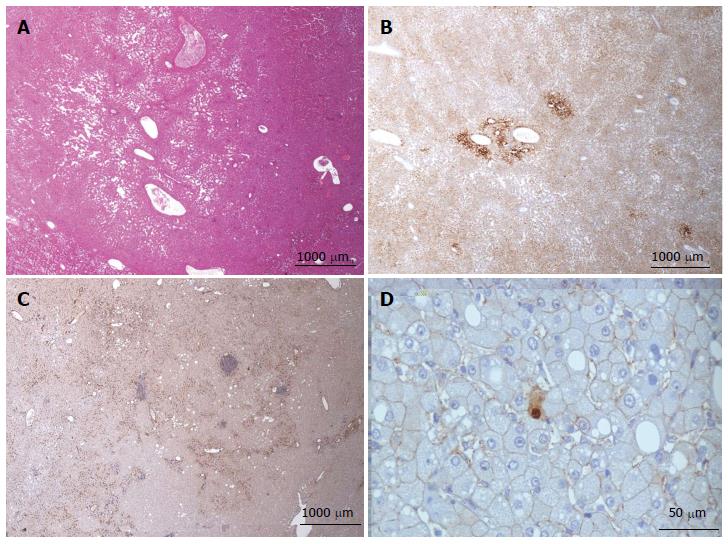
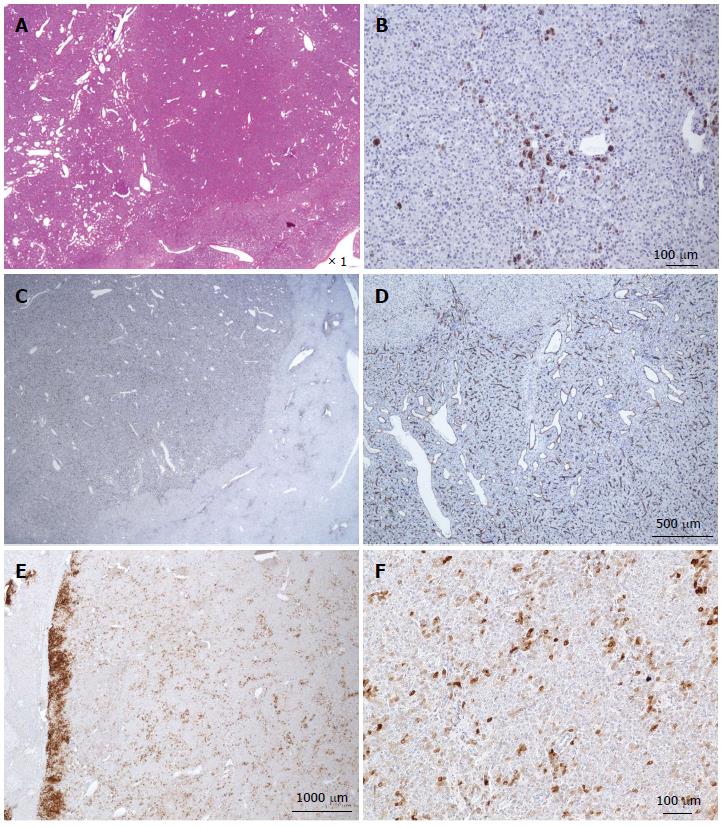
Figure 3 Focal nodular hyperplasia microscopic atypical features on Masson’s trichrome and glutamine synthase immunostaining.
A, B: Same patient as Figure 1E. A: Masson’s trichrome - large areas of sinusoidal dilatation (arrowhead), nearby solid hepatocytic areas (right) with thin, short fibrotic bands. B: Glutamine synthase (GS) immunostaining - typical aspect of focal nodular hyperplasia (FNH) with anastomosed positive areas in a “map-like pattern” in the nodular area (right); no staining in the sinusoidal dilation area (left). This aspect is very unusual. This nodule should not be interpreted as a mixed tumor (part FNH and part hepatocellular adenoma) and should not be called “telangiectatic FNH”[23,24]. A better term could be FNH with major sinusoidal dilatation. C, D: Woman born in 1969, 2003; 2 FNH: first hepatic resection (tumorectomy in 2003 for a 7-cm FNH). In 2004, persistence of abdominal discomfort (no change in size of the 7 cm FNH). Right hepatectomy. C: Typical GS staining (left); no GS staining in the area of sinusoidal dilatation (right). D: Obvious ductular reaction on the CK7 immunostaining. Although the 2 above cases are very rare, FNH with areas of sinusoidal dilatation are seen occasionally.
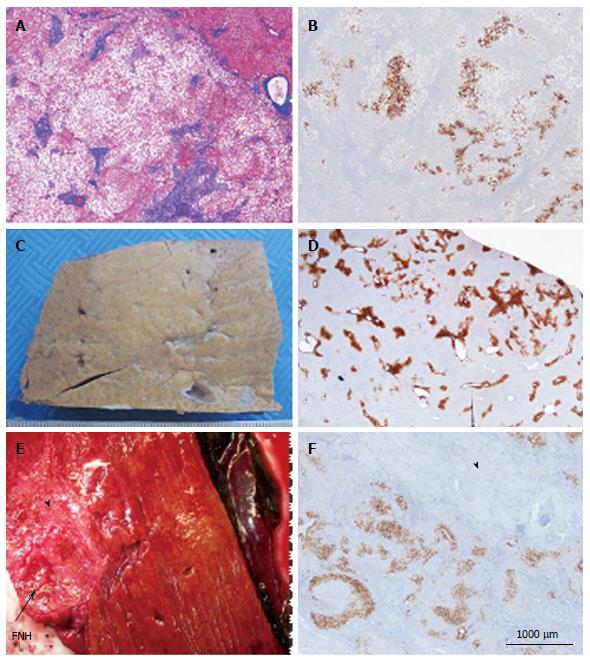
Figure 4 Other aspects (steatotic, pre-focal nodular hyperplasia, regressing focal nodular hyperplasia).
A, B: Woman born in 1959; heavy alcohol consumption, tobacco; hepatomegaly, no evidence for cirrhosis on liver function tests, virus negative. Computed tomography scan: nodule 3.5 cm and others (1.5, 0.3 and 0.8 cm). No firm radiological diagnosis: ?focal nodular hyperplasia (FNH)/hepatocellular adenoma (HCA)/HCC, left hepatectomy in 2001. A: Masson’s trichrome - the nodule is composed of benign steatotic hepatocytes separated by fibrous bands. Steatosis is not a rare event in FNH. B: Glutamine synthase (GS) immunostaining - anastomosed GS positive areas, favoring the diagnosis of steatotic FNH. The diagnosis of FNH remains doubtful, however. In this case, the diagnoses of HNF1A mutated HCA, inflammatory HCA, β-HCA and β-IHCA were ruled out (specific IHC markers were negative). The diagnosis of unclassified HCA cannot, however, be ruled out. Molecular analysis is necessary to confirm the diagnosis. C, D: Woman born in 1950; abdominal pain: gallbladder lithiasis; Magnetic resonance imaging (MRI): 2.5 cm nodule, probably an HCA. Left hepatectomy plus cholecystectomy in 2000. C: Fixed specimen - subcapsular, not well defined nodule barely visible, clearer than the surrounding liver. D: GS immunostaining - the positive perivenular areas are slightly but significantly larger than in the surrounding normal liver; this aspect is interpreted as a “pre-FNH” without fibrosis and nodulation. Pre FNH is probably not a rare entity but to-day there is no consensus concerning its denomination[9]. This type of lesion has been named in congenital vascular abnormalities such as Rendu-Osler disease. E, F: Woman born in 1948; intrahepatic hemorrhagic rupture of a large nodule of segment VII in 2009. MRI favors HCC, additional 4 nodules known since 2005 and interpreted as FNH. Size of the FNH nodule resected with the HCC has decreased significantly. Right hepatectomy (HCC + 1 additional not identified nodule, previously known as FNH). E: Fresh specimen: close to the hemorrhagic HCC, a 4 cm hard white/brown nodule, under the capsule (arrow), with irregular surface. F: GS immunostaining: limited positive areas (map-like pattern) surrounded by large areas of dense fibrous tissue (arrowhead). All these features are interpreted as a regressing FNH. This interpretation needs to be confirmed by additional cases.
Figure 5 Hepatocellular adenoma macroscopic aspects of different subtypes: A-C: Examples of HNF1A mutated hepatocellular adenoma (H-HCA).
A: Woman born in 1972; oral contraceptives for 8 years. Discovery of a liver nodule (3.5 cm). No regression after stopping contraceptives. Fear of complications by the patient. Left hepatectomy in 2008; fresh specimen: yellowish tumor, clearer than the surrounding liver. The diagnosis of H-HCA is likely but not self-evident. The diagnosis was confirmed by immunohistochemistry. B: Woman born in 1974; oral contraceptives for 12 years. Liver nodules discovered by chance on imaging and diagnosis of adenomatosis. Largest nodule 5 cm. Segmentectomy IVb 2006. Other small nodules. One nodule was an focal nodular hyperplasia (FNH). Fixed specimen of one nodule: yellowish, clear tumor, non encapsulated, contrasting with the surrounding liver. The diagnosis of H-HCA is likely. The diagnosis was confirmed by immunohistochemistry. C: Woman born in 1978. Massive right hepatomegaly discovered in the obstetric department (miscarriage at 6 wk). No oral contraception. Right hepatectomy in 2004. Fresh specimen: large irregular, mammillated tumor occupying the whole right liver. This is an exceptional case. The diagnosis was confirmed by immunohistochemistry. D-F: Examples of IHCA (D, E) and β-IHCA (F). D: Woman born in 1968; overweight, BMI > 40 kg/m2. Oral contraceptives for 18 years. Several nodules detected on imaging. Doubtful diagnosis. Surgical biopsies: HCA. Right hepatectomy in 2008 (largest nodule 10 cm). In 2009, another known HCA removed in the left liver (11 cm). Fresh specimen: reddish tumor with congestive areas. The diagnosis of inflammatory HCA (IHCA) is likely. The diagnosis was confirmed by immunohistochemistry. E: Woman born in 1956; oral contraceptives for 31 years. Biological abnormalities (inflammatory syndrome). CT scan and MRI multiple liver nodules (largest nodule 7 cm) in favor of IHCA. Surgery in 2007: bisegmentectomy VI-VII plus 2 tumorectomies. Fresh specimen: ill defined tumor with congestive strands. The diagnosis of IHCA is very likely. The diagnosis was confirmed by immunohistochemistry. F: Woman born in 1971; liver hemorrhage. Imaging: 5 nodules, largest 8 cm. No oral contraceptives; BMI 20.4 kg/m2. Segmentectomy III, VI, VIII 2007. Fixed specimen: large hematoma and a narrow viable tissue at the periphery. No obvious diagnosis. The diagnosis of HCC cannot be ruled out. The diagnosis was confirmed by immunohistochemistry. G: Example of β-catenin HCA. Woman born in 1981; one nodule 8 cm discovered by chance. Imaging HCA. Oral contraceptives for 8 years; BMI 21.1 kg/m2. Right hepatectomy 2005. Fresh specimen: well limited clear nodule. The diagnosis of HCA is likely. H-HCA and IHCA are unlikely. H: Example of HCC developed on β-IHCA. Woman born in 1934; intramuscular injection of hormones as contraceptive. Liver nodule interpreted as hemangioma, known for several years. Growth of the nodule. Segmentectomy in 2000. Fresh specimen: irregular, multinodular tumor, with a large necrotic and hemorrhagic area ( arrowhead) surrounded by a fibrous rim. The diagnosis of HCC is likely. I: Example of unclassified HCA. Woman born in 1983; abdominal pain. Imaging: one nodule 8 cm; no final diagnosis. Oral contraceptives for 8 years. BMI 20.2 kg/m2. Right hepatectomy 2007. Fresh specimen: well limited clear nodule with a pale reddish area. The diagnosis of HCA is likely. H-HCA and IHCA are unlikely.
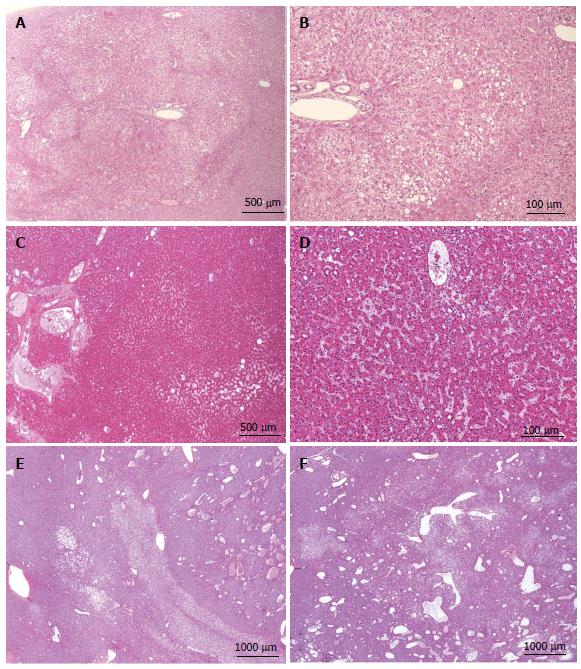
Figure 6 HNF1A mutated hepatocellular adenoma microscopic typical features.
A: Woman born in 1955; surgery in 2010 for an HCC (6 cm) developed on a non fibrotic liver. Discovery of another small nodule on the surgical specimen. No oral contraceptives; BMI 24.1 kg/m2. HE: typical aspect of HNF1A mutated hepatocellular adenoma (H-HCA): nodule with lobulated contours, made of benign hepatocytes with diffuse steatosis; some congestive areas ( arrowhead); sharp contrast with non steatotic surrounding liver. The diagnosis was confirmed by immunohistochemistry. B, C: Woman born in 1952; abdominal pain; imaging: nodule 1.8 cm, no firm diagnosis by magnetic resonance imaging. Oral contraceptives, 27 years; BMI 22.0 kg/m2. Tumorectomy in 2008. B: Nodule composed of benign, clear hepatocytes, sometimes steatotic, separated by thin strands of atrophic hepatocytes (arrow). C: Same nodule seen at higher magnification. Although clear hepatocytes are not the hallmark of H-HCA, the lobular pattern is very characteristic. It consists of steatotic or clear hepatocytes arranged in a lobular pattern separated by tumoral, atrophic, not steatotic hepatocytes. Commonly, arterioles/small arteries are seen in this space (not shown on this micrograph). The diagnosis was confirmed by immunohistochemistry.
Figure 7 HNF1a inactivated hepatocellular adenoma microscopic atypical feature.
A, B: Woman born in 1952; abnormal liver function tests; oral contraceptives 13 years; BMI 25.4; magnetic resonance imaging (MRI): adenomatosis, largest nodule 6 cm. Segmentectomy IV/VI in 2001. A: ill defined nodule composed of clear hepatocytes, with mild steatosis. B: Same nodule seen at a higher magnification. The normal portal tract from the periphery of non tumoral parenchyma is entrapped in the nodule. The diagnosis of HNF1A mutated hepatocellular adenoma (H-HCA) is less evident than in Figure 6B, C. The diagnosis was confirmed by immunohistochemistry. C, D: Woman born in 1950; abnormal liver function tests; imaging: probable FNH 12 cm. Right hepatectomy 2002. HE: proliferation of benign hepatocytes, no steatosis, mild sinusoidal dilatation. This is a rare case. The diagnosis of H-HCA cannot be suspected without performing immunohistochemistry. E, F: Woman born in 1965; past history of cancer. Abdominal pain. Oral contraceptives 10 years, BMI 21 kg/m2. MRI: Adenomatosis, largest nodule 3.9 cm. Segmentectomy and tumorectomy in 2011. HE: Proliferation of benign hepatocytes, no steatosis, mild sinusoidal dilatation, numerous ectatic vessels. As above, in this rare case the diagnosis of H-HCA cannot be suspected without immunohistochemistry, and malignant transformation has to be ruled out.
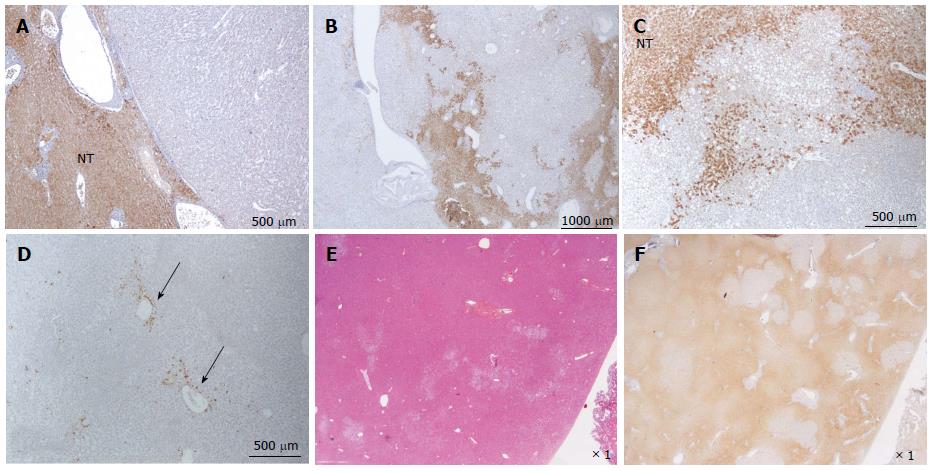
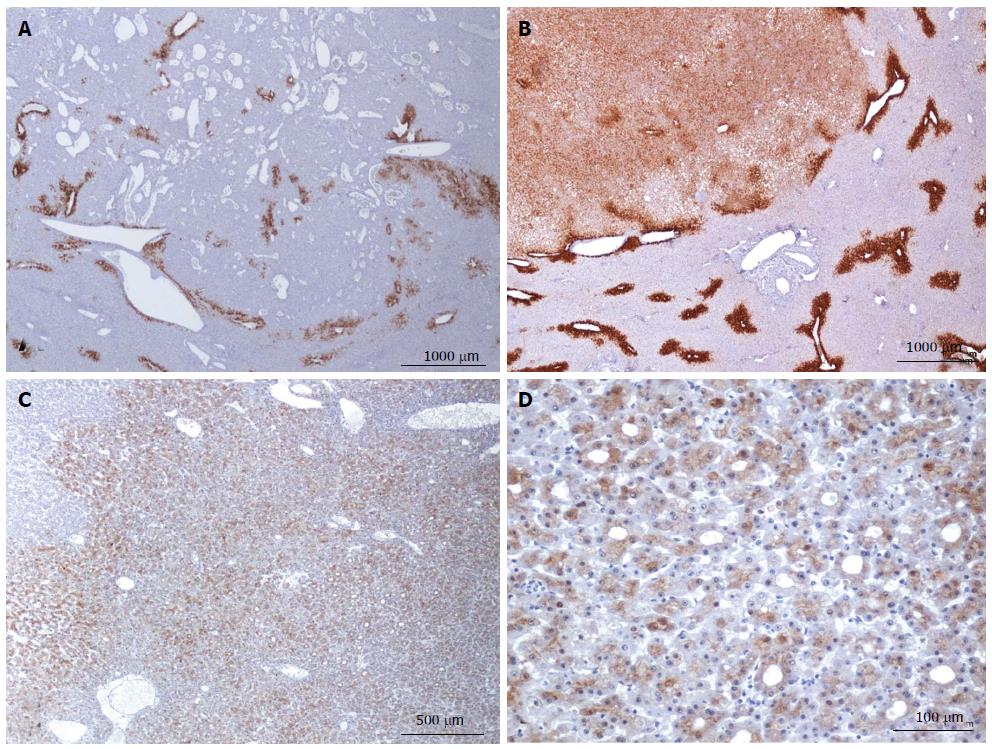
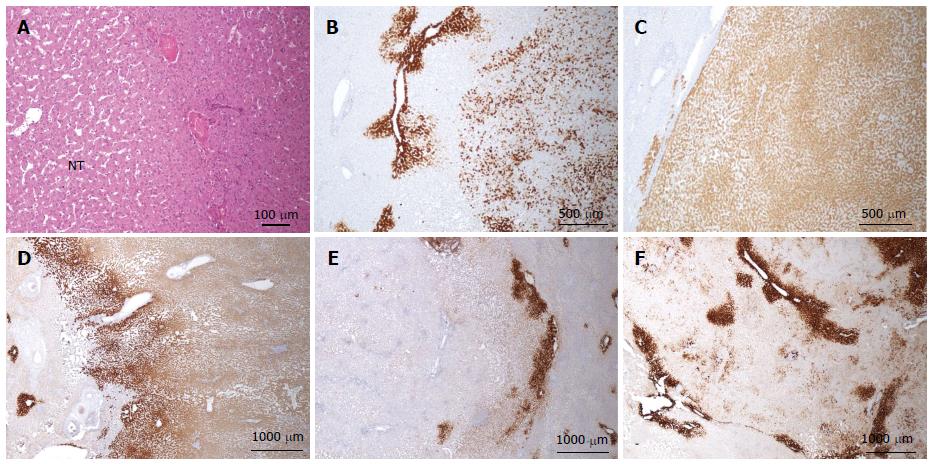
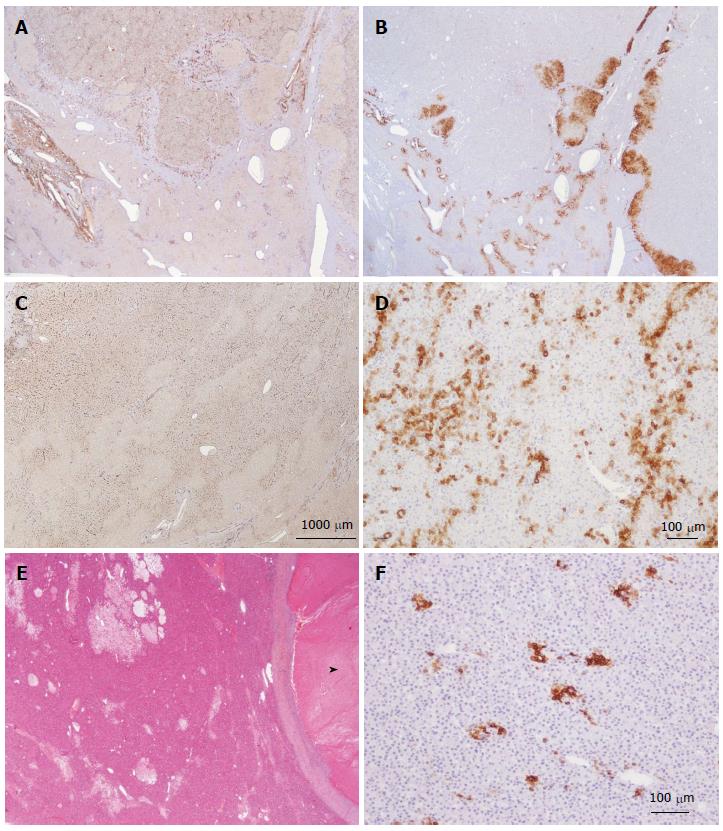
Figure 8 HNF1a inactivated hepatocellular adenoma different aspects of liver-fatty acid binding protein staining.
A: Same patient as Figure 7C, D. Typical aspect: absence of liver-fatty acid binding protein (LFABP) staining in tumor contrasting with normal expression in non tumoral liver (NT). B: Same patient as Figure 7E, F. Bands of non tumoral (NT) parenchyma normally expressing LFABP are penetrating within the unstained tumor. Indeed, the growth of HNF1A mutated hepatocellular adenoma (H-HCA) is due to the coalescence of several adenomatous liver nodules and it is not rare to find non adenomatous liver lobules squeezed in between. C: Woman born in 1962; abdominal pain. Oral contraceptives 25 years; BMI 25 kg/m2. Two liver nodules, largest 3.7 cm. Segmentectomy V/VI (2005). Bands of hepatocytes expressing LFABP within the unstained typical H-HCA. Same comment as above. D: Same patient as Figure 7E, F. LFABP expression in a few perivenular hepatocytes, particularly at the periphery of H-HCA, is a frequent observation, as well as a rim of positive hepatocytes at the border. E, F: Woman born in 1967; adenomatosis suspected during coelioscopy for extrauterine pregnancy. Segmentectomy for a 6 cm liver nodule. On HE (E), at distance from the main lesion, the presence of several steatotic small nodules well identified on LFABP staining (F). This is a very specific and characteristic aspect of H-HCA adenomatosis.s
Figure 9 HNF1a inactivated hepatocellular adenoma different aspects of glutamine synthase immunostaining.
A: Same patient as Figure 7E, F. There is no staining within the lesion, except around some veins, mainly at the borders where liver-fatty acid binding protein staining (not shown) demonstrates intermingled HNF1A mutated hepatocellular adenoma (H-HCA) and normal parenchyma areas. It was impossible on HE staining to clearly identify the border of the tumor. B: Woman born in 1964; one nodule discovered by chance, 5.5 cm; oral contraceptives 21 years; BMI 18.4 kg/m2. Biopsy: β-HCA. Segmentectomy VIII 2007. Diffuse, moderate glutamine synthase (GS) staining, contrasting with normal staining in non tumoral liver in which the GS staining is limited to 1-3 rows of centrilobular hepatocytes. Today, in the absence of β-catenin nuclear staining, the significance of this abnormal GS staining in HCA remains unknown and molecular analysis is mandatory to search for β-catenin mutations. C, D: Same patient as Figure 7C, D. Heterogeneous, mild staining in tumoral hepatocytes, sometimes arranged in rosettes. The presence of rosettes is often considered as a criterion suggesting a possible malignant transformation; it may also reflect cholestatic features. Same comment as above: in the absence of molecular analysis, it is not possible to conclude and a search for β-catenin mutations is mandatory.
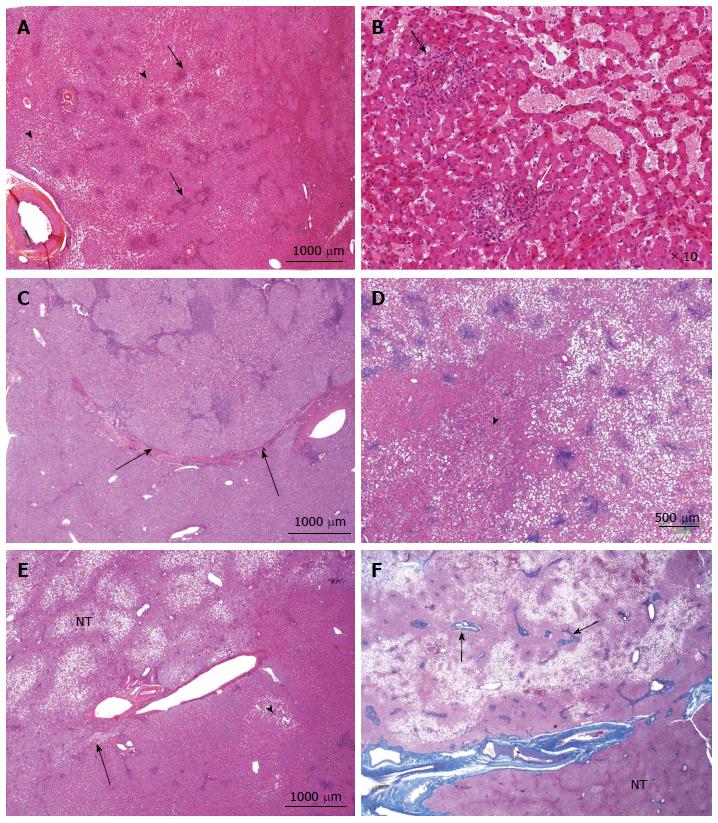
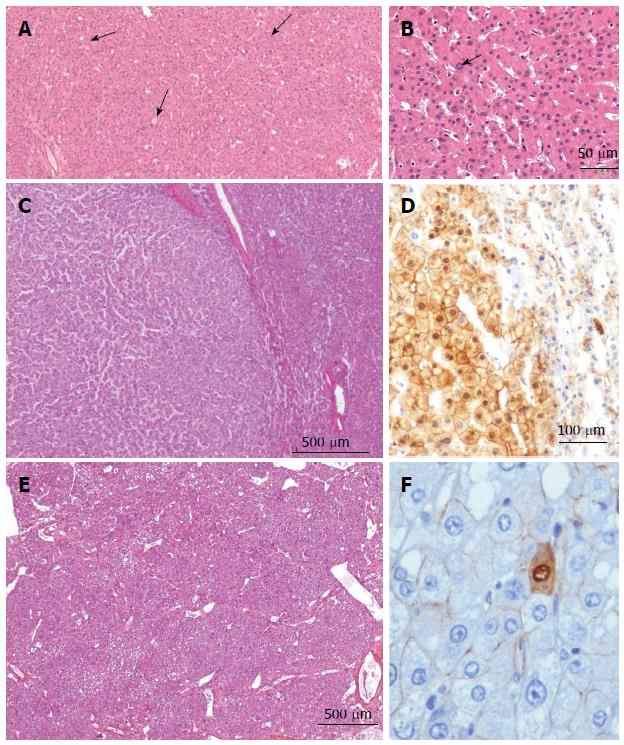
Figure 10 Inflammatory hepatocellular adenoma typical microscopic aspects.
A: Woman born in 1959; adenomatosis discovered by chance. Size of the largest nodule: 11 cm. Oral contraceptives for 23 years; BMI 27.0 kg/m2. Left hepatectomy 2008. HE: typical aspect of an inflammatory hepatocellular adenoma (IHCA) with many small inflammatory foci (arrows) dispersed within the tumor, associated with areas of moderate sinusoidal dilation ( arrowhead). In this area, sinusoids are dilated, another hallmark of this subgroup. B: Woman born in 1969; abnormal liver function tests. One liver nodule, 12 cm. Oral contraceptives for 16 years; BMI 26.0 kg/m2. Right hepatectomy 2004. HE: areas of sinusoidal dilatation and pseudo portal tracts with thick walled arteries and inflammatory cells (arrows), hallmarks of this subgroup. The diagnosis was confirmed by immunohistochemistry. C: Man born in 1968; abnormal liver function tests. One nodule 12 cm. BMI 30.0 kg/m2. Right hepatectomy 2011. HE: prominent inflammatory foci dispersed in the tumor; thick vessels at the border of the HCA (arrow). The diagnosis was confirmed by immunohistochemistry. D: Woman born in 1966; liver nodule, 3.5 cm discovered by chance. No oral contraceptives, BMI 24.5 kg/m2. Right hepatectomy 2004. HE: inflammatory foci, areas of sinusoidal dilatation; in this area, tumoral hepatocytes are steatotic. E: Woman born in 1973; overweight. Adenomatosis discovered by chance. Largest nodule 7 cm. Biopsy HCA. Tumorectomy IV, VI, VII 2003. HE: ill defined benign hepatocellular tumor (arrow); limited areas of sinusoidal dilatation (arrowhead) predominating at the periphery of the tumor. The non tumoral liver is steatotic, a frequent finding in this group of patients. F: Woman born in 1966; abnormal liver function tests. Several liver nodules. Biopsy HCA; oral contraceptives for 10 years, BMI 29.6 kg/m2. Left hepatectomy and tumorectomy IV and VI, 2007. Masson’s trichrome: pseudo portal tracts (arrows) with arteries in fibrous tissue; large areas of steatosis, within the tumor. The tumor is limited by thick arteries and veins from the non tumoral liver (NT).
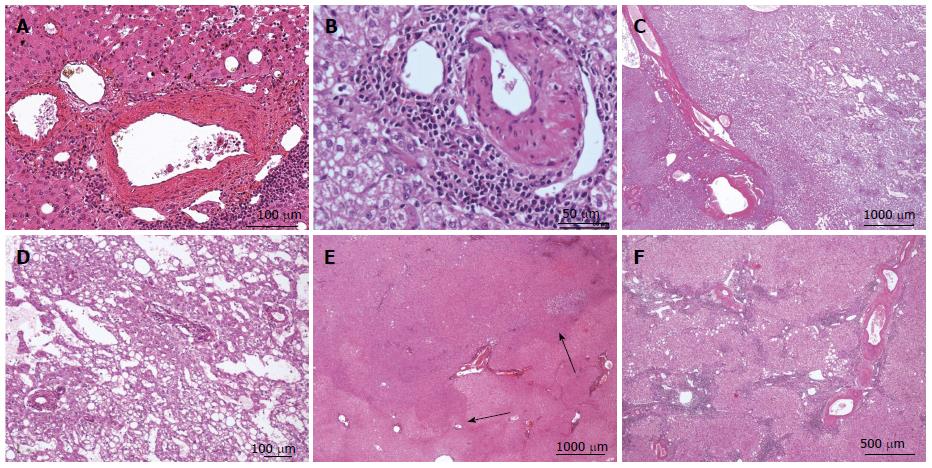
Figure 11 Inflammatory hepatocellular adenoma typical microscopic aspects.
A: Same patient as Figure 10 D. B: Same patient as Figure 10F. HE: thick-walled arteries surrounded by inflammatory cells. These pseudo portal tracts are very characteristic of inflammatory hepatocellular adenoma (IHCA). C, D: Woman born in 1972; oral contraceptives for 19 years. BMI 19.6 kg/m2. One nodule 10 cm discovered by chance. Magnetic resonance imaging: IHCA. Right hepatectomy 2009. HE: prominent sinusoidal dilatation. E, F: Same patient as Figure 10F, different tumors. E: HE - tumor ill-defined from the surrounding liver without any inflammation or sinusoidal dilatation. F: HE - the histological aspect is different with a more typical aspect of IHCA. Here, thick arteries are surrounded by inflammatory cells and fibrous tissue within the hepatocellular proliferation.
Figure 12 Inflammatory hepatocellular adenoma immunohistochemistry.
A, B: Same patient as Figure 10 F, different tumors. C-reactive protein (CRP): typical aspect of inflammatory hepatocellular adenoma (IHCA) with strong and diffuse expression in tumoral hepatocytes, with sharp demarcation from the surrounding non tumoral liver (A); more irregular CRP staining with limited areas remaining negative (B); C: Same patient as Figure 10D. CRP is expressed only in hepatocytes. D, E: Same patient as Figure 10F. D: Glutamine synthase: no abnormal staining; positivity only in some perivenous hepatocytes at the periphery of the nodule. E: CK7: faint staining around pseudo portal tracts, underlining ductular reaction, a common finding in IHCA. F: Same patient as Figure 10A. CK7 highlights the major ductular reaction at the periphery of pseudo portal tracts.
Figure 13 β-catenin activated, inflammatory hepatocellular adenoma.
A-F: Man born in 1971. BMI 21.6 kg/m2. By chance, discovery of one nodule 6 cm. Imaging: focal nodular hyperplasia. Right hepatectomy 2006. A: Fresh specimen: pigmented, irregular tumor. Non tumoral liver is normal. B: HE: Hepatocellular adenoma with sinusoidal dilatation and inflammatory infiltrate (on the left); large vessels at the junction with non tumoral liver. C: Diffuse expression of C-reactive protein by tumoral hepatocytes, with sharp demarcation from the non tumoral liver. D: Strong and diffuse glutamine synthase (GS) expression contrasting with normal staining of GS in adjacent non tumoral liver (in a few pericentrolobular hepatocytes). E: Large areas are positive for CD34, but not diffuse diffusely; F: Aberrant nuclear and cytoplasmic expression of β-catenin in quite numerous hepatocytes.
Figure 14 β-catenin activated, inflammatory hepatocellular adenoma.
A, B: Woman born in 1967. Oral contraceptives 20 years, BMI 20.0 kg/m2. By chance, discovery of one nodule 18 cm. Imaging hepatocellular adenoma (HCA). Segmentectomy IV and V 2005. A: HE: features of inflammatory HCA (IHCA): sinusoidal dilatation, thick vessels, mild inflammation. B: Glutamine synthase immunostaining is abnormal, but faint and heterogeneous with reinforcement around veins. C, D: Woman born in 1974; oral contraceptives 13 years. BMI 21.0 kg/m2. By chance, discovery of one nodule 6.5 cm. Imaging IHCA. Segmentectomy VI and VII 2009. C: Marked but not diffuse CD34 immunostaining. D: Very few tumoral hepatocytes expressed aberrant nuclear β-catenin.
Figure 15 β-catenin activated, inflammatory hepatocellular adenoma.
A-C: Woman born in 1971; liver hemorrhage. No oral contraceptives. BMI 20.4 kg/m2. Imaging 5 nodules, largest 8 cm. Segmentectomy III, VI, VIII 2007. A: HE - large arteries at the periphery of the nodule. Mild sinusoidal dilatation in the non tumoral liver (NT). B: Abnormal patchy GS staining in one nodule. C: Abnormal homogeneous glutamine synthase (GS) staining in another nodule. D-F: Woman born in 1959; oral contraceptives for 21 years. BMI 21.8 kg/m2. Abnormal liver function tests. Imaging: 3 nodules, largest 10 cm. Right hepatectomy 2005. In the three nodules, GS staining is different but definitely abnormal. D: Homogeneous. E: Extremely faint. F: Patchy GS staining. The difficulty in interpreting GS often comes from the positivity that can be found around veins. This perivenular staining is normal when it is strictly limited to 1 or 2 rows of perivenular hepatocytes; the interpretation of a GS staining larger than 2 or 3 rows of hepatocytes, even if faint or patchy, remains poorly understood in the absence of molecular analysis.
Figure 16 β-hepatocellular adenoma.
A, B: Man, 16 years old under androgen treatment for a hematological disorder. Imaging: several nodules; tumorectomy of one nodule 3 cm. HE: Hepatocellular adenoma (HCA) with some glandular arrangements ( arrowhead) and a few larger, irregular nuclei (arrow). According to the clinical context, the diagnosis of β-catenin is very likely. C, D: Woman born in 1953; oral contraceptives for 4 years. Danazol one year (endometriosis). BMI 19.5 kg/m2. Asthenia. Imaging: one nodule 3 cm. Right hepatectomy in 1989. C: HE: Well limited HCA with no features of H-HCA, or of IHCA. By default, the diagnosis of β-catenin is therefore a possibility. D: Aberrant cytoplasmic and nuclear expression of β-catenin in numerous hepatocytes confirms the diagnosis of β-HCA; E, F: Woman born in 1980; oral contraceptives for 12 years. BMI 20.4 kg/m2. Abdominal pain. Imaging: one nodule 15 cm: HCA. Right hepatectomy 2009. E: HE - numerous vessels within the HCA. This aspect seems to be quite characteristic of β-HCA and of some unclassified HCA; F: Aberrant expression of β-HCA in very few hepatocytes confirms the diagnosis of β-HCA.
Figure 17 β-hepatocellular adenoma different types of glutamine synthase immunostaining.
A: Same patient as Figure 16C, D. Strong and diffuse expression of glutamine synthase (GS) in hepatocellular adenoma (HCA) (left), contrasting with non tumoral liver (positivity in only few pericentrolobular hepatocytes). B-D: Same patient as Figure 16 E, F. Strong, heterogeneous (patchy) positivity of GS seen at different magnification, with a reinforcement rim at the periphery of the HCA (the rim positivity has no pathological significance). E, F: Woman born in 1986; abnormal liver function tests. Imaging: one nodule 9 cm, HCA. Left hepatectomy 2007. E: HE - no specific abnormalities. The presence of numerous vessels is, however, intriguing in this young patient. F: Patchy positivity of GS from mild to strong.
Figure 18 β-catenin hepatocellular adenoma.
A, F: Same patient as Figure 5G. A: HE: Numerous vessels dispersed within the hepatocellular proliferation. B: Quite numerous CK7+ cells dispersed within the tumor; some are small, looking like progenitor cells; others are larger as intermediate cells. C, D: Diffuse positivity of CD 34 within the tumor. E, F: Patchy positivity of glutamine synthase.
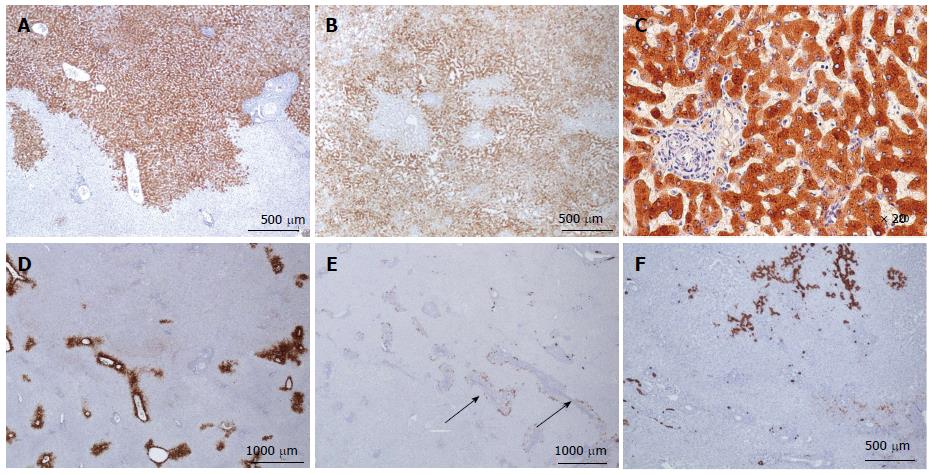
Figure 19 Unclassified hepatocellular adenoma.
A, B: Woman born in 1988; oral contraceptives for 4 years. BMI 16.8. Abdominal pain. Imaging: one nodule 11 cm. No final diagnosis. Left hepatectomy 2009. A: Diffuse expression of CD34 in hepatocellular adenoma (HCA) (top) contrasting with adjacent non tumoral liver (below). B: No expression of glutamine synthase (GS) except at the periphery of the HCA. Here the nodule is divided in 2 parts at its periphery by a thin band of normal tissue containing vessels. C, D: Woman born in 1975; oral contraceptives for 12 years. BMI 24.2 kg/m2. Hemorrhage. Imaging: one nodule 5 cm, HCA. Right hepatectomy 2003. C: Widespread but not diffuse expression of CD34 within the HCA. D: Numerous cells overexpressing CK7: small cells looking like progenitor cells and intermediate cells. GS was normal. E, F: Same patient as Figure 5I. E: HE: thick fibrous rim around a necrotic area (arrowhead); peliotic areas within the viable HCA. F: Some small CK7 positive cells dispersed within the HCA. GS (not shown) was normal. CD34 staining was more or less diffuse (not shown).
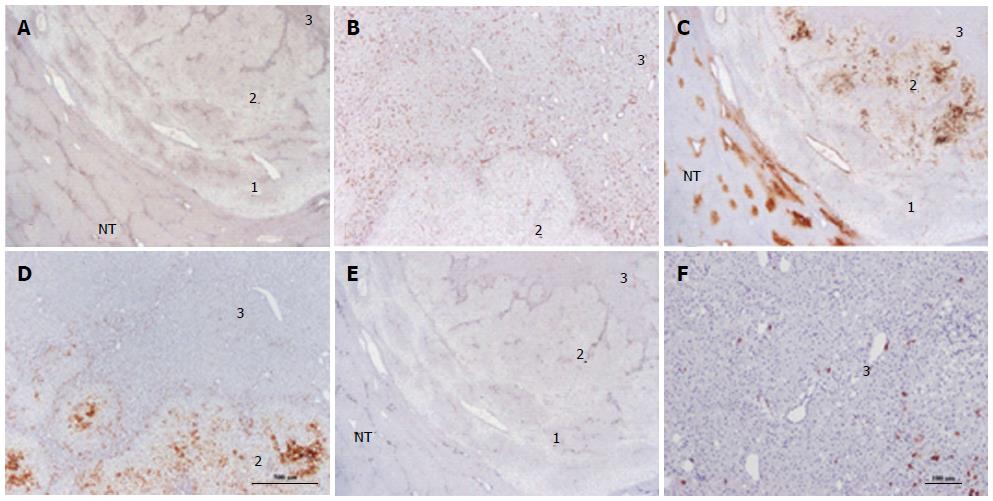
Figure 20 Unclassified hepatocellular adenoma.
Woman born in 1980; oral contraceptives for 6 years. BMI 22.2 kg/m2. Abdominal pain. Imaging: one nodule 3 cm, hepatocellular adenoma (HCA). Segmentectomy VI 2004. A, B: On CD34, three zones (1-3) are seen in this nodule. Zone 1 is the external limit of the nodule; zone 2 is intermediate and zone 3 represents the quasi totality of the nodule. Only zone 3 is diffusely positive. In zone 2, CD34 positivity is seen along vascular axis. C, D: Glutamine synthase (GS) staining: zone 3 is negative. Zone 1 is negative except around veins. In zone 2, GS staining is patchy. E, F: CK 7 - in zone 3, few cells, possibly progenitor cells are positive. In zone 2, positive cells are seen along vascular axis. This nodule has been classified as UHCA because all specific markers were negative. It is not rare to observe a thin peripheral rim which is CD34 negative /GS positive in unclassified HCA or β-HCA. In this case, the presence of 2 zones different from the bulk of the tumor remains unexplained but should not change the diagnosis.
- Citation: Sempoux C, Balabaud C, Bioulac-Sage P. Pictures of focal nodular hyperplasia and hepatocellular adenomas. World J Hepatol 2014; 6(8): 580-595
- URL: https://www.wjgnet.com/1948-5182/full/v6/i8/580.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i8.580