Published online May 26, 2025. doi: 10.4252/wjsc.v17.i5.104116
Revised: February 27, 2025
Accepted: April 18, 2025
Published online: May 26, 2025
Processing time: 167 Days and 5.8 Hours
Dental follicle stem cell (DFSC) sheets demonstrate strong extracellular secretion capabilities and efficacy in periodontal regeneration. However, existing methods for producing DFSC sheets lack a comprehensive discussion on the most efficient and cost-effective approaches at the good manufacturing practice (GMP) level.
To investigate the culture condition of GMP-compliant DFSC sheets and to compare the properties of DFSC sheets and cell suspensions.
This study explored the optimal conditions for culturing GMP-compliant DFSC sheets, focusing on four key factors: Cell passage, cell concentration, L-ascorbic acid content, and culture duration. We evaluated the characteristics of the cell sheets under varying culture conditions, including cell viability, cell count, appearance, osteogenesis, chondrogenesis, odontogenesis, aging, relative telomere length, and extracellular matrix secretion. A comparison was also made between the periodontal regeneration, osteogenesis, and paracrine capacity of cell sheets cultured under optimal conditions and those of the cell suspensions.
The GMP-compliant DFSC sheets cultured from passage 4 cells exhibited the highest viability (≥ 99%, P < 0.05) and optimal osteogenic differentiation capacity (optical density ≥ 0.126, P < 0.05). When cultured for 10 days, DFSC sheets demonstrated maximal expression of osteogenic, chondrogenic and periostin genes [alkaline phosphatase, Runt-related transcription factor 2, collagen type I, osteopontin, cartilage associated protein, and PERIOSTN (P < 0.001); osteocalcin (P < 0.01)]. Concurrently, they showed the lowest senescent cell count (P < 0.01) with no progression to late-stage senescence. At a seeding density of 2500 cells/cm2, GMP-compliant DFSC sheets achieved better osteogenic differentiation (P < 0.01) and maximal osteogenic, chondrogenic and periostin gene expression (P < 0.001), coupled with the highest hydroxyproline secretion (P < 0.001) and moderate sulfated glycosaminoglycan production. No statistically significant difference in senescent cell count was observed compared to DFSC sheets at a seeding density of 5000 cells/cm2. Supplementation with 25 μg/mL L-ascorbic acid significantly enhanced osteogenic gene expression (P < 0.001) and elevated hydroxyproline (P < 0.01) and sulfated glycosaminoglycan secretion to high ranges. Compared with the cell suspension, the cell sheet demonstrated improved osteogenic, paracrine, and periodontal regenerative capacities in Sprague-Dawley rats. The optimized DFSC sheets demonstrated significantly higher levels of vascular endothelial growth factor and angiopoietin-1 (P < 0.001) compared to DFSC suspensions, along with enhanced osteogenic induction outcomes (optical density = 0.1333 ± 0.01270 vs 0.1007 ± 0.0005774 in suspensions, P < 0.05). Following implantation into the rat periodontal defect model, micro-computed tomography analysis revealed superior bone regeneration metrics in the cell sheet group compared to both the cell suspension group and control group (percent bone volume, trabecular thickness, trabecular number), while trabecular spacing exhibited an inverse pattern.
Optimized DFSC sheets cultured under the identified conditions outperform DFSC suspensions. This study contributes to the industrial-scale production of DFSC sheets and establishes a foundation for cell therapy applications.
Core Tip: The existing culture method for dental follicle stem cell (DFSC) sheets is crude and not uniform. For clinical application, it is necessary to produce good manufacturing practice compliant DFSC sheets and determine a uniform culture method that can maintain the best performance of DFSC sheets and consume the least. In this study, the culture conditions of the good manufacturing practice-compliant DFSC sheet were optimized, focusing on four key factors: Cell passage, cell concentration, L-ascorbic acid content, and culture duration. Compared with the DFSC suspension, the cell sheet demonstrated superior capacities. These findings offer valuable insights and guidance for the clinical application of cell sheets.
- Citation: Yu JL, Yang C, Liu L, Lin A, Guo SJ, Tian WD. Optimal good manufacturing practice-compliant production of dental follicle stem cell sheet and its application in Sprague-Dawley rat periodontitis. World J Stem Cells 2025; 17(5): 104116
- URL: https://www.wjgnet.com/1948-0210/full/v17/i5/104116.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i5.104116
Periodontitis is a chronic infectious disease that can lead to various symptoms including tooth loosening, alveolar bone resorption, and periodontal pocket formation, posing a significant threat to public oral health. However, current periodontal treatment methods, such as scaling and root planning and guided tissue regeneration, can inhibit inflammation and partially restore the lost tissues. However, these approaches are only effective for certain types of bone defects and the outcomes of tissue regeneration remain uncertain. Long-term immune regulation and effective perio
Dental follicle stem cells (DFSCs) are precursor cells of periodontal tissue derived from the dental follicle of patients who require wisdom tooth extraction. Consequently, they are easier to obtain and pose fewer ethical concerns. DFSCs possess multidirectional differentiation potential (including osteogenesis, chondrogenesis, and neurogenesis) as well as immunomodulatory effects on macrophages, neutrophils, and helper T lymphocytes[7-9]. Moreover, our previous studies have shown that DFSCs exhibit superior osteogenic properties and greater extracellular matrix (ECM) secretion than those of periodontal ligament stem cells[10]. Numerous animal studies have demonstrated that DFSC sheets can effectively regenerate periodontal tissue[9].
The most common forms of cell therapy include cell suspension injection, three-dimensional (3D) scaffolds, and cell sheets[11]. The most commonly used stem cell therapy form is the cell suspension, which is injected into the periodontal defect area as a liquid reagent to promote local periodontal regeneration. However, owing to the fluid nature of the suspension, it is prone to leakage into the surrounding tissues and may even exude from the defect site through the injection point. The 3D scaffold approach introduces an exogenous scaffold, which is less safe and convenient than a cell sheet. In a light, thin solid preparation, the cell sheet can be easily secured in the defect area. Its rich ECM and cell surface proteins provide favorable biological characteristics, making it a superior option for periodontal tissue regeneration[12-14].
However, stem cell preparations still have a significant journey ahead, transitioning from animal studies to clinical trials before they become a fully developed product. Stem cell preparations are classified as a special category of drugs that require thorough exploration of the preparation process, quality standards, testing methods, stability, efficacy, and safety evaluations. Additionally, the safety, efficacy, and potential adverse reactions of these preparations should be investigated in phase I-III clinical trials. Therefore, determining a standardized production method and testing protocol for DFSC sheets that can be applied in clinical practice is crucial for their development as cell-based therapies.
In the pursuit of standardized production conditions for stem cell preparations, it is essential to ensure that all media and additives are of clinical-grade quality to meet the clinical application standards. In this study, serum-free mesen
All experiments involving human DFSCs cultures and rats in this study were approved and registered by the Animal Care and Use Committee and the Ethics Committee of the West China School of Stomatology, Sichuan University (No. WCHSIRB-D-2024-368 and No. WCHSIRB-D-2024-237). The animal experiments were designed and implemented in accordance with the guidelines outlined in the Guide for the Care and Use of Laboratory Animals, 8th edition (ISBN-13: 978-0-309-15400-0, National Academies Press, DC, United States, 2011).
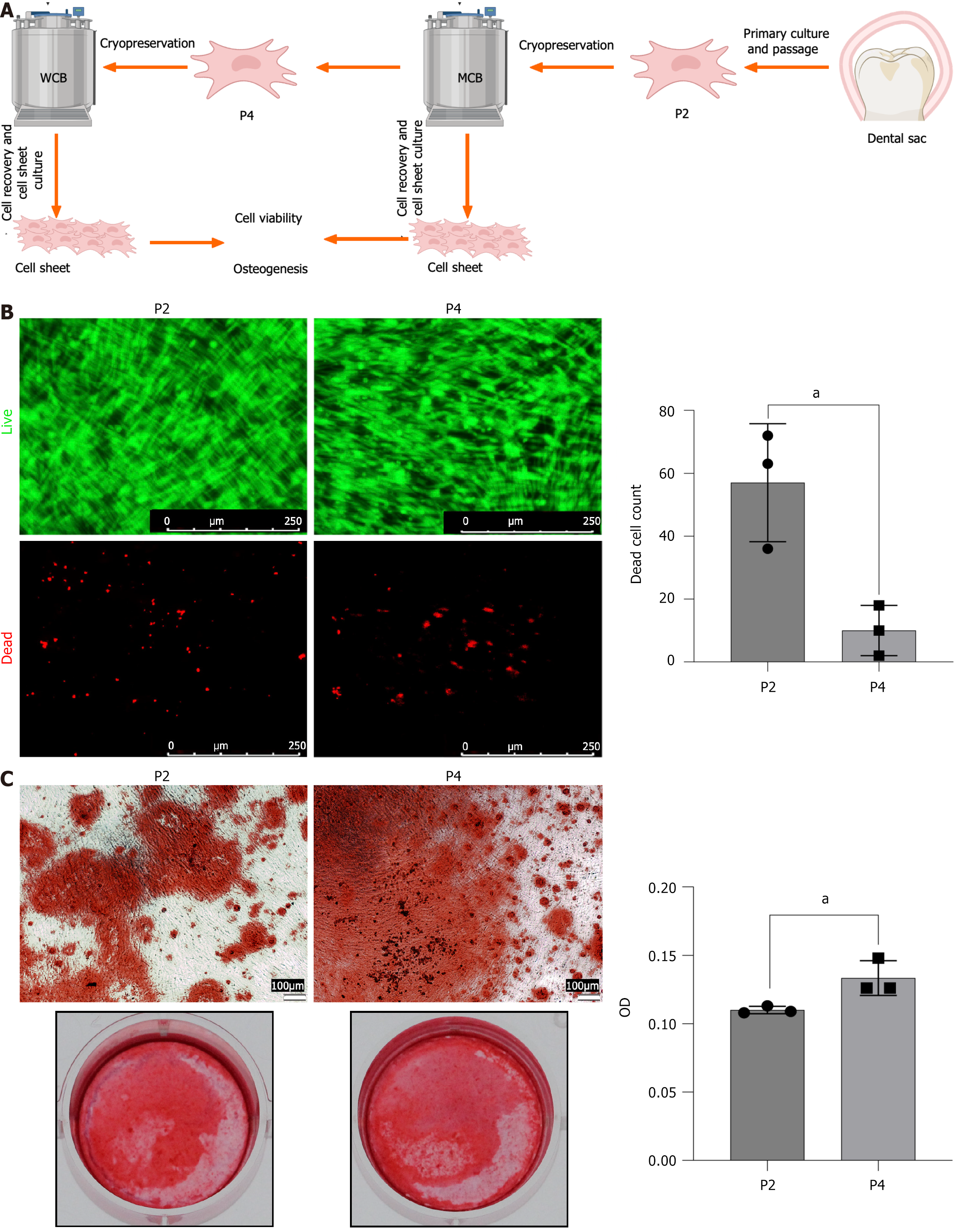
The cells used in this study were obtained from stem cell preparations (SLDSC001; Sichuan Cell Bank Co., China). Passage two (P2) DFSCs cryopreserved at a density of 5 × 106 cells/mL were designated as master cell banks (MCBs). DFSCs from MCB were thawed and cultured until they reached passage four (P4). P4 DFSCs were then cryopreserved and referred to as working cell banks (WCBs).
DFSCs from the MCB and WCB were revived and cultured at 37 °C with 5% CO2 in a 12-well plate using serum-free mesenchymal stem cell medium (Nuwacell Biotechnologies Co., Ltd., Anhui, China). Following cellular adhesion, the medium was replaced with a serum-free mesenchymal stem cell culture medium containing L-ascorbic acid (Clinical-grade drug, Huazhong Pharmaceutical Co., Hubei, China). The medium was refreshed every three days. Following a culture period of more than 10 days, DFSC sheets were successfully prepared. The sheets, observed on the bottom wall of the twelve-well plate, were white and slightly opaque, with wrinkled edges, and could be readily lifted.
Cells were suspended at densities of 2 × 106, 4 × 106, 6 × 106, 8 × 106, 10 × 106, 12 × 106, 14 × 106, and 16 × 106 cells/cm² in a 12-well plate and cultured for 4 hours to allow complete adherence. The initial solution was then removed, and Cell Counting Kit-8 (CCK-8) reagent (Keygen, Jiangsu, China) was added to the medium at a 1:9 ratio. Following next, 1 mL the medium was added to each well, and the plate was incubated in a 5% CO2 incubator at 37 °C for 1 hour. Then, 100 μL of the solution was transferred to a new 96-well plate, and the absorbance was measured at 450 nm to generate a standard curve. Using the above method, the CCK-8 solution was transferred to 96-well plates after co-incubation with the cell sheet for 1 hour and the OD value was measured at 450 nm. The cell density of the cell sheet can be calculated by substituting the standard curve.
The medium from each well was collected and the cells were washed with phosphate-buffered saline (PBS) for 10 s. Acridine orange (AO)/propidium iodide (PI) staining solution (Beyotime, Shanghai, China) was added and the cells were incubated at 37 °C for 30 minutes. The staining solution was discarded, and the cells were washed again with PBS for approximately 10 seconds. Finally, the stained cells were imaged using a fluorescence microscope and analyzed using ImageJ software.
The culture medium was removed and the cells were rinsed once with PBS before being incubated with 500 μL of β-galactosidase staining fixative at room temperature for 15 minutes. Following fixation, the cells were washed three times with PBS, with each wash lasting for 3 minutes. Next, 500 μL of β-galactosidase staining working solution (Solarbio, Beijing, China) was added to each well and the cells were incubated overnight at 37 °C. The stained cells were imaged using an optical microscope and analyzed using the ImageJ software.
Total RNA was extracted from the DFSC sheets using the FastPure Cell/Tissue Total RNA Isolation Kit V2 (Vazyme, Nanjing, China) and reverse-transcribed using an RNA reverse transcription kit (Vazyme, Nanjing, China). The cDNA was then analyzed by quantitative reverse-transcription polymerase chain reaction (qRT-PCR) using the SYBR Kit (Vazyme, Nanjing, China) with glyceraldehyde-3-phosphate dehydrogenase as the internal reference. Target gene expression was calculated using the 2-ΔΔCt method. Relative telomere length was assessed using a Telomere Relative Length Detection Kit (Biowing, Shanghai, China). Primers for genes related to the osteogenic, chondrogenic, odontogenic, and aging pathways are listed in Supplementary Table 1. Each experiment was conducted in triplicates.
Pipette tips and 12-well plates were pre-cooled to -20 °C for 2 hours. Subsequently, 300 μL of Matrigel (Corning, NY, United States) was added to each well of a 12-well plate at 4 °C. The coating liquid was gently shaken to ensure uniform coverage of the bottom of the well and the plate was incubated at room temperature for 30 minutes. After removing the excess coating liquid, the cell suspension was mixed to obtain concentrations of 2500, 5000, 7500, or 10000 cells/cm². The cells were allowed to adhere to the wells, followed by incubation in serum-free mesenchymal stem cell culture medium containing L-ascorbic acid (Huazhong Pharmaceutical Co., Hubei, China). The medium was refreshed every three days. After 10 days of culture, a cell sheet was formed, and the number of cells within the sheet was determined using the CCK-8 assay. Cells from identical cell sheets were seeded into a 12-well plate with Matrigel and allowed to adhere. Following this, osteogenic induction medium was added, with medium changes occurring every three days. At the end of three weeks, the supernatant was removed, and the cells were rinsed with saline, fixed with 4% paraformaldehyde for 10 minutes, and rinsed again with saline. Alizarin red reagent (Cyagen, Guangzhou, China) at a concentration of 600 μL/well was then applied for 30 minutes at 4 °C. The samples were washed thrice with distilled water. Microscopic images were captured at 4 × and 10 × magnification using a digital camera. After imaging, the supernatant was removed and a 10% cetylpyridinium chloride solution was added and incubated at 37 °C for 1 hour. The cetylpyridinium chloride solution was then diluted 20-fold and 100 μL of the diluted solution was transferred to each well of a 96-well plate. The absorbance was measured at 562 nm.
To digest the cell sheet, 300 μL of TrypLE was added to each well of a 12-well plate, followed by a 10-minute incubation. Digestion was halted by adding 1 mL serum-free medium, after which the supernatant and undigested cell sheet tissue were removed by centrifugation (400 r/minute, 5 minutes). The cell pellet was resuspended in 2 mL serum-free medium, evenly spread, and counted. Next, 200 cells were added to each well of a 6-well plate, cross-shaken, and incubated at 37 °C with 5% CO2 in a humidified atmosphere. The medium was refreshed every 2-3 days for 7 days. Following incubation, the cells were washed once with PBS, fixed with 1 mL of 4% paraformaldehyde per well for 30 minute, and stained with 1 mL of crystal violet dye for 20 minutes. The cells were subsequently rinsed several times with PBS, allowed to dry, and imaged using a digital camera.
Enzyme linked immunosorbent assay kits for vascular endothelial growth factor (VEGF) and angiopoietin-1 (ANG-1) (R&D Systems, Minneapolis, MN, United States) were used. The cell sheet was cultured as described in “Cell sheet preparation” and cell supernatants were collected from days 7 to 10. The cell count of the cell sheet was determined on day 7, as described in “Cell Counting Kit-8 assay”. An equal number of cells was then seeded in a 12-well plate and incubated at 37 °C with 5% CO2 for three days, after which the supernatant was collected. Enzyme linked immunosorbent assay was performed on the cell sheet and cell culture supernatant samples, according to the manufacturer’s instructions.
The cell sheet was gently separated from the 12-well plate by rinsing along its edges with 4 °C pre-cooled PBS and using tweezers. The stress-strain curve under tensile force was measured using an electronic universal testing machine, and the maximum tensile force was recorded.
On 10th, 12th, and 14th days, the cell sheet was digested as described in “Colony-forming units” to obtain a cell suspension. The suspension was agitated on a shaker, and anhydrous ethanol, pre-cooled to -20 °C, was added dropwise to achieve a 70% ethanol concentration. The samples were fixed at 4 °C for 30 minutes. After fixation, the cell suspension was washed twice with PBS, transferred to a centrifuge, and spun at 400 r/minute, after which the supernatant was discarded. Cells were then processed with reagents from the Cell Cycle Detection Kit (Beyotime, Beijing, China) following the manufacturer’s instructions. PI staining solution was added and the cells were incubated in the dark at 37 °C for 30 minutes. Finally, the cells were analyzed using flow cytometry.
To conduct the regulatory T cell (Treg) lymphocyte stimulation test, two experimental groups were established: A peripheral blood mononuclear cell (PBMC) group and a DFSC-PBMC co-culture group. Each group contained three wells. DFSCs were prepared by digesting the cell sheet as outlined in “Colony-forming units”, then resuspended at a concentration of 1 × 104 cells/mL. The cell suspension was added to each well of a 6-well plate and incubated for 4 hours to allow for cell adherence. The supernatant was removed and the wells were washed with normal saline. PBMCs from both the PBMC and co-culture groups were seeded at a density of 5 × 104 cells/mL in 1640 complete medium [90% 1640 medium, Corning, NY, United States; 10% fetal bovine serum (FBS, Gibco, NY, United States)]. The cells were cultured for 120 hours, after which the activators phorbol 12-myristate 13-acetate (25 ng/mL), ionomycin (1 μg/mL), and brefeldin A (10 μg/mL) were added and incubated for an additional 6 hours. The cells were collected and incubated with antibodies targeting CD8, CD4, CD25, and CD127 for 20 minutes. After antibody staining, the cells were washed and centrifuged before detection. A minimum of 10000 events were recorded on a flow cytometer, and the proportions of CD3(+)CD4(+)CD25(+)CD127(low) lymphocytes were analyzed using FlowJo software (BD Biosciences, Franklin Lakes, NJ, United States).
For type 1 T helper (Th1) and interleukin (IL)-17-producing T helper (Th17) lymphocyte inhibition experiments, two groups were established: Co-culture, and PBMC, with three wells per group. DFSCs were prepared by digesting the cell sheet as outlined in “Colony-forming units”, then resuspended to a concentration of 1 × 104 cells/mL. A 100 μL volume of this cell suspension was added to each well of a 6-well plate and cultured for 4 hours. The supernatant was removed, and the wells were washed once with normal saline. For the PBMC and co-culture groups, 1640 complete medium (90% 1640 medium, Corning, NY, United States; 10% FBS, Gibco, NY, United States) containing PBMCs at a density of 5 × 104 cells/mL was added. After 18 hours of culture, phorbol 12-myristate 13-acetate (25 ng/mL), ionomycin (1 μg/mL), and brefeldin A (10 μg/mL) were added and the cells were incubated for 6-hour incubation. The cells were then collected and incubated with CD3 and CD8 antibodies at room temperature in the dark for 20 minutes. The cells were subsequently fixed, permeabilized, and incubated with IL-17 or interferon-γ antibody for an additional 20 minutes. A minimum of 10000 events were recorded using a NovoCyte flow cytometer (Agilent, CA, United States). The proportions of CD3(+)CD8(-)IL-17(+) and CD3(+)CD8(-)interferon-γ(+) lymphocytes were analyzed using FlowJo software (BD, NJ, United States).
For the monocyte proliferation inhibition experiment, three groups were established: The DFSC group, co-culture group, and PBMC group, with three wells per group. Cells were prepared by digesting the cell sheet as described in “Colony-forming units”, then resuspended to a concentration of 1 × 104 cells/mL. A 100 μL volume of the cell suspension was added to each well of a 96-well plate and incubated for 4 hours. Following incubation, the supernatant was removed and the wells were washed once with normal saline. For the PBMC and co-culture groups, 1640 complete medium (90% 1640 medium, Corning, NY, United States; 10% FBS, Gibco, NY, United States) was added to achieve a PBMC density of 5 × 104 cells/mL. Three additional wells were filled with complete medium and served as controls. After a continuous 72-hour culture, 10 μL of CCK-8 solution was added to each well for 4-hour incubation. Subsequently, 100 μL of the supernatant from each well was collected to measure the absorbance at 450 nm. The antibodies used in the study are listed in Supplementary Table 2.
The cell sheet was removed as described in “Determination of the mechanical properties” and centrifuged at 100 g for 1 minute. The weights of the cell sheets were recorded.
Hydroxyproline content detection: The cell sheet was fragmented and combined with 1 mL of 6 mol/L hydrochloric acid, then digested at 110 °C for 2 hours. The pH was subsequently adjusted to 6-8 using approximately 0.5 mL of 10 mol/L NaOH. After centrifuging the solution at 16000 g at 25 °C for 20 minutes, the supernatant was collected, and hydroxyproline (HYP) content was determined using the HYP detection kit (Solarbio, Beijing, China) according to the manufacturer’s protocol.
Sulfated glycosaminoglycan content detection: The cell sheet was fragmented and combined with 1 mL of papain extraction reagent, then heated at 65 °C for 3 hours until no visible tissue debris remained. The solution was then centrifuged at 10000 g for 10 minutes, and the sulfated glycosaminoglycan (sGAG) content was measured using the sGAG assay kit (Biocolor, Carrickfergus, United Kingdom).
The cell sheet was carefully rinsed along its edges with pre-cooled PBS at 4 °C, then fixed in 4% paraformaldehyde for 4 hours. The subsequent dehydration process involved soaking in 70% ethanol for 1 hour, followed by additional dehydration steps in 80%, 90%, and 100% ethanol for 30 minutes each. The cell sheet was then immersed in xylene I and II for 15 minutes each before being embedded in wax for 24 hours. After excision, rat tissues were fixed in 4% paraformaldehyde for 24 hours and decalcified over a period of 30 days (Servicebio, Wuhan, China). The tissue was dehydrated in 70% ethanol for 2 hours and then further dehydrated in 80%, 90%, and 100% ethanol for 1 hour each. It was subsequently immersed in xylene I and II for 30 minutes each, followed by overnight immersion in wax. The tissues were embedded in paraffin, and serial sections of 6 μm thickness were prepared. These sections were then stained with hematoxylin and eosin (HE) (Solarbio Science & Technology Co., Beijing, China), Masson’s trichrome (Baso, Beijing, China), 4’,6-diamidino-2-phenylindole (Solarbio, Beijing, China), and tartrate-resistant acid phosphatase (Wako, Tokyo, Japan), according to the manufacturer’s protocols. Images were captured using either a confocal microscope or a standard microscope (Olympus, Tokyo, Japan).
The culture supernatant of DFSC sheet was collected, and bacteria test was performed to evaluate the results. The same supernatant was then used for endotoxin testing via the Gel Clot LAL Assay and be used for mycoplasma detection via the recombination-dependent rolling circle amplification method.
After centrifugation, the weight of the DFSC sheets was measured, and an extraction medium was prepared based on the ratio of sample weight (0.1 g) to the volume of extraction medium (including serum medium) (1 mL). Extraction was carried out at 37 °C for 72 hours. Once extraction was complete, the extract was stored at 4 °C to prevent microbial contamination. A periodontal ligament stem cell suspension was prepared, and the cell concentration was adjusted to 5 × 103 to 5 × 104 cells/mL. 100 μL of the cell suspension was added to each well of a 96-well plate. The blank group (without cells), control group (without extract), and experimental group were established. The 96-well plates were then incubated at 37 °C in a 5% CO2 incubator for 24 hours to allow the periodontal ligament stem cells to adhere to the plate. The pre-cultured medium was then aspirated. In the experimental group, the extract was added, while the control group received medium without extract. The culture plates were placed back into the incubator and cultured for 48 hours. Afterward, 10 μL of CCK-8 solution was added to each well, ensuring no bubbles were present. The culture plate was gently shaken to mix the CCK-8 solution with the medium. The plates were returned to the incubator for 1 hour. Absorbance (OD value) of each well was measured at 450 nm using an enzyme reader. Cell survival and inhibition rates were calculated using the following formulas:
Cell survival rate = (As - Ab)/(Ac - Ab) × 100%.
Inhibition rate = (Ac - As)/(Ac - Ab) × 100%.
As: Absorbance of experimental well (including cells, medium, CCK-8 solution, and extract). Ac: Absorbance of control well (including cells, medium, CCK-8 solution, no extract). Ab: Absorbance of blank well (including medium and CCK-8 solution, excluding cells and extract).
The animals used in this study were obtained from Chengdu Dossy Experimental Animals Co., China. The animal protocol was designed to minimize pain and discomfort experienced by the animals. The animal experiments were designed and implemented in accordance with the guidelines outlined in the Guide for the Care and Use of Laboratory Animals, 8th edition (ISBN-13: 978-0-309-15400-0, National Academies Press, DC, United States, 2011). This study was approved by the Ethics Committee of the State Key Laboratory of Oral Diseases, West China School of Stomatology, Sichuan University (approval number No. WCHSIRB-D-2024-368). All procedures complied with the regulations governing experimental animals and adhered to the guidelines set forth by the Animal Care and Use Committee of West China School of Stomatology, Sichuan University. The animals were maintained in a controlled environment (50% humidity, 25 °C, 12-hour light-dark cycle and free access to food and water) with unrestricted access to food and water. All animal experiments were conducted at the Animal Center of Sichuan University and the State Key Laboratory of Oral Diseases, West China School of Stomatology, Sichuan University, in line with ARRIVE guidelines 2.0.
Following a one-week acclimatization period, 18 healthy male SD rats, aged 8 weeks and meeting the inclusion and exclusion criteria, were randomly assigned to three groups. Specific inclusion and exclusion criteria are listed in Supplementary Table 3. In this study, we investigated the therapeutic effects of DFSC sheets and cell suspensions in promoting periodontal regeneration. The number of cells in the DFSC sheets was measured using the method described above. The cell concentration of the cell suspension was determined using a cell counter (Thermo Fisher Scientific, MA, United States). A cell suspension with the same cell count as the DFSC sheets was prepared. After centrifugation (400 r/minute, 5 minutes), 20 μL of normal saline was added to the suspension to ensure that the cell count used in the animal experiments matched that of the DFSC sheets. The rats were allocated as follows: Control group (received surgery without treatment, n = 6), cell group (received surgery and DFSC cell suspension implantation, n = 6), and cell sheet group (received surgery and DFSC cell sheet implantation, n = 6). The number of rats in each group was determined based on previous studies employing similar models[15].
In accordance with a previous study[9], a No. 11 blade was used to incise the mesial gingiva of the right maxillary first molar and the flap was elevated. The alveolar bone was then irrigated with PBS and dried using sterile cotton pellets. A periodontal defect measuring 1 mm × 2 mm × 1 mm (width × length × depth) was created using a ball drill with a diameter of 1 mm. Cell sheets (cell sheet group), a cell suspension containing the same number of cells as the cell sheets (cell group), or normal saline (control group) were placed in the defect area. The flap was closed with single interrupted sutures (Vicryl 6-0, Ethicon Products, Amersfoort, The Netherlands) to achieve primary closure. The animals were euthanized 30 days post-surgery with sodium pentobarbital (150 mg/kg) by intravenous injection for further analysis, aimed at investigating the therapeutic effects of DFSCs and DFSC sheets in promoting periodontal regeneration. Regeneration was assessed using micro-computed tomography (CT) scanning and tissue section analysis. Tissue sections were prepared from the maxillary alveolar bone as well as from the heart, liver, spleen, lungs, and kidneys of the rats. Individual rats were used as experimental units. Additionally, blood samples were collected from the rat tail vein before the operation, 3 days after the operation, and 1 month after the operation to detect routine blood and blood biochemical changes.
Blinding was maintained to the extent possible at all stages of animal experiments, including subject allocation, surgical procedures, and data analysis. Three researchers were involved in each animal: The first researcher performed the surgery and administered treatment based on a randomization table, with only the investigator aware of group assignments; the second investigator handled sample collection and processing; and the third researcher conducted the data analysis.
The quantification results are expressed as the mean ± SD of at least three replicates. The Shapiro-Wilk test was applied to verify the normality of data distribution. Non-parametric tests were employed for datasets that did not meet the criteria for normal distribution. For normally distributed data comparing two groups, an unpaired Student’s t-test was performed. When the variance between two datasets was unequal, an unpaired Student’s t-test with Welch correction was used. To analyze normally distributed data involving more than two groups, a one-way analysis of variance (ANOVA) was conducted. For datasets with unequal variance and more than two groups, the Brown-Forsythe and Welch ANOVA tests were utilized. Results were considered statistically significant only if the P value was less than 0.05. The data was analyzed and visualized using GraphPad Prism 9 software.
The biological properties of the cell sheets produced by cells from the WCB and the main cell bank require further comparison. Schematic are designed to outline operational processes and inspection items (Figure 1A). Cells from both the main and WCBs were cultured in cell sheets following resuscitation and AO/PI staining was performed. Cell sheets cultured from the WCB exhibited larger cell gaps and fewer dead cells (Figure 1B). Building on these structural observations, we further investigated functional competence through osteogenic differentiation assays. To determine whether passage number influenced this functional outcome, osteogenic differentiation was examined. Alizarin red staining revealed that the osteogenic effect of the cell sheets produced by P4 generation cells was superior to that produced by P2 generation cells (Figure 1C).
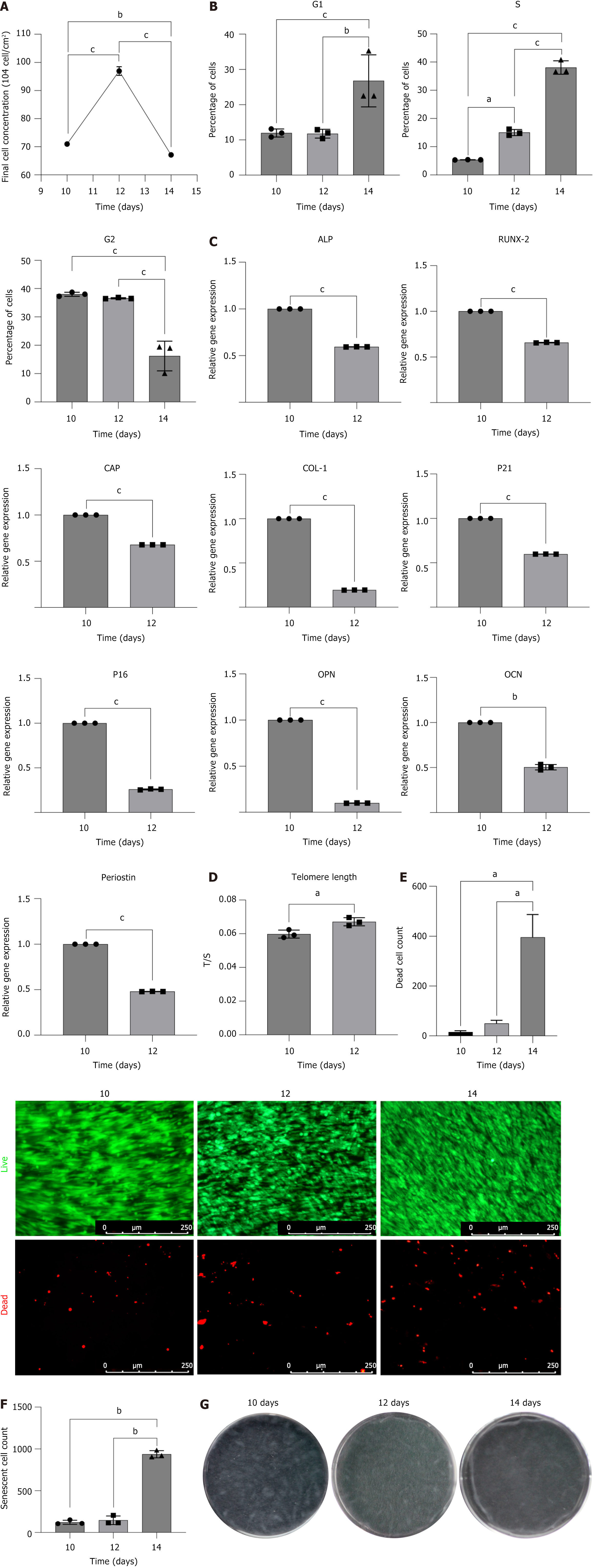
The number of cells in the cell sheet initially increased from the 10th day to the 12th day and subsequently decreased from the 12th day to the 14th day (P < 0.001) (Figure 2A), indicating that the cell population may have reached the Hayflick limit by the 12th day, indicating that the culture had achieved maximum population doubling[16]. Between the 10th and 12th days, some cells may have entered a stage of replicative senescence. Analysis of the cell cycle within the cell sheet showed an increase in the proportion of S-phase cells from the 10th day to the 12th day (P < 0.05), suggesting that some cells may have been arrested in the S-phase during this period (Figure 2B). However, qPCR analysis of cells in the cell sheet on the 10th day and the 12th day revealed that P16 and P21 gene expression was higher on the 10th day than on the 12th day (P < 0.001) (Figure 2C), and the relative telomere length was shorter on the 10th day than on the 12th day (P < 0.05) (Figure 2D). This discrepancy arises because qPCR measures the average gene expression across all cells rather than single-cell gene expression. Cell death is the eventual outcome of cellular aging, and the death of senescent cells results in a reduction in these cells[17]. Between the 10th day and the 12th day, the number of dead cells increased (Figure 2E) and the number of senescent cells decline (Figure 2F).
From the 12th day to the 14th day, β-galactosidase staining indicated a significant increase in the number of late senescent cells (P < 0.01) (Figure 2F). AO/PI staining also showed a marked increase in the number of dead cells (P < 0.05) (Figure 2E), while the proportion of S-phase and G1-phase cells increased, and the proportion of G2-phase cells decreased (P < 0.001) (Figure 2B). These results suggested that the cells were undergoing cellular aging.
Therefore, extending the culture period to 14 days could be excluded, and an earlier endpoint for cell sheet culture should be considered. Given these findings, we evaluated the optimal endpoint for functional cell sheet applications. RT-qPCR results revealed that the expression of osteogenic, chondrogenic, and odontogenic genes was the highest on 10th day (Figure 2C), coinciding with structural stability of the sheet (Figure 2G). By day 12, although the sheet remained intact with minimal wrinkling (Figure 2G), the decline in differentiation potential and onset of late senescence suggested functional compromise.
In summary, on the 10th day, the cell sheet exhibited a higher expression of osteogenic, chondrogenic, and odontogenic genes, had a stable structure, and had not yet entered the late senescence phase. Additionally, the time and cost of culture were minimized, making the 10th day as the optimal culture duration for DFSC sheets.
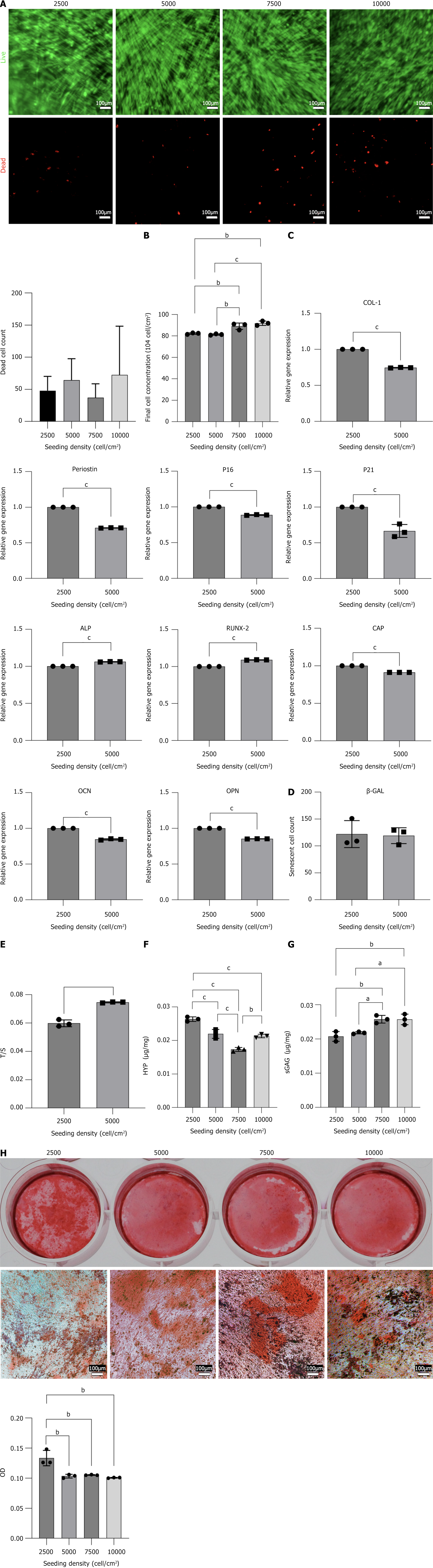
AO/PI staining was performed on cell sheets at inoculation concentrations of 2500/cm2, 5000/cm2, 7500/cm2, and 10000/cm2, revealing no statistically significant difference in the number of dead cells (Figure 3A). The cell sheet inoculated at 5000 cells/cm2 exhibited twice the cell density as the 2500 cells/cm2 group. However, there was no significant difference in the number of cells harvested on the 10th day between the two groups (Figure 3B). This suggests that the 2500 cells/cm2 group had a higher division rate than the 5000 cells/cm2 group, which might explain the slightly higher expression of senescent genes in the 2500 group (Figure 3C), along with a slightly shorter relative telomere length
To further characterize the functional implications of inoculation density, we analyzed ECM composition. The ECM is a non-cellular, 3D macromolecular network composed primarily of collagen, proteoglycans/GAGs, elastin, fibronectin, laminin, and several other glycoproteins, with collagen and GAG making up a large portion. Collagen, in particular, contains a high level of HYP[18]. The sGAG and HYP contents in the cell sheets from the 2500 and 5000 groups were measured, revealing that the cell sheets from the 2500 group contained more HYP than those from the 5000 group (P < 0.001) (Figure 3F). There was no significant difference in the sGAG content between the 2500 cells/cm2 and 5000 cells/cm2 groups (Figure 3G).
Expanding on these structural assessments, we investigated osteogenic differentiation potential using RT-qPCR and alizarin red staining. RT-qPCR was used to detect the expression of osteogenic genes in both groups, revealing different expression patterns across various osteogenic genes. The expression of cementum attachment protein, osteocalcin, and osteopontin genes was higher in the 2500 cells/cm2 group than in the 5000 cells/cm2 group, whereas the expression of alkaline phosphatase and runt-related transcription factor 2 genes was lower, which may be attributed to different stages of osteogenesis (Figure 3H). Alizarin red staining indicated that the osteogenic effect in the 2500 cells/cm2 group was better than that in the other groups (P < 0.01) (Figure 3H). Additionally, the expression of chondrogenic and odontogenic genes was higher in the 2500 cells/cm2 group than in the 5000 cells/cm2 group (P < 0.001) (Figure 3C).
In summary, selecting 2500 cells/cm2 as the inoculation concentration produced more cell sheets from the same number of cells, which is advantageous for large-scale industrial production. The 2500 cells/cm2 group showed higher expression of cartilage and odontoblastic genes, better osteogenic effects, and higher HYP content. There was no significant difference in the number of senescent or dead cells or the sGAG content. Therefore, 2500 cells/cm2 was determined to be the optimal culture concentration for producing the DFSC sheets.
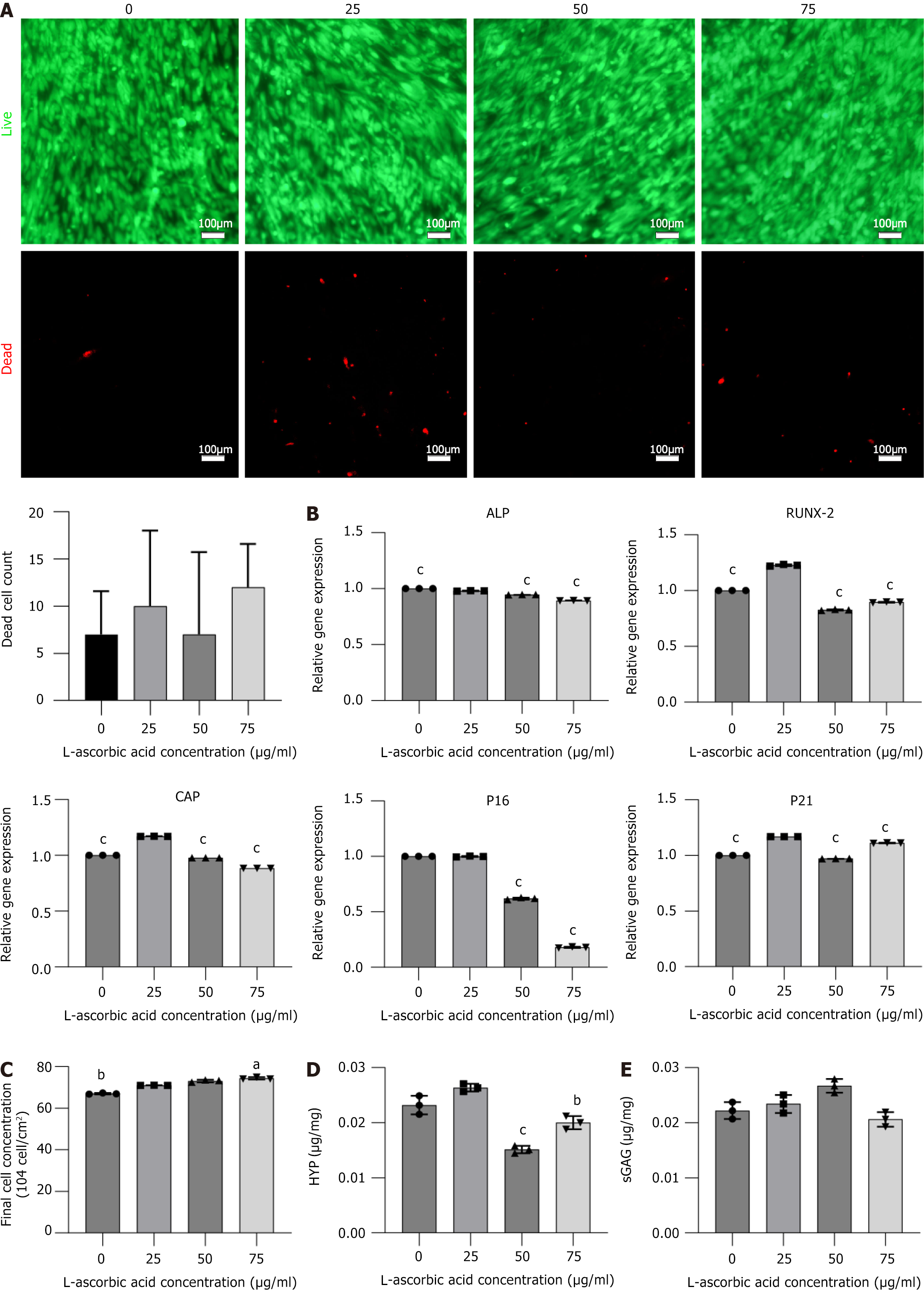
The effects of varying concentrations of L-ascorbic acid on cell sheet viability were initially assessed. No significant differences in dead cell counts were observed across different concentrations of L-ascorbic acid (Figure 4A), indicating that the compound did not exert cytotoxic effects at the tested concentrations. This foundational finding provided confidence to proceed with further analyses of osteogenic gene expression and cellular behavior under these conditions.
When the L-ascorbic acid concentration was 25 μg/mL, the expression of the osteogenic genes cementum attachment protein and runt-related transcription factor 2 was significantly higher, and the expression of alkaline phosphatase gene was also in a high range (P < 0.001) (Figure 4B). These results suggested that 25 μg/mL L-ascorbic acid may represent an optimal concentration for promoting osteogenic differentiation.
To further investigate the effects of L-ascorbic acid concentration on cellular senescence, we assessed the expression levels of senescence-related genes. As cells aged, the expression of P16 increased, while P21 expression initially increased and then decreased[19]. RT-qPCR results showed that P16 expression in the cell sheets decreased as the L-ascorbic acid concentration increased (P < 0.001) (Figure 4B), indicating that higher concentrations of L-ascorbic acid reduced the level of cell senescence.
In addition to its anti-aging effects, L-ascorbic acid can promote cell proliferation and stimulate cell secretion of ECM. An increased L-ascorbic acid concentration led to a higher final cell count in the cell sheets (Figure 4C). Furthermore, the ECM content of cells cultured in media containing different L-ascorbic acid concentrations was also measured. Although the trends in sGAG and HYP content varied, the ECM content remained consistently high when the L-ascorbic acid concentration was 25 μg/mL (Figure 4D and E).
As the DFSC sheet is intended to promote bone regeneration in periodontal defect areas, osteogenic performance serves as the primary criterion for selecting culture conditions. Therefore, 25 μg/mL L-ascorbic acid was identified as the optimal concentration, as it requires minimal L-ascorbic acid, promotes high expression of osteogenic genes, and maintains a high level of ECM content, making it the most suitable culture condition.
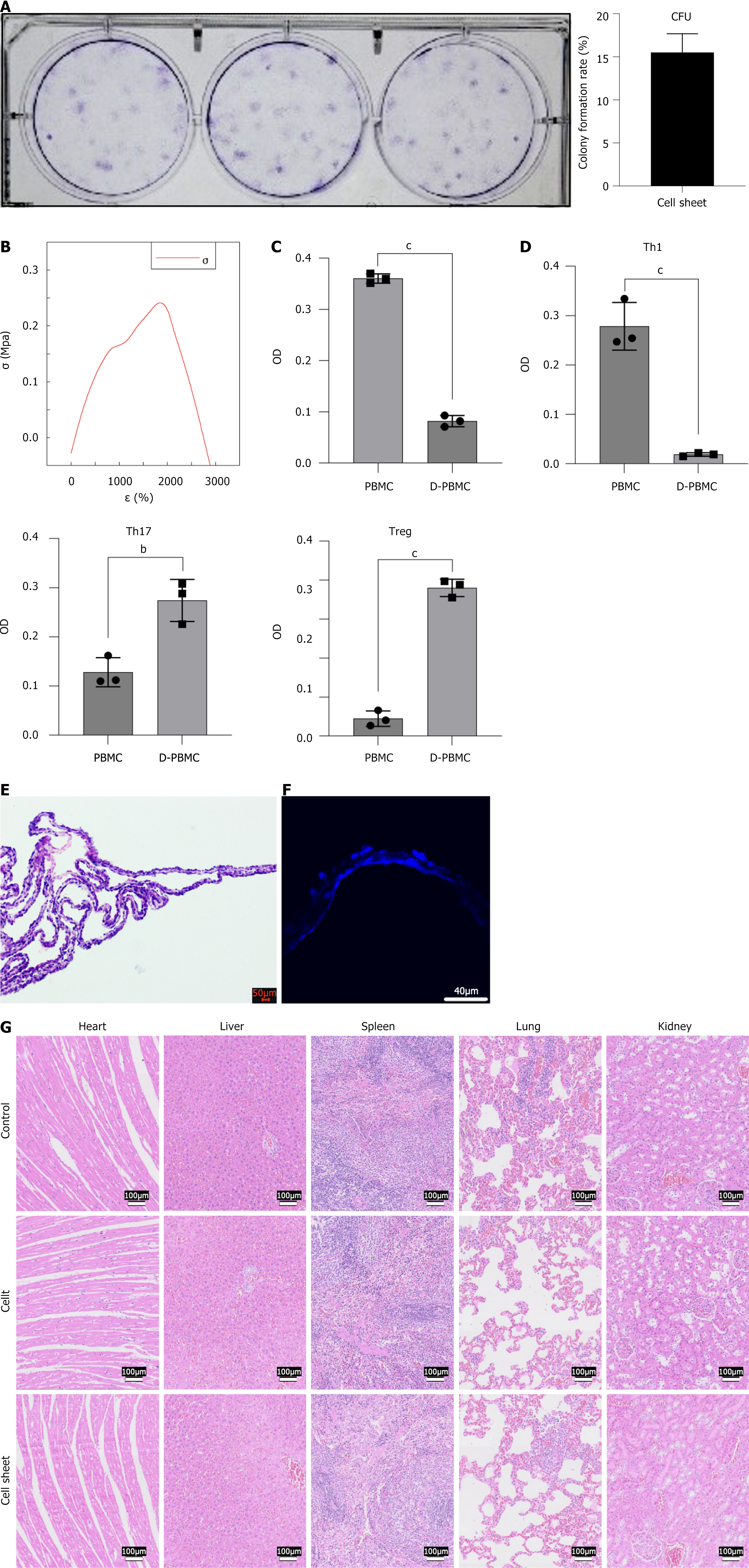
Based on the findings above, the optimal conditions for cell sheet culture were determined to be P4 cells, 10-day culture period, inoculation density of 2500 cells/cm2, and L-ascorbic acid concentration of 25 μg/mL. Under these optimized conditions, the colony formation ability of the standardized cultured cell sheet exceeded 10%, indicating strong proliferative capacity (Figure 5A).
To evaluate the mechanical properties of the cell sheets cultured under these conditions, physical characterization was performed using a universal testing machine. The stress-strain curve (Figure 5B) showed that the cell sheets could withstand a maximum tensile force of 0.0132 N, with an elastic modulus of 0.282 ± 0.113 MPa. Next, the biological interactions of the cell sheets were investigated. Co-culturing DFSCs from the cell sheet with peripheral blood mononuclear stem cells resulted in a significant decrease in the proliferation ability of the mononuclear cells (P < 0.001) (Figure 5C). Additionally, the proportion of Th1 and Th17 cells decreased, while the proportion of Tregs increased (Figure 5D).
Structural integrity was confirmed through histological analysis. HE staining and 4’,6-diamidino-2-phenylindole fluorescence staining confirmed that the cell sheet exhibited a uniform double-layer structure (Figure 5E and F). To further validate the safety and compatibility of the cell sheets, additional analyses were performed. When the cell sheet and cell suspension were implanted into rat periodontal defects, no abnormal cell proliferation was observed in the heart, liver, spleen, lungs, or kidneys, and no abnormal inflammatory responses, such as necrotic cells or macrophages, were detected (Figure 5G). After co-culture of periodontal ligament stem cells with the extraction DFSC sheets, the cell viability was 98.19%, and the inhibition rate was 1.81%. Generally, a relative cell viability of ≥ 70% is considered to indicate no cytotoxicity of the material, and an inhibition rate of < 30% is considered to indicate no significant inhibitory effect on cell growth. Therefore, DFSC sheets are deemed to be non-cytotoxic. Three days and 30 days after the implantation of cell sheets in SD rats, no statistically significant differences were observed between the cell sheet group and the control group in routine blood tests and biochemical blood analyses. The data are presented in Supplementary Table 4. No bacteria or fungi were detected in the culture supernatant of the cell sheets, and the endotoxin level was less than 0.5 EU/mL. The physical, chemical, and biological properties of the cell sheets cultured under standardized conditions were thoroughly characterized, resulting in the establishment of a proposed standard for DFSC sheets, as shown in Supplementary Table 5.
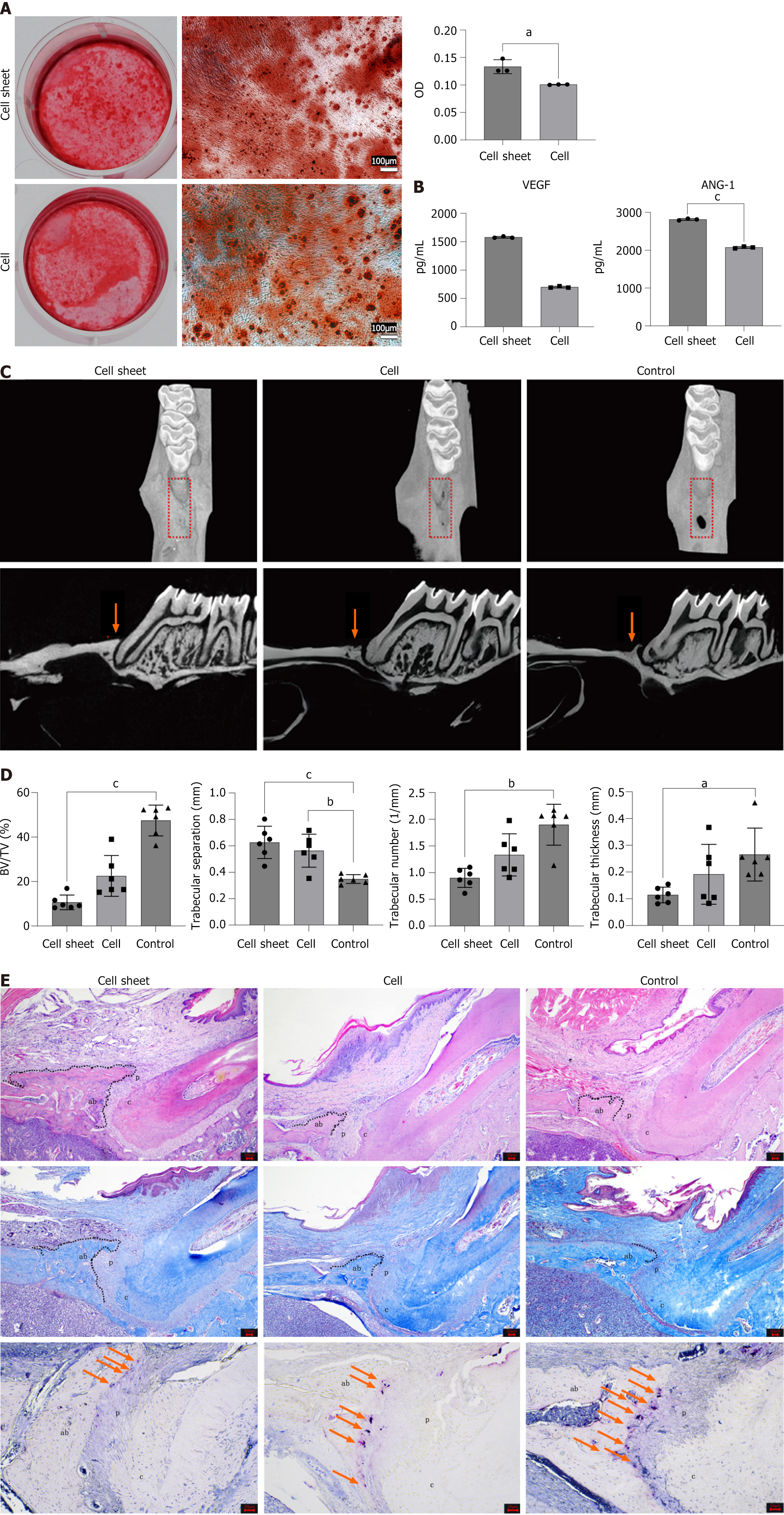
As a cell-based preparation, the cell sheet offers greater convenience for implantation into periodontal defect areas and significantly outperforms cell suspensions in terms of therapeutic efficacy. To initially compare the osteogenic potential of these two preparations, Alizarin red staining was employed. This method allowed for both qualitative and quantitative assessment of osteogenic effects, revealing that the osteogenic effect of the cell sheet was superior (P < 0.05) (Figure 6A).
Building on this finding, we further investigated the biological activities of the cell sheet and cell suspension by evaluating their paracrine functions. The cell sheet exhibited stronger exocrine activity than the cell suspension, with markedly higher secretion of the angiogenesis-related growth factors VEGF and ANG-1 (Figure 6B). This enhanced paracrine function likely contributes to the superior therapeutic efficacy of the cell sheet, as angiogenesis is critical for tissue regeneration.
To translate these in vitro observations into a preclinical setting, both the cell suspension and cell sheet were transplanted into periodontal defect areas of rats. After four weeks, bone formation was observed in the defect areas. HE staining and micro-CT 3D reconstruction demonstrated that the new bone height in the cell sheet group was greater than that in the cell suspension group, with both groups showing better results compared to the control group (Figure 6C and D).
Masson’s trichrome staining revealed that the new bone consisted of spongy cancellous bone rich in trabecular bone. Furthermore, the newly formed periodontal ligament was visible with fibers inserted vertically or obliquely into the new alveolar bone (Figure 6E). Micro-CT analysis also indicated that the trabecular separation in the cell sheet group was lower than that in the cell suspension group, and both were lower than that in the control group. The bone volume/tissue volume ratio, trabecular thickness, and trabecular number were higher in the cell sheet group than in the cell suspension group, and both were higher than in the control group (n = 6) (Figure 6D). Bone resorption is driven by osteoclasts[20]. Given that bone resorption is a critical counterbalance to bone formation, we also evaluated osteoclast activity. Tartrate-resistant acid phosphatase staining, which marks osteoclasts in red, indicated that osteoclasts were predominantly located at the junction between the alveolar bone and the periodontal ligament. The cell sheet group exhibited the lowest osteoclast density, whereas the control group displayed the highest density (Figure 6E).
The stem cell industry is rapidly emerging. A search of the ClinicalTrials.gov website using “Intervention/Treatment” as “Cell” revealed 37046 clinical studies, including 16 clinical trials investigating the use of cell injection to treat periodontitis. Various policies and regulations have been implemented to guide and standardize the development of the stem cell industry.
Unlike cell suspensions derived from enzymatic digestion, cell sheets exhibit dense networks of intercellular connections[13]. Various types of intercellular connections are involved in osteogenesis regulation[21]. This may explain the observed increase in bone regeneration. Pericellular matrix is believed to play a critical role in cell function and regulation. Pericellular matrix binds to growth factors, regulating their activation, synthesis, degradation, and local concentration[22]. This property could explain why the cell sheets in this study secreted higher levels of VEGF and ANG-1. Moreover, cell sheets are distinct from biological scaffolds because they contain no exogenous structures. Given their distinctive advantages, cell sheets have been widely used in research across multiple fields, including diabetes[23-25], liver failure[26-28], heart disease[29-31], retinopathy[32], periodontitis, skin injuries[33,34] and other conditions[35].
Current research on cell sheets has primarily focused on their application, with relatively little exploration of the mechanisms by which they exert therapeutic effects in vivo. Cell sheets are believed to function therapeutically through mechanisms such as angiogenesis[36], immune regulation[37], paracrine signaling[38] and the direct survival of transplanted cells[39]. However, the mechanism by which cell sheets promote healing can vary depending on the disease model and the cell source used. While expanding the clinical applications of cell sheets to address a wider range of medical issues, it is equally important to deepen our understanding of the mechanisms underlying their therapeutic effects in different disease models.
In addition to the culture process, several aspects of cell-sheet translation require further investigation. For example, the optimal conditions for transporting cell sheets from the production facility to the clinical site need to be identified to minimize any negative impact on cell viability. Determining the best reagents and transport conditions is critical for maintaining the cell quality. The ideal cryopreservation conditions for cell sheets warrant further exploration to enable large-scale production and batch usage. This would eliminate the need to produce cell sheets on-demand, which often requires starting the process up to two weeks in advance for clinical use, thereby reducing potential waste when patients’ treatment plans change unexpectedly. Cell sheets cultured in a low-dose, long-term manner contain more ECM and proteins than those produced in a large-dose, short-term manner. However, extending culture time also increases the risk of contamination and other production-related issues. Thus, determining the most effective production method for cell sheets is an important area of research.
As a cell product, the DFSC sheet adheres to GMP standards in its production process, with no exogenous growth factors added, and L-ascorbic acid included as a supplement. The cells used to produce the sheets were derived from the WCB, and each sheet required approximately 11250 cells. After 10 days of culture, the cell number increased by approximately 320-fold.
The number of cell passages can influence the morphology and differentiation potential of human mesenchymal stem cells[40]. DFSCs increase their secretion of ECM with additional cell passages; however, by the 14th passage, their osteogenic differentiation ability is inhibited[41,42]. Kim et al[43] found that the proliferation rate of embryonic stem cells decreased after the 9th passage, indicating that cell sheets should be generated before this point. In this study, cells were sourced from both the main and WCBs, and the cell sheets produced by the P2 and P4 generation cells were compared. It was found that an increased passage number in DFSCs led to greater ECM secretion, wider intercellular spaces, and increased cell viability. The osteogenic effect of the cell sheets cultured with P4 generation cells was superior to that of the sheets cultured with P2 generation cells.
There was an interaction between inoculation concentration and cell culture duration. Thus, increasing the inoculation concentration may reduce the required harvest time[43]. Wang et al[44] studied the production and transportation quality standards of human umbilical cord stem cell sheets. However, their approach involved the use of temperature-sensitive materials combined with high-density cell culture over a short period, which differs significantly from the traditional low-dose, long-term cell sheet culture approach. Additionally, the sources of umbilical cord mesenchymal stem cells and DFSCs differ, as do their biological characteristics, potentially leading to different quality standards for cell sheets. The typical culture conditions for DFSC sheets ranged from 5000 to 10000 cells/cm2 over approximately two weeks[10,45,46]. In this study, these conditions were referenced, and the optimal cell culture concentration and duration were determined based on osteogenesis, chondrogenesis, odontogenesis, cell aging, viability, and ECM secretion.
L-ascorbic acid is a common additive that is widely used in the culture of cell sheet[47-49]. It can promote cell growth[50,51], ECM secretion[52], osteogenic differentiation[47] and slow down senescence[53,54]. However, higher concentrations of L-ascorbic acid can be cytotoxic[55]. Most studies used 50 μg/mL L-ascorbic acid to culture cell sheets[56,57]. Wei et al[58] found that the optimal L-ascorbic acid concentration for culturing periodontal ligament cell sheets was 20 μg/mL. L-ascorbic acid concentrations between 20 and 50 μg/mL can successfully form cell sheets, but those cultured with lower concentrations of L-ascorbic acid tend to break easily and extend irregularly. In this study, the effects of L-ascorbic acid concentrations ranging from 0 to 75 μg/mL on DFSC sheets were compared. L-ascorbic acid inhibited aging while promoting cell proliferation and growth. At a concentration of 25 μg/mL, the expression of osteogenic, chondrogenic, and odontogenic genes was the highest, and the secretion of the ECM remained at an elevated level.
Colony-forming units analysis is commonly used to assess the proliferation capacity of stem cells isolated from tissues[59]. In this study, the clone formation rate of cells isolated from the cell sheet exceeded 10%, indicating that the cells retained good proliferative capacity. Under suboptimal culture conditions, cell sheets can become too fragile to be securely mounted on a testing apparatus, making it difficult to obtain tensile test data[52]. In this study, the cell sheet was easily and completely removed from the wells. To evaluate the physical properties of the cell sheet, tensile testing was performed to determine the maximum stress the sheet could withstand before breaking. The cell sheet could withstand a maximum tensile force of 0.0132 N. However, the mechanical properties of cell sheets can also be increased by altering cell orientation and increasing the production of growth factors, which is worthy of extensive and in-depth study[60]. Mesenchymal stem cells are known to inhibit the proliferation of PBMCs and reduce inflammation in vitro[61]. Within the body, Th1 and Th17 cells release pro-inflammatory factors, while Treg cells exert anti-inflammatory effects. Stem cells have been shown to reduce the proportion of Th1 and Th17 cells, while increasing the proportion of Treg cells, thereby achieving anti-inflammatory effects and improving periodontitis[62,63]. In this study, we utilized a periodontal defect model in SD rats to evaluate the periodontal regenerative capacities, safety, and biocompatibility of DFSC sheets and DFSC suspensions. To further investigate and validate these findings, future studies should employ clinically relevant validations to further explore and confirm the safety and efficacy of optimized DFSC sheets.
We developed a quality standard table for the DFSC sheets based on the data obtained in this study. It is important to note that variations in the culture medium and cell lineage may have slightly influenced the detection values. Therefore, other organizations may need to establish their own standard operating procedures and quality standards tailored to the specific medium and cell lineage they employ. Nonetheless, our research provides valuable detection methods and quality benchmarks for the translation of stem cell sheets, thereby contributing to the advancement and progress of stem cell-based therapies.
P4 DFSCs were cultured at a concentration of 2500 cells/cm² over a 10-day period with the addition of 25 μg/mL L-ascorbic acid, which was identified as the optimal condition for DFSC sheet formation. Compared with the DFSC suspension, the cell sheet demonstrated superior osteogenic, paracrine, immunoregulatory, and periodontal regenerative capacities. This study lays the groundwork for future clinical trials of DFSC sheets for periodontitis treatment and provides insights into the feasibility of industrial-scale production of these cell sheets.
| 1. | Mohammed E, Khalil E, Sabry D. Effect of Adipose-Derived Stem Cells and Their Exo as Adjunctive Therapy to Nonsurgical Periodontal Treatment: A Histologic and Histomorphometric Study in Rats. Biomolecules. 2018;8:167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 2. | Sun L, Du X, Kuang H, Sun H, Luo W, Yang C. Stem cell-based therapy in periodontal regeneration: a systematic review and meta-analysis of clinical studies. BMC Oral Health. 2023;23:492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 3. | Qiao X, Tang J, Dou L, Yang S, Sun Y, Mao H, Yang D. Dental Pulp Stem Cell-Derived Exosomes Regulate Anti-Inflammatory and Osteogenesis in Periodontal Ligament Stem Cells and Promote the Repair of Experimental Periodontitis in Rats. Int J Nanomedicine. 2023;18:4683-4703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 54] [Reference Citation Analysis (0)] |
| 4. | Tolouei AE, Oruji F, Tehrani S, Rezaei S, Mozaffari A, Jahri M, Nasiri K. Gingival mesenchymal stem cell therapy, immune cells, and immunoinflammatory application. Mol Biol Rep. 2023;50:10461-10469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Bharuka T, Reche A. Advancements in Periodontal Regeneration: A Comprehensive Review of Stem Cell Therapy. Cureus. 2024;16:e54115. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Nguyen-Thi TD, Nguyen-Huynh BH, Vo-Hoang TT, Nguyen-Thanh T. Stem cell therapies for periodontal tissue regeneration: A meta-analysis of clinical trials. J Oral Biol Craniofac Res. 2023;13:589-597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | Zhang J, Ding H, Liu X, Sheng Y, Liu X, Jiang C. Dental Follicle Stem Cells: Tissue Engineering and Immunomodulation. Stem Cells Dev. 2019;28:986-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Liu L, Wen Y, Chen L, Li M, Yu J, Tian W, Wu Y, Guo S. Xenogenous implanted dental follicle stem cells promote periodontal regeneration through inducing the N2 phenotype of neutrophils. Stem Cell Res Ther. 2024;15:270. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Wei X, Guo S, Liu Q, Liu L, Huo F, Wu Y, Tian W. Dental Follicle Stem Cells Promote Periodontal Regeneration through Periostin-Mediated Macrophage Infiltration and Reprogramming in an Inflammatory Microenvironment. Int J Mol Sci. 2023;24:6353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Guo S, Guo W, Ding Y, Gong J, Zou Q, Xie D, Chen Y, Wu Y, Tian W. Comparative study of human dental follicle cell sheets and periodontal ligament cell sheets for periodontal tissue regeneration. Cell Transplant. 2013;22:1061-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Khalili M, Asadi M, Kahroba H, Soleyman MR, Andre H, Alizadeh E. Corneal endothelium tissue engineering: An evolution of signaling molecules, cells, and scaffolds toward 3D bioprinting and cell sheets. J Cell Physiol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Chen M, Xu Y, Zhang T, Ma Y, Liu J, Yuan B, Chen X, Zhou P, Zhao X, Pang F, Liang W. Mesenchymal stem cell sheets: a new cell-based strategy for bone repair and regeneration. Biotechnol Lett. 2019;41:305-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Wibbe N, Ebnet K. Cell Adhesion at the Tight Junctions: New Aspects and New Functions. Cells. 2023;12:2701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 14. | Nakao M, Matsui M, Kim K, Nishiyama N, Grainger DW, Okano T, Kanazawa H, Nagase K. Umbilical cord-derived mesenchymal stem cell sheets transplanted subcutaneously enhance cell retention and survival more than dissociated stem cell injections. Stem Cell Res Ther. 2023;14:352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 15. | Yi G, Zhang S, Ma Y, Yang X, Huo F, Chen Y, Yang B, Tian W. Matrix vesicles from dental follicle cells improve alveolar bone regeneration via activation of the PLC/PKC/MAPK pathway. Stem Cell Res Ther. 2022;13:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 16. | Tkemaladze J, Chichinadze K. Centriole, differentiation, and senescence. Rejuvenation Res. 2010;13:339-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Ogrodnik M. Cellular aging beyond cellular senescence: Markers of senescence prior to cell cycle arrest in vitro and in vivo. Aging Cell. 2021;20:e13338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 18. | Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1588] [Article Influence: 176.4] [Reference Citation Analysis (0)] |
| 19. | Morsczeck C, Hullmann M, Reck A, Reichert TE. The cell cycle regulator protein P16 and the cellular senescence of dental follicle cells. Mol Cell Biochem. 2018;439:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Yahara Y, Nguyen T, Ishikawa K, Kamei K, Alman BA. The origins and roles of osteoclasts in bone development, homeostasis and repair. Development. 2022;149:dev199908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 21. | Izu Y, Ezura Y, Koch M, Birk DE, Noda M. Collagens VI and XII form complexes mediating osteoblast interactions during osteogenesis. Cell Tissue Res. 2016;364:623-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Vincent TL, McClurg O, Troeberg L. The Extracellular Matrix of Articular Cartilage Controls the Bioavailability of Pericellular Matrix-Bound Growth Factors to Drive Tissue Homeostasis and Repair. Int J Mol Sci. 2022;23:6003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 23. | Kim YH, Ko JH, Lee S, Oh JY, Jeong GS, Park SN, Shim IK, Kim SC. Long-term reversal of diabetes by subcutaneous transplantation of pancreatic islet cells and adipose-derived stem cell sheet using surface-immobilized heparin and engineered collagen scaffold. BMJ Open Diabetes Res Care. 2020;8:e001128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Narendran P, Tomlinson C, Beese S, Sharma P, Harris I, Adriano A, Maggs F, Burrows M, Nirantharakumar K, Thomas N, Price MJ, Andrews RC, Moore DJ. A systematic review and meta-analysis of interventions to preserve insulin-secreting β-cell function in people newly diagnosed with type 1 diabetes: Results from intervention studies aimed at improving glucose control. Diabet Med. 2022;39:e14730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Lee YN, Yi HJ, Kim YH, Lee S, Oh J, Okano T, Shim IK, Kim SC. Evaluation of Multi-Layered Pancreatic Islets and Adipose-Derived Stem Cell Sheets Transplanted on Various Sites for Diabetes Treatment. Cells. 2020;9:1999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Toya K, Tomimaru Y, Kobayashi S, Harada A, Sasaki K, Iwagami Y, Yamada D, Noda T, Takahashi H, Kado T, Imamura H, Takaichi S, Chijimatsu R, Asaoka T, Tanemura M, Miyagawa S, Doki Y, Eguchi H. Efficacy of Autologous Skeletal Myoblast Cell Sheet Transplantation for Liver Regeneration in Liver Failure. Transplantation. 2023;107:e190-e200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 27. | Nagamoto Y, Takayama K, Ohashi K, Okamoto R, Sakurai F, Tachibana M, Kawabata K, Mizuguchi H. Transplantation of a human iPSC-derived hepatocyte sheet increases survival in mice with acute liver failure. J Hepatol. 2016;64:1068-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 28. | Asadi M, Khalili M, Lotfi H, Vaghefi Moghaddam S, Zarghami N, André H, Alizadeh E. Liver bioengineering: Recent trends/advances in decellularization and cell sheet technologies towards translation into the clinic. Life Sci. 2021;276:119373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Guo R, Morimatsu M, Feng T, Lan F, Chang D, Wan F, Ling Y. Stem cell-derived cell sheet transplantation for heart tissue repair in myocardial infarction. Stem Cell Res Ther. 2020;11:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 30. | Fang J, Li JJ, Zhong X, Zhou Y, Lee RJ, Cheng K, Li S. Engineering stem cell therapeutics for cardiac repair. J Mol Cell Cardiol. 2022;171:56-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 31. | Matsuura K, Masuda S, Shimizu T. Cell sheet-based cardiac tissue engineering. Anat Rec (Hoboken). 2014;297:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Watari K, Yamasaki S, Tu HY, Shikamura M, Kamei T, Adachi H, Tochitani T, Kita Y, Nakamura A, Ueyama K, Ono K, Morinaga C, Matsuyama T, Sho J, Nakamura M, Fujiwara M, Hori Y, Tanabe A, Hirai R, Terai O, Ohno O, Ohara H, Hayama T, Ikeda A, Nukaya D, Matsushita K, Takahashi M, Kishino A, Kimura T, Kawamata S, Mandai M, Kuwahara A. Self-organization, quality control, and preclinical studies of human iPSC-derived retinal sheets for tissue-transplantation therapy. Commun Biol. 2023;6:164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 33. | Cerqueira MT, Pirraco RP, Martins AR, Santos TC, Reis RL, Marques AP. Cell sheet technology-driven re-epithelialization and neovascularization of skin wounds. Acta Biomater. 2014;10:3145-3155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Jackson CJ, Tønseth KA, Utheim TP. Cultured epidermal stem cells in regenerative medicine. Stem Cell Res Ther. 2017;8:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Hu D, Li X, Li J, Tong P, Li Z, Lin G, Sun Y, Wang J. The preclinical and clinical progress of cell sheet engineering in regenerative medicine. Stem Cell Res Ther. 2023;14:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 36. | Yamaguchi S, Higashi M, Kanetaka K, Maruya Y, Kobayashi S, Hashiguchi K, Hidaka M, Nakao K, Eguchi S. Rapid and chronological expression of angiogenetic genes is a major mechanism involved in cell sheet transplantation in a rat gastric ulcer model. Regen Ther. 2022;21:372-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 37. | Honda N, Watanabe Y, Tokuoka Y, Hanajima R. Roles of microglia/macrophage and antibody in cell sheet transplantation in the central nervous system. Stem Cell Res Ther. 2022;13:470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 38. | Xue F, Bai Y, Jiang Y, Liu J, Jian K. Construction and a preliminary study of paracrine effect of bone marrow-derived endothelial progenitor cell sheet. Cell Tissue Bank. 2022;23:185-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Shimizu R, Kamei N, Adachi N, Hamanishi M, Kamei G, Mahmoud EE, Nakano T, Iwata T, Yamato M, Okano T, Ochi M. Repair mechanism of osteochondral defect promoted by bioengineered chondrocyte sheet. Tissue Eng Part A. 2015;21:1131-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 40. | Yang YK, Ogando CR, Wang See C, Chang TY, Barabino GA. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res Ther. 2018;9:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 424] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 41. | Al-Bagdadi FA, Barona HM, Martinez-Ceballos E, Yao S. Ultrastructure Morphological Characterization of Different Passages of Rat Dental Follicle Stem Cells at In vitro Culture. J Microsc Ultrastruct. 2019;7:57-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 42. | Morsczeck C, Gresser J, Ettl T. The induction of cellular senescence in dental follicle cells inhibits the osteogenic differentiation. Mol Cell Biochem. 2016;417:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | Kim K, Bou-Ghannam S, Thorp H, Grainger DW, Okano T. Human mesenchymal stem cell sheets in xeno-free media for possible allogenic applications. Sci Rep. 2019;9:14415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 44. | Wang J, Gao S, Zhao Y, Fan T, Zhang M, Chang D. Manufacture and Quality Control of Human Umbilical Cord-Derived Mesenchymal Stem Cell Sheets for Clinical Use. Cells. 2022;11:2732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 45. | Yang H, Li J, Hu Y, Sun J, Guo W, Li H, Chen J, Huo F, Tian W, Li S. Treated dentin matrix particles combined with dental follicle cell sheet stimulate periodontal regeneration. Dent Mater. 2019;35:1238-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 46. | Yang B, Chen G, Li J, Zou Q, Xie D, Chen Y, Wang H, Zheng X, Long J, Tang W, Guo W, Tian W. Tooth root regeneration using dental follicle cell sheets in combination with a dentin matrix - based scaffold. Biomaterials. 2012;33:2449-2461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 47. | Ashok K, Thomas B, Manjappa AB, Shetty J, Rao S, Basavarajappa MK, Ramesh A. Characterization and evaluation of ascorbic acid-induced cell sheet formation in human periodontal ligament stem cells: An in vitro study. J Oral Biosci. 2021;63:429-435. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 48. | Taninaka A, Kabata T, Hayashi K, Kajino Y, Inoue D, Ohmori T, Ueoka K, Yamamuro Y, Kataoka T, Saiki Y, Yanagi Y, Ima M, Iyobe T, Tsuchiya H. Chondroprotective Effects of Chondrogenic Differentiated Adipose-Derived Mesenchymal Stem Cells Sheet on Degenerated Articular Cartilage in an Experimental Rabbit Model. Bioengineering (Basel). 2023;10:574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 49. | Pedroni ACF, Diniz IMA, Abe GL, Moreira MS, Sipert CR, Marques MM. Photobiomodulation therapy and vitamin C on longevity of cell sheets of human dental pulp stem cells. J Cell Physiol. 2018;233:7026-7035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 50. | Choi KM, Seo YK, Yoon HH, Song KY, Kwon SY, Lee HS, Park JK. Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation. J Biosci Bioeng. 2008;105:586-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 211] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 51. | Fujisawa K, Hara K, Takami T, Okada S, Matsumoto T, Yamamoto N, Sakaida I. Evaluation of the effects of ascorbic acid on metabolism of human mesenchymal stem cells. Stem Cell Res Ther. 2018;9:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 52. | Hasenzahl M, Müsken M, Mertsch S, Schrader S, Reichl S. Cell sheet technology: Influence of culture conditions on in vitro-cultivated corneal stromal tissue for regenerative therapies of the ocular surface. J Biomed Mater Res B Appl Biomater. 2021;109:1488-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Monacelli F, Acquarone E, Giannotti C, Borghi R, Nencioni A. Vitamin C, Aging and Alzheimer's Disease. Nutrients. 2017;9:670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 157] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 54. | Yang Y, Wang T, Zhang S, Jia S, Chen H, Duan Y, Wang S, Chen G, Tian W. Vitamin C alleviates the senescence of periodontal ligament stem cells through inhibition of Notch3 during long-term culture. J Cell Physiol. 2021;236:1237-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 55. | Wu YK, Tu YK, Yu J, Cheng NC. The Influence of Cell Culture Density on the Cytotoxicity of Adipose-Derived Stem Cells Induced by L-Ascorbic Acid-2-Phosphate. Sci Rep. 2020;10:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 56. | Guo P, Zeng JJ, Zhou N. A novel experimental study on the fabrication and biological characteristics of canine bone marrow mesenchymal stem cells sheet using vitamin C. Scanning. 2015;37:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Takewaki M, Kajiya M, Takeda K, Sasaki S, Motoike S, Komatsu N, Matsuda S, Ouhara K, Mizuno N, Fujita T, Kurihara H. MSC/ECM Cellular Complexes Induce Periodontal Tissue Regeneration. J Dent Res. 2017;96:984-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 58. | Wei F, Qu C, Song T, Ding G, Fan Z, Liu D, Liu Y, Zhang C, Shi S, Wang S. Vitamin C treatment promotes mesenchymal stem cell sheet formation and tissue regeneration by elevating telomerase activity. J Cell Physiol. 2012;227:3216-3224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 203] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 59. | Nguyen LT, Tran NT, Than UTT, Nguyen MQ, Tran AM, Do PTX, Chu TT, Nguyen TD, Bui AV, Ngo TA, Hoang VT, Hoang NTM. Optimization of human umbilical cord blood-derived mesenchymal stem cell isolation and culture methods in serum- and xeno-free conditions. Stem Cell Res Ther. 2022;13:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 60. | Xu W, Chen S, Yao M, Lu Q. Mechanical behavior of biomimetic oriented cell sheets from a perspective of living materials. Biomater Sci. 2022;10:3099-3109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 61. | Herzig MC, Christy BA, Montgomery RK, Delavan CP, Jensen KJ, Lovelace SE, Cantu C, Salgado CL, Cap AP, Bynum JA. Interactions of human mesenchymal stromal cells with peripheral blood mononuclear cells in a Mitogenic proliferation assay. J Immunol Methods. 2021;492:113000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 62. | Li A, Guo F, Pan Q, Chen S, Chen J, Liu HF, Pan Q. Mesenchymal Stem Cell Therapy: Hope for Patients With Systemic Lupus Erythematosus. Front Immunol. 2021;12:728190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 63. | Zhang Y, Chen J, Fu H, Kuang S, He F, Zhang M, Shen Z, Qin W, Lin Z, Huang S. Exosomes derived from 3D-cultured MSCs improve therapeutic effects in periodontitis and experimental colitis and restore the Th17 cell/Treg balance in inflamed periodontium. Int J Oral Sci. 2021;13:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |