Published online Sep 7, 2025. doi: 10.3748/wjg.v31.i33.108653
Revised: July 5, 2025
Accepted: August 5, 2025
Published online: September 7, 2025
Processing time: 128 Days and 0.1 Hours
Hepatic ischaemia-reperfusion injury (HIRI) is an unavoidable process in liver transplantation, where apoptosis plays a critical role. Human umbilical cord mesenchymal stem cell-derived exosomes (hucMSC-exos), which constitute a cell-free therapeutic approach, have garnered extensive attention in alleviating HIRI. However, the potential of hucMSC-exos in mitigating apoptosis and their underlying mechanisms remain largely unknown.
To investigate the effects of hucMSC-exos on apoptosis after HIRI and explore the underlying mechanisms.
The therapeutic effects of hucMSC-exos on HIRI and hypoxia/reoxygenation injury in L02 cells were investigated. RNA sequencing was used to detect differentially expressed genes in L02 cells after hucMSC-exo treatment, and the expression of apoptosis markers in L02 cells was analyzed. MicroRNA (miRNA) sequencing was performed to analyse the miRNA expression profiles of hucMSC-exos and L02 cells after hucMSC-exo treatment. Through a miRNA-mRNA integrated analysis, candidate miRNAs and their regulated target genes were identified. We subsequently studied the roles of these candidate miRNAs in mouse HIRI and L02 cell hypoxia/reoxygenation injury.
Fluorescence confocal microscopy revealed that hucMSC-exos effectively homed to the liver and were taken up by hepatocytes, likely due to the presence of anti-very late antigen-4 and anti-lymphocyte function-associated antigen-1 on the surface of hucMSC-exos. HucMSC-exos alleviate hepatocyte damage by inhibiting apoptosis. Specifically, let-7i-5p within hucMSC-exos inhibited the expression of the factor-related apoptosis ligand protein in L02 cells, leading to the upregulation of B-cell lymphoma-2 and the downregulation of B-cell lymphoma-2-associated X protein and cysteinyl aspartate specific proteinase-3, thereby inhibiting L02 cell apoptosis and enhancing cell proliferation activity. The overexpression of let-7i-5p effectively enhanced the antiapoptotic effects of hucMSC-exos both in vitro and in vivo.
Our findings indicate that hucMSC-exos alleviate HIRI by inhibiting apoptosis. We demonstrated that hucMSC-exos target apoptosis in L02 cells and mediate the let-7i-5p/factor-related apoptosis ligand pathway, thereby ameliorating HIRI. This study provides new insights into the role of hucMSC-exos in hepatocyte apoptosis and highlights the potential of hucMSC-exos as a therapeutic strategy for HIRI.
Core Tip: Human umbilical cord mesenchymal stem cell-derived exosomes mitigate hepatic ischaemia-reperfusion injury by targeting damaged hepatocytes via very late activation antigen-4/vascular cell adhesion molecule-1 and lymphocyte function-associated antigen-1/intercellular adhesion molecule-1 interactions. Their membrane proteins crucially mediate this specific protection, suppressing both the extrinsic and intrinsic apoptotic pathways. Central to this mechanism is the let-7i-5p/factor-related apoptosis ligand signaling axis.
- Citation: Gao Y, He M, Bian CW, Yu R, Luo JJ, Xiang YM, Yang YX, Huang HF, Zeng Z. Exosomes derived from human umbilical cord mesenchymal stem cells attenuate hepatic ischaemia-reperfusion injury via the let-7i-5p/Faslg axis. World J Gastroenterol 2025; 31(33): 108653
- URL: https://www.wjgnet.com/1007-9327/full/v31/i33/108653.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i33.108653
Hepatic ischaemia-reperfusion injury (HIRI) is an inevitable complication of liver transplants and hepatectomies. HIRI exacerbates perioperative complications, including reperfusion syndrome, primary graft nonfunction, delayed graft function recovery, and acute rejection responses, and causes damage to the lungs and brain[1-3]. Current interventions for HIRI include pharmacotherapy, ischaemic preconditioning, various mechanical perfusion techniques, and ischaemia-free liver transplantation[4,5]. Although animal studies suggest that ischaemic preconditioning and pharmacotherapy may mitigate HIRI, clinical trials have not yet definitively confirmed their effectiveness[6]. Moreover, the use of mechanical perfusion techniques in donation after cardiac death and ischaemia-free liver transplantation approaches do not entirely eliminate the adverse effects of HIRI. Consequently, more effective treatment strategies for this condition are needed. The primary pathological feature of HIRI is damage to hepatocytes in the periportal zone of the liver lobule[7]. These damaged hepatocytes release damage-associated molecular patterns, which in turn promote the formation of neutrophil extracellular traps, leading to microthrombus formation[8]. Thus, reducing hepatocyte damage is essential for improving the prognosis of patients with HIRI.
Mesenchymal stem cells (MSCs) have attracted substantial interest because of their biological functions, which include proliferation, nourishment, immunomodulation, multilineage differentiation, migration, and homing[9,10]. However, clinical trials have shown that MSC-based therapies can lead to complications such as fibrosis and thromboembolism[11]. Additionally, animal studies have revealed that intravenously administered MSCs predominantly accumulate in the lungs, exhibit short survival times, and face difficulties migrating to damaged tissues[12]. The low survival rate of MSCs suggests that their primary contribution to tissue repair is mediated through the paracrine secretion of exosomes or bioactive factors[13,14].
Exosomes, which are ubiquitously present in human tissues and bodily fluids, are characterized by low immunogenicity, the ability to easily cross biological barriers, diverse origins, and stable biological activity[15]. These attributes render exosomes potential carriers for disease treatment and valuable diagnostic and prognostic biomarkers for tumours[16-18]. Specifically, exosomes derived from human umbilical cord MSC-derived exosomes (hucMSC-exos) have substantial protective effects on animal models of HIRI. HucMSC-exos were shown to protect damaged liver cells by inhibiting inflammation, reducing apoptosis, and promoting hepatocyte regeneration[19]. Moreover, compared with MSCs, hucMSC-exos had a more pronounced effect on reducing hepatocyte apoptosis[20]. Therefore, the elucidation of the mechanisms by which hucMSC-exos treat HIRI is essential for their clinical application.
In various animal models of ischaemia/reperfusion (I/R) injury, the protective role of microRNAs (miRNAs) within exosomes has been highlighted[21-24]. Shi et al[25] demonstrated that miRNA-148b-3p, sourced from hucMSC-exos, can mitigate apoptosis in renal tubular cells by suppressing pyruvate dehydrogenase kinase 4 expression. However, the protective effect on renal tubules decreased when an miR-148b-3p inhibitor was used, underscoring the pivotal role of miRNAs in facilitating exosome-mediated renal protection. Conversely, Gao et al[26] reported that exosomes from bone marrow MSCs containing miRNA-125a-5p protect myocardial cells from injury without harming the liver or kidney. Consequently, we investigated the therapeutic effect of hucMSC-exos on damaged hepatic parenchymal cells and explored the involvement of specific miRNAs in this mechanism.
In this study, we utilized a mouse model of HIRI and a hypoxia/reoxygenation (H/R) injury model in L02 cells to assess the therapeutic potential of hucMSC-exos for H/R-induced injury. These findings indicate that hucMSC-exos effectively reduce apoptosis and increase cell proliferation, substantially mitigating HIRI. Through miRNA expression profiling and validation of specific miRNA target genes, we determined that hucMSC-exos protect L02 cells by modulating the let-7i-5p/factor-related apoptosis ligand (Faslg) signalling pathway. Consequently, our study identified hucMSC-exos as a novel and clinically promising therapeutic approach for the repair of hepatic cells after HIRI.
Supernatants were collected from human umbilical cord MSCs (hucMSCs) (Procell, Wuhan, China) at passages 3, 5, and 8, and the exosomes were extracted via ultracentrifugation. The specific steps were as follows: First, the supernatant was centrifuged at 2000 × g for 30 minutes, followed by centrifugation at 10000 × g for 45 minutes to remove larger vesicles. Next, the supernatant was filtered through a 0.45 μm filter membrane (Millipore, Darmstadt, Germany) and then ultracentrifuged at 100000 × g for 70 minutes. The pellet was subsequently resuspended in 10 mL of precooled phosphate-buffered saline (PBS) (Sangon Biotech, Shanghai, China). Finally, the suspension was ultracentrifuged again at 100000 × g for 70 minutes and resuspended in 100 μL of precooled PBS. The purified hucMSC-exos were stored at -80 °C.
For the identification of hucMSC-exos, transmission electron microscopy (Hitachi-HT7700, Tokyo, Japan) was used to observe their morphology, and nanoparticle tracking analysis (NanoFCM-N30E, Xiamen, China) was performed to measure the particle size and concentration. Additionally, fluorescence labelling of CD9 and CD81 (BD, Bergen, NJ, United States) was performed. Western blotting was performed to detect the markers ALG-2-interacting protein X (Alix), tumour susceptibility 101 (Tsg101), CD9, and CD63 (all antibodies were from Proteintech, Wuhan, China) in hucMSC-exos.
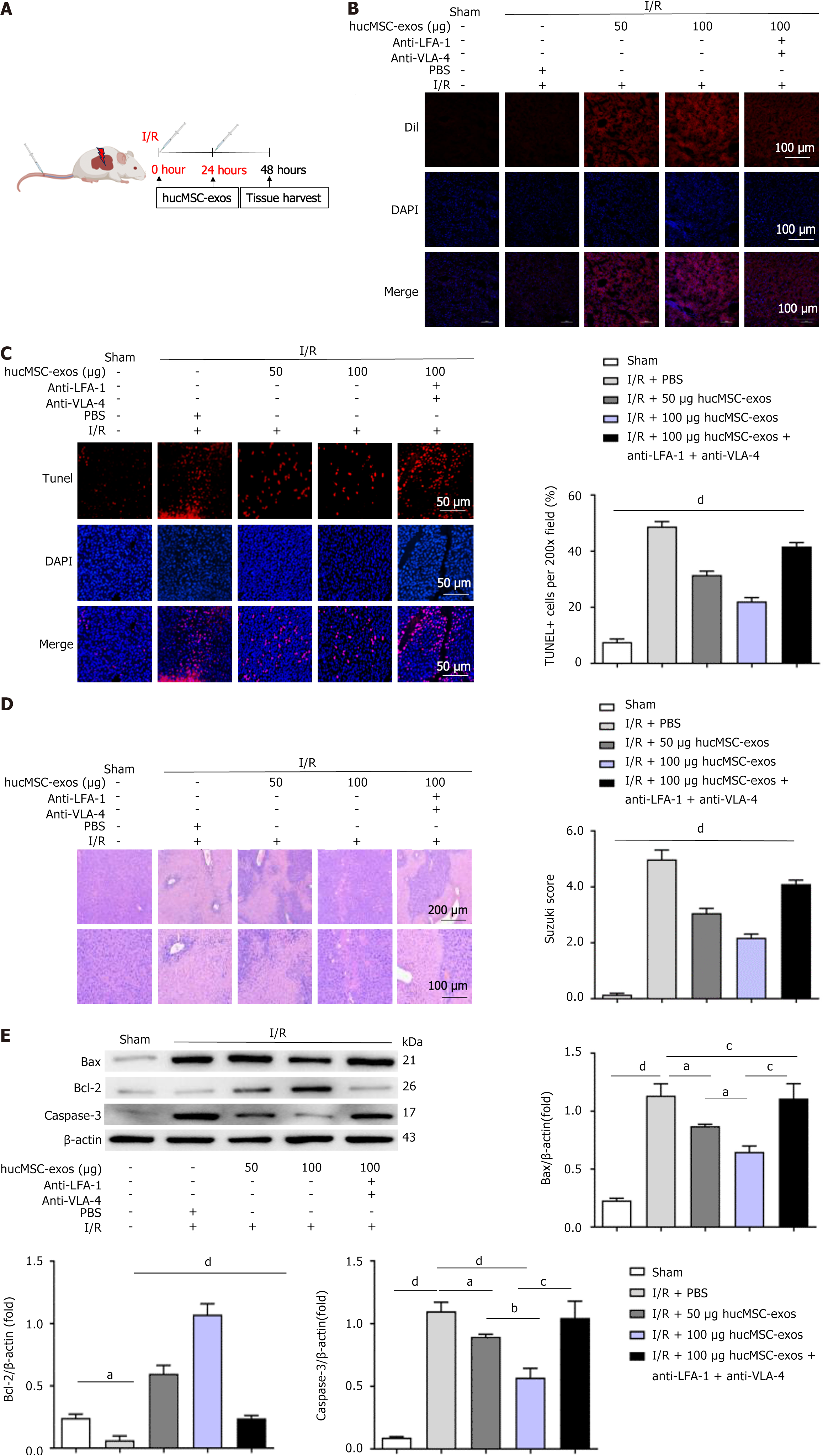
Adult male C57BL/6J mice (8-10 weeks old, weighing 21-24 g) were provided by the Animal Center of Kunming Medical University (Kunming, Yunnan Province, China). The animal experiments were approved by the Animal Experiment Ethics Committee of Kunming Medical University. HIRI was induced by occluding blood flow to the left lateral lobe and left medial lobe of the liver for 1 hour. Each group consisted of 6 mice at each time point. For the sham group, the mice underwent only abdominal incision and closure procedures. At 0 and 24 hours after reperfusion, the mice were intravenously injected with 100 μL of PBS (PBS group), 50 μg of hucMSC-exos (Exos1 group), 100 μg of hucMSC-exos (Exos2 group), or 100 μg of hucMSC-exos treated with anti-very late antigen (VLA)-4 and anti-lymphocyte function-associated antigen (LFA)-1 antibodies (Proteintech, Wuhan, China) (Exos3 group). The mice were euthanized 48 hours after HIRI.
hucMSCs were transfected with 100 nmol/L cyanine3 (Cy3)-let-7i-5 mimic (GenePharma, Shanghai, China) or negative control (Cy3-NC-mimic) to investigate the protective effect of let-7i-5p in hucMSC-exos on HIRI in mice. The isolated Cy3-let-7i-5 mimic-exos and Cy3-NC-mimic-exos were intravenously injected at 0 and 24 hours postischemia/reperfusion injury with 100 μg of Cy3-let-7i-5 mimic-exos, 100 μg of Cy3-NC-mimic-exos, or an equal volume of PBS (n = 6 mice per group).
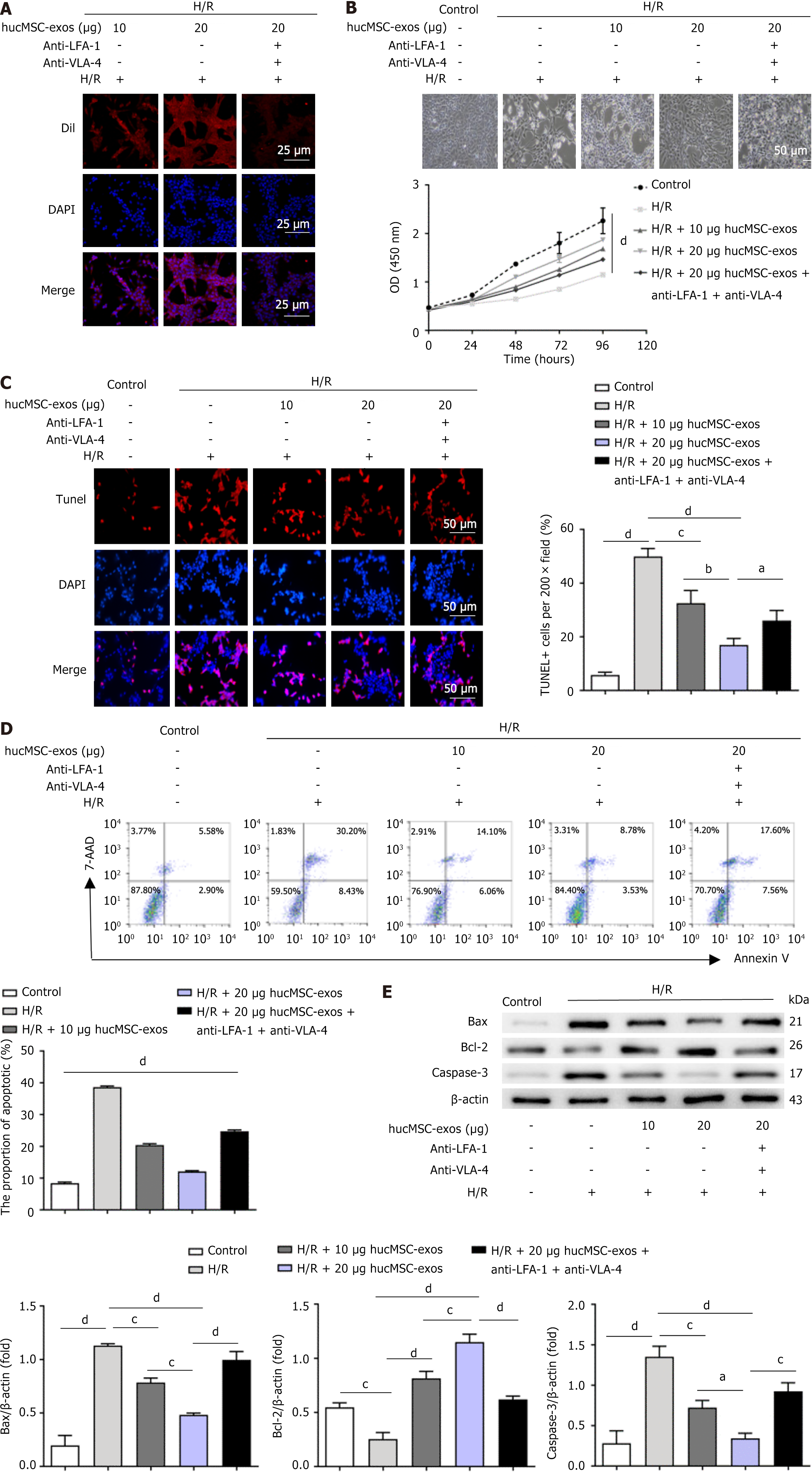
In the H/R group, L02 cells (Procell, Wuhan, China) were first incubated under hypoxic conditions (1% O2, 94% N2, and 5% CO2) in glucose-free and foetal bovine serum-free medium for 24 hours and then reoxygenated in complete medium for another 24 hours. Subsequently, 10 μg of hucMSC-exos (exos group), 20 μg of hucMSC-exos (exos1 group), or 20 μg of hucMSC-exos treated with anti-VLA-4 and anti-LFA-1 antibodies (exos2 group) were added to the medium and coincubated with H/R-treated L02 cells for 12 hours.
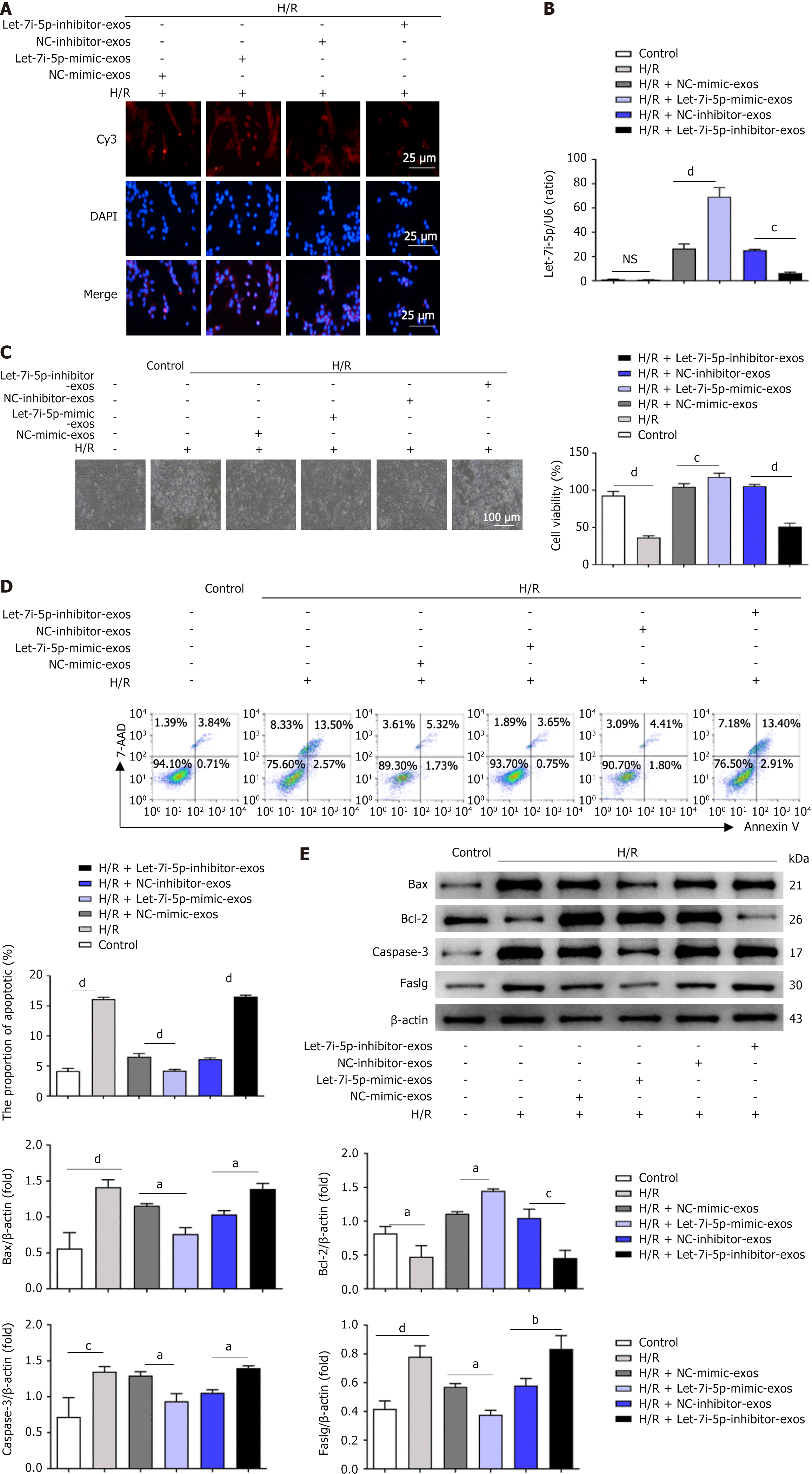
HucMSCs were transfected with 100 nmol/L Cy3-let-7i-5 mimic, negative control (Cy3-NC-mimic), 100 nmol/L Cy3-let-7i-5p inhibitor, or Cy3-NC-inhibitor to investigate the effects of let-7i-5p in hucMSC-exos on the function of L02 cells after H/R injury. Subsequently, Cy3-let-7i-5p mimic-exos, Cy3-NC-mimic-exos, Cy3-let-7i-5p inhibitor-exos, and Cy3-NC-inhibitor-exos were isolated. L02 cells were subjected to 2 hours of hypoxia and 12 hours of reoxygenation, followed by coincubation with 20 μg of Cy3-let-7i-5p mimic-exos, 20 μg of Cy3-NC-mimic-exos, 20 μg of Cy3-let-7i-5p inhibitor-exos, or 20 μg of Cy3-NC-inhibitor-exos for 12 hours.
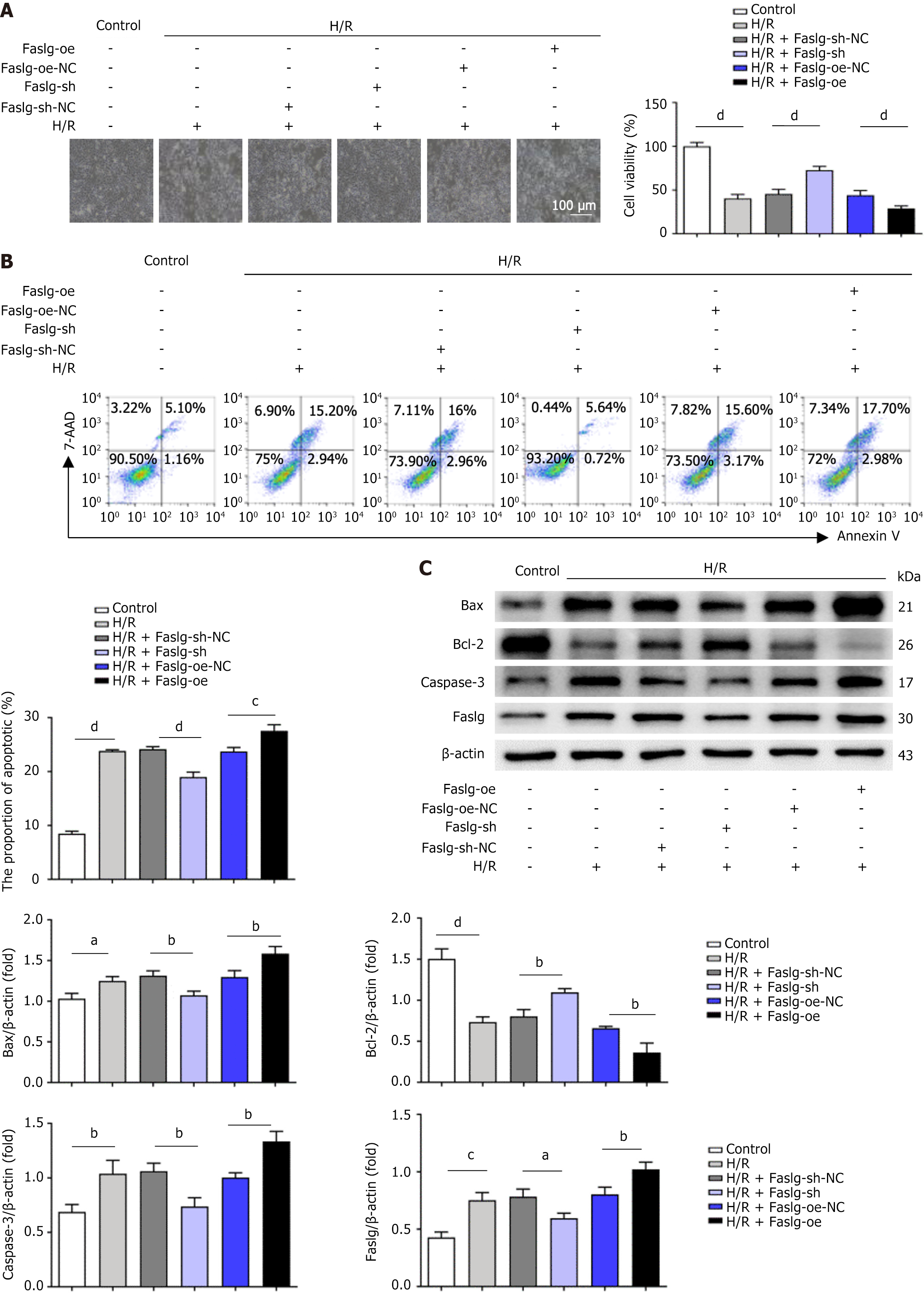
We used lentiviral vectors (GenePharma, Suzhou, China) overexpressing Faslg (Faslg-oe), a negative control (Faslg-oe-NC), Faslg short hairpin RNA (Faslg-sh) knockdown, or a negative control (Faslg-sh-NC) to infect L02 cells and explore the role of Faslg in mediating the reduction in apoptosis in H/R-treated L02 cells induced by let-7i-5p in hucMSC-exos. L02 cells were subjected to 24 hours of hypoxia and 24 hours of reoxygenation. Faslg-sh, Faslg-sh-NC, Faslg-oe, and Faslg-oe-NC were encapsulated in lentiviral vectors, which were prepared and incubated according to the manufacturer’s instructions.
Rescue experiments were conducted on L02 cells after H/R injury to investigate the regulatory relationship between let-7i-5p and Faslg in hucMSC-exos. L02 cells or L02 cells infected with a lentivirus (Faslg-oe or Faslg-oe-NC) were coincubated with 20 μg of hucMSC-exos for 2 hours under hypoxic conditions and 12 hours under reoxygenation. L02 cells were subsequently transfected with 100 nmol/L let-7i-5p mimic or mimic- negative control (mimic-NC).
In L02 cells from both the normal and H/R groups, hucMSC-exos labelled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI, Beyotime, Nanjing, China) or Sulfo-Cy3 were incubated at 4 °C for 2 hours. L02 cells were subsequently washed three times with sterile PBS to remove noninternalized hucMSC-exos, and the cells were observed via confocal microscopy (Motic, Xiamen, China). The liver tissues of the mice were processed for cryosectioning, and the nuclei were stained with 4',6-diamidino-2-phenylindole (Servicebio, Wuhan, China) to observe the biological distribution of hucMSC-exos in the livers of the mice. The tissues were observed via confocal microscopy. HucMSC-exos labelled with DiI or Cy3 appeared orange-red.
The viability of L02 cells was assessed using a cell counting kit-8 (CCK-8, Dojindo, Kyushu, Japan) assay. Briefly, 4 × 103 L02 cells were seeded into each well of a 96-well plate. Subsequently, 10 μL of CCK-8 solution was added to each well, followed by an incubation at 37 °C for 2 hours. The optical density values of each well were then measured at 450 nm using a microplate reader (Bio-Rad, Hercules, CA, United States).
Apoptosis was detected using an annexin V-phycoerythrin/7-aminoactinomycin D apoptosis detection kit (BD, NJ, United States). Briefly, 100 μL of the cell suspension was transferred into a centrifuge tube, to which 5 μL of annexin V-phycoerythrin and 5 μL of 7-aminoactinomycin D were added. The mixture was incubated in the dark at room temperature for 15 minutes, followed by the addition of 300 μL of 1 × binding buffer. The degree of apoptosis was then analysed with a flow cytometer (BD, NJ, United States) within 1 hour.
Paraffin sections of liver tissues were prepared to assess the level of apoptosis in liver tissues. Apoptotic cells were fluorescently stained with a tetramethylrhodamine red terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL, Servicebio, Wuhan, China) kit, and TUNEL-positive hepatocytes were counted under a fluorescence microscope, with apoptotic nuclei emitting red fluorescence. When the horseradish peroxidase-3,3’-diaminobenzidine TUNEL staining kit (Servicebio, Wuhan, China) was used, TUNEL-positive hepatocytes were counted under an optical microscope (Nikon, Tokyo, Japan), and the apoptotic nuclei were stained brownish-yellow. The percentage of TUNEL-positive nuclei in five randomly selected fields of each sample was calculated as the percentage of apoptotic cells (%).
Hematoxylin and eosin reagents (Servicebio, Wuhan, China) were used to stain paraffin-embedded liver tissue sections. Pathological damage to the liver tissue was observed under an optical microscope (Nikon, Tokyo, Japan). The Suzuki scoring method was used to evaluate the pathological damage caused by HIRI.
Proteins were extracted from L02 cells, liver tissues, and hucMSC-exos by adding lysis buffer containing protease inhibitors (Servicebio, Wuhan, China). The concentrations of the protein samples were determined using a bicinchoninic acid protein assay kit (Beyotime, Nanjing, China). After denaturation, equal amounts of protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10% gels (Solarbio, Beijing, China) and transferred onto polyvinylidene fluoride membranes (Millipore, MA, United States). The membranes were blocked with nonfat milk for 2 hours and then incubated overnight at 4 °C with the following primary antibodies: Anti-CD63, anti-Tsg101, anti-Alix, anti-CD9, anti-B-cell lymphoma (Bcl)-2 (Huabio, Hangzhou, China), anti-cysteinyl aspartate specific proteinase (Caspase)-3 (Affinity, Cincinnati, OH, United States), anti-Bcl-2-associated X protein (Huabio, Hangzhou, China), anti-β-actin (Proteintech, Wuhan, China), and anti-Faslg (Affinity, Cincinnati, OH, United States). The polyvinylidene fluoride membranes were subsequently incubated with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G secondary antibodies (Invitrogen, Carlsbad, CA, United States) for 1 hour and developed using enhanced chemiluminescence reagents (Affinity, Cincinnati, OH, United States). The grayscale values of the protein bands were analysed with ImageJ 7.0 software (NIH, Bethesda, MD, United States), and the relative protein expression levels were calculated using via β-actin as an internal reference.
Total RNA was extracted from mouse liver tissues or L02 cells with a TRIzol reagent kit (Ambion, Austin, TX, United States). The reverse transcription reaction was subsequently performed using the sweScript RT I First Strand cDNA Synthesis Kit (Servicebio, Wuhan, China). Quantitative polymerase chain reaction (PCR) was subsequently conducted using the 2 × Universal Blue SYBR Green quantitative PCR Master Mix Kit (Servicebio, Wuhan, China). The relative expression levels of mRNAs were normalized to those of glyceraldehyde-3-phosphate dehydrogenase, and the relative expression levels of miRNAs were normalized to those of U6. The relative expression levels of mRNAs and miRNAs were calculated using the 2-∆∆Ct method. The sequences of the primers used in this study are listed in Supplementary Table 1.
Mutations were introduced into the predicted binding site of let-7i-5p within the 3’ untranslated region (UTR) of Faslg to construct a mutated Faslg 3’ UTR sequence; this sequence and a wild-type Faslg 3’ UTR sequence were respectively inserted into the pmirGLO dual-luciferase reporter vector (GenePharma, Suzhou, China). The luciferase reporter vectors were cotransfected with let-7i-5p-mimic or mimic-NC (GenePharma, Suzhou, China) into 293T cells. After 48 hours of transfection, the relative firefly luciferase activity was measured, with Renilla luciferase activity used as a reference.
The detailed methods are provided in the Supplemental Material.
The quantitative data are presented as the mean ± SD. Comparisons among three or more experimental groups were performed using one-way analysis of variance, whereas comparisons between two experimental groups were conducted using two-tailed Student's t test. Both one-way analysis of variance and two-tailed Student's t tests were performed using GraphPad Prism 8.0 software (GraphPad Software, Inc., La Jolla, CA, United States). A P value < 0.05 was considered to indicate statistical significance in the intergroup comparisons.
We first cultured and identified hucMSCs to ensure the reliability of the subsequent experiments. As shown in Supplementary Figure 1, the hucMSCs grew well. The presence of red lipid droplets stained with Oil Red O within the cytoplasm of hucMSCs indicates a positive reaction, confirming successful differentiation into adipocytes. Supplementary Figure 1 also shows alizarin red staining for osteogenic induction, where the cells presented a distinct purplish-red colour visible to the naked eye, and clear purplish-red calcium nodules were observed under a microscope. These results indicate that hucMSCs can be used for exosome extraction.
The extracted exosomes were subsequently identified. We used ultracentrifugation to extract the exosomes and performed electron microscopy, particle size, concentration, and nanoflow fluorescence analyses of the extracted exosomes. Transmission electron microscopy (Supplementary Figure 1) produced clear images of the exosomes, indicating successful extraction. The particle size and concentration analyses (Supplementary Figure 1) revealed that the concentration of the extracted exosomes was 2.92 × 1010 particles/mL, with an average particle size of 82.93 nm, which falls within the 30-150 nm range typical of exosomes. Flow cytometry analysis revealed positive expression of the hucMSC-exo surface proteins CD9 and CD81 (Supplementary Figure 1). The western blot results (Supplementary Figure 1) demonstrated the expression of the exosome marker proteins Alix, CD63, Tsg101, and CD9. These results confirm that the extracted entities are indeed exosomes, which are suitable for subsequent experiments.
We first established a mouse model of HIRI to investigate the therapeutic effects of hucMSC-exos on HIRI in mice. At 0 and 24 hours postreperfusion, the mice were injected via the tail vein with 50 μg or 100 μg of hucMSC-exos, 100 μg of hucMSC-exos treated with anti-VLA-4 and anti-LFA-1 antibodies, or 100 μL of PBS (Figure 1A). The fluorescence confocal microscopy results (Figure 1B) revealed that, compared with that in the PBS control group, the fluorescence signal in the liver tissues of the Exos1 and Exos2 groups was greater, with a distinct orange-red colour. However, the fluorescence signal in the Exos3 group was lower than that in the Exos1 and Exos2 groups. These results indicate that DiI-labelled hucMSC-exos can be distributed within the liver and be absorbed by hepatocytes. However, blocking the expression of VLA-4 and LFA-1 in hucMSC-exos affects their ability to home to the liver and reduces their therapeutic effect on HIRI in mice. In the PBS control group, HIRI resulted in significant hepatocyte necrosis, extensive inflammatory cell infiltration, and increased apoptosis, further confirming the successful establishment of the mouse HIRI model (Figure 1C and D). However, these adverse effects were alleviated in a dose-dependent manner by hucMSC-exo treatment. We explored the potential mechanisms by which hucMSC-exos alleviate HIRI in mice. Immunoblotting of liver tissues (Figure 1E) revealed that hucMSC-exo treatment significantly affected the expression levels of apoptosis-related factors, suggesting that hucMSC-exos may improve HIRI in mice by regulating hepatocyte apoptosis.
Fluorescence confocal imaging (Figure 2A) revealed that the cells subjected to H/R had taken up DiI-labelled hucMSC-exos, which exhibited orange-red fluorescence. These findings indicate that hucMSC-exos can intervene in L02 cells after H/R injury. Light microscopy images (Figure 2B) revealed that L02 cells in the H/R group exhibited poor growth; however, the growth status of L02 cells in the exos and exos1 groups improved compared with that in the H/R group. Cell proliferation assays (Figure 2B) revealed that hucMSC-exos enhanced the proliferative capacity of L02 cells after H/R injury. TUNEL staining and flow cytometry results (Figure 2C and D) indicated that the therapeutic effect of hucMSC-exos was dose dependent and that blocking the expression of VLA-4 and LFA-1 in hucMSC-exos reduced their therapeutic efficacy. Additionally, western blot analysis (Figure 2E) confirmed that hucMSC-exos affected the expression levels of apoptosis-related factors, which was consistent with the immunoblotting results from the mouse HIRI models.
We first conducted an RNA sequencing analysis on the control group, H/R group, and exos1 group to explore the mechanism by which hucMSC-exos alleviate HIRI. In the Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis of differentially expressed genes between the exos1 group and the H/R group, we found that, compared with the H/R group, the exos1 group showed that hucMSC-exos may reduce H/R-induced L02 cell damage by affecting apoptosis-related signalling molecules (Supplementary Figure 2A and B).
We performed miRNA expression profiling to further investigate which miRNAs in hucMSC-exos are involved in ameliorating H/R injury in L02 cells. Our study revealed that the differentially expressed miRNAs between the H/R group and the exos1 group were enriched in the apoptosis pathway (Supplementary Figure 2C and D). Moreover, through a miRNA-mRNA correlation analysis, we further identified ten potential miRNAs in hucMSC-exos that may be involved in ameliorating H/R injury in L02 cells (Supplementary Table 2). Among these miRNAs, a negative correlation was observed between the let-7i-5p miRNA and Faslg mRNA.
We measured the relative expression levels of let-7i-5p and the Faslg mRNA in mouse liver tissues and L02 cells through quantitative reverse transcriptase PCR to verify the negative regulatory relationship between let-7i-5p and the Faslg mRNA. The results shown in Supplementary Figure 2E and F support the potential negative regulatory relationship between let-7i-5p and Faslg. Additionally, the dual-luciferase reporter assay further confirmed this relationship. Supplementary Figure 2G presents a schematic of the sequences of let-7i-5p targeting the wild-type or mutant 3’UTR of Faslg. The results in Supplementary Figure 2H show that, compared with mimic-NC, the overexpression of let-7i-5p significantly inhibited the luciferase activity of a wild-type Faslg 3’ UTR sequence but did not significantly affect the activity of the Faslg 3'-UTR mutant, indicateng that Faslg is a direct target of let-7i-5p. In summary, these results suggest that let-7i-5p contained within hucMSC-exos can regulate the expression of Faslg, which may in part affect the apoptotic process in L02 cells.
We subjected L02 cells to 2 hours of hypoxia followed by 12 hours of reoxygenation to further investigate the impact of let-7i-5p in hucMSC-exos on the function of L02 cells after H/R injury. These cells were subsequently treated with Cy3-let-7i-5p mimic-exos, Cy3-NC-mimic-exos, Cy3-let-7i-5p inhibitor-exos, or Cy3-NC-inhibitor-exos. The results from laser confocal microscopy revealed that the cells in all treatment groups exhibited significant orange-red fluorescence (Figure 3A). The quantitative reverse transcriptase PCR results (Figure 3B) revealed significant differences in the expression of let-7i-5p among the groups, confirming that hucMSC-exos effectively interfered with H/R-induced damage in L02 cells.
Subsequent cellular function experiments indicated that, compared with those in the control group, the growth conditions of L02 cells in the H/R group were poorer (Figure 3C). However, the growth conditions of the cells in the Cy3-let-7i-5p mimic-exos treatment group improved compared with those in the Cy3-NC-mimic-exos group. The results of the CCK-8 assay (Figure 3C) indicated that H/R injury significantly reduced the viability of L02 cells, but the viability of cells treated with Cy3-let-7i-5p mimic-exos was significantly greater than that of the cells treated with Cy3-NC-mimic-exos. Additionally, H/R injury significantly increased the apoptosis rate of L02 cells, whereas Cy3-let-7i-5p mimic-exos significantly reduced the apoptosis rate under H/R conditions (Figure 3D). Western blot analysis (Figure 3E) revealed that the expression of apoptosis-related factors in L02 cells was significantly increased after H/R injury but was significantly reversed after treatment with Cy3-let-7i-5p mimic-exos.
Conversely, in the Cy3-let-7i-5p inhibitor-exos group, inhibiting the expression of let-7i-5p in hucMSC-exos increased the sensitivity of L02 cells to H/R-induced damage, reversing the protective effect of overexpressed let-7i-5p in hucMSC-exos on L02 cells after H/R treatment. This process led to a decrease in cell viability and an increase in the level of apoptosis. Additionally, the expression levels of apoptosis-related proteins in L02 cells were significantly increased after H/R injury. Overall, these results suggest that the overexpression of let-7i-5p effectively enhances the ability of hucMSC-exos to reduce L02 cell apoptosis following H/R injury.
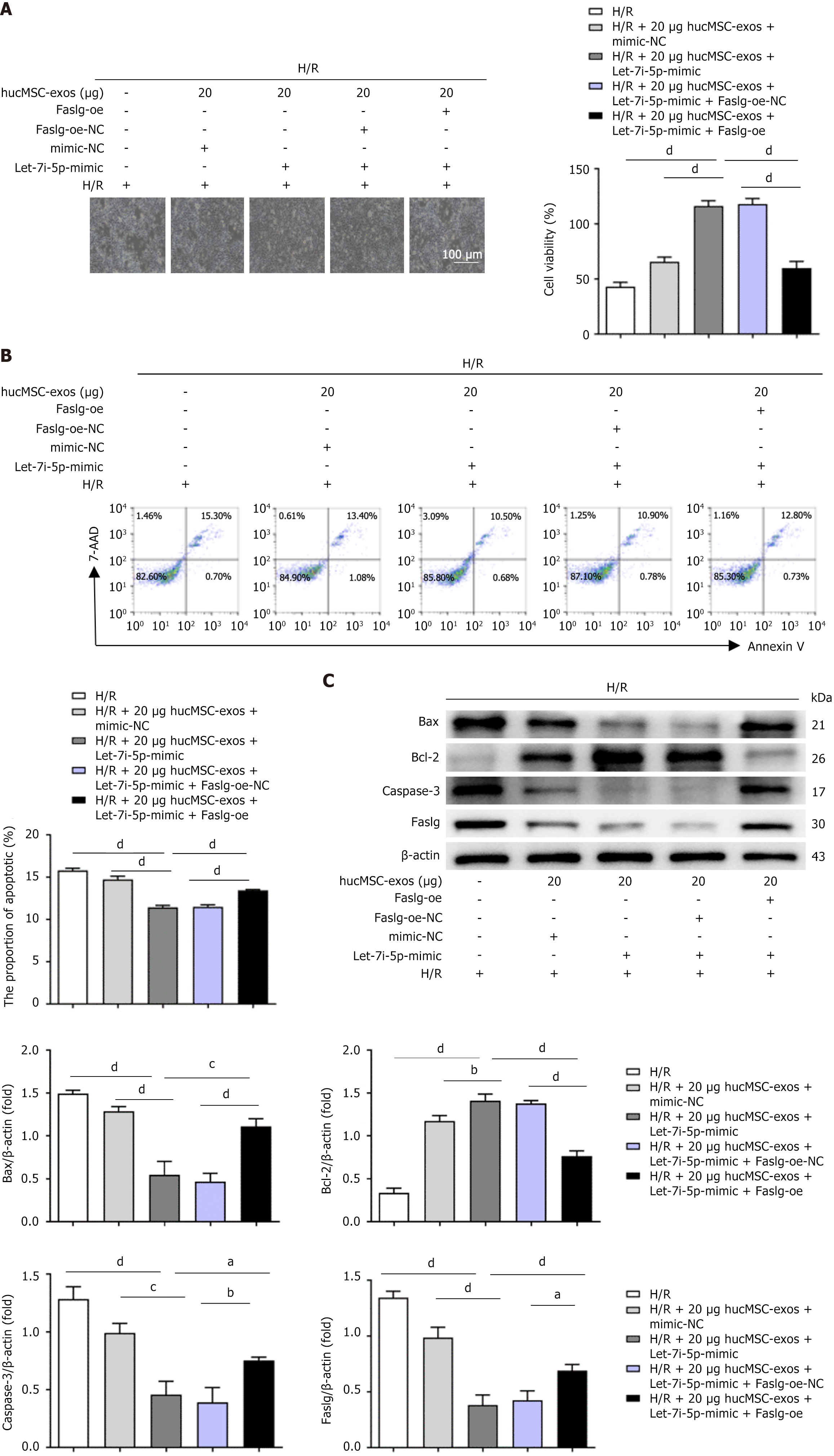
Given that Faslg is a target gene of let-7i-5p, we further investigated the role of Faslg in H/R-induced L02 cell apoptosis. First, we used the Faslg-knockdown (Faslg-sh) lentivirus to infect L02 cells. Compared with the control, Faslg knockdown reversed the H/R injury-induced reduction in L02 cell viability and improved the growth status of L02 cells (Figure 4A). Moreover, the level of H/R-induced L02 cell apoptosis was also significantly reduced (Figure 4B). Additionally, we examined the levels of apoptosis-related proteins in L02 cells. H/R treatment increased the expression of these proteins, whereas Faslg knockdown significantly decreased their levels (Figure 4C). Conversely, Faslg-oe increased the sensitivity of L02 cells to H/R injury, reversing the protective effects of Faslg knockdown on L02 cells after H/R injury and leading to decreased cell viability and increased apoptosis. Furthermore, after H/R injury, the expression levels of apoptosis-related proteins in L02 cells increased significantly. In summary, these results indicate that Faslg plays a crucial role in regulating the homeostasis of apoptosis in L02 cells following H/R injury.
We designed a rescue experiment using L02 cells to clarify the mechanism by which let-7i-5p in hucMSC-exos regulates apoptosis in L02 cells after H/R injury. In the experiment, L02 cells were subjected to 2 hours of hypoxia followed by 12 hours of reoxygenation and then treated with let-7i-5p mimic, mimic-NC, Faslg-oe lentivirus, negative control for Faslg (Faslg-NC) lentivirus, or a combination of Faslg and let-7i-5p overexpression. Compared with H/R-treated control cells, L02 cells overexpressing let-7i-5p were less sensitive to H/R injury and exhibited improved cell growth and viability (Figure 5A). Additionally, the percentage of apoptotic cells (Figure 5B) and the levels of apoptosis-related proteins (Figure 5C) in these cells were significantly reduced. However, when L02 cells were cotransfected with the lentiviral vector overexpressing Faslg, the protective effects of let-7i-5p overexpression were reversed, leading to decreased cell viability and increased levels of apoptosis. Following H/R injury, the expression levels of apoptosis-related proteins were significantly increased. Furthermore, the results from the dual-luciferase reporter gene assay indicated that hucMSC-exos can target and regulate Faslg levels through let-7i-5p, thereby affecting the extent of apoptosis in L02 cells after H/R injury. Overall, inhibiting the expression of let-7i-5p in hucMSC-exos upregulated Faslg expression, exacerbating H/R injury-induced apoptosis in L02 cells; conversely, increasing the expression of let-7i-5p downregulated Faslg expression, mitigating H/R injury-induced apoptosis in L02 cells.
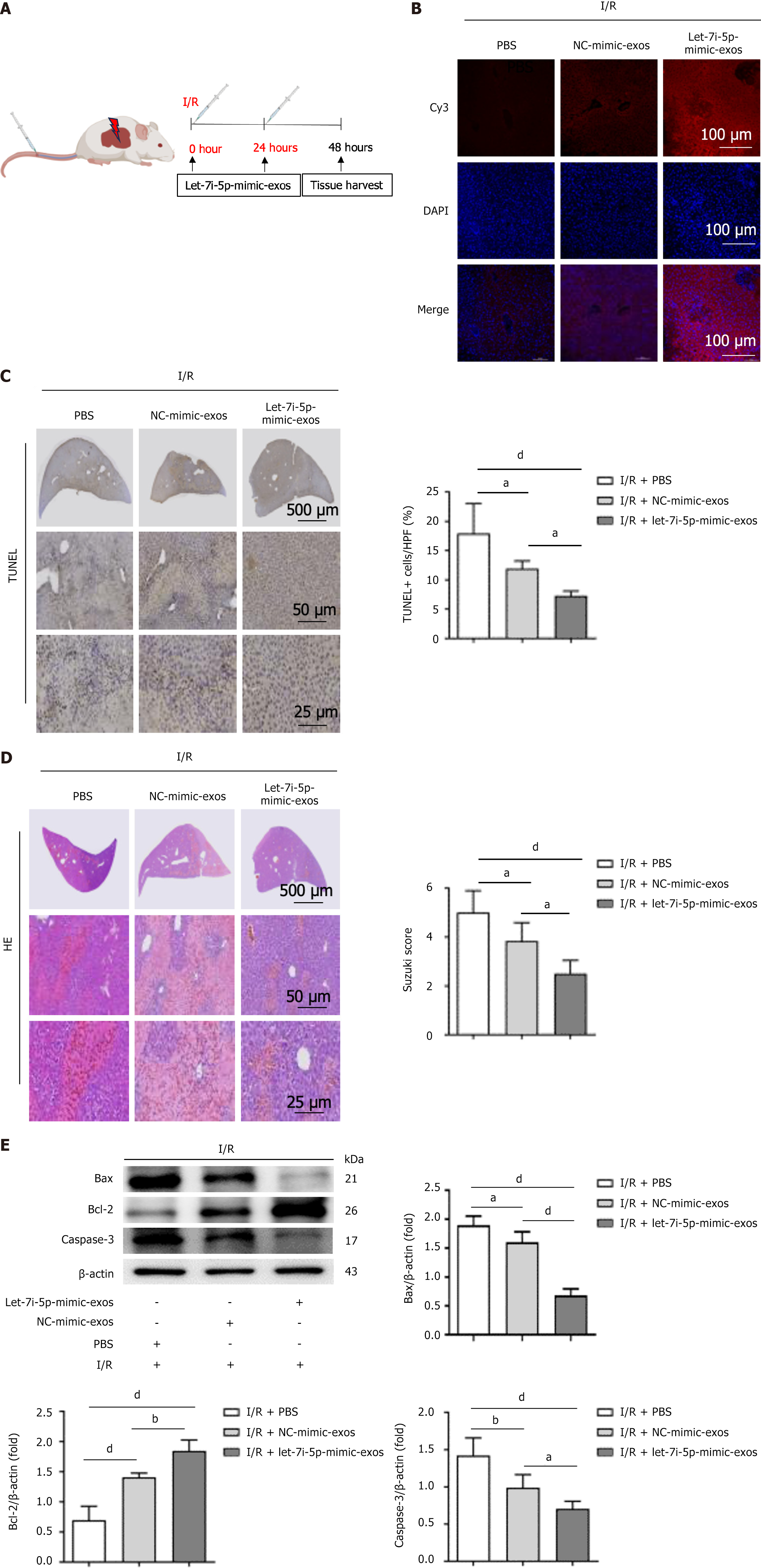
At 0 and 24 hours of liver reperfusion, the mice were injected via the tail vein with 100 μg of Cy3-let-7i-5p-mimic-exos, 100 μg of Cy3-NC-mimic-exos, or 100 μL of PBS to investigate the protective effect of let-7i-5p in hucMSC-exos on HIRI in mice (Figure 6A). The results of fluorescence confocal microscopy (Figure 6B) revealed increased cellular fluorescence signals in the liver tissues of the Cy3-NC-mimic-exos and Cy3-let-7i-5p-mimic-exos groups. The tissues displayed a distinct orange-red colour, indicating that Cy3-labelled hucMSC-exos successfully interacted with the liver tissue subjected to I/R injury. In the PBS control group, HIRI induced a greater degree of hepatocyte apoptosis and caused significant pathological changes, such as a disorganized hepatic lobule structure, disappearance of the hepatic cord structure, hepatocyte oedema, vacuolar degeneration, extensive coagulative necrosis, and inflammatory cell infiltration (Figure 6C and D). These results further confirmed the successful establishment of the mouse HIRI model.
However, in the Cy3-NC-mimic-exos and Cy3-let-7i-5p-mimic-exos groups, treatment with hucMSC-exos alleviated these adverse outcomes. In particular, in the Cy3-let-7i-5p-mimic-exos group, the therapeutic effect of hucMSC-exos was more pronounced, significantly ameliorating the histopathological changes and degree of hepatocyte apoptosis induced by HIRI. Additionally, this treatment significantly affected the expression levels of apoptosis-related proteins (Figure 6E). These results indicate that the let-7i-5p mimic enhances the protective effect of hucMSC-exos on HIRI mice.
Our study confirmed the protective efficacy of hucMSC-exos in HIRI. Importantly, we found that hucMSC-exos alleviate hepatocyte apoptosis and promote hepatocyte proliferation through the let-7i-5p/Faslg signalling pathway, thereby improving HIRI. Previous studies have shown that exosomes secreted by human liver stem cells primarily accumulate in the liver and are absorbed by hepatocytes[27,28]. The exosomal membrane proteins VLA-4 and LFA-1 secreted by hucMSCs play critical roles in mediating the specific protective effect of exosomes on kidney I/R injury[29]. In our study, we found that blocking the exosomal membrane proteins VLA-4 and LFA-1 secreted by hucMSCs reduced the uptake of exosomes by damaged hepatocytes and decreased the therapeutic effect of exosomes on HIRI. These data suggest that the exosomal membrane proteins VLA-4 and LFA-1 mediate the specific protective effect of hucMSC-exos on HIRI.
Exosomes derived from MSCs have therapeutic effects on organ I/R injury, and these effects are influenced by various factors, including the cell type from which the exosomes are derived, the dosage, and the administration route[30]. Among these factors, specific molecules expressed on the surface of exosomes, as well as the nucleic acids and active proteins they contain, are key factors determining their therapeutic efficacy[31].
This study revealed that, compared with that of normal liver cells, the uptake of exosomes by damaged liver cells increased. By blocking the exosomal membrane proteins VLA-4 and LFA-1, the uptake of exosomes by damaged liver cells decreased, and correspondingly, the protective effect of exosomes on liver cells was weakened. Previous research has shown that knocking down vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 in damaged renal tubular epithelial cells can reduce the uptake of exosomes. Another study revealed that exosomes derived from macrophages can effectively target inflamed kidneys through the surface membrane proteins LFA-1 and VLA-4[32]. Based on these findings, we speculate that exosomes can target damaged liver tissues and be taken up by liver cells, and this uptake may be related to the upregulated expression of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 in the damaged liver and the interaction of these molecules with the exosomal membrane proteins VLA-4 and LFA-1[33].
Notably, although the therapeutic effect of exosomes derived from human liver stem cells on HIRI does not occur in a dose-dependent manner, this effect may be related to the procoagulant activity of high doses of exosomes[34]. However, this study revealed that hucMSC-exos have a dose-dependent therapeutic effect on HIRI in mice, and no coagulation was observed in liver tissues. In addition, studies have shown that modifying exosomes with the CD47 molecule can inhibit the phagocytosis of liver macrophages, whereas the use of haem oxygenase oxygen-1-modified exosomes derived from bone marrow MSCs can enhance their protective effect on HIRI in mice[35,36]. In summary, these results suggest that specific molecular modifications of exosomes or their use as drug carriers may significantly enhance the therapeutic effect of hucMSC-exos on HIRI.
Compared with MSCs derived from fat and bone marrow, hucMSCs have several advantages, such as low immunogenicity and a high proliferative capacity[37]. Proteomic studies have further revealed that proteins secreted by hucMSCs in exosomes are related mainly to collagen metabolic processes, which may play a more significant role in tissue repair[38]. Previous data have shown that therapy using exosomes from hucMSCs has multiple benefits in mice with I/R injury, including reducing inflammation and oxidative stress responses, inhibiting apoptosis and fibrosis, and improving cell proliferation[39,40]. The data from this study clearly indicate that exosomes from hucMSCs have a similar therapeutic effect on inhibiting apoptosis in mice with I/R injury. This result is due to the synergistic effects of oxidative damage, inflammatory responses, and mitochondrial function, which can induce apoptosis[41]. We further speculate that hucMSC-exos may alleviate HIRI in mice through a comprehensive protective effect that reduces oxidation, inflammation, mitochondrial dysfunction, and apoptosis in addition to promoting tissue repair.
In this study, we performed mRNA transcriptome and miRNA sequencing on damaged liver cells pretreated with hucMSC-exos. Through the Kyoto Encyclopedia of Genes and Genomes analysis, we discovered that pretreatment with hucMSC-exos altered the biological pathways of damaged liver cells. This alteration may mitigate liver cell damage by regulating apoptotic pathways. After treatment with hucMSC-exos, the phenotype of the damaged liver cells changed, with reduced levels of apoptosis and increased cellular viability. These findings suggest that hucMSC-exos can protect liver cells by inhibiting apoptosis and increasing cell proliferation.
Cell death and inflammatory responses are the main pathological changes associated with HIRI[42,43]. Apoptosis, a type of cell death, includes the intrinsic pathway, which is mediated by mitochondria-dependent caspase activation, and the extrinsic pathway, which is mediated by the activation of factor-related apoptosis (Fas)/Faslg signals and is dependent on tumour superfamily members[44,45]. In this study, after overexpression of Faslg in hepatocytes following hypoxic injury, increases in the protein expression levels of Bcl-2-associated X protein, Caspase-3, and Faslg were observed, leading to increased apoptosis and suppressed cellular activity in injured hepatocytes. Similar changes were observed in cardiomyocytes after hypoxic injury[46]. These data suggest that Faslg can exert a protective effect on HIRI by regulating the extrinsic pathway mediated by Fas/Faslg signals and the mitochondria-dependent intrinsic pathway.
Previous data revealed the roles of several miRNAs, including miR-139-3p, miR-181a-1 and miR-21-5p, in targeting and regulating Fas/Faslg signalling to prevent organ I/R injury[47,48]. This study identified the regulatory role of let-7i-5p in mouse HIRI. By overexpressing let-7i-5p in hucMSC-exos, the level of apoptosis in liver cells postinjury was reduced, and cell viability was increased; similarly, this treatment also reduced the level of apoptosis in liver cells following I/R injury in mice. Research has shown that inhibiting the expression of let-7i-5p increases the level of apoptosis in injured liver cells and exacerbates liver function damage[49]. Additionally, overexpressing let-7i-5p in cardiomyocytes after hypoxic injury reduces their sensitivity to apoptosis and enhances their viability[50]. Based on these data, we propose that let-7i-5p can serve as a key antiapoptotic miRNA for preventing HIRI. Oligonucleotide modification may enhance the therapeutic effect of hucMSC-exos on HIRI, as the therapeutic effect of hucMSC-exos on HIRI is partly mediated by let-7i-5p.
We further investigated the role and mechanism of the let-7i-5p/Faslg signalling pathway in the treatment of HIRI with hucMSC-exos, and this study confirmed that let-7i-5p can inhibit apoptosis after HIRI by negatively regulating the proapoptotic gene Faslg. Research has shown that both let-7i-5p and miR-149 in exosomes derived from bone marrow MSCs can target and negatively regulate Faslg, affecting the level of myocardial apoptosis after hypoxic injury[51]. In this study, hucMSC-exos overexpressing let-7i-5p in the HIRI model significantly inhibited apoptosis by downregulating the expression level of the Faslg gene. However, before exosomes can be used clinically, several existing issues limit their clinical application, such as large-scale production, stability during storage, immune reactions after administration, and poor targeting effects of drug treatment[52]. These urgent problems need to be addressed in the current engineering development of exosomes.
Moreover, our study has several limitations. First, whether hucMSC-exos can produce similar preventive effects on I/R injury induced by different models, such as liver transplantation, is unclear. Second, the long-term effects of single or multiple administrations of hucMSC-exos remain to be elucidated. Third, the roles of other miRNAs, such as long noncoding RNAs and circular RNAs, in the effect of hucMSC-exos on preventing HIRI are still unclear. Fourth, whether exosomes from other MSCs can produce similar preventive effects on HIRI needs further research. Fifth, the targeting of hucMSC-exos to the liver and their role in reducing cell death, compared with other organs, require further study to understand their specificity.
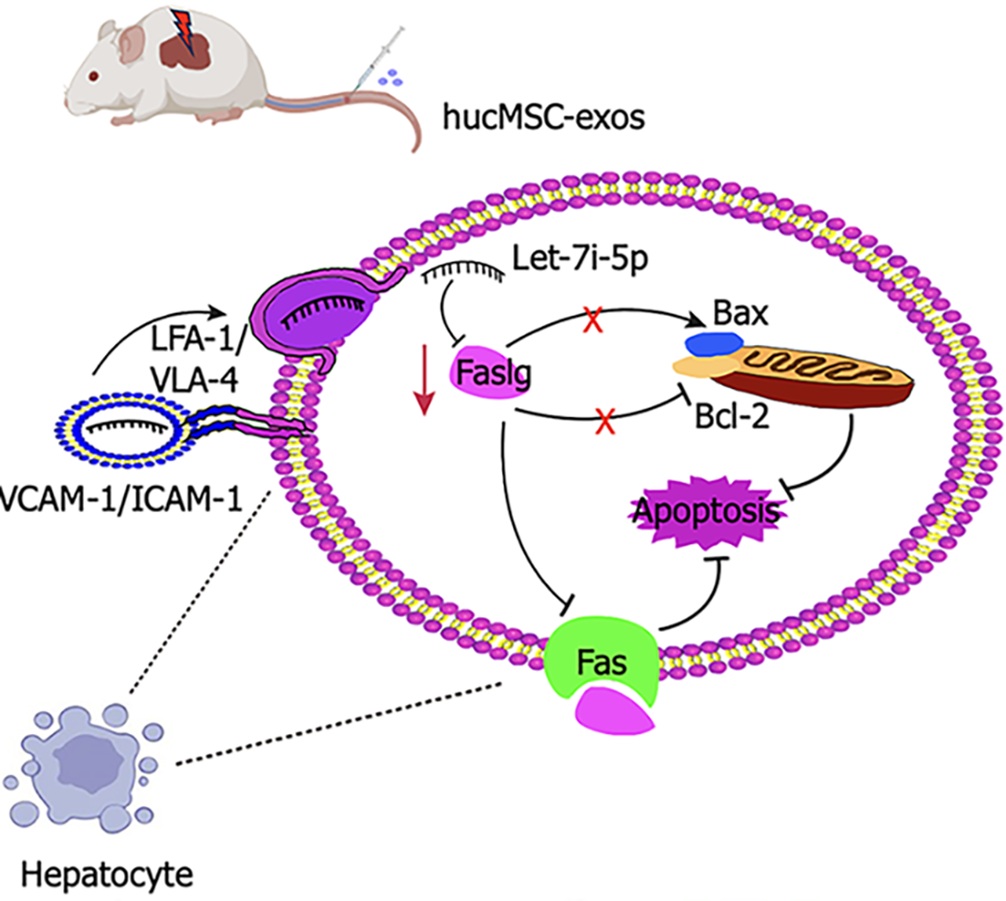
Overall, hucMSC-exos can specifically target damaged liver tissue and are effectively internalized by hepatocytes. Integrins on the surface of hucMSC-exos, such as VLA-4 and LFA-1, may increase their adhesion to hepatocytes, thereby improving their targeting and uptake efficiency. Mechanistically, hucMSC-exos promote hepatocyte proliferation and inhibit apoptosis by regulating the let-7i-5p/Faslg signalling pathway, which effectively alleviates HIRI. Elucidating this novel mechanism of hucMSC-exos is highly important for the development of innovative therapeutic strategies for HIRI.
HucMSC-exos can alleviate HIRI. The therapeutic effect is further enhanced by hucMSCs fortified with let-7i-5p, which inhibits Fas/Faslg-mediated apoptosis. We also discovered that Faslg is a candidate target gene of let-7i-5p and that its expression is suppressed by let-7i-5p. Additionally, the membrane proteins VLA-4 and LFA-1 from hucMSC-exos are involved in targeting the liver and are taken up by hepatocytes, thereby influencing their therapeutic effect on HIRI (Figure 7).
| 1. | Wang Y, Jia L, Wei M, Lyu J, Sheng M, Sun Y, Dong Z, Han W, Ren Y, Weng Y, Yu W. Circulating Exosomes Mediate Neurodegeneration Following Hepatic Ischemia-reperfusion Through Inducing Microglial Pyroptosis in the Developing Hippocampus. Transplantation. 2023;107:2364-2376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Czigany Z, Lurje I, Schmelzle M, Schöning W, Öllinger R, Raschzok N, Sauer IM, Tacke F, Strnad P, Trautwein C, Neumann UP, Fronek J, Mehrabi A, Pratschke J, Schlegel A, Lurje G. Ischemia-Reperfusion Injury in Marginal Liver Grafts and the Role of Hypothermic Machine Perfusion: Molecular Mechanisms and Clinical Implications. J Clin Med. 2020;9:846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 3. | Lyu J, Sheng M, Cao Y, Jia L, Zhang C, Weng Y, Yu W. Ischemia and reperfusion-injured liver-derived exosomes elicit acute lung injury through miR-122-5p regulated alveolar macrophage polarization. Int Immunopharmacol. 2024;131:111853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 4. | Nemeth N, Peto K, Magyar Z, Klarik Z, Varga G, Oltean M, Mantas A, Czigany Z, Tolba RH. Hemorheological and Microcirculatory Factors in Liver Ischemia-Reperfusion Injury-An Update on Pathophysiology, Molecular Mechanisms and Protective Strategies. Int J Mol Sci. 2021;22:1864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Guo Z, Zhao Q, Jia Z, Huang C, Wang D, Ju W, Zhang J, Yang L, Huang S, Chen M, Zhu X, Hu A, Ma Y, Wu L, Chen Y, Han M, Tang Y, Wang G, Wang L, Li L, Xiong W, Zhang Z, Shen Y, Tang Z, Zhu C, Chen X, Hu X, Guo Y, Chen H, Ma Y, Zhang T, Huang S, Zeng P, Lai S, Wang T, Chen Z, Gong J, Yu J, Sun C, Li C, Tan H, Liu Y, Dong Y, Sun C, Liao B, Ren J, Zhou Z, Andrea S, Björn N, Cai C, Gong F, Rong J, Huang W, Guan X, Clavien PA, Stefan TG, Huang J, He X. A randomized-controlled trial of ischemia-free liver transplantation for end-stage liver disease. J Hepatol. 2023;79:394-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 56] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 6. | Masior Ł, Grąt M. Methods of Attenuating Ischemia-Reperfusion Injury in Liver Transplantation for Hepatocellular Carcinoma. Int J Mol Sci. 2021;22:8229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Xin J, Yang T, Wu X, Wu Y, Liu Y, Liu X, Jiang M, Gao W. Spatial transcriptomics analysis of zone-dependent hepatic ischemia-reperfusion injury murine model. Commun Biol. 2023;6:194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Kaltenmeier C, Wang R, Popp B, Geller D, Tohme S, Yazdani HO. Role of Immuno-Inflammatory Signals in Liver Ischemia-Reperfusion Injury. Cells. 2022;11:2222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 69] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 9. | Naji A, Eitoku M, Favier B, Deschaseaux F, Rouas-Freiss N, Suganuma N. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. 2019;76:3323-3348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 375] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 10. | Rasmusson I. Immune modulation by mesenchymal stem cells. Exp Cell Res. 2006;312:2169-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 256] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 11. | Miceli V, Bulati M, Gallo A, Iannolo G, Busà R, Conaldi PG, Zito G. Role of Mesenchymal Stem/Stromal Cells in Modulating Ischemia/Reperfusion Injury: Current State of the Art and Future Perspectives. Biomedicines. 2023;11:689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, Baan CC, Dahlke MH, Hoogduijn MJ. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 2012;3:297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 439] [Cited by in RCA: 589] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 13. | Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG. Mesenchymal Stem Cell Migration and Tissue Repair. Cells. 2019;8:784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 562] [Cited by in RCA: 662] [Article Influence: 110.3] [Reference Citation Analysis (36)] |
| 14. | Hade MD, Suire CN, Suo Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells. 2021;10:1959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 316] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 15. | Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183-3195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 909] [Article Influence: 227.3] [Reference Citation Analysis (0)] |
| 16. | Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int J Nanomedicine. 2020;15:6917-6934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 566] [Cited by in RCA: 830] [Article Influence: 166.0] [Reference Citation Analysis (0)] |
| 17. | Li C, Ni YQ, Xu H, Xiang QY, Zhao Y, Zhan JK, He JY, Li S, Liu YS. Roles and mechanisms of exosomal non-coding RNAs in human health and diseases. Signal Transduct Target Ther. 2021;6:383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 245] [Article Influence: 61.3] [Reference Citation Analysis (1)] |
| 18. | Wang Y, Liu J, Ma J, Sun T, Zhou Q, Wang W, Wang G, Wu P, Wang H, Jiang L, Yuan W, Sun Z, Ming L. Exosomal circRNAs: biogenesis, effect and application in human diseases. Mol Cancer. 2019;18:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 493] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 19. | Xie K, Liu L, Chen J, Liu F. Exosomes derived from human umbilical cord blood mesenchymal stem cells improve hepatic ischemia reperfusion injury via delivering miR-1246. Cell Cycle. 2019;18:3491-3501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Wang Y, Piao C, Liu T, Lu X, Ma Y, Zhang J, Liu G, Wang H. Effects of the exosomes of adipose-derived mesenchymal stem cells on apoptosis and pyroptosis of injured liver in miniature pigs. Biomed Pharmacother. 2023;169:115873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 21. | Li H, Lin W, Zhang G, Liu R, Qu M, Zhang J, Xing X. BMSC-exosomes miR-25-3p Regulates the p53 Signaling Pathway Through PTEN to Inhibit Cell Apoptosis and Ameliorate Liver Ischemia‒reperfusion Injury. Stem Cell Rev Rep. 2023;19:2820-2836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 22. | Gong Y, Dai H, Liu W, Liao R, Chen H, Zhang L, Wang X, Chen Z. Exosomes derived from human adipose-derived stem cells alleviate hepatic ischemia-reperfusion (I/R) injury through the miR-183/ALOX5 axis. FASEB J. 2023;37:e22782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Xie K, Liu L, Chen J, Liu F. Exosomal miR-1246 derived from human umbilical cord blood mesenchymal stem cells attenuates hepatic ischemia reperfusion injury by modulating T helper 17/regulatory T balance. IUBMB Life. 2019;71:2020-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Tian X, Wu L, Li X, Zheng W, Zuo H, Song H. Exosomes derived from bone marrow mesenchymal stem cells alleviate biliary ischemia reperfusion injury in fatty liver transplantation by inhibiting ferroptosis. Mol Cell Biochem. 2024;479:881-894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 25. | Shi W, Zhou X, Li X, Peng X, Chen G, Li Y, Zhang C, Yu H, Feng Z, Gou X, Fan J. Human Umbilical Cord Mesenchymal Stem Cells Protect against Renal Ischemia-Reperfusion Injury by Secreting Extracellular Vesicles Loaded with miR-148b-3p That Target Pyruvate Dehydrogenase Kinase 4 to Inhibit Endoplasmic Reticulum Stress at the Reperfusion Stages. Int J Mol Sci. 2023;24:8899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 26. | Gao L, Qiu F, Cao H, Li H, Dai G, Ma T, Gong Y, Luo W, Zhu D, Qiu Z, Zhu P, Chu S, Yang H, Liu Z. Therapeutic delivery of microRNA-125a-5p oligonucleotides improves recovery from myocardial ischemia/reperfusion injury in mice and swine. Theranostics. 2023;13:685-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 75] [Reference Citation Analysis (0)] |
| 27. | Liu L, Xiao F, Sun J, Wang Q, Wang A, Zhang F, Li Z, Wang X, Fang Z, Qiao Y. Hepatocyte-derived extracellular vesicles miR-122-5p promotes hepatic ischemia reperfusion injury by regulating Kupffer cell polarization. Int Immunopharmacol. 2023;119:110060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 28. | Calleri A, Roggio D, Navarro-Tableros V, De Stefano N, Pasquino C, David E, Frigatti G, Rigo F, Antico F, Caropreso P, Patrono D, Bruno S, Romagnoli R. Protective Effects of Human Liver Stem Cell-Derived Extracellular Vesicles in a Mouse Model of Hepatic Ischemia-Reperfusion Injury. Stem Cell Rev Rep. 2021;17:459-470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 29. | Cao JY, Wang B, Tang TT, Wen Y, Li ZL, Feng ST, Wu M, Liu D, Yin D, Ma KL, Tang RN, Wu QL, Lan HY, Lv LL, Liu BC. Exosomal miR-125b-5p deriving from mesenchymal stem cells promotes tubular repair by suppression of p53 in ischemic acute kidney injury. Theranostics. 2021;11:5248-5266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 194] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 30. | Toghiani R, Abolmaali SS, Najafi H, Tamaddon AM. Bioengineering exosomes for treatment of organ ischemia-reperfusion injury. Life Sci. 2022;302:120654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Isaac R, Reis FCG, Ying W, Olefsky JM. Exosomes as mediators of intercellular crosstalk in metabolism. Cell Metab. 2021;33:1744-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 464] [Article Influence: 116.0] [Reference Citation Analysis (0)] |
| 32. | Tang TT, Lv LL, Wang B, Cao JY, Feng Y, Li ZL, Wu M, Wang FM, Wen Y, Zhou LT, Ni HF, Chen PS, Gu N, Crowley SD, Liu BC. Employing Macrophage-Derived Microvesicle for Kidney-Targeted Delivery of Dexamethasone: An Efficient Therapeutic Strategy against Renal Inflammation and Fibrosis. Theranostics. 2019;9:4740-4755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 33. | Gonda A, Kabagwira J, Senthil GN, Wall NR. Internalization of Exosomes through Receptor-Mediated Endocytosis. Mol Cancer Res. 2019;17:337-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 203] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 34. | Silachev DN, Goryunov KV, Shpilyuk MA, Beznoschenko OS, Morozova NY, Kraevaya EE, Popkov VA, Pevzner IB, Zorova LD, Evtushenko EA, Starodubtseva NL, Kononikhin AS, Bugrova AE, Evtushenko EG, Plotnikov EY, Zorov DB, Sukhikh GT. Effect of MSCs and MSC-Derived Extracellular Vesicles on Human Blood Coagulation. Cells. 2019;8:258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 35. | Liu S, Xiao X, Zhang L, Wang J, Zhao W, Liu H, Liao R, Li Z, Xu M, Guo J, Zhou B, Du C, Peng Q, Jiang N. Reprogramming Exosomes to Escape from Immune Surveillance for Mitochondrial Protection in Hepatic Ischemia-Reperfusion Injury. Theranostics. 2024;14:116-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 36. | Wu L, Tian X, Zuo H, Zheng W, Li X, Yuan M, Tian X, Song H. miR-124-3p delivered by exosomes from heme oxygenase-1 modified bone marrow mesenchymal stem cells inhibits ferroptosis to attenuate ischemia-reperfusion injury in steatotic grafts. J Nanobiotechnology. 2022;20:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 99] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 37. | Yaghoubi Y, Movassaghpour A, Zamani M, Talebi M, Mehdizadeh A, Yousefi M. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019;233:116733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 183] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 38. | Wang ZG, He ZY, Liang S, Yang Q, Cheng P, Chen AM. Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11:511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 166] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 39. | Rodrigues CE, Capcha JM, de Bragança AC, Sanches TR, Gouveia PQ, de Oliveira PA, Malheiros DM, Volpini RA, Santinho MA, Santana BA, Calado RD, Noronha IL, Andrade L. Human umbilical cord-derived mesenchymal stromal cells protect against premature renal senescence resulting from oxidative stress in rats with acute kidney injury. Stem Cell Res Ther. 2017;8:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 40. | Zhang L, Song Y, Chen L, Li D, Feng H, Lu Z, Fan T, Chen Z, Livingston MJ, Geng Q. MiR-20a-containing exosomes from umbilical cord mesenchymal stem cells alleviates liver ischemia/reperfusion injury. J Cell Physiol. 2020;235:3698-3710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 41. | Wang L, Feng ZJ, Ma X, Li K, Li XY, Tang Y, Peng C. Mitochondrial quality control in hepatic ischemia-reperfusion injury. Heliyon. 2023;9:e17702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 42. | Liu H, Man K. New Insights in Mechanisms and Therapeutics for Short- and Long-Term Impacts of Hepatic Ischemia Reperfusion Injury Post Liver Transplantation. Int J Mol Sci. 2021;22:8210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 43. | Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125:1246-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 442] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 44. | Newton K, Strasser A, Kayagaki N, Dixit VM. Cell death. Cell. 2024;187:235-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 376] [Reference Citation Analysis (0)] |
| 45. | Ranjan K, Pathak C. Cellular Dynamics of Fas-Associated Death Domain in the Regulation of Cancer and Inflammation. Int J Mol Sci. 2024;25:3228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 46. | Lu Y, Xi J, Zhang Y, Li C, Chen W, Hu X, Zhang M, Zhang F, Wei H, Li Z, Wang Z. MicroRNA-214-5p protects against myocardial ischemia reperfusion injury through targeting the FAS ligand. Arch Med Sci. 2020;16:1119-1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Aslan G, Atessahin A, Sahna E. The inhibition of apoptosis through myocardial postconditioning by affecting Fas/FasIg signaling through miR139-3p and miR181a-1. J Card Surg. 2020;35:564-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Tang M, Pan H, Zheng Z, Guo Y, Peng J, Yang J, Luo Y, He J, Yan S, Wang P, Zhang Y, Zhou Y. Prostaglandin E1 protects cardiomyocytes against hypoxia-reperfusion induced injury via the miR-21-5p/FASLG axis. Biosci Rep. 2019;39:BSR20190597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 49. | Chang CY, Chen KY, Shih HJ, Chiang M, Huang IT, Huang YH, Huang CJ. Let-7i-5p Mediates the Therapeutic Effects of Exosomes from Human Placenta Choriodecidual Membrane-Derived Mesenchymal Stem Cells on Mitigating Endotoxin-Induced Mortality and Liver Injury in High-Fat Diet-Induced Obese Mice. Pharmaceuticals (Basel). 2021;15:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 50. | Zhang H, Zou X, Liu F. Silencing TTTY15 mitigates hypoxia-induced mitochondrial energy metabolism dysfunction and cardiomyocytes apoptosis via TTTY15/let-7i-5p and TLR3/NF-κB pathways. Cell Signal. 2020;76:109779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 51. | Zou L, Ma X, Wu B, Chen Y, Xie D, Peng C. Protective effect of bone marrow mesenchymal stem cell-derived exosomes on cardiomyoblast hypoxia-reperfusion injury through the miR-149/let-7c/Faslg axis. Free Radic Res. 2020;54:722-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 52. | Sun Y, Liu G, Zhang K, Cao Q, Liu T, Li J. Mesenchymal stem cells-derived exosomes for drug delivery. Stem Cell Res Ther. 2021;12:561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 115] [Article Influence: 28.8] [Reference Citation Analysis (0)] |