Published online Mar 14, 2017. doi: 10.3748/wjg.v23.i10.1771
Peer-review started: November 23, 2016
First decision: February 19, 2016
Revised: January 22, 2017
Accepted: February 7, 2017
Article in press: February 8, 2017
Published online: March 14, 2017
Processing time: 111 Days and 12.8 Hours
To establish a severe acute cholangitis (SAC) model in mice.
Cholecystic catheterization was performed under the condition of bile duct ligation (BDL). Trans-cholecystic injection of lipopolysaccharide (LPS) was defined as the SAC animal model. Sham operation group, intraperitoneal injection of LPS without BDL group, intraperitoneal injection of LPS with BDL group and trans-cholecystic injection of normal saline with BDL group were defined as control groups. The survival rates and tissue injuries in liver, lungs and kidney were evaluated.
Mice in the SAC group showed a time-dependent mortality and much more severe tissue injuries in liver, lungs and kidney, compared with other groups. However, relieving biliary obstruction could effectively reduce mortality and attenuate liver injury in the SAC mouse model.
Trans-cholecystic injection of LPS under the condition of biliary obstruction could establish a repeatable and reversible mouse model of SAC.
Core tip: Severe acute cholangitis (SAC) is a severe biliary tract infection. Although mice are the most common experimental animal and have a similar anatomical construction of bile ducts to humans, there is still no valid study on establishing a SAC model in mice. To study SAC more easily and exactly, we established a repeatable and reversible mouse model of SAC through cholecystic catheterization under the condition of bile duct ligation.
- Citation: Yu JH, Tang HJ, Zhang WG, Zhu ZY, Ruan XX, Lu BC. Catheterization of the gallbladder: A novel mouse model of severe acute cholangitis. World J Gastroenterol 2017; 23(10): 1771-1779
- URL: https://www.wjgnet.com/1007-9327/full/v23/i10/1771.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i10.1771
Severe acute cholangitis (SAC) is a severe biliary tract infection with rapid progressing and a high mortality rate. Septicemia, septic shock and multiple-organ failure are always induced by this severe disease and result in a poor prognosis. For SAC, complete biliary obstruction is the initiation factor, and secondary infection from Gram-negative pathogen is the aggravating destroyer[1]. Pro-inflammatory cytokines, such as interleukin 1 beta (IL-1β), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), have been reported to have a close relationship with the process of the severe infectious diseases[2-4]. According to Tokyo guidelines 2013, associating with one or more other organs dysfunction is regarded as the diagnostic criteria to distinguish SAC from mild or moderate acute cholangitis[5,6]. Explosive inflammatory and subsequent multiple organ dysfunction syndrome (MODS) are important causes of poor prognosis.
To study the pathological process of SAC more accurately, a typical, stable, repeatable animal model is required. A few animal models of acute cholangitis have been established in species such as rats and rabbits[3,7,8]. However, mice are the most common experimental animal, and there is still no valid study on establishing an acute cholangitis model in mice. In the current study, we established a repeatable model of SAC in mice, based on common bile duct ligation and an indwelling catheter through cholecystostomy, with the aim to better understand the process of SAC.
Male C57Bl/6 mice weighing 20 to 25 g were purchased from the Zhejiang Province Experimental Animal Center. All procedures were approved by the Ethics Committee of Zhejiang University and conformed to the Care and Use of Laboratory Animals Guide published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). All surgery was performed under ether anesthesia, and all efforts were made to minimize suffering.
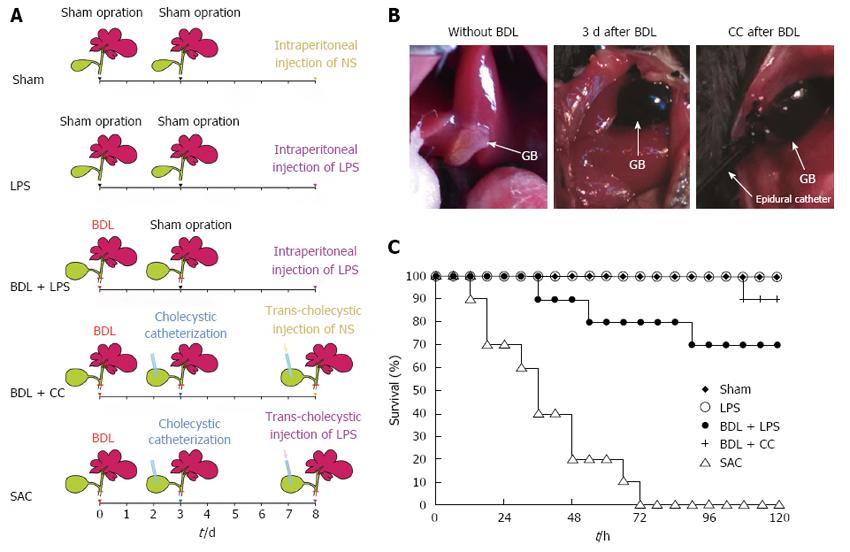
Mice were randomly assigned to the following five groups: sham (sham), lipopolysaccharide (LPS), bile duct ligation plus LPS (BDL + LPS), bile duct ligation plus cholecystic catheterization (BDL + CC), and severe acute cholangitis (SAC). Surgeries were performed under ether anesthesia. In the BDL + CC and SAC groups, common bile ducts were dissociated and ligated with 6-0 silk. Three days later, the gallbladders were greatly enlarged and mice received cholecystostomy in the BDL + CC and SAC groups (Figure 1B). Epidural catheters (format F3, diameter = 0.9 mm) were used as gallbladder drainage tubes and were outstretched from the skin. 6-0 silk was used to suture the incision of gallbladders and fix the epidural catheters. The distal ends of epidural catheters traveled through subcutaneous tissue and were finally fixed at the back of mice. The abdominal cavity was closed and the epidural catheters remained closed after cholecystostomy. Five days after cholecystic catheterization, 0.5 μg of LPS (from E. coli O111:B4, Sigma, St Louis, MO, United States) dissolved in 0.1 mL of normal saline (NS) was injected to the gallbladder through the epidural catheter in the SAC groups. An equivalent amount of NS was injected to the gallbladder through the epidural catheter in the BDL + CC groups. Before the catheter was sealed with a sealing cap, 0.1 mL air was injected and then the abdominal cavity was closed with silk sutures. In the BDL + LPS group, common bile ducts were ligated with 6-0 silk. At 72 h after bile duct ligation, another laparotomy, but no further operation, was performed. Eight days after the first operation, mice were administrated with 0.5 μg of LPS intraperitoneally. In the LPS and sham groups, two sham laparotomies were performed at the same time points as described above. Eight days after the first operation, mice in the LPS and sham groups were intraperitoneally administrated with 0.5 μg of LPS or 0.1 mL of NS, respectively.
Ten mice in each group were used to observe the mortality rate after the last operation. The other animals were sacrificed at the 24 h and 48 h after the last injection. Blood collected from the inferior vena cava and the serum was stored at -80 °C before analysis. Bile samples in the BDL + CC and SAC groups were obtained through catheterization of the gallbladder. The tissue samples were snap-frozen in liquid nitrogen and then stored at -80 °C.
The mice in the SAC group were used for these experiments. At 6 h or 12 h after LPS injection through cholecystic catheterization, the sealing caps of catheters were removed and bile was allowed to outflow from catheters. The mortality rate was analyzed at 24 h and 48 h after LPS injection. Mice that survived were sacrificed at 48 h after LPS injection and serum samples were used to evaluate liver injury.
Serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL), blood urea nitrogen (BUN), and serum creatinine (SC) were quantitated by an Automated Chemical Analyzer (Abbott, Chicago, IL, United States). Alkaline phosphatase (ALP) and γ-gltamyltranspeptidase (γ-GGT) using kits (Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer’s instruction.
Total RNA was isolated from tissues using TRIzol (Invitrogen Life Technologies, Carlsbad, CA, United States) and reverse transcribed into complementary DNA using the High-Capacity complementary DNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, United States), according to themanufacturer’s instructions. Quantitative real-time polymerase chain reaction (PCR) was performed using SYBR Green PCRMasterMix and the ABI 7500 Real-time PCR system (Applied Biosystems). Specific primers were designed, respectively, for IL-1β, IL-6 and TNF-α (Table 1). Mouse β-actin was used as an endogenous control. PCR was performed according to the manufacturer’s instructions. All assays were performed three times. Relative expression levels were then determined using the 2-ΔΔCt method[9].
| Name | Symbol | Forward (5'-3') | Reverse (5'-3') |
| β-actin | ACTB | CCACCATGTACCCAGGCATT | AGGGTGTAAAACGCAGCTCA |
| IL-6 | IL6 | TACCACTTCACAAGTCGGAGGC | CTGCAAGTGCATCATCGTTGTTC |
| TNF-α | TNF | GGTGCCTATGTCTCAGCCTCTT | GCCATAGAACTGATGAGAGGGAG |
| IL-1β | IL-1β | TGGACCTTCCAGGATGAGGACA | GTTCATCTCGGAGCCTGTAGTG |
Caspase 3 activity in cells proteins was detected by the Caspase 3 Activity Assay Kit (Beyotime, Nanjing, China), according to the manufacturer’s protocol. The absorbance was measured at 405 nm, and the relative activity of Caspase 3 was determined.
Primary antibodies, for B-cell CLL/lymphoma 2 (Bcl2), BCL2-associated X protein (Bax) and β-actin, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, United States). On a 10% SDS-PAGE (sodium dodecyl sulfate-poly-acrylamide gel) gel, 20 mg of total protein was electrophoresed, transferred onto to a polyvinylidene fluoride membranes, blocked, and incubated with primary antibody and then with horseradish peroxidase-conjugated secondary antibody. Immunoreactive bands were visualized using a chemiluminescence solution. β-actin was used as an endogenous control.
The formalin-fixed specimens were obtained from the mice which were sacrificed at the 48 h after the last injection. For histological evaluation, formalin-fixed specimens were stained with HE. Liver, lung and kidney tissues were examined for histopathological evidence of pathological damage.
Serum and bile samples were obtained from 18 male patients at the Shaoxing People’s Hospital from January 2014 to January 2016. Informed consent was obtained from patients and the tissue acquisition protocol was approved by the Shaoxing People’s Hospital Institutional Review Board. Among the patients, 6 patients in gallstone group suffered from gallstone and received cholecystectomy. Six patients in SAC group were diagnosed as SAC definitely, according to Tokyo guidelines 2013[6], with a condition characterized by fever, abdominal pain and jaundice (Charcot triad)[10]. Six patients in biliary obstruction group suffered from biliary obstructive disease (such as pancreaticobiliary tumour), with imaging findings of biliary dilation and elevated bilirubin, while without infection (such as fever or increase in leucocytes). Blood samples were obtained before surgery and bile samples were obtained during surgery.
IL-1β, IL-6 and TNF-α in serum and bile samples were measured by ELISA kit (eBioscience&Bender, San Diego, CA, United States) according to the manufacturer’s instructions. Each sample was tested in triplicate.
PCNA expression was detected immunohistochemistry (IHC) staining using paraffin-embedded specimens from different groups. After deparaffinization and rehydration of the sections, endogenous peroxidase activity was blocked using 0.3% hydrogen peroxide. The sections were incubated with primary anti-PCNA antibody (Santa Cruz) overnight at 4 °C, followed by incubation with appropriate horseradish peroxidase-conjugated secondary antibody for 1.5 h. After a thorough washing, the sections were developed in 3,3’-diaminobenzidine and counterstained with hematoxylin. Each stained sample was observed under high power magnification (× 200).
Data were presented as means standard deviation. Statistical significance between two groups was determined using the Student t-test. One-way analysis of variance followed by the TukeyeKramer adjustment was used to examine differences among multiple groups. P < 0.05 was considered to be significant. All statistical analyses were conducted using SPSS 11.0.
The mortality rate of the mice in the SAC group was 80% (8/10 animals) at the point of 48 h and all mice died within 72 h after LPS injection (Figure 1C). Mice in the BDL + CC and BDL + LPS groups showed 90% and 70% survival, respectively, at 5 d after the last operation (Figure 1C). However, none of mice in the sham group or LPS group died during the same period (Figure 1C).
Serum ALT, AST and TBIL levels were assessed to evaluate liver injury. Intraperitoneal injection with LPS induced a moderate rise in ALT and AST levels, but did not affect TBIL levels, compared with the sham group (Table 2). ALT, AST and TBIL levels in the BDL + LPS, BDL + CC and SAC groups were significantly higher because of bile duct ligation, compared with the sham group (Table 2). Interestingly, ALT, AST and TBIL levels in the SAC group were much higher compared with those in the BDL + LPS and BDL + CC groups (Table 2). However, although the data of BDL + LPS group were slightly higher than those of BDL + CC group, the differences were not significant. ALP and γ-GGT levels are regarded as important liver tests during acute cholangitis[10]. Our results also showed that ALP and γ-GGT levels in the SAC groups were significantly higher than those in the other groups (Table 2).
| Sham | LPS | BDL+LPS | BDL+CC | SAC | |
| TBIL (mg/dL) | 0.81 ± 0.15 | 1.26 ± 0.72 | 14.21 ± 2.10ac | 13.35 ± 1.71ac | 17.68 ± 2.66acef |
| ALT (IU/L) | 18.63 ± 1.46 | 106.4 ± 20.24a | 206.62 ± 25.92ac | 197.48 ± 30.85ac | 403.07 ± 74.92acef |
| AST (IU/L) | 126.12 ± 34.39 | 558.97 ± 111.35a | 1693.17 ± 313.73ac | 1462.85 ± 397.75ac | 2332.32 ± 531.64acef |
| γ-GGT (U/L) | 11.17 ± 2.27 | 24.67 ± 4.96a | 69.33 ± 9.74ac | 70.00 ± 7.24ac | 83.17 ± 9.08acef |
| ALP (U/L) | 114.33 ± 19.68 | 229.67 ± 27.51a | 2765.33 ± 250.51ac | 2544.17 ± 350.24ac | 3470.83 ± 522.35acef |
| Creatinine (mg/dL) | 0.27 ± 0.04 | 0.35 ± 0.07 | 0.32 ± 0.08 | 0.31 ± 0.08 | 0.45 ± 0.09aef |
| BUN (mg/dL) | 20.18 ± 5.43 | 25.77 ± 4.52 | 26.82 ± 7.80 | 25.17 ± 5.04 | 42.78 ± 9.76acef |
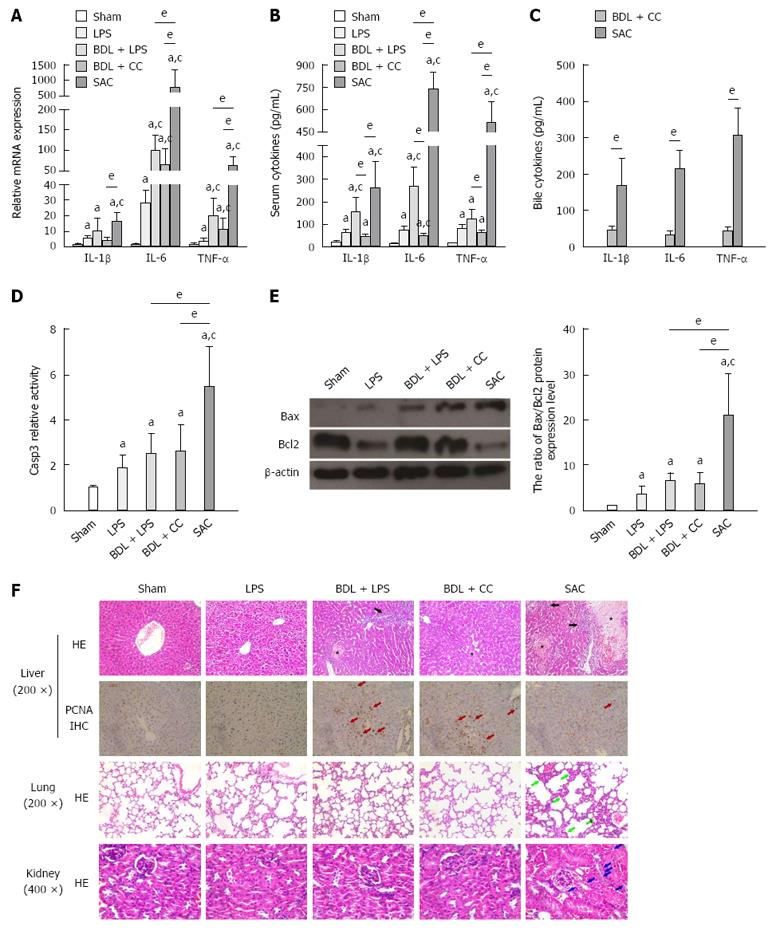
IL-1β, IL-6 and TNF-α are important pro-inflammatory cytokines in the process of liver injury and inflammation[4,11-14]. To evaluate the levels of inflammation and liver injury, the pro-inflammatory mediators’ level in serum and liver tissues were examined. The results showed that biliary obstruction was an important inducement to increase the mRNA expression of IL-6 and TNF-α (Figure 2A). However, SAC group showed a further increasing and much higher IL-6 and TNF-α mRNA expression after exposed to LPS, compared with that of BDL + LPS and BDL + CC groups (Figure 2A). IL-1β mRNA expression in SAC group also showed a significant increase, compared with that of LPS and BDL + CC groups (Figure 2A). The serum levels of IL-1β, IL-6 and TNF-α were also significantly increased in the SAC group, compared with those of other groups (Figure 2B). And not only that, SAC group also showed a higher level of pro-inflammatory cytokines in bile, compared with BDL + CC groups (Figure 2C). These results indicated that the inflammatory response was further activated because of LPS injection into the biliary system.
Caspase 3, Bcl2 and Bax are common molecular hallmarks of apoptosis[15,16]. The activity of caspase 3 and the ratio of Bax to Bcl2 are often used to evaluate tissue apoptosis. Detection of caspase 3 activity and the ratio of Bax to Bcl2 showed that both intraperitoneal injection of LPS and biliary obstruction induced moderate apoptosis of hepatocytes (Figure 2D and H). Caspase 3 activity and the ratio of Bax to Bcl2 in the SAC group were prominently higher than those in the other groups (Figure 2D and H). This finding indicated that injection of LPS through cholecystic catheterization induced more serious apoptosis in the liver.
Morphology was examined to further evaluate the degree of injury in mouse liver tissues. Pathological examination showed that intraperitoneal injection of LPS induced considerable vacuolar degenerative changes (Figure 2F). In the BDL + CC group, biliary obstruction caused an obvious dilatation of bile capillaries and local necrosis (Figure 2F). In the BDL + LPS group, dilatation of bile capillaries, vacuolar degenerative changes and inflammatory cell infiltration could be found (Figure 2F). Notably, the liver in the SAC group showed obvious vacuolar changes, more inflammatory cell infiltration, and more broad areas of necrosis, compared with the BDL + LPS and BDL + CC groups (Figure 2F).
Proliferating cell nuclear antigen (PCNA) is common molecular biological hallmarks of regeneration[17]. We detected the expression of PCNA in specimens from different groups using immunohistochemistry. Compared with SAC groups, there were more PCNA-positive cells in BDL + LPS and BDL + CC groups (Figure 2F). It indicated that injection of LPS through cholecystic catheterization significantly suppressed the reparative live regeneration induced by biliary obstruction.
We also examined BUN and serum creatinine to evaluate renal injury. BUN and creatinine levels were significantly elevated in the SAC group after injection of LPS through catheters, compared with the other four groups (Table 2). Conversely, in the other treatment groups, including the LPS, BDL + LPS, and BDL + CC groups, BUN and creatinine levels tended to be evaluated after exposure to LPS or ligation of the bile duct, but the differences were not significant (Table 2). On HE-stained sections, the kidney in the SAC group showed glomerular and tubular injury with obvious tubular vacuolization (Figure 2F). However, none of the other groups showed these pathological changes.
We also investigated pathological changes in the lungs in the different groups. As shown in Figure 2F, lung tissue in the SAC group displayed obvious inflammatory cells exudation and fibroblasts proliferation in the pulmonary interstitium and alveolar spaces (Figure 2F). Although LPS was administrated at the same dose in the LPS and BDL + LPS groups, the pathological changes in the lungs in these groups were not as obvious as those in the SAC group.
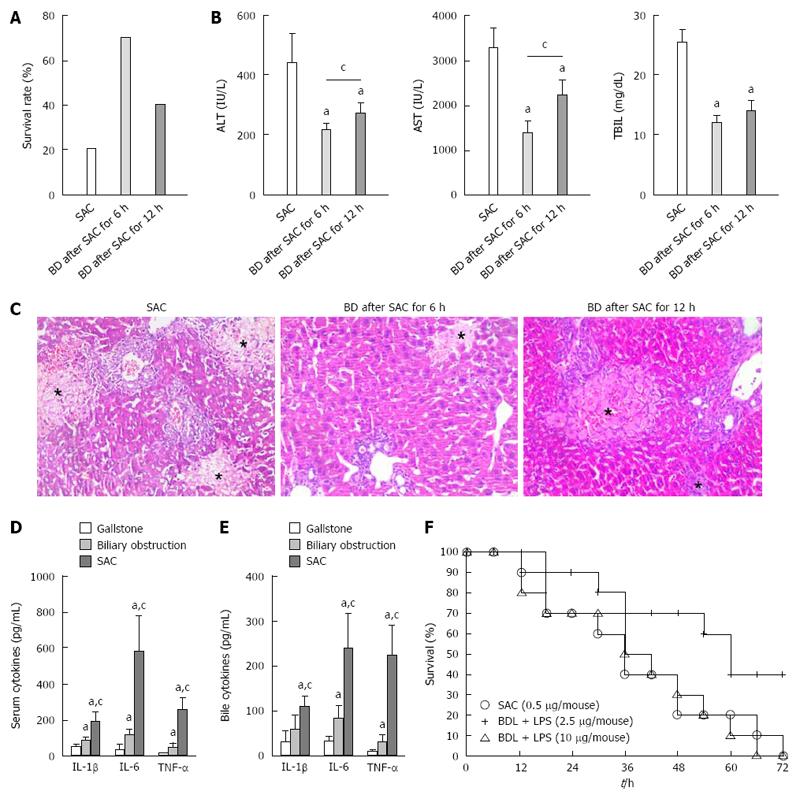
As shown in Figure 3A, relief of biliary obstruction at 6 or 12 h after LPS injection resulted in a higher survival at 48 h after LPS injection compared with the non-decompressing SAC group. Examination of injury to the liver showed that ALT, AST, and TBIL levels were significantly decreased because of relieving biliary obstruction (Figure 3B). At the same time, histological analysis also indicated that biliary drainage could mitigate SAC-induced liver injury (Figure 3C).
SAC is a bacterial infection in the setting of complete biliary obstruction and leads to serious systemic signs of infection. To study this disease, the animal model of SAC has been established in rodents, such as rats[8,18,19]. As the most commonly used experimental animal[20], the anatomical construction of bile ducts in mice is more similar to that in humans because rats do not have a gallbladder. The gallbladder slows down the tremendous increase in biliary pressure at the beginning of biliary obstruction. However, a high-tension and exceedingly full gallbladder will aggravate biliary pressure at the middle and later periods of biliary obstruction[21]. Therefore, mice are a more appropriate species to establish an animal model of SAC compared with rats. Nevertheless, the common bile duct of mice is too slim to complete successful injection. We performed cholecystic catheterization 3 d after ligation of the bile duct. At that time, the gallbladder was engorged and flexible enough to catheterize (Figure 1B). It’s worth noting that subsequent infection always arises after a period of cholestasis in the SAC patients[1,5]. Our model also simulated this process exactly. Catheterization of the gallbladder is the other core operation for our model. Benefited from it, in the SAC and BDL + CC groups, bile sample could be got through catheters conveniently. More importantly, biliary drainage is the main therapeutic method for SAC[1,10,22]. Catheterization of the gallbladder provided a convenient method to relieve biliary obstruction. Our study also showed that timely relief of biliary obstruction reduced mortality and alleviated liver injury effectively. The mouse model shows a clear advantage to simulate the rehabilitation process of SAC.
To validate the reliability of model, more examinations were performed. Exploding ALT, AST, TBIL, ALP, γ-GGT and death detection indicated serious liver injury arose. Increasing pro-inflammatory cytokines indicated obvious inflammatory response arose. In clinical practice, SAC patients always have a higher level of pro-inflammatory cytokines in serum and bile, compared with the patients those suffered from chronic biliary obstruction or gallstone (Figure 3D and E). Our mouse model showed a similar change as SAC patients. According to the obvious pathologic change of histological examination and repressed liver regeneration, the liver injury of our animal model was consistent with the characteristics of SAC-induced liver injury[1,10].
In clinical practice, systemic inflammatory response syndrome (SIRS) and MODS would arise if patients with SAC did not obtain timely medical treatment[1,22,23]. In our study, time-dependent mortality and obvious injury of the liver, lungs and kidney were observed in the SAC group. These finding also indicated that our animal model displays a similar disease progression to SAC patients.
The barrier between blood and bile always is dysfunction because of biliary high-pressure. It is noteworthy that mice in the LPS, BDL + LPS and BDL + CC groups did not show severe tissue injuries in liver, lungs and kidney, compared with the SAC group. Injection of LPS into a high-pressure biliary system accelerated further breakdown of the bile-blood barrier and induced severe endotoxemia should be an adequate explanation. Compared with LPS and BDL groups, BDL + LPS group also had the two requirements of SAC (injection of LPS and biliary obstruction) in the same time. However, intraperitoneal injection with LPS (0.5 μg) under the condition of biliary obstruction did not induce organs injury as serious as trans-cholecystic injection with LPS (BDL + LPS group vs SAC group). We also found that if the dose of LPS was increased to 10 μg/mouse, the mortality rate in the BDL + LPS group (10 μg/mouse) reached a similar level to that in the SAC group (Figure 3F). The main reason for this finding may be that intraperitoneal endotoxin enters the bloodstream depending on absorption through the peritoneum. This manner is different from the special manner of endotoxin entering the bloodstream during SAC, and the absorbing ability of the peritoneum would be influenced by other factors, such as abnormal liver function. We also injected LPS (10 μg/mouse i.p.) to mice without any other operation. However, no mouse died within 72 h after LPS injection. It indicated that biliary obstruction for long enough is also an essential condition for SAC model and always leads to poor prognosis.
In summary, we established a repeatable and reversible mouse model of SAC through cholecystic catheterization. This animal model can be used to study disease progression and treatment of SAC.
Severe acute cholangitis (SAC) is a severe biliary tract infection. Although mice are the most common experimental animal, there is still no valid study on establishing a SAC model in mice.
The animal model of SAC has been established in rodents, such as rats. However, the anatomical construction of bile ducts in mice is more similar to that in humans because rats do not have a gallbladder. To study SAC more easily and exactly, the authors established a repeatable and reversible mouse model of SAC.
This is the first study establishing a repeatable and reversible mouse model of SAC. To solve the technological difficulty that the common bile duct of mice is too slim to complete successful injection, the authors performed cholecystic catheterization 3 d after ligation of the bile duct. At that time, the gallbladder was engorged and flexible enough to catheterize.
The authors established a repeatable and reversible mouse model of SAC through cholecystic catheterization. This animal model can be used to study disease progression and treatment of SAC.
SAC is a severe biliary tract infection with rapid progressing and a high mortality rate. According to Tokyo guidelines 2013, associating with one or more other organs dysfunction is regarded as the diagnostic criteria to distinguish SAC from mild or moderate acute cholangitis.
The authors developed a novel mouse model of severe acute cholangitis. It is very interesting.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Abadi ATB, Nishino T S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Mosler P. Diagnosis and management of acute cholangitis. Curr Gastroenterol Rep. 2011;13:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 2. | Guldiken N, Kobazi Ensari G, Lahiri P, Couchy G, Preisinger C, Liedtke C, Zimmermann HW, Ziol M, Boor P, Zucman-Rossi J. Keratin 23 is a stress-inducible marker of mouse and human ductular reaction in liver disease. J Hepatol. 2016;65:552-559. [PubMed] |
| 3. | Watanabe K, Yokoyama Y, Kokuryo T, Kawai K, Kitagawa T, Seki T, Nakagawa A, Nagino M. 15-deoxy-delta 12,14-prostaglandin J2 prevents inflammatory response and endothelial cell damage in rats with acute obstructive cholangitis. Am J Physiol Gastrointest Liver Physiol. 2010;298:G410-G418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Plum W, Tschaharganeh DF, Kroy DC, Corsten E, Erschfeld S, Dierssen U, Wasmuth H, Trautwein C, Streetz KL. Lack of glycoprotein 130/signal transducer and activator of transcription 3-mediated signaling in hepatocytes enhances chronic liver injury and fibrosis progression in a model of sclerosing cholangitis. Am J Pathol. 2010;176:2236-2246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Kiriyama S, Takada T, Strasberg SM, Solomkin JS, Mayumi T, Pitt HA, Gouma DJ, Garden OJ, Büchler MW, Yokoe M. New diagnostic criteria and severity assessment of acute cholangitis in revised Tokyo Guidelines. J Hepatobiliary Pancreat Sci. 2012;19:548-556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Kiriyama S, Takada T, Strasberg SM, Solomkin JS, Mayumi T, Pitt HA, Gouma DJ, Garden OJ, Büchler MW, Yokoe M. TG13 guidelines for diagnosis and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci. 2013;20:24-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 197] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 7. | Higure A, Okamoto K, Hirata K, Todoroki H, Nagafuchi Y, Takeda S, Katoh H, Itoh H, Ohsato K, Nakamura S. Macrophages and neutrophils infiltrating into the liver are responsible for tissue factor expression in a rabbit model of acute obstructive cholangitis. Thromb Haemost. 1996;75:791-795. [PubMed] |
| 8. | Yang J, Lu B. Establishment of a novel rat model of severe acute cholangitis. Iran J Basic Med Sci. 2015;18:1124-1129. [PubMed] |
| 9. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149116] [Cited by in RCA: 133837] [Article Influence: 5576.5] [Reference Citation Analysis (1)] |
| 10. | Lee JG. Diagnosis and management of acute cholangitis. Nat Rev Gastroenterol Hepatol. 2009;6:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Yu H, Wu SD. Activation of TLR-4 and liver injury via NF-kappa B in rat with acute cholangitis. Hepatobiliary Pancreat Dis Int. 2008;7:185-191. [PubMed] |
| 12. | da Silva CG, Studer P, Skroch M, Mahiou J, Minussi DC, Peterson CR, Wilson SW, Patel VI, Ma A, Csizmadia E. A20 promotes liver regeneration by decreasing SOCS3 expression to enhance IL-6/STAT3 proliferative signals. Hepatology. 2013;57:2014-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Bavia L, de Castro ÍA, Cogliati B, Dettoni JB, Alves VA, Isaac L. Complement C5 controls liver lipid profile, promotes liver homeostasis and inflammation in C57BL/6 genetic background. Immunobiology. 2016;221:822-832. [PubMed] |
| 14. | Szabo G, Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol. 2015;12:387-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 464] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 16. | Zinkel S, Gross A, Yang E. BCL2 family in DNA damage and cell cycle control. Cell Death Differ. 2006;13:1351-1359. [PubMed] |
| 17. | Assy N, Gong Y, Zhang M, Pettigrew NM, Pashniak D, Minuk GY. Use of proliferating cell nuclear antigen as a marker of liver regeneration after partial hepatectomy in rats. J Lab Clin Med. 1998;131:251-256. [PubMed] |
| 18. | Huang YH, Chuang JH, Yang YL, Huang CC, Wu CL, Chen CL. Cholestasis downregulate hepcidin expression through inhibiting IL-6-induced phosphorylation of signal transducer and activator of transcription 3 signaling. Lab Invest. 2009;89:1128-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Matsumoto Y, Niimoto S, Katayama K, Hirose K, Yamaguchi A, Torigoe K. Effects of biliary drainage in obstructive jaundice on microcirculation, phagocytic activity, and ultrastructure of the liver in rats. J Hepatobiliary Pancreat Surg. 2002;9:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Vaquer G, Rivière F, Mavris M, Bignami F, Llinares-Garcia J, Westermark K, Sepodes B. Animal models for metabolic, neuromuscular and ophthalmological rare diseases. Nat Rev Drug Discov. 2013;12:287-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Center SA. Diseases of the gallbladder and biliary tree. Vet Clin North Am Small Anim Pract. 2009;39:543-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Wada K, Takada T, Kawarada Y, Nimura Y, Miura F, Yoshida M, Mayumi T, Strasberg S, Pitt HA, Gadacz TR. Diagnostic criteria and severity assessment of acute cholangitis: Tokyo Guidelines. J Hepatobiliary Pancreat Surg. 2007;14:52-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 185] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 23. | Zhang WZ, Chen YS, Wang JW, Chen XR. Early diagnosis and treatment of severe acute cholangitis. World J Gastroenterol. 2002;8:150-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |