Copyright
©The Author(s) 2025.
World J Gastroenterol. Jun 28, 2025; 31(24): 108234
Published online Jun 28, 2025. doi: 10.3748/wjg.v31.i24.108234
Published online Jun 28, 2025. doi: 10.3748/wjg.v31.i24.108234
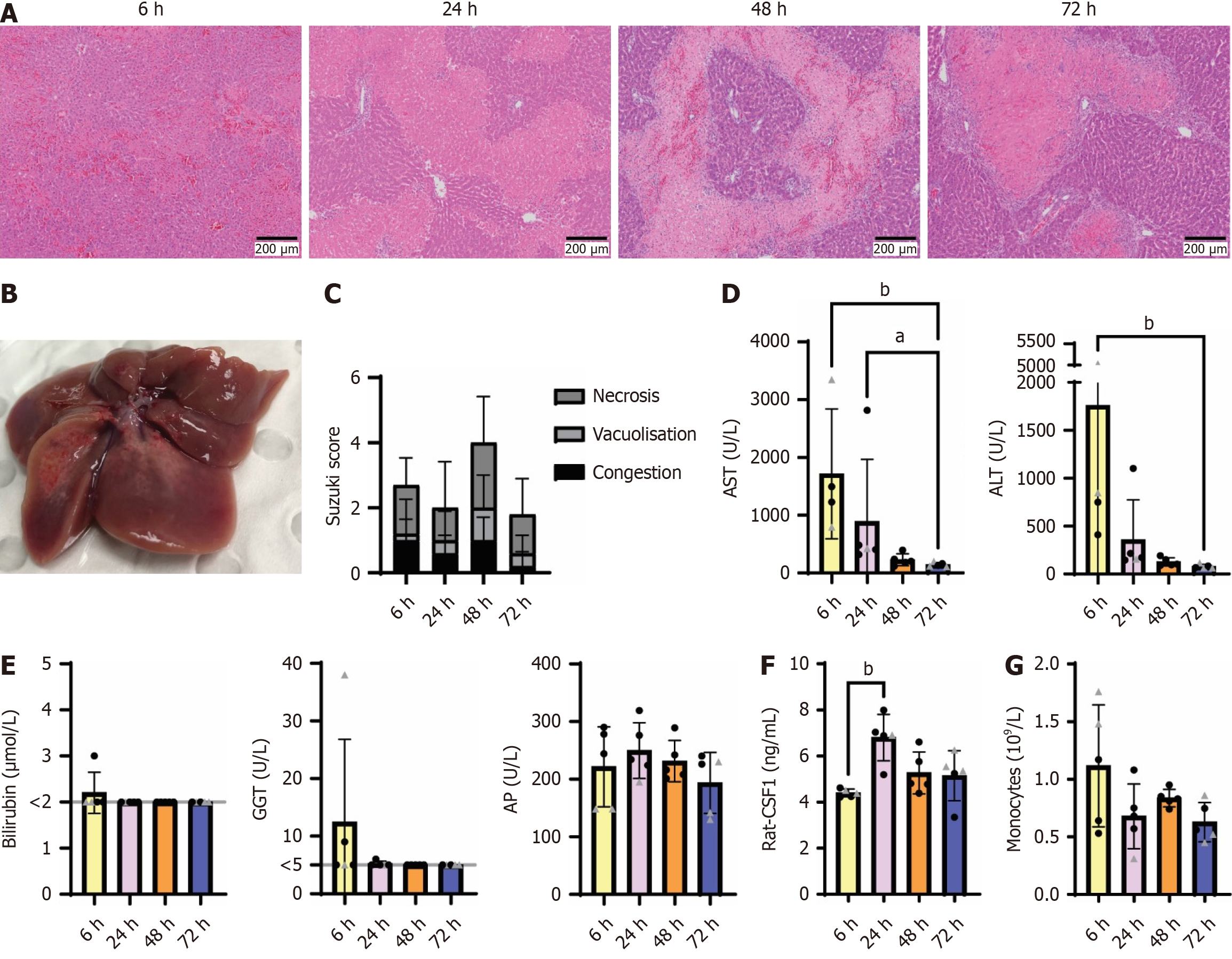
Figure 1 Partial liver ischemia-reperfusion injury induced endogenous colony stimulating factor 1 24 hours post reperfusion.
Groups of 4–6-week-old rats (n = 5/group, mixed gender, females indicated by triangle symbol) were subjected to 70% hepatic ischemia for 45 minutes and euthanized at 6-, 24-, 48- and 72-hours post reperfusion. A: Representative H&E sections of formalin-fixed paraffin-embedded livers showing different stages of ischemic injury at 6-72 hours; B: Macroscopic lesion on the left lateral and median liver lobes at 48 hours post ischemia-reperfusion; C-E: Suzuki Scores (C), serum aspartate aminotransferase and alanine aminotransferase (D), serum bilirubin, gamma glutamyl transferase and alkaline phosphatase (E). Grey line indicates limit of detection; F: Endogenous serum CSF1 was measured by ELISA; G: Monocyte count in the peripheral blood was quantified using a haematology analyser. Data show mean and standard deviation. Results were analysed with the Kruskal Wallis test; aP < 0.05, bP < 0.01. ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; CSF: Colony stimulating factor.
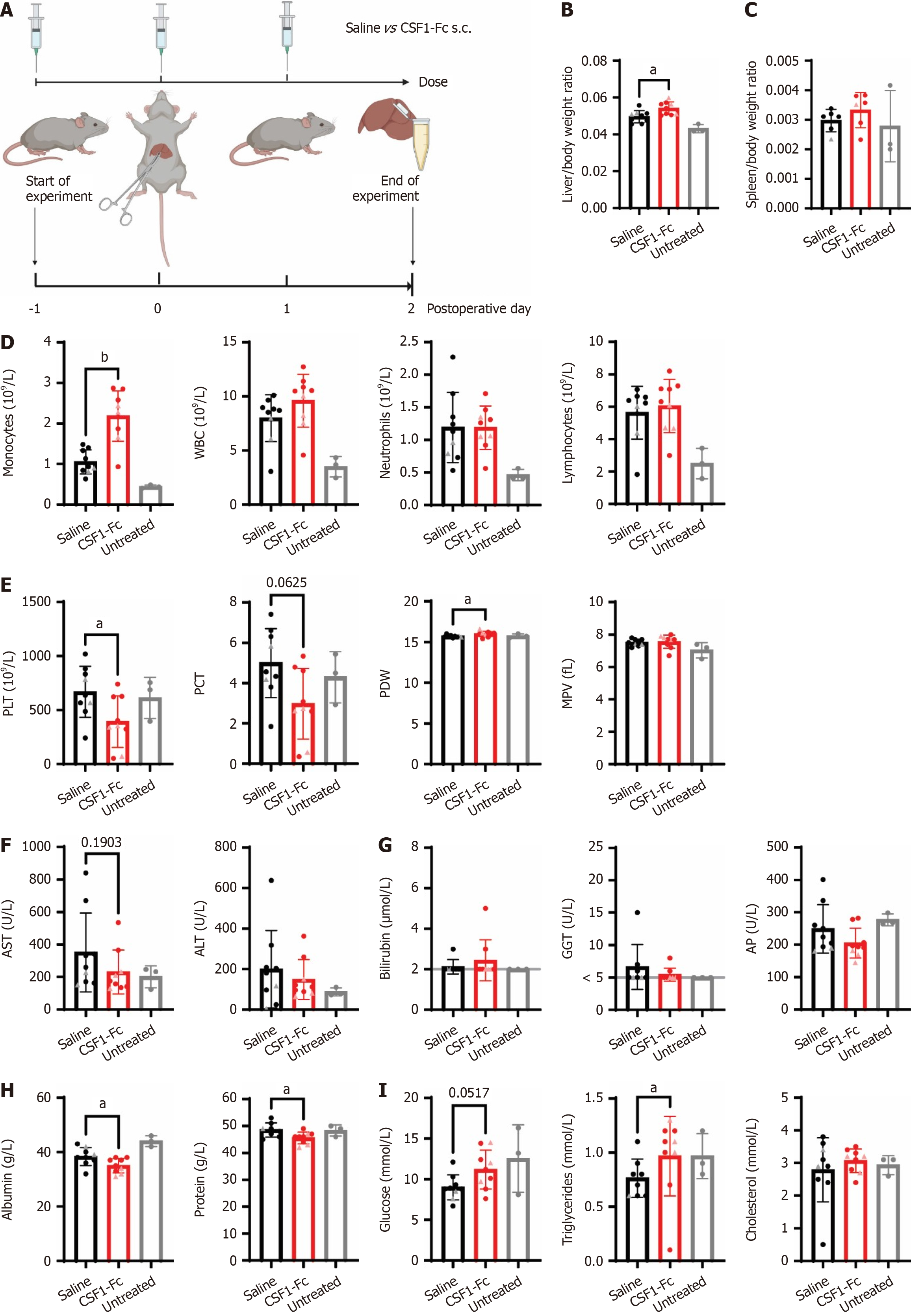
Figure 2 Perisurgical colony stimulating factor 1-Fc treatment increased circulating monocytes and promoted liver growth after ischemia-reperfusion injury.
A: Groups of rats (n = 9/group except spleen weight n = 6/group, females indicated by triangle symbol) were subjected to 60 minutes of ischemia, followed by 48 hours of reperfusion and treated with 3 × daily doses of 1 mg/kg of a human colony stimulating factor (CSF)1-Fc or saline control on postoperative day (POD) -1, 0 and 1; B: Liver/body weight ratio; C: Spleen/body weight ratio; D and E: Blood monocyte, white blood cell, neutrophil and lymphocyte count (D), platelets, plateletcrit, platelet distribution width and mean platelet volume (E) in the peripheral blood quantified using a haematology analyser; F-I: Aspartate aminotransferase and alanine aminotransferase (F), bilirubin, gamma glutamyl transferase and alkaline phosphatase levels (G), albumin and total protein (H) and glucose, triglycerides and cholesterol (I) were measured in the serum. Data show mean and standard deviation. Data were analysed using the Mann Whitney test for saline vs CSF1-Fc-treated groups. aP < 0.05, bP < 0.001. Data from age-matched Dark Agouti rats (n = 3) that had not been subjected to ischemia-reperfusion surgery collected at a different time are shown for reference (grey bars), but not included in statistical analysis. Grey line indicates limit of detection. s.c.: Subcutaneous; WBC: White blood cell; PLT: Platelet; PCT: Plateletcrit; PDW: Platelet distribution width; MPV: Mean platelet volume; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; GGT: Gamma glutamyl transferase; AP: Alkaline phosphatase; CSF: Colony stimulating factor.
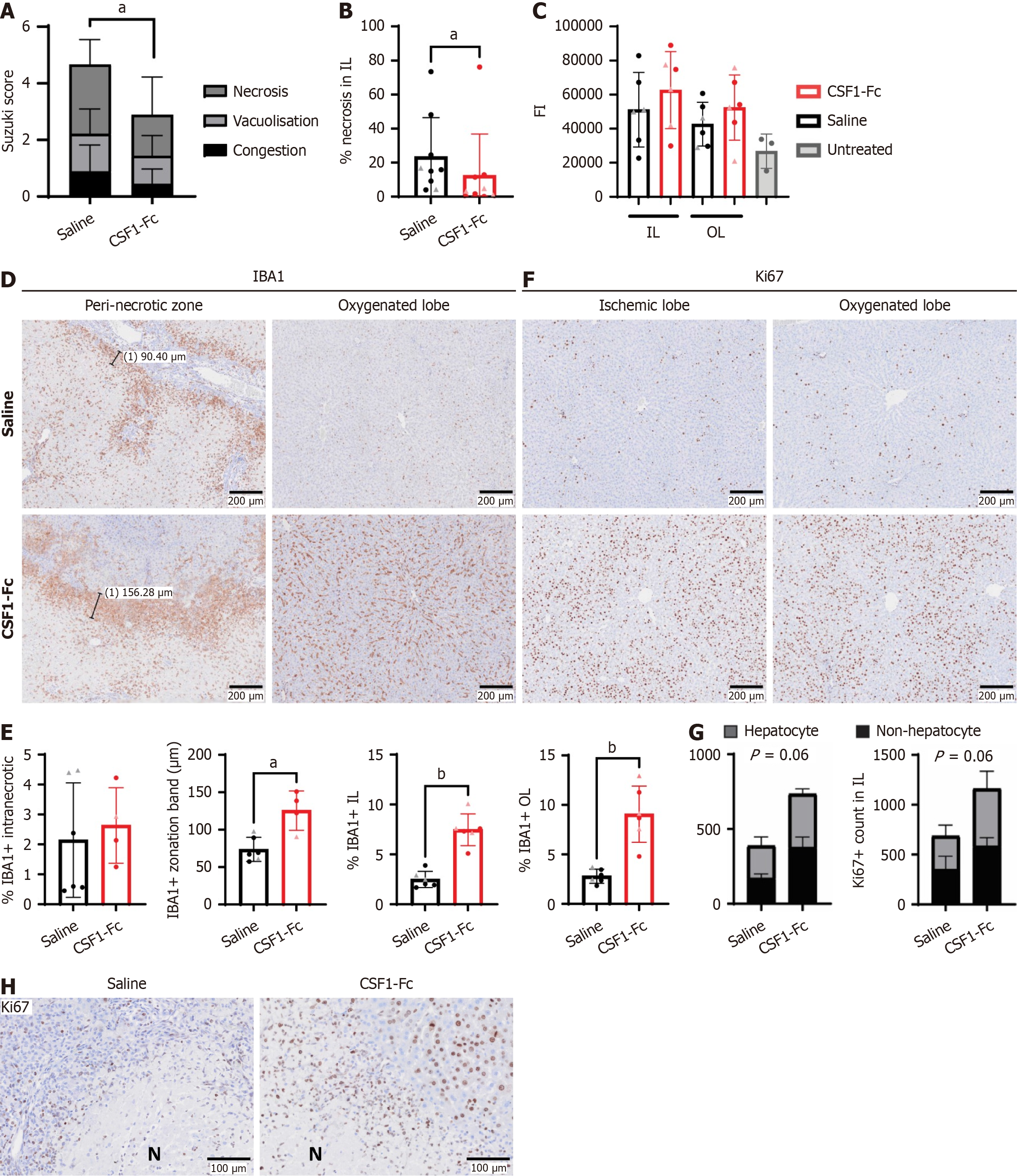
Figure 3 Colony stimulating factor 1-Fc treatment reduced the extent of histological necrosis, augmented liver macrophage numbers and promoted cell proliferation.
A and B: Suzuki Score (A) and necrotic area (B) assessed by image analysis in ischaemic lobes 48 hours post reperfusion; C: Caspase 3 activity in liver lysates, assessed cleavage of a fluorescent substrate. Liver lysates from age-matched Dark Agouti rats (n = 3) that had not been subjected to ischemia-reperfusion surgery collected at a different time were used as untreated controls. Kruskal Wallis test with Dunn’s multiple comparisons test (not significant); D and E: Representative immunohistochemistry images of liver IBA1+ cells (D) and quantification of IBA1+ intranecrotic staining area (E), zonation band thickness and IBA1+ staining area in the ischemic and oxygenated lobe (OL). The zonation band was not quantified in 3 rats -1 saline, 2 colony stimulating factor (CSF)1-Fc in which no distinct necrotic area was present; F and G: Representative immunohistochemistry images of liver Ki67 staining (F) and quantification of Ki67 positive hepatocytes and other cells in the ischemic lobe (IL) and OL (G); H: Representative immunohistochemistry images showing Ki67+ cells at a higher magnification in the peri-necrotic zone in the ischemic lobes for saline and CSF1-Fc treatment. Data show mean and standard deviation. Data were analysed using Mann Whitney test, except for (C). aP < 0.05, bP < 0.01. FI: Fluorescent intensity; IL: Ischemic lobe; OL: Oxygenated lobe; CSF: Colony stimulating factor.
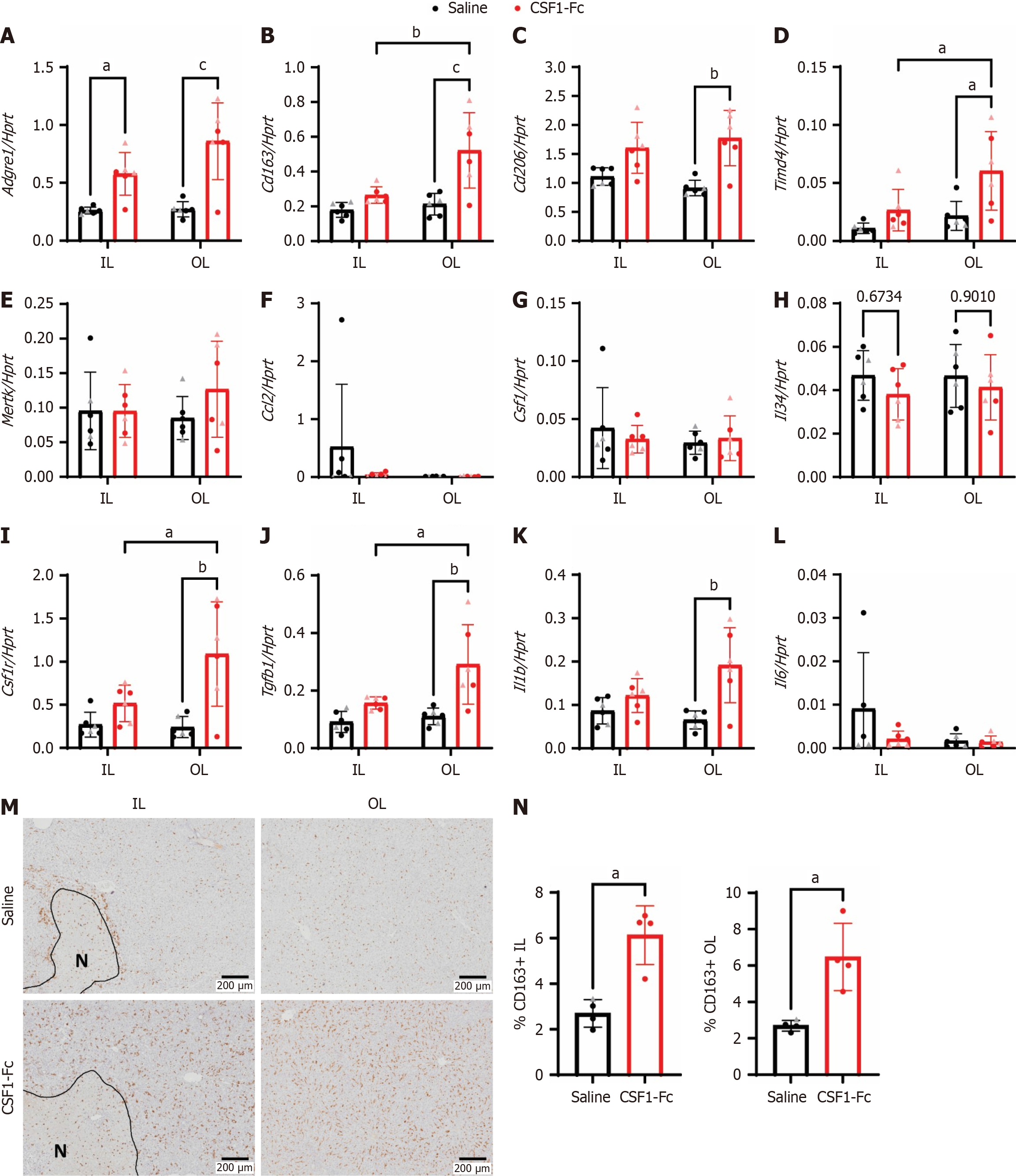
Figure 4 Perisurgical colony stimulating factor 1-Fc treatment increased macrophage and proinflammatory gene expression after liver ischemia-reperfusion.
A-L: Whole liver mRNA expression of Adgre1, Cd163, Cd206, Timd4, Mertk, Ccl2, Csf1, Il34, Csf1r, Tgfb1, Il1b and Il6 in the ischemic and oxygenated lobe 48 hours after ischemia-reperfusion; M: Representative immunohistochemistry images of CD163+ cells and necrosis outlined and indicated with ‘N’; N: Percentages of CD163+ cells were quantified, n = 4-6 for all groups and results were analysed with ordinary two-way ANOVA with Tukey’s multiple comparisons for A-L and Mann Whitney test for N. aP < 0.05, bP < 0.01, cP < 0.0001. Data show mean and standard deviation. IL: Ischemic lobe; OL: Oxygenated lobe; CSF: Colony stimulating factor.
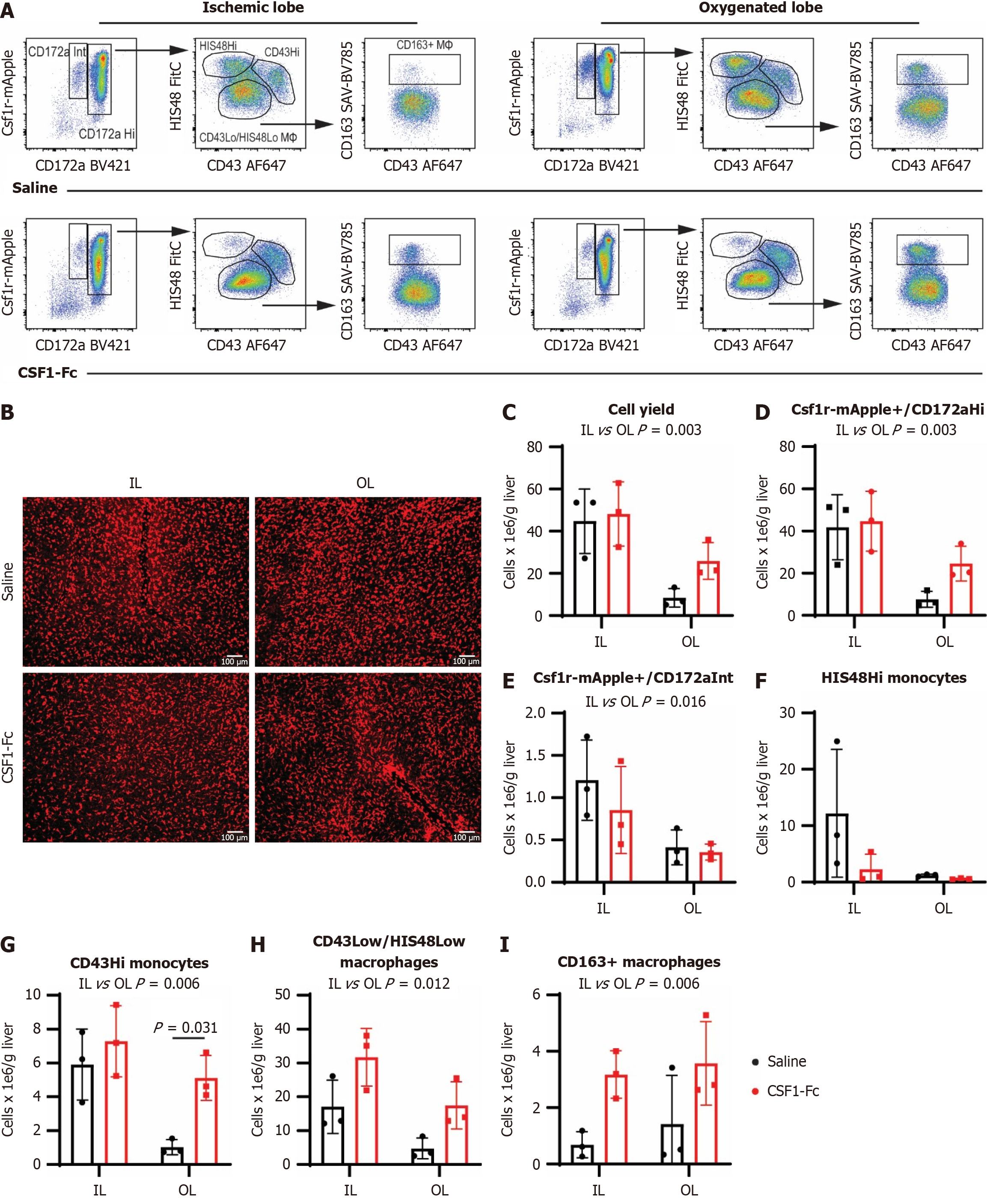
Figure 5 Colony stimulating factor 1-Fc mobilises non-classical monocytes into the liver and increases CD163+ macrophages.
Non-parenchymal cells were isolated from disaggregated livers harvested from Csf1r-mApple rats 48 hours post ischemia-reperfusion (I/R). A: Csf1r-mApple expression and CD172a (SIRPa) were used to gate myeloid cells, and further subdivided into CH172Hi (monocytes and macrophages) and CD172Intermediate (neutrophils); B: Representative whole-mount imaging of fresh unfixed tissues from Csf1r-mApple transgenic rats 48 hours post I/R using a spinning disc confocal microscope, n = 3 for all groups and results were analysed with ordinary two-way ANOVA with Tukey’s multiple comparisons for B-H; C-I: CD43 low/HIS48 low cells were gated as macrophages, HIS48Hi as classical monocytes and CD43Hi as non-classical monocytes. The liver macrophage marker CD163 was expressed on a subset of CD43 low/HIS48 low cells and the impact of colony stimulating factor 1 treatment on cell yield and the different subpopulations in the ischemic and oxygenated lobes were quantified; Data show mean and standard deviation. IL: Ischemic lobe; OL: Oxygenated lobe; CSF: Colony stimulating factor.
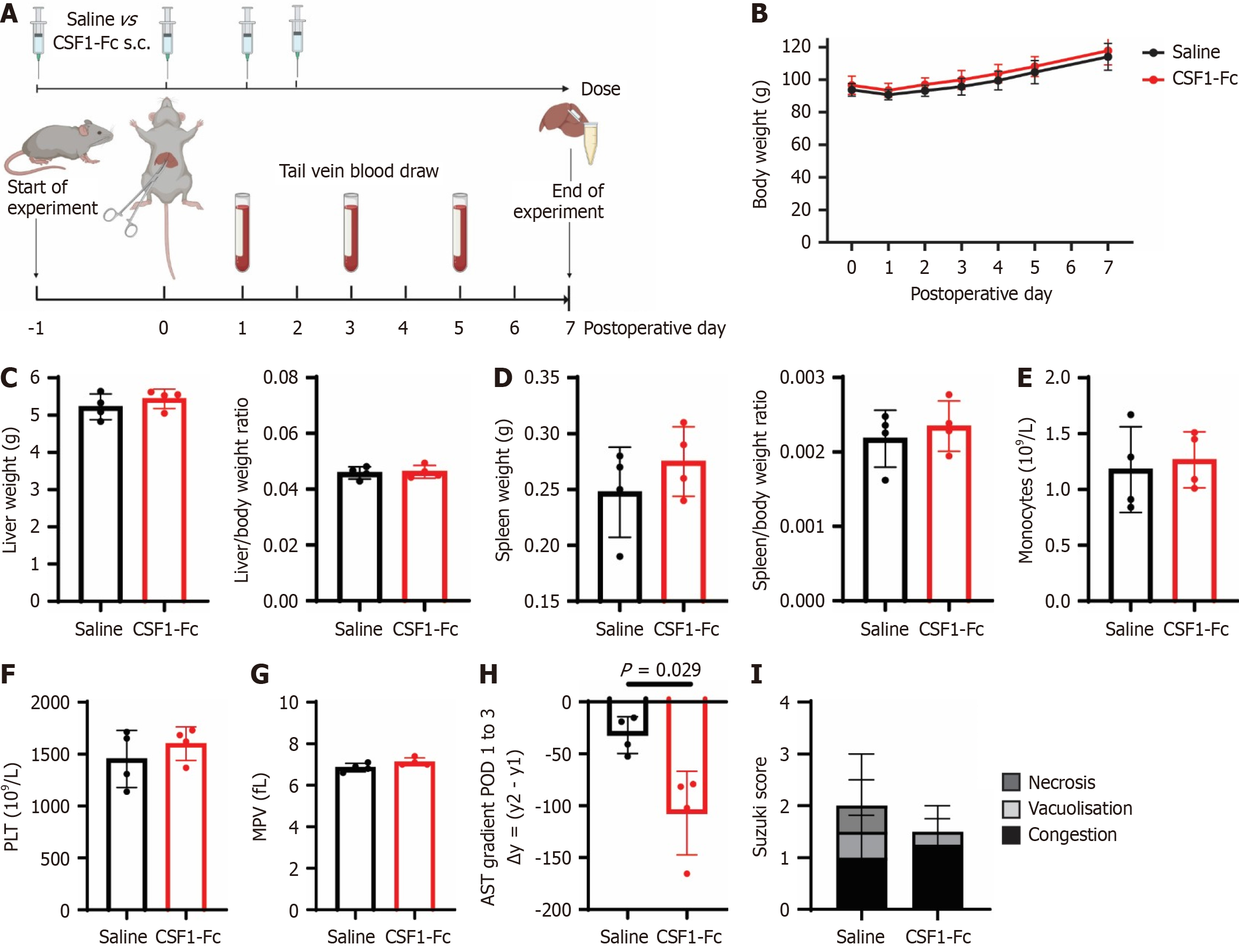
Figure 6 Colony stimulating factor 1-Fc treatment accelerates aspartate aminotransferase decline after ischemia-reperfusion injury.
A: Groups of male rats were subjected to 60 minutes of ischemia, followed by 7 days of reperfusion and treated with 4 × daily doses of 1 mg/kg of colony stimulating factor 1-Fc or saline control at postoperative day (POD) -1, 0, 1 and 2; B-D: Postoperative body weight (B), total liver weight, liver/body weight ratio (C) and total spleen and spleen/body weight ratio (D) were measured; E-G: Monocyte count (E), platelets (F) and mean platelet volume (G) in the peripheral blood were quantified; H: Aspartate aminotransferase gradient between POD 1 to 3; I: Suzuki scores on POD 7. n = 4 for all groups, results were analysed using the Mann Whitney test. Data show mean and standard deviation. Created in BioRender (Supplementary material). s.c.: Subcutaneous; CSF: Colony stimulating factor; PLT: Platelet; MPV: Mean platelet volume.
- Citation: Schulze S, Keshvari S, Miller GC, Bridle KR, Hume DA, Irvine KM. Perisurgical colony stimulating factor one treatment ameliorates liver ischaemia/reperfusion injury in rats. World J Gastroenterol 2025; 31(24): 108234
- URL: https://www.wjgnet.com/1007-9327/full/v31/i24/108234.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i24.108234