Published online Jul 26, 2021. doi: 10.12998/wjcc.v9.i21.6032
Peer-review started: March 15, 2021
First decision: April 4, 2021
Revised: April 14, 2021
Accepted: May 24, 2021
Article in press: May 24, 2021
Published online: July 26, 2021
Processing time: 127 Days and 21.3 Hours
Rosai–Dorfman disease (RDD) is a rare histiocytic proliferation of unknown etiology commonly found in children and adolescents. The common manifestation of RDD is massive and painless bilateral cervical lymphadenopathy with extranodal disease. While extranodal involvement in RDD is common, the spleen is an infrequent site of disease.
We report a 10-mo-old female infant with RDD presenting multiple splenic masses without cervical lymphadenopathy. She had fever, and blood tests showed leukocytosis, anemia, and elevated erythrocyte sedimentation rate and C-reactive protein. Ultrasound, computed tomography, and magnetic resonance images demonstrated multiple splenic masses. Despite antibiotic therapy, her symptoms were not relived. She underwent diagnostic splenectomy and was discharged with recovery.
In pediatric patients with refractory infectious symptoms or hematological abnormalities, clinicians should suspect RDD, even in patients without significant lymphadenopathy.
Core Tip: Rosai–Dorfman disease (RDD) is a rare histiocytic proliferation of unknown etiology, characterized by massive and painless bilateral cervical lymphadenopathy accompanied by fever, commonly found in children and adolescents. The spleen is an infrequent site of extranodal involvement in RDD. We report a 10-mo-old girl with RDD with extranodal involvement of the spleen without significant cervical lympha
- Citation: Ryu H, Hwang JY, Kim YW, Kim TU, Jang JY, Park SE, Yang EJ, Shin DH. Rosai-Dorfman disease in the spleen of a pediatric patient: A case report. World J Clin Cases 2021; 9(21): 6032-6040
- URL: https://www.wjgnet.com/2307-8960/full/v9/i21/6032.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i21.6032
Rosai–Dorfman disease (RDD), also called sinus histiocytosis with massive lymphadenopathy, is a rare histiocytic proliferation of unknown etiology, usually occurring in children and adolescents. It is characterized by massive and painless bilateral cervical lymphadenopathy accompanied by fever, leukocytosis, elevated erythrocyte sedimentation rate, and polyclonal hypergammaglobulinemia[1]. More than 40% of patients have an extranodal involvement, and commonly affected sites are the upper respiratory system, skin, eyes, bones, genitourinary system, oral cavity, central nervous system, and soft tissue[2,3]. The spleen is an infrequent site of disease, and if involved, combined nodal and extranodal disease is more frequent. Here we report a case of a 10-mo-old female infant with RDD with extranodal involvement of the spleen and provide a review of the available literature on extranodal RDD involvement in pediatric patients.
A 10-mo-old female infant visited our hospital due to fever.
The patient had persistent fever during the previous 10 d. Her body temperature was elevated to 39 ℃. The patient did not have any respiratory or gastrointestinal symptoms, except for mild rhinorrhea.
The patient had a free previous medical history.
No notable family history was found.
Physical examination showed no abnormal findings.
At presentation, blood tests showed leukocytosis (leukocyte count, 25.29 × 106/L), anemia (hemoglobin, 9.3 g/dL), a high erythrocyte sedimentation rate (ESR) (72 mm/h), and an elevated C-reactive protein level (CRP) (11.04 mg/dL). Peripheral blood smear showed normocytic normochromic anemia with spherocytes and tear drop cells and leukocytosis with monocytosis, basophilia, and neutrophilia.
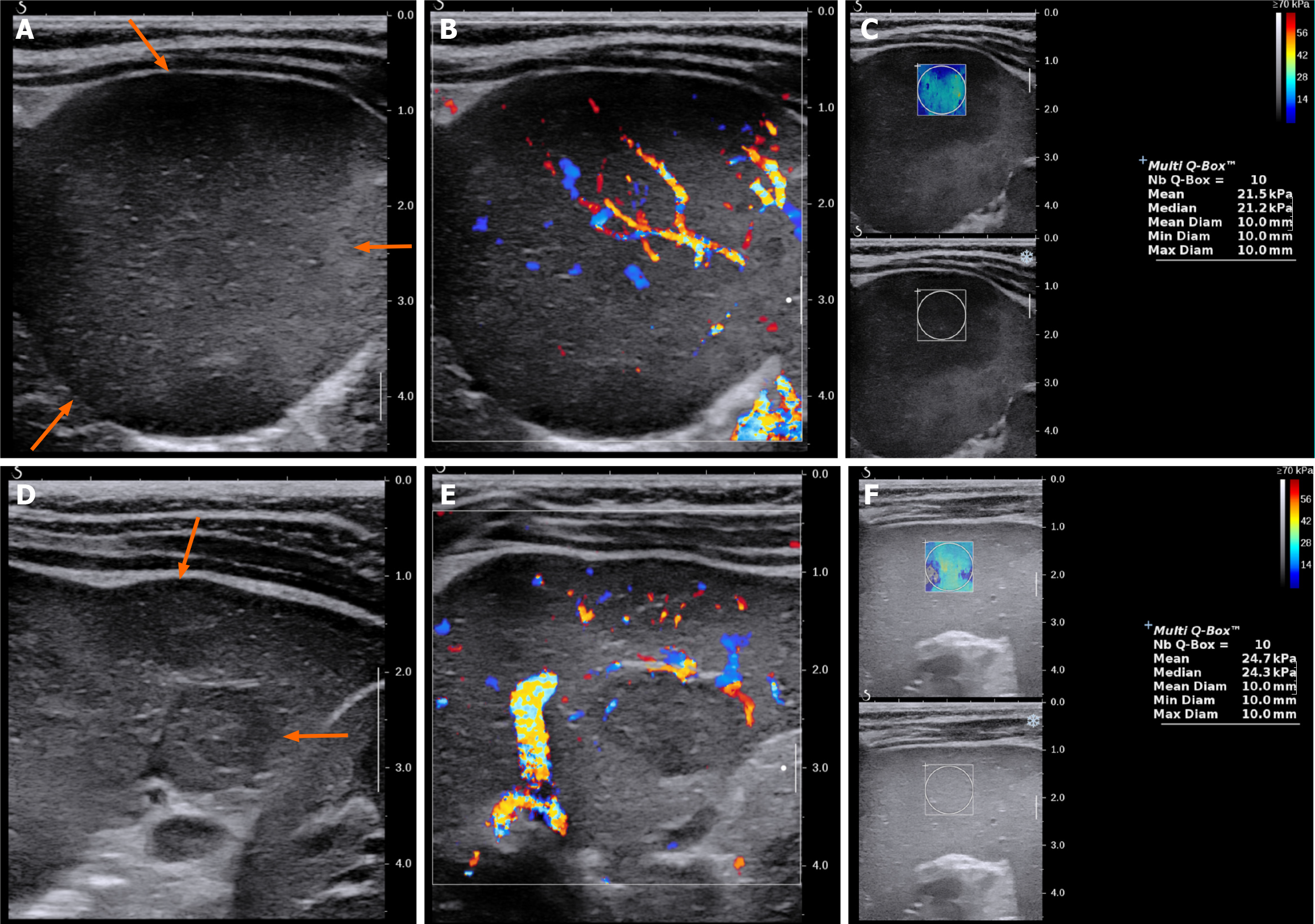
On plain radiography, an approximately 4-cm mass was observed in the left upper quadrant of the abdomen with displacement of the bowel loops. Ultrasonography showed two hypoechoic solid masses in the spleen with diameters between 1 cm and 1.5 cm. The lower pole of the spleen was enlarged, forming a 4.5-cm solid lump, which contained irregular and well-defined hypoechoic areas with intervening normal spleen tissue. The lesions did not show cystic portions or necrotic changes, but showed similar vascularity to the spleen on both color Doppler study and superb micro
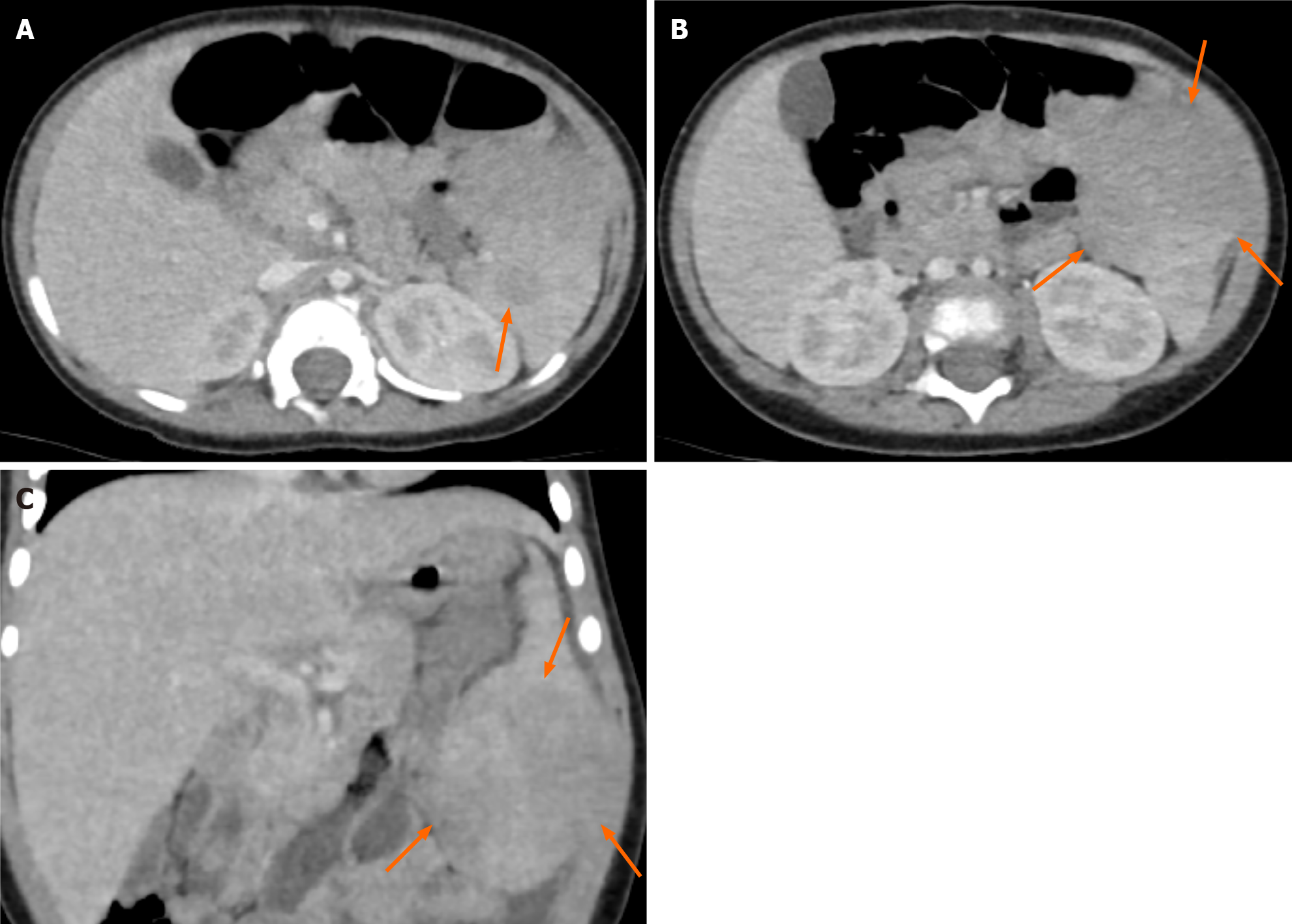
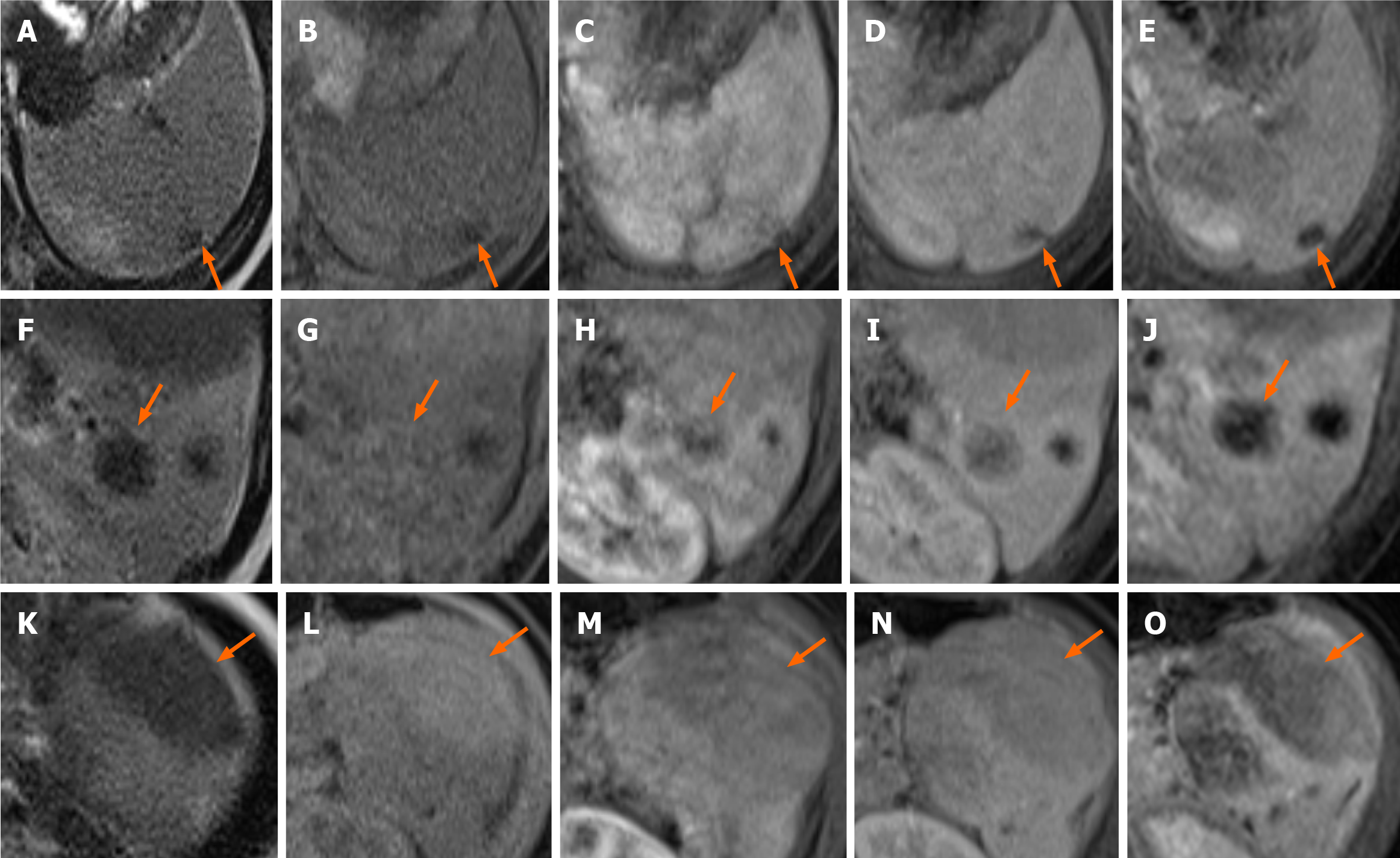
Contrast-enhanced computed tomography (CT) of the abdomen obtained in the portal venous phase showed hypo-enhanced splenic masses. The exophytic lesion at the inferior pole of the spleen showed heterogeneous enhancement with low attenuation and isoattenuation to the spleen. No significant lymphadenopathy was observed in the abdominal and pelvic cavities (Figure 2). Magnetic resonance imaging (MRI) was performed for further characterization of the masses. On T2-weighted images, the lesions showed low signal intensity to the spleen. However, on T1-weighted images, the lesions showed variable signal intensities: three lesions showed low signal intensity, one lesion showed iso signal intensity, and two lesions showed high signal intensity. After gadolinium administration, the lesions showed low signal intensity with peripheral enhancement in both the arterial and portal venous phases. The lesions showed persistent low signal intensity until 5 min after gadolinium administration (Figure 3).
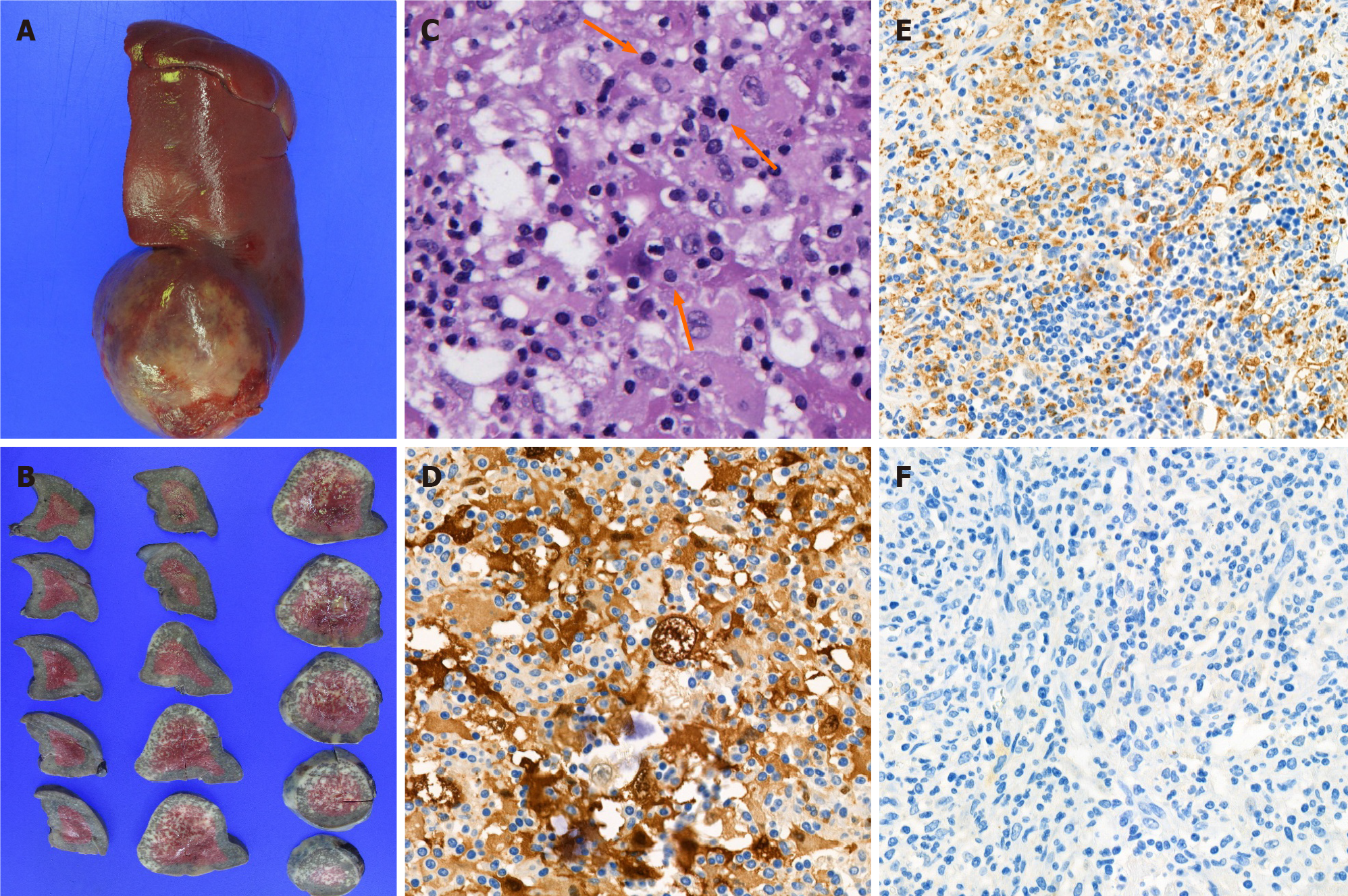
Histopathology showed histiocytic proliferation with emperipolesis in an ill-defined ovoid splenic mass and splenic hilar lymph nodes. Immunohistochemistry revealed that the proliferating histiocytes were positive for CD68 antigen and S-100 protein, but negative for CD1a (Figure 4). These findings were consistent with RDD. Epstein–Barr virus (EBV) was not detected in the specimen using EBV in situ hybridization for EBV-encoded RNA.
The patient was diagnosed with extranodal involvement of RDD in the spleen.
Although 1 mg of piperacillin-tazobactam were administered three times daily for five days, the patient had persistent fever and anemia. Considering laboratory and radiological findings, diagnostic splenectomy was performed. Following a laparotomy, a slightly hard and round-shaped exophytic mass approximately 6 cm in size was found at the inferior pole of the spleen, and a few enlarged lymph nodes around the splenic hilum and pancreas tail and along the gastroepiploic vessels were found. After splenectomy, one accessory spleen and a few enlarged lymph nodes along the gastoepiploic vessels were found and retrieved.
The patient’s symptoms, including fever, anemia, and leukocytosis, improved after splenectomy. The patient was discharged 14 d after the operation. At one month follow-up, the patient had no fever or other discomfort. The lab findings including leukocyte count, hemoglobin, ESR and CRP were within normal limit. The patient underwent contrast-enhanced CT exams one month, six months, and two year surgery. Those examinations demonstrated no abnormal finding except splenectomy state.
Histiocytoses are a group of hematological disorders characterized by pathological mononuclear phagocytic cell infiltration of normal tissues. Mononuclear phagocytes can be classified into two major classes: macrophages and dendritic cells. The World Health Organization has classified histiocytic disorders into three classes based on pathological cells: dendritic cell histiocytoses, non-dendritic cell histiocytoses, and malignant histiocytoses[4]. RDD, originally named sinus histiocytosis with massive lymphadenopathy, is a rare histiocytic disorder, but is the most common non-dendritic cell histiocytosis[5]. RDD was initially described by Rosai and Dorfman in 1969; however, its exact etiology and pathogenesis remained unknown. Some studies have proposed that RDD is a type of chronic inflammatory lymphoid hyperplasia due to antigenic stimuli, such as malignant tumors and acute lymphadenitis disorder[6,7]. Lu et al[8] have reported four cases in which RDD and malignant lymphoma were confirmed in the same lymph node. di Dio et al[9] have reported one case in which RDD and EBV infection were diagnosed in the same biopsied cervical lymph node specimen. In addition, Arakaki et al[5] have reported human herpesvirus 6 detection in a biopsied RDD renal sample. However, here, the patient had no simultaneous hematological disorder or definite infection.
RDD affects males more frequently than females[10] and commonly occurs in the first two decades of life[11]. The most commonly affected site is the lymph nodes, and the most common clinical symptom is painless and isolated lymphadenopathy[12]. Over 90% of patients with RDD present with cervical lymphadenopathy, but any group of lymph nodes can be invaded. Axillary, inguinal, mediastinal, hilar, and retroperitoneal lymph node involvements have been reported[9,10]. Extranodal involvement of RDD has been reported in up to 40% of all cases[2]; however, few cases are available in the literature. The most commonly affected extranodal sites are the skin and soft tissue. The involvement of the upper respiratory tract, eyes, salivary gland, central nervous system, bones, lungs, urogenital and gastrointestinal tracts, breast, thyroid, and heart has also been described[9,11-16]. Concurrent involvement of nodal and extranodal sites is more commonly observed. According to some studies, no correlation was observed between the type of RDD and clinical outcomes, but a significant difference was observed between the mean age of children and the type of RDD, with younger children more frequently affected by purely nodal RDD[9,17]. We summarized further case reports of RDD in pediatric patients in Table 1[4,5,7,9,12,14-16,18].
| Ref. | Age | Gender | Symptoms | Underlying disease | Nodal disease | Extranodal disease | Radiologic findings | |||
| Location | US | X-ray/CT | MRI | |||||||
| di Dioet al[9], 2016 | 14M | (-) | Swelling of the right parotid gland | Recent EBV infection | Bilateral cervical, hilar | Bilateral parotid gland | Multiple intraparotid LNs | Cystic and solid | (-) | Enhancement: Irregular |
| Rasool et al[4], 2015 | 15M | F | Pain and swelling of distal forearm | Not described | No | Physis of distal radius | Well-defined intramedullary lesion | (-) | Radiolucent lesion with scalloping and thin rim | (-) |
| Mantilla et al[12], 2016 | 2 | F | Proptosis, headache | H syndrome (SLC29A3 mutation) | No | Bilateral orbit | Numerous well-circumscribed intraorbital masses | (-) | (-) | T2WI: Moderately hyperintense; enhancement: Mild |
| Nandi et al[7], 2008 | 5 | M | Painless huge neck swelling, fever | HHV-6 infection | Bilateral cervical | No | (-) | (-) | (-) | |
| Nandi et al[7], 2008 | 5 | M | Bilateral proptosis, swelling over the mandibular area | No | Preauricular, mandibular | Bilateral orbit | Ill-defined irregular masses involving mainly intraconal parts | (-) | Enhancement: heterogeneous | (-) |
| Rodriguez-Galindo et al[14], 2004 | 6 | F | Orbital swelling, proptosis, and blindness | No | Ipsilateral cervical | Orbit | Retroconal and preseptal mass extended to cavernous sinus | (-) | (-) | Enhancement: Mild |
| Arakaki et al[5], 2012 | 7 | M | Abdominal pain and diarrhea | HHV-6 infection | No | Kidney | Mass at anterior portion of kidney | Homogeneous echogenecity | Soft tissue density; enhancement: Homogeneous | Enhancement: Homogeneous |
| Rodriguez-Galindo et al[14], 2004 | 9 | F | Left frontal swelling and tenderness | No | No | Skull | Well-defined lesions in frontal bone | Radiolucent lesions without sclerotic margin | (-) | |
| Sridhara et al[15], 2012 | 11 | M | Painless bilateral neck swelling | Recent EBV infection | No | Bilateral submandibular gland | Enlarged submandibular glands | (-) | Enhancement: Homogeneous | (-) |
| Tubbs et al[16], 2005 | 13 | M | Neck pain, headache, blurred vision | No | No | Right parietal bone | Intraosseous lesion | (-) | (-) | (-) |
| Suboccipital musculature | (-) | (-) | Enhancement: Mild | |||||||
| Rittner et al[18], 2012 | 15 | M | Fever, back and left hip pain | No | No | Multiple bone lesions (cranial bone, spine, pelvis, upper/lower extremities) | (-) | Lytic lesions | T1WI: Hypointense; T2WI: Hyperintense; enhancement: Strong | |
| Rodriguez-Galindo et al[14], 2004 | 15 | F | Headache, proptosis | Recent EBV infection | Mediastinal | Brain | Anterior and posterior falx | (-) | (-) | T1WI and T2WI: Isointense; Enhancement: Strong |
| Bilateral orbit | Retroconal, preseptal space | |||||||||
Few cases of splenic involvement of RDD have been reported to date. In the English literature, all patients were middle-aged women and presented with hematological symptoms, such as anemia and thrombocytopenia that improved after splenectomy[19,20]. To the best of our knowledge, splenic involvement of RDD in pediatric pa
Imaging examinations are not pathognomonic in the diagnosis of RDD because of their nonspecific findings. Furthermore, the appearance of extranodal RDD on imaging studies often mimics malignancy[12]. Therefore, biopsy is necessary to establish diagnosis[12,18]. In our case, CT images showed multiple hypodense masses in the spleen in accordance with other studies[19,20].
The definitive diagnosis of RDD can only be made by histological analysis. One of the pathognomonic findings is emperipolesis defined as the presence of phagocytized cells in a histiocyte. In immunohistochemical analysis, positivity for CD68 antigen and S-100 protein, but negativity for CD1a antigen is diagnostic for RDD. Emperipolesis can also be found in Langerhans cell histiocytosis (LCH), but the absence of Birbeck granules and negativity for CD1a antigen help differentiate RDD from LCH[9,12].
The clinical course of RDD is benign and may regress spontaneously. Given that fact, observation may be a good treatment option. However, treatment is required in cases of significant extranodal disease because locoregional recurrence or even dissemination can be possible. Compression of vital organs or severe constitutional symptoms, such as fever, is also indications for therapy[7,9]. Although there is no consensus for the optimum treatment option, surgery is probably the best curative treatment if complete resection is possible. In addition, medical therapy, including corticosteroids, antibiotics, antiviral agents, chemotherapy, and radiotherapy, is available[9].
In conclusion, although splenic involvement is rare, especially in pediatric patients with RDD, RDD should be considered a differential diagnosis, even in patients without significant lymphadenopathy, especially in the setting of hematological abnormality or infectious symptoms.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wen J S-Editor: Zhang H L-Editor: A P-Editor: Wang LYT
| 1. | Gupta P, Babyn P. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): a clinicoradiological profile of three cases including two with skeletal disease. Pediatr Radiol. 2008;38:721-8; quiz 821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Zhu F, Zhang JT, Xing XW, Wang DJ, Zhu RY, Zhang Q, Wang HT, Lang SY. Rosai-Dorfman disease: a retrospective analysis of 13 cases. Am J Med Sci. 2013;345:200-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Adeleye AO, Amir G, Fraifeld S, Shoshan Y, Umansky F, Spektor S. Diagnosis and management of Rosai-Dorfman disease involving the central nervous system. Neurol Res. 2010;32:572-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 4. | Rasool MN, Genzengane V. Rosai-Dorfman disease of the distal radius in a child: A case report and review of the literature. SA Orthopaedic Journa. 2015;14:67-70. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Arakaki N, Gallo G, Majluf R, Diez B, Arias E, Riudavets MA, Sevlever G. Extranodal rosai-dorfman disease presenting as a solitary mass with human herpesvirus 6 detection in a pediatric patient. Pediatr Dev Pathol. 2012;15:324-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Ambati S, Chamyan G, Restrepo R, Escalon E, Fort J, Pefkarou A, Khatib ZA, Dehner LP. Rosai-Dorfman disease following bone marrow transplantation for pre-B cell acute lymphoblastic leukemia. Pediatr Blood Cancer. 2008;51:433-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Nandi M, Mondal RK, Datta S, Karmakar BC, Mukherjee K, Dhibar TK. Rosai-Dorfman disease. Indian J Pediatr. 2008;75:290-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Lu D, Estalilla OC, Manning JT Jr, Medeiros LJ. Sinus histiocytosis with massive lymphadenopathy and malignant lymphoma involving the same lymph node: a report of four cases and review of the literature. Mod Pathol. 2000;13:414-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | di Dio F, Mariotti I, Coccolini E, Bruzzi P, Predieri B, Iughetti L. Unusual presentation of Rosai-Dorfman disease in a 14-month-old Italian child: a case report and review of the literature. BMC Pediatr. 2016;16:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Sodhi KS, Suri S, Nijhawan R, Kang M, Gautam V. Rosai-Dorfman disease: unusual cause of diffuse and massive retroperitoneal lymphadenopathy. Br J Radiol. 2005;78:845-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Foucar E, Rosai J, Dorfman R. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Semin Diagn Pathol. 1990;7:19-73. [PubMed] |
| 12. | Mantilla JG, Goldberg-Stein S, Wang Y. Extranodal Rosai-Dorfman Disease: Clinicopathologic Series of 10 Patients With Radiologic Correlation and Review of the Literature. Am J Clin Pathol. 2016;145:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Brenn T, Calonje E, Granter SR, Leonard N, Grayson W, Fletcher CD, McKee PH. Cutaneous rosai-dorfman disease is a distinct clinical entity. Am J Dermatopathol. 2002;24:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 136] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Rodriguez-Galindo C, Helton KJ, Sánchez ND, Rieman M, Jeng M, Wang W. Extranodal Rosai-Dorfman disease in children. J Pediatr Hematol Oncol. 2004;26:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Sridhara SK, Shah RK. Rosai-Dorfman in the submandibular salivary glands of a pediatric patient. Laryngoscope. 2010;120 Suppl 4:S228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 16. | Tubbs RS, Kelly DR, Mroczek-Musulman EC, Hammers YA, Berkow RL, Oakes WJ, Grabb PA. Spinal cord compression as a result of Rosai-Dorfman disease of the upper cervical spine in a child. Childs Nerv Syst. 2005;21:951-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Warrier R, Chauhan A, Jewan Y, Bansal S, Craver R. Rosai-Dorfman disease with central nervous system involvement. Clin Adv Hematol Oncol. 2012;10:196-198. [PubMed] |
| 18. | Rittner RE, Baumann U, Laenger F, Hartung D, Rosenthal H, Hueper K. Whole-body diffusion-weighted MRI in a case of Rosai-Dorfman disease with exclusive multifocal skeletal involvement. Skeletal Radiol. 2012;41:709-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Ha H, Kim KH, Ahn YJ, Kim JH, Kim JE, Yoon SS. A rare case of Rosai-Dorfman disease without lymphadenopathy. Korean J Intern Med. 2016;31:802-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Knox SK, Kurtin PJ, Steensma DP. Isolated splenic sinus histiocytosis (Rosai-Dorfman disease) in association with myelodysplastic syndrome. J Clin Oncol. 2006;24:4027-4028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |