Published online Apr 16, 2021. doi: 10.12998/wjcc.v9.i11.2584
Peer-review started: December 14, 2020
First decision: December 30, 2020
Revised: January 4, 2021
Accepted: February 9, 2021
Article in press: February 9, 2021
Published online: April 16, 2021
Processing time: 109 Days and 8.7 Hours
Emphysematous pyelonephritis (EPN) is a rare but fatal necrotic infection of the kidney, which usually leads to septic shock. Therefore, early diagnosis and optimized therapy are of paramount importance. In the past two decades, point-of-care ultrasound (POCUS) has been widely used in clinical practice, especially in emergency and critical care settings, and helps to rapidly identify the source of infection in sepsis. We report a rare case in which a “falls” sign on POCUS played a pivotal role in the early diagnosis of EPN.
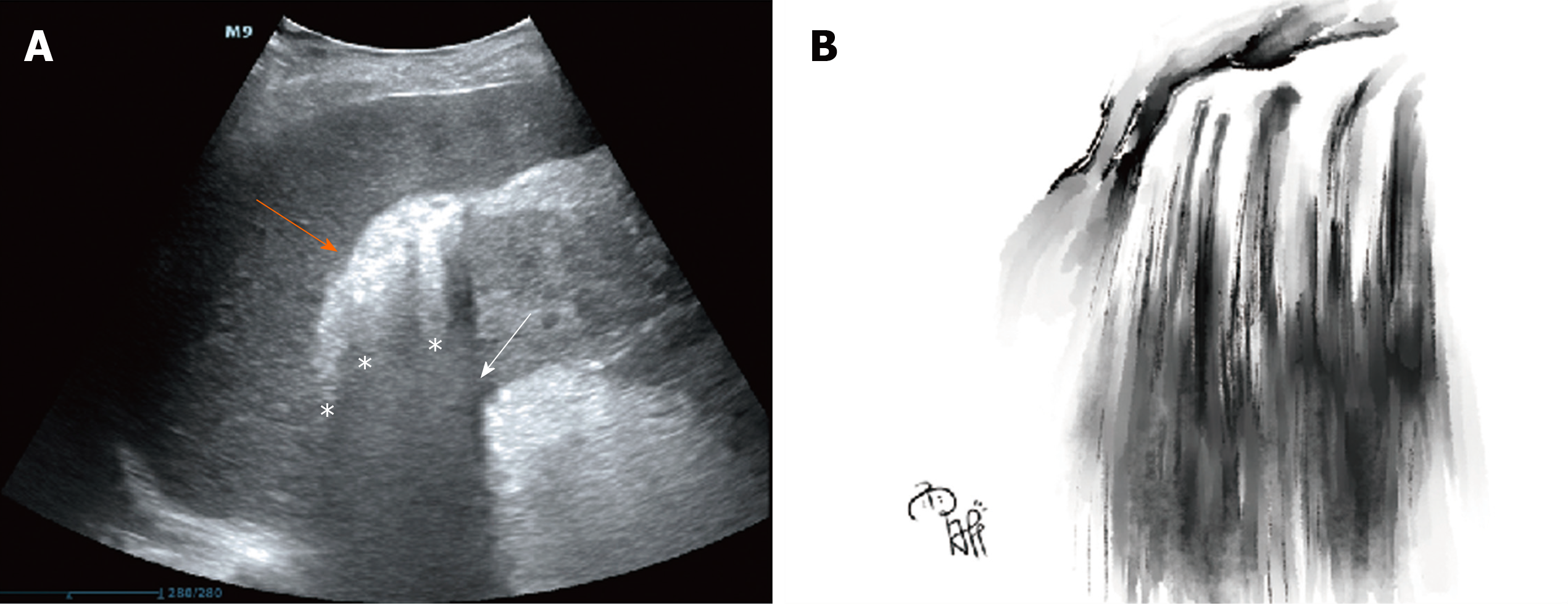
A 57-year-old man presented with fever and lumbago for 3 d prior to admission. He went to the emergency room, and the initial POCUS detected gas bubbles in the hepatorenal space showing a hyperechoic focus with dirty shadowing and comet-tail artifacts. This imaging feature was like a mini waterfall. His blood and urine culture demonstrated Escherichia coli bacteremia, and EPN associated with septic shock was diagnosed. The patient did not respond to broad-spectrum antibiotic treatment and a perirenal abscess developed. He subsequently underwent computed tomography-guided percutaneous catheter drainage, and fully recovered. We also review the literature on the sonographic features of POCUS in EPN.
This case indicates that a “falls” sign on POCUS facilitates the rapid diagnosis of severe EPN at the bedside.
Core Tip: Emphysematous pyelonephritis (EPN) is a rare but life-threatening infection, and its diagnosis and treatment remain challenging. Point-of-care ultrasound (POCUS) plays an important role in rapidly assessing critically ill patients at the bedside. Here, we report a “falls” sign on the initial POCUS examination in a patient diagnosed with EPN associated with septic shock. We suggest that the “falls” sign may act as an imaging feature for early diagnosis of EPN. The patient was successfully treated with computed tomography-guided percutaneous catheter drainage plus broad-spectrum antibiotic therapy.
- Citation: Xing ZX, Yang H, Zhang W, Wang Y, Wang CS, Chen T, Chen HJ. Point-of-care ultrasound for the early diagnosis of emphysematous pyelonephritis: A case report and literature review . World J Clin Cases 2021; 9(11): 2584-2594
- URL: https://www.wjgnet.com/2307-8960/full/v9/i11/2584.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i11.2584
Emphysematous pyelonephritis (EPN) is a lethal necrotic infection of the kidney with the key features of a collection of gas in the renal parenchyma, collecting system, as well as perinephric tissues[1]. More than 60% of patients with EPN have poorly controlled diabetes mellitus[2]. EPN usually presents with a fulminant clinical course and leads to sepsis and septic shock. Misdiagnosis of EPN and delayed management are associated with a mortality rate up to 80%[3]. Over the past decade, computed tomography (CT)-guided percutaneous catheter drainage (PCD), advanced antibiotic therapy, and intensive care medicine have improved the clinical outcome with a decreased mortality rate of 21%[4].
To date, point-of-care ultrasound (POCUS) is widely used in day-to-day clinical practice[5]. It seems more important in emergency and critical care where radiological examinations are time consuming or unavailable. POCUS has been defined as “the new stethoscope” challenging traditional diagnostic practice[6]. Sepsis is life-threatening organ dysfunction induced by infection, which remains a global health priority[7]. Bedside POCUS can be used to rapidly assess major organs, and helps to identify a septic source, especially acute pyelonephritis, and to speed up the diagnosis[8,9].
In the present case, we report a “falls” sign on POCUS examination, which contributed to the early diagnosis of EPN. We discuss the use of POCUS in EPN and review the relevant literature. To the best of our knowledge, this is the first study to report the “falls” sign in EPN and to systematically discuss the POCUS features of EPN.
A 57-year-old Chinese man complained of fever and lumbago for the last 3 d.
The patient presented to the emergency room with a history of sudden onset persistent right flank pain, fever and fatigue for 3 d. POCUS was performed immediately and EPN was initially diagnosed. The patient was transferred to the intensive care unit (ICU) due to septic shock and an abdominal CT scan was carried out.
The patient had a 10-year history of poorly controlled diabetes.
The patient had a 30-year history of smoking and drinking, which he had recently stopped.
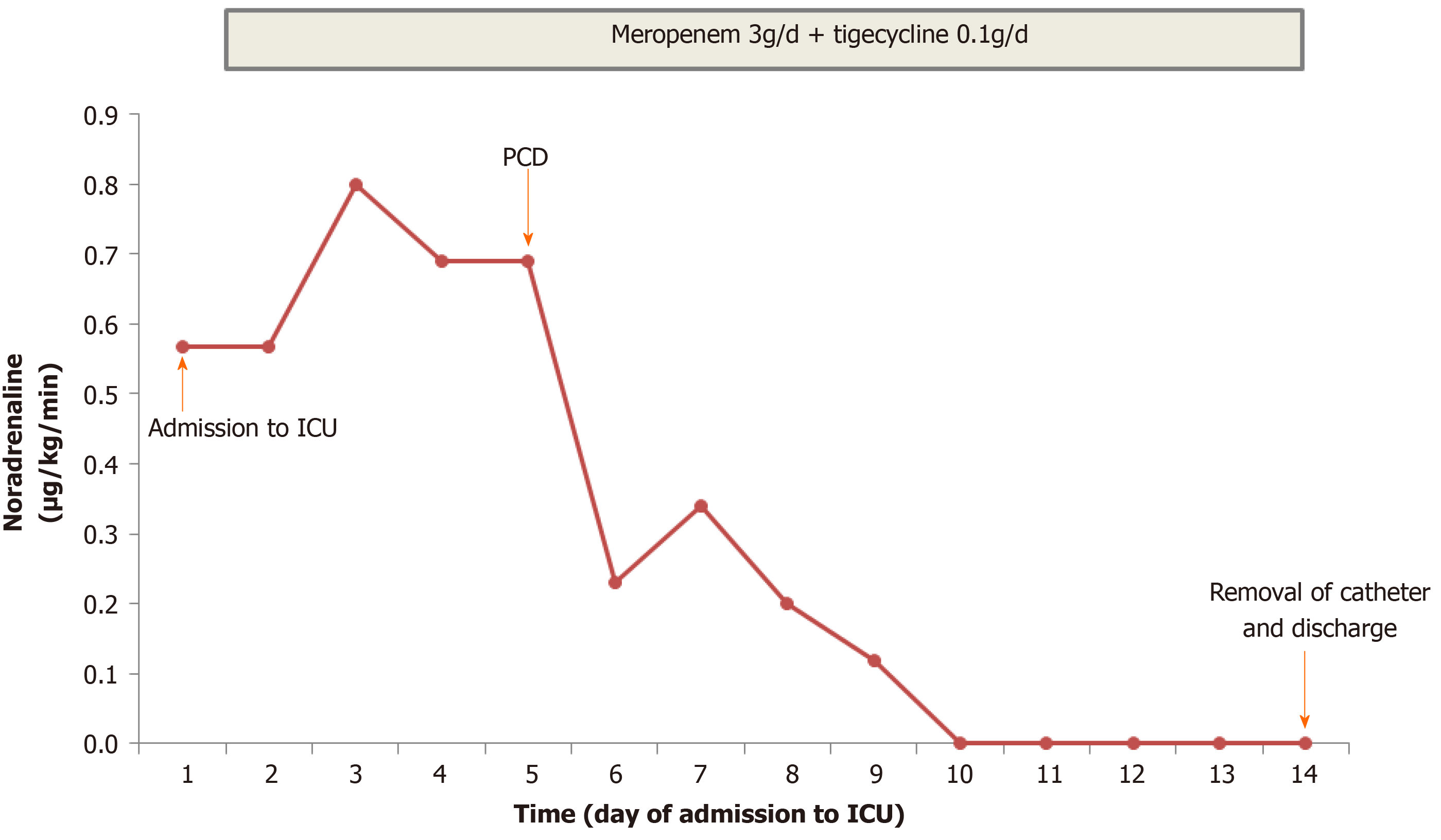
On admission to the ICU, physical examination revealed a temperature of 38.8 °C, heart rate of 130 bpm, and blood pressure of 108/74 mmHg with a moderate dose of continuously pumped norepinephrine (0.56 μg/kg/min) and respiratory rate of 22 breaths/min. His heart beat fast without murmurs and lungs sounded clear without crackles. His abdomen was soft and was not tender. He had severe knocking tenderness in the right flank. These findings indicated septic shock provoked by acute pyelonephritis.
Table 1 shows the initial laboratory findings. Blood analysis revealed leukocytosis of 10.37 × 109/L with neutrophils of 81%, hemoglobin of 11.9 g/dL, and thrombocytopenia (platelet count 69 × 109 /L) induced by sepsis. Alanine aminotrans
| Variables | Results | Normal range |
| White blood cells | 10.37 × 109/L | 4-10 × 109/L |
| Percentage of neutrophils | 81% | 50%-70% |
| Hemoglobin | 11.9 g/dL | 11.5-15 g/dL |
| Platelets | 69 × 109/L | 100-300 × 109/L |
| Alanine aminotransferase | 21 IU/L | 9-50 IU/L |
| Aspartate aminotransferase | 23 IU/L | 15-40 IU/L |
| Total bilirubin | 0.58 mg/dL | 0.29-1.2 mg/dL |
| Creatinine | 1.66 mg/dL | 0.3-1.0 mg/dL |
| CRP | 175.1 mg/L | 0.068-8.2 mg/L |
| PCT | > 100 ng/mL | < 0.05 ng/mL |
| Glycosylated hemoglobin | 9% | 4%-6% |
| Urine white blood cells | 325/μL | 0-5/μL |
Emergency POCUS on day 3 after symptom onset showed hyperechoic spotted or patchy foci in the right hepatorenal space with dirty shadowing and comet-tail artifacts (Figure 1A). We called this imaging feature a “falls” sign to describe the shadowing and “comet tails” radiating from the gas gathering in the hepatorenal space. It also presented a mini waterfall in Chinese landscape painting style (Figure 1B). The typical imaging findings speeded up the initial diagnosis of EPN.
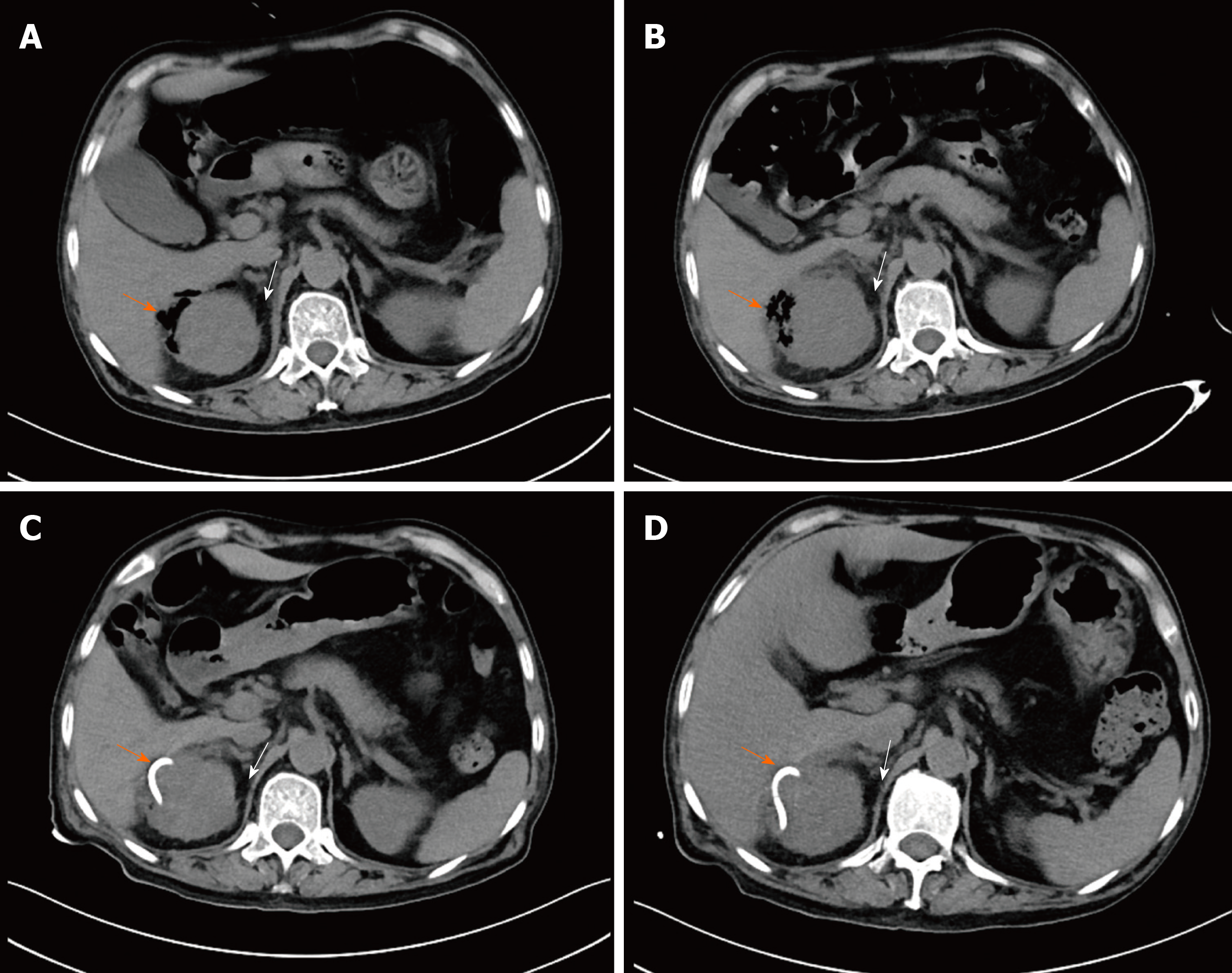
An abdominal CT scan on day 3 after symptom onset revealed gas collection in the right perirenal space, an enlarged right kidney with perinephric fat stranding (PFS) (Figure 2A) and mild right hydronephrosis without urinary stones. The CT scan confirmed the initial diagnosis of EPN based on emergency POCUS.
EPN associated with septic shock was the final diagnosis based on symptoms, physical examination, and imaging findings. Gas in the right perirenal space may result from necrotic pancreatitis and extraperitoneal hollow organ perforation, such as perforation of the descending duodenum[10,11]. The patient had a soft abdomen without symptoms of enteroparalysis, and further CT scan showed upper urinary tract infection. Hence, duodenal perforation and necrotic pancreatitis were unlikely to be the cause of gas in the right perirenal space.
The clinical course and vasopressor doses are shown in Figure 3. On admission to the ICU, the patient received fluid resuscitation, insulin infusion, vasopressor support, and 14 d of broad-spectrum antibiotic therapy including meropenem (3 g/d) and tigecycline (0.1 g/d). Septic shock did not respond to the initial therapy. A repeat CT scan was performed on day 7 after symptom onset (Figure 2B), and showed a more enlarged kidney with more PFS and gas plus an abscess in the right perirenal space. A urological and interventional radiological consultation was obtained, and urgent CT-guided PCD was recommended for the patient on day 5 after admission. The culture from pus also yielded E. coli bacteremia. Double-J catheter (DJB) stenting was not advocated due to mild hydronephrosis of the right kidney and the absence of urinary stones.
As shown in Figure 3, PCD associated with antibiotic therapy successfully reversed the clinical course. His clinical condition improved noticeably, and norepinephrine was discontinued within 5 d after initiating the combination therapy. CT reexaminations on days 9 and 11 after symptom onset (Figure 2C and D) revealed the pig-tail catheter in the right perirenal space and gas and abscess absorption. The patient was asymptomatic with a normal serum creatinine level and platelet count. The perirenal catheter was removed, and the patient was discharged with a 7 d course of oral levofloxacin (400 mg/d) on day 14 after admission. At 2 wk after discharge, a repeat urinary CT scan showed almost normal kidney imaging. The patient has been followed in an endocrinology clinic for his diabetes for 1.5 years. During follow-up, he remained healthy with stable blood glucose control and normal renal function. The patient was satisfied with his care.
EPN is a type of life-threatening upper urinary tract infection with a high mortality rate and the hallmark of the presence of gas[1]. It has become a challenge worldwide, especially in developing countries with poor health care access[12]. There is a growing amount of literature focusing on EPN; however, most is limited to case reports. The major predisposing factor of EPN is uncontrolled diabetes, which decreases renal tissues perfusion and impairs host immune response[13]. In addition, the hyperglycemic environment facilitates the growth of facultative anaerobes. The most common causative organism in EPN is facultative anaerobic Enterobacteriaceae, especially E. coli and Klebsiella pneumoniae, which is in common with urinary tract infections[14]. Gas is produced by the pathogenic organism via fermentation of glucose and lactate in necrotic tissues[14]. In addition to diabetes, other risk factors for EPN include obstructive nephropathy, urolithiasis, chronic renal failure, hypertension, and immunosuppression[15,16]. A CT scan is recommended for most patients with EPN during the clinical course[17].
In 2000, Huang published a pioneering clinicoradiological classification based on CT findings[18]. This classification has been assessed and used with widespread acceptance[12]. It classifies EPN into localized EPN (Classes 1 and 2) and extensive EPN (Classes 3 and 4), and shows the correlation between the class of EPN and its management[14,18]. Classes 1 and 2 indicate gas in the collecting system only and gas in the renal parenchyma only. Class 3A and B indicate the expansion of gas into the perinephric space and pararenal space, respectively. Class 4 indicates EPN in a solitary kidney or in bilateral kidneys[18]. In our case, the initial CT scan showed solitary gas in the right perirenal space only but no gas in renal parenchyma. The CT findings did not correspond with any of the classes in Huang’s radiologic classification. We suggest that EPN with solitary gas in the perirenal space is a special type of localized EPN, which can be successfully managed with PCD associated with antibiotics.
As this case shows, common clinical manifestations of EPN include fever, flank pain, pyuria and sepsis-associated presentations such as shock and thrombocyto
Huang has suggested that most Classes 1 and 2 EPN can be managed by PCD combined with antibiotic therapy, and Classes 3 and 4 EPN with a fulminant course (more than two risk factors) require nephrectomy[18]. However, there is increasing evidence to show that the priority of a more conservative approach decreases the mortality rate from 80% to 20%[13,32].With recent progress in medical care, most cases with extensive EPN can be successfully managed with PCD plus DJB stenting associated with antibiotic treatment[33-36]. Also, localized EPN responds well to antibiotic therapy alone with a good outcome[13,37]. A meta-analysis showed that emergency nephrectomy correlated with a higher mortality rate than a kidney-conserving therapeutic strategy[38]. Additionally, a standard management algorithm has been developed to optimize the treatment strategy to avoid aggressive nephrectomy[39]. Nephrectomy should be performed when there is no improvement with conservative therapy. As in our case, the patient with perinephric gas and abscess responds well to PCD plus aggressive antibiotic therapy. Prognostic factors for mortality in EPN include the need for hemodialysis, shock, altered mental status, thrombocytopenia, severe hypoalbuminemia and hyponatremia[36].
Although CT is the gold standard for diagnosing EPN[13,40], POCUS is portable and provides real-time information at the bedside without radiation exposure, and has become a promising tool facilitating rapid diagnosis in the past two decades[41]. The high acoustic impedance gradient between gas and renal tissues generates artifacts, which can be easily detected on POCUS at the bedside[42,43]. We performed a systematic literature search in PubMed using the key words “POCUS,” “point-of-care ultrasound,” “bedside ultrasound,” “emergency ultrasound,” “ultrasound,” and “emphysematous pyelonephritis.” A total of five other reports focusing on POCUS in EPN were identified[3,19,44-46] (Table 2). A hyperechoic focus with dirty acoustic shadowing is the most common sonographic feature on POCUS for the diagnosis of EPN[3,19,45]. However, other imaging features have also been reported, including poor delineation of the kidney, A-lines and B-lines[44-46]. Additionally, we report that the comet-tail artifacts and the “falls” sign are also imaging features on POCUS in EPN. But physicians should keep in mind that these air-related artifacts on POCUS vary in different cases. The variation not only results from multiple effects of gas bubbles such as volume, shape, position, and orientation, but also correlates with a mismatch of acoustic impedance between the gas bubbles and its surrounding renal tissues[43]. Moreover, the utility of POCUS remains a challenge as a result of its dependence on the skills and experience of the operators, especially non-imaging professionals[41]. So, we suggest that the standardization of the air-related artifacts on POCUS in EPN should be implemented on the basis of sufficient faculty training.
| Ref. | Age in yr | Sex | Diabetes/comorbidities | Class of EPN | Treatment strategy | Outcome | Location of gas on ultrasound | POCUS features |
| McCafferty et al[3], 2017 | 84 | Woman | Diabetes/CKD/hypertension | Class 2 | MM + nephrectomy | Recovered | Renal cortex | Hyperechoic focus/dirty shadowing |
| Stone et al[19], 2005 | 47 | Woman | Diabetes | Class 3A | MM + nephrectomy | Death | Renal parenchyma | Echogenic foci/dirty shadowing |
| Peng et al[44], 2017 | 68 | Woman | Diabetes | Class 3A | MM + nephrectomy | Recovered | Perirenal space | Poor delineation of the kidney |
| Koratala et al[45], 2019 | 22 | Woman | Diabetes | NM | NM | NM | Renal parenchyma | Hyperechoic focus/dirty shadowing/B-lines |
| Brown et al[46], 2019 | 60 | Man | Diabetes | Class 3A | MM + PCD + DBJ stenting | Recovered | Renal parenchyma/collecting system | A-lines |
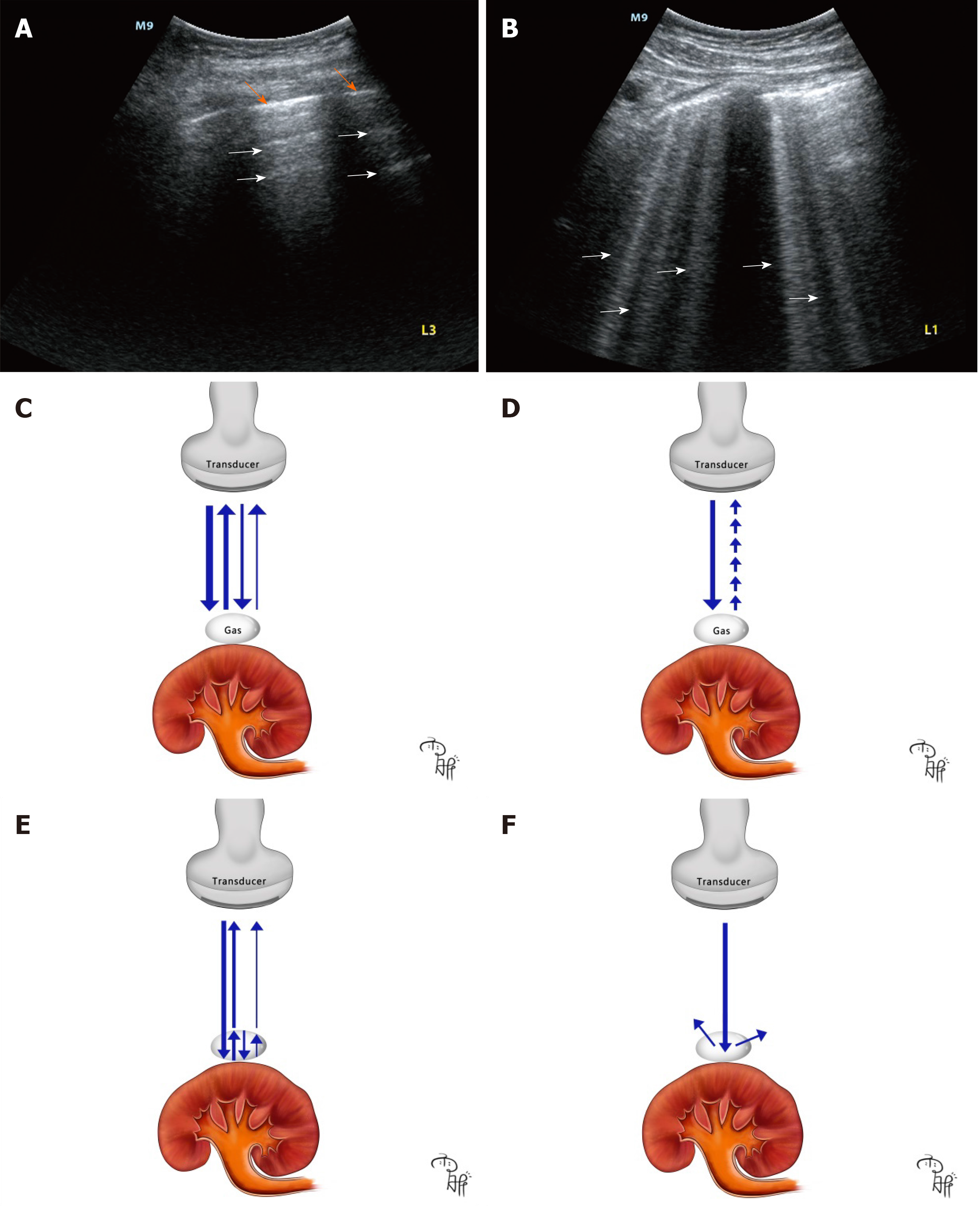
Air surrounding the perirenal space prevents the transduction of sound waves resulting in artifacts, decreased visualization of deeper structures and an obscure outline of the kidney[43]. A-lines (Figure 4A) and B-lines (Figure 4B) are basic signs on lung ultrasound for the diagnosis of acute respiratory failure[47]. Both are artifacts generated when air is struck by ultrasound beams. A-lines are repetitive horizontal artifacts derived by repetitive reflection from the tissue-gas interface to the transducer (Figure 4C)[48]. B-lines are well defined, vertical, laser-like artifacts, and are generated by a ring down effect when the sound waves pass through gas bubbles associated with fluid collection, and provokes resonance within the air-fluid interface, emitting continuous waves back to the transducer (Figure 4D)[49]. A comet-tail artifact is produced when ultrasound beams are repeatedly reflecting on the shallow and deep sides of gas bubbles (Figure 4E)[50], which usually looks like an inverted triangular hyperechoic lesion with reduced thickness and strength (Figure 1A).
Acoustic shadowing is a significantly reduced posterior echo, and it occurs when ultrasound waves pass through strongly reflecting or attenuating structures such as gas, bone, needles, calcifications and stones[51]. The “falls” sign should be differentiated between perirenal gas and perirenal calcification or renal wall calcification, which is non-specific pathology in renal wall tuberculosis[52], perirenal tumors, polycystic kidney disease and very rare diseases such as Erdheim-Chester disease and tumoral calcinosis[53-56]. In most cases, perirenal calcification and urinary stones present with clean shadowing which is an absolute anechoic band. However, gas in EPN generates dirty shadowing which is a heterogeneous echoic band with reduced signal intensity[45] (Figure 1A). Previously, it was thought that clean shadowing was associated with sound-absorbing materials, such as stones, and dirty shadowing results from sound-reflecting materials, such as gas. However, studies have indicated that clean shadowing and dirty shadowing in essence correlate with the properties of the surface of the subjects, curvature and roughness, rather than the inner nature[57]. Dirty shadowing is considered the hallmark of ultrasound in EPN, and it is generated by reflection of ultrasound waves in multiple directions into the gas bubbles[49] (Figure 4F). We suggest that knowledge of the sonographic features of air-related artifacts in EPN plays an important role in physicians making an early diagnosis. Given the limitation of the case report, further cohort studies are needed to assess the diagnostic accuracy of air-related artifacts on POCUS vs CT imaging for EPN.
EPN is a lethal gas-forming infection of the kidney. POCUS facilitates the timely diagnosis of EPN by the easily recognized hyperechoic focus associated with gas-related artifacts including A-lines, B-lines, comet-tail artifacts, dirty shadowing as well as a “falls” sign in our case. PCD plus antibiotic therapy can provide good clinical outcomes for most EPN cases.
We would like to acknowledge the assistance of Yu-Xin Wang in painting.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cabezuelo AS, Hussein Mohamed AH, Kadriyan H, Takahashi K S-Editor: Fan JR L-Editor: Filipodia P-Editor: Xing YX
| 1. | Song Y, Shen X. Diabetic ketoacidosis complicated by emphysematous pyelonephritis: a case report and literature review. BMC Urol. 2020;20:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Lu YC, Chiang BJ, Pong YH, Chen CH, Pu YS, Hsueh PR, Huang CY. Emphysematous pyelonephritis: clinical characteristics and prognostic factors. Int J Urol. 2014;21:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | McCafferty G, Shorette A, Singh S, Budhram G. Emphysematous Pyelonephritis: Bedside Ultrasound Diagnosis in the Emergency Department. Clin Pract Cases Emerg Med. 2017;1:92-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Yap XH, Ng CJ, Hsu KH, Chien CY, Goh ZNL, Li CH, Weng YM, Hsieh MS, Chen HY, Chen-Yeen Seak J, Seak CK, Seak CJ. Predicting need for intensive care unit admission in adult emphysematous pyelonephritis patients at emergency departments: comparison of five scoring systems. Sci Rep. 2019;9:16618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Koratala A. Focus on POCUS: it is time for the kidney doctors to upgrade their physical examination. Clin Exp Nephrol. 2019;23:982-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Singh Y, Tissot C, Fraga MV, Yousef N, Cortes RG, Lopez J, Sanchez-de-Toledo J, Brierley J, Colunga JM, Raffaj D, Da Cruz E, Durand P, Kenderessy P, Lang HJ, Nishisaki A, Kneyber MC, Tissieres P, Conlon TW, De Luca D. International evidence-based guidelines on Point of Care Ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit Care. 2020;24:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 360] [Article Influence: 72.0] [Reference Citation Analysis (1)] |
| 7. | Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392:75-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 1403] [Article Influence: 200.4] [Reference Citation Analysis (0)] |
| 8. | Cortellaro F, Ferrari L, Molteni F, Aseni P, Velati M, Guarnieri L, Cazzola KB, Colombo S, Coen D. Accuracy of point of care ultrasound to identify the source of infection in septic patients: a prospective study. Intern Emerg Med. 2017;12:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Chen KC, Hung SW, Seow VK, Chong CF, Wang TL, Li YC, Chang H. The role of emergency ultrasound for evaluating acute pyelonephritis in the ED. Am J Emerg Med. 2011;29:721-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Mehdi S, Singh V, Sinha RJ, Pandey S. Concealed diagnosis of duodenal perforation in a patient with emphysematous pyelonephritis: the dilemma of air in the right perirenal space. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Han SY, Tishler JM, Aldrete JS. Extraperitoneal gas: compartmental localization and identification of source. J Can Assoc Radiol. 1985;36:17-21. [PubMed] |
| 12. | Batirel A, Regmi SK, Singh P, Mert A, Konety BR, Kumar R. Urological infections in the developing world: an increasing problem in developed countries. World J Urol. 2020;38:2681-2691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Elawdy MM, Osman Y, Abouelkheir RT, El-Halwagy S, Awad B, El-Mekresh M. Emphysematous pyelonephritis treatment strategies in correlation to the CT classification: have the current experience and prognosis changed? Int Urol Nephrol. 2019;51:1709-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Ubee SS, McGlynn L, Fordham M. Emphysematous pyelonephritis. BJU Int. 2011;107:1474-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 15. | Watanabe H, Suzuki R, Asano T, Shio K, Iwadate H, Kobayashi H, Matsuoka T, Aikawa K, Ohira H. A case of emphysematous pyelonephritis in a patient with rheumatoid arthritis taking corticosteroid and low-dose methotrexate. Int J Rheum Dis. 2010;13:180-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Sokhal AK, Kumar M, Purkait B, Jhanwar A, Singh K, Bansal A, Sankhwar S. Emphysematous pyelonephritis: Changing trend of clinical spectrum, pathogenesis, management and outcome. Turk J Urol. 2017;43:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Cruz J, Figueiredo F, Matos AP, Duarte S, Guerra A, Ramalho M. Infectious and Inflammatory Diseases of the Urinary Tract: Role of MR Imaging. Magn Reson Imaging Clin N Am. 2019;27:59-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Huang JJ, Tseng CC. Emphysematous pyelonephritis: clinicoradiological classification, management, prognosis, and pathogenesis. Arch Intern Med. 2000;160:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 446] [Article Influence: 17.8] [Reference Citation Analysis (1)] |
| 19. | Stone SC, Mallon WK, Childs JM, Docherty SD. Emphysematous pyelonephritis: clues to rapid diagnosis in the Emergency Department. J Emerg Med. 2005;28:315-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Yeung A, Cheng CH, Chu P, Man CW, Chau H. A rare case of asymptomatic emphysematous pyelonephritis. Urol Case Rep. 2019;26:100962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Swami YK, Singh DV, Gupta SK, Pradhan A, Rana YP, Harkar S, Wani MS. Incidentally detected emphysematous pyelonephritis. Cent European J Urol. 2012;65:53-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 22. | Kazempour M, Oroei M, Shabani M, Faghihi T. Emphysematous Pyelonephritis and Hiccups, a Case Report. Iran J Kidney Dis. 2020;14:235-238. [PubMed] |
| 23. | Hsu CF, Chang H, Hu SC, Tsai MJ. Emphysematous pyelonephritis mimicking hollow organ perforation. Intern Med. 2012;51:2671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Sun JN, Zhang BL, Yu HY, Wang B. Severe emphysematous pyelonephritis mimicking intestinal obstruction. Am J Emerg Med 2015; 33: 1846.e3-1846. e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Chuang PH, Yii CY, Cheng KS, Chou JW, Chen CK, Lin YN. Emphysematous pyelonephritis concurrent with psoas muscle abscess. Intern Med. 2011;50:2859-2860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Wu CC, Hung SF. Severe emphysematous pyelonephritis combined with pneumobilia. Emerg Med J. 2012;29:938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Sodhi KS, Lal A, Vyas S, Verma S, Khandelwal N. Emphysematous pyelonephritis with emphysematous pancreatitis. J Emerg Med. 2010;39:e85-e87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Melgarejo-Segura MT, Morales-Martinez A, Arrabal-Polo MA. Pneumorachis and spondylodiscitis caused by emphysematous pyelonephritis. Int Urol Nephrol. 2021;53:91-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Sama S, Chandra N. Unusual presentation of emphysematous pyelonephritis. Intensive Care Med. 2019;45:525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Lai D, Tsai KC, Lin MS, Lin TK, Fan CM, Chang HC, Sun JT. A rare presentation of systemic emphysematous infections secondary to Klebsiella pneumoniae bacteremia in a diabetic patient. J Emerg Med. 2015;48:548-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Wu MY, Lee LC, Chen YL, Yeh YH, Li CJ, Yiang GT. Septic Pulmonary Emboli or Pulmonary Metastasis in a Patient with Diabetes Mellitus? J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Somani BK, Nabi G, Thorpe P, Hussey J, Cook J, N'Dow J; ABACUS Research Group. Is percutaneous drainage the new gold standard in the management of emphysematous pyelonephritis? J Urol. 2008;179:1844-1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 33. | Eswarappa M, Suryadevara S, John MM, Kumar M, Reddy SB, Suhail M. Emphysematous Pyelonephritis Case Series From South India. Kidney Int Rep. 2018;3:950-955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Chauhan V, Sharma R. Emphysematous pyelonephritis (class IIIa) managed with antibiotics alone. Hong Kong Med J. 2015;21:363-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Kuchay MS, Laway BA, Bhat MA, Mir SA. Medical therapy alone can be sufficient for bilateral emphysematous pyelonephritis: report of a new case and review of previous experiences. Int Urol Nephrol. 2014;46:223-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Lu YC, Chiang BJ, Pong YH, Huang KH, Hsueh PR, Huang CY, Pu YS. Predictors of failure of conservative treatment among patients with emphysematous pyelonephritis. BMC Infect Dis. 2014;14:418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Deoraj S, Zakharious F, Nasim A, Missouris C. Emphysematous pyelonephritis: outcomes of conservative management and literature review. BMJ Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Aboumarzouk OM, Hughes O, Narahari K, Coulthard R, Kynaston H, Chlosta P, Somani B. Emphysematous pyelonephritis: Time for a management plan with an evidence-based approach. Arab J Urol. 2014;12:106-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 39. | Jain A, Manikandan R, Dorairajan LN, Sreenivasan SK, Bokka S. Emphysematous pyelonephritis: Does a standard management algorithm and a prognostic scoring model optimize patient outcomes? Urol Ann. 2019;11:414-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Tasleem AM, Murray P, Anjum F, Sriprasad S. CT imaging is invaluable in diagnosing emphysematous pyelonephritis (EPN): a rare urological emergency. BMJ Case Rep. 2014;2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Bhagra A, Tierney DM, Sekiguchi H, Soni NJ. Point-of-Care Ultrasonography for Primary Care Physicians and General Internists. Mayo Clin Proc. 2016;91:1811-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 156] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 42. | Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008;134:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1152] [Cited by in RCA: 1193] [Article Influence: 70.2] [Reference Citation Analysis (0)] |
| 43. | Buttar S, Cooper D Jr, Olivieri P, Barca M, Drake AB, Ku M, Rose G, Siadecki SD, Saul T. Air and its Sonographic Appearance: Understanding the Artifacts. J Emerg Med. 2017;53:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Peng CZ, How CK. Diagnostic Challenge of Emphysematous Pyelonephritis. Am J Med Sci. 2017;353:93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 45. | Koratala A, Bejjanki H. Point-of-care ultrasound for the nephrologist: emphysematous pyelonephritis vs staghorn calculus. Clin Exp Nephrol. 2019;23:1257-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 46. | Brown N, Petersen P, Kinas D, Newberry M. Emphysematous Pyelonephritis Presenting as Pneumaturia and the Use of Point-of-Care Ultrasound in the Emergency Department. Case Rep Emerg Med. 2019;2019:6903193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 47. | Shin KC, Ha YR, Lee SJ, Ahn JH. Review of simulation model for education of point-of-care ultrasound using easy-to-make tools. World J Clin Cases. 2020;8:4286-4302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 48. | Mojoli F, Bouhemad B, Mongodi S, Lichtenstein D. Lung Ultrasound for Critically Ill Patients. Am J Respir Crit Care Med. 2019;199:701-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 277] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 49. | Wu WT, Chang KV, Hsu YC, Hsu PC, Ricci V, Özçakar L. Artifacts in Musculoskeletal Ultrasonography: From Physics to Clinics. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 50. | Oh SH, Han HY, Kim HJ. Comet tail artifact on ultrasonography: is it a reliable finding of benign gallbladder diseases? Ultrasonography. 2019;38:221-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Quien MM, Saric M. Ultrasound imaging artifacts: How to recognize them and how to avoid them. Echocardiography. 2018;35:1388-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 52. | Lu P, Li C, Zhou X. [Significance of the CT scan in renal tuberculosis]. Zhonghua Jie He He Hu Xi Za Zhi. 2001;24:407-409. [PubMed] |
| 53. | Levine E, Grantham JJ. Calcified renal stones and cyst calcifications in autosomal dominant polycystic kidney disease: clinical and CT study in 84 patients. AJR Am J Roentgenol. 1992;159:77-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Villatoro-Villar M, Koster MJ. Erdheim-Chester Disease with atrial mass and perinephric calcification. Clin Case Rep. 2017;5:2153-2154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 55. | Xia M, Liu C, Yang H, Yin K, Wang Y, Tong X, Zhang S, Shuang W. A case report: renal cystic tumoural calcinosis with ossification and bone marrow formation. BMC Urol. 2020;20:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 56. | Yoshino T, Sejima C, Oka Y, Taniguchi H, Nagami T, Wake K, Yamamoto T, Ohnuma H, Kodama K, Kanazawa A, Kawakami K. [Retroperitoneal Dedifferentiated Liposarcoma with Metaplastic Bone Formation: A Case Report and Review of the Literature]. Hinyokika Kiyo. 2019;65:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 57. | Rubin JM, Adler RS, Bude RO, Fowlkes JB, Carson PL. Clean and dirty shadowing at US: a reappraisal. Radiology. 1991;181:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 1.7] [Reference Citation Analysis (0)] |