Published online Oct 6, 2020. doi: 10.12998/wjcc.v8.i19.4603
Peer-review started: April 23, 2020
First decision: April 29, 2020
Revised: May 11, 2020
Accepted: August 25, 2020
Article in press: August 25, 2020
Published online: October 6, 2020
Processing time: 157 Days and 21.7 Hours
Hilar cholangiocarcinoma (CC) is a common malignant tumor with high malignancy and poor prognosis. Most patients have lost the opportunity to undergo radical surgery when diagnosed. Although palliative drainage or biliary stent placement is a preferable choice, the tumor cannot be controlled. This study aimed to develop a novel brachytherapy drainage tube for low-dose-rate brachytherapy with an effective drainage, thereby prolonging the survival time of patients.
A 54-year-old male patient had undergone choledochal stent implantation due to obstructive jaundice. He was admitted to the hospital because of the recurrence of jaundice. Preoperative imaging and pathological biopsy revealed hilar CC (Bismuth-Corlette type IIIa). First, the patient underwent percutaneous transhepatic cholangial drainage and the symptoms of jaundice gradually relieved. To further treat hilar CC and remove the biliary drainage tube as far as possible, the patient chose to use the novel brachytherapy drainage tube after a multi-disciplinary consultation. After 1 mo of brachytherapy, the re-examination revealed that the obstructive lesions disappeared, and the drainage tube was finally removed. During the following 10 mo of follow-up, the patient's hilar CC did not recur.
The novel brachytherapy drainage tube may be a new choice for patients with unresectable hilar CC.
Core Tip: This study aimed to develop a novel biliary drainage tube for low-dose-rate brachytherapy, which had a central cavity for drainage and bilateral cavities to fill 125I seeds. It was placed in a patient with unresectable hilar cholangiocarcinoma, and the obstruction was relieved 1 mo later. After 2 wk of observation, the biliary drainage tube was successfully removed, thereby improving the quality of life of the patient. No complications occurred, and obstructive jaundice did not recur during the follow-up. It was concluded that the novel brachytherapy drainage tube might be a novel choice for patients with unresectable hilar cholangiocarcinoma.
- Citation: Lei QY, Jiao DC, Han XW. Novel brachytherapy drainage tube loaded with double 125I strands for hilar cholangiocarcinoma: A case report. World J Clin Cases 2020; 8(19): 4603-4608
- URL: https://www.wjgnet.com/2307-8960/full/v8/i19/4603.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i19.4603
Hilar cholangiocarcinoma (CC) is highly malignant and difficult to treat. The 5-year survival rate of the disease is less than 30% due to the difficulty in early diagnosis, lack of effective targeted drugs, and surgical challenges caused by complex anatomical structures[1]. At the early stage (Bismuth-Corlette type I or type II), surgical resection is often used to pursue a radical cure. Some studies reported that neoadjuvant therapy before liver transplantation, namely, chemoradiotherapy combined with orthotopic liver transplantation, achieved good results in treating unresectable hilar CC[2].
However, considering that most patients have lost the opportunity to undergo radical surgery when diagnosed with CC, the number of liver donors is limited. The simple placement of the drainage tube or the biliary stent may be re-obstructed due to tumor invasion and growth. Hence, it is important to extend the effective drainage time as far as possible to prolong the survival of patients. Besides, for patients who cannot obtain a matched liver source in time, brachytherapy combined with chemotherapy to actively intervene and control tumor progression is also necessary, besides palliative drainage to relieve jaundice symptoms[3].
A 54-year-old male patient presented to the hospital due to worsening jaundice and a fever of 38.9°C lasting 2 d.
The patient had undergone choledochal stent implantation due to obstructive jaundice.
The patient had a history of hepatitis B.
The patient had no history of trauma or family tumors.
On a physical examination, the patient was observed with yellow sclera and skin, and mild tenderness in the right upper abdomen.
Serum parameters were as follows: Total bilirubin: 158 μmol/L; direct bilirubin: 126μmol/L; white blood cell count: 10.6 × 109/L; alanine transaminase: 66 U/L; and aspartate transaminase: 58 U/L. The renal and coagulation functions were normal.
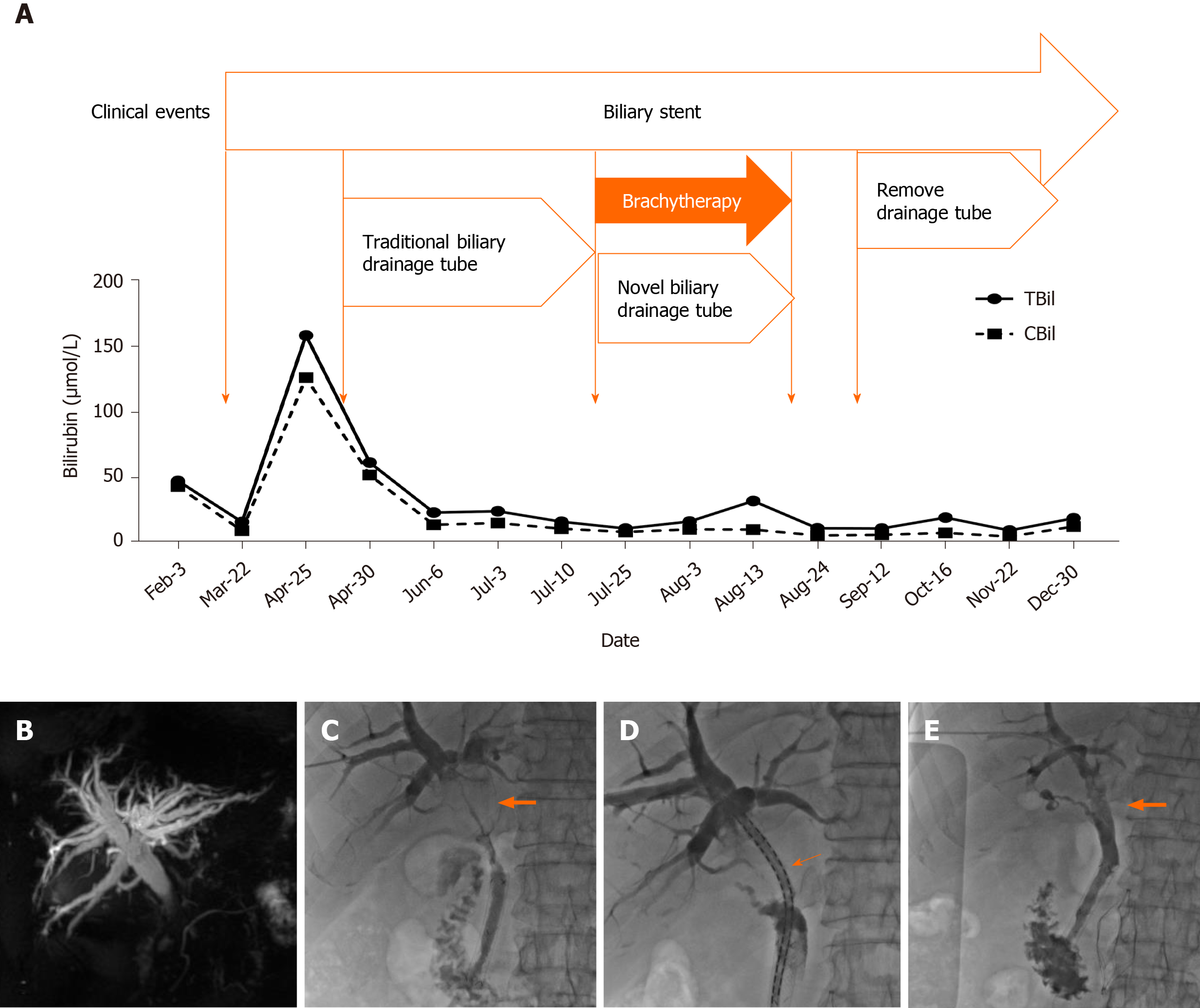
Magnetic resonance cholangiopancreatography indicated a significant expansion of the intrahepatic bile duct. Cholangiography indicated that the hilar lesion resulted in a linear passage of the contrast agent (Figure 1).
The patient was diagnosed with hilar CC and the pathological type was adenocarcinoma.
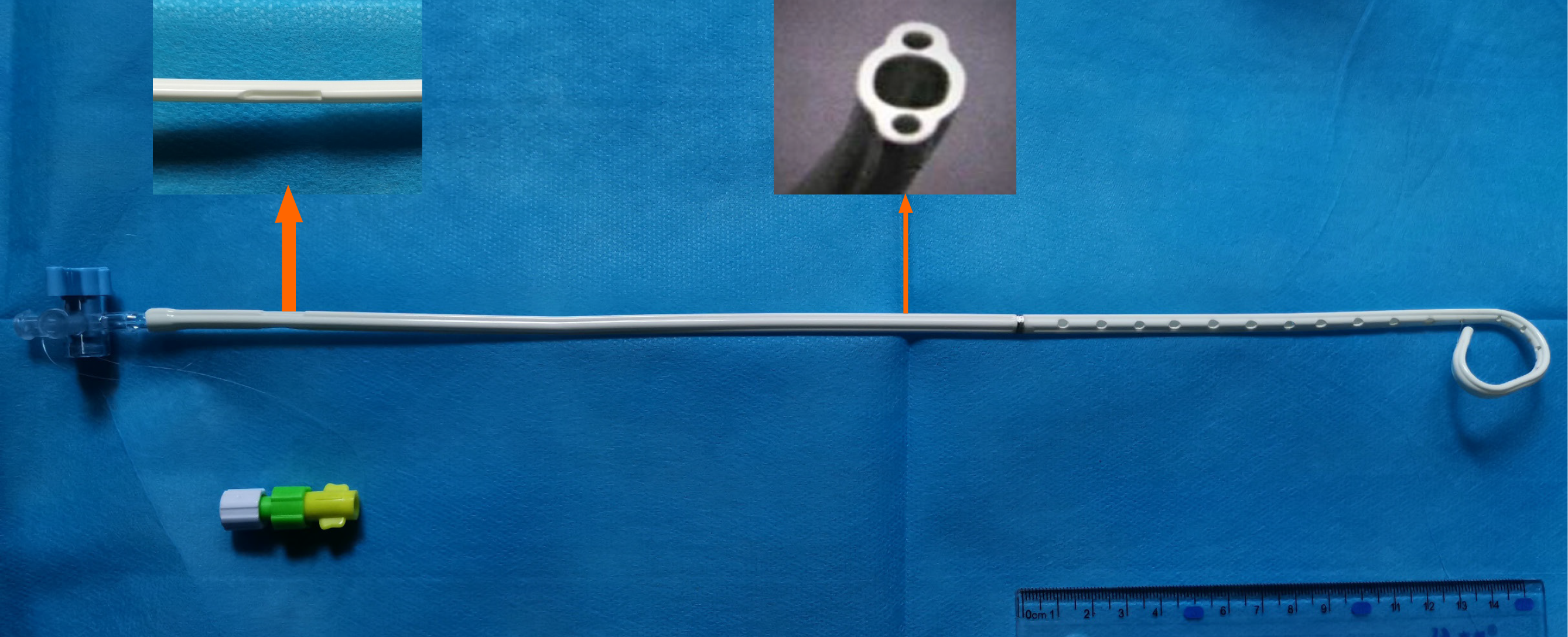
After percutaneous transhepatic cholangial drainage, the bilirubin level gradually returned to normal. Three months later, the patient was admitted for re-examination to replace the biliary drainage tube. Surgical removal and re-stent placement were refused, and the removal of biliary drainage tubes was strongly required to improve the quality of life. After discussion among interventional radiologists, oncologists, hepatobiliary surgeons, and radiotherapists, it was decided to try to place the novel brachytherapy drainage tube (Tuoren Medical Device Ltd. Co., China) loaded with double 125I strands developed by the center (Figure 2). The preoperative informed consent form was signed.
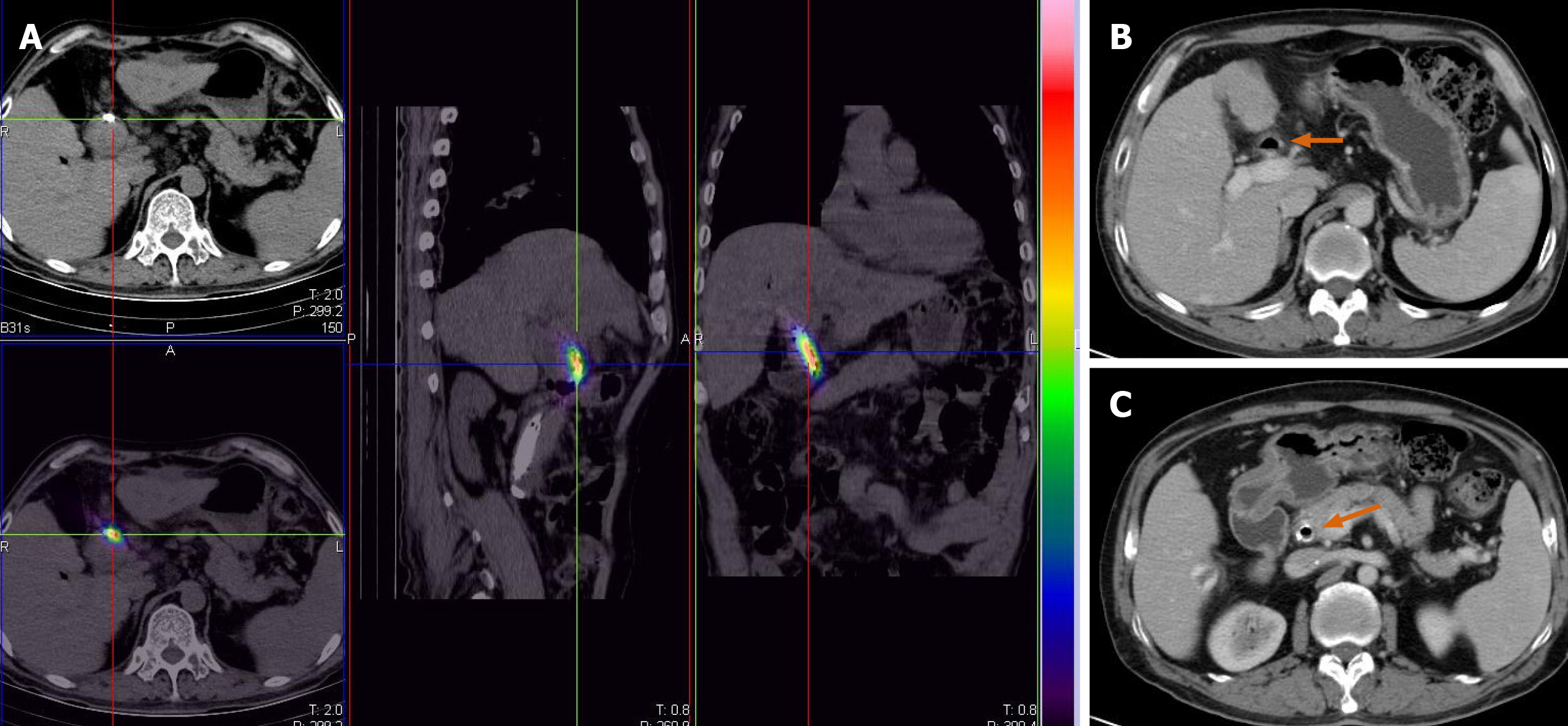
A guide wire (Terumo, Tokyo, Japan) was introduced along the original indwelling biliary drainage tube (Cook Medical, IN, USA). A novel brachytherapy drainage tube was then exchanged along the guide wire. The side hole area of the drainage tube was adjusted across the stenosis of the hilar lesion. The 125I seeds (Atom High Tech, Beijing, China) were pushed into lumens on both sides of the drainage tube, with a total of 30 seeds, ensuring that the double chain spanned 1 cm above and below the narrow zone (particle activity 29.6 MBq, size 0.45 cm × 0.08 cm) (Figure 1). Finally, a 0.018-inch guide wire (Cook Medical, IN, USA) of appropriate length was taken to fill both ends of the side cavity to avoid the replacement of radioactive seeds, and the catheter was fixed. The surgical time was 28 min.
After the surgery, the patient's computed tomography images were recorded into the treatment plan system (Yuan Bo, Beijing, China). The absorbed doses of the sites 0.5, 1.0, and 1.5 cm away from the particle chain were 90, 42, and 20 Gy, respectively.
Cholangiography showed that the hilar obstruction was eliminated. Therefore, the novel drainage tube was replaced with the traditional biliary drainage tube and kept closed for 2 wk. The patient did not have any symptoms after 2 wk of observation, and hence the drainage tube was completely removed. The bilirubin levels were normal after the monthly review, and the changes are shown in Figures 1 and 3. The patient was followed for more than 10 mo without any significant tumor progression.
Hilar CC originates from hilar biliary epithelial cells. The anatomical structure of the hepatobiliary system is complex; the rate of radical resection of the tumor is low, and the prognosis is also poor[4]. Bismuth-Corlette types I and II CC can undergo extrahepatic resection, while type III/IV hilar CC is generally considered unresectable[5]. For patients with type III, it is often necessary to remove the extrahepatic bile duct, the caudate lobe, and the corresponding left or right liver. However, for patients with type IV, it is necessary to combine hepatectomy and the reconstruction of the portal vein and hepatic artery, and also to eliminate the residual cancer cells at each margin to achieve radical resection[6]. Surgical resection alone is not the best method for the treatment of hilar CC[7,8]. For patients with unresectable hilar CC, standard combination therapy should be used, including intracavitary brachytherapy, chemotherapy with gemcitabine and cisplatin, or liver transplantation[1,2].
Multi-center studies have shown that, compared with the traditional stents, the application of 125I seeds loaded biliary stents can prolong the patency of the stents and the survival time of patients, thus proving the effectiveness of combined intracavitary brachytherapy for patients with CC[9]. But the anatomical structure of the hilar bile ducts restricts the application of conventional shape stents. It is difficult to implant custom Y-shaped or T-shaped stents, and the stents cannot be removed once implanted. Some studies used 192Ir to perform tumor irradiation through a percutaneous biliary catheter[10]. High-dose-rate (HDR)-192Ir was a common radioactive source for intraluminal brachytherapy. But HDR-192Ir after loading unit needs to be in an isolated, well-shielded room. To achieve high-dose-rate 192Ir intracavitary brachytherapy requires a high degree of protection. The bile duct tissue is very thin, and the risk of complications such as biliary tract perforation is higher with repeated procedures. The low-energy photons emitted by 125I seeds requires less shielding, and 125I seeds are characterized by a long half-life (59.6 d). This long half-life resulted in sustained injury to the tumor compared with the use of external irradiation. It is convenient to store and use, and reduces the damage to normal tissues around the tumor.
CC mainly invades and metastasizes along the long axis of the bile duct in the Glisson sheath. According to this feature, the present study used 125I seeds as a radioactive source and fused it with a traditional biliary drainage tube to design the proposed novel dual-function drainage tube. Based on a previous study, it is believed that sustained brachytherapy can minimize tumor progression before transplantation[11]. This case was consistent with previous results, and cholangiography showed no signs of recurrence[12]. After 1 mo of brachytherapy, the biliary drainage tube was successfully removed, greatly improving the quality of life of patients.
Compared with the previous brachytherapy, this patient was treated with the proposed novel radiotherapy drainage tube to relieve jaundice symptoms as soon as possible. The particle cavity on both sides was symmetrical. During the surgery, the doctor can freely adjust the position and number of 125I seeds according to cholangiography. This novel drainage tube has the following advantages. First, the tube reduces the infection rate compared with repeated 192Ir brachytherapy or photodynamic therapy. Second, due to the integrated design, the 125I seeds chain could be taken out or replaced with traditional drainage tube at any time, reducing the complications of severe brachytherapy. Last but not least, the dual-cavity design could achieve higher cumulative doses in a shorter period of time, expand the scope of treatment, shorten the time to carry the drainage tube, and improve the quality of life. The novel brachytherapy drainage tube can be used as palliative therapy alone or combined with chemotherapy as neoadjuvant therapy. It has a good application prospect in the future, but still needs further evaluation with a large number of cases and long-term clinical follow-up.
The novel biliary drainage tube combined with brachytherapy provides a new and effective treatment for patients with unresectable hilar CC.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, Nuclear Medicine and Medical Imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ferreira GSA, Mrzljak A, Perrotti S, Thanindratarn P S-Editor: Wang DM L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Mansour JC, Aloia TA, Crane CH, Heimbach JK, Nagino M, Vauthey JN. Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17:691-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 280] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 2. | Frosio F, Mocchegiani F, Conte G, Bona ED, Vecchi A, Nicolini D, Vivarelli M. Neoadjuvant therapy in the treatment of hilar cholangiocarcinoma: Review of the literature. World J Gastrointest Surg. 2019;11:279-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Tan Y, Zhu JY, Qiu BA, Xia NX, Wang JH. Percutaneous biliary stenting combined with radiotherapy as a treatment for unresectable hilar cholangiocarcinoma. Oncol Lett. 2015;10:2537-2542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Machairas N, Kostakis ID, Tsilimigras DI, Prodromidou A, Moris D. Liver transplantation for hilar cholangiocarcinoma: A systematic review. Transplant Rev (Orlando). 2020;34:100516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Lu J, Guo JH, Zhu HD, Zhu GY, Wang Y, Zhang Q, Chen L, Wang C, Pan TF, Teng GJ. Palliative treatment with radiation-emitting metallic stents in unresectable Bismuth type III or IV hilar cholangiocarcinoma. ESMO Open. 2017;2:e000242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Stremitzer S, Jones RP, Quinn LM, Fenwick SW, Diaz-Nieto R, Poston GJ, Malik HZ. Clinical outcome after resection of early-stage hilar cholangiocarcinoma. Eur J Surg Oncol. 2019;45:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Tran TB, Ethun CG, Pawlik TM, Schmidt C, Beal EW, Fields RC, Krasnick B, Weber SM, Salem A, Martin RCG, Scoggins CR, Shen P, Mogal HD, Idrees K, Isom CA, Hatzaras I, Shenoy R, Maithel SK, Poultsides GA. Actual 5-Year Survivors After Surgical Resection of Hilar Cholangiocarcinoma. Ann Surg Oncol. 2019;26:611-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Zheng WH, Yu T, Luo YH, Wang Y, Liu YF, Hua XD, Lin J, Ma ZH, Ai FL, Wang TL. Clinical efficacy of gemcitabine and cisplatin-based transcatheter arterial chemoembolization combined with radiotherapy in hilar cholangiocarcinoma. World J Gastrointest Oncol. 2019;11:489-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Zhu HD, Guo JH, Huang M, Ji JS, Xu H, Lu J, Li HL, Wang WH, Li YL, Ni CF, Shi HB, Xiao EH, Lv WF, Sun JH, Xu K, Han GH, Du LA, Ren WX, Li MQ, Mao AW, Xiang H, Zhang KX, Min J, Zhu GY, Su C, Chen L, Teng GJ. Irradiation stents vs. conventional metal stents for unresectable malignant biliary obstruction: A multicenter trial. J Hepatol. 2018;68:970-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 10. | Heimbach JK, Gores GJ, Haddock MG, Alberts SR, Pedersen R, Kremers W, Nyberg SL, Ishitani MB, Rosen CB. Predictors of disease recurrence following neoadjuvant chemoradiotherapy and liver transplantation for unresectable perihilar cholangiocarcinoma. Transplantation. 2006;82:1703-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 11. | Liu Y, Liu JL, Cai ZZ, Lu Z, Gong YF, Wu HY, Man XH, Jin ZD, Li ZS. A novel approach for treatment of unresectable extrahepatic bile duct carcinoma: design of radioactive stents and an experimental trial in healthy pigs. Gastrointest Endosc. 2009;69:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Wang J, Obulkasim H, Zou X, Liu B, Wu Y, Wu X, Ding Y. Neoadjuvant chemoradiotherapy followed by liver transplantation is a promising treatment for patients with unresectable hilar cholangiocarcinoma: A case report. Oncol Lett. 2019;17:2069-2074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |