Published online Oct 16, 2025. doi: 10.12998/wjcc.v13.i29.108046
Revised: May 5, 2025
Accepted: July 15, 2025
Published online: October 16, 2025
Processing time: 147 Days and 0.7 Hours
High-resolution optical coherence tomography (HR-OCT) has become an essential instrument in the screening and diagnosis of ocular surface neoplasms. Research demonstrates that HR-OCT possesses a diagnostic sensitivity ranging from 85% to 90% for ocular surface squamous neoplasia (OSSN). The connections between HR-OCT features and histological findings have consistently shown robustness, hence increasing the reliability of clinical diagnosis.
To examine the existing HR-OCT indicators employed in the identification of common non-benign ocular surface tumors, namely, basal cell carcinoma, OSSN, and melanocytic conjunctival lesions, and to assess their diagnostic efficacy, benefits, and prospective developments.
A thorough literature review was performed to assess the published research on HR-OCT in the diagnosis of ocular surface cancers. Significant attention was given to research that compares HR-OCT characteristics with histopathologic validation, as well as on publications addressing the integration of emerging technologies and artificial intelligence in ocular oncology imaging.
HR-OCT exhibits elevated diagnostic sensitivity (85%-90%) for identifying OSSN and presents distinct imaging patterns that align closely with histology results. This approach has substantial clinical advantages due to its non-invasive characteristics, improved axial resolution, and real-time imaging capabilities. HR-OCT has demonstrated potential in assessing various lesions, including basal cell carcinoma and melanocytic conjunctival malignancies.
HR-OCT assumes an increasingly vital role in the early identification and clinical management of ocular surface malignancies. With advancements in imaging technology and the integration of artificial intelligence, HR-OCT is anticipated to enhance individualized diagnosis and treatment planning in ocular oncology, hence improving patient outcomes.
Core Tip: The emergence of optical coherence tomography (OCT) has given healthcare systems a deeper understanding into tissue systems as related to both the abnormal and normal patient. In the eye, anterior segment OCT is gradually becoming the gold standard for diagnosis and documentation of pathologies in the front of the eye. An understanding of its development, application and uptake is important to the modern-day eye clinician.
- Citation: Enaholo E, Okoye G, Musa M, Suleman A, Ojo O, Foti R, D’Esposito F, Giglio R, Tognetto D, Gagliano C, Zeppieri M. High-resolution optical coherence tomography for screening ocular surface tumors: Historical markers and future directions. World J Clin Cases 2025; 13(29): 108046
- URL: https://www.wjgnet.com/2307-8960/full/v13/i29/108046.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i29.108046
Optical coherence tomography (OCT) is an advanced optical imaging technique that functions based on the measurement of phase difference relationships between a reflected beam of near-infrared light and its reference path (known as low-coherence interferometry)[1]. It is highly suitable for carrying out non-invasive in vivo studies on basal and sub-basal layers at a preferential micrometer scale. The high-performance image acquisition of modern OCT machinery is enabled via technological components such as high-speed microprocessors for quality signal processing, super luminescent diodes, and semiconductors incorporated into modern OCT systems.
OCT images signify computational reconstructions of tissue reflectance and spectrometric characteristics, rather than direct mirror representations of tissue. Over the years, several technologies have emerged, including time domain-OCT (TD-OCT) and Fourier domain-OCT (FD-OCT), each possessing improved capabilities of tissue penetration, axial resolution, and acquisition speeds[2]. The term “anterior segment optical coherence tomography” (AS-OCT) specifically pertains to OCT imaging of the anterior ocular structures; whereas “high-resolution optical coherence tomography” (HR-OCT) refers to standout machine capabilities to resolve images at 5 microns or finer, irrespective of anatomical location
FD-OCT technologies including spectral-domain-OCT and swept-source OCT (SS-OCT) utilize Fourier transform to enhance signal processing; they have been revolutionary to the field of ophthalmic imaging due to their long laser emission wavelength and more advanced tissue penetration capabilities[6]. Due to its cost and widefield tunable laser scanning capabilities, SS-OCT is mostly applied to studying vitreoretinal diseases[7].
OCT images detailing the cornea and anterior segment were first described in the mid-1990s[8]. Since its initial development, OCT has been most commonly applied in routine ophthalmic clinical practice for acquiring images of the anterior segment (including antero-posterior and lateral representations of the cornea and anterior chamber angles, respectively); and posterior segment imaging (also termed PS-OCT of the vitreoretinal interface, neurosensory retina and choroid).
Similar to all optical imaging technologies, the clinical applications of OCTs are limited in several scenarios. In ophthalmic clinical service delivery, its use is mostly contraindicated due to the non-penetrance of light waves through opaque tissue and poor signal acquisition from anatomical sites posterior to densely pigmented tissue[9]. These limitations hinder the use of OCT technology for clinical assessment of the posterior chamber and orbital space; thus, ultrasound imaging modalities remain practical[10].
With years of research and clinical practice, AS-OCT has evolved into a versatile modality with broader applications beyond imaging the corneal profile and iridocorneal angle only. Relatively novel clinical applications of AS-OCT have also been discovered, such as assessing patency following glaucoma drainage device implantation and detecting ocular surface tumors[11].
For in vivo studies of ocular surface tumors, classical sophisticated techniques such as confocal microscopy and impression cytology (IC) are usually applied[12,13]. Notwithstanding, conventional OCT imaging has been successfully applied for the non-invasive assessment of suspected malignant skin lesions in dermatology[14,15]. Research has also been undertaken into the usability of inverse spectroscopic OCT for the detection of structural tumor changes in greater detail post-excision[16].
In ocular oncology, OCT has typically been employed in detecting acquired metastatic tumors of the posterior segment[17]. Compared to other techniques available for in vivo tumor assessment, the ease of patient understanding and acquisition speed advantages associated with OCT make it more suitably integrated into bedside/clinical routines. The advent of improved modules has yielded higher resolution of the anterior segment. Although AS-OCT generally pertains to the imaging of anterior ocular structures, HR-OCT includes imaging systems with enhanced resolution capabilities. In clinical use, numerous high-resolution AS-OCT devices are designated as HR-AS-OCT, highlighting both anatomical emphasis and advancements in technological resolution.
HR-AS-OCT has emerged as an additional modality to resolve the conundrum associated with triaging external neoplastic lesions of the eye. Hence, timely referrals should be ensured before either severe tissue loss or aggressive orbital invasion occurs[18].
Ocular surface tumors are characterized as either benign, premalignant, or malignant presentations[19]. Inferences of malignancy are routinely made by clinicians based on tumor features such as asymmetry, irregular borders, atypical discoloration, and large lesion diameter[20]. Other features common with malignant lesions include history of chronic recurrence of inflammation, extensive presence of feeder vasculature, and madarosis with significant necrotic tissue in advanced palpebral presentations[21]. History of extensive unprotected exposure to ultraviolet sources seems to be a prominent risk factor for the development of ocular surface tumors[22]. A history of previous primary malignant tumors elsewhere on the body may also correlate well with the progression of ocular surface (particularly conjunctival) tumors[23]. Other factors implicated in the formation of ocular surface tumors include a history of human papillomavirus infection, major histocompatibility complex under expression, immuno-compromised state, sarcoidosis, and latent tuberculosis[24-27].
Excisional biopsy is a common recommendation for suspect lesions[28]. However, local excision and adjunctive therapies can pose unnecessary treatment burdens in non-malignant cases[28]. Hence, non-invasive procedures play a role in external ocular oncological exams. With capabilities such as rapid scan acquisition and near-histologic resolution, HR-AS-OCT has evolved into a highly useful practice tool[29]. The objective of this review is to explore the role of HR-OCT applications in ocular surface tumor characterization. It will focus on applications of HR-AS-OCT technology toward screening and detecting high-risk ocular surface lesions.
Articles were obtained from PubMed utilizing particular keywords pertaining to "optical coherence tomography" and "ocular surface malignancies". The inclusion criteria were: (1) Relevance to the review topic; (2) Availability of full text; (3) English language; and (4) Publication date between 2015 and 2025. The evaluation of quality was predicated on study design, population relevance, and data integrity. Two authors separately evaluated eligibility, with discrepancies addressed by a third reviewer. Results from qualifying studies were synthesized narratively. Diagnostic performance parameters (e.g., sensitivity, specificity) were extracted where applicable. Consistent imaging characteristics across trials were thematically categorized to discern signature HR-OCT patterns.
Inputting the wide search string on the PubMed database retrieved the following algorithm: (("ocul surf" [Journal] OR ("ocular" [All Fields] AND "surface" [All Fields]) OR "ocular surface" [All Fields]) AND ("tomographie" [All Fields] OR "tomography" [MeSH Terms] OR "tomography" [All Fields] OR "tomographies" [All Fields] OR "tomographys" [All Fields] OR "tomographys" [All Fields]) AND ("ocul surf" [Journal] OR ("ocular" [All Fields] AND "surface" [All Fields]) OR "ocular surface" [All Fields]) AND ("neoplasms" [All Fields] OR "neoplasms" [MeSH Terms] OR "neoplasms" [All Fields] OR "neoplasm" [All Fields])) AND (2015: 2025 [pdat])), returning 107 records.
Thirty-two other publications that were strictly relevant to the subject under review were independently hand-searched and evaluated by authors. Two of the authors scrutinized each record for consistency and relevance and when there was a tie on whether to include a record, a third author was invited to decide if it was included.
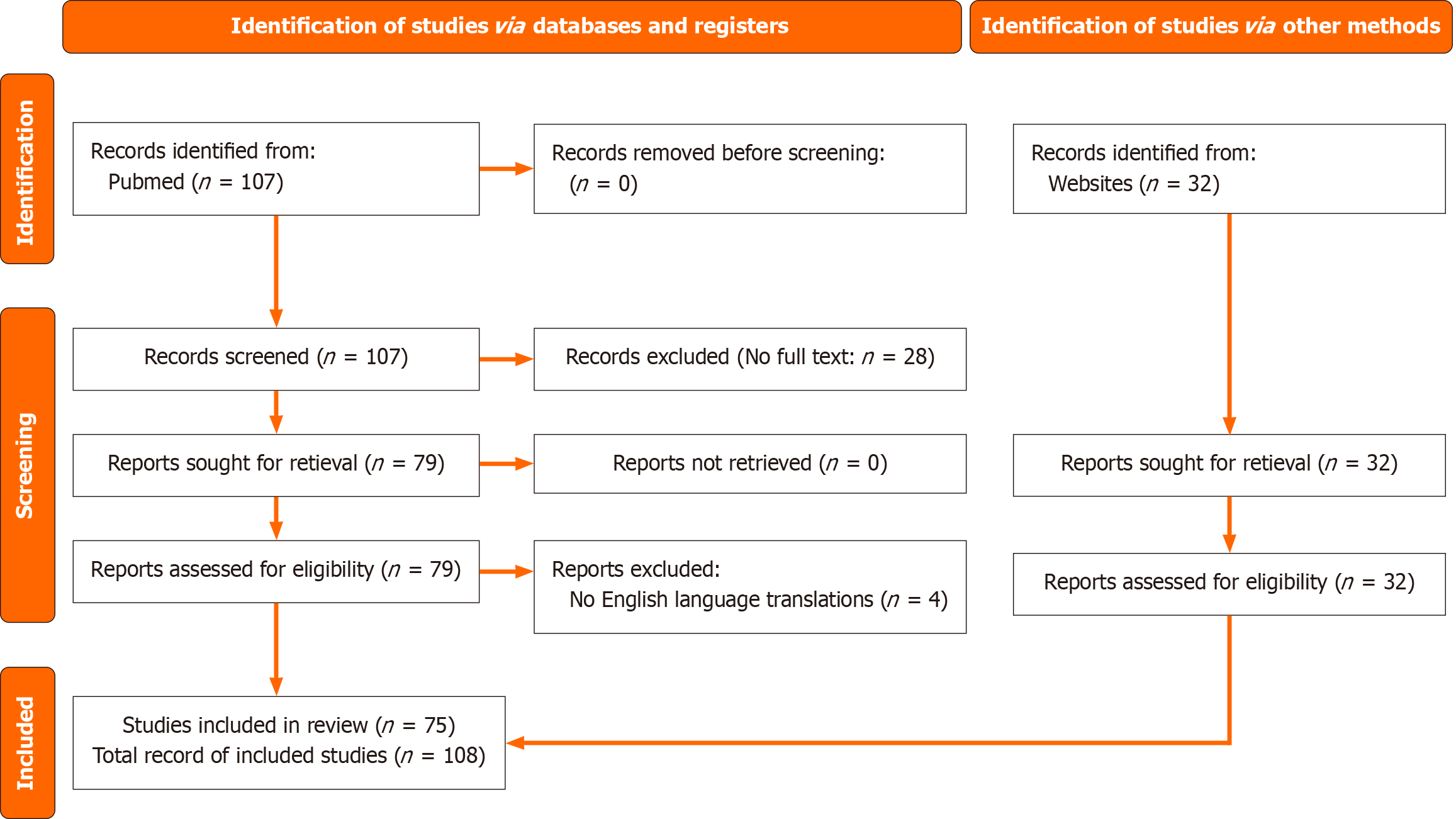
A total of 139 papers were initially obtained, with 64 full-text articles ultimately included following a screening for relevancy and quality. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses[30] flowchart (Figure 1) summarizes the research selection procedure.
Reviewing neoplasms of the ocular adnexa requires first, a description of ocular adnexal histology for ease of association. The ocular surface consists of structures visible within the palpebral fissure (palpebral and bulbar conjunctiva, cornea, caruncle), the periorbital soft tissue (palpebral margins, cilia, their associated secretory glands, and the lacrimal drainage orifice).
The palpebral apparatus: From outermost layers to innermost layers, the eyelids are composed of the epidermis, dermis, orbicularis oculi muscle, periorbital septum, tarsal plates, and palpebral conjunctiva.
The eyelid epidermis is composed of collagen surrounded by melanocytes, Langerhans cells, and Merkel cells. Beneath these structures lie a melanin-containing basal layer of single-rowed columnar cells. The dermis contains eyelid vasculature, lymphatics, cilia, and nerve fibers.
Facial nerve (CN7), and oculomotor nerve (CN3) fibers innervate the palpebral apparatus for motor functions such as voluntary eyelid closure; retraction and protraction[31]. The major protractor, orbicularis oculi muscle, receives its motor innervation from temporal and zygomatic branches of CN7; while the superior branch of CN3 innervates superior palpebral elevation. Weakness or laxity of the CN3-innervated levator palpebrae superioris muscle causes conventional blepharoptosis. Conversely, orbicularis oculi muscle laxity results in palpebral ectropion. Entropion (outward posture of the lids can be caused by either myogenic (mostly iatrogenic, traumatic, senescent) factors or insult to CN7 branches supplying the orbicularis oculi[31]. Common neurogenic causes of ectropion include: Bell's palsy, botulinum toxin exposure, and floppy eyelid syndrome.
Conjunctiva: The conjunctival epithelium consists of non-keratinized stratified squamous epithelium (around a few cells thick) containing goblet cells, melanocytes and Langerhans’ cells.
The conjunctival stroma (substantia propria) is a richly vascularized layer harboring fibrous and lymphoid tissues. The more superficial lymphoid layer consists of connective tissue lymphocytes while the underlying fibrous layer contains lymphatics, regular blood vessels, and conjunctival nerves[32].
Cornea: The cornea has slightly thicker diameter than sclera, and consists of six histologic cross-sections[32]: (1) Epithelium; (2) Bowman's membrane; (3) Stroma; (4) Pre-descemet's, ‘Dua’ layer; (5) Descemet's membrane; and (6) Endothelium.
Of these layers, the tear film-epithelial interface, corneal stroma, and posterior corneal (endothelial) interface can be inferred with OCT imaging. The epithelial basement membrane, Dua's layer, and endothelial basement membrane are not delineated on AS-OCT.
The corneal epithelial layer is composed of non-keratinized stratified squamous epithelium (about 5 cells thick) underlying the mucin tear film layer. The stroma, accounting for 90% of total corneal diameter, is made up of type 1 collagen fiber bundles organized in parallels for optimal transparency, mixed with fibroblasts and elastic fibers. The corneal endothelium is characterized by a single layer of simple cuboidal to simple squamous cells[33].
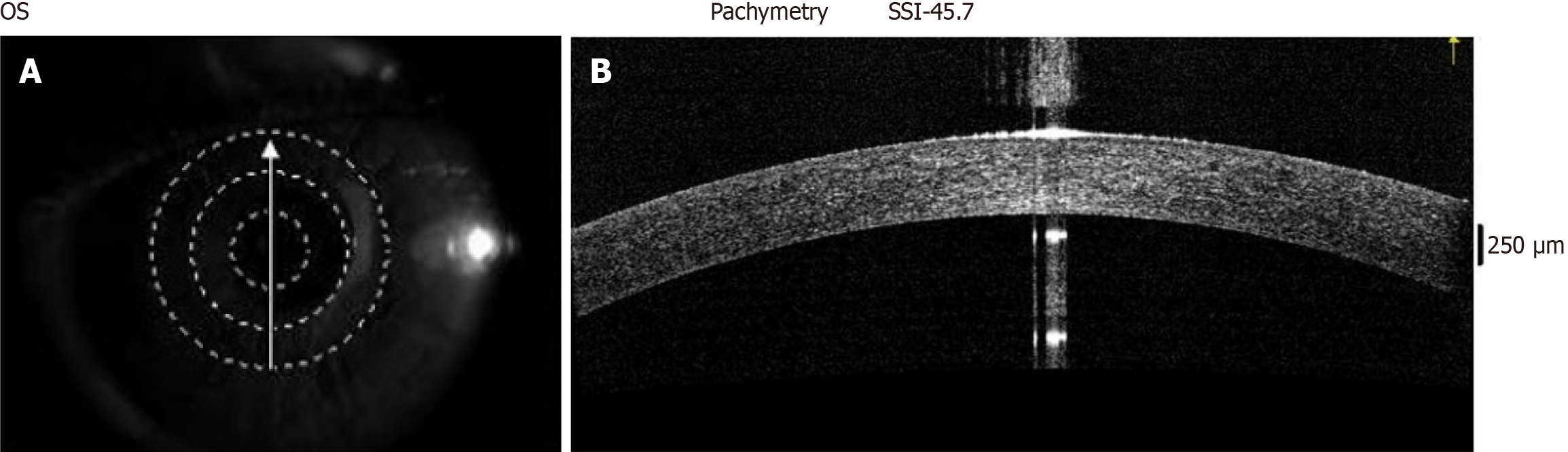
On normal AS-OCT images (Figure 2), both the corneal and conjunctival epithelium are hyporeflective layers beneath the tear film; with both epithelial layers overlying areas of hyperreflectivity.
The primary lacrimal gland and its drainage apparatus: The lacrimal gland is a tubuloacinar structure subdivided by connective tissue septa into lobules containing acinar cells and ducts. Lacrimal fluid drainage is accomplished via the following apparatus, with their histologic constitutions tabulated below (Table 1).
| Anterior segment structure | Histological appearance | Description |
| Lacrimal canaliculi | Stratified, non-keratinized squamous epithelium | Superior and inferior canals commencing from the nasal punctal orifices to the lacrimal sac |
| Lacrimal sac | Pseudostratified ciliated columnar epithelium and goblet cells | Dilated portion of the lacrimal fluid drainage system |
| Nasolacrimal duct | Pseudostratified ciliated columnar epithelium | Drains tear fluid toward the inferior meatus of the nasal cavity |
Sclera: The anterior one-third of the external eye is composed of the cornea, while the posterior two-thirds is composed of the sclera. The cornea and sclera are anatomically composed of similar collagen fibrils, with the only difference being their microfibrillar arrangement. The sclera's opaque morphology is explained by a non-lattice, radial arrangement of collagen fibers within its structure. The sclera has several landmarks that are of great importance to understanding its anatomy. These landmarks above are tabulated below (Table 2)[34,35].
| Histologic landmarks | Structural anatomy | Clinical anatomy |
| Episclera | Loose fibroelastic tissue layer overlying Tenon's capsule | Vascularized portion of sclera |
| Tenon’s capsule | Smooth muscle fiber and collagenous fascia | Transition zone from episclera to sclera |
| Stroma | Interlacing network of type 1 collagen and elastic fibers, oriented without parallelism. | Dense portion of sclera |
| Suprachoroidal lamina | Contains fibroblasts and melanocytes | Thin fibrous connective tissue between sclera and choroid |
| Scleral endothelium | Simple squamous epithelium | Covers deep suprachoroidal lamina at the limbus |
| Limbus | Scleral-corneal junction. Transition of scleral to corneal collagen bundles | Area of contact between conjunctival stroma and Tenon's capsule. Limbal stem cells (unipotent to superficial corneal layers) at palisades of Vogt |
Basal cell carcinoma (BCC) and ocular surface squamous neoplasia (OSSN) (comprised of a spectrum with squamous cell carcinoma, squamous cell carcinoma [SCC], at its extreme) represent the most common malignancies affecting the ocular adnexa, with BCC being the most prevalent external ocular malignancy[36]. BCCs account for between 85% and 90% of all non-melanotic eyelid malignancies[37], also constituting about 10% of all cutaneous extraocular malignancies. OSSN can account for as high as 10% of suspected ocular surface malignancies[38].
Epidemiologic data have reported the highest incidence of OSSN (about 0.16-0.34 per million persons/year) among sub-Saharan African populations[38]. SCC may be an indicator of poor viral suppression among persons living with the human immunodeficiency virus. Malignant melanocytic tumors of the conjunctiva or palpebral dermis and conjunctival lymphoma occur to a lesser extent compared with conjunctival OSSN[39]. In correlation with ocular surface histology, conjunctival lymphoma proliferates from the conjunctival stroma. Sebaceous gland carcinoma, Merkel cell carcinoma, microcystic adnexal carcinoma, and adenoid cystic carcinoma are examples of malignant tumors affecting periorbital soft tissue[40].
OSSN results from progressive atypical proliferation of stratified squamous epithelial cells along the conjunctival, limbal, or corneal epithelium. Thus, during early tumor phases of dysplasia, mimic findings can be quite common. Early-stage OSSN can be mistaken for several surface lesions like epithelial keratitis, hypertrophic corneal scars[41], conjunctival papilloma, and vascularized pinguecula or pterygium[41]. The very rare presentation of primary con
For dysplastic lesions with high potential for malignancy, delayed diagnosis and subsequent referral to secondary specialty services bear several consequences[44]. Aggressive tumor invasion of adjacent structures, including the anterior chamber and periorbita, may often result in ocular comorbidities such as recurrent/chronic blepharoconjunctivitis, secondary glaucoma, and periorbital tissue necrosis syndrome[45]. Orbital tumor invasion frequently necessitates exenteration[46]. However, this procedure remains high-risk.
Invasive techniques: Excisional biopsy: The gold standard for tumor characterization and confirmation, excisional biopsy, remains the most accurate method of tumor resection for histopathologic assessment[47]. It remains the most precise method for differentiating between OSSN and ocular surface squamous metaplasia[48]. For prognostication of ocular surface neoplasia, it is the sole technique for determining the histopathological depth of tumor invasion, as T1 and T2 stages of OSSN demonstrate histopathologic invasion beyond the epithelial basement membrane[49].
Conjunctival (excisional) biopsies are often taken prior to analysis of suspected ocular surface tumors[50]. Histopathologic evaluation following excisional biopsy is key in the early detection of ocular surface atypia, occasionally with immunohistochemistry to complement hematoxylin and eosin staining techniques[51]. The major drawback of the excisional biopsy technique includes the need for high-level expertise for specimen collection and histopathologic interpretation. It is also accompanied by a great degree of subjectivity, and hence, there is limited potential for improving its techniques using automated tools. With continuous improvement and optimization, non-invasive OCT techniques may, in the future, help reduce reliance on invasive diagnostic methods such as excisional biopsy and thus minimize their associated complications[52].
Non-invasive techniques: (1) Slit lamp biomicroscopic (SLB) exam of ocular surface lesions can be achieved using either a diffuser lens (for diffuse illumination) or the conical slit lamp beam[53]. Important features that can be observed using the SLB include madarosis (in cases of eyelid BCC), irregular contour, and dilated vasculature, which supply lesions of interest. A useful tactic involves measuring tumor sizes (largest horizontal, and vertical diameter) in comparison with the millimeter-scale of the slit lamp beam[54]. SLBs fitted with photographic units can also be employed for visual recording and monitoring progressive changes in ocular surface lesion morphology[55]. Slit lamp biomicroscopy features of OSSN have been described as: Leukoplakic (whitish plaque), gelatinous (rubbery), and papillomatous with greater predilection of tumor location along the nasal quadrant[56]; (2) Ultrasound biomicroscopy (UBM) gained popularity as a useful tool for imaging the anterior segment, the intraocular anterior and posterior chambers, and the ciliary body[57-59]. Due to the physical properties of high-frequency sound waves, UBM is the ideal modality for imaging pigmented (uveal) neoplastic lesions[60-62]. However, the practice of applying UBM for studies of bulbar tumors seems less common. Still, good visualization of highly pigmented conjunctival lesions has been achieved with UBM techniques[63]. Improved resolution of UBM output images can be achieved with higher frequency UBM devices, although such higher-frequency models yield lesser depths of signal penetration. Thus, attaining ultra-high-resolution may inhibit appropriate monitoring of ocular surface lesions with high risks for intraocular invasion (such as SCC). Similar to AS-OCT, UBM can be applied usefully in the determination of tumor thickness, although UBM is superior at delineating the posterior margins of these tumors[64]; (3) IC involves the collection of tumor specimens from superficial layers of OSSN. Its non-invasive technique consists of the use of adhesive biomaterial (e.g., biopore membrane, cellulose acetate paper) to obtain tumor samples for biological analysis[65]. IC evolved as a safer improvement upon previous, more invasive exfoliative and fine-needle techniques. However, greater reliance on surface samples eliminates the possibility of cross-sectional tissue evaluations achievable with gold-standard excisional biopsy. It exposes it to more false-positive and false-negative interpretations[66]; (4) In vivo confocal microscopy is an optical imaging technique that eliminates interference from back-scattered light to enhance the evaluation of the ocular adnexa[67]. The high axial and transverse resolution capabilities of confocal microscopes enable assessment of the ocular surface at high magnification[68]. It is most often applied to evaluations of the posterior cornea, particularly in the quantification of corneal endothelial cell count (CECC). Despite its popularity for healthy CECC determination, in vivo confocal microscopy is also a useful tool for screening neoplastic lesions invading the limbus and cornea[69]. Specialized skill requirements for efficient operation and image capturing remain the main limiting factor to large-scale adoption of in vivo confocal microscopy techniques[70]; (5) Multispectral autofluorescence imaging enables the assessment of spectral signals emitted from cancerous cells; as an added advantage, multispectral autofluorescence image analysis has also been optimized via machine learning (ML) protocols[71]. Two-photon auto-fluorescence microscopy is also employed for in vitro assessment of metabolic markers (on excised specimens)[72]; and (6) In vivo staining patterns such as ophthalmic dyes (e.g., rose bengal, methylene blue, lissamine green) can be applied directly onto suspicious lesions of the ocular surface to observe their staining characteristics[73]. However, this technique is grossly inferior to dye studies performed during the histopathologic exam, as basal layers are unstained. The fact, as mentioned earlier, also means that this technique is most suitable for assessing lesions suspected to be of the OSSN spectrum[74]. In vivo staining with rose bengal and other ocular surface dyes has low specificity, as multiple OSSN differentials can often yield generalized conjunctival staining characteristics.
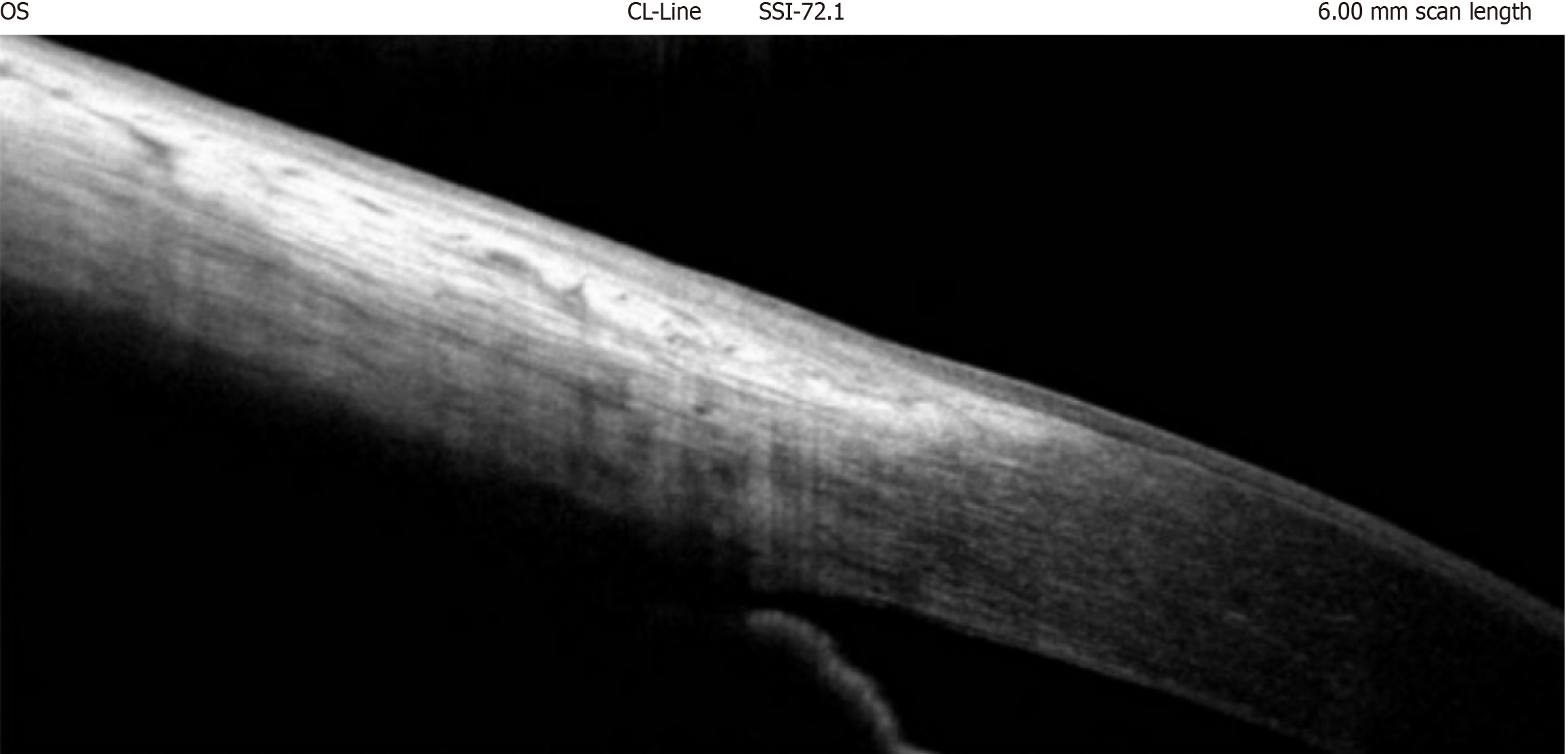
HR-AS-OCT is useful for screening suspicious and malignant ocular surface lesions at near-histologic resolution[75-77] and checking for normalcy, as seen in Figure 3, which shows the normal limbal margin.
Marked enhancement in the density of the corneal epithelium is becoming an associated feature of malignant lesions when corneal invasion is a clinical feature[78]. Research conducted by Maier et al[7] identified dark round structures linked to BCC telangiectasia, correlating them with vessel locations through high-definition OCT, yielding sensitivities of 81% for lesion characterization in 22 BCC samples[7]. On the other hand, Pelosini et al[79] detected perilesional dilated blood vessels in the OCT images of only 33% of 15 patients with BCC[79].
In the presence of corneal invasion by ocular surface tumors, marked enhancement of the corneal epithelial density is suggested to be associated with malignancy[80]. OSSNs of the conjunctiva and cornea often demonstrate subepithelial hyperreflectivity (i.e. augmented light reflectance beneath the epithelial layer) with posterior shadowing (i.e. a dark imaging artifact indicative of obstruction of light transmission through dense tissue) on high-resolution OCT[80-82]. More abrupt transitions from atypical to typical corneal epithelium are also often observed with OSSN[83,84]. However, benign conjunctival papilloma, keratotic plaque, and parakeratosis have been reported to reveal similar HR-AS-OCT appearance, especially epithelial surface corrugation[85,86]. The presence of overhanging edges of papilloma lesions, overlaying normal conjunctival epithelium, is the sole marker of differentiation from OSSN[87].
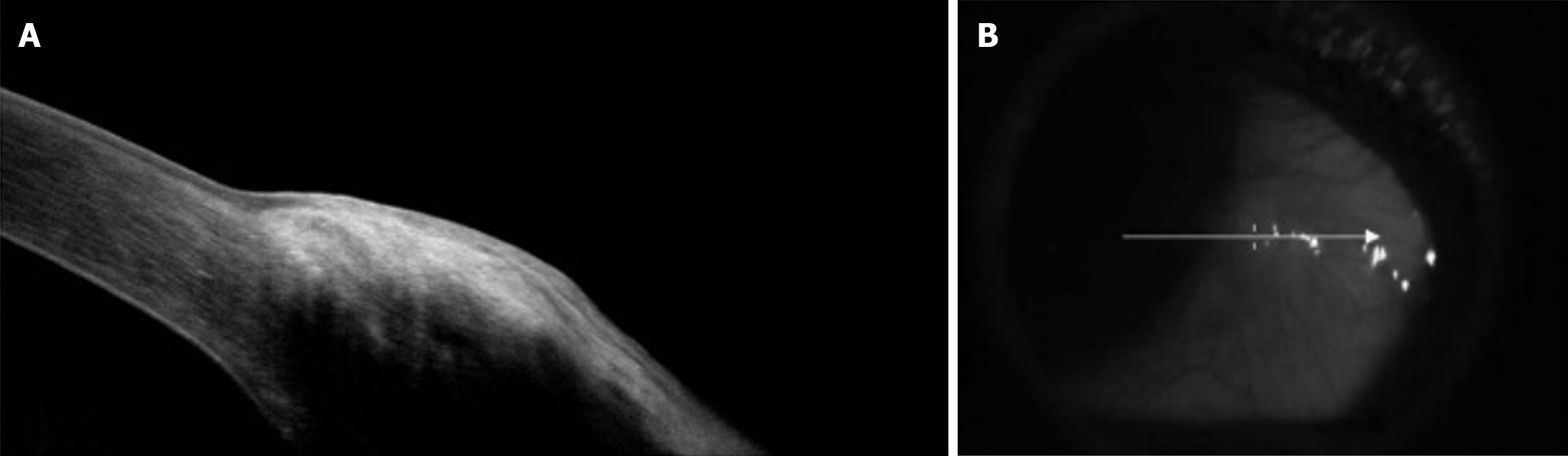
Pseudoepitheliomatous hyperplasia is another important differential that shares several HR-AS-OCT features of OSSN[88]. HR-AS-OCT signs can be used to differentiate between OSSN and benign lesions like pterygium (Figure 4)[89]. Nanji et al[88] described superficial thickening and hyperreflectivity of the corneal epithelium in cases of OSSN, while pterygium was found to yield subepithelial hyperreflectivity by invading the corneal Bowman’s layer[88]. In contrast, HR-AS-OCT signs of subepithelial conjunctival melanoma are distinctive; often demonstrating normal hyperreflectivity of the conjunctival epithelium (lacking corrugations) without abrupt transitions to normal epithelial tissue, variable hyperreflectivity of the basal epithelium, and diffuse posterior shadow effects[90,91]. Intralesional cysts on HR-AS-OCT are a hallmark sign that distinguishes conjunctival nevi from conjunctival melanoma[92].
HR-AS-OCT appearance of conjunctival lymphoma has been described as homogenous, and regular hyporeflective findings localized to the subepithelial area without abnormalities on the conjunctival epithelium[93].
AS-OCTA: OCTA technology was first applied to non-invasive investigations of retinal and choroidal vasculature without contrast dyes (e.g., fluorescein, indocyanine)[89]. AS-OCT angiography (AS-OCTA) is a powerful modality for detecting vascular networks on conjunctiva and episclera via blood flow motion contrast; hence, anterior segment-OCTA enables non-invasive assessment of conjunctival, limbal, and iris vasculature in great detail[90]. When applied expertly, AS-OCTA enables timely identification of ocular surface angiogenesis toward dysplastic lesions[91]. While OCTA has shown potential in identifying vascular alterations in ocular surface lesions, it has not yet attained the status of the gold standard for routine evaluations of the anterior segment or ocular surface tumors.
Notable differences on AS-OCTA have been found between the vasculature around benign and malignant ocular surface lesions[92]. Nampei et al[93] noted that AS-OCTA imaging revealed marked superficial and deep conjunctival vascular tortuosity defined as ‘meandering vessels’ in cases of early or advanced OSSN[93]. Subepithelial tissue underneath conjunctival OSSN lesions has also been reported to inhabit vasculature with greater-than-normal vessel area density[94]. Benign lesions often demonstrate a smaller feeder vessel diameter and depth than malignant ocular surface lesions[95]. Sea-fan-shaped intratumoral and/or conjunctival vasculature has also been described from OCTA studies of highly suggestive SCC lesions[96].
SCC is the most common primary conjunctival carcinoma. It often arises from mutations of non-keratinized squamous epithelia, invading the conjunctiva, limbus, or cornea. It is a malignant presentation belonging to the spectrum of OSSN. AS-OCT is also useful for clinical differentiation of OSSN from benign papillomas[84]. OSSNs are more invasive and aggressive among immunocompromised persons[97]. The American Joint Committee on Cancer classifies T3 OSSNs as those demonstrating invasion of adjacent extraocular tissues[98]. Histopathological studies are essential to confirm basement membrane tumor proliferation. However, T3-staged OSSNs can be diagnosed empirically upon detecting palpebral, canthal, forniceal, or corneal spread[98].
With HR-AS-OCT imaging of the corneal plane, several features of intraepithelial squamous neoplasia have been distinguished from those of invasive squamous cell carcinomas[99]. Aside from distinguishing non-invasive from invasive OSSNs, diagnostic outcomes have been highly agreeable with histopathological studies, as has been detailed earlier in this article (see section 1.2.). HR-AS-OCT imaging has evolved into an adjunctive tool for qualitative clinical detection of typical OSSN. Its application has encouraged less-invasive management strategies via topical immuno- and chemotherapeutic agents[100,101]. Common immuno-therapeutics and chemotherapeutics employed in OSSN management include interferon alpha 2b; and 5-fluorouracil and mitomycin-C, itemized in increasing order of ocular surface toxicity profiles[102,103]. In certain cases, less aggressive topical immunotherapy alone following surgical excision can be effective in the management of recurrent OSSN[104].
Attenuation of perilesional/subepithelial vascular network characteristics was suggested as a good measure of response to topical immuno- or chemotherapy[105]. OCT-guided surgical excision of OSSNs may lessen the need for adjuvant topical chemotherapy, due to more precise removal of tumor progenitor areas[106]. Adequate stage estimation using AS-OCTA could also be factored in by tertiary practitioners when deciding between either topical treatment options or aggressive management with cryotherapy and widespread tissue excision. Beyond their diagnostic application, dyes such as toluene blue, lissamine green, and rose bengal are occasionally used to stain and highlight OSSN tumor margins intraoperatively (for excision purposes)[107].
AS-OCTA may also be useful in confirming adequate ablation of angiogenic channels postoperatively; Indocyanine green angiography has been used to quantitative assessment of afferent and efferent bulbar feeder vasculature around SCCs and mimic lesions[95]. Irradiation treatment has been associated with less recurrence of microinvasive SCCs. However, the combined finding of OSSN and tortuous feeder vessels strongly suggest high-risk of intraocular invasion; irradiation is suboptimal in managing these cases[108].
The era of artificial intelligence (AI) presents huge potential for resolving issues of expertise, and use of ocular adnexal imaging tools. Before such models can be deployed, high-quality training datasets need to be applied to verification sets for the purpose of assessing algorithms’ statistical accuracy[109]. Deep learning (DL) algorithms differ from earlier ML frameworks in their application of large-scale neural networks, which give room for unsupervised learning via unlabeled data acquisition. Unsupervised learning enables AI systems to discern concealed patterns in data without pre-labeled outputs. Hence, DL is the preferred technique for identifying and prognosticating ocular tumor lesions[110]. Artificial neural networks (ANNs) are computational systems modeled after biological neural networks, whereas convolutional neural networks (CNNs) focus on the analysis of visual data via hierarchical feature extraction. As a result, AI-focused research in the field of ocular oncology involves the application of ANNs and CNNs.
Low-shot DL (CNN) models can be trained to detect conjunctival melanoma from smartphone images of three dimensional-printed anterior segment phantoms with high degrees of accuracy, sensitivity, and specificity[110]. Low-shot learning pertains to AI training methodologies that attain precise categorization with a restricted number of training samples. AS-OCT features characterized by ML datasets lend more sensitivity in screening for malignant ocular surface lesions. ANNs designed with feature extractor components will enable improved efficiency of AI training and validation sets by eliminating artifact and speckle noise on AS-OCT images acquired from corneal and limbal tissue[111,112]. As the most prevalent ocular surface malignancies, logical priority has been given to develop AI models for analyzing AS-OCT image scans of ocular surface tumors. Both reinforcement, and supervised learning models have been employed for inputting datasets[113]. Advancements in automated corneal lesion segmentation may serve as a useful supplement to ocular surface tumor detection and monitoring[114].
With the rapid acquisition of immensely large and complex datasets from neoplastic lesions using proteomics techniques, it is essential to leverage ML-based analysis capabilities in order to advance the emergence of precision medicine and the broader field of personalized healthcare[115]. An AI-based diagnostic system reportedly analyzed retrospective proteomics and metabolomics data acquired from eyelid tumors with strong predictive performance for eighteen proteins isolated: This outcome further reaffirms the transformative potential of AI in the field of ophthalmology-omics, including research relating to analyzing protein structure and compositions of ocular surface tumors[115].
This review was based on PubMed database searches and English-language articles, which could be limited and may have introduced selection bias. No formal meta-analysis or pooled quantitative synthesis was conducted due to the heterogeneity among the available studies. This review paper is also limited by the scope of the records sampled. While the authors appreciate that a wider range of years would result in a broader picture of HR-AS-OCT technology, we were mindful of using recent data as opposed to historical information. We also suggest that a comprehensive review of the technical principles of AS-OCT may be lacking in the body of knowledge presented, but that is beyond the scope of this paper.
Non-invasive tools that aid early detection of ocular surface malignancy need to be made more accessible, affordable, and deployable in underserved areas. For lower-resource clinical setups, especially in low-and-middle income countries, building frameworks for applying advanced diagnostic tools for tumor diagnosis may be unrealistic. TD-OCT is less expensive with lower axial resolution than FD-OCT; however, in attempts to improve global standards of practice, more research can be conducted to determine its suitability for qualitative external lesion analysis. High price margins of more novel and usable OCT machines contribute to promoting inaccessibility. Within such ophthalmic micro-systems, promoting a culture of professional diligence and referral to specialists after histopathologic confirmation may be most salient. Thus, continuous bolstering histopathology units within multidisciplinary health centers should be of priority. Primary eye care personnel who practice within underserved communities are bound to benefit from regular, advanced training and re-training modules focused on identifying suggestive markers of ocular surface malignancy. These measures may help diminish the impact of low access to technological advancements in OCT modalities.
Although HR-OCT is exceptionally beneficial for near-histologic assessment of superficial ocular lesions, its high-resolution focus may restrict light penetration depth, thereby diminishing efficacy in imaging very thick or deeply infiltrative tumors. Consequently, HR-AS-OCT is most beneficial for the diagnosis of early-stage lesions or tumors with good optical transmission properties. With systematic correlation of findings from the clinical assessment and imaging, intraoperative OCT-guided excision might improve rates of therapeutic success. As evident from literature, AS-OCT not only improves tumor screening and diagnostic algorithms but also assists in determining potential continuation or discontinuation of aggressive topical tumor treatment regimen. Although OCT-based pseudo-histological guidelines would not replace routine post-excisional biopsy, implementing its novel applications could transform present practice patterns employed in diagnosing, and also managing hard-to-determine external neoplastic ocular lesions. Adapting En-face OCT modalities for imaging and analyzing ocular surface lesions may also further improve diagnostic reliability. Present research is also limited in describing AS-OCT features of primary bulbar BCC, a tough mimic of OSSN during clinical assessment.
It remains essential to continue improving, and integrating predictive software into novel imaging systems. The advent of AI could translate into better decision support for practitioners involved in niche delivery of care for external ocular disease. However, in order to reassure the effectiveness of ML screening programs, AI models must ideally be trained using datasets of individuals similar in race and environmental exposure to demographics being studied.
| 1. | Zeppieri M, Marsili S, Enaholo ES, Shuaibu AO, Uwagboe N, Salati C, Spadea L, Musa M. Optical Coherence Tomography (OCT): A Brief Look at the Uses and Technological Evolution of Ophthalmology. Medicina (Kaunas). 2023;59:2114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 2. | Enaholo ES, Musa MJ, Zeppieri M. Optical Coherence Tomography. 2024 Oct 6. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2025. [PubMed] |
| 3. | Venkateswaran N, Sripawadkul W, Karp CL. The role of imaging technologies for ocular surface tumors. Curr Opin Ophthalmol. 2021;32:369-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Lim SH. Clinical applications of anterior segment optical coherence tomography. J Ophthalmol. 2015;2015:605729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Krema H, Santiago RA, Gonzalez JE, Pavlin CJ. Spectral-domain optical coherence tomography versus ultrasound biomicroscopy for imaging of nonpigmented iris tumors. Am J Ophthalmol. 2013;156:806-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Pavlin CJ, Vásquez LM, Lee R, Simpson ER, Ahmed II. Anterior segment optical coherence tomography and ultrasound biomicroscopy in the imaging of anterior segment tumors. Am J Ophthalmol. 2009;147:214-219.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Maier T, Braun-Falco M, Hinz T, Schmid-Wendtner MH, Ruzicka T, Berking C. Morphology of basal cell carcinoma in high definition optical coherence tomography: en-face and slice imaging mode, and comparison with histology. J Eur Acad Dermatol Venereol. 2013;27:e97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Hӧllhumer R, Williams S, Michelow P. Ocular surface squamous neoplasia: management and outcomes. Eye (Lond). 2021;35:1562-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 9. | Hau SC, Papastefanou V, Shah S, Sagoo MS, Restori M, Cohen V. Evaluation of iris and iridociliary body lesions with anterior segment optical coherence tomography versus ultrasound B-scan. Br J Ophthalmol. 2015;99:81-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Chuchvara N, Rao B, Liu X. Manually scanned single fiber optical coherence tomography for skin cancer characterization. Sci Rep. 2021;11:15570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Hӧllhumer R, Michelow P, Williams S. Comparison of non-invasive diagnostic modalities for ocular surface squamous neoplasia at a tertiary hospital, South Africa. Eye (Lond). 2024;38:1118-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Tanaka S, Kohanim S. The Role of Confocal Microscopy in Diagnosing Ocular Surface Tumors. Int Ophthalmol Clin. 2017;57:75-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Algarin YA, Pulumati A, Tan J, Zeitouni NC. Advances in non-invasive imaging for dermatofibrosarcoma protuberans: A review. Australas J Dermatol. 2024;65:610-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Boone MA, Suppa M, Dhaenens F, Miyamoto M, Marneffe A, Jemec GB, Del Marmol V, Nebosis R. In vivo assessment of optical properties of melanocytic skin lesions and differentiation of melanoma from non-malignant lesions by high-definition optical coherence tomography. Arch Dermatol Res. 2016;308:7-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Ulrich M, Themstrup L, de Carvalho N, Manfredi M, Grana C, Ciardo S, Kästle R, Holmes J, Whitehead R, Jemec GB, Pellacani G, Welzel J. Dynamic Optical Coherence Tomography in Dermatology. Dermatology. 2016;232:298-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 135] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 16. | Allphin MT, Ramasubramanian A. Bilateral familial retinoblastoma diagnosed via optical coherence tomography following a normal funduscopic exam. Fam Cancer. 2024;24:12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Córdoba-Ortega CM, Arias Aristizabal JD, Gómez Velasco MA, Martinez Pulgarín DF. Benign Lobular Inner Nuclear Layer Proliferations of the retina. Eur J Ophthalmol. 2025;35:NP65-NP69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Paul JP, McGuinness MB, Ashby BD, Tan J, Barber NM, Weisinger HS, Martin KR, van Wijngaarden P, Larsen PD. Increased Glaucoma Case-Finding Through Routine Optical Coherence Tomography in Optometry Practice. J Glaucoma. 2024;33:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Julius P, Siyumbwa SN, Moonga P, Maate F, Kaile T, Kang G, West JT, Wood C, Angeletti PC. Clinical and Pathologic Presentation of Primary Ocular Surface Tumors among Zambians. Ocul Oncol Pathol. 2021;7:108-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Spadafora M, Pampena R, Peris K, Del Regno L, Cornacchia L, Fargnoli MC, Pellegrini C, Quaglino P, Ribero S, Calzavara-Pinton PG, Arisi MC, Mirra M, Raucci M, Fusco A, Kaleci S, Chester J, Pellacani G, Longo C. Passive versus active educational interventions for nevus and melanoma classification: A randomized controlled study. J Eur Acad Dermatol Venereol. 2025;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Shields CL, Shields JA. Tumors of the conjunctiva and cornea. Indian J Ophthalmol. 2019;67:1930-1948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 22. | Hussain I, Khan BS. Ocular Surface Squamous Neoplasia: Clinicopathological Analysis and Treatment Outcome of 65 Cases. J Coll Physicians Surg Pak. 2020;30:970-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Williams BK Jr, Di Nicola M. Ocular Oncology-Primary and Metastatic Malignancies. Med Clin North Am. 2021;105:531-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Lloyd HCM, Arunga S, Twinamasiko A, Frederick MA, Onyango J. Predictors of Ocular Surface Squamous Neoplasia and Conjunctival Squamous Cell Carcinoma among Ugandan Patients: A Hospital-based Study. Middle East Afr J Ophthalmol. 2018;25:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 25. | Gichuhi S, Ohnuma S, Sagoo MS, Burton MJ. Pathophysiology of ocular surface squamous neoplasia. Exp Eye Res. 2014;129:172-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 26. | Ou S, Lin Y, Zhang Y, Shi K, Wu H. Epidemiology and tumor microenvironment of ocular surface and orbital tumors on growth and malignant transformation. Front Oncol. 2024;14:1388156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 27. | Atallah M, Joag M, Galor A, Amescua G, Nanji A, Wang J, Perez VL, Dubovy S, Karp CL. Role of high resolution optical coherence tomography in diagnosing ocular surface squamous neoplasia with coexisting ocular surface diseases. Ocul Surf. 2017;15:688-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 28. | Füst Á, Tóth J, Imre L, Nagy ZZ. Non-malignant conjunctival epithelial masses with ocular surface squamous neoplasia-like optical coherence tomography features. Int Ophthalmol. 2021;41:1827-1834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Chong YJ, Azzopardi M, Hussain G, Recchioni A, Gandhewar J, Loizou C, Giachos I, Barua A, Ting DSJ. Clinical Applications of Anterior Segment Optical Coherence Tomography: An Updated Review. Diagnostics (Basel). 2024;14:122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 30. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40601] [Article Influence: 10150.3] [Reference Citation Analysis (2)] |
| 31. | Hwang K, Wu XJ, Kim H, Kim DJ. Sensory Innervation of the Upper Eyelid. J Craniofac Surg. 2018;29:514-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Shumway CL, Motlagh M, Wade M. Anatomy, Head and Neck, Eye Conjunctiva. 2023 Aug 28. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2025. [PubMed] |
| 33. | Ludwig PE, Lopez MJ, Sevensma KE. Anatomy, Head and Neck, Eye Cornea. 2023 Aug 7. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2025. [PubMed] |
| 34. | Sridhar MS. Anatomy of cornea and ocular surface. Indian J Ophthalmol. 2018;66:190-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 304] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 35. | Pradeep T, Mehra D, Le PH. Histology, Eye. 2023 May 1. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2025. [PubMed] |
| 36. | Firnhaber JM. Basal Cell and Cutaneous Squamous Cell Carcinomas: Diagnosis and Treatment. Am Fam Physician. 2020;102:339-346. [PubMed] |
| 37. | Kim DP, Kus KJB, Ruiz E. Basal Cell Carcinoma Review. Hematol Oncol Clin North Am. 2019;33:13-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 38. | Hӧllhumer R, Michelow P, Williams S. Demographics, clinical presentation and risk factors of ocular surface squamous neoplasia at a tertiary hospital, South Africa. Eye (Lond). 2023;37:3602-3608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 39. | Volpini BMF, Maia M, Agi J, Vital J Filho, Lellis RF. Synchronous conjunctival melanoma and lentigo maligna melanoma. An Bras Dermatol. 2017;92:565-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Walsh NM. Merkel cell carcinoma of the eyelid and periocular region: A review. Saudi J Ophthalmol. 2021;35:186-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 41. | Watane A, Hansen ED, Vazquez LE, Karp CL. Ocular Surface Squamous Neoplasia Masquerading as Recalcitrant Epithelial Keratitis. Cornea. 2022;41:1185-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Brouwer NJ, Marinkovic M, Luyten GPM, Shields CL, Jager MJ. Lack of tumour pigmentation in conjunctival melanoma is associated with light iris colour and worse prognosis. Br J Ophthalmol. 2019;103:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Arya D, Das S, Gandhi A. Ipsilateral presentation of ocular surface squamous neoplasia and conjunctival melanoma in xeroderma pigmentosum: A rare occurrence. Indian J Ophthalmol. 2019;67:2068-2071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 44. | Alharbi I, Alfawaz AM, Otaif W, Al-Dahmash SA, Alkatan HM. Variable presentations of six conjunctival/limbal ocular surface squamous neoplasia (OSSN) cases: How good is our clinical judgment evidenced by the correlation to the histopathological findings and diagnosis? Int J Surg Case Rep. 2024;116:109359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 45. | Camp DA, Yadav P, Dalvin LA, Shields CL. Glaucoma secondary to intraocular tumors: mechanisms and management. Curr Opin Ophthalmol. 2019;30:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 46. | Maheshwari A, Finger PT. Cancers of the eye. Cancer Metastasis Rev. 2018;37:677-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 47. | Nanji AA, Mercado C, Galor A, Dubovy S, Karp CL. Updates in Ocular Surface Tumor Diagnostics. Int Ophthalmol Clin. 2017;57:47-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 48. | Stevens SM, Reyes-Capo DP, Patel U, Choudhary A, Khzam RA, Tang V, Galor A, Karp CL, Dubovy S. Clinical and Optical Coherence Tomography Comparison Between Ocular Surface Squamous Neoplasia and Squamous Metaplasia. Cornea. 2023;42:429-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 49. | Chauhan S, Sen S, Sharma A, Tandon R, Kashyap S, Pushker N, Vanathi M, Sharma N. American Joint Committee on Cancer Staging and clinicopathological high-risk predictors of ocular surface squamous neoplasia: a study from a tertiary eye center in India. Arch Pathol Lab Med. 2014;138:1488-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 50. | Polski A, Sibug Saber M, Kim JW, Berry JL. Extending far and wide: the role of biopsy and staging in the management of ocular surface squamous neoplasia. Clin Exp Ophthalmol. 2019;47:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Lin Y, Liu X, Zhang Y, Xie Z, Fang X, Shi K, Zhong Y, Su S, Cai M, Wu H, Ou S. The clinicopathological analysis of ocular and orbit tumors in southeast of China. Front Oncol. 2023;13:1118862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 52. | Vempuluru VS, Jakati S, Godbole A, Mishra DK, Mohamed A, Kaliki S. Spectrum of AS-OCT features of ocular surface tumors and correlation of clinico-tomographic features with histopathology: a study of 70 lesions. Int Ophthalmol. 2021;41:3571-3586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Kaur K, Gurnani B. Slit-Lamp Biomicroscope. 2023 Jun 11. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2025. [PubMed] |
| 54. | Muth DR, Blaser F, Foa N, Scherm P, Mayer WJ, Barthelmes D, Zweifel SA. Smartphone Slit Lamp Imaging-Usability and Quality Assessment. Diagnostics (Basel). 2023;13:423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 55. | Dubey S, Jain K, Fredrick TN. Quality assurance in ophthalmic imaging. Indian J Ophthalmol. 2019;67:1279-1287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 56. | Karp CL, Mercado C, Venkateswaran N, Ruggeri M, Galor A, Garcia A, Sivaraman KR, Fernandez MP, Bermudez A, Dubovy SR. Use of High-Resolution Optical Coherence Tomography in the Surgical Management of Ocular Surface Squamous Neoplasia: A Pilot Study. Am J Ophthalmol. 2019;206:17-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 57. | Mansoori T. Qualitative ultrasound biomicroscopy of the normal anterior segment. Indian J Ophthalmol. 2023;71:2323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 58. | Biçer Ö, Hoşal MB. The Diagnostic Value of Ultrasound Biomicroscopy in Anterior Segment Diseases. Turk J Ophthalmol. 2023;53:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 59. | Martin R. Cornea and anterior eye assessment with slit lamp biomicroscopy, specular microscopy, confocal microscopy, and ultrasound biomicroscopy. Indian J Ophthalmol. 2018;66:195-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 60. | Medina CA, Plesec T, Singh AD. Optical coherence tomography imaging of ocular and periocular tumours. Br J Ophthalmol. 2014;98 Suppl 2:ii40-ii46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 61. | Thomas BJ, Galor A, Nanji AA, El Sayyad F, Wang J, Dubovy SR, Joag MG, Karp CL. Ultra high-resolution anterior segment optical coherence tomography in the diagnosis and management of ocular surface squamous neoplasia. Ocul Surf. 2014;12:46-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 62. | Fernández-Nogueira P, Linzoain-Agos P, Cueto-Remacha M, De la Guia-Lopez I, Recalde-Percaz L, Parcerisas A, Gascon P, Carbó N, Gutierrez-Uzquiza A, Fuster G, Bragado P. Role of semaphorins, neuropilins and plexins in cancer progression. Cancer Lett. 2024;606:217308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 63. | Alzahrani YA, Kumar S, Abdul Aziz H, Plesec T, Singh AD. Primary Acquired Melanosis: Clinical, Histopathologic and Optical Coherence Tomographic Correlation. Ocul Oncol Pathol. 2016;2:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | Yeşiltaş YS, Zhou M, Zabor EC, Oakey Z, Singh N, Sedaghat A, Yeaney G, Singh AD. Iris freckle: a distinct entity. Br J Ophthalmol. 2024;108:1749-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 65. | Barros Jde N, Almeida SR, Lowen MS, Cunha MC, Gomes JÁ. Impression cytology in the evaluation of ocular surface tumors: review article. Arq Bras Oftalmol. 2015;78:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Barros JD, Lowen MS, Moraes-Filho MN, Martins MC. Use of impression cytology for the detection of unsuspected ocular surface squamous neoplasia cells in pterygia. Arq Bras Oftalmol. 2014;77:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Yu M, Liu C, Mehta JS, Liu YC. A review of the application of in-vivo confocal microscopy on conjunctival diseases. Eye Vis (Lond). 2024;11:43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 68. | Cabrera-Aguas M, Watson SL. Updates in Diagnostic Imaging for Infectious Keratitis: A Review. Diagnostics (Basel). 2023;13:3358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 69. | Ong SS, Vora GK, Gupta PK. Anterior Segment Imaging in Ocular Surface Squamous Neoplasia. J Ophthalmol. 2016;2016:5435092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 70. | Teo AWJ, Mansoor H, Sim N, Lin MT, Liu YC. In Vivo Confocal Microscopy Evaluation in Patients with Keratoconus. J Clin Med. 2022;11:393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 71. | Knab A, Anwer AG, Pedersen B, Handley S, Marupally AG, Habibalahi A, Goldys EM. Towards label-free non-invasive autofluorescence multispectral imaging for melanoma diagnosis. J Biophotonics. 2024;17:e202300402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 72. | Lee HJ, Zhang L, Zhang S, Yi J. Detection of Malignancy in Ocular Surface Lesions by Inverse Spectroscopic Optical Coherence Tomography and Two-Photon Autofluorescence. Transl Vis Sci Technol. 2019;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 73. | Blalock TD, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Release of membrane-associated mucins from ocular surface epithelia. Invest Ophthalmol Vis Sci. 2008;49:1864-1871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 74. | Steffen J, Rice J, Lecuona K, Carrara H. Identification of ocular surface squamous neoplasia by in vivo staining with methylene blue. Br J Ophthalmol. 2014;98:13-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 75. | Ray Chaudhuri B, Bhaduri A, Sengupta M. The ocular surface after simple limbal epithelial transplant (SLET): A high-resolution OCT study of the early postoperative period. Indian J Ophthalmol. 2019;67:1348-1350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 76. | Kieval JZ, Karp CL, Abou Shousha M, Galor A, Hoffman RA, Dubovy SR, Wang J. Ultra-high resolution optical coherence tomography for differentiation of ocular surface squamous neoplasia and pterygia. Ophthalmology. 2012;119:481-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 77. | Mangahas LJ, Reyes RW, Siazon R. Primary Conjunctival Basal Cell Carcinoma Mimicking an Ocular Surface Squamous Neoplasia in a Young Adult Filipino: A Case Report and Literature Review. Case Rep Ophthalmol Med. 2024;2024:3113342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 78. | Başkan C, Kılıcarslan A. How Can We Diagnose Ocular Surface Squamous Neoplasia With Optical Coherence Tomography? Cureus. 2023;15:e36320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 79. | Pelosini L, Smith HB, Schofield JB, Meeckings A, Dithal A, Khandwala M. A novel imaging approach to periocular basal cell carcinoma: in vivo optical coherence tomography and histological correlates. Eye (Lond). 2015;29:1092-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Kaliki S, Maniar A, Jakati S, Mishra DK. Anterior segment optical coherence tomography features of pseudoepitheliomatous hyperplasia of the ocular surface: a study of 9 lesions. Int Ophthalmol. 2021;41:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 81. | Aboumourad RJ, Galor A, Karp CL. Case Series: High-resolution Optical Coherence Tomography as an Optical Biopsy in Ocular Surface Squamous Neoplasia. Optom Vis Sci. 2021;98:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 82. | Agarwal A, Farhan MH, Mishra DK, Kaliki S. Corneal ocular surface squamous neoplasia: Case series and review of literature. Oman J Ophthalmol. 2024;17:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 83. | Agarwal A, Kaliki S, Murthy SI. Corneal squamous neoplasia: masquerades and management outcomes at a rural eyecare centre. BMJ Case Rep. 2023;16:e254365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 84. | Sripawadkul W, Khzam RA, Tang V, Zein M, Dubovy SR, Galor A, Karp CL. Anterior segment optical coherence tomography characteristics of conjunctival papilloma as compared to papilliform ocular surface squamous neoplasia. Eye (Lond). 2023;37:995-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 85. | Nibandhe A, Kaliki S, Jakati S, Shanbhag S, Basu S, Donthineni PR. Ocular surface pseudoepitheliomatous hyperplasia secondary to allergic eye disease: clinical features and management. Eye (Lond). 2024;38:1320-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 86. | Venkateswaran N, Mercado C, Wall SC, Galor A, Wang J, Karp CL. High resolution anterior segment optical coherence tomography of ocular surface lesions: A review and handbook. Expert Rev Ophthalmol. 2021;16:81-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 87. | Gündüz AK, Mirzayev I, Okcu Heper A, Kuzu I, Gahramanli Z, Cansiz Ersöz C, Gündüz ÖÖ, Ataoğlu Ö. Anterior segment optical coherence tomography in ocular surface tumours and simulating lesions. Eye (Lond). 2023;37:925-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 88. | Nanji AA, Sayyad FE, Galor A, Dubovy S, Karp CL. High-Resolution Optical Coherence Tomography as an Adjunctive Tool in the Diagnosis of Corneal and Conjunctival Pathology. Ocul Surf. 2015;13:226-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 89. | Pacheco RR, Yaghy A, Shields CL. OCT Angiography of Squamous Cell Carcinoma in Situ. Ophthalmology. 2020;127:688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 90. | Lee BJH, Tey KY, Cheong EZK, Wong QY, Chua CSQ, Ang M. Anterior Segment Optical Coherence Tomography Angiography: A Review of Applications for the Cornea and Ocular Surface. Medicina (Kaunas). 2024;60:1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 91. | Steger B. Ocular surface angiography: from neovessels to neoplasia. BMJ Open Ophthalmol. 2021;6:e000829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 92. | Binotti WW, Mills H, Nosé RM, Wu HK, Duker JS, Hamrah P. Anterior segment optical coherence tomography angiography in the assessment of ocular surface lesions. Ocul Surf. 2021;22:86-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 93. | Nampei K, Oie Y, Kiritoshi S, Morota M, Satoh S, Kawasaki S, Nishida K. Comparison of ocular surface squamous neoplasia and pterygium using anterior segment optical coherence tomography angiography. Am J Ophthalmol Case Rep. 2020;20:100902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 94. | Liu Z, Karp CL, Galor A, Al Bayyat GJ, Jiang H, Wang J. Role of optical coherence tomography angiography in the characterization of vascular network patterns of ocular surface squamous neoplasia. Ocul Surf. 2020;18:926-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 95. | Brunner M, Steger B, Romano V, Hodson M, Zheng Y, Heimann H, Kaye SB. Identification of Feeder Vessels in Ocular Surface Neoplasia Using Indocyanine Green Angiography. Curr Eye Res. 2018;43:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 96. | Sun Y, Hua R. Ocular surface squamous neoplasia: angiographic characteristics and response to subconjunctival/perilesional 5-fluorouracil injections. Drug Des Devel Ther. 2019;13:1323-1334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 97. | Kamal S, Kaliki S, Mishra DK, Batra J, Naik MN. Ocular Surface Squamous Neoplasia in 200 Patients: A Case-Control Study of Immunosuppression Resulting from Human Immunodeficiency Virus versus Immunocompetency. Ophthalmology. 2015;122:1688-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 98. | Singh S, Mohamed A, Kaliki S. Ocular surface squamous neoplasia: analysis based on the 8th American Joint Committee on Cancer classification. Int Ophthalmol. 2019;39:1283-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 99. | Singh S, Mittal R, Ghosh A, Tripathy D, Rath S. High-Resolution Anterior Segment Optical Coherence Tomography in Intraepithelial Versus Invasive Ocular Surface Squamous Neoplasia. Cornea. 2018;37:1292-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 100. | Monroy D, Serrano A, Galor A, Karp CL. Medical treatment for ocular surface squamous neoplasia. Eye (Lond). 2023;37:885-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 101. | Kozma K, Dömötör ZR, Csutak A, Szabó L, Hegyi P, Erőss B, Helyes Z, Molnár Z, Dembrovszky F, Szalai E. Topical pharmacotherapy for ocular surface squamous neoplasia: systematic review and meta-analysis. Sci Rep. 2022;12:14221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 102. | Sripawadkul W, Reyes-Capo D, Zein M, Wylegala A, Albayyat G, Galor A, Karp CL. Long term study of topical interferon α-2b eye drops as primary treatment of ocular surface squamous neoplasia. Ocul Surf. 2023;28:108-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 103. | Wylegala A, Sripawadkul W, Zein M, Alvarez OP, Al Bayyat G, Galor A, Karp CL. Topical 1% 5-fluorouracil eye drops as primary treatment for ocular surface squamous neoplasia: Long-term follow-up study. Ocul Surf. 2023;27:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 104. | Singh S, Jakati S, Pasari A, Basu S. Isolated keratinising corneal ocular surface squamous neoplasia with multifocal recurrence. BMJ Case Rep. 2021;14:e243925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 105. | Theotoka D, Liu Z, Wall S, Galor A, Al Bayyat GJ, Feuer W, Jianhua W, Karp CL. Optical coherence tomography angiography in the evaluation of vascular patterns of ocular surface squamous neoplasia during topical medical treatment. Ocul Surf. 2022;25:8-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 106. | Hӧllhumer R, Williams S, Michelow P. Observational study of ocular surface squamous neoplasia: Risk factors, diagnosis, management and outcomes at a tertiary eye hospital in South Africa. PLoS One. 2020;15:e0237453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 107. | Yeoh CHY, Lee JJR, Lim BXH, Sundar G, Mehta JS, Chan ASY, Lim DKA, Watson SL, Honavar SG, Manotosh R, Lim CHL. The Management of Ocular Surface Squamous Neoplasia (OSSN). Int J Mol Sci. 2022;24:713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 108. | Feizi S, Esfandiari H. Recurrent Conjunctival Squamous Cell Carcinoma and Intraocular Tumor Extension after Topical Erythropoietin: A Case Report. Case Rep Ophthalmol. 2022;13:89-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 109. | Zhang Z, Wang Y, Zhang H, Samusak A, Rao H, Xiao C, Abula M, Cao Q, Dai Q. Artificial intelligence-assisted diagnosis of ocular surface diseases. Front Cell Dev Biol. 2023;11:1133680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 110. | Koseoglu ND, Corrêa ZM, Liu TYA. Artificial intelligence for ocular oncology. Curr Opin Ophthalmol. 2023;34:437-440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 111. | Sinha S, Ramesh PV, Nishant P, Morya AK, Prasad R. Novel automated non-invasive detection of ocular surface squamous neoplasia using artificial intelligence. World J Methodol. 2024;14:92267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (6)] |
| 112. | Ouyang J, Mathai TS, Lathrop K, Galeotti J. Accurate tissue interface segmentation via adversarial pre-segmentation of anterior segment OCT images. Biomed Opt Express. 2019;10:5291-5324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 113. | Wawer Matos PA, Reimer RP, Rokohl AC, Caldeira L, Heindl LM, Große Hokamp N. Artificial Intelligence in Ophthalmology - Status Quo and Future Perspectives. Semin Ophthalmol. 2023;38:226-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 114. | Garcia Marin YF, Alonso-Caneiro D, Vincent SJ, Collins MJ. Anterior segment optical coherence tomography (AS-OCT) image analysis methods and applications: A systematic review. Comput Biol Med. 2022;146:105471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 115. | Wang L, Dai X, Liu Z, Zhao Y, Sun Y, Mao B, Wu S, Zhu T, Huang F, Maimaiti N, Cai X, Li SZ, Sheng J, Guo T, Ye J. AI-driven eyelid tumor classification in ocular oncology using proteomic data. NPJ Precis Oncol. 2024;8:289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |