Published online May 19, 2025. doi: 10.5498/wjp.v15.i5.103628
Revised: March 14, 2025
Accepted: April 8, 2025
Published online: May 19, 2025
Processing time: 77 Days and 0.9 Hours
Thyroid malignancies, while accounting for a small proportion of cancer diag
To analyse the factors influencing the poor prognosis of patients surviving long-term differentiated thyroid cancer with psychiatric disorders and to construct a prediction model.
Forty-eight patients with mental disorders combined with differentiated thyroid cancer who were treated in our hospital during the period of March 2018 to March 2023 were retrospectively selected as the study subjects (thyroid cancer group), and 30 cases each of patients with mental disorders combined with benign thyroid nodules (benign nodules group) and patients with mental disorders alone (mental disorders group), who were treated during the same time period, were selected as controls. The patients with differentiated thyroid cancer were further divided into a poor prognosis group (10 cases) and a good prognosis group (38 cases). The study outcome was poor prognosis as shown by whole body bone imaging within 2 years after thyroid cancer surgery. Factors influencing poor prognosis in survivors of differentiated thyroid cancer were analyzed by univariate and multivariate logistic regression analyses, receiver operating characteristic (ROC) curve analysis was used to assess the predictive efficacy of these factors for poor prognosis, and the DeLong test was used to determine whether there was a statistically significant difference in the area under the curve (AUC) of the model.
One-way logistic regression analysis showed that tumour diameter [odds ratio (OR) = 19.190, P = 0.002], T-stage (OR = 7.692, P = 0.018), extra-glandular infiltration (OR = 37.000, P = 0.003), degree of differentiation (OR = 24.667, P = 0.008), serum free T3 (OR = 22.348, P = 0.025), serum free T4 (FT4) (OR = 1.158, P = 0.002), total bilirubin (TBil) (OR = 1.792, P = 0.004), albumin (OR = 0.675, P = 0.003), cortisol (OR = 1.180, P = 0.003), norepinephrine (OR = 1.047, P = 0.002), angiotensin II (OR = 1.975, P = 0.002), and superoxide dismutase (OR = 0.515, P = 0.005) all increased the risk of poor prognosis in patients with psychiatric disorders and long-term differentiated thyroid cancer. Multifactorial logistic regression analysis showed that tumour diameter (OR = 16.570, P = 0.021), extra-glandular infiltration (OR = 53.145, P = 0.010), FT4 (OR = 1.186, P = 0.007), and TBil (OR = 2.823, P = 0.048) were independent risk factors for poor prognosis of patients with psychiatric disorders with long-term differentiated thyroid cancer, and the regression equation was: Y = 2.808 × tumour diameter + 3.973 × extra-glandular infiltration + 0.171 × FT4 + 1.038 × TBil - 88.138. ROC analysis showed that the predictive power of the overall model (AUC = 0.992, P = 0.000) was significantly higher than that of independent risk factors (DeLong test P < 0.05).
Tumour diameter, extra-glandular infiltration, FT4, and TBil are independent risk factors for poor prognosis in patients with psychiatric disorders with long-term differentiated thyroid cancer, and the combination of these factors is of higher value in predicting the prognosis of patients. These risk factors can be used as a basis to develop a reasonable prognostic management plan in clinical practice for patients with long-term differentiated thyroid cancer with mental disorders, so as to improve the prognosis and quality of life of patients.
Core Tip: The innovative and significant finding of this study is that the combination of tumour diameter, extra-glandular infiltration, serum free T4 levels, and total bilirubin serves as an independent risk factor for poor prognosis in patients with psychiatric disorders and long-term differentiated thyroid cancer, providing higher predictive value for patient prognosis and serving as a basis for developing a reasonable prognostic management plan in clinical practice.
- Citation: Jia JL, Han JH, Pang R, Bi W, Liu B, Yang K. Predictors of poor prognosis in long-term survivors of differentiated thyroid cancer with psychiatric disorders. World J Psychiatry 2025; 15(5): 103628
- URL: https://www.wjgnet.com/2220-3206/full/v15/i5/103628.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i5.103628
Thyroid cancer is relatively rare, but its incidence continues to rise worldwide[1]. According to the statistics of the World Health Organization, thyroid cancer occupies an increasingly prominent position among all cancers, especially among women, and its incidence is increasing year by year[2]. Although thyroid cancer is generally regarded as a type of cancer with a good prognosis, the quality of life after treatment and the mental health of long-term survivors have gradually attracted the attention of the medical community. Mental disorders are a range of disorders that affect an individual’s thinking, feelings, and behavior. These disorders can affect patients’ quality of life, social skills, and physical health, and in severe cases even affect survival rates. Studies have shown that patients with cancer are often accompanied by anxiety, depression, and other psychological problems in the process of treatment and recovery[3]. For survivors of thyroid cancer, the effect of mental disorders is particularly significant. Some patients still experience mood swings and cognitive dysfunction after treatment, causing them to face more challenges in life. Studies have shown that the disease of patients with mental disorders is more serious than that of patients with the tumour alone, which affects the survival rate and quality of life[4]. Therefore, identifying and evaluating prognostic factors in thyroid cancer survivors with psychiatric disorders is an important part of improving treatment outcomes and quality of life of patients. Therefore, the purpose of this study was to analyze the influencing factors of long-term survival of patients with differentiated thyroid cancer complicated with mental illness, and establish a prediction model to provide valuable reference for clinical practice, with an aim to provide a new perspective for the comprehensive treatment of thyroid cancer to improve the prognosis and quality of life of patients.
Patients with mental disorders combined with differentiated thyroid cancer admitted to our hospital between March 2018 and March 2023 were selected for the study.
The inclusion criteria were: (1) Meeting the diagnostic criteria for thyroid cancer[5], with differentiated thyroid cancer confirmed by pathological examination; (2) Being at least 18 years old and not more than 80 years old; (3) Primary tumour with initial surgical treatment; (4) Presence of a mental disorder; (5) Complete clinical data; and (6) The expected survival time after admission was more than 3 months.
The exclusion criteria included: (1) Surgical contraindications; (2) Combined with other malignant tumours; (3) Distant metastasis; (4) Receiving relevant treatment for the disease before admission; (5) Coagulation dysfunction; and (6) Pregnant or lactating women.
According to the inclusion and exclusion criteria, a total of 48 cases were selected and included in the thyroid cancer group. At the same time, 30 patients with mental disorders combined with thyroid benign nodule (benign nodule group) and 30 patients with mental disorders (mental disorder group) were selected as controls.
The primary endpoint of this study was poor prognosis in patients with thyroid cancer within 1 year after surgery. All patients were followed by telephone and outpatient review, and the outcomes of patients within 2 years after surgery were assessed by whole body bone imaging (WBS) criteria[6]. In WBS, grade I has a good prognosis and grade II has a poor prognosis.
Electronic medical record information was collected on all patients, mainly including patients’ baseline information and laboratory indicators.
Baseline data included age, sex, body mass index (BMI), history of smoking, alcohol consumption, and duration of psychiatric disorders in all patients, as well as duration of tumour, side of onset, pathological type, number of foci, tumour diameter, T-stage, lymph node metastasis, extra-glandular infiltration, and degree of differentiation in patients with thyroid cancer.
For measuring laboratory parameters, eight milliliters of fasting peripheral venous blood was drawn the morning following admission and centrifuged for 10 minutes at 3000 r/minute in a centrifuge with an 11 cm centrifuge radius. Thyroid stimulating hormone, serum free T3 (FT3), serum free T4 (FT4), total triiodothyronine, and total thyroxine levels were measured using an automatic chemiluminescence analyzer after serum was collected. An automatic biochemical analyzer was used to measure the levels of alanine aminotransferase (ALT), aspartine aminotransferase, alkaline phosphatase, total bilirubin (TBil), total protein (TP), and albumin (ALB). Using enzyme-linked immunosorbent assay, stress response indices were measured, such as superoxide dismutase (SOD), angiotensin II (Ang II), cortisol, and norepinephrine (NE).
All data in the study were analysed using SPSS 27.0. Univariate and multivariate logistic regression analysis models were used to investigate the relationship of tumour diameter, extra-glandular invasion, FT4, and TBil with poor postoperative prognosis in survivors of differentiated thyroid cancer, with results expressed as odds ratios (ORs) and 95% confidence intervals. The area under the receiver operating characteristic (ROC) curve (AUC) and its 95% confidence interval were calculated to assess the predictive efficacy of different clinical factors for poor postoperative prognosis, and the DeLong test was used to determine whether there was a statistically significant difference in AUC values. A P value of less than 0.05 was considered statistically significant.
All patients were divided into a thyroid cancer group (48 cases), a benign nodule group (30 cases), and a mental disorder group (30 cases) according to the type of disease. The basic information of the included patients was: 50 males and 58 females; mean age (56.27 ± 6.51) years; BMI (23.21 ± 3.48) kg/m2; history of smoking in 24 cases; history of alcohol consumption in 32 cases; and duration of mental disorder (3.48 ± 0.62) years. There was no statistical difference in the basic information among the three groups (P > 0.05) (Figure 1 and 2). However, there were significant differences in FT3, FT4, ALT, TBil, ALB, cortisol, NE, Ang II, and SOD among the three groups (P < 0.05) (Table 1).
| Clinical feature | Total number of patients (n = 108) | Thyroid cancer group (n = 48) | Benign nodule group (n = 30) | Mental disorder group (n = 30) | χ2/F | P value |
| Age (years) | 56.27 ± 6.51 | 56.67 ± 6.25 | 56.17 ± 7.06 | 55.76 ± 6.58 | 0.183 | 0.833 |
| Gender | 0.158 | 0.924 | ||||
| Male | 50 (46.30) | 23 (47.92) | 13 (43.33) | 14 (46.67) | ||
| Female | 58 (53.70) | 25 (52.08) | 17 (56.67) | 16 (53.33) | ||
| BMI (kg/m2) | 23.21 ± 3.48 | 23.11 ± 3.57 | 23.26 ± 3.62 | 23.35 ± 3.29 | 0.046 | 0.955 |
| Smoking history | 24 (22.22) | 14 (29.17) | 6 (20.00) | 4 (13.33) | 2.796 | 0.247 |
| Drinking history | 32 (29.63) | 19 (39.58) | 7 (23.33) | 6 (20.00) | 4.185 | 0.123 |
| Duration of mental disorders (years) | 3.48 ± 0.62 | 3.54 ± 0.21 | 3.49 ± 0.32 | 3.41 ± 0.24 | 2.440 | 0.092 |
| FT3 (pmol/L) | 16.20 ± 4.66 | 18.96 ± 2.54 | 16.25 ± 5.33 | 11.75 ± 2.85 | 36.979 | 0.000 |
| FT4 (pmol/L) | 38.87 ± 20.20 | 52.23 ± 18.41 | 39.52 ± 12.33 | 16.85 ± 2.65 | 59.105 | 0.000 |
| TSH (mU/L) | 2.94 ± 0.47 | 2.96 ± 0.46 | 2.89 ± 0.54 | 2.94 ± 0.41 | 0.206 | 0.814 |
| TT3 (nmol/L) | 2.04 ± 0.28 | 2.03 ± 0.24 | 2.05 ± 0.28 | 2.04 ± 0.19 | 0.065 | 0.937 |
| TT4 (nmol/L) | 107.58 ± 21.31 | 106.33 ± 22.41 | 111.31 ± 19.63 | 105.85 ± 21.36 | 0.637 | 0.531 |
| ALT (U/L) | 27.73 ± 10.81 | 32.65 ± 11.52 | 28.45 ± 7.69 | 19.13 ± 6.34 | 19.555 | 0.000 |
| AST (U/L) | 27.75 ± 5.38 | 28.13 ± 5.65 | 27.95 ± 4.98 | 26.95 ± 5.41 | 0.468 | 0.628 |
| ALP (U/L) | 84.27 ± 22.42 | 87.15 ± 21.74 | 84.96 ± 22.14 | 78.98 ± 23.54 | 1.252 | 0.290 |
| TBil (μmol/L) | 15.86 ± 5.69 | 18.13 ± 5.58 | 16.23 ± 4.16 | 11.85 ± 5.11 | 14.151 | 0.000 |
| TP (g/L) | 72.76 ± 5.76 | 73.26 ± 6.35 | 72.99 ± 5.18 | 71.72 ± 5.34 | 0.691 | 0.504 |
| ALB (g/L) | 43.22 ± 5.84 | 41.54 ± 6.94 | 43.79 ± 5.29 | 45.33 ± 3.18 | 4.345 | 0.015 |
| Cortisol (nmol/L) | 178.77 ± 28.43 | 198.41 ± 16.98 | 179.87 ± 14.13 | 146.23 ± 24.11 | 72.976 | 0.000 |
| NE (ng/L) | 277.50 ± 69.03 | 326.85 ± 56.31 | 249.85 ± 44.18 | 226.17 ± 54.25 | 39.491 | 0.000 |
| Ang II (ng/mL) | 18.89 ± 5.84 | 23.68 ± 4.98 | 15.79 ± 2.54 | 14.32 ± 3.15 | 64.523 | 0.000 |
| SOD (nU/mL) | 92.99 ± 22.98 | 76.25 ± 6.21 | 95.34 ± 6.41 | 117.41 ± 27.54 | 66.166 | 0.000 |
Among the 48 patients with long-term differentiated thyroid cancer with psychiatric disorders included in this study, 38 (79.17%) had a good prognosis and 10 (20.83%) had a poor prognosis. Comparing the clinical data of the two groups of patients, it can be found that the tumour diameter, FT3, FT4, ALT, TBil, TP, cortisol, NE, and Ang II of the patients in the good prognosis group before treatment were significantly lower than those of the bad prognosis group, and the levels of ALB and SOD before treatment were significantly higher than those of the poor prognosis group. Moreover, the percentage of the patients in the poor prognosis group who had T3-T4 stage, extra-glandular infiltration, and moderate and high differentiation was significantly higher than that of the poor prognosis group (P < 0.05) (Table 2).
| Clinical feature | Total number of patients (n = 48) | Good prognosis group | Poor prognosis group | χ2/t | P value |
| Age (years) | 56.67 ± 6.25 | 57.21 ± 6.17 | 54.60 ± 6.47 | 1.179 | 0.245 |
| Gender | 0.254 | 0.614 | |||
| Male | 23 (47.92) | 17 (44.74) | 6 (60.00) | ||
| Female | 25 (52.08) | 21 (55.26) | 4 (40.00) | ||
| BMI (kg/m2) | 23.11 ± 3.57 | 22.92 ± 3.50 | 23.81 ± 3.95 | 0.697 | 0.489 |
| Tumour duration (months) | 18.15 ± 2.32 | 18.34 ± 2.13 | 17.40 ± 2.84 | 1.157 | 0.253 |
| Incidence side | 1.044 | 0.307 | |||
| Unilateral | 37 (77.08) | 31 (81.58) | 6 (60.00) | ||
| Bilateral | 11 (22.92) | 7 (18.42) | 4 (40.00) | ||
| Pathological type | 2.695 | 0.101 | |||
| Papillocarcinoma | 36 (75.00) | 31 (81.58) | 5 (50.00) | ||
| Lecarcinome folliculaire | 12 (25.00) | 7 (18.42) | 5 (50.00) | ||
| Number of lesions | 0.393 | 0.531 | |||
| Single | 21 (43.75) | 18 (47.37) | 3 (30.00) | ||
| Multiple | 27 (56.25) | 20 (52.63) | 7 (70.00) | ||
| Tumour diameter (cm) | 2.71 ± 0.84 | 2.48 ± 0.73 | 3.62 ± 0.62 | 4.519 | 0.000 |
| T stage | 5.013 | 0.025 | |||
| T1-T2 | 27 (56.25) | 25 (65.79) | 2 (20.00) | ||
| T3-T4 | 21 (43.75) | 13 (34.21) | 8 (80.00) | ||
| Lymph node metastasis | 42 (87.50) | 34 (89.47) | 8 (80.00) | 0.072 | 0.788 |
| Extra-glandular infiltration | 6 (12.50) | 1 (2.63) | 5 (50.00) | 12.199 | 0.001 |
| Degree of differentiation | 8.181 | 0.004 | |||
| Moderate-high differentiation | 43 (89.58) | 37 (97.37) | 6 (60.00) | ||
| Low differentiation | 5 (10.42) | 1 (2.63) | 4 (40.00) | ||
| FT3 (pmol/L) | 18.96 ± 2.54 | 18.04 ± 1.86 | 22.45 ± 1.47 | 6.931 | 0.000 |
| FT4 (pmol/L) | 52.23 ± 18.41 | 46.77 ± 15.04 | 72.96 ± 15.42 | 4.875 | 0.000 |
| TSH (mU/L) | 2.96 ± 0.46 | 2.97 ± 0.48 | 2.95 ± 0.42 | 0.120 | 0.905 |
| TT3 (nmol/L) | 2.03 ± 0.24 | 2.03 ± 0.27 | 2.06 ± 0.14 | 0.338 | 0.737 |
| TT4 (nmol/L) | 106.33 ± 22.41 | 106.98 ± 21.54 | 103.82 ± 26.61 | 0.393 | 0.696 |
| ALT (U/L) | 32.65 ± 11.52 | 29.57 ± 10.15 | 44.37 ± 8.73 | 4.211 | 0.000 |
| AST (U/L) | 28.13 ± 5.65 | 27.79 ± 5.74 | 29.40 ± 5.43 | 0.797 | 0.429 |
| ALP (U/L) | 87.15 ± 21.74 | 86.58 ± 23.42 | 89.34 ± 14.38 | 0.354 | 0.725 |
| TBil (μmol/L) | 18.13 ± 5.58 | 16.34 ± 4.49 | 24.90 ± 3.96 | 5.485 | 0.000 |
| TP (g/L) | 73.26 ± 6.35 | 72.34 ± 6.08 | 76.79 ± 6.41 | 2.037 | 0.047 |
| ALB (g/L) | 41.54 ± 6.94 | 43.51 ± 6.07 | 34.06 ± 4.60 | 4.575 | 0.000 |
| Cortisol (nmol/L) | 198.41 ± 16.98 | 193.77 ± 15.47 | 216.07 ± 9.09 | 4.344 | 0.000 |
| NE (ng/L) | 326.85 ± 56.31 | 309.14 ± 43.96 | 394.17 ± 47.51 | 5.355 | 0.000 |
| Ang II (ng/mL) | 23.68 ± 4.98 | 22.22 ± 4.47 | 29.25 ± 1.92 | 4.827 | 0.000 |
| SOD (nU/mL) | 76.25 ± 6.21 | 78.35 ± 4.59 | 68.31 ± 5.13 | 6.010 | 0.000 |
Indicators that differed in the comparison of clinical characteristics of patients with different prognoses were further analysed by univariate logistic regression, which showed that tumour diameter (OR = 19.190, P = 0.002), T-stage (OR = 7.692, P = 0.018), extra-glandular infiltration (OR = 37.000, P = 0.003), degree of differentiation (OR = 24.667 P = 0.008), FT3 (OR = 22.348, P = 0.025), FT4 (OR = 1.158, P = 0.002), TBil (OR = 1.792, P = 0.004), ALB (OR = 0.675, P = 0.003), cortisol (OR = 1.180, P = 0.003), NE (OR = 1.047, P = 0.002), Ang II (OR = 1.975, P = 0.002), and SOD (OR = 0.515, P = 0.005) all increased the risk of poor prognosis in patients with psychiatric disorders with long-term differentiated thyroid carcinoma (Table 3 and 4).
| Independent variable | Assign values |
| Tumour diameter | Original value |
| T stage | T1-T2 = 1, T3-T4 = 2 |
| Lymph node metastasis | No = 0, yes = 1 |
| Degree of differentiation | Moderate-high differentiation = 1, low differentiation = 2 |
| FT3 | Original value |
| FT4 | Original value |
| TBil | Original value |
| TP | Original value |
| ALB | Original value |
| Cortisol | Original value |
| NE | Original value |
| Ang II | Original value |
| SOD | Original value |
| Independent variable | B | S.E. | Wald | P value | OR | 95% confidence interval | |
| Lower limit | Upper limit | ||||||
| Tumour diameter | 2.954 | 0.948 | 9.719 | 0.002 | 19.190 | 2.995 | 122.949 |
| T stage | 2.042 | 0.861 | 5.610 | 0.018 | 7.692 | 1.422 | 41.614 |
| Lymph node metastasis | 3.611 | 1.195 | 9.137 | 0.003 | 37.000 | 3.559 | 384.620 |
| Degree of differentiation | 3.205 | 1.202 | 7.117 | 0.008 | 24.667 | 2.341 | 259.933 |
| FT3 | 3.107 | 1.389 | 5.001 | 0.025 | 22.348 | 1.468 | 340.259 |
| FT4 | 0.147 | 0.048 | 9.467 | 0.002 | 1.158 | 1.055 | 1.272 |
| TBil | 0.583 | 0.202 | 8.330 | 0.004 | 1.792 | 1.206 | 2.663 |
| TP | 0.120 | 0.063 | 3.608 | 0.057 | 1.127 | 0.996 | 1.275 |
| ALB | -0.393 | 0.134 | 8.617 | 0.003 | 0.675 | 0.520 | 0.878 |
| Cortisol | 0.166 | 0.057 | 8.540 | 0.003 | 1.180 | 1.056 | 1.319 |
| NE | 0.046 | 0.015 | 9.904 | 0.002 | 1.047 | 1.018 | 1.078 |
| Ang II | 0.680 | 0.224 | 9.187 | 0.002 | 1.975 | 1.272 | 3.066 |
| SOD | -0.663 | 0.235 | 7.956 | 0.005 | 0.515 | 0.325 | 0.817 |
The patients’ prognosis was taken as the dependent variable (good prognosis = 0, poor prognosis = 1), and based on the results of univariate analysis, multifactorial logistic regression analysis was performed with tumour diameter, T-stage, extra-glandular infiltration, degree of differentiation, FT3, FT4, ALB, TBil, cortisol, NE, Ang II, and SOD as the independent variables. FT3, ALB, Ang II, and SOD were excluded by the variance inflation factor > 10, and tumour diameter (OR = 16.570, P = 0.021), extra-glandular infiltration (OR = 53.145, P = 0.010), FT4 (OR = 1.186, P = 0.007), and TBil (OR = 2.823, P = 0.048) were identified to be independent risk factors for poor prognosis in patients with psychiatric disorders with long-term differentiated thyroid cancer. The regression equation was: Y = 2.808 × tumour diameter + 3.973 × extra-glandular infiltration + 0.171 × FT4 + 1.038 × TBil - 88.138 (Table 5).
| Independent variable | B | S.E. | Wald | P value | OR | 95% confidence interval | |
| Lower limit | Upper limit | ||||||
| Tumour diameter | 2.808 | 1.220 | 5.296 | 0.021 | 16.570 | 1.516 | 181.048 |
| T stage | 1.270 | 1.115 | 1.298 | 0.255 | 3.560 | 0.401 | 31.643 |
| Lymph node metastasis | 3.973 | 1.549 | 6.579 | 0.010 | 53.145 | 2.553 | 1106.354 |
| Degree of differentiation | 1.012 | 1.428 | 0.502 | 0.479 | 2.750 | 0.167 | 45.186 |
| FT4 | 0.171 | 0.063 | 7.323 | 0.007 | 1.186 | 1.048 | 1.343 |
| TBil | 1.038 | 0.525 | 3.900 | 0.048 | 2.823 | 1.008 | 7.907 |
| Cortisol | 0.240 | 0.128 | 3.521 | 0.061 | 1.271 | 0.989 | 1.632 |
| NE | 0.039 | 0.029 | 1.791 | 0.181 | 1.040 | 0.982 | 1.102 |
| Constant | -88.138 | 43.255 | 4.152 | 0.042 | 0.000 | ||
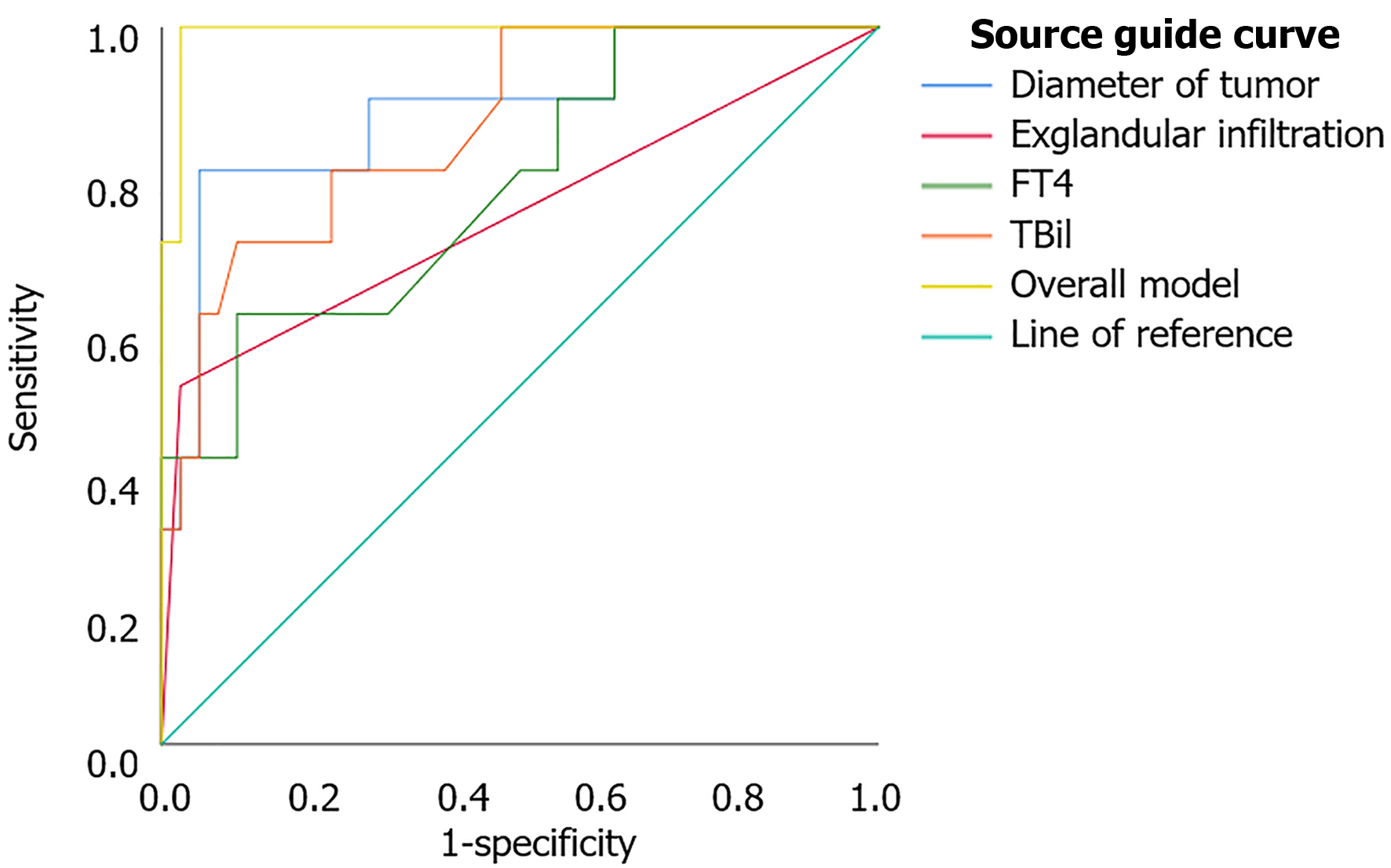
ROC analysis of the independent risk factors in the results of the multifactorial analysis showed an AUC of 0.992 (P = 0.000) for the overall model, 0.884 (P = 0.000) for the tumour diameter variable, 0.737 (P = 0.022) for the extra-glandular infiltration variable, 0.779 (P = 0.007) for the FT4 variable, and 0.863 (P = 0.003) for the TBil variable. The predictive power of the overall model was significantly higher than that of the independent risk factors (DeLong test P < 0.05) (Table 6 and Figure 3).
| Index | AUC | Sensitivity (%) | Specificity (%) | 95% confidence interval | Youden index |
| Tumour diameter | 0.884 | 80.00 | 94.70 | 0.756-1.000 | 0.747 |
| Extra-glandular infiltration | 0.737 | 50.00 | 97.40 | 0.530-0.943 | 0.474 |
| FT4 | 0.779 | 60.00 | 89.50 | 0.614-0.944 | 0.495 |
| TBil | 0.863 | 70.00 | 89.50 | 0.740-0.986 | 0.595 |
| Overall model | 0.992 | 100.00 | 97.40 | 0.974-1.000 | 0.974 |
As the early symptoms of thyroid cancer are not obvious, the patients are often in the stage of poor disease differentiation when they are diagnosed, and surgical treatment is the main treatment[7], which leads to the increase of patients’ anxiety, depression, and other adverse emotions, thus leading to the emergence of mental disorders. Thyroid cancer, especially differentiated thyroid cancer, is a common type of endocrine tumour[8]. Even though these individuals have a more promising prognosis, certain patients are nonetheless susceptible to a bad outcome. However, research has shown that patients’ mental health might also influence the prognostic effect[9]. Furthermore, current research has demonstrated that the incidence of mental illnesses is intimately linked to the immune system and endocrine system, among other factors, and that these aspects may also have an indirect impact on tumour patients’ prognosis[10,11]. In this study, there was no significant difference in sex, BMI, smoking and drinking history, or course of disease among the case group, the benign nodule group, and the mental disorder group, which ruled out the influence of clinical factors on the variables in this study and made the results more consistent with the disease itself. FT3, FT4, ALT, TBil, cortisol, NE, and Ang II in the case group were higher than those in the other two groups, while ALB and SOD were lower than those of the other groups, reflecting that the mental disorders in patients with differentiated thyroid cancer were closely related to thyroid function, liver function, stress response, and neuroendocrine function.
Thyroid hormone levels and liver function indexes are frequently used in clinical practice to assess the prognosis of patients with thyroid cancer. According to earlier research, FT4 can help better judge the illness of individuals with thyroid problems[12]. However, research has shown that thyroid hormone levels and liver function indicators are strongly linked, and that patients with thyroid dysfunction have considerably elevated levels of the liver function indicator TBil[13]. Furthermore, research has indicated that tumour diameter[14] and extra-glandular invasion[15] are also associated with a poor prognosis for tumour patients. In order to assess the poor prognosis of patients with differentiated thyroid carcinoma who also had mental issues, this study evaluated tumour diameter, extra-glandular invasion, FT4, and TBiL as indicators.
In this study, it was found that tumour diameter, extra-glandular infiltration, FT4, and TBiL were independent risk factors for poor prognosis in patients with long-term differentiated thyroid cancer with mental disorders after surgery. This suggests that the size of the tumour in thyroid cancer patients, even after surgery, increases the risk of a poor prognosis. The main reasons are as follows: When the tumour diameter is larger, it is often accompanied by higher tumour cell invasiveness and metastasis ability, thus affecting its sensitivity to treatment to a certain extent. In addition, large tumours are difficult to be completely removed by surgery, and the surgical technique is more complicated, which will increase the surgical risk and the incidence of postoperative complications, thus affecting the therapeutic effect. Therefore, tumour size itself is an important risk factor for poor prognosis. Furthermore, relevant studies have shown that extra-glandular infiltration is a risk factor for the prognosis of thyroid cancer, and the recurrence rate in patients with extra-glandular infiltration visible to the naked eye is significantly higher than that of patients without extra-glandular infiltration or those with extra-glandular infiltration only detected under the microscope[16]. The reason may be that the infiltrated cells are usually highly invasive and can invade the surrounding tissues and structures by penetrating the thyroid capsule. In this process, tumour cells can spread to distant lymph nodes through lymphatic vessels and blood vessels, thereby increasing the risk of recurrence[17] and thus affecting the prognosis of patients. Therefore, the assessment and management of extra-glandular infiltration is essential to improve patient outcomes. Moreover, studies have found that thyroid hormones can affect the response of cancer patients to treatment, thus affecting the prognosis of patients[18]. The reason is that FT4, as an important thyroid hormone, can affect the expression of transcription factors in its downstream Wnt/β-catenin signaling pathway by binding to its receptors THRα and THRβ, thereby regulating the functions of natural killer cells and T cells. As a result, the secretion of interferon-γ, tumour necrosis factor-α, interleukin-10, and other cytokines is abnormal, thus affecting the regulatory function of immune cells[19,20] and the prognosis of patients to a certain extent. Therefore, for cancer patients, monitoring and managing thyroid function, especially FT4 levels, is of great significance for improving prognosis.
In addition, studies have found that abnormal thyroid function is usually closely related to the progression of thyroid cancer and liver function[21]. In this study, it can be found in further clinical observation that some patients with poor prognosis have abnormal thyroid function and liver function simultaneously. By analyzing the predictive value of these risk factors for the prognosis of patients, it can be found that the combination of tumour diameter, extra-glandular infiltration, FT4, and TBil levels has higher predictive value. The reason may be that the liver is an important place for thyroid hormone metabolism. When TBil level in the liver is abnormal, the liver function will also be damaged, which will lead to inhibition of the enzyme activity in the liver, resulting in the increase of FT4 level, thus leading to the aggravation of thyroid dysfunction[22]. This will further lead to the disturbance of basic metabolism in the body, increasing the oxygen consumption of tissues and organs in the body, causing the proliferation of macrophages, leading to the infiltration of polynuclear white blood cells such as neutrophils, aggravating the inflammatory response in the body[23], and enhancing the proliferation and invasiveness of tumour cells, thus affecting the prognosis of patients.
Although this study revealed the predictors of poor prognosis in long-term survivors of differentiated thyroid cancer with mental disorders, there were still some limitations: (1) The sample size in this study was small, which affected the generalizability of the results; (2) This was a retrospective study with data from patients’ clinical records, and the analysis of results may be incomplete; (3) In the prognosis observation, only the prognosis effect in patients 1 year after surgery was analyzed, and long-term follow-up was not carried out; and (4) This study only conducted preliminary clinical observation, and the specific mechanism of action of influencing factors is still unclear. Therefore, in the future research, the sample size can be further expanded, the multi-center, multi-indicator prospective design can be adopted, and the basic research can be verified, so as to improve the accuracy and scientificity of the research.
In summary, tumour diameter, extra-glandular infiltration, FT4, and TBil are independent risk factors for poor prognosis in patients with mental disorders with long-term differentiated thyroid cancer, and these factors have high value in predicting prognosis. Therefore, the tumour size, extra-glandular infiltration, FT4, and TBil levels of such patients should be effectively evaluated, monitored, and managed before operation, and individualized treatment schemes should be implemented to prevent the occurrence of poor prognosis. These important results provide valuable reference for clinical practice, and also provide a new perspective for the comprehensive treatment of thyroid cancer, so as to improve the prognosis and quality of life of patients.
Tumour diameter, extra-glandular infiltration, FT4, and TBil are independent risk factors for poor prognosis in patients with mental disorders with long-term differentiated thyroid cancer, and the combination of these factors is of high value in predicting the prognosis of patients. These independent risk factors can be used as a basis to develop a reasonable prognostic management plan for patients with long-term differentiated thyroid cancer with mental disorders, so as to improve the prognosis and quality of life of patients.
We would like to express our sincere gratitude to all the patients who participated in this study, as well as their families, for their cooperation and support. We also extend our thanks to the medical and nursing staff at Harbin Medical University Cancer Hospital for their assistance in data collection and patient care. Special appreciation goes to our colleagues in the Department of Head and Neck Surgery and the Department of Anesthesiology for their valuable contributions and insights. Finally, we acknowledge the support from the Hospital’s Ethics Committee for approving this research. This study would not have been possible without the collective effort and dedication of everyone involved.
| 1. | Nabhan F, Dedhia PH, Ringel MD. Thyroid cancer, recent advances in diagnosis and therapy. Int J Cancer. 2021;149:984-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 2. | Haymart MR. Progress and Challenges in Thyroid Cancer Management. Endocr Pract. 2021;27:1260-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 3. | Evans EE, Dougherty A, Jensen CB, Sinco B, Robinson N, Ozkan M, Khan I, Roche K, Saucke MC, Bushaw KJ, Antunez AG, Voils CI, Pitt SC. Thyroid Cancer-Related Fear & Anxiety in Patients With Benign Thyroid Nodules: A Mixed-Methods Study. J Surg Res. 2024;302:805-813. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Wootten JC, Wiener JC, Blanchette PS, Anderson KK. Cancer incidence and stage at diagnosis among people with psychotic disorders: Systematic review and meta-analysis. Cancer Epidemiol. 2022;80:102233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Perros P, Boelaert K, Colley S, Evans C, Evans RM, Gerrard Ba G, Gilbert J, Harrison B, Johnson SJ, Giles TE, Moss L, Lewington V, Newbold K, Taylor J, Thakker RV, Watkinson J, Williams GR; British Thyroid Association. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf). 2014;81 Suppl 1:1-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 780] [Article Influence: 70.9] [Reference Citation Analysis (0)] |
| 6. | Hannoush ZC, Palacios JD, Kuker RA, Casula S. False Positive Findings on I-131 WBS and SPECT/CT in Patients with History of Thyroid Cancer: Case Series. Case Rep Endocrinol. 2017;2017:8568347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Boucai L, Zafereo M, Cabanillas ME. Thyroid Cancer: A Review. JAMA. 2024;331:425-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 203] [Reference Citation Analysis (0)] |
| 8. | Richmond BK, Gallimore J. Genetic Considerations in the Tumorigenesis, Diagnosis, and Treatment of Differentiated Thyroid Cancer: Current State of the Science. Am Surg. 2023;89:4853-4859. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Hjell G, Rokicki J, Szabo A, Holst R, Tesli N, Bell C, Fischer-Vieler T, Werner MCF, Lunding SH, Ormerod MBEG, Johansen IT, Djurovic S, Ueland T, Andreassen OA, Melle I, Lagerberg TV, Mørch-Johnsen L, Steen NE, Haukvik UK. Impulsivity across severe mental disorders: a cross-sectional study of immune markers and psychopharmacotherapy. BMC Psychiatry. 2023;23:659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 10. | Herweijer E, Wang J, Hu K, Valdimarsdóttir UA, Adami HO, Sparén P, Sundström K, Fang F. Overall and Cervical Cancer Survival in Patients With and Without Mental Disorders. JAMA Netw Open. 2023;6:e2336213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 11. | Jordan KR, Loman BR, Bailey MT, Pyter LM. Gut microbiota-immune-brain interactions in chemotherapy-associated behavioral comorbidities. Cancer. 2018;124:3990-3999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Wang X, Liu X, Li Y, Tang M, Meng X, Chai Y, Zhang L, Zhang H. The causal relationship between thyroid function, autoimune thyroid dysfunction and lung cancer: a mendelian randomization study. BMC Pulm Med. 2023;23:338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Zeng B, Yuan L, Chu J, Yang Y, Lin S. Challenges in early identification of causes and treatment of cholestasis in patients with hyperthyroidism: a case report and literature review. J Int Med Res. 2020;48:300060519891018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Al-Qurayshi Z, Foggia MJ, Pagedar N, Lee GS, Tufano R, Kandil E. Thyroid cancer histological subtypes based on tumor size: National perspective. Head Neck. 2020;42:2257-2266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Nishino M, Jacob J. Invasion in thyroid cancer: Controversies and best practices. Semin Diagn Pathol. 2020;37:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Li C, Zhang J, Dionigi G, Sun H. The relationship between subclinical hypothyroidism and invasive papillary thyroid cancer. Front Endocrinol (Lausanne). 2023;14:1294441. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Machens A, Lorenz K, Weber F, Dralle H. Metastatic Risk Profile of Microscopic Lymphatic and Venous Invasion in Medullary Thyroid Cancer. Horm Metab Res. 2021;53:588-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 18. | Foster JR, Tinwell H, Melching-Kollmuss S. A review of species differences in the control of, and response to, chemical-induced thyroid hormone perturbations leading to thyroid cancer. Arch Toxicol. 2021;95:807-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Tao Y, Li P, Feng C, Cao Y. New Insights into Immune Cells and Immunotherapy for Thyroid Cancer. Immunol Invest. 2023;52:1039-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Cunha LL, Ward LS. Translating the immune microenvironment of thyroid cancer into clinical practice. Endocr Relat Cancer. 2022;29:R67-R83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Barnhoorn S, Meima ME, Peeters RP, Darras VM, Leeuwenburgh S, Hoeijmakers JHJ, Vermeij WP, Visser WE. Decreased hepatic thyroid hormone signaling in systemic and liver-specific but not brain-specific accelerated aging due to DNA repair deficiency in mice. Eur Thyroid J. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 22. | Chi HC, Tsai CY, Tsai MM, Yeh CT, Lin KH. Molecular functions and clinical impact of thyroid hormone-triggered autophagy in liver-related diseases. J Biomed Sci. 2019;26:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 23. | Bruinstroop E, van der Spek AH, Boelen A. Role of hepatic deiodinases in thyroid hormone homeostasis and liver metabolism, inflammation, and fibrosis. Eur Thyroid J. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |