Published online Jul 24, 2025. doi: 10.5306/wjco.v16.i7.107781
Revised: April 15, 2025
Accepted: June 3, 2025
Published online: July 24, 2025
Processing time: 115 Days and 23 Hours
Gall bladder cancer (GBC) remains a highly aggressive disease, with an overall 5-year dismal survival rate of 15%-20%. Its asymptomatic nature in very early stages and non-specific clinical presentations pose significant challenges to timely detection. Consequently, GBC often presents late, making it one of the most challenging cancers to manage. Surgery offers the best chance for long-term survival; however, only 10% of GBC patients are candidates for upfront resection, with the majority presenting in locally advanced or metastatic stages. Further
Core Tip: Gall bladder cancer is an aggressive disease with a dismal prognosis. Surgery offers the best treatment. However, most of the patients present late due to nonspecific symptoms and at a stage where resection is not feasible. Therefore, early detection is crucial for better outcomes. Identifying high-risk features, utilizing various imaging modalities, use of biomarkers, liquid biopsy and newer tools like artificial intelligence can aid in the early diagnosis.
- Citation: Sarangi Y, Kumar A. Early detection of gallbladder cancer: Current status and future perspectives. World J Clin Oncol 2025; 16(7): 107781
- URL: https://www.wjgnet.com/2218-4333/full/v16/i7/107781.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i7.107781
According to global data, gall bladder cancer (GBC) ranks 22nd in incidence and 20th in mortality worldwide, making it the most common biliary tract cancer. The age-standardized rate (ASR) for GBC incidence is 1.2 per 100000. The highest reported ASR rates have been observed in subpopulations of Northeastern India and Southern Chile. In 2022, China, India, and Japan had the highest numbers of GBC cases and deaths[1]. It’s estimated that only 10% of GBCs are upfront resectable, and most are at an advanced stage[2]. The five-year survival is only 25%-40% in early-stage GBC and 2% for metastatic GBC[3]. In one of the population-based cohort studies from India, the authors found that 1–year and 3-year survival for GBC was 29.0% (22.6%-35.8%) and 5.4% (2.5%-9.8%), with no 5-year survival[4]. Patkar et al[5] found the 3-year overall survival rates for stages I, II and III were 94.1%, 82.6% and 48.2%, respectively. In a large Japanese study, patients with T1 GBC had an 85.9% 5-year survival rate, while those with T3 tumors and T4 tumors had 19.2% and 14.1%respectively[6]. Most patients with GBC are diagnosed at the locally advanced stage, leading to a poor prognosis. As a result, it’s necessary to diagnose at an early stage. GBC lacks specific symptoms, and the existing biomarkers and diagnostic modalities often fail to diagnose it in the early stage. The nonspecific symptoms of GBC, such as right hy
This underscores the critical need for early diagnosis of this dreaded disease. The lack of nonspecific symptoms results in further delays in diagnosis. Surgical resection offers the most effective treatment; however, as mentioned earlier, only a small percentage of patients present at a stage where resection is possible. No definitive diagnostic techniques or biomarkers exist today to aid in early diagnosis. Early diagnosis and timely intervention in patients who are at high risk of developing GBC is the best strategy to confront this dreadful disease. In this review, we discuss various modalities that can be utilized for the early diagnosis of GBC. We have categorized these methods into distinct sections, including susceptible genetic markers, biochemical and molecular markers, in addition to clinical and radiological features.
We systematically reviewed published literature on GBC between 1995 and 2024 from online search engines PubMed and MEDLINE using the search terms GBC, early diagnosis, clinical and radiological features, biomarkers, molecular markers, genetic markers, liquid biopsy, and bullion operators like “AND, OR, and NOT”. We included only those publications relevant to early diagnosis. Secondary sources retrieved from these publications were identified through manual searches and assessed for relevance. The results are discussed in detail.
It is crucial to recognize the risk factors that predispose individuals to GBC. Identifying patients at high risk enables early diagnosis, proactive surgical intervention(cholecystectomy), increased awareness, and better symptom recognition. The various high-risk factors associated with the development of GBC are detailed in Table 1[7-24]. Recent data indicate that the highest GBC incidence rates are found in women from India (21.5 per 100000), Chile (18.1 per 100000), Pakistan (13.8 per 100000), and Ecuador (12.9 per 100000). Females in northern India, particularly in Delhi, have an equal ASR compared to Chile and Bolivia[2]. Numerous studies have indicated that populations living in high-risk areas are associated with an increased likelihood of developing GBC. Mhatre et al[25] observed that individuals born in high-risk regions have 4.82 times greater odds of developing GBC [95% confidence interval (CI): 3.87-5.99] compared to those born in low-risk regions. Moreover, a dose-response relationship was identified between the risk of GBC and the duration of residence in high-risk areas, resulting in an odds ratio of 5.58 (95%CI: 4.42-7.05) along with a significant P value (≤ 0.001). The ASR of GBC in Indian females is similar to that in the Chilean population. So, the population from these high-risk areas should be carefully followed and evaluated promptly if required. Similarly, various studies found an increased risk of GBC in populations residing near Indian rivers - Sutlej, Ganges, Yamuna, and Brahmaputra due to heavy metals and industrial activity contaminations. Chhabra et al[26] found that chromium, lead, arsenic, and zinc were significantly higher in Indian patients compared to Japanese patients.
| Serial number | High risk factors | Ref. |
| 1 | Age (≥ 50 years), socioeconomic status (≤ below poverty line), bowel habits (≤ once a day), tap water drinking, number of pregnancies (≥ 3 pregnancies), multiparity (≥ 3 babies) | [7] |
| 2 | Female sex | [8] |
| 3 | Residence in the Gangetic belt, consumption of tea, tobacco, joint family structure, chemical exposure, fried food, high levels of secondary bile salts | [9] |
| 4 | Females with more than two children, mustard oil consumption, low socioeconomic strata age of menarche less than 14 years, age of the first child birth less than 20 years, Presence of gall stone | [10] |
| 5 | Long-standing gallstone disease, female | [11] |
| 6 | Female, older age, solitary stones and stones size more than one centimetre | [12] |
| 7 | Increase in the number and size of the stones | [13] |
| 8 | Women, long history of gall stone disease | [14] |
| 9 | Weight, volume and size of the stones increases the changes in the gall bladder mucosa changes from cholecystitis, hyperplasia, metaplasia, dysplasia, to carcinoma | [15] |
| 10 | Stone size, solitary polyps with a size of greater than 1 cm that are echogenic, sessile, and high cell density, AJPBD, porcelain gallbladder | [16] |
| 11 | Patients with history of salmonella and helicobacter infection | [17] |
| 12 | Primary sclerosing cholangitis | [18] |
| 13 | Cholelithiasis, females with gallstones in their sixth decade, multiple stones | [19] |
| 14 | Higher stone volume | [20] |
| 15 | High soil arsenic levels, residence in gangetic belt | [21] |
| 16 | Smoking, cholelithiasis, alcohol consumption, typhoid in the past, post-menopausal women | [22] |
| 17 | Helicobacter pylori infection | [23] |
| 18 | Females, consumption of mustard oil, family history, low socioeconomic status and drinking water from hand pump | [24] |
However, screening all these high-risk populations is practically challenging. Therefore, these groups should raise the threshold of suspicion for GBC when they are symptomatic or have asymptomatic gallstone disease. In asymptomatic patients, the evidence does not support that the presence of gallstones leads to GBC[27,28]. There should be a tailored approach for the workup of both symptomatic patients and asymptomatic patients with high-risk characteristics to carefully evaluate for malignancy and detect early cancer should any suspicion arise on clinical or imaging evaluation.
GBC often presents with nonspecific signs and symptoms. Patients diagnosed with GBC often present with pain located in the right upper quadrant as well as the epigastric region of the abdomen. Many patients give a history of a change in the character of pain, with previous long-standing intermittent pain becoming continuous, dull aching pain. Pain may be persistent and may not respond to routine analgesics. At times, it may be associated with unexplained weight loss. They may be presented with jaundice and features of gastric outlet obstruction in advanced lesions[2]. Pandey et al[29] found that weight loss was the most common symptom (reported in 201 patients, 99%), followed by loss of appetite (reported in 197 patients, 97%). Other symptoms included lower right abdominal pain (143 patients, 70%), lower right abdominal mass (107 patients, 53%), jaundice (79 patients, 39%), and nausea and vomiting (21 patients, 10%). In the authors’ institute, located in an endemic GBC area, almost all patients presented with weight loss or appetite issues. Therefore, in the absence of specific symptoms, patients with unexplained weight loss or appetite should be evaluated for GBC in endemic regions.
Ultrasonography (USG) is the most widely used method for evaluating the gallbladder (GB). The most common early finding of GBC is thickening of the GB wall. However, USG does not reliably distinguish between benign and malignant GB wall thickening. In this section, we discuss various imaging features of traditional imaging modalities, including USG, contrast-enhanced computer tomography (CECT), magnetic resonance imaging (MRI), and positron emission tomo
Ultrasound: For most patients, USG is the initial investigation they undergo. Therefore, it’s crucial to recognize the features of USG that raise suspicion about GBC. Many patients get diagnosed late due to missed findings or an inability to identify suspicious findings on USG. USG identifies multiple abnormalities, including wall thickness, polyps, associated gallstones, masses, ascites, metastases, liver infiltration, and involvement of adjacent structures. Gallstones are often present along with a mass in 60%-90% of cases[30]. Various USG findings that suggest malignant GB lesions include focal and asymmetric wall thickening, discontinuous mucosa, loss of the layered pattern of the GB wall, high peak systolic velocity in doppler, high shear velocity in elastography, and a mass filling the GB lumen[30]. Gupta et al[31] introduced a GB reporting and data system based on GB wall features observed on ultrasound (US). These features include the symmetry and extent (focal vs circumferential) of involvement, intramural characteristics (such as intramural cysts and echogenic foci), layered appearance, and the interface with the liver. This system, based on risk stratification, recommends six categories (GB reporting and data system: 0-5) of GB wall thickening, indicating a progressively increasing risk of malignancy. Batra et al[32] found that the most frequent finding on US was GB mass replacement, observed in 73% of cases, followed by the presence of both a gallstone and a mass in 54% of cases. Rana et al[33] proposed a sonographic “Cervix Sign” for GB neck malignancy. So, early identification of mass in the GB results in a prompt referral to an expert center. As USG is the most basic investigation, the question arises if it can be used as a screening strategy in high-risk populations like North India, Chile and Pakistan.
CEUS: Kong et al[34] found that a higher rate of CEUS had higher sensitivity than USG in detecting GB malignancy. Xu et al[35] found that focal GB wall thickening, inner wall discontinuity, and outer wall discontinuity are distinctive features of GBC. Figueiredo et al[36] found higher sensitivity of CEUS than MRI in detecting GB cancer. Features in CEUS suggestive of malignancy include non-homogeneous arterial phase enhancement, dotted intralesional vascularity, washout time of less than 40 seconds, and liver parenchymal infiltration.
Contrast-enhanced computed tomography: Contrast-enhanced computed tomography (CECT) is the preferred diagnostic modality for staging the disease. Kalra et al[37] found CECT showed a sensitivity of 72.7%, a specificity of 100%, and an overall accuracy of 85% in assessing the resectability of GBC. In detecting hepatic and vascular invasion by the tumor, computed tomography (CT) findings were in complete agreement with surgical findings. Kumaran et al[38] found that CECT had an accuracy of 93.3% in the staging of GBC. Pruthi et al[39] studied the role of dual-energy CT and found that asymmetrical mural thickening and non-layered mural thickening were observed in all patients on iodine maps, as well as at 80 keV and 140 keV. Iodine maps showed heterogeneous enhancement in all patients. The author concluded that dual-energy CT has a clear benefit in identifying malignant GB thickening. CECT findings that directly indicate GBC include irregular GB wall contours, submucosal edema, localized wall thickening, interruption of the mucosal lining, presence of a polypoidal mass, and evidence of direct invasion into the neighboring organs, etc[40]. The indirect signs of malignancy include biliary obstruction, regional lymphadenopathy, paraaortic lymphadenopathy, and distant metastasis. Corwin et al[41] based on CT findings, classified incidental focal fundal GB wall thickening into six types: type 1 (nodular/pinched intramural low attenuation), type 2 (intramural low attenuation), type 3 (homogeneous enhancement), type 4 (nodular/pinched homogeneous enhancement), type 5 (intramural cystic spaces), and type 6 (hyper-enhancing/heterogeneous enhancement). It was found that types 3 and 6 thickening were malignant.
MRI: Kalage et al[42] found that MRI has higher sensitivity than CT in detecting malignant GB thickening. Han et al[43] proposed a scoring system based on nine MRI features indicative of malignant transformation: Diffuse GB wall thickening, mucosal retraction, loss of mucosal uniformity, presence of gallstones, intramural T2 hyperintensity, inter
PET scan: (18)F-fluorodeoxyglucose (FDG) uptake in GB may result from both inflammatory and cancerous conditions. PET-CT is usually indicated for evaluating distant metastasis but can also be used when there is GB wall thickening and any diagnostic dilemma with conventional imaging methods. Both the GB wall thickness and the standardized uptake value (SUVmax) are important in diagnosing GBC and play complementary roles in diagnosis[47]. Benign conditions usually have a lower SUVmax value compared to malignant lesions[48]. Ramos-Font et al[47] found that at a cutoff value of 3.62, diagnostic accuracy was 95.9%. Gupta et al[49] reported a sensitivity and specificity of 92% and 79%, respectively, at a cutoff value of SUVmax of 5.95 and 94% and 67% when using a cutoff value of 8.5 mm.
In Table 2[50-68], we have summarized the various imaging features of malignant GB lesions from various studies. In addition to diagnostic values, other factors such as cost and the availability of various imaging modalities also influence detection in resource-poor countries like India. USG is the most widely available modality, being both easily accessible and the least expensive option; it is the first investigation that most patients undergo. Therefore, it is essential for radiologists and clinicians to understand the various features indicative of malignant changes. Table 3[45,55,57,60,61,69-82] summarizes the sensitivity, specificity and limitations of conventional imaging modalities in the detection of early GBC.
| Imaging modalities | Features suggestive of malignancy | References |
| USG | Localized thickening, mass and a stone GB lumen with localized thickening | [50] |
| Mass along with thickening | [51] | |
| Polypoidal mass with wall thickening > 1 cm, hypoechogenicity, internal hypoechoic foci | [52] | |
| Loss of layered structure and enhancement of wall | [53] | |
| Solitary gallstone, displaced stone, intraluminal mass, GB-replacing or invasive mass, discontinuity of the mucosal echo | [54] | |
| CEUS | Arterial phase inhomogeneous hyperenhancement, venous phase hypoenhancement and disruption of GB wall layer structure | [55] |
| Rapid GB wall blood flow and the irregularity of color signal patterns on doppler imaging, and heterogeneous enhancement in the venous phase, focal thickening, discontinuity and irregularity of the innermost hyperechoic layer and irregular or disrupted GB wall layer structure | [56] | |
| Differences in enhancement direction, vascular morphology, serous layer continuity, wash-out time and mural layering in the venous phase | [57] | |
| An irregular shape, branched intralesional vessels, and hypo-enhancement in the late phase | [58] | |
| Homogeneous enhancement in the arterial phase, followed by interrupted inner layer, early washout (≤ 40 seconds), and wall thickness > 1.6 cm | [59] | |
| CECT | The thicknesses of the inner and outer layers (“thick” enhancing inner layer > or = 2.6 mm, “thin” outer layer < or = 3.4 mm), strong enhancement of the inner wall, Irregular contour GB wall, layering pattern - two-layer pattern with a strongly enhancing thick inner layer and weakly enhancing or nonenhancing outer layer and the one-layer pattern with a heterogeneously enhancing thick layer | [60] |
| Wall irregularity, focal wall thickening, discontinuous mucosa, submucosal edema, polypoid mass, direct invasion to adjacent organ, biliary obstruction, regional and paraaortic lymphadenopathy and distant metastasis | [61] | |
| The thickened GB wall with one-layer heterogeneous enhancement (type 1) | ||
| Mass replacing GB, diffuse/focal GB wall thickening and polypoidal mass, associated cholelithiasis, liver infiltration, intra hepatic biliary dilatation, liver metastases, portal vein invasion, antroduodenal and hepatic flexure involvement | [62] | |
| Heterogeneous peripheral and central enhancement in arterial phase | [63] | |
| MRI | Heterogeneous enhancement, indistinct interface with the liver, and diffusion restriction | |
| T2 moderate hyperintensity of the thickened wall, papillary appearance, and diffusion restriction | [44] | |
| Discontinuous enhancing mucosal line, earlier enhancement of wall, diffusion restriction, lower mean ADC in malignant wall | [64] | |
| Diffusion restriction | [65] | |
| Low ADC and diffusion restriction | [66] | |
| Diffusion-weighted examination with a high b value | [67] | |
| PET Scan | Higher SUV uptake | [68] |
| Delayed uptake in dual phase PET |
| Imaging modalities | SN | SP | Accuracy | Advantages | Limitations | References |
| USG | 65%-94% | 70%-95% | 80%-90% | Inexpensive, non-invasive, portable, and widely available | Low accuracy in differentiating benign vs malignant GB wall thickening | [45,69,70] |
| Can flag suspicious case | Malignant small sessile polyps | |||||
| Operator dependent | ||||||
| Nodal involvement | ||||||
| CEUS | 90%-100% | 90%-95% | 55%-90% | Portable | Smaller lesion gives false positive results so less sensitive for smaller lesions | [55,57,71-74] |
| Better modality for detecting vascularity and lesion characterization | Operator dependent | |||||
| Artefacts | ||||||
| Not available widely | ||||||
| CECT | 70%-100% | 40%-100% | 75%-95% | Better characterization staging | Limited sensitivity in small polyps, T1 lesions, thick walled | [60,61,75-77] |
| Better anatomical detail | High cost | |||||
| Lymph node involvement | Radiation exposure | |||||
| Poor specificity to differentiate XGC, AC and GBC | ||||||
| Not available in rural areas | ||||||
| MRI | 70%-88% | 60%-70% | 92% | Excellent soft tissue contrast, good for delineating bile duct involvement | Miss early lesions with subtle findings | [78-80] |
| Overdiagnosis of benign lesions | ||||||
| High cost | ||||||
| Not readily available in rural areas | ||||||
| PET | 70%-80% | 80%-85% | 50%-70% | Only for distant metastasis | Cannot accurately differentiate benign inflammation and malignant thickening | [81,82] |
| High cost and not readily available |
Traditional imaging modalities, like USG, CT, and MRI, are often used when there are obvious symptoms, and the disease is in an advanced stage. In addition, they lack the specificity to differentiate between benign and malignant lesions. The newer imaging modalities may be useful tools for early diagnosis.
Real-time elastography (RTE) differentiates tissues according to their rigidity, proving to be a promising method for distinguishing malignant from benign tissue. Kapoor et al[83] found that, at a cut-off value of 2.7 m/second, elastography showed an overall accuracy of 92.8% with sensitivity and specificity of 100% and 91.3%, respectively, for diagnosing GB carcinoma. RTE leveraging acoustic radiation force impulse is effective in distinguishing benign from malignant GB masses. This technique relies on the concept that malignant tissues exhibit significantly greater stiffness, resulting from higher cell density, in contrast to tissues affected by chronic inflammation and fibrosis. Teber et al[84] studied the RTE of GB polyps and discovered that benign GB polyps on successive RTE images exhibited a high-strain pattern. Soun
Aside from detecting malignant GB conditions, imaging also helps to differentiate early from late GBC. Some of the imaging findings are specific (Table 4) for the early detection of GBC. Based on the imaging features above, in the next section, we describe various conditions, such as polyps, thick-walled GB, XGC, and calcified GB, that are at risk of developing cancer or are associated with cancer. We have delineated important features that differentiate them from malignancy.
| Modalities | Imaging characteristics |
| USG | Polyp |
| Focal thickening | |
| Asymmetric thickening | |
| Subtle GB wall thickening | |
| Irregular thickening | |
| CECT | Subtle wall thickening |
| Localized thickening > 4 mm | |
| Small polyp | |
| CEUS | Dotted intralesional blood vessels |
| Arterial phase inhomogeneous hyperenhancement, venous phase hypoenhancement and disruption of GB wall layer structure | |
| RTE | Higher mean shear wave elasticity |
| PET scan | Higher SUV uptake |
GB polyps can be divided into neoplastic and non-neoplastic polyps. Neoplastic polyps are adenomas and adenocarcinomas, and non-neoplastic polyps are cholesterol polyps, inflammatory polyps and adenomyomatosis, etc. Among newer modalities, endoscopic US (EUS) can distinguish the two-layered architecture of the GB wall and offers superior resolution for detecting small polypoid lesions. Choi et al[86], based on five parameters (layer structure, the margin of the polyps, echo patterns, and stalk and polyp numbers), proposed a scoring system to detect malignant polyps. Similarly, Sadamoto et al[87], based on EUS, proposed a formula: maximum diameter (in millimeters) + internal echo pattern score (heterogenous = 4, homogenous = 0) + hyperechoic spot (5) to differentiate benign vs malignant polyps. The PET scan can also be used to diagnose malignant GB polyps. Lee et al[88] found that the SUVmax of GB polyp and the ratio of SUVmax of polyp mean SUV of the liver (GP/L ratio) were high predictors of malignancy. Another study found that delayed (18) F-FDG PET is more helpful than early (18) F-FDG PET for evaluating malignant lesions because of heightened uptake within the lesion and improved contrast between the lesion and surrounding tissue[68]. Various other commonly used investigations that were used to differentiate benign from malignant GB polyps are described in Table 5[52,74,89-100].
| Modalities | Factors favoring malignant polyps | References |
| Clinical | Endemic areas, associated with PSC, old age > 60 years | [90-94] |
| USG | Single lobular surface, vascular core, hypo-echoic polyp and hypoechoic foci or a polyp size of greater than 1 cm, associated gall stones, sessile polyp, localized GB wall thickening, associated gall stone disease, focal gallbladder wall thickening > 4 mm, growth 2-4 mm/year | [92,95-99] |
| CEUS | Fast-in and “fast-out” enhancement pattern, hyper-enhancement in comparison to the GB wall in the arterial phase, wash-out time ≤ 40 seconds, GB wall destruction, and hepatic parenchymal infiltration, diffuse and branched types of contrast enhancement | [74,100] |
| CECT | Enhancement of polyp, mass filling GB lumen, liver infiltration, surrounding lymphadenopathy, associated asymmetric thickening, | [52] |
| PET scan | High SUV max | [89] |
Many cases of GBC present with only wall thickening without any mass or associated stone. Wall thickening usually refers to a thickness greater than 3 mm. In Table 6, we have summarized the findings that differentiate benign from malignant wall thickening from the literature.
| Modalities | Benign wall thickening | Malignant wall thickening |
| USG | Diffuse and symmetric | Focal and asymmetric |
| Intact mucosa | Discontinuous mucosa | |
| Layered GB wall | Loss of layering | |
| Low mean flow velocity and peak systolic velocity | High mean flow velocity and Peak systolic velocity | |
| Low shear wave velocity | High shear wave velocity | |
| Liver parenchyma infiltration absent | Liver parenchyma infiltration present | |
| CEUS | Homogenous arterial phase enhancement | Non-homogenous arterial phase enhancement |
| Tortuous intralesional vascularity | Dotted intralesional vascularity | |
| Delayed washout | Early washout | |
| CECT | Homogenous enhancement | Heterogenous enhancement |
| If layering present inner layer is enhancing | If layering present inner layer enhancing | |
| Lymphadenopathy usually absent | Present | |
| Symmetric | Asymmetric | |
| MRI | On T2, thin hypointense inner layer and thick hyperintense outer layer or multiple T2 hyperintense foci in wall | Diffuse nodular thickening without |
| Delayed enhancement | Layering | |
| High ADC | Early enhancement in contrast phase | |
| Low ADC |
Various studies have found that pancreaticobiliary reflux is a significant risk factor for biliary tract cancer in patients with pancreatic-biliary malunion (PBM). Kamisawa et al[100] found that among 49 patients with PBM, without biliary dilatation, GBC was identified in only 33 cases. Misra et al[101] also found that an abnormally long common channel (greater than or equal to 8 mm) was seen more frequently in carcinoma of the GB compared with normal subjects and patients with gallstones. Sugiyama et al[102] also found a similar finding that PBM without biliary dilatation is associated with an increased risk of malignancy. Therefore, when identified, it should be kept in mind that these patients have a very high risk of malignancy and carefully further evaluated.
The presence of mural calcification is considered premalignant, and previous studies have documented a malignancy rate of 7%-60%[103]. Recently, however, it has become clear that not all patients with porcelain GBs are pre-malignant. Stephen et al[104] noted that there are two categories of calcified GBs: One exhibiting complete intramural calcification and the other showing selective mucosal calcification. The likelihood of cancer developing in a GB with selective mucosal wall calcification is about 7%, while none of the patients with diffuse intramural calcification have been found to develop cancer. According to current evidence, the incidence of GBC, in general, is around 2% to 8%, particularly among those with partial presence of calcification adherent to intact mucosa. There appears to be no GBC risk in specimens where the GB wall is replaced with calcium, and there is no intact mucosa[103,105].
XGC is a malignant masquerade of GBC. This is a long-lasting inflammatory condition of the GB, linked to GBC in 8.5% to 30.5% of instances[106-108]. The association is crucial because the presence of both lesions in the same specimen can lead to overlooking the carcinoma entirely. Therefore, it is essential to identify the XGC pre-operatively. The Presence of hypoechoic nodules or bands in the thickened wall is regarded as a characteristic finding of XGC. Hypoechoic nodules on sonography have been observed in 15% and 73% of cases by Parra et al[109] and Kim et al[110], respectively. CECT commonly shows symmetrical diffuse thickening in XGC, though 22.2% of cases display asymmetrical thickening. The intramural nodules were detected in 85.7% and 61.1%, respectively, by Zhao et al[111] and Goshima et al[112]. XGC affects the pathology affecting the GB wall, where the mucosal surface remains either intact or slightly damaged. In contrast, GB carcinoma develops from the GB epithelium, leading to significant mucosal disruption in most cases. Mucosal disruption in the XGC mucosal lining is more likely to indicate malignant change. Enhancement of the luminal surface (LSE) is characterized by continuous mucosal lines or mucosal lines showing focal breaches linked to XGC. LSE was noted in 70% of cases by Shuto et al[113]. LSE and preservation of the epithelial layer point towards benign disease[113]. So, it’s important to identify the subset of the population with XGC with a risk of malignant features for early evaluation.
Recent research has focused on refining the diagnostic and prognostic utility of existing biomarkers, developing new biomarkers such as liquid biopsy tools, and enhancing imaging techniques that can more effectively distinguish between benign and malignant pathologies. In this section, we have described various markers and their clinical utility in the early diagnosis of GBC.
Serological markers: Previous studies have failed to demonstrate the utility of biomarkers like carbohydrate antigen (CA) 19-9 (CA19-9), carcinoembryonic antigen (CEA), CA125, and CA242 in diagnosing GBC due to low sensitivity and specificity. Wang et al[114] studied CEA, CA125, CA242, and CA19-9 and found that CA19-9 had the highest sensitivity (71.7%), while CA242 had the highest specificity (98.7%). Furthermore, inflammatory disorders did not impact CA242 levels. The author also found that a combination of CA19.9, CA242, and CA125 had the highest diagnostic accuracy (69.2%). In Table 7, we have described the diagnostic utility of serum biomarkers in GBC[115-122].
| Serial number | Biological markers | Cut off value | Sensitivity | Specificity | Ref. |
| 1 | CEA | 5 ng/mL | 52% | 55% | [115] |
| CA19-9 | 37 U/mL | 74% | 82% | ||
| CYFRA 21-1 | 2.7 ng/mL | 76% | 79% | ||
| MMP7 | 7.5 ng/mL | 78% | 77% | ||
| Combination of all four | - | 92% | 96% | ||
| 2 | CEA | 1.95 ng/mL | 90.2% | 35.29% | [116] |
| CA19-9 | 26 U/mL | 58.82% | 83.82% | ||
| Combination of the two | - | 90.2% | 88.24% | ||
| 3 | CEA | 10 μg/L | 11.5% | 97.4% | [117] |
| CA19-9 | 39 U/mL | 71.7% | 96.1% | ||
| CA242 | 15 U/mL | 64.1% | 98.7% | ||
| CA125 | 35 U/mL | 44.8% | 96.2% | ||
| 4 | CA 242 | 20 U/mL | 64% | 84% | [118] |
| CEA | 5 U/mL | 61% | 44% | ||
| CA 19–9 | 35 U/mL | 17% | 67% | ||
| 5 | CYFRA 21-1 | 3.27 ng/mL | 93.7% | 96.2% | [119] |
| CYFRA 21-1 | 2.61 ng/mL | 74.6% | 84.6% | ||
| CYFRA 21-1 | 3.27 ng/mL | 75.6% | 96.2% | ||
| CYFRA 21-1 | 2.27 ng/mL | 71.0% | 71.2 % | ||
| 6 | CA 19-9 | 39 U/mL | 71.3% | 90.0% | [120] |
| CA 125 | 36 U/mL | 38.8% | 93.3% | ||
| CEA | 10.36 U/mL | 12.5% | 92.5% | ||
| CA 242 | 15 U/mL | 86.3% | 90.0% | ||
| 7 | CA 19-9 | 252.31 U/mL | 100% | 98.90% | [121] |
| CA 125 | 92.19 U/mL | 100% | 94.50% | ||
| 8 | CA 19-9 | 250 U/mL | 76.3% | 70.8% | [122] |
Among some newer biomarkers, Oshikiri et al[123] found high receptor-binding cancer antigen expressed on SiSo cells (RCAS1) expression in 32 of the 46 GBC specimens (70%) but not in cases of cholecystitis, adenomyomatosis and adenomas. However, the author found that RCAS1 expression was believed to promote tumor progression and to be a relatively late event in GB carcinogenesis, thereby undermining the role of RCAS1 in the early-stage diagnosis. Giaginis et al[124] also suggested the role of RCAS1 expression only in tumor prognosis, not in early diagnosis. Koopmann et al[125] evaluated the performance of Mac-2 binding protein and its ligand, galectin-3, as diagnostic markers of biliary carcinoma and found that it can differentiate between malignant and benign biliary diseases with sensitivity and specificity of 69% and 67%, and the AUC was 0.70. Ghimire et al[126] evaluated the levels of cytokeratin-19 fragment antigen 21-1 (CYFRA 21-1) and CA19-9 in 61 patients diagnosed with biliary tract cancers, most notably GBC as the prevalent type of group. They found that CYFRA 21-1 had a sensitivity of 80.3%, and CA19-9 had a sensitivity of 68.9 % for detecting biliary tract cancer. More recently, Huang et al[119] discovered that CYFRA 21-1 at a cut-off value of 3.27 (ng/mL) outperformed CEA and CA19-9 in diagnosing GB carcinoma and intrahepatic cholangiocarcinoma.
Susceptible genetic markers: The pathophysiology of GBC is multifactorial and influenced by several risk factors, including predisposing conditions, gallstones, chronic cholecystitis, porcelain GB, GB polyps, obesity, and exposure to toxic minerals. Genetic predisposition also plays a crucial role, as highlighted in various studies. Somatic mutations are present in 90.0 % of GBC[127]. This highlights the importance of detecting somatic genetic mutations in GBC in suspected early-stage disease, as it may aid in identifying high-risk individuals and potential therapeutic targets. By analyzing genetic factors, researchers have identified critical genes and specific patient subgroups at increased risk of developing GBC, thus facilitating early detection and timely intervention. Genome-wide association studies have identified numerous genetic variants associated with a heightened risk of developing GBC. These molecular genetic markers are based on histological specimen analysis and may play a significant role in early diagnosis. Various genetic markers are described in Table 8[128-171].
| Types of genetic markers | Genes | Ref. |
| Inflammatory markers | CR1, PTGS2 (cyclooxygenase), TLR, sTNFR2, IL-6, sTNFR1, CCL20, VCAM-1, IL-16, G-CSF, TGFb1, IL-8, MMP-2,7,9 | [128-134] |
| Metabolic pathway genes | CYP1A1, Ile462Val (rs1048943), (Japanese and Hungarian population), IVS1 + 606 (rs2606345) T allele of CYP1A1 (Chinese population), GSTM1 (Bolivian population), MTHFR, APOB, NAT2, GSTT1, GSTP1 CYP17, LDLR, LPL, ALOX5, ABCG8, CETP, LAPAP1, ApoB, CYP17A1ADRB3 | [135-146] |
| DNA repair pathway genes | CC genotype of TP53 (India), ERCC2, IVS1 + 9G > C in the MSH2, Ser326Cys in the 8-OGG1, EX5-25C>T in the O6-aky guanine DNA acyltransferase (MGMT), APEX1, RAD23B, FEN1 | [147-150] |
| Hormone pathway genes | CCKAR, ESR1, ESR2, CYP1A1, CYP19A, HSD3B2, RXR- a PPARD | [151-156] |
| Apoptosis pathway | DR4 haplotype C rs20575 A rs20576 A rs6557634, caspase-8 | [157-158] |
| Cancer Stem cell gene | CD44, NANOG, ALCAM, EpCAM, SOX-2, OCT-4, and NANOG | [159] |
| MiRNA | hsa-miR-146a, hsa-mir-196a2, hsa-mir-499 miR-27a (rs895819) A>G | [160,161] |
| WNT signalling pathway | SFRP4, DKK2, DKK3, APC, AXIN-2, Β-CATENIN, GLI-1 | [162] |
| Genome-wide association study | SERPINB5, BCL10, CD44, ARHGEF11 SERPINB2, RELA, PAK4, PPARD and BUB1B. SNP rs7504990 in the DCC (Japan), SNPs in ABCB1 and ABCB4 genes (India) | [163-165] |
| Others | KRAS (codon 12,13,61), c-erb-B2, ACE I/D, DNMT3B, VDR | [166-171] |
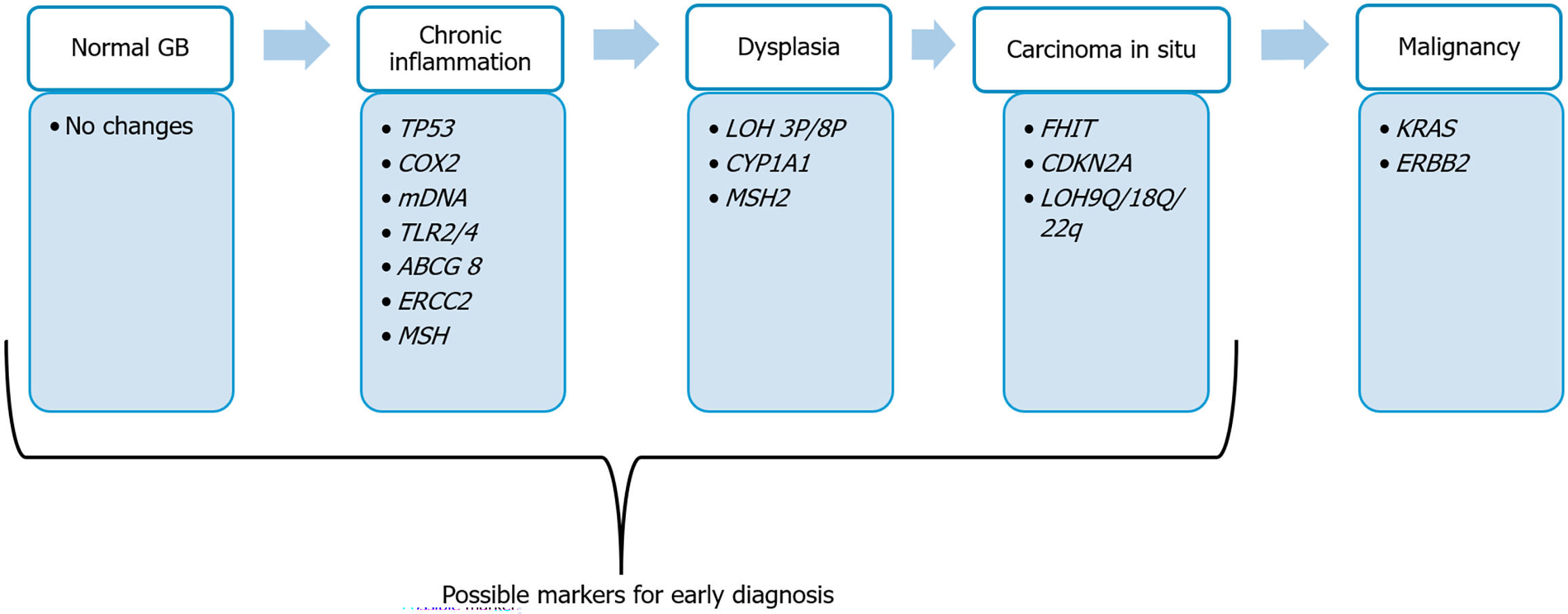
Barreto et al[172] proposed a genetic model similar to the adenoma-carcinoma sequence for GBC, illustrating how genetic changes shift from chronic inflammation to malignant transformation (Figure 1). Genetic mutations at different stages of pathogenesis, such as from inflammation to carcinoma in situ, can be used to detect GBC at an early stage. These mutations can be investigated in high-risk populations for the early diagnosis of GBC, such as individuals with chronic cholecystitis of long duration and from the high GBC belt, and also for screening purposes. In addition to diagnosis, these markers can be used for various diagnostic and therapeutic applications. In Table 9[131,136,137,149,168,173-179], we have summarized some of the genes that have demonstrated clinical utility in the early diagnosis of GBC in small cohort studies and can be considered for testing in high-risk conditions, as outlined in section 1 for early identification and risk stratification.
| Genes | Mutation | Method of identification | Role in early diagnosis | Ref. |
| TLR2, TLR4 | Polymorphism | PCR-RFLP | TLR4 Ex4 + 936C>T polymorphism (g.14143C>T; rs4986791) significantly associated with the overall higher risk of GBC in north Indian population | [131] |
| CYP1A1 (rs2606345) | Polymorphism | TaqMan assay | Associated with increased risk of biliary tract cancer in Chinese populations | [136] |
| TP 53 | Point mutation/loss of heterozygosity | PCR/RFLP | Found in both early and advanced GBC, associated with pre malignant conditions in Indian, Japanese Chile and bolivian population | [137,173-175] |
| KRAS | Mutation (codon 12/13) | PCR-RFLP | Seen in early lesions; potential marker for pre-malignant transformation | [176] |
| CDKN2A (p16) | Promoter methylation or loss of homozygosity | PCR-RFLP | Acquisition of hypermethylation contribute to tumor formation and progression within the chronically inflamed gallbladder at early stage of carcinogenesis | [177,178] |
| ABCG8 | Polymorphism | GWAS/PCR-RFLP | Increased risk of GBC in patients with gall stone disease | [179] |
| ERCC2, MSH2 | - | PCR-RFLP | Associated with early steps of carcinogenesis | - |
While identifying genetic markers, such as various genes mentioned above, has diagnostic utility for early identification of high-risk groups, there are several limitations to their use as diagnostic tools. These include the absence of a universally accepted genetic panel for GBC diagnosis, the high cost and requirement for molecular laboratories, low awareness and accessibility in resource-poor settings, Genetic predisposition varies widely among populations and a lack of extensive data regarding real-world clinical utility.
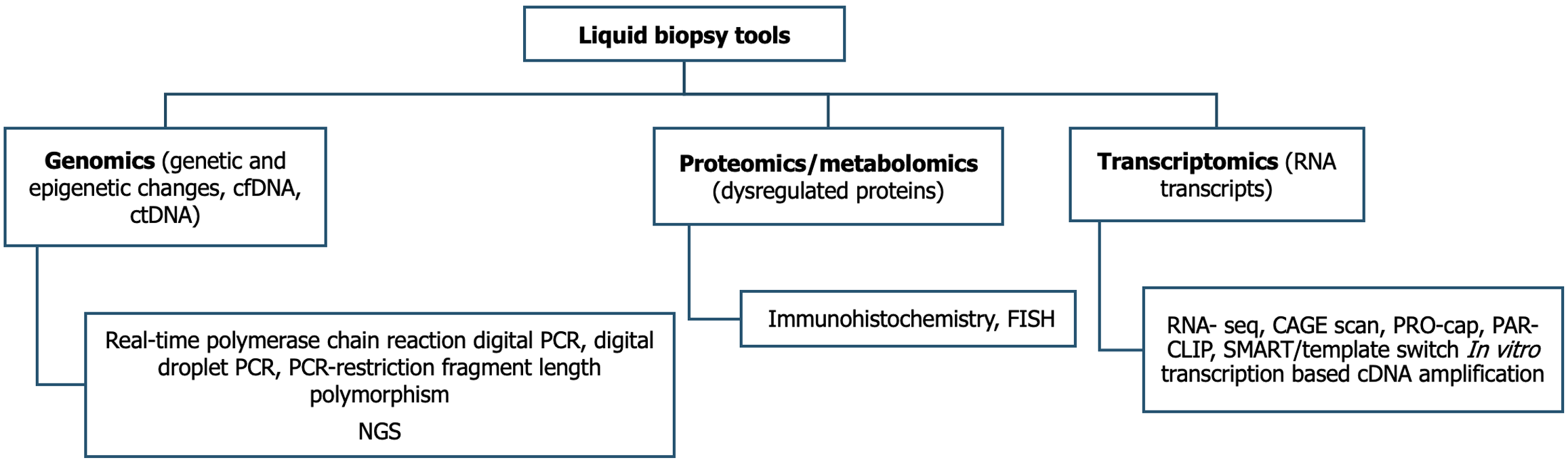
Liquid biopsy tools in early diagnosis: Over the last decade, the concept of liquid biopsy has evolved, which involves detecting and analyzing circulating tumor DNA (ctDNA), cell-free DNA (cfDNA), and microRNA (miRNA), circulating tumor cells (CTCs), and extracellular tumor vesicles released into blood and other body fluids like bile and urine[180]. One method in liquid biopsy, which provides information about the tumor through CTCs, circulating tumor DNA, miRNAs, and exosomes released by the tumor, collectively known as genomics. Another method is proteomics/metabolomics, which reflects specific conditions in the tumor. The third method is transcriptomics, which involves the study of RNA transcripts. Genomics uses various genes, DNA, mRNA, etc., for analysis via quantitative real-time polymerase chain reaction (PCR), digital PCR, digital droplet PCR, polymerase chain reaction-restriction fragment length polymorphism, and next-generation sequencing (NGS). Proteomics measures various proteins derived from tumor cells via immunohistochemistry and fluorescence in situ hybridization methods (Figure 2)[181].
The role of liquid biopsy as a non-invasive diagnostic biomarker compared to tissue biopsy has recently emerged. Assessing blood, urine, and saliva is simpler than evaluating tissue samples, leading to a faster process and simpler method for diagnosis[175,176]. Liquid biopsy, although highly useful in metastatic testing, has a less studied role in early diagnosis and faces many constraints. Tumors in early stages exhibit a low variety of alleles, resulting in diminished diagnostic accuracy for their detection variants[182]. Similarly, ctDNA utilized in genetic testing constitutes a minuscule fraction of the tumor, occasionally dropping to as low as 0.01%. CTC are typically present in low quantities in early-stage tumors, making their detection and quantification particularly challenging compared to late-stage or metastatic GBC. Another constraint involves the higher blood volume needed for CTC detection. Moreover, elements like sample integrity, the fleeting nature of CTCs, and interference from circulating nucleic acids can greatly impact result reliability[127]. Moreover, sample integrity, the short lifespan of CTCs, and the influence of circulating nucleic acids impact the outcome results. As a result, the role in early diagnosis is not well established because of the low sensitivity of liquid biopsy methods.
Increased levels of cfDNA have already been reported in various malignancies, including colon, head and neck, gastric, pancreatic, and hepatocellular cancers[183]. Kumari et al[184] found that cfDNA was significantly higher in cancer patients versus cholecystitis and control groups, with a cut-off of > 218.55 ng/mL showing 100% sensitivity (95%CI: 89.6-100; P < 0.001) and specificity (95%CI: 80.3-100; P < 0.001) for GBC compared to controls. For GBC versus patients with cholecystitis, the cut-off of > 372.92 ng/mL provided 88.24% sensitivity (95%CI: 72.5-96.6; P < 0.001) and 100.00% specificity (95%CI: 84.4-100.0; P < 0.001) for cholecystitis. Another study by Kumari et al[185] studied the role of cfDNA as a liquid biopsy in the diagnosis of GBC utilizing levels of long DNA fragments (ALU247) derived from tumor necrosis, short apoptotic fragments (ALU115) denoting total cfDNA and cfDNA integrity denoting the ratio of ALU247 and ALU115. They found that the sensitivity and specificity of ALU247 in discriminating GBC from controls were highest, with a sensitivity, specificity and diagnostic accuracy of 80.0%, 86.1% and 82.2%, respectively. Shen et al[186] studied ten biliary tract cancer patients, four with GB carcinomas and six with cholangiocarcinomas, aiming to identify individual actionable mutations derived from bile cfDNA using targeted deep sequencing. The study found high sensitivity (94.7%) and specificity (99.9%) when comparing bile cfDNA and tumor DNA for single nucleotide variation/insertion and deletion. For copy number variation, sensitivity was 75.0%, with a specificity of 98.9%. The percutaneous or operative method of bile sampling showed no significant difference in single nucleotide variation/insertion and deletion or copy number variation detection sensitivity. Mishra et al[187] found that NGS-based cfDNA mutation profiling can be used to diagnose GBC before surgery and guide treatment decisions. Currently, ctDNA/cfDNA has no proven role in early diagnosis, but newer tools and technology, in future research, may come with its usefulness in early GBC.
Since ctDNA/cfDNA sensitivity is low in early-stage cancer patients, large-scale epigenetic changes that are specific to tissue and cancer types may be more effective in detecting and classifying cancers in early-stage disease. Various studies have also demonstrated that epigenetic alterations in cfDNA, which are tissue and cancer-type specific have a more remarkable ability to detect and classify cancers in patients with early-stage disease. NGS can detect these epigenetic changes easily[188-190].
It is rare to find CTCs in peripheral blood (approximately 1 CTC per 106-10 leukocytes); however, their detection and quantification may identify cancer patients at high risk of metastasis and indicate tumor progression rather than early diagnosis[127]. In a study by Awasthi et al[191], CTC was found in 92.59% (25/27) of GBC cases, with a median count of 4 CTCs/ml (range: 0-20), and a sensitivity of 92.6% and specificity of 91.7%, respectively. Yan et al[192] reported an overall CTC detection rate of 19.80% among 101 GBC patients, with CTC positivity observed in 61.54% (8 out of 13) of patients in the inoperable group and 13.64% (12 out of 88) in the surgical group. Due to the extremely rare peripheral smear, the role of CTC in early detection is still to be proved and only helps in prognostication rather than early diagnosis.
Various long noncoding RNAs and miRNA are epigenetic changes that can serve as early diagnostic markers. These RNAs influence cancer-related signalling pathways in GBC, including WNT/-catenin, phosphatidylinositol 3-kinase/protein kinase B, epidermal growth factor receptor, Notch, mammalian target of rapamycin and TP53[127]. Around 30% of global gene expression is regulated by miRNA, and abnormal expression of various miRNAs has been linked to numerous human cancers[193]. Their high stability enables detection in various body fluids, making them promising biomarkers for early diagnosis and prognosis[194]. Additionally, miRNAs, short non-coding RNAs, play a role in tumor initiation[195]. Yang et al[196] found that suggested miRNAs could be utilized in diagnosing and screening GBC, with their study revealing that the sensitivity of miR-4433a-3p was the highest at 96.67%, and the specificity of miR-551b-3p also reached 96.67%. Srivastava et al[197] studied the role of tumor-associated miRNA expression for the early diagnosis of GBC and found that serum levels of miR-21 and miR-182 were upregulated, while miR-130, miR-146, and miR-182 were downregulated in GBC compared to normal cholecystitis. They discovered that miR-1 exhibited the greatest sensitivity at 85.71%, while miR-21 demonstrated the highest specificity at 92.73%. Mishra et al[198] studied the role of long noncoding RNAs in early diagnosis of GBC and found that the expression of serum hox antisense intergenic RNA, antisense non-coding RNA in the ink4 locus and H19 were upregulated, and colon cancer associated transcript 1 and maternally expressed 3 were downregulated in GBC compared to normal control and cholecystitis. Ueta et al[199] studied the role of miRNAs in serum extracellular vesicles in early diagnosis of GBC and found that serum extracellular tumor vesicles miR-1246 were significantly upregulated and miR-451a was downregulated, respectively, in GBC patients (P = 0.005 and P = 0.001).
Although the field of research in liquid biopsy is expanding, there are still some technical limitations in adopting liquid biopsy in the diagnosis of GBC. The isolation of ctDNA from cfDNA - which comprises a mixture of normal DNA, non-mutant tumor DNA, and tumor DNA - can be technically challenging. The ctDNA utilized for genetic testing constitutes a minimal fraction of the tumor, occasionally amounting to as little as 0.01%. CTC are difficult to detect in early tumors and is seen more commonly in metastatic settings. A larger blood volume is also required for CTC detection, and it represents a minor tumor environment. There is a need to enhance the diagnostic tests for liquid biopsy for the early diagnosis of GBC. Table 10 summarizes the current status of liquid biopsy in GBC[193,198-201].
| Liquid biopsy | Detection method | Sensitivity and specificity | Implementation barrier in early diagnosis | Ref. |
| CTC | Flow cytometric detection, nano microfluid chip, immunoaffinity (EpCAM), microfiltration (ISET) | 55.6%, 100.0% | Very low detection rate in early stage | [193] |
| CTCs in peripheral blood (approximately 1 CTC per 106-10 Leukocytes) | ||||
| Large blood volume required to detect CTC | ||||
| Short half-life of CTC makes it difficult to analyse | ||||
| miRNAs | qRT-PCR, microarrays, NGS | 80%-90%, 80%-90% | miRNA expression can vary depending on samples | [198,199] |
| Delay in processing, and temperature fluctuations can alter miRNA levels | ||||
| Low abudance, platform dependency, lack of standardization, high cost | ||||
| LncRNA | qRT-PCR, microarrays, NGS | 84%-100% | Low abundance, delay in processing, and temperature fluctuations can alter miRNA levels | [198,199] |
| Platform dependency, lack of standardization, high cost | ||||
| ctDNA/cfDNA | qPCR, dPCR, ddPCR, NGS, high-throughput quantitative methylation assays | 78%-100%, 80%-100% | Separation of ctDNA from the cfDNA (mixture of non-mutant tumor DNA, normal DNA and tumor DNA) is technically challenging | [200,201] |
| RBBS | High false negative rate because of tumor heterogenicity | |||
| ctDNA used for genetic testing only 0.01% of tumor | ||||
| Limited representation of tumor environment | ||||
| No standardization and no universally accepted cutoff for detection | ||||
| High cost | ||||
| Low availability | ||||
| Bile as liquid biopsy | qRT-PCR, microarrays, NGS | 45.8% and 99.9% | Invasive methods to aspirate bile from GB | [202] |
| No standardized methods and cutoff value |
In biliary tract cancer, bile is in direct contact with the tumor, so it’s expected that protein, genetic material, and tumor cells are continuously shed in the bile, which helps in the detection of cancer. However, various methods to sample bile are invasive, and there is also a small risk of needle tract seedings. Various methods are percutaneous approaches like aspiration, Percutaneous transhepatic cholangiography, Percutaneous cholecystostomy, and various endoscopic approaches are Endoscopic retrograde cholangiopancreatography, Endoscopic naso-biliary drainage, EUS-guided aspiration, etc. Bile can also be used to detect cfDNA and ctDNA. In GBC, Kinugasa et al[202] using NGS, analyzed the cytologic and biliary ctDNA. They found sensitivities of 45.8% and 58.3%, respectively, with an agreement rate of 87.5%, indicating a higher sensitivity of bile ctDNA in GBC.
Nuclear magnetic resonance spectroscopy (MRS) and liquid chromatography-tandem mass spectrometry are commonly used to differentiate bile composition, aiding in distinguishing benign conditions from malignant ones. However, research is limited, and nuclear magnetic resonance could be further investigated for the early diagnosis of GBC[203].
Many new innovations are upcoming in the field of diagnostics, and they will be useful in early detection soon.
Alternative splicing may result in oncogenic protein isoforms or the absence of tumor suppressors. Around 65% of human genes are alternatively spliced. Splice variants of key genes involved in cell cycle regulation, apoptosis, and immune evasion can drive GBC progression. Some splice variants may result in epithelial-mesenchymal transition and give rise to metastasis (CD44, FGFR2). Some splice variants confer resistance to chemotherapy and targeted therapies by altering drug targets or apoptotic pathways. Circulating splice variants of RNA in blood or bile may lead to the early detection of GBC. Splice variants of some candidate genes like TP-53, BCL-X, and MCL 1 may aid in the early detection of GBC[193,204].
The tumor microenvironment, which consists of tumor cells, inflammatory cells, epithelial cells, and stromal cells, plays a crucial role in the pathogenesis of GBC and helps in the early detection of GBC. Various tumor-associated macrophages like CD163+ tumor-associated macrophages, T cell exhaustion markers like programmed death-ligand 1, cytotoxic T lymphocyte-associated antigen-4, various cytokines like interleukin 10, transforming growth factor and hypoxia and angiogenesis markers such as hypoxia-inducible factor 1-alpha and vascular endothelial growth factor, as well as endothelial markers like CD31 and angiopoietin-2, can facilitate the early detection of GBC. Additionally, cancer-associated fibroblasts and extracellular matrix markers such as alpha-smooth muscle actin, fibronectin, matrix metalloproteinases-9, and matrix metalloproteinases-2 can also be utilized for early detection and prognostication. These potential markers can be detected through liquid biopsy, enzyme-linked immunosorbent assay, multiplex assays, and AI-based prediction models for early detection[193,205].
MRS has emerged as a valuable technique for assessing biochemical changes linked to cancer tissues. MRS enables a non-invasive evaluation of metabolite levels in tissues, shedding light on cellular processes frequently disrupted in cancers such as GBC[206]. The utilization of MRS to assess choline levels in GBC represents a significant research focus. MRS allows for a non-invasive evaluation of metabolite concentrations in tissues, such as choline, which is part of cell membranes. elevated choline levels are linked to increased cell proliferation and malignant transformation, suggesting that MRS could serve as a valuable tool for identifying higher choline levels as a biomarker for GBC detection. There are not many advances in the use of MRS in GBC, but the use of MRS alone or in combination with other modalities may enhance the early detection of GBC[207].
Artificial intelligence (AI)-based algorithms can be integrated into CT scans to analyze GBC. Deep learning AI can examine CT scans and deliver accurate diagnoses. Deep learning algorithms have demonstrated exceptionally promising results, significantly improving the efficacy and accuracy in early diagnosis of GBC[208]. Fujita et al[209] achieved accuracy rates of 98.35% by utilizing deep neural networks and MobileNet architectures. Radiomics analysis and computer-aided diagnosis programs can also be used to improve diagnostic efficacy[210]. AI models based on demo
The authors proposed a Scoring system for the further evaluation of patients to identify early GBC. Based on demographics, clinical features, and imaging findings, the authors propose a scoring system to further evaluate patients for early detection of GBC. This scoring system (Table 11) aims to ensure that individuals presenting with biliary colic and specific imaging findings are considered for additional evaluation.
| Patient and imaging factors | Presence or absence of factor | Score |
| Area from patients belongs | High Endemic area (Chile, Gangetic belt in India, Pakistan, Bolivia) | 3 |
| Moderate endemic area (Japan, Korea, Latin America) | 2 | |
| Low endemic area (United States) | ||
| Non endemic area | 1 | |
| Middle or old aged female | Yes | 2 |
| No | 1 | |
| Long standing biliary colicky pain | Yes | 2 |
| No | 1 | |
| Recent change in character of pain | Yes | 2 |
| No | 1 | |
| Unexplained weight loss present | Yes | 2 |
| No | 1 | |
| Any associated risk factors like PSC, PBM, calcific gall bladder | Yes | 1 for each |
| Gall stone size | > 3 cm | 2 |
| < 3 cm | 1 | |
| Polyp | Polyp with lobular surface, vascular core or hypo-echoic polyp or hypoechoic foci or a polyp size of greater than 1 cm or sessile polyp | 2 |
| Other polyps | 1 | |
| Mass and texture of gall bladder wall | Mass filling GB | 3 |
| Asymmetrical wall thickness | 1 | |
| Loss of layered structure of wall localized GB wall thickening > 4 mm | 1 | |
| Normal GB wall | 1 |
Based on the scoring system, patients can be divided into three groups: Very high risk (> 15), high risk (11-14), inter
Despite several advances in treatment modalities for GBC, the early detection rate remains very poor and presents a challenge for surgeons. Most GBCs are identified at a late stage due to non-specific signs and symptoms. So, it’s important to identify high-risk populations, such as patients with GB wall thickening or polyps who have a long duration of symptoms and are from high GBC belt areas, along with those experiencing weight loss or appetite changes, for further evaluation to detect early GBC. Currently, the diagnosis is mainly based on a high index of clinical suspicion and on imaging modalities. So, every surgeon and radiologist should be aware of the early imaging features of GBC, such as asymmetrical wall thickening, loss of layering on USG, discontinuity, and irregularity of the innermost enhancing layer CECT. Judicious use of newer imaging tools, molecular genetics, and biomarkers - alone or in combination - is essential for detecting early disease. Single diagnostic tools are still not very effective in early diagnosis; a multimodal approach is necessary in suspected cases. Further research is required to validate and standardize the real-world applicability of the newer imaging modalities, markers, tools, and technology in the early detection of GBC.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 8084] [Article Influence: 8084.0] [Reference Citation Analysis (2)] |
| 2. | Kumar A, Sarangi Y, Gupta A, Sharma A. Gallbladder cancer: Progress in the Indian subcontinent. World J Clin Oncol. 2024;15:695-716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 3. | Zhu X, Zhang X, Hu X, Ren H, Wu S, Wu J, Wu G, Si X, Wang B. Survival analysis of patients with primary gallbladder cancer from 2010 to 2015: A retrospective study based on SEER data. Medicine (Baltimore). 2020;99:e22292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Shridhar K, Kapoor R, Goodman M, Kondal D, Narang K, Singh P, Thakur JS, Dhillon PK. Lung and gallbladder cancer survival in north India: an ambidirectional feasibility cohort study using telephone interviews. J Glob Health Rep. 2020;4:e2020053. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Patkar S, Patel S, Gupta A, Ostwal V, Ramaswamy A, Shetty N, Goel M. Lessons learnt from 1300 consecutive gallbladder cancer surgeries: Evolving role of peri-operative chemotherapy in the treatment paradigm. Eur J Surg Oncol. 2023;49:107035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Miyakawa S, Ishihara S, Horiguchi A, Takada T, Miyazaki M, Nagakawa T. Biliary tract cancer treatment: 5,584 results from the Biliary Tract Cancer Statistics Registry from 1998 to 2004 in Japan. J Hepatobiliary Pancreat Surg. 2009;16:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 7. | Mishra K, Behari A, Shukla P, Tsuchiya Y, Endoh K, Asai T, Ikoma T, Nakamura K, Kapoor VK. Risk factors for gallbladder cancer development in northern India: A gallstones-matched, case-control study. Indian J Med Res. 2021;154:699-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 8. | Barbhuiya MA, Singh TD, Poojary SS, Gupta S, Kakkar M, Shrivastav BR, Tiwari PK. Gallbladder cancer incidence in Gwalior district of India: Five-year trend based on the registry of a regional cancer center. Indian J Cancer. 2015;52:430-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Jain K, Sreenivas V, Velpandian T, Kapil U, Garg PK. Risk factors for gallbladder cancer: a case-control study. Int J Cancer. 2013;132:1660-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Gupta S, Kori C, Kumar V, Misra S, Akhtar N. Epidemiological Study of Gallbladder Cancer Patients from North Indian Gangetic Planes--a High-Volume Centre's Experience. J Gastrointest Cancer. 2016;47:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Mohandas KM, Patil PS. Cholecystectomy for asymptomatic gallstones can reduce gall bladder cancer mortality in northern Indian women. Indian J Gastroenterol. 2006;25:147-151. [PubMed] |
| 12. | Alvi AR, Siddiqui NA, Zafar H. Risk factors of gallbladder cancer in Karachi-a case-control study. World J Surg Oncol. 2011;9:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (37)] |
| 13. | Csendes A, Becerra M, Rojas J, Medina E. Number and size of stones in patients with asymptomatic and symptomatic gallstones and gallbladder carcinoma: a prospective study of 592 cases. J Gastrointest Surg. 2000;4:481-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Vitetta L, Sali A, Little P, Mrazek L. Gallstones and gall bladder carcinoma. Aust N Z J Surg. 2000;70:667-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Mathur SK, Duhan A, Singh S, Aggarwal M, Aggarwal G, Sen R, Singh S, Garg S. Correlation of gallstone characteristics with mucosal changes in gall bladder. Trop Gastroenterol. 2012;33:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Tazuma S, Kajiyama G. Carcinogenesis of malignant lesions of the gall bladder. The impact of chronic inflammation and gallstones. Langenbecks Arch Surg. 2001;386:224-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Caygill CP, Hill MJ, Braddick M, Sharp JC. Cancer mortality in chronic typhoid and paratyphoid carriers. Lancet. 1994;343:83-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 130] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Lewis JT, Talwalkar JA, Rosen CB, Smyrk TC, Abraham SC. Prevalence and risk factors for gallbladder neoplasia in patients with primary sclerosing cholangitis: evidence for a metaplasia-dysplasia-carcinoma sequence. Am J Surg Pathol. 2007;31:907-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Bhattacharjee PK, Nanda D. Prospective observational study on cholelithiasis in patients with carcinoma gall bladder in a tertiary referral hospital of Eastern India. J Cancer Res Ther. 2019;15:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Roa I, Ibacache G, Roa J, Araya J, de Aretxabala X, Muñoz S. Gallstones and gallbladder cancer-volume and weight of gallstones are associated with gallbladder cancer: a case-control study. J Surg Oncol. 2006;93:624-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Madhawi R, Pandey A, Raj S, Mandal M, Devi S, Sinha PK, Singh RK. Geographical pattern of carcinoma gallbladder in Bihar and its association with river Ganges and arsenic levels: Retrospective individual consecutive patient data from Regional Cancer Centre. South Asian J Cancer. 2018;7:167-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Tyagi BB, Manoharan N, Raina V. Risk factors for gallbladder cancer : A population based case-control study in Delhi. Indian J Med Paediatr Oncol. 2008;29:16-26. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Bansal VK, Misra MC, Chaubal G, Datta Gupta S, Das B, Ahuja V, Sagar S. Helicobacter pylori in gallbladder mucosa in patients with gallbladder disease. Indian J Gastroenterol. 2012;31:57-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Kumar JR, Tewari M, Rai A, Sinha R, Mohapatra SC, Shukla HS. An objective assessment of demography of gallbladder cancer. J Surg Oncol. 2006;93:610-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Mhatre SS, Nagrani RT, Budukh A, Chiplunkar S, Badwe R, Patil P, Laversanne M, Rajaraman P, Bray F, Dikshit R. Place of birth and risk of gallbladder cancer in India. Indian J Cancer. 2016;53:304-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Chhabra D, Oda K, Jagannath P, Utsunomiya H, Takekoshi S, Nimura Y. Chronic heavy metal exposure and gallbladder cancer risk in India, a comparative study with Japan. Asian Pac J Cancer Prev. 2012;13:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Gracie WA, Ransohoff DF. The natural history of silent gallstones: the innocent gallstone is not a myth. N Engl J Med. 1982;307:798-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 339] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 28. | McSherry CK, Ferstenberg H, Calhoun WF, Lahman E, Virshup M. The natural history of diagnosed gallstone disease in symptomatic and asymptomatic patients. Ann Surg. 1985;202:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 223] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Pandey M, Sood BP, Shukla RC, Aryya NC, Singh S, Shukla VK. Carcinoma of the gallbladder: role of sonography in diagnosis and staging. J Clin Ultrasound. 2000;28:227-232. [PubMed] [DOI] [Full Text] |
| 30. | Zatonski WA, Lowenfels AB, Boyle P, Maisonneuve P, Bueno de Mesquita HB, Ghadirian P, Jain M, Przewozniak K, Baghurst P, Moerman CJ, Simard A, Howe GR, McMichael AJ, Hsieh CC, Walker AM. Epidemiologic aspects of gallbladder cancer: a case-control study of the SEARCH Program of the International Agency for Research on Cancer. J Natl Cancer Inst. 1997;89:1132-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 179] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Gupta P, Dutta U, Rana P, Singhal M, Gulati A, Kalra N, Soundararajan R, Kalage D, Chhabra M, Sharma V, Gupta V, Yadav TD, Kaman L, Irrinki S, Singh H, Sakaray Y, Das CK, Saikia U, Nada R, Srinivasan R, Sandhu MS, Sharma R, Shetty N, Eapen A, Kaur H, Kambadakone A, de Haas R, Kapoor VK, Barreto SG, Sharma AK, Patel A, Garg P, Pal SK, Goel M, Patkar S, Behari A, Agarwal AK, Sirohi B, Javle M, Garcea G, Nervi F, Adsay V, Roa JC, Han HS. Gallbladder reporting and data system (GB-RADS) for risk stratification of gallbladder wall thickening on ultrasonography: an international expert consensus. Abdom Radiol (NY). 2022;47:554-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 32. | Batra Y, Pal S, Dutta U, Desai P, Garg PK, Makharia G, Ahuja V, Pande GK, Sahni P, Chattopadhyay TK, Tandon RK. Gallbladder cancer in India: a dismal picture. J Gastroenterol Hepatol. 2005;20:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Rana P, Pruthi H, Gupta P, Chhabra M, Soundararajan R, Singh S, Gulati A, Das CK, Yadav TD, Gupta V, Gupta P, Saikia UN, Dutta U, Sandhu M. Sonographic "Cervix Sign": A New Ancillary Sign of Gallbladder Neck Malignancy. J Clin Exp Hepatol. 2023;13:972-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Kong WT, Shen HY, Qiu YD, Han H, Wen BJ, Wu M. Application of contrast enhanced ultrasound in gallbladder lesion: is it helpful to improve the diagnostic capabilities? Med Ultrason. 2018;20:420-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Xu JM, Guo LH, Xu HX, Zheng SG, Liu LN, Sun LP, Lu MD, Wang WP, Hu B, Yan K, Hong D, Tang SS, Qian LX, Luo BM. Differential diagnosis of gallbladder wall thickening: the usefulness of contrast-enhanced ultrasound. Ultrasound Med Biol. 2014;40:2794-2804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Negrão de Figueiredo G, Mueller-Peltzer K, Schwarze V, Zhang L, Rübenthaler J, Clevert DA. Performance of contrast-enhanced ultrasound (CEUS) compared to MRI in the diagnostic of gallbladder diseases. Clin Hemorheol Microcirc. 2019;73:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Kalra N, Suri S, Gupta R, Natarajan SK, Khandelwal N, Wig JD, Joshi K. MDCT in the staging of gallbladder carcinoma. AJR Am J Roentgenol. 2006;186:758-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Kumaran V, Gulati S, Paul B, Pande K, Sahni P, Chattopadhyay K. The role of dual-phase helical CT in assessing resectability of carcinoma of the gallbladder. Eur Radiol. 2002;12:1993-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Pruthi H, Chabbra M, Soundararajan R, Rana P, Gupta P, Dutta U, Sandhu M. Role of dual energy computed tomography in evaluation of suspected wall thickening type of gallbladder cancer. Clin Exp Hepatol. 2022;8:92-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Gupta SK, Shukla VK. Silent gallstones: a therapeutic dilemma. Trop Gastroenterol. 2004;25:65-68. [PubMed] |
| 41. | Corwin MT, Khera SS, Loehfelm TW, Yang NT, Fananapazir G. Incidentally Detected Focal Fundal Gallbladder Wall Thickening at Contrast-Enhanced Computed Tomography: Prevalence and Computed Tomography Features of Malignancy. J Comput Assist Tomogr. 2019;43:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Kalage D, Gupta P, Gulati A, Reddy KP, Sharma K, Thakur A, Yadav TD, Gupta V, Kaman L, Nada R, Singh H, Irrinki S, Gupta P, Das CK, Dutta U, Sandhu M. Contrast Enhanced CT Versus MRI for Accurate Diagnosis of Wall-thickening Type Gallbladder Cancer. J Clin Exp Hepatol. 2024;14:101397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 43. | Han S, Lee YH, Kim YR, Soh EG. Usefulness of MRI Scoring System for Differential Diagnosis between Xanthogranulomatous Cholecystitis and Wall-Thickening Type Gallbladder Cancer. J Korean Soc Radiol. 2024;85:147-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 44. | Cha SY, Kim YK, Min JH, Lee J, Cha DI, Lee SJ. Usefulness of noncontrast MRI in differentiation between gallbladder carcinoma and benign conditions manifesting as focal mild wall thickening. Clin Imaging. 2019;54:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Gupta P, Marodia Y, Bansal A, Kalra N, Kumar-M P, Sharma V, Dutta U, Sandhu MS. Imaging-based algorithmic approach to gallbladder wall thickening. World J Gastroenterol. 2020;26:6163-6181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (3)] |
| 46. | Jung SE, Lee JM, Lee K, Rha SE, Choi BG, Kim EK, Hahn ST. Gallbladder wall thickening: MR imaging and pathologic correlation with emphasis on layered pattern. Eur Radiol. 2005;15:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Ramos-Font C, Gómez-Rio M, Rodríguez-Fernández A, Jiménez-Heffernan A, Sánchez Sánchez R, Llamas-Elvira JM. Ability of FDG-PET/CT in the detection of gallbladder cancer. J Surg Oncol. 2014;109:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 48. | Kunishima S, Taniguchi H, Yamaguchi A, Koh T, Yamagishi H. Evaluation of Abdominal Tumors with. Clin Positron Imaging. 2000;3:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Gupta V, Vishnu KS, Yadav TD, Sakaray YR, Irrinki S, Mittal BR, Kalra N, Vaiphei K. Radio-pathological Correlation of 18F-FDG PET in Characterizing Gallbladder Wall Thickening. J Gastrointest Cancer. 2019;50:901-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 50. | Zevallos Maldonado C, Ruiz Lopez MJ, Gonzalez Valverde FM, Alarcon Soldevilla F, Pastor Quirante F, Garcia Medina V. Ultrasound Findings Associated to Gallbladder Carcinoma. Cirugía Española (English Edition). 2014;92:348-355. [DOI] [Full Text] |
| 51. | Chhabra M, Kalage D, Gupta P, Siddiqui R, Singh S, Yadav TD, Gupta V, Kaman L, Singh H, Irrinki S, Das C, Prakash G, Gupta P, Saikia UN, Nada R, Dutta U, Sandhu MS. Proposal for a new morphological "combined type" of gallbladder cancer: description of radiopathological characteristics and comparison with other morphological types. Abdom Radiol (NY). 2024;49:703-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 52. | Kim JH, Lee JY, Baek JH, Eun HW, Kim YJ, Han JK, Choi BI. High-resolution sonography for distinguishing neoplastic gallbladder polyps and staging gallbladder cancer. AJR Am J Roentgenol. 2015;204:W150-W159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 53. | Yu MH, Kim YJ, Park HS, Jung SI. Benign gallbladder diseases: Imaging techniques and tips for differentiating with malignant gallbladder diseases. World J Gastroenterol. 2020;26:2967-2986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (5)] |
| 54. | Wibbenmeyer LA, Sharafuddin MJ, Wolverson MK, Heiberg EV, Wade TP, Shields JB. Sonographic diagnosis of unsuspected gallbladder cancer: imaging findings in comparison with benign gallbladder conditions. AJR Am J Roentgenol. 1995;165:1169-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 55. | Dong Y, Xu B, Cao Q, Zhang Q, Qiu Y, Yang D, Yu L, Wang WP. Incidentally detected focal fundal gallbladder wall thickening: Differentiation contrast enhanced ultrasound features with high-resolution linear transducers. Clin Hemorheol Microcirc. 2020;74:315-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 56. | Okaniwa S. How Can We Manage Gallbladder Lesions by Transabdominal Ultrasound? Diagnostics (Basel). 2021;11:784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 57. | Zhu L, Li N, Zhu Y, Han P, Jiang B, Li M, Luo Y, Clevert DA, Fei X. Value of high frame rate contrast enhanced ultrasound in gallbladder wall thickening in non-acute setting. Cancer Imaging. 2024;24:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 58. | Zhuang B, Li W, Wang W, Lin M, Xu M, Xie X, Lu M, Xie X. Contrast-enhanced ultrasonography improves the diagnostic specificity for gallbladder-confined focal tumors. Abdom Radiol (NY). 2018;43:1134-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Chen LD, Huang Y, Xie XH, Chen W, Shan QY, Xu M, Liu JY, Nie ZQ, Xie XY, Lu MD, Shen SL, Wang W. Diagnostic nomogram for gallbladder wall thickening mimicking malignancy: using contrast-enhanced ultrasonography or multi-detector computed tomography? Abdom Radiol (NY). 2017;42:2436-2446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 60. | Kim SJ, Lee JM, Lee JY, Kim SH, Han JK, Choi BI, Choi JY. Analysis of enhancement pattern of flat gallbladder wall thickening on MDCT to differentiate gallbladder cancer from cholecystitis. AJR Am J Roentgenol. 2008;191:765-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 61. | Tongdee R, Maroongroge P, Suthikeree W. The value of MDCT scans in differentiation between benign and malignant gallbladder wall thickening. J Med Assoc Thai. 2011;94:592-600. [PubMed] |
| 62. | Jindal G, Singal S, Nagi B, Mittal A, Mittal S, Singal R. Role of Multidetector Computed Tomography (MDCT) in Evaluation of Gallbladder Malignancy and its Pathological Correlation in an Indian Rural Center. Maedica (Bucur). 2018;13:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 63. | Kalage D, Sajjana G, Hegde S, Gulati A, Yadav TD, Kaman L, Irrinki S, Singh H, Gupta P, Nahar Saikia U, Nada R, Dutta U, Gupta P. Enhancement Patterns of Malignant Gallbladder Masses at Multiphasic Contrast-enhanced CT: Associations With Clinicoradiopathological Features. J Clin Exp Hepatol. 2024;14:102377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 64. | Kang TW, Kim SH, Park HJ, Lim S, Jang KM, Choi D, Lee SJ. Differentiating xanthogranulomatous cholecystitis from wall-thickening type of gallbladder cancer: added value of diffusion-weighted MRI. Clin Radiol. 2013;68:992-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 65. | Kim SJ, Lee JM, Kim H, Yoon JH, Han JK, Choi BI. Role of diffusion-weighted magnetic resonance imaging in the diagnosis of gallbladder cancer. J Magn Reson Imaging. 2013;38:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Kitazume Y, Taura S, Nakaminato S, Noguchi O, Masaki Y, Kasahara I, Kishino M, Tateishi U. Diffusion-weighted magnetic resonance imaging to differentiate malignant from benign gallbladder disorders. Eur J Radiol. 2016;85:864-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 67. | Solak A, Solak I, Genç B, Sahin N. The role of diffusion-weighted examination in non-polyploid gallbladder malignancies: A preliminary study. Turk J Gastroenterol. 2013;24:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 68. | Nishiyama Y, Yamamoto Y, Fukunaga K, Kimura N, Miki A, Sasakawa Y, Wakabayashi H, Satoh K, Ohkawa M. Dual-time-point 18F-FDG PET for the evaluation of gallbladder carcinoma. J Nucl Med. 2006;47:633-638. [PubMed] |
| 69. | Khanduri S, Goyal A, Usmani T, Jain S, Goyal A, Chaudhary M, Sharma H, Sabharwal T, Yadav S, Khanduri S, Singh T. Role of ultrasonography in the diagnosis of gall-bladder carcinoma: A boon for low-resource settings. Asian J Oncol. 2018;04:001-005. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 70. | Lee JS, Kim JH, Kim YJ, Ryu JK, Kim YT, Lee JY, Han JK. Diagnostic accuracy of transabdominal high-resolution US for staging gallbladder cancer and differential diagnosis of neoplastic polyps compared with EUS. Eur Radiol. 2017;27:3097-3103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 71. | Negrão de Figueiredo G, Mueller-Peltzer K, Zengel P, Armbruster M, Rübenthaler J, Clevert DA. Contrast-enhanced ultrasound (CEUS) and gallbladder diseases - A retrospective mono-center analysis of imaging findings with histopathological correlation. Clin Hemorheol Microcirc. 2019;71:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 72. | Prasad N, Sen S. Gall bladder carcinoma: the facts and the mimics. Egypt J Radiol Nucl Med. 2021;52:1. [DOI] [Full Text] |
| 73. | Cheng Y, Wang M, Ma B, Ma X. Potential role of contrast-enhanced ultrasound for the differentiation of malignant and benign gallbladder lesions in East Asia: A meta-analysis and systematic review. Medicine (Baltimore). 2018;97:e11808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 74. | Zhang X, Tang S, Huang L, Jin H, Wang Y, Wang Y, Liu Z, Lu C. Value of contrast-enhanced ultrasound in diagnosis and differential diagnosis of polypoid lesions of gallbladder ≥ 1 cm. BMC Gastroenterol. 2022;22:354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 75. | Prasad N, Goyal A. MDCT in Characterizing Gall Bladder Carcinoma: Experience From an Endemic Region. Int J Contemp Med. Surg Radiol. 2018;3. [DOI] [Full Text] |
| 76. | Ahmed M, Nag HH, Meena P. Clinical approach to patients with thick wall gallbladder. Egypt J Radiol Nucl Med. 2023;54:184. [DOI] [Full Text] |
| 77. | Wasnik AP, Davenport MS, Kaza RK, Weadock WJ, Udager A, Keshavarzi N, Nan B, Maturen KE. Diagnostic accuracy of MDCT in differentiating gallbladder cancer from acute and xanthogranulomatous cholecystitis. Clin Imaging. 2018;50:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 78. | Kim JH, Kim TK, Eun HW, Kim BS, Lee MG, Kim PN, Ha HK. Preoperative evaluation of gallbladder carcinoma: efficacy of combined use of MR imaging, MR cholangiography, and contrast-enhanced dual-phase three-dimensional MR angiography. J Magn Reson Imaging. 2002;16:676-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 79. | Kaza RK, Gulati M, Wig JD, Chawla YK. Evaluation of gall bladder carcinoma with dynamic magnetic resonance imaging and magnetic resonance cholangiopancreatography. Australas Radiol. 2006;50:212-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 80. | Lopes Vendrami C, Magnetta MJ, Mittal PK, Moreno CC, Miller FH. Gallbladder Carcinoma and Its Differential Diagnosis at MRI: What Radiologists Should Know. Radiographics. 2021;41:78-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 81. | Annunziata S, Pizzuto DA, Caldarella C, Galiandro F, Sadeghi R, Treglia G. Diagnostic accuracy of fluorine-18-fluorodeoxyglucose positron emission tomography in gallbladder cancer: A meta-analysis. World J Gastroenterol. 2015;21:11481-11488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 82. | Pericleous S, Doran SLF, Wotherspoon A, Terlizzo M, Riddell A, Brown G, Shur J, Chua S, Hujairi N, Middleton N, Cunningham D, Kumar S, Bhogal RH. The Diagnostic Accuracy of (18) F-FGD-PET/CT for Cancer of the Gallbladder: A Retrospective Study. World J Nucl Med. 2022;21:112-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 83. | Kapoor A, Kapoor A, Mahajan G. Differentiating malignant from benign thickening of the gallbladder wall by the use of acoustic radiation force impulse elastography. J Ultrasound Med. 2011;30:1499-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 84. | Teber MA, Tan S, Dönmez U, İpek A, Uçar AE, Yıldırım H, Aslan A, Arslan H. The use of real-time elastography in the assessment of gallbladder polyps: preliminary observations. Med Ultrason. 2014;16:304-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 85. | Soundararajan R, Dutta U, Bhatia A, Gupta P, Nahar U, Kaman L, Bhattacharya A, Kumar A, Sandhu MS. Two-dimensional Shear Wave Elastography: Utility in Differentiating Gallbladder Cancer From Chronic Cholecystitis. J Ultrasound Med. 2023;42:1577-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 86. | Choi WB, Lee SK, Kim MH, Seo DW, Kim HJ, Kim DI, Park ET, Yoo KS, Lim BC, Myung SJ, Park HJ, Min YI. A new strategy to predict the neoplastic polyps of the gallbladder based on a scoring system using EUS. Gastrointest Endosc. 2000;52:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 87. | Sadamoto Y, Oda S, Tanaka M, Harada N, Kubo H, Eguchi T, Nawata H. A useful approach to the differential diagnosis of small polypoid lesions of the gallbladder, utilizing an endoscopic ultrasound scoring system. Endoscopy. 2002;34:959-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 88. | Lee J, Yun M, Kim KS, Lee JD, Kim CK. Risk stratification of gallbladder polyps (1-2 cm) for surgical intervention with 18F-FDG PET/CT. J Nucl Med. 2012;53:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 89. | Wiles R, Varadpande M, Muly S, Webb J. Growth rate and malignant potential of small gallbladder polyps--systematic review of evidence. Surgeon. 2014;12:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 90. | Babu BI, Dennison AR, Garcea G. Management and diagnosis of gallbladder polyps: a systematic review. Langenbecks Arch Surg. 2015;400:455-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 91. | Bhatt NR, Gillis A, Smoothey CO, Awan FN, Ridgway PF. Evidence based management of polyps of the gall bladder: A systematic review of the risk factors of malignancy. Surgeon. 2016;14:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 92. | Mainprize KS, Gould SW, Gilbert JM. Surgical management of polypoid lesions of the gallbladder. Br J Surg. 2000;87:414-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 93. | Rafaelsen SR, Otto PO, Pedersen MRV. Long-term ultrasound follow-up in patients with small gallbladder polyps. Dan Med J. 2020;67:A06200414. [PubMed] |
| 94. | Chattopadhyay D, Lochan R, Balupuri S, Gopinath BR, Wynne KS. Outcome of gall bladder polypoidal lesions detected by transabdominal ultrasound scanning: a nine year experience. World J Gastroenterol. 2005;11:2171-2173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 95. | Wennmacker SZ, van Dijk AH, Raessens JHJ, van Laarhoven CJHM, Drenth JPH, de Reuver PR, Nagtegaal ID. Polyp size of 1 cm is insufficient to discriminate neoplastic and non-neoplastic gallbladder polyps. Surg Endosc. 2019;33:1564-1571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 96. | Kamaya A, Fung C, Szpakowski JL, Fetzer DT, Walsh AJ, Alimi Y, Bingham DB, Corwin MT, Dahiya N, Gabriel H, Park WG, Porembka MR, Rodgers SK, Tublin ME, Yuan X, Zhang Y, Middleton WD. Management of Incidentally Detected Gallbladder Polyps: Society of Radiologists in Ultrasound Consensus Conference Recommendations. Radiology. 2022;305:277-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 97. | Foley KG, Lahaye MJ, Thoeni RF, Soltes M, Dewhurst C, Barbu ST, Vashist YK, Rafaelsen SR, Arvanitakis M, Perinel J, Wiles R, Roberts SA. Management and follow-up of gallbladder polyps: updated joint guidelines between the ESGAR, EAES, EFISDS and ESGE. Eur Radiol. 2022;32:3358-3368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 98. | Terzioğlu SG, Kılıç MÖ, Sapmaz A, Karaca AS. Predictive factors of neoplastic gallbladder polyps: Outcomes of 278 patients. Turk J Gastroenterol. 2017;28:202-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 99. | Hattori M, Inui K, Yoshino J, Miyoshi H, Okushima K, Nakamura Y, Naito T, Imaeda Y, Horibe Y, Hattori T, Nakazawa S. [Usefulness of contrast-enhanced ultrasonography in the differential diagnosis of polypoid gallbladder lesions]. Nihon Shokakibyo Gakkai Zasshi. 2007;104:790-798. [PubMed] |
| 100. | Kamisawa T, Takuma K, Anjiki H, Egawa N, Kurata M, Honda G, Tsuruta K, Sasaki T. Pancreaticobiliary maljunction. Clin Gastroenterol Hepatol. 2009;7:S84-S88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 101. | Misra SP, Gulati P, Thorat VK, Vij JC, Anand BS. Pancreaticobiliary ductal union in biliary diseases. An endoscopic retrograde cholangiopancreatographic study. Gastroenterology. 1989;96:907-912. [PubMed] [DOI] [Full Text] |
| 102. | Sugiyama M, Atomi Y. Anomalous pancreaticobiliary junction without congenital choledochal cyst. Br J Surg. 1998;85:911-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 103. | Machado NO. Porcelain Gallbladder: Decoding the malignant truth. Sultan Qaboos Univ Med J. 2016;16:e416-e421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 104. | Stephen AE, Berger DL. Carcinoma in the porcelain gallbladder: a relationship revisited. Surgery. 2001;129:699-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 105. | Schnelldorfer T. Porcelain gallbladder: a benign process or concern for malignancy? J Gastrointest Surg. 2013;17:1161-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 106. | Krishnani N, Shukla S, Jain M, Pandey R, Gupta RK. Fine needle aspiration cytology in xanthogranulomatous cholecystitis, gallbladder adenocarcinoma and coexistent lesions. Acta Cytol. 2000;44:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 107. | Kim PN, Lee SH, Gong GY, Kim JG, Ha HK, Lee YJ, Lee MG, Auh YH. Xanthogranulomatous cholecystitis: radiologic findings with histologic correlation that focuses on intramural nodules. AJR Am J Roentgenol. 1999;172:949-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 108. | Benbow EW. Xanthogranulomatous cholecystitis associated with carcinoma of the gallbladder. Postgrad Med J. 1989;65:528-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 109. | Parra JA, Acinas O, Bueno J, Güezmes A, Fernández MA, Fariñas MC. Xanthogranulomatous cholecystitis: clinical, sonographic, and CT findings in 26 patients. AJR Am J Roentgenol. 2000;174:979-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 110. | Kim PN, Ha HK, Kim YH, Lee MG, Kim MH, Auh YH. US findings of xanthogranulomatous cholecystitis. Clin Radiol. 1998;53:290-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 111. | Zhao F, Lu PX, Yan SX, Wang GF, Yuan J, Zhang SZ, Wang YX. CT and MR features of xanthogranulomatous cholecystitis: an analysis of consecutive 49 cases. Eur J Radiol. 2013;82:1391-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 112. | Goshima S, Chang S, Wang JH, Kanematsu M, Bae KT, Federle MP. Xanthogranulomatous cholecystitis: diagnostic performance of CT to differentiate from gallbladder cancer. Eur J Radiol. 2010;74:e79-e83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 113. | Shuto R, Kiyosue H, Komatsu E, Matsumoto S, Kawano K, Kondo Y, Yokoyama S, Mori H. CT and MR imaging findings of xanthogranulomatous cholecystitis: correlation with pathologic findings. Eur Radiol. 2004;14:440-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 114. | Wang YF, Feng FL, Zhao XH, Ye ZX, Zeng HP, Li Z, Jiang XQ, Peng ZH. Combined detection tumor markers for diagnosis and prognosis of gallbladder cancer. World J Gastroenterol. 2014;20:4085-4092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 88] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (2)] |
| 115. | Lumachi F, Lo Re G, Tozzoli R, D'Aurizio F, Facomer F, Chiara GB, Basso SM. Measurement of serum carcinoembryonic antigen, carbohydrate antigen 19-9, cytokeratin-19 fragment and matrix metalloproteinase-7 for detecting cholangiocarcinoma: a preliminary case-control study. Anticancer Res. 2014;34:6663-6667. [PubMed] |
| 116. | Li Y, Huang Y, Chen J. Diagnostic Value of Serum Biomarkers for Intrahepatic Cholangiocarcinoma. J Coll Physicians Surg Pak. 2019;29:962-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 117. | Wang QH, Ji ZG, Chen ZG, Li HZ, Fan H, Fan XR, Shi BB, Fang Y. Serum CA 19-9 as a good prognostic biomarker in patients with bladder cancer. Int J Surg. 2015;15:113-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 118. | Rana S, Dutta U, Kochhar R, Rana SV, Gupta R, Pal R, Jain K, Srinivasan R, Nagi B, Nain CK, Singh K. Evaluation of CA 242 as a tumor marker in gallbladder cancer. J Gastrointest Cancer. 2012;43:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 119. | Huang L, Chen W, Liang P, Hu W, Zhang K, Shen S, Chen J, Zhang Z, Chen B, Han Y, Meng F, DeMorrow S, Yin X, Lai J, Liang L. Serum CYFRA 21-1 in Biliary Tract Cancers: A Reliable Biomarker for Gallbladder Carcinoma and Intrahepatic Cholangiocarcinoma. Dig Dis Sci. 2015;60:1273-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 120. | Sinha SR, Prakash P, Singh RK, Sinha DK. Assessment of tumor markers CA 19-9, CEA, CA 125, and CA 242 for the early diagnosis and prognosis prediction of gallbladder cancer. World J Gastrointest Surg. 2022;14:1272-1284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 121. | Bind MK, Mishra RR, Kumar V, Misra V, Singh PA. Serum CA 19-9 and CA 125 as a diagnostic marker in carcinoma of gallbladder. Indian J Pathol Microbiol. 2021;64:65-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 122. | Liu F, Hu HJ, Ma WJ, Yang Q, Wang JK, Li FY. Prognostic significance of neutrophil-lymphocyte ratio and carbohydrate antigen 19-9 in patients with gallbladder carcinoma. Medicine (Baltimore). 2019;98:e14550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 123. | Oshikiri T, Hida Y, Miyamoto M, Hashida H, Katoh K, Suzuoki M, Nakakubo Y, Hiraoka K, Shinohara T, Itoh T, Kondo S, Katoh H. RCAS1 as a tumour progression marker: an independent negative prognostic factor in gallbladder cancer. Br J Cancer. 2001;85:1922-1927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 124. | Giaginis C, Margeli A, Kouraklis G, Zira A, Tsourouflis G, Theocharis S. Diagnostic and prognostic utility of serum receptor-binding cancer antigen expressed on SiSo cells (RCAS1) levels in colon cancer patients. Int J Biol Markers. 2009;24:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 125. | Koopmann J, Thuluvath PJ, Zahurak ML, Kristiansen TZ, Pandey A, Schulick R, Argani P, Hidalgo M, Iacobelli S, Goggins M, Maitra A. Mac-2-binding protein is a diagnostic marker for biliary tract carcinoma. Cancer. 2004;101:1609-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 126. | Ghimire B, Kansakar PBS, Sngh YP. Comparison of serum biomarkers cifra 21-1 and ca 19-9 in biliary tract cancers. J Nepal Health Res Counc. 2021;19:626-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 127. | Mishra S, Kumari S, Husain N. Liquid biopsy in gallbladder carcinoma: Current evidence and future prospective. J Liq Biopsy. 2024;6:100280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 128. | Sakoda LC, Gao YT, Chen BE, Chen J, Rosenberg PS, Rashid A, Deng J, Shen MC, Wang BS, Han TQ, Zhang BH, Cohen-Webb H, Yeager M, Welch R, Chanock S, Fraumeni JF Jr, Hsing AW. Prostaglandin-endoperoxide synthase 2 (PTGS2) gene polymorphisms and risk of biliary tract cancer and gallstones: a population-based study in Shanghai, China. Carcinogenesis. 2006;27:1251-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 129. | Srivastava K, Srivastava A, Pandey SN, Kumar A, Mittal B. Functional polymorphisms of the cyclooxygenase (PTGS2) gene and risk for gallbladder cancer in a North Indian population. J Gastroenterol. 2009;44:774-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 130. | Srivastava K, Srivastava A, Kumar A, Mittal B. Significant association between toll-like receptor gene polymorphisms and gallbladder cancer. Liver Int. 2010;30:1067-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 131. | Koshiol J, Gao YT, Corbel A, Kemp TJ, Shen MC, Hildesheim A, Hsing AW, Rashid A, Wang B, Pfeiffer RM, Pinto LA. Circulating inflammatory proteins and gallbladder cancer: Potential for risk stratification to improve prioritization for cholecystectomy in high-risk regions. Cancer Epidemiol. 2018;54:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 132. | Vishnoi M, Pandey SN, Choudhury G, Kumar A, Modi DR, Mittal B. Do TNFA -308 G/A and IL6 -174 G/C gene polymorphisms modulate risk of gallbladder cancer in the north Indian population? Asian Pac J Cancer Prev. 2007;8:567-572. [PubMed] |
| 133. | Vishnoi M, Pandey SN, Choudhuri G, Mittal B. IL-1 gene polymorphisms and genetic susceptibility of gallbladder cancer in a north Indian population. Cancer Genet Cytogenet. 2008;186:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 134. | Vishnoi M, Pandey SN, Modi DR, Kumar A, Mittal B. Genetic susceptibility of epidermal growth factor +61A>G and transforming growth factor beta1 -509C>T gene polymorphisms with gallbladder cancer. Hum Immunol. 2008;69:360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 135. | Park SK, Andreotti G, Sakoda LC, Gao YT, Rashid A, Chen J, Chen BE, Rosenberg PS, Shen MC, Wang BS, Han TQ, Zhang BH, Yeager M, Chanock S, Hsing AW. Variants in hormone-related genes and the risk of biliary tract cancers and stones: a population-based study in China. Carcinogenesis. 2009;30:606-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 136. | Tsuchiya Y, Kiyohara C, Sato T, Nakamura K, Kimura A, Yamamoto M. Polymorphisms of cytochrome P450 1A1, glutathione S-transferase class mu, and tumour protein p53 genes and the risk of developing gallbladder cancer in Japanese. Clin Biochem. 2007;40:881-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 137. | Srivastava A, Pandey SN, Pandey P, Choudhuri G, Mittal B. No association of Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism in susceptibility to gallbladder cancer. DNA Cell Biol. 2008;27:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 138. | Srivastava A, Tulsyan S, Pandey SN, Choudhuri G, Mittal B. Single nucleotide polymorphism in the ABCG8 transporter gene is associated with gallbladder cancer susceptibility. Liver Int. 2009;29:831-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 139. | Pandey SN, Srivastava A, Dixit M, Choudhuri G, Mittal B. Haplotype analysis of signal peptide (insertion/deletion) and XbaI polymorphisms of the APOB gene in gallbladder cancer. Liver Int. 2007;27:1008-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 140. | Pandey SN, Modi DR, Choudhuri G, Mittall B. Slow acetylator genotype of N-acetyl transferase2 (NAT2) is associated with increased susceptibility to gallbladder cancer: the cancer risk not modulated by gallstone disease. Cancer Biol Ther. 2007;6:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 141. | Pandey SN, Jain M, Nigam P, Choudhuri G, Mittal B. Genetic polymorphisms in GSTM1, GSTT1, GSTP1, GSTM3 and the susceptibility to gallbladder cancer in North India. Biomarkers. 2006;11:250-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 142. | Hou L, Xu J, Gao YT, Rashid A, Zheng SL, Sakoda LC, Shen MC, Wang BS, Deng J, Han TQ, Zhang BH, Meyers DA, Fraumeni JF Jr, Hsing AW. CYP17 MspA1 polymorphism and risk of biliary tract cancers and gallstones: a population-based study in Shanghai, China. Int J Cancer. 2006;118:2847-2853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 143. | Rai R, Sharma KL, Misra S, Kumar A, Mittal B. CYP17 polymorphism (rs743572) is associated with increased risk of gallbladder cancer in tobacco users. Tumour Biol. 2014;35:6531-6537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 144. | Kimura A, Tsuchiya Y, Lang I, Zoltan S, Nakadaira H, Ajioka Y, Kiyohara C, Oyama M, Nakamura K. Effect of genetic predisposition on the risk of gallbladder cancer in Hungary. Asian Pac J Cancer Prev. 2008;9:391-396. [PubMed] |
| 145. | Sharma KL, Agarwal A, Misra S, Kumar A, Kumar V, Mittal B. Association of genetic variants of xenobiotic and estrogen metabolism pathway (CYP1A1 and CYP1B1) with gallbladder cancer susceptibility. Tumour Biol. 2014;35:5431-5439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 146. | Andreotti G, Chen J, Gao YT, Rashid A, Chen BE, Rosenberg P, Sakoda LC, Deng J, Shen MC, Wang BS, Han TQ, Zhang BH, Yeager M, Welch R, Chanock S, Fraumeni JF Jr, Hsing AW. Polymorphisms of genes in the lipid metabolism pathway and risk of biliary tract cancers and stones: a population-based case-control study in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2008;17:525-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 147. | Asai T, Tsuchiya Y, Mishra K, Behari A, Shukla P, Ikoma T, Kapoor VK, Nakamura K. Carcinogen Metabolism Pathway and Tumor Suppressor Gene Polymorphisms and Gallbladder Cancer Risk in North Indians: A Hospital-Based Case-Control Study. Asian Pac J Cancer Prev. 2019;20:3643-3647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 148. | Srivastava K, Srivastava A, Mittal B. Polymorphisms in ERCC2, MSH2, and OGG1 DNA repair genes and gallbladder cancer risk in a population of Northern India. Cancer. 2010;116:3160-3169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 149. | Zhang M, Huang WY, Andreotti G, Gao YT, Rashid A, Chen J, Sakoda LC, Shen MC, Wang BS, Chanock S, Hsing AW. Variants of DNA repair genes and the risk of biliary tract cancers and stones: a population-based study in China. Cancer Epidemiol Biomarkers Prev. 2008;17:2123-2127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 150. | Jiao X, Wu Y, Zhou L, He J, Yang C, Zhang P, Hu R, Luo C, Du J, Fu J, Shi J, He R, Li D, Jun W. Variants and haplotypes in Flap endonuclease 1 and risk of gallbladder cancer and gallstones: a population-based study in China. Sci Rep. 2015;5:18160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 151. | Chang SC, Rashid A, Gao YT, Andreotti G, Shen MC, Wang BS, Han TQ, Zhang BH, Sakoda LC, Leitzmann MF, Chen BE, Rosenberg PS, Chen J, Chanock SJ, Hsing AW. Polymorphism of genes related to insulin sensitivity and the risk of biliary tract cancer and biliary stone: a population-based case-control study in Shanghai, China. Carcinogenesis. 2008;29:944-948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 152. | Srivastava A, Pandey SN, Dixit M, Choudhuri G, Mittal B. Cholecystokinin receptor A gene polymorphism in gallstone disease and gallbladder cancer. J Gastroenterol Hepatol. 2008;23:970-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 153. | Xu HL, Hsing AW, Vogtmann E, Chu LW, Cheng JR, Gao J, Tan YT, Wang BS, Shen MC, Gao YT. Variants in CCK and CCKAR genes to susceptibility to biliary tract cancers and stones: a population-based study in Shanghai, China. J Gastroenterol Hepatol. 2013;28:1476-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 154. | Srivastava A, Sharma KL, Srivastava N, Misra S, Mittal B. Significant role of estrogen and progesterone receptor sequence variants in gallbladder cancer predisposition: a multi-analytical strategy. PLoS One. 2012;7:e40162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 155. | Park SK, Andreotti G, Rashid A, Chen J, Rosenberg PS, Yu K, Olsen J, Gao YT, Deng J, Sakoda LC, Zhang M, Shen MC, Wang BS, Han TQ, Zhang BH, Yeager M, Chanock SJ, Hsing AW. Polymorphisms of estrogen receptors and risk of biliary tract cancers and gallstones: a population-based study in Shanghai, China. Carcinogenesis. 2010;31:842-846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 156. | Meyer TE, O'Brien TG, Andreotti G, Yu K, Li Q, Gao YT, Rashid A, Shen MC, Wang BS, Han TQ, Zhang BH, Niwa S, Fraumeni JF Jr, Hsing AW. Androgen receptor CAG repeat length and risk of biliary tract cancer and stones. Cancer Epidemiol Biomarkers Prev. 2010;19:787-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 157. | Rai R, Sharma KL, Sharma S, Misra S, Kumar A, Mittal B. Death receptor (DR4) haplotypes are associated with increased susceptibility of gallbladder carcinoma in north Indian population. PLoS One. 2014;9:e90264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 158. | Srivastava K, Srivastava A, Mittal B. Caspase-8 polymorphisms and risk of gallbladder cancer in a northern Indian population. Mol Carcinog. 2010;49:684-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 159. | Yadav A, Gupta A, Rastogi N, Agrawal S, Kumar A, Kumar V, Mittal B. Association of cancer stem cell markers genetic variants with gallbladder cancer susceptibility, prognosis, and survival. Tumour Biol. 2016;37:1835-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 160. | Srivastava K, Srivastava A, Mittal B. Common genetic variants in pre-microRNAs and risk of gallbladder cancer in North Indian population. J Hum Genet. 2010;55:495-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 161. | Rai R, Sharma KL, Misra S, Kumar A, Mittal B. PSCA gene variants (rs2294008 and rs2978974) confer increased susceptibility of gallbladder carcinoma in females. Gene. 2013;530:172-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 162. | Yadav A, Gupta A, Yadav S, Rastogi N, Agrawal S, Kumar A, Kumar V, Misra S, Mittal B. Association of Wnt signaling pathway genetic variants in gallbladder cancer susceptibility and survival. Tumour Biol. 2016;37:8083-8095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 163. | Kim JH, Kim HN, Lee KT, Lee JK, Choi SH, Paik SW, Rhee JC, Lowe AW. Gene expression profiles in gallbladder cancer: the close genetic similarity seen for early and advanced gallbladder cancers may explain the poor prognosis. Tumour Biol. 2008;29:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 164. | Mhatre S, Wang Z, Nagrani R, Badwe R, Chiplunkar S, Mittal B, Yadav S, Zhang H, Chung CC, Patil P, Chanock S, Dikshit R, Chatterjee N, Rajaraman P. Common genetic variation and risk of gallbladder cancer in India: a case-control genome-wide association study. Lancet Oncol. 2017;18:535-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 165. | Cha PC, Zembutsu H, Takahashi A, Kubo M, Kamatani N, Nakamura Y. A genome-wide association study identifies SNP in DCC is associated with gallbladder cancer in the Japanese population. J Hum Genet. 2012;57:235-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 166. | Boekstegers F, Marcelain K, Barahona Ponce C, Baez Benavides PF, Müller B, de Toro G, Retamales J, Barajas O, Ahumada M, Morales E, Rojas A, Sanhueza V, Loader D, Rivera MT, Gutiérrez L, Bernal G, Ortega A, Montalvo D, Portiño S, Bertrán ME, Gabler F, Spencer L, Olloquequi J, González Silos R, Fischer C, Scherer D, Jenab M, Aleksandrova K, Katzke V, Weiderpass E, Moradi T, Fischer K, Bossers W, Brenner H, Hveem K, Eklund N, Völker U, Waldenberger M, Fuentes Guajardo M, Gonzalez-Jose R, Bedoya G, Bortolini MC, Canizales S, Gallo C, Ruiz Linares A, Rothhammer F, Lorenzo Bermejo J. ABCB1/4 gallbladder cancer risk variants identified in India also show strong effects in Chileans. Cancer Epidemiol. 2020;65:101643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 167. | Singh MK, Chetri K, Pandey UB, Kapoor VK, Mittal B, Choudhuri G. Mutational spectrum of K-ras oncogene among Indian patients with gallbladder cancer. J Gastroenterol Hepatol. 2004;19:916-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 168. | Kumari N, Kapoor VK, Krishnani N, Kumar K, Baitha DK. Role of C-erbB2 expression in gallbladder cancer. Indian J Pathol Microbiol. 2012;55:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 169. | Pramanik V, Sarkar BN, Kar M, Das G, Malay BK, Sufia KK, Lakkakula BV, Vadlamudi RR. A novel polymorphism in codon 25 of the KRAS gene associated with gallbladder carcinoma patients of the eastern part of India. Genet Test Mol Biomarkers. 2011;15:431-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 170. | Srivastava K, Srivastava A, Mittal B. Angiotensin I-converting enzyme insertion/deletion polymorphism and increased risk of gall bladder cancer in women. DNA Cell Biol. 2010;29:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 171. | Srivastava K, Srivastava A, Mittal B. DNMT3B -579 G>T promoter polymorphism and risk of gallbladder carcinoma in North Indian population. J Gastrointest Cancer. 2010;41:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 172. | Barreto SG, Dutt A, Chaudhary A. A genetic model for gallbladder carcinogenesis and its dissemination. Ann Oncol. 2014;25:1086-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 173. | Mishra S, Kumari S, Srivastava P, Pandey A, Shukla S, Husain N. Genomic profiling of gallbladder carcinoma: Targetable mutations and pathways involved. Pathol Res Pract. 2022;232:153806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (1)] |
| 174. | Moreno M, Pimentel F, Gazdar AF, Wistuba II, Miquel JF. TP53 abnormalities are frequent and early events in the sequential pathogenesis of gallbladder carcinoma. Ann Hepatol. 2005;4:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 175. | Wee A, Teh M, Raju GC. Clinical importance of p53 protein in gall bladder carcinoma and its precursor lesions. J Clin Pathol. 1994;47:453-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 176. | Tanno S, Obara T, Fujii T, Mizukami Y, Shudo R, Nishino N, Ura H, Klein-Szanto AJ, Kohgo Y. Proliferative potential and K-ras mutation in epithelial hyperplasia of the gallbladder in patients with anomalous pancreaticobiliary ductal union. Cancer. 1998;83:267-275. [PubMed] [DOI] [Full Text] |
| 177. | House MG, Wistuba II, Argani P, Guo M, Schulick RD, Hruban RH, Herman JG, Maitra A. Progression of gene hypermethylation in gallstone disease leading to gallbladder cancer. Ann Surg Oncol. 2003;10:882-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 178. | Tadokoro H, Shigihara T, Ikeda T, Takase M, Suyama M. Two distinct pathways of p16 gene inactivation in gallbladder cancer. World J Gastroenterol. 2007;13:6396-6403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 179. | Bustos BI, Pérez-Palma E, Buch S, Azócar L, Riveras E, Ugarte GD, Toliat M, Nürnberg P, Lieb W, Franke A, Hinz S, Burmeister G, von Schönfels W, Schafmayer C, Völzke H, Völker U, Homuth G, Lerch MM, Santos JL, Puschel K, Bambs C, Roa JC, Gutiérrez RA, Hampe J, De Ferrari GV, Miquel JF. Variants in ABCG8 and TRAF3 genes confer risk for gallstone disease in admixed Latinos with Mapuche Native American ancestry. Sci Rep. 2019;9:772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 180. | Alix-Panabières C, Pantel K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021;11:858-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 581] [Article Influence: 145.3] [Reference Citation Analysis (0)] |
| 181. | Arechederra M, Rullán M, Oyón D, Ávila MA, Urman JM, Berasain C. Bile as a liquid biopsy matrix: potential applications and limitations. Explor Dig Dis. 2024;3:5-21. [DOI] [Full Text] |
| 182. | Cree IA. Liquid biopsy for cancer patients: Principles and practice. Pathogenesis. 2015;2:1-4. [RCA] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 183. | Sisodiya S, Kasherwal V, Khan A, Roy B, Goel A, Kumar S, Arif N, Tanwar P, Hussain S. Liquid Biopsies: Emerging role and clinical applications in solid tumours. Transl Oncol. 2023;35:101716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 184. | Kumari S, Tewari S, Husain N, Agarwal A, Pandey A, Singhal A, Lohani M. Quantification of Circulating Free DNA as a Diagnostic Marker in Gall Bladder Cancer. Pathol Oncol Res. 2017;23:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 185. | Kumari S, Husain N, Agarwal A, Neyaz A, Gupta S, Chaturvedi A, Lohani M, Sonkar AA. Diagnostic Value of Circulating Free DNA Integrity and Global Methylation Status in Gall Bladder Carcinoma. Pathol Oncol Res. 2019;25:925-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 186. | Shen SY, Singhania R, Fehringer G, Chakravarthy A, Roehrl MHA, Chadwick D, Zuzarte PC, Borgida A, Wang TT, Li T, Kis O, Zhao Z, Spreafico A, Medina TDS, Wang Y, Roulois D, Ettayebi I, Chen Z, Chow S, Murphy T, Arruda A, O'Kane GM, Liu J, Mansour M, McPherson JD, O'Brien C, Leighl N, Bedard PL, Fleshner N, Liu G, Minden MD, Gallinger S, Goldenberg A, Pugh TJ, Hoffman MM, Bratman SV, Hung RJ, De Carvalho DD. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 617] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 187. | Mishra S, Srivastava P, Pandey A, Shukla S, Agarwal A, Husain N. Diagnostic Utility of Next-Generation Sequencing in Circulating Free DNA and a Comparison With Matched Tissue in Gallbladder Carcinoma. Lab Invest. 2024;104:100301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 188. | Tekcham DS, Poojary SS, Bhunia S, Barbhuiya MA, Gupta S, Shrivastav BR, Tiwari PK. Epigenetic regulation of APC in the molecular pathogenesis of gallbladder cancer. Indian J Med Res. 2016;143:S82-S90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 189. | Sharma P, Bhunia S, Poojary SS, Tekcham DS, Barbhuiya MA, Gupta S, Shrivastav BR, Tiwari PK. Global methylation profiling to identify epigenetic signature of gallbladder cancer and gallstone disease. Tumour Biol. 2016;37:14687-14699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 190. | Singh TD, Gupta S, Shrivastav BR, Tiwari PK. Epigenetic profiling of gallbladder cancer and gall stone diseases: Evaluation of role of tumour associated genes. Gene. 2016;576:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 191. | Awasthi NP, Kumari S, Neyaz A, Gupta S, Agarwal A, Singhal A, Husain N. EpCAM-based Flow Cytometric Detection of Circulating Tumor Cells in Gallbladder Carcinoma Cases. Asian Pac J Cancer Prev. 2017;18:3429-3437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 192. | Yan C, Xiao Y, Zhang W, Sun Y, Lin Y, Cai W. Circulating Tumor Cells are an Independent Risk Factor for Poor Prognosis in Patients with Gallbladder Adenocarcinoma. Ann Surg Oncol. 2023;30:7966-7975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 193. | Srivastava K, Srivastava A, Mittal B. Potential biomarkers in gallbladder cancer: present status and future directions. Biomarkers. 2013;18:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 194. | Zhong Y, Wu X, Li Q, Ge X, Wang F, Wu P, Deng X, Miao L. Long noncoding RNAs as potential biomarkers and therapeutic targets in gallbladder cancer: a systematic review and meta-analysis. Cancer Cell Int. 2019;19:169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 195. | Li M, Zhang Z, Li X, Ye J, Wu X, Tan Z, Liu C, Shen B, Wang XA, Wu W, Zhou D, Zhang D, Wang T, Liu B, Qu K, Ding Q, Weng H, Ding Q, Mu J, Shu Y, Bao R, Cao Y, Chen P, Liu T, Jiang L, Hu Y, Dong P, Gu J, Lu W, Shi W, Lu J, Gong W, Tang Z, Zhang Y, Wang X, Chin YE, Weng X, Zhang H, Tang W, Zheng Y, He L, Wang H, Liu Y, Liu Y. Whole-exome and targeted gene sequencing of gallbladder carcinoma identifies recurrent mutations in the ErbB pathway. Nat Genet. 2014;46:872-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 326] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 196. | Yang G, Lu Z, Meng F, Wan Y, Zhang L, Xu Q, Wang Z. Circulating miR-141 as a potential biomarker for diagnosis, prognosis and therapeutic targets in gallbladder cancer. Sci Rep. 2022;12:10072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 197. | Srivastava P, Mishra S, Agarwal A, Pandey A, Husain N. Circulating microRNAs in gallbladder cancer: Is serum assay of diagnostic value? Pathol Res Pract. 2023;242:154320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 198. | Mishra S, Srivastava P, Pandey A, Agarwal A, Shukla S, Husain N. Panel of serum long non-coding RNAs as potential non-invasive biomarkers for gallbladder carcinoma. Noncoding RNA Res. 2024;9:583-593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 199. | Ueta E, Tsutsumi K, Kato H, Matsushita H, Shiraha H, Fujii M, Matsumoto K, Horiguchi S, Okada H. Extracellular vesicle-shuttled miRNAs as a diagnostic and prognostic biomarker and their potential roles in gallbladder cancer patients. Sci Rep. 2021;11:12298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 200. | Ying H, Fengying S, Feng H, Yanhong W, Xianru X, Xiaolei T. Diagnostic value of quantification of circulating free DNA for gall bladder cancer using a chemiluminescence DNA biosensor system based on DNA G-quadruplex/ hemin enzyme. Transl Oncol. 2021;14:100928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 201. | Sakamoto K, Ogawa K, Tamura K, Honjo M, Sogabe K, Ito C, Iwata M, Sakamoto A, Shine M, Nishi Y, Uraoka M, Nagaoka T, Funamizu N, Takada Y. Diagnostic value of quantification of cell-free DNA for suspected gallbladder cancer. JGH Open. 2023;7:748-754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 202. | Kinugasa H, Nouso K, Ako S, Dohi C, Matsushita H, Matsumoto K, Kato H, Okada H. Liquid biopsy of bile for the molecular diagnosis of gallbladder cancer. Cancer Biol Ther. 2018;19:934-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 203. | Nagana Gowda GA, Shanaiah N, Cooper A, Maluccio M, Raftery D. Visualization of bile homeostasis using (1)H-NMR spectroscopy as a route for assessing liver cancer. Lipids. 2009;44:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 204. | Mironov AA, Fickett JW, Gelfand MS. Frequent alternative splicing of human genes. Genome Res. 1999;9:1288-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 363] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 205. | Okada K, Kijima H, Imaizumi T, Hirabayashi K, Matsuyama M, Yazawa N, Oida Y, Dowaki S, Tobita K, Ohtani Y, Tanaka M, Inokuchi S, Makuuchi H. Stromal laminin-5gamma2 chain expression is associated with the wall-invasion pattern of gallbladder adenocarcinoma. Biomed Res. 2009;30:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 206. | Srivastava M, Sharma A, Kapoor VK, Nagana Gowda GA. Stones from cancerous and benign gallbladders are different: A proton nuclear magnetic resonance spectroscopy study. Hepatol Res. 2008;38:997-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 207. | Tognarelli JM, Dawood M, Shariff MI, Grover VP, Crossey MM, Cox IJ, Taylor-Robinson SD, McPhail MJ. Magnetic Resonance Spectroscopy: Principles and Techniques: Lessons for Clinicians. J Clin Exp Hepatol. 2015;5:320-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 208. | Sehrawat A, Gopi VP, Gupta A. A Systematic Review on Role of Deep Learning in CT scan for Detection of Gall Bladder Cancer. Arch Computat Methods Eng. 2024;31:3303-3311. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 209. | Fujita H, Wakiya T, Ishido K, Kimura N, Nagase H, Kanda T, Matsuzaka M, Sasaki Y, Hakamada K. Differential diagnoses of gallbladder tumors using CT-based deep learning. Ann Gastroenterol Surg. 2022;6:823-832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 210. | Ardali Duzgun S, Durhan G, Basaran Demirkazik F, Irmak I, Karakaya J, Akpinar E, Gulsun Akpinar M, Inkaya AC, Ocal S, Topeli A, Ariyurek OM. AI-Based Quantitative CT Analysis of Temporal Changes According to Disease Severity in COVID-19 Pneumonia. J Comput Assist Tomogr. 2021;45:970-978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |