Published online May 24, 2025. doi: 10.5306/wjco.v16.i5.105341
Revised: February 27, 2025
Accepted: March 24, 2025
Published online: May 24, 2025
Processing time: 121 Days and 14.6 Hours
Rosmarinic acid (RA) is a natural polyphenol carboxylic acid known for its role in chemoprevention. Given its widespread use as a food additive, we are interested in whether RA affects the development of colorectal cancer (CRC).
To examine the anti-tumor effects of RA on various CRC cell lines, and to further investigate the possible mechanisms.
Cell Counting Kit-8 assay and optical microscopy imaging were used to evaluate the viability of CRC cell lines. Western blot, quantitative real-time polymerase chain reaction, and flow cytometry analyses were performed to assess cell viability and activation of nuclear factor-kappa B (NF-κB) signaling. Molecular modeling was used to assess the interaction between RA and inhibitory kappa B kinase beta. Luciferase assay was used to examine the activity of NF-κB-driven transcription. The combinations of RA with 5-fluorouracil or oxaliplatin were utilized to evaluate the potential synergistic action of RA with the chemotherapeutics.
RA exerted potent cytotoxic actions on all six CRC cell lines examined. RA was docked nicely into the binding pocket of inhibitory kappa B kinase beta by molecular modeling. The activity of NF-κB-driven luciferase and the phosphorylation of NF-κB p65 were decreased after exposure to the compound. Lipopolysaccharide-induced NF-κB activation was effectively inhibited by RA, too. Further, RA downregulated the expression of cell proliferation-related cyclin D1 and MYC, which are target genes of NF-κB. Of note, the cytotoxic actions of 5-fluorouracil and oxaliplatin were markedly enhanced by RA in those CRC cells.
Our results indicate that RA inhibits NF-κB signaling and induces apoptosis in CRC cells. It enhances the cytotoxic actions of chemotherapeutics and might help to improve the chemotherapy of CRC.
Core Tip: Given the widespread use of rosmarinic acid (RA) as a food additive, this study aimed to investigate whether RA affected colorectal cancer (CRC) development. We verified that RA exerted its anti-tumor activity through effectively suppressing nuclear factor-kappa B signaling, and enhanced the cytotoxic actions of 5-fluorouracil and oxaliplatin in CRC cells, which indicated that RA might help to improve the chemotherapy of CRC.
- Citation: Liu WY, Wang H, Xu X, Wang X, Han KK, You WD, Yang Y, Zhang T. Natural compound rosmarinic acid displays anti-tumor activity in colorectal cancer cells by suppressing nuclear factor-kappa B signaling. World J Clin Oncol 2025; 16(5): 105341
- URL: https://www.wjgnet.com/2218-4333/full/v16/i5/105341.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i5.105341
Colorectal cancer (CRC) is one of the most common malignant tumors in the world[1], and the incidence and mortality of CRC in China are constantly increasing[2]. In China, the incidence of CRC ranks second among all malignant tumors, and its mortality ranks fourth[2]. It is known that CRC initially presents as colonic intraepithelial polyps and gradually transforms into cancerous tissues[3]. Polyps can be detected in the early screening of diseases, but most cases of CRC patients are only discovered after transitioning to a late stage[4]. The primary treatment methods for CRC include surgery, radiation, chemotherapy, and targeted therapy[5]. While these approaches have shown some success, their efficacy remains limited, particularly for patients with advanced-stage tumors[6]. This underscores the urgent need for the development of novel therapeutic strategies to improve outcomes of CRC patients.
In recent years, traditional Chinese medicine (TCM) has emerged as a pivotal resource for novel drug development, with its therapeutic potential garnering increasing attention. Notably, specific monomeric compounds obtained from TCM have been reported to display significant anti-cancer properties. For example, Pien Tze Huang, a traditional formulation, has been shown to suppress the carcinogenesis of CRC by restoring gut microbiota and metabolites[7]. Key components of Pien Tze Huang, such as ginsenoside-F2 and ginsenoside-Re, exhibited potent inhibitory effects on CRC cells and primary organoids[7]. Similarly, oridonin induced the death of CRC cells by upregulating TP53 expression, inhibiting transcription factor 4 transactivation, and dysregulating endoplasmic reticulum (ER) stress[8]. Additionally, curcumin, a major bioactive compound in Curcuma longa, has been widely studied for its multifaceted anti-tumor activities, mediated through the modulation of key cancer-related signaling pathways[9]. Such information underscores the critical roles of monomeric compounds from TCM in advancing innovative cancer therapies.
Rosmarinic acid (RA), a natural phenolic acid compound derived from rosemary, is a potent antioxidant with de
The CRC cell lines HCT116, HT29, LoVo, RKO, SW480 and SW620 were maintained in our laboratory. All the cells were cultured in Dulbecco’s Modified Eagle Medium (Hyclone, UT, United States) with 10% fetal bovine serum (Gibco, NY, United States), 100 units/mL penicillin and 100 μg/mL streptomycin (Beyotime, Beijing, China). RA, oxaliplatin (OXA), and 5-fluorouracil (5-FU) were purchased from Selleck, Houston, TX, United States. Lipopolysaccharide (LPS) was purchased from Sigma-Aldrich, St. Louis, MO, United States.
CRC cells were cultured in 96-well plates overnight, and then cells were incubated with indicated compounds for 24 hours. Then, the cells were taken photos under a microscope, and the cell viability was measured by using the Cell Counting Kit-8 (CCK-8) reagent according to the manufacturer’s instruction (Selleck, #B34304).
Quantitative real-time polymerase chain reaction (qRT-PCR) was conducted as stated previously[13]. Total RNA was isolated with Trizol reagent (Takara), and the mRNA was reversely transcribed into cDNA with the PrimeScriptTM RT reagent Kit (Takara). Then, qRT-PCR was performed to assess the mRNA levels of genes of interest with TB Green Premix Ex Taq II (Takara) followed by the manufacturer’s instruction. Primers used in this study are listed in Supplementary Table 1.
Western blot was performed following the protocol described in our previous study[14,15]. Briefly, cells were harvested and lysed for total protein isolation. The isolated protein was quantified, denatured, and separated by sodium-dodecyl sulfate gel electrophoresis. Then, the separated protein was transferred to polyvinylidene fluoride membranes (Millipore). The membranes were blocked by non-fat milk and then incubated with primary antibodies. The primary antibodies against cyclin D1, MYC, α-Tublin, and GAPDH were purchased from Selleck, Houston, TX, United States. Anti-phosphorylated nuclear factor-kappa B (NF-κB) P65 (p-p65, Ser536) antibody, anti-NF-κB P65 antibody, anti-Bcl-2 antibody, anti-caspase-3 antibody, and anti-phosphorylated protein kinase B (p-AKT, Ser473) antibody were purchased from Cell Signaling Technology, Danvers, MA, United States.
Flow cytometry analysis was performed to assess the cell apoptosis. In brief, HT29 cells were incubated with 0, 200, and 400 μg/mL RA for 24 hours, and then harvested and stained with Annexin V-FITC and propidium iodide (Beyotime, Beijing, China), followed by flow cytometry detection (BD FACSCalibur).
Molecular docking was performed using the Schrodinger suite (2017-1) with default parameters. To be specific, the protein structure file of inhibitory kappa B kinase beta (IKKβ) (PDB entry: 4KIK) was obtained from the Protein Data Bank (http://www.rcsb.org), and preprocessed with the Protein Preparation Wizard program and the Receptor Grid Generation program. The compound was prepared via the LigPrep program and docked into the original active site of IKKβ utilizing the Ligand Docking program of the Schrodinger suite with the XP (extra precision) mode.
Luciferase assay was conducted according to our previous study[16]. HT29 and SW480 cells transfected with NF-κB-derived firefly luciferase reporter gene (pNF-κB-Luc) along with Renilla luciferase were incubated with increasing concentrations of RA overnight. Then, the cells were lysed for luciferase assay by using the Dual-Luciferase® Reporter Assay System kit (Promega, WI, United States).
The pictures in the study were generated using the software GraphPad Prism 8.0.2. All data are presented as the mean ± SD. Student’s t test was used to compare the differences between two groups. One-way ANOVA with Dunnett’s post hoc test was performed to analyze differences among multiple groups. A P value less than 0.05 was considered statistically significant.
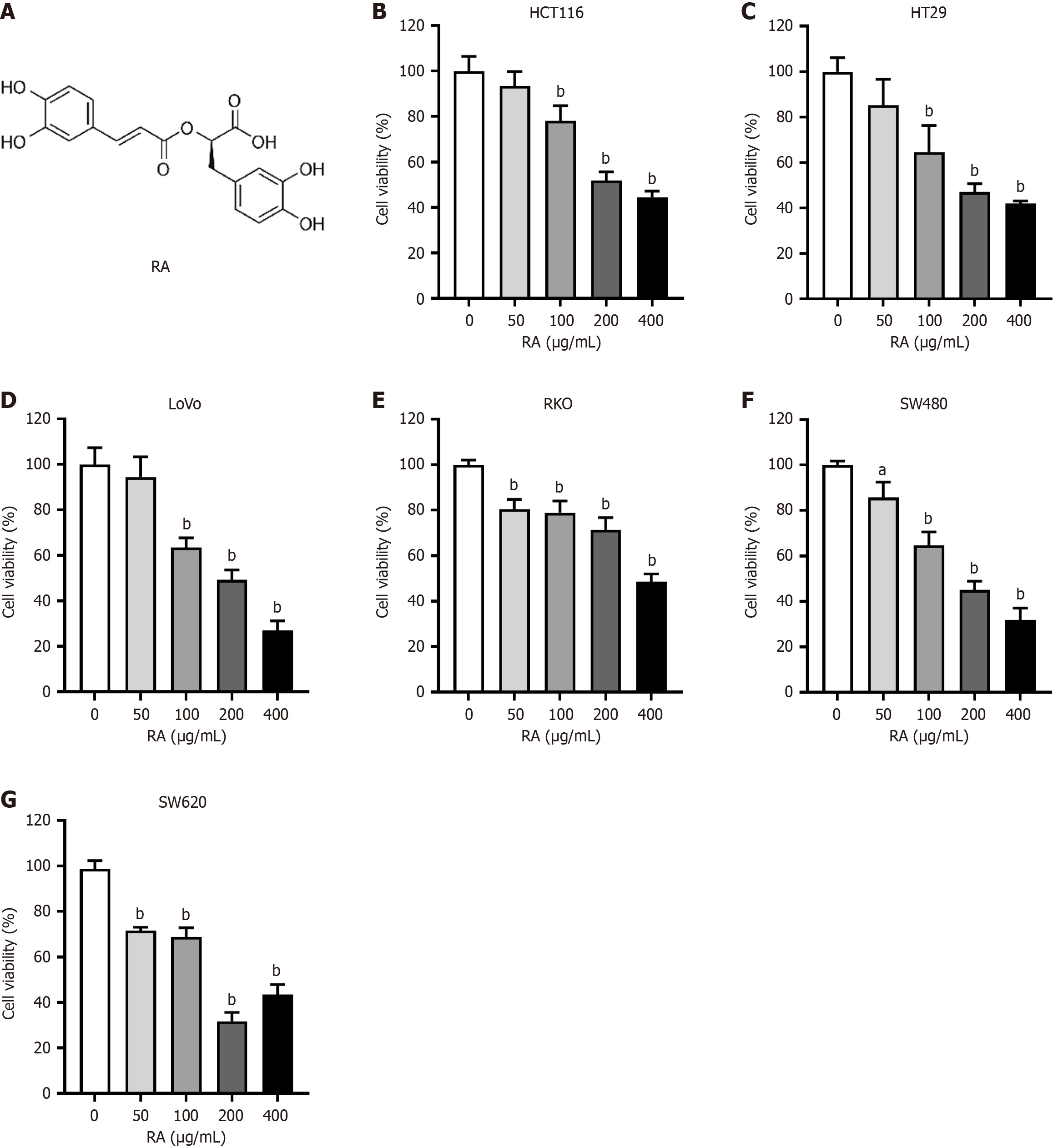
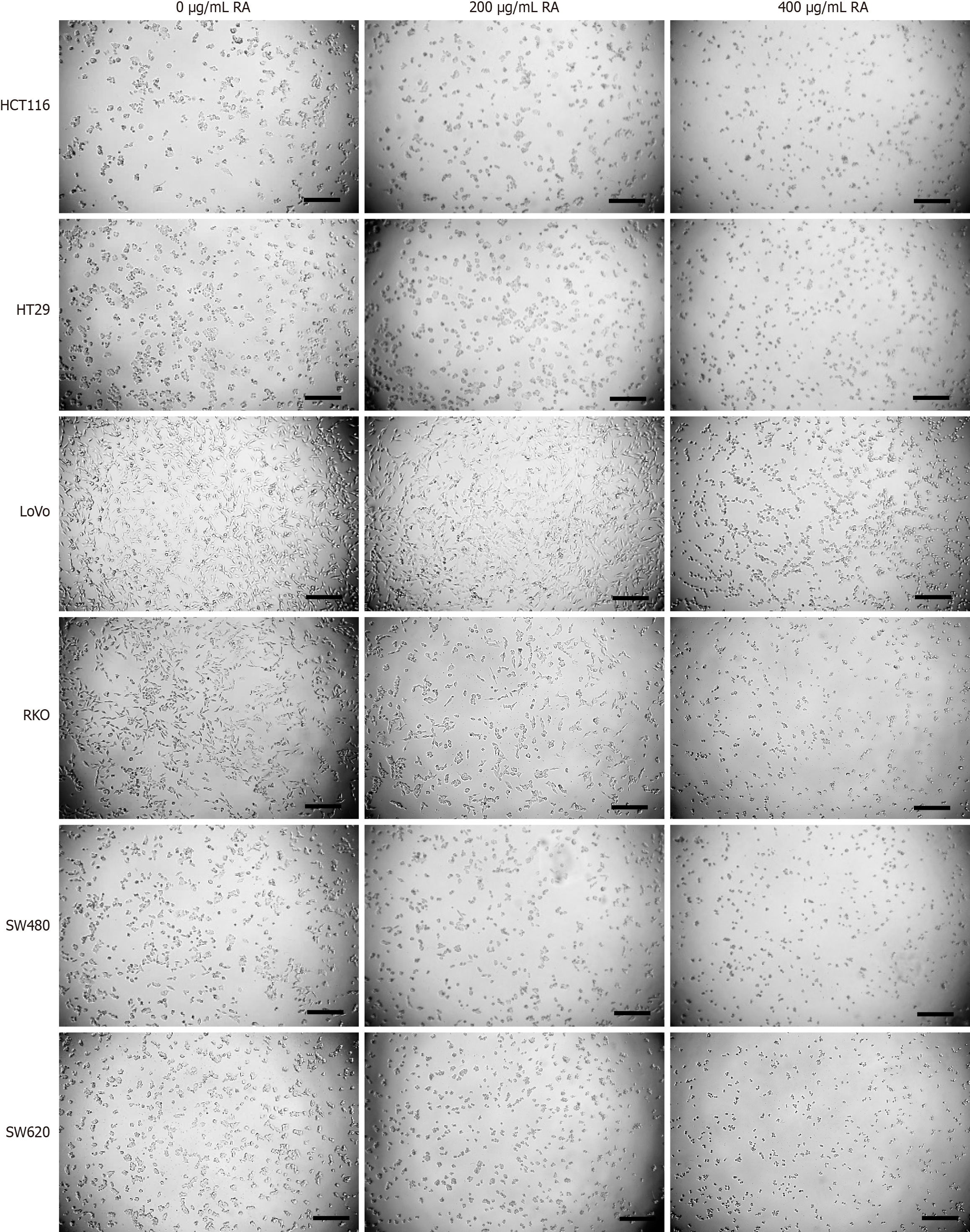
Six CRC cell lines were incubated with escalating concentrations of RA. Twenty-four hours later, the cells were prepared for CCK-8 assay to assess cell viability. As shown in Figure 1, the CCK-8 assay results indicated that RA could suppress the viability of all six CRC cell lines in a dose-dependent manner. In addition, after 24 hours of cell culture with RA, optical microscopy imaging was carried out. The microscopic images, shown in Figure 2, reveal that the quantity of the CRC cells was significantly decreased after RA treatment. This visual evidence further corroborated the inhibitory effect of RA on CRC cell proliferation demonstrated by the CCK-8 assay.
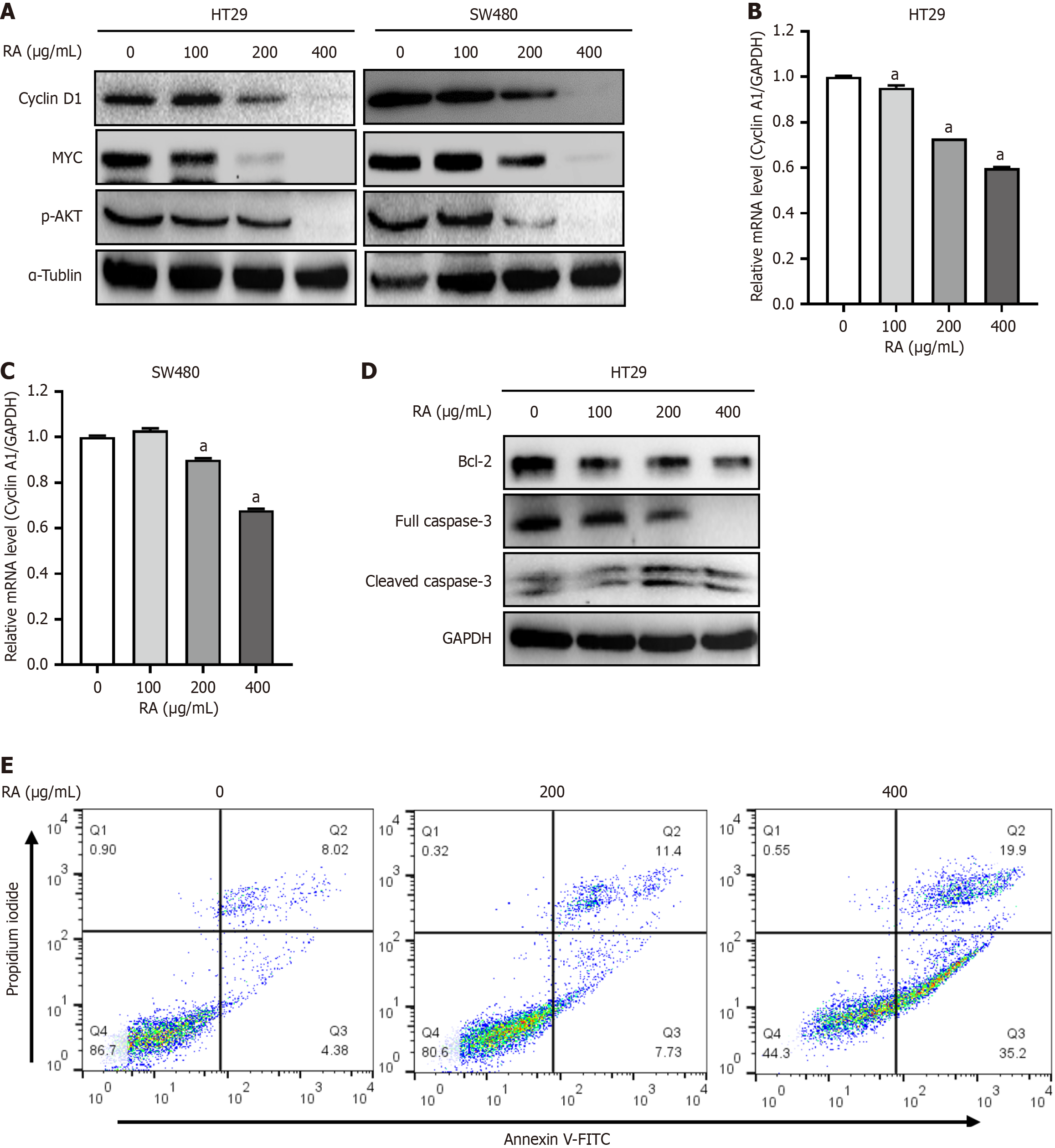
It is well-established that numerous proteins exhibit tumor-promoting functions in CRC cells, such as cyclin A1, cyclin D1, and MYC. Subsequently, HT29 and SW480 cells were incubated with increasing concentrations of RA for 24 hours, and then harvested and processed for subsequent analysis of target gene expression using Western blot and qRT-PCR techniques. As shown in Figure 3A, the results of Western blot showed that RA markedly decreased the expression of cyclin D1, MYC, and p-AKT. In addition, qRT-PCR also showed that RA decreased the expression of cyclin A1 in HT29 and SW480 cells (Figure 3B and C). Moreover, we found that RA downregulated the expression of anti-apoptotic Bcl-2, and induced the cleavage of caspase 3, a biomarker of cell apoptosis (Figure 3D). And flow cytometry also revealed that RA induced the apoptosis of CRC cells (Figure 3E). These results above further indicated that RA inhibited CRC cell survival by inducing cell apoptosis.
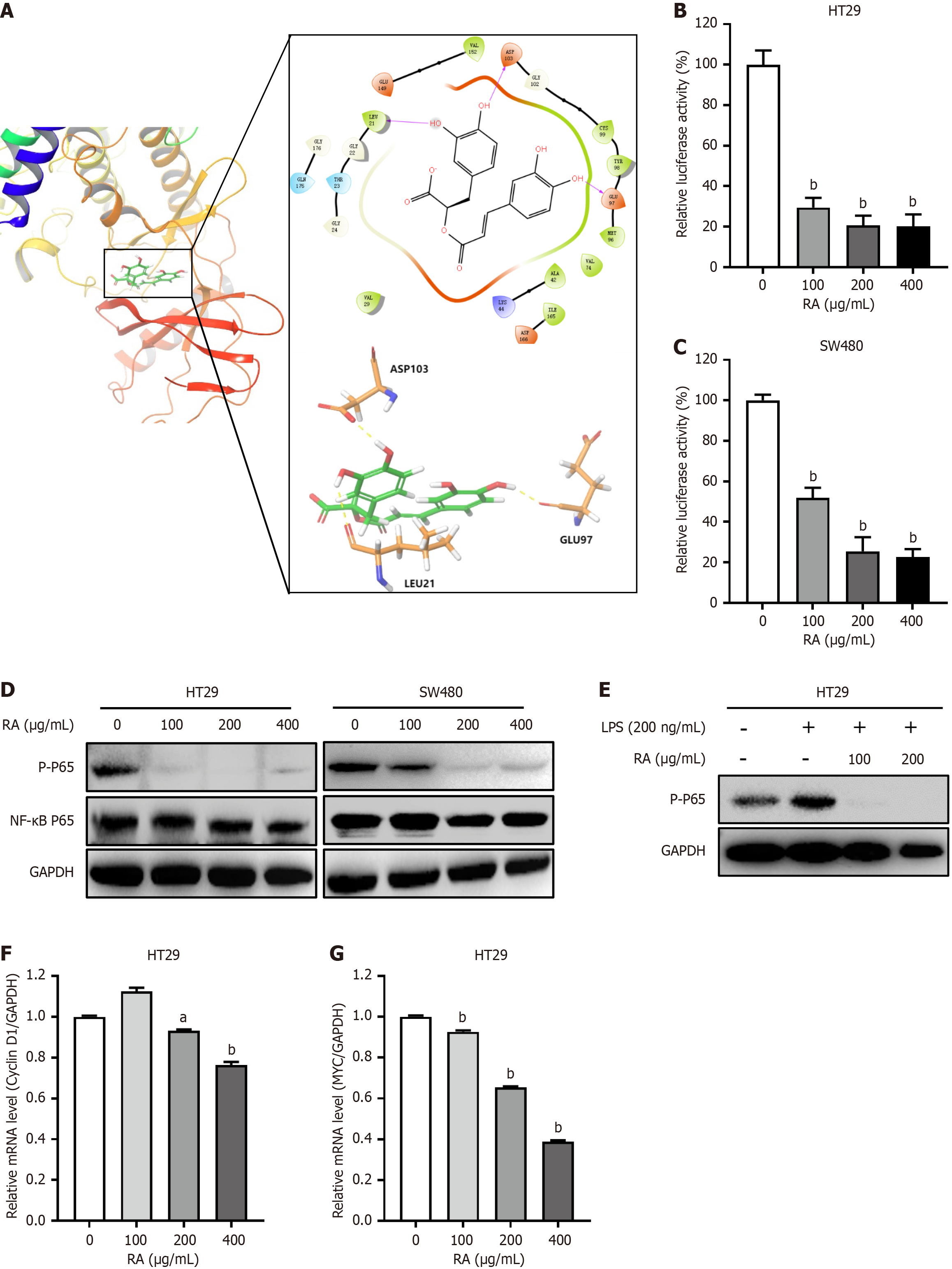
Activation of the NF-κB signaling pathway mediates the tumorigenesis of CRC, and NF-κB activation is dependent on IKK complexes, particularly IKKβ. Given these, we analyzed the interaction between RA and IKKβ by molecular modeling. As shown in Figure 4A, RA was docked nicely into the binding pocket of IKKβ, which indicated that RA may specifically modulate NF-κB signaling. Subsequently, our results from luciferase assay showed that RA significantly downregulated NF-κB-derived luciferase activity (Figure 4B and C). To further validate this phenomenon, HT29 and SW480 cells were incubated with RA for 24 hours, and then lysed for Western blot analysis. As shown in Figure 4D, RA obviously downregulated the phosphorylation of NF-κB P65. Moreover, RA also abolished LPS-induced NF-κB P65 phosphorylation in CRC cells (Figure 4E). We further assessed the transcriptional profiles of NF-κB downstream effector genes implicated in cell cycle progression and oncogenesis. Quantitative analysis revealed that RA induced marked downregulation of cyclin D1 and MYC expression in CRC cells. Collectively, our results above indicated that RA inhibited NF-κB signaling in CRC cells (Figure 4F and G).
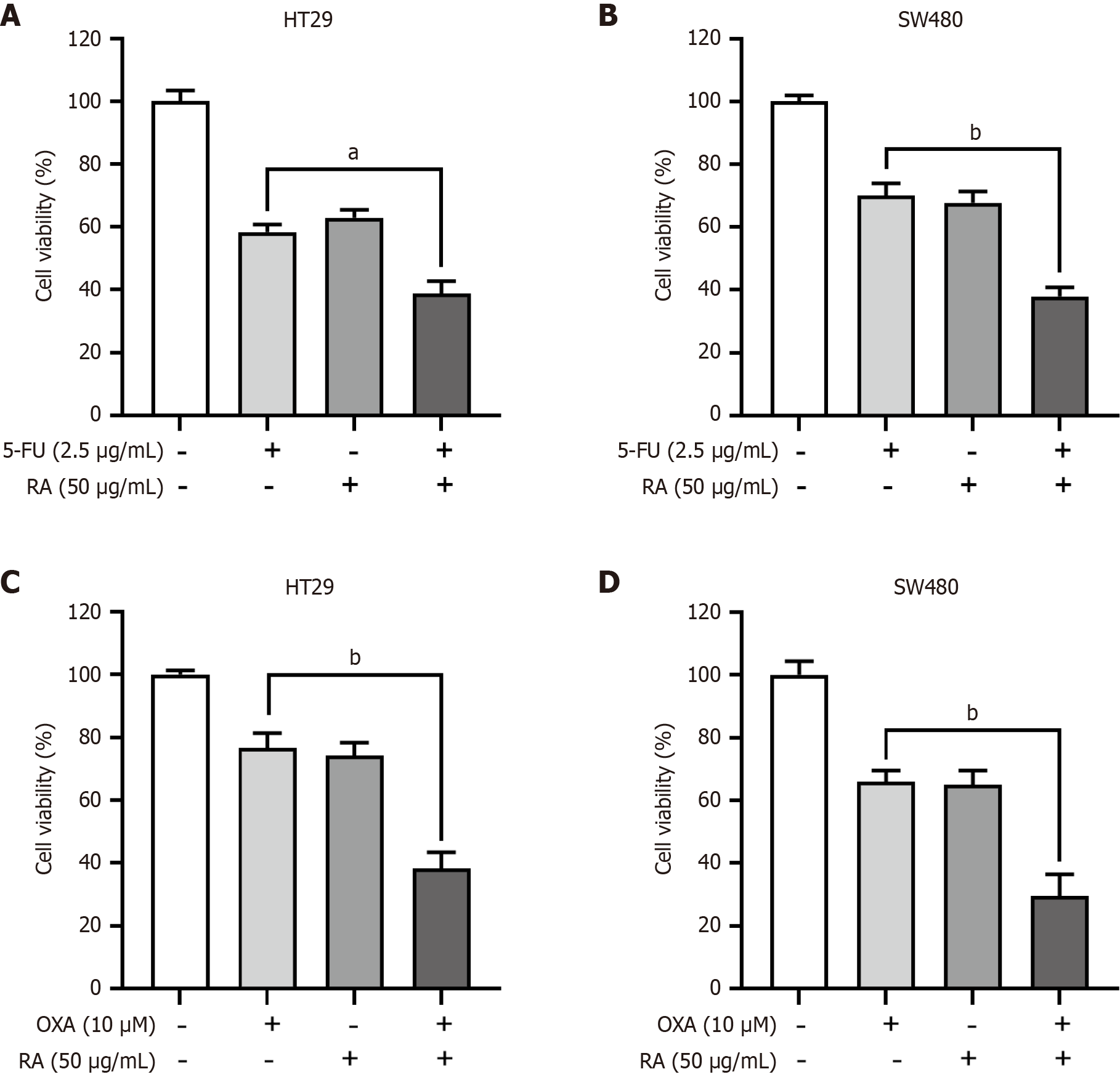
5-FU and OXA are used as the cornerstone drugs for CRC therapy in the first line setting. However, the frequent acquired resistance compromises their therapeutic efficacy, which underscores the critical need for developing novel drugs for CRC therapy. In this study, CRC cells were treated with RA in combination with the first-line chemotherapeutic drugs 5-FU or OXA. As shown in Figure 5A and B, the results from CCK-8 assay indicated that RA significantly enhanced the cytotoxicity of 5-FU in both of HT29 and SW480 cells. In addition, RA also enhanced the cytotoxicity of OXA in both of HT29 and SW480 cells (Figure 5C and D). Collectively, our findings demonstrated that RA exhibited significant potential as a synergistic agent in combinatorial anti-cancer regimens.
Extensive research has established that RA exhibits potent anti-tumor effects across multiple malignancies. In glio
Activated NF-κB can bind to the promoter regions of many genes, and then initiates the transcription of genes[19]. It is not only related to the cell proliferation, cell differentiation, cell cycle, and immune response of the body, but also closely related to the development, metastasis, and infiltration of tumors[20]. It has been also demonstrated that specific inhibition of NF-κB can effectively suppress the development of tumors and improve the prognosis of patients with tumors[21]. In the present study, through structure-based virtual screening, we found that RA formed a stable interaction with the ATP binding pocket of IKKβ in the molecular docking model. It is worth noting that IKKβ is the core regulatory kinase of the classical NF-κB signaling pathway. Based on this, we speculated that RA may specifically suppress the activation of classical NF-κB signaling. Subsequently, a NF-κB-responsive luciferase reporter system was established in CRC cells. Results from luciferase assays indicated that RA significantly suppressed the transcriptional activity of NF-κB. And results from Western blot analysis also revealed that RA markedly inhibited both basal and LPS-induced activation of NF-κB in CRC cells. Moreover, results from qRT-PCR analysis also showed that RA significantly suppressed the transcription of NF-κB downstream effector genes in CRC cells, including cyclin D1 and MYC. These findings above strongly supported that RA exerted its anti-cancer effects in CRC cells by selectively blocking NF-κB signaling.
OXA and 5-FU are the cornerstone chemotherapeutical drugs for CRC treatment[22]. However, almost all chemotherapeutical agents, including 5-FU and OXA, inevitably lead to drug resistance during prolonged therapeutic cycles[23]. A promising strategy, involving identifying novel bioactive natural compounds for synergistic combination therapy with conventional chemotherapeutics, has been considered to be potential in overcoming drug resistance and enhancing therapeutic efficacy in the treatment of CRC[24]. Based on this information, we conducted combination therapy of RA with chemotherapeutic drugs. And our present study demonstrated that co-administration of RA with the first-line chemotherapeutics OXA or 5-FU synergistically enhanced their anti-CRC efficacy. Mechanistically, RA-mediated su
RA exerts its anti-tumor effects in CRC cells by inhibiting the NF-κB signaling pathway and holds great promise for the chemotherapeutical combination in CRC.
We would like to thank Dr. Daylen for her help in this work.
| 1. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 3016] [Article Influence: 502.7] [Reference Citation Analysis (3)] |
| 2. | Wang Z, Dan W, Zhang N, Fang J, Yang Y. Colorectal cancer and gut microbiota studies in China. Gut Microbes. 2023;15:2236364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 3. | Patel SG, Karlitz JJ, Yen T, Lieu CH, Boland CR. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol. 2022;7:262-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 386] [Article Influence: 128.7] [Reference Citation Analysis (6)] |
| 4. | Mahmoud NN. Colorectal Cancer: Preoperative Evaluation and Staging. Surg Oncol Clin N Am. 2022;31:127-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 54] [Reference Citation Analysis (0)] |
| 5. | Abedizadeh R, Majidi F, Khorasani HR, Abedi H, Sabour D. Colorectal cancer: a comprehensive review of carcinogenesis, diagnosis, and novel strategies for classified treatments. Cancer Metastasis Rev. 2024;43:729-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 50] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 6. | Shinji S, Yamada T, Matsuda A, Sonoda H, Ohta R, Iwai T, Takeda K, Yonaga K, Masuda Y, Yoshida H. Recent Advances in the Treatment of Colorectal Cancer: A Review. J Nippon Med Sch. 2022;89:246-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 112] [Reference Citation Analysis (1)] |
| 7. | Gou H, Su H, Liu D, Wong CC, Shang H, Fang Y, Zeng X, Chen H, Li Y, Huang Z, Fan M, Wei C, Wang X, Zhang X, Li X, Yu J. Traditional Medicine Pien Tze Huang Suppresses Colorectal Tumorigenesis Through Restoring Gut Microbiota and Metabolites. Gastroenterology. 2023;165:1404-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 77] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 8. | Zhou F, Gao H, Shang L, Li J, Zhang M, Wang S, Li R, Ye L, Yang S. Oridonin promotes endoplasmic reticulum stress via TP53-repressed TCF4 transactivation in colorectal cancer. J Exp Clin Cancer Res. 2023;42:150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 9. | Weng W, Goel A. Curcumin and colorectal cancer: An update and current perspective on this natural medicine. Semin Cancer Biol. 2022;80:73-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 186] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 10. | Dahchour A. Anxiolytic and antidepressive potentials of rosmarinic acid: A review with a focus on antioxidant and anti-inflammatory effects. Pharmacol Res. 2022;184:106421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 11. | Chen C, Liu Y, Shen Y, Zhu L, Yao L, Wang X, Zhang A, Li J, Wu J, Qin L. Rosmarinic acid, the active component of Rubi Fructus, induces apoptosis of SGC-7901 and HepG2 cells through mitochondrial pathway and exerts anti-tumor effect. Naunyn Schmiedebergs Arch Pharmacol. 2023;396:3743-3755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Jin BR, Chung KS, Hwang S, Hwang SN, Rhee KJ, Lee M, An HJ. Rosmarinic acid represses colitis-associated colon cancer: A pivotal involvement of the TLR4-mediated NF-κB-STAT3 axis. Neoplasia. 2021;23:561-573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 13. | Li J, Mo R, Zheng L. MicroRNA-490-3p inhibits migration and chemoresistance of colorectal cancer cells via targeting TNKS2. World J Surg Oncol. 2021;19:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Zhang P, Li R, Xiao H, Liu W, Zeng X, Xie G, Yang W, Shi L, Yin Y, Tao K. BRD4 Inhibitor AZD5153 Suppresses the Proliferation of Colorectal Cancer Cells and Sensitizes the Anticancer Effect of PARP Inhibitor. Int J Biol Sci. 2019;15:1942-1954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Li Y, Li Y, Liu Y, Xie P, Li F, Li G. PAX6, a novel target of microRNA-7, promotes cellular proliferation and invasion in human colorectal cancer cells. Dig Dis Sci. 2014;59:598-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Zhu G, Cheng Z, Huang Y, Zheng W, Yang S, Lin C, Ye J. TRAF6 promotes the progression and growth of colorectal cancer through nuclear shuttle regulation NF-kB/c-jun signaling pathway. Life Sci. 2019;235:116831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Şengelen A, Önay-Uçar E. Rosmarinic acid attenuates glioblastoma cells and spheroids' growth and EMT/stem-like state by PTEN/PI3K/AKT downregulation and ERK-induced apoptosis. Phytomedicine. 2024;135:156060. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Liu H, Deng R, Zhu CW, Han HK, Zong GF, Ren L, Cheng P, Wei ZH, Zhao Y, Yu SY, Lu Y. Rosmarinic acid in combination with ginsenoside Rg1 suppresses colon cancer metastasis via co-inhition of COX-2 and PD1/PD-L1 signaling axis. Acta Pharmacol Sin. 2024;45:193-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 19. | Zhu G, Cheng Z, Huang Y, Zheng W, Yang S, Lin C, Ye J. MyD88 mediates colorectal cancer cell proliferation, migration and invasion via NFκB/AP1 signaling pathway. Int J Mol Med. 2020;45:131-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 20. | Patel M, Horgan PG, McMillan DC, Edwards J. NF-κB pathways in the development and progression of colorectal cancer. Transl Res. 2018;197:43-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 186] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 21. | Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2313] [Cited by in RCA: 2531] [Article Influence: 210.9] [Reference Citation Analysis (0)] |
| 22. | Mitchell EP. Oxaliplatin with 5-FU or as a single agent in advanced/metastatic colorectal cancer. Oncology (Williston Park). 2000;14:30-32. [PubMed] |
| 23. | Van der Jeught K, Xu HC, Li YJ, Lu XB, Ji G. Drug resistance and new therapies in colorectal cancer. World J Gastroenterol. 2018;24:3834-3848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 448] [Cited by in RCA: 413] [Article Influence: 59.0] [Reference Citation Analysis (5)] |
| 24. | Islam MR, Akash S, Rahman MM, Nowrin FT, Akter T, Shohag S, Rauf A, Aljohani ASM, Simal-Gandara J. Colon cancer and colorectal cancer: Prevention and treatment by potential natural products. Chem Biol Interact. 2022;368:110170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 111] [Reference Citation Analysis (0)] |