Published online Jun 5, 2025. doi: 10.4292/wjgpt.v16.i2.105335
Revised: April 10, 2025
Accepted: May 13, 2025
Published online: June 5, 2025
Processing time: 136 Days and 1.8 Hours
Patients with inflammatory bowel disease (IBD) are at an increased risk of bacterial pneumonia, contributing to significant morbidity and mortality. While previous studies have identified various risk factors, including medications and comorbidities, the independent contribution of IBD to pneumonia risk remains unclear. We hypothesized that the increased pneumonia risk is primarily driven by factors other than IBD itself.
To investigate the relative contributions of IBD, comorbidities, and medications to pneumonia risk in patients with IBD.
We conducted a retrospective cohort study using the All of Us Research Program database (2010-2022). We matched 2810 participants with IBD 1:1 with controls using four propensity score models: (1) Demographics/Lifestyle only; (2) Plus comorbidities; (3) Plus medications; and (4) All factors combined. Then we used Cox proportional hazards models to assess pneumonia risk and logistic regression to evaluate risk factors.
In the primary analysis of 5620 matched participants, IBD was not independently associated with increased pneumonia risk [hazard ratio (HR) = 1.07, 95%CI: 0.84-1.35] when matched for all factors. However, participants with IBD had significantly higher risk (HR = 2.08, 95%CI: 1.56-2.78) when matched only for demographics and lifestyle factors. Within the IBD cohort, a high comorbidity burden (Charlson Comorbidity Index ≥ 10) [odds ratio (OR) = 12.20, 95%CI: 6.69-23.00] and systemic steroid use (OR = 2.26, 95%CI: 1.21-4.64) were independently associated with increased pneumonia risk.
Comorbidities and systemic steroids, rather than IBD itself, drive pneumonia risk. Management should focus on these factors and prioritize vaccination in high-risk patients.
Core Tip: While increased pneumonia risk in inflammatory bowel disease (IBD) is well-documented, this large propensity-matched study of 5620 participants provides novel insights into its underlying drivers. Using multiple propensity score models, we demonstrate that the heightened pneumonia risk is primarily driven by comorbidities and systemic steroid use, rather than IBD itself. Most notably, patients with a high Charlson Comorbidity Index (≥ 10) showed a 12-fold increased risk, while systemic steroid use doubled the risk. These findings suggest that pneumonia prevention strategies in IBD should prioritize comorbidity management and judicious steroid use, particularly in high-risk patients.
- Citation: Eun Y, Culpepper-Morgan J, Akanmode AM, Thu MB, Sta Lucia AA, Thearle MS, Trousdale RK. Comorbidities and systemic steroids drive pneumonia risk in inflammatory bowel disease: Propensity score-matched cohort study. World J Gastrointest Pharmacol Ther 2025; 16(2): 105335
- URL: https://www.wjgnet.com/2150-5349/full/v16/i2/105335.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v16.i2.105335
Inflammatory bowel disease (IBD) is a chronic condition characterized by recurring inflammation of the gastrointestinal tract, driven by an abnormal immune response to gut microflora. IBD affects an estimated 2.4 million individuals in the United States and is frequently accompanied by extraintestinal manifestations[1]. Infections are a leading cause of mortality in this population, with infection-related hospitalizations significantly contributing to increased morbidity, mortality, and healthcare costs[2,3]. Among these infections, pneumonia stands out as a major concern, carrying a particularly high risk of mortality[2].
Previous studies have demonstrated an increased risk of invasive pneumococcal infections following an IBD diagnosis[4]. Several factors contribute to the heightened risk of pneumonia in people with IBD, including advanced age, comorbidities, and a prior history of pneumonia[5]. While medications like corticosteroids and anti-tumor necrosis factor (TNF) inhibitors have been associated with elevated pneumonia risk, the effect of immunomodulators is less clear[5-7]. Calcineurin inhibitors, such as cyclosporine and tacrolimus, commonly used in combination therapy for IBD, are reportedly associated with Pneumocystis jirovecii pneumonia, while another study reported an association with lower risk of bacterial pneumonia[7,8].
Given this heightened risk, pneumococcal vaccination with both the 13-Valent Pneumococcal Conjugate Vaccine (PCV13) and 23-Valent Pneumococcal Polysaccharide Vaccine (PPSV23) is recommended for adults with IBD receiving immunosuppressive therapy, according to national guidelines[9]. However, large database studies have not shown that pneumococcal vaccination reduces pneumonia risk in patients with IBD[5]. This might be due to impaired immune responses to pneumococcal vaccination in patients receiving anti-TNF inhibitor or combination therapy[10,11].
Most studies investigating infection risks in IBD have focused on IBD cohorts, leaving the question of whether IBD itself, independent of treatment or comorbidities, directly increases the risk of pneumonia unanswered. One study showed an increased risk of pneumonia in IBD compared to matched controls, but it did not account for differences in immunosuppressive use, a major risk factor for pneumonia[7]. Furthermore, studies exploring the impact of medications on pneumonia risk have often been restricted to specific populations, such as veterans or people with certain commercial insurances, limiting the generalizability of the findings[5-7].
This study assessed the risk of bacterial pneumonia-related hospitalization in people with IBD compared to a matched control group. It seeks to disentangle the contributions of IBD itself, comorbidities, and immunosuppressive medications to pneumonia risk. Utilizing data from the All of Us Research Program, which provides a diverse and inclusive dataset representative of various racial and demographic groups, this study aims to offer a comprehensive and generalizable analysis.
This retrospective cohort study used the Controlled Tier Dataset version 7 from the All of Us Research Program, a large-scale cohort initiated by the National Institutes of Health. This dataset includes longitudinal medical information from 413000 adults, of whom 287012 consented to share their electronic health records (EHRs). The data include health questionnaires, physical measurements, EHRs, and biospecimen data, accessible through the cloud-based Researcher Workbench platform. The study protocol was reviewed and approved by the Institutional Review Board (IRB) of the All of Us Research Program. Detailed protocols have been previously published[12].
The follow-up period was from January 1, 2010 to June 30, 2022. Records prior to 2010 were excluded due to limited biologic therapy use and incomplete EHR integration. Participants who consented to share EHRs and had at least two clinical visits spaced 90 days or more apart within the follow-up period were included in the analysis. We identified people with a diagnosis of IBD based on at least two IBD-related International Classification of Diseases, Ninth and Tenth Revision (ICD-9/10) codes (K50.x, K51.x, 555.x, 556.x), with at least one from an outpatient visit. This method has been validated with a positive predictive value of 0.91 and a miss rate of 0.13[13]. The onset of IBD for each patient was defined as the date of the first recorded IBD diagnosis in their EHR.
Demographic and socioeconomic factors, along with lifestyle variables (current smoking, alcohol use), were collected from enrollment surveys. Medication data were extracted from EHRs which included systemic steroids (e.g., prednisone, prednisolone, methylprednisolone, dexamethasone), anti-TNF inhibitors (e.g., adalimumab, certolizumab pegol, golimumab, infliximab), calcineurin inhibitors (e.g., cyclosporine, tacrolimus), and immunomodulators (e.g., azathioprine, 6-mercaptopurine, methotrexate). Pneumococcal vaccination status was confirmed with vaccination records (pneumococcal conjugate vaccines or pneumococcal polysaccharide vaccine) given after age 5 and prior to any pneumonia diagnosis, based on a validated method[14]. Comorbidities were identified using Charlson Comorbidity Index (CCI) components, based on ICD-9/10 codes[15-17]. CCI scores were calculated and categorized into three levels (0-4, 5-9, ≥ 10) to represent patients’ comorbidity burden.
Bacterial pneumonia-related hospitalization was defined as having at least one inpatient visit with ICD-9/10 codes for bacterial pneumonia (481.x, 482.x, 483.x, 484.x, 485.x, 486.x, J13.x, J14.x, J15.x, J16.x, J17.x, J18.x). This approach has been validated with a positive predictive value of 0.97[18].
Continuous variables are summarized as the mean and SD, while categorical variables are reported as counts and percentages. In accordance with the All of Us Data and Statistics Dissemination Policy, participant counts ranging from 1 to 20 were not displayed.
A sample size calculation indicated that 44 pneumonia events would allow for a power greater than 0.8 with an alpha of 0.05 to detect a difference in incidence rates between the IBD group and the control group using a Cox proportional hazards analysis.
Statistical analyses were performed using R version 4.4.0 (R Core Team, Vienna, Austria) in a Jupyter Notebook environment. Propensity score (PS) matching was conducted using the MatchIt package. The statistical methods of this study were reviewed by Marie S Thearle from NYC Health + Hospitals/Harlem (New York, NY, United States).
To assess the independent impact of IBD on the risk of bacterial pneumonia, we first created a baseline PS–matched cohort. We matched 1:1 on age, sex, race/ethnicity, income, education, current tobacco use, alcohol use, all 18 com
To further delineate the individual contributions of comorbidities and immunosuppressive medications to pneumonia risk, we constructed three additional PS models to compare to the base model: Model 1 matched participants for demographics and lifestyle factors alone; model 2 matched for demographics, lifestyle factors, and comorbidities, excluding immunosuppressive medications; and model 3 matched for demographics, lifestyle factors, and immunosuppressive medications, excluding comorbidities. A detailed list of the variables included in each model is provided in Table 1.
| Propensity score model | Factors used for matching |
| Base model | Age, sex, race/ethnicity, income status, educational attainment, current smoking status, and alcohol consumption, all 18 components of the CCI, use of systemic steroids, anti-TNF inhibitors, calcineurin inhibitors, and immunomodulators |
| Model 1 | Age, sex, race/ethnicity, income status, educational attainment, current smoking status, and alcohol consumption |
| Model 2 | Age, sex, race/ethnicity, income status, educational attainment, current smoking status, and alcohol consumption, all 18 components of the CCI |
| Model 3 | Age, sex, race/ethnicity, income status, educational attainment, current smoking status, and alcohol consumption, use of systemic steroids, anti-TNF inhibitors, calcineurin inhibitors, and immunomodulators |
For people with IBD, follow-up started at the time of IBD diagnosis or the first study-period visit. For the non-IBD group, follow-up started at the first study-period visit. Follow-up ended at the earliest at the time of bacterial pneumonia diagnosis, last recorded visit, or study end. Follow-up time, measured in days, was reported as the median and interquartile range (IQR) due to non-normality (Shapiro-Wilk test, P < 0.001). The Wilcoxon rank-sum test with continuity correction was used to compare the follow-up time between groups.
We estimated the univariable risk of bacterial pneumonia using Cox proportional hazards models for the matched cohort and multivariable models adjusted for vaccination status. The proportional hazard assumption was verified via Schoenfeld residuals. Hazard ratios (HRs) and 95%CI were calculated. By comparing HRs across different PS models, we determined the extent to which the increased pneumonia risk in IBD patients is attributable to IBD, associated comorbidities, or immunosuppressive medications use.
Logistic regression models (univariable and multivariable) were used to evaluate risk factors for bacterial pneumonia within the IBD cohort. Risk factors analyzed included age at IBD diagnosis, duration of IBD, sex, race/ethnicity, annual income, education, current smoking status, alcohol consumption, CCI category, use of systemic steroids, anti-TNF inhibitors, calcineurin inhibitors, immunomodulators, and pneumococcal vaccination.
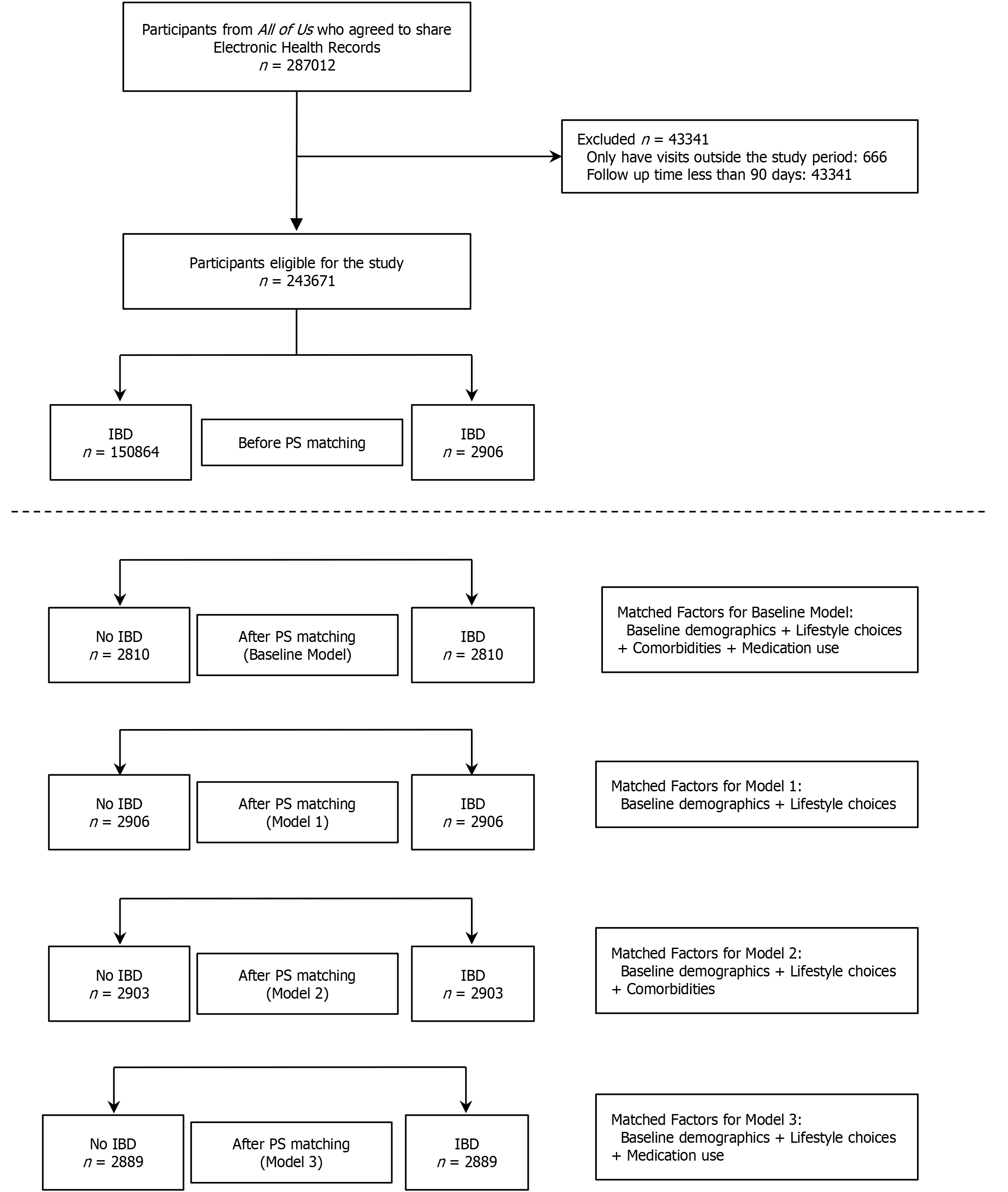
Among the 287012 participants with available EHR data, 243671 had qualifying visits during the study period, and 2906 were diagnosed with IBD. After 1:1 PS matching for baseline demographics, lifestyle factors, comorbidities, and medication use, 96 participants in the IBD group were excluded due to the lack of appropriate matched controls. The base model included 2810 participants in both the IBD and non-IBD groups (n = 5620 total). The number of participants included in each group varied slightly across the additional PS models: 2906 in model 1, 2903 in model 2, and 2889 in model 3 (Figure 1).
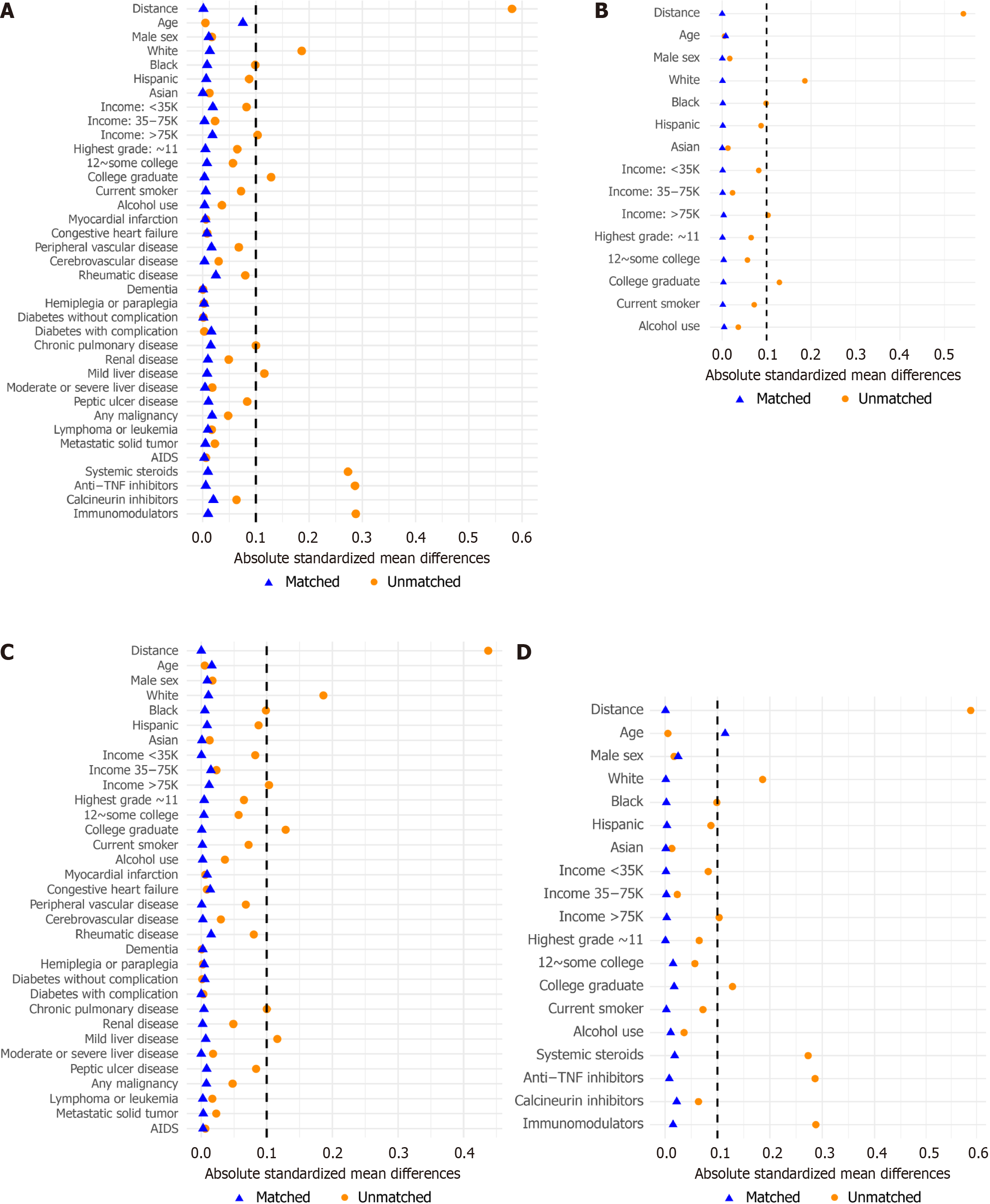
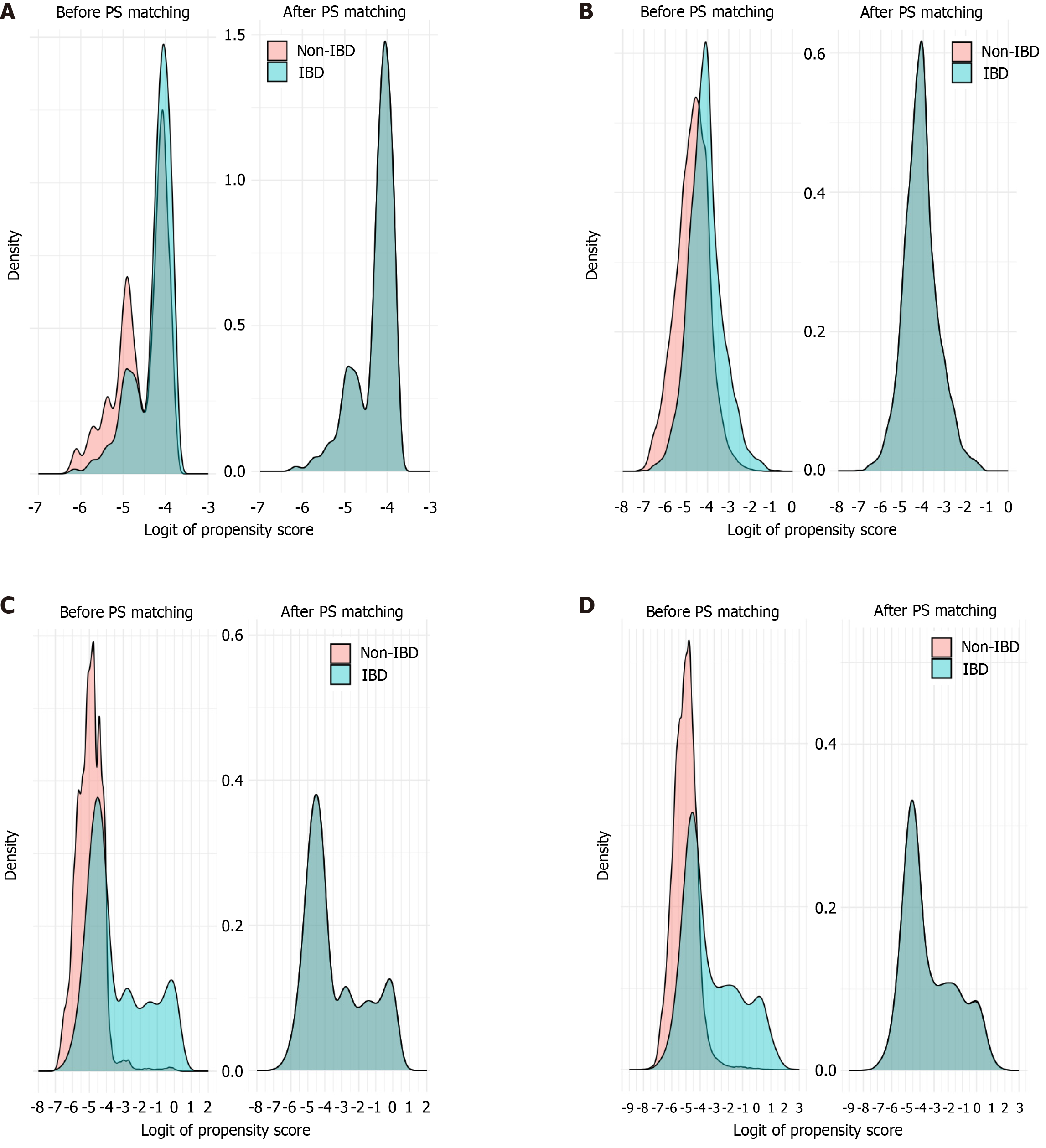
Baseline characteristics of each cohort before and after matching, including standardized differences for all variables, are summarized in Supplementary Table 1. After PS matching, most baseline characteristics achieved an acceptable balance, with the exception of age in model 3, where the standardized mean difference exceeded 10% (Figure 2). The mean CCI score and prevalence of 17 of the 18 comorbidities showed standardized differences less than 10% in model 3, which did not include comorbidities as a matching factor. Figure 3 illustrates the distribution of PSs for each model before and after matching.
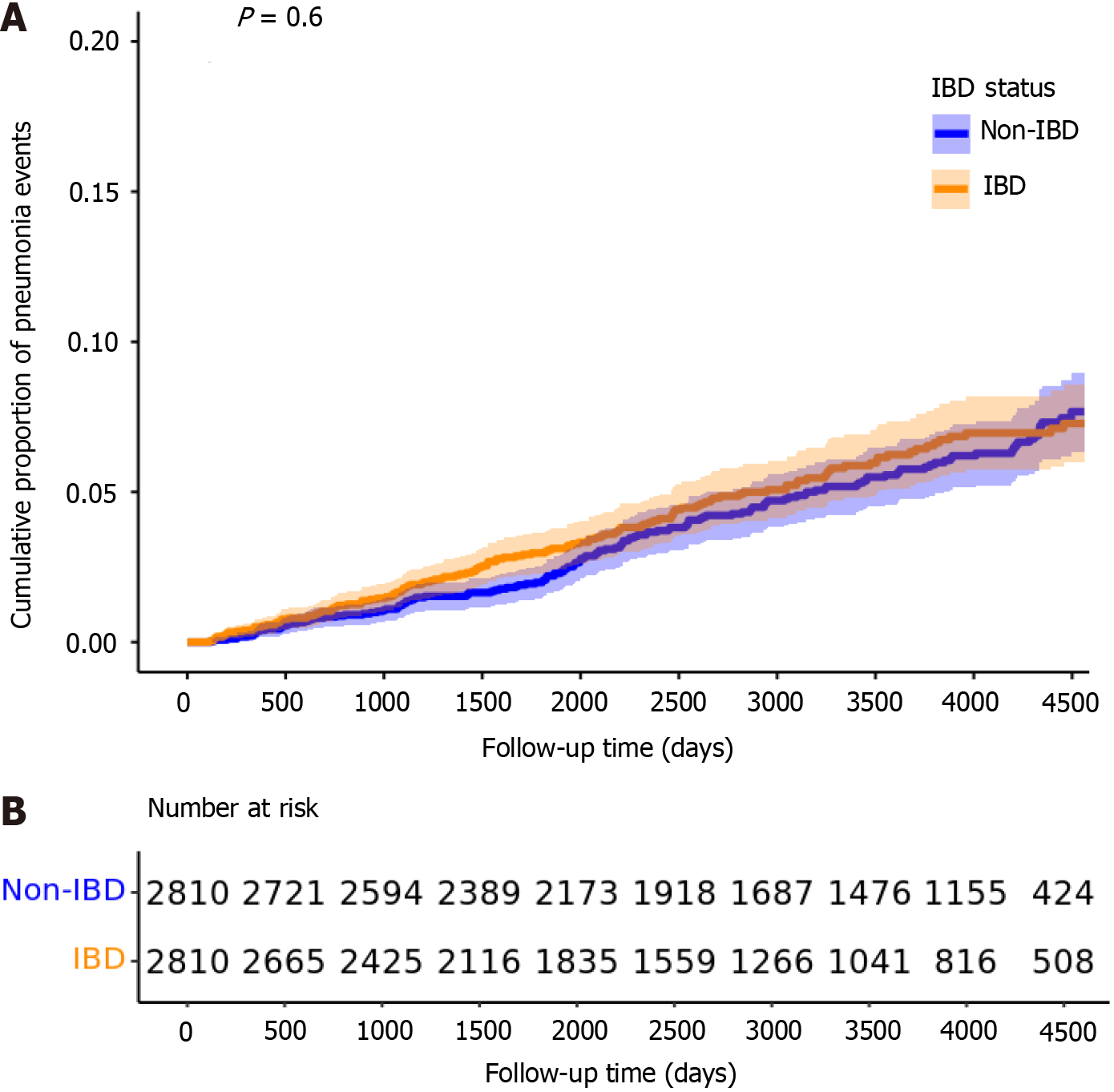
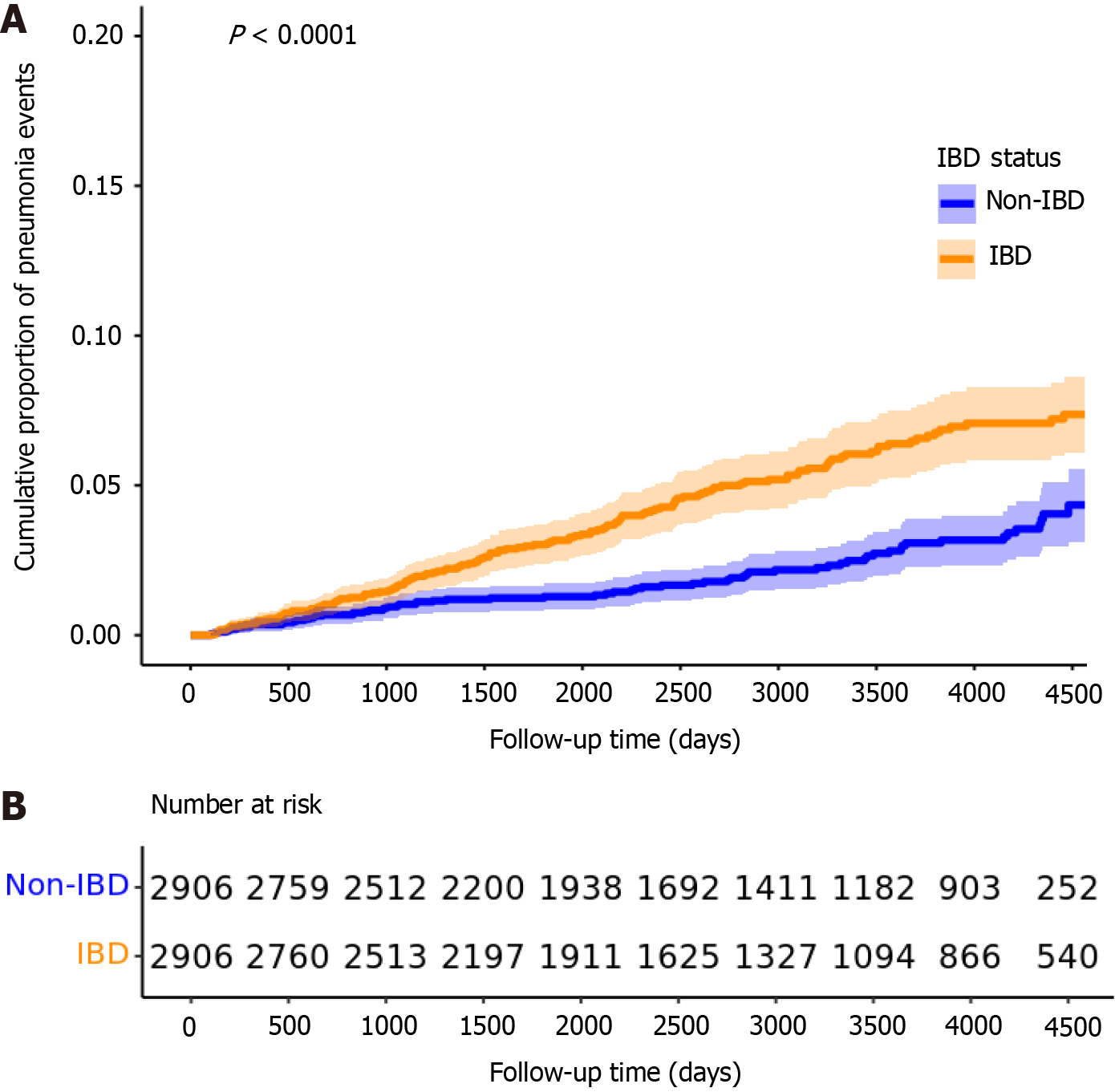
In the baseline PS-matched cohort (n = 5620), there were 132 bacterial pneumonia events in the IBD group (incidence rate: 62.3 per 10000 patient-years) and 145 events in the non-IBD group (59.1 per 10000 patient-years). No significant difference in pneumonia risk was observed between the two groups, either before or after adjusting for pneumococcal vaccination status (univariable HR = 1.07, 95%CI: 0.84-1.35; P = 0.596; multivariable HR = 0.97, 95%CI: 0.82-1.31; P = 0.779) (Figure 4). The median follow-up was 2784 days for IBD patients (IQR: 1515-4244) and 3626 days for non-IBD patients (IQR: 2119-4416). The median time to pneumonia event was 1528 days (IQR: 800-2490) in the IBD group and 2073 days (IQR: 1113-2951) in the non-IBD group. Both differences were statistically significant on the Wilcoxon rank-sum test (P < 0.05).
By contrast, when using model 1—accounting only for demographics and lifestyle factors (n = 5812)—the IBD group showed a significantly increased risk of bacterial pneumonia compared to the non-IBD group (univariable HR = 2.08, 95%CI: 1.56-2.78, P < 0.001; multivariable = HR 1.95, 95%CI: 1.46-2.62, P < 0.001) (Figure 5).
However, when comorbidities were included (model 2, n = 5806) but medications were not, the association was no longer significant (univariable HR = 1.12, 95%CI: 0.88-1.41, P = 0.353; multivariable HR = 1.09, 95%CI: 0.86-1.37, P = 0.489). Similarly, when medications were included but comorbidities were not (model 3, n = 5778), the association was again non-significant (univariable HR = 1.17, 95%CI: 0.92-1.49, P = 0.203; multivariable HR = 1.08, 95%CI: 0.85-1.38, P = 0.522) (Table 2).
| Characteristics | IBD cohort, n = 2810 | |
| No pneumonia, n = 2678 | Pneumonia, n = 132 | |
| Demographic | ||
| Age in year, mean (SD) | 58.0 (17.1) | 65.4 (14.9) |
| Male sex | 1017 (38) | 55 (41.7) |
| Race/ethnicity | ||
| Non-Hispanic White | 1937 (72.3) | 92 (69.7) |
| Non-Hispanic Black | 268 (10) | > 201 |
| Hispanic | 244 (9.1) | < 201 |
| Non-Hispanic Asian | 39 (1.5) | < 201 |
| Other | 190 (7.1) | < 201 |
| Annual income | ||
| < $35000 | 626 (23.4) | 52 (39.4) |
| $35-75000 | 550 (20.5) | 22 (16.7) |
| > $75000 | 1059 (39.5) | 27 (20.5) |
| Education | ||
| Highest grade: About 11 | 73 (2.7) | < 201 |
| Highest grade: 12 to some college | 1019 (38.1) | 69 (52.3) |
| Highest grade: College graduate | 1519 (56.7) | > 201 |
| Current smoker | 231 (8.6) | < 201 |
| Alcohol use | 2447 (91.4) | 118 (89.4) |
| Baseline comorbid conditions | ||
| Myocardial infarction | 143 (5.3) | 36 (27.3) |
| Congestive heart failure | 233 (8.7) | 59 (44.7) |
| Peripheral vascular disease | 441 (16.5) | 72 (54.5) |
| Cerebrovascular disease | 338 (12.6) | 53 (40.2) |
| Chronic pulmonary disease | 986 (36.8) | 99 (75) |
| Rheumatic disease | 366 (13.7) | 32 (24.2) |
| Dementia | 29 (1.1) | < 201 |
| Hemiplegia or paraplegia | 57 (2.1) | < 201 |
| Diabetes mellitus without complication | 288 (10.8) | 26 (19.7) |
| Diabetes mellitus with complication | 265 (9.9) | 40 (30.3) |
| Renal disease | 378 (14.1) | 47 (35.6) |
| Mild liver disease | 633 (23.6) | 51 (38.6) |
| Moderate to severe liver disease | 68 (2.5) | < 201 |
| Peptic ulcer disease | 288 (10.8) | 31 (23.5) |
| Any solid tumor | 391 (14.6) | 27 (20.5) |
| Lymphoma or leukemia | 103 (3.8) | < 201 |
| Metastatic solid tumor | 154 (5.8) | 23 (17.4) |
| AIDS | 23 (0.9) | < 201 |
| CCI score (SD) | 3.66 (3.55) | 4.56 (4.04) |
| CCI score 0-4 | 1606 (60) | 25 (18.9) |
| CCI score 5-9 | 678 (25.3) | 32 (24.2) |
| CCI score ≥ 10 | 394 (14.7) | 75 (56.8) |
| Baseline medications | ||
| Systemic steroids | 1992 (74.4) | 121 (91.7) |
| Anti-TNF inhibitor | 722 (27) | 40 (30.3) |
| Calcineurin inhibitor | 261 (9.7) | 24 (18.2) |
| Immunomodulators | 772 (28.8) | 40 (30.3) |
Characteristics of the 132 patients with both IBD and bacterial pneumonia, compared to the 2678 patients with IBD but no pneumonia, are summarized in Table 3. After adjusting for other factors, Hispanic patients had a significantly lower risk of pneumonia compared to non-Hispanic White patients [adjusted odds ratio (OR) = 0.38, 95%CI: 0.14-0.85]. A higher annual income (> $75000) was associated with a reduced risk of pneumonia compared to the lowest income bracket (< $35000) (adjusted OR = 0.46, 95%CI: 0.26-0.81), and a college degree was also protective compared to having completed grade 11 or lower (adjusted OR = 0.36, 95%CI: 0.15-0.97).
| Category | Variable | Adjusted ORs (95%CI) |
| Age at IBD diagnosis (per decade) | 0.90 (0.77-1.06) | |
| Duration of IBD (per decade) | 0.97 (0.72-1.30) | |
| Sex | Female | 0.83 (0.57-1.23) |
| Race/Ethnicity | Non-Hispanic White | |
| Non-Hispanic Black | 1.33 (0.77-2.23) | |
| Hispanic | 0.38 (0.14-0.85)a | |
| Non-Hispanic Asian | 1.09 (0.06-5.50) | |
| Other | 0.97 (0.39-2.08) | |
| Annual income | < $35000 | - |
| $35-75000 | 0.61 (0.35-1.06) | |
| > $75000 | 0.46 (0.26-0.81)b | |
| Education | Highest grade: About 11 | - |
| Highest grade: 12 to some college | 0.48 (0.21-1.22) | |
| Highest grade: College graduate | 0.36 (0.15-0.97)a | |
| Lifestyle choices | Current smoker | 0.93 (0.48-1.66) |
| Alcohol use | 1.03 (0.56 - 2.03) | |
| CCI | CCI score 0-4 | - |
| CCI score 5-9 | 3.00 (1.63-5.62)b | |
| CCI score ≥ 10 | 12.20 (6.69-23.00)b | |
| Medication use | Systemic steroids | 2.26 (1.21-4.64)a |
| Anti-TNF inhibitor | 1.39 (0.88-2.15) | |
| Calcineurin inhibitor | 1.36 (0.81-2.20) | |
| Immunomodulators | 0.87 (0.56-1.34) | |
| Pneumococcal vaccination | 1.00 (0.65-1.52) |
Comorbidity burden, measured by the CCI, was a key driver of pneumonia risk. Patients with a CCI ≥ 10 had a markedly increased risk (adjusted OR = 12.20, 95%CI: 6.69-23.00) relative to those with a CCI score of 0-4. Among medication variables, systemic steroid use was significantly associated with higher pneumonia risk (adjusted OR = 2.26, 95%CI: 1.21-4.64). By contrast, other immunosuppressive therapies, including anti-TNF inhibitors, calcineurin inhibitors, and immunomodulators, did not significantly alter pneumonia risk. Notably, pneumococcal vaccination was not associated with a reduced risk of bacterial pneumonia in this cohort (adjusted OR = 1.00, 95%CI: 0.65-1.52).
In this PS-matched study of a large United States cohort, we evaluated the risk of bacterial pneumonia among patients with IBD using four different PS-matched models. In our primary base model, which accounted for sociodemographic factors, lifestyle choices, comorbidities, and medication use, we found no significant increase in pneumonia risk among patients with IBD compared to matched controls. Similarly, results were not significant when we controlled for comorbidities without controlling for medication use and vice versa. Notably, the only PS model that showed significantly increased pneumonia risk for patients with IBD was the model matched only for sociodemographic factors and lifestyle choices, and not matching for comorbidities or medication use.
The comparable pneumonia risk in the IBD group and the matched control group in the base model, in contrast to the increased risk in the model not accounting for comorbidities or treatment, suggests that the increased pneumonia risk previously associated with IBD is primarily driven by comorbidities and immunosuppressive medication use, not by IBD itself. The question of whether inflammation itself acts as an independent risk factor for infection has been debated. Animal models suggest chronic inflammation may impair CD8 T-cell function, memory development, and alveolar macrophage phagocytic activity, predisposing to pneumonia[20,21]. However, similar evidence in humans is lacking.
An increased pneumonia risk in people with IBD compared to those without IBD has been demonstrated when adjusting for healthcare utilization and comorbidities but not immunosuppressive medication use[7]. By contrast, model 2 of our analysis, which controlled for sociodemographic factors, lifestyle factors, and comorbidities, but not treatment, showed no significant increase in pneumonia risk. This discrepancy may be due to our analysis including more sociodemographic and lifestyle factors in the baseline matching, as the prior study did not account for race/ethnicity, income, education, or cigarette/alcohol use, or to differences in the databases used given that the prior study used a commercial health plans claims database.
Risk factor regression analysis within the IBD cohort provided further evidence supporting our primary findings. Even after adjusting for potential confounders, patients with higher comorbidity burdens and those receiving systemic steroids had increased odds of bacterial pneumonia. This aligns with existing literature identifying comorbidities and corticosteroid therapy as key factors elevating infection risk in IBD[4-7].
By contrast, other immunosuppressive treatments, including anti-TNF inhibitors and immunomodulators, did not significantly influence pneumonia risk in our cohort. While previous research has suggested a link between anti-TNF therapy and increased pneumonia risk, the magnitude of this risk has generally been modest[5-7]. A nationwide Danish cohort study found a limited impact of anti–TNF inhibitors on invasive pneumococcal disease risk[4]. In our analysis, strict confounder adjustment may have minimized any detectable effect of anti–TNF inhibitors. Consistent with previous literature, immunomodulators were not significantly associated with pneumonia risk in this study[5]. Calcineurin inhibitors, previously associated with lower risk of bacterial pneumonia, showed no association with pneumonia risk in our analysis[7].
Consistent with prior work, vaccination against Streptococcus pneumoniae did not demonstrate a protective effect on our cohort. This may be due to the impaired immune responses to PPSV23 in patients receiving anti-TNF therapy or combination therapy[10,11]. In support of this hypothesis, it has been shown that vaccination response was decreased in patients with IBD on immunomodulators, anti-TNF inhibitors, or combination therapy[22]. Thus, recommendations have been made to immunize people early in the disease course, before the initiation of immunosuppressive therapy, in a sequential vaccination schedule of PCV13 followed by PPSV23[9,22]. The low vaccination rate (19%) in our IBD cohort may have also influenced the observed results, which is consistent with prior research showing low pneumococcal vaccination rate in the IBD population[23]. These results highlight the need to educate patients with IBD for optimal timing of vaccination and strategies for improving uptake, particularly in high-risk patients with multiple comorbidities.
The current study had several strengths. We utilized a nationwide, population-based All of Us database, a diverse dataset that attempts to properly represent traditionally underrepresented minorities in research, independent of insurance status. The width and depth of the All of Us data allowed us to account for various sociodemographic and lifestyle variables, integrated with EHR data. Robust methodology for defining exposure and outcomes have been implemented. Multiple PS models were used, allowing us to balance critical baseline characteristics between IBD and non-IBD groups and evaluate the combined effects of comorbidities and medication use on pneumonia risk.
First, the reliance on EHR data may introduce potential biases related to disparities in healthcare access, which may affect data accuracy and completeness. Additionally, differing medical institutions have varying recording standards and practices, which likely introduces data heterogeneity. Nonetheless, this approach allowed us to sample a broader and more diverse United States population than would be possible with traditional prospective studies. Importantly, our ability to replicate the previously documented increased pneumonia risk in IBD patients in model 1 validates our dataset's consistency with prior literature. To address the issue of data accuracy, we also required the diagnosis of IBD to be documented twice in the EHR. Although this is a conservative approach, it is considered best practices when defining phenotypes in the All of Us dataset.
Second, we only included participants with at least two clinical visits 90 days or apart, potentially selecting for patients with more established care and limiting generalizability to all patients with IBD. However, this requirement was necessary to ensure longitudinal follow-up and reliable time-to-event data.
Third, in PS model 3, although comorbidity status was not a distinct matching factor, the mean CCI scores and prevalence of all but one comorbidity were balanced between groups. This unintended balance may reflect collinearity between certain immunosuppressive medication use and comorbidity status.
Fourth, the follow-up time and the time to pneumonia were shorter in the IBD group compared to the non-IBD group. This difference likely arose from our study design, where the control group's follow-up began at their first study-period visit, while IBD patients' follow-up started at their IBD diagnosis or first study-period visit, whichever came later. While this difference in follow-up time could affect the observed outcomes, our use of time-to-event analysis methods helps mitigate this limitation.
Fifth, despite using multiple PS models and multivariable analysis, the presence of residual confounding factors cannot be ruled out. For instance, we did not account for other medications that may increase the risk of pneumonia, such as proton pump inhibitors or opioids, to avoid overfitting our statistical models.
Finally, while medication use was considered, detailed data on dosage and duration were unavailable within this database, which might have influenced infection risk. Nevertheless, we had access to a relatively comprehensive list of prescribed medications, allowing us to capture the overall patterns of immunosuppressive therapy use in this population. Future studies with more granular medication data are needed to refine our understanding of dose-dependent risks.
This large propensity-matched study demonstrates that the increased pneumonia risk in patients with IBD is primarily driven by comorbidities and systemic steroid use, rather than IBD itself. Through the novel application of four distinct PS models, we isolated the contributions of different risk factors, showing that when matched for all factors, IBD patients had similar pneumonia risk to non-IBD controls. Most notably, high comorbidity burden (CCI ≥ 10) increased pneumonia risk 12-fold, while systemic steroid use doubled the risk. These findings have important clinical implications, suggesting that pneumonia prevention strategies in IBD should prioritize comorbidity management and judicious steroid use, particularly in high-risk patients. The low pneumococcal vaccination rates observed in our cohort highlight the need for optimizing vaccination timing and improving vaccination rates. Future research should focus on developing steroid-sparing treatment regimens, investigating the impact of early vs delayed vaccination, and evaluating targeted preventive strategies for patients with multiple comorbidities. These efforts could significantly reduce the burden of pneumonia in the IBD population.
We gratefully acknowledge All of Us participants for their contributions, without whom this research would not have been possible. We also thank the National Institutes of Health’s All of Us Research Program for making available the participant data examined in this study.
| 1. | Lewis JD, Parlett LE, Jonsson Funk ML, Brensinger C, Pate V, Wu Q, Dawwas GK, Weiss A, Constant BD, McCauley M, Haynes K, Yang JY, Schaubel DE, Hurtado-Lorenzo A, Kappelman MD. Incidence, Prevalence, and Racial and Ethnic Distribution of Inflammatory Bowel Disease in the United States. Gastroenterology. 2023;165:1197-1205.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 167] [Article Influence: 83.5] [Reference Citation Analysis (1)] |
| 2. | Ananthakrishnan AN, McGinley EL. Infection-related hospitalizations are associated with increased mortality in patients with inflammatory bowel diseases. J Crohns Colitis. 2013;7:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 3. | Jess T, Frisch M, Simonsen J. Trends in overall and cause-specific mortality among patients with inflammatory bowel disease from 1982 to 2010. Clin Gastroenterol Hepatol. 2013;11:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 4. | Kantsø B, Simonsen J, Hoffmann S, Valentiner-Branth P, Petersen AM, Jess T. Inflammatory Bowel Disease Patients Are at Increased Risk of Invasive Pneumococcal Disease: A Nationwide Danish Cohort Study 1977-2013. Am J Gastroenterol. 2015;110:1582-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Gregory MH, Ciorba MA, Wiitala WL, Stidham RW, Higgins P, Morley SC, Hou JK, Feagins LA, Govani SM, Cohen-Mekelburg SA, Waljee AK. The Association of Medications and Vaccination with Risk of Pneumonia in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2020;26:919-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Khan N, Patel D, Trivedi C, Pernes T, Kavani H, Xie D, Yang YX. The impact of IBD medications on risk of pneumonia and pneumonia-related hospitalisation: a nationwide cohort study of 56 410 IBD patients. Aliment Pharmacol Ther. 2022;55:64-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 7. | Long MD, Martin C, Sandler RS, Kappelman MD. Increased risk of pneumonia among patients with inflammatory bowel disease. Am J Gastroenterol. 2013;108:240-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 8. | Vieujean S, Moens A, Hassid D, Rothfuss K, Savarino EV, Vavricka SR, Reenaers C, Jacobsen BA, Allez M, Ferrante M, Rahier JF. Pneumocystis jirovecii Pneumonia in Patients with Inflammatory Bowel Disease-a Case Series. J Crohns Colitis. 2023;17:472-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (1)] |
| 9. | Farraye FA, Melmed GY, Lichtenstein GR, Kane SV. ACG Clinical Guideline: Preventive Care in Inflammatory Bowel Disease. Am J Gastroenterol. 2017;112:241-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 354] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 10. | Fiorino G, Peyrin-Biroulet L, Naccarato P, Szabò H, Sociale OR, Vetrano S, Fries W, Montanelli A, Repici A, Malesci A, Danese S. Effects of immunosuppression on immune response to pneumococcal vaccine in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. 2012;18:1042-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (3)] |
| 11. | Melmed GY, Agarwal N, Frenck RW, Ippoliti AF, Ibanez P, Papadakis KA, Simpson P, Barolet-Garcia C, Ward J, Targan SR, Vasiliauskas EA. Immunosuppression impairs response to pneumococcal polysaccharide vaccination in patients with inflammatory bowel disease. Am J Gastroenterol. 2010;105:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 12. | All of Us Research Program Investigators; Denny JC, Rutter JL, Goldstein DB, Philippakis A, Smoller JW, Jenkins G, Dishman E. The "All of Us" Research Program. N Engl J Med. 2019;381:668-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1103] [Cited by in RCA: 1364] [Article Influence: 227.3] [Reference Citation Analysis (0)] |
| 13. | Hou JK, Tan M, Stidham RW, Colozzi J, Adams D, El-Serag H, Waljee AK. Accuracy of diagnostic codes for identifying patients with ulcerative colitis and Crohn's disease in the Veterans Affairs Health Care System. Dig Dis Sci. 2014;59:2406-2410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Ng DQ, Jia S, Wisseh C, Cadiz C, Nguyen M, Lee J, McBane S, Nguyen L, Chan A, Hurley-Kim K. Sociodemographic characteristics differ across routine adult vaccine cohorts: An All of Us descriptive study. J Am Pharm Assoc (2003). 2023;63:582-591.e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32099] [Cited by in RCA: 38296] [Article Influence: 1007.8] [Reference Citation Analysis (0)] |
| 16. | Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7532] [Cited by in RCA: 8646] [Article Influence: 262.0] [Reference Citation Analysis (0)] |
| 17. | Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6122] [Cited by in RCA: 8379] [Article Influence: 419.0] [Reference Citation Analysis (0)] |
| 18. | Wiese AD, Griffin MR, Stein CM, Schaffner W, Greevy RA, Mitchel EF Jr, Grijalva CG. Validation of discharge diagnosis codes to identify serious infections among middle age and older adults. BMJ Open. 2018;8:e020857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Benedetto U, Head SJ, Angelini GD, Blackstone EH. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg. 2018;53:1112-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 189] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 20. | Roquilly A, Jacqueline C, Davieau M, Mollé A, Sadek A, Fourgeux C, Rooze P, Broquet A, Misme-Aucouturier B, Chaumette T, Vourc'h M, Cinotti R, Marec N, Gauttier V, McWilliam HEG, Altare F, Poschmann J, Villadangos JA, Asehnoune K. Alveolar macrophages are epigenetically altered after inflammation, leading to long-term lung immunoparalysis. Nat Immunol. 2020;21:636-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 167] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 21. | Stelekati E, Shin H, Doering TA, Dolfi DV, Ziegler CG, Beiting DP, Dawson L, Liboon J, Wolski D, Ali MA, Katsikis PD, Shen H, Roos DS, Haining WN, Lauer GM, Wherry EJ. Bystander chronic infection negatively impacts development of CD8(+) T cell memory. Immunity. 2014;40:801-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | van Aalst M, Garcia Garrido HM, van der Leun J, Meek B, van Leeuwen EMM, Löwenberg M, D'Haens GR, Ponsioen CYI, Grobusch MP, Goorhuis A. Immunogenicity of the Currently Recommended Pneumococcal Vaccination Schedule in Patients With Inflammatory Bowel Disease. Clin Infect Dis. 2020;70:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Chan W, Salazar E, Lim TG, Ong WC, Shim HH. Vaccinations and inflammatory bowel disease - a systematic review. Dig Liver Dis. 2021;53:1079-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |