Published online Jun 28, 2025. doi: 10.4329/wjr.v17.i6.107205
Revised: April 11, 2025
Accepted: May 18, 2025
Published online: June 28, 2025
Processing time: 100 Days and 21.8 Hours
Obstructed defecation syndrome (ODS) represents an important cause of consti
Core Tip: Obstructed defecation syndrome (ODS) represents an important cause of chronic constipation, resulting from various functional and anatomical abnormalities of the pelvic floor. Magnetic resonance defecography (MRD) owing to its exceptional spatial resolution and dynamic imaging capabilities, facilitates an in-depth assessment of the pelvic floor's supporting structures. It provides direct visualization of the pelvic floor musculature and an indirect evaluation of the endopelvic fascia. MRD plays a pivotal role in the management of ODS by elucidating critical anatomical and functional impairments, thereby guiding therapeutic interventions.
- Citation: Parry AH, Rehaman B, Bhat SA, Wani AH, Jehangir M, Baba AA. Role of magnetic resonance defecography in the assessment of obstructed defecation syndrome. World J Radiol 2025; 17(6): 107205
- URL: https://www.wjgnet.com/1949-8470/full/v17/i6/107205.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i6.107205
Defecation is an important physiological process that entails the precise coordination of various elements, including bowel motility, rectal sensation, the contraction of the rectum, and the relaxation of the anal sphincters, as well as the coordinated function of the pelvic floor muscles[1]. Functional constipation refers to a common condition where no physical obstruction exists in the defecation pathway. This disorder is frequently seen by physicians, gastroenterologists, and colorectal surgeons. It is estimated that 16% of the adult population worldwide is affected by functional constipation, with the prevalence rising to 33.5% among individuals aged 60 to 100 years[2,3]. Given its widespread occurrence, func
Diagnosing the pelvic floor dysfunction associated with ODS through physical examination alone is challenging[5]. Studies show that physical assessments often fail to accurately identify pelvic floor abnormalities in 45%–90% of patients, leading to misdiagnosis. This diagnostic gap frequently results in inappropriate treatments and recurrent symptoms in 10%–30% of patients following surgery[7,9].
Magnetic resonance defecography (MRD) is a dynamic real time imaging of pelvic floor using fast imaging sequences to study the functional and structural disorders of various pelvic organs. It is particularly valuable due to its multiplanar acquisition, lack of ionising radiation, high soft tissue contrast resolution, and its capacity to concurrently assess abnor
MRD has been investigated in the assessment of ODS, with evidence suggesting that a substantial proportion of patients with ODS display various structural abnormalities. In the study conducted by RB Thapar et al[7], only 28 (14.5%) of the 192 patients with ODS exhibited normal MRD, while the remaining 164 (85.5%) demonstrated one or more abnor
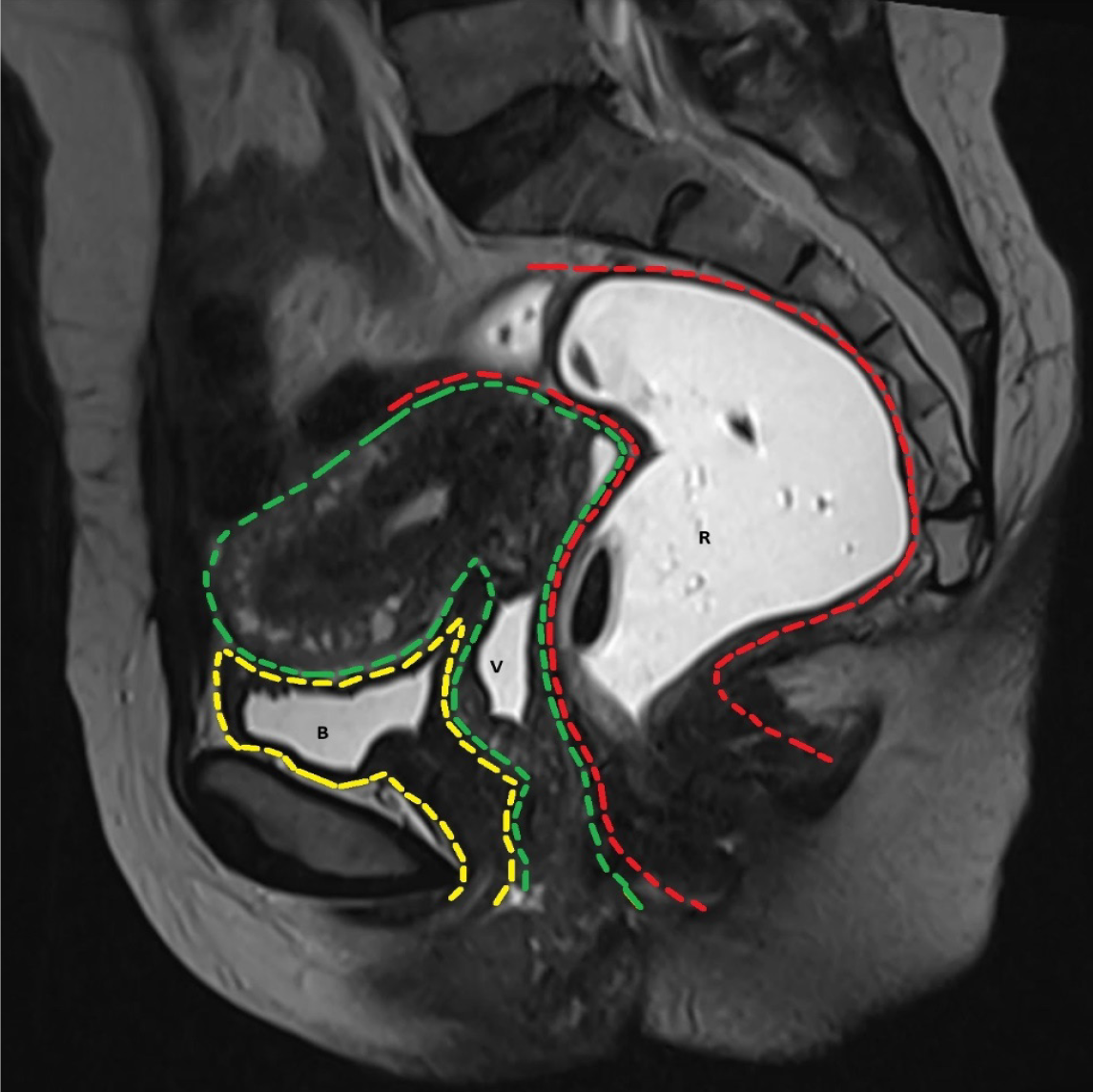
The pelvic is anatomically divided into three compartments: The anterior, middle, and posterior compartments[1,2]. The anterior compartment includes the urinary bladder and urethra, the middle compartment contains the uterus and vagina, and the posterior compartment houses the rectum and anal canal (Figure 1). Pelvic floor is a complex structure, made up of fascia, ligaments, and muscles, organized into three distinct layers, each contributing to the support and proper func
This is the most superior layer, composed of connective tissue, and wraps around the pelvic organs and levatorani mu
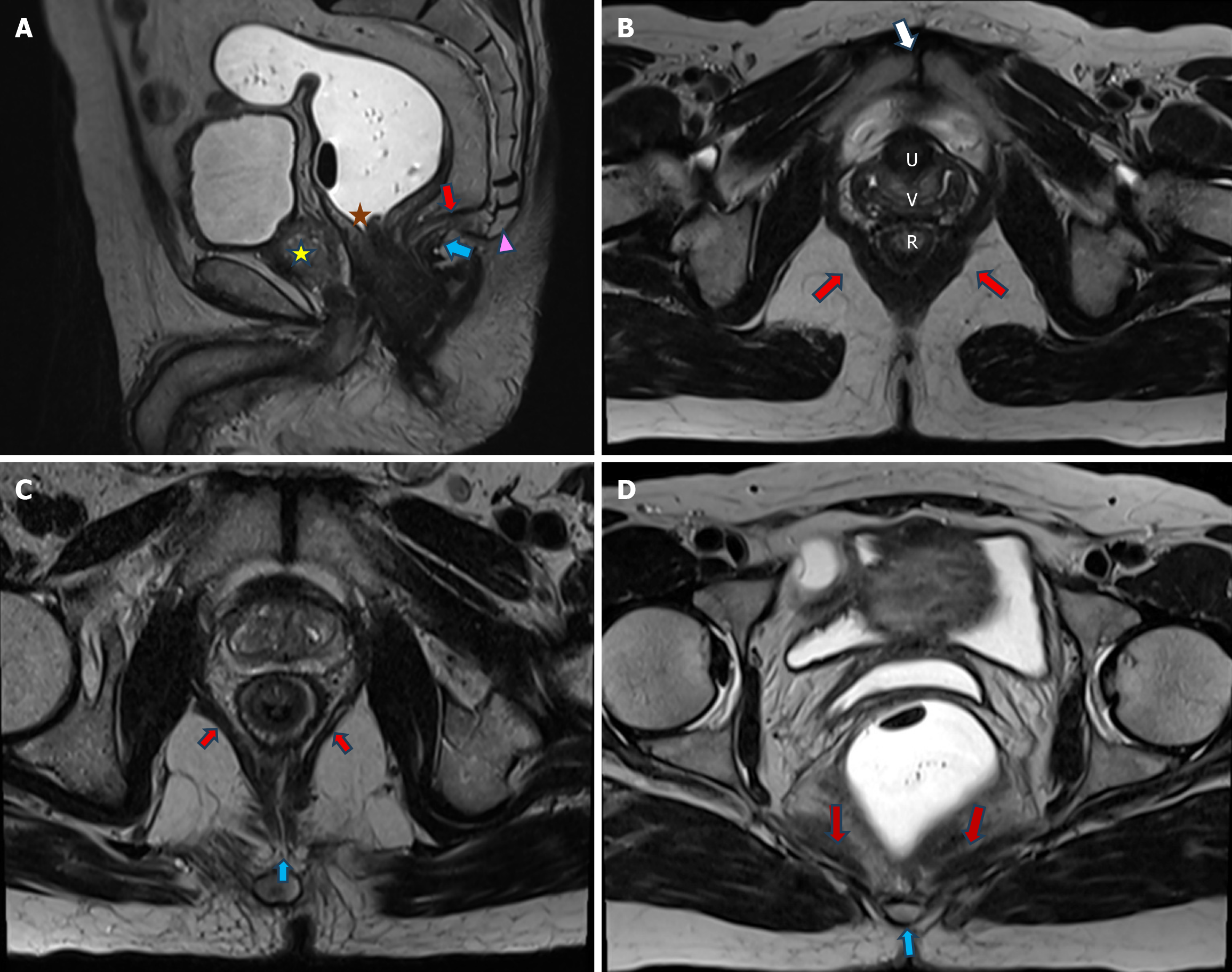
The second layer consists of the pelvic diaphragm, which is made up of the levatorani and coccygeus muscles. These muscles are visible on MRI. The levatorani is a group of three muscles: The puborectalis, pubococcygeus and iliococcygeus (Figure 2). The levator plate is composed of the posterior fibers of the pubococcygeus, which fuse in the midline to create a median raphe (Figure 2). The puborectalis muscle is visualised on T2-weighted axial images, while the iliococcygeus and pubococcygeus muscles are more clearly depicted in T2-weighted coronal images[13,14].
The third and lowest layer consists of the urogenital diaphragm, made of connective tissue and the deep transverse peri
The anal sphincter comprises of internal anal sphincter which is formed by the longitudinal muscle fibres within the anal wall, and the external anal sphincter, which is the inferior or caudal continuation of the puborectalis muscle.
MRD examinations are typically conducted using widely available MRI scanners, employing closed magnets with field strengths of 1.5 Tesla or 3 Tesla. MRD acquisition can be performed with the patient in either a sitting position on an open configuration MRI or a supine position on a closed configuration MRI. Several studies have yielded conflicting results regarding the impact of patient positioning on MRD outcomes. Some studies suggest that MRD performed in the supine position tends to underestimate the degree of organ prolapse, particularly in cases of posterior compartment dysfunction, when compared to sitting MRD, which more accurately simulates the physiological process of defecation. However, numerous other studies have shown no significant difference between sitting and supine MRD in terms of diagnosing clinically relevant pelvic floor dysfunctions. Regardless of patient positioning, ensuring adequate defecatory efforts to facilitate thorough rectal emptying is crucial in minimizing false-negative results.
Patient education regarding the procedure is essential to ensure the acquisition of a good-quality scan. Prior to the examination, patients must be thoroughly instructed on the procedural steps to promote cooperation during the pro
A multichannel body coil is positioned around the patient's pelvis. Static images are obtained to evaluate the pelvic floor anatomy, focusing on the muscles, including the puborectalis and iliococcygeus. T2-weighted fast spin-echo (FSE) or fast recovery FSE sequences without fat saturation are obtained in all three orthogonal planes.
Dynamic imaging is then performed using fast T2-weighted sequences in the sagittal plane. Dynamic cine imaging utilizing balanced steady-state free precession sequences is conducted in the mid-sagittal plane, with cine loops captured to visualize the movement of the pelvic floor and the expulsion of the gel. These images are acquired during the rest, squeeze, strain, and defecation phases. During the squeeze phase, the patient is instructed to contract the anal sphincter inward to elevate the pelvic floor (Kegel maneuver), enabling an evaluation of puborectalis muscle function. In the defecation phase, the patient is instructed to expel the gel from the rectum, a process crucial for assessing the coordina
To ensure optimal results, the defecation or evacuation sequence should be repeated a minimum of three times to allow for adequate gel expulsion. The defecation phase is the most critical component of MRD, as it provides the most accurate insights into the presence and severity of pelvic floor dysfunction.
MRD interpretation commences with a brief assessment of the anatomical supporting structures within the pelvis on static images. The evaluation includes assessment of the levator ani muscle and external anal sphincter, noting any changes in signal intensity, atrophic changes, surgical scars, or tears. Special attention is given to the puborectalis muscle, which is frequently observed to be thinned in individuals with stress or mixed urinary incontinence, and thickened in those with pelvic floor dyssynergia. While no standardized reference values exist for the normal thickness of the pubo
Subsequent analysis involves the evaluation of defecatory effort using dynamic sagittal cine sequences, which assess the level of defecatory effort (classified as good, moderate, or poor). The amount of instilled rectal gel expelled is quantified as none, one-third, two-thirds, or nearly all[1]. The cine sequence demonstrating maximal strain is selected for interpretation, in comparison to a baseline resting image. Several reference lines are then drawn to assess the functiona
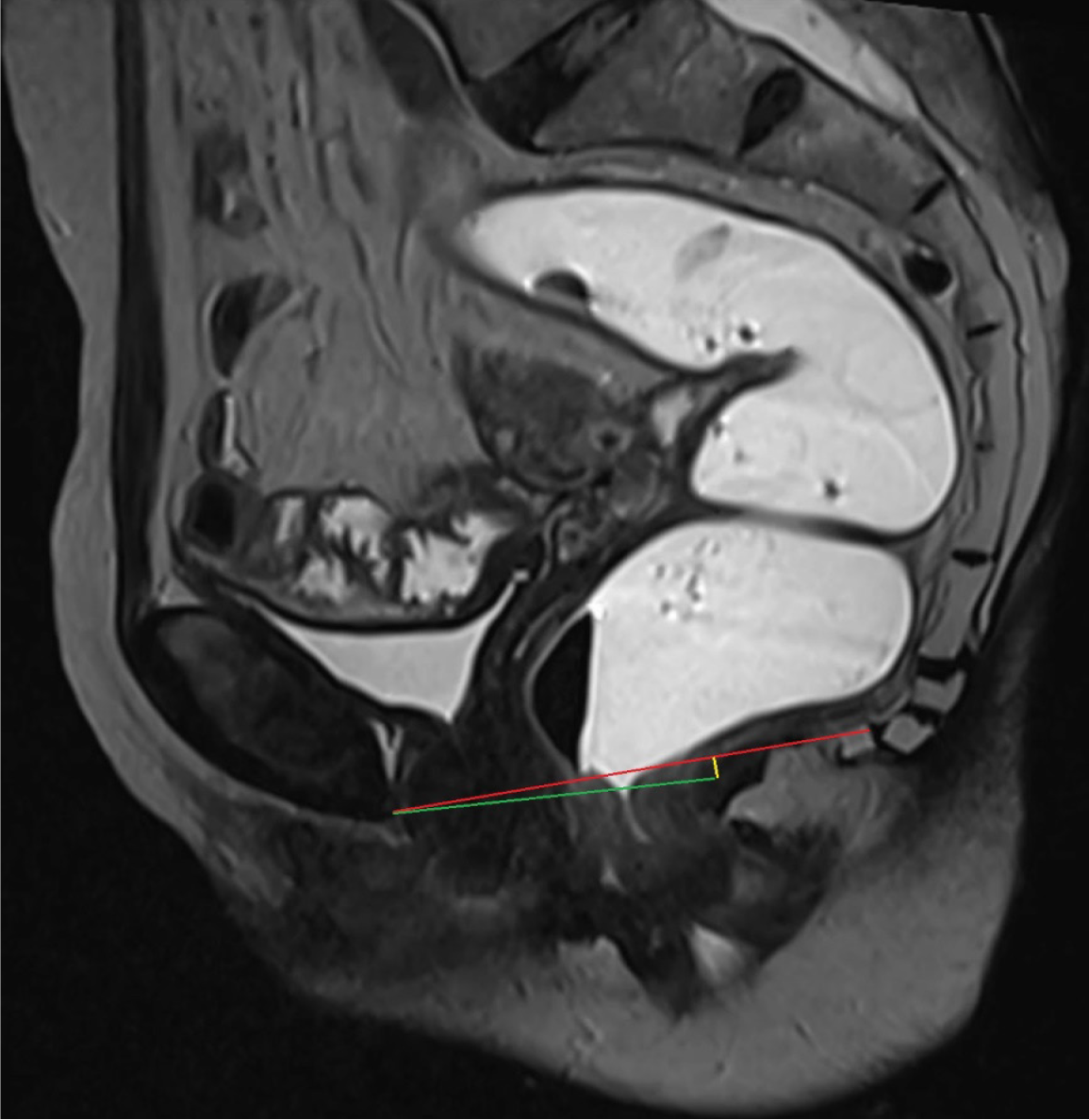
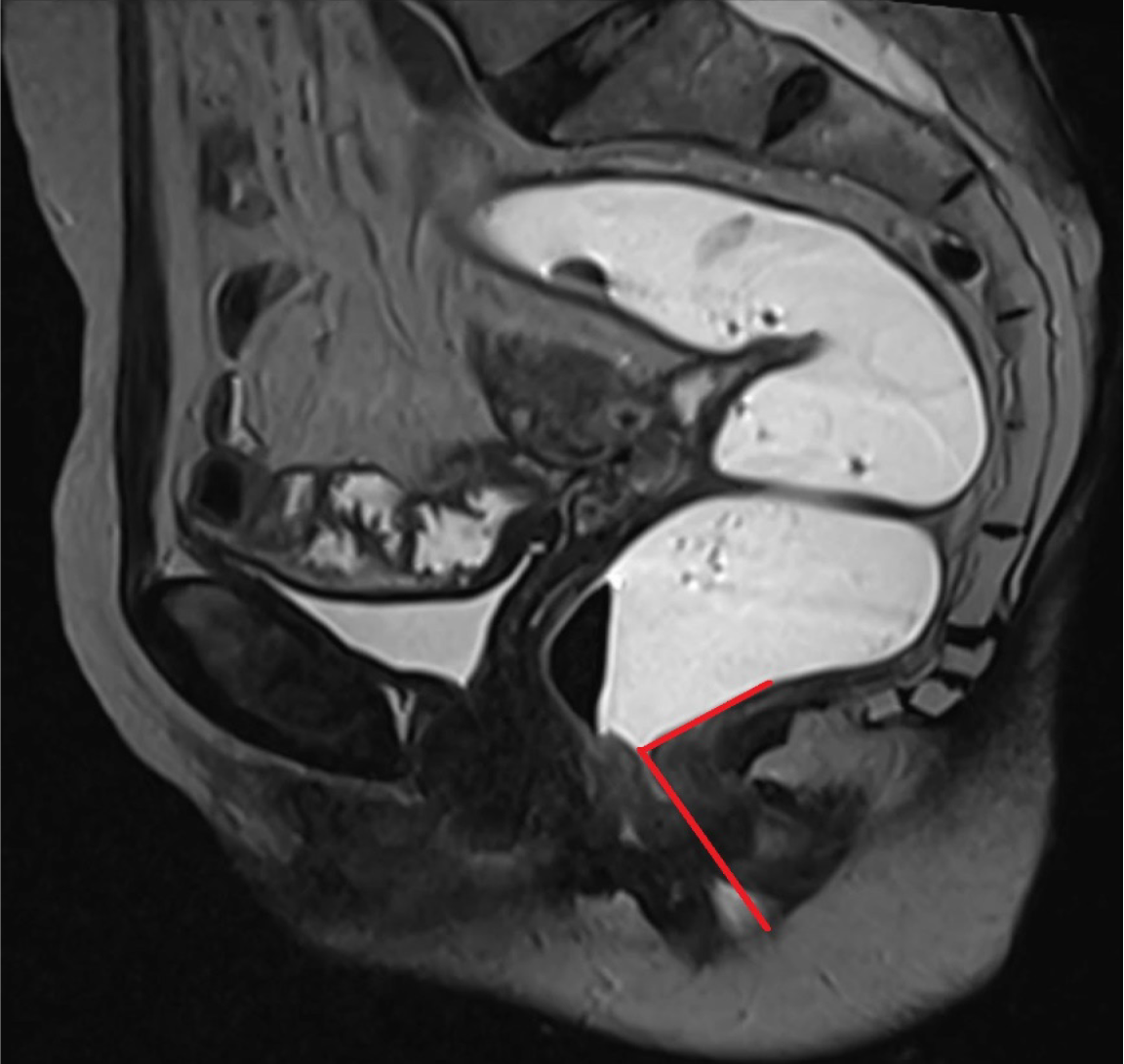
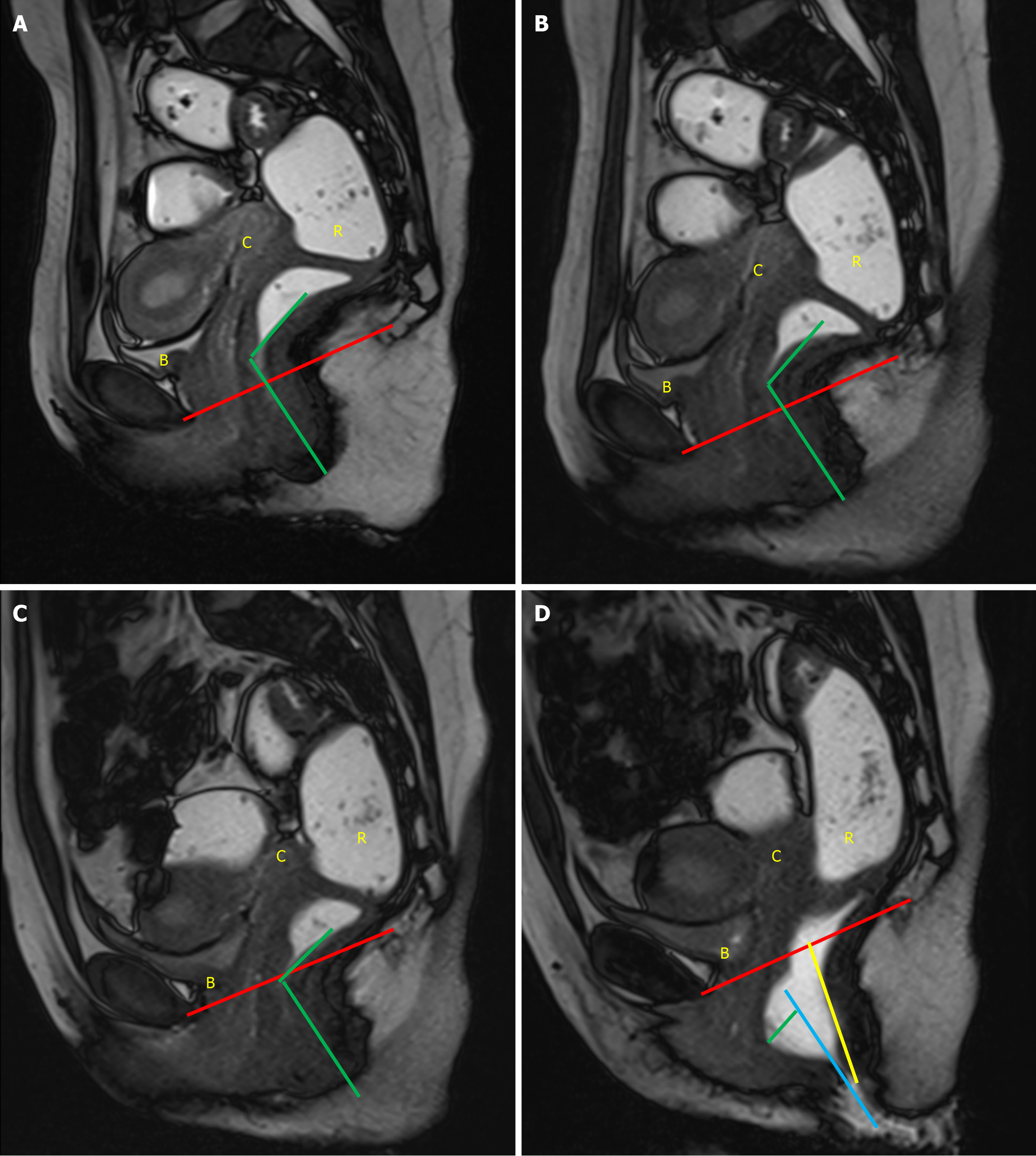
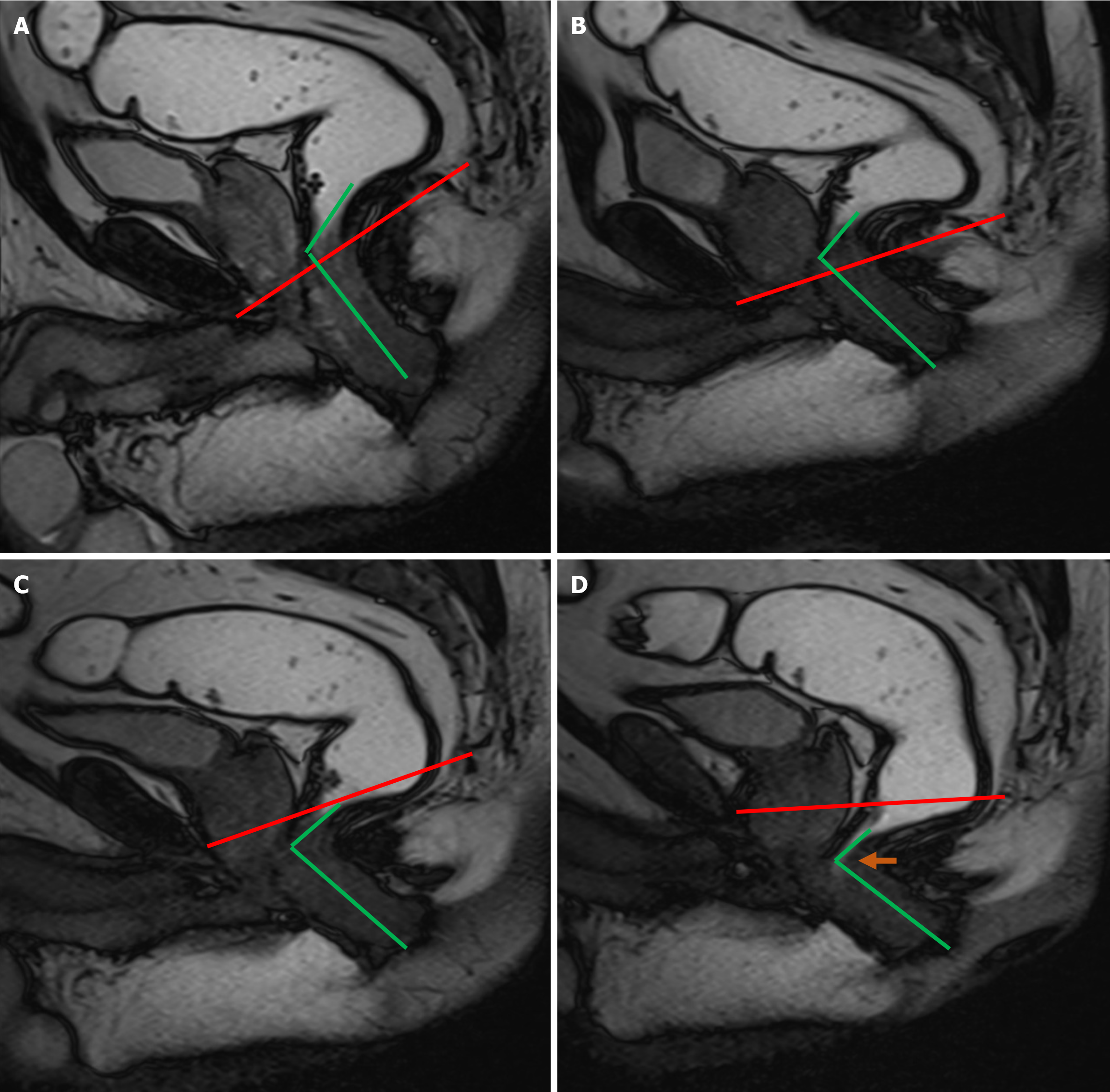
A vertical line extending from the PCL superiorly to the neck of urinary bladder inferiorly provides an estimate of anterior compartment descent. The urethral angle is estimated by drawing a line along the long axis of the urethra and the patient’s body axis, both at rest and under maximal strain. Urethral hypermobility is identified when the urethral angle increases by more than 30 degrees during strain as compared to rest, indicating weakness, laxity, or disruption of the urethral ligaments[2,9]. Additionally, a line is drawn from the PCL superiorly to the anterior cervical lip or superior vaginal cuff inferiorly to measure the degree of descent of the middle compartment[1]. A similar line from the PCL superiorly to the anorectal junction inferiorly is used to calculate the descent of the anorectal junction. Another line is drawn along the most anterior aspect of the rectal wall to its anticipated location to evaluate the presence and severity of an anterior rectocele. The anorectal angle (ARA) is measured to evaluate pelvic floor dyssynergia. The angle is calculated by drawing two lines: One parallel to the long axis of the anal canal and the other along the posterior rectal wall. The intersection of these lines gives the ARA (Figure 4). At rest, the ARA ranges between 108° and 127°[1,2]. During the squeeze maneuver, the ARA decreases by 15%–35% as a result of the contraction of the puborectalis muscle, which pulls the anorectal junction anteriorly and superiorly. During the defecation phase, the puborectalis muscle relaxes, moving the anorectal junction posteriorly and inferiorly, which increases the ARA by 15°–20° from the resting phase, signifying the opening of the anal canal[1,2]. Once all these measurements are obtained, the dynamic cine images are revisited to identify any abnormalities within the cul-de-sac. The rectum is also scrutinized for signs of intussusception, which is often most conspicuous toward the completion of the evacuation process[16].
At rest, the anal canal remains closed, with the puborectalis muscle creating an impression on the posterior wall of anorectal junction. Effective defecation requires the coordinated interplay between intra-abdominal pressure, the sphincter complex, and the pelvic floor musculature. During defecation there is rise in intra-abdominal pressure, which is followed by a rise in rectal pressure. This culminates in the relaxation of the anal sphincters and pelvic floor which facilitates the opening of the anal canal. At MRD, the rectum should evacuate the majority of the contrast medium within 60 seconds. However, some individuals with normal defecatory function may experience difficulty expelling the rectal gel when positioned supine[1,2,17].
Pelvic floor dysfunction, refers to a range of abnormalities due to weakened or deficient muscles or tissues of pelvic floor. It may encompass both pelvic floor relaxation and pelvic organ prolapse. As a result, one or more than one compartments may descend during defecation. This condition leads to a descent of the pelvic floor itself, along with an expansion of the levator hiatus. These alterations are assessed by using H and M lines, with the grading system detailed in Table 1. Patients with pelvic organ prolapse, demonstrate abnormal descent of the pelvic organs—such as the bladder, cervix, or bowel—through the hiatus. To quantify the extent of pelvic organ prolapse, PCL is used as a reference line (Table 2).
| Grade | H-line (measure of hiatal enlargement) | M-line (measure of pelvic floor descent) |
| Normal | < 6 cm | < 2 cm |
| Mild | 6-8 cm | 2-4 cm |
| Moderate | 8-10 cm | 4-6 cm |
| Severe | > 10 cm | > 6 cm |
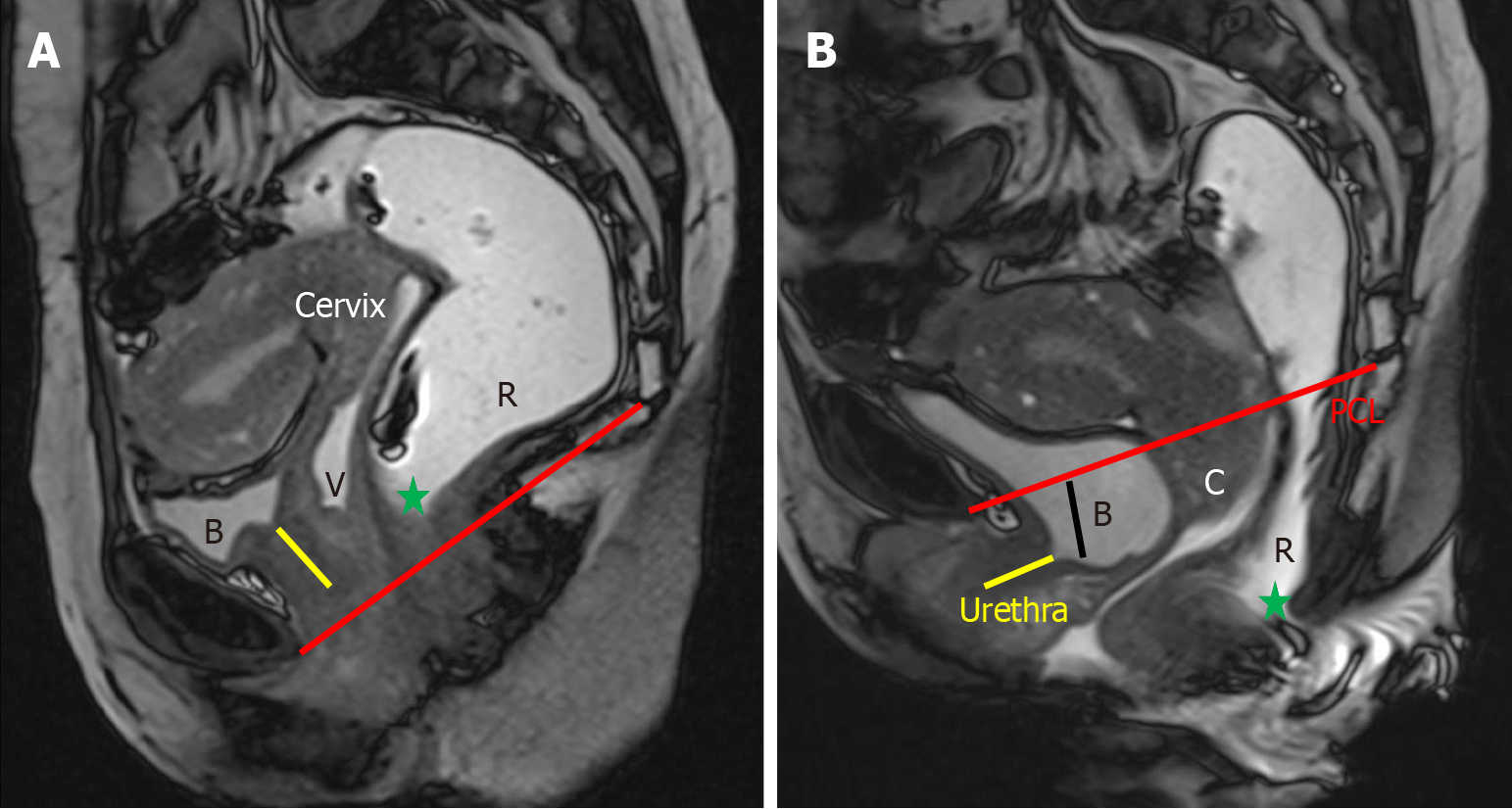
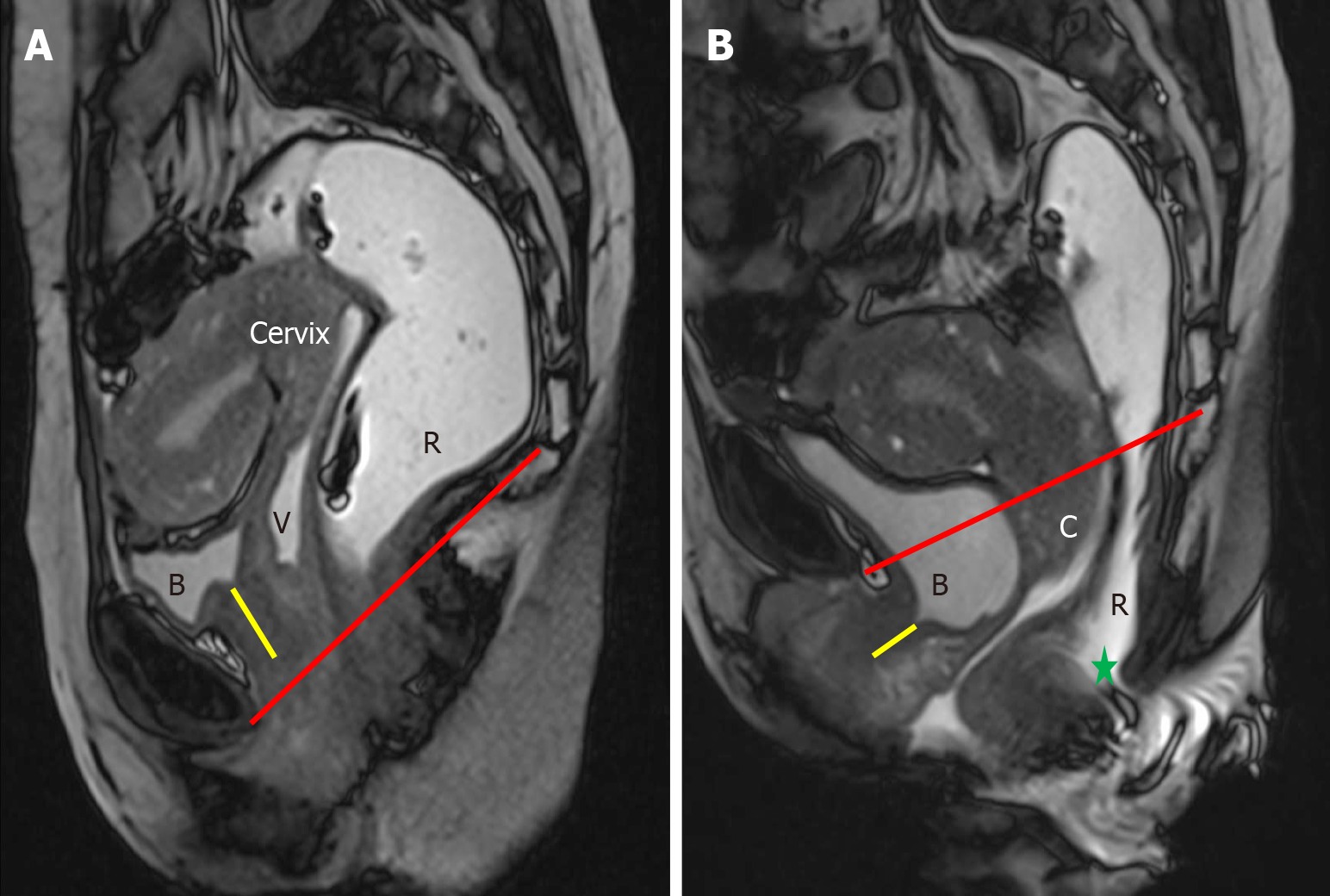
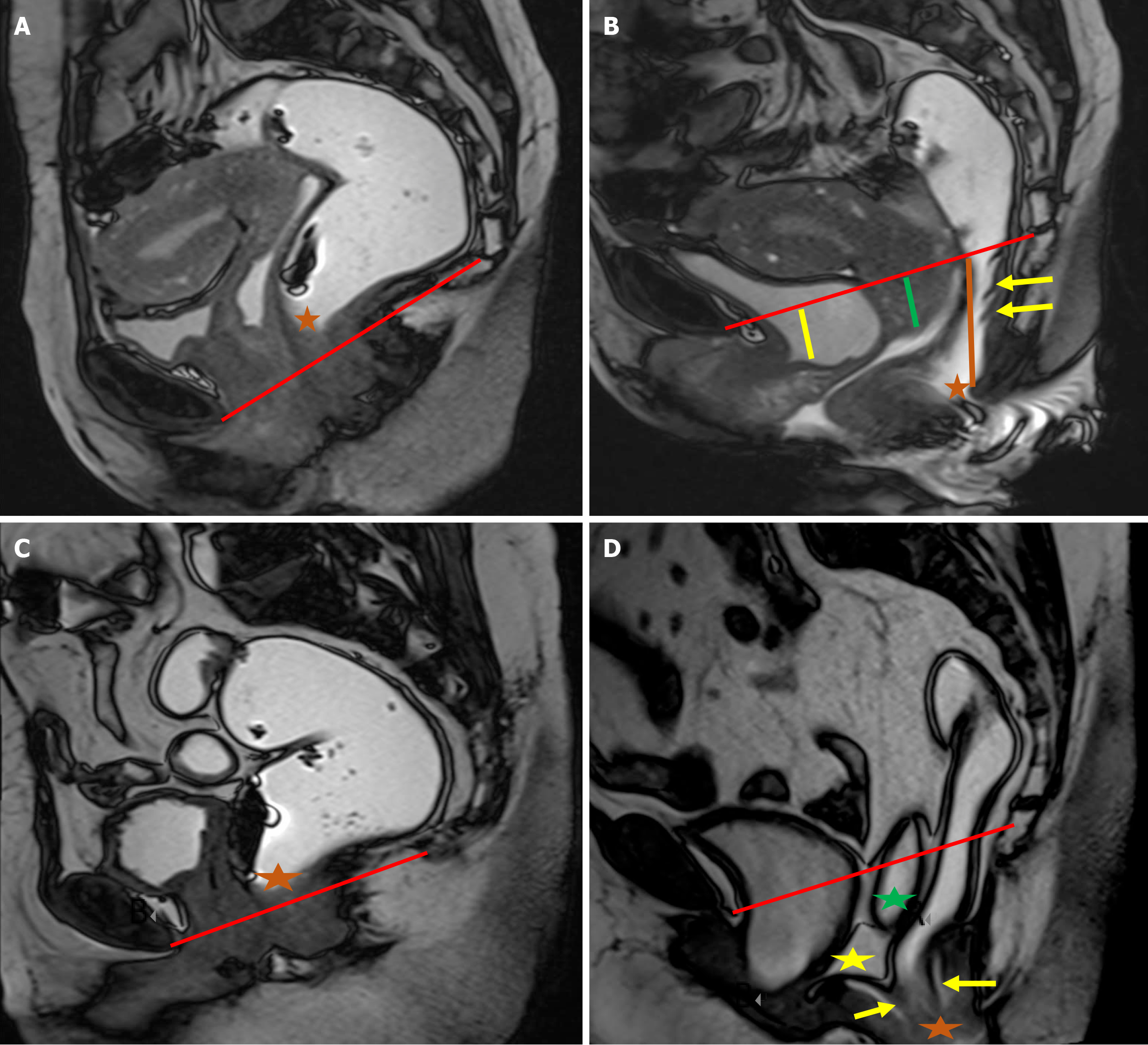
Disorders affecting the anterior compartment, such as cystocele and urethral hypermobility, primarily involve the urinary system and do not have a direct impact on defecation[1,2]. Although these two conditions can sometimes occur together, they are distinct clinical entities, and each should be evaluated and documented individually. Under normal conditions, the urethra is positioned vertically and lies posterior to the pubic symphysis[1]. When the ligaments supporting the urethra become weakened or disrupted, it leads to urethral hypermobility, characterized by a posterior tilt of the urethra during strain[18,19]. This phenomenon is most clearly observed on sagittal MRI images, where the urethra demonstrates a posteriorly inclined orientation at rest and a downward shift with strain. The hypermobility of urethra is often linked with the downward displacement of both the urethra, which may move below the pubic symphysis, and the bladder, which can descend past the PCL, a key reference for the pelvic floor level (Figure 5). Recognizing urethral hypermobility is crucial, as it typically requires surgical intervention, often through a sling procedure[20,21]. A cystocele occurs when the bladder experiences insufficient support from the anterior abdominal wall or due to defects in the pubocervical fascia, leading to abnormal bladder descent. Clinically, this is observed as bulging of the anterior vaginal wall, with or without full-blown vaginal prolapse[22]. On MRI, the bladder normally rests above the PCL at rest, and may descend to or just below the PCL during strain. However, in cases of cystocele, the bladder moves in a curved, posterior, and downward direction, often dropping significantly below the PCL, resulting in a mass effect that distorts the anterior vaginal wall (Figure 5).
Uterine or vaginal prolapse can occur due to damage to the pubocervical fascia, cardinal and uterosacral ligaments, and other supportive tissues. Like other pelvic floor disorders, the likelihood of genital prolapse increases with factors such as previous pregnancies, aging, obesity, and activities that involve excessive abdominal pressure[4]. Normally, the cervix should be located above the PCL, and the lower vagina should maintain a vertical alignment[1]. On MRD imaging, it is considered abnormal for any part of the uterus, cervix, or vaginal apex to descend below the PCL (Figure 6). Uterine prolapse is often observed alongside cystocele. In cases of posterior compartment disorders, uterine descent can con
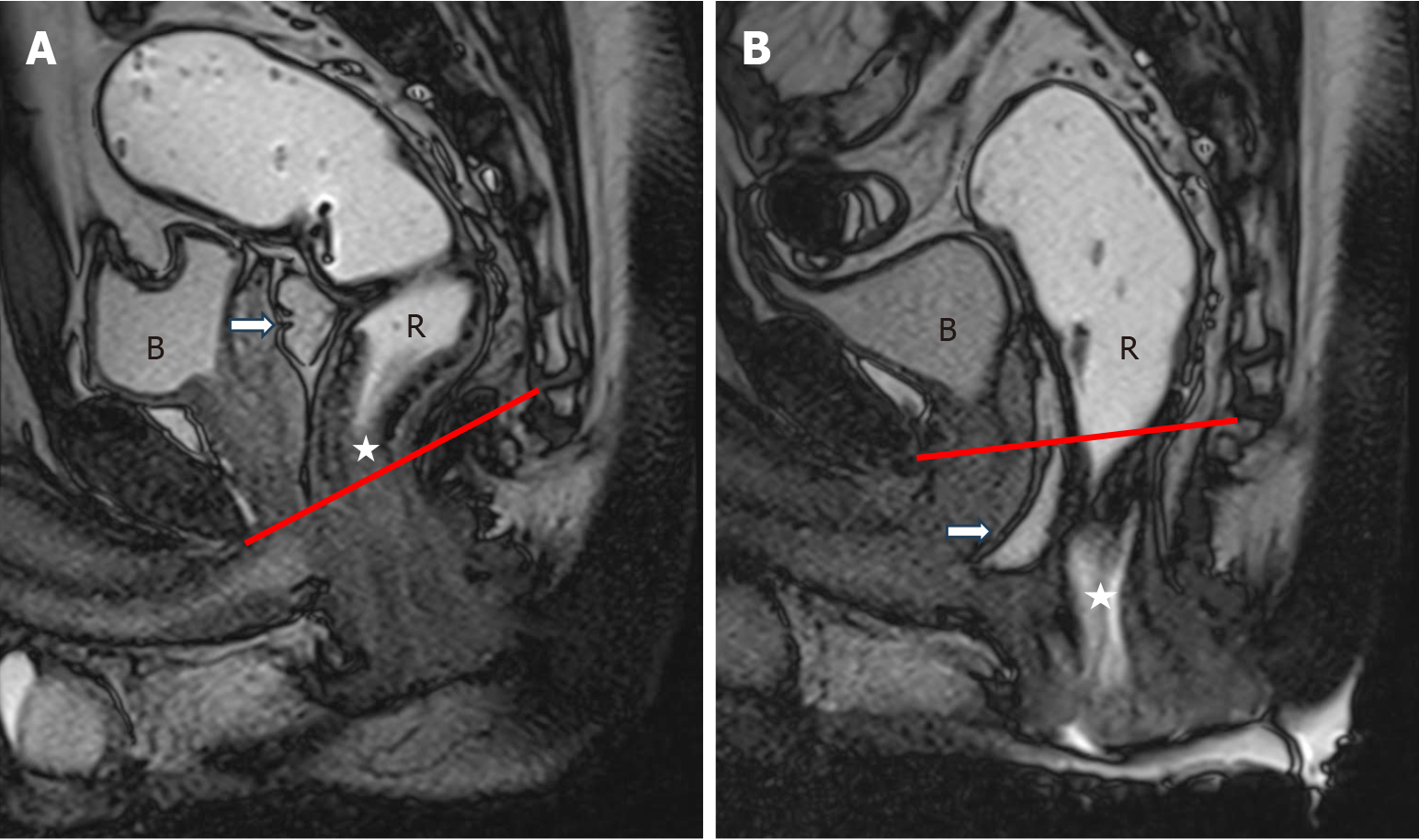
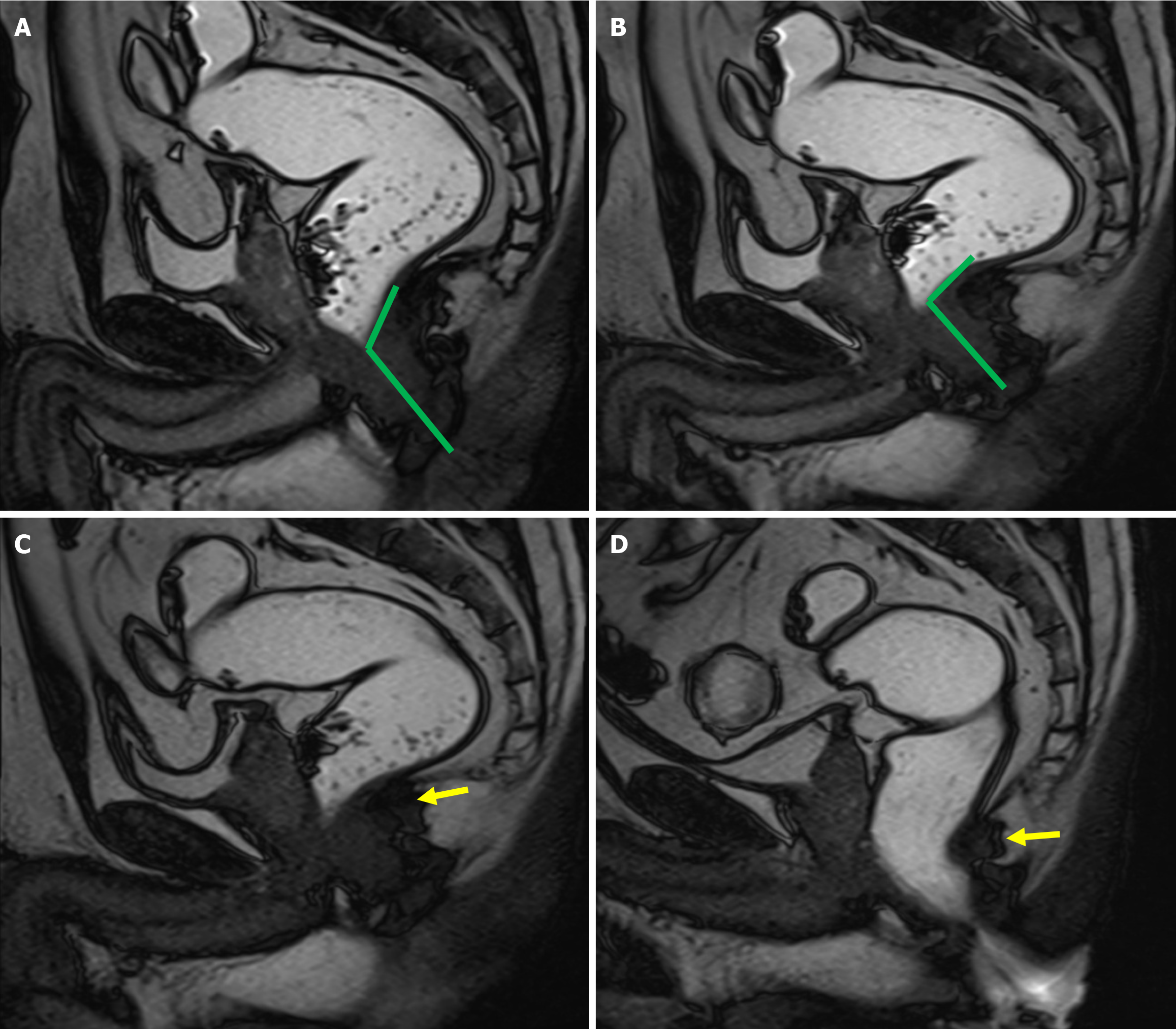
Cul-de-sac hernias, also called pouch of Douglas hernias, develop as a result of herniation of abdominal contents into the rectovaginal space. It occurs due to the weakening or disruption of the supporting structures of vaginal wall. These hernias are more frequently encountered in patients who have had a hysterectomy, as this surgical procedure leads to damage to the endopelvic fascia. These hernias are categorized according to their contents, such as enterocele, peritoneocele, and sigmoidocele (Figures 7 and 8). It has been estimated that 37% of patients with pelvic floor disorders also have cul-de-sac hernias, however this statistic may be underestimated due to the difficulty in diagnosing these hernias during a routine clinical examination[23,24]. MRD is useful for assessing whether these hernias contribute to obstructed defecation, which can then guide decisions regarding the need for surgery. On MRD, cul-de-sac hernias are seen as a widening and deepening of the rectovaginal space and its filling with bowel and/or peritoneum. These hernias may not be apparent in the initial defecatory MRD images, but become more evident in post-evacuation images, after the rectum and bladder have emptied. When reporting cul-de-sac hernias, it is important to include information about the type of hernia, its contents, and whether it obstructs rectal evacuation[25,26].
The posterior compartment is comprised of the rectum and anal canal, which are anatomically connected at the anorectal junction. It is crucial to run the defecation phase over a sufficient duration in order to fully capture the range of posterior compartment disorders[16]. MRD reveals a variety of abnormalities within the posterior compartment in patients with ODS, including anorectal junction descent, rectocele, intussusception, pelvic dyssynergia and solitary rectal ulcer syn
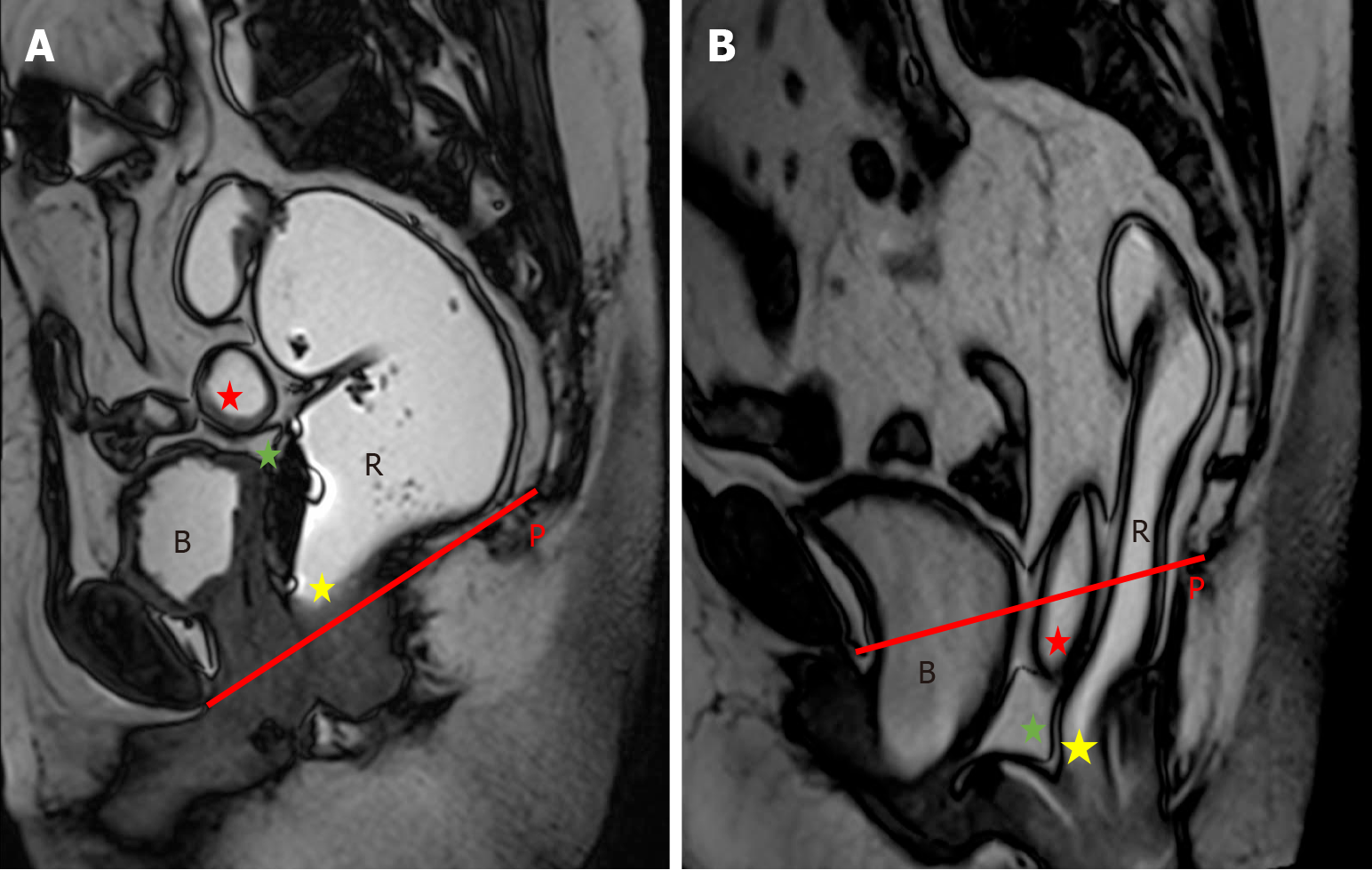
In the resting state, the anorectal junction should ideally lie above the PCL (Figure 9). Anorectal junction descent is generally considered excessive when the junction descends more than 2 cm below the PCL[27,28]. Various studies have documented anorectal junction descent as a common finding in anorectal dysfunction, with rates of 70% in Saraya et al[22], 60% in Rentsch et al[28], and 65% in El-Nashar et al[29].
Rectocele refers to the protrusion or outward bulging of the rectal wall during defecation, arising as a consequence of weakening the pelvic floor’s supportive structures, particularly the recto-vaginal fascia. Rectoceles may be directed anteriorly or posteriorly, with anterior rectoceles being significantly more prevalent than posterior ones. Clinically, anterior rectoceles may present as a bulge or fullness against the posterior vaginal wall. However, clinical examination proves to be suboptimal for detecting anterior rectoceles, with sensitivity ranging from 30% to 80%[27]. Furthermore, it can be challenging to differentiate between anterior rectoceles and cul-de-sac hernias during clinical evaluation. Recto
Rectal intussusception refers to telescoping or invagination of the rectal wall into the lumen of the distal rectum or anal canal during the process of evacuation[1,2]. Depending on the extent and length of the intussusception, it can be classified into three categories: Intrarectal (where the tip of the intussusception remains confined within the rectum), intra-anal (where the tip extends into the anal canal), and extra-anal (where the tip protrudes externally beyond the anal orifice). The latter is also referred to as rectal prolapse. Rectal intussusception can further be categorized based on the thickness of the intussuscepted wall into partial-thickness (mucosal) intussusception, which involves only the mucosal layer, and full-thickness intussusception, which encompasses all layers of the rectal wall[30-32].
MRD reveals mucosal intussusceptions as thin, hypointense curvilinear folds on the rectal wall during defecation. In contrast, full-thickness intussusceptions appear as the entire rectal wall folding upon itself (Figure 10).
The pathophysiology of rectal intussusception is often attributed to chronic straining during defecation, which results in damage to the supporting fascial structures[4]. Patients typically present with a range of symptoms, including a sensa
MRD, with a reported sensitivity of 70%, is capable of detecting rectal intussusception, classifying it as partial or full-thickness, and assessing its vertical extension (whether intra-anal or extra-anal)[1]. These distinctions are crucial when determining the appropriate management strategy.
Management decisions primarily depend on the thickness of the intussusception, in addition to the clinical severity of symptoms. For instance, partial-thickness intussusceptions may be amenable to conservative treatment, such as a high-fiber diet and the use of laxatives, or through transanal resection of redundant mucosa. Conversely, full-thickness intussusceptions typically require surgical intervention, such as rectopexy. While intrarectal and intra-anal intussusceptions may respond to conservative measures, patients with extra-anal intussusception frequently necessitate surgical correction[33,34].
Solitary rectal ulcer syndrome (SRUS) is characterized by chronic, localized inflammation and thickening of the distal rectum[33]. On endoscopy, it may present as mucosal hyperemia, a solitary ulcer, or multiple ulcers. Patients commonly exhibit symptoms consistent with ODS, including constipation, excessive straining, rectal bleeding[33]. MRD often re
Pelvic floor dyssynergia, also referred to as spastic pelvic floor or anismus, is a functional cause of constipation characterized by the uncoordinated interaction between the pelvic floor and abdominal muscles involved in defecation[34]. Dyssynergia accounts for approximately 7% of cases of ODS[35]. It arises from inadequate relaxation of the anal canal, resulting in increased resistance to evacuation. Patients with dyssynergia typically report excessive straining, incomplete evacuation, and the need for digital manipulation during defecation[36].
Dyssynergia is considered a functional cause of ODS and may occur due to the paradoxical contraction of the puborectalis muscle (paradoxical puborectalis syndrome) or failure of the anal sphincter to relax properly[1]. The Rome IV criteria provide the diagnostic framework for dyssynergic defecation, which includes an increased contraction of the pelvic floor muscles (as measured by anal electromyography or manometry) in the presence of adequate rectal propulsive forces[2]. For a diagnosis to be made, these criteria must be met for a minimum duration of three months[2].
MRD assists in making a confident diagnosis and also helps in assessing any coexisting abnormalities, such as intussusception, rectocele or cul-de-sac hernias[37]. On dynamic MRD dyssynergia is evidenced by the failure of the ARA to open despite repeated attempts at defecation, due to the abnormal contraction of the puborectalis (Figure 11).
MRD is capable of detecting incomplete and prolonged evacuation, defined as the failure to completely expel the rectal gel during the examination or to eliminate two-thirds of the inserted gel within 60 seconds, caused by the anal canal's failure to open (Figure 12). This 60-second time frame is derived from studies conducted on conventional defecography[2].
A study by Reiner et al[20], conducted on patients (n = 48) with clinically suspected dyssynergic defecation revealed that impaired evacuation of rectal jelly exhibited an exceptionally high sensitivity of 100% for diagnosing dyssynergia. In contrast, paradoxical contraction of the puborectalis muscle demonstrated a sensitivity of 83%. The failure of the ARA to open displayed a lower sensitivity of 50%, but a high specificity of 97% for diagnosing dyssynergic defecation. The combination of failure to open the ARA and paradoxical puborectalis contraction further increased the diagnostic sensi
Thanaracthanon et al[21] reported that various findings on MRD aid in diagnosing dyssynergic defecation, including the descent of the anorectal junction, alterations in the ARA, prominence of the puborectalis muscle, and incomplete or failed evacuation. Notably, changes in the ARA and the prominent indentation of the anorectal junction by the puborec
The primary treatment approach for dyssynergia defecation includes dietary modifications and biofeedback therapy[37]. Although myectomy and botulinum toxin injections have been investigated as potential treatments for dyssynergia, they have not demonstrated superior outcomes compared to conservative management.
According to the American College of Radiology, only two imaging modalities are deemed appropriate for the evaluation of pelvic floor dysfunction: MRD and Fluoroscopic Cystocolpoproctography (CCP), both of which have been assigned an appropriateness rating of 7 to 9—scores that indicate a high level of clinical utility[38]. As such, dynamic CCP serves as a viable alternative imaging modality for assessing pelvic floor disorders. Dynamic CCP entails fluoroscopic imaging during the act of defecation, performed with the patient seated in a physiologically upright position on a specialized fluoroscopic commode. Image acquisition occurs at multiple functional phases: Rest, kegel maneuver (pelvic floor muscle contraction), straining, and defecation. CCP is capable of identifying clinically occult prolapse that may otherwise go undetected on physical assessment. A principal advantage of dynamic CCP lies in its capacity to provide functional evaluation in the upright, seated posture, closely mirroring normal physiological conditions.
A study conducted by Poncelet et al[39] compared the diagnostic performance of CCP and MRD in the evaluation of posterior compartment disorders. The investigators assessed the sensitivity of both dynamic CCP and MRD against a composite reference standard. The results of the combination of CCP and MRD were used as the standard of reference.
According to the study, X-ray defecography demonstrated a sensitivity of 90.9% for detecting peritoneocele, 71.4% for rectocele, 81.1% for rectal prolapse, and 63.6% for anismus. MRD, in comparison, showed a sensitivity of 86.4% for peritoneocele, 78.6% for rectocele, 62.2% for rectal prolapse, and 63.6% for anismus.
These findings revealed no statistically significant differences in sensitivity between the two imaging modalities across the spectrum of posterior compartment abnormalities. Consequently, the authors concluded that either X-ray defecography or MRD may be employed in clinical practice, with the choice largely dependent upon local availability and the expertise of the interpreting radiologist. In complex or ambiguous cases, the combined use of both modalities may be used to enhance diagnostic precision and guide management.
However, several limitations are inherent to fluoroscopic CCP. Notably, it lacks soft-tissue contrast resolution and is incapable of directly visualizing pelvic floor musculature, fascial structures, or post-surgical anatomical changes. Furthermore, the procedure requires the administration of contrast media into the bladder and vagina, in addition to oral contrast ingestion prior to the examination—elements that may render the study cumbersome and uncomfortable for patients. Finally, dynamic CCP involves exposure to ionizing radiation, with an estimated dose ranging from 1 to 10 mSv[38].
MRD offers several distinct advantages over X-ray defecography. MRD is free of ionizing radiation, rendering MRD a safer option for patients. In addition, MRD enables detailed visualization of pelvic organ morphology and facilitates a more precise evaluation of key pelvic floor musculature and ligaments. Perhaps most critically, it permits a comprehensive, simultaneous assessment of all three pelvic compartments within a single examination—an essential consi
MRD offers direct visualization of pelvic floor dysfunction through cine loop imaging. This dynamic representation enhances diagnostic clarity and serves as a valuable tool in multidisciplinary team discussions, thereby facilitating more informed and collaborative treatment planning. Furthermore, MRD is increasingly accessible and allows for repetition or acquisition of additional sequences in cases of suboptimal or inconclusive studies, thereby enhancing diagnostic relia
However, MRD has certain limitations. Primarily, it is typically conducted with the patient in a supine position, which does not replicate the physiological posture assumed during natural defecation. This discrepancy may restrict the accu
In conclusion, pelvic floor disorders represent a significant and multifaceted clinical challenge, manifesting through a broad spectrum of symptoms. ODS is an important cause of chronic constipation associated with a range of pelvic floor abnormalities. MRD serves as a versatile diagnostic tool in the management of ODS by offering detailed visualization of the pelvic floor abnormalities underpinning ODS. Its dynamic imaging capabilities enable the identification of multicompartmental pelvic disorders, thereby playing a crucial role in the accurate diagnosis and formulation of an appropriate management plan tailored to the patient’s needs. Further research is needed to deepen our understanding of the complex pathophysiology underlying pelvic floor dysfunction. Such efforts are essential to advance diagnostic accuracy and optimize clinical management.
| 1. | Revels JW, Mansoori B, Fadl S, Wang SS, Olson MC, Moran SK, Terrazas MF, Fletcher JG, Perry WRG, Chernyak V, Mileto A. MR Defecating Proctography with Emphasis on Posterior Compartment Disorders. Radiographics. 2023;43:e220119. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Kanmaniraja D, Arif-Tiwari H, Palmer SL, Kamath A, Lewis SC, Flusberg M, Kobi M, Lockhart ME, Chernyak V. MR defecography review. Abdom Radiol (NY). 2021;46:1334-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Grossi U, Di Tanna GL, Heinrich H, Taylor SA, Knowles CH, Scott SM. Systematic review with meta-analysis: defecography should be a first-line diagnostic modality in patients with refractory constipation. Aliment Pharmacol Ther. 2018;48:1186-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Hallock JL, Handa VL. The Epidemiology of Pelvic Floor Disorders and Childbirth: An Update. Obstet Gynecol Clin North Am. 2016;43:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 5. | Podzemny V, Pescatori LC, Pescatori M. Management of obstructed defecation. World J Gastroenterol. 2015;21:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 6. | Roos JE, Weishaupt D, Wildermuth S, Willmann JK, Marincek B, Hilfiker PR. Experience of 4 years with open MR defecography: pictorial review of anorectal anatomy and disease. Radiographics. 2002;22:817-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Thapar RB, Patankar RV, Kamat RD, Thapar RR, Chemburkar V. MR defecography for obstructed defecation syndrome. Indian J Radiol Imaging. 2015;25:25-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Piloni V, Bergamasco M, Melara G, Garavello P. The clinical value of magnetic resonance defecography in males with obstructed defecation syndrome. Tech Coloproctol. 2018;22:179-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Ahmed F. Role of MR Defecography in the Assessment of Obstructed Defecation Syndrome. Med J Cairo Univ. 2018;86:927-931. [DOI] [Full Text] |
| 10. | Kim NY, Kim DH, Pickhardt PJ, Carchman EH, Wald A, Robbins JB. Defecography: An Overview of Technique, Interpretation, and Impact on Patient Care. Gastroenterol Clin North Am. 2018;47:553-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Wu JM, Vaughan CP, Goode PS, Redden DT, Burgio KL, Richter HE, Markland AD. Prevalence and trends of symptomatic pelvic floor disorders in U.S. women. Obstet Gynecol. 2014;123:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 593] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 12. | Heinrich H, Sauter M, Fox M, Weishaupt D, Halama M, Misselwitz B, Buetikofer S, Reiner C, Fried M, Schwizer W, Fruehauf H. Assessment of Obstructive Defecation by High-Resolution Anorectal Manometry Compared With Magnetic Resonance Defecography. Clin Gastroenterol Hepatol. 2015;13:1310-1317.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 13. | Flusberg M, Kobi M, Bahrami S, Glanc P, Palmer S, Chernyak V, Kanmaniraja D, El Sayed RF. Multimodality imaging of pelvic floor anatomy. Abdom Radiol (NY). 2021;46:1302-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Lakhoo J, Khatri G, Elsayed RF, Chernyak V, Olpin J, Steiner A, Tammisetti VS, Sundaram KM, Arora SS. MRI of the Male Pelvic Floor. Radiographics. 2019;39:2003-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Piloni V, Tosi P, Vernelli M. MR-defecography in obstructed defecation syndrome (ODS): technique, diagnostic criteria and grading. Tech Coloproctol. 2013;17:501-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 16. | Flusberg M, Sahni VA, Erturk SM, Mortele KJ. Dynamic MR defecography: assessment of the usefulness of the defecation phase. AJR Am J Roentgenol. 2011;196:W394-W399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Flusberg M. Magnetic Resonance Defecography for Obstructed Defecation. Radiol Clin North Am. 2025;63:465-475. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Hassan HH, Elnekiedy AM, Elshazly WG, Naguib NN. Modified MR defecography without rectal filling in obstructed defecation syndrome: Initial experience. Eur J Radiol. 2016;85:1673-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Li M, Jiang T, Peng P, Yang X. MR Defecography in Assessing Functional Defecation Disorder: Diagnostic Value of the Defecation Phase in Detection of Dyssynergic Defecation and Pelvic Floor Prolapse in Females. Digestion. 2019;100:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Reiner CS, Tutuian R, Solopova AE, Pohl D, Marincek B, Weishaupt D. MR defecography in patients with dyssynergic defecation: spectrum of imaging findings and diagnostic value. Br J Radiol. 2011;84:136-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Thanaracthanon P, Sasiwimonphan K, Sunthornram A, Harisinghani MG, Chulroek T. Diagnostic performance of dynamic MR defecography in assessment of dyssynergic defecation. Abdom Radiol (NY). 2023;48:3458-3468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Saraya S, Awad A, El Bakry RE. MR defecography in ano-rectal dysfunction: a clinical-radiological correlation study. Egypt J Radiol Nucl Med. 2020;51:173. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Al-Najar MS, Ghanem AF, AlRyalat SAS, Al-Ryalat NT, Alhajahjeh SO. The usefulness of MR defecography in the evaluation of pelvic floor dysfunction: our experience using 3T MRI. Abdom Radiol (NY). 2017;42:2219-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Schawkat K, Heinrich H, Parker HL, Barth BK, Mathew RP, Weishaupt D, Fox M, Reiner CS. How to define pathologic pelvic floor descent in MR defecography during defecation? Abdom Radiol (NY). 2018;43:3233-3240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Lalwani N, El Sayed RF, Kamath A, Lewis S, Arif H, Chernyak V. Imaging and clinical assessment of functional defecatory disorders with emphasis on defecography. Abdom Radiol (NY). 2021;46:1323-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Colarieti A, Stuto A, Cellerino P, Sardanelli F. Clinical value of MR defecography: What additional knowledge is provided by the radiologist to the surgeon? Eur J Radiol. 2024;181:111760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Lalwani N, Khatri G, El Sayed RF, Ram R, Jambhekar K, Chernyak V, Kamath A, Lewis S, Flusberg M, Scholz F, Arif-Tiwari H, Palmer SL, Lockhart ME, Fielding JR. MR defecography technique: recommendations of the society of abdominal radiology's disease-focused panel on pelvic floor imaging. Abdom Radiol (NY). 2021;46:1351-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Rentsch M, Paetzel C, Lenhart M, Feuerbach S, Jauch KW, Fürst A. Dynamic magnetic resonance imaging defecography: a diagnostic alternative in the assessment of pelvic floor disorders in proctology. Dis Colon Rectum. 2001;44:999-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | El-nashar S, Occhino J, Trabuco E, Gebhart J, Klingele C. Descending Perineum Syndrome: A Fresh Look at an Interesting and Complex Pelvic Floor Disorder. J Minim Invasive Gynecol. 2014;21:S16-S17. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Ramage L, Georgiou P, Qiu S, McLean P, Khan N, Kontnvounisios C, Tekkis P, Tan E. Can we correlate pelvic floor dysfunction severity on MR defecography with patient-reported symptom severity? Updates Surg. 2018;70:467-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Maccioni F, Alt CD. MRI of the Pelvic Floor and MR Defecography. 2018 Mar 21. In: Diseases of the Abdomen and Pelvis 2018-2021: Diagnostic Imaging - IDKD Book [Internet]. Cham (CH): Springer, 2018–. [PubMed] |
| 32. | Çıvgın E, Yiğit H, Doğulu FF, Koşar PN, Uysal Ramadan S. Agreement analysis of the magnetic resonance defecography and clinical examination findings in the evaluation of pelvic floor disorders. ACH Med J. 2023;2:117-124. [DOI] [Full Text] |
| 33. | Zhu QC, Shen RR, Qin HL, Wang Y. Solitary rectal ulcer syndrome: clinical features, pathophysiology, diagnosis and treatment strategies. World J Gastroenterol. 2014;20:738-744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (5)] |
| 34. | Rao SS. Dyssynergic defecation. Gastroenterol Clin North Am. 2001;30:97-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Sadeghi A, Akbarpour E, Majidirad F, Bor S, Forootan M, Hadian MR, Adibi P. Dyssynergic Defecation: A Comprehensive Review on Diagnosis and Management. Turk J Gastroenterol. 2023;34:182-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 36. | Sharma A, Herekar A, Yan Y, Karunaratne T, Rao SSC. Dyssynergic Defecation and Other Evacuation Disorders. Gastroenterol Clin North Am. 2022;51:55-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Haliloglu N, Erden A. Magnetic resonance defecography findings of dyssynergic defecation. Pol J Radiol. 2022;87:e181-e185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 38. | Expert Panel on GYN and OB Imaging, Khatri G, Bhosale PR, Robbins JB, Akin EA, Ascher SM, Brook OR, Dassel M, Glanc P, Henrichsen TL, Learman LA, Sadowski EA, Saphier CJ, Wasnik AP, Maturen KE. ACR Appropriateness Criteria® Pelvic Floor Dysfunction in Females. J Am Coll Radiol. 2022;19:S137-S155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Poncelet E, Rock A, Quinton JF, Cosson M, Ramdane N, Nicolas L, Feldmann A, Salleron J. Dynamic MR defecography of the posterior compartment: Comparison with conventional X-ray defecography. Diagn Interv Imaging. 2017;98:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |