Published online Jun 26, 2025. doi: 10.4330/wjc.v17.i6.106717
Revised: April 2, 2025
Accepted: May 16, 2025
Published online: June 26, 2025
Processing time: 107 Days and 10.4 Hours
There is no available data about the trajectory of heart failure (HF) with improved ejection fraction (EF) and patient clinical outcomes in Qatar.
To explore the difference in characteristics and outcomes between patients with transient and sustained improvement in left ventricular ejection fraction (LVEF) and to determine the independent predictors for sustained improvement in LVEF.
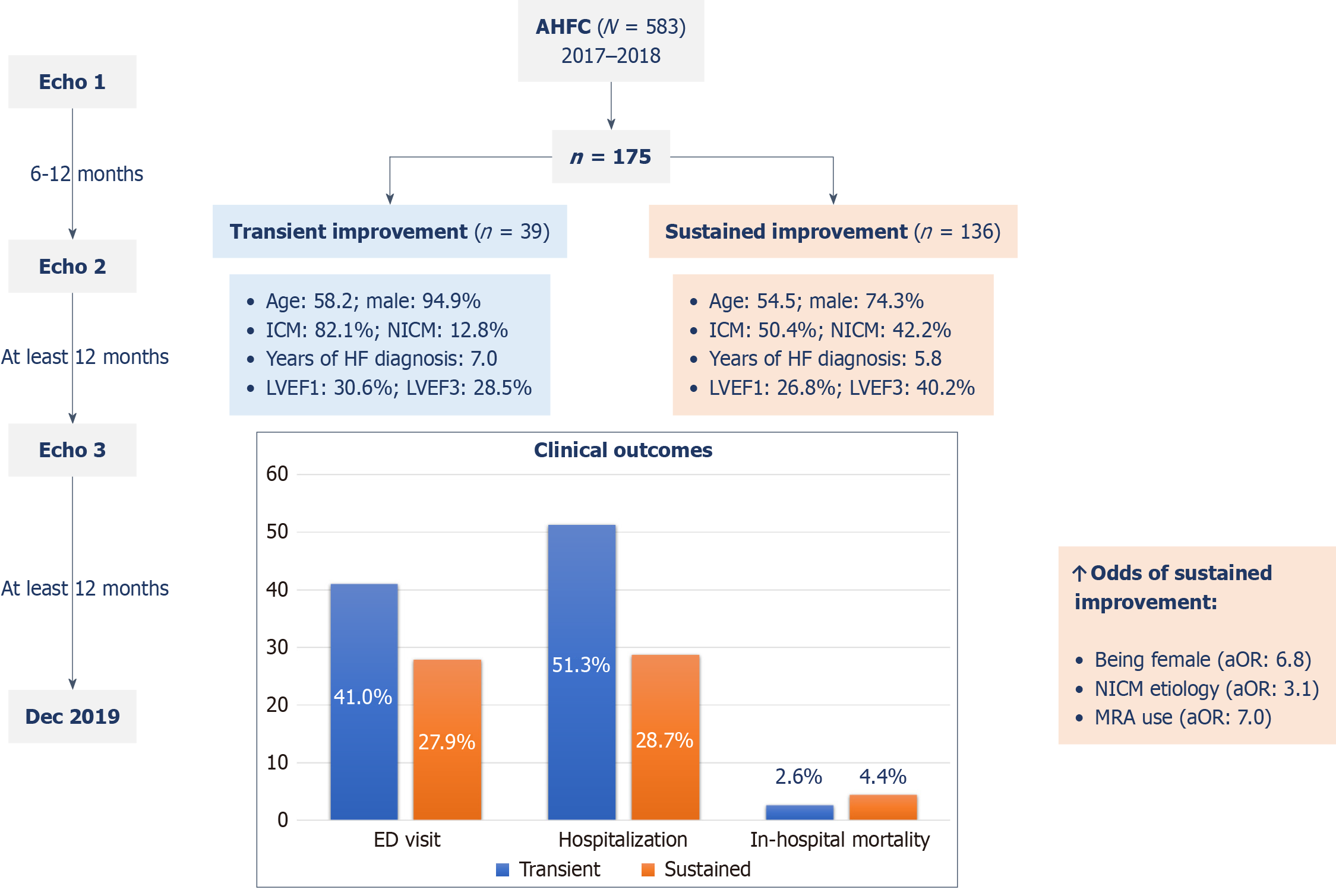
This is a retrospective cohort study that was conducted at the advanced HF clinic of a tertiary care hospital in Qatar between January 2017 and December 2018. This study included adult patients with improved LVEF and had at least three echocardiographic studies. The patients were divided into two groups: HF with transient improvement in EF (HFtimpEF) and HF with sustained improvement in EF (HFsimpEF).
A total of 175 patients with HF and improved EF were included. Among them 136 (77.7%) patients showed sustained improvement in LVEF. The remaining patients with HFtimpEF were predominantly males [37 (94.9%) vs 101 (74.3%), P = 0.005] with a higher incidence of ischemic cardiomyopathy [32 (82.1%) vs 68 (50.4%), P = 0.002], dyslipidemia [24 (61.5%) vs 54 (39.7%), P = 0.03], and hypertension [34 (87.2%) vs 93 (68.4%), P = 0.03] than those with HFsimpEF. The latter experienced significantly lower rates of hospitalization [39 (28.7%) vs 20 (51.3%), P = 0.01] and diagnosis of new cardiovascular conditions during the follow-up (e.g., acute coronary syndrome, stroke, decompensated HF, and atrial fibrillation) [14 (10.3%) vs 10 (25.6%), P = 0.03] without a difference in emergency department visits or in-hospital death. Sustained improvement in LVEF was positively associated with being female [adjusted odds ratio (aOR) = 6.8, 95% confidence interval (CI): 1.4-32.3, P = 0.02], having non-ischemic etiology of HF (aOR = 3.1, 95%CI: 1.03-9.3, P = 0.04), and using a mineralocorticoid receptor antagonist (aOR = 7.0, 95%CI: 1.50-31.8, P = 0.01).
Patients with HFsimpEF experienced significantly lower rates of hospitalization and diagnosis of new cardiova
Core Tip: Our study reported observations from a population with heart failure in Qatar, a Middle Eastern country that is usually underrepresented in major clinical trials. This study was the first in the Middle East to characterize the clinical features and outcomes of patients with heart failure who demonstrate either sustained or temporary improvement in left ventricular ejection fraction.
- Citation: Kaddoura R, Chapra A, Shah J, Izham M, Singh R, Alsadi H, Al-Amri M, Hamamyh T, Fallouh M, Elasad F, Abdelghani M, Alsaadi Alyafei S, Badr A, Patel A. Beyond initial recovery: Heart failure with transient vs sustained improvement in left ventricular ejection fraction. World J Cardiol 2025; 17(6): 106717
- URL: https://www.wjgnet.com/1949-8462/full/v17/i6/106717.htm
- DOI: https://dx.doi.org/10.4330/wjc.v17.i6.106717
Heart failure (HF) remains one of the key causes of cardiovascular mortality[1]. The current guidelines propose strati
Yet this improvement is not always sustained; reverse remodeling is often accompanied by detrimental neurohormonal changes that can precipitate fluctuations in EF[9]. This can lead to variations in EF over time and highlights the impor
The factors that determine whether patients with HFrEF go on to become HFsimpEF or HFtimpEF has been an area of interest yet poorly studied. There is also a need to assess the long-term outcomes and prognosis of these patients. There is a lack of available data on the trajectory of HFimpEF and patient clinical outcomes in the Middle East. Hence, this study was conducted to explore several aspects related to patients with HFimpEF and their sustained improvement in LVEF. The hypothesis was that patients with sustained improvement in LVEF would have better clinical outcomes compared with those with transient improvement building on preliminary findings from previous studies exploring outcomes of patients with HFimpEF, who showed a generally favorable prognosis[10]. The main objective of this study was to explore the difference in characteristics and outcomes between patients with transient and sustained improvement in LVEF and to determine the independent predictors for sustained improvement in LVEF.
This was a retrospective cohort study that was conducted at the advanced HF clinic in a tertiary care hospital in Qatar between January 1, 2017 and December 31, 2018. The study was approved by the Institutional Review Board of the Heart Hospital and the Medical Research Center, approval No. MRC-01-20-139. An informed consent was waived due to the retrospective nature of the study. The study was in line with the principles of the Declaration of Helsinki, Good Clinical Practice and the laws and regulations of the Ministry of Public Health in Qatar. This study included all adult patients (≥ 18 years) who were initially diagnosed with HFrEF, defined as LVEF < 40.0% at baseline, who demonstrated an initial improvement in LVEF and had at least three echocardiographic studies from their initial visit to the clinic.
The study population was selected from our previously published study that described the characteristics of patients with HFrEF who had improvement in their LVEF and analyzed the independent predictors of LVEF improvement. Our previous study screened all patients who visited the advanced HF clinic during the study period and included adult patients aged 18 years or older with a diagnosis of HFrEF and who had two echocardiographic examinations performed at least 6 months apart[11].
For the current study, all patients had a first (or baseline) echocardiogram (Echo-1) where the diagnosis of HFrEF was established and showed improvement in LVEF of at least 1.0% on the second echocardiogram (Echo-2) performed at least six months after Echo-1. Hence, Echo-2 would identify the HFimpEF cohort. The specific cutoff values for the improve
Furthermore, the improvement in LVEF was considered large if the increase or change in LVEF (∆ LVEF) was 10.0% or more and modest if ∆ LVEF was 1.0%-9.0%[13]. A subgroup analysis for the patients with an initial large LVEF improve
LVEF was measured using the Simpson biplane method, unless the Simpson method was not possible. This study excluded patients with specific cardiomyopathies (e.g., hypertrophic, infiltrative, restrictive, stress-induced, or chemothe
Within the specified study duration, 582 patients with HFrEF underwent at least two echocardiograms. Of them, 175 patients (30.1%) showed any improvement in LVEF (i.e. ≥ 1.0%) between Echo-1 and Echo-2 and who had a second follow-up echocardiogram (i.e. Echo-3) performed at least 12 months after Echo-2. Among them, 136 patients (77.7%) showed sustained improvement in LVEF on Echo-3 while the remaining had reworsening of the LVEF (i.e. had transient improvement).
Patients with HFtimpEF were predominantly males (94.9% vs 74.3%, P = 0.005) with ischemic cardiomyopathy (82.1% vs 50.4%, P = 0.002). There were no differences between the groups in terms of age, body mass index, or duration of HF diagnosis. Patients with HFtimpEF had a higher incidence of dyslipidemia (61.5% vs 39.7%, P = 0.03) and hypertension (87.2% vs 68.4, P = 0.03) than those with HFsimpEF.
Table 1 presents details of baseline characteristics. At baseline, there were no differences between the groups in terms of blood pressure measurements, heart rate values, and the presence of left bundle branch block.
| Variable | Transient improvement (n = 39) | Sustained improvement (n = 136) | P value |
| Age (years), mean ± SD | 58.15 ± 11.69 | 54.51 ± 13.52 | 0.13 |
| BMI (kg/m2), mean ± SD | 28.36 ± 5.67 | 30.49 ± 7.20 | 0.09 |
| Male | 37 (94.9) | 101 (74.3) | 0.005 |
| Female | 2 (5.1) | 35 (25.7) | - |
| HF etiology | |||
| Ischemic | 32 (82.1) | 68 (50.4) | 0.002 |
| Dilated | 5 (12.8) | 57 (42.2) | |
| Others | 2 (5.1) | 10 (7.4) | |
| Duration of HF diagnosis, mean ± SD | 7.05 ± 3.81 | 5.79 ± 4.0 | 0.08 |
| Comorbidities | |||
| Dyslipidemia | 24 (61.5) | 54 (39.7) | 0.03 |
| Hypertension | 34 (87.2) | 93 (68.4) | 0.03 |
| Diabetes | 28 (71.8) | 90 (66.2) | 0.57 |
| Atrial fibrillation | 8 (20.5) | 25 (18.4) | 0.82 |
| Ventricular arrhythmia | 5 (12.8) | 18 (13.2) | 1.00 |
| CVA/TIA | 5 (12.8) | 16 (11.8) | 0.79 |
| PAD | 2 (5.1) | 8 (5.9) | 1.00 |
| Renal impairment | 17 (43.6) | 35 (25.7) | 0.05 |
| Liver disease | 2 (5.1) | 5 (3.7) | 0.65 |
| Anemia | 10 (25.6) | 38 (27.9) | 0.84 |
| Sleep apnea | 0 (0) | 9 (6.6) | 0.21 |
| Chronic lung disease | 6 (15.4) | 21 (15.4) | 1.00 |
| Cancer | 0 (0) | 7 (5.1) | 0.35 |
| Dementia | 2 (5.1) | 3 (2.2) | 0.31 |
| Depression | 0 (0) | 6 (4.4) | 0.34 |
| Hypothyroidism | 2 (5.1) | 11 (8.1) | 0.74 |
| Hyperthyroidism | 0 (0) | 4 (2.9) | 0.58 |
Similarly, there was no difference in the previous parameter measurements between the groups at follow-up except for the heart rate, which was significantly lower in patients with HFsimpEF [72.84 ± 12.90 vs 79.41 ± 15.45 beats per minute (bpm), P = 0.02] as shown in Table 2. Blood test parameters did not differ between the two groups at baseline and follow-up except for the estimated glomerular filtration rate, which was significantly higher in the sustained improvement group at both baseline (79.68 ± 33.27 vs 66.47 ± 27.79 mL/minute, P = 0.03) and follow-up (70.35 ± 29.24 vs 59.89 ± 28.49 mL/minute, P = 0.05) (Table 3).
| Variable | Transient improvement (n = 39) | Sustained improvement (n = 136) | P value |
| Baseline (Echo-1), mean ± SD | |||
| Systolic BP (mmHg) | 124.38 ± 22.41 | 125.30 ± 20.1 (n = 134) | 0.81 |
| Diastolic BP (mmHg) | 72.64 ± 12.36 | 75.97 ± 12.99 (n = 134) | 0.16 |
| Heart rate (bpm) | 86.43 ± 18.02 (n = 37) | 89.69 ± 19.12 (n = 132) | 0.36 |
| Achieved target BP | 26 (66.7) | 87 (64.9) | 1.00 |
| Sinus rhythm | 28 (90.3) | 103 (86.6) | 0.77 |
| LBBB | 7 (14.1) | 19 (17.1) | 0.42 |
| QRS (ms), mean ± SD | 109.45 ± 26.29 (n = 29) | 109.43 ± 44.04 (n = 129) | 1.0 |
| QTc (ms), mean ± SD | 468.86 ± 43.48 (n = 29) | 462.84 ± 52.19 (n = 109) | 0.57 |
| Second follow-up (Echo-3), mean ± SD | |||
| Systolic BP (mmHg) | 127.87 ± 21.43 | 133.47 ± 98.18 (n = 129) | 0.73 |
| Diastolic BP (mmHg) | 73.85 ± 13.62 | 73.81 ± 11.49 (n = 129) | 0.99 |
| Heart rate (bpm) | 79.41 ± 15.45 | 72.84 ± 12.90 (n = 128) | 0.02 |
| Achieved target BP | 26 (66.7) | 90 (69.8) | 0.67 |
| Sinus rhythm | 33 (89.2) | 111 (88.8) | 1.00 |
| LBBB | 7 (19.4) | 21 (17.4) | 0.81 |
| QRS (ms), mean ± SD | 112.00 ± 26.87 (n = 37) | 110.07 ± 26.34 (n = 120) | 0.70 |
| QTc (ms), mean ± SD | 455.08 ± 31.57 (n = 37) | 448.91 ± 42.92 (n = 120) | 0.35 |
| Variable | Transient improvement (n = 39) | Sustained improvement (n = 136) | P value |
| Baseline (Echo-1) | |||
| NT-pro-BNP (pg/mL) | 5106.63 ± 6035.19 (n = 27) | 4730.48 ± 6792.82 (n = 93) | 0.80 |
| Hemoglobin (g/dL) | 13.16 ± 2.22 (n = 38) | 14.20 ± 12.89 (n = 132) | 0.62 |
| Urea (mmol/L) | 8.16 ± 4.53 (n = 38) | 7.15 ± 4.17 (n = 133) | 0.20 |
| Creatinine (µmol/L) | 120.03 ± 56.87 (n = 38) | 113.55 ± 114.84 (n = 132) | 0.74 |
| eGFR (mL/min) | 66.47 ± 27.79 (n = 38) | 79.68 ± 33.27 (n = 132) | 0.03 |
| HbA1c (%) | 8.38 ± 2.34 (n = 26) | 7.54 ± 2.19 (n = 102) | 0.09 |
| LDL-C (mmol/L) | 2.69 ± 0.92 (n = 35) | 2.57 ± 1.18 (n = 120) | 0.56 |
| Iron (µmol/L) | 8.87 ± 3.69 (n = 10) | 10.43 ± 6.49 (n = 37) | 0.47 |
| TSAT (%) | 16.77 ± 5.42 (n = 10) | 20.37 ± 15.74 (n = 35) | 0.48 |
| Ferritin (ug/L) | 325.75 ± 347.95 (n = 8) | 208.50 ± 277.71 (n = 30) | 0.32 |
| TSH (pmol/L) | 1.76 ± 1.51 (n = 22) | 2.38 ± 2.18 (n = 92) | 0.21 |
| T4 (mIU/L) | 13.51 ± 1.70 (n = 23) | 14.87 ± 3.58 (n = 87) | 0.08 |
| Second follow-up (Echo-3) | |||
| NT-pro-BNP (pg/mL) | 7672.15 ± 13459.32 (n = 27) | 3184.52 ± 6564.79 (n = 81) | 0.11 |
| Hemoglobin (g/dL) | 12.23 ± 2.25 (n = 37) | 12.87 ± 2.08 (n = 120) | 0.11 |
| Urea (mmol/L) | 10.28 ± 7.27 (n = 38) | 8.50 ± 8.94 (n = 125) | 0.26 |
| Creatinine (µmol/L) | 142.84 ± 88.49 (n = 38) | 127.25 ± 126.01 (n = 128) | 0.48 |
| eGFR (mL/min) | 59.89 ± 28.49 (n = 38) | 70.35 ± 29.24 (n = 127) | 0.05 |
| HbA1c (%) | 7.66 ± 2.04 (n = 33) | 7.90 ± 4.88 (n = 103) | 0.79 |
| LDL-C (mmol/L) | 1.81 ± 0.99 (n = 33) | 2.03 ± 0.93 (n = 99) | 0.25 |
| Iron (µmol/L) | 9.64 ± 4.33 (n = 18) | 11.23 ± 6.24 (n = 41) | 0.33 |
| TSAT (%) | 17.44 ± 8.69 (n = 18) | 23.48 ± 16.53 (n = 41) | 0.15 |
| Ferritin (ug/L) | 276.17 ± 489.76 (n = 14) | 244.41 ± 429.58 (n = 30) | 0.83 |
| TSH (pmol/L) | 2.43 ± 1.45 (n = 22) | 3.22 ± 3.12 (n = 83) | 0.26 |
| T4 (mIU/L) | 15.73 ± 2.23 (n = 19) | 15.05 ± 3.86 (n = 73) | 0.46 |
Upon initial diagnosis, mean LVEF was significantly lower in the sustained improvement group (26.77 ± 7.05% vs 30.56 ± 6.95%, P = 0.003) with no difference in other echocardiographic parameters. At follow-up, LVEF became significantly higher in the sustained improvement group (40.18 ± 0.12 vs 28.46 ± 6.59%, P = 0.001) with significant improvement in other parameters such as lower left atrium volume index (34.30 ± 11.26 vs 41.41 ± 12.92 mL/m2, P = 0.003), left ventricular end-systolic diameter (4.50 ± 0.95 vs 4.99 ± 0.75 cm, P = 0.004), and right ventricular systolic pressure (RVSP; 33.10 ± 11.82 vs 38.78 ± 14.61 mmHg, P = 0.04). The initial improvement in LVEF (between Echo-1 and Echo-2) at almost 20 months of follow-up did not differ in magnitude between the groups (Echo-2 - Echo-1: ∆ LVEF: 9.54 ± 7.69% vs 8.05 ± 6.53%, P = 0.27). After another 20 months of follow-up, the overall improvement in LVEF differed significantly between the groups (Echo-3 - Echo-1: ∆ LVEF: 13.43% ± 9.09% vs 2.10% ± 5.05%, P = 0.001) (Table 4).
| Variable | Transient improvement (n = 39) | Sustained improvement (n = 136) | P value |
| Baseline (Echo-1) | |||
| LVEF (%) | 30.56 ± 6.95 | 26.77 ± 7.05 | 0.003 |
| Average E/e’ | 15.01 ± 5.88 (n = 20) | 14.52 ± 5.78 (n = 67) | 0.74 |
| LA volume index (mL/m2) | 35.97 ± 12.51 (n = 25) | 37.09 ± 11.35 (n = 82) | 0.68 |
| LVEDD (cm) | 5.76 ± 0.79 (n = 37) | 6.70 ± 4.17 (n = 130) | 0.18 |
| LVESD (cm) | 4.76 ± 0.84 (n = 34) | 5.08 ± 0.89 (n = 115) | 0.80 |
| RVSP (mmHg) | 33.46 ± 11.06 | 37.35 ± 12.89 (n = 130) | 0.13 |
| Second follow-up (Echo-3) | |||
| LVEF (%) | 28.46 ± 6.59 | 40.18 ± 0.12 | 0.001 |
| Average E/e’ | 13.12 ± 5.46 (n = 19) | 12.08 ± 5.86 (n = 77) | 0.49 |
| LA volume index (mL/m2) | 41.41 ± 12.92 (n = 31) | 34.30 ± 11.26 (n = 114) | 0.003 |
| LVEDD (cm) | 6.06 ± 0.62 (n = 38) | 5.81 ± 0.89 (n = 134) | 0.05 |
| LVESD (cm) | 4.99 ± 0.75 (n = 37) | 4.50 ± 0.95 (n = 131) | 0.004 |
| RVSP (mmHg) | 38.78 ± 14.61 (n = 37) | 33.10 ± 11.82 (n = 129) | 0.04 |
| LVEF (Echo-2) | 38.59 ± 7.57 | 36.33 ± 7.69 | 0.11 |
| ∆ LVEF (Echo-2 - Echo-1) (%) | 8.05 ± 6.53 | 9.54 ± 7.69 | 0.27 |
| Duration between Echo-1 and Echo-2 (months) | 19.82 ± 26.61 | 17.24 ± 21.15 | 0.53 |
| ∆ LVEF (Echo-3 - Echo-1) (%) | -2.10 ± 5.05 | 13.43 ± 9.09 | 0.001 |
| Duration between Echo-2 and Echo-3 (months) | 20.49 ± 13.40 | 21.79 ± 23.39 | 0.74 |
At the time of diagnosis, more than half of the patients in both groups were on beta-blockers and renin-angiotensin-aldosterone system inhibitors without a difference between the two groups. Significantly more patients in the sustained improvement group were on mineralocorticoid receptor antagonists (MRA; 21.3% vs 5.1%, P = 0.02). At the second follow-up (Echo 3), the use of guideline-directed medical therapy for HF increased in both groups without a difference between them (Table 5).
| Variable | Transient improvement (n = 39) | Sustained improvement (n = 136) | P value |
| Baseline (Echo-1) | |||
| Beta-blockers | 19 (48.7) | 78 (57.4) | 0.37 |
| ACEI/ARB | 25 (64.1) | 81 (59.6) | 0.71 |
| MRA | 2 (5.1) | 29 (21.3) | 0.02 |
| Diuretics | 13 (33.3) | 62 (45.6) | 0.20 |
| HDZ/ISDN | 1 (2.6) | 4 (2.9) | 1.00 |
| Ivabradine | 0 (0) | 6 (4.4) | 0.34 |
| Digoxin | 0 (0) | 9 (6.6) | 0.21 |
| Antiplatelet therapy | 24 (61.5) | 78 (57.4) | 0.71 |
| LLA | 24 (61.5) | 72 (52.9) | 0.38 |
| Iron (IV) | 7 (17.9) | 17 (12.5) | 0.43 |
| Inotropes (hospital) | 4 (10.3) | 23 (16.9) | 0.45 |
| Second follow-up (Echo-3) | |||
| Beta-blockers | 36 (92.3) | 126 (95.5) | 0.43 |
| ACEI/ARB | 30 (76.9) | 110 (80.9) | 0.58 |
| MRA | 13 (33.3) | 54 (40.9) | 0.46 |
| Diuretics | 31 (79.5) | 99 (75.0) | 0.67 |
| HDZ/ISDN | 4 (10.3) | 10 (7.6) | 0.53 |
| Ivabradine | 3 (7.7) | 11 (8.3) | 1.00 |
| Digoxin | 2 (5.1) | 7 (5.3) | 1.00 |
| Antiplatelet therapy | 34 (87.2) | 97 (73.5) | 0.09 |
| Lipid-lowering agents | 36 (92.3) | 106 (80.3) | 0.08 |
| Iron (IV) | 4 (10.3) | 5 (3.7) | 0.12 |
| Inotropes (hospital) | 3 (7.7) | 3 (2.2) | 0.13 |
Patients with sustained improvement in LVEF demonstrated a significantly lower rate of all-cause hospitalization compared with those with transient improvement [39 (28.7%) vs 20 (51.3%) patients, P = 0.01]. Among all the hospitali
Multivariate nominal logistic regression analysis was performed to identify factors associated with sustained improve
| Variable | Adjusted OR (95%CI) | P value |
| Age in years | 0.99 (0.95-1.0) | 0.42 |
| Female | 6.8 (1.4-32.3) | 0.02 |
| HF etiology | ||
| Ischemic | 1 (reference) | - |
| Dilated | 3.1 (1.03-9.3) | 0.04 |
| Others | 1.7 (0.30-9.3) | 0.56 |
| Hyperlipidemia | 0.50 (0.21-1.11) | 0.09 |
| Hypertension | 0.40 (0.13-1.16) | 0.09 |
| MRA use | 7.0 (1.50-31.8) | 0.01 |
A subgroup analysis was performed to identify the characteristics and outcomes of patients with HFimpEF and a large improvement in LVEF between the baseline (Echo-1) and first follow-up echocardiogram (Echo-2). Seventy-one (40.6%) patients had a large improvement in LVEF (i.e. ∆ LVEF ≥ 10.0%) at Echo-2. Of them, 14 (19.3%) and 57 (80.3%) patients showed transient and sustained improvement in LVEF, respectively. Patients with transient improvement were signifi
Guideline-directed medications for HF did not differ between the sustained and transient improvement subgroups. The clinical outcome findings of the subgroups were in line with the overall study findings. Patients with sustained improvement in LVEF experienced a significantly lower incidence of new cardiovascular conditions (28.6% vs 7.0%, P = 0.022) without a difference in the emergency department visits (35.7% vs 29.8%, P = 0.750) or in-hospital deaths (7.1% vs 5.3%, P = 1.000). However, there was a trend towards lower hospitalizations in those with sustained improvement (50.0% vs 22.8%, P = 0.054).
This study explored the independent predictors for sustained improvement in LVEF at the advanced HF clinic in Qatar. One-quarter of our study population had reworsening in LVEF despite initial improvement. These patients with HFtimpEF were predominantly males with ischemic cardiomyopathy and higher incidence of hypertension and dysli
Although the current diagnosis and management of HF depends on LVEF, it is important to consider that LVEF may vary over time. Many patients may erroneously be managed in the long-term as HFimpEF based on the initial im
Currently, there is no consensus in the international guidelines on the exact cutoffs for LVEF improvement to define HFimpEF[12]. Due to the small sample size, we included all patients who demonstrated any (rather than large) improve
Patients with HFsimpEF made up 77.7% of the total cohort in our study. Similarly, in a study that enrolled patients with dilated cardiomyopathy (n = 188), 46.0% of them showed an initial improvement in their LVEF (from 26.0 ± 7.0% to 48.0 ± 10.0%). At 36 months of follow-up after Echo-2, most patients had Echo-3 (n = 183), which also showed sustained EF improvement in 70.0% of patients[15]. In contrast the reported frequency of HFtimpEF varies between 11.0% and 38.0% in prior studies[16]. This variation is thought to be due to different definitions of LVEF reworsening and variations in the timing and length of the follow-up echocardiograms. This led to increased rates of reported reworsening as the follow-up period was extended.
In our study patients with HFsimpEF showed concomitant improvement in echocardiographic parameters such as left atrium volume index, left ventricular end-systolic diameter, and RVSP despite having an initially lower LVEF than those with HFtimpEF. Similar findings have been observed in a study by Blechman et al[15]. In our study patients with HFsimpEF had a lower mean heart rate than those with HFtimpEF at follow-up, highlighting the continued use of beta-blockers despite initial improvement in patients with HFrEF. However, another study found no significant association between continued beta blocker therapy and sustained improvement in LVEF[17]. Hence, further studies are needed to confirm the long-term benefit of beta-blockers in HFsimpEF, especially to determine whether the benefits exist beyond just the lowering of heart rate.
Understanding the factors that lead to long-term sustained vs transient improvement followed by deterioration in LVEF is crucial for individualizing patient care. By recognizing the characteristics of such patients in each group, we can improve risk stratification and identify the vulnerable patients who may benefit from more aggressive treatment approaches. Currently, there is substantial evidence on the characteristics and outcomes of patients with HFimpEF. It is also known that patients with recovered EF have an improved prognosis after recovery[6,7,18]. However, it is not an entirely benign outlook. Despite experiencing improvement in their LVEF, these patients tend to have increased rates of all-cause hospitalizations and hospitalizations for HF[8]. Hence, HFimpEF should not be considered recovered. However, data is lacking about whether it is due to reworsening of the LVEF or whether there is a difference in the characteristics and clinical outcomes between those with HFtimpEF and those with HFsimpEF.
Our patients with HFsimpEF had favorable outcomes in terms of hospitalization rates and diagnosis of new cardio
In a recent study by McElderry et al[17], who recruited 7070 patients with HFimpEF, White race (OR = 1.31, 95%CI: 1.17-1.48) and continued use of renin-angiotensin-aldosterone system inhibitors or angiotensin receptor-neprilysin inhibitor (ARNi) (OR = 1.13, 95%CI: 1.03-1.25) were associated with increased odds of maintaining an improved LVEF. Male sex (OR = 0.84, 95%CI: 0.76-0.93), use of loop diuretics (OR = 0.79, 95%CI: 0.72-0.87), atrial fibrillation (OR = 0.85, 95%CI: 0.77-0.94), and history of myocardial infarction (OR = 0.76, 95%CI: 0.67-0.85) were correlated with a decline in LVEF over time in patients with HFimpEF[17].
Another study (n = 183) concluded that sustained improvement in LVEF was associated with a favorable long-term prognosis and reported the factors that were associated with the sustained improvement in patients with dilated cardio
To the best of our knowledge, only a few studies have investigated the factors associated with sustained improvement in LVEF despite the accumulating studies examining the characteristics of patients with HFimpEF[17]. Enzan et al[20] concluded that beta-blocker therapy prevented the deterioration of LVEF (i.e. decrease in LVEF ≥ 10.0%) in patients with recovered dilated cardiomyopathy (i.e. mean LVEF was 49.3 ± 8.2%) at the 2-year follow-up (OR = 0.77, 95%CI: 0.63-0.95; P = 0.013). Chen et al[21] prospectively determined the safety of withdrawing spironolactone in patients with idiopathic dilated cardiomyopathy with improved LVEF (i.e. mean 44.0%-47.0%). Withdrawing spironolactone increased the likelihood of relapse (i.e. reduction in LVEF < 10.0%) within 12 months (relative risk = 4.31, 95%CI: 1.67-11.11; P < 0.001).
Halliday et al[22] in their pilot study concluded that tapering medications gradually can lead to relapses in patients with recovered dilated cardiomyopathy. Additionally, recent research identified echocardiographic parameters, namely global longitudinal strain (GLS), that could predict sustained improvement in LVEF. Adamo et al[23] reported that a normal GLS was an indicator of maintaining a stable LVEF after its recovery and abnormal GLS was a predictor of a decrease in LVEF during follow-up. However, the authors defined a reduced LVEF as < 50.0%.
The results of our study should be interpreted in the context of the following potential limitations. Firstly, the retro
Despite these limitations, the key strength of our study was the inclusion of all patients from the largest tertiary care center for cardiovascular disease in Qatar, a country with a predominantly South East Asian and Middle Eastern/North African populations who are underrepresented in most HF studies. Other peripheral hospitals in Qatar share the same electronic medical records, hence the potential to miss a clinical outcome was reduced. Our study offers further baseline data for long-term longitudinal assessment of HFrEF patients with regular follow-up echocardiograms to prevent lack of recognition of HFtimpEF.
Further prospective studies are needed for the validation of the associated factors of improvement mentioned in this study to allow the creation of a risk stratification model and to identify patients at risk of reworsening LVEF to intensify their management strategy. In an era marked by extensive research demonstrating the benefits of ARNi and sodium-glucose cotransporter-2 in patients with HF, our study reported favorable outcomes despite the majority of patients not receiving these novel therapies. This highlights the need for future studies focusing on patients with improved LVEF who are treated with contemporary, guideline-directed HF therapies.
Patients with HFsimpEF experienced significantly lower rates of hospitalization and diagnosis of new cardiovascular conditions than patients with HFtimpEF. Sustained improvement in LVEF was positively associated with being female, having non-ischemic etiology of HF, and using an MRA.
| 1. | Roger VL. Epidemiology of Heart Failure: A Contemporary Perspective. Circ Res. 2021;128:1421-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 509] [Article Influence: 127.3] [Reference Citation Analysis (0)] |
| 2. | Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW; ACC/AHA Joint Committee Members. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895-e1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1289] [Cited by in RCA: 1209] [Article Influence: 403.0] [Reference Citation Analysis (0)] |
| 3. | Xu Y, Li W, Wan K, Liang Y, Jiang X, Wang J, Mui D, Li Y, Tang S, Guo J, Guo X, Liu X, Sun J, Zhang Q, Han Y, Chen Y. Myocardial Tissue Reverse Remodeling After Guideline-Directed Medical Therapy in Idiopathic Dilated Cardiomyopathy. Circ Heart Fail. 2021;14:e007944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Savarese G, Vedin O, D'Amario D, Uijl A, Dahlström U, Rosano G, Lam CSP, Lund LH. Prevalence and Prognostic Implications of Longitudinal Ejection Fraction Change in Heart Failure. JACC Heart Fail. 2019;7:306-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 154] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 5. | Su K, Li M, Wang L, Tian S, Su J, Gu J, Chen S. Clinical characteristics, predictors, and outcomes of heart failure with improved ejection fraction. Int J Cardiol. 2022;357:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 6. | Park CS, Park JJ, Mebazaa A, Oh IY, Park HA, Cho HJ, Lee HY, Kim KH, Yoo BS, Kang SM, Baek SH, Jeon ES, Kim JJ, Cho MC, Chae SC, Oh BH, Choi DJ. Characteristics, Outcomes, and Treatment of Heart Failure With Improved Ejection Fraction. J Am Heart Assoc. 2019;8:e011077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 7. | Gulati G, Udelson JE. Heart Failure With Improved Ejection Fraction: Is it Possible to Escape One's Past? JACC Heart Fail. 2018;6:725-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Basuray A, French B, Ky B, Vorovich E, Olt C, Sweitzer NK, Cappola TP, Fang JC. Heart failure with recovered ejection fraction: clinical description, biomarkers, and outcomes. Circulation. 2014;129:2380-2387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 256] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 9. | Hartupee J, Mann DL. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol. 2017;14:30-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 432] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 10. | He Y, Ling Y, Guo W, Li Q, Yu S, Huang H, Zhang R, Gong Z, Liu J, Mo L, Yi S, Lai D, Yao Y, Liu J, Chen J, Liu Y, Chen S. Prevalence and Prognosis of HFimpEF Developed From Patients With Heart Failure With Reduced Ejection Fraction: Systematic Review and Meta-Analysis. Front Cardiovasc Med. 2021;8:757596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Kaddoura R, Shah JZ, Ibrahim MIM, Singh R, Chapra A, Alsadi H, Amri MA, Hamamyh T, Fallouh M, Elasad F, Abdelghani MS, Alyafei SA, Badr A, Patel A. Characteristics and Outcomes of Heart Failure Outpatients with Improvement in Ejection Fraction in Qatar. Heart Views. 2024;25:117-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 12. | Oommen SG, Man RK, Talluri K, Nizam M, Kohir T, Aviles MA, Nino M, Jaisankar LG, Jaura J, Wannakuwatte RA, Tom L, Abraham J, Siddiqui HF. Heart Failure With Improved Ejection Fraction: Prevalence, Predictors, and Guideline-Directed Medical Therapy. Cureus. 2024;16:e61790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Chew DS, Heikki H, Schmidt G, Kavanagh KM, Dommasch M, Bloch Thomsen PE, Sinnecker D, Raatikainen P, Exner DV. Change in Left Ventricular Ejection Fraction Following First Myocardial Infarction and Outcome. JACC Clin Electrophysiol. 2018;4:672-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | de Groote P, Fertin M, Duva Pentiah A, Goéminne C, Lamblin N, Bauters C. Long-term functional and clinical follow-up of patients with heart failure with recovered left ventricular ejection fraction after β-blocker therapy. Circ Heart Fail. 2014;7:434-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Blechman I, Arad M, Nussbaum T, Goldenberg I, Freimark D. Predictors and outcome of sustained improvement in left ventricular function in dilated cardiomyopathy. Clin Cardiol. 2014;37:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Nabeta T, Inomata T, Ishii S, Yazaki M, Fujita T, Iida Y, Ikeda Y, Maekawa E, Naruke T, Koitabashi T, Ako J. Dilated cardiomyopathy with re-worsening left ventricular ejection fraction. Heart Vessels. 2019;34:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | McElderry B, O'Neill T, Griffin BP, Kalahasti V, Barzilai B, Brateanu A. Factors Associated With Maintenance of an Improved Ejection Fraction: An Echocardiogram-Based Registry Study. J Am Heart Assoc. 2023;12:e031093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 18. | Jørgensen ME, Andersson C, Vasan RS, Køber L, Abdulla J. Characteristics and prognosis of heart failure with improved compared with persistently reduced ejection fraction: A systematic review and meta-analyses. Eur J Prev Cardiol. 2018;25:366-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Park JJ, Mebazaa A, Hwang IC, Park JB, Park JH, Cho GY. Phenotyping Heart Failure According to the Longitudinal Ejection Fraction Change: Myocardial Strain, Predictors, and Outcomes. J Am Heart Assoc. 2020;9:e015009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Enzan N, Matsushima S, Ide T, Kaku H, Tohyama T, Funakoshi K, Higo T, Tsutsui H. Beta-Blocker Use Is Associated With Prevention of Left Ventricular Remodeling in Recovered Dilated Cardiomyopathy. J Am Heart Assoc. 2021;10:e019240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Chen Y, Qiu Z, Jiang J, Su X, Huang F, Tang J, Jin W. Outcomes of Spironolactone Withdrawal in Dilated Cardiomyopathy With Improved Ejection Fraction. Front Cardiovasc Med. 2021;8:725399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 22. | Halliday BP, Wassall R, Lota AS, Khalique Z, Gregson J, Newsome S, Jackson R, Rahneva T, Wage R, Smith G, Venneri L, Tayal U, Auger D, Midwinter W, Whiffin N, Rajani R, Dungu JN, Pantazis A, Cook SA, Ware JS, Baksi AJ, Pennell DJ, Rosen SD, Cowie MR, Cleland JGF, Prasad SK. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): an open-label, pilot, randomised trial. Lancet. 2019;393:61-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 419] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 23. | Adamo L, Perry A, Novak E, Makan M, Lindman BR, Mann DL. Abnormal Global Longitudinal Strain Predicts Future Deterioration of Left Ventricular Function in Heart Failure Patients With a Recovered Left Ventricular Ejection Fraction. Circ Heart Fail. 2017;10:e003788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Evangelista LS, Doering LV, Dracup K. Usefulness of a history of tobacco and alcohol use in predicting multiple heart failure readmissions among veterans. Am J Cardiol. 2000;86:1339-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |