Published online Aug 27, 2025. doi: 10.4240/wjgs.v17.i8.105112
Revised: March 24, 2025
Accepted: July 10, 2025
Published online: August 27, 2025
Processing time: 194 Days and 4.7 Hours
Acute pancreatitis (AP) is a potentially life-threatening complication of pancrea
To retrospectively assess the prognostic value of preoperative IL-17a levels in predicting AP and related POPF following pancreaticoduodenectomy.
Retrospective analysis of pancreaticoduodenectomies performed on patients 150 patients between 2017 and 2023. Clinical data including pre-operative IL-17a levels were collected. The primary composite outcomes were postoperative AP and postoperative pancreatic (PP), and the predictive performances of IL-17a levels and fluid load status for postoperative complications were evaluated by statistical analysis.
A total of 150 patients were included, and 26 patients (17.3%) developed postope
Preoperative IL-17a levels and intravascular volume status may serve as useful predictors of AP and subsequent PP following PD. These parameters provide means to evaluate preoperative risk and may guide clinical decision making to enhance postoperative recovery.
Core Tip: Preoperative interleukin-17A (IL-17a) levels independently predict post pancreatectomy acute pancreatitis and related postoperative pancreatic fistula after pancreaticoduodenectomy. Elevated IL-17a reflects inflammatory status, aiding risk stratification and guiding preoperative optimization and postoperative monitoring. Combining IL-17a with fluid management improves predictive accuracy, offering a practical tool for reducing complications.
- Citation: Zheng J, Ye WK, Wang J, Zhou YN, Yu TT. Preoperative interleukin-17a as a predictor of acute pancreatitis after pancreaticoduodenectomy. World J Gastrointest Surg 2025; 17(8): 105112
- URL: https://www.wjgnet.com/1948-9366/full/v17/i8/105112.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v17.i8.105112
The standard surgical treatment for tumors of the pancreatic head, lower bile duct, and ampulla is pancreaticoduodenectomy, but anatomical nuances and the surgical difficulty for this procedure result in a high rate of postoperative complications[1,2]. However, postoperative acute pancreatitis (PAP) is a more severe complication that can aggravate the patient's condition, prolong hospital stays, rise medical costs, and even threaten life[3,4]. In addition, postoperative pancreatic fistula (POPF), which has an incidence of as high as 20%, is one of the most frequent complications, leading to increased rates of infection, reoperation, and mortality[5,6]. POPF is related to multiple influencing factors, including pre-existing inflammatory status, surgical methods, and basic diseases of patients. The pathophysiological changes in POPF predominantly include exocrine abnormalities. Although pancreatic fluid is similar to interstitial fluid, excessive loss of pancreatic fluid may result in electrolyte imbalances and acid-base disorders, which may lead to hypoproteinemia. In addition, poor drainage around an external pancreatic fistula may cause the surrounding skin to become congested (swollen), eroded (damaged), ulcerated, or bleed; it may also result in pseudo-cysts or infections. Secondary infections can lead to purulent peritonitis, further systemic infection and local abscess formation[7,8]. PAP is not only responsible for patient suffering and economic burden but also prolonging hospitalization and delaying postoperative recovery, as well as possibly increasing mortality risk. Also, postoperative challenges like pancreatic and biliary leaks are significant problems after pancreaticoduodenectomy.
The exact mechanism behind the development of PAP is not fully understood, although there are suggestions that pancreatic injury caused by surgery, inflammatory response, and obstruction in drainage of pancreatic fluid may be responsible. The incidence of PAP has been observed to correlate with multiple factors such as pre-existing inflammatory states, surgical techniques, and underlying patient factors[9,10]. Two previous studies found that preoperative biliary obstruction, diabetes, hypoproteinemia, and anemia, etc. could be related to postoperative bile leak, Masterson has demonstrated the interaction of these risk factors, including radiological confirmation of hepatic bile duct injury prior to resection, so the timing of surgery and intraoperative management is crucial to preventing postoperative bile leak. However, in light of a clearer understanding of PAP, the International Study Group for Pancreatic Surgery (ISGPS) has recently called for standardized definitions and grading of PAP and the assessment of PAP as an independent complica
This retrospective study aimed to evaluate the potential role of preoperative IL-17a in the prediction of PAP and POPF in patients undergoing pancreaticoduodenectomy. A better understanding of how these factors are associated with postoperative complications may allow clinicians to risk stratify better, mitigate intraoperative fluid management stra
Between January 2021 and December 2023, 150 patients aged ≥ 18 years who received a surgical operation of pancreaticoduodenectomy were included in this study. Post pancreatectomy acute pancreatitis (PPAP) was defined according to the 2022 criteria proposed by ISGPS as serum amylase levels above the upper limit of normal (> 100 U/L) on post
The Whipple procedure was performed using pancreatojejunostomy with duct-to-mucosa double-layer anastomosis. An oblique incision below the right costal margin was used for abdominal exploration. The second part of the duodenum and the pancreatic head were mobilized anteriorly from the retroperitoneum, with both duodenum and pancreatic head being freed. Changes in the pancreas and its relationship to the mass were examined to assess tumor resectability. Gastrectomy was performed following Hoffmeister's method, and lymphatic-fatty tissue around the arteries was separated to remove the pancreas and duodenum with the tumor. After resection, digestive tract reconstruction included choledochojejunostomy, pancreatojejunostomy, and gastrojejunostomy. Drains were placed in the abdominal cavity for postoperative fluid drainage and monitoring. Lymph node dissection was performed to reduce tumor recurrence risk. Postoperatively, antibiotics and somatostatin analogs were routinely administered. Routine CT scans were conducted on postoperative days 3 and 7 to assess recovery, with urgent scans for patients suspected of having severe complications[15-17].
A retrospective analysis examined patient characteristics including demographics [age, sex, body mass index (BMI)], ASA classification, albumin values, and jaundice indicators (total bilirubin and biliary status before surgery). The study also evaluated preoperative inflammatory markers such as WBC count, NE levels, CRP, and various cytokines. T-cell subset analysis and cytokine measurement employed enzyme-linked immunosorbent assay techniques. The cytokine panel consisted of seven markers: Interleukins 2, 4, 6, 10, and 17A, along with IFN-γ and tumor necrosis factor-α (TNF-α). All inflammatory parameters were obtained from initial assessments conducted within 3 days prior to surgical intervention. Certain patients underwent preoperative optimization, such as management of severe jaundice, to ensure surgical fitness. Surgical data collection encompassed main pancreatic duct (MPD) diameter, pancreatic consistency, operative approach (open pancreaticoduodenectomy vs robotic pancreaticoduodenectomy), vein resection necessity, procedure duration, blood loss estimation, tumor dimensions, and pathological findings. The fistula risk score (FRS) was subsequently calculated to facilitate further statistical evaluation.
According to ISGPS criteria, diagnoses were established for POPF, and delayed gastric emptying. Chylous leakage was also identified using these standards. For bile leakage, diagnostic confirmation relied on endoscopic or radiological assessment of drainage fluid abnormalities. Intra-abdominal infection was recognized through sustained fever with culture-positive drainage samples or imaging evidence. The management approach included post-surgical drainage procedures performed under radiological or endoscopic guidance. Data collection encompassed surgical re-exploration incidence, length of hospitalization following surgery, and mortality within 90 days as the perioperative mortality indicator. The Clavien-Dindo classification system categorized postoperative complications, with grades III-V designated as significant complications.
We conducted a subgroup analysis for risk factors of POPF in PPAP and non-PPAP groups. For continuous outcomes such as mechanical ventilation time or infusion of inflammatory markers, Analysis of Variance (ANOVA), assuming data normality and homoscedasticity, was used to evaluate the influence of categorical independent variables. Post hoc tests following ANOVA were used to investigate differences between individual group means when an overall ANOVA (Type III sum of squares) indicated an overall significant effect. Our findings were obtained from a robust healthcare dataset and highlighted critical risk factors for PPAP and other complications following PD, which can be invaluable as a guide for subsequent research and advances in clinical practice.
This study compared patients who received PPAP (n = 45) and those who did not receive PPAP (n = 105). The non-PPAP group had higher rates of soft pancreatic texture (61.0% vs 34.0%, P < 0.001) and MPD ≤ 3 mm (81.0% vs 60.0%, P = 0.012), as well as higher FRS risk area (P < 0.001). Regarding complications, the non-PPAP group performed significantly worse, with higher rates of POPF (55.6% vs 7.4%, P < 0.001), bile leak (9.1% vs 2.1%, P = 0.006), intra-abdominal infection (26.7% vs 9.5%, P = 0.001), major complications (17.2% vs 3.8%, P = 0.001), need for interventional drainage (12.2% vs 1.4%, P < 0.001), and reoperation (5.6% vs 0.7%, P = 0.021). Additionally, the non-PPAP group had significantly longer postope
Overall, despite the PPAP group having higher bilirubin levels and more frequent pancreatic ductal adenocarcinoma (PDAC) or chronic pancreatitis (CP), the application of PPAP significantly reduced the incidence of postoperative compli
| Variables | No PPAP (n = 105) | PPAP (n = 45) | P value |
| Age [median (IQR)], years | 63 (55-70) | 63 (56.5–70) | 0.549 |
| Sex, n (%), male | 230 (59.7%) | 42 (51.2%) | 0.155 |
| Total bilirubin level (μmol/L) | 12.0 (7.8-20.0) | 19.5 (12.6-35.0) | 0.04 |
| Incidence of soft pancreatic texture (%) | 61.0 | 34.0 | < 0.001 |
| Incidence of MPD ≤ 3 mm (%) | 81.0 | 60.0 | 0.012 |
| Incidence of PDAC or CP (%) | 26.7 | 58.7 | 0.001 |
| FRS risk area | Higher | Lower | < 0.001 |
| Incidence of POPF (%) | 55.6 | 7.4 | < 0.001 |
| Incidence of bile leak (%) | 9.1 | 2.1 | 0.006 |
| Incidence of intra-abdominal infection (%) | 26.7 | 9.5 | 0.001 |
| Incidence of major complications (%) | 17A.2 | 3.8 | 0.001 |
| Incidence of interventional drainage (%) | 12.2 | 1.4 | < 0.001 |
| Incidence of reoperation (%) | 5.6 | 0.7 | 0.021 |
| Postoperative hospital stay (days) | 28 (21-35) | 14 (10-20) | < 0.001 |
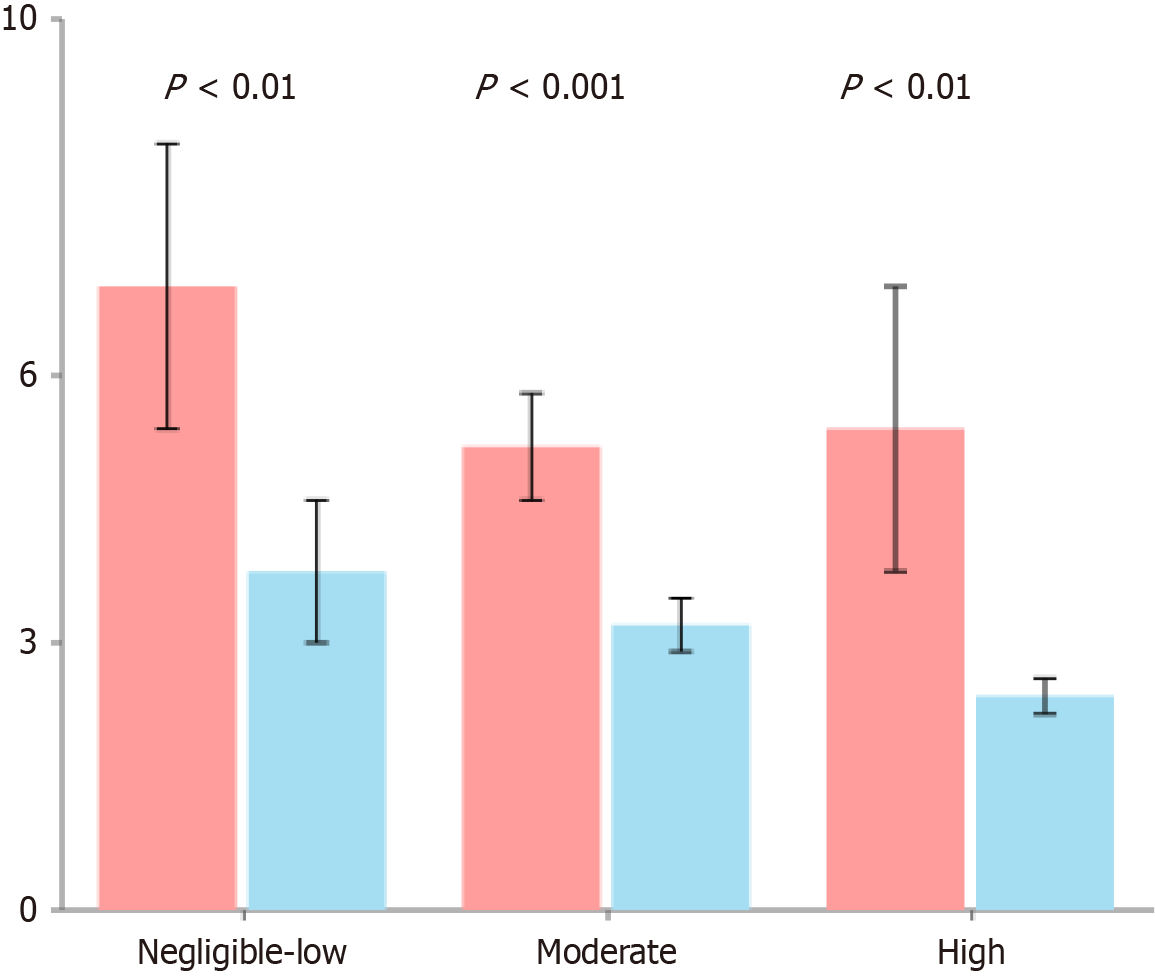
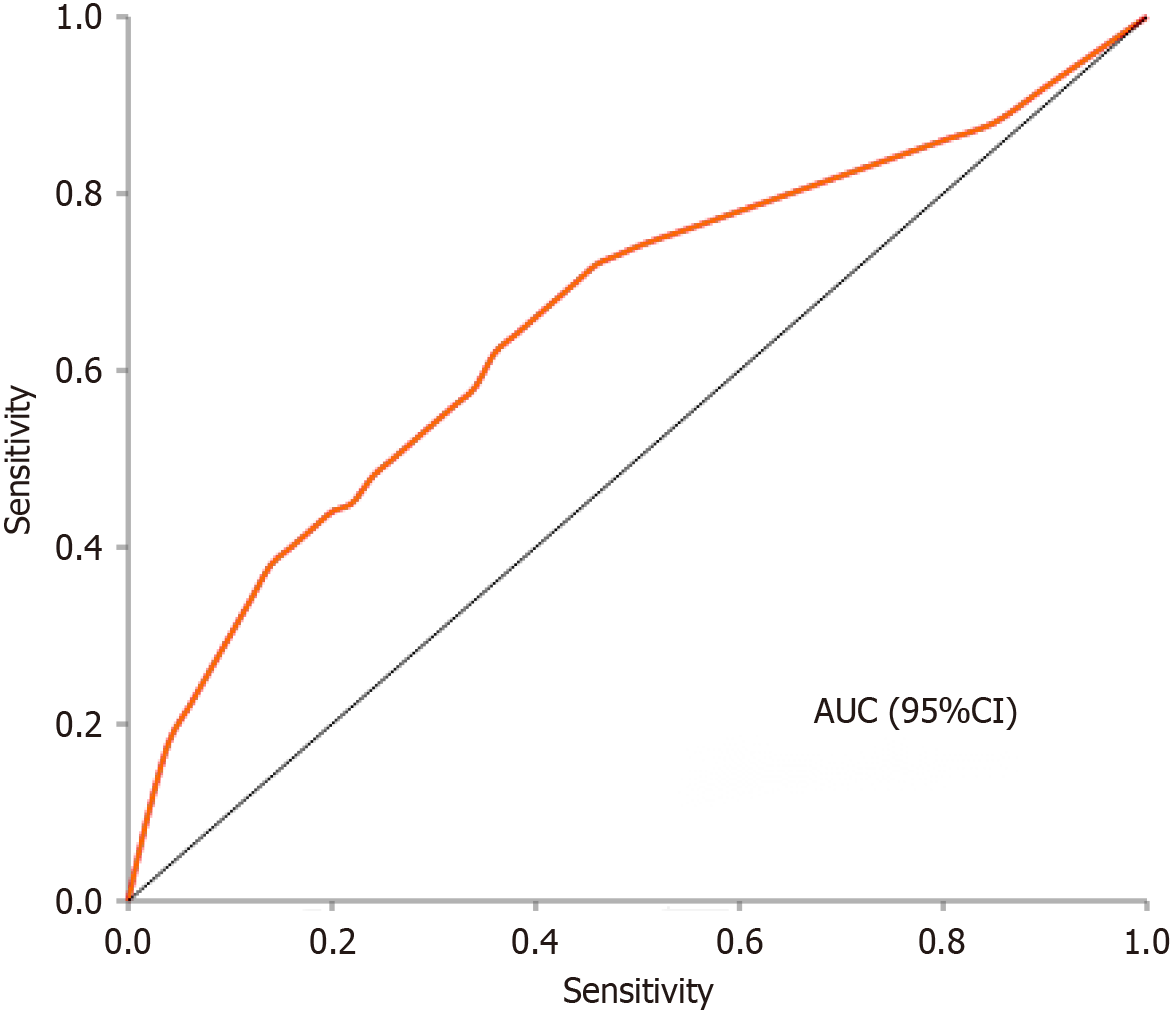
This analysis compared the immune and inflammatory markers between patients who received PPAP (n = 45) and those who did not receive PPAP (n = 105). The results showed no significant differences between the two groups in most inflammatory markers, including CRP (2.12 vs 2.11 mg/L, P = 0.214), IFN-α (0.2 vs 0.2 pg/mL, P = 0.472), IFN-γ (3.4 vs 3.6 pg/mL, P = 0.578), IL-2 (0.2 vs 0.2 pg/mL, P = 0.467), IL-4 (0.2 vs 0.2 pg/mL, P = 0.401), and IL-5 (0.2 vs 0.2 pg/mL, P = 0.469). WBC also showed no statistical difference (5.96 vs 5.66 × 109/L, P = 0.070). However, there was a significant difference in IL-17a levels between the groups, with the PPAP group showing markedly lower levels than the non-PPAP group (3.5 vs 12.5 pg/mL, P < 0.001). Additionally, the PPAP group had significantly higher daily fluid load compared to the non-PPAP group (800 vs 600 mL/day, P < 0.001). These findings suggest that while the two groups were similar in most inflammatory markers, the PPAP intervention was associated with significantly reduced levels of IL-17a (a pro-inflammatory cytokine) along with higher fluid load. This may be related to the previously observed lower incidence of complications in the PPAP group, indicating that PPAP might work by modulating specific immune responses and optimizing fluid management (Table 2). Conversely, interleukin-6 (IL-6) levels were higher in all PPAP groups compared to non-PPAP groups (P < 0.01), consistent across each FRS risk area (Figure 1). Further receiver operating characteristic (ROC) analysis (Figure 2) demonstrated that preoperative IL-17a and fluid load have predictive value for PPAP (Table 3).
| Variables | No PPAP (n = 105) | PPAP (n = 45) | P value |
| CRP [median (IQR)], mg/L | 2.11 (1.12–7.92) | 2.12 (1.13–5.24) | 0.214 |
| IFN-α [median (IQR)], pg/mL | 0.2 (0.2–0.2) | 0.2 (0.2–0.2) | 0.472 |
| IFN-γ [median (IQR)], pg/mL | 3.6 (0.1–6.9) | 3.4 (0.1–6.2) | 0.578 |
| IL-2 [median (IQR)], pg/mL | 0.2 (0.2–0.2) | 0.2 (0.2–0.2) | 0.467 |
| IL-4 [median (IQR)], pg/mL | 0.2 (0.2–0.2) | 0.2 (0.2–0.2) | 0.401 |
| IL-5 [median (IQR)], pg/mL | 0.2 (0.4–3.6) | 0.2 (0.1–3.6) | 0.469 |
| IL-17a [median (IQR)], pg/mL | 12.5 (5.8–28.7) | 3.5 (1.5–8.0) | < 0.001 |
| WBC [median (IQR)], × 109/L | 5.66 (4.64–6.67) | 5.96 (5.06–7.42) | 0.070 |
| Fluid load [median (IQR)], mL/day | 600 (500-700) | 800(700-900) | < 0.001 |
| Variables | IL-17a ≥ 4.5 pg/mL (n = 65) | IL-17a < 4.5 pg/mL (n = 84) | P value |
| CRP > 5 mg/L | 43.5 | 30.0 | 0.053 |
| CRP [median (IQR)], mg/L | 3.50(1.50-10.5) | 1.75 (1.00-4.5) | < 0.001 |
| WBC > 10 × 109/L | 10.0 | 12.0 | 0.765 |
| WBC [median (IQR)], × 109/L | 6.20 (5.10-7.80) | 5.30 (4.40-6.20) | 0.002 |
| NE > 7 × 109/L | 5.0 | 6.0 | 0.924 |
| NE [median (IQR)], × 109/L | 4.00 (3.20-5.10) | 3.20 (2.60-4.10) | 0.002 |
| PPAP | 31.3 | 7.5 | < 0.001 |
| POPF | 29.8 | 12.9 | < 0.001 |
| Biliary leak | 4 (6.5) | 3 (4.5) | 0.678 |
| PPH | 10 (15.0) | 12 (18.0) | 0.543 |
| Intra-abdominal infections | 18 (27.0) | 20 (30.0) | 0.789 |
| DGE | 6 (9.0) | 8 (12.0) | 0.654 |
| Chyle leak | 2 (3.0) | 4 (6.0) | 0.567 |
| Interventional drains | 12 (18.0) | 15 (22.5) | 0.456 |
| Major complications | 25 (37.5) | 28 (42.0) | 0.784 |
| Relaparotomy | 8 (12.0) | 10 (15.0) | 0.679 |
| 90-day mortality | 3 (4.5) | 5 (7.5) | 0.564 |
| Postoperative hospital stays, days | 18 (15-22) | 20 (17-25) | 0.453 |
Elevated preoperative IL-17a levels (≥ 4.5 pg/mL) were the strongest independent risk factor, with an odds ratio of 3.85 in univariate analysis (P < 0.001) and an even higher odds ratio of 4.67 in multivariate analysis (P < 0.001). Pathology other than PDAC or CP was also significant, with an odds ratio of 2.08 in univariate analysis (P = 0.004) and 1.89 in multivariate analysis (P = 0.025). All other factors, including WBC, NE count, age, sex, BMI, ASA score, bilirubin levels, venous resection, surgical procedure type, operative time, and estimated blood loss did not reach statistical significance as inde
| Risk factors | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| IL-17a ≥ 4.5 pg/mL | 3.85 (2.14-6.91) | < 0.001 | 4.67 (2.49-8.72) | < 0.001 |
| WBC > 10 × 109/L | 1.86 (0.64-5.36) | 0.252 | ||
| NE > 7 × 109/L | 2.12 (0.82-5.12) | 0.148 | ||
| Age, years | 1.11 (0.99-1.02) | 0.382 | ||
| Sex, male vs female | 0.70 (0.43-1.13) | 0.168 | ||
| BMI, kg/m2 | 1.06 (0.97-1.03) | 0.194 | ||
| ASA ≥ III | 0.75 (0.45-1.19) | 0.268 | ||
| Total bilirubin ≥ 24 μmol/L | 0.64 (0.38-1.04) | 0.068 | ||
| Venous resection, yes vs no | 1.34 (0.74-2.36) | 0.384 | ||
| Surgical procedure, RPD vs OPD | 0.96 (0.55-1.69) | 0.889 | ||
| Operative time > 300 minutes | 1.36 (0.84-2.26) | 0.265 | ||
| Estimated blood loss > 200 mL | 1.38 (0.86-2.22) | 0.247 | ||
| Pathology (not PDAC/CP) | 2.08 (1.26-3.44) | 0.004 | 1.89 (1.08-3.30) | 0.025 |
IL-17a levels (pg/mL) were identified as a significant risk factor in both univariate and multivariate analyses. In uni
Albumin levels below 35 g/L showed significance in univariate analysis (OR: 2.56, 95%CI: 1.37-4.77, P = 0.002) but were not included in the final multivariate model. Similarly, estimated blood loss > 200 mL (OR: 2.78, 95%CI: 1.51-5.12, P = 0.001) and surgical procedure type (OR: 2.08, 95%CI: 1.26-3.44, P = 0.004) were significant in univariate analysis but did not remain independent predictors in multivariate analysis. Other factors including CRP, IFN-α, IFN-γ, IL-2, age, sex, BMI, pancreatic biliary drainage, pancreatic texture, MPD diameter, venous resection, and operative time showed no significant association with the outcome (Table 5).
| Risk factors | Univariate analysis | Multivariate analysis | ||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| CRP, mg/L | 0.85 (0.72-1.34) | 0.17A9 | ||
| IFN-α, pg/mL | 3.84(2.25-6.84) | 0.164 | ||
| IFN-γ, pg/mL | 1.86 (0.64-5.36) | 0.252 | ||
| IL-2, pg/mL | 2.12 (0.82-5.12) | 0.148 | ||
| IL-17a, pg/mL | 1.24 (1.12-1.36) | < 0.001 | 1.12 (1.11-1.16) | < 0.001 |
| Age, years | 0.75 (0.45-1.19) | 0.268 | ||
| Sex, male vs female | 0.64 (0.38-1.04) | 0.068 | ||
| BMI, kg/m2 | 1.06 (0.58-2.17) | 0.896 | ||
| Albumin < 35 g/L | 2.56 (1.37-4.77) | 0.002 | ||
| PBD, yes vs no | 1.34 (0.74-2.36) | 0.384 | ||
| Pancreatic texture, soft vs firm | 0.96 (0.55-1.69) | 0.889 | ||
| MPD ≤ 3 mm | 1.36 (0.84-2.26) | 0.265 | ||
| Venous resection, yes vs no | 1.38 (0.86-2.22) | 0.247 | ||
| Surgical procedure, RPD vs OPD | 2.08 (1.26-3.44) | 0.004 | ||
| Operative time > 300 minutes | 1.19 (0.65-1.86) | 0.782 | ||
| Estimated blood loss > 200 mL | 2.78 (1.51-5.12) | 0.001 | ||
PPAP is significantly and independently correlated with individual postoperative complications. The negative implications of PPAP not only aggravate the patient suffering and the economic burden, but also can lead to longer hospitalization, delayed postoperative recovery, and increased risk of death[11,18,19]. PPAP pathogenesis is complex, being attributable to factors such as pancreatic damage during surgical procedure, inflammatory responses, and occlusion obstruction of pancreatic juice outflow. In the treatment of PPAP, the principle of early fluid resuscitation is currently advocated to do the "targeted therapy" strategy, pay attention to the ratio of crystalloid to colloid for fluid infusion, and control the infusion speed. Antimicrobial drugs and body fluid bacterial culture also play a critical role in prevention of drug-resistant bacteria. Serum amylase measurements on days 1-2 are essential for diagnosing PAP according to the 2022 ISGPS criteria mentioned in the text. Without these values, researchers couldn't reliably determine if patients developed PAP. CT scans within 10 days are needed to identify radiological changes associated with PAP, which is another com
POPF is one of the most common complications after pancreatoduodenectomy, the incidence rate can be up to 20%-60% and even the mortality rate can be as high as 45%. POPF is associated with multiple parameters, such as the preoperative inflammatory state, the type of surgical techniques, and the severity of the patient's underlying diseases. It is important to correctly manage POPF by treating anaemia and optimising nutritional status pre-operatively. Selecting pancreatic gastrointestinal reconstruction during operation plays a great role in preventing POPF, and the surgeon can choose the most reliable method of pancreatic anastomosis based on personal experience and the texture of pancreas. Somatostatin drugs and POPF prevention Somatostatin drugs are effective POPF prevention methods following surgical procedures[20-22].
This study was conducted to assess the values of preoperative 17a levels and fluid load for predicting PPAP and its associated POPF after pancreatoduodenectomy. Analysis of data from 150 patients revealed that high preoperative 17a levels were significantly correlated with the development of PPAP, and this factor was found to be independent risk factor for PPAP. Furthermore, we found a significant close correlation between the increased preoperative levels of 17a and the incidence of POPF due to PPAP.
17A serves as an IFN-γ inducing factor that influences both Th1 and Th2 immune responses while activating NK cells and macrophages. Its impact extends across various pathological conditions, including inflammatory diseases and cancer. In the context of pancreatic pathologies specifically, 17A plays a significant role in acute and chronic pancreatitis as well as pancreatic cancer.
Research has demonstrated that elevated serum 17A levels in acute pancreatitis (AP) patients correlate significantly with both APACHE II scores at admission and overall prognosis. The positive statistical correlation between serum 17A concentration and APACHE II scores in these patients indicates that 17A actively participates in the inflammatory cascade during AP, making it a potential predictor of disease severity.
17A functions in concert with other inflammatory mediators in AP, particularly TNF-α and IL-6. These factors collectively drive the pathogenesis of AP, from initial local immune responses to systemic inflammatory response syn
The established relationship between 17A and AP severity suggests its potential as a novel biomarker for monitoring and predicting disease progression. This discovery opens new therapeutic possibilities, including strategies to inhibit 17A levels to counteract pancreatic acinar cell autophagy, reduce proenzyme activation, and minimize pancreatic tissue edema, hemorrhage, and necrosis during AP episodes[23-25]. In terms of nutritional support, differentiated regimens can be developed based on IL-17a levels, providing enhanced immune nutrition support for patients with high levels, including nutrients with immunomodulatory effects such as ω-3 fatty acids, arginine, and glutamine. Additionally, appropriately timing the surgery preoperatively to ensure inflammatory markers return to relatively low levels before proceeding with the operation is also a potential optimization strategy.
Our findings show that patients with high preoperative 17a levels have a greater risk of PPAP, and this association is stronger in patients with POPF owing to the PPAP,” the authors wrote. 17a, as a pro-inflammatory cytokine, has been implicated in inflammatory responses and immune regulation. Elevated 17a levels may reflect the inflammatory status of the patients before surgery, which may aggravating pancreatic damage after surgery, thus increasing the risk of PPAP and POPF. This result is in line with past studies that preoperative inflammatory markers are linked with a higher like
Moreover, preoperative 17a levels can be a strong predictive factor for identifying patients at risk of PPAP and the POPF that could follow in the postoperative period. ROC analysis demonstrated a good predictive efficacy of 17a for PPAP with an area under the curve of 0.75. Such a finding would indicate that the preoperative determination of 17a may help clinicians evaluate the risk of patients prior to surgical intervention and allow for proper prophylactic measures.
Considering the relationship between preoperative inflammatory status and the elevated risk of PPAP and POPF, managing preoperative inflammation plays a crucial role. Preoperative correction of anemia, as well as improvement of nutritional status, may significantly reduce the occurrence of POPF in patients. Furthermore, in patients with elevated 17a levels, more intensive monitoring and treatment strategies postoperatively may be necessary to decrease the risk of postoperative complications.
The limitations of our study are its retrospective design and the relatively small sample size, which can limit the generalization and extrapolation of results. Prospective, multicenter, large-sample studies should be considered in future studies to further confirm the predictive value of 17a in PPAP and POPF. Additionally, future studies should investigate 17a levels in conjunction with other inflammatory markers to evaluate their joint predictive capacity across different patient populations.
A major limitation of this study is the focus solely on IL-17a without fully utilizing the data collected on other inflammatory markers for multivariate analysis. Cytokines such as IL-6 and TNF-α play different but complementary roles in pancreatic inflammatory responses, and assessing IL-17a alone may not capture the complexity of the inflammatory network. Furthermore, the dynamic changes of inflammatory markers may have greater predictive value than measure
In conclusion, our findings reveal that elevated preoperative 17A levels serve as a significant predictor for both PPAP and PPAP-related POPF. This discovery has substantial clinical implications for perioperative care. Integrating 17A level assessment into preoperative risk stratification protocols could identify high-risk patients who might benefit from modified surgical approaches or enhanced postoperative monitoring.
| 1. | González-Abós C, Lorenzo C, Rey S, Salgado F, Ausania F. High-Risk Biliary Anastomosis During Robotic Pancreaticoduodenectomy: Initial Experience with Biodegradable Biliary Stent. Medicina (Kaunas). 2024;60:1798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 2. | Li D, Wang S, Zhang H, Cao Y, Chu Q. Impact of overweight on patients undergoing laparoscopic pancreaticoduodenectomy: analysis of surgical outcomes in a high-volume center. BMC Surg. 2024;24:372. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Gajda M, Grudzińska E, Szmigiel P, Czopek P, Rusinowski C, Putowski Z, Mrowiec S. Risk Factors of Postoperative Acute Pancreatitis and Its Impact on the Postoperative Course after Pancreaticoduodenectomy-10 Years of Single-Center Experience. Life (Basel). 2023;13:2344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Wu Z, Zong K, Zhou B, Yin K, Zhang A, Li M. Incidence and risk factors of postoperative acute pancreatitis after pancreaticoduodenectomy: a systematic review and meta-analysis. Front Surg. 2023;10:1150053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Jiang K, Chen H, Wang J, Zhou S, Qiu K, Wang H. Laparoscopic distal pancreatectomy with pancreatic remnant-gastric coverage: a modified technique to reduce postoperative pancreatic fistula. Surg Endosc. 2025;39:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Zhong YQ, Zhu XX, Huang XT, Luo YJ, Huang CS, Xu QC, Yin XY. Prediction of clinically relevant postoperative pancreatic fistula after pancreatoduodenectomy based on multifrequency magnetic resonance elastography. J Gastrointest Surg. 2025;29:101886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Doussot B, Doussot A, Ayav A, Santucci N, Deguelte S, Sow AK, El Amrani M, Duvillard L, Piessen G, Girard E, Mabrut JY, Garnier J, Ortega-Deballon P, Fournel I, Facy O. Diagnostic Accuracy of Lipase as Early Predictor of Postoperative Pancreatic Fistula: Results from the LIPADRAIN study. Ann Surg Open. 2024;5:e492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Ji Y, Chen H, Xu Z, Zhou Y, Fu N, Li H, Zhai S, Deng X, Shen B. The proinflammatory status, based on preoperative interleukin-6, predicts postpancreatectomy acute pancreatitis and associated postoperative pancreatic fistula after pancreaticoduodenectomy. J Gastroenterol Hepatol. 2025;40:315-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Hu Y, Dong Y, Yang Z, Qi J, Zhang X, Hou G, Lv Y, Tian Y. Incidence, clinical features, and risk factors for acute pancreatitis following posterior instrumented fusion surgery for lumbar degenerative disease: a single-center, retrospective analysis of 20,929 patients. Eur Spine J. 2023;32:3218-3229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Kandhala S, Kumar N, Goswami AG, Rai A, Mallik D, Chauhan U, Basu S. Severe acute pancreatitis in the early postoperative period due to afferent loop syndrome following gastrectomy for gastric cancer. Ann R Coll Surg Engl. 2022;104:e252-e254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Quero G, Fiorillo C, Massimiani G, Lucinato C, Menghi R, Longo F, Laterza V, Schena CA, De Sio D, Rosa F, Papa V, Tortorelli AP, Tondolo V, Alfieri S. The Impact of Post-Pancreatectomy Acute Pancreatitis (PPAP) on Long-Term Outcomes after Pancreaticoduodenectomy: A Single-Center Propensity-Score-Matched Analysis According to the International Study Group of Pancreatic Surgery (ISGPS) Definition. Cancers (Basel). 2023;15:2691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 12. | Theijse RT, Stoop TF, Hendriks TE, Suurmeijer JA, Smits FJ, Bonsing BA, Lips DJ, Manusama E, van der Harst E, Patijn GA, Wijsman JH, Meerdink M, den Dulk M, van Dam R, Stommel MWJ, van Laarhoven K, de Wilde RF, Festen S, Draaisma WA, Bosscha K, van Eijck CHJ, Busch OR, Molenaar IQ, Groot Koerkamp B, van Santvoort HC, Besselink MG; Dutch Pancreatic Cancer Group. Nationwide Outcome after Pancreatoduodenectomy in Patients at very High Risk (ISGPS-D) for Postoperative Pancreatic Fistula. Ann Surg. 2023;281:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Zhou P, Yu J, Yan B. The serum IL-17A levels in patients with traumatic bowel rupture post-surgery and its predictive value for patient prognosis. Open Med (Wars). 2025;20:20241135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Razooqi OA, Ghazi HF, Khudair MS. Evaluation of serum IL 18 / IL 18 binding protein ratio and their relation with IL- 18 gene polymorphisms in sample of Iraqi type 2 diabetes mellitus patients. A case control study. J Pak Med Assoc. 2024;74:S181-S185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Balzano G, Zerbi A, Aleotti F, Capretti G, Melzi R, Pecorelli N, Mercalli A, Nano R, Magistretti P, Gavazzi F, De Cobelli F, Poretti D, Scavini M, Molinari C, Partelli S, Crippa S, Maffi P, Falconi M, Piemonti L. Total Pancreatectomy With Islet Autotransplantation as an Alternative to High-risk Pancreatojejunostomy After Pancreaticoduodenectomy: A Prospective Randomized Trial. Ann Surg. 2023;277:894-903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 16. | Dubois E, Geelen R. An unusual case of high gastrointestinal bleeding after Whipple surgery. Acta Gastroenterol Belg. 2024;87:430-432. [PubMed] [DOI] [Full Text] |
| 17. | Martinez-Cabrera C, Martinez-Esteban A, Barron-Cervantes NM, Bandin-Musa A, Chan C. Delayed Gastric Emptying and Other Adverse Outcomes in Patients Undergoing Classic Whipple Versus Pylorus-Sparing Pancreatoduodenectomy. Cureus. 2024;16:e69406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Keita-Perse O, Bruyère F, Goux CL, Slim K. Picture of Peri-Operative Antisepsis Practices (PPAP Survey) in France. Surg Infect (Larchmt). 2023;24:468-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Labrousse G, Vande Perre P, Parra G, Jaffrelot M, Leroy L, Chibon F, Escudie F, Selves J, Hoffmann JS, Guimbaud R, Lutzmann M. The hereditary N363K POLE exonuclease mutant extends PPAP tumor spectrum to glioblastomas by causing DNA damage and aneuploidy in addition to increased mismatch mutagenicity. NAR Cancer. 2023;5:zcad011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Aoyama Y, Matsunobu Y, Etoh T, Suzuki K, Fujita S, Aiba T, Fujishima H, Empuku S, Kono Y, Endo Y, Ueda Y, Shiroshita H, Kamiyama T, Sugita T, Morishima K, Ebe K, Tokuyasu T, Inomata M. Artificial intelligence for surgical safety during laparoscopic gastrectomy for gastric cancer: Indication of anatomical landmarks related to postoperative pancreatic fistula using deep learning. Surg Endosc. 2024;38:5601-5612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Ju JW, Jang HS, Lee M, Lee HJ, Kwon W, Jang JY. Early postoperative fever as a predictor of pancreatic fistula after pancreaticoduodenectomy: a single-center retrospective observational study. BMC Surg. 2024;24:229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Ryu T, Nomura Y, Takeishi K, Yamamoto G, Wada Y, Takami Y. Efficacy of Reinforced Stapler for Preventing Postoperative Pancreatic Fistula After Minimally Invasive Distal Pancreatectomy. Anticancer Res. 2024;44:3655-3661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Kandikattu HK, Manohar M, Verma AK, Kumar S, Yadavalli CS, Upparahalli Venkateshaiah S, Mishra A. Macrophages-induced IL-18-mediated eosinophilia promotes characteristics of pancreatic malignancy. Life Sci Alliance. 2021;4:e202000979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Sun Q, Fan G, Zhuo Q, Dai W, Ye Z, Ji S, Xu W, Liu W, Hu Q, Zhang Z, Liu M, Yu X, Xu X, Qin Y. Pin1 promotes pancreatic cancer progression and metastasis by activation of NF-κB-IL-18 feedback loop. Cell Prolif. 2020;53:e12816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 25. | Zhang HR, Li TJ, Yu XJ, Liu C, Wu WD, Ye LY, Jin KZ. The GFPT2-O-GlcNAcylation-YBX1 axis promotes IL-18 secretion to regulate the tumor immune microenvironment in pancreatic cancer. Cell Death Dis. 2024;15:244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |