Published online Dec 15, 2016. doi: 10.4239/wjd.v7.i20.572
Peer-review started: June 27, 2016
First decision: July 27, 2016
Revised: September 27, 2016
Accepted: October 17, 2016
Article in press: October 18, 2016
Published online: December 15, 2016
Processing time: 178 Days and 3.2 Hours
Though the pathophysiology of clinical obesity is undoubtedly multifaceted, several lines of clinical evidence implicate an important functional role for glucagon-like peptide 1 (GLP-1) signalling. Clinical studies assessing GLP-1 responses in normal weight and obese subjects suggest that weight gain may induce functional deficits in GLP-1 signalling that facilitates maintenance of the obesity phenotype. In addition, genetic studies implicate a possible role for altered GLP-1 signalling as a risk factor towards the development of obesity. As reductions in functional GLP-1 signalling seem to play a role in clinical obesity, the pharmacological replenishment seems a promising target for the medical management of obesity in clinical practice. GLP-1 analogue liraglutide at a high dose (3 mg/d) has shown promising results in achieving and maintaining greater weight loss in obese individuals compared to placebo control, and currently licensed anti-obesity medications. Generally well tolerated, provided that longer-term data in clinical practice supports the currently available evidence of superior short- and long-term weight loss efficacy, GLP-1 analogues provide promise towards achieving the successful, sustainable medical management of obesity that remains as yet, an unmet clinical need.
Core tip: Several lines of clinical evidence implicate an important functional role for glucagon-like peptide 1 (GLP-1) signalling in the pathophysiology of clinical obesity. Here we critically evaluate such findings in way that as yet has been unexplored; using the well established roles of GLP-1 as an incretin and meal to meal satiety signal to go some way toward explaining findings from interventional and observational clinical data that suggest functional deficits of GLP-1 to be a contributor to the obesity phenotype. We also explore the promise shown by GLP-1 analogues in achieving and maintaining significant weight loss in obese individuals, and use findings to discuss to what extent they too may support a role for GLP-1 in obesity pathophysiology. We conclude by exploring what an association with functional GLP-1 deficit could mean for the clinical management of obesity; conducting cost and risk benefit analyses to evaluate the extent to which GLP-1 analogues may provide a successful and sustainable option for the medical management of obesity that remains as yet, an unmet clinical need.
- Citation: Anandhakrishnan A, Korbonits M. Glucagon-like peptide 1 in the pathophysiology and pharmacotherapy of clinical obesity. World J Diabetes 2016; 7(20): 572-598
- URL: https://www.wjgnet.com/1948-9358/full/v7/i20/572.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i20.572
Obesity is a global epidemic, perhaps the greatest challenge to global and public health of our time. With a doubling in prevalence from 1980 to 2008[1], 13% of the world’s population at present are obese [body mass index (BMI) ≥ 30 kg/m2] and 39% overweight (BMI ≥ 27 kg/m2)[2]. If recent trends continue, by 2030 up to 57.8% of the world’s adult population will be overweight or obese[3] (Figure 1). The World Health Organisation (WHO) has estimated that 44% of the global diabetes burden, and 23% and 7%-41% of the burdens for ischaemic heart disease and specific cancers respectively can be attributed to being overweight or obese[4]. Psychosocially, stigma and discrimination toward obese people can have consequences for psychological as well as physical health[5], with impaired quality-of life[6] and increased rates of depression[7] reported in this group. Even modest losses of 5%-10% of total body weight are associated with reduced risk of comorbidities in obese individuals[8-10]. Therefore, effectively managing rates of obesity is a major goal in public health policy.
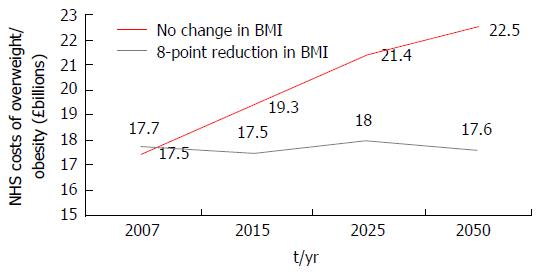
In addition to its physical and psychological burdens, obesity and its comorbidities impose disproportionately high healthcare and economic demands at individual and societal levels[11]. Affecting the wider economy indirectly through increased rates of worker illness absenteeism and resultant losses in productivity, healthcare systems are burdened from direct healthcare related costs; obese individuals on average incurring healthcare related costs 30% greater that their healthy weight peers[12-16]. A global systematic review has estimated the direct costs of obesity related diseases to account for between 0.7% and 2.8% of a country’s total healthcare expenditure[16]. In the United Kingdom alone, direct costs to the National Health Service (NHS) of treating overweight and obesity, and related co-morbidities were estimated at £5.1 billion in July 2006; representing around 5% of total NHS spending[17,18]. A computer based micro-simulation model predicting the direct healthcare related costs of overweight and obesity in the United Kingdom should 2001 prevalence remain constant, has forecasted the NHS spending £15.4 billion and £22.5 billion in 2015 and 2050 respectively[18,19] on the direct health costs of treating overweight and obesity and related co-morbidities in England alone. An upward trajectory prevented by significant weight loss in those currently obese (Figure 2), findings imply that whilst the prevention of obesity is the strategic imperative, the effective management of those already obese is an immediate priority.
Current medical management of obesity involves lifestyle, pharmacological and surgical interventions[20]. Lifestyle intervention, in the form of dietary, behavioural and exercise counselling, are currently the suggested first line treatment for obesity; however, whilst a recent meta-analysis reports such interventions to show small but significant benefits on weight loss maintenance, weight loss achieved and sustained with lifestyle intervention alone remains suboptimal[20-24]. In the face of such challenges, a number of pharmaceuticals have been marketed to assist weight management over the years[25,26] (Table 1). However, adverse effects of some and the transient weight losses associated with others[27] mean that the pharmacological management of obesity remains suboptimal. The only proven treatment to achieve and maintain weight loss in obesity is bariatric surgery[28-30]. However, surgical and anaesthetic risks associated with overweight and obese status sees these invasive procedures reserved to those patients classed morbidly obese (BMI ≥ 40 kg/m2) or as a last resort in those failing more conservative management[20,31,32]. The minimally invasive and efficacious management of obesity therefore, remains an unmet clinical need.
| Drug | Mechanism | Year | Clinical use and limitations | Suspension reason |
| Currently FDA licenced drugs | ||||
| Diethylpropion | NA releasing agent | 1959 | FDA approved for short term use (3 mo); not recommended with uncontrolled hypertension or heart disease | - |
| Phentermine | 1959 | FDA and EMA approved for long term use; treatment dependent weight loss | - | |
| Orlistat (Xenical) | - | 1999 | - | |
| Orlistat (Alli) | Pancreatic lipase inhibitor | 2007 | - | |
| Phentermine-topamirate (Qysmia) | - | - | Approved for long term use; treatment dependent weight loss | - |
| Lorcaserin (Belviq) | 5HT2c-R antagonist | 2012 | FDA approved for long term use, recommended in those with cardiovascular disease; treatment dependent weight loss | - |
| Liraglutide (Saxenda) | GLP-1 analogue | 2014 | FDA and EMA (2015) approved | - |
| Previously FDA licenced drugs | ||||
| Dinitrophenol | Unknown | 1938 | - | Dermatitis, neuropathy, agranulocytosis, visual impairment, death |
| Aminorex | Unknown | 1968 | - | Chronic pulmonary hypertension |
| Amphetamines | Monoamine reuptake inhibitor | 1971 | - | Addiction, hypertension, myocardial toxicity |
| Fenfluramine | Serotonin reuptake inhibitor | 1997 | - | Valvular heart disease |
| Phenylpropanolamine | NA-R and DA-R agonist | 2000 | - | Haemorrhagic stroke |
| Rimonabant | CB1R antagonist | 2009 | - | Psychiatric disorders, depression, suicidal ideation |
| Sibutramine | Serotonin-NA reuptake inhibitor | 2010 | - | Risk of major cardiovascular events |
The ideal management of any illness involves an understanding of its underlying pathophysiology; greater understanding facilitating the development of targeted pharmacotherapies to either replete physiological factors pathologically depleted, or antagonize pathological processes. The pathophysiology of obesity however, remains poorly understood. The WHO has defined the current obesity crisis epidemiologically, as the consequence of an increasing imbalance between energy intake and expenditure[33]. Physiologically, energy balance is a closely regulated system involving interactions between peripheral endocrine, nutritional and neural signals acting on regulatory central hypothalamic and hedonic brain regions[34-36]. Clinical obesity has been associated with deregulations in both homeostatic and hedonic controls of energy balance potentially facilitated by impaired glucagon-like peptide 1 (GLP-1) signalling[35-37] (a role for GLP-1 in the pathophysiology of clinical obesity). Pharmacologically targeting GLP-1 therefore, may go some way towards achieving the successful and sustainable medical management of clinical obesity that as yet remains to be achieved.
GLP-1 is a 31 amino acid polypeptide primarily synthesized by the enteroendocrine L cells of the terminal ileum. Amongst its pleotropic central and peripheral effects, GLP-1 acts as a potent incretin first clinically used in the medical management of overweight or obese individuals with type 2 diabetes mellitus (T2DM)[38]. The repeatedly demonstrated ability of GLP-1 analogues to induce weight loss in this cohort[39,40] prompted phase III trials studying the weight loss efficacy of the GLP-1 analogue liraglutide (3 mg; trade name Saxenda) vs placebo[41] and the pancreatic lipase inhibitor orlistat[42,43] (the only anti-obesity drug licensed in the United Kingdom) in non-diabetic overweight and obese adults. The greater weight loss efficacy achieved and maintained by GLP-1 analogues prompting the Food and Drug Administration (FDA) in 2014 to approve Saxenda as the first GLP-1 analogue for use as a weight loss aid in obese adults and overweight adults with at least one weight related co-morbidity[44]. March 2015 saw the European Medical Association (EMA) grant marketing authorization for 3 mg liraglutide under the FDA approved criteria in all 28 European Union (EU) states[45]. However, launching in April 2015 in the United States at a cost of over $1000 per patient a month, cost-benefit is of greater issue in EU nations such as the United Kingdom where health care is primarily socially funded; undoubtedly contributing to the uncertainty of launch plans in the United Kingdom at present[46]. Clinical evidence however implicates a role for functional impairments in GLP-1 signalling in the pathophysiology of obesity, GLP-1 agonism therefore may be the first truly targeted therapeutic in the medical management of clinical obesity. Therefore, with its superior clinical efficacy to currently United Kingdom licensed therapies benefiting patients through greater achieved and maintained weight loss and the economy through the potential to reduce long-term financial burdens of obesity, the cost-benefit spectrum may therefore be swayed, favouring the use of GLP-1 analogues in the medical management of obesity in the United Kingdom[46].
Physiologically, energy balance is a closely regulated system involving interactions between peripheral endocrine, nutritional and neural signals acting on regulatory central hypothalamic[34] and hedonic[35,36] brain regions. Where previously the neurocircuits mediating the homeostatic and hedonistic control of energy balance were considered distinct entities, it has now emerged that considerable cross talk exists with implications for the pathophysiology of clinical obesity.
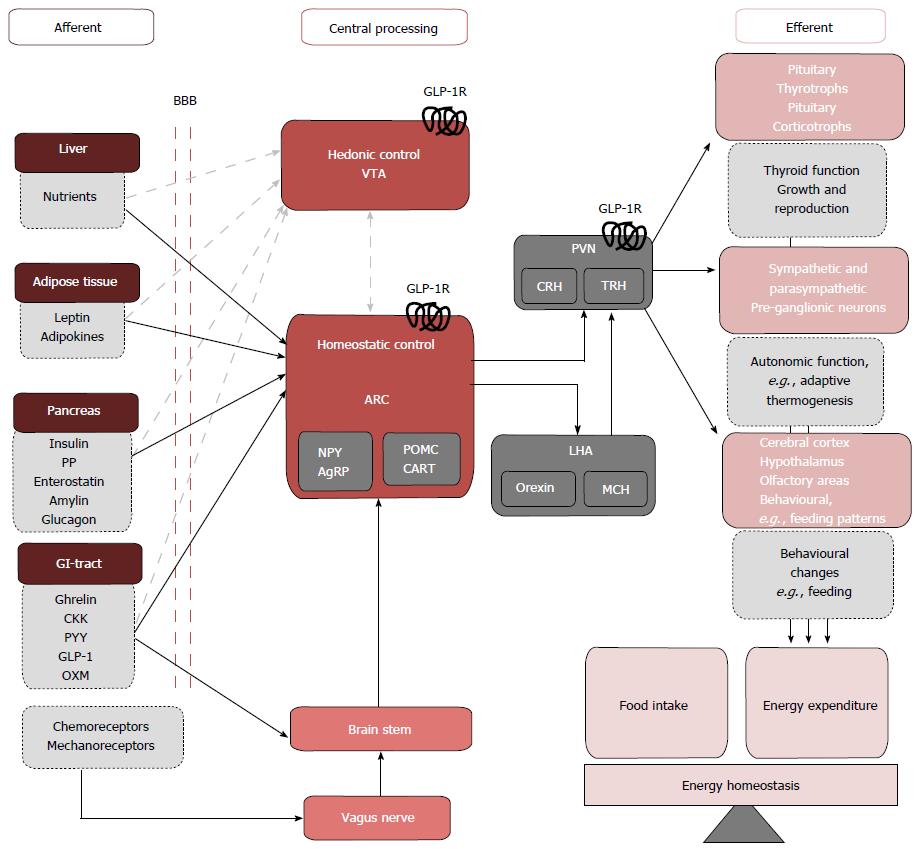
Peripheral signals involved in energy homeostasis are often stratified as long or short acting. Long acting signals provide information about available energy stores, and in response, the brain makes corrective adjustments to food intake and energy expenditure to maintain body weight[47]. The white adipocyte hormone leptin[48] and pancreatic hormone insulin are the two major afferents governing long-term energy balance and act primarily as anorexigens. Food intake and energy expenditure in the short term are modulated by a wide variety of situational and meal-related factors, among the most important are short-term gut derived hormones such as GLP-1 that act to signal acute energy status. Originally thought to exert their effects on energy balance through modulating homeostatic hypothalamic circuits, both long and short term afferents may also modulate the hedonic drive toward food consumption, though these pathways remain less extensively studied[49] (Figure 3).
The homeostatic control of food intake: The hypothalamic arcuate nucleus (ARC) is believed to play a crucial role in the homeostatic control of energy balance. At a cellular level, the ARC contains two distinct neural populations exerting antagonistic effects on food intake; a medially located orexigenic (appetite stimulating) population consisting of neurons co-expressing Agouti related peptide (AgRP) and neuropeptide Y and a laterally located anorexigenic (appetite suppressing) population consisting of neurons co-expressing pro-opiomelanocortin (POMC) and cocaine and amphetamine related transcript (CART)[55-58]. Both neural subsets project to melanocortin 4 receptor (MC4R) positive neurons located in intra- and extra-hypothalamic sites. POMC is cleaved to produce α-MSH an agonist of MC4R whereas AgRP acts an inverse agonist[59-61]. The ARC may exert its effects on energy homeostasis by direct cortical projections or indirectly via second order neurons in adjacent hypothalmic nuceli of which the paraventricular nucleus (PVN) is believed to be play a crucial role[62,63]. GLP-1 receptors (GLP1-Rs) have been localized pre-clinically in the ARC and PVN[50,51] and stimulation of theses receptors reduce food intake to induce weight loss in rodents. Targeting the homeostatic controls of energy balance may therefore be the means by which GLP-1 agonism achieves its weight loss effects in the clinic, suggesting an underlying deregulation in GLP-1 signalling contributing to the multifactorial pathophysiology of human obesity.
The hedonic control of food intake: Despite a robust homeostatic system governing energy balance, feeding and meal termination are also influenced by hedonic, reward-related factors such as palatability and the perceived rewards associated with meal consumption. The drive to pursue such pleasurable experiences largely mediated by the mesolimbic rewards system originating from dopaminergic neurons in ventral tegmental area (VTA) that terminate on neurons in the nucleus accumbens. Though the relationship between peripheral afferents signalling acute and long term energy status and central hedonic control centres are less well defined, GLP-1Rs have been located in the dopaminergic neurons of the VTA[64] where activation inhibits neural firing, potentially reducing hedonic drives toward food consumption. Interestingly, where the homeostatic control of energy balance modulates food intake to regulate the amount of body fat an individual maintains[65], in obesity, despite an overall positive energy balance, hyperphagia is the norm. Where previously, the neurocircuits mediating the homeostatic and hedonistic control of energy balance were considered distinct entities, it has now emerged that considerable cross talk exists and central GLP-1 signalling has been implicated as a mediator of such interactions (detailed in a number of excellent reviews[36,37,54]). A skew toward hedonic and away from homeostatic controls of energy balance may explain the pathological hyperphagia seen in obesity; restoring the balance between homeostatic and hedonic drives towards food consumption may therefore be the means by which GLP-1 agonism achieves its sustained weight loss effects in the clinic, suggesting an underlying deregulation in GLP-1 signalling contributing to the multifactorial pathophysiology of human obesity.
GLP-1 is a 31 amino acid polypeptide derived from post-translational processing of the native 160 amino acid peptide proglucagon by the enzyme prohormone convertase 1 (PC1/3). Peripheral proglucagon gene expression has been localized to the enteroendocrine L cells and pancreatic α-cells whilst centrally, proglucagon expressing neurons have been localized to brainstem regions such as the nucleus of the solitary tract (NTS)[66-68]. Tissue specific post-translational processing liberates different pro-glucagon derived peptides[69] depending on subtype of PC enzyme present. Figure 4 details the different post-translational products following PC1/3 and 2 cleavage.
GLP-1 is primarily synthesized by PC1/3 activity in the intestinal L cells[75]; open-type epithelial cells most densely located in the ileum and colon[76-78]. Long apical processes that extend toward the intestinal lumen[77] allow direct nutrient sensing by L cells, of which glucose has been implicated as the most potent GLP-1 secretagogue in both healthy and T2DM humans[79]. Being in close proximity to neurons of the enteric nervous system and the intestinal microvasculature[80,81], L cells also receive neural and hormonal signals that act as indirect nutrient sensors. Following synthesis, GLP-1 is secreted from the L-cells via secretory granules located in the basolateral membrane. GLP-1 secretion in response to nutrient sensing is biphasic; an initial rapid rise occurring within 10-15 min post-prandial, followed by a second longer phase peaking at 30-60 min[82]. The early phase of GLP-secretion has traditionally been attributed to signals from the parasympathetic vagal nerve and neurotransmitters such as gastrin-releasing peptide (GRP) and acetylcholine. However, more recently, GLP-1 secreting cells that show direct secretory responses to nutrient stimulation have been localised in significant numbers in the proximal small intestine implicating a role for this albeit sparser population of proximal GLP-1 releasing cells in the rapid postprandial rises of plasma GLP-1[83-85]. The second phase is mediated via direct nutrient contact with subsequent membrane depolarization or activation of second messenger systems mediating GLP-1 release. Figure 5 depicts the major nutrient, neural and hormonal secretagogues of GLP-1.
Secreted GLP-1 is rapidly degraded at its N-terminal residue by the ubiquitously expressed enzyme dipeptidyl peptidase IV (DPPV) to yield residues GLP-1 (9-36 amide) and GLP-1 9-37[88,89]. The majority of GLP-1 degradation is attributed to membrane-bound DPPV in the hepatic portal system resulting in an extremely short half-life (about 2 min)[81,90]. As such, only about 10%-15% of GLP1 secreted from intestinal L cells reaches peripheral downstream targets. The amount of GLP-1 reaching potential central targets involved in energy balance is unknown. As parenteral administration of GLP-1 avoids the physiological first-pass effect of hepatic DPPV, the supraphysiological plasma concentrations achieved by subcutaneous (SC) administration may explain the weight loss efficacy achieved by 3 mg liraglutide in obese and overweight patients in the clinic. Findings also go some way to suggest either a reduction in secretion of, or sensitivity to, physiological GLP-1 secretion as a contributor to the multifactorial pathophysiology of human obesity.
GLP-1 exerts its effects by intracellular signalling pathways activated after binding to the G-protein coupled receptor GLP-1R[91]. The extensive central and peripheral expression of the GLP-R reflects the pleotropic physiological roles of GLP-1 that are summarised in Figure 6 and extensively reviewed elsewhere[70,86]. From this point onward the review will focus on exploring the evidence surrounding a role for physiological GLP-1 signalling in the regulation of energy balance and deregulations of this signalling as one contributor to the multifactorial pathophysiology of clinical obesity.
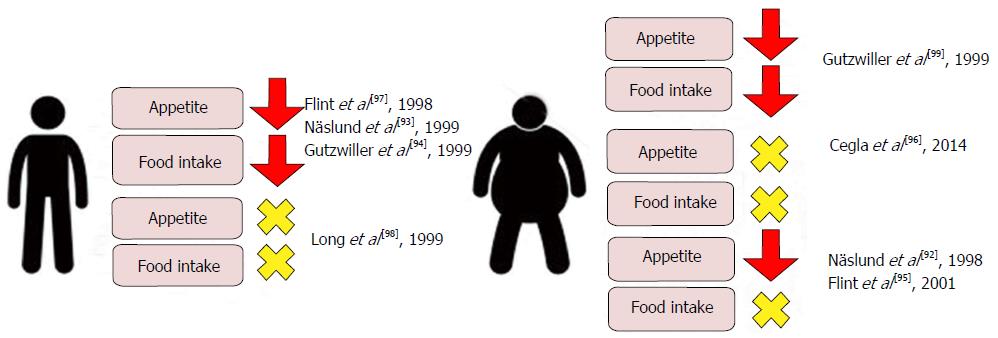
Numerous clinical studies have examined the relationship between acute physiological and supraphysiological doses of GLP-1 with measurements of food intake and feelings of hunger and satiety in healthy normal weight and obese adults with and without T2DM[92-99]. The main findings of these studies have been summarized in Figure 7. Though individual studies are conflicting, a meta-analysis reports that acute GLP-1 infusion induces a mean 11.7% decrease in food intake when compared with saline control in man[100]. Interestingly, whilst supraphysiological doses of GLP-1 reduces appetite and food intake in both lean and obese subjects, physiological GLP-1 doses reduces appetite and food intake in only lean subjects[93,94,97,99]. Findings go some way to suggest a role for resistance to physiological GLP-1 signalling as a factor contributing to obesity pathophysiology. Interestingly, whilst physiological GLP-1 infusions in obese subjects induce appetite reductions[92,95] similar to those observed in their lean peers, this is not translated into a reduction in food intake, suggesting pathological alterations of GLP-1 signalling in obesity that reinforce feeding despite a reduced physiological drive to food intake. One mechanism that this may be achieved is through a pathological skew toward hedonic and away from homeostatic controls of energy balance in obesity, potentially mediated by deregulated central GLP-1 signalling (a role for GLP-1 in the pathophysiology of clinical obesity).
Whilst evidence from clinical interventional studies suggests that physiological GLP-1 contributes to negative energy balance by decreasing food intake. The effects of GLP-1 on energy expenditure are less clear. Fasting plasma GLP-1 concentrations have been positively associated with increased rates of energy expenditure in man[101]. Clinical evidence regarding the effects of acute GLP-1 administration on energy expenditure however is conflicting. Physiological infusions of GLP-1 have been reported to reduce energy expenditure in lean and non-diabetic obese patients[95,102] associated with reduced carbohydrate metabolism. Others, however, have observed that supraphysiological infusions of GLP-1 increase energy expenditure in lean individuals in an insulin dependent manner[103].
Evidence from clinical interventional studies suggests that acute post-meal rises in GLP-1 contribute to negative energy balance primarily through an anorexigenic effect. The long-acting GLP-1 analogue liraglutide (3 mg) has recently been approved as a once daily bolus SC injection for the medical management of obesity. The sustained anorectic effect of a long term agonist combined with supraphysiological dosing perhaps the mechanism of the clinical weight loss efficacy achieved by liraglutide 3 mg. Unfortunately, to date, clinical studies assessing the comparative efficacy of acute vs continuous GLP-1 administration on appetite reduction and weight loss remain scarce. Näslund et al[104] compared the effects of 4 doses of acute GLP-1 infused 30 min prior to meals [prandial subcutaneous infusion (PSI)] to an equivalent dose of continuous SC GLP-1 infusion (CSI) on food intake and weight loss in non diabetic obese patients. Though both acute and continuous GLP-1 infusion produced significant reductions in food intake when compared to placebo (P = 0.02 PSI and CSI), a statistically significant weight loss compared to placebo was only observed following PSI. With respect to the clinic, findings suggest that lowered dose; more frequent GLP-1 administration may prove more efficacious in inducing weight loss in obese patients. Nevertheless, in view of the negative impact of SC drug administration on patient adherence and the potential biases associated with the significantly greater peak plasma GLP-1 concentrations achieved following PSI compared to CSI (269.4 pmol vs 88.7 pmol) once daily bolus administration at present, seems to be the most clinically efficacious means of therapeutic GLP-1 analogue delivery.
Clinical and pre-clinical evidence suggests that targeting peripherally and centrally located GLP-1Rs may exert the anorectic effects of physiological GLP-1 signalling.
Peripheral effectors: Histological studies in man have shown GLP-1Rs to be expressed in cells of the gastric mucosa and in pancreatic islet cells[105,106]. Pre-clinically, stimulation of gastric and pancreatic GLP1-Rs are associated with reductions in food intake that occurs alongside activation of hedonic and homeostatic brain regions[47,63,86]. Findings suggest physiological GLP-1 signalling may induce its anorectic effects in man by indirectly activating central controllers of appetite through gastric and pancreatic receptors.
Gastric mechanoreceptors are activated by gastric distension following acute nutrient intake, and gastric mechanoreceptor signalling plays an important role as a meal-to-meal satiety signal, activating the NTS which in turn modulates neural activity in both the ARC the VTA[107] (the homeostatic and hedonic control of energy balance). By relaying to the NTS, mechanoreceptor induced anorectic effects may therefore be exerted through modulation of both homeostatic and hedonic appetite control. The amount of gastric distension in response to a given meal is negatively associated with the rate of gastric emptying; delayed gastric emptying positively associated with increased satiety and fullness in both healthy and obese patients[108-112]. GLP-1 has been found to delay gastric emptying in healthy lean, obese and T2DM subjects, and histological studies in man have shown that GLP-1Rs are expressed in gastric mucosa[92,105,113-116]. Post-prandial GLP-1 secretion may therefore exert its anorectic effect through activating GLP-1Rs in gastric mucosa, which in turn increase mechanoreceptor firing and signalling to the NTS. Though the neurotransmitters involved in relaying signals from the NTS to homeostatic and hedonic appetite controls remain to be defined, physiological gastric distension in rodents has been shown to up-regulate GLP-1 gene expression in the NTS associated with central proglucagon processing[117], implicating a role for centrally synthesised GLP-1.
In the fasted state, the stomach is empty and so gastric motility is reduced to basal levels. That reductions in appetite after GLP-1 administration have been observed in fasting human subjects[99], suggests that mechanisms other delaying gastric motility contribute to the physiological anorectic effect of GLP-1. The glucoregulatory hormone insulin, traditionally viewed as an anorectic signal involved in the regulation of long-term energy balance[47,63], displays both basal and acute meal-related secretion[118]. With acute insulin administration associated with reduced ad libitum food intake in healthy lean individuals[119], findings implicate a role for insulin as an anorexigen involved in the regulation of short term energy balance. Insulin receptors are widely expressed in the ARC and VTA[120-122], thus modulation of both homeostatic and hedonic appetite control may be the means by which insulin exerts its anorectic effects on short-term energy balance.
The most extensively studied of GLP-1’s physiological roles is as a positive modulator of insulin secretion from pancreatic β-cells[123] (evidence: Effects of GLP-1 administration on food intake and energy expenditure in man). Whilst GLP-1 has been shown to increase energy expenditure in healthy lean individuals in an insulin dependent manner[103], no clinical evidence to date exists exploring the role of insulin as a mediator of GLP-1 anorexigenic signalling. Studies assessing the effects of GLP-1 interactions with the oriexigen ghrelin however, suggest that this may indeed be the case. Ghrelin receptors have been localised preclinically in Agrp/NpY neurons of the ARC and dopaminergic neurons of the VTA[124,125], with activation of neurons in either brain region producing orexigenic effects. GLP-1 infusion in healthy lean humans is associated with significant suppression of postprandial rises in ghrelin[126]; the decline in orexigenic signalling a potential indirect mediator of GLP-1s anorexigenic effect. Interestingly, the reductions in ghrelin concentration observed with GLP-1 infusion inversely correlate with coinciding rises in insulin concentration and elsewhere, insulin infusion has been shown to display a reciprocal relationship with ghrelin secretion in man[127]. Together, findings suggest that GLP-1’s anorectic effects may be mediated secondary to its incretin effect that in turn that suppresses ghrelin release, thus orexigenic signalling.
Central controllers: Histological and in vivo studies in rodents have shown that GLP-1Rs are expressed in anorexigenic POMC/CART neurons of the ARC and in dopaminergic neurons of the VTA[59,61,18,128] where they stimulate and inhibit neural firing respectively. Preclinical studies have shown that the stimulation of the POMC/ARC neurons of the hypothalamus and inhibition of the dopaminergic neurons of the VTA reduce food intake. Findings suggest that GLP-1 may exert its negative energy balance effects in man through direct activation of central GLP-1 receptors in the ARC and VTA; activating the anorexigenic homeostatic and inhibiting the hedonic hyperphagic drives to food intake. With the development of neuroimaging techniques, in vivo clinical studies substantiate the effects of GLP-1 on brain regions involved in the homeostatic and hedonic controls of energy balance. Whether these effects are mediated by direct central GLP-1R activation or indirectly via peripherally located GLP-1Rs however, remain to be defined.
Using fluorodeoxyglucose positron emission tomography Alvarez et al[129] demonstrate that GLP-1 infusion in lean individuals reduces glucose metabolism in the hypothalamus and brainstem. With patients fasted during the study and with no changes in peripheral hormone profiles observed, the effects of gastric mechanoreceptor activation or other hormonal influences respectively on observed effects are negated. Elsewhere, correlations between PET assessed increases in hypothalamic blood flow and physiological post-prandial rises in serum GLP-1 have been observed[130]. Both findings may represent altered neural activity in brain regions associated with homeostatic energy balance secondary to direct or indirect GLP-1/GLP-1R signalling. The effects of this alteration in central neural activity on food intake and appetite however, have not been explored. Using functional magnetic resonance imaging (fMRI), De Silva et al[131] demonstrate that GLP-1 infusion in lean individuals attenuates neuronal activity in 6 brain regions involved in rewards processing and hedonic feeding accompanied with reductions in food intake. Though neither parameter reached statistical significance vs placebo, results support the idea that central GLP-1 signalling may at least in part exert its negative energy balance effects through modulations in hedonic appetite control centres, potentially by reducing the hedonic value associated with food and food-driven motivation.
Clinical evidence exists to suggest that the SNS modulates energy expenditure through increased thermogenesis assessed in vivo as muscle sympathetic nerve activity (MSNA)[132,133]; increased MSNA positively associated with increased short and longer term energy expenditure in otherwise healthy human subjects[134,135]. Peripheral GLP-1 infusion has been shown to significantly increase MSNA in healthy human controls[136] and suggest that GLP-1 signalling may produce its negative energy balance effects not only through anorexigenic signalling, but also by increasing energy output.
Genetic analyses in man suggest clinical obesity is associated with a lack of functional GLP-1 signalling that may contribute to the development of the obesity phenotype.
Monogenic human obesity: Monogenic human obesity is a rare form of clinical obesity that shows Mendelian patterns of inheritance; the obesity phenotype attributed to the loss or gain of function in a single gene[137]. Two broad classes of Mendelian human obesity exist; syndromic obesity encompasses about 30 Mendelian disorders wherein obesity co-presents alongside characteristic physical and developmental anomalies. Though causative genes have been identified, the mechanisms through which the genetic mutations induce obesity are not completely understood in all cases[138]. Non-syndromic obesity is characterized by a severe, early onset hyperphagic obesity attributed to loss of function mutations in 1 of 11 genes[139-141]. Interestingly, 8 of these genes have physiological roles in the central control of energy balance[142]. One such gene is PCSK1 encoding the enzyme PC1/3 involved in the proteolytic processing of proglucagon, to yield, amongst other peptides, GLP-1 (GLP-1). Six studies to date document the relationship between autosomal recessive, compound heterozygous or homozygous[143-148] mutations in PCSK1 in 21 probands associated with reduced or absent function of PC1/3. Table 2 details the phenotypes of probands, all of whom presented with an early onset hyperphagic obesity and malabsorptive diarrhoea with varying, though extensively overlapping endocrine phenotypes.
| Ref. | Jackson et al[144], 1997 | Jackson et al[143], 2003 | Farooqi et al[148], 2007 | Frank et al[145], 2013 | Parker et al[147], 2013 | Bandsma et al[146], 2013 |
| Obesity phenotype | ||||||
| Hyperphagic, early-onset | Yes | Yes | Yes | Yes | Yes | Yes |
| Endocrine phenotype | ||||||
| Abnormal glucose metabolism | Yes | Yes | Yes | Yes | Yes | |
| Hypogonadotrophic hypogonadism | Yes | Yes | Yes | |||
| Hypocortisolaemia | Yes | Yes | Yes | Yes | ||
| Hypothyroidism | Yes | Yes | Yes | |||
| Central diabetes insipidus | Yes | |||||
| Others | ||||||
| Early onset malabsorptive diarrhoea | Yes | Yes | Yes | Yes | Yes | Yes |
Though the cause of the obesity and endocrine phenotypes associated with PCSK1 mutation are unknown, they may well be attributed to the loss of PC1/3 pro-hormone processing function. Signs of impaired intestinal[146] pro-glucagon processing have been described in probands with PC1/3 deficiency and may contribute to the development of the obesity phenotype secondary to reduced GLP-1 synthesis. Disappointingly, only 2 of 6 studies detailing the phenotypes PCSK1 mutant probands assess post-prandial GLP-1 secretory responses and report conflicting results; whilst an oral glucose load (OGTT) yields significantly reduced GLP-1 response in three child probands compared to age matched controls, post-prandial responses in a 40-year-old proband match those of healthy age-matched controls. One interpretation of such findings may be that whilst other PCs may compensate for lacking PC1/3 to allow for GLP-1 synthesis in response to mixed nutrient secretagogues, PC1/3 is necessary and essential for GLP-1 synthesis in response to its most potent secretagogue, glucose. An alternative interpretation comes from observations that GLP-1 secretion following OGTT in the 3 child probands studied by Bandsma et al[146] seem to show an age dependent impairment improving with increasing age. Following follows reports by Parker et al[147] who observed that the pattern of endocrinopathy in probands with PSCK1 mutant monogenic obesity change with age, perhaps GLP-1 secretion too may show an age-dependent alteration, potentially compensated for over time. One way to test this hypothesis would be to histologically examine the enteroendocrine expression of GLP-1 in adult PCSK1-mutant probands; enteroendocrine expression of GLP-1 is significantly reduced compared to control in children with PCSK1 monogenic obesity[146], if indeed the normal post-prandial GLP-1 responses seen in adulthood are a reflection of the activation of redundant PC activity in intestinal cells up regulation of enteroendocrine GLP-1 expression would be observed. Though the cause of the hyperphagic obesity in PCSK1 mutant human monogenic obesity remains ill defined, monogenic obesity implicates a role for deregulated GLP-1 signalling in the development of the obesity phenotype.
Polygenic obesity and Genome Wide Association Studies: Monogenic obesity is a rare form of clinical obesity, accounting for less than 1% of total cases of obesity worldwide. The obesity epidemic of the past 10-50 years has been largely attributed to environmental and societal changes facilitating a positive energy balance; “the obesogenic environment”[149,150]. However evidence from adoption, twin and family studies suggest the genetic contribution to BMI ranges between 60% and 84%[151]. As such, the current obesity epidemic may be defined as the interaction between a genetic predisposition and the “obesogenic environment”[149,150,152-154]. Genome wide association studies have identified 119 independent gene loci implicated as risk factors toward “common” obesity[155,156], as such today’s obesity epidemic may be referred to as a polygenic obesity. One such susceptibility gene is PCSK1 encoding the enzyme PC1/3 involved in the proteolytic processing of proglucagon, to yield, amongst other peptides, GLP-1.
Single-nucleotide polymorphisms (SNPs) at three independent PCSK1 loci have been consistently linked to an increased risk of obesity[157-161]. Though it is unclear how these minor alleles predispose to obesity, in vitro studies suggest that the encoded PC1/3 variants may not be as enzymatically active or physiologically available as the common form, potentially resulting in a partial PC1/3 deficiency. Decreased GLP-1 synthesis secondary to reduced proglucagon processing by PC1/3 in enteroendocrine L cells may therefore be the mechanism by which identified PCSK1 SNPs confer an increased risk toward the obesity phenotype.
Intestinal neuroendocrine gene expression: Neuroendocrine signals from the gut play an important role in the physiological control of energy balance. Findings from a recent study by Ritze et al[162] studying the gene expression of several proteins in the intestinal neuroendocrine network go some way to suggest intestinal GLP-1 expression and/or function may be altered in obesity. Though GLP-1 was not directly tested in the study, the anorectic neuropeptide PYY shown to co-localise and be co-secreted with GLP-1 in enteroendocrine cells[163] was tested. Taking PYY levels as proxy measures of GLP-1, Ritze et al[162] report significant correlations between GLP-1 with the GLP-1R in non-obese subjects (suggesting physiological ligand-receptor signalling), a correlation lost in obese subjects and replaced by correlations with the orexigen ghrelin (P < 0.01). Ritze et al[162] also observed correlations between the long-term satiety signal leptin and GLP-1R in obese subjects not seen in their lean counterparts.
A recent in vitro study on human L cells has shown that ghrelin is a positive modulator of GLP-1 release[164]. Ghrelin levels have also been reported to be reduced in humans with obesity[165,166]. The correlations between intestinal PYY (GLP-1) and ghrelin reported in obese subjects suggests that ghrelin decreases in obesity coincide with decreased GLP-1 levels, the latter potentially antagonising the anorexigenic effects of the former and may explain the difficulty to attain and maintain weight loss observed by many obese patients. Intestinal GLP-1 signalling has been suggested to promote small-intestinal motility in humans[167] and preclinically, central administration of leptin has been shown to increase the satiating effect of GLP-1, possibly through enhancing GLP-1R signalling. Correlations between the leptin and GLP-1R in obese subjects may therefore reflect a leptin-mediated enhancement of intestinal GLP-1/GLP-1R increasing intestinal motility to promote increased gastric emptying and reduced gastric mechanoreceptor activation in response to a given meal; the resultant decrease in anorexigenic signalling potentially explaining the persistent hyperphagia seen in obesity despite an overall positive energy balance.
Interventional and observational clinical evidence suggests that malfunctioning of GLP-1 contributes to the development and/or maintenance of the obesity phenotype, rationalizing the use of GLP-1 analogues as novel therapeutic agents in the medical management of obesity.
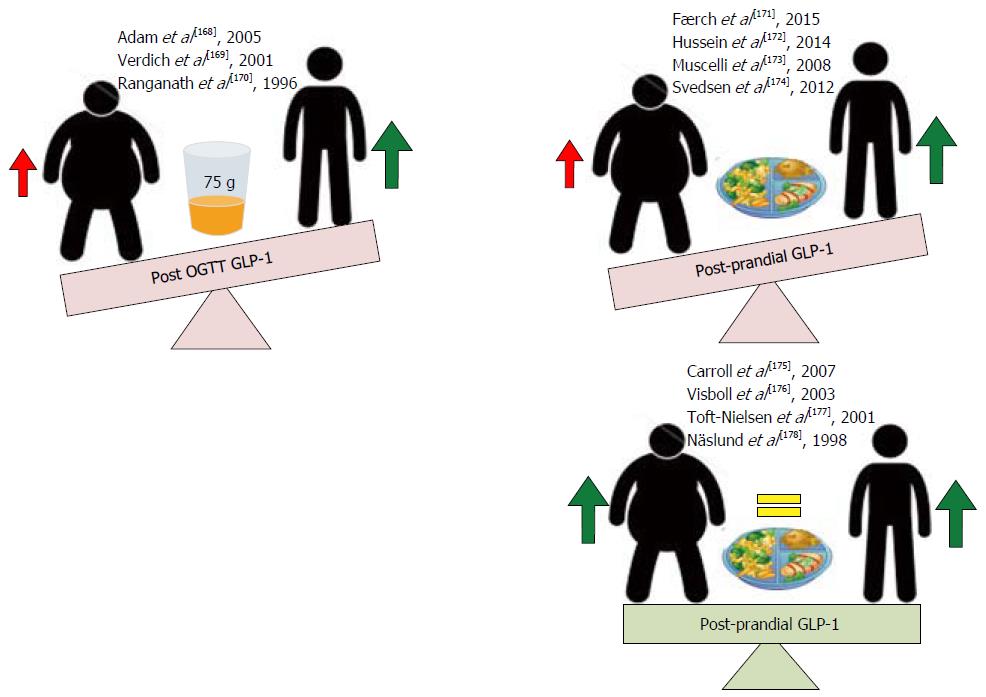
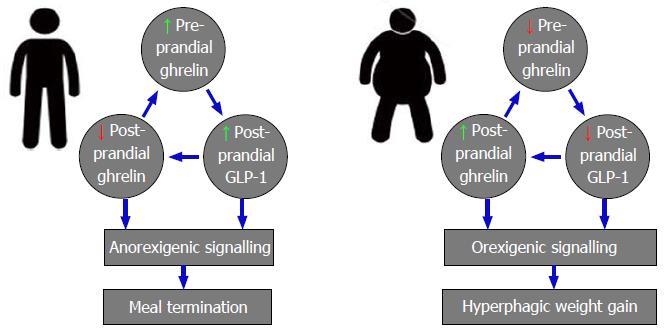
Post meal and oral glucose GLP-1 secretory responses: A number of clinical studies have assessed the effect of physiological GLP-1 secretion responses in obese and lean subjects following an oral 75 g glucose load (OGTT) or post-prandial following a balanced meal. Where an OGTT consistently demonstrates a reduced GLP-1 secretion in obese subjects compared to their lean control post-prandial GLP-1 responses are conflicting; some observing significant reductions and others no change[168-178] in obese subjects when compared to their lean counterparts (Figure 8). That oral glucose, the most powerful GLP-1 secretagogue consistently demonstrates reduced GLP-1 responses in obese subjects may suggest that the impaired GLP-1 response observed are secondary to a reduced L-cell glucose sensing capacity in obesity. Support for such a postulate comes from findings by Ranganath et al[170] who demonstrate that whilst GLP-1 secretion to an oral fat load remains intact, GLP-1 secretion in response to an oral carbohydrate load is decreased in obesity. However, reports by Adam et al[168] that demonstrate reductions in GLP-1 response in obesity to a balance meal with retained responses to an oral carbohydrate load challenge such an interpretation. Interestingly however, in their observational study, Adam et al[168] also demonstrate that whilst carbohydrates stimulate post-prandial GLP-1 release similarly in obese and non-obese subjects, these rises are positively correlated with increased satiety only in lean subjects and put forward an alternate hypothesis that rather than impaired GLP-1 secretion, downstream receptor resistance may be the root of GLP-1 dyshomeostasis in obesity. That deranged GLP-1 signalling is observed only in obese subjects suggests that obesity induces changes in functional GLP-1 signalling that through resultant reductions of signalling at central and peripherally located receptors (GLP-1) may facilitate the maintenance of the obesity phenotype. Pharmacologically targeting GLP-1 to restore physiological signalling may therefore be an efficacious method to prevent the propagation of, and potentially reverse weight gain in obesity.
Evidence from clinical studies suggests that weight gain induces alterations in functional GLP-1 signalling that facilitates and propagates the obesity phenotype. Though the mechanisms of reduced functional post-prandial GLP-1 signalling in obesity remain to be defined, clinical evidence implicates a role for interactions between GLP-1 and the long-term satiety signals insulin and leptin, and short-term orexigen ghrelin. The most extensively studied of post-prandial GLP-1’s physiological roles is as a positive modulator of pancreatic β-cell insulin secretion[123]. Hyperinsulinaemia is positively associated increased BMI in individuals with normal glucose tolerance and increased BMI and increasing glucose intolerance have been shown to independently and additively impair GLP-1 secretion[171-173,175,179]. The chronic hyperinsulinaemia positively associated with increasing levels of obesity therefore, may acting as a negative feedback signal to inhibit physiological post-prandial GLP-1 release observed in obese subjects when compared with healthy lean control[171-173]. The long term adiposity signal leptin acts as a satiety signal governing long term energy balance and clinically, increased BMI has been shown to be positively correlated with fasted leptin, however obese subjects are thought to be resistant to leptin’s effects[180,181]. In vitro studies of human intestinal L-cells have shown that leptin acts a GLP-1 secretogogue and go some way to suggest that the leptin resistance associated with obesity may account for the decreased post-prandial GLP-1 secretion observed in obese humans[168-170,182]. Ghrelin is the only orexigenic gut derived hormone[183]; released pre-prandial, ghrelin promotes meal initiation and increases food intake, and complex reciprocal interactions exist between GLP-1 and ghrelin that have implications for obesity pathophysiology. Preclinically, physiological ghrelin signalling has been shown to enhance post-prandial GLP-1 release[164], clinical obesity has however been associated with reductions in fasting ghrelin levels that may contribute to the reduced post-prandial GLP-1 release observed[168-170]. Conversely, clinical data exists to suggest that suppression of late post-prandial rises in ghrelin is one mechanism by which GLP-1 exerts its anorexigenic effect[126]; reduced post-prandial GLP-1 secretion in obesity potentially explaining the attenuated decreases of post-prandial serum ghrelin observed in this cohort[168-170,184,185] (Figure 9).
Together, evidence exists to suggest that the hyperinsulinaemia, leptin resistance and impaired ghrelin secretion occurring secondary to obesity cause functional deficits in GLP-1 signalling; the resultant reductions in GLP-1 mediated anorexigenic signalling facilitating post-meal hyperphagia, weight gain and thus perhaps the obesity phenotype. Pharmacologically targeting GLP-1 to restore homeostatic signalling may therefore be an efficacious method to prevent the propagation of, and potentially reverse the weight gain in obesity.
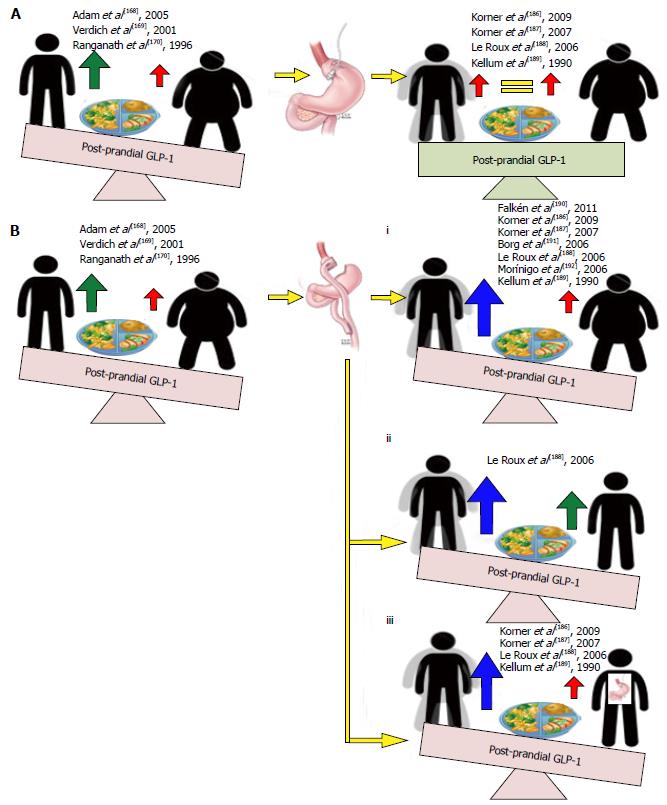
GLP-1 secretion post RYGB: Weight losses following bariatric surgery, pharmacotherapy or diet and lifestyle modification are all associated with decreases in circulating leptin and improved insulin sensitivity. The resultant reductions in anorexigenic signalling potentially facilitating weight gain and may explain the difficulty obese subjects have in attaining and maintaining weight loss. Bariatric surgery remains the most effective treatment modality for morbid obesity, with a meta-analysis reporting the Roux-en-y gastric bypass (RYGB) to produce a greater and more sustained weight loss than currently available pharmacotherapeutics, diet and lifestyle interventions or other bariatric options. Prospective studies assessing the effects of RYGB on post-prandial GLP-1 responses in non-diabetic obese patients consistently report statistically significant increases in post-prandial GLP-1 when compared to the pre-operative state, following equivalent weight losses with GB[186-193] and when compared with healthy lean control[188] (Figure 10). This post-operative supraphysiological GLP-1 secretory response therefore may explain the greater short- and long-term weight loss efficacy achieved with this treatment modality.
Evidence from clinical studies implicate the supraphysiological[188] post-prandial GLP-1 responses achieved following RYGB in the superior weight loss efficacy of this treatment modality. Though the mechanisms by which RYGB may induce increases in GLP-1 secretion remain poorly understood, clinical studies implicate a role for altered gut mechanics and L cell resensitisation.
That increases in post-prandial GLP-1 responses following RYGB are observed as early as 3 d post-operatively[190] suggest physical changes associated with RYGB, rather than gene-mediated up-regulations GLP-1 synthesis play a role in the increased GLP-1 secretory responses observed. Where both RYGB and GB induce weight loss through volume restriction, the former also redirects nutrient flow from the upper stomach directly into the distal jejunum. The exaggerated GLP-1 response following RYGB likely secondary to the increased glucose load delivered to the distal small intestine where L-cells are more densely populated. Such a concept is supported by the observed reductions in foregut and increases in hindgut hormones following RYGB and the hyperplasia of GLP-1 containing ileal cells in biopsy samples of obese humans after bypass[194-196].
RYGB induces a weight loss greater time-for-time and reaches a plateau more successfully maintained when compared with weight loss following GB[186-189]. Post-prandial GLP-1 responses following RYGB are also significantly greater than those following GB (that show no change from pre-operative levels[186,190,191]) however this response does not plateau but instead shows a tendency to increase with time past surgery. A relationship between the exponentially increasing post-prandial GLP-1 response and greater weight loss maintenance achieved post-RYGB may be explained by findings observed Kellum et al[189] who report that at 1 year post RYGB, alongside significantly greater achieved and maintained weight loss when compared to GB, GLP-1 responses were significantly increased in response to a carbohydrate meal in subjects post RYGB; a response positively associated with amount of weight lost. With derangements in L-cell carbohydrate sensing implicated in the pathophysiology of human obesity (see 5.1.1B, 5.2.1B), and with no alterations in response to a protein-fat meal observed following weight loss with RYGB and no altered response to either meal following weight loss with GB, findings suggest that weight loss following RYGB may be associated with a restoration of L cell sensitivity to the most potent GLP-1 secretagogue; a resensitisation that may occur proportionately to the amount of weight lost, the feed forward effect of weight loss on increased GLP-1 secretion resulting in supraphysiological GLP-1 signalling with the potential to antagonize the increased orexigenic drives of decreased leptin and insulin signalling associated with weight loss. Such a concept may explain the long-term weight loss efficacy associated with RYGB.
Together, evidence exists to suggest that the supraphysiological[188] upregulation in GLP-1 signalling seen following RYGB contributes to the superior short and long term weight loss efficacy observed with this treatment modality. Not only do findings go some way to suggest a role for impaired GLP-1 signalling in the pathophysiology of human obesity, findings support the potential for pharmacological mimicry of this supraphysiological GLP-1 secretion as a minimally invasive, thus risk reducing and cost-effective alterative of achieving and maintaining similarly significant weight loss in obese subjects in the clinic.
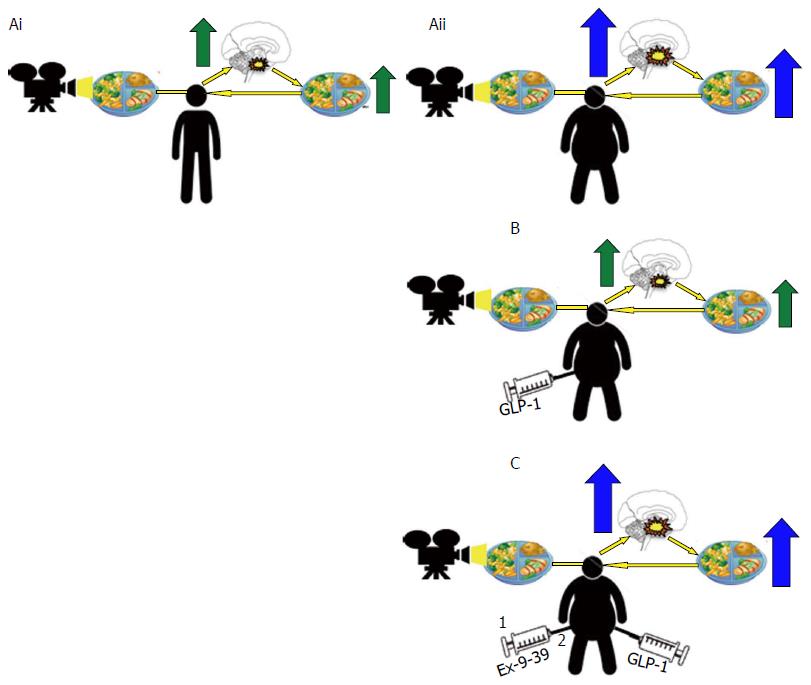
Functional neuroimaging and self-assessments of appetite: fMRI studies have provided evidence in vivo to suggest that central nervous system responses in brain regions involved in rewards processing are altered in obese individuals; reduced brain activity in response to the consumption of, and increased activity in response to the anticipation of palatable food consistently observed when compared with healthy lean controls[197-199]. Interestingly, GLP-1 agonism reverses these functional brain changes to match those of lean control with associated reductions in ad libitum food intake[199], an effect prevented by pre-treatment with a GLP-1 antagonist[200] (Figure 11). Together, findings suggests that obesity induced decreases in functional GLP-1 signalling contribute to altered rewards processing in obesity to facilitate hyperphagic weight gain despite an overall positive energy balance.
Further support for a role for GLP-1 and altered rewards processing in obesity pathophysiology comes from self-assessments of appetite. Subjectively assessed emotional eating scores have been defined as hedonic markers of appetite that display strong positive associations with the degree of human obesity, and, in obese subjects, relate to the extent to which GLP-1 receptor activation in brain regions involved in rewards processing are reduced[201-203]. Together, findings suggests that obesity induced decreases in functional GLP-1 signalling creates a feed-forward loop of hyperphagic weight gain despite an overall positive energy balance, an effect perhaps secondary to a GLP-1 deficit mediated skew toward hedonic and away from homeostatic controls of food intake. Together, findings from fMRI and subjective appetite assessment scores implicate obesity-associated reductions in functional GLP-1/GLP-1R signalling in the pathophysiology of hyperphagic weight gain in obesity. As such, findings support the role of the GLP-1R as a novel therapeutic target in the medical management of obesity, providing rationale for the use of liraglutide 3 mg in the pharmacotherapy of obesity in the clinic.
The balance between drug efficacy and cost determines the selection of a pharmacological agent for use in the medical management of any disease; greater understanding of underlying disease pathophysiology facilitating the development of targeted therapeutics with the potential for greater efficacy. Several lines of clinical evidence implicate a role for altered GLP-1 function in the pathophysiology of human obesity and a number of recent clinical trials have validated the clinical efficacy of long-term once daily SC 3 mg liraglutide (Saxenda) as an adjunct to calorie-restriction and exercise counselling in obese and overweight individuals with at least one weight related comorbidity. Significant improvements in clinical outcome measures such as body weight, anthropometric and cardiometabolic parameters, and indices of glucose tolerance have been observed and recently reviewed elsewhere[204]. Though March 2015 saw the EMA grant marketing authorization for 3 mg liraglutide as a weight-management agent in all 28 EU states[45], cost-benefit of funding treatment on the NHS undoubtedly contributes to the uncertainty of launch plans in the United Kingdom at present[46].
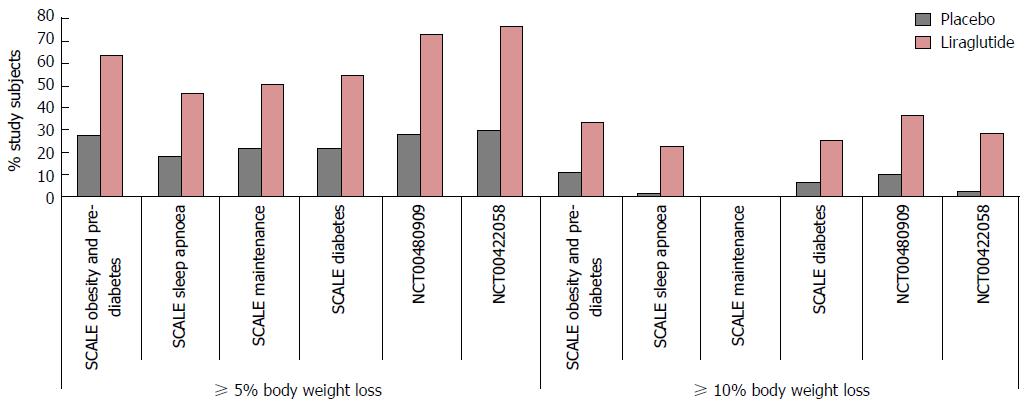
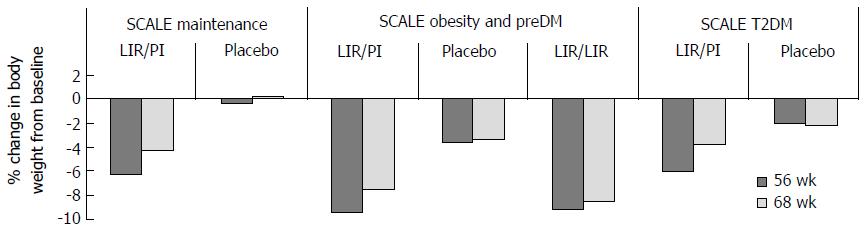
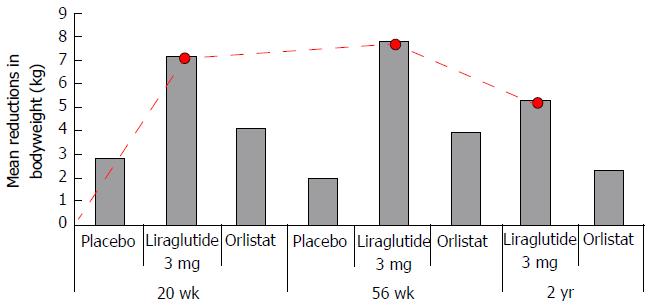
Evidence: One phase II (NCT00422058)[43], and a number of phase III multi-national double-blinded randomized control trials conducted in non-diabetic obese adults (NCT00480909)[42], overweight adults with at least one weight related co-morbidity (SCALE Obesity and Pre-diabetes, and SCALE Maintenance[205,206]), non-diabetic obese adults with obstructive sleep apnoea (OSA) (SCALE OSA[41]) and obese adults with T2DM (SCALE diabetes[207]) have established the efficacy of once daily 3 mg SC liraglutide as an adjunct to an energy-deficient low-calorie diet and physical activity counselling for weight management in this cohort. Results from the first study; a 20-wk phase II trial in non-diabetic obese subjects showed that weight loss with liraglutide is dose-dependent up to 3.0 mg once daily[42,206]. Significantly more liraglutide 3 mg/d recipients than placebo or orlistat recipients achieved a 5% or 10% reduction of body weight at 20 wk. In a 2-year phase III extension of the same study[42], double-blind treatment (liraglutide 1.2-3 mg/d) was continued until week 52, after which all liraglutide (< 2.4 mg/d) and placebo recipients were switched to liraglutide 2.4 mg, then 3.0 mg (week 70-96) based on 20-wk and 1-year results respectively (Figure 12) that indicated this was the optimal dosage. At 2 years, mean bodyweight reductions in those randomized to liraglutide were significantly greater than pancreatic lipase inhibitor orlistat, the only alternative licenced weight loss agent in the United Kingdom. Results from the SCALE maintenance and SCALE obesity and prediabetes[205,206] trials report similarly significant reductions in bodyweight in subjects randomized to 3 mg liraglutide when compared with placebo at 56 wk (P < 0.0001) alongside increased 5% and 10% responder rates. Findings are supported by results of the 32 wk SCALE Sleep Apnoea trial[41] in obese non-diabetic subjects with moderate to severe OSA and in the 56-68 wk SCALE Diabetes[207] trial in obese subjects with T2DM (Figure 13). With even modest losses of 5%-10% of total body weight associated with reduced risk of comorbidities in obese individuals[8-10], findings provide rationale for the licensing and funding of 3 mg liraglutide as an adjunct to lifestyle alteration as the first line anti-obesity pharmaceutical agent for weight management in obese and comorbid overweight adults in the United Kingdom.
Interpretations - obesity pathophysiology: Excessive consumption of palatable food can trigger neuroadaptive responses in brain reward circuits similar to that of alcohol and drugs of abuse[208] and clinical studies provide evidence to suggest that human obestiy is associated with altered rewards processing mediated in part by altered GLP-1 function that may render the hyperphagia of obesity the manifestation of a “food addiction”. Whilst 3 mg liraglutide has been shown to induce weight loss in man by reductions appetite pre-clinically, liraglutide attenuates the reinforcing properties of alcohol in vivo[209,210]. As such, perhaps GLP-1 agonism may attenuate produce its weight loss effects in part, by attenuating the negative reinforcement of hyperphagia in obesity. Interestingly, though all aforementioned trials[41-43,205-207] advise participants to restrict food consumption throughout the treatment period, adherence rates are not reported. If indeed GLP-1 agonism induces its weight loss effects by modulating food related rewards that potentially reverses the negative reinforcement of hyperphagia in obesity, an increased adherence to caloric restriction would be expected. It would be interesting to see if this were the case.
Interpretations - cost-benefit of 3 mg liraglutide as an anti-obesity agent on the NHS: Follow-up period (FUP) assessments in the SCALE Maintenance, and SCALE Diabetes[205-207] trials suggest that weight loss with 3 mg liraglutide is treatment dependent; weight gains in excess to those seen in placebo control and subjects re-randomized to treatment observed in liraglutide treated participants upon treatment cessation (Figure 12).
Though reductions in bodyweight have been shown to significantly improve health outcomes and thereby reduce healthcare costs long term[211], in 2013, 24.9% of the United Kingdom adult population were classed obese and 90% of the £2.7 million T2DM adults in the United Kingdom were overweight. Following FDA and EMA approval criteria, all these individuals are potential candidates for treatment with liraglutide 3 mg. Costing in excess of $1000 per patient a month, and with prevalence of obesity and overweight predicted to rise (introduction), prolonged treatment seems unsustainable. A potential solution comes from longitudinal observations from Astrup et al[43] who observed that subjects randomized to liraglutide 3 mg achieve maximal rates of weight loss in the initial 0-20 wk treatment period with a tendency toward weight gain beyond 36 wk[42,43]. Findings suggest that whilst initially treatment with a GLP-1 analogue may compensate for functional deficits in obesity, treatment beyond 20 wk may be associated with the development of treatment resistance, most apparent 36 wk from initiation (Figure 14). Based on this, perhaps treatment with liraglutide 3 mg should be prescribed for 20 to a maximum of 36 wk alongside behavioural therapies promoting lifestyle changes and developing strategies to combat the addiction driven hyperphagia implicated in obesity pathophysiology (GLP-1 secretion post RYGB); combing behavioural therapies in the initial 20 wk of drug induction where weight loss is most pronounced may act as a positive reinforcer of sustained behavioural change facilitating continuation of these behaviours. This approach, integrating the psychosocial empowerment associated with patient self-management of chronic illness, alongside cost benefits associated with limited in-treatment period seems an attractive one, especially if sustainable long-term weight losses with resultant reductions in the socioeconomic impacts of weight-related comorbidities can be achieved.
Evidence: Clinical studies in obese subjects with pre-diabetes consistently demonstrate a greater reversion to normal glycaemic control following treatment with liraglutide 3 mg coinciding reduced T2DM incidence[42,206,212].
Interpretations - obesity pathophysiology: Being overweight or obese is the main modifiable risk factor for T2DM and increasing BMI is positively associated with hyperinsulinaemia even in those with normal glycaemic control[175,179,213] suggesting a common pathophysiology to both conditions. That increased BMI and impaired glucose tolerance have been shown to independently and additively impair GLP-1 secretory responses following an OGTT[171-173] suggests that this common pathophysiology may lie in a functional deficit of GLP-1. Support for the existence of a common pathophysiology between obesity and T2DM comes from observations that treatment with the insulin sensitizer metformin (currently the first line pharmacotherapeutic agent in the management of T2DM) upregulates GLP-1 secretory response following an OGTT, the restoration of physiological anorexigenic and incretin effects perhaps explaining the weight loss, and insulin sensitising properties of the drug seen in the clinic respectively[214,215].
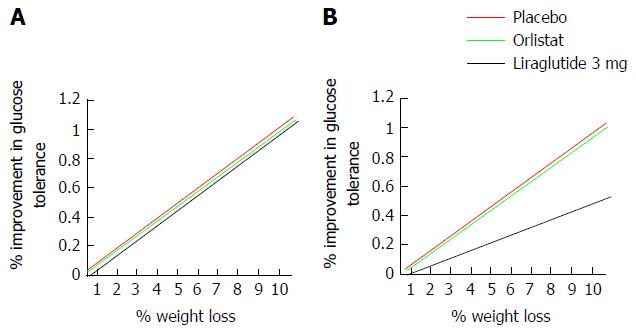
Interpretations: Cost-benefit of 3 mg liraglutide as an anti-obesity agent on the NHS: Being overweight or obese is the main modifiable risk factor for T2DM[213] and T2DM is one of the major indirect financial burdens of obesity and overweight. Treating T2DM and its complications alone current costs the NHS £8.8 billion a year with indirect costs estimated at £13 billion[216]. With the incidence of obesity projected to rise (introduction), so too can be expected the incidence of T2DM, the management of which therefore, may become unsustainable on the NHS. Though costly, treatment with liraglutide 3 mg is associated with reductions in the rate of development of T2DM in overweight and obese subjects[42,206,212] and goes some way to suggest that treatment may reduce both direct burdens of obesity and overweight and the large indirect burden posed by new incidences of T2DM. However, weight loss in itself is associated with an improvement in glycaemic control. It may be argued therefore, that true cost-benefit of funding liraglutide 3 mg on the rationale of T2DM prevention in overweight and obese subjects exists only if the improved glucose tolerance achieved following a given weight loss with liraglutide 3 mg exceeds those attained following other, arguably cheaper treatment modalities. Calculating correlation coefficients between percentage weight lost and percentage changes in glucose tolerance in non-diabetic obese subjects randomized to liraglutide 3 mg compared to those administered orlistat or placebo[41-43,205,206] in aforementioned phase III trials may be one way in which this could be assessed. Figure 15 interprets the possible findings of such a test.
If indeed pharmacological GLP-1 agonism improves glucose tolerance independent of weight lost, the potential curb in prevalence, thus socio-economic burden of T2DM achieved with treatment provides a second rationale for the licensing and funding of liraglutide 3 mg as an adjunct to lifestyle alteration as the first line anti-obesity agent for weight management in obese and co-morbid overweight individuals in the United Kingdom.
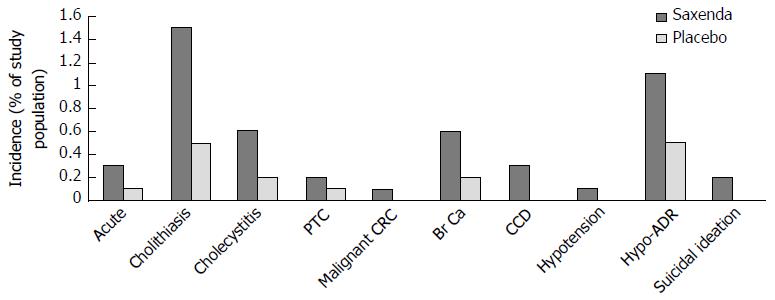
Evidence - in-treatment tolerability: Whilst evidence exists to suggest GLP-1 agonism may be a targeted agent with long term cost-benefit for use in the medical management of obesity, tolerability and safety are important considerations in determining the choice of any pharmaceutical, especially in the management of chronic disease. The safety and efficacy of liraglutide 3 mg has been evaluated in 5 phase III double-blinded placebo controlled trials comprising 3384 overweight or obese subjects receiving liraglutide 3 mg and 1941 placebo controls for a treatment periods of 32, 52 and 56 wk[42,42,205-207]. In a pooled analysis of the 5 aforementioned trials, liraglutide 3 mg in obese and overweight subjects was generally well tolerated, with most adverse drug events gastrointestinal in nature, transient and of mild to moderate intensity[42,43,205-207]. However, 9.8% of liraglutide and 4.3% of placebo recipients discontinued treatment because of an adverse event[217]. Figure 16 details adverse reactions occurring with a higher incidence to placebo with an incidence of ≥ 10% in liraglutide 3 mg recipients, stratified by system.
Of interest, 0.6% of subjects receiving liraglutide 3 mg experienced increases in mean heart rate (an average baseline increase of 2.5 beats/min) compared to 0.1% of placebo recipients[218]. Potentially a manifestation of GLP-1 induced increases in SNS activity (potential effectors of GLP-1s negative energy balance effects: Central controllers) contributing to GLP-1 induced weight loss via increases energy expenditure, elsewhere tachycardia associated with 3 mg liraglutide treatment in non-diabetic obese subjects yields no associated increases in 24 h energy expenditure[209]. Thus, whilst the clinical significance of this finding remains to be determined, observations may warrant more intense monitoring in patients with pre-existing cardiovascular disease.
Interpretations - long term risk-benefit of liraglutide 3 mg as an anti-obesity agent on the NHS: Though generally well tolerated in the acute setting, safety concerns have been raised regarding the potential risk of pancreatitis and pancreatic and thyroid cancer with long-term use of GLP-1 analogues[219,220]. Confirmed cases of acute pancreatitis and papillary thyroid carcinoma were reported in 0.3% of liraglutide 3 mg treated compared to 0.2% of placebo treated participants, however the relative rarity of events means the relationship between treatment with disease incidence and severity remains to be defined. On-going clinical experience and thorough post-marketing surveillance should help clarify any such associations and also identify other potential adverse drug events. To this end, episodes of acute renal failure and medullary thyroid carcinoma (not observed during in-treatment and FUP period assessments[42,43,205-207]) have been reported in the post-marketing period, though again, insufficient data exists to establish or exclude a causal relationship. Figure 17 details other potentially serious medical conditions observed in during in-treatment and FUP assessments[42,43,205-207].
Though potentially associated with serious long-term adverse effects, the rarity of incidence and lack of causal relationship mean that current knowledge supports benefit over risk, supporting the licensing and funding of liraglutide 3 mg as the first line anti-obesity pharmaceutical agent for weight management in obese and overweight adults with at-least one weight related co-morbidity in the United Kingdom.
Obesity is a global epidemic, perhaps the greatest challenge to global and public health of our time. Whilst public health initiatives should continue to focus on curbing the projected upward trends in the incidence of obesity and overweight, effective management of those individuals already obese remains an important and as yet unmet clinical need. The current medical management of obesity in the United Kingdom is suboptimal, with the only treatment modality with proven long-term efficacy being bariatric surgery. Both risky and costly, this treatment option is not viable for the widespread management of obesity, and remains reserved for extreme cases. The ideal medical management of any illness utilizes a targeted pharmacotherapy that either repletes physiological factors pathologically depleted, or antagonizes pathological processes, the development of such an agent requiring an understanding of the pathophysiology underpinning a disease. Though the pathophysiology of clinical obesity is undoubtedly multifaceted, several lines of clinical evidence implicate a role for functional impairments in GLP-1 signalling. Whilst genetic studies implicate a role for primary altered GLP-1 signalling as a risk factor towards development of the obesity phenotype, clinical studies assessing physiological GLP-1 responses in normal weight and obese subjects suggest weight gain may induce functional deficits in GLP-1 that facilitates maintenance of the obesity phenotype. Whatever the relationship, cause or effect, reductions in functional GLP-1 signalling seems to play a role in clinical obesity, as such, the pharmacological replenishment of this functional deficit seems a promising target for the medical management of obesity in the clinic. Indeed, the GLP-1 analogue liraglutide 3 mg has shown promising results in achieving and maintaining greater weight loss in obese individuals when compared to control or currently licensed anti-obesity medication. Though results from extended phase III and phase IV studies report the development of potentially fatal adverse drug events in those randomized to or prescribed liraglutide 3 mg respectively, the scarcity of incidence and lack of causal relationship sees such potential risks overshadowed by the proven superior weight loss efficacy of treatment. Cost-benefit, however, may pose a barrier toward viable NHS funding, though this may be overcome by strategic treatment delivery; combining short-term liraglutide 3 mg treatment (≤ 36 wk) with behavioural therapies targeted toward promoting healthy lifestyle changes. With drug induced weight loss potentially reinforcing adherence to long-term lifestyle changes, if successful, shortened in-treatment period alongside decreases in direct and indirect socioeconomic burdens of obesity and overweight secondary to achievement and maintenance of significant weight loss associates a long-term cost-benefit to funding treatment. Such a concept supports the use of liraglutide 3 mg as the first line anti-obesity agent on the NHS when conservative lifestyle management alone has failed in achieving clinically significant weight loss in comorbid overweight or obese adults.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Beltowski J, Tarantino G, Takebayashi K S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Organisation TWH. Global health observatory data; Situation and trends, 2016. Available from: http://www.who.int/gho/ncd/risk_factors/obesity_text/en/. |
| 2. | Organisation TWH. Obesity and Overweight fact sheet. Jun 2016. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/. |
| 3. | Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond). 2008;32:1431-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 2074] [Article Influence: 122.0] [Reference Citation Analysis (2)] |
| 4. | Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2406] [Cited by in RCA: 2538] [Article Influence: 158.6] [Reference Citation Analysis (0)] |
| 5. | Puhl RM, Heuer CA. Obesity stigma: important considerations for public health. Am J Public Health. 2010;100:1019-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 952] [Cited by in RCA: 1064] [Article Influence: 70.9] [Reference Citation Analysis (0)] |
| 6. | Kolotkin RL, Meter K, Williams GR. Quality of life and obesity. Obes Rev. 2001;2:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 413] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 7. | Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, Zitman FG. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3235] [Cited by in RCA: 2850] [Article Influence: 190.0] [Reference Citation Analysis (0)] |
| 8. | Vidal J. Updated review on the benefits of weight loss. Int J Obes Relat Metab Disord. 2002;26 Suppl 4:S25-S28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 130] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397-415. [PubMed] |
| 10. | Blackburn G. Effect of degree of weight loss on health benefits. Obes Res. 1995;3 Suppl 2:211s-216s. [PubMed] |
| 11. | Grieve E, Fenwick E, Yang HC, Lean M. The disproportionate economic burden associated with severe and complicated obesity: a systematic review. Obes Rev. 2013;14:883-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Trogdon JG, Finkelstein EA, Hylands T, Dellea PS, Kamal-Bahl SJ. Indirect costs of obesity: a review of the current literature. Obes Rev. 2008;9:489-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 179] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Howard JT, Potter LB. An assessment of the relationships between overweight, obesity, related chronic health conditions and worker absenteeism. Obes Res Clin Pract. 2014;8:e1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Ricci JA, Chee E. Lost productive time associated with excess weight in the U.S. workforce. J Occup Environ Med. 2005;47:1227-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Specchia ML, Veneziano MA, Cadeddu C, Ferriero AM, Mancuso A, Ianuale C, Parente P, Capri S, Ricciardi W. Economic impact of adult obesity on health systems: a systematic review. Eur J Public Health. 2015;25:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Withrow D, Alter DA. The economic burden of obesity worldwide: a systematic review of the direct costs of obesity. Obes Rev. 2011;12:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 750] [Cited by in RCA: 632] [Article Influence: 45.1] [Reference Citation Analysis (1)] |
| 17. | Scarborough P, Bhatnagar P, Wickramasinghe KK, Allender S, Foster C, Rayner M. The economic burden of ill health due to diet, physical inactivity, smoking, alcohol and obesity in the UK: an update to 2006-07 NHS costs. J Public Health (Oxf). 2011;33:527-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 18. | McPherson K, Marsh TN. Foresight Tackling Obesities: Future Choices - Modelling Future Trends in Obesity and the Impact on Health (GOV UK). 2007;. |
| 19. | Collins B, Capewell S, O’Flaherty M, Timpson H, Razzaq A, Cheater S, Ireland R, Bromley H. Modelling the Health Impact of an English Sugary Drinks Duty at National and Local Levels. PLoS One. 2015;10:e0130770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | National Institute for Clinical Excellence T. Obesity: Identification, assessment and management of overweight and obesity in children, young people and adults. 2014;. |
| 21. | Dombrowski SU, Knittle K, Avenell A, Araújo-Soares V, Sniehotta FF. Long term maintenance of weight loss with non-surgical interventions in obese adults: systematic review and meta-analyses of randomised controlled trials. BMJ. 2014;348:g2646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 609] [Cited by in RCA: 547] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 22. | Holzapfel C, Hauner H. [Weight maintenance after weight loss - how the body defends its weight]. Dtsch Med Wochenschr. 2011;136:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Loveman E, Frampton GK, Shepherd J, Picot J, Cooper K, Bryant J, Welch K, Clegg A. The clinical effectiveness and cost-effectiveness of long-term weight management schemes for adults: a systematic review. Health Technol Assess. 2011;15:1-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 176] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 24. | Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes (Lond). 2015;39:1188-1196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 285] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 25. | Derosa G, Maffioli P. Anti-obesity drugs: a review about their effects and their safety. Expert Opin Drug Saf. 2012;11:459-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 26. | Kang JG, Park CY. Anti-Obesity Drugs: A Review about Their Effects and Safety. Diabetes Metab J. 2012;36:13-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 393] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 27. | Yanovski SZ, Yanovski JA. Obesity. N Engl J Med. 2002;346:591-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 414] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 28. | Golomb I, Ben David M, Glass A, Kolitz T, Keidar A. Long-term Metabolic Effects of Laparoscopic Sleeve Gastrectomy. JAMA Surg. 2015;150:1051-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 29. | Hirth DA, Jones EL, Rothchild KB, Mitchell BC, Schoen JA. Laparoscopic sleeve gastrectomy: long-term weight loss outcomes. Surg Obes Relat Dis. 2015;11:1004-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Costa RC, Yamaguchi N, Santo MA, Riccioppo D, Pinto-Junior PE. Outcomes on quality of life, weight loss, and comorbidities after Roux-en-Y gastric bypass. Arq Gastroenterol. 2014;51:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Pasulka PS, Bistrian BR, Benotti PN, Blackburn GL. The risks of surgery in obese patients. Ann Intern Med. 1986;104:540-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 224] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Dority J, Hassan ZU, Chau D. Anesthetic implications of obesity in the surgical patient. Clin Colon Rectal Surg. 2011;24:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Nishida C, Uauy R, Kumanyika S, Shetty P. The joint WHO/FAO expert consultation on diet, nutrition and the prevention of chronic diseases: process, product and policy implications. Public Health Nutr. 2004;7:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 424] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 34. | Woods SC, D’Alessio DA. Central control of body weight and appetite. J Clin Endocrinol Metab. 2008;93:S37-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 308] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 35. | Berthoud HR. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr Opin Neurobiol. 2011;21:888-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 320] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 36. | Williams DL. Neural integration of satiation and food reward: role of GLP-1 and orexin pathways. Physiol Behav. 2014;136:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Skibicka KP. The central GLP-1: implications for food and drug reward. Front Neurosci. 2013;7:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 38. | Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context. 2015;4:212283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 253] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 39. | Monami M, Dicembrini I, Marchionni N, Rotella CM, Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on body weight: a meta-analysis. Exp Diabetes Res. 2012;2012:672658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 40. | Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 737] [Cited by in RCA: 660] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 41. | Collier A, Blackman A, Foster G, Zammit G, Rosenberg R, Wadden T, Aronne L, Claudius B, Jensen T, Mignot E. S28 Liraglutide 3.0 Mg Reduces Severity Of Obstructive Sleep Apnoea And Body Weight In Obese Individuals With Moderate Or Severe Disease: Scale Sleep Apnoea Trial. Thorax. 2014;69 Suppl 2:A16-A17. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 42. | Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean ME, Niskanen L, Rasmussen MF, Rissanen A, Rössner S. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond). 2012;36:843-854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 479] [Article Influence: 34.2] [Reference Citation Analysis (1)] |
| 43. | Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF, Lean ME. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 743] [Cited by in RCA: 793] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 44. | Liraglutide 3.0 mg for Weight Management; briefing document. 2014;. |
| 45. | Novo Nordisk. Saxenda® approved in Europe for the treatment of obesity. 2015, March 23. Available from: http://www.novonordisk.com/bin/getPDF.1905678.pdf. |
| 46. | UK Medicines Information. New drugs online: Liraglutide. 2015;. |
| 47. | Gao Q, Horvath TL. Neurobiology of feeding and energy expenditure. Annu Rev Neurosci. 2007;30:367-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 254] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 48. | Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9119] [Cited by in RCA: 8855] [Article Influence: 285.6] [Reference Citation Analysis (0)] |
| 49. | Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci. 2011;15:37-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 989] [Cited by in RCA: 877] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 50. | Secher A, Jelsing J, Baquero AF, Hecksher-Sørensen J, Cowley MA, Dalbøge LS, Hansen G, Grove KL, Pyke C, Raun K. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124:4473-4488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 677] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 51. | Katsurada K, Maejima Y, Nakata M, Kodaira M, Suyama S, Iwasaki Y, Kario K, Yada T. Endogenous GLP-1 acts on paraventricular nucleus to suppress feeding: projection from nucleus tractus solitarius and activation of corticotropin-releasing hormone, nesfatin-1 and oxytocin neurons. Biochem Biophys Res Commun. 2014;451:276-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 52. | Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4037] [Cited by in RCA: 3696] [Article Influence: 246.4] [Reference Citation Analysis (0)] |
| 53. | Figlewicz DP. Adiposity signals and food reward: expanding the CNS roles of insulin and leptin. Am J Physiol Regul Integr Comp Physiol. 2003;284:R882-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 54. | Ulrich-Lai YM, Ryan KK. Neuroendocrine circuits governing energy balance and stress regulation: functional overlap and therapeutic implications. Cell Metab. 2014;19:910-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 55. | Broberger C, Johansen J, Johansson C, Schalling M, Hökfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci USA. 1998;95:15043-15048. [PubMed] |
| 56. | Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1:271-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 838] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 57. | Millington GW. The role of proopiomelanocortin (POMC) neurones in feeding behaviour. Nutr Metab (Lond). 2007;4:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 228] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 58. | Dhillo WS, Small CJ, Stanley SA, Jethwa PH, Seal LJ, Murphy KG, Ghatei MA, Bloom SR. Hypothalamic interactions between neuropeptide Y, agouti-related protein, cocaine- and amphetamine-regulated transcript and alpha-melanocyte-stimulating hormone in vitro in male rats. J Neuroendocrinol. 2002;14:725-730. [PubMed] |
| 59. | Haskell-Luevano C, Hendrata S, North C, Sawyer TK, Hadley ME, Hruby VJ, Dickinson C, Gantz I. Discovery of prototype peptidomimetic agonists at the human melanocortin receptors MC1R and MC4R. J Med Chem. 1997;40:2133-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 60. | Nijenhuis WA, Oosterom J, Adan RA. AgRP(83-132) acts as an inverse agonist on the human-melanocortin-4 receptor. Mol Endocrinol. 2001;15:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 61. | Haskell-Luevano C, Monck EK. Agouti-related protein functions as an inverse agonist at a constitutively active brain melanocortin-4 receptor. Regul Pept. 2001;99:1-7. [PubMed] |
| 62. | Broberger C, Hökfelt T. Hypothalamic and vagal neuropeptide circuitries regulating food intake. Physiol Behav. 2001;74:669-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 63. | Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24:155-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 477] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 64. | Richard JE, Anderberg RH, Göteson A, Gribble FM, Reimann F, Skibicka KP. Activation of the GLP-1 receptors in the nucleus of the solitary tract reduces food reward behavior and targets the mesolimbic system. PLoS One. 2015;10:e0119034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 65. | Kennedy GC. The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B Biol Sci. 1953;140:578-596. [PubMed] |
| 66. | Drucker DJ, Asa S. Glucagon gene expression in vertebrate brain. J Biol Chem. 1988;263:13475-13478. [PubMed] |
| 67. | Lee YC, Brubaker PL, Drucker DJ. Developmental and tissue-specific regulation of proglucagon gene expression. Endocrinology. 1990;127:2217-2222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 68. | Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261-280. [PubMed] |
| 69. | Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem. 1986;261:11880-11889. [PubMed] |
| 70. | Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2461] [Cited by in RCA: 2662] [Article Influence: 147.9] [Reference Citation Analysis (0)] |
| 71. | Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2055] [Cited by in RCA: 2349] [Article Influence: 130.5] [Reference Citation Analysis (1)] |
| 72. | Pocai A. Unraveling oxyntomodulin, GLP1's enigmatic brother. J Endocrinol. 2012;215:335-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 73. | Orskov C, Holst JJ, Knuhtsen S, Baldissera FG, Poulsen SS, Nielsen OV. Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology. 1986;119:1467-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 337] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 74. | Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology. 1999;140:1687-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 265] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 75. | Tucker JD, Dhanvantari S, Brubaker PL. Proglucagon processing in islet and intestinal cell lines. Regul Pept. 1996;62:29-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 76. | Bryant MG, Bloom SR, Polak JM, Hobbs S, Domschke W, Domschke S, Mitznegg P, Ruppin H, Demling L. Measurement of gut hormonal peptides in biopsies from human stomach and proximal small intestine. Gut. 1983;24:114-119. [PubMed] |
| 77. | Eissele R, Göke R, Willemer S, Harthus HP, Vermeer H, Arnold R, Göke B. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest. 1992;22:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 453] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 78. | Knudsen JB, Holst JJ, Asnaes S, Johansen A. Identification of cells with pancreatic-type and gut-type glucagon immunoreactivity in the human colon. Acta Pathol Microbiol Scand A. 1975;83:741-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 79. | O’Donovan DG, Doran S, Feinle-Bisset C, Jones KL, Meyer JH, Wishart JM, Morris HA, Horowitz M. Effect of variations in small intestinal glucose delivery on plasma glucose, insulin, and incretin hormones in healthy subjects and type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3431-3435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 80. | Anini Y, Hansotia T, Brubaker PL. Muscarinic receptors control postprandial release of glucagon-like peptide-1: in vivo and in vitro studies in rats. Endocrinology. 2002;143:2420-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 81. | Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology. 1999;140:5356-5363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 360] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 82. | Herrmann C, Göke R, Richter G, Fehmann HC, Arnold R, Göke B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion. 1995;56:117-126. [PubMed] |
| 83. | Pais R, Gribble FM, Reimann F. Signalling pathways involved in the detection of peptones by murine small intestinal enteroendocrine L-cells. Peptides. 2016;77:9-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 84. | Theodorakis MJ, Carlson O, Michopoulos S, Doyle ME, Juhaszova M, Petraki K, Egan JM. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab. 2006;290:E550-E559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 273] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 85. | Habib AM, Richards P, Cairns LS, Rogers GJ, Bannon CA, Parker HE, Morley TC, Yeo GS, Reimann F, Gribble FM. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology. 2012;153:3054-3065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 302] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 86. | Cho YM, Fujita Y, Kieffer TJ. Glucagon-like peptide-1: glucose homeostasis and beyond. Annu Rev Physiol. 2014;76:535-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 87. | Lim GE, Brubaker PL. Glucagon-like peptide 1 secretion by the L-cell. Diabetes. 2006;55 Suppl 2:S70. |
| 88. | Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136:3585-3596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 649] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 89. | Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr Rev. 1999;20:876-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 601] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 90. | Deacon CF, Pridal L, Klarskov L, Olesen M, Holst JJ. Glucagon-like peptide 1 undergoes differential tissue-specific metabolism in the anesthetized pig. Am J Physiol. 1996;271:E458-E464. [PubMed] |
| 91. | Donnelly D. The structure and function of the glucagon-like peptide-1 receptor and its ligands. Br J Pharmacol. 2012;166:27-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 92. | Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2461] [Cited by in RCA: 2662] [Article Influence: 147.9] [Reference Citation Analysis (0)] |
| 93. | Cho YM, Fujita Y, Kieffer TJ. Glucagon-like peptide-1: glucose homeostasis and beyond. Annu Rev Physiol. 2014;76:535-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 94. | Näslund E, Gutniak M, Skogar S, Rössner S, Hellström PM. Glucagon-like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am J Clin Nutr. 1998;68:525-530. [PubMed] |
| 95. | Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 951] [Cited by in RCA: 993] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 96. | Cegla J, Troke RC, Jones B, Tharakan G, Kenkre J, McCullough KA, Lim CT, Parvizi N, Hussein M, Chambers ES. Coinfusion of low-dose GLP-1 and glucagon in man results in a reduction in food intake. Diabetes. 2014;63:3711-3720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 97. | Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 951] [Cited by in RCA: 993] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 98. | Long SJ, Sutton JA, Amaee WB, Giouvanoudi A, Spyrou NM, Rogers PJ, Morgan LM. No effect of glucagon-like peptide-1 on short-term satiety and energy intake in man. Br J Nutr. 1999;81:273-279. [PubMed] |
| 99. | Gutzwiller JP, Drewe J, Göke B, Schmidt H, Rohrer B, Lareida J, Beglinger C. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol. 1999;276:R1541-R1544. [PubMed] |
| 100. | Verdich C, Flint A, Gutzwiller JP, Näslund E, Beglinger C, Hellström PM, Long SJ, Morgan LM, Holst JJ, Astrup A. A meta-analysis of the effect of glucagon-like peptide-1 (7-36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab. 2001;86:4382-4389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 157] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 101. | Pannacciulli N, Bunt JC, Koska J, Bogardus C, Krakoff J. Higher fasting plasma concentrations of glucagon-like peptide 1 are associated with higher resting energy expenditure and fat oxidation rates in humans. Am J Clin Nutr. 2006;84:556-560. [PubMed] |
| 102. | Flint A, Raben A, Rehfeld JF, Holst JJ, Astrup A. The effect of glucagon-like peptide-1 on energy expenditure and substrate metabolism in humans. Int J Obes Relat Metab Disord. 2000;24:288-298. [PubMed] |
| 103. | Shalev A, Holst JJ, Keller U. Effects of glucagon-like peptide 1 (7-36 amide) on whole-body protein metabolism in healthy man. Eur J Clin Invest. 1997;27:10-16. [PubMed] |
| 104. | Näslund E, Barkeling B, King N, Gutniak M, Blundell JE, Holst JJ, Rössner S, Hellström PM. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int J Obes Relat Metab Disord. 1999;23:304-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 316] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 105. | Broide E, Bloch O, Ben-Yehudah G, Cantrell D, Shirin H, Rapoport MJ. GLP-1 receptor is expressed in human stomach mucosa: analysis of its cellular association and distribution within gastric glands. J Histochem Cytochem. 2013;61:649-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 106. | Tornehave D, Kristensen P, Rømer J, Knudsen LB, Heller RS. Expression of the GLP-1 receptor in mouse, rat, and human pancreas. J Histochem Cytochem. 2008;56:841-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 199] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 107. | Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Annu Rev Physiol. 2006;68:279-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 388] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 108. | Hunt JN. A possible relation between the regulation of gastric emptying and food intake. Am J Physiol. 1980;239:G1-G4. [PubMed] |
| 109. | Di Lorenzo C, Williams CM, Hajnal F, Valenzuela JE. Pectin delays gastric emptying and increases satiety in obese subjects. Gastroenterology. 1988;95:1211-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 96] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 110. | Clegg ME, Ranawana V, Shafat A, Henry CJ. Soups increase satiety through delayed gastric emptying yet increased glycaemic response. Eur J Clin Nutr. 2013;67:8-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 111. | Hlebowicz J, Darwiche G, Björgell O, Almér LO. Effect of cinnamon on postprandial blood glucose, gastric emptying, and satiety in healthy subjects. Am J Clin Nutr. 2007;85:1552-1556. [PubMed] |
| 112. | Hlebowicz J, Hlebowicz A, Lindstedt S, Björgell O, Höglund P, Holst JJ, Darwiche G, Almér LO. Effects of 1 and 3 g cinnamon on gastric emptying, satiety, and postprandial blood glucose, insulin, glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1, and ghrelin concentrations in healthy subjects. Am J Clin Nutr. 2009;89:815-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 113. | Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, Ritzel R, Schmiegel WH. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol. 1997;273:E981-E988. [PubMed] |
| 114. | Schirra J, Wank U, Arnold R, Göke B, Katschinski M. Effects of glucagon-like peptide-1(7-36)amide on motility and sensation of the proximal stomach in humans. Gut. 2002;50:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 115. | Little TJ, Pilichiewicz AN, Russo A, Phillips L, Jones KL, Nauck MA, Wishart J, Horowitz M, Feinle-Bisset C. Effects of intravenous glucagon-like peptide-1 on gastric emptying and intragastric distribution in healthy subjects: relationships with postprandial glycemic and insulinemic responses. J Clin Endocrinol Metab. 2006;91:1916-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 116. | Meier JJ, Gallwitz B, Salmen S, Goetze O, Holst JJ, Schmidt WE, Nauck MA. Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:2719-2725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 262] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 117. | Vrang N, Phifer CB, Corkern MM, Berthoud HR. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol. 2003;285:R470-R478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 160] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 118. | Polonsky KS, Given BD, Hirsch L, Shapiro ET, Tillil H, Beebe C, Galloway JA, Frank BH, Karrison T, Van Cauter E. Quantitative study of insulin secretion and clearance in normal and obese subjects. J Clin Invest. 1988;81:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 302] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 119. | Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab. 2008;93:1339-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 221] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 120. | Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG, Seeley RJ, Schwartz MW. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes. 2003;52:227-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 358] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 121. | Watanabe M, Hayasaki H, Tamayama T, Shimada M. Histologic distribution of insulin and glucagon receptors. Braz J Med Biol Res. 1998;31:243-256. [PubMed] |
| 122. | Iñiguez SD, Warren BL, Neve RL, Nestler EJ, Russo SJ, Bolaños-Guzmán CA. Insulin receptor substrate-2 in the ventral tegmental area regulates behavioral responses to cocaine. Behav Neurosci. 2008;122:1172-1177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 123. | Meloni AR, DeYoung MB, Lowe C, Parkes DG. GLP-1 receptor activated insulin secretion from pancreatic β-cells: mechanism and glucose dependence. Diabetes Obes Metab. 2013;15:15-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 268] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 124. | Wang Q, Liu C, Uchida A, Chuang JC, Walker A, Liu T, Osborne-Lawrence S, Mason BL, Mosher C, Berglund ED. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol Metab. 2014;3:64-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 125. | Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience. 2011;180:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 261] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 126. | Hagemann D, Holst JJ, Gethmann A, Banasch M, Schmidt WE, Meier JJ. Glucagon-like peptide 1 (GLP-1) suppresses ghrelin levels in humans via increased insulin secretion. Regul Pept. 2007;143:64-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 127. | Saad MF, Bernaba B, Hwu CM, Jinagouda S, Fahmi S, Kogosov E, Boyadjian R. Insulin regulates plasma ghrelin concentration. J Clin Endocrinol Metab. 2002;87:3997-4000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 249] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 128. | Wei Y, Mojsov S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 1995;358:219-224. [PubMed] |
| 129. | Alvarez E, Martínez MD, Roncero I, Chowen JA, García-Cuartero B, Gispert JD, Sanz C, Vázquez P, Maldonado A, de Cáceres J. The expression of GLP-1 receptor mRNA and protein allows the effect of GLP-1 on glucose metabolism in the human hypothalamus and brainstem. J Neurochem. 2005;92:798-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 214] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 130. | Pannacciulli N, Le DS, Salbe AD, Chen K, Reiman EM, Tataranni PA, Krakoff J. Postprandial glucagon-like peptide-1 (GLP-1) response is positively associated with changes in neuronal activity of brain areas implicated in satiety and food intake regulation in humans. Neuroimage. 2007;35:511-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 131. | De Silva A, Salem V, Long CJ, Makwana A, Newbould RD, Rabiner EA, Ghatei MA, Bloom SR, Matthews PM, Beaver JD. The gut hormones PYY 3-36 and GLP-1 7-36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab. 2011;14:700-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 259] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 132. | Astrup A. Thermogenesis in human brown adipose tissue and skeletal muscle induced by sympathomimetic stimulation. Acta Endocrinol Suppl (Copenh). 1986;278:1-32. [PubMed] |
| 133. | Astrup A, Bülow J, Madsen J, Christensen NJ. Contribution of BAT and skeletal muscle to thermogenesis induced by ephedrine in man. Am J Physiol. 1985;248:E507-E515. [PubMed] |
| 134. | Welle S. Sympathetic nervous system response to intake. Am J Clin Nutr. 1995;62:1118S-1122S. [PubMed] |
| 135. | Spraul M, Ravussin E, Fontvieille AM, Rising R, Larson DE, Anderson EA. Reduced sympathetic nervous activity. A potential mechanism predisposing to body weight gain. J Clin Invest. 1993;92:1730-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 193] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 136. | Bharucha AE, Charkoudian N, Andrews CN, Camilleri M, Sletten D, Zinsmeister AR, Low PA. Effects of glucagon-like peptide-1, yohimbine, and nitrergic modulation on sympathetic and parasympathetic activity in humans. Am J Physiol Regul Integr Comp Physiol. 2008;295:R874-R880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 137. | Huvenne H, Dubern B. Molecular Mechanisms Underpinning the Development of Obesity; Monogenic Forms of Obesity. Switzerland: Springer International Publishing 2014; . [DOI] [Full Text] |
| 138. | Chung WK. An overview of mongenic and syndromic obesities in humans. Pediatr Blood Cancer. 2012;58:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 139. | Doche ME, Bochukova EG, Su HW, Pearce LR, Keogh JM, Henning E, Cline JM, Saeed S, Dale A, Cheetham T. Human SH2B1 mutations are associated with maladaptive behaviors and obesity. J Clin Invest. 2012;122:4732-4736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 140. | Mencarelli M, Dubern B, Alili R, Maestrini S, Benajiba L, Tagliaferri M, Galan P, Rinaldi M, Simon C, Tounian P. Rare melanocortin-3 receptor mutations with in vitro functional consequences are associated with human obesity. Hum Mol Genet. 2011;20:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 141. | Yazdi FT, Clee SM, Meyre D. Obesity genetics in mouse and human: back and forth, and back again. PeerJ. 2015;3:e856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 142. | Farooqi IS. Monogenic human obesity. Front Horm Res. 2008;36:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 143. | Jackson RS, Creemers JW, Farooqi IS, Raffin-Sanson ML, Varro A, Dockray GJ, Holst JJ, Brubaker PL, Corvol P, Polonsky KS. Small-intestinal dysfunction accompanies the complex endocrinopathy of human proprotein convertase 1 deficiency. J Clin Invest. 2003;112:1550-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 144. | Jackson RS, Creemers JW, Ohagi S, Raffin-Sanson ML, Sanders L, Montague CT, Hutton JC, O’Rahilly S. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet. 1997;16:303-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 809] [Cited by in RCA: 695] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 145. | Frank GR, Fox J, Candela N, Jovanovic Z, Bochukova E, Levine J, Papenhausen PR, O’Rahilly S, Farooqi IS. Severe obesity and diabetes insipidus in a patient with PCSK1 deficiency. Mol Genet Metab. 2013;110:191-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 146. | Bandsma RH, Sokollik C, Chami R, Cutz E, Brubaker PL, Hamilton JK, Perlman K, Zlotkin S, Sigalet DL, Sherman PM. From diarrhea to obesity in prohormone convertase 1/3 deficiency: age-dependent clinical, pathologic, and enteroendocrine characteristics. J Clin Gastroenterol. 2013;47:834-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 147. | Parker JA, McCullough KA, Field BC, Minnion JS, Martin NM, Ghatei MA, Bloom SR. Glucagon and GLP-1 inhibit food intake and increase c-fos expression in similar appetite regulating centres in the brainstem and amygdala. Int J Obes (Lond). 2013;37:1391-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 148. | Lake A, Townshend T. Obesogenic environments: exploring the built and food environments. J R Soc Promot Health. 2006;126:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 331] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 149. | Mackenbach JD, Rutter H, Compernolle S, Glonti K, Oppert JM, Charreire H, De Bourdeaudhuij I, Brug J, Nijpels G, Lakerveld J. Obesogenic environments: a systematic review of the association between the physical environment and adult weight status, the SPOTLIGHT project. BMC Public Health. 2014;14:233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 150. | Mackenbach JD, Rutter H, Compernolle S, Glonti K, Oppert JM, Charreire H, De Bourdeaudhuij I, Brug J, Nijpels G, Lakerveld J. Obesogenic environments: a systematic review of the association between the physical environment and adult weight status, the SPOTLIGHT project. BMC Public Health. 2014;14:233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 151. | Maes HH, Neale MC, Eaves LJ. Genetic and environmental factors in relative body weight and human adiposity. Behav Genet. 1997;27:325-351. [PubMed] |
| 152. | O’Rahilly S, Farooqi IS. Human obesity as a heritable disorder of the central control of energy balance. Int J Obes (Lond). 2008;32 Suppl 7:S55-S61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 153. | O’Rahilly S, Farooqi IS. Human obesity: a heritable neurobehavioral disorder that is highly sensitive to environmental conditions. Diabetes. 2008;57:2905-2910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 154. | Hebebrand J, Hinney A. Environmental and genetic risk factors in obesity. Child Adolesc Psychiatr Clin N Am. 2009;18:83-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 155. | Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2987] [Cited by in RCA: 3253] [Article Influence: 325.3] [Reference Citation Analysis (0)] |
| 156. | Choquet H, Meyre D. Genetics of Obesity: What have we Learned? Curr Genomics. 2011;12:169-179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 157. | Benzinou M, Creemers JW, Choquet H, Lobbens S, Dina C, Durand E, Guerardel A, Boutin P, Jouret B, Heude B. Common nonsynonymous variants in PCSK1 confer risk of obesity. Nat Genet. 2008;40:943-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 215] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 158. | Choquet H, Kasberger J, Hamidovic A, Jorgenson E. Contribution of common PCSK1 genetic variants to obesity in 8,359 subjects from multi-ethnic American population. PLoS One. 2013;8:e57857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 159. | Kilpeläinen TO, Bingham SA, Khaw KT, Wareham NJ, Loos RJ. Association of variants in the PCSK1 gene with obesity in the EPIC-Norfolk study. Hum Mol Genet. 2009;18:3496-3501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 160. | Qi Q, Li H, Loos RJ, Liu C, Hu FB, Wu H, Yu Z, Lin X. Association of PCSK1 rs6234 with obesity and related traits in a Chinese Han population. PLoS One. 2010;5:e10590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 161. | Chang YC, Chiu YF, Shih KC, Lin MW, Sheu WH, Donlon T, Curb JD, Jou YS, Chang TJ, Li HY. Common PCSK1 haplotypes are associated with obesity in the Chinese population. Obesity (Silver Spring). 2010;18:1404-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 162. | Ritze Y, Hengelhaupt C, Bárdos G, Ernst B, Thurnheer M, D’Haese JG, Bischoff SC, Schultes B. Altered intestinal neuroendocrine gene expression in humans with obesity. Obesity (Silver Spring). 2015;23:2278-2285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 163. | Roth KA, Kim S, Gordon JI. Immunocytochemical studies suggest two pathways for enteroendocrine cell differentiation in the colon. Am J Physiol. 1992;263:G174-G180. [PubMed] |
| 164. | Gagnon J, Baggio LL, Drucker DJ, Brubaker PL. Ghrelin Is a Novel Regulator of GLP-1 Secretion. Diabetes. 2015;64:1513-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 165. | Marzullo P, Verti B, Savia G, Walker GE, Guzzaloni G, Tagliaferri M, Di Blasio A, Liuzzi A. The relationship between active ghrelin levels and human obesity involves alterations in resting energy expenditure. J Clin Endocrinol Metab. 2004;89:936-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 131] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 166. | Rosická M, Krsek M, Matoulek M, Jarkovská Z, Marek J, Justová V, Lacinová Z. Serum ghrelin levels in obese patients: the relationship to serum leptin levels and soluble leptin receptors levels. Physiol Res. 2003;52:61-66. [PubMed] |
| 167. | Jeppesen PB, Hartmann B, Thulesen J, Hansen BS, Holst JJ, Poulsen SS, Mortensen PB. Elevated plasma glucagon-like peptide 1 and 2 concentrations in ileum resected short bowel patients with a preserved colon. Gut. 2000;47:370-376. [PubMed] |
| 168. | Adam TC, Westerterp-Plantenga MS. Glucagon-like peptide-1 release and satiety after a nutrient challenge in normal-weight and obese subjects. Br J Nutr. 2005;93:845-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 169. | Verdich C, Toubro S, Buemann B, Lysgård Madsen J, Juul Holst J, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety--effect of obesity and weight reduction. Int J Obes Relat Metab Disord. 2001;25:1206-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 307] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 170. | Ranganath LR, Beety JM, Morgan LM, Wright JW, Howland R, Marks V. Attenuated GLP-1 secretion in obesity: cause or consequence? Gut. 1996;38:916-919. [PubMed] |
| 171. | Færch K, Torekov SS, Vistisen D, Johansen NB, Witte DR, Jonsson A, Pedersen O, Hansen T, Lauritzen T, Sandbæk A. GLP-1 Response to Oral Glucose Is Reduced in Prediabetes, Screen-Detected Type 2 Diabetes, and Obesity and Influenced by Sex: The ADDITION-PRO Study. Diabetes. 2015;64:2513-2525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 239] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 172. | Hussein MS, Abushady MM, Refaat S, Ibrahim R. Plasma level of glucagon-like peptide 1 in obese Egyptians with normal and impaired glucose tolerance. Arch Med Res. 2014;45:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 173. | Muscelli E, Mari A, Casolaro A, Camastra S, Seghieri G, Gastaldelli A, Holst JJ, Ferrannini E. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes. 2008;57:1340-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 292] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 174. | Svendsen PF, Jensen FK, Holst JJ, Haugaard SB, Nilas L, Madsbad S. The effect of a very low calorie diet on insulin sensitivity, beta cell function, insulin clearance, incretin hormone secretion, androgen levels and body composition in obese young women. Scand J Clin Lab Invest. 2012;72:410-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 175. | Carroll JF, Kaiser KA, Franks SF, Deere C, Caffrey JL. Influence of BMI and gender on postprandial hormone responses. Obesity (Silver Spring). 2007;15:2974-2983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 176. | Vilsbøll T, Krarup T, Sonne J, Madsbad S, Vølund A, Juul AG, Holst JJ. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88:2706-2713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 375] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 177. | Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717-3723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 546] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 178. | Näslund E, Grybäck P, Backman L, Jacobsson H, Holst JJ, Theodorsson E, Hellström PM. Distal small bowel hormones: correlation with fasting antroduodenal motility and gastric emptying. Dig Dis Sci. 1998;43:945-952. [PubMed] |
| 179. | McKeigue PM, Pierpoint T, Ferrie JE, Marmot MG. Relationship of glucose intolerance and hyperinsulinaemia to body fat pattern in south Asians and Europeans. Diabetologia. 1992;35:785-791. [PubMed] |
| 180. | Mannucci E, Ognibene A, Cremasco F, Bardini G, Mencucci A, Pierazzuoli E, Ciani S, Fanelli A, Messeri G, Rotella CM. Glucagon-like peptide (GLP)-1 and leptin concentrations in obese patients with Type 2 diabetes mellitus. Diabet Med. 2000;17:713-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 181. | Zhang Y, Scarpace PJ. The role of leptin in leptin resistance and obesity. Physiol Behav. 2006;88:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 182. | Anini Y, Brubaker PL. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes. 2003;52:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 200] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 183. | Bewick GA, Kent A, Campbell D, Patterson M, Ghatei MA, Bloom SR, Gardiner JV. Mice with hyperghrelinemia are hyperphagic and glucose intolerant and have reduced leptin sensitivity. Diabetes. 2009;58:840-846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 184. | Daghestani MH. A preprandial and postprandial plasma levels of ghrelin hormone in lean, overweight and obese Saudi females. Journal of King Saud University - Science. 2009;21:119-124. [DOI] [Full Text] |
| 185. | English PJ, Ghatei MA, Malik IA, Bloom SR, Wilding JP. Food fails to suppress ghrelin levels in obese humans. J Clin Endocrinol Metab. 2002;87:2984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 278] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 186. | Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, Taveras C, Schrope B, Bessler M. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond). 2009;33:786-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 187. | Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 280] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 188. | le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 821] [Cited by in RCA: 734] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 189. | Kellum JM, Kuemmerle JF, O'Dorisio TM, Rayford P, Martin D, Engle K, Wolf L, Sugerman HJ. Gastrointestinal hormone responses to meals before and after gastric bypass and vertical banded gastroplasty. Ann Surg. 1990;211:763-770; discussion 770-771. [PubMed] |
| 190. | Falkén Y, Hellström PM, Holst JJ, Näslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab. 2011;96:2227-2235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 239] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 191. | Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg. 2006;93:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 240] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 192. | Morínigo R, Moizé V, Musri M, Lacy AM, Navarro S, Marín JL, Delgado S, Casamitjana R, Vidal J. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91:1735-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 293] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 193. | Maggard MA, Shugarman LR, Suttorp M, Maglione M, Sugerman HJ, Sugarman HJ, Livingston EH, Nguyen NT, Li Z, Mojica WA. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142:547-559. [PubMed] |
| 194. | Sarson DL, Scopinaro N, Bloom SR. Gut hormone changes after jejunoileal (JIB) or biliopancreatic (BPB) bypass surgery for morbid obesity. Int J Obes. 1981;5:471-480. [PubMed] |
| 195. | Holst JJ, Sørensen TI, Andersen AN, Stadil F, Andersen B, Lauritsen KB, Klein HC. Plasma enteroglucagon after jejunoileal bypass with 3: 1 or 1: 3 jejunoileal ratio. Scand J Gastroenterol. 1979;14:205-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 64] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 196. | Buchan AM, Pederson RA, Koop I, Gourlay RH, Cleator IG. Morphological and functional alterations to a sub-group of regulatory peptides in human pancreas and intestine after jejuno-ileal bypass. Int J Obes Relat Metab Disord. 1993;17:109-113. [PubMed] |
| 197. | Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322:449-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 648] [Cited by in RCA: 589] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 198. | Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924-935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 621] [Cited by in RCA: 550] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 199. | van Bloemendaal L, Veltman DJ, Ten Kulve JS, Groot PF, Ruhé HG, Barkhof F, Sloan JH, Diamant M, Ijzerman RG. Brain reward-system activation in response to anticipation and consumption of palatable food is altered by glucagon-like peptide-1 receptor activation in humans. Diabetes Obes Metab. 2015;17:878-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 200. | van Bloemendaal L, IJzerman RG, Ten Kulve JS, Barkhof F, Konrad RJ, Drent ML, Veltman DJ, Diamant M. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes. 2014;63:4186-4196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 269] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 201. | Witt AA, Raggio GA, Butryn ML, Lowe MR. Do hunger and exposure to food affect scores on a measure of hedonic hunger? An experimental study. Appetite. 2014;74:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 202. | Singh M. Mood, food, and obesity. Front Psychol. 2014;5:925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 214] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 203. | van Bloemendaal L, Veltman DJ, ten Kulve JS, Drent ML, Barkhof F, Diamant M, IJzerman RG. Emotional eating is associated with increased brain responses to food-cues and reduced sensitivity to GLP-1 receptor activation. Obesity (Silver Spring). 2015;23:2075-2082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 204. | Scott LJ. Liraglutide: a review of its use in the management of obesity. Drugs. 2015;75:899-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 205. | Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, Lau DC, le Roux CW, Violante Ortiz R, Jensen CB. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N Engl J Med. 2015;373:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1103] [Cited by in RCA: 1576] [Article Influence: 157.6] [Reference Citation Analysis (0)] |
| 206. | Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, Aronne L. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013;37:1443-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 513] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 207. | Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjøth TV, Andreasen AH, Jensen CB, DeFronzo RA. Efficacy of Liraglutide for Weight Loss Among Patients With Type 2 Diabetes: The SCALE Diabetes Randomized Clinical Trial. JAMA. 2015;314:687-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 711] [Article Influence: 71.1] [Reference Citation Analysis (0)] |
| 208. | Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011;69:664-679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 551] [Cited by in RCA: 493] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 209. | van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond). 2014;38:784-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 354] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 210. | Vallöf D, Maccioni P, Colombo G, Mandrapa M, Jörnulf JW, Egecioglu E, Engel JA, Jerlhag E. The glucagon-like peptide 1 receptor agonist liraglutide attenuates the reinforcing properties of alcohol in rodents. Addict Biol. 2016;21:422-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 211. | Bray GA. Why do we need drugs to treat the patient with obesity? Obesity (Silver Spring). 2013;21:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 212. | Pi-Sunyer FX, Astrup A, Fujioka K, Greenway FL, Halpern A, Krempf M, Lau DC, le Roux C, Ortiz RV, Jensen CB. Liraglutide 3.0 Mg Reduces the Prevalence of Prediabetes and Delays Onset of Type 2 Diabetes in Overweight and Obese Adults: Results from Scale Obesity and Prediabetes, a Randomized, Double-Blind and Placebo-Controlled 56-Week Trial. 2014. Available from: http://press.endocrine.org/doi/abs/10.1210/endo-meetings.2014.OABA.19.PP05-4#sthash.adiubGqI.dpuf. |
| 213. | Public Health England. Adult obesity and type 2 diabetes. 2014: p6-p17. [accessed 2015 Nov 17]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/338934/Adult_obesity_and_type_2_diabetes_.pdf. |
| 214. | Mannucci E, Ognibene A, Cremasco F, Bardini G, Mencucci A, Pierazzuoli E, Ciani S, Messeri G, Rotella CM. Effect of metformin on glucagon-like peptide 1 (GLP-1) and leptin levels in obese nondiabetic subjects. Diabetes Care. 2001;24:489-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 232] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 215. | Campbell IW, Howlett HC. Worldwide experience of metformin as an effective glucose-lowering agent: a meta-analysis. Diabetes Metab Rev. 1995;11 Suppl 1:S57-S62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 216. | Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of Type 1 and Type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med. 2012;29:855-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 568] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 217. | Novo Nordisk. Saxenda; injectable liraglutide for obesity. Drugs information leaflet ONLINE. Revised 2015. Available from: https://www.saxenda.com/. |
| 218. | European Medicines Agency. Assessment report: Saxenda. 2015. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/003780/WC500185788.pdf. |
| 219. | Haluzík M, Mráz M, Svačina Š. Balancing benefits and risks in patients receiving incretin-based therapies: focus on cardiovascular and pancreatic side effects. Drug Saf. 2014;37:1003-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 220. | Nauck MA, Friedrich N. Do GLP-1-based therapies increase cancer risk? Diabetes Care. 2013;36 Suppl 2:S245-S252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |