Published online Aug 15, 2025. doi: 10.4239/wjd.v16.i8.108101
Revised: May 8, 2025
Accepted: July 2, 2025
Published online: August 15, 2025
Processing time: 131 Days and 18.7 Hours
Diabetic cystopathy (DCP) is a complication affecting the lives of people with diabetes. However, the pathogenesis of DCP is not well known.
To investigate the potential mechanisms by which cAMP-responsive element-binding protein 3 like 3 (CREB3 L3) promotes the occurrence and development of DCP.
High-throughput sequencing was used to analyze differentially expressed genes (DEGs) in bladder urothelium from patients with DCP and healthy controls. Gene enrichment analysis was conducted to assess the biological functions of DEG. Small interfering RNA technology was performed to silence the CREB3 L3 gene in both in vitro and in vivo experiments. Morphological changes in bladder uro
We identified significant DEGs through high-throughput sequencing. These genes were primarily enriched in inflammatory activation, epithelial-mesenchymal transition (EMT) and tight junction organization. Upregulated expression of both CREB3 L3 and C-reactive protein (CRP) in bladder urothelium from patients with DCP was accompanied by upregulated EMT markers including N-cadherin and vimentin proteins, but downregulated E-cadherin. Silencing CREB3 L3 attenuated the protein expression of CRP and EMT in SV-HUC-1 urothelial cells under hyperglycemic conditions and in the diabetes mellitus rat model at 4, 8, and 12 weeks. CREB3 L3 knockdown also reversed downregulation of the tight junction proteins occludin and claudin 1.
Hyperglycemia induces the upregulation of CREB3 L3 expression, thereby promoting the EMT and impairing tight junctions in bladder urothelial cells. Targeting CREB3 L3 in bladder urothelial cells is likely to be a key approach in preventing and treating DCP.
Core Tip: Diabetic cystopathy (DCP), a debilitating complication of diabetes mellitus, involves hyperglycemia-driven bladder dysfunction. This study identified cAMP-responsive element-binding protein 3 like 3 (CREB3 L3) as a novel mediator of DCP pathogenesis. Chronic hyperglycemia induced CREB3 L3 overexpression in bladder urothelium, pro
- Citation: Wu QG, Zhang MJ, Lan YB, Ma CL, Fu WJ. Hyperglycemia-induced overexpression of CREB3 L3 promotes the epithelial-to-mesenchymal transition in bladder urothelial cells in diabetes mellitus. World J Diabetes 2025; 16(8): 108101
- URL: https://www.wjgnet.com/1948-9358/full/v16/i8/108101.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i8.108101
Diabetes mellitus (DM), a chronic non-communicable disease, has become a global epidemic, severely threatening human health and imposing substantial socioeconomic burdens. It is characterized by chronic hyperglycemia due to insulin deficiency (type 1 DM) or insulin resistance type 2 DM (T2DM). Chronic hyperglycemia can result in a variety of complications, including peripheral neuropathy, retinopathy, and cardiac pathologies including myocardial fibrosis, coronary artery disease, and diabetic cardiomyopathy[1,2]. Diabetic cystopathy (DCP), also known as diabetic bladder dysfunction (DBD), is a bladder dysfunction disorder that occurs in patients with DM when stimulated by chronically high blood glucose. Lower urinary tract symptoms, including overactive bladder (OAB) and voiding dysfunction, are the characteristics of DCP[3]. DCP can be divided into compensated and decompensated stages. The compensated stage is characterized by the symptoms of OAB such as urinary frequency, urgency, and urge urinary incontinence. The decompensated stage is characterized by bladder contraction disorder, difficulty urinating, bladder sensory function impairment, and urinary retention.
The bladder urothelium senses stimuli through associated receptor proteins and releases neurotransmitters such as acetylcholine and adenosine triphosphate, which transmit signals to submucosal nerves, connective tissue, and muscle tissue, and participates in the regulation of bladder contraction function[4]. Stimulation due to hyperglycemia can lead to peripheral neuropathy and a reduction in the release of associated neurotransmitters, resulting in dysfunction of the nerves innervating the bladder[5,6]. Simultaneously, hyperglycemia can lead to alterations in, for example, cholinergic receptors in the inferior detrusor and calcium channels manipulated by the calcium depot, which affect detrusor function[7,8]. Impairment of bladder urothelial integrity and function contributes to the development of DCP and leads to dysfunctional bladder urothelium perception and signaling, in turn affecting bladder voiding function[9]. In addition, related studies have also found decreased numbers of Cajal interstitial cells in the detrusor of patients with DCP, impacting bladder autonomic rhythm as well as contraction and diastolic function[10].
Studies have shown abnormal activation of the inflammatory response in the bladder urothelium in DCP and an increase in inflammatory cells compared with healthy controls (HCs)[11]. In a rat model of DCP, remission of symptoms in the decompensated phase of DCP was accompanied by a reduction in inflammatory cell infiltration in the bladder urothelium[12]. The inflammatory response has previously been shown to promote the phenotypic transition of epithelial cells to mesenchymal cells, a process known as the epithelial-mesenchymal transition (EMT)[13,14]. The EMT can disrupt cellular tight junctions, leading to impaired epithelial integrity and even triggering the development of tissue fibrosis and causing diabetes-related complications[15,16].
The pathogenesis of DCP is complex and involves multiple mechanisms such as the detrusor muscle of the bladder, urothelium, and nerves. The hyperglycemic state induced by diabetes can damage the integrity of the bladder uro
This study focused on the effects of related inflammatory pathways on the bladder urothelium, and RNA sequencing analyses were performed to identify genes that were differentially expressed between patients with DCP and HCs. One of the significantly upregulated genes, cAMP-responsive element-binding protein 3 Like 3 (CREB3 L3), also known as CREBH, was found to be associated with the inflammatory reaction, the EMT, and impaired cellular tight junctions in patients with DCP. These effects of CREB3 L3 in DCP were confirmed by both in vitro and in vivo experiments. As the effects of CREB3 L3 Lead to bladder urothelium damage, promoting DCP development and progression, CREB3 L3 is likely a promising target for the prevention and treatment of DCP.
Inclusion criteria for patients with DCP were as follows[3]: (1) Confirmed T2DM; (2) Lower urinary tract symptoms such as frequent urination, urgent urination, and dysuria; and (3) Abnormal urodynamic test.
Exclusion criteria were: (1) Prostate cancer, bladder cancer or other lower urinary tract malignancies; (2) History of pelvic surgery such as radical prostatectomy, radical cystectomy, colorectal surgery or hysterectomy; (3) Complicated by central nervous system diseases such as Parkinson's disease, multiple sclerosis, and cerebral hemorrhage; (4) Spinal-related diseases such as spinal meningocele or meningocele; (5) Recurrent urinary tract infections; and (6) Complications of bladder outlet obstruction such as benign prostatic hyperplasia, urethral stricture, and bladder neck contracture.
The HC group consisted of volunteers who underwent cystoscopy for non-bladder diseases (such as indwelling ureteral stents before percutaneous nephrolithotomy), and those with urinary system diseases were strictly excluded.
This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (Ethics Approval No. 2023-E046-01). All participants provide written informed consent. A total of 6 patients with DCP and 6 HCs were included in the study. All procedures were performed in accordance with the Helsinki Declaration and ethical approval.
A biopsy of the posterior wall of the bladder approximately 2 cm above the ureteral orifice was performed to obtain human bladder urothelium. Only the bladder mucosa and submucosa were taken to prevent bladder perforation. The normal control (NC) group underwent the same biopsy procedure. The bladder was spread flat on a sterile filter paper (with the mucosal surface facing upward), and the bladder mucosal epithelial layer was scraped along the submucosa in parallel using a blunt dissector, following a previously published method[17].
Next, we obtained bladder urothelium from Sprague-Dawley (SD) rats. The anesthetized rats were sacrificed by cervical dislocation, and the complete bladder was surgically removed and was longitudinally incised. The bladder urothelial layer was carefully lifted. Separation was carried out along its natural interface, and the connective tissue connecting the detrusor muscle below it was cut off, thereby achieving separation of the urothelial layer from the bladder detrusor muscle. This was achieved by following a previously published method[18].
Samples of both human and animal bladder tissues were collected using random sampling in this study. RNA isolated from bladder urothelium from patients with DCP (n = 3) and the HC group (n = 3) was subjected to second-generation mRNA sequencing. Total RNA was extracted from the urothelium of each group using the HaiGene Extreme RNA Extraction Kit (Harbin,China) , and RNA integrity was determined by 1% agarose gel electrophoresis. Contaminated ribosomes were removed from the samples using the Epicentre Ribo-ZeroTM Kit(Merck Life Science,Germany). For cDNA synthesis and end repair of purified cDNA, oligo(dT)-20 primers were used according to the instructions provided for the 1st Strand cDNA Synthesis Kit (PrimeScript II; TaKaRa Bio Inc., Shiga, Japan). The cDNA libraries were con
Finally, we used StringTie v2.1.1 software to analyze spliced transcripts and to perform expression calculations. Transcripts per kilobase of exon model per million mapped reads were used to correct for the relative expression levels of different transcripts.
The DESeq2 package in RStudio (V.4.0.2) was used to screen for differentially expressed genes (DEGs) with a log2|fold change| greater than 4 and an adjusted P value less than 0.05. Volcano maps and heatmaps were constructed using the ggplot2 and the pheatmap package, respectively. The Gene Ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis were performed on DEGs using the DAVID database (https://david.ncifcrf.gov/tools.jsp). The data were visualized using the Microbiology Letter online website (https://www.bioinformatics.com.cn/).
In this study, a rat model of DCP caused by T2DM was selected as the research model[19]. We obtained adult male SD rats (6-8 weeks old, 250 ± 50 g) from the Animal House Centre in Guangxi Medical University (Guangxi, China).
The DCP rat model was prepared following a previously published method[20]. A total of 45 male SD rats were randomly divided into the following three groups: NC group, DCP group (DM) and DCP + CREB3 L3 knockdown (KD) group (DM + KD), and each group consisted of 15 rats.
The rats were housed in a specific pathogen-free animal house. To establish the DCP model, the animals were given a high-fat and high-sugar diet (containing 1% sodium cholate, 2% cholesterol, 10% lard, 20% sucrose, and 67% basal diet). The NC group was given basal feed. In addition, the rats in the DM and DM + KD groups were injected intraperitoneally with 1% streptozotocin (STZ) dissolved in 0.1 M citrate buffer solution at a dose of 30 mg/kg; rats in the NC group were injected with 0.1 M citrate buffer solution. After 3 days of STZ injection, the fasting blood glucose was greater than 16.7 mmol/L, suggesting that the T2DM model was successfully established. The high-fat diet was continued for 8 weeks, and model rats were assessed using a urodynamic test, and rats in the DM and DM + KD groups exhibited irregular micturition patterns and significantly increased bladder capacity.
Blood glucose and body weight of the rats in each group were monitored once a week. At the designated time points of 4, 8, and 12 weeks, five rats in each group were euthanized by carbon dioxide (CO2) inhalation, and the bladder tissues were removed for weighing and histopathological examination.
All procedures were conducted in accordance with ARRIVE guidelines. Animal experiments were approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (No. 2023-S753-01) and all methods were carried out in accordance with relevant guidelines and regulations.
SV-HUC-1 cells are derived from normal human ureteral epithelium, and not from the bladder. However, these cells retain the characteristics of non-cancerous urothelial cells (e.g., barrier function and cell differentiation markers), and are suitable for simulating the physiological and pathological changes in the urothelium of the diabetic bladder (e.g., hyperglycemia-induced oxidative stress, inflammation and barrier damage), making it a reasonable alternative model[21,22].
Human SV-HUC-1 ureteral immortalized urothelial cells (Cat No. CC4009; Cellcook, Guangzhou,China) were cultured in Dulbecco's modified Eagle's medium/F12 (DMEM/F12; Invitrogen, Carlsbad, CA, United States) supplemented with 10% fetal bovine serum (FBS; Invitrogen) at 37 °C with 5% CO2. The DMEM/F12 in the NC group contained 5 mmol/L glucose, whereas the medium in the high-glucose (HG) group contained 15 mmol/L and 30 mmol/L glucose.
Cells cultured in vitro were treated directly with RIPA lysis buffer (G2002; Servicebio, Hubei, China). Tissue samples were processed with a homogenizer (KZ-II; Servicebio) after the addition of RIPA lysis buffer. The protein concentration was measured using a protein concentration assay kit (G2026; Servicebio). The protein solution was denatured in a boiling water bath for 15 minutes after adding 5 × protein loading buffer (G2013; Servicebio) at a 4:1 ratio. Gels were prepared according to the instructions provided for the sodium dodecyl sulfate polyacrylamide gel electrophoresis gel preparation kit (G2003; Servicebio). For membrane transfer, the voltage was set to 75 volts for the concentration gels and 120 volts for the separation gels. The conditions for polyvinylidene fluoride membrane transfer (G6015-0.45 µm; Servicebio) were set to a constant current of 300 mA for 30 minutes. Then we incubated the sections with primary and secondary antibodies according to the antibody instructions. Antibody information is detailed in Table 1. β-actin (ACTIN) was selected as the internal reference protein based on its stable expression. Primary antibody dilutions (e.g., ACTIN at 1:1000) followed the manufacturer-recommended protocols. Three independent biological replicates were included for each experimental group (n = 3). ECL solution (G2019; Servicebio) was used for color development.
| Antibody name | Manufacturers | CAS | Molecular weight (kDa) | Species | Dilution ratio |
| ACTIN | Servicebio | GB15003 | 42 | Rabbit | 1:1000 |
| Occludin | Servicebio | GB111401 | 59 | Rabbit | 1:500 |
| Claudin 1 | servicebio | GB11032 | 20 | Rabbit | 1:500 |
| CRP | ABCLONAL | A0224 | 25 | Rabbit | 1:500 |
| CREB3 L3 | Biorbyt | orb382910 | 49 | Rabbit | 1:500 |
| N-cadherin | servicebio | GB111273 | 140 | Rabbit | 1:500 |
| Vimentin | Servicebio | GB111308 | 54 | Rabbit | 1:500 |
| E-cadherin | Servicebio | GB11082 | 135 | Rabbit | 1:500 |
Total RNA from bladder tissues and urothelial cells was extracted using TRIzol reagent (Invitrogen). The cells were cultured for 24, 48, 72, 96, and 120 hours, and the mRNA expression of CREB3 L3 was determined. The mRNA was reverse transcribed to cDNA using the PrimeScript RT Kit (Takara). Primers were designed using Oligo 7.0 software. The primers used in this experiment were synthesized by Shanghai Sangon Biotech (Shanghai, China), and the upstream and downstream primer sequences are shown in Table 2. Quantitative PCR (qPCR) assays were performed using the ChamQ Universal SYBR qPCR Master Mix (Q711-02; Novozymes, Bagsværd, Denmark) according to the manufacturer’s instructions. The 7900 Sequence Detection System (Applied Biosystems, Foster City, CA, United States) was used for DNA amplification. The real-time fluorescent qPCR protocol was as follows: 50 °C for 2 minutes, 95 °C for 5 minutes, 95 °C for 15 seconds, 56 °C for 20 seconds, and 72 °C for 40 seconds for 40 cycles. The relative expression levels of target genes were calculated using the 2-ΔΔCt method.
| Genes | Direction | Sequence (5’→3’) |
| CREB3 L3 | Forward | ATCCTGGCAACTCTTGCTCC |
| CREB3 L3 | Reverse | AGGTGATGCTGTTGCAGGTC |
| GAPDH | Forward | GAGTCAACGGATTTGGTCGT |
| GAPDH | Reverse | GACAAGCTTCCCGTTCTCAG |
SV-HUC-1 cells were inoculated in 6-well plates at 5 × 105 cells per well prior to transfection. The cells were divided into NC, scrambled small interfering RNA NC (si-NC), and siRNA-CREB3 L3 groups. Then the cells were transfected according to the manufacturer’s instructions for Lipofectamine 3000 (Shanghai Jiya Biotechnology Co., Ltd., Shanghai, China). When the cells had grown to approximately 50% confluence, the cell medium was spiked with 5 μL Lipofectamine 3000 pmol/mL and 50 pmol/μL siRNA-CREB3 L3, and incubation was continued for 6 hours. The medium was then replaced with fresh DMEM/F12, and incubation was continued for 48 hours for subsequent experiments. The siRNAs were synthesized by Girma Biotechnology (Shanghai, China), and their sequences are shown in Table 3.
| Gene name | Sense sequence (5'--3') | Antisense sequence (5'--3') |
| si-NC | ACGUGACACGUUCGGAGAA | UUCUCCGAACGUGUCACGU |
| si-CREB3 L3-1 | GGAAGAAGAAGAAGGAAUAUA | UAUUCCUUCUUCUUCUUCCUG |
| si-CREB3 L3-2 | CGAGAAGAGUCUCCAGGAAGC | UUCCUGGAGACUCUUCUCGGG |
| si-CREB3 L3-3 | GUACGAGUGUUCUCCAGAACU | UUCUGGAGAACACUCGUACAG |
For CREB3 L3 KD in rat bladder, the siRNA1-CREB3 L3 was enclosed in Lentivirus (Genepharma, Shanghai, China), forming the Lentivirus-siRNA1-CREB3 L3 vector. CREB3 L3 KD was induced by intravesical instillation of the Lentivirus-siRNA1-CREB3 L3 (108-10 IU), according to a previously published method[23].
SV-HUC-1 cells were divided into the NC group and HG group. DMEM/F12 in the NC group contained 5 mmol/L glucose, whereas DMEM/F12 in the high-glucose group contained 15 mmol/L and 30 mmol/L glucose. The cells in each group were incubated for 24, 48, 72, 96, and 120 hours, and then 10 μL Cell Counting Kit-8 reagent was added to each well. The cells were incubated at 37 °C for 2 hours. After incubation, absorbance values were measured at 450 nm, and relative cell viability was calculated.
Bladder tissue was fixed in a 4% paraformaldehyde solution for 24 hours for fixation, followed by dehydration and transparency using a gradient of alcohol and xylene, respectively. The bladder tissues were placed in an embedding machine for paraffin embedding. The bladder tissues were cut into 4 μm sections and baked at 60 °C overnight. The sections were then dewaxed using xylene and an alcohol gradient. The sections were washed for 5 minutes using distilled water. The staining was carried out according to the instructions provided for the hematoxylin and eosin (H&E) staining kit. Finally, the sections were observed under an inverted microscope and photographed.
Bladder tissue was dehydrated, hyalinized, paraffin embedded, and sectioned as described above for the H&E staining procedure. For antigen repair, the tissue was covered with proteinase K working solution and incubated at 37 °C for 25 minutes. The tissues were then incubated in a solution of Triton X-100 (0.1%) in phosphate-buffered saline (PBS) for 20 minutes at ambient temperature. The sections were blocked in a 3% BSA solution for 5 minutes. A primary antibody diluted at the ratio indicated in the antibody instructions was then added dropwise to the sections. The sections were washed three times with PBS, covered with secondary antibodies and incubated for 50 minutes at room temperature in the dark. The sections were again washed three times with PBS. The nuclei were then stained with DAPI and incubated for 10 min at ambient temperature, protected from light. SV-HUC-1 cells were transfected with si-CREB3 L3-1 after 24 hours of treatment with 15 mmol/L glucose and then cultured in DMEM/F12 with 15 mmol/L glucose for 48 hours. Subsequently, the cells were examined for Vimentin, N-cadherin and E-cadherin by immunofluorescence microscopy (DM5500 B; Leica, Mannheim, Germany), and images were acquired.
ImageJ software applied automated intensity thresholds to define regions of interest (ROIs), ensuring consistent signal detection across replicates. In bladder tissues, ROIs were restricted to the urothelial layer (excluding submucosa and detrusor muscle) to focus on epithelial-specific changes. For cellular analysis, only intact, adherent cells with clear nuclei (DAPI-stained) were included. For tissue sections, 5-10 non-overlapping fields per slide were randomly selected across the entire urothelial layer to avoid selection bias.
GraphPad Prism 8.0 was used for statistical analyses. The results of the experimental data are expressed as the mean ± SD. The statistical significance of the differences between the two groups was determined with an unpaired two-tailed student's t test. One-way analysis of variance was used to make comparisons between more than two groups. P < 0.05 was considered statistically significant.
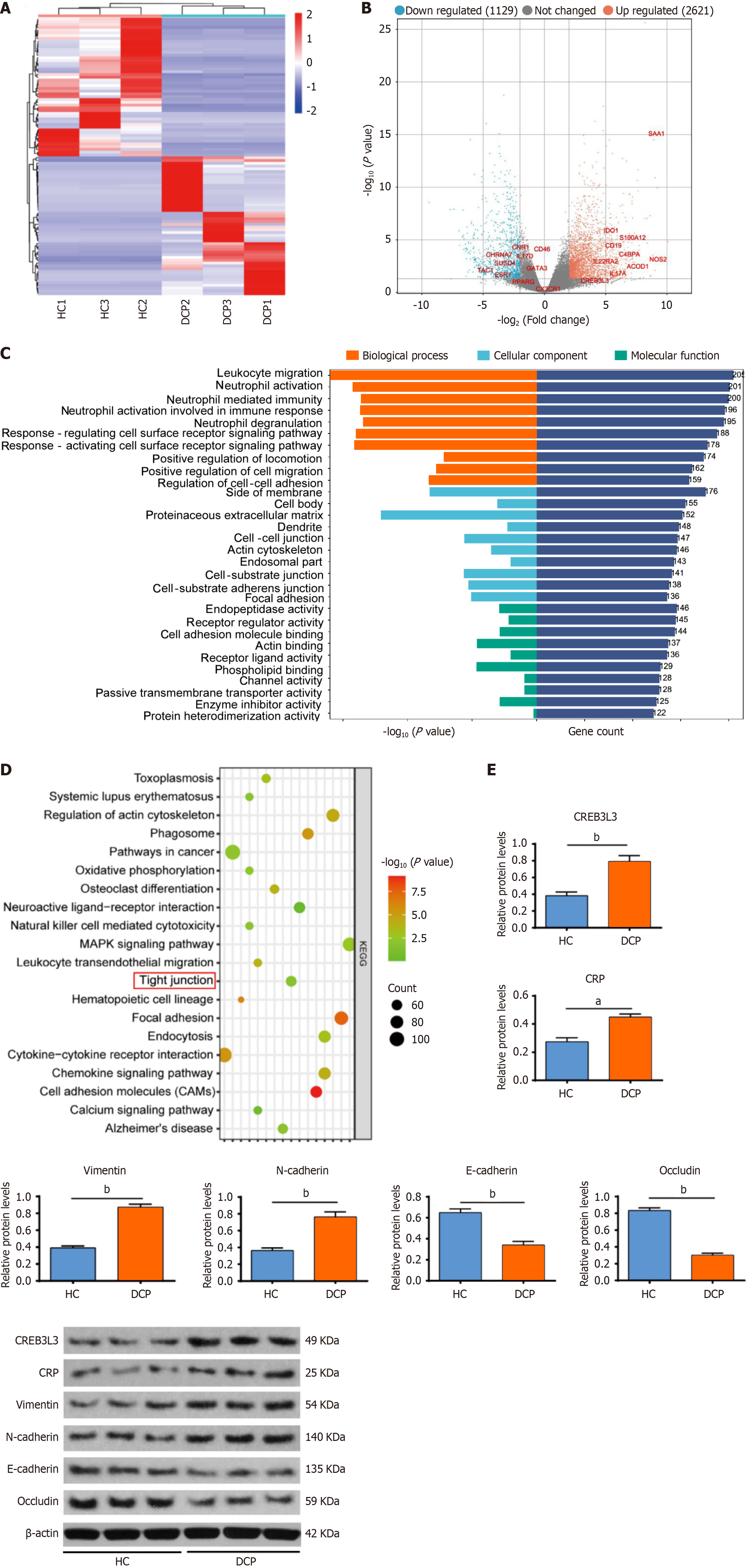
Second-generation sequencing was conducted to detect genes that were differentially expressed in bladder urothelium between patients with DCP and HCs. We identified a total of 3750 differentially expressed mRNAs, including 2621 upregulated genes and 1129 downregulated genes in bladder urothelium from patients with DCP, based on a log2 |fold change| > 4 and an adjusted P < 0.05 (Figure 1A and B). The upregulated genes were subjected to GO enrichment analysis.
The upregulated genes were associated with biological processes, such as inflammation processes (e.g., leukocyte migration, neutrophil activation and neutrophil mediated immunity), the EMT, and tight junction organization (Figure 1C). The results of KEGG enrichment analysis also showed that the upregulated genes were associated with pathways such as tight junctions, focal adhesion, and cell adhesion (Figure 1D). We then screened 168 mRNAs that may be involved in regulating inflammatory responses, including 10 mRNAs with more than a 2-fold difference in up
| Ensemble Gene ID | Gene name | Log2Fold change | P value | Regulation |
| ENSG00000173432 | SAA1 | 9.14073 | 0.00000 | Up |
| ENSG00000007171 | NOS2 | 7.36349 | 0.00141 | Up |
| ENSG00000102794 | ACOD1 | 7.16781 | 0.00226 | Up |
| ENSG00000163221 | S100A12 | 7.06422 | 0.00001 | Up |
| ENSG00000123838 | C4BPA | 6.64444 | 0.00071 | Up |
| ENSG00000112115 | IL17A | 6.23562 | 0.01454 | Up |
| ENSG00000164485 | IL22RA2 | 6.02855 | 0.00162 | Up |
| ENSG00000060566 | CREB3 L3 | 5.75447 | 0.01491 | Up |
| ENSG00000177455 | CD19 | 5.62606 | 0.00004 | Up |
| ENSG00000131203 | IDO1 | 5.55962 | 0.00000 | Up |
| ENSG00000006128 | TAC1 | -3.38685 | 0.00907 | Down |
| ENSG00000143502 | SUSD4 | -2.17976 | 0.00717 | Down |
| ENSG00000175344 | CHRNA7 | -2.17060 | 0.00039 | Down |
| ENSG00000118432 | CNR1 | -2.08059 | 0.00014 | Down |
| ENSG00000172458 | IL17D | -2.01522 | 0.00173 | Down |
| ENSG00000091831 | ESR1 | -1.97610 | 0.01013 | Down |
| ENSG00000117335 | CD46 | -1.69696 | 0.00128 | Down |
| ENSG00000132170 | PPARG | -1.65880 | 0.01276 | Down |
| ENSG00000107485 | GATA3 | -1.53300 | 0.00175 | Down |
| ENSG00000168329 | CX3CR1 | -1.47299 | 0.02335 | Down |
CREB3 L3 plays important roles in metabolic inflammation and inflammation-related diseases partially by regulating the transcription of CRP[23,24]. The inflammatory response has previously been shown to promote EMT[13,14]. The EMT can disrupt cellular tight junctions, leading to impaired epithelial integrity[15,16]. Therefore, we speculated that the CREB3 L3-mediated inflammatory response promotes EMT and further disrupts cellular tight junctions.
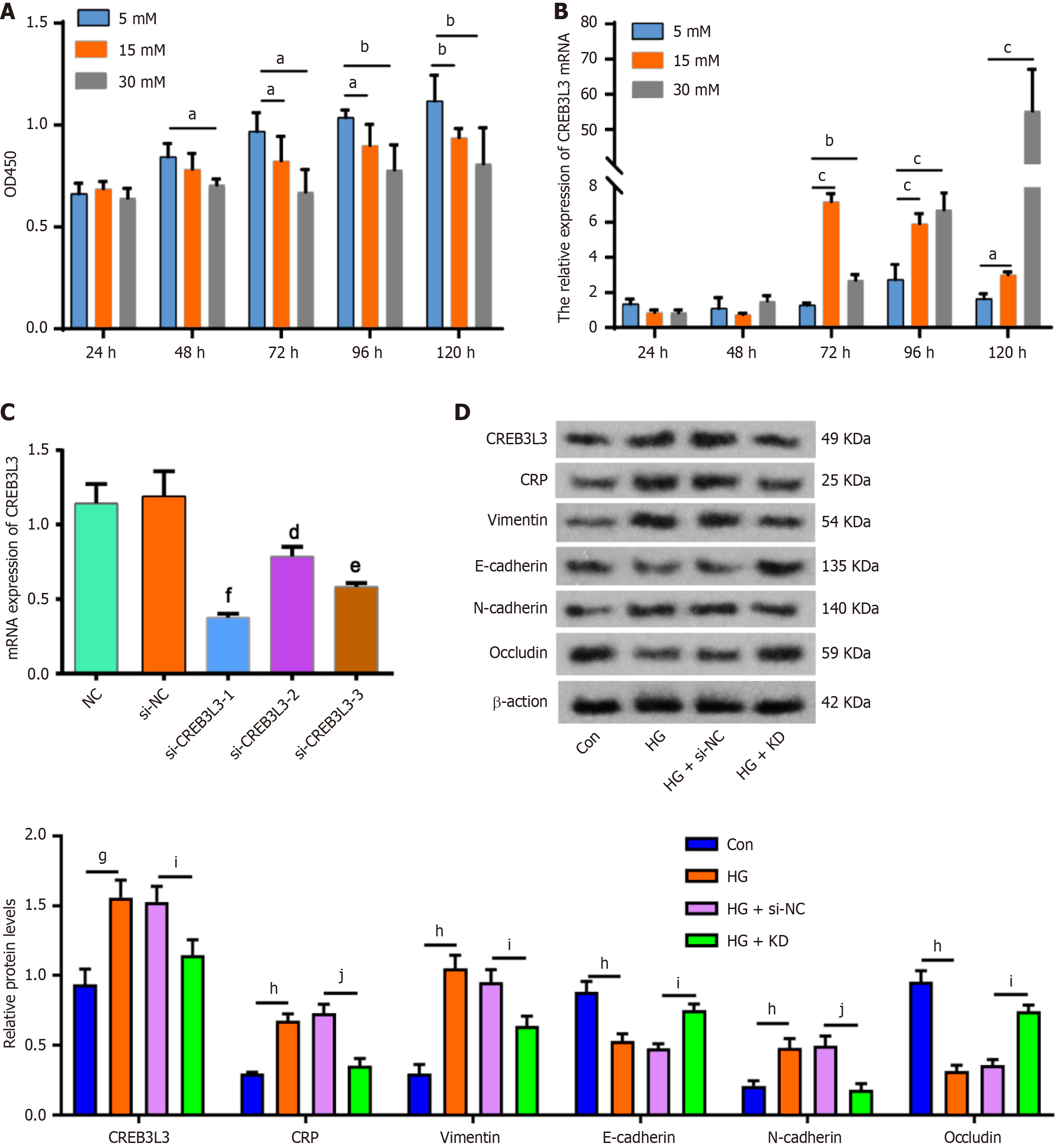
To confirm this speculation, we conducted a series of in vitro and in vivo studies. The in vitro study showed that hyperglycemia, especially at 30 mmol/L, inhibited SV-HUC-1 cell viability from 48 hours to 120 hours (Figure 2A). Subsequently, we examined CREB3 L3 expression in SV-HUC-1 cells following exposure to hyperglycemia for different time periods using qPCR. Compared with 5 mmol/L glucose, CREB3 L3 mRNA expression was significantly increased by 15 mmol/L glucose stimulation for 72, 96 and 120 hours with the highest expression following stimulation for 72 hours. The mRNA expression of CREB3 L3 was also upregulated upon 30 mmol/L glucose stimulation for 72, 96, and 120 hours, but the highest expression by stimulation for 120 hours (Figure 2B). These findings indicated that hyperglycemia can promote the mRNA expression of CREB3 L3.
Subsequently, we silenced the expression of CREB3 L3 using three siRNAs. Of these siRNAs, si-CREB3 L3-1 most effectively downregulated CREB3 L3 mRNA expression (Figure 2C).
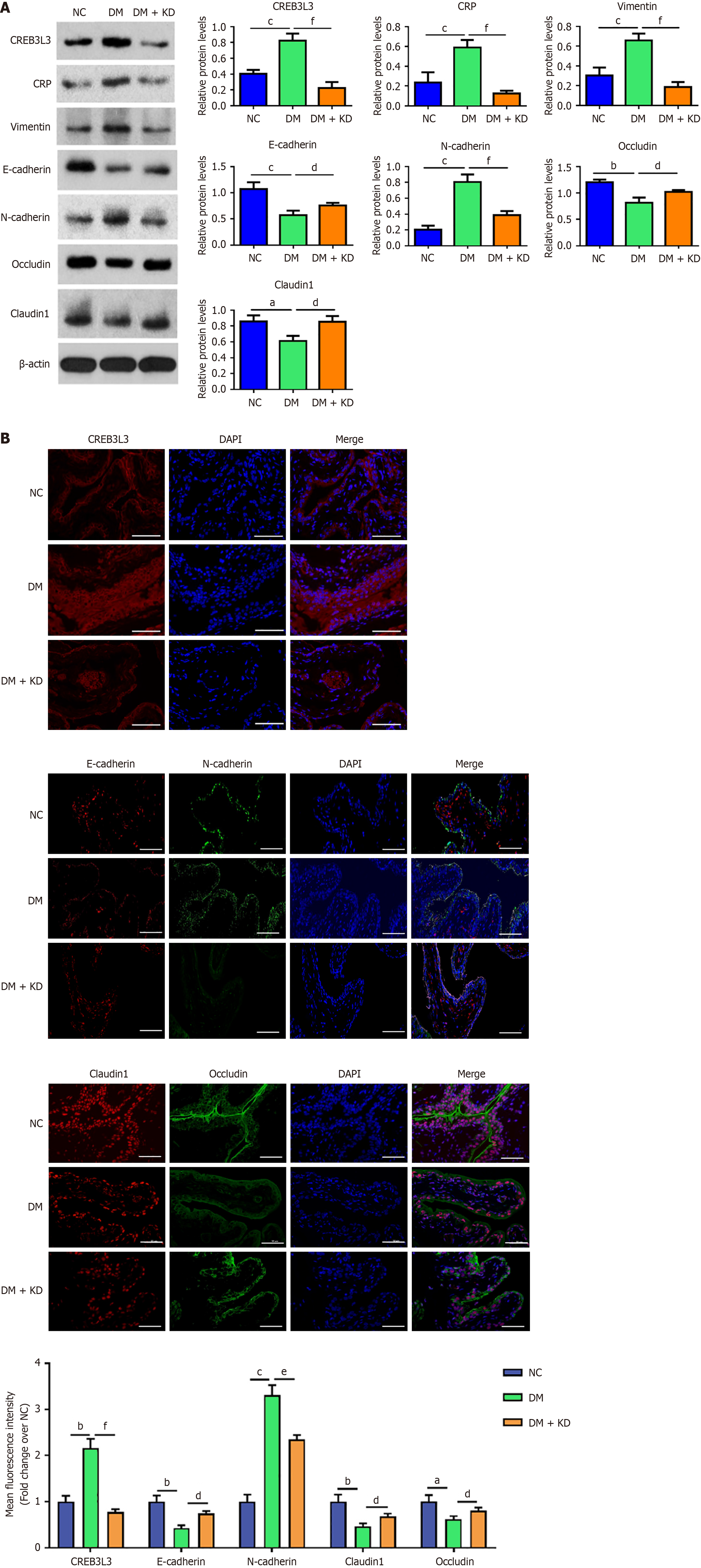
As indicated by western blot analysis, CREB3 L3, CRP, vimentin and N-cadherin proteins were upregulated in the HG group, while E-cadherin and occludin proteins were downregulated. However, the upregulation of CRP, vimentin, and N-cadherin proteins was attenuated in the HG + KD group, and E-cadherin and occludin proteins were upregulated (Figure 2D).
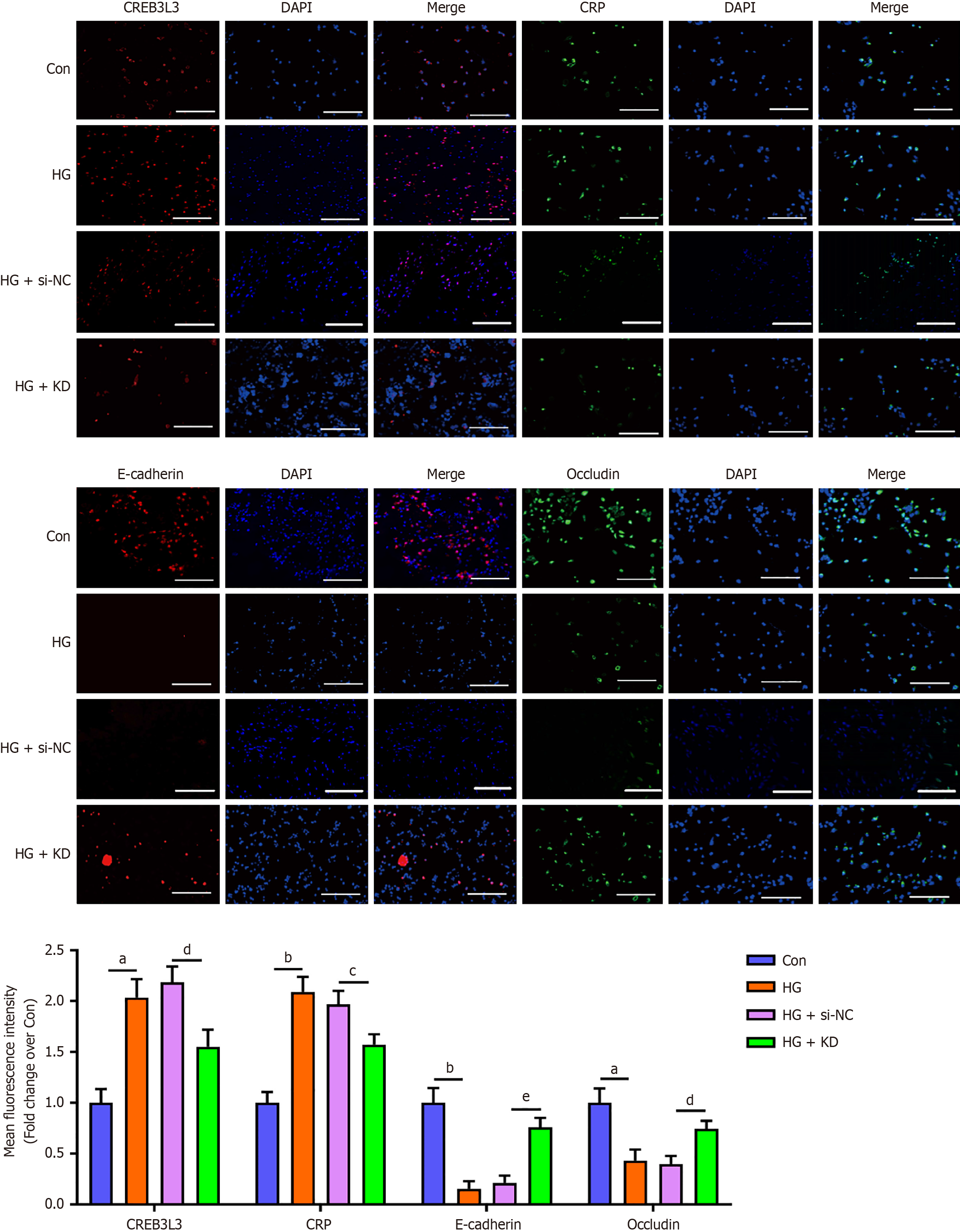
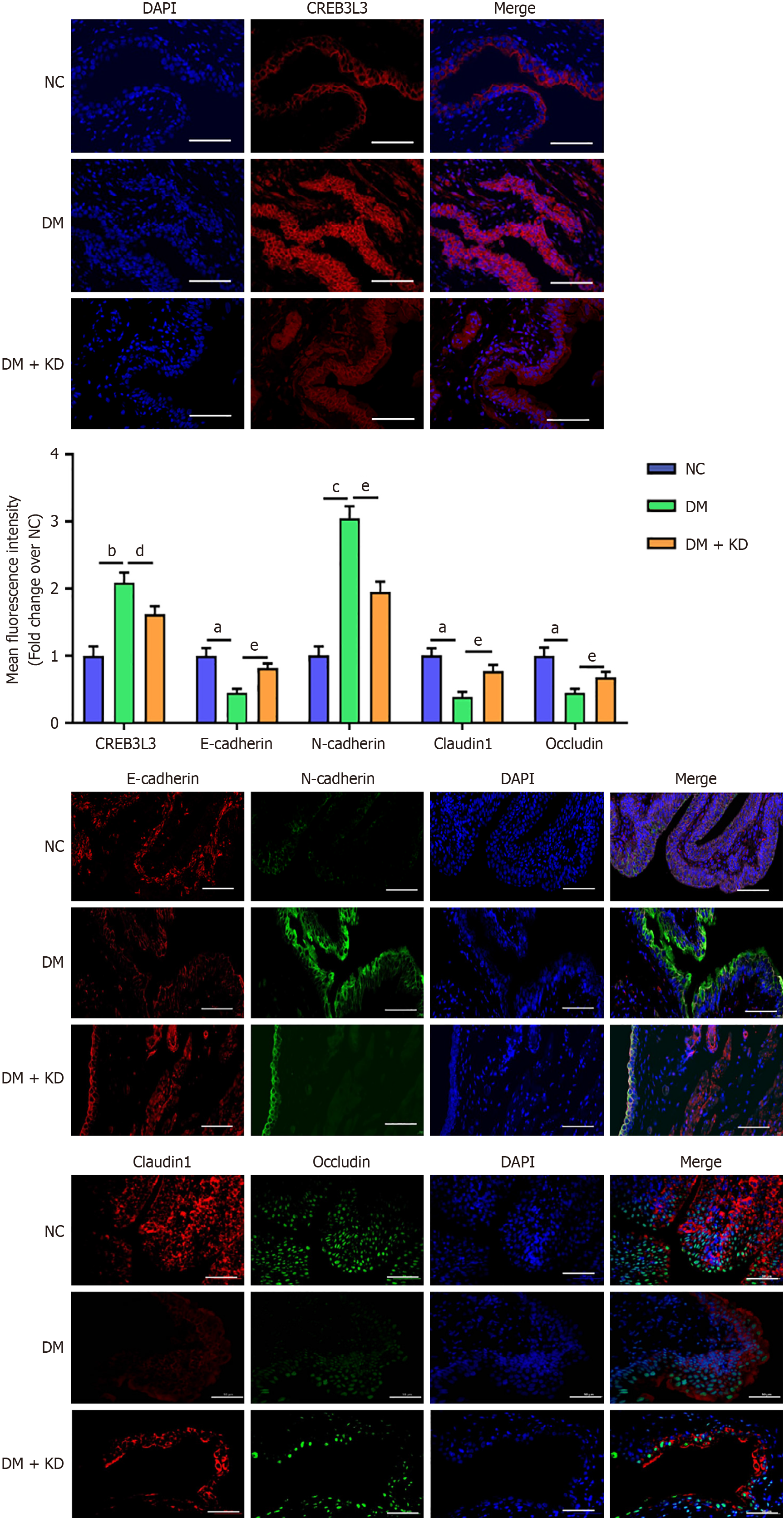
These results were also confirmed by the immunofluorescence assay (Figure 3). These findings suggested that CREB3 L3 positively regulated CRP expression, inducing the EMT and impaired cellular tight junctions under hyperglycemic conditions.
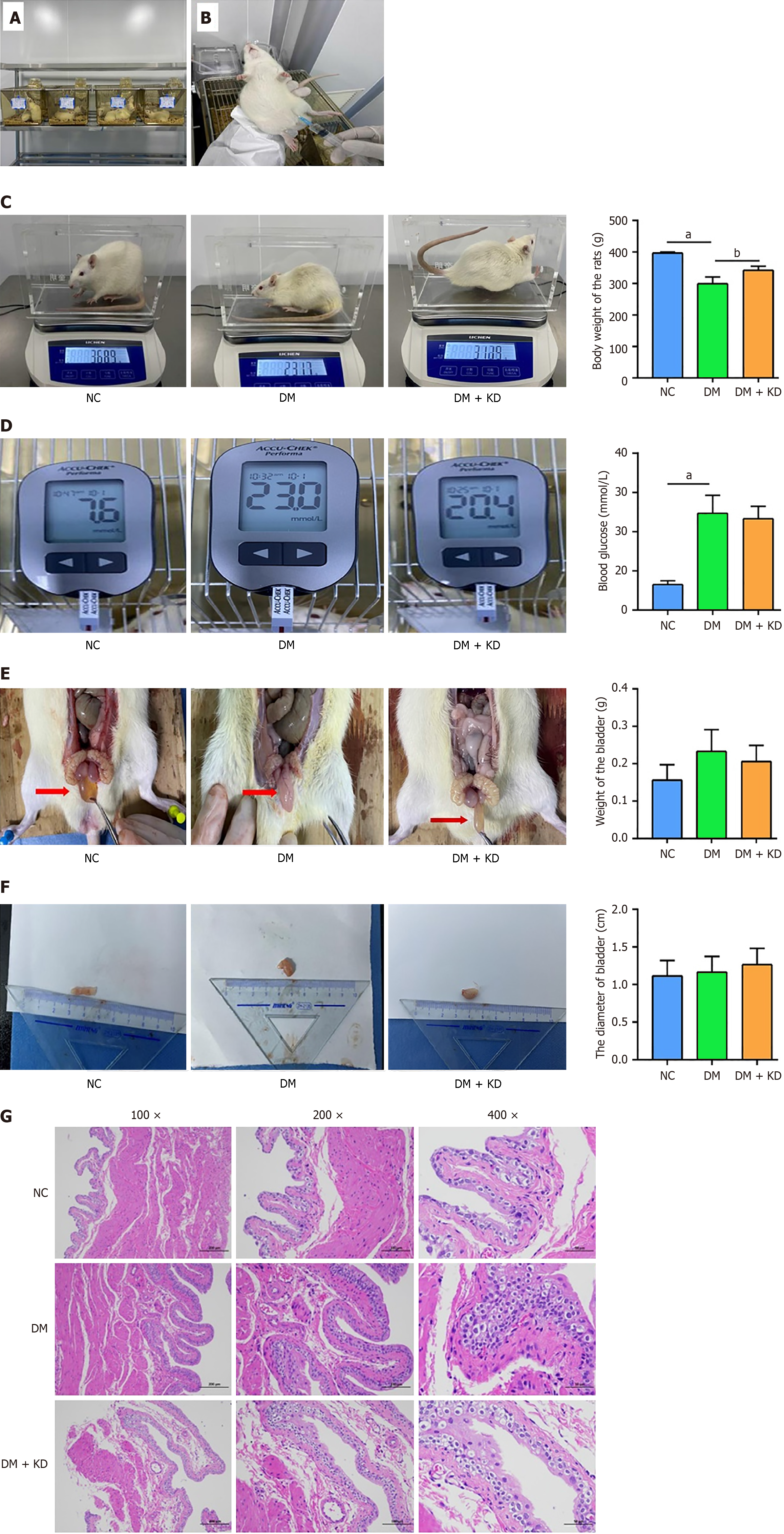
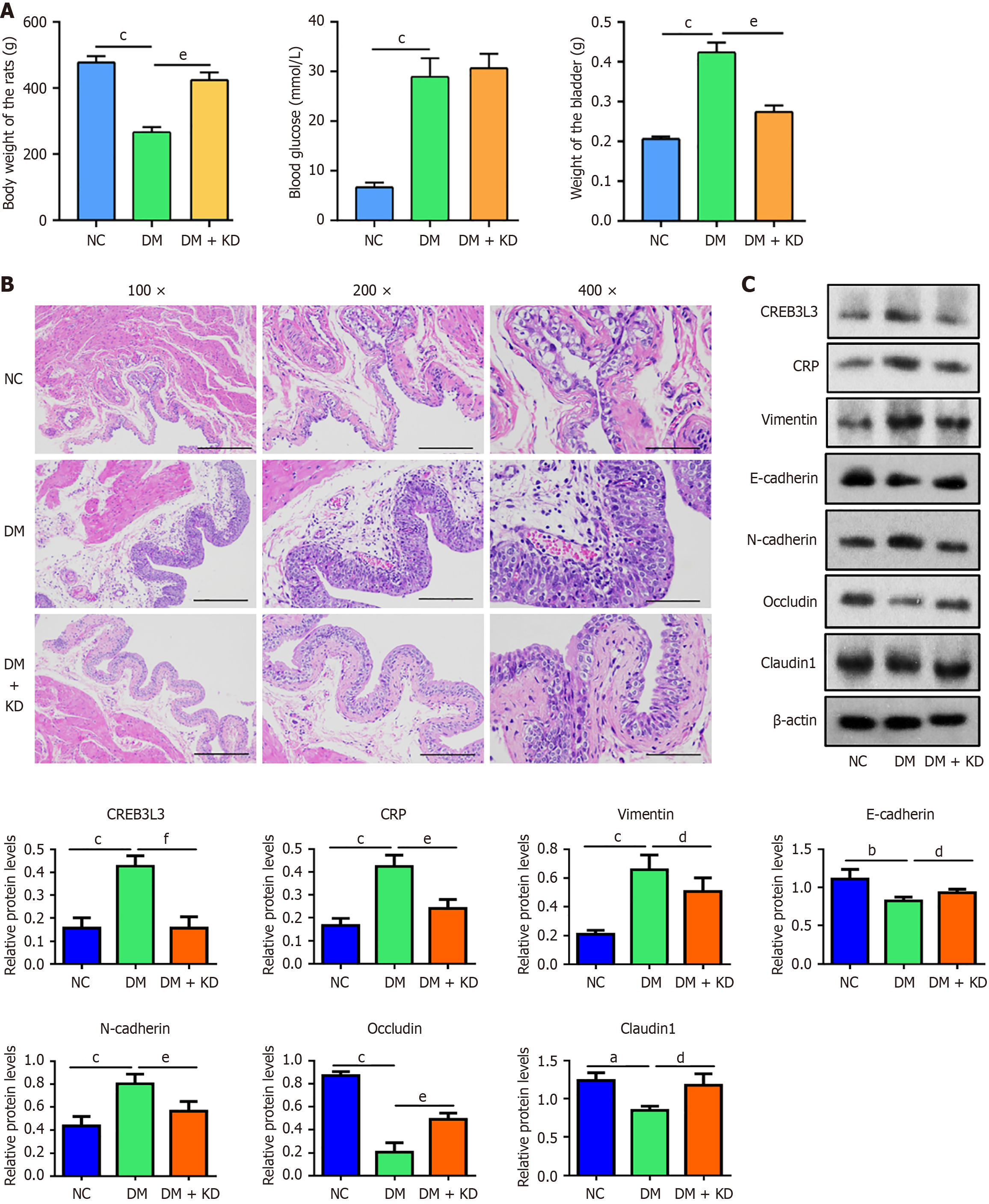
In the in vivo study, we established DCP rat models by feeding a high-fat and high-sugar diet and intraperitoneally injecting 1% STZ (Figure 4A and B). Following the high-fat and high-sugar diet for 4 weeks, the rats showed a significant decrease in body weight (Figure 4C) but a significant increase in blood glucose (Figure 4D) compared with the NC group. The weight (Figure 4E) and diameter (Figure 4F) of bladder urothelial tissues remained unchanged. Body weight was partially restored in the DM + KD group, but blood glucose and the weight and length of bladder tissues showed no changes. Following H&E staining of bladder tissues, we observed that the DM group showed significant morphological changes, such as hyperplasia and edema of the urothelium compared with the NC group (Figure 4G). In the DM + KD group, partial reversal of these morphological changes was observed.
The changes in protein levels were determined by western blotting. The expression of CREB3 L3, CRP, vimentin, and N-cadherin proteins were upregulated in the DM group, while E-cadherin, claudin 1 and occludin protein levels were downregulated compared with the NC group (Figure 5A). By contrast, the upregulation of CREB3 L3, CRP, and N-cadherin proteins was attenuated in the DM + KD group, promoting the upregulation of E-cadherin. The downregulation of claudin 1 and occludin proteins was partially restored (Figure 5A). Abnormal changes in the proteins at the onset of DCP was prevented in the DM + KD group.
Consistent with the aforementioned findings, immunofluorescence analysis yielded similar results (Figure 5B). These findings suggest that CREB3 L3 expression increased in the early stage of DCP, and upregulated CREB3 L3 contributed to the EMT in bladder urothelium and led to the impairment of cell tight junctions.
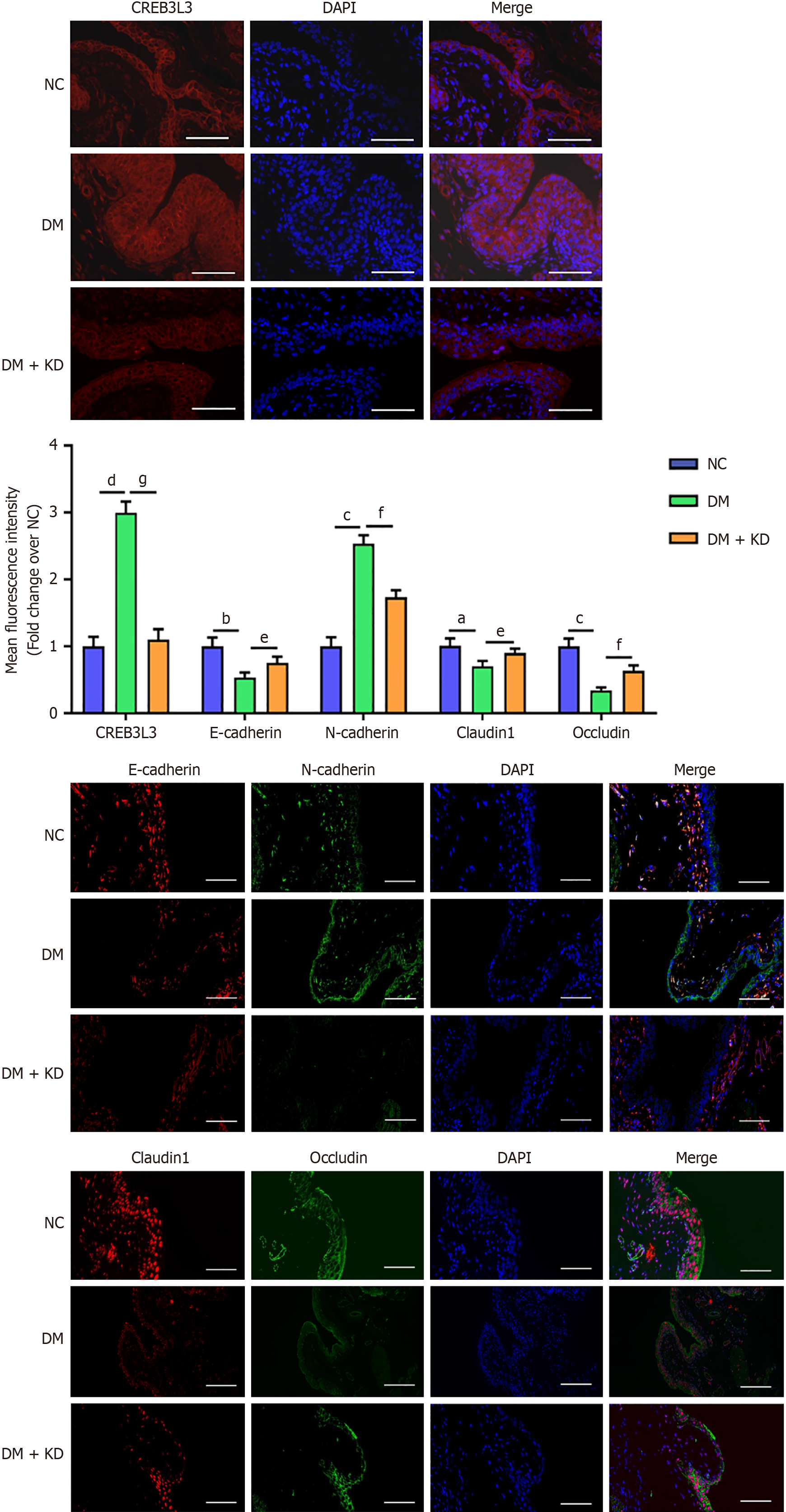
By the end of the 8-week period, the DM group showed a significant increase in blood glucose and bladder weight but a significant decrease in body weight compared with rats in the NC group (Figure 6A). H&E staining showed that the bladder urothelium from the DM group was significantly thickened (Figure 6B). Conversely, the increase in bladder weight and the decrease in body weight was prevented in the DM + KD group, but blood glucose levels were unaffected (Figure 6A). Moreover, abnormal thickening of bladder urothelium was attenuated in the DM + KD group (Figure 6B). Western blotting showed that CREB3 L3, CRP, vimentin, and N-cadherin proteins were upregulated in the DM group, while E-cadherin, occludin and claudin 1 protein levels were downregulated (Figure 6C). In the DM + KD group, the upregulation of CREB3 L3, CRP, and N-cadherin expression was attenuated compared with the DM group, while E-cadherin, claudin 1 and occludin proteins were partially upregulated (Figure 6C).
Immunofluorescence measurements demonstrated significant concordance with western blotting assay results, by the end of the 8-week period, CREB3 L3 protein levels remained upregulated in the DM group compared with the NC group (Figure 7). These results demonstrated that the upregulation of CREB3 L3 expression continuously induced EMT and impaired cellular tight junctions with the progression of DCP.
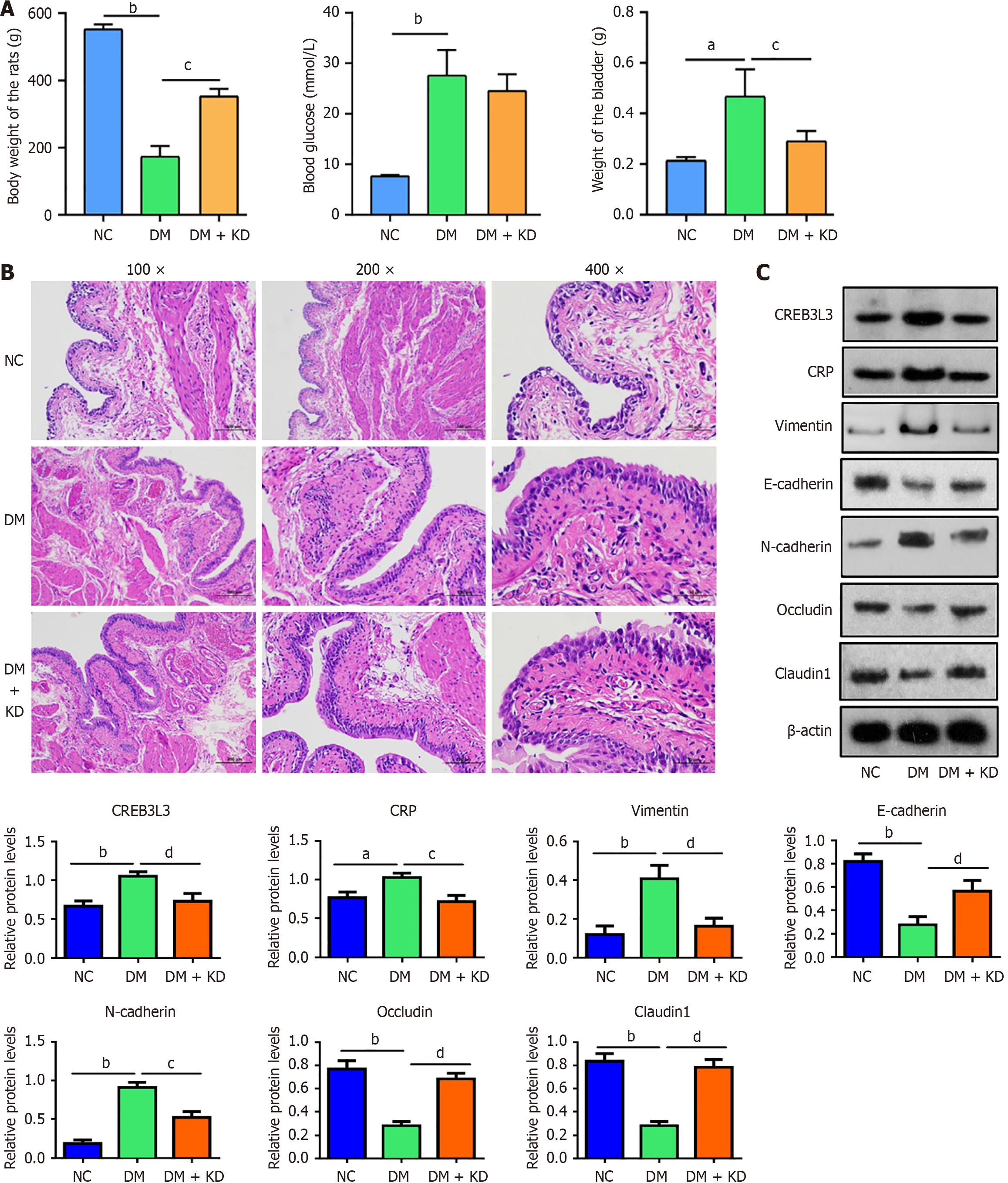
At the 12-week time point, when the rats were moribund, this phase was classified as the late stage of DCP. Body weight in rats from the DM group was only one-third of that in the HC group (Figure 8A). Blood glucose levels were markedly elevated in the DM group, concomitant with increasing bladder weight.
Compared with the DM group, rat body weight was restored and increasing bladder weight was attenuated in the DM + KD group (Figure 8A). H&E staining revealed significant thickening of the bladder urothelium and increased extracellular matrix in the DM group. In the DM + KD group, partial suppression of thickening bladder urothelium was observed (Figure 8B). Western blotting also indicated that CREB3 L3, CRP, vimentin, and N-cadherin proteins were upregulated in the DM group, while E-cadherin, occludin, and claudin 1 proteins were downregulated (Figure 8C). These protein changes were attenuated in the DM + KD group.
Results of the immunofluorescence assays were concordant with the western blot analyses (Figure 9). These results suggest that CREB3 L3 still promotes the EMT and impairs tight junctions during late-stage DCP.
Previous studies have shown that hyperglycemia can induce the EMT in renal tubular epithelial cells, leading to diabetic nephropathy[25]. In addition, hyperglycemia promotes the EMT in bladder urothelial carcinoma by increasing the expression of Yes-associated protein 1[26]. However, whether hyperglycemia also leads to the EMT in bladder urothelial cells remains to be fully elucidated.
Our high-throughput sequencing and bioinformatics showed that highly expressed CRP in the bladder urothelium of patients with DCP was associated with the protein implicated in the EMT. Our study demonstrated that CREB3 L3 was upregulated in the bladder urothelium of patients with DCP, in hyperglycemia-treated SV-HUC-1 cells, and in the bladder urothelium of DCP rats. These findings reveal that hyperglycemia is an important inducement of CREB3 L3 expression. Additionally, we proved that CREB3 L3 can promote CRP expression. In hepatocytes under endothelial reticulum stress (ERS), CREB3 L3 and activating transcription factor 6 bind to a conserved promoter element in the CRP gene and synergistically activate the transcription of CRP. We speculate that CREB3 L3 may induce the transcription of CRP in a similar manner in bladder urothelial cells under hyperglycemic conditions. Furthermore, CRP has been found to facilitate the EMT through Wnt/beta-catenin and extracellular signal-regulated kinase 1/2 (ERK1/2) signaling, in STZ-induced diabetic nephropathy. Therefore, CREB3 L3 promotes bladder urothelial EMT probably via the CRP-mediated signaling pathway, which results in bladder urothelial damage and cell tight junction impairment, thus contributing to the development of DCP. Conversely, CREB3 L3 KD alleviated DCP, as the increase in bladder weight in DCP rats was prevented after 8 weeks.
In addition to its role in the EMT, CREB3 L3 is an important component of the CREB3 transcription factor family and is involved in maintaining cellular homeostasis[27]. In liver tissues, the transcription factor CREB3 L3 regulates glucose and lipid metabolism and controls the homeostasis of systemic energy metabolism[28,29]. In addition, CREB3 L3 maintains glucose homeostasis by regulating glycogenolysis and glycogen synthesis in hepatocytes[30,31]. In terms of mechanism, CREB3 L3 regulates the rhythmic expression of genes encoding the rate-limiting enzymes for glycogenolysis and gluconeogenesis, including liver glycogen phosphorylase, phosphoenolpyruvate carboxykinase 1, and the glucose-6-pho
Interestingly, CREB3 L3 has a dual role in the regulation of the inflammatory response, reducing ERS at the onset of inflammation and enhancing the inflammatory response when inflammation persists[33]. For example, CREB3 L3 inhibits nuclear factor kappa B, thereby attenuating concanavalin A-induced hepatitis by reducing neutrophil recruitment to the liver. Mechanistically, CREB3 L3 alleviates mitochondrial oxidative stress. Moreover, CREB3 L3 facilitates the deace
However, we found that CRP was also expressed in the bladder urothelium of healthy individuals, and the reason for this expression is unclear. Emerging evidence suggests that low-level constitutive expression of CRP in non-pathological states may have homeostatic functions. For example, previous studies have found that the CRP-cAMP complex can activate the Cas operon to play a role in immune surveillance[37]. Bound Factor H-related protein 1 (FHR-1) and FHR-5 on apoptotic cells can recruit CRP and pentraxin 3 to participate in the clearance of apoptotic cells[38]. We speculate that CRP may be induced by normal expression of CREB3 L3, but under normal conditions, its action is antagonized by the associated anti-inflammatory proteins. We observed significantly elevated CREB3 L3 Levels in bladder urothelium in both patients with DCP and the DCP rat model. The chronic overexpression of CREB3 L3 could lead to the inflammatory response in bladder urothelium. On Day 4 of induction in the DM rat model, hyperglycemia significantly induced mitochondrial dysfunction and suppressed nuclear factor erythroid 2-related factor 2 defense, leading to increased release of reactive oxygen species (ROS) from the mitochondria in bladder tissues[39]. The excessive increase of ROS in cells causes ERS[40]. ERS then leads to CREB3 L3 overexpression, inducing a systemic inflammatory response. These findings are consistent with our results that CREB3 L3 and inflammatory response factor CRP were elevated in hyperglycemia-treated urothelial cells.
Previous studies on diabetic nephropathy have indicated that CRP promotes the EMT through Wnt/β-catenin and ERK signaling, which is achieved by CRP binding to Fc gamma receptor II[25]. Similar to renal tubular epithelial cells (human kidney 2 cells, HK-2), CREB3 L3-induced CRP overexpression also promoted the EMT in the bladder urothelium and SV-HUC-1 cells in our study. The EMT mainly occurs in epithelial and tumor cells and is an important process in tissue cell remodeling and cell migration. The occurrence of the EMT leads to dysfunctions in epithelial sensing and intercellular transmission. E-cadherin is an important molecule for maintaining cellular morphology. During the EMT, E-cadherin expression is decreased, allowing for enhanced cell migration and invasion[41]. Simultaneously, N-cadherin and vimentin play roles in promoting the transformation of cells during EMT[42]. Our results showed that N-cadherin and vimentin expression was upregulated but E-cadherin expression was downregulated in bladder urothelium of the DCP rat model. However, CREB3 L3 KD prevented downregulation of E-cadherin and upregulation of N-cadherin and vimentin, suggesting that CREB3 L3 can promote the EMT.
A limitation of the present study is that the molecular mechanisms underlying the EMT regulation by CREB3 L3 remain to be fully elucidated. Particularly, the upstream mechanisms activating CREB3 L3 (e.g., exclusive reliance on ERS vs ROS/advanced glycation end products pathways) and its downstream effectors beyond CRP require further exploration. We hypothesize that CREB3 L3 may promote the EMT in bladder urothelial cells under hyperglycemic conditions through CRP activation and other downstream signaling pathways. Finally, developing tissue-specific CREB3 L3 inhibitors while preserving its metabolic roles will be essential for therapeutic translation. Addressing these gaps will advance mechanistic understanding and guide targeted interventions for DCP in the future.
CREB3 L3 plays a key role in the development of DCP, and it may be a critical target for DCP therapy. This is of great importance for the early prevention and treatment of DCP. However, given that CREB3 L3 has protective effects in regulating glucose metabolism, lipid metabolism, insulin resistance, and hepatic steatosis, considerable attention should be given to improving its targeting of the bladder urothelium when developing relevant drugs in the future.
| 1. | Pitale PM, Gorbatyuk MS. Diabetic Retinopathy: From Animal Models to Cellular Signaling. Int J Mol Sci. 2022;23:1487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 2. | Méndez-Morales ST, Pérez-De Marcos JC, Rodríguez-Cortés O, Flores-Mejía R, Martínez-Venegas M, Sánchez-Vera Y, Tamay-Cach F, Lomeli-Gonzaléz J, Emilio Reyes A, Lehman-Mendoza R, Martínez-Arredondo HA, Vazquez-Dávila RA, Torres-Roldan JF, Correa-Basurto J, Arellano-Mendoza MG. Diabetic neuropathy: Molecular approach a treatment opportunity. Vascul Pharmacol. 2022;143:106954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Bolgeo T, Maconi A, Bertolotti M, Roveta A, Betti M, Gatti D, Boccafoschi C. Physiopathology of the diabetic bladder. Arch Ital Urol Androl. 2020;92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Yuan Z, Tang Z, He C, Tang W. Diabetic cystopathy: A review. J Diabetes. 2015;7:442-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Langdale CL, Thor KB, Marson L, Burgard EC. Maintenance of bladder innervation in diabetes: a stereological study of streptozotocin-treated female rats. Auton Neurosci. 2014;185:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Xue J, Liu Y, Zhang S, Ding L, Shen B, Shao Y, Wei Z. Caffeine improves bladder function in diabetic rats via a neuroprotective effect. Exp Ther Med. 2021;21:501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Leiria LO, Mónica FZ, Carvalho FD, Claudino MA, Franco-Penteado CF, Schenka A, Grant AD, De Nucci G, Antunes E. Functional, morphological and molecular characterization of bladder dysfunction in streptozotocin-induced diabetic mice: evidence of a role for L-type voltage-operated Ca2+ channels. Br J Pharmacol. 2011;163:1276-1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Yang S, Wang D, Cao X, Zhang X, Yuan X, Yang T, Mi Y. Store operated calcium channels are associated with diabetic cystopathy in streptozotocin‑induced diabetic rats. Mol Med Rep. 2018;17:6612-6620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Lee S, Rose'meyer R, McDermott C, Chess-Williams R, Sellers DJ. Diabetes-induced alterations in urothelium function: Enhanced ATP release and nerve-evoked contractions in the streptozotocin rat bladder. Clin Exp Pharmacol Physiol. 2018;45:1161-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Dong X, Song Q, Zhu J, Zhao J, Liu Q, Zhang T, Long Z, Li J, Wu C, Wang Q, Hu X, Damaser M, Li L. Interaction of Caveolin-3 and HCN is involved in the pathogenesis of diabetic cystopathy. Sci Rep. 2016;6:24844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Hughes FM Jr, Hirshman NA, Inouye BM, Jin H, Stanton EW, Yun CE, Davis LG, Routh JC, Purves JT. NLRP3 Promotes Diabetic Bladder Dysfunction and Changes in Symptom-Specific Bladder Innervation. Diabetes. 2019;68:430-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Inouye BM, Hughes FM Jr, Jin H, Lütolf R, Potnis KC, Routh JC, Rouse DC, Foo WC, Purves JT. Diabetic bladder dysfunction is associated with bladder inflammation triggered through hyperglycemia, not polyuria. Res Rep Urol. 2018;10:219-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Zhang Q, Liu X, Sullivan MA, Shi C, Deng B. Protective Effect of Yi Shen Pai Du Formula against Diabetic Kidney Injury via Inhibition of Oxidative Stress, Inflammation, and Epithelial-to-Mesenchymal Transition in db/db Mice. Oxid Med Cell Longev. 2021;2021:7958021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Korhonen A, Gucciardo E, Lehti K, Loukovaara S. Proliferative diabetic retinopathy transcriptomes reveal angiogenesis, anti-angiogenic therapy escape mechanisms, fibrosis and lymphatic involvement. Sci Rep. 2021;11:18810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Zheng X, Peng M, Li Y, Wang X, Lu W, Wang X, Shan Y, Li R, Gao L, Qiu C. Cathelicidin-related antimicrobial peptide protects against cardiac fibrosis in diabetic mice heart by regulating endothelial-mesenchymal transition. Int J Biol Sci. 2019;15:2393-2407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Zang L, Gao F, Huang A, Zhang Y, Luo Y, Chen L, Mao N. Icariin inhibits epithelial mesenchymal transition of renal tubular epithelial cells via regulating the miR-122-5p/FOXP2 axis in diabetic nephropathy rats. J Pharmacol Sci. 2022;148:204-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Wang CC, Kuo HC. Urothelial Dysfunction and Chronic Inflammation in Diabetic Patients with Overactive Bladder. Low Urin Tract Symptoms. 2017;9:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Wang Y, Tar MT, Fu S, Melman A, Davies KP. Diabetes attenuates urothelial modulation of detrusor contractility and spontaneous activity. Int J Urol. 2014;21:1059-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Liang CC, Shaw SW, Huang YH, Lee TH. Human amniotic fluid stem cell therapy can help regain bladder function in type 2 diabetic rats. World J Stem Cells. 2022;14:330-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Han X, Chen Y, Ha L, Yang J, Wang F, Chen H, Zhou Q, Long C, Qiu X, Chen Q. Effects of electroacupuncture on bladder dysfunction and the expression of PACAP38 in a diabetic rat model. Front Physiol. 2022;13:1008269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Wu Q, Qin B, Wu X, Zhang M, Gan Z, Lan Y, Ma C, Fu W. Allograft inflammatory factor-1 enhances inflammation and oxidative stress via the NF-κB pathway of bladder urothelium in diabetic rat model. Cytokine. 2024;173:156438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Chen PC, Ho CH, Fan CK, Liu SP, Cheng PC. Antimicrobial Peptide LCN2 Inhibited Uropathogenic Escherichia coli Infection in Bladder Cells in a High-Glucose Environment through JAK/STAT Signaling Pathway. Int J Mol Sci. 2022;23:15763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 23. | Noh JR, Kim JH, Na SY, Lee IB, Seo YJ, Choi JH, Seo Y, Lee TG, Choi HS, Kim YH, Lee CH. Hepatocyte CREBH deficiency aggravates inflammatory liver injury following chemokine-dependent neutrophil infiltration through upregulation of NF-κB p65 in mice. Arch Toxicol. 2020;94:509-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, Back SH, Kaufman RJ. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 643] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 25. | Zhang L, Shen ZY, Wang K, Li W, Shi JM, Osoro EK, Ullah N, Zhou Y, Ji SR. C-reactive protein exacerbates epithelial-mesenchymal transition through Wnt/β-catenin and ERK signaling in streptozocin-induced diabetic nephropathy. FASEB J. 2019;33:6551-6563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Li S, Zhu H, Chen H, Xia J, Zhang F, Xu R, Lin Q. Glucose promotes epithelial-mesenchymal transitions in bladder cancer by regulating the functions of YAP1 and TAZ. J Cell Mol Med. 2020;24:10391-10401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Sampieri L, Di Giusto P, Alvarez C. CREB3 Transcription Factors: ER-Golgi Stress Transducers as Hubs for Cellular Homeostasis. Front Cell Dev Biol. 2019;7:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 28. | Zheng Z, Kim H, Qiu Y, Chen X, Mendez R, Dandekar A, Zhang X, Zhang C, Liu AC, Yin L, Lin JD, Walker PD, Kapatos G, Zhang K. CREBH Couples Circadian Clock With Hepatic Lipid Metabolism. Diabetes. 2016;65:3369-3383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 29. | Nakagawa Y, Satoh A, Yabe S, Furusawa M, Tokushige N, Tezuka H, Mikami M, Iwata W, Shingyouchi A, Matsuzaka T, Kiwata S, Fujimoto Y, Shimizu H, Danno H, Yamamoto T, Ishii K, Karasawa T, Takeuchi Y, Iwasaki H, Shimada M, Kawakami Y, Urayama O, Sone H, Takekoshi K, Kobayashi K, Yatoh S, Takahashi A, Yahagi N, Suzuki H, Yamada N, Shimano H. Hepatic CREB3L3 controls whole-body energy homeostasis and improves obesity and diabetes. Endocrinology. 2014;155:4706-4719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Kim H, Zheng Z, Walker PD, Kapatos G, Zhang K. CREBH Maintains Circadian Glucose Homeostasis by Regulating Hepatic Glycogenolysis and Gluconeogenesis. Mol Cell Biol. 2017;37:e00048-e00017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | Nakagawa Y, Shimano H. CREBH Regulates Systemic Glucose and Lipid Metabolism. Int J Mol Sci. 2018;19:1396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 32. | Krumm CS, Xu X, Bare CJ, Holman CD, Kersten S, Dow LE, Lee AH, Cohen DE. Inducible hepatic expression of CREBH mitigates diet-induced obesity, insulin resistance, and hepatic steatosis in mice. J Biol Chem. 2021;297:100815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Wade H, Pan K, Su Q. CREBH: A Complex Array of Regulatory Mechanisms in Nutritional Signaling, Metabolic Inflammation, and Metabolic Disease. Mol Nutr Food Res. 2021;65:e2000771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Zhang J, Zhao Y, Wang S, Li G, Xu K. CREBH alleviates mitochondrial oxidative stress through SIRT3 mediating deacetylation of MnSOD and suppression of Nlrp3 inflammasome in NASH. Free Radic Biol Med. 2022;190:28-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Li Y, Zhang H, Long W, Gao M, Guo W, Yu L. Inhibition of NLRP3 and Golph3 ameliorates diabetes-induced neuroinflammation in vitro and in vivo. Aging (Albany NY). 2022;14:8745-8762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 36. | Song Y, Zhao M, Cheng X, Shen J, Khound R, Zhang K, Su Q. CREBH mediates metabolic inflammation to hepatic VLDL overproduction and hyperlipoproteinemia. J Mol Med (Berl). 2017;95:839-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Patterson AG, Chang JT, Taylor C, Fineran PC. Regulation of the Type I-F CRISPR-Cas system by CRP-cAMP and GalM controls spacer acquisition and interference. Nucleic Acids Res. 2015;43:6038-6048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 38. | Kárpáti É, Papp A, Schneider AE, Hajnal D, Cserhalmi M, Csincsi ÁI, Uzonyi B, Józsi M. Interaction of the Factor H Family Proteins FHR-1 and FHR-5 With DNA and Dead Cells: Implications for the Regulation of Complement Activation and Opsonization. Front Immunol. 2020;11:1297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Lin CF, Chueh TH, Chung CH, Chung SD, Chang TC, Chien CT. Sulforaphane improves voiding function via the preserving mitochondrial function in diabetic rats. J Formos Med Assoc. 2020;119:1422-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Gao L, Jin N, Ye Z, Ma T, Huang Y, Li H, Du J, Li Z. A possible connection between reactive oxygen species and the unfolded protein response in lens development: From insight to foresight. Front Cell Dev Biol. 2022;10:820949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 41. | Zhao GX, Xu YY, Weng SQ, Zhang S, Chen Y, Shen XZ, Dong L, Chen S. CAPS1 promotes colorectal cancer metastasis via Snail mediated epithelial mesenchymal transformation. Oncogene. 2019;38:4574-4589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 42. | Ishiguro Y, Sakihama H, Yoshida T, Ichikawa N, Homma S, Fukai M, Kawamura H, Takahashi N, Taketomi A. Prognostic Significance of Circulating Tumor Cells with Mesenchymal Phenotypes in Patients with Gastric Cancer: A Prospective Study. Ann Surg Oncol. 2021;28:1178-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |