Published online Jul 15, 2025. doi: 10.4239/wjd.v16.i7.107647
Revised: April 18, 2025
Accepted: June 3, 2025
Published online: July 15, 2025
Processing time: 110 Days and 10 Hours
Decreased renal function is a well-known risk factor for cardiovascular diseases (CVD) and death. However, the impact of diabetes duration and the glomerular filtration rate (GFR) on cardiovascular complications in patients with type 2 dia
To investigate the complex impact of longer diabetes duration and GFR on CVD and mortality.
Subjects with diabetes age ≥ 20 years, who underwent health check-ups from 2015 to 2016 were identified in the Korean National Health Insurance Service database. Based on diabetes duration, subjects were grouped into new-onset, < 5 years, 5–9 years, or ≥ 10 years. The new-onset diabetes group [estimated GFR (eGFR): ≥ 90 mL/min/1.73 m2] was the reference group. A Cox proportional hazards model adjusted for potential confounders was used to estimate the risk for myocardial infarction (MI), ischemic stroke (IS), and mortality.
During a 3.9-year follow-up of 2105228 patients, 36003 (1.7%) MIs, 46496 (2.2%) ISs, and 73549 (3.5%) deaths were documented. Both longer diabetes duration and lower eGFR were independently associated with higher risks of MI, IS, and mortality, which were further amplified when these factors coexisted. Even patients with new-onset diabetes had elevated MI and IS risk at mildly reduced eGFR (60–90 mL/min/1.73 m²). Mortality risk rose appreciably once eGFR declined below 60 mL/min/1.73 m², particularly in those with longer diabetes duration. eGFR ≥ 90 mL/min/1.73 m2 subgroups had higher death risk than eGFR 60–90 mL/min/1.73 m2 subgroups regardless of diabetic duration.
Increasing diabetes duration and decreasing eGFR are associated with increased risk of MI, IS, and mortality. For cardiovascular risk estimation, diabetes duration should be considered an important risk factor.
Core Tip: This study investigated the complex impact of longer diabetes duration and glomerular filtration rate (GFR) on cardiovascular diseases and mortality. We reveal an association between longer diabetes duration and an increased risk of myocardial infarction and ischemic stroke, even when the GFR is within the normal range. With increasing diabetes duration, mortality increased in subjects with GFR < 60 mL/min/1.73 m2. These findings highlight that for cardiovascular risk estimation, diabetes duration should be considered an important risk factor.
- Citation: Choi HS, Kim B, Han KD, Suh SH, Kim CS, Bae EH, Ma SK, Kim SW. Impact of longer diabetes duration and lower estimated glomerular filtration rate on cardiovascular complications and mortality: A nationwide population-based study. World J Diabetes 2025; 16(7): 107647
- URL: https://www.wjgnet.com/1948-9358/full/v16/i7/107647.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i7.107647
Diabetes mellitus (DM) accounts for a large portion of the global disease burden, and as of 2022, its worldwide prevalence was approximately 14% in the adult population, double the prevalence about 30 years ago[1]. DM causes various complications, including diabetic kidney disease, diabetic foot, and cardiovascular disease (CVD)[2], the leading cause of morbidity and mortality in people with diabetes[3]. Diabetes is the most common cause of chronic kidney disease (CKD) in adults, with 20%–40% of people with diabetes developing kidney disease[4]. In people with DM, CKD markedly increases CV risk and socioeconomic burden[5]. Like DM, CKD increases future coronary risk and their coexistence leads to greater risk[5].
With the recent emphasis on individualized DM treatment, interest in each patient’s characteristics is increasing. A previous study reported that in patients with DM, adding diabetic duration to classic risk factors improves CV risk asse
Here, we aimed to investigate the impact of diabetes duration on the association between GFR and the risk of CVD and mortality. We analyzed large-scale nationally representative data from the Korean National Health Insurance System (NHIS).
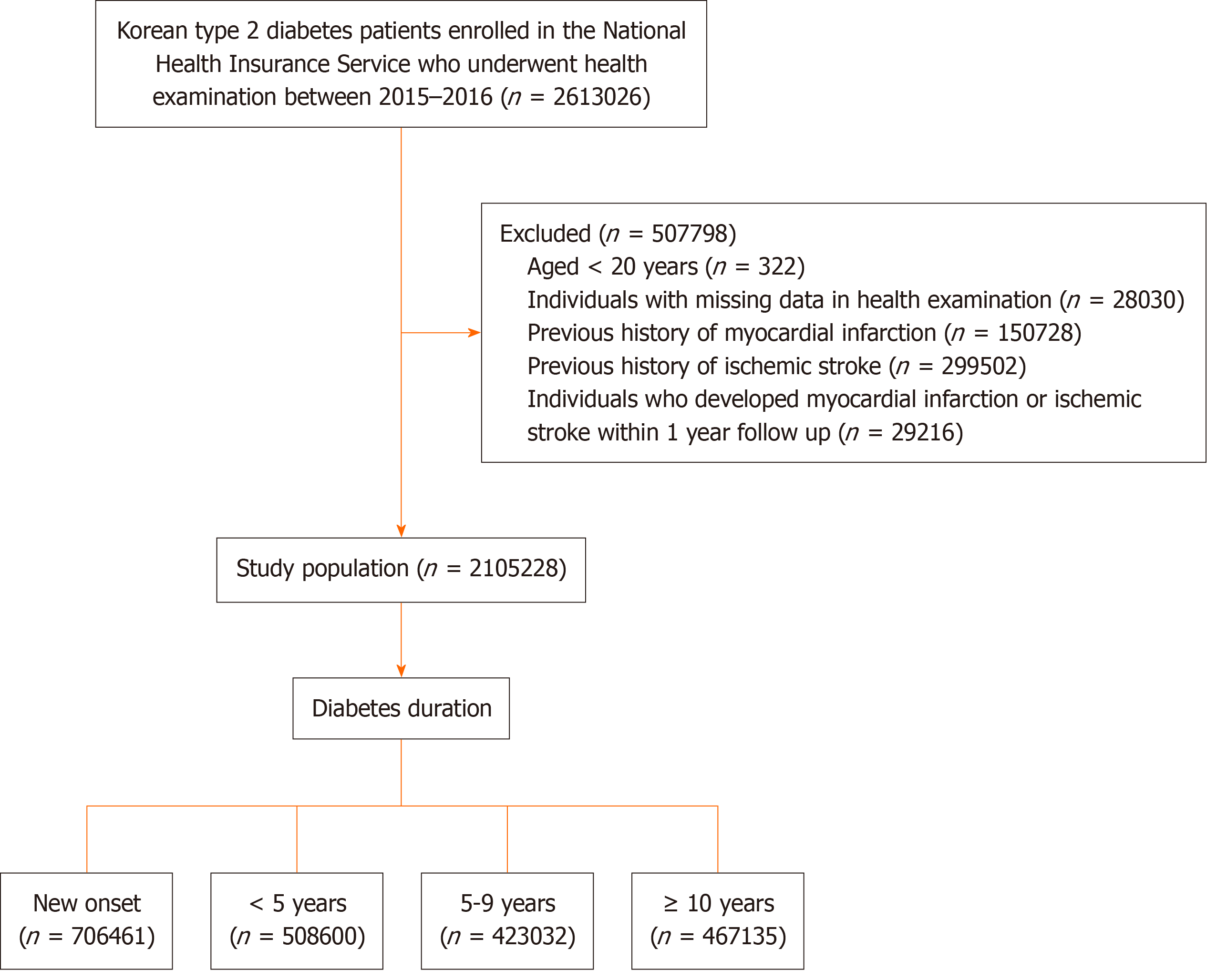
Information on the Korean NHIS has been published previously[11]. Figure 1 provides an overview of the patient selection process. Among 2613026 individuals with diabetes, who had undergone health examination provided by the Korean NHIS between January 1, 2015, and December 31, 2016, those aged < 20 years (n = 322), those with missing variables (n = 28030), and those with prior myocardial infarction (MI) and ischemic stroke (IS) diagnoses (n = 150728 and 299502, respectively) were excluded. To avoid confounding by pre-existing disease and to minimize possible reverse causality effects, individuals with newly diagnosed MI or IS in the first year of follow-up, or those who died during the first year of follow-up were excluded (n = 29216). Finally, 2105228 individuals with type 2 DM (1291933 men and 813295 women), who had estimated GFR (eGFR) monitoring at baseline and data on diabetes duration, were enrolled for analyses and follow-up until the date of death or end of follow-up (December 31, 2020; mean follow-up duration: 3.9 years).
Type 2 diabetes was defined based on the following criteria: at least one claim per year under International Classification of Disease, 10th Revision (ICD-10) codes E11–14 and at least one claim per year for antidiabetic medication prescription or fasting serum glucose levels of ≥ 126 mg/dL in the health examination database. Based on diabetes duration, subjects were categorized into the new-onset (those with no previously recorded disease code or history of antidiabetic drug prescription, but with a fasting blood glucose (FBG) level ≥ 126 mg/dL at health examination), < 5 years, 5–9 years, or ≥ 10 years groups. This categorization of diabetes duration is based on observations from previous studies[12-14].
Covariate definitions were based on data from health examination of the index years (2015–2016), and they included age, sex, socioeconomic status, body mass index (BMI; kg/m2), and systolic/diastolic blood pressure (mmHg). Smoking status, alcohol consumption, and physical activity data were obtained from a health examination questionnaire. Stan
The study endpoints were newly diagnosed MI, IS, or death after a 1-year lag period. MI was defined by ICD-10 codes I21 or I22, with more than one diagnosis during hospitalization. IS was defined as the recording of ICD-10 codes I63 or I64 during hospitalization, and concomitant brain imaging studies using magnetic resonance imaging or computed to
Continuous and categorical variables are presented as mean ± SD and n (%), respectively. Intergroup differences were estimated using a χ2 test or analysis of variance. MI, IS, or death incidence rates are presented per 1000 person-years. The hazard ratios (HRs) and 95% confidence intervals (CIs) of the risk of CV events or death were estimated using mul
The Institutional Review Board of Chonnam National University Hospital (No. CNUH-EXP-2024-136) waived the ethical approval and informed consent requirements for this study. Hence, consent was not obtained because the participants’ records and information were anonymized and de-identified before analysis.
Table 1 shows the patients’ baseline characteristics based on diabetes duration. A total of 2 105 228 individuals with type 2 diabetes were enrolled. Of these, 33.6% had new-onset diabetes, 24.2% had diabetes for < 5 years, 20.1% had diabetes for 5–9 years, and 22.2% had diabetes for ≥ 10 years. Patients with longer duration were older, had higher hypertension and dyslipidemia prevalences, and were more likely to be non- or ex-smokers, nondrinkers, and to have regular physical activity. The longer the diabetes duration, the higher the likelihood that subjects were taking > 3 antidiabetic medications or using insulin. FBG was lowest in the < 5 years diabetes duration group and gradually increased with duration. However, total cholesterol, LDL cholesterol, and triglycerides decreased with diabetes duration. In particular, as diabetes duration increased, the proportion of patients with eGFR ≥ 90 mL/min/1.73 m2 decreased, while the number of patients with eGFR < 90 mL/min/1.73 m2 increased gradually (Table 1).
| New-onset (n = 706461) | < 5 yr (n = 508600) | 5–9 yr (n = 423032) | ≥ 10 yr (n = 467135) | P | |
| Age (yr) | 52.98 ± 11.9 | 57.52 ± 11.1 | 60.56 ± 10.6 | 63.93 ± 9.9 | < 0.0001 |
| Sex (male) | 481589 (68.2) | 299076 (58.8) | 246708 (58.3) | 264560 (56.6) | < 0.0001 |
| Smoking | < 0.0001 | ||||
| Non-smokers | 332142 (47.01) | 276207 (54.31) | 238244 (56.32) | 281535 (60.27) | |
| Ex-smokers | 157675 (22.32) | 112509 (22.12) | 95514 (22.58) | 105528 (22.59) | |
| Current smokers | 216644 (30.67) | 119884 (23.57) | 89274 (21.1) | 80072 (17.14) | |
| Alcohol consumption | < 0.0001 | ||||
| None | 300444 (42.53) | 291682 (57.35) | 253053 (59.82) | 308113 (65.96) | |
| Mild | 311423 (44.08) | 170781 (33.58) | 134214 (31.73) | 128087 (27.42) | |
| Heavy | 94594 (13.39) | 46137 (9.07) | 35765 (8.45) | 30935 (6.62) | |
| Regular exercise | 140064 (19.83) | 111534 (21.93) | 96503 (22.81) | 114805 (24.58) | < 0.0001 |
| Low income | 154498 (21.87) | 116919 (22.99) | 100476 (23.75) | 105547 (22.59) | < 0.0001 |
| Hypertension | 310325 (43.93) | 286258 (56.28) | 258243 (61.05) | 302440 (64.74) | < 0.0001 |
| Dyslipidemia | 272583 (38.58) | 317279 (62.38) | 260692 (61.62) | 286680 (61.37) | < 0.0001 |
| No. of oral GLD ≥ 3 | 133 (0.02) | 85760 (16.86) | 130444 (30.84) | 226545 (48.5) | < 0.0001 |
| Insulin use | 623 (0.09) | 29453 (5.79) | 29086 (6.88) | 84052 (17.99) | < 0.0001 |
| BMI (kg/m2) | 25.74 ± 3.74 | 25.74 ± 3.62 | 25.25 ± 3.41 | 24.52 ± 3.23 | < 0.0001 |
| WC (cm) | 86.34 ± 9.27 | 86.63 ± 9.12 | 86.02 ± 8.82 | 85.02 ± 8.61 | < 0.0001 |
| Systolic BP (mmHg) | 129.33 ± 15.22 | 127.23 ± 14.38 | 127.75 ± 14.69 | 128.15 ± 15.19 | < 0.0001 |
| Diastolic BP (mmHg) | 80.45 ± 10.3 | 78.24 ± 9.53 | 77.5 ± 9.46 | 75.9 ± 9.51 | <.0001 |
| Fasting glucose (mg/dL) | 151.14 ± 41.23 | 136.54 ± 43.02 | 144.22 ± 47.37 | 150.1 ± 52.12 | < 0.0001 |
| Total cholesterol (mg/dL) | 209.39 ± 41.85 | 181.72 ± 41.28 | 177.47 ± 40.03 | 171.98 ± 38.99 | < 0.0001 |
| HDL-C (mg/dL) | 52.46 ± 16.27 | 50.72 ± 14.25 | 50.69 ± 13.83 | 50.37 ± 14.08 | < 0.0001 |
| LDL-C (mg/dL) | 121.35 ± 38.42 | 100.06 ± 36.78 | 97.02 ± 35.69 | 93.73 ± 34.61 | <.0001 |
| Triglyceride (mg/dL) | 158.94 (158.72–159.16) | 137.31 (137.1–137.52) | 131.6 (131.39–131.82) | 122.67 (122.48–122.86) | < 0.0001 |
| eGFR (mL/min/1.73 m2) | < 0.0001 | ||||
| ≥ 90 | 427078 (60.45) | 291515 (57.32) | 219064 (51.78) | 201984 (43.24) | |
| 60-90 | 259089 (36.67) | 193292 (38) | 173265 (40.96) | 200657 (42.95) | |
| 30-60 | 18770 (2.66) | 21810 (4.29) | 27807 (6.57) | 55283 (11.83) | |
| < 30 | 767 (0.11) | 904 (0.18) | 1442 (0.34) | 5010 (1.07) | |
| ESKD | 757 (0.11) | 1079 (0.21) | 1454 (0.34) | 4201 (0.9) |
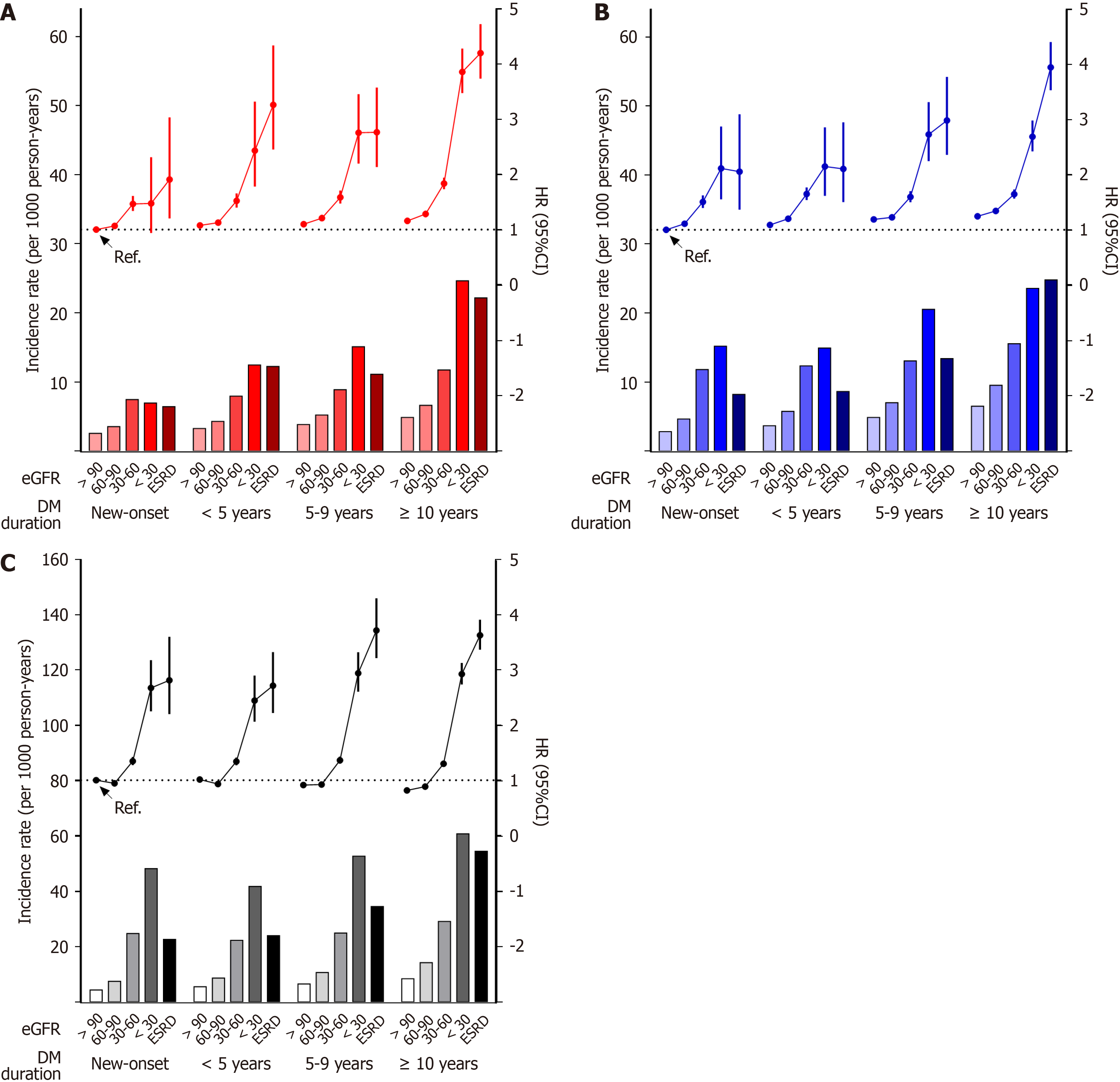
During 3.9 years of follow-up, 36 003 (1.7%) MI cases and 46 496 (2.2%) IS cases were identified. The new-onset diabetes group with eGFR ≥ 90 mL/min/1.73 m2 was used as the reference group. Incidence rates and risk of MI and IS increased gradually with increasing diabetes duration (Figure 2, Tables 2 and 3). In each diabetes duration group, the lower the eGFR, the higher the risk of MI or IS. In each diabetes duration group, MI or IS risk in the groups with eGFR of 60–90 mL/min/1.73 m2, which implies preserved renal function, was higher than in those with eGFR ≥ 90 mL/min/1.73 m2. (Tables 2 and 3, Supplementary Table 1). Even in the new-onset diabetes group, MI or IS risk began to increase at an eGFR of 60–90 mL/min/1.73 m2 (adjusted HR: 1.067 or 1.111; 95%CI: 1.02–1.117 or 1.066–1.158, respectively). In the same eGFR subgroup, longer diabetes duration was associated with higher MI or IS risk. Even patients with eGFR ≥ 90 mL/min/1.73 m2 in the < 5 years, 5–9 years, and > 10 years groups had a significantly higher risk of MI or IS than those in the reference group. Patients with the longest diabetes duration and the lowest eGFR experienced the highest rates of MI and IS, indicating an amplified risk when these factors coexisted. Similar trends were revealed by the associations between eGFR on a continuous scale and the risk of MI or IS according to diabetes duration (Supplementary Figure 1). As the duration of diabetes increased, the increase in MI or IS risk due to a decrease in eGFR tended to be steeper.
| Diabetes duration | eGFR, | n | Events (n) | Incidence rate (per 1000 person-years) | HR (95%CI) | ||
| Model 11 | Model 22 | Model 33 | |||||
| New-onset | ≥ 90 | 427078 | 4191 | 2.56 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| 60–90 | 259089 | 3530 | 3.54 | 1.384 (1.323-1.447) | 1.076 (1.028-1.126) | 1.067 (1.020-1.117) | |
| 30–60 | 18770 | 525 | 7.46 | 2.916 (2.663-3.193) | 1.520 (1.385-1.668) | 1.466 (1.336-1.609) | |
| < 30 | 767 | 19 | 6.96 | 2.726 (1.737-4.278) | 1.523 (0.970-2.391)4 | 1.474 (0.939-2.314)4 | |
| ESKD | 757 | 18 | 6.44 | 2.519 (1.585-4.002) | 1.916 (1.205-3.044) | 1.909 (1.201-3.034) | |
| < 5 yr | ≥ 90 | 291515 | 3744 | 3.28 | 1.279 (1.224-1.336) | 1.103 (1.055-1.154) | 1.077 (1.029-1.126) |
| 60–90 | 193292 | 3264 | 4.31 | 1.679 (1.604-1.758) | 1.150 (1.096-1.206) | 1.125 (1.072-1.180) | |
| 30–60 | 21810 | 661 | 7.95 | 3.104 (2.86-3.369) | 1.590 (1.461-1.730) | 1.522 (1.398-1.656) | |
| < 30 | 904 | 40 | 12.47 | 4.888 (3.58-6.674) | 2.657 (1.945-3.629) | 2.431 (1.779-3.322) | |
| ESKD | 1079 | 48 | 12.25 | 4.805 (3.615-6.386) | 3.655 (2.749-4.860) | 3.262 (2.452-4.338) | |
| 5–9 yr | ≥ 90 | 219064 | 3290 | 3.84 | 1.498 (1.432-1.568) | 1.176 (1.122-1.232) | 1.098 (1.047-1.151) |
| 60–90 | 173265 | 3538 | 5.23 | 2.039 (1.949-2.132) | 1.285 (1.226-1.347) | 1.207 (1.150-1.266) | |
| 30–60 | 27807 | 941 | 8.89 | 3.469 (3.232-3.724) | 1.716 (1.594-1.848) | 1.584 (1.470-1.706) | |
| < 30 | 1442 | 77 | 15.11 | 5.926 (4.73-7.424) | 3.140 (2.504-3.937) | 2.756 (2.197-3.457) | |
| ESKD | 1454 | 59 | 11.13 | 4.359 (3.371-5.636) | 3.282 (2.537-4.245) | 2.764 (2.135-3.577) | |
| ≥ 10 yr | ≥ 90 | 201984 | 3803 | 4.87 | 1.904 (1.822-1.989) | 1.356 (1.296-1.419) | 1.158 (1.104-1.215) |
| 60–90 | 200657 | 5104 | 6.62 | 2.585 (2.482-2.693) | 1.505 (1.440-1.573) | 1.283 (1.224-1.344) | |
| 30–60 | 55283 | 2410 | 11.75 | 4.597 (4.373-4.834) | 2.240 (2.122-2.366) | 1.835 (1.733-1.943) | |
| < 30 | 5010 | 422 | 24.65 | 9.695 (8.772-10.716) | 5.039 (4.550-5.582) | 3.858 (3.476-4.281) | |
| ESKD | 4201 | 319 | 22.18 | 8.731 (7.792-9.784) | 5.721 (5.099-6.419) | 4.202 (3.737-4.725) | |
| Diabetes duration | eGFR, | n | Events (n) | Incidence rate (per 1000 person-years) | HR (95%CI) | ||
| Model 11 | Model 22 | Model 33 | |||||
| New-onset | ≥ 90 | 427078 | 4615 | 2.82 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| 60–90 | 259089 | 4627 | 4.65 | 1.650 (1.584-1.718) | 1.123 (1.077-1.170) | 1.111 (1.066-1.158) | |
| 30–60 | 18770 | 826 | 11.83 | 4.196 (3.897-4.519) | 1.578 (1.462-1.702) | 1.505 (1.395-1.624) | |
| < 30 | 767 | 41 | 15.21 | 5.413 (3.980-7.361) | 2.215 (1.628-3.013) | 2.114 (1.553-2.876) | |
| ESKD | 757 | 23 | 8.21 | 2.913 (1.934-4.389) | 2.048 (1.360-3.086) | 2.057 (1.365-3.099) | |
| < 5 yr | ≥ 90 | 291515 | 4185 | 3.67 | 1.297 (1.244-1.353) | 1.092 (1.047-1.139) | 1.087 (1.042-1.134) |
| 60–90 | 193292 | 4344 | 5.75 | 2.031 (1.949-2.117) | 1.205 (1.154-1.258) | 1.200 (1.149-1.253) | |
| 30–60 | 21810 | 1020 | 12.35 | 4.374 (4.087-4.681) | 1.694 (1.580-1.817) | 1.651 (1.539-1.771) | |
| < 30 | 904 | 48 | 14.93 | 5.312 (3.997-7.059) | 2.289 (1.721-3.043) | 2.150 (1.617-2.860) | |
| ESKD | 1079 | 34 | 8.63 | 3.072 (2.192-4.305) | 2.251 (1.606-3.155) | 2.105 (1.502-2.951) | |
| 5–9 yr | ≥ 90 | 219064 | 4171 | 4.88 | 1.726 (1.655-1.800) | 1.257 (1.204-1.311) | 1.188 (1.138-1.240) |
| 60–90 | 173265 | 4720 | 7.00 | 2.474 (2.376-2.577) | 1.293 (1.239-1.349) | 1.228 (1.176-1.282) | |
| 30–60 | 27807 | 1373 | 13.07 | 4.625 (4.355-4.913) | 1.704 (1.600-1.815) | 1.597 (1.499-1.702) | |
| < 30 | 1442 | 104 | 20.53 | 7.309 (6.018-8.877) | 3.012 (2.478-3.661) | 2.730 (2.246-3.319) | |
| ESKD | 1454 | 71 | 13.39 | 4.758 (3.764-6.015) | 3.403 (2.691-4.303) | 2.986 (2.361-3.778) | |
| ≥ 10 yr | ≥ 90 | 201984 | 5058 | 6.50 | 2.304 (2.214-2.398) | 1.445 (1.387-1.505) | 1.245 (1.193-1.300) |
| 60–90 | 200657 | 7305 | 9.53 | 3.375 (3.253-3.502) | 1.561 (1.500-1.624) | 1.342 (1.287-1.399) | |
| 30–60 | 55283 | 3170 | 15.55 | 5.520 (5.276-5.776) | 1.988 (1.894-2.088) | 1.647 (1.565-1.733) | |
| < 30 | 5010 | 405 | 23.57 | 8.412 (7.599-9.311) | 3.416 (3.081-3.787) | 2.689 (2.422-2.985) | |
| ESKD | 4201 | 356 | 24.79 | 8.863 (7.957-9.871) | 5.208 (4.672-5.807) | 3.948 (3.535-4.409) | |
A total of 73 549 (3.5%) deaths were identified during follow-up. Incidence rates and risk of death increased gradually with increasing diabetes duration (Figure 2, Table 4). In each diabetes duration groups, the risk of death in the groups with eGFR of 60–90 mL/min/1.73 m2 was significantly lower than in the reference group. When the group with eGFR ≥ 90 mL/min/1.73 m2 in each diabetes duration group was set as the reference, the risk of MI or IS was higher in the groups with eGFR 60–90 mL/min/1.73 m2 than in the groups with eGFR ≥ 90 mL/min/1.73 m2 only in the new-onset and < 5 years groups (Supplementary Table 1). The risk of death began to increase at eGFR of 30–60 mL/min/1.73 m2 in each diabetes duration group and the risk gradually increased as GFR decreased. The association between eGFR on a continuous scale and the risk of death according to diabetes duration exhibited a U shape in all duration groups (Supplementary Figure 1).
| Diabetes duration | eGFR, | n | Events (n) | Incidence rate (per 1000 person-years) | HR (95%CI) | ||
| Model 11 | Model 22 | Model 33 | |||||
| New-onset | ≥ 90 | 427078 | 7135 | 4.33 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| 60–90 | 259089 | 7509 | 7.49 | 1.724 (1.669-1.780) | 0.957 (0.926-0.989) | 0.943 (0.913-0.975) | |
| 30–60 | 18770 | 1765 | 24.78 | 5.695 (5.406-5.999) | 1.406 (1.332-1.483) | 1.345 (1.275-1.420) | |
| < 30 | 767 | 133 | 48.28 | 11.150 (9.392-13.236) | 2.800 (2.357-3.326) | 2.673 (2.250-3.176) | |
| ESKD | 757 | 64 | 22.63 | 5.215 (4.077-6.670) | 2.839 (2.219-3.631) | 2.814 (2.200-3.600) | |
| < 5 yr | ≥ 90 | 291515 | 6400 | 5.57 | 1.276 (1.234-1.320) | 1.056 (1.020-1.092) | 1.013 (0.979-1.048)4 |
| 60–90 | 193292 | 6614 | 8.66 | 1.980 (1.915-2.047) | 0.971 (0.938-1.005)4 | 0.932 (0.900-0.965) | |
| 30–60 | 21810 | 1881 | 22.33 | 5.110 (4.857-5.376) | 1.439 (1.365-1.516) | 1.339 (1.270-1.412) | |
| < 30 | 904 | 137 | 41.82 | 9.651 (8.15-11.429) | 2.787 (2.352-3.302) | 2.444 (2.063-2.897) | |
| ESKD | 1079 | 96 | 24.01 | 5.565 (4.550-6.807) | 3.307 (2.703-4.046) | 2.716 (2.220-3.324) | |
| 5–9 yr | ≥ 90 | 219064 | 5639 | 6.54 | 1.498 (1.446-1.551) | 1.000 (0.965-1.036)4 | 0.913 (0.881-0.947) |
| 60–90 | 173265 | 7305 | 10.70 | 2.445 (2.367-2.526) | 1.003 (0.969-1.037)4 | 0.921 (0.889-0.953) | |
| 30–60 | 27807 | 2683 | 24.99 | 5.714 (5.466-5.974) | 1.514 (1.445-1.586) | 1.362 (1.300-1.428) | |
| < 30 | 1442 | 275 | 52.80 | 12.192 (10.808-13.753) | 3.548 (3.143-4.005) | 2.942 (2.605-3.322) | |
| ESKD | 1454 | 187 | 34.57 | 7.978 (6.900-9.224) | 4.685 (4.051-5.419) | 3.717 (3.213-4.301) | |
| ≥ 10 yr | ≥ 90 | 201984 | 6632 | 8.43 | 1.936 (1.872-2.002) | 1.006 (0.972-1.041)4 | 0.816 (0.787-0.845) |
| 60–90 | 200657 | 11112 | 14.26 | 3.270 (3.174-3.368) | 1.091 (1.057-1.126) | 0.885 (0.856-0.915) | |
| 30–60 | 55283 | 6091 | 29.16 | 6.706 (6.480-6.939) | 1.688 (1.627-1.752) | 1.300 (1.250-1.351) | |
| < 30 | 5010 | 1081 | 60.88 | 14.153 (13.276-15.087) | 4.115 (3.854-4.393) | 2.926 (2.738-3.128) | |
| ESKD | 4201 | 810 | 54.56 | 12.711 (11.820-13.669) | 5.540 (5.147-5.963) | 3.629 (3.367-3.912) | |
This study investigated the association between diabetes duration, GFR, and the risk of CVD and mortality in individuals with type 2 diabetes. Our findings indicate that prolonged diabetes duration is a significant predictor of increased MI, IS, and all-cause mortality risk. Notably, even among individuals with normal or near-normal GFR (≥ 90 mL/min/1.73 m²), extended diabetes duration correlated with increased MI and IS risk. Additionally, a GFR decline to < 60 mL/min/
Our results are consistent with existing literature emphasizing the pivotal role of diabetes duration in CVD risk prediction. For instance, using United Kingdom Biobank data, a study demonstrated that participants with diabetes durations of 10–15 years had a 1.5-fold risk increase, and those with durations of ≥ 15 years had a 2.22-fold increase in fatal and nonfatal CVD event risk when compared with those with durations of < 5 years[6]. Similarly, the Atherosclerosis Risk in Communities study revealed that compared with nondiabetic counterparts, individuals with diabetes duration ≥ 15 years exhibited a 2.82-fold increase in heart failure risk and each 5-year increase in duration was associated with a 17% relative increase in heart failure risk, independent of traditional risk factors[16]. Collectively, these findings highlight the need to integrate diabetes duration into routine CV risk assessments to enhance predictive accuracy and guide therapeutic interventions. In the same context as diabetes duration, age at diabetes onset has also emerged as a crucial determinant of disease trajectory and complication risk. Individuals diagnosed with type 2 diabetes before the age of 40 years face a higher CVD risk and cardiac 10-year expected risk than those diagnosed later[17]. A prospective study by Chan et al[18] found that compared with patients with late-onset diabetes, young-onset patients diagnosed with type 2 diabetes before 40 years of age had a higher risk for CV and renal events[18]. However, after adjustment for diabetes duration, the association between young-onset type 2 diabetes and CVD was not significant[18]. Since complications, including retinopathy and nephropathy, progress rapidly, these demographic characteristics highlight the need for early, aggressive management strategies and ongoing monitoring to mitigate long-term adverse effects.
A notable observation from our study is the U-shaped association between GFR and mortality risk. While reduced GFR (< 60 mL/min/1.73 m²) is traditionally linked to higher mortality, our findings indicate that those with preserved or high normal GFR also face increased mortality rates. This paradox may be attributed to several factors. First, individuals with low muscle mass, malnutrition, or frailty may present with artificially elevated GFR because of lower serum creatinine levels, masking underlying health issues and leading to actual renal impairment underestimation; a phenomenon known as reverse epidemiology[19]. Second, early diabetic nephropathy stages often involve glomerular hyperfiltration, which, despite normal or elevated GFR readings, signifies renal pathology and correlates with increased CV events and mortality[5]. These insights suggest that GFR alone may be an insufficient renal function marker and highlight the need to include additional parameters, such as albuminuria, for a more reasonable risk assessment. Current CKD guidelines, including by KDIGO, primarily use GFR and albuminuria levels to stratify CV risk. Albuminuria is one of the important indicators of renal damage, and it is well known that the amount of albuminuria shows a linear relationship with not only renal prognosis but also CVD risk[20]. GFR and albuminuria are currently the main markers used for risk estimation of CKD[9]. However, our study advocates for the incorporation of diabetes duration as an independent risk factor in these models. Although diabetes duration cannot be changed, it is a crucial indicator of cumulative glycemic exposure and vascular damage, much like age reflects accumulated risk. Therefore, incorporating diabetes duration into risk asse
The mechanism of CVD development due to renal dysfunction represented by low GFR is well known. This includes mechanisms involving traditional risk factors such as hypertension, hyperglycemia, and dyslipidemia, and nontraditional factors such as vascular calcification, chronic systemic inflammation, and uremic milieu[21]. However, the mechanism by which longer diabetes duration increases CV complications is unclear. However, through previous studies, several causes can be hypothesized. A prospective cohort study involving patients with diabetes undergoing diagnostic coronary angiography and intravascular ultrasound revealed that a longer diabetes duration was associated with intravascular ultrasound-defined thin-cap fibroatheroma, which is believed to be associated with plaque rupture and events related to coronary heart disease [22]. Oxidized LDL is a key factor in atherosclerosis progression, and compared with newly diagnosed patients and healthy participants, oxidized LDL cholesterol levels were significantly higher in patients with diabetes duration > 5 years, while total LDL cholesterol was significantly lower in patients with prolonged diabetes when compared with newly diagnosed patients[23]. In the Northern Manhattan Study, a prospective population-based cohort study designed to determine stroke incidence, risk factors, and prognosis in an urban multiethnic population, diabetes duration was associated with an increased risk of IS (adjusted HR: 1.03, 95%CI: 1.02–1.04). Patients with a diabetes duration of > 10 years showed a 3.2-fold higher IS risk (adjusted HR: 3.2, 95%CI: 2.4–4.5)[24]. The authors suggest that in longstanding diabetes, endothelial dysfunction and fibrinogen and clotting mechanism abnormalities may contribute to ischemic vascular complications.
To our knowledge, this is the first study to demonstrate in a nationwide cohort that prolonged diabetes duration magnifies the CV risks associated with CKD. These findings build upon prior evidence of diabetes duration as a risk factor and extend it by illustrating the interactive impact of diabetes duration and renal function on CVD and mortality risk. Despite the strengths of our large population-based study, several limitations warrant consideration. First, diabetes duration was determined using claims data, which may introduce misclassification bias. Diabetes duration calculation may have some errors, especially in patients with late diagnoses or those who did not visit the hospital. However, because of the characteristics of the Korean NHIS, in which most citizens are enrolled, and biennial mandatory free health check-ups, it seems a fairly accurate calculation was made. Second, the lack of albuminuria measurements, a pivotal diabetic kidney disease marker, limits the renal risk assessment’s comprehensiveness. If we had known the albuminuria level of the patients in our study, we could have observed the joint association of diabetes duration and CVD according to more detailed risk status including albuminuria as well as GFR. In future research, incorporating albuminuria data might enhance the understanding of the interplay between renal function and CV outcomes. Third, because this was a retrospective cohort study, causal inferences cannot be definitively established. Future studies will need to evaluate diabetes duration as one of the major risk factors for complications and design interventions based on this risk stratification. Additionally, evaluating the potential benefits of modifying risk assessment frameworks to include diabetes duration may inform clinical practice and guideline development. For example, diabetes duration can be used to adjust baseline risk or coefficients in multivariable models, or to guide the timing of using specific classes of drugs, such as sodium-glucose cotransporter-2 inhibitors or glucagon-like peptide-1 receptor agonists, based on risk assessment using diabetes duration.
Our study elucidated a profound impact of diabetes duration on CV risk and mortality, independent of GFR status. In all diabetes duration groups, lower eGFR was associated with higher risk of MI or IS. The association between eGFR and mortality revealed a U shape, especially in new-onset diabetes and diabetes duration < 5 years. These findings highlight the need for considering diabetes duration as an essential factor in CV risk assessment and CKD management. Diabetes duration integration into clinical guidelines may facilitate the identification of high-risk individuals early, enabling more personalized and effective interventions.
| 1. | NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: a pooled analysis of 1108 population-representative studies with 141 million participants. Lancet. 2024;404:2077-2093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 135] [Article Influence: 135.0] [Reference Citation Analysis (0)] |
| 2. | Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2249] [Cited by in RCA: 3395] [Article Influence: 485.0] [Reference Citation Analysis (0)] |
| 3. | Rawshani A, Rawshani A, Franzén S, Eliasson B, Svensson AM, Miftaraj M, McGuire DK, Sattar N, Rosengren A, Gudbjörnsdottir S. Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. N Engl J Med. 2017;376:1407-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 883] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 4. | American Diabetes Association Professional Practice Committee. 11. Chronic Kidney Disease and Risk Management: Standards of Care in Diabetes-2025. Diabetes Care. 2025;48:S239-S251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 5. | Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, Lee BJ, Perkins RM, Rossing P, Sairenchi T, Tonelli M, Vassalotti JA, Yamagishi K, Coresh J, de Jong PE, Wen CP, Nelson RG; Chronic Kidney Disease Prognosis Consortium. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 877] [Cited by in RCA: 899] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 6. | Li FR, Yang HL, Zhou R, Zheng JZ, Chen GC, Zou MC, Wu XX, Wu XB. Diabetes duration and glycaemic control as predictors of cardiovascular disease and mortality. Diabetes Obes Metab. 2021;23:1361-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 7. | Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Jüni P, Lettino M, Marx N, Mellbin LG, Östgren CJ, Rocca B, Roffi M, Sattar N, Seferović PM, Sousa-Uva M, Valensi P, Wheeler DC; ESC Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1670] [Cited by in RCA: 2636] [Article Influence: 659.0] [Reference Citation Analysis (0)] |
| 8. | Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2022;102:S1-S127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 571] [Article Influence: 190.3] [Reference Citation Analysis (0)] |
| 9. | Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024;105:S117-S314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1064] [Article Influence: 1064.0] [Reference Citation Analysis (0)] |
| 10. | Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7995] [Cited by in RCA: 8524] [Article Influence: 405.9] [Reference Citation Analysis (0)] |
| 11. | Choi HS, Han KD, Oh TR, Suh SH, Kim M, Kim CS, Bae EH, Ma SK, Kim SW. Trends in the incidence and prevalence of end-stage renal disease with hemodialysis in entire Korean population: A nationwide population-based study. Medicine (Baltimore). 2021;100:e25293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Chaiken RL, Khawaja R, Bard M, Eckert-Norton M, Banerji MA, Lebovitz HE. Utility of untimed urinary albumin measurements in assessing albuminuria in black NIDDM subjects. Diabetes Care. 1997;20:709-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Kim JJ, Hwang BH, Choi IJ, Choo EH, Lim S, Kim JK, Koh YS, Kim DB, Jang SW, Cho EJ, Lee JM, Kim PJ, Cho JH, Jung JI, Seung KB, Min JK, Chang K. Impact of diabetes duration on the extent and severity of coronary atheroma burden and long-term clinical outcome in asymptomatic type 2 diabetic patients: evaluation by Coronary CT angiography. Eur Heart J Cardiovasc Imaging. 2015;16:1065-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Wan Nazaimoon WM, Letchuman R, Noraini N, Ropilah AR, Zainal M, Ismail IS, Wan Mohamad WB, Faridah I, Singaraveloo M, Sheriff IH, Khalid BA. Systolic hypertension and duration of diabetes mellitus are important determinants of retinopathy and microalbuminuria in young diabetics. Diabetes Res Clin Pract. 1999;46:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20319] [Cited by in RCA: 20141] [Article Influence: 1258.8] [Reference Citation Analysis (0)] |
| 16. | Echouffo-Tcheugui JB, Zhang S, Florido R, Hamo C, Pankow JS, Michos ED, Goldberg RB, Nambi V, Gerstenblith G, Post WS, Blumenthal RS, Ballantyne CM, Coresh J, Selvin E, Ndumele CE. Duration of Diabetes and Incident Heart Failure: The ARIC (Atherosclerosis Risk In Communities) Study. JACC Heart Fail. 2021;9:594-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Magliano DJ, Sacre JW, Harding JL, Gregg EW, Zimmet PZ, Shaw JE. Young-onset type 2 diabetes mellitus - implications for morbidity and mortality. Nat Rev Endocrinol. 2020;16:321-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 279] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 18. | Chan JC, Lau ES, Luk AO, Cheung KK, Kong AP, Yu LW, Choi KC, Chow FC, Ozaki R, Brown N, Yang X, Bennett PH, Ma RC, So WY. Premature mortality and comorbidities in young-onset diabetes: a 7-year prospective analysis. Am J Med. 2014;127:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 19. | Kovesdy CP, Anderson JE. Reverse epidemiology in patients with chronic kidney disease who are not yet on dialysis. Semin Dial. 2007;20:566-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J; Chronic Kidney Disease Prognosis Consortium. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80:93-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 690] [Cited by in RCA: 643] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 21. | Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation. 2021;143:1157-1172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 531] [Cited by in RCA: 1083] [Article Influence: 270.8] [Reference Citation Analysis (2)] |
| 22. | Lindsey JB, House JA, Kennedy KF, Marso SP. Diabetes duration is associated with increased thin-cap fibroatheroma detected by intravascular ultrasound with virtual histology. Circ Cardiovasc Interv. 2009;2:543-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Nakhjavani M, Khalilzadeh O, Khajeali L, Esteghamati A, Morteza A, Jamali A, Dadkhahipour S. Serum oxidized-LDL is associated with diabetes duration independent of maintaining optimized levels of LDL-cholesterol. Lipids. 2010;45:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Banerjee C, Moon YP, Paik MC, Rundek T, Mora-McLaughlin C, Vieira JR, Sacco RL, Elkind MS. Duration of diabetes and risk of ischemic stroke: the Northern Manhattan Study. Stroke. 2012;43:1212-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |