Published online Aug 15, 2025. doi: 10.4251/wjgo.v17.i8.108007
Revised: May 23, 2025
Accepted: July 8, 2025
Published online: August 15, 2025
Processing time: 113 Days and 21.1 Hours
Rectal cancer is one of the common digestive system malignant tumors around the world. Its early diagnosis and staging are crucial for rectal cancer treatment and prognosis. In recent years, tumor markers have gradually received attention in early screening, treatment monitoring and prognostic evaluation of cancer, but their predictive role in rectal cancer staging and differentiation is still unclear.
To assess the prognostic value of tumor markers alpha-fetoprotein (AFP) cancer antigen 72-4 (CA72-4), carbohydrate antigen 19-9 (CA19-9), and carcinoembryonic antigen (CEA), alongside multimodal magnetic resonance imaging (MRI), for staging and differentiating rectal cancer in patients.
This study retrospectively analyzed 167 patients with rectal cancer who were treated at our institution from January 2020 to December 2024. Each patient underwent serological testing and multimodal MRI for diagnosis. Histopathological examination after surgical resection or imaging based on follow-up was used as the gold standard. According to the T stage and differentiation degree, patients were divided into low stage group (T1-T2) and high stage group (T3-T4). In addition, they were divided into low-differentiation groups and high-differentiation groups according to their differentiation degree. We compared the accuracy, sensitivity and specificity of tumor marker levels and MRI in rectal cancer stage and differentiation.
The study's findings indicate that in the context of rectal cancer T staging, there is substantial concordance between MRI and clinicopathological assessments, with a Kappa coefficient of 0.789 (P < 0.001). Similarly, for various degrees of tumor differentiation, MRI and clinicopathological evaluations demonstrated substantial agreement, with a Kappa coefficient of 0.651 (P < 0.001). Notably, the concentrations of tumor markers CA19-9, CA72-4, CEA, and AFP were significantly elevated in the T3-T4 stage compared to the T1-T2 stage. Furthermore, these markers were significantly higher in the low-differentiation group compared to the high-differentiation group (P < 0.05). The combined use of tumor markers and MRI for preoperative T staging of rectal cancer yielded a diagnostic sensitivity of 93.7% and a specificity of 94.6%, as evidenced by the receiver operating characteristic analysis, with an area under the curve of 0.947. For tumor differentiation, the diagnostic sensitivity and specificity were 93.6% and 97.1%, respectively, with an area under the curve of 0.978 (95% confidence interval: 0.946-1.000), surpassing the accuracy of individual detection methods.
The CA19-9, CA72-4, CEA and AFP tumor markers combined with multimodal MRI have high sensitivity and specificity in diagnosing rectal cancer stage and differentiation. Their diagnostic efficacy is significantly better than that of single tests, which can effectively improve the predictive ability of rectal cancer stage and differentiation, provide a more reliable diagnostic reference for clinical practice, and have important clinical significance.
Core Tip: This study included 167 rectal cancer patients who underwent serological tests and multimodal magnetic resonance imaging. Results showed significant concordance between magnetic resonance imaging and pathological T-staging. Combined use of tumor markers (alpha-fetoprotein cancer antigen 72-4, carbohydrate antigen 19-9, and carcinoembryonic antigen) and magnetic resonance imaging had high sensitivity/specificity for staging/differentiation, outperforming single tests, thus enhancing diagnostic accuracy for clinical practice.
- Citation: Wang P, Han J, Zhao WN, Wu F, Zhang SH, Huang YJ. Tumor markers and multimodal magnetic resonance imaging in predicting rectal cancer stage and differentiation. World J Gastrointest Oncol 2025; 17(8): 108007
- URL: https://www.wjgnet.com/1948-5204/full/v17/i8/108007.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i8.108007
Rectal cancer is a frequently occurring malignant tumor in the digestive system, with its incidence increasing annually. Early detection and precise staging are crucial for its prognosis[1]. Approximately 1.8 million new cases are diagnosed annually, leading to 900000 related deaths[2]. Improvements in diagnostic and treatment methods have led to a 5-year survival rate of around 90% for early-stage rectal cancer patients, but this decreases to 13.1% for advanced stage cases[3]. Metastatic colorectal cancer is identified in about 40% to 50% of colorectal cancer patients[4]. In China, there is a growing trend in rectal cancer cases with an earlier age of onset[5]. Early stage rectal cancer is frequently asymptomatic, complicating diagnosis. Patients usually have poor prognoses when they show more advanced symptoms, such as rectal bleeding and abdominal pain[6]. Therefore, prompt identification, diagnosis, and treatment of rectal cancer is vital for improving patient survival and quality of life.
Colonoscopy is the main method for diagnosing rectal cancer, whereas computed tomography (CT) and positron emission tomography (PET) scans are commonly used to assess the spread of rectal cancer tumors[7]. Magnetic resonance imaging (MRI) is highly effective in diagnosing rectal cancer, particularly lesions under 1 cm, due to its excellent soft tissue resolution, various scanning techniques, and lack of radiation risks[8]. Multi-modal MRI has recently become crucial for diagnosing and staging rectal cancer because of its excellent resolution and tissue contrast. Imaging techniques alone may not completely uncover the biological traits of the tumor, particularly when evaluating rectal cancer staging and differentiation[9]. Tumor markers are crucial for cancer diagnosis and treatment monitoring. Blood markers like alpha-fetoprotein (AFP), cancer antigen 72-4 (CA72-4), carbohydrate antigen 19-9 (CA19-9), and carcinoembryonic antigen (CEA) are overexpressed in various tumors, indicating growth, invasion, and metastasis[10]. These biomarkers correlate with tumor behavior and clinical progression, aiding in diagnosis and staging[11]. However, relying solely on imaging or biomarker detection can lead to misdiagnosis or missed diagnosis in rectal cancer. This study aims to evaluate the diagnostic value of combining tumor markers (AFP, CA19-9, CEA, and CA72-4) with enhanced MRI for preoperative staging and differentiation of rectal cancer, thereby improving preoperative assessment.
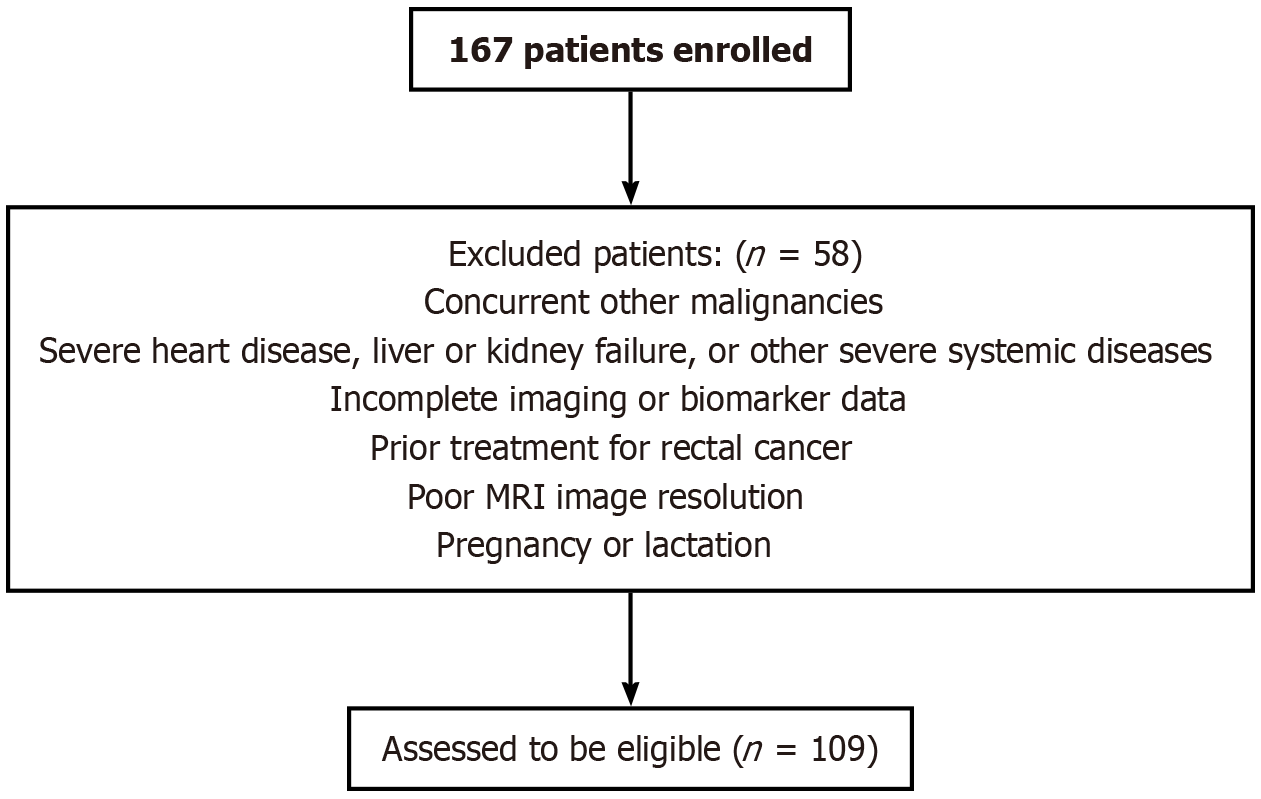
This retrospective study involved the selection of 167 patients diagnosed with rectal cancer via colonoscopy and proctoscopy at our institution between January 2020 and December 2024. These patients underwent surgical resection within 1 week following imaging examinations. After applying the inclusion and exclusion criteria, 109 patients were deemed eligible and subsequently screened, as depicted in the patient screening flowchart in Figure 1. Oncological variables and outcomes were documented, with tumors staged according to the tumor-node-metastasis classification of the Union for International Cancer Control. Patients were categorized into low-stage (T1-T2, n = 59) and high-stage (T3-T4, n = 50) groups. Furthermore, tumors were classified into poorly differentiated (n = 52) and well-differentiated (n = 57) groups, with poorly and moderately differentiated tumors being collectively categorized as poorly differentiated.
Inclusion criteria: (1) Diagnosis in accordance with the “National Comprehensive Cancer Network Guidelines for Diagnosis and Treatment of Rectal Cancer”[12]; (2) Availability of comprehensive clinical, pathological, and imaging data for assessment; (3) Underwent preoperative multimodal MRI; and (4) Absence of prior chemotherapy, radiotherapy, or any other treatments before study enrollment.
Exclusion criteria: (1) Presence of other malignant tumors; (2) Severe cardiovascular disease, hepatic or renal failure, or other significant systemic diseases; (3) Incomplete imaging or biomarker data; (4) Prior treatment for rectal cancer; (5) Suboptimal MRI image resolution; and (6) Pregnant or lactating women.
The ethical standards of the local ethics committee and the Declaration of Helsinki (1964) and its subsequent updates were adhered to in all procedures. Informed consent was not required for retrospective image data, as approved by the institutional review board.
All patients fasted for 8-12 hours before the examination. Patients with intestinal obstruction symptoms underwent bowel preparation.
Venous blood samples were obtained from patients, and serum was isolated via centrifugation. CA199, CA72-4, CEA, and AFP concentrations were quantified using an enzyme-linked immunosorbent assay (ELISA). On the second day following admission, 4-5 mL of fasting blood was collected and centrifuged at 3000 rpm for 10 minutes within 1 hour of collection.
Patients were positioned supine on the scanning table for initial localization imaging[13]. The primary imaging sequences included T2-weighted imaging for axial and sagittal plane scans to assess the tumor’s location, size, and penetration through the intestinal wall, utilizing scanning parameters of repetition time = 4000 millisecond/echo time = 80 milliseconds and a 3 mm slice thickness. MRI examinations were performed using a 3.0-T scanner (GE Discovery MR750), with a spatial resolution of 0.5 mm × 0.5 mm × 3 mm for T2-weighted sequences. Diffusion-weighted imaging was used to differentiate tumor tissue, with b values set at 0, 500, and 1000 second/mm2 and a 3 mm slice thickness. Dynamic contrast-enhanced T1-weighted imaging was conducted following intravenous administration of a contrast agent to evaluate the degree of tumor vascularization, using scanning parameters of repetition time = 3.5 millisecond/echo time = 1.5 milliseconds and a 3 mm slice thickness. Upon completion of all imaging sequences, two radiologists, each with over 10 years of experience, conducted meticulous image analysis. The evaluation was based on the tumor’s morphology, size, invasion, and vascular characteristics, leading to a comprehensive assessment of T stage and degree of differentiation. Detailed diagnostic reports were subsequently prepared, outlining the specific location, size, stage, and degree of differentiation of the tumor as determined by MRI results. These reports provide a precise foundation for clinical treatment decisions (Table 1).
| MRI staging criteria | Description |
| T1 stage | The tumor is limited to the mucosal layer, presenting as a polypoid structure within the lumen or as a local mass |
| T2 stage | The thickness of the wall exceeds 0.5 cm, but the tumor has not yet extended to the surrounding fat tissue, with a clear distinction from surrounding fat |
| T3 stage | The tumor extends to the surrounding fat tissue but has not reached the fascia. This stage is defined as tumor penetration through the wall layer and invasion into surrounding fat |
| T4 stage | The tumor further invades the pelvic region and may be accompanied by distant metastasis |
Pathological specimens were preserved in formalin and diagnosed by two pathologists, each with over 10 years of experience. Imaging results were compared with the gold standard, which comprised surgical intervention with histopathological analysis and follow-up imaging. The follow-up and comparative imaging modalities included CT, MRI, and fluorine-18-fluorodeoxyglucose-positron emission tomography (PET) CT. Tumor differentiation was classified based on World Health Organization 2019 criteria[14]: (1) High-grade: G1 (well-differentiated) and G2 (moderately differentiated); and (2) Low-grade: G3 (poorly differentiated) and G4 (undifferentiated).
The following comparisons were made: (1) Concordance between MRI findings and the pathological diagnostic gold standard in patients with varying stages and degrees of differentiation of rectal cancer; (2) The comparison of CA199, CA72-4, CEA, and AFP levels in patients with different stages and degrees of differentiation of rectal cancer; (3) The construction of receiver operating characteristic (ROC) curves, with the calculation of the area under the curve (AUC) and 95% confidence intervals to assess diagnostic accuracy. The sensitivity and specificity of various detection methods were compared, with a P value less than 0.05 considered statistically significant.
Data processing was performed in SPSS version 26.0. For normally distributed quantitative data, the results were articulated as mean ± SD, with intergroup comparisons conducted via independent samples t-test. For data deviating from a normal distribution, results were represented as the median accompanied by the interquartile range, and the Mann-Whitney U test was utilized for intergroup comparisons. Categorical variables were delineated as frequency and percentage, with group differences evaluated using the χ² test or Fisher’s exact test.
The baseline characteristics of the 109 rectal cancer patients were as follows: Most patients (62.39%) were 60 years of age or older, while 37.61% were under 60. There was a slight male predominance (55.96%) compared to females (44.04%). In terms of lymph node involvement, 36.70% of patients exhibited lymphatic metastasis, whereas 63.30% did not. Vascular and nerve infiltration were relatively rare, occurring in 6.42% and 5.50% of cases, respectively. Most tumors were moderately differentiated (84.40%), with 11.93% classified as poorly differentiated and 3.67% as highly differentiated. The distribution of T staging was as follows: T1 (17.43%), T2 (42.20%), T3 (33.94%), and T4 (6.42%). Further details are provided in Table 2. MRI and clinicopathological examination showed a high degree of consistency between the two T staging methods (Kappa coefficient = 0.789, P < 0.001). Similarly, the diagnostic results for the degree of differentiation were largely consistent between the two methods (Kappa coefficient = 0.651, P < 0.001), as demonstrated in Tables 3 and 4.
| Clinicopathological parameters | Cases |
| Age | |
| < 60 | 41 (37.61) |
| ≥ 60 | 68 (62.39) |
| Gender | |
| Male | 61 (55.96) |
| Female | 48 (44.04) |
| Lymphatic metastasis | |
| Yes | 40 (36.70) |
| No | 69 (63.30) |
| Vascular invasion | |
| Yes | 7 (6.42) |
| No | 102 (93.58) |
| Nerve infiltration | |
| Yes | 6 (5.50) |
| No | 103 (94.50) |
| Degree of differentiation | |
| Poorly | 13 (11.93) |
| Moderately | 92 (84.40) |
| Highly | 4 (3.67) |
| T staging | |
| T1 | 19 (17.43) |
| T2 | 46 (42.20) |
| T3 | 37 (33.94) |
| T4 | 7 (6.42) |
| MRI staging | Pathological staging (gold standard) | Total (n) | |
| T1-T2 | T3-T4 | ||
| T1-T2 | 60 | 6 | 66 |
| T3-T4 | 5 | 38 | 43 |
| Total (n) | 65 | 44 | 109 |
| MRI grading | Pathological grading (gold standard) | Total | |
| High-grade | Low-grade | ||
| High-grade | 3 | 2 | 5 |
| Low-grade | 1 | 103 | 104 |
| Total | 4 | 105 | 109 |
AFP, CEA, CA72-4, and CA19-9 Levels were significantly higher in patients at the T3-T4 stages compared to those at the T1-T2 stages (P < 0.05), as presented in Table 5.
| Indicator | T1-T2 (n = 65) | T3-T4 (n = 44) | P value |
| CA19-9 (kU/L) | 10.37 ± 2.05 | 43.31 ± 4.52 | < 0.001 |
| CA72-4 (kU/L) | 6.43 ± 1.71 | 12.62 ± 2.14 | < 0.001 |
| CEA (μg/L) | 13.28 ± 3.75 | 23.26 ± 2.32 | < 0.001 |
| AFP (μg/L) | 16.12 ± 3.83 | 21.16 ± 2.53 | < 0.001 |
During the T3-T4 stages, AFP, CEA, CA72-4, and CA19-9 Levels were markedly elevated compared to the T1-T2 stages, as illustrated in Table 6.
| Indicator | Low differentiation (n = 105) | High differentiation (n = 4) | P value |
| CA19-9 (kU/L) | 55.43 ± 5.12 | 9.62 ± 2.71 | < 0.001 |
| CA72-4 (kU/L) | 14.15 ± 3.63 | 7.34 ± 1.25 | < 0.001 |
| CEA (μg/L) | 14.82 ± 2.16 | 7.73 ± 1.48 | < 0.001 |
| AFP (μg/L) | 3.94 ± 0.58 | 3.26 ± 0.51 | 0.022 |
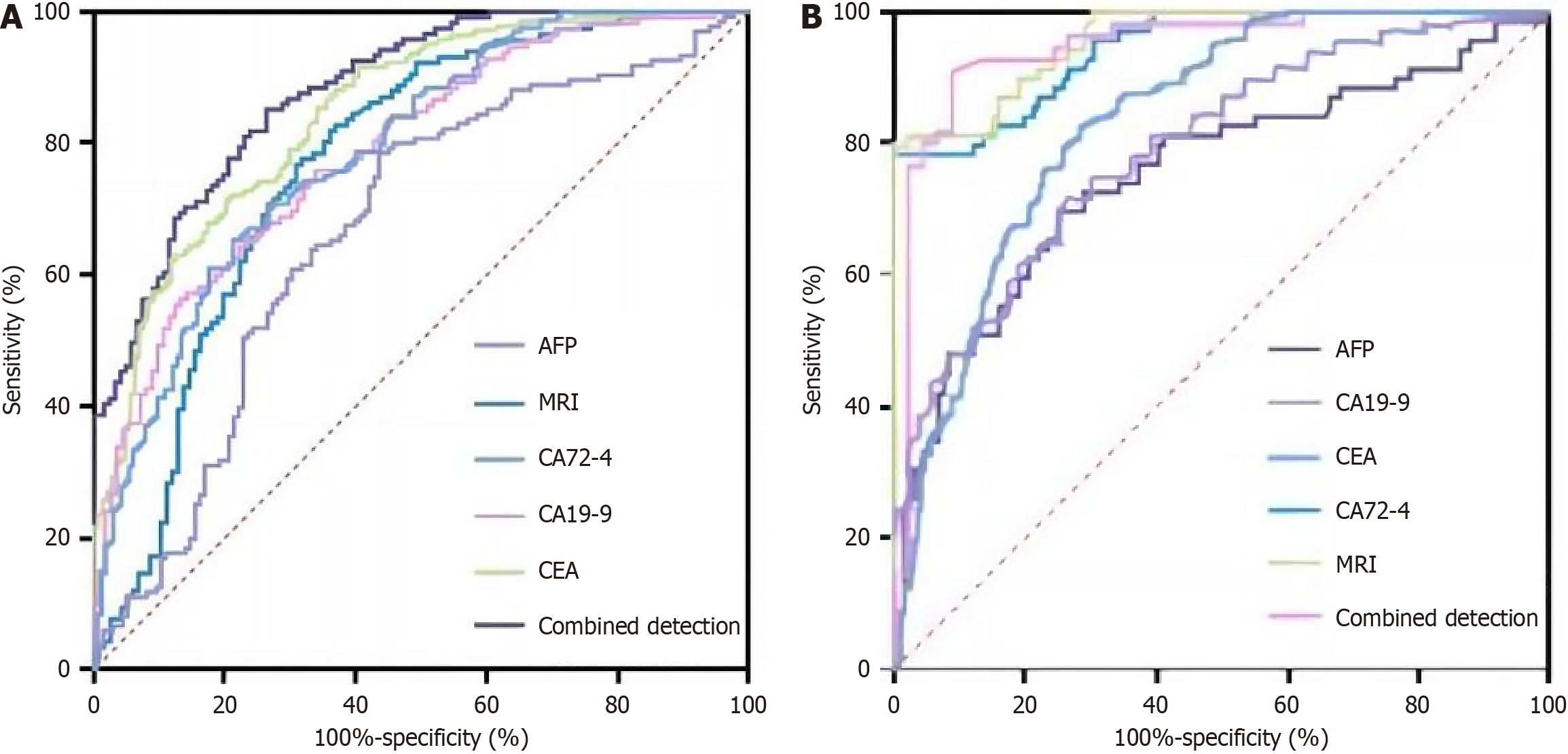
ROC analysis revealed that the combination of tumor markers with MRI for preoperative T staging of rectal cancer achieved a diagnostic sensitivity and specificity of 93.7% and 94.6%, respectively, with an AUC of 0.947, as depicted in Table 7 and Figure 2A.
| Indicator | AUC | 95%CI | P value | Sensitivity (%) | Specificity (%) |
| AFP | 0.669 | 0.604-0.735 | < 0.001 | 73.5 | 76.7 |
| MRI | 0.771 | 0.709-0.833 | < 0.001 | 83.1 | 85.8 |
| CA72-4 | 0.789 | 0.741-0.836 | < 0.001 | 65.2 | 78.6 |
| CA19-9 | 0.846 | 0.802-0.889 | < 0.001 | 89.5 | 82.6 |
| CEA | 0.879 | 0.838-0.921 | < 0.001 | 85.3 | 91.2 |
| Combined detection | 0.947 | 0.899-0.994 | < 0.001 | 93.7 | 94.6 |
ROC analysis indicated that the combination of tumor markers and MRI achieved a diagnostic sensitivity of 93.6% and specificity of 97.1% for rectal cancer differentiation, with an AUC of 0.978, as illustrated in Table 8 and Figure 2B.
| Indicator | AUC | 95%CI | P value | Sensitivity (%) | Specificity (%) |
| AFP | 0.815 | 0.721-0.896 | < 0.001 | 75.4 | 76.2 |
| CA19-9 | 0.887 | 0.817-0.955 | < 0.001 | 89.2 | 91.2 |
| CEA | 0.894 | 0.842-0.964 | < 0.001 | 90.5 | 88.1 |
| CA72-4 | 0.916 | 0.866-0.975 | < 0.001 | 89.6 | 89.4 |
| MRI | 0.923 | 0.873-0.975 | < 0.001 | 91.3 | 93.1 |
| Combined detection | 0.978 | 0.946-0.998 | < 0.001 | 93.6 | 97.1 |
This study reveals that MRI exhibits certain inconsistencies compared to conventional pathological staging in the preoperative T-staging of rectal cancer. Notably, MRI has a conservative bias in diagnosing T1-T2 stage tumors, largely attributable to its limitations in detecting micro-tumor invasion, particularly in evaluating tumor penetration of the muscularis propria. Additionally, MRI often underestimates tumor aggressiveness in T3-T4 staging. This finding aligns with previous research that identified limitations in the ability of MRI to assess rectal cancer invasion depth[15-17]. Therefore, it is important to recognize the potential restrictions of MRI when using it for T-staging and to consider combining it with other diagnostic methods to improve overall assessment accuracy[18].
Regarding the comparison of tumor marker levels between T1-T2 and T3-T4 stages, AFP, CEA, CA72-4, and CA19-9 Levels were significantly higher in T3-T4 patients than in T1-T2 patients, potentially reflecting larger tumor size and aggressiveness. These data suggest a close relationship between tumor marker levels and its biological behavior, making them valuable indicators for tumor invasiveness and prognosis. This finding is consistent with previous research[19-21]. Therefore, incorporating tumor marker detection can provide more information for rectal cancer staging and treatment decisions.
In diagnosing differentiation grades, MRI successfully identified 103 out of 105 pathologically confirmed low-differentiation cases, and 3 out of 4 pathologically diagnosed high-differentiation cases. This indicates that MRI has relatively high accuracy in diagnosing the differentiation grade of rectal cancer. Previous studies found that MRI is valuable in assessing extramural vascular invasion in rectal cancer, especially in predicting distant metastasis risk[22-25]. Results of this study demonstrate that CA19-9, CA72-4, CEA, and AFP levels in the low-differentiation group were significantly higher than those in the high-differentiation group, potentially reflecting higher tumor burden and aggressiveness. This result suggests that tumor markers can be used both for tumor detection and monitoring and as auxiliary indicators for assessing tumor invasiveness and predicting patient prognosis. Studies have shown that elevated tumor marker levels are associated with aggressive features of rectal cancer[26].
ROC analysis further demonstrates that the combination of tumor markers and multi-modal MRI has high diagnostic efficacy in T-staging and differentiation assessment of rectal cancer. This suggests that a comprehensive application of MRI and tumor marker detection can more accurately assess the invasiveness and differentiation degree of rectal cancer, thereby providing patients with more appropriate treatment plans[27]. The synergy arises from MRI’s ability to delineate anatomical invasion (e.g., muscularis propria penetration) and tumor markers’ reflection of tumor biological aggressiveness (e.g., angiogenesis, proliferation). This combined approach may particularly benefit clinical decisions requiring both structural and molecular insights. The effectiveness of this comprehensive diagnostic approach is consistent with previous research findings[14]. The application of comprehensive diagnostic methods can improve diagnostic sensitivity and specificity and also provide important information for the personalized treatment of rectal cancer patients.
As a retrospective single-center study, this study has the following limitations: Firstly, because the data for this study comes from the electronic medical records of patients from a single center in China, there may be bias in the admission rate, potentially impacting the accuracy of the results. Secondly, laboratory indicator data may have differences due to environmental influences. Finally, due to the relatively small sample size, the findings of this study should be approached with caution. Future studies should include more centers and larger cohorts to eliminate selection bias and improve generalizability. Additionally, integrating high-resolution MRI or artificial intelligence-assisted image segmentation could enhance micro-invasion detection in T1-T2 stages.
In conclusion, this research investigated the usefulness of combining tumor markers with multi-modal MRI for preoperative T-staging and differential diagnosis of rectal cancer. The findings demonstrate that this combination method has significant advantages in improving the sensitivity and specificity of preoperative diagnosis of rectal cancer. This approach could help clinicians more precisely formulate treatment strategies, such as avoiding overtreatment in early-stage patients while optimizing neoadjuvant therapy for advanced cases. However, the study also revealed the limitations of MRI in accurately identifying early tumor stages and completely matching pathological diagnoses. Future research should further optimize imaging techniques and evaluation criteria, and increase sample size to improve diagnostic accuracy and reliability, so as to better guide personalized treatment strategies for rectal cancer.
| 1. | Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Jeck W, Johung KL, Kirilcuk N, Krishnamurthi S, Maratt JK, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stotsky-Himelfarb E, Tavakkoli A, Willett CG, Gregory K, Gurski L. Rectal Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:1139-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 410] [Article Influence: 136.7] [Reference Citation Analysis (0)] |
| 2. | Zhao P, Zhou P, Tang T, Si R, Ji Y, Hu X, Li A, Jiang Y. Levels of circulating mast cell progenitors and tumourinfiltrating mast cells in patients with colorectal cancer. Oncol Rep. 2022;47:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Shang Y, Zhang Y, Liu J, Chen L, Yang X, Zhu Z, Li D, Deng Y, Zhou Z, Lu B, Fu CG. Decreased E2F2 Expression Correlates with Poor Prognosis and Immune Infiltrates in Patients with Colorectal Cancer. J Cancer. 2022;13:653-668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 4. | Zlobec I, Molinari F, Martin V, Mazzucchelli L, Saletti P, Trezzi R, De Dosso S, Vlajnic T, Frattini M, Lugli A. Tumor budding predicts response to anti-EGFR therapies in metastatic colorectal cancer patients. World J Gastroenterol. 2010;16:4823-4831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Jiang H, Kang B, Huang X, Yan Y, Wang S, Ye Y, Shen Z. Cancer IgG, a potential prognostic marker, promotes colorectal cancer progression. Chin J Cancer Res. 2019;31:499-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Zwaans BMM, Nicolai HE, Chancellor MB, Lamb LE. Prostate cancer survivors with symptoms of radiation cystitis have elevated fibrotic and vascular proteins in urine. PLoS One. 2020;15:e0241388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Zheng H, Yuan C, Cai J, Pu W, Wu P, Li C, Li G, Zhang Y, Zhang J, Guo J, Huang D. Early diagnosis of breast cancer lung metastasis by nanoprobe-based luminescence imaging of the pre-metastatic niche. J Nanobiotechnology. 2022;20:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Anzai Y, Minoshima S, Lee VS. Enhancing Value of MRI: A Call for Action. J Magn Reson Imaging. 2019;49:e40-e48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Wald LL. Ultimate MRI. J Magn Reson. 2019;306:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Yousaf T, Dervenoulas G, Politis M. Advances in MRI Methodology. Int Rev Neurobiol. 2018;141:31-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 11. | Hu X, Bao M, Huang J, Zhou L, Zheng S. Corrigendum: Identification and Validation of Novel Biomarkers for Diagnosis and Prognosis of Hepatocellular Carcinoma. Front Oncol. 2020;10:617539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Park JH, Baek JH, Sym SJ, Lee KY, Lee Y. A data-driven approach to a chemotherapy recommendation model based on deep learning for patients with colorectal cancer in Korea. BMC Med Inform Decis Mak. 2020;20:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Pham TT, Liney G, Wong K, Rai R, Lee M, Moses D, Henderson C, Lin M, Shin JS, Barton MB. Study protocol: multi-parametric magnetic resonance imaging for therapeutic response prediction in rectal cancer. BMC Cancer. 2017;17:465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Zheng Z, He R, Lin C, Huang C. Multimodal Magnetic Resonance Imaging to Diagnose Knee Osteoarthritis under Artificial Intelligence. Comput Intell Neurosci. 2022;2022:6488889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Ștefan PA, Coțe A, Csutak C, Lupean RA, Lebovici A, Mihu CM, Lenghel LM, Pușcas ME, Roman A, Feier D. Texture Analysis in Uterine Cervix Carcinoma: Primary Tumour and Lymph Node Assessment. Diagnostics (Basel). 2023;13:442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Peng Y, Li J, Hu X, Shen Y, Hu D, Li Z, Kamel I. Assessing the histopathological features of rectal adenocarcinoma with chemical shift-encoded sequence (CSE)-MRI and diffusion-weighted imaging (DWI). Quant Imaging Med Surg. 2023;13:3199-3212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Zhang Z, Zhang C, Xiao L, Zhang S. Diagnosis of Early Cervical Cancer with a Multimodal Magnetic Resonance Image under the Artificial Intelligence Algorithm. Contrast Media Mol Imaging. 2022;2022:6495309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Ma X, Shen F, Jia Y, Xia Y, Li Q, Lu J. MRI-based radiomics of rectal cancer: preoperative assessment of the pathological features. BMC Med Imaging. 2019;19:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 19. | Zhang SQ, Zhang J, Yu Y, Yu MM, Wei J, Tang YH. APOBEC3B expression has prognostic significance in cervical cancer. Int J Clin Exp Pathol. 2023;16:48-56. [PubMed] |
| 20. | Ding F, Chen RY, Hou J, Guo J, Dong TY. Efficacy and prognostic factors of neoadjuvant chemotherapy for triple-negative breast cancer. World J Clin Cases. 2022;10:3698-3708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (2)] |
| 21. | Li Z, Qian Y, Li W, Liu L, Yu L, Liu X, Wu G, Wang Y, Luo W, Fang F, Liu Y, Song F, Cai Z, Chen W, Huang W. Human Lung Adenocarcinoma-Derived Organoid Models for Drug Screening. iScience. 2020;23:101411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 22. | dePrisco G. MRI Local Staging and Restaging in Rectal Cancer. Clin Colon Rectal Surg. 2015;28:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Kokelaar RF, Jones HG, Williamson J, Williams N, Griffiths AP, Beynon J, Jenkins GJ, Harris DA. DNA hypermethylation as a predictor of extramural vascular invasion (EMVI) in rectal cancer. Cancer Biol Ther. 2018;19:214-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Zhang J, Liu X. MRI is more valuable than CT in the diagnosis of cervical cancer. Am J Transl Res. 2023;15:2970-2976. [PubMed] |
| 25. | Xu J, Ma Y, Mei H, Wang Q. Diagnostic Value of Multimodal Magnetic Resonance Imaging in Discriminating Between Metastatic and Non-Metastatic Pelvic Lymph Nodes in Cervical Cancer. Int J Gen Med. 2022;15:6279-6288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 26. | Ding R, Chen Z, He M, Cen H, Liu Z, Su Y. Application Value of Combined Detection of NLR, PNI, D-Dimer, CD3(+) T Lymphocytes, and CEA in Colorectal Cancer Screening. Dis Markers. 2022;2022:7913025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Yin P, Zhong J, Liu Y, Liu T, Sun C, Liu X, Cui J, Chen L, Hong N. Clinical-radiomics models based on plain X-rays for prediction of lung metastasis in patients with osteosarcoma. BMC Med Imaging. 2023;23:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |