Published online Aug 15, 2025. doi: 10.4251/wjgo.v17.i8.107453
Revised: May 6, 2025
Accepted: June 9, 2025
Published online: August 15, 2025
Processing time: 136 Days and 22.9 Hours
Deoxycholic acid (DCA), a secondary bile acid, is associated with colorectal carcinogenesis, but its mechanisms remain unclear.
To investigate how DCA regulates apoptosis in colorectal cancer (CRC) cells.
SW480 and DLD-1 CRC cell lines were used to investigate the mechanism of apoptosis by western blotting, flow cytometry, confocal microscopy, and other methods.
DCA significantly induced apoptosis, with rates increasing to 7.2% ± 1.5% in SW480 cells and 14.3% ± 0.6% in DLD-1 cells after treatment, compared to 4.7% ± 1.0% and 11.6% ± 0.8% in controls (P < 0.05). Western blot analysis showed upregulation of pro-apoptotic proteins Bax and Cleaved-PARP, with a significant increase in the Cleaved-PARP/PARP ratio (P < 0.001). DCA treatment also increased the intracellular reactive oxygen species (ROS) levels of SW480 and DLD-1 cells to 1.2-fold and 1.3-fold, respectively (P < 0.01), while the increase of mitochondrial ROS levels in these cells was statistically significant under confocal microscopy. Additionally, cytosolic and mitochondrial Ca2+ levels increased 1.3-fold and 1.2-fold, respectively, in SW480 cells (P < 0.01), and 1.1-fold and 1.1-fold, respectively, in DLD-1 cells compared with controls (P < 0.05). p-CaMKII protein levels were also elevated (P < 0.01), indicating activation of the Ca2+-CaMKII signaling pathway. Pharmacological inhibition with BAPTA-AM (1 μM) reduced mitochondrial Ca2+ accumulation and ROS levels in SW480 cells (P < 0.05), and suppressed apoptosis.
DCA activates the Ca2+-CaMKII pathway, leading to ROS-mediated apoptosis in CRC cells, providing insights for potential therapeutic targets.
Core Tip: This study reveals that deoxycholic acid (DCA) induces colorectal cancer (CRC) cell apoptosis via CaMKII-Ca2+ signaling. Using DLD-1/SW480 models, DCA elevated mitochondrial calcium and reactive oxygen species (ROS) levels, driving apoptosis. Calcium chelation reversed these effects, confirming calcium-ROS interplay as the core mechanism. The findings highlight disrupted calcium homeostasis and oxidative stress in DCA-associated carcinogenesis, providing new therapeutic targets for CRC interventions.
- Citation: Chen JY, Wen JY, Lin JL, Li Y, Wu YZ, Lou LQ, Lou YL, Zuo ZG, Li X. Deoxycholic acid induces reactive oxygen species accumulation and promotes colorectal cancer cell apoptosis through the CaMKII-Ca2+ pathway. World J Gastrointest Oncol 2025; 17(8): 107453
- URL: https://www.wjgnet.com/1948-5204/full/v17/i8/107453.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i8.107453
Colorectal cancer (CRC), ranking as the third most prevalent malignancy globally and the second leading cause of cancer-related mortality, represents a major public health challenge in gastrointestinal oncology. Epidemiological data from the International Agency for Research on Cancer indicate that in 2020 alone, there were over 1.9 million newly diagnosed CRC cases and 930000 associated fatalities worldwide, constituting approximately 10% of total cancer incidence and mortality[1]. The pathogenesis of CRC involves multifactorial interactions, including genetic predisposition, dietary patterns, and environmental exposures[2,3]. Notably, high-fat dietary intake has been widely recognized as a significant risk factor for both the development of premalignant lesions (e.g., adenomatous polyps) and the progression of colon cancer[4]. Mechanistically, experimental evidence demonstrates that chronic consumption of high-fat diets elevates colonic bile acid concentrations to approximately 1 mmol/L through enterohepatic cycling[5,6]. In human populations, this process amplifies the levels of deoxycholic acid (DCA), a secondary bile acid with well-documented tumor-promoting properties in colorectal carcinogenesis[7,8].
As a secondary bile acid synthesized through hepatic conversion of cholesterol-derived primary bile acids, DCA constitutes the predominant bile acid species in the colonic lumen. Bile acids exhibit concentration-dependent cytotoxicity to mammalian cells that has been mechanistically linked to hepatobiliary disorders and colorectal carcinogenesis[9]. Recent studies have delineated two distinct mechanisms underlying bile acid cytotoxicity[10]. At supraphysiological concentrations, bile acids induce plasma membrane disintegration through dissolution of lipid bilayer components, coupled with excessive reactive oxygen species (ROS) generation, culminating in necrotic cell death and secondary apoptosis[11,12]. In contrast, sublytic concentrations of hydrophobic bile acids (e.g., chenodeoxycholic acid and DCA) also increase ROS production, leading to a state of oxidative stress that triggers apoptosis by disrupting the mitochondrial membrane potential and activating the mitochondrial permeability transition (MPT)[13,14]. Crucially, ROS-mediated genotoxic stress not only directly damages nuclear DNA but also impairs DNA damage response mechanisms, establishing a carcinogenic microenvironment[15-17]. This is particularly significant given that mitochondria-derived ROS overproduction may trigger cellular damage associated with cancer progression[18]. Experimental evidence from Glinghammar et al[19] confirmed the presence of DCA-induced DNA strand breaks in colonic epithelial cells, while Bernstein et al[12] elucidated a novel nitrosative stress axis wherein DCA-generated reactive oxygen/nitrogen species inhibit DNA repair enzymes by producing NO, thereby potentiating oxidative DNA damage and initiating apoptosis.
Calcium ions serve as critical intracellular secondary messengers that regulate diverse signaling pathways that are essential for cellular proliferation and multifunctional responses[20]. Mitochondrial calcium (mtCa2+) homeostasis exerts regulatory control over nearly all mitochondrial functions, including modulation of mitochondrial ROS production through metabolic adjustments[21]. Dysregulation of mtCa2+ dynamics has been pathologically linked to numerous disease states, particularly malignancies where it constitutes an emerging oncogenic hallmark[22]. Notably, CaMKII activation demonstrates spatial and functional specificity through differential calcium store mobilization[23]. Centuori et al[24] demonstrated the pivotal role of the Ca2+/CaMKII-MAPK pathway in DCA-triggered cellular responses; however, its regulatory network at the suborganelle level remains unclear.
DCA induces calcium mobilization in CRC cells, and its capacity to trigger apoptosis is directly mediated through calcium signaling. Nevertheless, the impact of calcium-associated signaling on other cellular processes in CRC cells (e.g., ROS elevation) has not been systematically examined. This study aimed to investigate the pro-apoptotic mechanisms of DCA in CRC cells. Preliminary experiments confirmed significant apoptosis induction (P < 0.05) in CRC cells exposed to 100 μM DCA. Mechanistic analysis revealed that DCA-mediated apoptosis involves increased cytoplasmic and mtCa2+ concentrations, coupled with upregulated expression of phosphorylated CaMKII proteins. These coordinated events ultimately drive the accumulation of ROS. Our findings suggest that the calcium-dependent molecular cascade elicited by elevated DCA levels in the colon may represent a mechanism for inducing apoptosis in CRC cells.
The CRC cell lines DLD-1 and SW480 were sourced from the American Type Culture Collection. For routine culture, DLD-1 cells were maintained in Roswell Park Memorial Institute-1640 medium, whereas SW480 cells were cultured in Dulbecco’s Modified Eagle Medium. Both media were supplemented with 10% (v/v) fetal bovine serum (Invitrogen, Waltham, MA, United States), 100 μg/mL streptomycin (Sangon Biotech, Shanghai, China), and 100 U/mL penicillin G (Sangon Biotech). The cells were incubated at 37 °C in a humidified 5% CO2 atmosphere.
DCA (D2510) was procured from Sigma-Aldrich (Shanghai, China). BAPTA-AM was sourced from Cayman Chemical (Beijing, China). Sodium tauro-β-muricholate was purchased from MedChemExpress (Shanghai, China). MitoSOX™ Red and Rhod-2, AM were acquired from Thermo Fisher Scientific Inc. (Waltham, MA, United States). These reagents were dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich) and prepared to the desired concentrations for stock solutions. All chemicals used were of analytical grade purity.
Cells were seeded at a density of 4 × 105 cells/well in six-well plates and treated with either 100 μM DCA or 0.1% DMSO for 24 hours. Following treatment, cells from each group were harvested using EDTA-free trypsin, resuspended in annexin V-binding buffer, and incubated for 10 minutes at 25 °C ± 1 °C under light-protected conditions with 5 μL of Annexin V-PE and 10 μL of 7-aminoactinomycin D. Apoptosis was quantified using a FACSAria III flow cytometer (BD Biosciences).
ROS generation in cells treated with an endomorphin-2 analog was quantified using flow cytometry and 2′,7′-dichlorofluorescein-diacetate (DCFH-DA) fluorescent dye. Briefly, cells were seeded in six-well plates at a density of 4 × 105 cells/well and incubated with either 100 μM DCA or 0.1% DMSO for 24 hours. Following treatment, cells were harvested, washed three times with phosphate-buffered saline (PBS), resuspended with 200 μL serum-free basal medium containing DCFH-DA, and incubated for 20 minutes at 37 °C. ROS levels were analyzed using a FACSAria III flow cytometer (BD Biosciences), and data were processed using FACSDiva software.
For laser scanning confocal microscopy, cells were seeded in six-well plates at a density of 1× 105 cells/well and incubated with either 100 μM DCA or 0.1% DMSO for 24 hours. Following treatment, the cells were stained with diluted MitoSOX™ (1:2000) and MitoTracker® (1:10000) for 20 minutes at 37 °C. The stained cells were then gently washed twice with serum-free basal medium. All steps were performed under light-protected conditions, and fluorescence was visualized using a laser scanning confocal microscope.
To assess intracellular free Ca2+ levels, the fluorescent probe Fluo-3 AM (Beyotime Biotechnology, China) was utilized. Briefly, cells were resuspended in binding buffer and incubated with 5 μM Fluo-3 AM for 30 minutes at 37 °C in a 5% CO2 incubator. Following incubation, the cells were washed three times with PBS and analyzed using a flow cytometer.
For flow cytometry analysis, cells were seeded in six-well plates at a density of 4 × 105 cells/well and incubated with either 100 μM DCA or 0.1% DMSO for 24 hours. Following treatment, the cell suspension was collected and incubated with 2 μM Rhod-2 AM fluorescent dye for 30 minutes at 37 °C in a humidified 5% CO2 incubator. The stained cells were then washed three times with PBS and analyzed using a flow cytometer.
For laser scanning confocal microscopy, 1 × 105 cells were seeded onto confocal dishes overnight. Post-adherent cells were exposed to 100 μM DCA for 24 hours. Following triple PBS washes, the cells were incubated with 200 μL serum-free medium containing 2 μM Rhod-2 AM at 37 °C for 30 minutes. Finally, nuclear counterstaining was performed using 500 μL Hoechst 33258 for 5 minutes before imaging acquisition.
Protein from colon tissues and treated cells were extracted using RIPA buffer containing protease inhibitor (PMSF, Beyotime Biotechnology, Shanghai, China) and phosphatase inhibitor (PhosSTOP, Roche). The protein concentration in the collected supernatants was determined by a bicinchoninic acid (BCA) protein assay kit (Solarbio, Beijing, China). For western blot analysis, equal amounts of protein samples were separated by SDS-PAGE and transferred to nitrocellulose membranes (Pall Corporation, New York, United States), which were incubated with the specific primary antibodies (1:1000 dilution) for Cleaved-PARP, PARP, Bax, Cyt-c, p-CaMKII, TGR5, and β-actin at 4 °C overnight after blocking in 5% milk. Membranes were subsequently incubated with HRP-conjugated secondary antibodies (Beyotime Institute of Biotechnology). β-actin served as the loading control. Exposure images were performed using Chemiluminescence imaging system (Bio-Rad).
TGR5-specific short hairpin RNAs (shRNAs) were obtained from QingKe Biotechnology (Beijing, China). The lentiviral packaging system components, including psPAX2 and pMD2.G, along with the pBABE-Puro transfer vector, were co-transfected into HEK293T cells at 2:1:2 mass ratio. At 48-72 hours post-transfection, the viral supernatants were filter-sterilized through 0.45 μm cellulose acetate membranes and concentrated via ultracentrifugation. SW480 cells seeded in six-well plates (1 × 105 cells/well) were infected with lentivirus for 24 hours. Cells stably expressing lentiviral TGR5-shRNA were selected with 2 mg/mL puromycin for 21-28 days, with the medium refreshed every 72 hours. Knockdown efficiency was validated by western blotting. All experiments were repeated at least three times.
Data analysis and statistical graph production were conducted using GraphPad Prism 7.0 software. The representative outcomes are expressed as mean ± SD. One-way analysis of variance (ANOVA) was used for between-group comparisons, whereas comparisons among multiple groups was made using ANOVA and the least significant difference test. P < 0.05 was considered as significant.
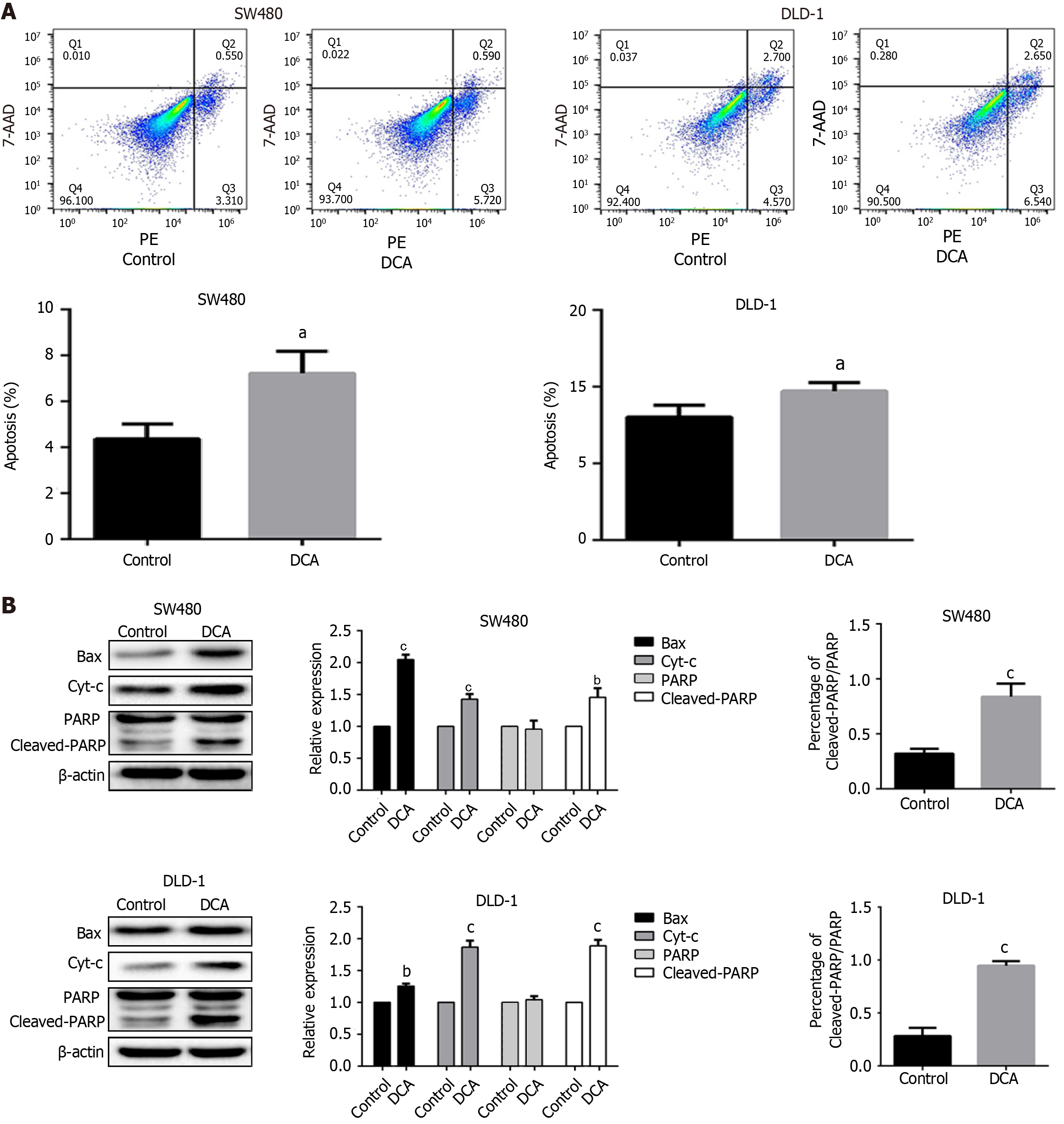
To investigate the effects of DCA on apoptotic regulation in CRC cells, SW480 and DLD-1 cell lines were exposed to 100 μM DCA and subjected to flow cytometric analysis. As illustrated in Figure 1A, after incubation with DCA, the apoptosis rates of SW480 and DLD-1 cells were significantly increased to 7.2% ± 1.5% and 14.3% ± 0.6%, respectively, compared with 4.7% ± 1.0% and 11.6% ± 0.8% in the control group (P < 0.05), and showed pro-apoptotic effects mainly in the early apoptotic stage. To elucidate the mechanistic basis of DCA-induced apoptosis, western blotting was employed to profile key apoptotic pathway components. Bax, a critical mediator of mitochondrial apoptosis, exerts its pro-apoptotic function through oligomerization-dependent augmentation of mitochondrial membrane permeability. This permeabilization event facilitates Cyt-c efflux, thereby activating caspase cascades and leading to apoptosis. PARP is an important factor for repairing DNA to maintain cell viability, and its sheared form, Cleaved-PARP, can represent the apoptosis level. As shown in Figure 1B, the protein expression level of Bax, Cyt-c, and Cleaved-PARP increased significantly in SW480 and DLD-1 treated groups compared with the control group (P < 0.001). In addition, the ratio of Cleaved-PARP/PARP was significantly increased in SW480 and DLD-1 treated groups compared with the control group (P < 0.001), which showed that PARP was increased by shearing. The results indicated that DCA could promote apoptosis of SW480 and DLD-1 cells through mitochondria.
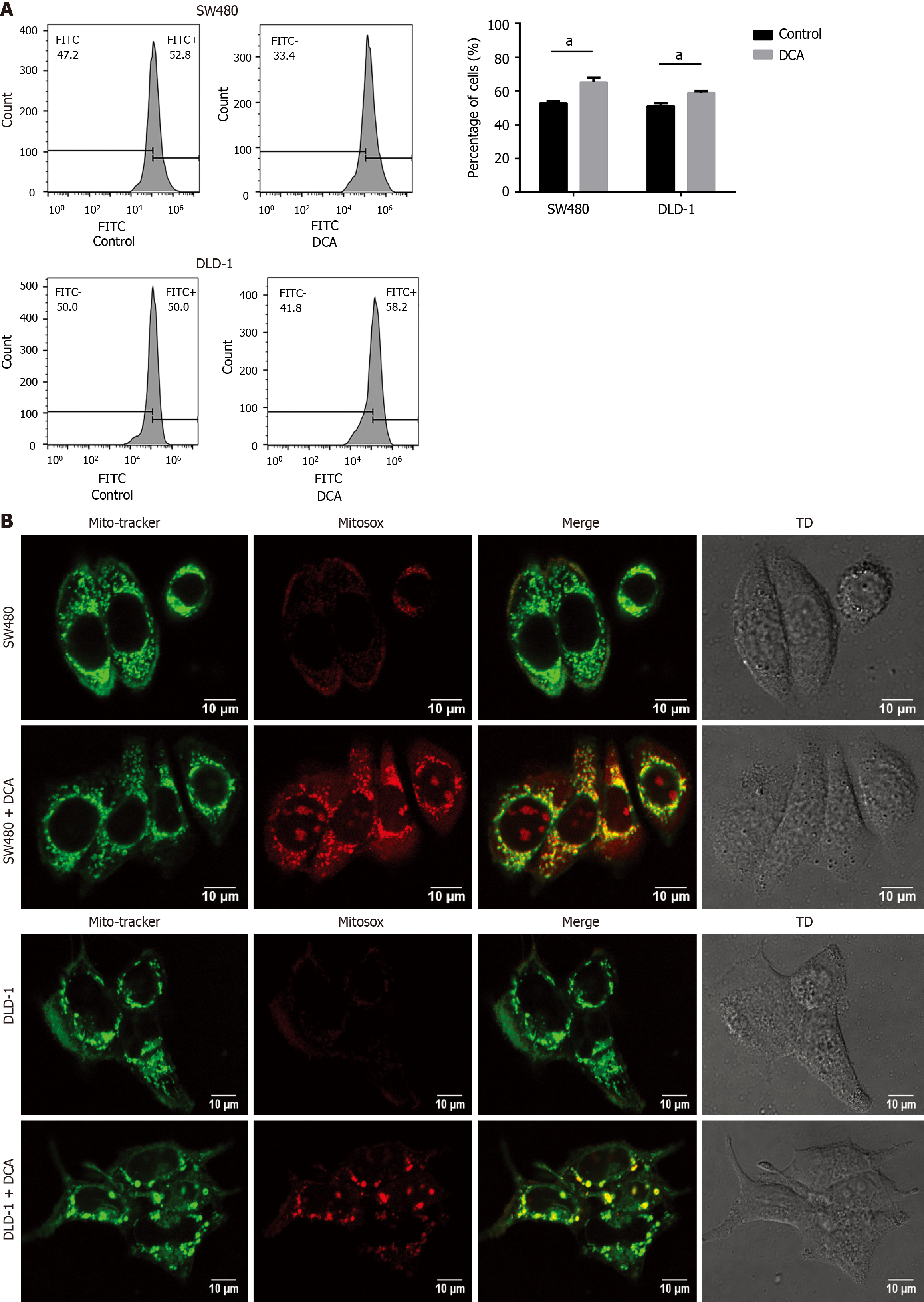
Mitochondrial membranes constitute a major contributor to intracellular ROS generation, with excessive ROS accumulation recognized as a critical mediator of apoptotic pathways. To investigate the potential involvement of ROS in DCA-induced apoptosis, we quantified intracellular ROS levels using flow cytometric analysis. As illustrated in Figure 2A, DCA treatment increased intracellular ROS levels in SW480 and DLD-1 cells by 1.2-fold and 1.3-fold, respectively, compared with the control group (P < 0.01). Subsequent mitochondrial-specific ROS detection via confocal microscopy revealed intensified red fluorescence signals in both cell lines following DCA exposure (Figure 2B), demonstrating a statistically significant increase in mitochondrial ROS accumulation relative to control conditions. These findings collectively indicate that DCA triggered apoptosis in SW480 and DLD-1 cells by promoting intracellular and mitochondrial ROS accumulation.
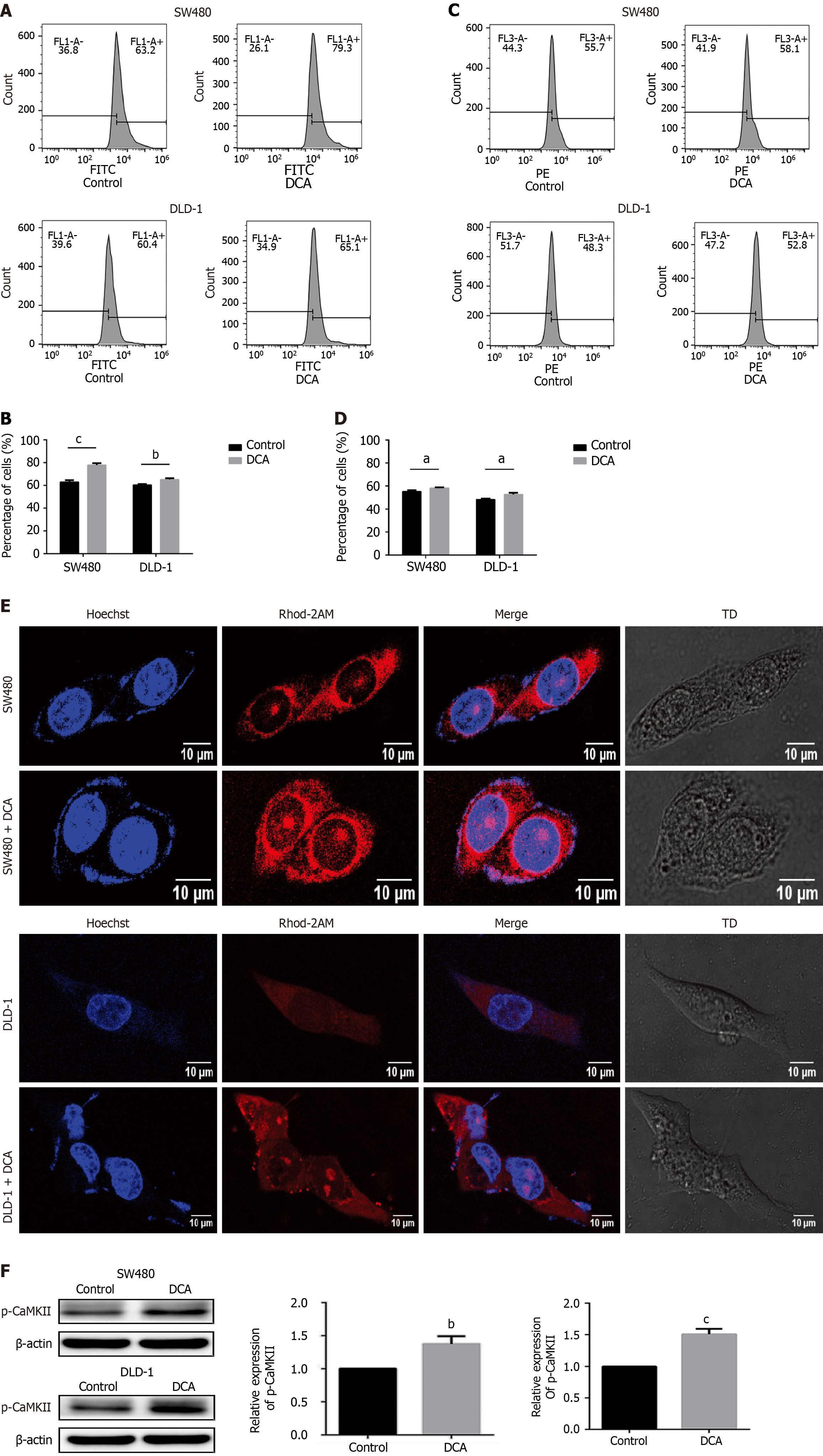
Ca2+ serves as a pivotal intracellular secondary messenger governing fundamental cellular processes including proliferation and apoptosis. To quantify cytosolic Ca2+ dynamics, we employed the fluorescent probe Fluo-3 AM (5 μM) with subsequent quantification via flow cytometric analysis. As shown in Figure 3A and B, DCA-treated SW480 and DLD-1 cells increased cytoplasmic Ca2+ levels by 1.3-fold and 1.1-fold, respectively, compared to the control group (P < 0.01). Given the established role of mitochondria as principal intracellular Ca2+ storage organelles, we further investigated the changes in intracellular calcium concentration in mitochondria using flow cytometry. As shown in Figure 3C and D, the mtCa2+ ion content in the SW480 and DLD-1 treatment groups was significantly increased 1.2-fold and 1.1-fold compared with the control group (P < 0.05). Confocal microscopy with Hoechst nuclear counterstaining enabled simultaneous visualization of mitochondrial Ca2+ flux, showing characteristic punctate fluorescence patterns that correlated with flow cytometry findings. As demonstrated in Figure 3E, mitochondrial Ca2+ content exhibited elevation in DCA-treated SW480 and DLD-1 cells compared to controls (P < 0.01). Concurrently, DCA-exposed DLD-1 cells displayed marked nuclear morphological alterations. These findings collectively demonstrate that DCA can increase the intracellular and intra-mitochondrial Ca2+ content. CaMKII, a multifunctional serine/threonine kinase family, transduces Ca2+/calmodulin signals through autophosphorylation. Phosphorylated CaMKII has kinase activity and induces “CaM trapping,” leading to increased affinity for CaM binding under low Ca2+ concentration conditions and resulting in time-dependent CaMKII activity. Western blot analysis revealed that the expression levels of p-CaMKII protein in SW480 and DLD-1 treated groups were significantly increased compared with the control group (P < 0.01; Figure 3F). These data indicate that DCA could up-regulate the expression level of p-CaMKII protein in SW480 and DLD-1 cells and establish DCA as a potent activator of the Ca2+/CaMKII signaling axis in CRC models.
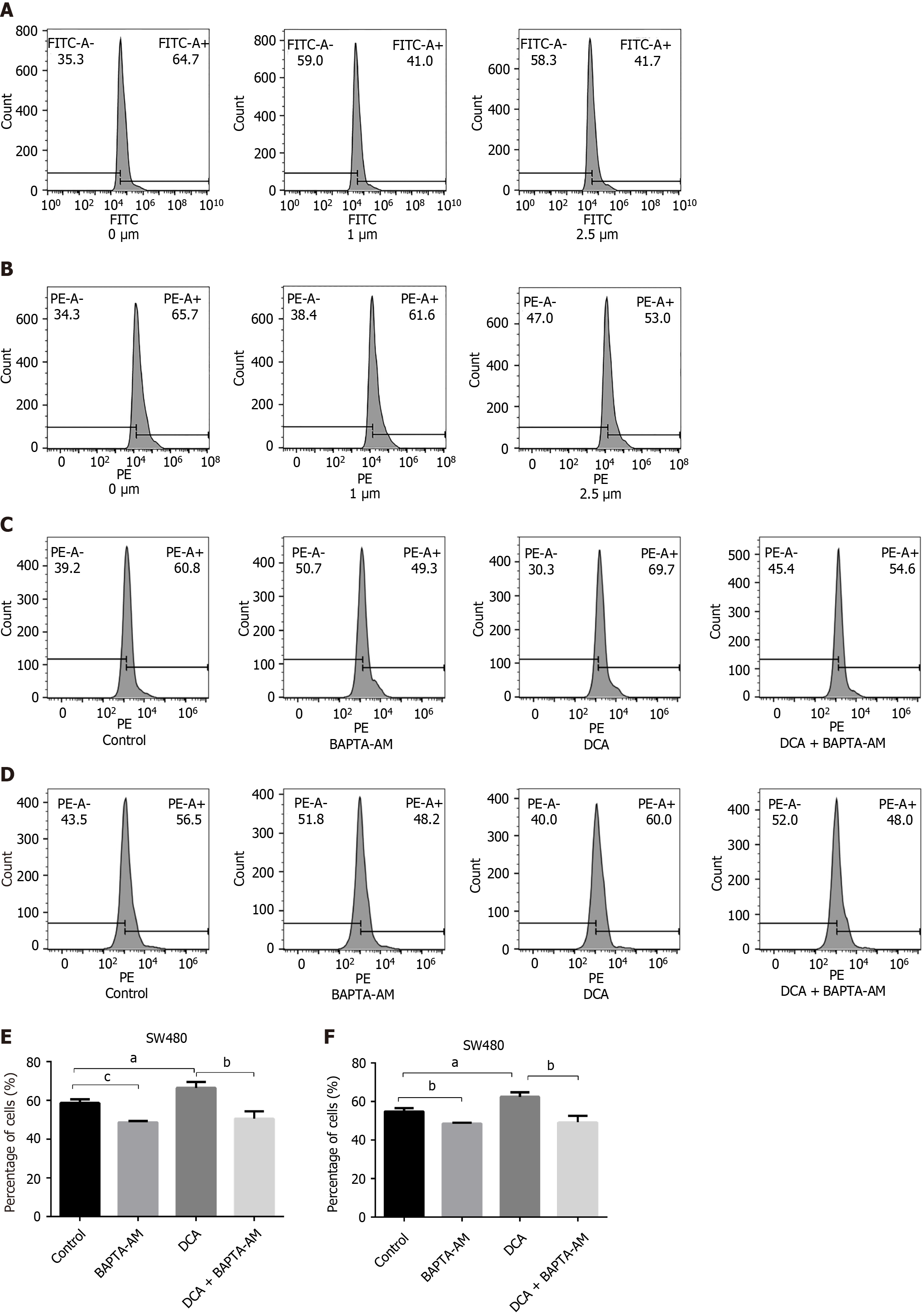
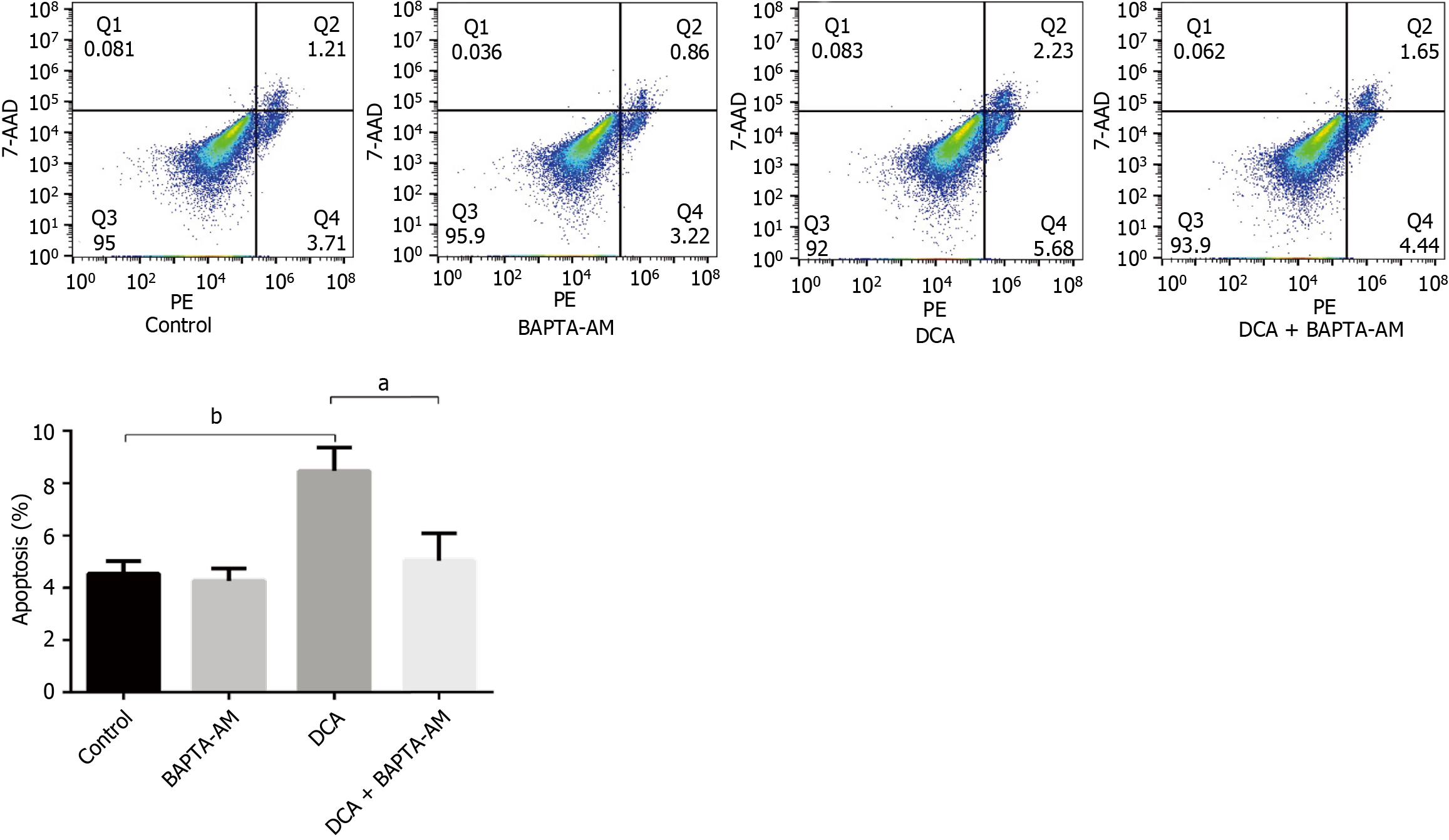
To verify whether DCA triggers a series of intracellular reactions by affecting intracellular calcium ion rearrangement, we subjected SW480 colorectal carcinoma cells to graded BAPTA-AM pretreatment (0, 1, 2.5 μM; 24 hours) and used flow cytometry to detect the intracellular and intra-mitochondrial Ca2+ contents. Quantitative analysis revealed reduction in cytosolic Ca2+ levels in BAPTA-AM treated cells vs untreated controls (P < 0.05; Figure 4A), and the intracellular Ca2+ content did not decrease further as the concentration of BAPTA-AM increased. Concomitantly, mitochondrial matrix Ca2+ content decreased in chelator-treated groups (P < 0.05; Figure 4B), confirming mtCa2+ mobilization as a DCA-dependent phenomenon. Based on these dose-response characteristics, 1 μM BAPTA-AM was selected for subsequent mechanistic studies due to its sufficient calcium chelation efficiency. To investigate whether DCA induces alterations in mitochondrial Ca2+ and ROS levels following pretreatment with the intracellular calcium chelator BAPTA-AM, we conducted flow cytometric analyses of mitochondrial Ca2+ and ROS dynamics in SW480 cells subjected to four experimental conditions: (1) Untreated controls; (2) BAPTA-AM-only treatment; (3) DCA-only treatment; and (4) Combined DCA + BAPTA-AM treatment. Quantification of mitochondrial Ca2+ levels revealed a significant elevation in DCA-treated cells compared to controls (P < 0.05). In contrast, no statistically significant differences in mitochondrial Ca2+ content were observed between the DCA + BAPTA-AM co-treatment group and the BAPTA-AM-only group (P > 0.05; Figure 4C and D). Assessment of mitochondrial ROS via flow cytometry demonstrated higher content in DCA-treated cells compared to controls. In the BAPTA-AM group, the content decreased, and the difference of mitochondrial ROS content between the DCA and BAPTA-AM co-treatment groups and the BAPTA-AM group was not statistically significant (P > 0.05; Figure 4E and F). Therefore, we speculated that the intracellular Ca2+ chelation could effectively reduce the mitochondrial Ca2+ and ROS content while offsetting the increase of mitochondrial Ca2+ and ROS content caused by the effect of DCA.
To elucidate the calcium-dependent mechanisms underlying DCA-induced mitochondrial ROS accumulation and subsequent apoptosis, we quantified apoptotic indices across four experimental cohorts using flow cytometry: (1) Controls; (2) BAPTA-AM-treated group; (3) DCA-treated group; and (4) DCA + BAPTA-AM co-treatment. As illustrated in Figure 5, DCA monotherapy significantly elevated apoptotic rates compared to controls (P < 0.01). DCA with the calcium chelator BAPTA-AM attenuated this pro-apoptotic effect, demonstrating a reduction in apoptosis relative to DCA monotherapy (P < 0.05), and no significant difference in apoptosis was detected between the DCA + BAPTA-AM and BAPTA-AM groups (P > 0.05).
These data conclusively demonstrate that intracellular Ca2+ sequestration abrogates DCA-driven mitochondrial ROS accumulation and apoptotic execution. Our findings establish a causal linkage between dysregulation of the Ca2+/CaMKII/ROS signaling axis and programmed cell death in CRC cells, thereby providing mechanistic validation of this pathway as a therapeutic target.
We investigated the intricate relationship between DCA, the Ca2+-CaMKII pathway, and the pathogenesis of CRC. DCA, a secondary bile acid, has long been implicated in colorectal carcinogenesis, yet its precise role and underlying mechanisms have remained elusive. The biological characteristics of DCA pro-apoptosis present a unique “carcinogenic-cancer-suppressor” biphasic regulatory pattern in colorectal carcinogenesis[25-28]. In vitro studies have established a significant positive correlation between the tumorigenic potential of bile acids and their apoptosis-inducing potency[29]. Powell et al[30], utilizing a chronic DCA exposure model, demonstrated that HCT116 cells surviving an initial apoptotic surge progressively evolve into apoptosis-resistant subclones characterized by BCL-2 overexpression and p53 mutations. This selective pressure-driven clonal evolution culminates an elevation in malignant transformation rates[31], corroborating the oncogenic risk of sustained apoptotic stimuli within genomically unstable microenvironments[32]. Our findings demonstrate that DCA induces apoptosis in CRC cells through the activation of the Ca2+-CaMKII signaling pathway, leading to the accumulation of ROS and subsequent mitochondrial dysfunction.
The significance of our study lies in its elucidation of the molecular mechanisms by which DCA contributes to CRC development and progression. CRC, ranking as the third most prevalent malignancy globally, involves multifactorial interactions including genetic predisposition, dietary patterns, and environmental exposures. High-fat dietary intake, a well-established risk factor for CRC, elevates colonic bile acid concentrations, predominantly amplifying DCA levels. DCA, through its hydrophobic properties, disrupts mitochondrial membrane potential and activates the MPT, leading to increased ROS production and oxidative stress[25,26]. This, in turn, triggers apoptosis by activating the Bax-dependent mitochondrial apoptotic pathway, as evidenced by the upregulation of Bax and Cleaved-PARP pro-apoptotic proteins observed in our study (Figure 1).
In the context of CRC diagnosis and therapy, our findings provide novel insights into the potential diagnostic and therapeutic applications of targeting the DCA-Ca2+-CaMKII-ROS axis. Currently, CRC diagnosis relies heavily on colonoscopy and biopsy, which are invasive and costly procedures. The identification of biomarkers associated with DCA-induced apoptosis, such as elevated ROS levels or altered calcium homeostasis, could lead to the development of non-invasive diagnostic tools. For instance, the detection of mitochondrial ROS accumulation or changes in intracellular calcium concentrations in patient samples could serve as early indicators of CRC risk or progression.
Furthermore, our study highlights the therapeutic potential of targeting the Ca2+-CaMKII-ROS axis in CRC. Strategies aimed at modulating calcium homeostasis or ROS levels could offer alternative therapeutic approaches. For example, calcium chelators like BAPTA-AM effectively reduce mtCa2+ overload and ROS generation, thereby attenuating DCA-induced apoptosis (Figures 4 and 5).
While our study provides compelling evidence for the role of DCA in promoting CRC cell apoptosis via the Ca2+-CaMKII-ROS axis, several questions remain unanswered. Future research should focus on elucidating the precise mechanisms by which DCA modulates mtCa2+ uniporter phosphorylation to influence Ca2+ influx, as well as investigating the potential synergistic effects of DCA with other therapeutic agents in CRC models. Additionally, the evaluation of targeted strategies against the Ca2+/CaMKII/ROS axis in patient-derived organoids and patient-derived xenograft models will be crucial to validate the therapeutic potential of these approaches.
Incorporating Supplementary Figure 1, we found that farnesoid X receptor signaling does not mediate DCA-induced apoptosis in CRC cells, suggesting alternative pathways are involved. Conversely, Supplementary Figure 2 revealed that TGR5 knockdown paradoxically enhances DCA-induced apoptosis, implying TGR5 may function as a parallel regulatory node rather than a direct mediator. These findings underscore the complexity of the action of DCA in CRC cells.
Our study sheds light on the complex interplay between DCA, the Ca2+-CaMKII pathway, and CRC pathogenesis. By elucidating the molecular mechanisms underlying DCA-induced apoptosis in CRC cells, we provide a foundation for the development of novel diagnostic tools and therapeutic strategies aimed at targeting this critical pathway. Further research in this area holds promise for improving CRC diagnosis, prognosis, and treatment outcomes.
Our findings establish a causal linkage between dysregulation of the Ca2+/CaMKII/ROS signaling axis and programmed cell death in CRC cells, thereby providing mechanistic validation of this pathway as a therapeutic target.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64702] [Article Influence: 16175.5] [Reference Citation Analysis (177)] |
| 2. | Seol JE, Kim J, Lee BH, Hwang DY, Jeong J, Lee HJ, Ahn YO, Lee JE, Kim DH. Folate, alcohol, ADH1B and ALDH2 and colorectal cancer risk. Public Health Nutr. 2020;24:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Carr PR, Weigl K, Edelmann D, Jansen L, Chang-Claude J, Brenner H, Hoffmeister M. Estimation of Absolute Risk of Colorectal Cancer Based on Healthy Lifestyle, Genetic Risk, and Colonoscopy Status in a Population-Based Study. Gastroenterology. 2020;159:129-138.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 4. | Ocvirk S, O'Keefe SJD. Dietary fat, bile acid metabolism and colorectal cancer. Semin Cancer Biol. 2021;73:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 158] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 5. | Bernstein C, Bernstein H, Garewal H, Dinning P, Jabi R, Sampliner RE, McCuskey MK, Panda M, Roe DJ, L'Heureux L, Payne C. A bile acid-induced apoptosis assay for colon cancer risk and associated quality control studies. Cancer Res. 1999;59:2353-2357. [PubMed] |
| 6. | Weinberg DS, Strom BL. Screening for colon cancer: a review of current and future strategies. Semin Oncol. 1995;22:433-447. [PubMed] |
| 7. | Hori T, Matsumoto K, Sakaitani Y, Sato M, Morotomi M. Effect of dietary deoxycholic acid and cholesterol on fecal steroid concentration and its impact on the colonic crypt cell proliferation in azoxymethane-treated rats. Cancer Lett. 1998;124:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Centuori SM, Martinez JD. Differential regulation of EGFR-MAPK signaling by deoxycholic acid (DCA) and ursodeoxycholic acid (UDCA) in colon cancer. Dig Dis Sci. 2014;59:2367-2380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Amaral JD, Viana RJ, Ramalho RM, Steer CJ, Rodrigues CM. Bile acids: regulation of apoptosis by ursodeoxycholic acid. J Lipid Res. 2009;50:1721-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 264] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 10. | Ignacio Barrasa J, Olmo N, Pérez-Ramos P, Santiago-Gómez A, Lecona E, Turnay J, Antonia Lizarbe M. Deoxycholic and chenodeoxycholic bile acids induce apoptosis via oxidative stress in human colon adenocarcinoma cells. Apoptosis. 2011;16:1054-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Perez MJ, Briz O. Bile-acid-induced cell injury and protection. World J Gastroenterol. 2009;15:1677-1689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 469] [Cited by in RCA: 520] [Article Influence: 32.5] [Reference Citation Analysis (3)] |
| 12. | Bernstein H, Bernstein C, Payne CM, Dvorakova K, Garewal H. Bile acids as carcinogens in human gastrointestinal cancers. Mutat Res. 2005;589:47-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 458] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 13. | Araki Y, Katoh T, Ogawa A, Bamba S, Andoh A, Koyama S, Fujiyama Y, Bamba T. Bile acid modulates transepithelial permeability via the generation of reactive oxygen species in the Caco-2 cell line. Free Radic Biol Med. 2005;39:769-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Solá S, Brito MA, Brites D, Moura JJ, Rodrigues CM. Membrane structural changes support the involvement of mitochondria in the bile salt-induced apoptosis of rat hepatocytes. Clin Sci (Lond). 2002;103:475-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Boonstra J, Post JA. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene. 2004;337:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 533] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 16. | Colin DJ, Limagne E, Ragot K, Lizard G, Ghiringhelli F, Solary É, Chauffert B, Latruffe N, Delmas D. The role of reactive oxygen species and subsequent DNA-damage response in the emergence of resistance towards resveratrol in colon cancer models. Cell Death Dis. 2014;5:e1533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 17. | Pathania AS, Kumar S, Guru SK, Bhushan S, Sharma PR, Aithagani SK, Singh PP, Vishwakarma RA, Kumar A, Malik F. The synthetic tryptanthrin analogue suppresses STAT3 signaling and induces caspase dependent apoptosis via ERK up regulation in human leukemia HL-60 cells. PLoS One. 2014;9:e110411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Koppenol WH, Bounds PL, Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2023] [Cited by in RCA: 2461] [Article Influence: 175.8] [Reference Citation Analysis (0)] |
| 19. | Glinghammar B, Inoue H, Rafter JJ. Deoxycholic acid causes DNA damage in colonic cells with subsequent induction of caspases, COX-2 promoter activity and the transcription factors NF-kB and AP-1. Carcinogenesis. 2002;23:839-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Hansford RG. Physiological role of mitochondrial Ca2+ transport. J Bioenerg Biomembr. 1994;26:495-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 157] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 21. | Pathak T, Trebak M. Mitochondrial Ca(2+) signaling. Pharmacol Ther. 2018;192:112-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 158] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 22. | Paupe V, Prudent J. New insights into the role of mitochondrial calcium homeostasis in cell migration. Biochem Biophys Res Commun. 2018;500:75-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 23. | Mishra S, Gray CB, Miyamoto S, Bers DM, Brown JH. Location matters: clarifying the concept of nuclear and cytosolic CaMKII subtypes. Circ Res. 2011;109:1354-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Centuori SM, Gomes CJ, Trujillo J, Borg J, Brownlee J, Putnam CW, Martinez JD. Deoxycholic acid mediates non-canonical EGFR-MAPK activation through the induction of calcium signaling in colon cancer cells. Biochim Biophys Acta. 2016;1861:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21373] [Article Influence: 2137.3] [Reference Citation Analysis (3)] |
| 26. | Martinez-Diez MC, Serrano MA, Monte MJ, Marin JJ. Comparison of the effects of bile acids on cell viability and DNA synthesis by rat hepatocytes in primary culture. Biochim Biophys Acta. 2000;1500:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Rust C, Karnitz LM, Paya CV, Moscat J, Simari RD, Gores GJ. The bile acid taurochenodeoxycholate activates a phosphatidylinositol 3-kinase-dependent survival signaling cascade. J Biol Chem. 2000;275:20210-20216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 145] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 28. | Patel T, Bronk SF, Gores GJ. Increases of intracellular magnesium promote glycodeoxycholate-induced apoptosis in rat hepatocytes. J Clin Invest. 1994;94:2183-2192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 194] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Martinez JD, Stratagoules ED, LaRue JM, Powell AA, Gause PR, Craven MT, Payne CM, Powell MB, Gerner EW, Earnest DL. Different bile acids exhibit distinct biological effects: the tumor promoter deoxycholic acid induces apoptosis and the chemopreventive agent ursodeoxycholic acid inhibits cell proliferation. Nutr Cancer. 1998;31:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 149] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Powell AA, LaRue JM, Batta AK, Martinez JD. Bile acid hydrophobicity is correlated with induction of apoptosis and/or growth arrest in HCT116 cells. Biochem J. 2001;356:481-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Hu Y, Chau T, Liu HX, Liao D, Keane R, Nie Y, Yang H, Wan YJ. Bile acids regulate nuclear receptor (Nur77) expression and intracellular location to control proliferation and apoptosis. Mol Cancer Res. 2015;13:281-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Garewal H, Bernstein H, Bernstein C, Sampliner R, Payne C. Reduced bile acid-induced apoptosis in "normal" colorectal mucosa: a potential biological marker for cancer risk. Cancer Res. 1996;56:1480-1483. [PubMed] |