Published online Jun 26, 2025. doi: 10.4252/wjsc.v17.i6.104367
Revised: March 21, 2025
Accepted: May 13, 2025
Published online: June 26, 2025
Processing time: 189 Days and 1 Hours
Hypertrophy obesity is closely associated with obesity-related metabolic diseases. The senescence of adipose-derived mesenchymal stem cells (ASCs) is believed to play a significant role in the development of hypertrophy obesity.
To investigate the relationship between ASC senescence, endoplasmic reticulum (ER) stress, and nuclear factor erythroid-derived 2 (NRF2) activity in a mouse model of hypertrophy obesity. Additionally, we explored the mechanism through which NRF2 affects ASC senescence via mitofusin-2 (MFN2).
We observed the senescent phenotype and ER stress (ERS) in ASCs from hyper
The study found significant increases in senescence and ERS, accompanied by decreased NRF2 activity in ASCs from hypertrophic obese mouse models. Simultaneously, chromatin immunoprecipitation-qPCR analysis revealed a reduction in NRF2 transcriptional activity on Mfn2. The downregulation of NRF2 activity and Mfn2 expression promoted senescence and ERS in ASCs, subsequently impacting the anti-insulin resistance effect of ASC transplantation. Furthermore, there exists a direct or indirect binding between MFN2 and binding immunoglobulin protein.
The research outcomes suggest that NRF2 may regulate ERS and senescence in subcutaneous ASCs of hypertrophic obese mice by modulating Mfn2. These discoveries offer new insights into understanding metabolic diseases associated with hypertrophic obesity and potentially provide a foundation for intervention strategies.
Core Tip: We observed increased adipose-derived mesenchymal stem cell (ASC) senescence and endoplasmic reticulum (ER) stress, along with decreased nuclear factor erythroid-derived 2 (NRF2) activity, in a hypertrophic obese mouse model. Chromatin immunoprecipitation-quantitative polymerase chain reaction analysis revealed reduced NRF2 transcriptional activity against mitofusin-2 (Mfn2). The downregulation of NRF2 activity and MFN2 expression promoted senescence and ER stress in ASCs. Additionally, co-immunoprecipitation showed that MFN2 interacts with binding immunoglobulin protein. These findings suggest that NRF2 may regulate ER stress and senescence via MFN2 in ASCs of hypertrophic obese mice.
- Citation: Fang J. Reduced NRF2/Mfn2 activity promotes endoplasmic reticulum stress and senescence in adipose-derived mesenchymal stem cells in hypertrophic obese mice. World J Stem Cells 2025; 17(6): 104367
- URL: https://www.wjgnet.com/1948-0210/full/v17/i6/104367.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i6.104367
Hypertrophic obesity, characterized by an increase in adipocyte size rather than the number of adipocytes needed to store excess fat, is associated with insulin resistance and metabolic-related diseases[1]. The senescence of adipocyte progenitor cells may contribute to hypertrophic obesity by limiting adipocyte proliferation. Adipose-derived mesenchymal stem cells (ASCs) in the subcutaneous adipose tissue of individuals with hypertrophic obesity exhibit a senescent phenotype[2]. Our previous research demonstrated that the senescent phenotype of ASCs can be alleviated by suppressing endoplasmic reticulum (ER) stress, and this effect is further promoted by the activation of nuclear factor erythroid-derived 2 (NRF2)[3,4]. NRF2, which is typically bound to Kelch-like ECH-associated protein 1 in the cytoplasm, becomes activated during oxidative stress. NRF2 then translocated to the nucleus, where it recognizes the antioxidant response element (ARE) region and induces the expression of protective genes. This process not only helps maintain the cellular redox balance but also reduces ER stress (ERS). Persistent ERS and excessive oxidative stress interact and mutually reinforce each other in a positive feedback manner. NRF2 alleviates oxidative stress by promoting the transcription of antioxidant enzymes, indirectly mitigating ERS and cellular senescence[5,6].
As key organelles for reactive oxygen species generation, mitochondria indirectly link the antioxidant effects of NRF2 with ERS mechanisms. In addition to regulating the expression of antioxidant-related enzymes, I postulated that NRF2 may also indirectly modulate ERS by regulating the expression of mitochondrial membrane protein mitofusin-2 (MFN2)[7]. Previous studies have demonstrated that the absence of MFN2 leads to persistent activation of protein kinase-like ER kinase (PERK) and ERS[8], whereas NRF2 activation can increase MFN2 expression[9]. Although a potential ARE region exists in Mfn2[10], further research has not yet confirmed the transcriptional regulatory effect of NRF2 on Mfn2. The molecular mechanism by which MFN2 regulates ERS also remains poorly understood. Given that I detected decreased NRF2 transcriptional activity and reduced Mfn2 expression in ASCs from individuals with hypertrophic obesity, I hypothesize that reduced NRF2 activity may increase ERS and senescence by decreasing Mfn2 transcription. Furthermore, through the analysis of MFN2-interacting proteins, I explored the molecular mechanisms involved in the participation of MFN2 in ERS signaling.
In this study, I identified the NRF2 binding region on the Mfn2 promoter through chromatin immunoprecipitation (ChIP)-quantitative polymerase chain reaction (qPCR) experiments. Disrupting the expression of either Nrf2 or Mfn2 can reduce the therapeutic effect of ASC transplantation in insulin-resistant individuals. I further explored the impact of MFN2 on ERS using IP-mass spectrometry (MS) and reported that MFN2 may directly or indirectly bind to binding immunoglobulin protein (BIP), a signaling molecule involved in ERS. These findings suggest that the reduced NRF2 activity in ASCs from individuals with hypertrophic obesity may promote ERS and senescence by decreasing Mfn2 transcription.
Eight-month-old male C57BL/6 mice were used in this research. The mice were kept in a clean environment with constant temperature and humidity, with alternating light and dark cycles every 12 hours. The mice in the control group were fed a maintenance diet containing 75.9% carbohydrates, 14.7% protein, and 9.4% fat, whereas the mice in the obesity group were fed a high-fat diet containing 20.8% carbohydrates, 18.3% protein, and 60.9% fat (in % kcal) for 16 weeks.
After 16 weeks, the mice were weighed, and subcutaneous fat was collected for paraffin sectioning and hematoxylin and eosin (H&E) staining. The procedure for H&E staining of paraffin sections of adipose tissue followed standard embedding, sectioning, and staining methods. The diameter of adipocytes in the H&E-stained images was measured using ImageJ software (National Institutes of Health, Bethesda, MD, United States). The “set scale” function was utilized to establish the picture scale. The widest part of the cell diameter was subsequently outlined with a straight line, and the cell diameter was measured using the “measure” function.
The inguinal subcutaneous adipose tissue of mice was isolated and snipped under sterile conditions. Stromal vascular fraction (SVF) was isolated by incubating the tissue with type 1 collagenase (Gibco, Grand Island, NY, United States) for 1 hour at 37 °C. SVF was obtained by centrifugation at 1000 rpm for 10 minutes. Adherent ASCs were obtained after 24 hours by culturing SVF in adherent culture dishes. The ASCs were cultured in α-MEM medium (Gibco) supplemented with 10% fetal bovine serum. The identification of ASCs was carried out through flow cytometry analysis of surface markers, differentiation into osteoblasts, chondrocytes, and adipocytes, as previously described[3].
To calculate the number of ASCs in adipose tissue, the adipose tissue was excised from the subcutaneous area of the groin and weighed. After the SVF was cultured on the plate for 10 hours, the ASCs were subsequently harvested, and the number of ASCs per unit weight of adipose tissue was determined via a hemocytometer. For adipogenic differentiation, ASCs were cultured in a medium containing 1 μM dexamethasone, 1 μg/mL insulin, 2 μM rosiglitazone, and 0.1 mmol/L indomethacin (Solarbio, China) for 14 days. Adipocytes were identified using Oil Red O staining. All of the experiments below were performed using the second to fourth passages. Cell differentiation was calculated as the percentage of positive Oil Red O-stained areas to the number of cells. The data were normalized to the control group. The area of positive staining and the number of cells were analyzed by ImageJ software.
Staining ASCs from different treatment groups were seeded into 12-well plates at a density of 2 × 104 cells per well before staining. Three replicate wells were used for each treatment group. Staining began when the cell density reached 50% confluence. The cells were fixed and incubated overnight in the staining solution following the instructions provided in the senescence-associated β-galactosidase staining (SA-B-Gal staining) kit (Beyotime, Beijing, China). The cells were stained in the dye solution for 12 hours at 37 °C. Three independent fields of view were captured for each treatment group, and the number of positive cells was statistically analyzed using ImageJ software.
The proliferative capacity of ASCs was calculated using the formula population doubling time = (log 2/([og Nt - logN0]) × t, where N0 and Nt represent the cell numbers at initiation and after t hours of culture, respectively. A total of 5 × 104 cells were seeded into a 35 mm cell culture dish. After 24 hours, the cells were suspended using trypsin and counted using cell counting plates. Data from three independent experiments were statistically analyzed.
The plasmid pSIH-H1-CopGFP-shRNA was utilized for this purpose, with the target sequences listed in Supplementary Table 1. The plasmids pSIH-H1-CopGFP-shRNA, pLP1, pLP2, and pLP VSV-G were packaged in HEK293T cells. The cell supernatants containing lentiviruses were collected after 48 hours and then inoculated with 60% confluent ASCs at the third passage in a 60 mm cell culture dish. Cells were harvested 48 hours later for further analysis.
The plasmid pLenti-puro was used. The coding sequence region of Mfn2 gene (gene ID: 170731) was cloned into the plasmid vector and transfected into HEK293T cells with lentivirus packaging vectors pLP1, pLP2, pLP VSV-G. After 48 hours, the virus was collected and used to infect ASCs. The third passage ASCs were infected with lentivirus in 60 mm dishes at 60% confluence. Cells were collected 48 hours later for analysis.
The activity of NRF2 was analyzed using the pGL3-ARE-luc plasmid. The pRL-TK Renilla luciferase plasmid (Promega, Madison, WI, United States) was used as a control. Plasmid pGL3-ARE-luc and plasmid pRL-TK were transfected into ASCs in a ratio of 1:100 using transfection reagents Lipofectamine 2000 (Thermo Fisher Scientific, Walthma, MA, United States) according to the instructions. Thirty-six hours after transfection, the activities of firefly and Renilla luciferase were determined using the dual-luciferase reporter assay system (Promega) following the manufacturer’s instructions.
The cells were fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, blocked in 4% bovine serum albumin, and incubated with primary NRF2 antibody (PA5-27882; Thermo Fisher Scientific), MFN2 antibody (12186-1-AP; Proteintech, Rosemont, IL, United States), BIP antibody (66574-1; Proteintech) and secondary antibodies (ab69899, ab150113, ab150080; Abcam, Cambridge, MA, United States). Nuclear counterstaining was performed with 1 μg/mL Hoechst 33342 (Sigma-Aldrich, Marlborough, MA, United States). Fluorescence images were captured using a fluorescence microscope (Olympus, Tokyo, Japan).
cDNA was synthesized from ASCs after RNA extraction (Trizol reagent; Takara, Shiga, Japan) and reverse transcription using the Reverse Transcript Reagent Kit (Thermo Fisher Scientific) following the instructions. qPCR was performed using the ABI StepOne Real-Time PCR-Cycler and qRT-PCR Kit (Power SYBR™ Green PCR Master Mix; Thermo Fisher Scientific) according to the manufacturer’s instructions. β-actin was used as the reference gene. The 2-ΔΔCt method was employed to determine the relative expression of target genes. Primers are listed in Supplementary Table 2.
Total cell extracts were prepared in 1 × sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample loading buffer. Nuclear and cytoplasmic proteins were extracted using the Cell Fractions Extraction Kit (Beyotime). Cell proteins were separated by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and probed with antibodies against β-actin (20536-1-AP), H3 (17168-1-AP), GAPDH (10494-1-AP), MFN2 (12186-1-AP) (1:1000; Proteintech, Wuhan, China), BIP (3177), inositol-requiring enzyme-1 alpha (IRE1α) (3294), p16 (29271) (1:1000; Cell Signaling Technology, Danvers, MA, United States), NRF2 (1:1000, PA5-27882; Thermo Fisher Scientific), and interleukin 1 beta (IL-1β) (TA5103), P21 (T55088), NAD(P)H:quinone oxidoreductase 1 (T56710), glutathione peroxidase 4 (T56959) (1:1000; Abmart, Shanghai, China). Secondary antibodies were anti-rabbit and anti-mouse antibodies (1:3000, M21003, Abmart). Detection was carried out using the Thermo Scientific Pierce Enhanced Chemiluminescence Western Blotting Substrate (Thermo Fisher Scientific). Results were analyzed using the ChemiDoc XRS+ Chemiluminescence Imaging System (Bio-Rad, Hercules, CA, United States).
ChIP assays for NRF2 were performed with 1 × 107 ASCs according to the protocol from the Pierce Magnetic ChIP Kit (26157; Thermo Fisher Scientific) with slight modifications. After 10 minutes of formaldehyde crosslinking, an ultrasonic crusher (SCIENTZ, Zhejiang, China) was used to sonicate the genomic DNA. Sonicated cell lysates were subjected to immunoprecipitation with antibodies against NRF2 (PA5-27882; Thermo Fisher Scientific), and normal rabbit immunoglobulin G (MA5-42729; Thermo Fisher Scientific). The purified DNA was analyzed via qPCR. The ChIP DNA values were calculated as a proportional expression over the isotype-specific input DNA values of each primer set. The primer sequences for the Mfn2 promoter region were “GTTAGGGTGAAGTAATTGTCCTTCA” and “CAGTGCAATGACTGTCACCTAGTAG”. Comparative ΔΔCt analytical methods were used to analyze the results. The fold change in occupancy was calculated as 2-ΔΔCt(S2-S1). ΔΔCt(S2-S1) = ΔCtS2 - ΔCtS1, ΔCtS2 = IP2Ct - (Input2Ct - log2Dilution times of the input). IP2Ct: Ct value of the obesity group NRF2 antibodies IP product. Input2Ct: Ct value of the obesity group input. ΔCtS1 = IP1Ct - (Input1Ct - log2Dilution times of the input), IP1Ct: Ct value of the control group NRF2 antibodies IP product. Input1Ct: Ct value of the control group input.
When third passage ASCs reached 60% confluence in a 35 mm cell culture dish, the media was removed from the dish and prewarmed (37 °C) fetal bovine serum-free mesenchymal stem cell culture medium (Yocon, Beijing, China) containing 100 nM MitoTracker® Red CMXRos (M7512; Thermo Fisher Scientific) was added and incubated for 20 minutes. After staining was complete, the staining solution was replaced with fresh mesenchymal stem cell culture medium and the cells were observed via a fluorescence microscope (Olympus).
Coimmunoprecipitation assays were performed according to standard protocols. Lysates of 1 × 107 ASCs were collected with a cell scraper using the immunoprecipitation lysis buffer NP-40 (P0013F; Beyotime) with phenylmethanesulfonyl fluoride and protease inhibitors. The cell lysate was incubated with MFN2 antibody (1:50, 12186-1-AP; Proteintech, China) or immunoglobulin G (MA5-42729; Thermo Fisher Scientific) overnight and then with DynaGreen™ Protein A/G magnetic beads (80104G; Thermo Fisher Scientific) for 2 hours. The proteins were collected according to the instructions of the magnetic beads and examined by MS or subjected to SDS-PAGE and Western blot analysis. The antibodies used for Western blotting included MFN2 antibodies (1:1000, 12186-1-AP; Proteintech, China) and BIP antibodies (1:1000, 3177; Cell Signaling Technology) avoiding heavy or light chain secondary antibodies (1:3000, M21008; Abmart).
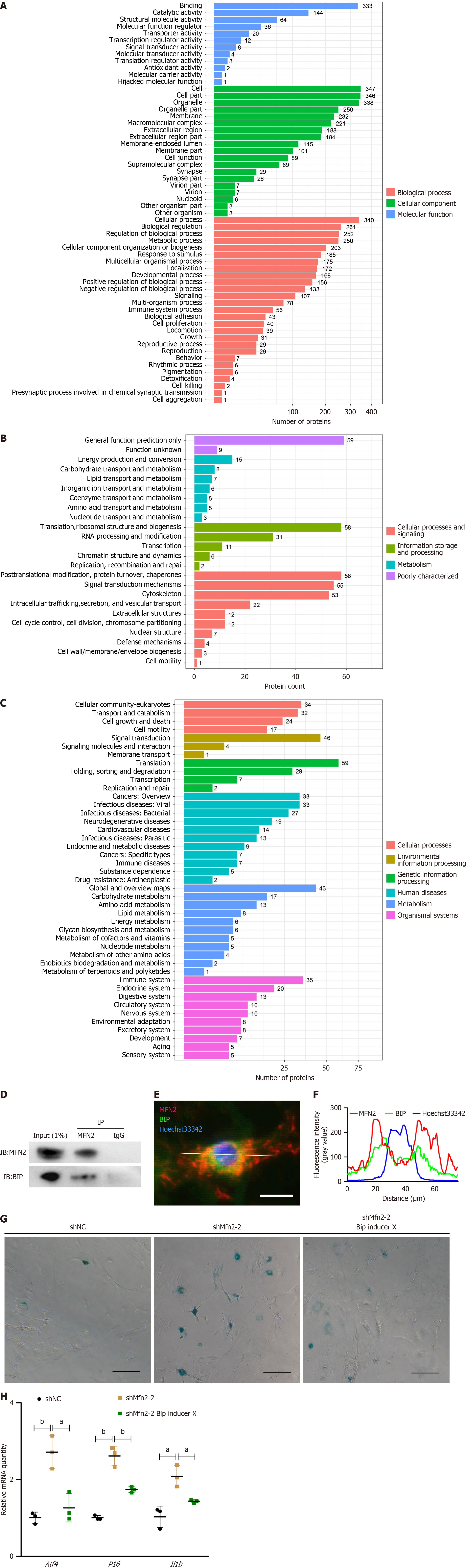
After sample processing, MS data were collected using the Triple TOF 5600 mass spectrometer (AB SCIEX, Framingham, MA, United States). The mass spectral data were compared with the UniProt database (Proteome ID: UP000000589) to identify proteins. The IP product-specific proteins of the MFN2 group were analyzed by Eukaryotic Orthologous Group, Kyoto Encyclopedia of Genes and Genomes and Gene Ontology with the R language.
Hypertrophic obesity model mice with significantly elevated blood glucose at 30 minutes and 120 minutes of the glucose tolerance test (GTT) were used for ASC subcutaneous inguinal transplantation. The cells were divided into the shNC-interfered ASC transplantation group, shNrf2-interfered ASC transplantation group, and shMfn2-interfered ASC transplantation group. The normal control group and the hypertrophic obesity model group served as controls, simu
All graphs are presented as the mean ± standard error of the mean. Two-tailed Student’s t-test was used when comparing two means. One-way analysis of variance was performed for comparisons among multiple groups. All statistical analyses were calculated using Prism 10.1.2 (GraphPad Software Inc., La Jolla, CA, United States). Sample sizes for all statistical evaluations are indicated in the figure legends. No statistical method was used to predetermine sample size. No data were excluded from the analyses. All sample images were acquired in random order within the sets and all cells were chosen at random sites. Experimenters were blinded to each group allocation during the image analysis by removing any sample information in file names. aP < 0.05; bP < 0.01. And “n” denoted the number of independent experiments.
The hypertrophic obesity mouse model was established via the consumption of a high-fat diet. After 16 weeks of high-fat diet feeding, the body weight of hypertrophic obese mice was significantly greater than that of the control group (Figure 1A), and the diameter of subcutaneous adipocytes was notably greater than that of the control group (Figure 1B and C). ASCs were isolated and cultured from the subcutaneous adipose tissue of the inguinal region. The SVF was cultured in adherent culture dishes for 10 hours to determine the number of ASCs in the adipose tissue. The results revealed no significant difference in the number of ASCs per gram of adipose tissue between the control group and the obese model group (Figure 1D). The population doubling time was significantly prolonged in the ASCs of the model group (Figure 1E). SA-B-Gal staining revealed a significantly greater positive rate in hypertrophic obese group mice than in control group mice (Figure 1F and G). After the induction of ASC differentiation into adipocytes, Oil Red O staining was performed (Figure 1H). The proportion of Oil Red O-stained areas in the hypertrophic obesity group was significantly lower than that in the control group (Figure 1I). The expression of senescence-related genes (p16 and p21), senescence-associated secretory phenotype genes (IL-6, C-C motif ligand 2, and tumor necrosis factor alpha), and ERS-related genes (Bip, X-box binding protein 1 [Xbp1], and spliced Xbp1 [sXbp1]) was significantly greater in the ASCs of the model group than in those of the control group (Figure 1J). The levels of the ERS-related protein IRE1α and the senescence-related proteins p16 and p21 were notably greater in the ASCs of the model group than in those of the control group (Figure 1K and L).
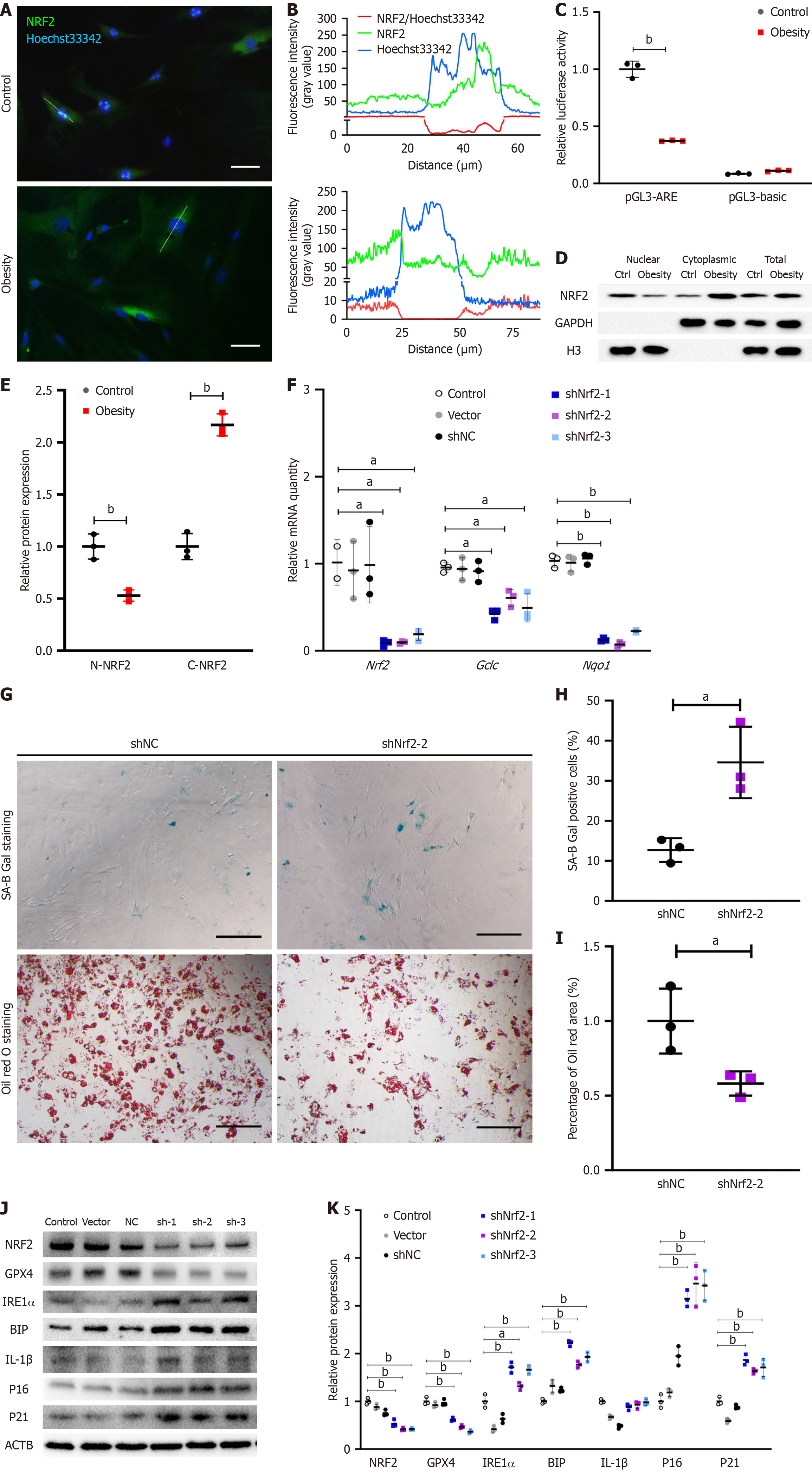
NRF2 is considered a remarkable transcription factor capable of delaying aging. In my previous studies, I discovered that NRF2 activity also regulates ERS levels. To explore the transcriptional activity of NRF2 in the ASCs of hypertrophic obese mice and investigate whether its reduced activity affects the aging and ERS levels of these cells, I conducted the following experiments. Immunofluorescence staining revealed that NRF2 was localized in the nucleus of ASCs in the control group and in the cytoplasm in the obesity model group (Figure 2A). The fluorescence intensity of NRF2 was greater in the nuclear region of control ASCs than in the nonnuclear region of obese model ASCs (Figure 2B). A dual-luciferase assay demonstrated that the transcriptional activity of NRF2 in the ASCs of the model group was significantly lower than that in the control group (Figure 2C). By isolating and extracting cytoplasmic and nuclear proteins from ASCs, I observed that the level of NRF2 protein in the nucleus of the model group was significantly lower than that in the control group (Figure 2D and E). To verify the relationship between NRF2 and the senescence phenotype of ASCs in the model group, Nrf2-knockdown ASCs were generated, and the expression of Nrf2 and its downstream target genes was verified via qPCR (Figure 2F). The percentage of SA-B-Gal stained-positive ASCs in the Nrf2-knockdown group was greater than that in the shNC control group (Figure 2G and H). Oil Red O staining after inducing ASCs to differentiate into adipocytes revealed that the staining area of the control group was larger than that of the Nrf2-knockdown group, indicating a lower differentiation potential of ASCs in the Nrf2-knockdown group than in the control group (Figure 2G and I). The levels of senescence-related proteins (p16, p21, and IL-1β) and ERS-related proteins (IRE1α and BIP) in the ASCs of the Nrf2-knockdown group were significantly greater than those in the control group (Figure 2J and K).
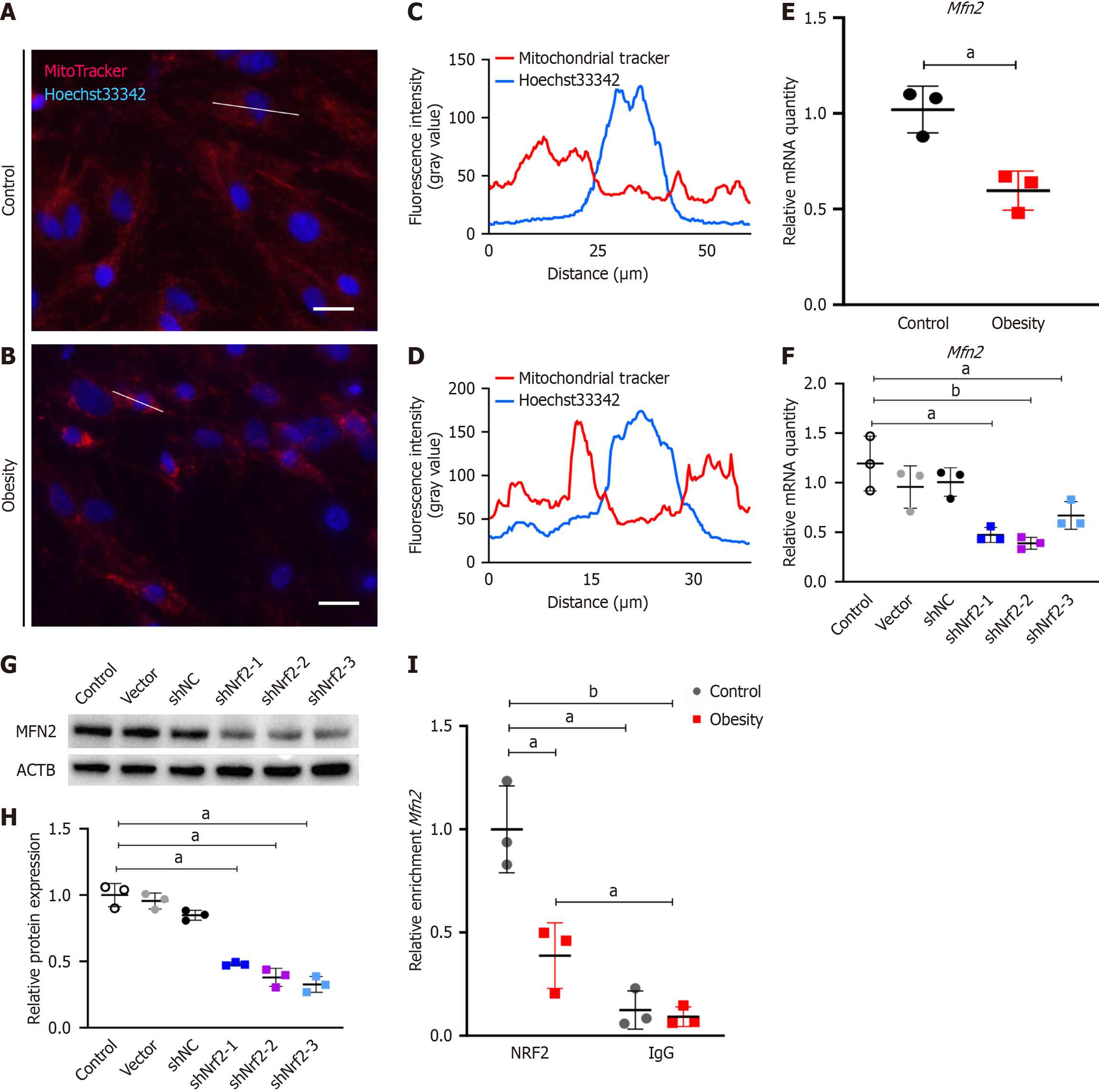
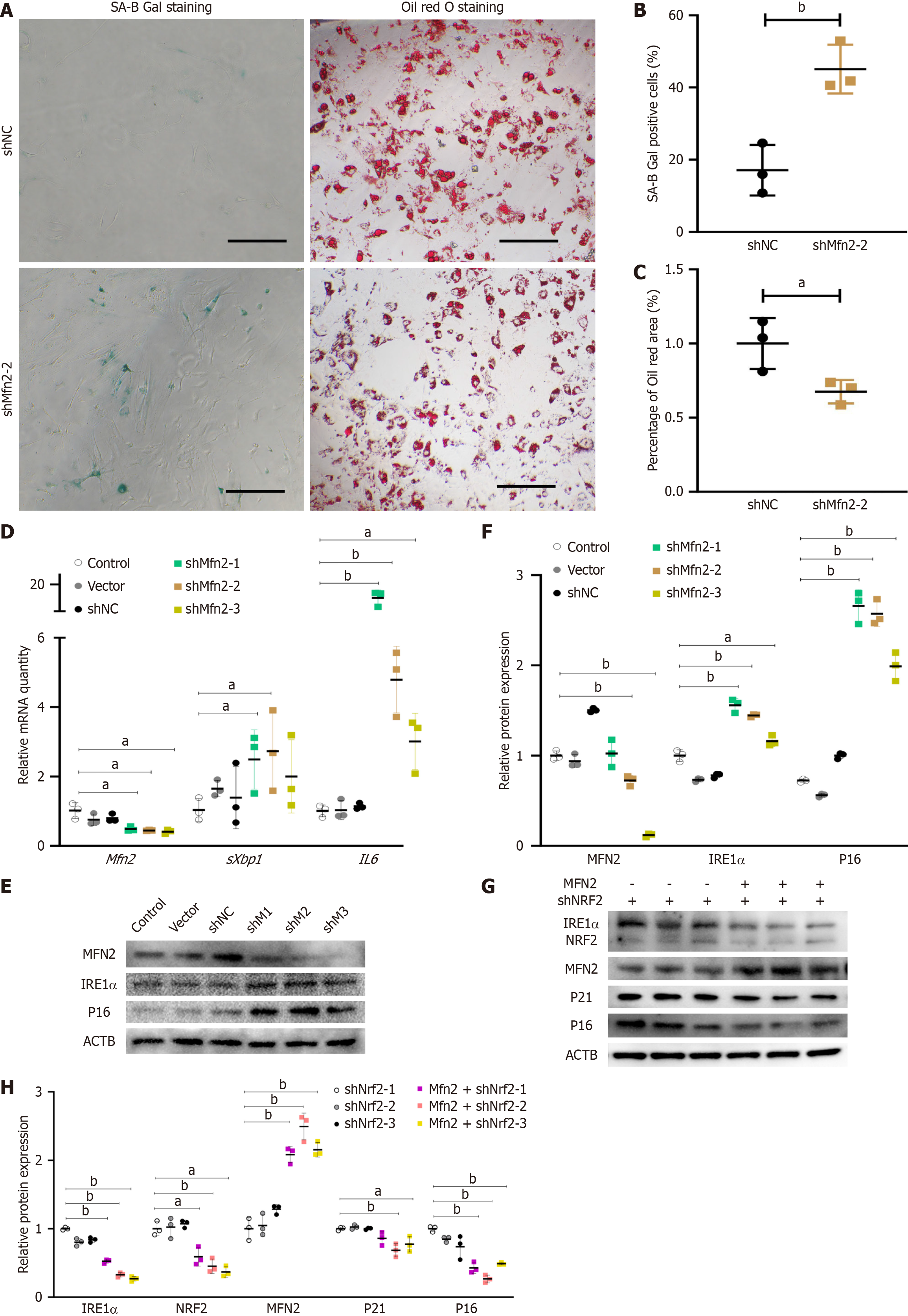
Considering that mitochondria are the primary site for reactive oxygen species production, the antiaging effects of NRF2 might indirectly regulate mitochondrial homeostasis. Thus, I observed morphological changes in the mitochondria of hypertrophic obese ASCs via the use of fluorescent dyes. Mitochondria were stained with MitoTracker® Red CMXRos (M7512, Thermo Fisher Scientific) (Figure 3A and B). Mitochondrial staining revealed greater and more concentrated fluorescence intensity in the model group (Figure 3C and D), suggesting a more concentrated distribution of mitochondria in the ASCs of the model group or possible mitochondrial fusion. Since MFN2 is a protein that regulates mitochondrial fusion, I examined its expression. The relative gene expression of Mfn2 was significantly lower in the model group than in the control group (Figure 3E). The gene and protein expression levels of MFN2 in the ASCs of the Nrf2-knockdown group were also notably downregulated (Figure 3F-H). To confirm the regulatory effect of NRF2 on Mfn2 transcription, the binding of NRF2 to the Mfn2 promoter region was detected via a ChIP-qPCR assay. The results indicated weaker binding of NRF2 to the Mfn2 promoter region of ASCs in the hypertrophic obesity model group than in the control group (Figure 3I). I then knocked down Mfn2 expression using shRNA in ASCs and assessed its impact on the senescence phenotype. The percentage of SA-B-Gal-stained-positive ASCs in the Mfn2-knockdown group was greater than that in the control group (Figure 4A and B). The adipocyte differentiation potential of ASCs in the shMfn2 group was reduced compared with that in the control group (Figure 4A and C). The gene expression levels of the senescence-related gene Il-6 and the ERS-related gene sXbp1 were greater in the shMfn2 group than in the control group (Figure 4D). Additionally, the protein levels of p16 and IRE1α in the shMfn2 group were significantly greater than those in the control group (Figure 4E and F). To investigate whether NRF2 can regulate senescence and ERS by activating Mfn2 expression, I overexpressed the Mfn2 gene in Nrf2-knockdown cell lines. Mfn2 overexpression led to decreased expression of the ERS-related protein IRE1α and the senescence-related proteins p21 and p16 (Figure 4G and H).
To elucidate the mechanism by which MFN2 regulates the senescence phenotype of ASCs, I conducted a coimmunoprecipitation assay to identify MFN2-binding proteins and analyze the binding proteins. The list of binding proteins identified through IP-MS is provided in Supplementary Table 3. Eukaryotic Orthologous Group (Figure 5A), Gene Ontology (Figure 5B) and Kyoto Encyclopedia of Genes and Genomes (Figure 5C) analyses revealed that MFN2-binding proteins were involved primarily in translation, posttranslational modification, protein turnover, and other cellular processes. MS analysis identified the ERS marker BIP as a binding protein of MFN2, which was confirmed through Western blotting (Figure 5D) and immunofluorescence containing of MFN2 and BIP (Figure 5E and F). Immunofluorescence analysis revealed overlapping fluorescence of MFN2 and BIP in certain areas, suggesting that MFN2 may physically interact with BIP either directly or indirectly.
To explore the molecular mechanism by which MFN2 regulates the senescence of ASCs through BIP, I examined the senescence phenotypes of Mfn2 knockdown ASCs and Mfn2-knockdown ASCs treated with BIP inducer X (MedChemExpress, Monmouth Junction, NJ, United States), an inducer of BIP. The concentration of BIP inducer X used to treat Mfn2-knockdown ASCs was 5 μM for 12 hours. Compared with shNC, Mfn2 knockdown resulted in a higher positivity rate for SA-B-Gal staining (Figure 5G) and elevated expression levels of the ERS-related gene activating transcription factor 4 (Atf4) and the senescence-related genes p16 and IL-1β (Figure 5H). However, the senescence phenotype and expression of the ERS-related gene Atf4 were alleviated after treatment with the BIP inducer.
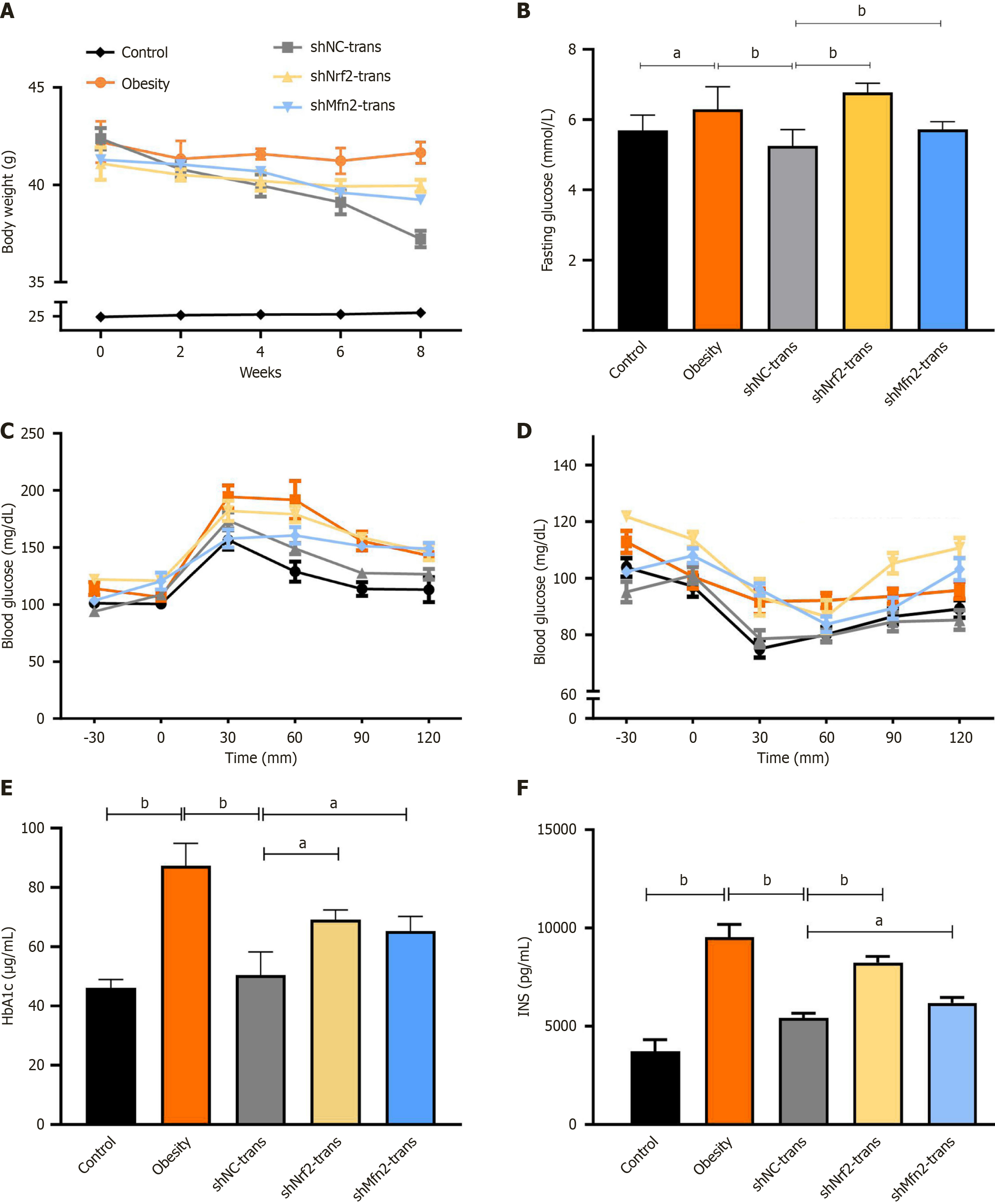
To explore the impact of Nrf2 or Mfn2 knockdown on the therapeutic efficacy of ASCs, Nrf2 or Mfn2 in ASCs derived from control mice was knocked down using shRNA. Owing to the large differences in blood glucose between individuals in the hypertrophic obesity model, I selected individuals with significantly higher blood glucose values at 30 minutes and 120 minutes for the GTT than those in the control group for the cell transplantation test. Additionally, I simulated transplantation in the normal control group and the hypertrophic obesity model group by injecting PBS. Eight weeks after cell transplantation, I observed significant weight loss in the shNC ASC transplantation group (Figure 6A). Blood glucose was significantly greater in the Nrf2- or Mfn2-knockdown ASC-transplanted group than in the shNC ASC-transplanted group (Figure 6B). The results of the GTT (Figure 6C) and ITT (Figure 6D) indicated that insulin and glucose tolerance were lower in mice transplanted with Nrf2- or Mfn2-knockdown ASCs than in those transplanted with shNC-transfected ASCs. In the Nrf2- or Mfn2-knockdown ASC transplantation group, the hemoglobin A1c and insulin levels were significantly greater than those that in the shNC ASC transplantation group (Figure 6E and F).
The hypertrophy of adipocytes in adult individuals leads to decreased insulin sensitivity and altered secretory function, promoting the development of metabolic-related diseases. A deeper understanding of the expansion mechanism of adult adipocytes is crucial for revealing the key factors involved in hypertrophic obesity-related metabolic diseases. Our study revealed that ASCs from hypertrophic obese mice presented senescence-related phenotypes, such as increased expression of senescence-associated genes and an elevated SA-B-Gal staining rate, along with a reduced potential for differentiation into adipocytes. These findings are consistent with previous reports indicating a decrease in the stemness of ASCs in individuals with metabolic diseases[11]. To investigate whether the number of ASCs plays a role in the increased volume of adipose tissue in individuals with obesity, I calculated the number of ASCs per gram of adipose tissue in both the obesity model group and the control group. No significant difference was observed. In adults with hypertrophic obesity, the number of mesenchymal progenitor/precursor cells was also found to be unchanged, but their differentiation potential was reduced[12]. The senescence of ASCs, decreased differentiation potential, and maintained cell number suggest that lipids cannot be stored through an increase in the number of adipocytes in individuals with hypertrophic obesity. Exploring the molecular mechanisms of ASC senescence is critical for the treatment of hypertrophic obesity-related metabolic diseases.
In the subcutaneous ASCs of a hypertrophic obesity mouse model, I observed concurrent upregulation of ERS levels and a senescent phenotype. Our previous research demonstrated that ERS can drive senescent phenotypes in ASCs[4]. Other related studies have shown that the activation of molecules in the unfolded protein response pathway can amplify the senescent phenotype. For example, activated IRE1α can enhance the senescence-associated secretory phenotype through the nuclear factor-kappa B signaling pathway[13]. Additionally, ATF4 has been proven to directly regulate the expression of p16[14]. Identifying the molecular mechanisms that alleviate ERS may help control the senescence of ASCs.
Our previous research indicated that the activation of NRF2 can alleviate ERS and senescence in ASCs[4]. Activating NRF2 increases several metabolic pathways, which in turn fuel the tricarboxylic acid cycle and mitochondrial respiration, leading to increased energy production (ATP), oxygen consumption, and mitochondrial membrane potential. Persistent ERS and excess oxidative stress interact and intensify in a positive feedback manner, which can lead to mitochondrial dysfunction and apoptosis. NRF2, by reducing oxidative stress, indirectly protects against mitochondrial dysfunction and apoptosis[15,16]. In this study, I observed that Nrf2 knockdown in ASCs promoted ERS and senescence. Interestingly, Mfn2 overexpression partially offset the increase in ERS and senescence-related protein expression caused by Nrf2 knockdown. Furthermore, the expression level of Mfn2 in ASCs from hypertrophic obesity model mice was lower than that in control ASCs, and interfering with Nrf2 expression also reduced Mfn2 levels. These results suggest that Mfn2 may be a key player in the NRF2 mediated regulation of ERS and senescence.
Studies have reported the presence of a potential ARE region in the Mfn2 gene locus[10], but this has not been verified through ChIP assays. To clarify the regulatory effect of NRF2 on Mfn2, I performed ChIP-qPCR and found an NRF2 binding region near the Mfn2 promoter, indicating that NRF2 has a transcriptional regulatory effect on Mfn2. With respect to the regulatory role of NRF2 in Mfn2, other studies have shown that peroxisome proliferator-activated receptor gamma coactivator 1 alpha can upregulate Mfn2 expression by enhancing heme oxygenase-1[17]. Since heme oxygenase-1 is a target gene that can be transcriptionally regulated by NRF2[18] and peroxisome proliferator-activated receptor gamma coactivator 1 alpha can also increase the expression of various transcription factors including NRF2[19], NRF2 may also promote Mfn2 expression through other indirect mechanisms.
MFN2 is an important molecule for maintaining ER homeostasis and the structure of mitochondria-associated membranes. Mitochondria-associated membranes (MAMs) mediate oxidative stress, ERS, Ca2+ transport, autophagy, mitochondrial fusion and fission, and apoptosis. An increase in Ca2+ flux from the ER to the mitochondria can augment ATP production, thereby counteracting ERS. Increasing the degree of mitochondria–ER coupling can alleviate the degree of ERS[20]. Studies have shown that the absence of Mfn2 can lead to ER expansion, morphological disorders[21], and Ca2+ homeostasis imbalances[22]. MFN2 can physically interact with the PERK protein structure, preventing PERK activation and maintaining ERS at a low level[23]. Knocking out Mfn2 also disrupts mitochondrial-ER contact, inducing ERS[24]. These findings suggest that MFN2 may play a role in regulating ERS within the MAM structure. In our study, MFN2 may directly or indirectly bind to BIP, which could be a part of how MFN2 helps maintain low levels of ERS. Other studies have emphasized that BIP is an important component of MAM[25], suggesting that MFN2 and BIP may spatially colocalize in the MAM region to participate in the regulation of ERS levels. I treated Mfn2-knockdown ASCs with a BIP inducer and found that inducing BIP expression can alleviate the senescent phenotype and reduce the expression of the ERS-related gene Atf4 in ASCs caused by Mfn2 knockdown. I hypothesize that MFN2 can stabilize the binding of BIP to the ERS signal transduction molecules PERK, IRE1, and ATF6, thereby maintaining ERS at a low level. However, the specific mechanism of action requires further investigation in subsequent studies. Since the MAM structure also contains other ERS-related proteins, such as Sig-1R[26], MFN2 may also regulate ERS levels through other mechanisms, which requires further exploration in future research.
The expression of Mfn2 is closely related to adipocyte differentiation and obesity-related metabolic diseases[27]. Changes in Mfn2 expression often occur under altered glucose oxidation conditions, such as diabetes, obesity, insulin resistance, physical activity, and weight fluctuations[28-31]. Decreased Mfn2 expression is associated with obesity and negatively correlated with body mass index[32]. Additionally, low Mfn2 expression is linked to senescence in multiple cell types[33,34], and the mechanism may involve reduced autophagy[35]. In our study, the expression level of Mfn2 in ASCs from hypertrophic obesity model mice was lower than that in control ASCs, which is consistent with the literature. Interference with Mfn2 also led to increased expression of senescence and ERS-related proteins. Transplantation of ASCs into insulin-resistant, hypertrophic obesity model mice alleviated insulin resistance. However, shNrf2 or shMfn2 reduced the therapeutic effect of ASCs. These findings suggest that regulating Mfn2 levels may be a potential target for the treatment of hypertrophic obesity-related metabolic diseases.
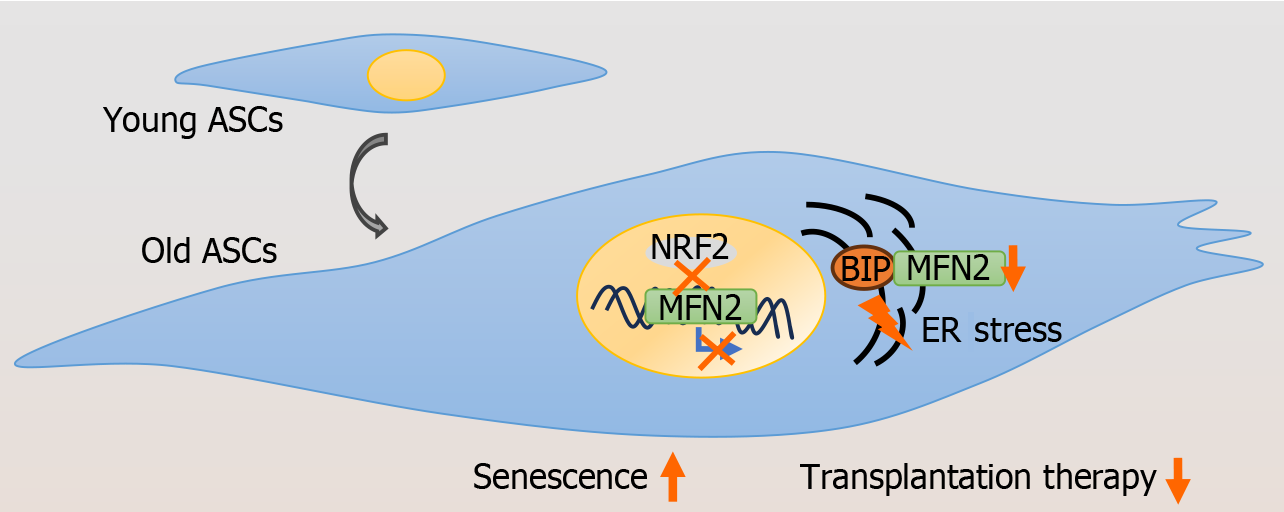
Our findings indicate that senescence and increased ERS levels occur in obese ASCs, possibly due to the decreased transcriptional activity of NRF2 on Mfn2 (Figure 7). Controlling NRF2 activity or Mfn2 expression in ASCs may have beneficial effects on preventing and treating obesity-related metabolic diseases.
| 1. | White U. Adipose tissue expansion in obesity, health, and disease. Front Cell Dev Biol. 2023;11:1188844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 38] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 2. | Nerstedt A, Smith U. The impact of cellular senescence in human adipose tissue. J Cell Commun Signal. 2023;17:563-573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 3. | Fang J, Li L, Cao X, Yue H, Fu W, Chen Y, Xu Z, Zhao Q, Zhao J, Wang Y, Liang W. Transmissible Endoplasmic Reticulum Stress Mediated by Extracellular Vesicles from Adipocyte Promoting the Senescence of Adipose-Derived Mesenchymal Stem Cells in Hypertrophic Obesity. Oxid Med Cell Longev. 2022;2022:7175027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Fang J, Yan Y, Teng X, Wen X, Li N, Peng S, Liu W, Donadeu FX, Zhao S, Hua J. Melatonin prevents senescence of canine adipose-derived mesenchymal stem cells through activating NRF2 and inhibiting ER stress. Aging (Albany NY). 2018;10:2954-2972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 5. | Ulasov AV, Rosenkranz AA, Georgiev GP, Sobolev AS. Nrf2/Keap1/ARE signaling: Towards specific regulation. Life Sci. 2022;291:120111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 302] [Article Influence: 100.7] [Reference Citation Analysis (0)] |
| 6. | Digaleh H, Kiaei M, Khodagholi F. Nrf2 and Nrf1 signaling and ER stress crosstalk: implication for proteasomal degradation and autophagy. Cell Mol Life Sci. 2013;70:4681-4694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Gottschalk B, Koshenov Z, Bachkoenig OA, Rost R, Malli R, Graier WF. MFN2 mediates ER-mitochondrial coupling during ER stress through specialized stable contact sites. Front Cell Dev Biol. 2022;10:918691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Muñoz JP, Ivanova S, Sánchez-Wandelmer J, Martínez-Cristóbal P, Noguera E, Sancho A, Díaz-Ramos A, Hernández-Alvarez MI, Sebastián D, Mauvezin C, Palacín M, Zorzano A. Mfn2 modulates the UPR and mitochondrial function via repression of PERK. EMBO J. 2013;32:2348-2361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 348] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 9. | Chen QM. Nrf2 for protection against oxidant generation and mitochondrial damage in cardiac injury. Free Radic Biol Med. 2022;179:133-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 146] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 10. | Cho HY, Miller-DeGraff L, Blankenship-Paris T, Wang X, Bell DA, Lih F, Deterding L, Panduri V, Morgan DL, Yamamoto M, Reddy AJ, Talalay P, Kleeberger SR. Sulforaphane enriched transcriptome of lung mitochondrial energy metabolism and provided pulmonary injury protection via Nrf2 in mice. Toxicol Appl Pharmacol. 2019;364:29-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Alicka M, Major P, Wysocki M, Marycz K. Adipose-Derived Mesenchymal Stem Cells Isolated from Patients with Type 2 Diabetes Show Reduced "Stemness" through an Altered Secretome Profile, Impaired Anti-Oxidative Protection, and Mitochondrial Dynamics Deterioration. J Clin Med. 2019;8:765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 12. | Gustafson B, Nerstedt A, Smith U. Reduced subcutaneous adipogenesis in human hypertrophic obesity is linked to senescent precursor cells. Nat Commun. 2019;10:2757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 13. | Tam AB, Mercado EL, Hoffmann A, Niwa M. ER stress activates NF-κB by integrating functions of basal IKK activity, IRE1 and PERK. PLoS One. 2012;7:e45078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 276] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 14. | Sun Y, Lin X, Liu B, Zhang Y, Li W, Zhang S, He F, Tian H, Zhu X, Liu X, Wu J, Cai J, Li M. Loss of ATF4 leads to functional aging-like attrition of adult hematopoietic stem cells. Sci Adv. 2021;7:eabj6877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;73:3221-3247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1641] [Cited by in RCA: 1919] [Article Influence: 213.2] [Reference Citation Analysis (0)] |
| 16. | He F, Ru X, Wen T. NRF2, a Transcription Factor for Stress Response and Beyond. Int J Mol Sci. 2020;21:4777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 1076] [Article Influence: 215.2] [Reference Citation Analysis (0)] |
| 17. | Singh SP, Schragenheim J, Cao J, Falck JR, Abraham NG, Bellner L. PGC-1 alpha regulates HO-1 expression, mitochondrial dynamics and biogenesis: Role of epoxyeicosatrienoic acid. Prostaglandins Other Lipid Mediat. 2016;125:8-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Ghareghomi S, Moosavi-Movahedi F, Saso L, Habibi-Rezaei M, Khatibi A, Hong J, Moosavi-Movahedi AA. Modulation of Nrf2/HO-1 by Natural Compounds in Lung Cancer. Antioxidants (Basel). 2023;12:735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 19. | Rius-Pérez S, Torres-Cuevas I, Millán I, Ortega ÁL, Pérez S. PGC-1α, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxid Med Cell Longev. 2020;2020:1452696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 400] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 20. | Tong D, Zhou J, Zhou J, Wang X, Gao B, Rui X, Liu L, Chen Q, Huang C. LAMC2 mitigates ER stress by enhancing ER-mitochondria interaction via binding to MYH9 and MYH10. Cancer Gene Ther. 2024;31:43-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Zorzano A, Hernández-Alvarez MI, Sebastián D, Muñoz JP. Mitofusin 2 as a driver that controls energy metabolism and insulin signaling. Antioxid Redox Signal. 2015;22:1020-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Kowaltowski AJ, Menezes-Filho SL, Assali EA, Gonçalves IG, Cabral-Costa JV, Abreu P, Miller N, Nolasco P, Laurindo FRM, Bruni-Cardoso A, Shirihai OS. Mitochondrial morphology regulates organellar Ca(2+) uptake and changes cellular Ca(2+) homeostasis. FASEB J. 2019;33:13176-13188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 23. | Xin Y, Wu W, Qu J, Wang X, Lei S, Yuan L, Liu X. Inhibition of Mitofusin-2 Promotes Cardiac Fibroblast Activation via the PERK/ATF4 Pathway and Reactive Oxygen Species. Oxid Med Cell Longev. 2019;2019:3649808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Schneeberger M, Dietrich MO, Sebastián D, Imbernón M, Castaño C, Garcia A, Esteban Y, Gonzalez-Franquesa A, Rodríguez IC, Bortolozzi A, Garcia-Roves PM, Gomis R, Nogueiras R, Horvath TL, Zorzano A, Claret M. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell. 2013;155:172-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 423] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 25. | Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1345] [Cited by in RCA: 1452] [Article Influence: 80.7] [Reference Citation Analysis (0)] |
| 26. | Mao H, Chen W, Chen L, Li L. Potential role of mitochondria-associated endoplasmic reticulum membrane proteins in diseases. Biochem Pharmacol. 2022;199:115011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 27. | Rocha N, Bulger DA, Frontini A, Titheradge H, Gribsholt SB, Knox R, Page M, Harris J, Payne F, Adams C, Sleigh A, Crawford J, Gjesing AP, Bork-Jensen J, Pedersen O, Barroso I, Hansen T, Cox H, Reilly M, Rossor A, Brown RJ, Taylor SI, McHale D, Armstrong M, Oral EA, Saudek V, O'Rahilly S, Maher ER, Richelsen B, Savage DB, Semple RK. Human biallelic MFN2 mutations induce mitochondrial dysfunction, upper body adipose hyperplasia, and suppression of leptin expression. Elife. 2017;6:e23813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 28. | Chandhok G, Lazarou M, Neumann B. Structure, function, and regulation of mitofusin-2 in health and disease. Biol Rev Camb Philos Soc. 2018;93:933-949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 187] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 29. | Mancini G, Pirruccio K, Yang X, Blüher M, Rodeheffer M, Horvath TL. Mitofusin 2 in Mature Adipocytes Controls Adiposity and Body Weight. Cell Rep. 2019;26:2849-2858.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 30. | Hu L, Ding M, Tang D, Gao E, Li C, Wang K, Qi B, Qiu J, Zhao H, Chang P, Fu F, Li Y. Targeting mitochondrial dynamics by regulating Mfn2 for therapeutic intervention in diabetic cardiomyopathy. Theranostics. 2019;9:3687-3706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 169] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 31. | Lv Y, Cheng L, Peng F. Compositions and Functions of Mitochondria-Associated Endoplasmic Reticulum Membranes and Their Contribution to Cardioprotection by Exercise Preconditioning. Front Physiol. 2022;13:910452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 32. | Bach D, Naon D, Pich S, Soriano FX, Vega N, Rieusset J, Laville M, Guillet C, Boirie Y, Wallberg-Henriksson H, Manco M, Calvani M, Castagneto M, Palacín M, Mingrone G, Zierath JR, Vidal H, Zorzano A. Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes. 2005;54:2685-2693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 297] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 33. | Sebastián D, Zorzano A. When MFN2 (mitofusin 2) met autophagy: A new age for old muscles. Autophagy. 2016;12:2250-2251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Wang L, Song S, Liu X, Zhang M, Xiang W. Low MFN2 expression related to ageing in granulosa cells is associated with assisted reproductive technology outcome. Reprod Biomed Online. 2019;38:152-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Hu Y, Chen H, Zhang L, Lin X, Li X, Zhuang H, Fan H, Meng T, He Z, Huang H, Gong Q, Zhu D, Xu Y, He P, Li L, Feng D. The AMPK-MFN2 axis regulates MAM dynamics and autophagy induced by energy stresses. Autophagy. 2021;17:1142-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 195] [Article Influence: 48.8] [Reference Citation Analysis (0)] |