Published online May 26, 2025. doi: 10.4252/wjsc.v17.i5.105266
Revised: March 17, 2025
Accepted: May 9, 2025
Published online: May 26, 2025
Processing time: 104 Days and 1.4 Hours
Diabetes mellitus (DM) and metabolic-associated fatty liver disease (MAFLD) are common metabolic disorders, and their coexistence can exacerbate the pro
To investigate how hUC-MSCs affect liver metabolism in diabetic rats with MAFLD and assess their therapeutic potential and underlying mechanisms.
A streptozotocin-induced rat model of DM with MAFLD was established, and hUC-MSCs were administered via tail vein injection. Changes in body weight, fasting blood glucose (FBG), and serum triglyceride (TG), alanine aminotransferase, aspartate aminotransferase levels, and pathological changes of liver were evaluated. Receiver operating characteristic analysis was used to assess the diagnostic value of differential metabolites and their ability to predict the therapeutic effects of hUC-MSCs. Spearman correlation was employed to analyze the relationships between liver metabolites and key biochemical markers.
hUC-MSC treatment significantly reduced FBG and TG levels in diabetic rats with MAFLD and improved histological steatosis and injury in the liver. Metabolomic analysis indicated that hUC-MSCs significantly ameliorated liver metabolic disturbances via their regulatory effect on several key metabolic pathways related to carbohydrate, amino acid, and lipid metabolism. Receiver operating characteristic curve analysis revealed that 70 differential metabolites had good diagnostic value for DM with MAFLD and could effectively predict the therapeutic effect of hUC-MSCs. Moreover, Spearman correlation analysis confirmed that significant correlations existed between differential liver metabolites and the concentrations of biochemical markers (FBG, TG, alanine aminotransferase, aspartate aminotransferase).
hUC-MSCs alleviate liver metabolic disturbances in diabetic rats with MAFLD, thereby mitigating the pathological state of DM and slowing the progression of MAFLD.
Core Tip: Current treatment methods for diabetes mellitus (DM) with metabolic-associated fatty liver disease (MAFLD) remain limited, and there is an urgent need to identify novel and effective therapeutic strategies. In this study, a rat model of DM with MAFLD was established using streptozotocin, and human umbilical cord mesenchymal stem cell were admi
- Citation: Zhou KB, Nie L, Wang ML, Xiao DH, Zhang HY, Yang X, Liao DF, Yang XF. Human umbilical cord mesenchymal stem cells ameliorate liver metabolism in diabetic rats with metabolic-associated fatty liver disease. World J Stem Cells 2025; 17(5): 105266
- URL: https://www.wjgnet.com/1948-0210/full/v17/i5/105266.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v17.i5.105266
Diabetes mellitus (DM) and metabolic-associated fatty liver disease (MAFLD) are prevalent metabolic disorders with a rising global incidence, posing a significant public health concern that urgently needs to be addressed[1]. MAFLD, formerly known as non-alcoholic fatty liver disease, can further progress to non-alcoholic steatohepatitis (NASH), characterized by hepatic fat accumulation, hepatocyte damage, and an inflammatory response. Without effective intervention, NASH can develop into liver fibrosis, cirrhosis, or even hepatocellular carcinoma[2]. Studies have shown that there is a close association between MAFLD and DM. The former represents the most frequently diagnosed liver disease among patients with DM, affecting approximately 55% of these individuals[3]. The coexistence of DM and MAFLD significantly increases the pathological burden of affected patients, primarily due to metabolic disorders, especially insulin resistance[4]. Insulin resistance not only affects carbohydrate metabolism but also promotes abnormal fat accumulation in the liver, driving the occurrence and progression of MAFLD. Meanwhile, the dysregulation of hepatic carbohydrate and lipid metabolism further exacerbates the pathological state of DM[5,6].
Current treatment options for DM and MAFLD primarily rely on pharmacological intervention and lifestyle modifications; however, these conventional therapies have limitations. Although progress has been made in blood glucose control for patients with DM, many relevant medications have significant side effects, which severely influences patient adherence to treatment[7]. For MAFLD, there are currently no approved therapeutic drugs, and treatment strategies still mainly focus on lifestyle improvements[8]. These observations highlight the urgent need to find new treatment strategies that can effectively improve the pathological state of patients with DM complicated with MAFLD.
Mesenchymal stem cells (MSCs) are mesoderm-derived adult stem cells widely distributed in tissues such as bone marrow, umbilical cord, and adipose tissue. MSCs have the potential for self-renewal and multilineage differentiation and have shown broad application prospects, especially in immune modulation, tissue repair, and regenerative medicine[9]. Over recent years, MSCs have garnered significant attention in the treatment of metabolic diseases, particularly DM and MAFLD. For instance, bone marrow-derived MSCs can effectively lower blood glucose levels, improve liver steatosis and function, and restore disrupted carbohydrate and lipid metabolism[10]. Compared to other types of MSCs, those obtained from human umbilical cord (hUC-MSCs) exhibit better clinical application potential owing to their ease of procurement and reduced risk of immunogenic rejection[11]. Additionally, hUC-MSCs have demonstrated therapeutic efficacy in NASH by improving metabolic disorders, alleviating liver steatosis, and reducing liver inflammation and fibrosis[12]. This suggests that the use of hUC-MSCs may constitute a novel therapeutic strategy for DM with MAFLD through metabolic modulation.
In this study, we investigated the therapeutic effects of hUC-MSCs in DM with MAFLD and sought to identify the potential mechanisms of action. Additionally, we evaluated the impact of hUC-MSCs on body weight, fasting blood glucose (FBG), serum triglyceride (TG) levels, and pathological changes in the livers of rats. Employing non-targeted hepatic metabolomics, we further explored the regulatory effects of hUC-MSCs on liver metabolism. Our findings provide novel insights and strategies for the treatment of DM with MAFLD.
Primary hUC-MSCs, derived from human umbilical cord Wharton’s jelly, were purchased from Original Cloud Medical Technology Co., Ltd (Foshan, China). These cells expressed CD44, CD73, and CD105, while being negative for HLA-DR, CD45, and CD34, and were found to be free of microbial contamination. The hUC-MSCs were cultured in α-MEM complete medium (PM150421, Procell, Wuhan, China) containing 10% fetal bovine serum (IC-1908, InCellGene, Germany) and 1% penicillin-streptomycin (PB180120, Procell, Wuhan, China). The cells were cultured in an incubator at 37 °C and with 5% CO2. At 80%-90% confluence, the cells were passaged at a 1:2 ratio, with the medium being replaced every 3 days. hUC-MSCs at generations three and five were selected for tail vein injection in rats.
After discarding the cell culture medium, the cells were washed 2-3 times with phosphate buffered saline, the residual liquid was removed, and 1 mL of trypsin was added for digestion. After 2 minutes of digestion, 2 mL of α-MEM complete medium was added to terminate the reaction. The cells were then transferred to a centrifuge tube and centrifuged at 3000 rpm/minutes for 5 minutes. The supernatant was then discarded and the cells were resuspended in phosphate buffered saline to a concentration of 1 × 106 cells/mL. The prepared cell suspension was used for tail vein injection in rats and was administered promptly to ensure cell viability.
Specific pathogen-free-grade male Sprague-Dawley rats, aged 6-8 weeks and weighing 200 ± 20 g, were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd (101, Beijing, China). The rats were housed in a standard environment (temperature: 23 ± 2 °C; relative humidity: 55%-60%; 12-hour/12-hour light/dark cycle) and had free access to food and water. Before experiments, all the rats underwent a one-week acclimatization period. This study was approved by the Animal Ethics Committee of the Affiliated Nanhua Hospital, University of South China (Ethics Number: 2024-KY-239), and strictly followed the guidelines of the Guide for the Care and Use of Laboratory Animals.
Before the experiment, all rats were fasted for 12 hours while retaining free access to water. Subsequently, the body weight of each rat was recorded using an electronic scale, and their blood glucose levels in tail-tip blood samples were measured using a Roche glucometer (ACCU-CHEK, Shanghai, China). Twenty-four rats were then randomly divided into a control group (n = 8) and a DM group (n = 16).
All the rats in the DM group underwent DM model induction using the following procedure: Citric acid buffer (pH = 4.5) was prepared by mixing a citric acid solution [2.1 g of citric acid (60347ES25, YEASEN, Shanghai, China) in 100 mL of distilled water] and a sodium citrate solution [2.94 g of sodium citrate (60348ES25, YEASEN, Shanghai, China) in 100 mL of distilled water] at a ratio of 1:1.32. Then, streptozotocin (STZ; S8050, Solarbio, Beijing, China) was dissolved in the citric acid buffer, yielding a 2% (w/v) STZ solution. After filtering through a 0.22-μm filter membrane (84301ES03, YEASEN, Shanghai, China) for sterilization, the STZ solution was covered in aluminum foil and stored on ice. Rats in the DM group were given a single intraperitoneal injection of freshly prepared STZ solution (60 mg/kg) and subsequently allowed to resume feeding. A Roche glucometer was used to measure blood glucose levels in rat tail-tip blood samples after 72 hours of injection and then consecutively for 3 days. Blood glucose levels ≥ 16.7 mmol/L on three measurements indicated that the DM model had been successfully established, which occurred with all the rats in the DM group in this study. Rats in the control group were simultaneously given an equal volume of citrate buffer via intraperitoneal injection. After modeling, the rats in the DM group were provided a high-fat, high-sugar diet (12451M, Beijing Boaigang Biological Technology Co., Ltd, Beijing, China), while those in the control group continued receiving a normal diet until the end of the experiment.
On week 10 of the experiment, the 16 rats in the DM group were randomly divided into a DM group (n = 8) and a DM + hUC-MSCs group (n = 8). Rats in the DM group received no treatment, while those in the DM + hUC-MSCs group were injected via the tail vein with 1 mL of a hUC-MSC suspension at a concentration of 1 × 106 cells/mL. Before injection, the cell suspension was gently mixed using a pipette to avoid cell clumping or sedimentation, which could lead to vascular embolism. During the entire experiment, all the rats were fasted for 12 hours every Wednesday, their body weights were recorded, and their FBG levels were measured using the Roche glucometer. If the blood glucose level exceeded the upper limit of the glucometer (33.3 mmol/L), it was recorded as 33.3 mmol/L. During the experiment, one rat from the DM group died due to complications, including ketosis and infection.
At the end of week 14 of the experiment, all the rats were euthanized with an overdose of sodium pentobarbital. Blood samples were collected from the heart, and serum was separated for the measurement of the levels of TG, alanine aminotransferase (ALT), and aspartate aminotransferase (AST). Liver tissue was also collected, with one portion placed in an Eppendorf tube and stored at -80 °C for metabolomics analysis, and the other fixed in 4% paraformaldehyde (P0099, Beyotime, Shanghai, China) for histological examination.
After fixation in 4% paraformaldehyde, liver tissue was routinely paraffin-embedded, sectioned into 5-μm-thick slices, and subjected to hematoxylin and eosin staining using a hematoxylin and eosin staining kit (C0105S, Beyotime, Shanghai, China). Briefly, after deparaffinization and rehydration, the sections were first stained with hematoxylin for 3 minutes, washed with tap water, differentiated in hydrochloric acid ethanol (C0163S, Beyotime, Shanghai, China) for 8 seconds, washed with distilled water for 8 seconds, and treated with 0.25% ammonia water for 8 seconds. The sections were then stained with eosin for 1 minutes, dehydrated, cleared, mounted, and finally observed and imaged under an optical microscope (Olympus, Tokyo, Japan).
Frozen liver samples were thawed on ice and homogenized for 20 seconds at 30 Hz using a grinding machine. Twenty milligrams of the sample was mixed with 400 μL of a solution containing internal standards (methanol:water = 7:3), and the mixture was vortexed at 1500 rpm/minutes for 5 minutes, left to stand on ice for 15 minutes, and centrifuged at 12000 rpm/minutes for 10 minutes at 4 °C. A 300-μL volume of the supernatant was stored at -20 °C for 30 minutes and then centrifuged again at 12000 rpm/minute for 3 minutes at 4 °C. Finally, 200 μL of the supernatant was collected for liquid chromatography-mass spectrometry analysis.
For mass spectrometry, all the samples were analyzed in both positive and negative ion modes. Solvent A was 0.1% formic acid in water and solvent B was 0.1% formic acid in acetonitrile. A Waters ACQUITY Premier HSS T3 Column (1.8 μm, 2.1 mm × 100 mm) was used for gradient elution. Data were collected using Analyst TF 1.7.1 software (Sciex, Concord, ON, Canada) in information-dependent acquisition mode. The gradient elution program and data acquisition parameters were as previously described[13].
After performing unit variance scaling on the data, principal component analysis was conducted using the prcomp function in R software. Next, the data were log2-transformed and mean-centered, and then subjected to orthogonal partial least squares discriminant analysis (OPLS-DA) using the MetaboAnalystR package in R software, with 200 permutation tests conducted to prevent overfitting. The criteria for the selection of differential metabolites were VIP > 1 and P < 0.05. Correlation heatmaps, chord diagrams, and correlation network diagrams of the top 50 differential metabolites ranked by VIP value were generated using the ComplexHeatmap, circlize, and igraph packages in R software, respectively. Differential metabolites were annotated using the Kyoto Encyclopedia of Genes and Genomes (KEGG) compound database (http://www.kegg.jp/kegg/compound/), and pathway enrichment analysis was per
Quantitative data were analyzed using GraphPad Prism 10.1.2. Data are presented as means ± SE. For comparisons between two groups, t-tests were used, while for comparisons among three or more groups, one-way analysis of variance (ANOVA) with non-parametric tests was employed. Associations were assessed using Spearman correlation analysis. For comparisons between two groups, P < 0.05 was considered statistically significant.
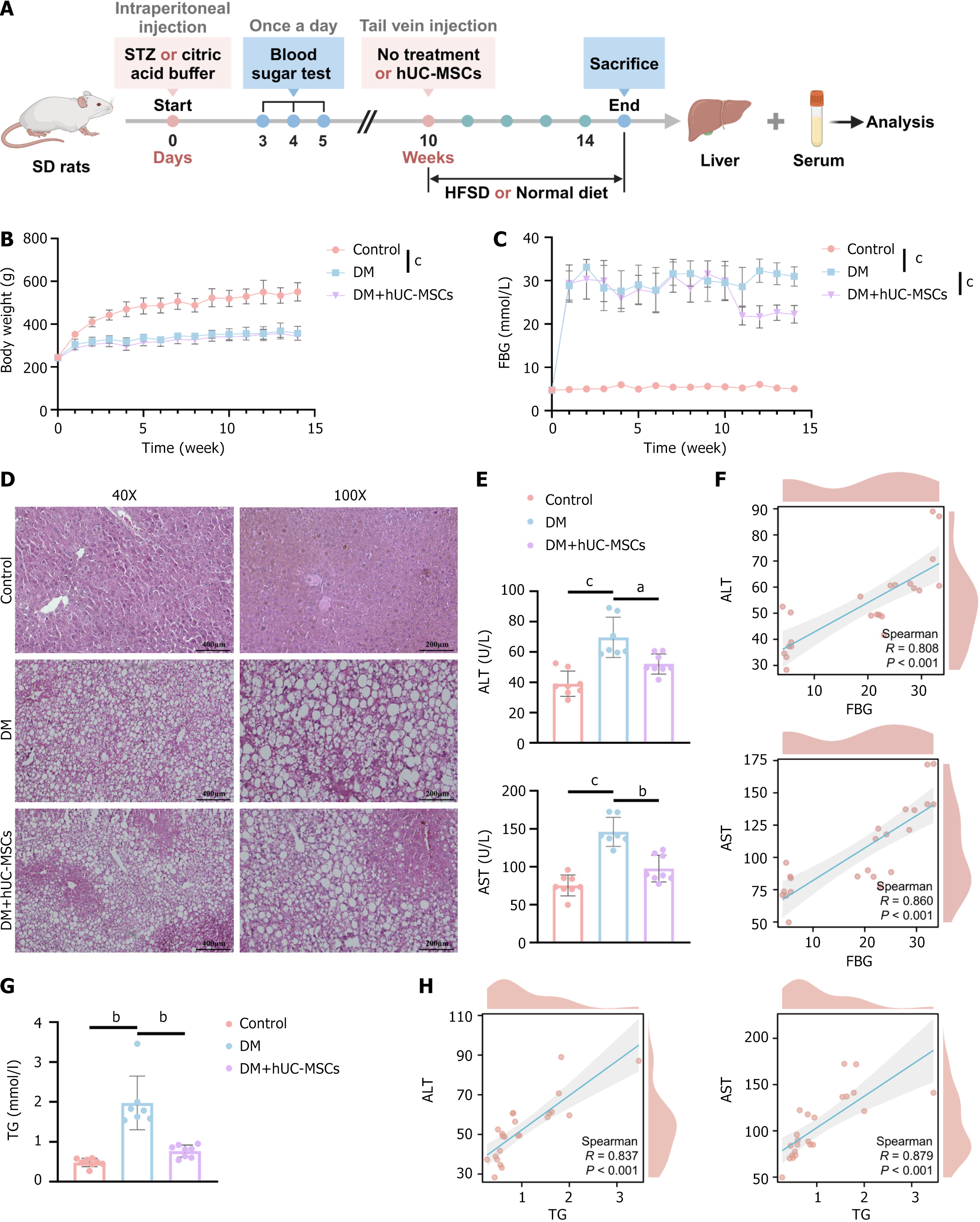
To assess the effects of hUC-MSCs on body weight and FBG levels in DM rats, we first established a DM rat model via the intraperitoneal injection of STZ, and then administered hUC-MSCs through tail vein injection (Figure 1A). Compared to control animals, rats in the DM group showed a significant decrease in body weight and a marked increase in FBG levels (Figure 1B and C), indicating that the DM model had been successfully established. However, hUC-MSC treatment significantly reduced FBG levels in DM rats, but did not affect their body weight (Figure 1B and C). Notably, fat vacuoles were apparent in the livers of DM rats (Figure 1D), while the serum levels of the liver injury markers ALT and AST were significantly elevated relative to those detected in the controls (Figure 1E), indicative of the presence of MAFLD in the DM rats. Compared to the DM group, the DM + hUC-MSCs group showed a significant reduction in the number of liver fat vacuoles and a marked decrease in ALT and AST concentrations (Figure 1D and E). These results suggested that hUC-MSCs can inhibit the progression of DM-related MAFLD. Additionally, Spearman correlation analysis showed that FBG levels were significantly and positively correlated with ALT (r = 0.808, P < 0.001) and AST (r = 0.860, P < 0.001) contents (Figure 1F), indicating that hUC-MSCs can alleviate liver injury by lowering blood glucose levels. Furthermore, hUC-MSC intervention was found to reduce the abnormally elevated TG levels in DM rats (Figure 1G). As anticipated, TG levels exhibited a significant positive correlation with ALT (r = 0.837, P < 0.001) and AST (r = 0.879, P < 0.001) concentrations. These findings suggested that hUC-MSCs can attenuate liver injury by lowering TG levels (Figure 1H). In conclusion, the above data suggested that hUC-MSCs can effectively reduce blood glucose and TG levels and improve steatosis and injury in the livers of rats with coexisting DM and MAFLD.
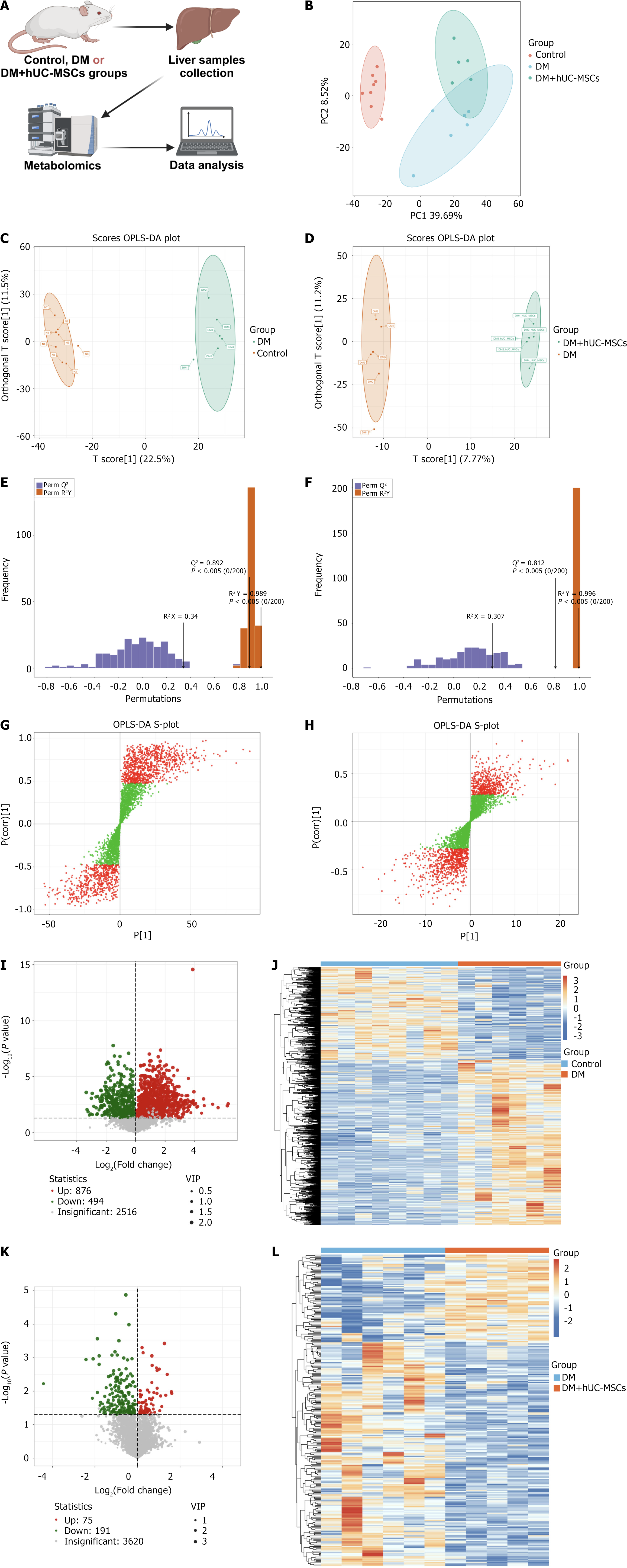
We performed an untargeted metabolomics analysis on liver samples from the control, DM, and DM + hUC-MSCs groups to further evaluate the regulatory effects of hUC-MSCs on liver metabolism in DM rats with MAFLD (Figure 2A). Principal component analysis showed clear clustering within each group and significant separation between groups, reflecting the high stability and reliability of the metabolomic data (Figure 2B). OPLS-DA further confirmed that there were marked metabolic differences between the control and DM groups (Figure 2C) and between the DM and DM + hUC-MSCs groups (Figure 2D). The stability and reliability of the model were validated through OPLS-DA (Figure 2E and F). Based on this analysis, we initially screened metabolites with VIP values > 1 between groups (Figure 2G and H) and further selected those with P values of < 0.05 as significant differential metabolites. A total of 1370 and 266 significant differential metabolites were identified between the control and DM groups (Figure 2I and J) and between the DM and DM + hUC-MSCs groups, respectively (Figure 2K and L).
In addition, we performed a correlation analysis on the top 50 differential metabolites, ranked by VIP value, in the different comparison groups, and constructed correlation heatmaps, chord diagrams, and network graphs (Supplementary Figure 1). We found that the network of interrelationships among the differential metabolites in the DM vs control comparison group mainly consisted of fatty acids (FAs), amino acids, and their metabolites, while the network of differential metabolites in the DM vs DM + hUC-MSCs comparison group mainly involved amino acids and their metabolites, glycerophospholipids, and FAs (Supplementary Figure 1). These results further revealed the metabolic characteristics of DM with MAFLD and suggested that hUC-MSC treatment exerted its effect by modulating the networks of these significantly differential metabolites. Taken together, these results indicated that hUC-MSC intervention significantly modulated the liver metabolic profile in rats with coexisting DM and MAFLD, suggesting that these cells may significantly contribute to improving abnormal metabolism.
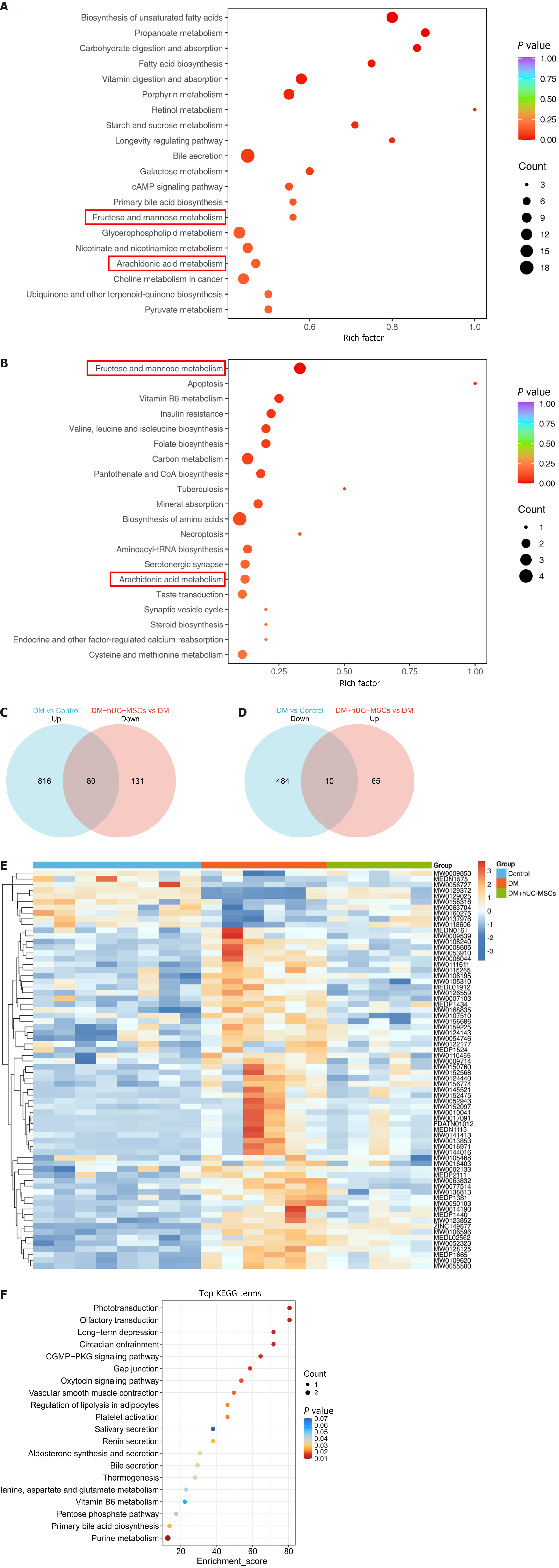
To elucidate the mechanisms underlying the development of DM-related MAFLD and the metabolic characteristics associated with hUC-MSC intervention, we performed a KEGG pathway enrichment analysis on the differential metabolites among the different groups. The results showed that the differential metabolites in both the DM group vs control group comparison and the DM + hUC-MSCs group vs DM group comparison were significantly enriched in two metabolic pathways, namely, fructose and mannose metabolism and arachidonic acid metabolism (Figure 3A and B). This suggested that hUC-MSCs improve abnormal liver metabolism in rats with DM and MAFLD through their regulatory effects on these key metabolic pathways.
We further clarified the regulatory effects of hUC-MSC treatment on specific metabolites through Venn diagram analysis. The results showed that hUC-MSC treatment reversed the increase in the levels of 60 metabolites whose abundance was elevated in the DM group (Figure 3C) and restored the levels of 10 metabolites whose contents were reduced in the DM group (Figure 3D). The changes in abundance and the regulation (up/down) trends for these 70 metabolites are shown in Figure 3E and Table 1. The above findings suggested that hUC-MSCs improve the abnormal liver metabolism observed in rats with coexisting DM and MAFLD by modulating the contents of these key metabolites.
| Index1 | Compound2 | DM vs control | DM + hUC-MSCs vs DM | ||
| Log2FC3 | Type4 | Log2FC3 | Type4 | ||
| MW0017091 | Cholic acid | 6.224 | Up | -1.595 | Down |
| MW0150760 | Herbimycin | 0.779 | Up | -0.508 | Down |
| MEDL01912 | Succinic acid semialdehyde | 0.259 | Up | -0.401 | Down |
| MW0109620 | Ser-Leu | 2.086 | Up | -0.753 | Down |
| MW0107510 | Ile-Ser | 0.564 | Up | -1.202 | Down |
| MW0108240 | Methoxyacetic acid | 1.678 | Up | -1.124 | Down |
| MEDL02562 | Euscaphic acid | 0.592 | Up | -0.211 | Down |
| MEDN1113 | 12-ketolithocholic acid | 5.594 | Up | -1.757 | Down |
| MW0002133 | 2,3-dcpe hydrochloride | 0.380 | Up | -0.226 | Down |
| MW0110455 | Val-Val | 0.590 | Up | -0.369 | Down |
| FDATN01012 | Sodium cholate | 2.152 | Up | -1.258 | Down |
| MW0124440 | Ipriflavone | 1.105 | Up | -1.073 | Down |
| MEDP2111 | 2’-hydroxy-4,4’,6’-trimethoxychalcone | 0.444 | Up | -0.389 | Down |
| MW0052323 | Diatoxanthin | 0.396 | Up | -0.164 | Down |
| MEDP1440 | Carnitine C5:0 | 1.640 | Up | -0.819 | Down |
| MW0105468 | Ala-Ile | 0.444 | Up | -0.543 | Down |
| MW0106195 | Cyanophos | 0.596 | Up | -0.332 | Down |
| MW0053910 | Carnitine C6:0 | 1.386 | Up | -1.118 | Down |
| MW0010041 | Vardenafil | 1.858 | Up | -1.180 | Down |
| MW0055500 | Levocarnitine propionate | 2.527 | Up | -0.808 | Down |
| MEDP1665 | Carnitine C3:0 | 2.093 | Up | -0.950 | Down |
| MW0123852 | Erythrosine | 1.865 | Up | -0.943 | Down |
| MW0063832 | Tetrahydropersin | 2.502 | Up | -1.804 | Down |
| MW0052943 | Fulvestrant | 2.365 | Up | -2.123 | Down |
| MW0006044 | Anastrozole | 2.351 | Up | -2.422 | Down |
| MW0122177 | (6aR,10aR)-9-(hydroxymethyl)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6a,7,10,10a-tetrahydrobenzo[c]chromen-1-ol | 0.793 | Up | -0.745 | Down |
| MW0014190 | 3-methylglutarylcarnitine | 1.446 | Up | -0.787 | Down |
| MW0111511 | D-fructofuranose | 1.023 | Up | -0.630 | Down |
| MW0016403 | Carnitine C5:1 | 0.826 | Up | -0.826 | Down |
| MEDP1381 | Carnitine C20:2 | 1.525 | Up | -0.847 | Down |
| MEDP1524 | Carnitine C16:2 isomer 1 | 0.950 | Up | -0.671 | Down |
| MEDP1434 | Carnitine C4:DC | 1.891 | Up | -0.786 | Down |
| MW0124143 | Fluphenazine sulfoxide | 0.284 | Up | -0.098 | Down |
| MW0145521 | Arg-Thr-Leu-Ser-Asp | 2.949 | Up | -1.640 | Down |
| MW0050103 | 1,2-dioleoyl-sn-glycerol | 3.323 | Up | -1.571 | Down |
| ZINC149577 | 2-methoxycinnamic acid | 3.876 | Up | -0.198 | Down |
| MW0159225 | Val-Tyr-Pro-Glu-Leu | 0.230 | Up | -0.128 | Down |
| MW0152568 | Leu-Val-Phe-Ala-Ile | 2.991 | Up | -1.349 | Down |
| MW0156774 | Ser-His-Glu-Ala-Glu | 0.955 | Up | -0.383 | Down |
| MW0156686 | Ser-Asp-Ser-Gly-Val | 0.537 | Up | -0.767 | Down |
| MW0126559 | SIN-1 hydrochloride | 0.337 | Up | -0.547 | Down |
| MW0105310 | 5-hydroxy-2-oxo-4-ureido-2,5-dihydro-1H-imidazole-5-carboxylate | 0.226 | Up | -0.306 | Down |
| MW0077514 | TG(18:3(9Z,12Z,15Z)/14:0/20:5(5Z,8Z,11Z,14Z,17Z)) | 1.519 | Up | -1.887 | Down |
| MW0054746 | Melleolide M | 0.218 | Up | -0.116 | Down |
| MW0013853 | 3,7,11,15,23-pentaoxolanost-8-en-26-oic acid | 4.610 | Up | -1.426 | Down |
| MW0106596 | Enterobactin | 1.636 | Up | -0.400 | Down |
| MW0009714 | Salmeterol | 0.206 | Up | -0.227 | Down |
| MW0007103 | F-amidine (trifluoroacetate salt) | 0.314 | Up | -0.328 | Down |
| MW0138813 | Loxoprofen | 0.605 | Up | -0.436 | Down |
| MW0168835 | Beta-D-galactose | 0.719 | Up | -0.458 | Down |
| MW0008605 | N-[(4-hydroxy-3-methoxyphenyl)methyl]octanamide | 0.290 | Up | -0.260 | Down |
| MW0016971 | Celastrol | 3.881 | Up | -1.410 | Down |
| MW0115265 | Sedoheptulose 7-phosphate | 0.817 | Up | -0.783 | Down |
| MW0141413 | 15(R)-17-phenyl trinor prostaglandin F 2alpha isopropyl ester | 2.776 | Up | -1.706 | Down |
| MEDN0161 | Guanosine 3’,5’-cyclic monophosphate | 0.286 | Up | -0.278 | Down |
| MW0152475 | Leu-Pro-Val-Leu-Glu | 3.248 | Up | -1.645 | Down |
| MW0009539 | Tyrphostin A25 | 0.322 | Up | -0.294 | Down |
| MW0152097 | L-a-lysophosphatidylserine | 2.594 | Up | -1.768 | Down |
| MW0128125 | {3-[8-(1-{2,4-dihydroxyphenyl}-3-{3,4-dihydroxyphenyl}-2-hydroxypropyl)-3,5,7-trihydroxy-3,4-dihydro-2H-1-benzopyran-2-yl]phenyl}oxidanesulfonic acid | 1.722 | Up | -0.535 | Down |
| MW0144016 | 8,12-diethyl-3-vinylbacteriochlorophyllide d | 3.297 | Up | -1.267 | Down |
| MW0056727 | Paeoniflorin | -2.291 | Down | 0.768 | Up |
| MW0009853 | Telmisartan | -0.497 | Down | 0.469 | Up |
| MW0129372 | {6-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-6-yl]-4,5-dihydroxy-2-(hydroxymethyl)oxan-3-yl}oxidanesulfonic acid | -1.983 | Down | 1.615 | Up |
| MW0063704 | Sulfaphenazole | -0.186 | Down | 0.168 | Up |
| MEDN1575 | Rhapontigenin | -1.550 | Down | 0.605 | Up |
| MW0137976 | Dihydromyricetin | -0.258 | Down | 0.214 | Up |
| MW0158316 | Trp-Val | -0.367 | Down | 0.177 | Up |
| MW0118606 | 2-hydroxy-4,7-dimethoxy-2H-1,4-benzoxazin-3(4H)-one | -0.156 | Down | 0.128 | Up |
| MW0160275 | D-Glycero-D-manno-heptose 1-phosphate | -0.559 | Down | 0.298 | Up |
| MW0129025 | {2-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-6-yl]-3-hydroxy-6-methyl-5-oxooxan-4-yl}oxidanesulfonic acid | -1.801 | Down | 1.597 | Up |
Further KEGG pathway enrichment analysis revealed that these key differential metabolites were primarily associated with multiple metabolic pathways, including: (1) Alanine, aspartate and glutamate metabolism; (2) Pentose phosphate pathway; (3) Primary bile acid biosynthesis; and (4) Purine metabolism (Figure 3F). These pathways are involved in important biological processes such as amino acid metabolism, carbohydrate metabolism, lipid metabolism, and nucleotide metabolism (Supplementary Table 1). These results further supported that hUC-MSCs improve the patho
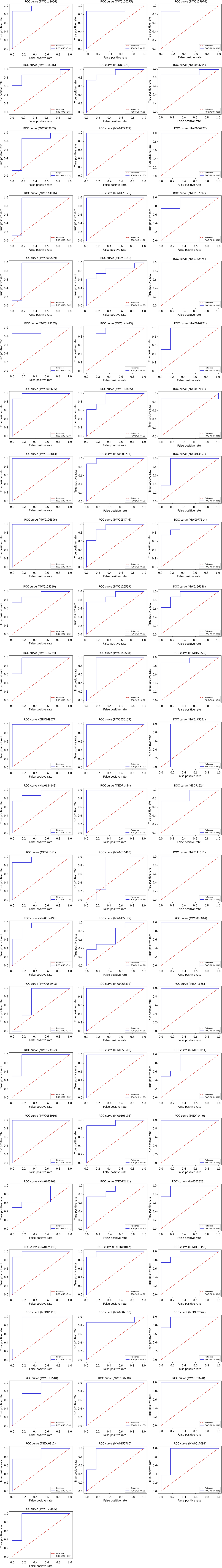
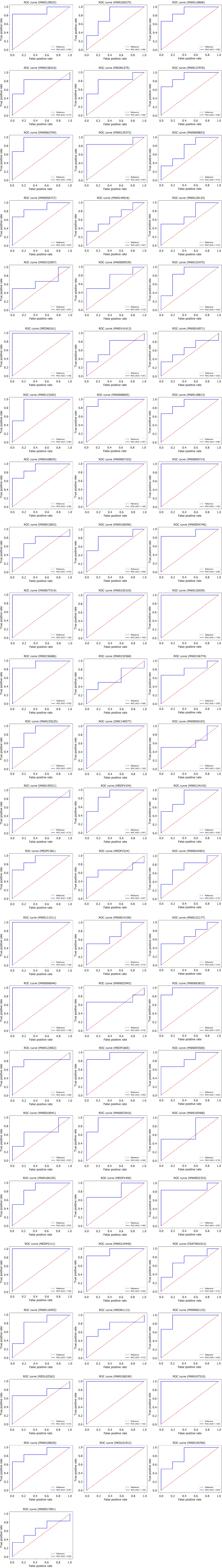
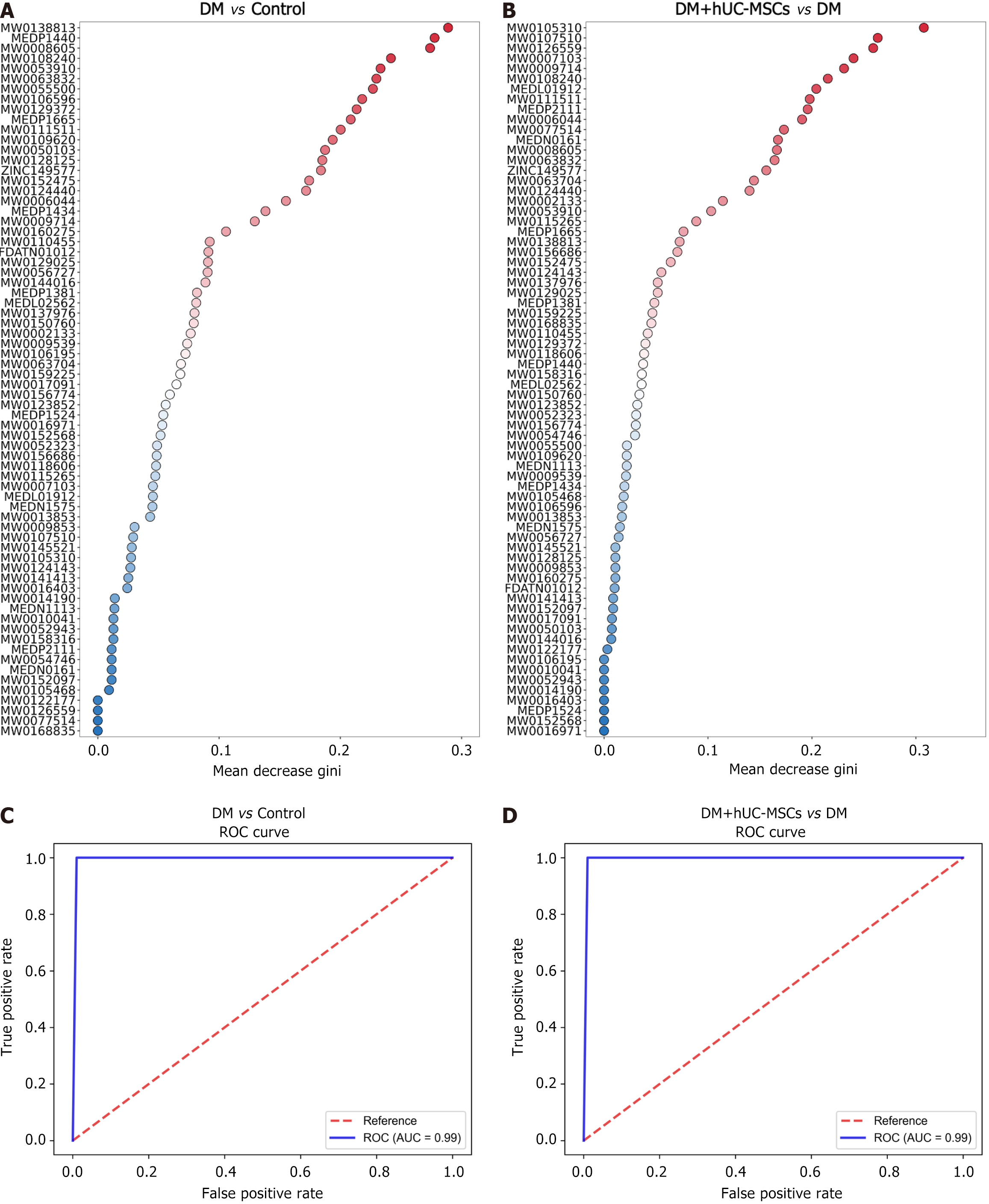
Receiver operating characteristic curve analysis showed that the 70 key differential metabolites exhibited high sensitivity and specificity in predicting DM with MAFLD, highlighting their potential as biomarkers [Figure 4, area under the curve (AUC) > 0.7]. However, some metabolites demonstrated relatively limited predictive ability when distinguishing the DM + hUC-MSCs treatment group from the DM group (Figure 5, 0.6 ≤ AUC < 0.7). Accordingly, we next applied a random forest algorithm to analyze the importance of these key differential metabolites (Figure 6A and B) and constructed a composite receiver operating characteristic curve for these metabolites based on this model. The analysis revealed that the model accurately distinguished the control group from the DM group (Figure 6C, AUC = 0.99) and predicted the metabolic changes after hUC-MSC treatment with high sensitivity (Figure 6D, AUC = 0.99), thereby significantly improving the diagnostic accuracy for DM with MAFLD as well as the prediction of the therapeutic effects of hUC-MSCs.
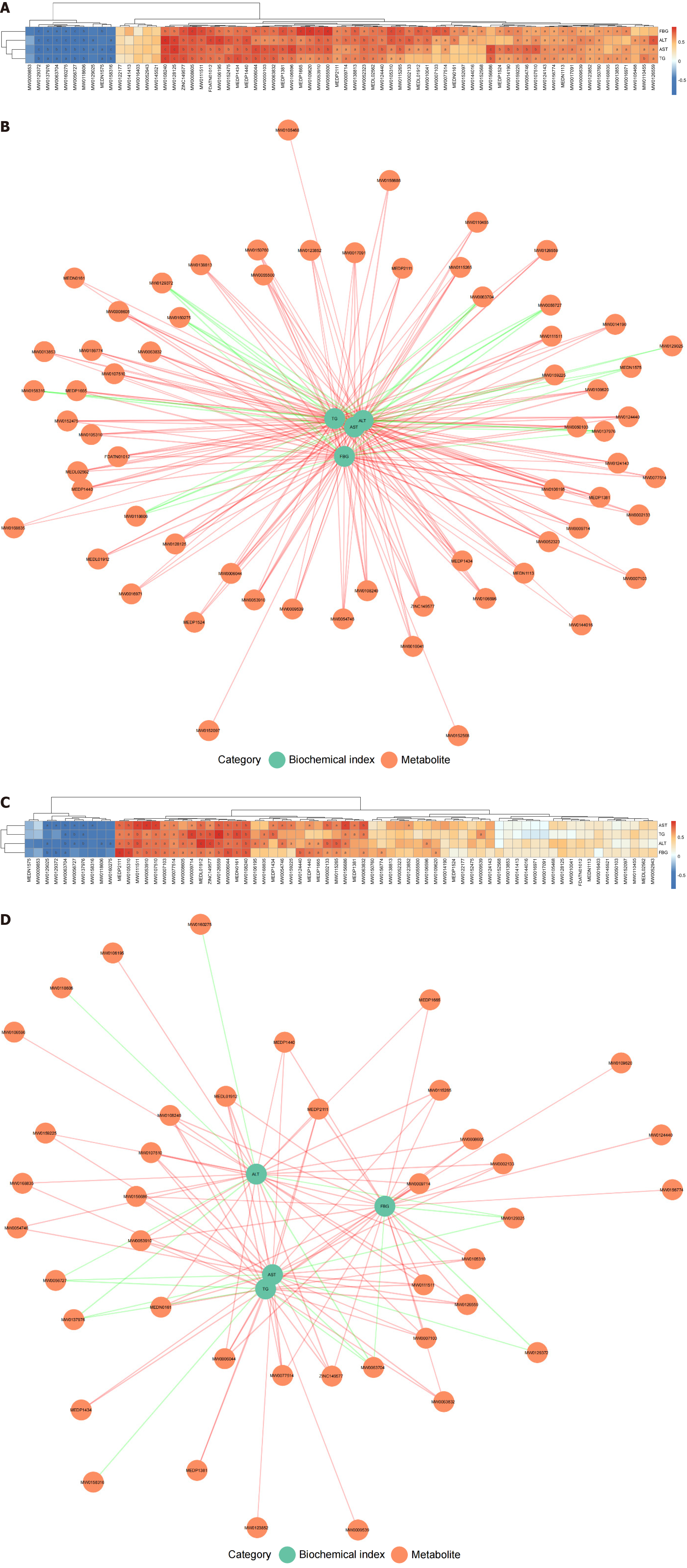
Subsequently, we performed a Spearman correlation analysis to assess the relationship between biochemical indicators (FBG, TG, ALT, and AST) and changes in liver metabolite abundance in rats with DM and MAFLD. In the DM and control groups, FBG, TG, ALT, and AST levels were significantly correlated with 58, 53, 55, and 57 differential metabolites, respectively (P < 0.05) (Figure 7A and B, Supplementary Table 2). In the DM + hUC-MSCs and DM groups, the FBG, TG, ALT, and AST contents were significantly correlated with 27, 24, 31, and 30 differential metabolites, respectively (P < 0.05) (Figure 7C and D, Supplementary Table 3). These results suggested that liver metabolites play an important regulatory role in the occurrence of hyperglycemia, hyperlipidemia, and MAFLD-related liver injury while also significantly contributing to the therapeutic effects of hUC-MSCs.
DM and MAFLD are among the most common chronic metabolic diseases worldwide[1]. The coexistence of DM with MAFLD greatly diminishes the quality of life of affected patients and, given its increasing incidence, imposes a significant burden on healthcare systems. Current treatment options remain limited, underscoring the urgent need to identify novel and effective therapeutic strategies for this condition[3]. hUC-MSCs have attracted considerable interest as a potential treatment for metabolic diseases owing to their wide availability and strong differentiation potential, along with limited ethical concerns[11]. However, their therapeutic effects against DM with MAFLD have not been fully explored, and the potential underlying mechanisms remain poorly understood. In this study, we sought to fill this knowledge gap by systematically evaluating the effects of hUC-MSCs on liver metabolism in DM rats with MAFLD, thus providing novel intervention strategies for the treatment of this condition.
It has been shown that blood glucose levels are significantly elevated in DM rats, while the body weight of the animals is markedly reduced. Additionally, hyperglycemia caused by DM can lead to liver damage and fat deposition[14]. In this study, we observed liver steatosis in DM rats, along with elevated levels of the liver injury markers ALT and AST. These changes confirmed the successful establishment of the rat model of DM with MAFLD, consistent with prior research findings[15]. However, hUC-MSC treatment significantly mitigated the increase in FBG levels recorded in the model rats and improved liver steatosis and liver injury. These results align with the findings of Xu et al[16] and suggest that hUC-MSCs exert a significant therapeutic effect in DM-related MAFLD, inhibiting its progression. However, a single injection of hUC-MSCs did not significantly affect the body weight of the model rats, suggesting that weight recovery may require increased frequency of cell injections, greater cell numbers, or prolonged treatment duration. Notably, we observed that FBG levels were positively correlated with the concentrations of the liver injury markers ALT and AST, further suggesting that hUC-MSCs can effectively alleviate liver damage by regulating blood glucose levels.
There is increasing evidence that metabolic dysregulation is a key driver of DM with MAFLD[15,17-19]. Under physiological conditions, elevated blood glucose stimulates insulin secretion, which promotes glycogen synthesis and lipogenesis while inhibiting hepatic gluconeogenesis[20]. Gluconeogenesis is the primary pathway for endogenous glucose production[21]. However, in the insulin-resistant state induced by DM with MAFLD, the liver cannot effectively utilize insulin for carbohydrate metabolism, leading to increased gluconeogenesis and FA synthesis, which results in the characteristic hyperglycemia and hyperlipidemia of DM. The excessive entry of free FAs into the liver promotes fat accumulation, further exacerbating the occurrence and progression of MAFLD[22,23]. We found that hUC-MSCs can reverse the elevated TG levels in diabetic rats with MAFLD. Moreover, TG levels exhibited a positive correlation with the ALT and AST contents, further indicating that hUC-MSCs can effectively attenuate liver injury by ameliorating hyperlipidemia. Amino acid metabolism also plays an important role in maintaining blood glucose balance by promoting protein synthesis and influencing insulin sensitivity[24]. In this study, employing untargeted metabolomics, we found that hUC-MSCs significantly mitigated the abnormal increase in the levels of liver metabolites in DM with MAFLD model rats. These metabolites were primarily associated with key biological processes such as amino acid, carbohydrate, and lipid metabolism. Notably, the liver metabolites exhibiting differential abundance were significantly correlated with FBG, TG, ALT, and AST concentrations. This further revealed that hUC-MSCs can effectively alleviate the pathological state of DM with MAFLD by restoring the metabolic balance in the liver, particularly in terms of amino acid, carbohydrate, and lipid metabolism.
Although this study demonstrated the therapeutic potential of hUC-MSCs in a rat model of DM with MAFLD, it nevertheless had some limitations. First, validation was limited to rats, and the efficacy of hUC-MSCs in DM with MAFLD requires further confirmation through the use of other animal models and clinical trials. Second, this study primarily focused on the short-term therapeutic effects of hUC-MSCs, and their long-term efficacy and potential side effects need further evaluation.
In conclusion, hUC-MSCs effectively improve liver metabolic disorders in a rat model of DM with MAFLD, thereby inhibiting the progression of the condition. This study provides a theoretical basis for the clinical application of hUC-MSCs in the treatment of DM with MAFLD.
| 1. | Chew NWS, Ng CH, Tan DJH, Kong G, Lin C, Chin YH, Lim WH, Huang DQ, Quek J, Fu CE, Xiao J, Syn N, Foo R, Khoo CM, Wang JW, Dimitriadis GK, Young DY, Siddiqui MS, Lam CSP, Wang Y, Figtree GA, Chan MY, Cummings DE, Noureddin M, Wong VW, Ma RCW, Mantzoros CS, Sanyal A, Muthiah MD. The global burden of metabolic disease: Data from 2000 to 2019. Cell Metab. 2023;35:414-428.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 352] [Article Influence: 176.0] [Reference Citation Analysis (0)] |
| 2. | Peiseler M, Schwabe R, Hampe J, Kubes P, Heikenwälder M, Tacke F. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease - novel insights into cellular communication circuits. J Hepatol. 2022;77:1136-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 293] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 3. | Kapoor N, Kalra S. Metabolic-Associated Fatty Liver Disease and Diabetes: A Double Whammy. Endocrinol Metab Clin North Am. 2023;52:469-484. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Nevola R, Epifani R, Imbriani S, Tortorella G, Aprea C, Galiero R, Rinaldi L, Marfella R, Sasso FC. GLP-1 Receptor Agonists in Non-Alcoholic Fatty Liver Disease: Current Evidence and Future Perspectives. Int J Mol Sci. 2023;24:1703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 127] [Reference Citation Analysis (0)] |
| 5. | Gastaldelli A, Cusi K. From NASH to diabetes and from diabetes to NASH: Mechanisms and treatment options. JHEP Rep. 2019;1:312-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 298] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 6. | Meex RCR, Watt MJ. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat Rev Endocrinol. 2017;13:509-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 458] [Article Influence: 57.3] [Reference Citation Analysis (2)] |
| 7. | Jiang Y, Yue R, Liu G, Liu J, Peng B, Yang M, Zhao L, Li Z. Garlic (Allium sativum L.) in diabetes and its complications: Recent advances in mechanisms of action. Crit Rev Food Sci Nutr. 2024;64:5290-5340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 8. | Venkatesan K, Haroon NN. Management of Metabolic-Associated Fatty Liver Disease. Endocrinol Metab Clin North Am. 2023;52:547-557. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG. Mesenchymal Stem Cell Migration and Tissue Repair. Cells. 2019;8:784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 562] [Cited by in RCA: 660] [Article Influence: 110.0] [Reference Citation Analysis (36)] |
| 10. | Wang W, Wu RD, Chen P, Xu XJ, Shi XZ, Huang LH, Shao ZL, Guo W. Liraglutide combined with human umbilical cord mesenchymal stem cell transplantation inhibits beta-cell apoptosis via mediating the ASK1/JNK/BAX pathway in rats with type 2 diabetes. Diabetes Metab Res Rev. 2020;36:e3212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Xie Q, Liu R, Jiang J, Peng J, Yang C, Zhang W, Wang S, Song J. What is the impact of human umbilical cord mesenchymal stem cell transplantation on clinical treatment? Stem Cell Res Ther. 2020;11:519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 12. | Yang Z, Xia Q, Lu D, Yue H, Zhang J, Li Y, Zhang B, Li X, Cao M. Human mesenchymal stem cells treatment improved hepatic lesions and reversed gut microbiome disorder in non-alcoholic steatohepatitis. Aging (Albany NY). 2020;12:21660-21673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Liang R, Shi W, Li T, Gao H, Wan T, Li B, Zhou X. Effect of exogenous calcitriol on myopia development and axial length in guinea pigs with form deprivation myopia. Sci Rep. 2024;14:11382. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Son Y, Lee H, Son SY, Lee CH, Kim SY, Lim Y. Ameliorative Effect of Annona muricata (Graviola) Extract on Hyperglycemia Induced Hepatic Damage in Type 2 Diabetic Mice. Antioxidants (Basel). 2021;10:1546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Zou W, Zhang C, Gu X, Li X, Zhu H. Metformin in Combination with Malvidin Prevents Progression of Non-Alcoholic Fatty Liver Disease via Improving Lipid and Glucose Metabolisms, and Inhibiting Inflammation in Type 2 Diabetes Rats. Drug Des Devel Ther. 2021;15:2565-2576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 16. | Xu X, Wang W, Lin L, Chen P. Liraglutide in combination with human umbilical cord mesenchymal stem cell could improve liver lesions by modulating TLR4/NF-kB inflammatory pathway and oxidative stress in T2DM/NAFLD rats. Tissue Cell. 2020;66:101382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Ferguson D, Finck BN. Emerging therapeutic approaches for the treatment of NAFLD and type 2 diabetes mellitus. Nat Rev Endocrinol. 2021;17:484-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 325] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 18. | Yuan Z, Qiao H, Wang Z, Wang H, Han M, Zhang W, Zhou Y, Hassan HM, Zhao W, Qin T. Taohe Chengqi decoction alleviated metabolic-associated fatty liver disease by boosting branched chain amino acids catabolism in the skeletal muscles of type 2 diabetes mellitus. Phytomedicine. 2024;126:155315. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Zhou M, Liu X, Wu Y, Xiang Q, Yu R. Liver Lipidomics Analysis Revealed the Protective mechanism of Zuogui Jiangtang Qinggan Formula in type 2 diabetes mellitus with non-alcoholic fatty liver disease. J Ethnopharmacol. 2024;329:118160. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Kubota T, Kubota N, Kadowaki T. Imbalanced Insulin Actions in Obesity and Type 2 Diabetes: Key Mouse Models of Insulin Signaling Pathway. Cell Metab. 2017;25:797-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 21. | Li H, Xu Q, Xu C, Hu Y, Yu X, Zhao K, Li M, Li M, Xu J, Kuang H. Bicyclol Regulates Hepatic Gluconeogenesis in Rats with Type 2 Diabetes and Non-alcoholic Fatty Liver Disease by Inhibiting Inflammation. Front Pharmacol. 2021;12:644129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Dewidar B, Kahl S, Pafili K, Roden M. Metabolic liver disease in diabetes - From mechanisms to clinical trials. Metabolism. 2020;111S:154299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 23. | Mu W, Cheng XF, Liu Y, Lv QZ, Liu GL, Zhang JG, Li XY. Potential Nexus of Non-alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Insulin Resistance Between Hepatic and Peripheral Tissues. Front Pharmacol. 2018;9:1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 24. | Gao C, Hou L. Branched chain amino acids metabolism in heart failure. Front Nutr. 2023;10:1279066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |