Published online Aug 14, 2025. doi: 10.3748/wjg.v31.i30.110401
Revised: June 26, 2025
Accepted: July 21, 2025
Published online: August 14, 2025
Processing time: 62 Days and 22.3 Hours
Acute-on-chronic liver failure (ACLF) is characterized by severe metabolic disturbances; however, the specific metabolomic features and their predictive value on 90-day prognosis remain unclear.
To identify serum metabolomic changes in patients with ACLF with different prognoses to support clinical prediction of outcomes and treatment decisions.
This non-interventional, observational case-control study enrolled 58 patients with ACLF. Fasting venous blood samples were analyzed using targeted metabolomics. Univariate and multivariate statistical analyses identified differential metabolites among 18 amino acids, 11 fatty acids, 5 gut microbiota-related metabolites, and 4 bile acid metabolites. Binary logistic regression identified independent mortality risk factors, visualized via forest plots and receiver operating characteristic curves.
Significant differences (P < 0.05) were observed between the death and survival groups in baseline age, model for end-stage liver disease score, model for end-stage liver disease with sodium, neutrophil-to-lymphocyte ratio (NLR), total bilirubin, serum creatinine, blood urea nitrogen, and platelet count. Metabolites, including L-carnitine, creatinine, alanine, arginine (Arg), proline, choline, and oleic acid, also showed statistically significant differences between the groups. Multivariate analysis identified age, NLR, and Arg as independent risk factors for 90-day mortality in patients with ACLF. The predictive model, age-NLR-Arg = -15.481 + 0.135 × age + 0.156 × NLR + 0.203 × Arg, with a cutoff of 0.759, achieved an area under the receiver operating characteristic curve of 0.945 with sensitivity of 84.0% and specificity of 87.9%.
The age-NLR-Arg model demonstrates a strong predictive value for 90-day mortality risk in patients with ACLF.
Core Tip: Acute-on-chronic liver failure (ACLF) is a rapidly progressing condition with high mortality and limited treatment options. Traditional prognostic models fail to capture its dynamic metabolic disturbances. This study identifies seven key metabolites linked to 90-day ACLF prognosis, with Arginine as an independent risk factor. Age- neutrophil-to-lymphocyte ratio-arginine model expressed perfect predictive efficiency for 90-day prognosis of patients with ACLF. In addition, artificial liver blood purification system treatment modulated alanine and L-carnitine, reducing inflammation and promoting liver regeneration. These findings highlight the potential of metabolomics to enhance ACLF prognosis, offering a more precise approach for clinical assessment and management.
- Citation: Liu Y, Xiao Y, Ai LF, Zhang JJ, Zhang JD, Qi ZQ, Dong L, Wang YD. Serum metabolomic characteristics and their predictive value for ninety-day prognosis in patients with acute-on-chronic liver failure. World J Gastroenterol 2025; 31(30): 110401
- URL: https://www.wjgnet.com/1007-9327/full/v31/i30/110401.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i30.110401
Acute-on-chronic liver failure (ACLF) is a critical clinical syndrome characterized by rapid deterioration in patients with relatively stable chronic liver disease due to acute insults, manifesting as jaundice, coagulopathy, hepatorenal syndrome, hepatic encephalopathy, and ascites[1]. ACLF is a severe, rapidly progressing condition with no effective pharmacological treatments, resulting in a 28/90-day mortality rate of 20%-80%[2,3], considerably impacting patients’ quality of life. Early and accurate prediction of ACLF prognosis is crucial for developing evidence-based, individualized treatments, optimizing resource allocation, and improving clinical outcomes. Metabolomics, which uses advanced analytical platforms to identify and quantify small-molecule metabolites in biological samples, facilitates early biomarker discovery and guides clinical management, making it the omics discipline closest to the biological phenotype[4]. The liver regulates the metabolism of carbohydrates, lipids, proteins, and bile acids, and severe metabolic disruption is a key mechanism in the pathogenesis of ACLF[5]. Our previous study found that as Child-Turcotte-Pugh grades increase, 3-phosphopyruvate levels rise, peaking at Child-Turcotte-Pugh-B before declining. This indicates a shift from glucose or pyruvate oxidation to lipid oxidation with disease progression driving cellular growth reliance from glycolysis to lipid and amino acid metabolism[6]. Therefore, metabolomics is valuable for understanding liver metabolic functions and supporting early diagnosis and prognosis of liver diseases.
Current clinical practice relies on traditional indicators and scoring systems, such as the model for end-stage liver disease (MELD), chronic liver failure-sequential organ failure assessment, and Chinese group on the study of severe hepatitis B models to assess ACLF prognosis. Given the severe immune, inflammatory, and metabolic disturbances in ACLF, integrating metabolomic characteristics can more precisely reflect dynamic disease changes, enhancing the sensitivity and specificity of prognostic predictions. This study aimed to develop a more scientific and accurate prognostic prediction model to guide the clinical management of patients with ACLF by assessing baseline-serum metabolite differences in patients with ACLF with varying 90-day outcomes and their correlation with the efficacy of artificial liver blood purification system (ALBPS) treatment using tandem mass spectrometry.
This single-center, prospective, non-interventional, observational case-control study included 58 patients with ACLF hospitalized at the Hebei Medical University Third Hospital between January 2023 and December 2024. ACLF diagnosis adhered to the 2019 Asia-Pacific Association for the Study of the Liver criteria[1]. All patients received standardized medical treatment (SMT), including etiology control, hepatoprotective therapy, and complication prevention. Treatment was individualized based on etiology, recovery status, tolerance, and adverse reactions, with adjustments per guideline. Of the 58 patients, 23 received ALBPS in addition to SMT, with treatment modalities and efficacy assessed per the 2022 Chinese Medical Association Hepatology Branch consensus[7]. Exclusion criteria included: (1) Presence of liver or other extrahepatic solid organ malignancies; (2) Coexisting metabolic disorders (e.g., metabolic syndrome, hemochromatosis, alpha-1 antitrypsin deficiency, glycogen storage disease, tyrosinemia, gout, and phenylketonuria); and (3) Pregnant or lactating women. This study was approved by the Medical Ethics Committee of The Hebei Medical University Third Hospital, approval No. W2023-043-1, and all procedures complied with the Declaration of Helsinki. Informed consent was obtained and documented from all participants before enrollment.
Clinical data included age, sex, underlying liver disease etiology, treatment regimen, and 90-day prognostic outcomes. Laboratory indices at initial ACLF diagnosis were collected, including: (1) Hematology: White blood cell count, neutrophils, lymphocytes, hemoglobin, platelets (PLT); (2) Serum biochemistry: Albumin, alanine transaminase, aspartate transaminase, alkaline phosphatase, γ-glutamyl transpeptidase, total bilirubin (TBil), serum creatinine (Scr), serum sodium (Na+); and (3) Coagulation: Activated partial thromboplastin time, international normalized ratio (INR).
MELD scores, MELD with Na (MELD-Na) scores, and neutrophil-to-lymphocyte ratio (NLR) were calculated based on these indices. The MELD score formula was: 9.6 × ln [Scr (mg/dL)] + 3.8 × ln [TBil (mg/dL)] + 11.2 × ln (INR) + 6.4 × etiology (0 for cholestatic or alcoholic cirrhosis, 1 for other causes). The MELD-Na score formula was: MELD score + 1.59 × [135 - Na+ (mmol/L)], with Na+ capped at 135 mmol/L if > 135 mmol/L, floored at 120 mmol/L if < 120 mmol/L, and otherwise calculated as the actual value. The NLR was: Neutrophil count/Lymphocyte count.
Sample collection: To eliminate dietary interference, 2 mL of peripheral venous blood was collected after 12 hours fast via elbow venipuncture into tubes with inert separation gel and coagulant. Samples were centrifuged at 4000 rpm for 5 minutes at 20-25 °C within 8 hours, and the supernatant was collected and stored at -80 °C until analysis. Samples were prepared through extraction and reconstitution for targeted metabolomics analysis of serum fatty acids, bile acids, amino acids, and trimethylamine N-oxide (TMAO) levels.
Instrumentation and testing conditions: Fatty and bile acids were analyzed using the Xevo TQ-S Cronos LC-MS (Waters Corporation, United States); amino acids and TMAO were analyzed using the ACQUITY UPLC-Xevo TQ-S tandem quadrupole mass spectrometer (Waters Corporation, United States). Sample pretreatment and analysis conditions are detailed in Supplementary material 1.
Metabolomics data analysis: Metabolomics data were analyzed using SPSS 26.0 (International Business Machines Corporation, Chicago, IL, United States) for t tests and P value calculations. Fold change (FC) was used to assess differential metabolite expression between the groups, and volcano plots were generated using the OmicStudio online tool (https://www.omicstudio.cn/tool). Multivariate statistical analysis was performed using the SIMCA 14.1 software. Principal component (PC) analysis was used to assess overall trends and outliers, visualized via PC score plots, with each point representing an independent sample. Orthogonal partial least squares-discriminant analysis was applied to identify group-specific differences, visualized via variation coefficient plots. Variable importance in projection (VIP) scores were calculated, with VIP > 1 defining differential metabolites. Model reliability was evaluated using R2X, R2Y, and Q2Y values.
Data were analyzed using SPSS 26.0. Normally distributed continuous data were expressed as the mean ± SD deviation and compared using two-sample t tests; non-normally distributed data were presented as the median (quartiles) and compared using the Mann–Whitney U test. Categorical data were reported as counts (percentages), n (%) and analyzed with χ2 tests or Fisher’s exact test. Binary logistic regression identified independent risk factors for 90-day prognosis in patients with ACLF. Receiver operating characteristic curves and area under the curve (AUC) assessed model predictive value, with forest plots visualized using the R 4.4.0 forest plot package. All tests were two-sided, and statistical significance was set at P < 0.05.
This study enrolled 58 patients with ACLF and the data collected were complete. The mean age of the included population was 47 ± 11 years (range: 28-73), 44 males (75.86%) and 14 females (24.14%). Collected data is complete. Hepatitis B virus (HBV) infection was the predominant etiology (28 cases, 48.28%). Based on 90-day outcomes, patients were categorized into survival (n = 33; male:female = 25:8; mean age: 44 ± 10 years) and death (n = 25; male:female = 19:6; mean age: 51 ± 11 years) groups. The death group exhibited significantly higher age, MELD, MELD-Na, NLR, TBil, Scr, and blood urea nitrogen, and lower PLT than did the survival group (P < 0.05). Other indices showed no significant differences (P > 0.05) (Table 1).
| Characteristic | Survival group (n = 33) | Death group (n = 25) | Statistic | P value |
| Age (years) | 44 ± 10 | 51 ± 11 | t = -2.520 | 0.015 |
| Male/female (n) | 25/8 | 19/6 | Z = -0.021 | 0.983 |
| Liver disease etiology | ||||
| HBV | 15 | 9 | NA | NA |
| Alcohol | 13 | 10 | NA | NA |
| Other | 5 | 6 | NA | NA |
| MELD | 18.75 ± 6.65 | 22.89 ± 6.03 | t = -2.440 | 0.018 |
| MELD-Na | 21.98 ± 8.01 | 28.36 ± 8.41 | t = -2.940 | 0.005 |
| ALBPS sessions | 1.36 ± 2.09 | 1.61 ± 2.14 | t = -0.494 | 0.623 |
| Laboratory tests | ||||
| WBC | 6.26 (4.36-9.85) | 8.23 (5.23-13.40) | Z = -1.382 | 0.167 |
| NLR | 5.98 ± 6.56 | 11.46 ± 9.78 | t = -2.548 | 0.014 |
| Hb | 104.47 ± 25.31 | 95.92 ± 28.91 | t = 1.198 | 0.236 |
| PLT | 132.96 ± 84.89 | 83.96 ± 58.93 | t = 2.468 | 0.017 |
| ALB | 31.18 ± 6.20 | 30.35 ± 4.24 | t = 0.575 | 0.567 |
| ALT | 55.00 (28.00-212.50) | 47.00 (25.50-76.00) | Z = -0.864 | 0.388 |
| AST | 107.00 (61.00-275.00) | 105.00 (55.00-150.00) | Z = -1.083 | 0.279 |
| ALP | 132.00 (105.50-186.50) | 137.00 (119.00-213.00) | Z = -0.958 | 0.338 |
| GGT | 68.00 (49.00-145.50) | 76.00 (40.50-164.50) | Z = -0.196 | 0.844 |
| TBil | 235.10 (198.40-310.15) | 297.50 (235.45-378.50) | Z = -2.206 | 0.027 |
| Scr | 53.00 (47.50-72.50) | 68.00 (56.00-89.50) | Z = -2.223 | 0.026 |
| BUN | 5.25 ± 3.84 | 10.16 ± 8.38 | t = -2.978 | 0.004 |
| Na+ | 134.33 ± 4.14 | 132.24 ± 6.19 | t = 1.505 | 0.138 |
| APTT | 42.61 ± 8.49 | 45.97 ± 10.70 | t = -1.334 | 0.188 |
| INR | 2.35 ± 0.59 | 2.53 ± 0.87 | t = -0.977 | 0.333 |
Based on treatment strategies, 23 patients received ALBPS. Compared with the SMT-only group, no significant differences were observed in age or sex ratio (P > 0.05); however, the SMT group had lower hemoglobin, Albumin, alanine transaminase, aspartate transaminase, TBil, and MELD scores (P < 0.05). Other indices showed no significant differences (P > 0.05) (Table 2).
| Characteristic | Total (n = 35) | SMT group | Total (n = 23) | ALBPS group | P valuea | ||
| Survival (n = 22) | Death (n = 13) | Survival (n = 11) | Death (n = 12) | ||||
| Age (years) | 45 ± 11 | 42 ± 10 | 50 ± 11 | 50 ± 11 | 47 ± 9 | 52 ± 12 | 0.128 |
| Male/female (n) | 27/8 | 17/5 | 10/3 | 17/6 | 8/3 | 9/3 | 0.780 |
| Liver disease etiology | |||||||
| HBV | 10 | 8 | 2 | 17 | 7 | 7 | NA |
| Alcohol | 18 | 10 | 8 | 5 | 3 | 2 | NA |
| Other | 7 | 4 | 3 | 1 | 1 | 3 | NA |
| MELD | 18.83 ± 6.13 | 17.42 ± 5.86 | 21.21 ± 6.03 | 23.13 ± 6.74 | 21.41 ± 7.60 | 24.70 ± 5.73 | 0.015 |
| MELD-Na | 24.92 ± 10.00 | 21.61 ± 8.15 | 30.51 ± 10.65 | 24.44 ± 6.47 | 22.71 ± 8.05 | 26.02 ± 4.37 | 0.841 |
| ALBPS sessions | - | - | - | 3.74 ± 1.60 | 4.09 ± 1.30 | 3.42 ± 1.83 | NA |
| Laboratory tests | |||||||
| WBC | 6.75 (4.57-11.05) | 6.51 (4.31-9.47) | 7.96 (4.32-13.47) | 7.32 (5.15-12.81) | 6.03 (4.17-12.81) | 9.38 (5.76-13.68) | 0.465 |
| NLR | 5.88 (2.68-9.15) | 5.18 (2.03-7.80) | 9.15 (4.40-25.11) | 7.09 ± 6.31 | 5.55 ± 5.82 | 8.50 ± 6.66 | 0.541 |
| Hb | 94.64 ± 28.37 | 97.83 ± 26.77 | 87.54 ± 30.64 | 110.13 ± 22.28 | 115.73 ± 18.33 | 105.00 ± 25.04 | 0.031 |
| PLT | 106.24 ± 81.53 | 127.02 ± 91.56 | 71.08 ± 45.07 | 120.35 ± 73.66 | 144.82 ± 72.28 | 97.92 ± 70.38 | 0.506 |
| ALB | 28.73 ± 5.31 | 29.34 ± 6.02 | 27.71 ± 3.82 | 34.00 ± 3.87 | 34.86 ± 4.97 | 33.21 ± 2.46 | 0.000 |
| ALT | 40.00 (20.00-70.00) | 36.50 (19.50-124.50) | 44.00 (22.50-70.00) | 79.00 (47.00-203.00) | 124.00 (79.00-222.00) | 64.00 (30.25-81.50) | 0.004 |
| AST | 75.00 (43.00-161.00) | 77.50 (44.75-384.25) | 75.00 (30.50-141.50) | 148.00 (71.00-176.00) | 167.00 (95.00-179.00) | 126.50 (65.00-152.00) | 0.048 |
| ALP | 149.69 ± 55.04 | 146.19 ± 54.91 | 155.62 ± 56.98 | 133.00 (115.00-203.00) | 127.00 (107.00-203.00) | 133.00 (117.00-210.75) | 0.899 |
| GGT | 68.00 (36.00-165.00) | 89.50 (36.75-171.25) | 53.00 (30.50-164.50) | 76.00 (49.00-108.00) | 65.00 (50.00-108.00) | 88.00 (44.25-172.25) | 0.844 |
| TBil | 249.98 ± 80.28 | 237.03 ± 79.44 | 271.89 ± 79.93 | 287.00 (223.30-353.70) | 225.00 (208.00-317.20) | 312.25 (279.38-392.23) | 0.046 |
| Scr | 70.00 ± 37.37 | 58.14 ± 20.57 | 90.08 ± 50.22 | 62.00 (52.00-79.00) | 53.00 (51.00-78.00) | 66.50 (60.25-83.50) | 0.426 |
| BUN | 5.01 (3.75-9.00) | 4.43 (3.46-5.71) | 6.73 (4.50-17.04) | 6.64 ± 5.57 | 4.54 ± 2.22 | 8.57 ± 7.16 | 0.830 |
| Na+ | 132.34 ± 6.29 | 134.23 ± 5.31 | 129.15 ± 6.72 | 135.09 ± 2.66 | 134.55 ± 1.75 | 135.58 ± 3.29 | 0.053 |
| APTT | 43.22 ± 8.91 | 41.93 ± 8.70 | 45.39 ± 9.17 | 45.33 ± 10.58 | 43.96 ± 8.31 | 46.59 ± 12.55 | 0.415 |
| INR | 2.33 ± 0.59 | 2.26 ± 0.52 | 2.46 ± 0.69 | 2.57 ± 0.88 | 2.53 ± 0.69 | 2.61 ± 1.05 | 0.216 |
Targeted metabolomics detected 38 metabolites in serum samples, including 18 amino acids, 11 fatty acids, 5 gut microbiota-related metabolites (TMAO), and 4 bile acids (Supplementary material 2). All data were collected completely.
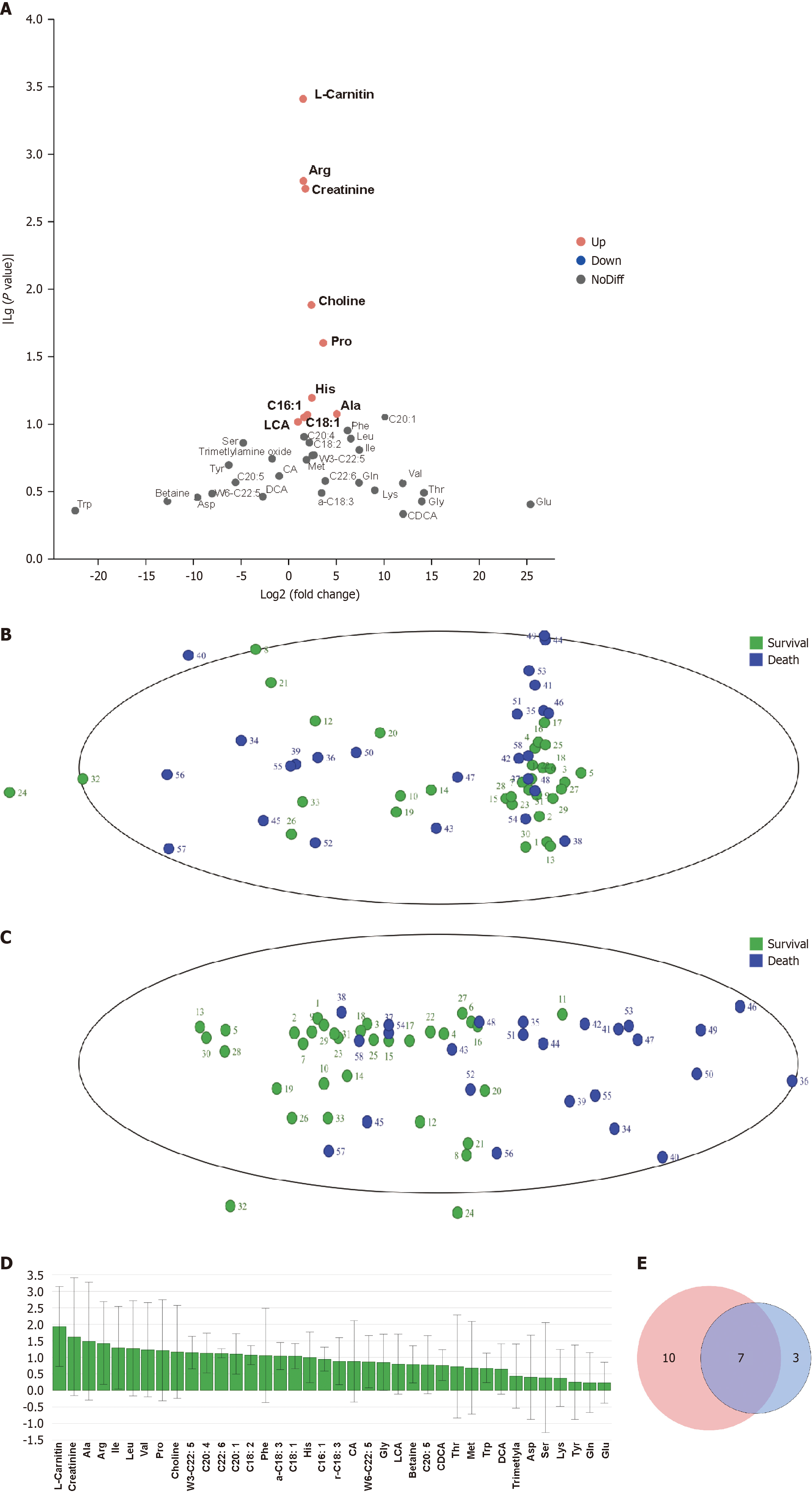
Univariate analysis using FC and t-tests filtered metabolites with |log2FC| > 0.58 and P < 0.1, yielding 10 differentially expressed metabolites between the death and survival groups, visualized in a volcano plot (Figure 1A). Owing to the multidimensional and highly correlated nature of metabolomics data, traditional univariate analysis struggled to capture latent information effectively. Thus, multivariate analysis was conducted using the SIMCA 14.1 software for dimensiona
| Metabolite | Survival | Death | |Log2FC| | P value | VIP |
| Carnitine | 50.55 ± 27.06 | 78.89 ± 35.86 | 1.51 | 0 | 1.94 |
| Creatinine | 61.75 ± 25.55 | 92.07 ± 49.32 | 1.74 | 0 | 1.62 |
| Ala | 405.67 ± 110.92 | 465.51 ± 211.05 | 5.04 | 0.08 | 1.49 |
| Arg | 14.56 ± 8.41 | 22.81 ± 11.93 | 1.55 | 0 | 1.43 |
| Pro | 266.13 ± 78.89 | 322.25 ± 133.30 | 3.62 | 0.03 | 1.21 |
| Choline | 57.26 ± 27.04 | 76.70 ± 37.78 | 2.37 | 0.01 | 1.17 |
| C18:1 | 182.34 ± 221.42 | 279.69 ± 322.04 | 1.62 | 0.09 | 1.05 |
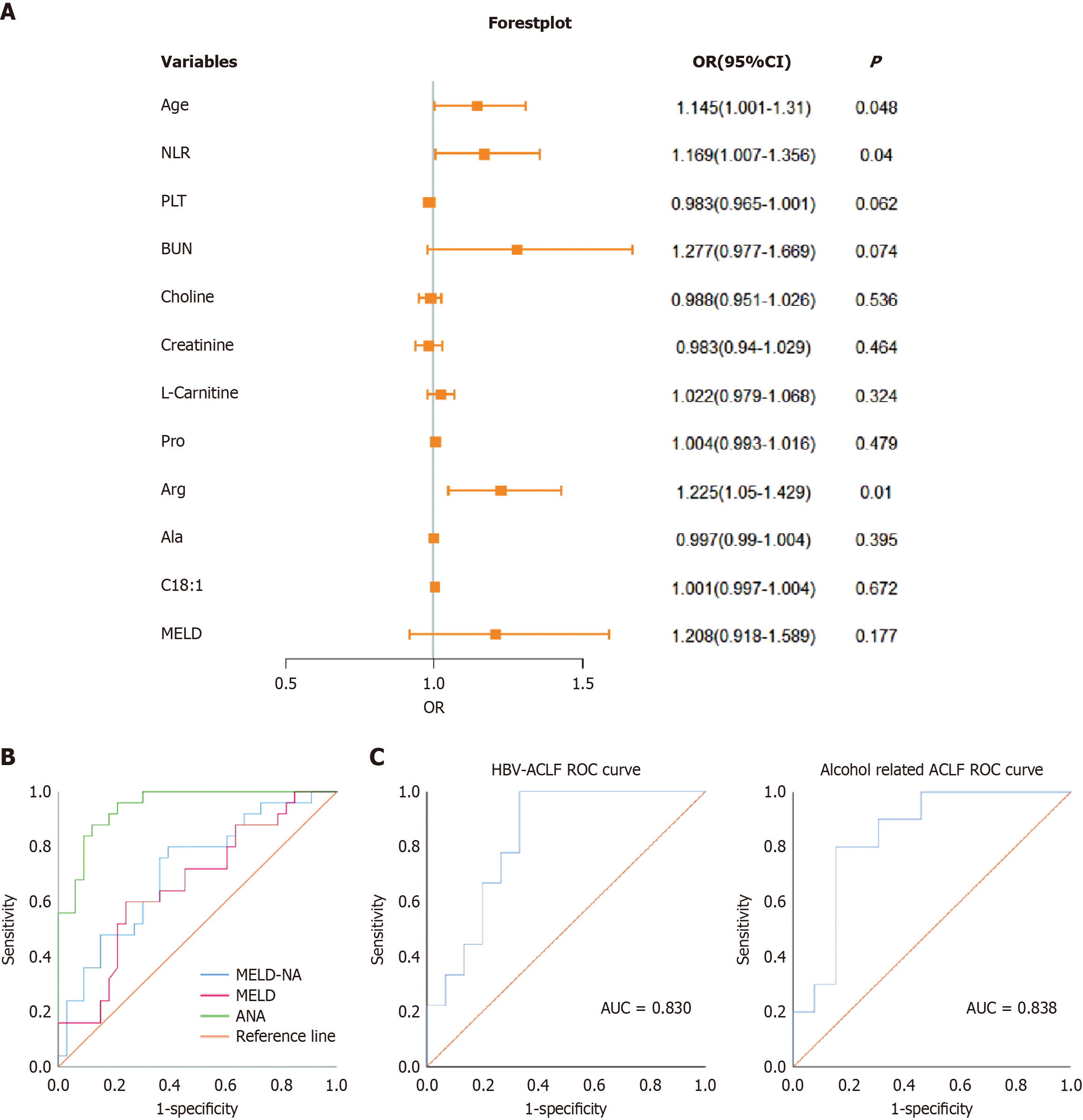
Baseline variables differing between the survival and death groups (age, MELD, NLR, blood urea nitrogen, and PLT) and differential metabolites (L-carnitine, creatinine, Ala, Arg, Pro, choline, and C18:1) were included in a binary logistic regression model to identify independent predictors of 90-day prognosis (survival = 0, death = 1). The results showed that high age [odds ratio (OR) = 1.145, 95% confidence interval (CI): 0.001-1.31, P < 0.05], high NLR (OR = 1.169, 95%CI: 1.007-1.356, P < 0.05), and high Arg (OR = 1.225, 95%CI: 1.05-1.429, P < 0.05) were independent risk factors for 90-day mortality in patients with ACLF (Table 4).
| Variable | Coefficient | SE | Wald χ2 | P value | OR value | 95%CI |
| Age | 0.135 | 0.069 | 3.893 | 0.048 | 1.145 | 1.001-1.31 |
| NLR | 0.156 | 0.076 | 4.22 | 0.04 | 1.169 | 1.007-1.356 |
| MELD | 0.189 | −0.140 | 1.824 | 0.177 | 1.208 | 0.918-1.589 |
| PLT | -0.017 | 0.009 | 3.492 | 0.062 | 0.983 | 0.965-1.001 |
| BUN | 0.244 | 0.137 | 3.201 | 0.074 | 1.277 | 0.977-1.669 |
| Choline | -0.012 | 0.019 | 0.384 | 0.536 | 0.988 | 0.951-1.026 |
| Creatinine | -0.017 | 0.023 | 0.536 | 0.464 | 0.983 | 0.940-1.029 |
| L-Carnitine | 0.022 | 0.022 | 0.973 | 0.324 | 1.022 | 0.979-1.068 |
| Pro | 0.004 | 0.006 | 0.5 | 0.479 | 1.004 | 0.993-1.016 |
| Arg | 0.203 | 0.079 | 6.648 | 0.01 | 1.225 | 1.05-1.429 |
| Ala | -0.003 | 0.004 | 0.723 | 0.395 | 0.997 | 0.990-1.004 |
| C18:1 | 0.001 | 0.002 | 0.18 | 0.672 | 1.001 | 0.997-1.004 |
| Constant | -15.481 | 6.044 | 6.561 | 0.01 | 0 | - |
Logistic regression forest plots visually depicted risk factors potentially influencing 90-day prognosis in patients with ACLF (Figure 2A). The predictive values for 90-day mortality were AUC_age = 0.682, AUC_NLR = 0.704, and AUC_Arg = 0.721. MELD and MELD-Na scores yielded AUC_MELD = 0.663 and AUC_MELD-Na = 0.714. A combined predictive ANA model comprising age, NLR, and Arg was developed, with the formula ANA = -15.481 + 0.135 × age + 0.156 × NLR + 0.203 × Arg. The cutoff was 0.759, with AUC_ANA = 0.945, a sensitivity and positive predictive value of 84.0%, and specificity and negative predictive value of 87.9%, outperforming MELD and MELD-Na scores (Figure 2B, Table 5). Stratified analysis of the etiology revealed that the ANA model predicted a 90-day prognostic AUC of 0.830 for patients with HBV-ACLF and 0.838 for those with alcohol-related ACLF (Figure 2C).
| Parameter | AUC | Sensitivity (%) | Specificity (%) | Optimal cut-off | 95%CI | P value |
| Age | 0.682 | 68 | 66.7 | 0.347 | 0.541-0.823 | 0.019 |
| NLR | 0.704 | 64 | 66.3 | 0.337 | 0.569-0.840 | 0.008 |
| Arg | 0.721 | 76 | 69.7 | 0.457 | 0.584-0.858 | 0.004 |
| MELD | 0.663 | 60 | 75.8 | 0.358 | 0.521-0.805 | 0.035 |
| MELD-Na | 0.714 | 80 | 60.6 | 0.406 | 0.580-0.847 | 0.006 |
| ANA | 0.945 | 88 | 87.9 | 0.759 | 0.893-0.997 | 0 |
To further validate ALBPS effects on the seven metabolites, VIP values were compared before (within 2 hours) and after (within 2 hours) the first and last ALBPS sessions. Only Ala and L-carnitine showed statistically significant changes (VIP > 1) post-treatment (Table 6).
| Metabolite | ALBPS | P value | VIP | |
| Before | After | |||
| Ala | 370.58 ± 143.66 | 548.53 ± 339.56 | 0.025 | 1.88 |
| L-carnitine | 69.79 ± 40.90 | 101.68 ± 70.24 | 0.067 | 1.36 |
| Arg | 22.48 ± 9.56 | 22.95 ± 9.57 | 0.87 | 0.91 |
| Creatinine | 90.21 ± 44.44 | 112.29 ± 69.86 | 0.208 | 0.88 |
| Pro | 303.03 ± 106.05 | 324.23 ± 171.56 | 0.617 | 0.79 |
| C18:1 | 459.80 ± 304.72 | 350.58 ± 243.92 | 0.186 | 0.7 |
| Choline | 81.41 ± 39.88 | 89.30 ± 61.74 | 0.609 | 0.41 |
Advancements in informatics and analytical technologies, coupled with integrative biological approaches, have empowered metabolomics to detect subtle early changes in biological pathways, providing insights into disease mechanisms[4]. ACLF is marked by heightened systemic catabolism, driving extensive lipolysis, glycogenolysis, and proteolysis. These processes release fatty acids, glucose, and amino acids into the bloodstream, fueling immune responses and peripheral organ dysfunction, leading to a cascade of immune–inflammatory–metabolic dysregulation[8]. Accurately predicting survival in patients with ACLF is paramount, as liver transplantation markedly improves outcomes for those unresponsive to SMT or ALBPS. Patients with poorer predicted prognoses are prioritized for transplantation. This study employed Xevo TQ-S Cronos LC-MS and ACQUITY UPLC-Xevo TQ-S tandem quadrupole mass spectrometry to analyze serum levels of fatty acids, bile acids, amino acids, and TMAO in patients with ACLF experiencing divergent 90-day outcomes. Through combined univariate and multivariate analyses, we identified seven differential metabolites-L-carnitine, creatinine, Ala, Arg, Pro, choline, and C18:1 - distinguishing the survival from death groups. Logistic regression confirmed Arg as an independent predictor of 90-day mortality risk. In addition, ALBPS treatment modulated Ala and L-carnitine levels. Although clinical evidence underscores ALBPS’s role as an effective adjunct to SMT and a bridge to transplantation, this study did not establish a definitive link between ALBPS-mediated metabolite change and improved prognosis, possibly owing to the limited sample size. Larger, prospective studies are needed to clarify this relationship.
The liver orchestrates amino acid metabolism through specialized transporters, converting portal vein-derived amino acids into glucose and fatty acids to meet energy demands and maintain amino acid homeostasis[9]. Ala, a critical gluconeogenic amino acid, is exclusively metabolized in the liver. Its heightened catabolism in ACLF contributes to hypergly
Univariate and multivariate logistic regression analyses pinpointed age, NLR, and Arg as significant predictors of 90-day mortality in patients with ACLF. These findings align with prior studies identifying age and INR as independent risk factors for poor 28- and 90-day outcomes in patients with HBV-ACLF meeting the Chinese group on the study of severe hepatitis B-ACLF criteria[18]. Lashen et al[19] similarly validated NLR as a predictor of 30-day and 6-month mortality in ACLF. Notably, integrating these clinical markers with metabolites into the ANA model yielded a robust AUC of 0.945, with superior sensitivity, specificity, and predictive accuracy compared with those of the MELD and MELD-Na scores, offering a powerful tool for early detection of adverse ACLF outcomes.
Furthermore, this study compared clinical parameters between the ALBPS and SMT groups, reflecting real-world practice: ALBPS-treated patients exhibited more severe baseline conditions (for example, higher MELD scores). Although ALBPS frequency or application did not independently predict 90-day mortality, its effectiveness in improving outcomes in critically ill patients with ACLF underscores its value as an adjunctive therapy. Treatment modality significantly influences ACLF prognosis. Yadav et al[20] conducted plasma metabolomics in 45 patients with ACLF categorized into SMT, hemoperfusion adsorption (HA), and therapeutic plasma exchange (TPE) groups using high-resolution mass spectrometry. HA reduced Arg-Pro and TMAO metabolites after 7 days, while TPE transiently elevated phenylalanine and serine levels. Compared with TPE and SMT, HA more effectively curbed inflammation and secondary energy metabolism pathways, improving the plasma environment (about 80% probability). In our study, ALBPS increased Ala and L-carnitine post-treatment, which suggesting its capacity to clear inflammatory mediators, ameliorate metabolic dysregulation, and support hepatocyte regeneration. However, differing blood purification techniques may influence metabolite profiles, potentially explaining ALBPS’s limited prognostic impact. This limitation, combined with a small sample size, variable treatment modalities, and patient-specific metabolic differences, underscores the need for further investigation to elucidate ALBPS’s role in modulating metabolites and improving clinical outcomes. In the future, untargeted metabolomics and spatial metabolomics need to be used to further elucidate the all-round and dynamic effects of ALBPS therapy on metabolites in patients with ACLF, and to further assess the impact on patient prognosis.
In conclusion, using high-performance liquid chromatography-mass spectrometry, we identified seven metabolites - L-carnitine, creatinine, Ala, Arg, Pro, choline, and C18:1 - linked to 90-day ACLF prognosis, with Arg emerging as an independent mortality risk factor. ALBPS treatment modulated Ala and L-carnitine, reducing inflammation and promoting hepatocyte regeneration. However, this study had limitations: (1) A single-center case-control design with a small sample size; (2) A restricted scope of metabolite detection, potentially skewing results; and (3) Variability in baseline metabolism, complications, and disease severity despite standardized treatment, influencing indices and outcomes. In future, multicenter, large-scale studies should be carried out to refine metabolite profiling based on stratified ACLF severity and treatment regimens, enabling more precise diagnosis and management.
| 1. | Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, Saigal S, Saraf N, Soin AS, Devarbhavi H, Kim DJ, Dhiman RK, Duseja A, Taneja S, Eapen CE, Goel A, Ning Q, Chen T, Ma K, Duan Z, Yu C, Treeprasertsuk S, Hamid SS, Butt AS, Jafri W, Shukla A, Saraswat V, Tan SS, Sood A, Midha V, Goyal O, Ghazinyan H, Arora A, Hu J, Sahu M, Rao PN, Lee GH, Lim SG, Lesmana LA, Lesmana CR, Shah S, Prasad VGM, Payawal DA, Abbas Z, Dokmeci AK, Sollano JD, Carpio G, Shresta A, Lau GK, Fazal Karim M, Shiha G, Gani R, Kalista KF, Yuen MF, Alam S, Khanna R, Sood V, Lal BB, Pamecha V, Jindal A, Rajan V, Arora V, Yokosuka O, Niriella MA, Li H, Qi X, Tanaka A, Mochida S, Chaudhuri DR, Gane E, Win KM, Chen WT, Rela M, Kapoor D, Rastogi A, Kale P, Rastogi A, Sharma CB, Bajpai M, Singh V, Premkumar M, Maharashi S, Olithselvan A, Philips CA, Srivastava A, Yachha SK, Wani ZA, Thapa BR, Saraya A, Shalimar, Kumar A, Wadhawan M, Gupta S, Madan K, Sakhuja P, Vij V, Sharma BC, Garg H, Garg V, Kalal C, Anand L, Vyas T, Mathur RP, Kumar G, Jain P, Pasupuleti SSR, Chawla YK, Chowdhury A, Alam S, Song DS, Yang JM, Yoon EL; APASL ACLF Research Consortium (AARC) for APASL ACLF working Party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:353-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 614] [Cited by in RCA: 591] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 2. | Trebicka J, Hernaez R, Shawcross DL, Gerbes AL. Recent advances in the prevention and treatment of decompensated cirrhosis and acute-on-chronic liver failure (ACLF) and the role of biomarkers. Gut. 2024;73:1015-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 3. | Mezzano G, Juanola A, Cardenas A, Mezey E, Hamilton JP, Pose E, Graupera I, Ginès P, Solà E, Hernaez R. Global burden of disease: acute-on-chronic liver failure, a systematic review and meta-analysis. Gut. 2022;71:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 142] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 4. | Marchev AS, Vasileva LV, Amirova KM, Savova MS, Balcheva-Sivenova ZP, Georgiev MI. Metabolomics and health: from nutritional crops and plant-based pharmaceuticals to profiling of human biofluids. Cell Mol Life Sci. 2021;78:6487-6503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 5. | Li J, Liang X, Jiang J, Yang L, Xin J, Shi D, Lu Y, Li J, Ren K, Hassan HM, Zhang J, Chen P, Yao H, Li J, Wu T, Jin L, Ye P, Li T, Zhang H, Sun S, Guo B, Zhou X, Cai Q, Chen J, Xu X, Huang J, Hao S, He J, Xin S, Wang D, Trebicka J, Chen X, Li J; Chinese Group on the Study of Severe Hepatitis B (COSSH). PBMC transcriptomics identifies immune-metabolism disorder during the development of HBV-ACLF. Gut. 2022;71:163-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 83] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 6. | Xiao Y, Lu J, Xu S, Wu Z, Wang W, Ji R, Guo T, Qi Z, Tong H, Wang Y, Zhao C. Metabolic Differences among Patients with Cirrhosis Using Q Exactive Hybrid Quadrupole Orbitrap Mass Spectrometry Technology. J Proteome Res. 2024;23:5352-5359. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Chen Y, Han T, Duan Z; Severe Liver Disease and Artificial Liver Group, Chinese Society of Hepatology, Chinese Medical Association. Clinical application of artificial liver and blood purification: expert consensus recommendations. Hepatol Int. 2023;17:4-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Valainathan SR, Xie Q, Arroyo V, Rautou PE. Prognosis algorithms for acute decompensation of cirrhosis and ACLF. Liver Int. 2025;45:e15927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 9. | Paulusma CC, Lamers WH, Broer S, van de Graaf SFJ. Amino acid metabolism, transport and signalling in the liver revisited. Biochem Pharmacol. 2022;201:115074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 10. | Okun JG, Rusu PM, Chan AY, Wu Y, Yap YW, Sharkie T, Schumacher J, Schmidt KV, Roberts-Thomson KM, Russell RD, Zota A, Hille S, Jungmann A, Maggi L, Lee Y, Blüher M, Herzig S, Keske MA, Heikenwalder M, Müller OJ, Rose AJ. Liver alanine catabolism promotes skeletal muscle atrophy and hyperglycaemia in type 2 diabetes. Nat Metab. 2021;3:394-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 11. | Ding Z, Ericksen RE, Lee QY, Han W. Reprogramming of mitochondrial proline metabolism promotes liver tumorigenesis. Amino Acids. 2021;53:1807-1815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Zhang Q, Song Q, Liu S, Xu Y, Gao D, Lu P, Liu Y, Zhao G, Wu L, Zhao C, Yang J. Integrated transcriptomic and metabolomic analysis reveals the metabolic programming of GM-CSF- and M-CSF- differentiated mouse macrophages. Front Immunol. 2023;14:1230772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 13. | Matuszyk E, Sierka E, Rodewald M, Bae H, Meyer T, Kus E, Chlopicki S, Schmitt M, Popp J, Baranska M. Differential response of liver sinusoidal endothelial cells and hepatocytes to oleic and palmitic acid revealed by Raman and CARS imaging. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Zhou X, Huang G, Wang L, Zhao Y, Li J, Chen D, Wei L, Chen Z, Yang B. L-carnitine promotes liver regeneration after hepatectomy by enhancing lipid metabolism. J Transl Med. 2023;21:487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Piccinin E, Cariello M, De Santis S, Ducheix S, Sabbà C, Ntambi JM, Moschetta A. Role of Oleic Acid in the Gut-Liver Axis: From Diet to the Regulation of Its Synthesis via Stearoyl-CoA Desaturase 1 (SCD1). Nutrients. 2019;11:2283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 16. | Haines RW, Fowler AJ, Wan YI, Flower L, Heyland DK, Day A, Pearse RM, Prowle JR, Puthucheary Z. Catabolism in Critical Illness: A Reanalysis of the REducing Deaths due to OXidative Stress (REDOXS) Trial. Crit Care Med. 2022;50:1072-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 17. | Chen X, Qiu W, Ma X, Ren L, Feng M, Hu S, Xue C, Chen R. Roles and Mechanisms of Choline Metabolism in Nonalcoholic Fatty Liver Disease and Cancers. Front Biosci (Landmark Ed). 2024;29:182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 18. | Ma S, Xie Z, Zhang L, Yang Y, Jiang H, Ouyang X, Zhao Y, Liu Q, Xu X, Li L. Identification of a Potential miRNA-mRNA Regulatory Network Associated With the Prognosis of HBV-ACLF. Front Mol Biosci. 2021;8:657631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Lashen SA, Salem P, Ibrahim E, Abd Elmoaty D, Yousif WI. Hematological ratios in patients with acute decompensation and acute-on-chronic liver failure: prognostic factors. Eur J Gastroenterol Hepatol. 2024;36:952-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Yadav M, Maiwal R, Kumar Br V, Tripathi G, Sharma N, Sharma N, Bindal V, Mathew B, Pandey S, Singh SP, Tevathia HV, Maras JS, Sarin SK. Comparative metabolome analysis reveals higher potential of haemoperfusion adsorption in providing favourable outcome in ACLF patients. Liver Int. 2024;44:1189-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |