Published online Jul 14, 2025. doi: 10.3748/wjg.v31.i26.105656
Revised: April 2, 2025
Accepted: May 26, 2025
Published online: July 14, 2025
Processing time: 159 Days and 19.8 Hours
Kushenol I (KSCI) exhibits potential anti-inflammatory and antioxidant activities. However, its therapeutic effects and mechanisms in ulcerative colitis (UC) remain unclear.
To investigate the therapeutic effects and mechanisms of KSCI in alleviating UC.
Therapeutic targets for KSCI in treating UC were identified using network pharmacology. Molecular docking and dynamics simulations confirmed the interactions between KSCI and these targets. In a murine UC model induced by dextran sodium sulfate (DSS), the anti-inflammatory and antioxidant effects of KSCI were evaluated following oral administration, as well as its impact on intestinal barrier function and immune response modulation. Finally, changes in gut microbiota composition were analyzed using 16S ribosomal RNA sequencing.
A total of 192 potential targets of KSCI in treating UC were identified using network pharmacology. KSCI bound stably to core targets including protein kinase B (AKT), p38 mitogen-activated protein kinase (p38 MAPK), NOD-like receptor thermal protein domain associated protein 3 (NLRP3), phosphoinositide 3-kinase (PI3K), forkhead box O1 (FOXO1), and Toll-like receptor 4 (TLR4). The oral administration of KSCI improved colon length and body weight, and reduced disease activity in a mouse model of DSS-induced UC. KSCI suppressed pro-inflammatory cytokines (interleukin [IL-1β], IL-6, IL-17, and tumor necrosis factor alpha) and promoted the expression of the anti-inflammatory cytokine IL-10. It also inhibited key signaling molecules and modulated the expression of IL-1β, AKT, p38 MAPK, NLRP3, PI3K, AKT, FOXO1, and TLR4. KSCI exhibited potent antioxidant effects, ameliorating colonic inflammation and tissue damage. It improved intestinal barrier function, influenced gut microbiota composition, and increased splenic T-cell percentages.
KSCI alleviated DSS-induced UC by modulating gut microbiota, enhancing the intestinal barrier, reducing inflammation and oxidative stress, and regulating the immune response.
Core Tip: Kushenol I (KSCI) from Sophora flavescens shows promise for treating ulcerative colitis (UC). Network pharmacology reveals 192 potential targets, including protein kinase B, NOD-like receptor thermal protein domain associated protein 3, Toll-like receptor 4, phosphoinositide 3-kinase, and forkhead box O1. In UC mice, KSCI alleviates symptoms, reduces pro-inflammatory cytokines, enhances anti-inflammatory activity, and inhibits the activation of key signaling pathways. It also exerts antioxidant effects, repairs intestinal barrier damage, and modulates the gut microbiota. Additionally, KSCI regulates T-cell balance. These findings highlight KSCI’s multi-target mechanism offering potential as a therapeutic agent for UC.
- Citation: He XD, Li M, Zuo XD, Ni HY, Han YX, Hu YK, Yu J, Yang XX. Kushenol I combats ulcerative colitis via intestinal barrier preservation and gut microbiota optimization. World J Gastroenterol 2025; 31(26): 105656
- URL: https://www.wjgnet.com/1007-9327/full/v31/i26/105656.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i26.105656
Ulcerative colitis (UC) is a chronic inflammatory disease of the gastrointestinal tract. Its incidence and prevalence are increasing worldwide, particularly among children and adolescents[1]. UC is usually characterized by specific clinical signs such as persistent abdominal pain, diarrhea, rectal bleeding, and severe weight loss. It is widely accepted that the occurrence and development of UC are attributable to damage to the intestinal barrier, intestinal microbiota, and mucosal immunity[2]. Pharmacological therapy with 5-aminosalicylic acid and corticosteroids is currently the primary approach for managing UC. However, many of these therapeutic agents are associated with side effects. Consequently, the prevention of the development of UC has emerged as a significant global challenge in the medical community.
Sophora flavescens Aiton is a widely used traditional Chinese medicine known for its therapeutic effects such as clearing heat, drying dampness, eliminating parasites, and promoting diuresis[3]. Recent research has revealed that S. flavescens exhibits significant efficacy in reducing the inflammatory response associated with UC and in effectively controlling disease progression[4]. Notably, the active components responsible for these therapeutic effects are primarily alkaloids and flavonoids. These findings highlight the potential of S. flavescens as a promising therapeutic agent in the management of UC. Extensive research has demonstrated the antioxidant and anti-inflammatory activities of the isopentenyl flavonoids of S. flavescens, including kushenol F, kurarinone, kuraridin, kushenol B, and kushenol A[4]. Collectively, these findings underscore the favorable anti-inflammatory and antioxidant properties of isopentenyl flavonoids found in S. flavescens. Kushenol I (KSCI) is a representative compound of the isoprenylated flavonoids found in S. flavescens and is a significant constituent among the numerous flavonoids of S. flavescens. Previous studies have demonstrated its pharmacological effects in treating lipid-metabolism disorders[5]. However, the specific anti-inflammatory and antioxidant effects and the UC-mitigation ability of KSCI remain unclear.
In this study, we explored the therapeutic effects and mechanisms of KSCI in alleviating UC. First, we used a combination of network pharmacology, molecular docking, and molecular dynamics (MD) simulations to predict the therapeutic effects of KSCI and its underlying mechanisms in a mouse model of UC. Subsequently, we conducted a comprehensive evaluation of the therapeutic effects and the mechanism of KSCI in a dextran sodium sulfate (DSS)-induced mouse model of UC. KSCI was significantly effective in alleviating the symptoms of UC by adjusting the composition of the gut microbiota, maintaining the integrity of the intestinal mucosal barrier, decreasing inflammation and oxidative stress, and fine-tuning the immune response. These multidimensional mechanisms of action underscore the role of KSCI as a treatment approach in the management of UC.
DSS was manufactured by MP Biomedicals (Santa Ana, CA, United States) and procured from Guangzhou Yining Biotechnology Co., Ltd. (Guangzhou, Guangdong Province, China). Enzyme-linked immunosorbent assay (ELISA) kits for the determination of IL-1β (220409-072M), IL-6 (220409-056M), IL-10 (220409-003M), IL-17 (220409-017M), and tumor necrosis factor alpha (TNF-α) (220409-081M) were obtained from Jiangsu Enzyme-Linked Biotechnology Co., Ltd. (Nanjing, Jiangsu Province, China). Antibodies targeting forkhead box O1 (FOXO1), p38 mitogen-activated protein kinase (p38 MAPK), phosphorylated p38 MAPK (p-p38 MAPK), protein kinase B (AKT), phosphorylated protein kinase B (p-AKT), and β-actin were sourced from Proteintech (Proteintech, Rosemont, IL, United States). Antibodies against nuclear factor kappa B (NF-κB) p65, phosphorylated NF-κB p65 (NF-κB p-p65), NOD-like receptor thermal protein domain associated protein 3 (NLRP3), phosphoinositide 3-kinase (PI3K), and phosphorylated PI3K (p-PI3K) were purchased from Cell Signaling Technology Inc. (Danvers, MA, United States). Antibodies targeting occludin and zonula occludens-1 (ZO-1) were obtained from Wuhan Sanying Biotechnology Co., Ltd. (Wuhan, Hubei Province, China). PerCP/Cyanine5.5 anti-mouse cluster of differentiation 3 (CD3), fluorescein isothiocyanate anti-mouse CD4, and polyethylene anti-mouse CD8 were acquired from BioLegend (San Diego, CA, United States). Bicinchoninic acid (BCA) protein quantification kit, radio immunoprecipitation assay lysis buffer, and protein phosphatase inhibitors were procured from Shanghai Beyotime Biotechnology Co., Ltd (Shanghai, China). High-purity deionized water was purified using a Milli-Q system (Millipore, Bedford, MA, United States). All other reagents used in this study were of analytical reagent grade or higher.
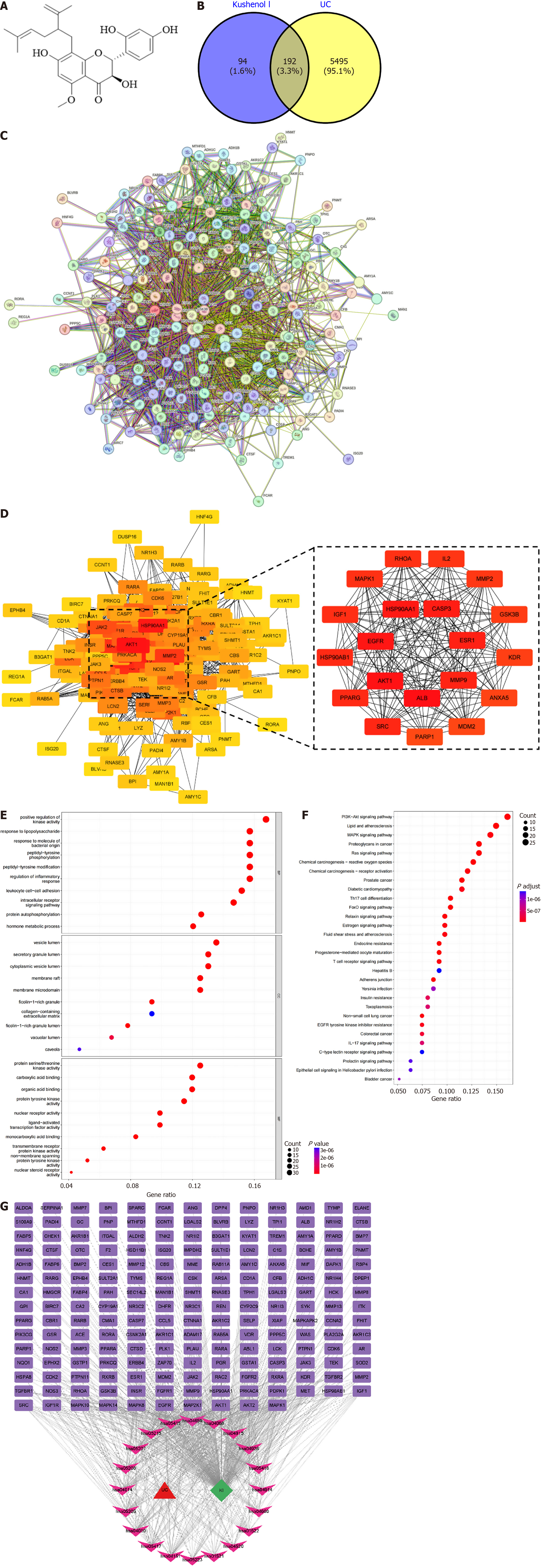
Air-dried S. flavescens was subjected to a series of three consecutive reflux extractions using 80% methanol, with each extraction lasting for 1 hour. The extracts were combined and subjected to solvent evaporation under reduced pressure until no odor of alcohol was detected. Next, the crude extract was subjected to adsorption resin column chromatography and sequentially eluted with 40% and 80% methanol, and the 80% fraction was collected. Quality control at this stage involved thin-layer chromatography (TLC) (silica gel GF254, visualized under ultraviolet 254 nm) to monitor batch consistency and intermediate purity. Silica gel column chromatography was performed using a gradient elution of petroleum ether and ethyl acetate (8:1 to 1:1 ratio). Fractions were analyzed by TLC (Rf = 0.35, ethyl acetate: Petroleum ether = 3:1), and only those containing KSCI (confirmed by co-spotting with a reference standard) were pooled. The fractions containing KSCI were collected while monitoring by TLC and were then concentrated and dried. Subsequently, reverse-phase preparative liquid chromatography was performed using a gradient of acetonitrile and water as the mobile phase. Purity was assessed by peak area normalization (≥ 98% at 290 nm), validated via external standard calibration using authenticated KSCI (≥ 95% purity; Sigma-Aldrich, St. Louis, MO, United States). Fractions with a target peak purity of ≥ 98% were collected, concentrated, and dried to obtain a light-yellow powder. The structure of the compound (Figure 1A) was determined using nuclear magnetic resonance spectroscopy and by comparison with previously reported data, and then elucidated as KSCI[6]. S. flavescens samples were authenticated by Professor Yang XX, and voucher specimens were deposited in the Key Laboratory of Preventing Metabolic Diseases of Traditional Chinese Medicine (Yunnan University of Chinese Medicine, Kunming, Yunnan Province, China).
A comprehensive search was conducted in three databases, namely the Online Mendelian Inheritance in Man database, DisGeNET database, and GeneCards database, using the keyword “ulcerative colitis” to identify disease targets associated with UC. The specific score thresholds used were GeneCards score ≥ 50 and DisGeNET score ≥ 0.1. The potential mechanisms of KSCI were explored by importing its molecular structure into the PharmMapper database. To generate multiple conformations, the structure of KSCI was optimized within the database. Prior to conducting the analysis, the human protein target database was used to obtain disease targets specifically associated with UC. Using the Universal Protein Resource database, the targets based on the structural characteristics of KSCI were identified and converted into the corresponding gene names for further mechanistic research. Common target proteins between KSCI targets and UC disease targets were identified using Venn diagram analysis. The overlapping target proteins were then imported into the search tool for the retrieval of interacting genes/proteins database to construct a visual protein-protein interaction (PPI) network using Homo sapiens as the model organism. Consequently, a PPI network diagram illustrating the potential interactions of KSCI in the treatment of UC was obtained. Lastly, the CytoHubba plugin in Cytoscape was used to calculate and identify the core disease targets of KSCI.
The core disease targets of KSCI were imported into the online software David (https://david.ncifcrf.gov/) and the species was set to “human”. The significance level was set at P< 0.05. Gene Ontology (GO) biological processes, GO molecular functions, GO Cellular Components, and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway were selected to analyze the obtained results. Subsequently, bar charts and bubble plots were generated to visualize the enrichment results from the GO and KEGG pathways based on the number of genes. The “drug-target-pathway” table was imported into Cytoscape to generate a network pharmacology diagram.
The crystal structure of the target protein and the three-dimensional (3D) structure of KSCI were retrieved from the Protein Data Bank (http://www.pdb.org/) and the PubChem database (http://pubchem.ncbi.nlm.nih.gov/), respectively. Subsequently, the binding affinity of KSCI with the target protein molecule was determined using AutoDock tools 4 using the semiflexible docking protocol. A binding energy result of ≤ 0 kcal/mol indicates that the compound can bind to and interact with the target protein molecule, whereas a binding energy of < -5 kcal/mol suggests a strong binding affinity between them.
The protein-ligand complex obtained from molecular docking was subjected to MD simulation using Gromacs2020 software. The Charmm36 force field was used for the protein and the Gaff2 force field was used for KSCI. The transferable interatomic potential with three points model water model was applied to solvate the protein-ligand system, and a periodic boundary box with dimensions of 1.2 nm was established. Sodium and chloride ions were introduced to balance the charge of the system and replicate the experimental environment as closely as possible. Prior to the formal MD simulation, the complex was subjected to energy minimization using the conjugate gradient algorithm for 50000 steps. Subsequently, further equilibration of the system was conducted for 100 ps under the canonical ensemble at a constant temperature of 310 K and under the isothermal-isobaric ensemble at a constant pressure of 1 atm. Lastly, 100 ns MD simulation was performed at ambient temperature and pressure.
Animals, diet, and experimental design: According to the National Guidelines for Experimental Animal Welfare, all animal experiment protocols were approved by the Ethics Committee of the Experimental Animal Center at Yunnan University of Chinese Medicine (Permission No. R-062023111). Thirty male C57BL/6 mice aged 6-8 weeks and weighing 16-18 g were selected and housed at the Experimental Animal Center of Yunnan University of Chinese Medicine (Kunming, Yunnan Province, China). The mice were kept in a specific-pathogen-free-grade animal room with humidity maintained at 50% ± 5% and temperature of 21 ± 1 °C and were subjected to a 12-hour light/12-hour dark cycle. The mice were provided unrestricted access to food and water during the one-week acclimatization period.
The mice were randomly divided into the following groups: Normal control group (control), DSS-induced UC group (model), positive control group (sulfasalazine), 100 mg/kg KSCI group (KSCI-100), and 50 mg/kg KSCI group (KSCI-50)
| Weight loss (%) | Stool consistency | Stool bleeding | Score |
| No loss | Normal | Occult blood test-negative | 0 |
| 1.0 to < 5.0 | Soft but formed | Occult blood test-positive | 1 |
| 5.0 to < 10.0 | Soft | Slight | 2 |
| 10.0 to < 15.0 | Slight diarrhea | Blood traces in stool | 3 |
| More than 15.0 | Watery diarrhea | Gross rectal bleeding | 4 |
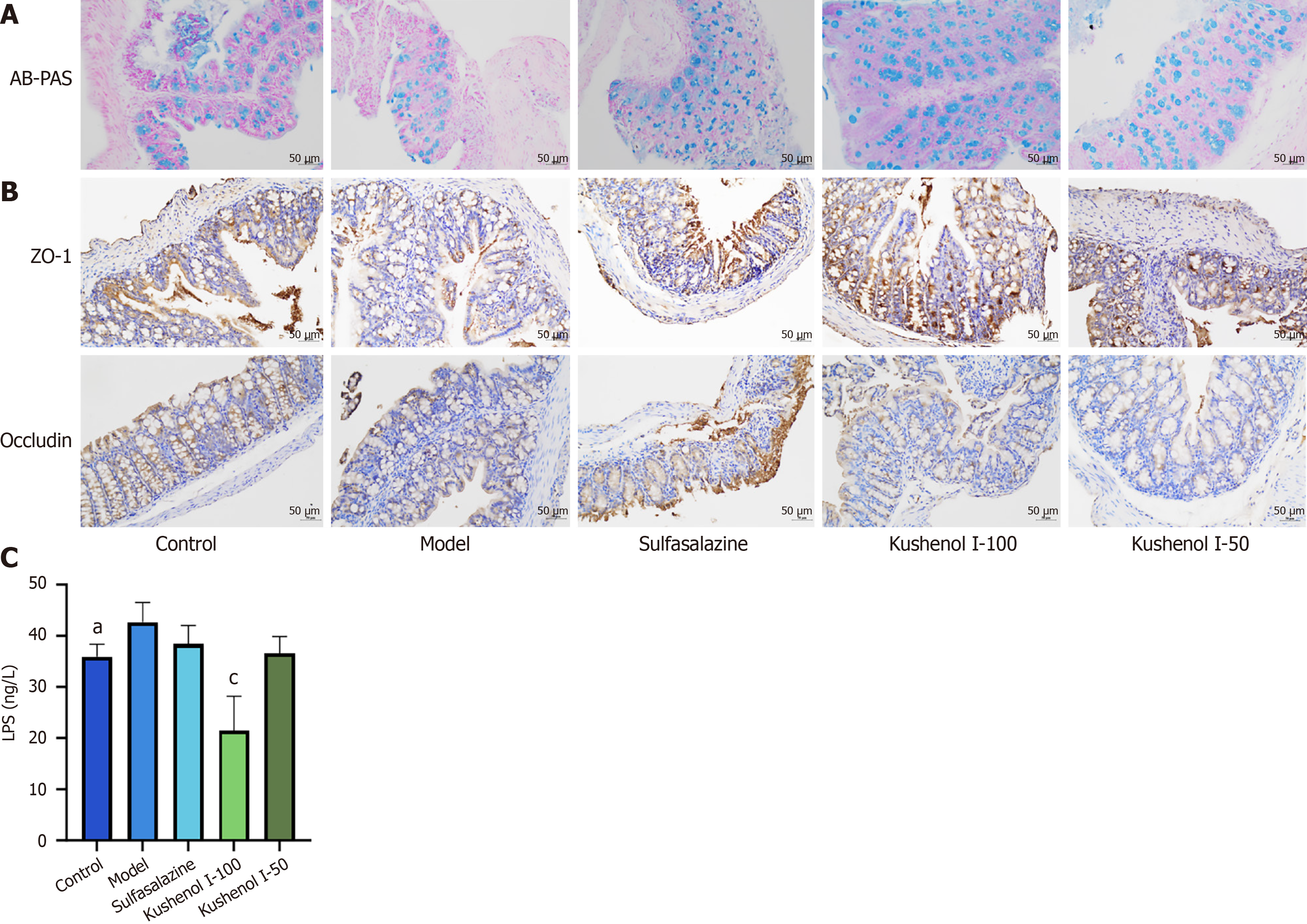
Histological analysis: Fresh colons were removed, fixed in formalin at 4 °C for 12 hours, and embedded in paraffin after dehydrating with gradient alcohol. Hematoxylin and eosin staining and Alcian blue and periodic acid-Schiff (AB-PAS) staining were conducted following the established protocols described previously[9].
Measurement of inflammatory biomarkers and oxidant system in the colon: After the mice were anesthetized, their eyeballs were promptly extracted to obtain blood samples. The samples were then centrifuged at 4 °C for 10 minutes at a low rotational speed and the upper serum layer was collected carefully. The levels of cytokines (lipopolysaccharide [LPS], IL-6, IL-8, IL-10, IL-1β, and TNF-α) in the serum were measured using the corresponding ELISA kits, following the manufacturers’ protocols.
The colon tissues of mice were homogenized in saline solution (running speed: 70 Hz; running time: 60 seconds; pause time: 20 seconds; cycles: 4) and centrifuged at 12000 × g for 15 minutes at 4 °C, and the supernatant was collected. The protein concentration in the supernatant was determined using commercially available kits, following the manufacturers’ instructions. The levels of IL-6, IL-8, IL-10, IL-1β, and TNF-α in the colon were determined using commercial ELISA kits, and their values were normalized to the total protein content. Furthermore, the activity of myeloperoxidase (MPO), malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GSH-PX) in the colon were determined using the respective commercial kits.
Immunohistochemical analyses: Immunohistochemical analyses were conducted following previously established protocols[10]. Briefly, colon sections were incubated with primary antibodies against occludin and ZO-1 for 60 minutes, followed by incubation with secondary antibodies and streptavidin-biotin peroxidase for 120 minutes. Then the sections were visualized by light microscopy using 3,3-diaminobenzidine tetrahydrochloride, which resulted in a brown coloration. Imaging of the sections was performed using a panoramic scanner (Pannoramic MIDI; 3D HISTECH, Budapest, Hungary).
Quantitative real-time polymerase chain reaction: Total RNA was extracted from the colon using a commercial kit following the manufacturer’s protocol. The concentration and purity of RNA samples were determined using the NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, United States). Subsequently, the extracted RNA was reverse-transcribed into complementary DNA using a commercial kit. Quantitative real-time polymerase chain reaction (RT-qPCR) was performed using the SYBR Green PCR Master Mix kit on an RT-qPCR system (Bio-Rad Laboratories, Hercules, CA, United States). The relative mRNA expression of UC-associated genes was normalized to the expression of the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase. The primer sequences for these genes are listed in Table 2.
| Target gene | Amplified fragment length/bp | Primer sequences |
| IL-17 | 75 | Upstream: 5’-TGATGCTGTTGCTGCTGCTGAG-3’ |
| Downstream: 5’-GACACGCTGAGCTTTGAGGGATG-3’ | ||
| IL-10 | 81 | Upstream: 5’-GGACAACATACTGCTAACCGACTC-3’ |
| Downstream: 5’-TGGATCATTTCCGATAAGGCTTGG-3’ | ||
| TNF-α | 118 | Upstream: 5’-CCACCACGCTCTTCTGTCTACTG-3’ |
| Downstream: 5’-TGGTTTGTGAGTGTGAGGGTCTG-3’ | ||
| IL-1β | 145 | Upstream: 5’-CACTACAGGCTCCGAGATGAACAAC-3’ |
| Downstream: 5’-TGTCGTTGCTTGGTTCTCCTTGTAC-3’ | ||
| IL-6 | 91 | Upstream: 5’-CTTCTTGGGACTGATGCTGGTGAC-3’ |
| Downstream: 5’-TCTGTTGGGAGTGGTATCCTCTGTG-3’ |
Western blotting: For protein extraction, the colon tissues were lysed and homogenized in ice-cold radio immunoprecipitation assay buffer (containing phenylmethylsulfonyl fluoride and phosphatase inhibitors) and centrifuged at 12000 r/minute for 5 minutes at 4 °C. The supernatant was collected for protein quantification using the BCA protein assay kit. The protein samples were separated using 8%-12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto a polyvinylidene difluoride membrane (Millipore, MA, United States). The membrane was blocked for 2 hours at 22-28 °C in Tris-buffered saline with Tween 20 (TBST) containing 5% nonfat milk (blocking solution) while shaking. The membrane was incubated overnight at 4 °C with p-PI3K, PI3K, p-AKT, AKT, FOXO1, IL-1β, TLR4, NF-κB p-p65, NF-κB p65, and NLRP3 antibodies (1:1000). The membrane was washed with TBST and incubated at room temperature for 2 hours with horseradish peroxidase-conjugated diluted secondary antibodies (1:5000). Lastly, the protein bands were visualized using an enhanced chemiluminescence imaging system (Bio-Rad Laboratories) and the results were quantified using ImageJ software (Bio-Rad Laboratories).
Determination of T-cell subsets in the spleen: The spleens were dissected and thoroughly ground under aseptic conditions. The cell suspension was obtained by rinsing the cell strainer with a solution of whole blood and tissue dilution. Subsequently, the collected cell suspension was mixed with 3 mL lymphocyte separation solution and centrifuged at room temperature at a speed of 500-900 g for 20-30 minutes. The supernatant was discarded and the cells were resuspended. The resuspended cells were placed in a flow tube and incubated with a premixed solution of primary antibodies (0.5 μL CD3 antibody, 0.5 μL CD4 antibody, and 0.5 μL CD8 antibody) at room temperature with gentle agitation for 30 minutes while being protected from light. After incubation, the cells were further incubated for an additional 15 minutes while being protected from light. Following the addition of 2 mL of phosphate-buffered saline (PBS) for washing, the cells were centrifuged at 1500 rpm for 5 minutes and the supernatant was discarded. An appropriate amount of PBS was added to resuspend the cells. To determine the proportions of CD3+, CD4+, and CD8+ cells, as well as to calculate the CD4+/CD8+ ratio, cell counting was conducted using 123-count eBeads (Thermo Fisher Scientific). Flow cytometry data were acquired using the LSRFortessa cell analyzer (BD Biosciences, Franklin Lakes, NJ, United States) and analyzed using FlowJo software (Tree Star Inc., Ashland, OR, United States).
Bioinformatics analysis of 16S ribosomal RNA gene sequences: Genomic DNA from frozen feces was extracted using a DNA isolation kit following the manufacturer’s protocols. 16S ribosomal RNA (rRNA) genes, specifically the V3-V4 region, were amplified by PCR using the forward primer (338F) and reverse primer (806R). Am pure magnetic purification beads were used to purify the PCR products, and the products were subsequently pooled into equal concentrations. The quantity of the sequencing libraries was assessed using the Qubit 2.0 Fluorometer (Thermo Fisher Scientific). Lastly, the libraries were sequenced on the Illumina MiSeq platform.
Raw data from high-throughput sequencing were demultiplexed and quality-filtered using the QIIME2 platform. Operational taxonomic units (OTUs) were assigned to the results using UCLUST with a threshold of 97%. Principal component analysis plots were generated using R software (v4.1.2) for data visualization. STAMP software (v2.1.3) was used to identify the gut microbial phylotypes that were significantly different among groups.
Data are presented as the mean ± standard error of the mean using Prism 7.0 (GraphPad Software, La Jolla, CA, United States). Statistical analyses were performed using one-way analysis of variance. The significance of the differences in alpha diversity of microbial communities were verified using the Kruskal-Wallis test. Significant differences were indicated as P < 0.05.
A total of 289 targets for KSCI and 6790 targets for UC were identified. Duplicate targets were removed, resulting in 5687 targets remaining for KSCI and 286 for UC. A total of 192 common targets between KSCI and UC were pinpointed (Figure 1B). The PPI network for these 192 common targets is depicted in Figure 1C. Notably, among these shared targets, the top 20 targets affected by KSCI in UC were identified as AKT1, epidermal growth factor receptor, heat shock protein 90 alpha family class A member 1 (HSP90AA1), caspase-3, estrogen receptor 1, matrix metalloproteinase 9 (MMP9), albumin, Src, peroxisome proliferator-activated receptor gamma, HSP90AB1, insulin-like growth factor 1, MAPK1, Ras homolog family member A, interleukin 2, MMP2, glycogen synthase kinase 3 beta, kinase insert domain receptor, annexin A5, mouse double minute 2 homolog, and poly(ADP-ribose) polymerase 1 (Figure 1D).
As shown in Figure 1E, the core targets are predominantly engaged in positively regulating kinase activity and the inflammatory response, and in responding to LPS and bacterial molecular processes. Figure 1E provides a comprehensive overview of the 208 molecular functions identified within these biological processes, encompassing protein serine/threonine kinase activity, carboxylic acid binding, organic acid binding, protein tyrosine kinase activity, and nuclear receptor activity. Moreover, this analysis identified 61 cellular components, including vesicle lumen, secretory granule lumen, and cytoplasmic vesicle lumen. Figure 1F shows that the core targets are mainly concentrated within signaling pathways such as the PI3K/AKT signaling pathway, lipid and atherosclerosis, MAPK signaling pathway, cancer-related proteoglycans, and Ras signaling pathway. By connecting the top 20 ranked signaling pathways in KEGG with their corresponding target genes, the UC target-KSCI-signaling pathway network diagram (Figure 1G) was constructed.
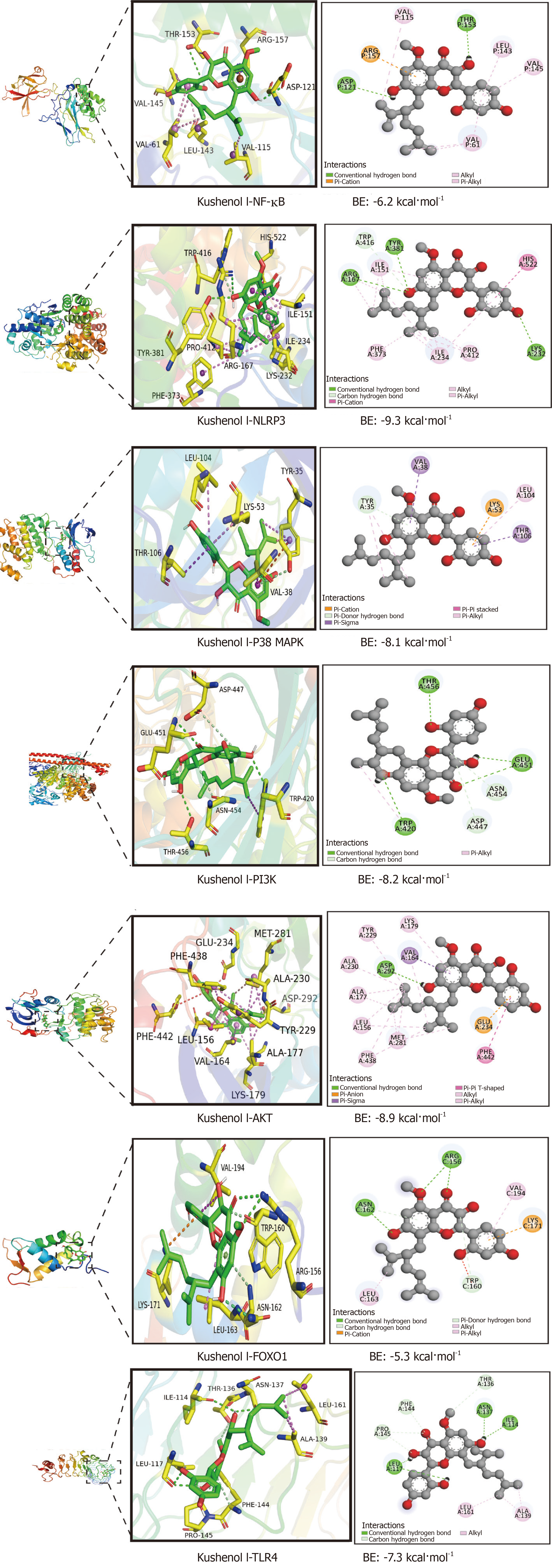
Based on the comprehensive analysis of the KSCI-UC target network, PPI network, KSCI-UC target pathway network, as well as GO and KEGG pathway enrichment analyses, we identified the following seven essential genes: AKT, p38 MAPK, NLRP3, PI3K, AKT, FOXO1, and TLR4. The 3D structures of AKT, p38 MAPK, NLRP3, PI3K, AKT, FOXO1, and TLR4 protein receptors were obtained from the Protein Data Bank. The 2D structure of the small molecule compound was retrieved from the PubChem database and transformed into a 3D structure using ChemOffice software. To ensure accuracy, PyMOL 2.4.0 was used to eliminate water molecules and ligands bound to these protein receptors. The 3D binding interactions between KSCI and Akt, p38 MAPK, NLRP3, PI3K, AKT, FOXO1, and TLR4 protein receptors were visualized using AutoDock tools and AutoDock Vina. Lower binding energy values indicated more stable molecular structures. Docking simulations were conducted for a total of seven pairs. Molecular docking revealed that KSCI exhibited spontaneous binding to the key targets in the AKT, p38 MAPK, NLRP3, PI3K, AKT, FOXO1, and TLR4 protein receptors. Notably, the binding energies of AKT, p38 MAPK, NLRP3, PI3K, AKT, FOXO1, and TLR4 were all < -5 kcal/mol, as depicted in Figure 2.
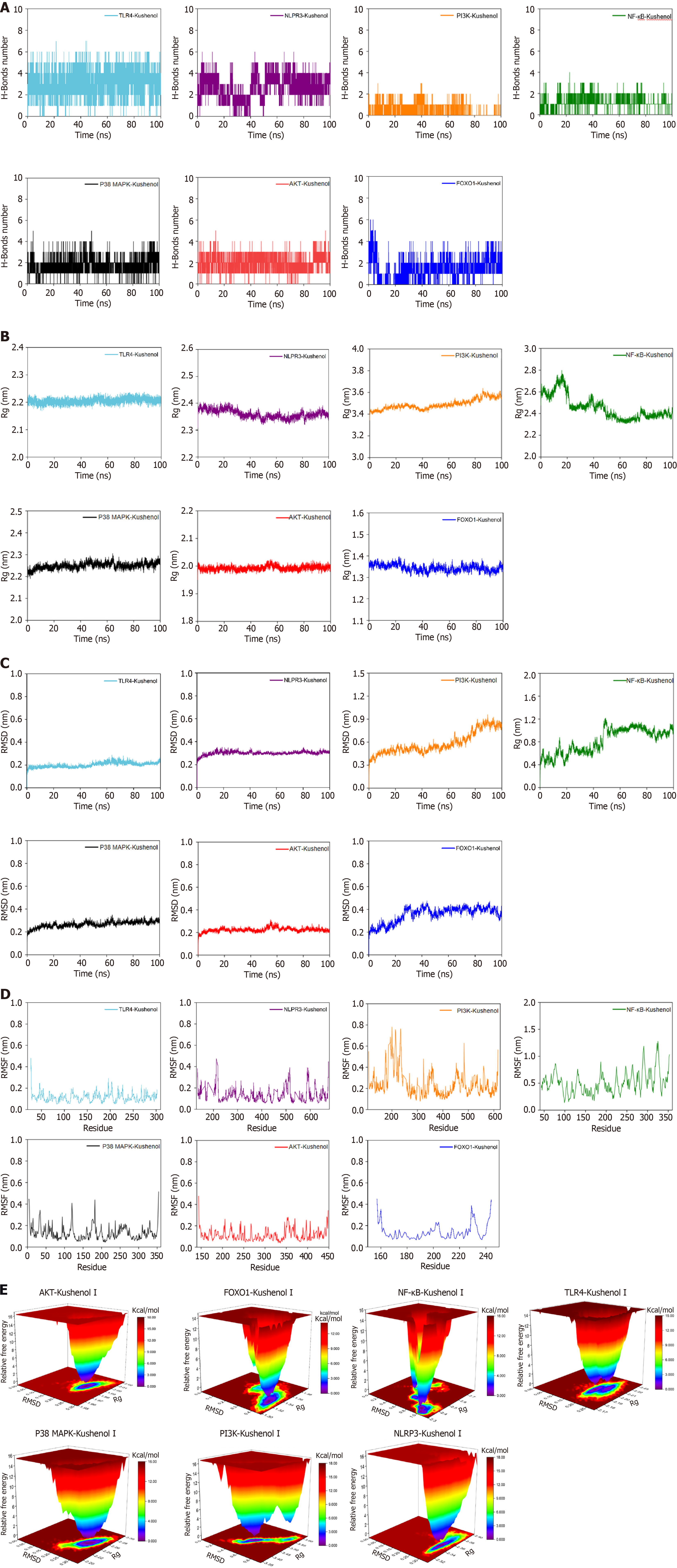
To provide a more comprehensive understanding of the binding affinity and stability between KSCI and the protein, a 100 ns MD simulation was used for each docking result. The simulation meticulously analyzed crucial dynamic parameters, such as root mean square deviation (RMSD), root mean square fluctuation (RMSF), and gyration radius (Rg), alongside monitoring the number of hydrogen bonds and the Gibbs free energy landscape between the protein and KSCI throughout the simulation trajectory.
Figure 3A reveals the formation of several hydrogen bonds in the AKT, NLRP3, p38 MAPK, and TLR4 complexes, with the quantity of these bonds displaying steady fluctuations. This finding indicates that KSCI interacts favorably with AKT, NLRP3, p38 MAPK, and TLR4 via the formation of stable hydrogen bonds, thereby demonstrating a high level of stability. The number of hydrogen bonds in the FOXO1 complex also exhibits consistent fluctuations after the 30 ns mark, suggesting stable binding. Conversely, the NF-κB and PI3K complexes have fewer hydrogen bonds, and their corresponding curves fluctuate in an unstable manner, indicating a lower level of complex stability.
Rg is used to characterize the compactness and stability of a molecule. A higher Rg indicates a more significant expansion during dynamic simulations. Conversely, a smaller Rg suggests that the system maintains its compactness and stability throughout the simulations. As shown in Figure 3B, the Rg curves for the AKT, FOXO1, NLRP3, p38 MAPK, and TLR4 complexes demonstrate fluctuations within a range of 3 nm, suggesting that the incorporation of KSCI enhances the stability of these complexes. By contrast, the Rg curves for the NF-κB and PI3K complexes exhibit substantial fluctuations, suggesting lower stability of the NF-κB and PI3K complexes.
RSMD provides insights into the fluctuation of these complexes, with a smoother RMSD curve indicating a higher level of stability. The RMSD curves of AKT, NLRP3, p38 MAPK, and TLR4 complexes demonstrate fluctuations within a narrow range of 0.5 nm, without any significant variations, suggesting the stable binding of these four complexes (Figure 3C). The RMSD curve of the FOXO1 complex exhibits stable fluctuations after 50 ns, indicating a favorable level of stability. By contrast, the RMSD curves of the NF-κB and PI3K complexes show noteworthy fluctuations, indicating poor stability in the binding of KSCI with FOXO1, NF-κB, and PI3K proteins.
RMSF is a metric used to assess the extent of fluctuations in amino acid residues of proteins during dynamic simulations. A higher RMSF value indicates more fluctuations in these residues, whereas a lower RMSF value suggests limited fluctuations. The RMSF curves of the AKT, FOXO1, NLRP3, p38 MAPK, and TLR4 complexes show fluctuations within the range of 0.5 nm (Figure 3D), implying that the addition of KSCI does not have a significant influence on the stability of amino acid residues or the overall protein structure of these complexes. Moreover, the RMSF curves of the NF-κB and PI3K complexes exhibit substantial fluctuations, indicating that the addition of KSCI has a notable impact on the overall protein stability of NF-κB and PI3K.
Gibbs free energy landscapes were plotted based on the calculated RMSD and Rg values. The X-axis represents the RMSD, the Y-axis represents the Rg, and the Z-axis represents Gibbs free energy. The free energy landscapes were plotted to illustrate the energetically favored conformations in the MD simulations. In this representation, the dark purple/blue spots indicate the lowest energy values, representing the most stable structures, whereas the red/yellow spots indicate unstable structures. Figure 3E displays the free energy landscapes of the AKT, NLRP3, and p38 MAPK complexes, which exhibit relatively singular clusters of minimum energy. This suggests that KSCI forms stable complexes with AKT, NLRP3, and p38 MAPK. By contrast, the free energy landscape of the TLR4 complex shows two clusters of minimum energy that are concentrated and possess smooth cluster surfaces. These characteristics indicate good stability of the KSCI-TLR4 complex. By contrast, the free energy landscape of the FOXO1 complex shows two adjacent but relatively dispersed clusters of minimum energy, indicating moderate stability. Furthermore, the free energy landscapes of the NF-κB and PI3K complexes exhibit multiple dispersed clusters of minimum energy which suggests poorer stability of the complexes formed by KSCI with NF-κB and PI3K.
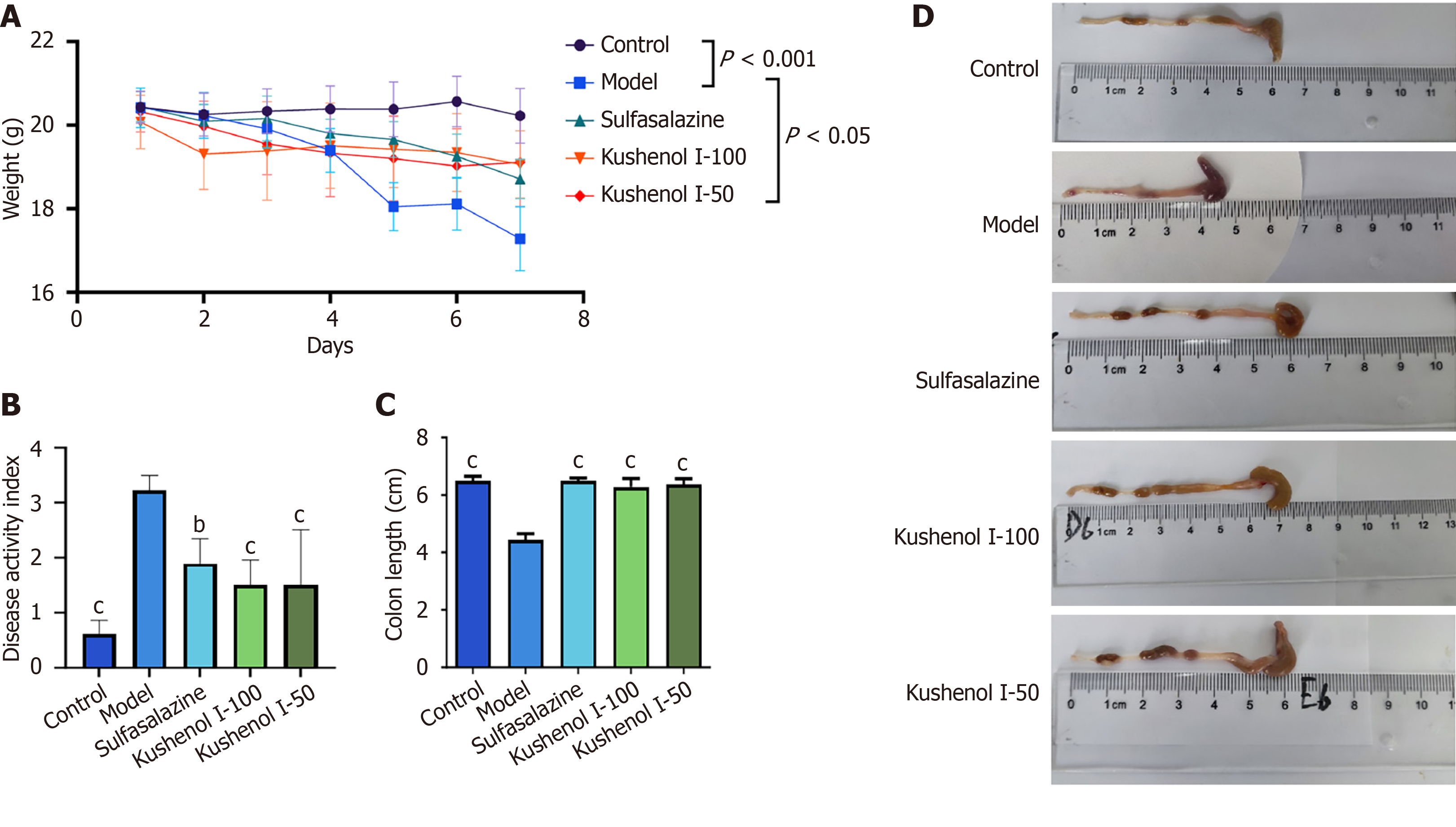
As shown in Figure 4A, the model group of mice demonstrated a significant reduction in body weight after 7 days of DSS administration (P < 0.001). However, the oral administration of KSCI effectively delayed and mitigated the DSS-induced loss in body weight (P < 0.05). Moreover, the DAI score was used to assess the impact of KSCI on mice with UC. The mice in the model group exhibited a substantial increase in the DAI score after DSS induction compared with those in the control group (P < 0.001) (Figure 4B). Interestingly, KSCI intervention successfully reversed the DSS-induced trends (P < 0.001). The colons of mice in the model group were notably shorter than those of mice in the control group (P < 0.001) (Figure 4C and D). The oral administration of KSCI restored the shortened colon length in mice with UC (P < 0.001). Therefore, KSCI intervention could effectively alleviate the symptoms of UC, including weight loss, DAI score, and colon shortening.
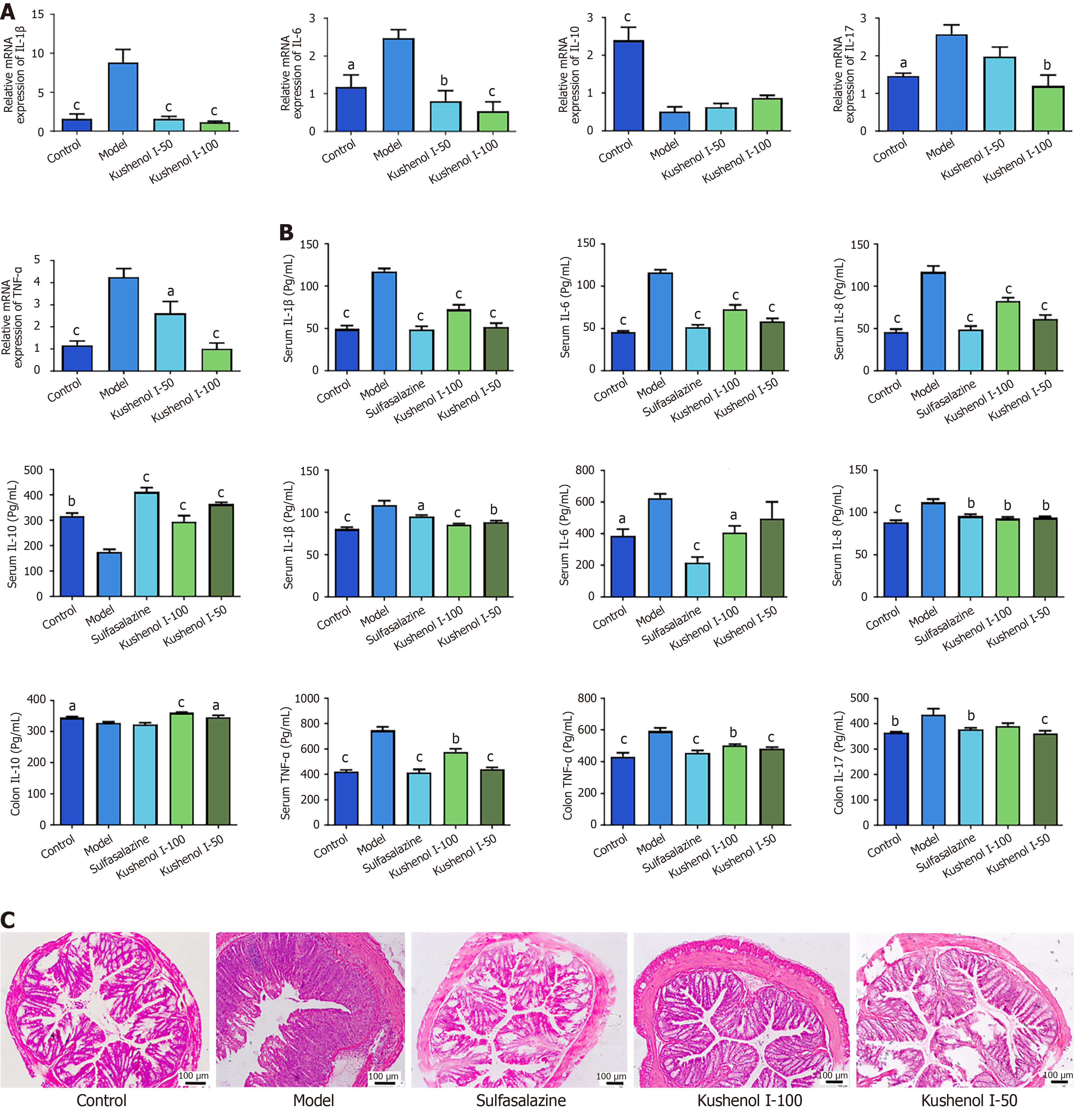
RT-qPCR was used to quantify the transcription levels of genes associated with UC (Figure 5A). The transcription levels of IL-1β, IL-6, IL-17, and TNF-α were significantly elevated in the model group (P < 0.05 or 0.001) and that of IL-10 was notably reduced (P < 0.001) compared with those in the control group. However, KSCI intervention markedly suppressed the transcription of IL-1β, IL-6, IL-17, and TNF-α in mice with UC (P < 0.05, P < 0.01, or P < 0.001) and increased the transcription level of IL-10 in the colon. These findings indicate that KSCI can potentially enhance anti-inflammatory cytokine production, suppress pro-inflammatory cytokine production, and consequently alleviate DSS-induced colitis.
As illustrated in Figure 5B, both the colon and serum concentrations of TNF-α, IL-6, IL-1β, and IL-8 were significantly higher in the model group than in the control group (P < 0.05, P < 0.01, or P < 0.001). By contrast, IL-17 was significantly elevated only in the colon (P < 0.01), while the levels of IL-10 in both colon and serum were notably reduced (P < 0.05 or P < 0.01). After KSCI intervention, a significant reduction in colon and serum concentrations of TNF-α, IL-6, IL-1β, and IL-8 was observed (P < 0.05, P < 0.01, or P < 0.001). By contrast, IL-17 was significantly reduced in the colon (P < 0.001), while IL-10 concentration in the colon and serum was substantially increased (P < 0.05 or P < 0.001). These findings suggest that KSCI stimulated the secretion of anti-inflammatory cytokines while inhibiting the release of pro-inflammatory cytokines, thereby alleviating DSS-induced UC.
Detailed histological analysis of the lesions in the colon revealed that DSS exposure led to extensive distortion of crypts, inflammatory cell infiltration, and loss of epithelial and goblet cells. However, KSCI intervention significantly reversed the DSS-induced pathological changes by reducing inflammatory cell infiltration, inhibiting the foci of crypt loss, and decreasing goblet cell damage (Figure 5C). Thus, KSCI could effectively mitigate DSS-induced colon damage.
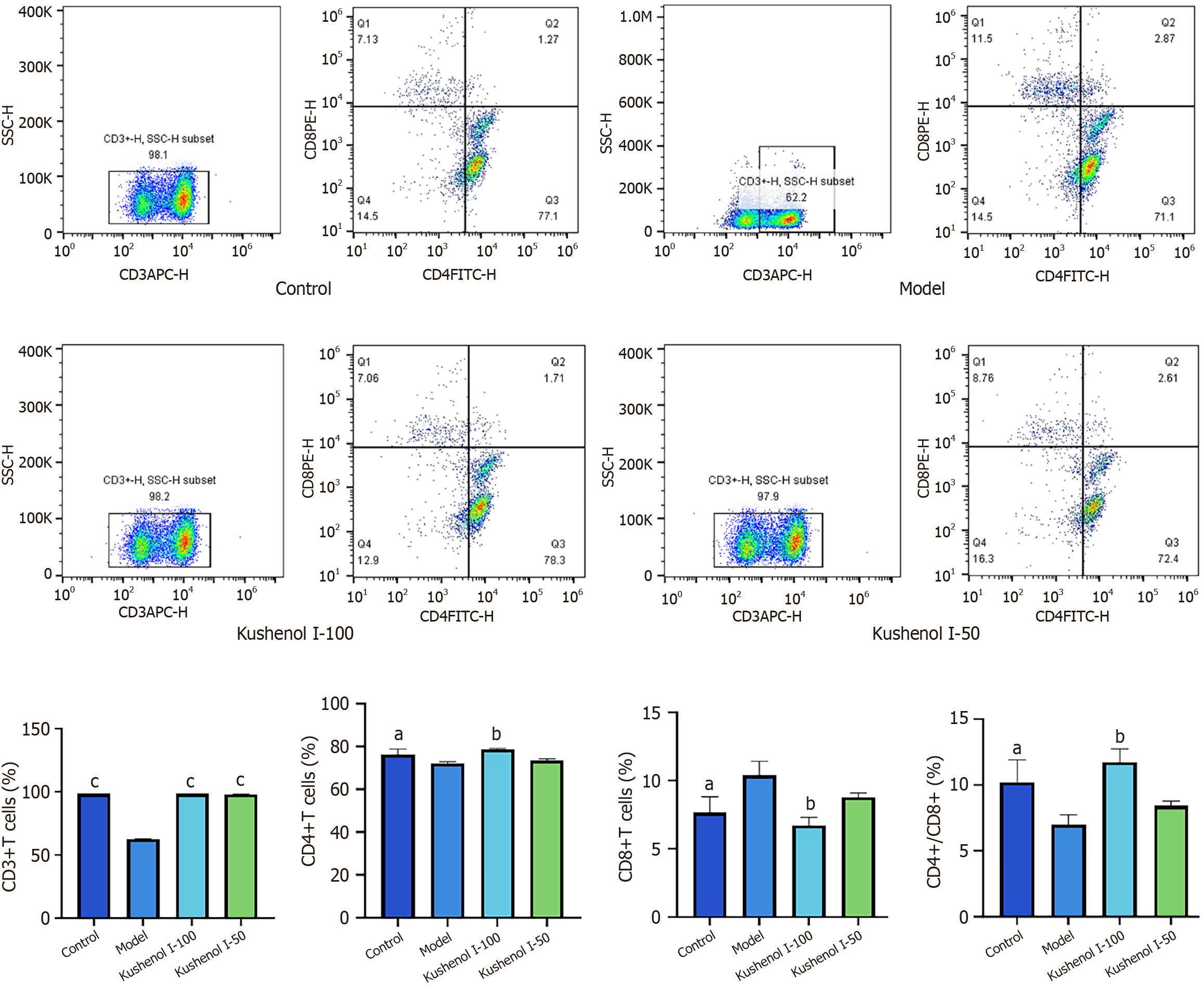
As shown in Figure 6, the proportions of CD3+ and CD4+ cells, as well as the CD4+/CD8+ ratio, were significantly reduced in the model group following DSS induction (P < 0.05 or P < 0.001), whereas the proportion of CD8+ cells was significantly increased (P < 0.05) compared with those in the control group. The proportions of CD3+ and CD4+ cells in the spleens of mice with UC, as well as the CD4+/CD8+ ratio, increased significantly (P < 0.01 or P < 0.001), and the proportion of CD8+ cells decreased significantly (P < 0.01) following KSCI intervention. These findings suggested that KSCI may exert anti-inflammatory effects by modulating the proportions of immune cells.
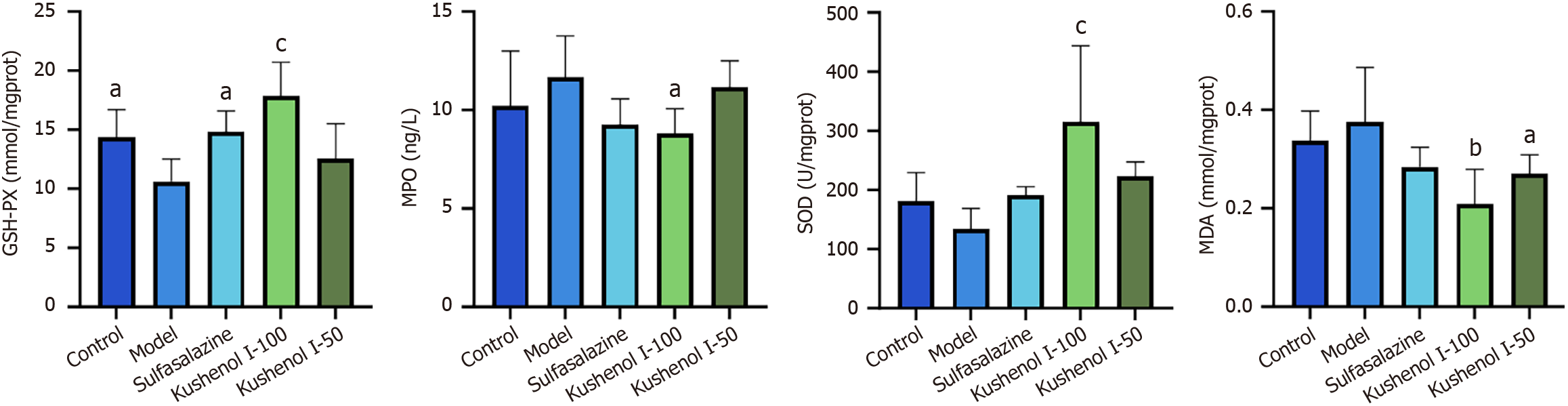
MPO and MDA levels in the colons of mice exhibited an increasing trend after DSS exposure, indicating increased oxidative stress (Figure 7). However, KSCI intervention significantly reduced MPO and MDA levels in the colon (P < 0.05 or P < 0.01). Additionally, mice in the model group demonstrated a decreasing trend in SOD activity and a significant reduction in GSH-PX activity (P < 0.05), whereas KSCI intervention resulted in a significant increase in GSH-PX and SOD activities in mice with DSS-induced UC (P < 0.001). These results indicated that KSCI improved the ability of mice with DSS-induced colitis to scavenge free radicals and prevent oxidative stress.
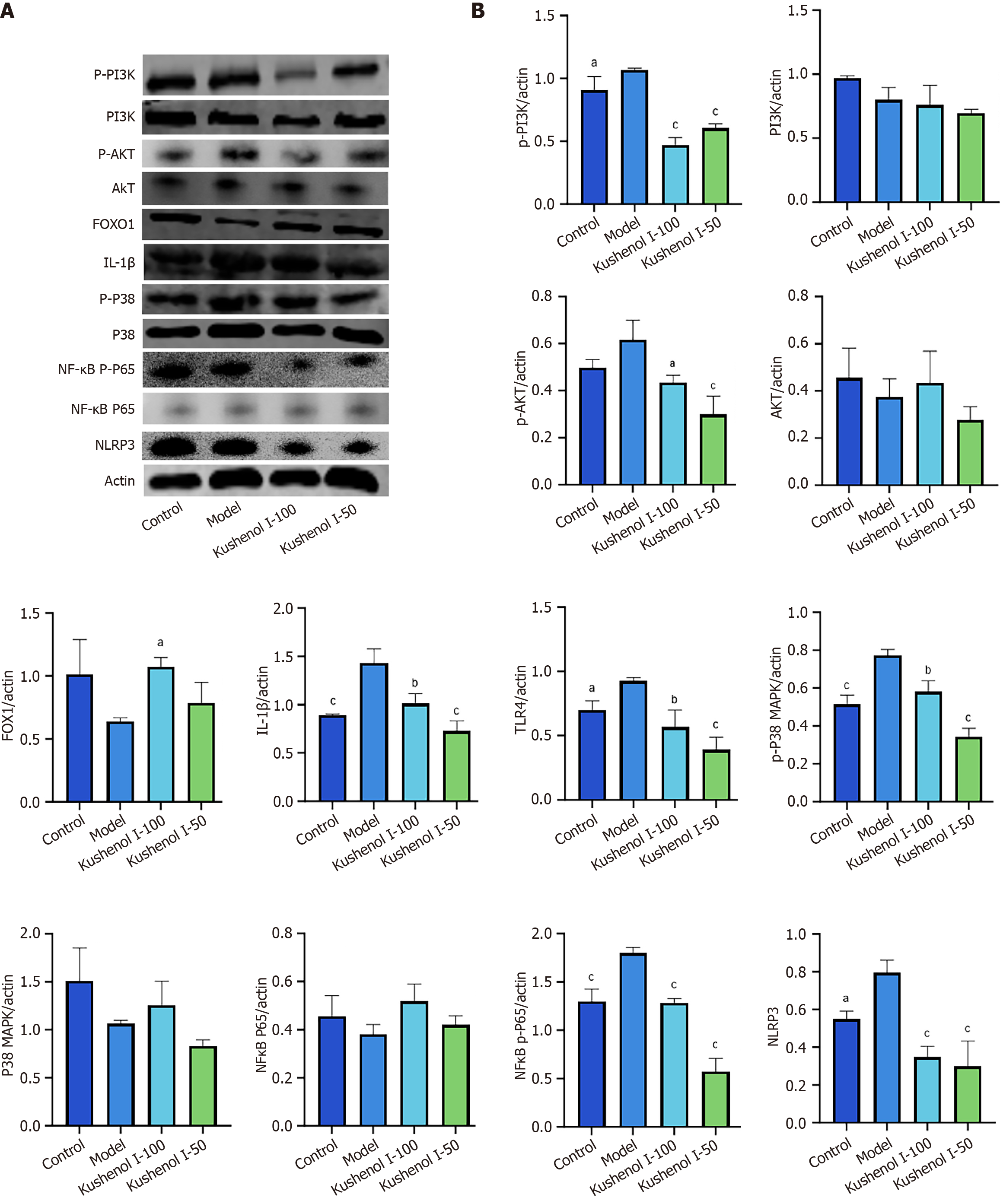
Western blotting was used to quantify UC-related proteins (Figure 8). The phosphorylation of p-PI3K, p-AKT, p-p38 MAPK, and NF-κB p-p65 was significantly increased in the model group (P < 0.05 or P < 0.001); the expression of IL-1β, TLR4, and NLRP3 was notably elevated (P < 0.05 or P < 0.001); and the expression of FOXO1 exhibited a downward trend compared with that in the control group. However, KSCI intervention significantly inhibited the phosphorylation levels of p-PI3K, p-AKT, p-p38 MAPK, and NF-κB p-p65 in mice with UC (P < 0.05, P < 0.01, or P < 0.001), markedly reduced the expression of IL-1β, TLR4, and NLRP3 (P < 0.01 or P < 0.001), and increased the expression level of colonic FOXO1 (P < 0.05). Thus, KSCI could alleviate DSS-induced UC by regulating the inflammatory signaling pathway associated with UC.
The integrity of the colonic barrier of mice with UC is compromised, thereby stimulating an inflammatory response and promoting the progression of UC. AB-PAS staining was used to assess the effect of KSCI on the colonic barrier. As shown in Figure 9A, the number of goblet cells reduced significantly after DSS exposure. However, KSCI intervention effectively alleviated the damage to the mucus layer.
As shown in Figure 9B and C, the distribution of occludin and ZO-1 proteins in the colonic mucosa of mice in the model group exhibited irregularity, reduced staining intensity, and decreased expression compared with that in normal mice. Moreover, serum LPS levels were significantly increased (P < 0.05) after DSS induction. By contrast, KSCI intervention led to a more uniform distribution of occludin and ZO-1 proteins in the colon, accompanied by an increase in their expression. Moreover, serum LPS levels decreased significantly (P < 0.001). These findings provide evidence supporting the effectiveness of KSCI in enhancing the integrity of the colonic mucosa in mice with UC.
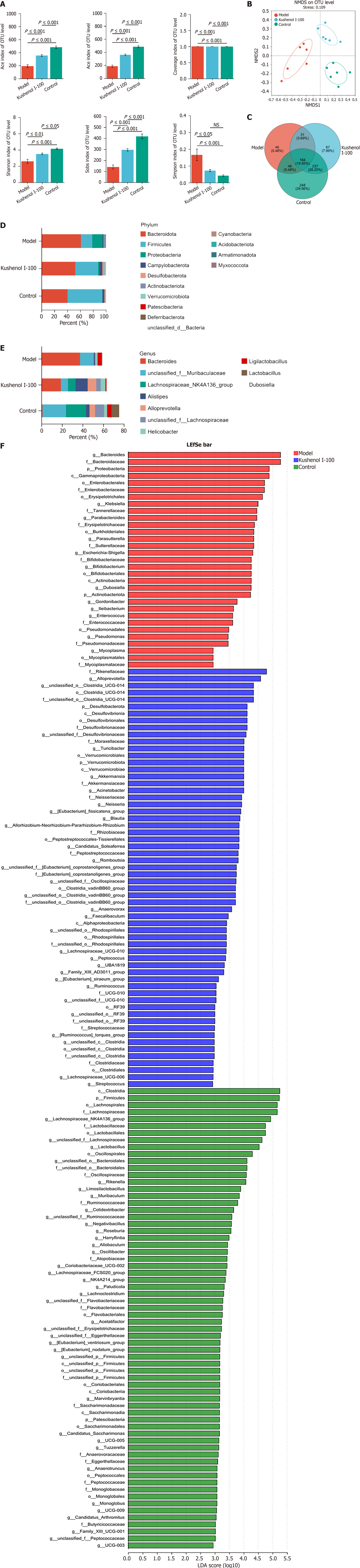
Alpha diversity on the OTU levels: Alpha diversity refers to the microbial diversity in a specific area or ecosystem, and the generally used metrics are the Chao, Shannon, Ace, Coverage, Simpson, and Sobs indices[11]. The higher the value of these indices, the greater the abundance of microbial species in the ecosystem. However, Simpson’s index contradicts this notion. As shown in Figure 10A, the alpha diversity of the model group was significantly reduced compared with that of the normal group. This was likely because DSS-induced inflammation promotes the overgrowth of certain harmful intestinal microbiota and inhibits the growth of normal flora, thereby reducing the abundance of the normal flora. However, KSCI intervention significantly increased the Chao, Shannon, Ace, Coverage, and Sobs indices while significantly decreasing the Simpson index. These findings suggest the potential of KSCI in enhancing the diversity and abundance of the intestinal microbiota in mice with UC, thereby promoting a more balanced and healthy microbial community.
Beta diversity at the OTU level: Beta diversity analysis explores similarities in the community composition by analyzing the species diversity of different microbial communities. In this study, we used the Bray-Curtis statistical algorithm to execute non-metric multidimensional scaling (NMDS) to determine the beta diversity of each group. The NMDS data (Figure 10B) indicated significant disparities in the intestinal flora between the control and model groups, and the KSCI group tended to be far away from the model group. Statistically, the analysis of similarities confirmed significant differences between the KSCI-treated and DSS groups (P < 0.001).
Abundance of intestinal flora: The total number of OTUs in fecal samples serves as an indicator of the diversity of the composition of the intestinal microbiota. As depicted in Figure 10C, the Venn diagram visually represents the shared and unique OTU data across three distinct groups. Specifically, the proportion of shared species between the KSCI treatment group and the control group is 28.25%, that between the KSCI treatment group and the model group is 3.69%, and that between the control and model groups is 5.48%. These findings suggest that DSS-induced UC led to a reduction in intestinal microbial diversity, while KSCI intervention ameliorated the microbial imbalance in UC-afflicted mice.
An analysis of the alterations in gut microbiota at the phylum level was conducted across all groups (Figure 10D). Specifically, four predominant bacterial phyla, namely Bacteroidota, Firmicutes, Proteobacteria, and Actinobacteriota, collectively accounting for > 90% of all phyla and prevalent in all samples, were identified. Following DSS treatment, a notable increase in the proportions of Bacteroidota, Proteobacteria, and Actinobacteriota was observed (P < 0.05 or P < 0.001), whereas a declining trend in Firmicutes was noted. By contrast, KSCI treatment resulted in a significant reduction in the relative abundance of Bacteroidota, Proteobacteria, and Actinobacteriota (P < 0.05 or P < 0.001) and an upward trend in the relative abundance of Firmicutes, thereby indicating the regulation of the gut microbiota by KSCI.
The abundance of the top 10 genera of gut microbiota is depicted in Figure 10E. Compared with the control group, significant alterations in the abundance of Bacteroides, unclassified Lachnospiraceae, and Lachnospiraceae NK4A136 group were observed in the DSS group (P < 0.05, P < 0.01, or P < 0.001). Notably, mitigation of the imbalance of Bacteroides, Helicobacter, and Parabacteroides by KSCI was significant (P < 0.05 or P < 0.01). Conversely, treatment with KSCI resulted in a significant reduction in the relative abundance of Bacteroides while showing an increasing trend in the relative abundance of Lachnospiraceae NK4A136 group and unclassified Lachnospiraceae. These findings are suggestive of the beneficial effects of KSCI on gut health.
Linear discriminant analysis (LDA) effect size was used to evaluate the taxonomic composition and relative abundance of the gut microbiota in mice subjected to various treatments (Figure 10F). Based on the LDA values, the normal group displayed significant differences in microbial taxa, including Clostridiales (LDA = 5.25; P < 0.01) and Firmicutes (LDA = 5.23; P < 0.01). After DSS exposure, mice with UC exhibited notable alterations in Bacteroidaceae (LDA = 5.27, P < 0.001) and Bacteroides (LDA = 5.27; P < 0.001). Conversely, oral administration of KSCI to mice resulted in the most pronounced changes in Rikenellaceae (LDA = 4.79; P < 0.05) and Alloprevotella (LDA = 4.58; P < 0.05). The diverse array of bacterial flora, such as Bacteroidaceae and Bacteroides, were classified within the phylum Bacteroidota. Likewise, Rikenellaceae and Alloprevotella were also assigned to the phylum Bacteroidota. By contrast, Clostridiales, which are part of the Firmicutes phylum, were highly prevalent in the intestinal microbiota. These results were in agreement with the phylum-level distribution of microbiota.
The recovery from UC is influenced by multiple factors, including restoration of the intestinal barrier, suppression of pro-inflammatory cytokines, and proliferation and differentiation of epithelial cells[12]. Drug therapy is the primary treatment for UC. Network pharmacology analysis indicated the potential of therapeutic intervention and the targets of KSCI on UC. Binding stability between KSCI and its targets was predicted using molecular docking and MD simulations, and the findings indicated that the therapeutic effects of KSCI in UC may involve the PI3K/AKT and p38 MAPK signaling pathways.
The protective effect of KSCI was determined using a DSS-induced male mouse model of UC. Sulfasalazine was employed as a positive control for the assurance of phenotypic validity; however, its application was omitted in mechanistic studies to avoid potential interference with the mechanisms under investigation. Notably, studies show that UC incidence is higher in males than females, possibly due to estrogen’s protective effects through receptor beta activation, which promotes mucosal healing by modulating branched-chain amino acid transport and epithelial cell migration[13,14]. Therefore, male mice were used in this study to obtain clear pathological manifestations, though KSCI’s therapeutic effects in females may be more pronounced due to estrogen synergy. Notable features of the model animals include weight loss and an increased DAI score[12]. The prevention of weight loss and reduction of the DAI score can decelerate the progression of UC. Additionally, UC is closely linked to elevated levels of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6[15]. IL-1β is acted upon as a central regulator for triggering the inflammatory response, and the secretion of TNF-α is modulated by IL-1β[16]. IL-6 is characteristic cytokine expressed in colitis and is a key pathophysiological regulator of the onset, progression, and eventual resolution of inflammation[17]. IL-10 is a crucial immunoregulatory cytokine that can ameliorate the inflammatory response by inhibiting the production of CD4+ and CD8+ T cells[18]. CD3+ and CD4+ cells are generally recognized as regulators of the immune response, and their reduction may lead to impaired immune-system function[19]; conversely, CD8+ cells play a vital role in the inflammatory response, with their increase indicating an aggravated inflammatory state. KSCI intervention effectively alleviated weight loss and reduced the DAI score in mice with UC, and also lowered the mRNA levels and secretion levels of colonic
The inflamed intestinal cells generate an excess of reactive oxygen species, resulting in tissue damage and oxidative stress[16]. Abnormal oxidative stress may expedite the progression of UC and potentially lead to rectal cancer[20]. The abnormal oxidative stress state can be gauged by MDA levels and by MPO, SOD, and GSH-PX activities. KSCI intervention decreased MDA levels and MPO activity, concurrently increasing SOD and GSH-PX activities, suggesting that this compound might mitigate UC by modulating the activity of the antioxidant enzymes. Nevertheless, these findings solely represent phenotypic observations, and the underlying mechanisms necessitate further exploration.
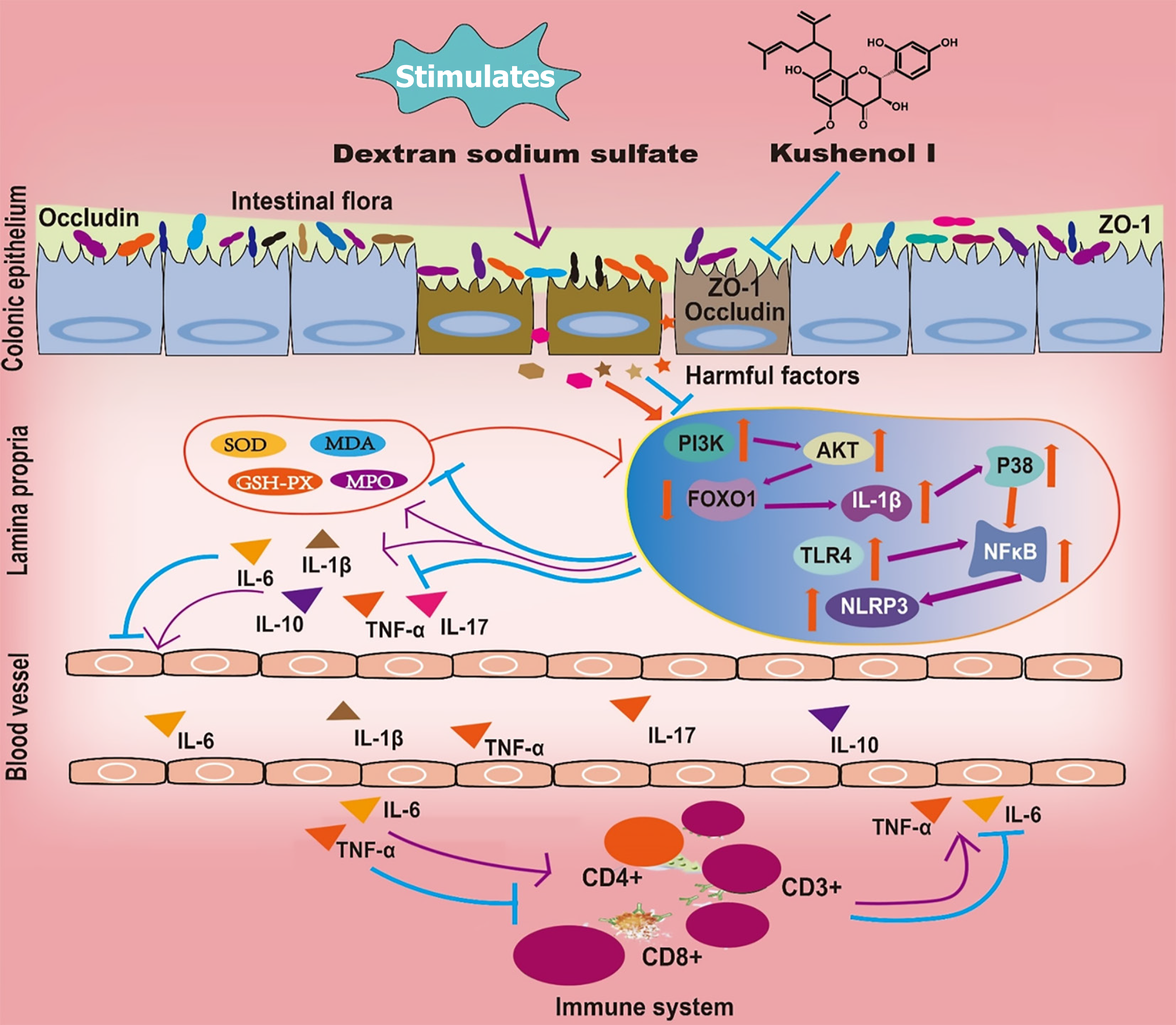
The activation of PI3K triggers AKT activation, leading to the promotion of inflammatory cell activation and the production of inflammatory factors[21]. Furthermore, activated AKT negatively regulates FOXO1 expression, resulting in a further increase in IL-1β expression[22-24] IL-1β activates p38 MAPK, thereby enhancing the inflammatory response. Next, activated p38 MAPK recruits and subsequently enhances the nuclear translocation of NF-κB, triggering the downstream activation of the NF-κB pathway and secretion of pro-inflammatory mediators, and positively regulates NLRP3, leading to intestinal inflammation[16]. Activation of the NF-κB p65/NLRP3 signaling pathway in the colonic mucosa of mice with UC stimulates the release of inflammatory cytokines by neutrophils and macrophages[16]. The inflamed intestinal cells generate an excess of reactive oxygen species, resulting in tissue damage and oxidative stress, subsequently culminating in increased intestinal epithelial cell-barrier permeability and facilitating LPS absorption into the bloodstream. The end effect is the inflammatory response[25]. Western blotting was used to evaluate the protein levels of UC-related factors, such as p-PI3K, p-AKT, IL-1β, p-p38 MAPK, NF-κB p-p65, FOXO1, and NLRP3. The expression of colonic p-PI3K, p-AKT, IL-1β, p-p38 MAPK, NF-κB p-p65, and NLRP3 was significantly inhibited by KSCI in mice with colitis, whereas the expression of FOXO1 increased, which may be the reason for the decrease in colonic IL-1β, IL-6, IL-17, and TNF-α levels and oxidative stress levels.
The protective effect of KSCI against DSS-induced UC has been confirmed based on the findings related to weight loss, DAI score, inflammatory response, and oxidative status. However, the mechanism underlying the protective effect of KSCI remains to be elucidated. DSS-induced damage to the intestinal barrier is characterized by a reduction in the thicknesses of the mucus layer and tight junction proteins, and by the disruption of mucosal and crypt structures[25]. Intestinal barrier damage is observed in colitis, which results in increased intestinal permeability. Subsequently, toxins and microbial antigens can cross the lamina propria and enter the systemic circulation, leading to disease states[25]. Therefore, restoring the altered barrier function is a crucial aspect in treating UC. The mucus layer is an indicator of the integrity of the intestinal barrier and safeguards epithelial cells from harmful substances[26]. To elucidate the potential mechanisms of KSCI in alleviating DSS-induced UC, immunohistochemistry was used to assess the expression of ZO-1 and occludin in colon tissues. A decrease in the levels of the tight junction proteins ZO-1 and occludin is a clear symptom of UC. The findings of our study suggest that KSCI intervention augmented the expression of ZO-1 and occludin and decreased LPS absorption into the bloodstream, indicating the role of KSCI in restoring the intestinal barrier and preserving its function.
Intestinal dysbiosis is commonly observed in patients with UC and is believed to be involved in disease development[12]. Previous studies have demonstrated that an increase in the relative abundance of Proteobacteria, Bacteroidota, and Actinobacteriota, along with a decrease in the relative abundance of Firmicutes, is indicative of intestinal inflammation in animals including mice with DSS-induced UC[16,17]. Additionally, there was a significant increase in the abundance of the genus Bacteroides, along with a noticeable decrease in the abundance of the genus Lachnospiraceae NK4A136 group and unclassified Lachnospiraceae in mice with DSS-induced UC[15,16]. The present study demonstrated that KSCI intervention significantly modulated gut microbiota composition, marked by reduced Bacteroidota, Actinobacteriotaand Proteobacteria at the phylum level. Concurrently, KSCI elevated Bacteroides with decreased Lachnospiraceae NK4A136 group/unclassified Lachnospiraceae at the genus level. These microbial shifts correlated with therapeutic improvements, including attenuated colonic inflammation, enhanced barrier function, and elevated anti-inflammatory IL-10 levels, consistent with the known roles of these bacterial taxa in maintaining mucosal homeostasis. These findings suggested that KSCI intervention may have an impact on the gut microbiota, which could be associated with the alleviation of UC. However, further research is warranted to elucidate the molecular basis of the functional activities of intestinal bacteria. The isolation and cultivation of specific gut bacteria pose significant challenges.
Compared to sulfasalazine, KSCI showed significant advantages in reducing weight loss and modulating colonic inflammation, with decreased levels of pro-inflammatory cytokines (IL-1β, IL-17) and increased levels of the anti-inflammatory cytokine IL-10 at lower doses of 50-100 mg, which might be attributed to the regulation of multiple pathways. These findings strongly suggest KSCI as a promising therapeutic candidate for UC. To achieve clinical translation of KSCI for UC therapy, the long-term toxicity of KSCI for chronic use should be systematically evaluated. Functional insights beyond 16S rRNA sequencing are also worth revealing using metabolomics or metagenomics. Additionally, KSCI’s efficacy and mechanism in human-relevant models (e.g., organoids/clinical samples) should be evaluated.
KSCI significantly alleviated the symptoms of DSS-induced UC by adjusting the composition of the gut microbiota, maintaining the integrity of the intestinal mucosal barrier, decreasing inflammation and oxidative stress, and fine-tuning the immune response (Figure 11). These multidimensional mechanisms of action underscore the importance of KSCI as a potential treatment approach in the management of UC. Therefore, KSCI is anticipated to be an effective drug in the treatment of UC.
| 1. | Colombel JF, Mahadevan U. Inflammatory Bowel Disease 2017: Innovations and Changing Paradigms. Gastroenterology. 2017;152:309-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 2. | Vindigni SM, Zisman TL, Suskind DL, Damman CJ. The intestinal microbiome, barrier function, and immune system in inflammatory bowel disease: a tripartite pathophysiological circuit with implications for new therapeutic directions. Therap Adv Gastroenterol. 2016;9:606-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 3. | Li JJ, Zhang X, Shen XC, Long QD, Xu CY, Tan CJ, Lin Y. Phytochemistry and biological properties of isoprenoid flavonoids from Sophora flavescens Ait. Fitoterapia. 2020;143:104556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Peng Z, Zheng Z, Gao M, Qin L, Xiong L, Li X, Xie J. Mechanisms of Compound Sophora flavescens (Kushen) Decoction for the Treatment of Ulcerative Colitis based on Network Pharmacology and Molecular Docking Technology. Curr Comput Aided Drug Des. 2022;18:228-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Li FJ, Zhang F, He XD, Liu X, Mu JK, Yang M, Li YQ, Gu W, Yu J, Yang XX. A Novel Method for Identifying Parkin Binding Agents in Complex Preparations of Herbal Medicines. Oxid Med Cell Longev. 2022;2022:3260243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Wu L, Miyase T, Ueno A, Kuroyanagi M, Noro T, Fukushima S, Sasaki S. Studies on the Constituents of Sophora flavescens AIT. V. YAKUGAKU ZASSHI. 1986;106:22-26. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Xie L, Gong W, Chen J, Xie HW, Wang M, Yin XP, Wu W. The flavonoid kurarinone inhibits clinical progression of EAE through inhibiting Th1 and Th17 cell differentiation and proliferation. Int Immunopharmacol. 2018;62:227-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | He J, Wan C, Li X, Zhang Z, Yang Y, Wang H, Qi Y. Bioactive Components and Potential Mechanism Prediction of Kui Jie Kang against Ulcerative Colitis via Systematic Pharmacology and UPLC-QE-MS Analysis. Evid Based Complement Alternat Med. 2022;2022:9122315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 9. | Wlodarska M, Thaiss CA, Nowarski R, Henao-Mejia J, Zhang JP, Brown EM, Frankel G, Levy M, Katz MN, Philbrick WM, Elinav E, Finlay BB, Flavell RA. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell. 2014;156:1045-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 563] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 10. | Guo WL, Guo JB, Liu BY, Lu JQ, Chen M, Liu B, Bai WD, Rao PF, Ni L, Lv XC. Ganoderic acid A from Ganoderma lucidum ameliorates lipid metabolism and alters gut microbiota composition in hyperlipidemic mice fed a high-fat diet. Food Funct. 2020;11:6818-6833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 11. | Zhang X, Zhang N, Kan J, Sun R, Tang S, Wang Z, Chen M, Liu J, Jin C. Anti-inflammatory activity of alkali-soluble polysaccharides from Arctium lappa L. and its effect on gut microbiota of mice with inflammation. Int J Biol Macromol. 2020;154:773-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 12. | Mao B, Guo W, Cui S, Zhang Q, Zhao J, Tang X, Zhang H. Blautia producta displays potential probiotic properties against dextran sulfate sodium-induced colitis in mice. Food Sci Hum Wellness. 2024;13:709-720. [DOI] [Full Text] |
| 13. | Goodman WA, Erkkila IP, Pizarro TT. Sex matters: impact on pathogenesis, presentation and treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17:740-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 14. | Guo Y, Zhu Y, Zhang J, He Y, Zhao M, Lin H, Wei Z, Xia Y, Dai Y. Facilitation of mucosal healing by estrogen receptor β in ulcerative colitis through suppression of branched-chain amino acid transport and subsequent triggering of autophagy in colonic epithelial cells. Acta Pharm Sin B. 2025;15:168-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Li X, Lv H, Shi F, Song J, Zhang Z. The potential therapeutic effects of hydroxypropyl cellulose on acute murine colitis induced by DSS. Carbohydr Polym. 2022;289:119430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Li L, Qiu N, Meng Y, Wang C, Mine Y, Keast R, Guyonnet V. Preserved egg white alleviates DSS-induced colitis in mice through the reduction of oxidative stress, modulation of infl ammatory cytokines, NF-κB, MAPK and gut microbiota composition. Food Sci Hum Wellness. 2023;12:312-323. [RCA] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 17. | Gong H, Gan X, Qin B, Chen J, Zhao Y, Qiu B, Chen W, Yu Y, Shi S, Li T, Liu D, Li B, Wang S, Wang H. Structural characteristics of steamed Polygonatum cyrtonema polysaccharide and its bioactivity on colitis via improving the intestinal barrier and modifying the gut microbiota. Carbohydr Polym. 2024;327:121669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 18. | Blackburn SD, Wherry EJ. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 2007;15:143-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 178] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 19. | Xiang J, Hu R, Li Q, Zhang Y, Li S, Wang X, Song Y. Total Glucosides of Paeony Attenuates Ulcerative Colitis via Inhibiting TLR4/NF-κB Signaling Pathway. Tohoku J Exp Med. 2022;258:225-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 20. | Li HL, Wei YY, Li XH, Zhang SS, Zhang RT, Li JH, Ma BW, Shao SB, Lv ZW, Ruan H, Zhou HG, Yang C. Diosmetin has therapeutic efficacy in colitis regulating gut microbiota, inflammation, and oxidative stress via the circ-Sirt1/Sirt1 axis. Acta Pharmacol Sin. 2022;43:919-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 21. | Liu J, Liu J, Tong X, Peng W, Wei S, Sun T, Wang Y, Zhang B, Li W. Network Pharmacology Prediction and Molecular Docking-Based Strategy to Discover the Potential Pharmacological Mechanism of Huai Hua San Against Ulcerative Colitis. Drug Des Devel Ther. 2021;15:3255-3276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 154] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 22. | Kawamori D, Kaneto H, Nakatani Y, Matsuoka TA, Matsuhisa M, Hori M, Yamasaki Y. The forkhead transcription factor Foxo1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation. J Biol Chem. 2006;281:1091-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 211] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 23. | Martinez SC, Tanabe K, Cras-Méneur C, Abumrad NA, Bernal-Mizrachi E, Permutt MA. Inhibition of Foxo1 protects pancreatic islet beta-cells against fatty acid and endoplasmic reticulum stress-induced apoptosis. Diabetes. 2008;57:846-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 24. | Cho MJ, Kim DH, Ha S, Bang E, Jung HJ, Moon HR, Chung HY. MHY2013 Modulates Age-related Inflammation and Insulin Resistance by Suppressing the Akt/FOXO1/IL-1β Axis and MAPK-mediated NF-κB Signaling in Aged Rat Liver. Appl Immunohistochem Mol Morphol. 2020;28:579-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Li H, Cao W, Xie J, Che H, Liu L, Dong X, Song L, Xie W. α-D-1,6-glucan from Castanea mollissima Blume alleviates dextran sulfate sodium-induced colitis in vivo. Carbohydr Polym. 2022;289:119410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 26. | Rupani B, Caputo FJ, Watkins AC, Vega D, Magnotti LJ, Lu Q, Xu DZ, Deitch EA. Relationship between disruption of the unstirred mucus layer and intestinal restitution in loss of gut barrier function after trauma hemorrhagic shock. Surgery. 2007;141:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |