Published online Mar 14, 2018. doi: 10.3748/wjg.v24.i10.1167
Peer-review started: January 9, 2018
First decision: February 5, 2018
Revised: February 18, 2018
Accepted: February 15, 2018
Article in press: February 15, 2018
Published online: March 14, 2018
Processing time: 63 Days and 15.3 Hours
To perform a systematic review and meta-analysis for the diagnostic accuracy of in vivo lesion characterization in colonic inflammatory bowel disease (IBD), using optical imaging techniques, including virtual chromoendoscopy (VCE), dye-based chromoendoscopy (DBC), magnification endoscopy and confocal laser endomicroscopy (CLE).
We searched Medline, Embase and the Cochrane library. We performed a bivariate meta-analysis to calculate the pooled estimate sensitivities, specificities, positive and negative likelihood ratios (+LHR, -LHR), diagnostic odds ratios (DOR), and area under the SROC curve (AUSROC) for each technology group. A subgroup analysis was performed to investigate differences in real-time non-magnified Kudo pit patterns (with VCE and DBC) and real-time CLE.
We included 22 studies [1491 patients; 4674 polyps, of which 539 (11.5%) were neoplastic]. Real-time CLE had a pooled sensitivity of 91% (95%CI: 66%-98%), specificity of 97% (95%CI: 94%-98%), and an AUSROC of 0.98 (95%CI: 0.97-0.99). Magnification endoscopy had a pooled sensitivity of 90% (95%CI: 77%-96%) and specificity of 87% (95%CI: 81%-91%). VCE had a pooled sensitivity of 86% (95%CI: 62%-95%) and specificity of 87% (95%CI: 72%-95%). DBC had a pooled sensitivity of 67% (95%CI: 44%-84%) and specificity of 86% (95%CI: 72%-94%).
Real-time CLE is a highly accurate technology for differentiating neoplastic from non-neoplastic lesions in patients with colonic IBD. However, most CLE studies were performed by single expert users within tertiary centres, potentially confounding these results.
Core tip:In vivo lesion characterization in colonic inflammatory bowel disease presents many challenges. Lesions tend to be morphologically different and potentially associated with surrounding/overlying inflammation, obscuring the pit pattern. The ability to accurately characterize lesions in vivo could reduce costs and complications by decreasing the need for polypectomies. Virtual chromoendoscopy (VCE) and dye-based chromoendoscopy currently cannot be recommended for lesion characterization. Confocal laser endomicroscopy is an accurate technology at differentiating neoplastic from non-neoplastic lesions but studies within this meta-analysis involved single expert center with single advanced endoscopic operators, reducing its generalizability. Larger studies are required specifically looking at lesion characterization, especially with rapid technological advancements in VCE (Narrow band imaging, i-scan, Fujinon intelligence chromoendoscopy).
- Citation: Lord R, Burr NE, Mohammed N, Subramanian V. Colonic lesion characterization in inflammatory bowel disease: A systematic review and meta-analysis. World J Gastroenterol 2018; 24(10): 1167-1180
- URL: https://www.wjgnet.com/1007-9327/full/v24/i10/1167.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i10.1167
The association between colonic inflammatory bowel disease (IBD) and colorectal cancer (CRC) has been acknowledged for almost 100 years[1]. Several meta-analyses have attempted to estimate this increased risk with varying results, reflecting the heterogeneity of studies included[2-4]. Nevertheless all agree that disease duration, disease activity and extent of IBD increase the risk for developing CRC. In response, surveillance colonoscopy is recommended by most gastroenterology societies worldwide. Yet, there is still disparity amongst the societies with regards timing of surveillance intervals.
Most CRC within IBD is thought to develop along the inflammation-dysplasia-cancer pathway; however in rare cases it may not always evolve in this stepwise fashion, and its rate of transition could potentially be accelerated in some lesions[5].
With advancements in endoscopic technology and recommendations for surveillance during inactive disease, most dysplasia is now believed to be visible[6]. Gastroenterological societies currently advocate targeted biopsies for detection of dysplasia, owing to the low yield from random biopsies, with evidence supporting dye-based chromoendoscopy (DBC) for enhancing lesion detection[6-8]. An international consensus group in 2015 recommended that dysplastic polypoid or non-polypoid lesions within a colitic segment should be treated as significant and that well circumscribed lesions with no endoscopic features of submucosal invasion can now be resected[6]. The risk for developing CRC following complete endoscopic resection is now thought to be lower than previous studies suggested[9].
Novel technologies, including narrow band imaging (NBI), fujinon intelligence chromoendoscopy (FICE), i-scan, magnification endoscopy and confocal laser endomicroscopy (CLE), have been studied to obtain an in-vivo optical diagnosis of colorectal lesions. DBC using contrast agents, such as indigo-carmine, or absorptive agents, like methylene blue, are customarily applied via a spray catheter to provide mucosal enhancement. Virtual chromoendoscopy (NBI, FICE, i-scan) are dye-less enhancement technologies that are built into the colonoscope or processor. NBI uses optical filter enhancement at the distal end of the endoscope, narrowing the light bandwidth, thereby improving visualization of the mucosa. FICE and i-scan use digital post-processing technology with spectral estimation to achieve mucosal enhancement. Magnification endoscopy possesses a variable lens, providing magnification up to 150-fold, permitting detailed examination of the mucosal pit patterns. Whilst CLE technology involves focusing laser light onto the mucosa and the reflected light is returned via a pinhole. This filters out non-focused light, giving a highly magnified, real-time histological diagnosis. CLE can either be integrated (iCLE) within the endoscope or via a probe (pCLE), which can be passed through the biopsy channel.
In patients without colitis, multiple studies have looked at in-vivo optical diagnosis of colorectal lesions using these technologies, allowing differentiation between neoplastic and non-neoplastic lesions. The hope that this would be cost-effective, reduce risk associated with polypectomy and provide instant determination of polyp surveillance intervals for the patient. A recent meta-analysis by the ASGE group looked at novel technologies to allow a “diagnose and leave” and “resect and discard” strategy[10]. To achieve a “diagnose and leave” strategy, (a decision to leave in-situ diminutive rectosigmoid polyps), the technology had to achieve > 90% NPV for adenomatous histology. To achieve a “resect and discard” strategy, (remove diminutive adenomatous polyps without histological assessment), the technology should provide > 90% agreement in post-polypectomy surveillance intervals. The meta-analysis showed that this could only be achieved with NBI technology, in endoscopists that were experienced and that the assessment of the polyp was made with high confidence. Recently a large multicenter prospective study evaluated the use of NBI assisted optical diagnosis in non-expert endoscopists for small colonic polyps and was found to not achieve the above criteria[11].
The accuracy of these technologies during surveillance colonoscopy in colonic IBD is unclear with the majority of studies being small and assessed as secondary outcomes. With additional hurdles to overcome in patients with colitis, such as active inflammation and the fact that lesions tend to be morphologically different (flatter rather than polypoid), how precise are we at characterizing lesions in IBD with the current technologies available. Our objective was to perform the first systematic review and meta-analysis for the diagnostic accuracy of optical imaging techniques for in-vivo lesion characterization in colonic IBD. We aimed to calculate the pooled estimated sensitivities, specificities, positive and negative likelihood ratios (+LHR, -LHR), diagnostic odd ratios (DOR), and area under summary receiver-operator characteristic (AUSROC) curve for each technology type, with histopathology as the reference standard. We also planned to perform a subgroup analysis looking at the accuracy of studies using real-time non-magnified Kudo pit pattern (Kudo PP) and real-time CLE[12].
We performed a meta-analysis in concordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines[13]. RL searched Medline (from 1946 to May 2017) and Embase (from 1974 to May 2017), using the healthcare databases advanced search (HDAS) system. The search terms used included: ((((“high definition”).ti,ab OR (HD).ti,ab OR (“white light”).ti,ab OR (WL).ti,ab OR (chromoendoscop*).ti,ab OR (CE).ti,ab OR (NBI).ti,ab OR (“narrow band”).ti,ab OR (FICE).ti,ab OR (“fujinon intelligent chromoendoscopy”).ti,ab OR (“I-scan”).ti,ab OR (AFI).ti,ab OR (autofluorescence).ti,ab OR (CLE).ti,ab OR (“confocal laser”).ti,ab OR (“real time histology”).ti,ab) AND ((“colon imag*”).ti,ab OR (“intestinal imag*”).ti,ab OR (colonoscop*).ti,ab)) AND ((“inflammatory bowel disease”).ti,ab OR (IBD).ti,ab OR (coliti*).ti,ab OR (uc).ti,ab OR (“ulcerative coliti*”).ti,ab OR (“crohns coliti*”).ti,ab OR (“crohn’s coliti*”).ti,ab)) AND ((lesion*).ti,ab OR (polyp*).ti,ab OR (dysplas*).ti,ab OR (neoplas*).ti,ab)”. A Cochrane Library search for any systematic reviews relevant to this area was also performed. No language restrictions were used. The results for each database were combined and any duplicates removed.
Study inclusion and exclusion was determined by predefined criteria.
Inclusion criteria: (1) Studies using novel technologies to provide in-vivo optical characterization of lesions in patients with colonic IBD during colonoscopy; (2) characterized lesions into neoplastic and non-neoplastic using histology as the reference standard; (3) able to extract data to obtain a 2 × 2 contingency table to calculate the true positive (TP), false positive (FP), false negative (FN) and true negative (TN); and (4) Real-time characterization or retrospective image-review.
Exclusion criteria: (1) Case studies or case series; (2) studies not involving patients with colonic ibd; (3) inability to construct a 2 × 2 contingency table from the data given; (4) inability to differentiate detection from characterization studies; and (5) not used histology as reference standard (6) children (age < 16).
RL and NB identified study eligibility using the above inclusion and exclusion criteria. We searched the combined list of results for relevant studies, looking at the abstract or if supplementary information required, the full article. Reference lists of selected papers were also checked for potential missed articles. Abstract or articles for clinical trials or observational studies were eligible for inclusion if characterization of lesions by NBI, FICE, i-scan, DBC, magnification endoscopy or CLE, differentiated neoplastic from non-neoplastic lesions in colonic IBD, using histopathology as the gold standard. From this, data was extracted using a 2 × 2 contingency table. If exact figures for the true positive (TP), false positive (FP), false negative (FN) and true negative (TN) were not represented in the articles, it was calculated from the documented sensitivity, specificity, accuracy, positive predictive value (PPV) or negative predictive value (NPV). RL and VS performed data ascertainment and calculations. If TP, FP, FN and TN couldn’t be calculated from the article data, attempts were made to contact relevant authors by email for clarification of figures.
As studies included were diagnostic, RL and NM used the QUADAS-2 (quality assessment of diagnostic accuracy studies) tool to independently assess the degree of study validity[14]. This looks at the risk of bias and applicability regarding four domains: patient selection, index test, reference standard, flow and timing. Risk of bias (involved all four domains) and applicability (involved three domains) is scored using low risk, high risk or unclear. Any indifference on determining risk between RL and NM was discussed and clarified with VS, who made the final decision.
In performing a systematic review for diagnostic studies, a bivariate meta-analysis using a random effects model was performed, allowing for the assumption of heterogeneity between the studies[15]. A random effects model was used in order to provide a more conservative result due to differences between study methods such as endoscopic expertise, classification model, study type and the population studied. We obtained summary estimates for sensitivity, specificity, +LHR, -LHR and DOR, with their 95% confidence intervals. A hierarchical summary receiver-operator characteristic (ROC) curve was plotted, with its summary point estimate, and a dashed line around representing its 95% confidence interval. The area under the SROC curve (AUROC) served as a marker of test accuracy. Forest plots were also calculated to demonstrate study sensitivity and specificity.
Heterogeneity between studies was assessed using the Cochrane Q and I2 tests. Cochrane Q is established upon the chi-squared test, providing a weighted sum of the squared differences of each study estimate from the overall pooled estimate. P valves are given. I2 describes the percentage of variation between studies that is due to heterogeneity rather than chance and is not dependent on the number of studies included. I2 quantifies the impact of heterogeneity on the meta-analysis rather than just the extent of heterogeneity. Results range from 0-100%: 0% indicates there is no heterogeneity between the studies, whereas scores > 50% equate to moderate heterogeneity and > 75% high heterogeneity.
To help determine factors that may account for heterogeneity, we performed a subgroup analysis concentrating on real-time mucosal characterization, dividing into two groups: non-magnified Kudo PP (using VCE and DBC) and CLE. We also pooled results for all studies (real-time and retrospective image-capture) looking at non-magnified Kudo PP.
The Deeks et al[16] funnel plot assessed for publication bias. This uses regression of diagnostic log odds ratio against1/sqrt (effective sample size), weighting by sample size with a P < 0.10 for the slope coefficient as an indicator of substantial asymmetry.
All data analysis was done using Stata version 13 (Stata Corp, Texas, United States) using the user written command Midas (Dwamena, 2009)[17].
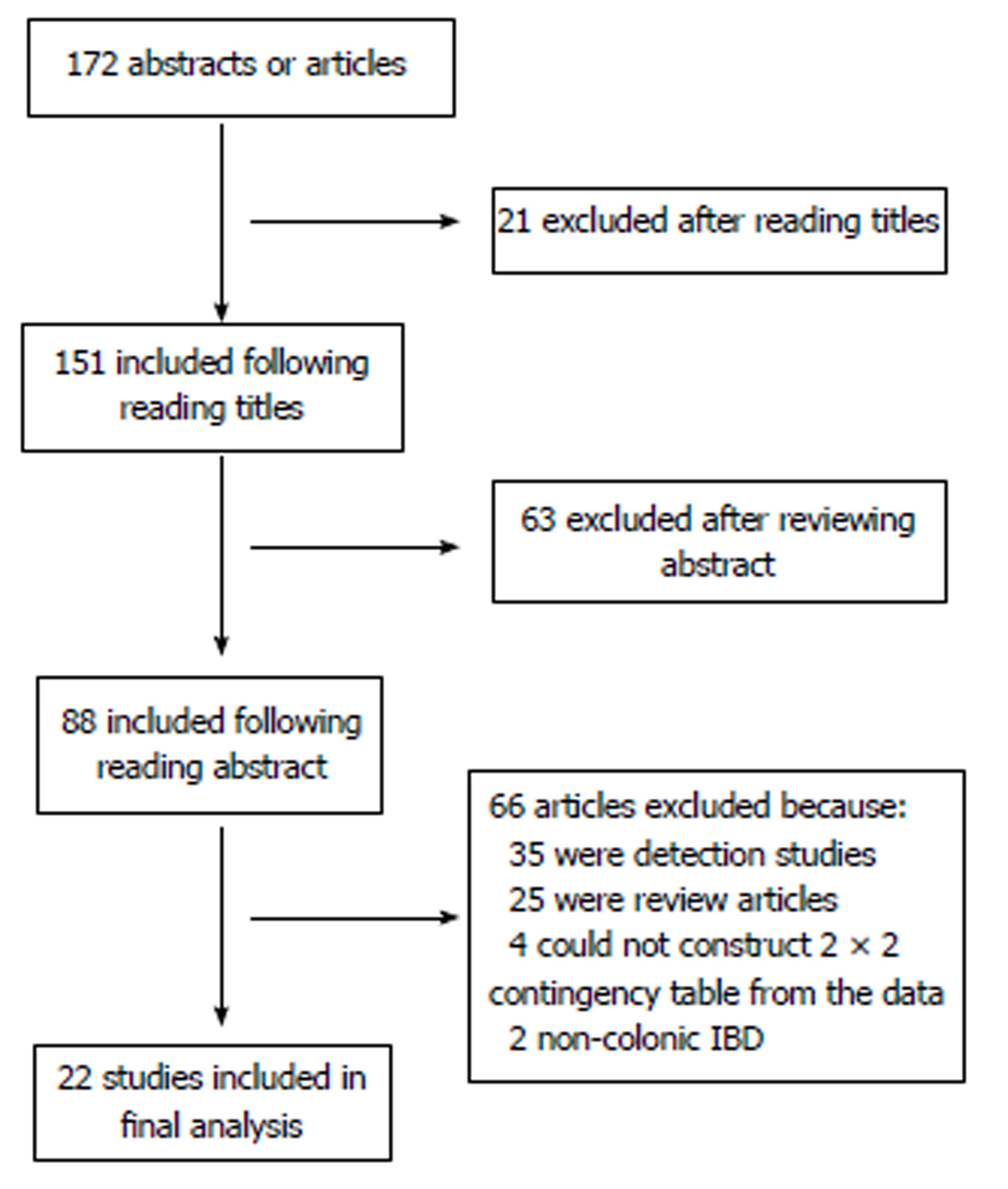
One hundred and seventy-two abstracts and articles were obtained following the initial keyword search, following removal of duplicates (Figure 1). 21 studies were excluded following screening of the title, leaving 151 citations. A further 63 studies were excluded following review of the abstract, leaving 88 citations. 66 more studies were excluded following review of papers as a result of: 35 being detection studies, 25 were review articles, 2 involved patients without colonic IBD and 4 we were unable to construct a 2 × 2 contingency table.
The characteristics of the 22 studies included are presented in Table 1[18-39]. Twenty-one studies included 1491 patients, with one study not reporting the number of patients included, and 4674 lesions, of which 539 (11.5%) were neoplastic.
| Authors | Year | Abstract/article | Technology | Number of endoscopists | Study design | Real time vs Image review | No. of Patients | No. of Polyps | Mucosal classification method |
| Virtual Chromoendoscopy | |||||||||
| Cassinotti et al[22] | 2016 | Abstract | i-scan HD | / | Single centre/prospective cohort | Real time | 40 | 287 | Kudo PP + other endoscopic features |
| Prospective cohort | |||||||||
| Efthymiou et al[21] | 2013 | Article | NBI HD | 2 | Single centre/prospective cohort | Real time | 44 | 121 | Kudo PP + low level magnification |
| Van den broek et al[23] | 2011 | Article | NBI HD | 4 | Single centre/randomized cross-over | Real time | 48 | 153 | Kudo PP |
| Cassinotti et al[24] | 2015 | Abstract | FICE HD | 1 | Single centre/randomized parallel | Real time | 41 | 261 | Kudo PP |
| Cassinotti et al[25] | 2015 | Abstract | FICE HD | 1 | Single centre/prospective cohort | Real time | 59 | 205 | Kudo PP |
| Dye-based Chromoendoscopy | |||||||||
| Carballal et al[26] | 2016 | Article | IC 0.4% SD/HD | 15 | Multi-centre/prospective cohort | Real time | 350 | 595 | Kudo PP + 10 other items |
| 1Buchner et al[27] | 2016 | Abstract | MB 0.1% HD | / | Prospective cohort | Real time | 22 | 21 | / |
| 2Wanders et al[20] | 2016 | Article | MB 0.1% SD | > 1 | Multi-centre/prospective cohort | Real time | 61 | 66 | Kudo PP |
| Munoz et al [28] | 2016 | Abstract | IC 0.2%-0.4% HD | > 1 | Multi-centre/retrospective cohort | Real time | 243 | 953 | Kudo PP |
| Wanders et al[29] | 2015 | Article | MB 0.1% or IC 0.3% | 17 | Multi-centre/retrospective questionnaire | Image review | / | 30 | / |
| 3Hlavaty et al [18] | 2011 | Article | IC 0.4% SD | 2 | Single centre/prospective cohort | Real time | 30 | 100 | Kudo PP |
| Confocal Laser Endomicroscopy | |||||||||
| 2Wanders et al[20] | 2016 | Article | iCLE | > 1 | Multi-centre/prospective cohort | Real time | 61 | 60 | Mainz criteria |
| Dlugosz et al[30] | 2016 | Article | pCLE | 1 endoscopist (2 reviewed images) | Single centre/retrospective cohort | Image review | 69 | 644 | Crypt + vessel architecture |
| 1Buchner et al[27] | 2016 | Abstract | pCLE | / | Prospective cohort | Real time | 22 | 20 | Miami classification |
| Freire et al[31] | 2014 | Article | iCLE | 1 | Single centre/randomized trial | Real time | 72 | 104 | Mainz criteria |
| Rispo et al[32] | 2012 | Article | pCLE | 1 | Single centre/prospective cohort | Real time | 51 | 15 | De Palma classification |
| Shahid et al[33] | 2011 | Abstract | pCLE | 3 reviewed images | Single centre/retrospective cohort | Image review | 25 | 61 | / |
| 3Hlavaty et al[18] | 2011 | Article | iCLE | 2 | Single centre/prospective cohort | Real time | 30 | 68 | Mainz classification |
| 4Van den broek et al[19] | 2011 | Article | pCLE | 4 endoscopists (2 reviewing images) | Single centre/retrospective cohort | Image review | 22 | 48 | Crypt + vessel architecture |
| Keisslich et al[34] | 2007 | Article | iCLE | / | Single centre/randomized trial | Real time | 80 | 134 | Mainz classification |
| Magnification endoscopy | |||||||||
| Nishiyama et al[35] | 2016 | Article | NBI | 5 reviewed images | Single centre/retrospective cohort | Image review | 27 | 33 | Surface + vessel patterns |
| 4Van den broek et al[19] | 2011 | Article | NBI | 4 | Single centre/prospective cohort | Real time | 22 | 48 | Kudo PP + vascular patterns |
| Van den broek et al[36] | 2008 | Article | NBI | 3 | Single centre/randomized trial | Real time | 50 | 98 | Kudo PP |
| Matsumoto et al[37] | 2007 | Article | NBI | 1 | Single centre/prospective cohort | Real time | 46 | 296 | Surface structure |
| Keisslich et al[38] | 2003 | Article | MB 0.1% | 1 | Single centre/randomized trial | Real time | 84 | 118 | Kudo PP |
| Studies using combined technologies | |||||||||
| Bisschops et al[39] | 2013 | Abstract | Dye-based chromo/NBI | 10 reviewed images | Multi-centre / Retrospective cohort | Image review | 27 | 50 | Kudo PP |
The VCE group consisted of five studies, with one study looking at i-scan technology, two studies involved NBI and a further two used FICE. Three of the papers were abstracts and two being articles. All of these studies used endoscopic real-time diagnosis of lesions.
The DBC group entailed six studies, using either indigo-carmine (0.2%-0.4%) or methylene blue (0.1%) as the contrast agent. One of these studies performed endoscopic lesion diagnosis using a retrospective image-captured questionnaire, whilst the others used real-time diagnosis. Two were abstracts with the others being articles.
The CLE group comprised of nine studies; four studies used iCLE and five studies used pCLE. Three studies were retrospective image based, with the remaining being real-time studies. Two were abstracts and the others being articles.
The magnification endoscopy group consisted of five studies, four of which being used in conjunction with NBI and one used with DBC. One study was retrospective image-based, with the others being real-time diagnosis. All were articles.
For the subgroup analysis, real-time non-magnified Kudo PP involved ten studies and real-time CLE involved six studies. The “all study” Kudo PP included twelve studies of which two were retrospective image-based abstracts.
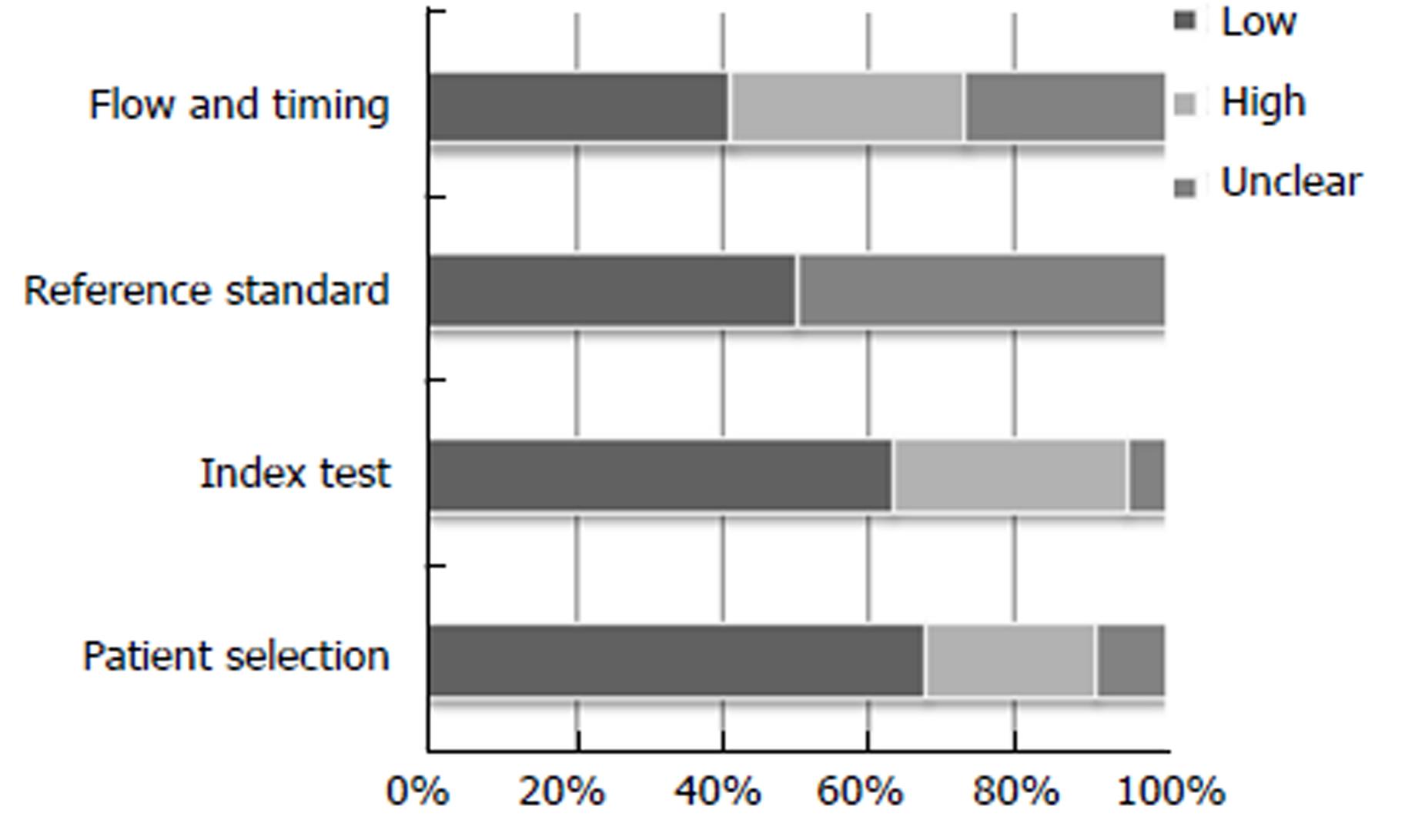
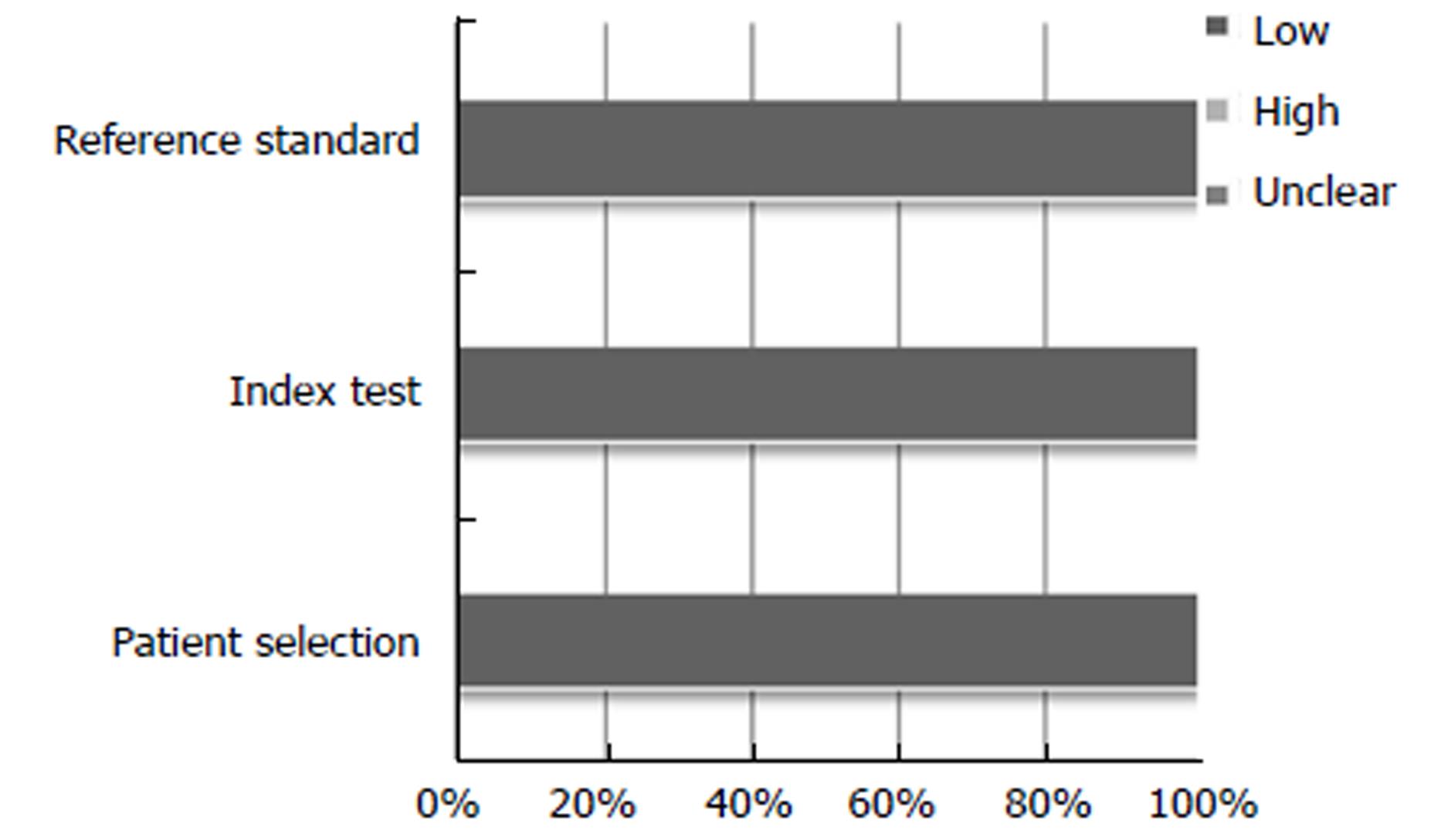
The results for the study quality assessment using the QUADAS 2 tool are presented using stacked bar charts (Figures 2 and 3), displaying risk of bias and applicability. Individual study quality assessment can be seen in the supplementary table (Table 2). Results varied across the twenty-two studies. Abstracts predominantly scored “unclear” for domains associated with “risk of bias”, due to lack of in-depth information within the abstract. However studies also scored “unclear” for “risk of bias” with regards “reference standard” if it did not clearly state if the histopathologist was blinded to the endoscopic diagnosis. Papers scoring “high” for “patient selection”, “index test” and “flow and timing” for “risk of bias”, were generally associated with retrospective image-captured studies which selected and reviewed only clear images of lesions, thereby introducing attrition bias. All studies scored “low” for all three domains with regards “applicability”.
| Cassinotti et al[22] 2016 | Efthymiou et al[21]2013 | Van den broek et al[23]2011 | Cassinottiet al[24]2015 | Cassinottiet al[25]2015 | Carballel et al[26]2016 | Buchner et al[27]2016 | Wanders et al[20]2016 | Munoz et al[28]2016 | Wanders et al[29]2015 | Hlavaty et al[18]2011 | Dlugosz et al[30]2016 | Freireet al[31]2014 | Rispoet al[32]2012 | Shahidet al[33]2011 | Van den broek et al[19] 2011 | Keisslichet al[34]2007 | Nishiyamaet al[35]2016 | Van den Broek et al[36]2008 | Matsumotoet al[37]2007 | Keisslich et al[38]2003 | Bisschopset al[39]2013 | |
| DOMAIN 1 | ||||||||||||||||||||||
| Patient selection | ||||||||||||||||||||||
| Risk of bias | ||||||||||||||||||||||
| Could selection of patients introduced bias? | L | L | L | L | L | L | U | L | L | H | U | L | L | L | H | H | L | H | L | L | L | H |
| Concerns regarding applicability | ||||||||||||||||||||||
| Concern included patients don’t match review question? | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L |
| DOMAIN 2 | ||||||||||||||||||||||
| Index test | ||||||||||||||||||||||
| Risk of bias | ||||||||||||||||||||||
| Conduct or interpretation of index test introduced bias? | L | L | L | L | L | L | L | L | U | H | H | H | L | L | H | H | L | H | L | L | L | H |
| Concerns regarding applicability | ||||||||||||||||||||||
| Concern index test, its conduct or interpretation differs from review question? | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L |
| DOMAIN 3 | ||||||||||||||||||||||
| Reference standard | ||||||||||||||||||||||
| Risk of bias | ||||||||||||||||||||||
| Could reference standard, conduct or interpretation have introduced bias? | U | U | L | U | U | U | U | L | U | U | L | L | L | L | U | L | L | U | L | L | L | U |
| Concerns regarding applicability | ||||||||||||||||||||||
| Concern target condition as defined by reference standard not match review question? | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L | L |
| DOMAIN 4 | ||||||||||||||||||||||
| Flow and timing | ||||||||||||||||||||||
| Risk of bias | ||||||||||||||||||||||
| Could patient flow introduced bias? | U | L | H | U | U | L | U | L | U | H | H | U | L | L | H | H | L | H | L | L | L | H |
A summary for the pooled diagnostic accuracy estimates for the different technologies and for the subgroup analysis is outlined in Table 3.
| Analysis groups | No. of studies | Pooled estimates (95%CI) | Likelihood ratios (95%CI) | Diagnostic odds ratio (95%CI) | Area under SROC curve (95%CI) | ||
| Sensitivity | Specificity | LHR+ | LHR- | DOR | |||
| All | |||||||
| VCE | 5 | 0.86 (0.62-0.95) | 0.87 (0.72-0.95) | 6.7 (2.6-17.8) | 0.17 (0.05-0.53) | 41 (6-297) | 0.93 (0.90-0.95) |
| DBC | 6 | 0.67 (0.44-0.84) | 0.86 (0.72-0.94) | 4.9 (2.1-11.3) | 0.38 (0.20-0.73) | 13 (3-48) | 0.84 (0.81-0.87) |
| Magnification | 5 | 0.90 (0.77-0.96) | 0.87 (0.81-0.91) | 7.0 (4.6-10.7) | 0.11 (0.05-0.28) | 62 (18-209) | 0.93 (0.91-0.95) |
| CLE | 9 | 0.87 (0.71-0.95) | 0.94 (0.87-0.97) | 14.0 (6.1-32.4) | 0.14 (0.06-0.33) | 101 (23-442) | 0.96 (0.94-0.97) |
| Real-time | |||||||
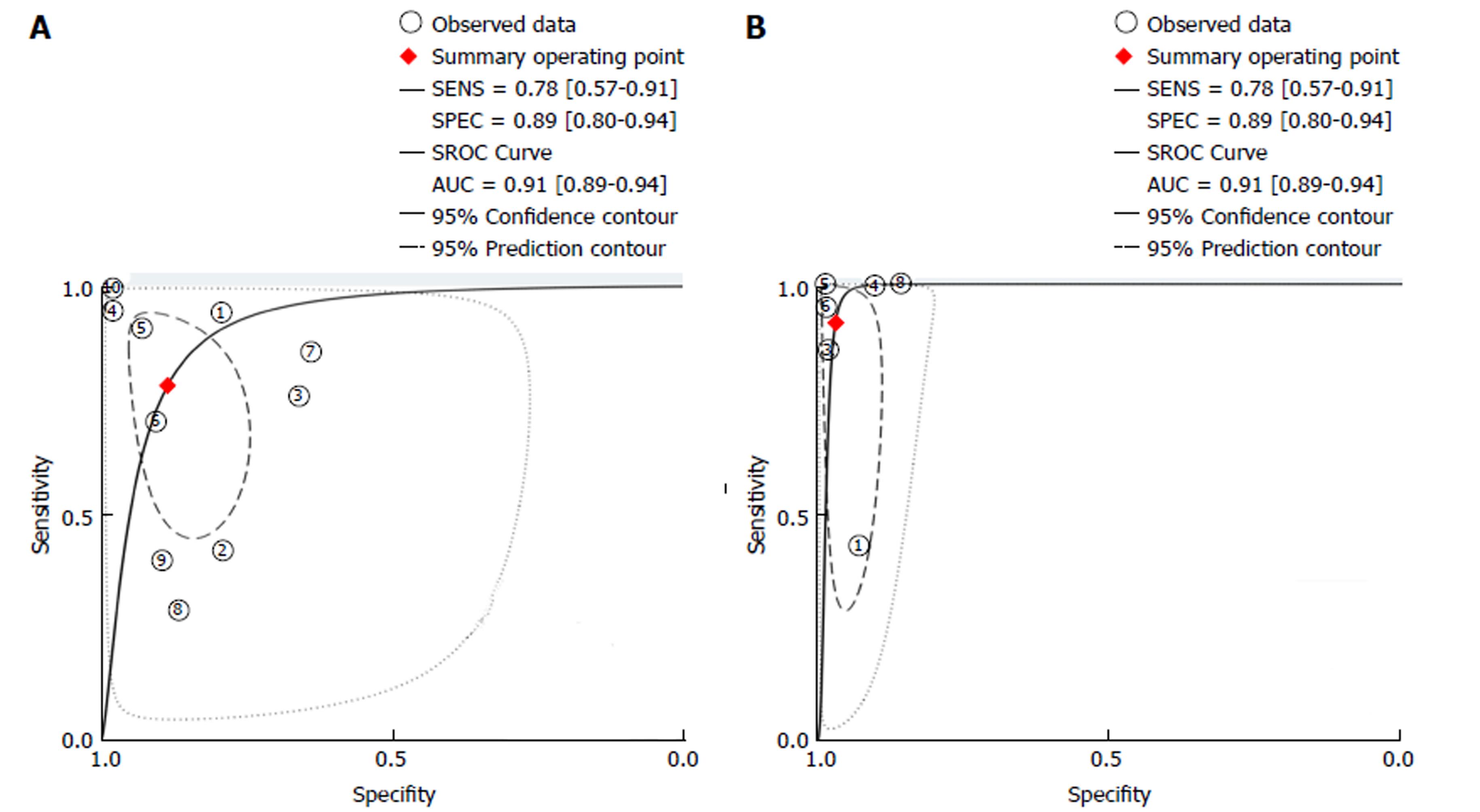
| Kudo PP | 10 | 0.78 (0.57-0.91) | 0.89 (0.80-0.94) | 6.9 (3.5-13.5) | 0.24 (0.11-0.55) | 28 (7-110) | 0.91 (0.89-0.94) |
| CLE | 6 | 0.91 (0.66-0.98) | 0.97 (0.94-0.98) | 28.4 (13.6-59.1) | 0.09 (0.02-0.43) | 322 (41-2529) | 0.98 (0.97-0.99) |
| All Kudo PP | 12 | 0.78 (0.61-0.88) | 0.86 (0.76-0.92) | 5.5 (2.9-10.1) | 0.26 (0.14-0.50) | 21 (7-66) | 0.89 (0.86-0.92) |
The meta-analysis for the five studies involving VCE showed it was fairly accurate at differentiating neoplastic from non-neoplastic lesions with a pooled sensitivity of 86% (95%CI: 62%-95%), specificity of 87% (95%CI: 72%-95%), and the area under the SROC curve was 0.93 (95%CI: 0.90-0.95).
Pooled results of the six studies for DBC revealed the least accurate results for lesion characterization, with a sensitivity of 67% (95%CI: 44%-84%), specificity of 86% (95%CI: 72%-94%) and an area under the SROC curve was 0.84 (95%CI: 0.81-0.87). Most of the studies within this group were multi-centre with more than one endoscopist.
Results of the five studies for magnification endoscopy showed a pooled sensitivity of 90% (95%CI: 77%-96%), specificity of 87% (95%CI: 81%-91%), and an area under the SROC curve was 0.93 (95%CI: 0.91-0.95). The results are similar to those of VCE; however these were mainly single centre, single expert endoscopist studies.
Meta-analysis of nine studies for CLE showed a sensitivity of 87% (95%CI: 71%-95%), specificity of 94% (95%CI: 87%-97%), with an area under the SROC curve of 0.96 (95%CI: 0.94-0.97). Again these mere all single centre, single expert endoscopist studies.
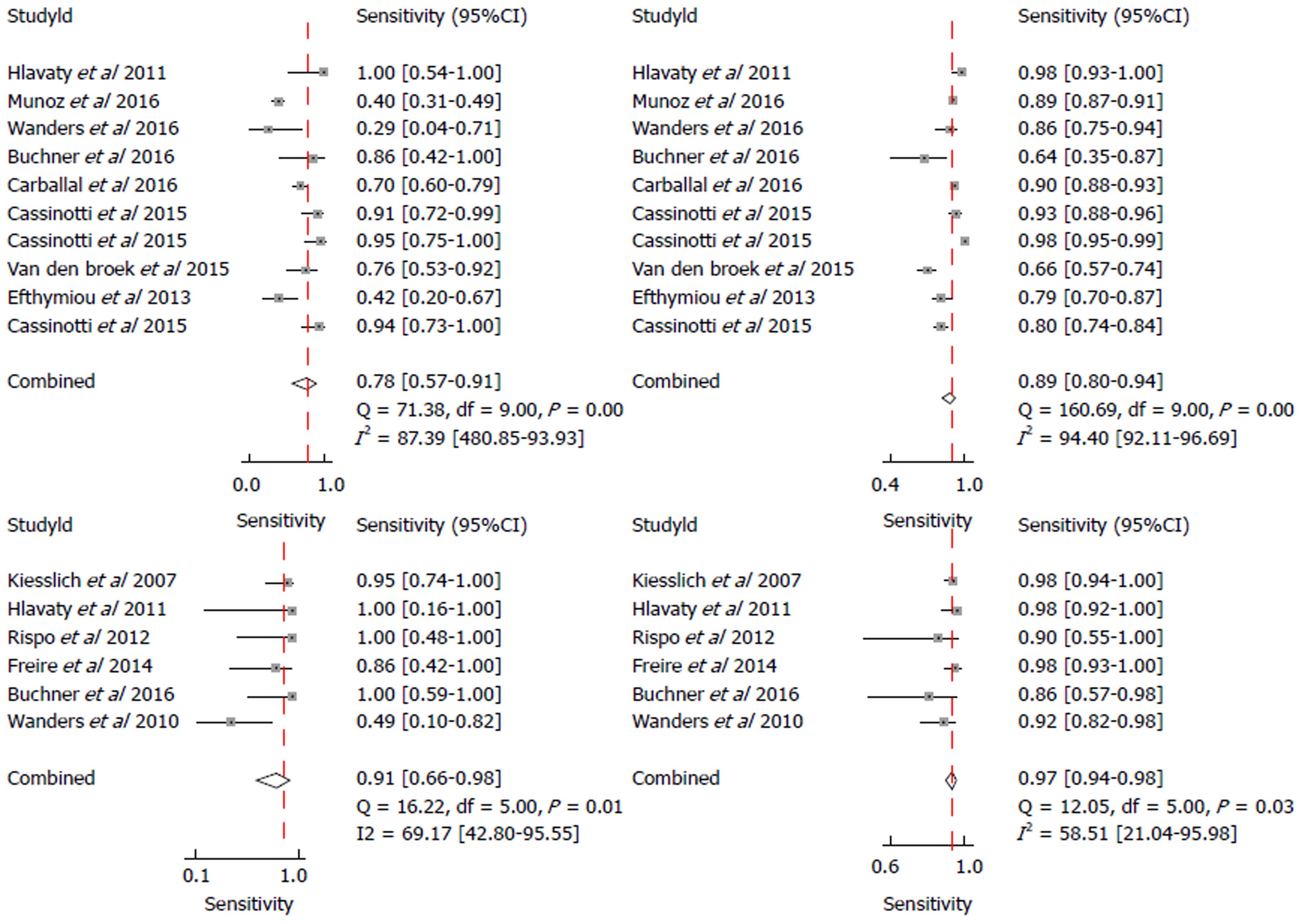
A subgroup analysis was performed involving studies using real-time endoscopic mucosal characterisation of lesions, divided into real-time non-magnified Kudo PP (with VCE and DBC) and real-time CLE. Both the forest plots and SROC curves for real-time non-magnified Kudo PP and real-time CLE are given in Figures 4 and 5. The subgroup for real-time Kudo PP included ten studies, with a pooled estimate sensitivity of 78% (95%CI: 57%-91%), specificity of 89% (95%CI: 80%-94%), with an area under the SROC of 0.91 (95%CI: 0.89-0.94). The subgroup analysis looking at real-time CLE included 6 studies. The pooled estimated sensitivity was 91% (95%CI: 66%-98%), specificity was 97% (95%CI: 94%-98%), and the area under the AUSROC was 0.98 (0.97-0.99).
A further subgroup analysis was performed looking at all (real-time and image review) non-magnified Kudo PP. This included twelve studies. The pooled estimate sensitivity was 78% (95%CI: 61%-88%), specificity of 86% (95%CI: 76%-92%), and an area under the SROC of 0.89 (95%CI: 0.86-0.92).
I2 and Cochrane Q were used to test for heterogeneity. Heterogeneity for VCE was moderate to high with an I2 = 63% (95%CI: 16%-100%) and Q = 5.347 (P = 0.034). DBC showed extremely high levels of heterogeneity between studies with an I2 = 89% (95%CI: 78%-100%) and Q = 18.573 (P = 0.00). Magnification [I2 = 0 (95%CI: 0%-100%) and Q = 0.607 (P = 0.369)] and CLE [I2 = 40% (95%CI: 0%-100%) and Q = 3.335 (P = 0.094)] represented low levels of heterogeneity between studies, however had very broad 95% confidence intervals.
Real-time non-magnified Kudo PP had an I2 = 96% (95%CI: 92-99) and Q = 45.575 (P < 0.001) showing exceptionally high levels of heterogeneity. Real-time CLE studies had low levels of heterogeneity, with an I2 = 0 (95%CI: 0-100%) and Q = 1.697 (P = 0.214).
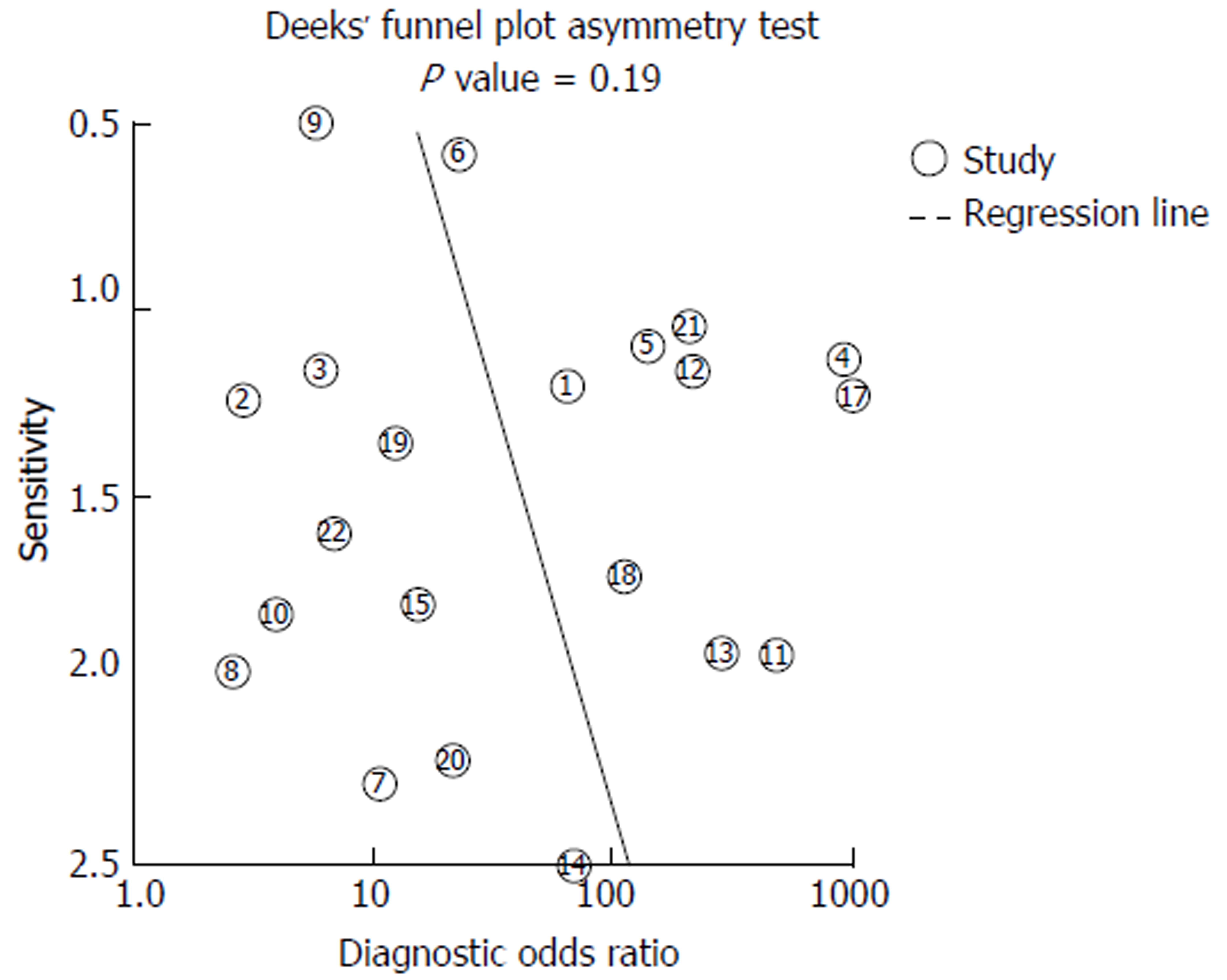
Deeks et al[15] funnel plot, seen in Figure 6, was used to assess publication bias. The funnel plot has slope coefficient of 9.84 (P = 0.194). The non-significant P valve would suggest a low likelihood of publication bias in this meta-analysis.
Our meta-analysis illustrates that real-time CLE currently appears to be the best technology in performing in-vivo lesion characterization in patients with colonic IBD, with an impressive AUSROC of 0.98 (95%CI: 0.97-0.99). It demonstrates an extremely high specificity, 97% (95%CI: 94%-98%), and sensitivity, 91% (95%CI: 66%-98%), in differentiating neoplastic from non-neoplastic lesions. Using all study types (real-time and image capture) CLE again out-performs the other technologies, with an area under SROC cure of 0.96 (95%CI: 0.94-0.97). Magnification and VCE technologies also show a good accuracy with a SROC of 0.93 (95%CI: 0.91-0.95) and 0.93 (95%CI: 0.90-0.95), respectively.
Despite CLE being a highly accurate technology in lesion characterization, there are several concerns with regards applicability. Most of the studies in our meta-analysis for CLE involved a single endoscopy operator within a single-centre. They were vastly experienced in IBD surveillance endoscopy and in using CLE technology. Studies in which inexperienced operators used this technology, they themselves did not make real-time lesion diagnosis. Instead, people trained in the interpretation of the histology reviewed the images retrospectively. This is because CLE is not a routinely used modality. It requires expertise in handling, positioning of the colonoscope/probe onto the lesion and in analysing/interpreting in-vivo histology. Bowel preparation has to be meticulous, as any faecal material can interfere with image capture and lesion interrogation. This is unlikely to be achieved consistently during “real-life” surveillance lists. In one study, 32% of lesions were not accessible to CLE evaluation and a second study, 1.5% of lesions the histology was not visualised by CLE. These unclassified lesions aren’t accounted for in the final results, contributing to attrition bias in the observed results. In addition, IV fluorescein injection is required before lesion analysis, further adding to procedure time. One study showed the mean additional time per procedure being 20 min. Adoption of this technology in throughout less experienced centres is doubtful. It would demand vast resources for training, education and require new guidance for endoscopic competence.
A further concern with CLE was equipment failure. In one multi-centre study, four of the five centres had to send the equipment back to the manufactures as the lens on the endomicroscope broke. Repair took the teams months to address, significantly affecting recruitment, resulting in the study being underpowered. With concerns over equipment failure, costs of purchasing the technology and repairs, CLE could in fact be a financial burden, negating any benefit obtained from the reduction in polypectomies and histological analysis. Therefore, questions still remain unanswered with regards practicalities and applicability for this technology.
VCE showed relatively good accuracy although fell short of reaching the 90% mark for sensitivity and specificity. One major limitation for this technology was the small number of studies for VCE technology. We therefore combined the NBI, FICE and i-scan to obtain pooled results. Although the technologies have been grouped as one, there are obvious differences in the way they achieve the modified image and the modes used with that technology. NBI endoscopes contain a rotating filter in front of the light source at the end of the endoscope, allowing a narrow wavelength of light to strike the mucosa resulting in image enhancement, whereas both FICE and i-scan use a post-processing technology built within the processer to provide a coloured-enhanced image. There were several other drawbacks with the VCE group analysis. One study in our meta-analysis used the first generation NBI technology, resulting in images being less bright, undoubtedly having an impact on lesion characterization when compared with newer generation technology. Three of the five studies for VCE were abstracts making critical analysis for the quality of these studies difficult to determine. From our results we cannot currently recommend using VCE solely as an accurate technology for lesion characterization in IBD. However, with newer generation endoscopes, further evaluation is clearly warranted as these technologies continue to improve. In comparison with CLE, VCE is potentially less complicated to use, more robust, economical as they are almost universal in newer endoscope processors, and training is more likely to be attainable.
Magnification endoscopy achieves similar accuracy to VCE technology. However in the majority of these studies magnification was used in combination with NBI, predominantly using older NBI technology. This makes it challenging to differentiate the two technologies. With new colonoscopes delivering digital magnification, like “near focus” technology, it is questionable the additional information optical magnification will provide. A threshold may be reached at which further magnification provides no additional benefit for differentiating neoplastic from non-neoplastic pit patterns. However, this meta-analysis cannot necessarily address that question.
DBC pooled results were suboptimal for lesion characterization. However, more than half of the studies used standard-definition colonoscopies, reducing image resolution, and therefore impacting on lesion interpretation. With most centres now using high-definition colonoscopies accuracy is likely to improve. Another confounding factor was that the majority of the studies were multi-centred, with multiple operators, undoubtedly accounting for a diverse range of endoscopic experience and therefore skill at lesion classification.
A subgroup analysis was performed in order to look for potential sources of heterogeneity and to determine whether it was the type of mucosal classification used that influenced the accuracy rather than the technology. Real-time studies were used as this provided the most clinically authentic evaluation of lesions and minimises bias as a result of photographic selection and time for analysis. Most studies used Kudo PP or a variation on the Kudo PP (Kudo PP plus additional features) and therefore we pooled the results for both real-time VCE and DBC. Real-time Kudo PP had an area under the SROC curve of 0.91 (95%CI: 0.89-0.94), with a reasonable specificity of 89% (95%CI: 80%-94%) but a sensitivity of 78% (95%CI: 57%-91%). The poor sensitivity likely reflects inclusion of the DBC group with the majority involving standard-definition scopes. The use of Kudo PP and Kudo PP plus did not seem to influence the accuracy of lesion characterisation, independent of the technology. Caution however, has to be noted for combining DBC and VCE using Kudo PP as a mucosal classification system. Studies have shown a lack of pit pattern agreement between chromoendoscopy and NBI[40]. This has leading to the adoption of new classification systems, such as NICE for NBI[41]. Further mucosal classification systems may need to be studied, especially for i-scan and FICE. However, determining the ideal post-processing mode for these software systems could be challenging as these technologies have multiple combination options of modes.
Another important issue that wasn’t clearly stated for studies in this meta-analysis was the degree of mucosal inflammation in which the lesions resided. Varying degrees of mucosal inflammation unquestionably contribute to difficulties in pit pattern and vasculature interpretation and therefore diagnostic accuracy. Future studies looking at in-vivo lesion diagnostic accuracy could stratify patients depending on the degree of inflammation surrounding the lesions.
As with any meta-analysis there are limitations. The number of studies for each technology group was fairly limited, except for the CLE group. Seven of the twenty-two studies were abstracts introducing concerns with regards data extraction and interrogation for study validity.
Despite an extensive literature review, no papers had direct head-to-head studies, comparing the different technologies against each other. However, this would require a very large cohort looking specifically at lesion characterisation and all endoscopists participating being familiar with the different technologies. Endoscopic familiarity with certain technologies in such a study could potentially confound the accuracy of lesion interpretation.
In the majority of studies, lesion characterization was a secondary outcome, therefore in some studies the number of lesions being characterised was small. Some studies didn’t clearly state the TP, FP, FN and TN, therefore calculations had to be performed in order to achieve this.
There was also a large degree of heterogeneity within the VCE and DBC groups that was further increased when we performed real-time Kudo PP assessment. Further areas of subgroup classification that were not explored within this meta-analysis were the number of endoscopists performing the procedures in each study and also whether it was a single centre or multi-centre study. This undoubtedly will have an impact on the accuracy of the technology being used. Single-centre, single endoscopist studies are more likely to achieve better results.
Suggested avenues to explore in future studies looking at in-vivo lesion characterization in colonic IBD include: accuracy according to varying endoscopic experience, accuracy dependent on the degree of surrounding mucosal inflammation, whether the endoscopist confidence (high or low) in lesion characterization impacts accuracy and exploring new mucosal lesion classification for different technologies.
Real-time CLE appears to be currently the best commercially available technology at differentiating neoplastic from non-neoplastic lesions in patients with colonic IBD, with an area under the SROC of 0.98 (95%CI: 97%-99%). However, most CLE studies were single centered with single expert users, which could significantly confound the results, and some studies not reporting non-interpretable images, contributing to attrition bias. Clinical applicability for this technology is likely to be a challenge. VCE technology performed well but currently cannot be recommended for in-vivo lesion characterization in such a high-risk group. However, with improved endoscopes and newer generation technologies further studies are required to assess their real-time performance in clinical settings with trained colonoscopists.
Patients with inflammatory bowel disease (IBD) colitis are known to have an increased risk of colorectal cancer compared to that of non-colitic patients. This is thought to progress along the inflammation-dysplasia-carcinoma pathway. Many studies and meta-analyses have been performed for lesion detection in IBD but few studies have looked into in-vivo lesion characterization. This is the first meta-analysis on lesion characterization in colonic IBD.
Characterization of colonic lesions in IBD maybe more challenging because they tend to be flatter and their pit-pattern maybe obscured by inflammation. Some patients also have numerous pseudopolyps throughout the colon, making polypectomy impractical, time-consuming, costly and potentially associated with increased risk. If we are able to characterize these lesions with a high accuracy without needing to perform polypectomy, we could potentially circumvent these problems.
Our objective was to perform the first systematic review and meta-analysis for the diagnostic accuracy of optical imaging techniques for in-vivo lesion characterization in colonic IBD.
We conducted a review of the current literature and included studies which characterized lesions in-vivo into neoplastic and non-neoplastic, using histology as the gold standard. Data was pooled for each technology using a bivariate meta-analysis with a random effects model to account for study differences. Sensitivities, specificities, positive and negative likelihood ratios, diagnostic odds ratio, and area under summary receiver-operator characteristic curve, were calculated for each technology type.
Confocal laser endomicroscopy (CLE) had the greatest accuracy for differentiating neoplastic from non-neoplastic lesions in-vivo. Magnification and virtual chromoendoscopy (VCE) performed well, whilst dye-based chromoendoscopy (DBC) had suboptimal accuracy.
CLE is highly accurate at in-vivo lesion characterization but studies are within experienced centres with mainly single expert users limiting its generalizability.
Future studies should look at newer generation virtual chromoendoscopic technology [narrow band imaging (NBI), i-scan, fujinon intelligence chromoendoscopy (FICE)] for lesion characterization. A standardised mucosal lesion classification system specific for lesions in IBD colitis accounting for the technology being used should be explored.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
PRISMA 2009 checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
P- Reviewer: Espinel J, Guerra IG, Tang ST S- Editor: Wang XJ L- Editor: A E- Editor: Ma YJ
| 1. | Crohn B, Rosenberg H. The sigmoidoscopic picture of chronic ulcerative colitis (non- specific). AM J MED SCI. 1925;170:220-227. [RCA] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 200] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 2079] [Article Influence: 86.6] [Reference Citation Analysis (1)] |
| 3. | Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 660] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 4. | Lutgens MW, van Oijen MG, van der Heijden GJ, Vleggaar FP, Siersema PD, Oldenburg B. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis. 2013;19:789-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 376] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 5. | Zisman TL, Rubin DT. Colorectal cancer and dysplasia in inflammatory bowel disease. World J Gastroenterol. 2008;14:2662-2669. |
| 6. | Laine L, Kaltenbach T, Barkun A, McQuaid KR, Subramanian V, Soetikno R; SCENIC Guideline Development Panel. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology. 2015;148:639-651.e28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 390] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 7. | Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, Eaden JA, Rutter MD, Atkin WP, Saunders BP. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010;59:666-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 808] [Article Influence: 53.9] [Reference Citation Analysis (2)] |
| 8. | Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, Ferrante M, Götz M, Katsanos KH, Kießlich R. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 584] [Article Influence: 48.7] [Reference Citation Analysis (1)] |
| 9. | Wanders LK, Dekker E, Pullens B, Bassett P, Travis SP, East JE. Cancer risk after resection of polypoid dysplasia in patients with longstanding ulcerative colitis: a meta-analysis. Clin Gastroenterol Hepatol. 2014;12:756-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 10. | ASGE Technology Committee, Abu Dayyeh BK, Thosani N, Konda V, Wallace MB, Rex DK, Chauhan SS, Hwang JH, Komanduri S, Manfredi M, Maple JT, Murad FM, Siddiqui UD, Banerjee S. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2015;81:502.e1-502.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 249] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 11. | Rees CJ, Rajasekhar PT, Wilson A, Close H, Rutter MD, Saunders BP, East JE, Maier R, Moorghen M, Muhammad U. Narrow band imaging optical diagnosis of small colorectal polyps in routine clinical practice: the Detect Inspect Characterise Resect and Discard 2 (DISCARD 2) study. Gut. 2017;66:887-895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 151] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 12. | Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 709] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 13. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13358] [Article Influence: 834.9] [Reference Citation Analysis (0)] |
| 14. | Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6953] [Cited by in RCA: 9591] [Article Influence: 685.1] [Reference Citation Analysis (0)] |
| 15. | Dwamena BA. MIDAS: Stata module for meta-analytical integration of diagnostic test accuracy studies. Statistical Software Components S456880. Boston, MA: Boston College Department of Econonics 2009; . |
| 16. | Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58:882-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1792] [Cited by in RCA: 2229] [Article Influence: 111.5] [Reference Citation Analysis (1)] |
| 17. | StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP 2013; . |
| 18. | Hlavaty T, Huorka M, Koller T, Zita P, Kresanova E, Rychly B, Toth J. Colorectal cancer screening in patients with ulcerative and Crohn’s colitis with use of colonoscopy, chromoendoscopy and confocal endomicroscopy. Eur J Gastroenterol Hepatol. 2011;23:680-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | van den Broek FJ, van Es JA, van Eeden S, Stokkers PC, Ponsioen CY, Reitsma JB, Fockens P, Dekker E. Pilot study of probe-based confocal laser endomicroscopy during colonoscopic surveillance of patients with longstanding ulcerative colitis. Endoscopy. 2011;43:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Wanders LK, Kuiper T, Kiesslich R, Karstensen JG, Leong RW, Dekker E, Bisschops R. Limited applicability of chromoendoscopy-guided confocal laser endomicroscopy as daily-practice surveillance strategy in Crohn’s disease. Gastrointest Endosc. 2016;83:966-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Efthymiou M, Allen PB, Taylor AC, Desmond PV, Jayasakera C, De Cruz P, Kamm MA. Chromoendoscopy versus narrow band imaging for colonic surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:2132-2138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Gordon H, Langholz E. The EpiCom Survey-Registries Across Europe, Epidemiological Research and Beyond. J Crohns Colitis. 2017;11:1019-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | van den Broek FJ, Fockens P, van Eeden S, Stokkers PC, Ponsioen CY, Reitsma JB, Dekker E. Narrow-band imaging versus high-definition endoscopy for the diagnosis of neoplasia in ulcerative colitis. Endoscopy. 2011;43:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Cassinotti A, Ardizzone S, Buffoli F, Fociani P, Villanacci V, Nebuloni M, Fichera M, Salemme M, Lombardini M, Molteni P. Virtual chromoendoscopy with FICE is superior to standard colonoscopic surveillaillance for flat visibile dysplasic lesions and raised lesions (polyps and pseudopolyps) evaluation in long-standing ulcerative colitis: a prospective, randomized, trial Suppl 1. J CROHNS COLITIS. 2015;9:S1-S479. |
| 25. | Gordon H, Langholz E. The EpiCom Survey-Registries Across Europe, Epidemiological Research and Beyond. J Crohns Colitis. 2017;11:1019-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Carballal S, Maisterra S, López-Serrano A, Gimeno-García AZ, Vera MI, Marín-Garbriel JC, Díaz-Tasende J, Márquez L, Álvarez MA, Hernández L. Real-life chromoendoscopy for neoplasia detection and characterisation in long-standing IBD. Gut. 2018;67:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 27. | Buchner AM, Ma GK, Ginsberg GG, Lichtenstein GR, Kerner C. Chromoendoscopy-Guided Probe Based Confocal Laser Endomicroscopy: A Novel Approach for Dysplasia Evaluation in IBD Surveillance. Gastroenterology. 2016;150:S627. [DOI] [Full Text] |
| 28. | Muñoz J, García M, Sicilia B, Sierra M, Fernandez N, Arias L, Barrio J, Velayos B, Hernandez-Villalba L. Results of dysplasia surveillance programme with chromoendoscopy for inflammatory bowel disease Suppl. Journal of Crohn’s and Colitis. 2016;10:158. |
| 29. | Wanders LK, Mooiweer E, Wang J, Bisschops R, Offerhaus GJ, Siersema PD, D’Haens GR, Oldenburg B, Dekker E. Low interobserver agreement among endoscopists in differentiating dysplastic from non-dysplastic lesions during inflammatory bowel disease colitis surveillance. Scand J Gastroenterol. 2015;50:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Dlugosz A, Barakat AM, Björkström NK, Öst Å, Bergquist A. Diagnostic yield of endomicroscopy for dysplasia in primary sclerosing cholangitis associated inflammatory bowel disease: a feasibility study. Endosc Int Open. 2016;4:E901-E911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Freire P, Figueiredo P, Cardoso R, Donato MM, Ferreira M, Mendes S, Silva MR, Cipriano A, Ferreira AM, Vasconcelos H. Surveillance in ulcerative colitis: is chromoendoscopy-guided endomicroscopy always better than conventional colonoscopy? A randomized trial. Inflamm Bowel Dis. 2014;20:2038-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Rispo A, Castiglione F, Staibano S, Esposito D, Maione F, Siano M, Salvatori F, Masone S, Persico M, De Palma GD. Diagnostic accuracy of confocal laser endomicroscopy in diagnosing dysplasia in patients affected by long-standing ulcerative colitis. World J Gastrointest Endosc. 2012;4:414-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Shahid MW, Wang YR, Cangemi JR, Wallace MB, Picco MF. The Role of Probe-Based Confocal Laser Endomicroscopy in Detection of Neoplasia in Polypoid Lesions in Ulcerative Colitis: An Exploratory Pilot Study. Gastrointest Endosc. 2011;73:AB302-AB3. [DOI] [Full Text] |
| 34. | Kiesslich R, Goetz M, Lammersdorf K, Schneider C, Burg J, Stolte M, Vieth M, Nafe B, Galle PR, Neurath MF. Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology. 2007;132:874-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 376] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 35. | Nishiyama S, Oka S, Tanaka S, Sagami S, Hayashi R, Ueno Y, Arihiro K, Chayama K. Clinical usefulness of narrow band imaging magnifying colonoscopy for assessing ulcerative colitis-associated cancer/dysplasia. Endosc Int Open. 2016;4:E1183-E1187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | van den Broek FJ, Fockens P, van Eeden S, Reitsma JB, Hardwick JC, Stokkers PC, Dekker E. Endoscopic tri-modal imaging for surveillance in ulcerative colitis: randomised comparison of high-resolution endoscopy and autofluorescence imaging for neoplasia detection; and evaluation of narrow-band imaging for classification of lesions. Gut. 2008;57:1083-1089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 37. | Matsumoto T, Kudo T, Jo Y, Esaki M, Yao T, Iida M. Magnifying colonoscopy with narrow band imaging system for the diagnosis of dysplasia in ulcerative colitis: a pilot study. Gastrointest Endosc. 2007;66:957-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | Kiesslich R, Fritsch J, Holtmann M, Koehler HH, Stolte M, Kanzler S, Nafe B, Jung M, Galle PR, Neurath MF. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003;124:880-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 557] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 39. | Bisschops R, Bessissow T, Bhandari P, Dekker E, East JE, Ignjatovic A, Parra-Blanco A, Ragunath K, Rutter MD, Schoon EJ. Chromo-Endoscopy and NBI for Ruling out Neoplasia in Ulcerative Colitis: an International Multicenter Interobserver Study. Gastrointest Endosc. 2013;77:AB444-AB455. [DOI] [Full Text] |
| 40. | East JE, Suzuki N, Saunders BP. Comparison of magnified pit pattern interpretation with narrow band imaging versus chromoendoscopy for diminutive colonic polyps: a pilot study. Gastrointest Endosc. 2007;66:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 41. | Hewett DG, Kaltenbach T, Sano Y, Tanaka S, Saunders BP, Ponchon T, Soetikno R, Rex DK. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology. 2012;143:599-607.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 417] [Article Influence: 32.1] [Reference Citation Analysis (0)] |