Published online Aug 28, 2016. doi: 10.3748/wjg.v22.i32.7353
Peer-review started: April 8, 2016
First decision: May 12, 2016
Revised: June 9, 2016
Accepted: July 21, 2016
Article in press: July 21, 2016
Published online: August 28, 2016
Processing time: 138 Days and 17.7 Hours
To study the effects of different diets on intestinal microbiota and nonalcoholic fatty liver disease (NAFLD) development at the same caloric intake.
Thirty male Sprague-Dawley rats were randomized into five groups (six rats each). The control diet (CON) group and free high-fat diet (FFAT) group were allowed ad libitum access to a normal chow diet and a high-fat diet, respectively. The restrictive high-fat diet (RFAT) group, restrictive high-sugar diet (RSUG) group, and high-protein diet (PRO) group were fed a high-fat diet, a high-sugar diet, and a high-protein diet, respectively, in an isocaloric way. All rats were killed at 12 wk. Body weight, visceral fat index (visceral fat/body weight), liver index (liver/body weight), insulin resistance, portal lipopolysaccharide (LPS), serum alanine aminotransferase (ALT), serum aspartate aminotransferase (AST), and liver triglycerides were measured. The intestinal microbiota in the different groups of rats was sequenced using high-throughput sequencing technology.
The FFAT group had higher body weight, visceral fat index, liver index, peripheral insulin resistance, portal LPS, serum ALT, serum AST, and liver triglycerides compared with all other groups (P < 0.05). Taking the same calories, the RFAT and RSUG groups demonstrated increased body weight, visceral fat index, peripheral insulin resistance and liver triglycerides compared with the PRO group (P < 0.05). The RFAT group also showed increased portal LPS compared with the PRO group (P < 0.05). Unweighted UniFrac principal coordinates analysis of the sequencing data revealed that the intestinal microbiota structures of the CON, FFAT, RSUG and PRO groups were roughly separated away from each other. Taxon-based analysis showed that, compared with the CON group, the FFAT group had an increased abundance of Firmicutes, Roseburia and Oscillospira bacteria, a higher ratio of Firmicutes to Bacteroidetes, and a decreased abundance of Bacteroidetes, Bacteroides and Parabacteroides bacteria (P < 0.05). The RFAT group showed an increased abundance of Firmicutes and decreased abundance of Parabacteroides bacteria (P < 0.05). The RSUG group showed an increased abundance of Bacteroidetes and Sutterella bacteria, higher ratio of Bacteroidetes to Firmicutes, and a decreased abundance of Firmicutes (P < 0.05). The PRO group showed an increased abundance of Bacteroidetes, Prevotella, Oscillospira and Sutterella bacteria, and a decreased abundance of Firmicutes (P < 0.05). Compared with the FFAT group, the RFAT group had an increased abundance of Bacteroidetes, higher ratio of Bacteroidetes to Firmicutes, and decreased abundance of Firmicutes and Oscillospira bacteria (P < 0.05).
Compared with the high-protein diet, the NAFLD-inducing effects of high-fat and high-sugar diets are independent from calories, and may be associated with changed intestinal microbiota.
Core tip: Diet plays an important role in development of nonalcoholic fatty liver disease (NAFLD), and can shape intestinal microbiota, which is closely linked to NAFLD. We studied the effects of high-fat, high-sugar and high-protein diets on intestinal microbiota and NAFLD development in an isocaloric way. NAFLD-inducing effects of high-fat and high-sugar diets, compared with high-protein diet, are independent from calories, and these diets can alter intestinal microbiota independently from calories. The effects of these diets on NAFLD development at the same caloric intake may be associated with changes in intestinal microbiota. These findings are meaningful for appropriate dietary therapy for NAFLD.
- Citation: Liu JP, Zou WL, Chen SJ, Wei HY, Yin YN, Zou YY, Lu FG. Effects of different diets on intestinal microbiota and nonalcoholic fatty liver disease development. World J Gastroenterol 2016; 22(32): 7353-7364
- URL: https://www.wjgnet.com/1007-9327/full/v22/i32/7353.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i32.7353
Nonalcoholic fatty liver disease (NAFLD) is a chronic liver disease in which triglycerides accumulate within the hepatocytes of patients with minimal or no alcohol intake and without any other known cause. It comprises a wide spectrum of liver damage ranging from steatosis to nonalcoholic steatohepatitis (NASH), advanced fibrosis and cirrhosis[1,2]. With the increasing incidence of obesity and diabetes mellitus, NAFLD has been recognized as a burgeoning health burden which affects 10%-24% of the general population and 70% of obese patients[3,4]. Approximately 30%-40% of individuals with simple steatosis progress to NASH, and NASH can progress to cirrhosis, which is a major risk factor for hepatocellular carcinoma[5,6]. Studies also have reported that NAFLD is a strong independent risk factor for cardiovascular disease[7].
Although many genetic and environmental factors contribute to the development of NAFLD, diet is an important environmental factor that can affect the development of NAFLD. High-fat diet is a widely-studied diet that can induce NAFLD[8] and is often used to induce animal models of the disease[9]. Recently, high-sugar diet, mainly as high-fructose diet, has been found to play an important role in the development of NAFLD[10-12]. However, unlike high-fat and high-sugar diets, high-protein diet can ameliorate NAFLD[13]. The results of these former studies suggest that different types of diets may have different effects on the development of NAFLD, and high-fat and high-sugar diets are NAFLD-inducing diets.
To understand the effects of diet on the development of NAFLD, the intestinal microbiota must be considered because it is the interface between diet and the liver. Germ-free mice are resistant to NAFLD induced by high-fat diet[14]. However, when the intestinal microbiota was introduced into germ-free mice, the mice showed a rapid increase in body fat content and liver triglycerides. When a high-fat diet induces NAFLD, it also causes dysbiosis of the intestinal microbiota[15]. Moreover, small bowel bacterial overgrowth is associated with NAFLD, and patients with NASH have a lower percentage of Bacteroidetes[16,17]. These studies show a close link between the intestinal microbiota and NAFLD. As diet can affect the development of NAFLD through the intestinal microbiota, it may be useful for us to understand the relationship between diet, intestinal microbiota, and NAFLD in order to prevent or treat this disease.
High-fat and high-sugar diets are associated with hyperphagia; however, high-protein diet can reduce caloric intake[18-20]. In order to understand the effects of different diets on the development of NAFLD more clearly, we restricted the caloric intake of rats in the high-fat and high-sugar diet groups to the same levels as rats in the high-protein diet group, to exclude caloric intake as a confounder. Given the important role that the intestinal microbiota plays in the pathogenesis of NAFLD, and that the intestinal microbiota is greatly influenced by diet, we examined the dietary effects on the intestinal microbiota.
Thirty 5-wk-old male Sprague-Dawley rats (Hunan SJA Laboratory Animal Co. Ltd., China) were housed at a regulated temperature (21 ± 1.6 °C), humidity (45% ± 10%), and an alternating 12-h light and dark cycle. After 1 wk of acclimation, rats were randomized into five groups (six rats each). The control diet (CON) group was allowed ad libitum access to a normal chow diet and the free high-fat diet (FFAT) group was allowed ad libitum access to a high-fat diet. The restrictive high-fat diet (RFAT) group, restrictive high-sugar diet (RSUG) group and high-protein diet (PRO) group had access to a high-fat diet, a high-sugar diet and a high-protein diet, respectively. The caloric intake of the RFAT and RSUG groups was calibrated to the same caloric intake as the PRO group. The energy density and percentage of energy derived from protein, sugar and fat in the different diets are shown in Table 1. Dietary foods were irradiated by Co60 and stored at 4 °C, protected from air. Fresh diets were administered daily and any remaining food was weighed and discarded. Food intake was measured every day. The study was approved by the Animal Ethics Committee of Central South University and all efforts were made to minimize animal suffering.
| Normal | High protein | High sugar | High fat | |
| Protein (casein) | 19.2% | 60.0% | 19.2% | 19.2% |
| Sugar (fructose) | 10.5% | 10.5% | 60.0% | 10.5% |
| Fat (lard) | 16.5% | 16.5% | 16.5% | 60.0% |
| Energy density (kcal/kg) | 3810 | 3810 | 3810 | 5179 |
Two days before the rats were sacrificed, Oral glucose tolerance test (OGTT) was performed. Briefly, 12-h fasted rats were administered 2 g/kg glucose orally. Blood samples were taken from the tail to measure blood glucose levels before and 30, 60, 90 and 120 min after glucose administration by using an OneTouchUltraSmart Blood Glucose Monitoring System (LifeScan, Milpitas, United States).
After the rats were killed, the livers were isolated and weighed. Liver index was calculated as the ratio of liver to body weight. Mesenteric, retroperitoneal, and epididymal fat was isolated and weighed as visceral fat mass. The visceral fat index was calculated as the ratio of visceral fat to body weight.
At the end of the experiment, all the animals were killed after a 12-h overnight fast. Blood samples were collected via cardiac puncture and centrifuged to obtain serum. The concentrations of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured by standard procedures. The level of serum insulin was assayed with an ELISA kit (Mercodia, Uppsala, Sweden). Insulin resistance (IR) was determined by the homeostasis model assessment (HOMA) formula: [HOMA-IR = fasting insulin (μU/mL) × plasma glucose (mmol/L)/22.5)][21]. One milliliter of portal blood was collected into an apyrogenic tube for the lipopolysaccharide (LPS) assay. The level of portal LPS was measured with a chromogenic limulus amoebocyte lysate test kit (Bokang, Zhanjiang, China).
Liver lipids were extracted using a modified chloroform/methanol method[22,23]. A small fragment of snap-frozen liver tissue was pulverized to a fine powder in liquid nitrogen. Approximately 50 mg of liver tissue was extracted twice in 1 mL of 2:1 (v/v) chloroform: methanol solution. The organic extracts were air dried and resuspended in 1 mL of 2% Triton X-100 solution. Liver triglycerides were measured colorimetrically using a Triglyceride Reagent Kit (Dongou, Wenzhou, China). Frozen sections stained with Oil Red O were also used for liver lipid detection.
Before the rats were killed, fecal samples were collected and stored at -80 °C. 16S rRNA genes of V4 hypervariable region were amplified using specific primers (515F: 5’-GTGCCAGCMGCCGCGGTAA-3’, 806R: 5’-GGACTACHVGGGTWTCTAAT-3’) with the barcode. All polymerase chain reactions (PCRs) were carried out with Phusion High-Fidelity PCR Master Mix (New England Biolabs, Ipswich, United States). PCR products were mixed in equidensity ratios. The mixture of PCR products was purified using a Qiagen Gel Extraction Kit (Qiagen, Hilden, Germany). Sequencing libraries were generated using the TruSeq DNA PCR-Free Sample Preparation Kit (Illumina, San Diego, United States). Lastly, the library was sequenced on an Illumina HiSeq2500 platform and 250 bp paired-end reads were generated.
QIIME pipeline (1.9.1) was used to process and analyze the 16S raw data for all samples[24]. Paired-end reads were merged using join_paired_ends.py script, and then the raw reads were demultiplexed and quality filtered (at Phred ≥ Q20) using split_libraries_fastq.py script. The high quality reads were clustered into operational taxonomic units (OTUs) at 97% similarity using pick_open_reference_otus.py script. This script also picked a representative sequence for each OTU, and all representative sequences were taxonomically classified using the Greengene database gg_13_8. Sequence alignment was conducted using the PyNAST method and a phylogenetic tree was constructed. The phylogenetic tree was then used for Unweighted UniFrac principal coordinates analysis (PCoA). The chimera sequences were removed with pick_open_reference_otus.py script. To remove sampling depth heterogeneity, OTU abundance information was normalized using a standard sequence number corresponding to the sample with the least sequences. Subsequent analyses were all performed based on these normalized data.
Data are presented as mean ± SE. The differences in quantitative data were statistically analyzed using one-way analysis of variance. When differences were significant, post hoc comparisons were made using a Bonferroni multiple-comparison test. The relative abundance of different phyla and genera was compared between groups using the Mann-Whitney test. SPSS version 20.0 software was used for statistical analyses of the data. The results were considered significant at P < 0.05.
Figure 1A shows the mean caloric intake of the different groups of rats. The FFAT group, which was fed a diet containing 60% of calories from fat, consumed more calories than the other groups. The RFAT, RSUG and PRO groups, which were restricted to the same caloric intake, consumed fewer calories than the CON group. Figure 1B shows the body weight of each group of rats over time. At the end of the experiment, the FFAT group had the highest body weight, visceral fat index, and liver index among all the groups (P < 0.05, Figure 1C-E). The RFAT and RSUG groups displayed no significant differences in body weight and visceral fat index compared with the CON group (P > 0.05); however, the body weight and visceral fat index of these two groups were higher than those in the PRO group (P < 0.05, Figure 1C and D). Compared with the CON group, the PRO group showed a decrease in body weight (P < 0.05) but not visceral fat index (P > 0.05). There were no significant differences in liver index among the other groups (P > 0.05, Figure 1E).
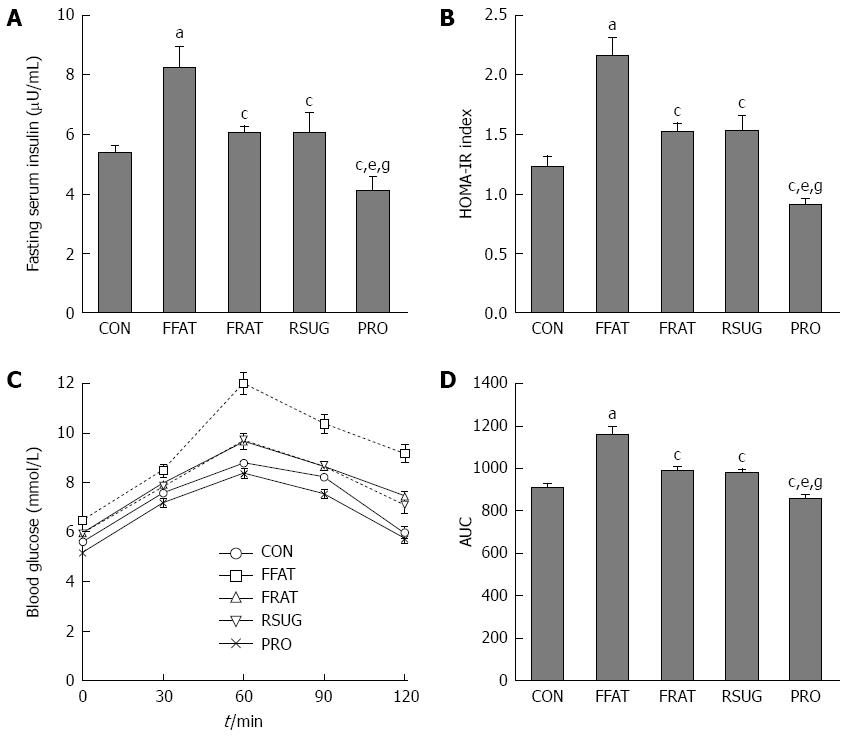
There were no significant differences in fasting blood glucose levels among the different groups (data not shown). However, compared with other groups, the FFAT group exhibited elevated fasting serum insulin and HOMA-IR values (P < 0.05; Figure 2A and B). Although the RFAT, RSUG and PRO groups showed no significant differences in fasting serum insulin and HOMA-IR values compared with the CON group (P > 0.05), the PRO group showed decreased fasting serum insulin and HOMA-IR values compared with the RFAT and RSUG groups (P < 0.05; Figure 2A and B). The OGTT confirmed the impaired glucose tolerance of the FFAT group (Figure 2C and D). The area under the curve (AUC) of the OGTT showed that the PRO group had decreased AUCOGTT when compared with the RFAT and RSUG groups (P < 0.05), but these three groups showed no significant differences in AUCOGTT values when compared with the CON group (P > 0.05; Figure 2D).
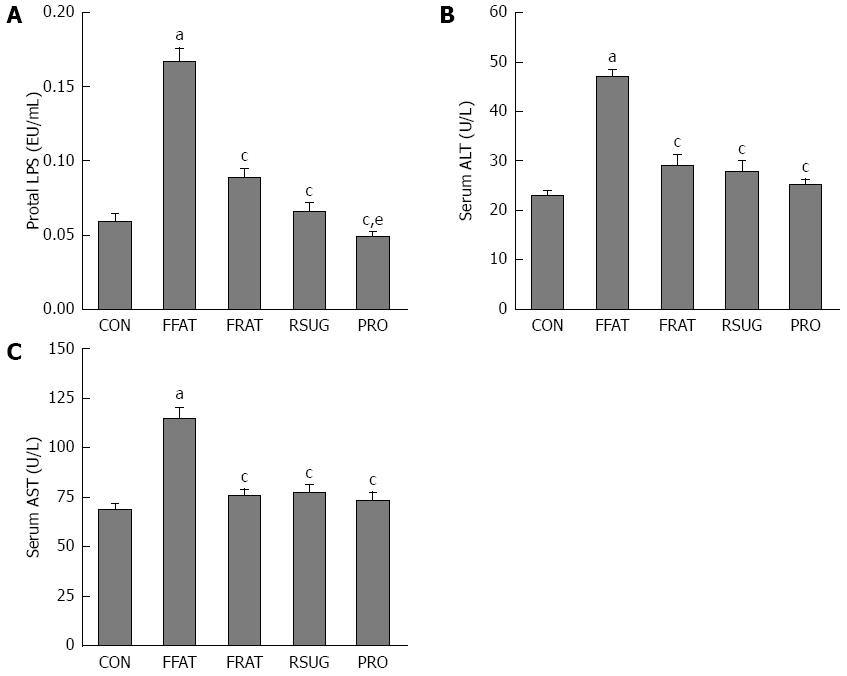
At the end of the trial, portal LPS was measured. The FFAT group had higher levels of portal LPS compared with the other groups (P < 0.05; Figure 3A). Taking the same calories, the RFAT group showed higher levels of portal LPS than the PRO group (P < 0.05; Figure 3A). Serum ALT and AST are biomarkers of liver function, which can reflect liver injury. Only the FFAT group had higher serum ALT and AST than the other groups (P < 0.05), and other groups had the same level of serum ALT and AST (Figure 3B and C).
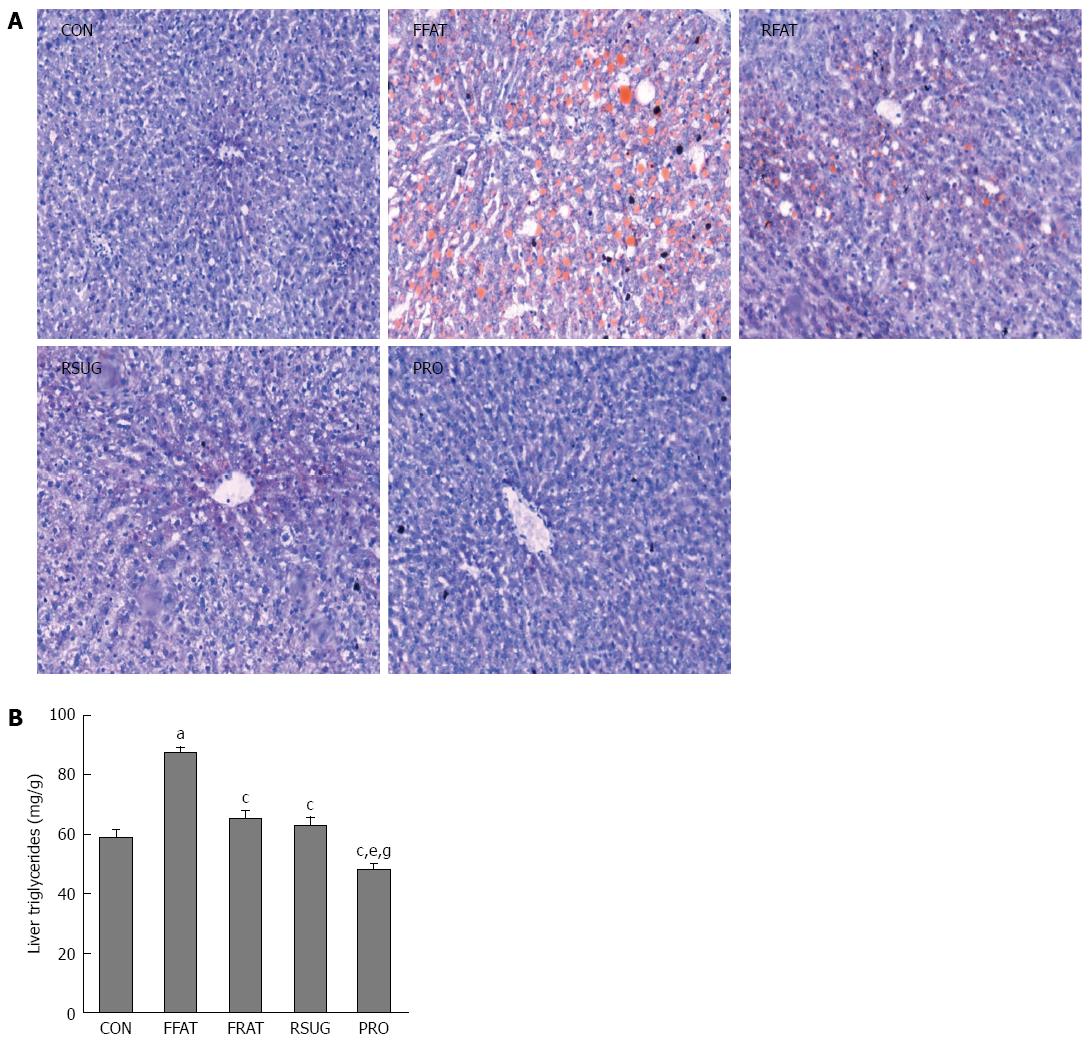
The results of oil red O staining showed that the FFAT group had obvious accumulation of triglycerides in the liver (Figure 4A). However, the RFAT and RSUG groups had significantly less triglyceride accumulation in the liver than the FFAT group. The CON and PRO groups had no detectable triglyceride accumulation in the liver by oil red O staining. Colorimetric measurement confirmed the oil red O staining findings. Using colorimetric measurement, the FFAT group also showed increased liver triglyceride levels compared with the other groups (P < 0.05; Figure 4B). Among the three isocaloric groups, the level of liver triglycerides was higher in the RFAT and RSUG groups than in the PRO group (P < 0.05); however, when compared with the CON group, no significant differences were found (P > 0.05).
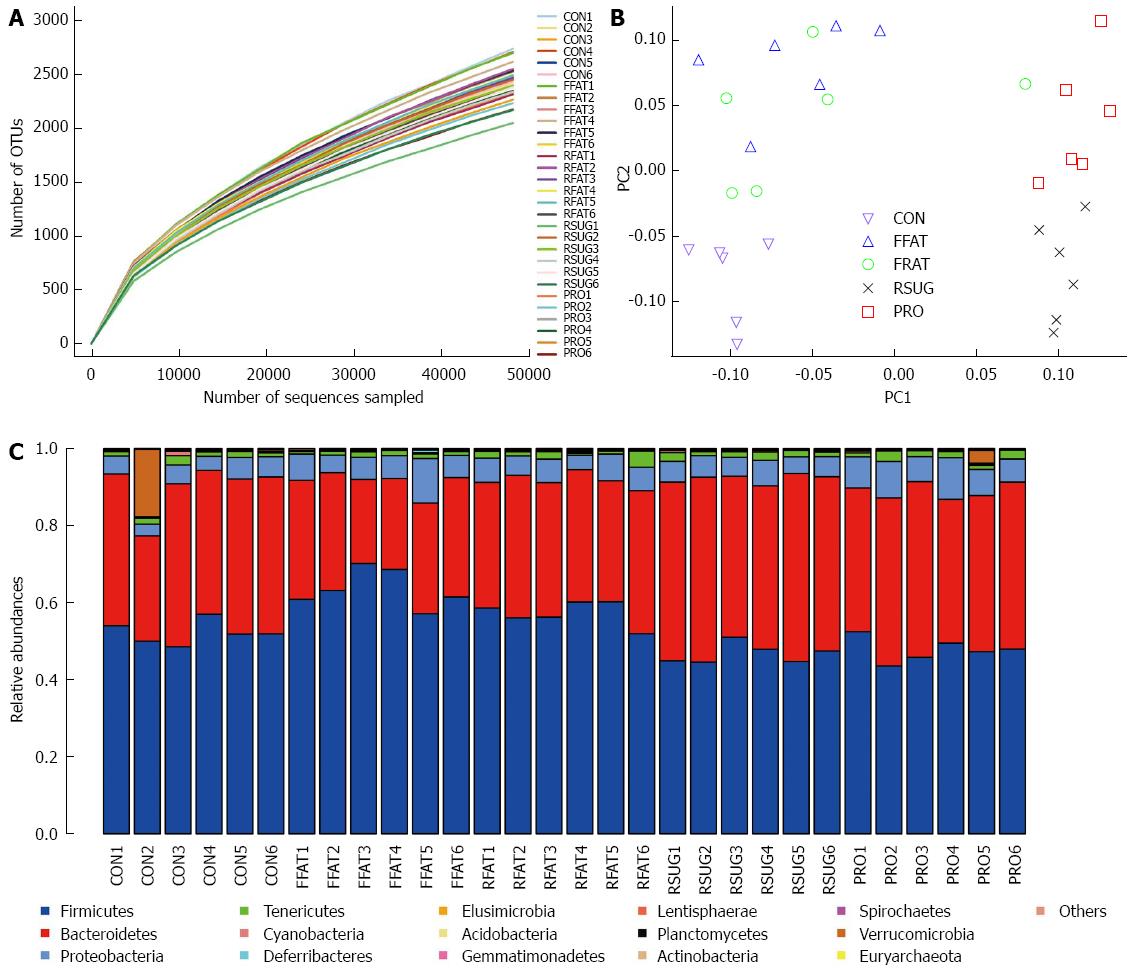
A total of 1705045 high-quality reads (average of 56 835 sequences per sample) were delineated into OTUs at the 97% similarity level. Rarefaction curves revealed that all the samples had a similar pattern, which indicates that some new OTU would be expected with additional sequencing (Figure 5A). However, the good-coverage index, an indicator of sequencing depth, of all samples ranged from 0.968 to 0.977. Combining the results of the rarefaction curves and the good-coverage index, we demonstrated that the sequencing depth in the present study was sufficient to reflect the bacterial composition of different group samples.
Unweighted UniFrac PCoA revealed that despite inter-individual variation, the intestinal microbiota structures of the CON, FFAT, RSUG and PRO groups were roughly separated away from each other (Figure 5B). However, the intestinal microbiota structure of the RFAT group was not well separated.
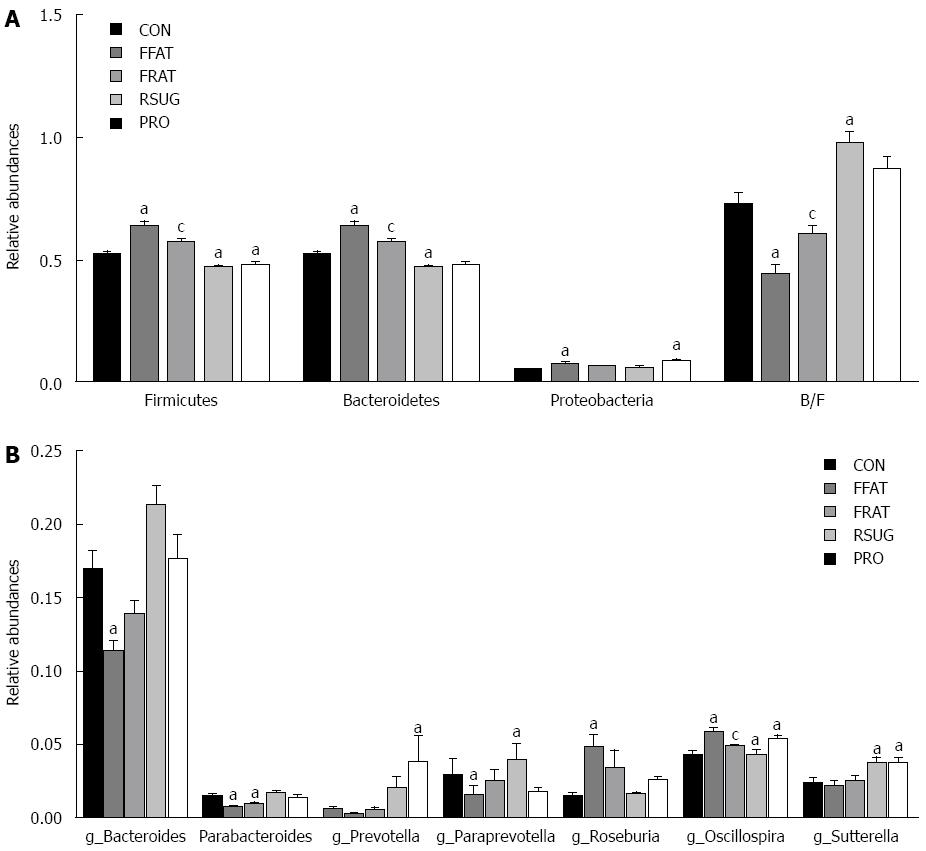
For taxon-based analysis, a total of 15 phyla were detected and the most abundant phyla included Firmicutes, Bacteroidetes, Proteobacteria and Tenericutes (Figure 5C). Compared with the CON group samples, the FFAT group samples showed an increased abundance of Firmicutes, decreased abundance of Bacteroidetes, and lower ratio of Bacteroidetes to Firmicutes (P < 0.05; Figure 6A). The RFAT group samples showed only an increased abundance of Firmicutes (P < 0.05; Figure 6A). The RSUG group samples showed an increased abundance of Bacteroidetes, higher ratio of Bacteroidetes to Firmicutes, and decreased abundance of Firmicutes (P < 0.05; Figure 6A). The PRO group samples showed an increased abundance of Bacteroidetes and decreased abundance of Firmicutes (P < 0.05; Figure 6A). The FFAT and RFAT groups had the same high-fat diet, and the only difference between these two groups was the total caloric intake. However, compared with the FFAT group samples, the RFAT group samples showed an increased abundance of Bacteroidetes, higher ratio of Bacteroidetes to Firmicutes, and decreased abundance of Firmicutes (P < 0.05; Figure 6A).
At the genus level, a total of 109 genera were found, and we only selected the genera with a relative abundance > 1% for analysis. Compared with the CON group samples, the FFAT group samples showed an increased abundance of Roseburia and Oscillospira, and a decreased abundance of Bacteroides and Parabacterioides (P < 0.05; Figure 6B). The RFAT group samples showed a decreased abundance of Parabacterioides (P < 0.05; Figure 6B); the RSUG group samples showed an increased abundance of Sutterella (P < 0.05; Figure 6B); the PRO group samples showed an increased abundance of Prevotella, Oscillospira, and Sutterella (P < 0.05; Figure 6B). Compared with the FFAT group samples, the RFAT group samples showed a decreased abundance of Oscillospira (P < 0.05; Figure 6B).
We investigated the effects of different diets on the development of NAFLD, excluding caloric intake as a confounder. We demonstrated that high-fat and high-sugar diets in rats increased body weight, IR, and liver triglycerides than a high-protein diet, and that these effects were independent of caloric intake. We also found that different diets changed the intestinal microbiota composition independently from caloric intake. The different effects of these diets on NAFLD development, at the same caloric intake, may be associated with changes in the intestinal microbiota.
Obesity and IR are recognized as risk factors for NAFLD[25,26]. In most cases, NAFLD is a complication of obesity[4]. Peripheral IR may cause accumulation of liver triglycerides via three main mechanisms: increased peripheral lipolysis (which increases free fatty acids in the liver), hyperinsulinemia (which stimulates activity of the key lipogenic transcription factors) and hyperglycemia (which increases glucose concentration in the liver and provides substrates for lipogenesis)[27]. Free availability of a high-fat or high-sugar diet can cause rodent obesity, IR, and accumulation of liver triglycerides[28,29]. However, free availability of a high-protein diet has the opposite effect[14]. In the present study, we restricted the caloric intake of these three diets to the same level, and found that high-fat and high-sugar diets caused experimental rats to have greater body weight, peripheral IR, and accumulation of liver triglycerides than a high-protein diet. This suggests that, compared with a high-protein diet, the NAFLD-inducing effects of high-fat and high-sugar diets are independent from caloric intake. The results of the present study showed no significant difference in body weight, peripheral IR, and accumulation of liver triglycerides between the RFAT and CON groups. However, de Meijer et al[30] found that mice with a restrictive high-fat diet showed higher body weight, peripheral IR, and accumulation of liver triglycerides than the control diet mice. The main reason for this discrepancy may be the different restrictive levels that we chose. The study by de Meijer et al[30] restricted the caloric intake of the high-fat diet group to the same level as the control diet group; however, we restricted the caloric intake of the high-fat diet group to the same level as the high-protein diet group, which consumed fewer calories than the control diet group. This discrepancy may reflect the role that calories play in the development of NAFLD. Compared with the FFAT group, the RFAT group showed decreased body weight, peripheral IR, and accumulation of liver triglycerides. This confirms the role of calories in the development of NAFLD. We conclude that dietary composition and caloric intake are two independent factors that can affect the development of NAFLD.
LPS is a constituent of Gram-negative bacteria and can trigger the secretion of proinflammatory cytokines, such as tumor necrosis factor-α, when it binds to Toll-like receptor (TLR) 4 on the membrane surface of monocytes, macrophages, adipocytes and hepatocytes. After continuous subcutaneous infusion of LPS for 4 wk, wild-type mice exhibited obesity, adipose tissue weight gain, steatosis, and fasting hyperglycemia[31]. However, mice mutant for CD14 (a key multifunctional receptor that mediates the combination of LPS and TLR4) resisted most of the LPS-induced features of metabolic diseases. Therefore, LPS is considered a triggering factor in the development of metabolic disorders. In NAFLD patients, elevated LPS levels have been observed[32]. However, the relationship between different diets and LPS is unclear. In the present study, with the same caloric intake, rats fed a high-fat diet showed higher portal LPS levels than those fed a high-protein diet, suggesting that compared with a high-protein diet, a high-fat diet can elevate portal LPS level independently from caloric intake. Unexpectedly, although the RFAT group showed increased portal LPS levels, serum ALT and AST levels in the RFAT and PRO groups did not differ significantly. A possible explanatory hypothesis is that there is a threshold level of LPS concentration in the liver that induces obvious injury, and the liver can handle the elevated LPS which does not reach the threshold level. The higher levels of portal LPS and serum ALT and AST in the FFAT group compared with the RFAT group partly confirm this hypothesis.
The human intestine harbors > 2000 species of commensal bacteria that define the intestinal microbiota. This community consists of 10 times more bacteria than human cells and encodes 100-200-fold more genes than our own genome[33,34]. The intestinal microbiota provides the host with enhanced metabolic capabilities (fermentation of nondigestible dietary residue, production of vitamin K, and absorption of ions), protection against pathogens, education of the immune system, and modulation of gastrointestinal development. So the intestinal microbiota can be viewed as a “metabolic organ”. This organ can affect host metabolic processes[35,36]. Although the composition of the intestinal microbiota is influenced by multiple factors, diet plays an important role in shaping it[37,38]. In the present study, Unweighted UniFrac PCoA results showed that the intestinal microbiota structures of the CON, FFAT, RSUG and PRO groups were roughly separated away from each other; however, the intestinal microbiota of the RFAT group was not well separated. However, taxon-based analysis found that intestinal microbiota composition of the RFAT, RSUG and PRO groups differed from that of the CON group. These results suggest that different diets can affect the composition of the intestinal microbiota independently from caloric intake. This important finding may be helpful in better understanding the effect of diet on the composition of the intestinal microbiota. In the present study, the RSUG group showed an increased abundance of Bacteroidetes. Some species of bacteria in this phylum, such as Bacteroides thetaiotaomicron, can encode adequate carbohydrate active enzymes for carbohydrate metabolism of food[39]. This enables the host to extract more energy from the diet, which will be deposited in the liver in the form of triglycerides. Unlike the RSUG group, the RFAT group showed an increased abundance of Firmicutes; however, how the bacteria in this phylum affect NAFLD development is not clear. The effect of a high-protein diet on the composition of the intestinal microbiota is not well studied. We found that the PRO group had an increased abundance of Bacteroidetes and Sutterella bacteria, and decreased abundance of Firmicutes. This change in intestinal microbiota was similar to that in the RSUG group; however, the PRO group also had an increased abundance of Prevotella and Oscillospira. Kovatcheva-Datchary et al[40] found that Prevotella is associated with improvement in glucose metabolism. Oscillospira has never been cultured, so it is an enigmatic bacterial genus and little is known of it role in the intestinal tract. However, recent studies found that Oscillospira is positively associated with leanness, and is reduced in pediatric NASH[41,42]. We conclude that the beneficial effects of high-protein diet on NAFLD may be closely associated with Prevotella and Oscillospira, which requires further study. Changes in intestinal microbiota are one mechanism by which diet affects NAFLD development, so we can conclude that the different effects of these diets on NAFLD development, at the same caloric intake, may be associated with changes in intestinal microbiota. However, the exact role that these different bacteria play in the development of NAFLD remains unclear and needs further study. Previous studies have found that high-fat diet reduces Bacteroides and increases Firmicutes and Proteobacteria. The ratio of Bacteroidetes to Firmicutes was also decreased by a high-fat diet[43,44]. The results of the present study confirmed these findings. Moreover, we found that the high-fat diet increased Roseburia and Oscillospira spp. However, Neyrinck et al[45] have reported that the number of Roseburia spp. was decreased when mice were fed a high-fat diet. As host genotype is also an important factor that can affect the intestinal microbiota, the reason for this contrary result may be mainly due to the different animals that were used[21]. Taxon-based analysis also found that intestinal microbiota composition of the RFAT group was different from that of the FFAT group, suggesting that, except for different dietary composition, caloric intake is an independent factor that can shape the intestinal microbiota.
Because of the limitations of the bacterial 16S rRNA gene sequencing technique that we adopted, we could not classify sequences at the species level, and the relationship between intestinal bacteria and portal LPS levels was undefined. It should be pointed out that the results of the present study were obtained from rats and host genotype also can affect intestinal microbiota composition, so it may not be appropriate to apply our results directly to humans.
In conclusion, our present study found that compared with the high-protein diet, the NAFLD-inducing effect of the high-fat and high-sugar diets is independent from caloric intake. This helps in understanding the effects of diet on the development of NAFLD. In addition, the effects of diet on the intestinal microbiota in the present study extend our knowledge of the relationship between diet and the intestinal microbiota. In the future, we can manipulate the intestinal microbiota by diet to prevent or treat NAFLD. Overall, our findings shed some light on the desirability of dietary therapy for NAFLD.
Although several studies have elaborated the relationship between different diets and nonalcoholic fatty liver disease (NAFLD) development, those studies had the confounder of caloric intake. The intestinal microbiota is the interface between diet and the liver; however, the relationship between diet, intestinal microbiota and NAFLD is unclear.
Previous studies have shown that high-fat and high-sugar diets can induce NAFLD; however, a high-protein diet can ameliorate it. Diet is also an important factor that can shape the intestinal microbiota, and NAFLD patients are always associated with changes in intestinal microbiota.
To the best of the authors’ knowledge, this is the first study to evaluate the effects of different diets on the intestinal microbiota and NAFLD development at the same caloric intake. This study suggests that compared with a high-protein diet, the NAFLD-inducing effect of high-fat and high-sugar diets is independent from caloric intake. The different effects of these diets on NAFLD development, at the same caloric intake, may be associated with changes in the intestinal microbiota.
This study extends the authors’ knowledge of the relationship between diet, intestinal microbiota, and NAFLD. It sheds some light on the desirability of dietary therapy for NAFLD.
Unweighted UniFrac principal coordinates analysis is a method used to discriminate the microbiota composition of the different groups based on evolutionary distance.
In this paper, the authors examined the effects of different diets on NAFLD and intestinal microiota. They found that high-fat diet and high-sugar diet but not high-protein diet induced NAFLD independently from calories. Also diet affects the microbiota.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hirasawa N, Mulholland MW, Sugimura H, Skrypnyk I S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Ma S
| 1. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3655] [Cited by in RCA: 3720] [Article Influence: 161.7] [Reference Citation Analysis (2)] |
| 2. | Wang JT, Liu YL. Non-alcoholic fatty liver disease: the problems we are facing. Hepatobiliary Pancreat Dis Int. 2003;2:334-337. [PubMed] |
| 3. | Angulo P. GI epidemiology: nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2007;25:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 261] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 4. | Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40 Suppl 1:S5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 234] [Reference Citation Analysis (0)] |
| 5. | McCullough AJ. Pathophysiology of nonalcoholic steatohepatitis. J Clin Gastroenterol. 2006;40 Suppl 1:S17-S29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 139] [Reference Citation Analysis (0)] |
| 6. | Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519-1523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1720] [Cited by in RCA: 1660] [Article Influence: 118.6] [Reference Citation Analysis (2)] |
| 7. | Kim D, Choi SY, Park EH, Lee W, Kang JH, Kim W, Kim YJ, Yoon JH, Jeong SH, Lee DH. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology. 2012;56:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 254] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 8. | Vilar L, Oliveira CP, Faintuch J, Mello ES, Nogueira MA, Santos TE, Alves VA, Carrilho FJ. High-fat diet: a trigger of non-alcoholic steatohepatitis? Preliminary findings in obese subjects. Nutrition. 2008;24:1097-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Lieber CS, Leo MA, Mak KM, Xu Y, Cao Q, Ren C, Ponomarenko A, DeCarli LM. Model of nonalcoholic steatohepatitis. Am J Clin Nutr. 2004;79:502-509. [PubMed] |
| 10. | Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008;48:993-999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 678] [Cited by in RCA: 606] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 11. | Faeh D, Minehira K, Schwarz JM, Periasamy R, Park S, Tappy L. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes. 2005;54:1907-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 12. | Roglans N, Vilà L, Farré M, Alegret M, Sánchez RM, Vázquez-Carrera M, Laguna JC. Impairment of hepatic Stat-3 activation and reduction of PPARalpha activity in fructose-fed rats. Hepatology. 2007;45:778-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Yang HY, Tzeng YH, Chai CY, Hsieh AT, Chen JR, Chang LS, Yang SS. Soy protein retards the progression of non-alcoholic steatohepatitis via improvement of insulin resistance and steatosis. Nutrition. 2011;27:943-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718-15723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4530] [Cited by in RCA: 4417] [Article Influence: 210.3] [Reference Citation Analysis (4)] |
| 15. | Yin X, Peng J, Zhao L, Yu Y, Zhang X, Liu P, Feng Q, Hu Y, Pang X. Structural changes of gut microbiota in a rat non-alcoholic fatty liver disease model treated with a Chinese herbal formula. Syst Appl Microbiol. 2013;36:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 630] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 17. | Mouzaki M, Comelli EM, Arendt BM, Bonengel J, Fung SK, Fischer SE, McGilvray ID, Allard JP. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology. 2013;58:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 556] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 18. | Savastano DM, Covasa M. Adaptation to a high-fat diet leads to hyperphagia and diminished sensitivity to cholecystokinin in rats. J Nutr. 2005;135:1953-1959. [PubMed] |
| 19. | Sclafani A. Carbohydrate-induced hyperphagia and obesity in the rat: effects of saccharide type, form, and taste. Neurosci Biobehav Rev. 1987;11:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Weigle DS, Breen PA, Matthys CC, Callahan HS, Meeuws KE, Burden VR, Purnell JQ. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr. 2005;82:41-48. [PubMed] |
| 21. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22373] [Cited by in RCA: 24521] [Article Influence: 613.0] [Reference Citation Analysis (1)] |
| 22. | Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497-509. [PubMed] |
| 23. | Ables GP, Perrone CE, Orentreich D, Orentreich N. Methionine-restricted C57BL/6J mice are resistant to diet-induced obesity and insulin resistance but have low bone density. PLoS One. 2012;7:e51357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 179] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 24. | Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29318] [Cited by in RCA: 23751] [Article Influence: 1583.4] [Reference Citation Analysis (0)] |
| 25. | Bellentani S, Saccoccio G, Masutti F, Crocè LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Choudhury J, Sanyal AJ. Insulin resistance and the pathogenesis of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:575-94, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Lockman KA, Nyirenda MJ. Interrelationships between hepatic fat and insulin resistance in non-alcoholic fatty liver disease. Curr Diabetes Rev. 2010;6:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Meli R, Mattace Raso G, Irace C, Simeoli R, Di Pascale A, Paciello O, Pagano TB, Calignano A, Colonna A, Santamaria R. High Fat Diet Induces Liver Steatosis and Early Dysregulation of Iron Metabolism in Rats. PLoS One. 2013;8:e66570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 29. | Park DY, Ahn YT, Huh CS, McGregor RA, Choi MS. Dual probiotic strains suppress high fructose-induced metabolic syndrome. World J Gastroenterol. 2013;19:274-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | de Meijer VE, Le HD, Meisel JA, Akhavan Sharif MR, Pan A, Nosé V, Puder M. Dietary fat intake promotes the development of hepatic steatosis independently from excess caloric consumption in a murine model. Metabolism. 2010;59:1092-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4095] [Cited by in RCA: 4565] [Article Influence: 253.6] [Reference Citation Analysis (1)] |
| 32. | Harte AL, da Silva NF, Creely SJ, McGee KC, Billyard T, Youssef-Elabd EM, Tripathi G, Ashour E, Abdalla MS, Sharada HM. Elevated endotoxin levels in non-alcoholic fatty liver disease. J Inflamm (Lond). 2010;7:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 295] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 33. | Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1071] [Cited by in RCA: 921] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 34. | Ashida H, Ogawa M, Kim M, Mimuro H, Sasakawa C. Bacteria and host interactions in the gut epithelial barrier. Nat Chem Biol. 2012;8:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 239] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 35. | Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3394] [Cited by in RCA: 3546] [Article Influence: 177.3] [Reference Citation Analysis (5)] |
| 36. | Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2194] [Cited by in RCA: 2114] [Article Influence: 96.1] [Reference Citation Analysis (0)] |
| 37. | Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2376] [Cited by in RCA: 2165] [Article Influence: 135.3] [Reference Citation Analysis (0)] |
| 38. | Zhang C, Zhang M, Wang S, Han R, Cao Y, Hua W, Mao Y, Zhang X, Pang X, Wei C. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010;4:232-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 651] [Cited by in RCA: 831] [Article Influence: 51.9] [Reference Citation Analysis (1)] |
| 39. | Martens EC, Koropatkin NM, Smith TJ, Gordon JI. Complex glycan catabolism by the human gut microbiota: the Bacteroidetes Sus-like paradigm. J Biol Chem. 2009;284:24673-24677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 434] [Cited by in RCA: 478] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 40. | Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, Hallen A, Martens E, Björck I, Bäckhed F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015;22:971-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 927] [Cited by in RCA: 1246] [Article Influence: 124.6] [Reference Citation Analysis (2)] |
| 41. | Verdam FJ, Fuentes S, de Jonge C, Zoetendal EG, Erbil R, Greve JW, Buurman WA, de Vos WM, Rensen SS. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity (Silver Spring). 2013;21:E607-E615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 427] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 42. | Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1283] [Article Influence: 106.9] [Reference Citation Analysis (1)] |
| 43. | Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, Gibson GR, Delzenne NM. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374-2383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1237] [Cited by in RCA: 1296] [Article Influence: 72.0] [Reference Citation Analysis (1)] |
| 44. | Murphy EF, Cotter PD, Healy S, Marques TM, O’Sullivan O, Fouhy F, Clarke SF, O’Toole PW, Quigley EM, Stanton C. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut. 2010;59:1635-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 709] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 45. | Neyrinck AM, Possemiers S, Druart C, Van de Wiele T, De Backer F, Cani PD, Larondelle Y, Delzenne NM. Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS One. 2011;6:e20944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 322] [Cited by in RCA: 353] [Article Influence: 25.2] [Reference Citation Analysis (0)] |