Copyright
©The Author(s) 2018.
World J Gastroenterol. Jan 21, 2018; 24(3): 323-337
Published online Jan 21, 2018. doi: 10.3748/wjg.v24.i3.323
Published online Jan 21, 2018. doi: 10.3748/wjg.v24.i3.323
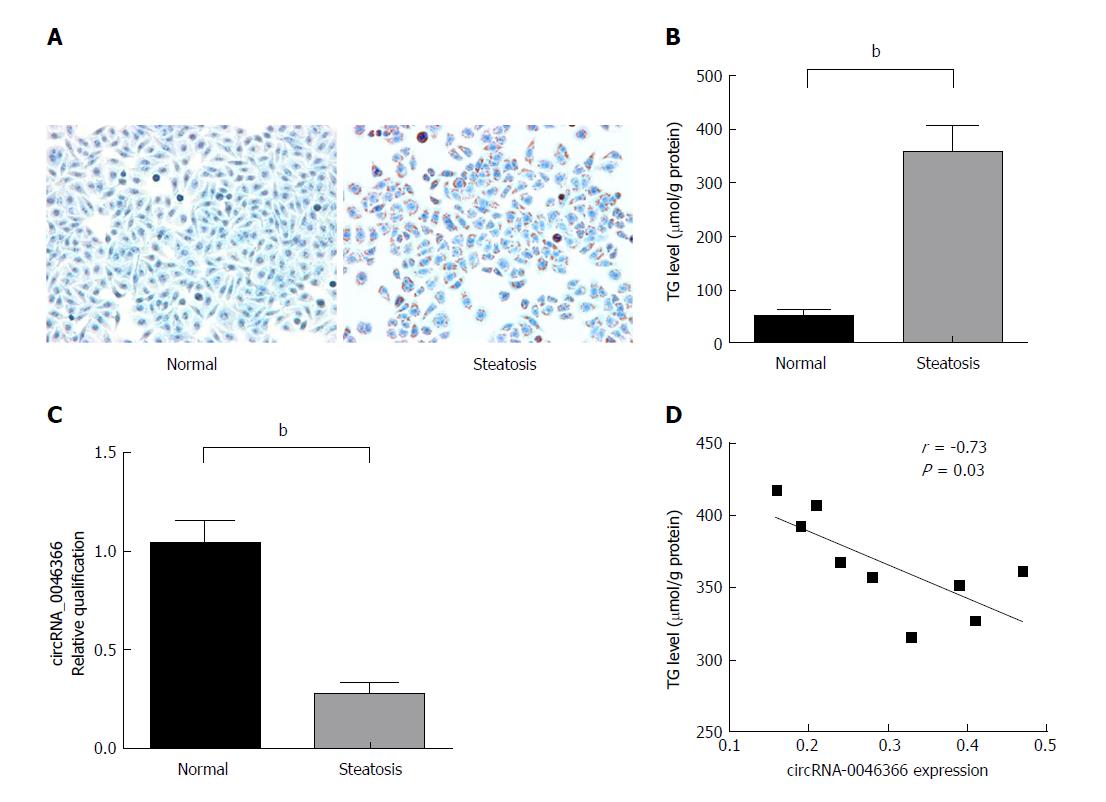
Figure 1 circRNA_0046366 loss demonstrates the epigenetic characteristic of FFA-induced hepatocellular steatosis.
A: Oil Red O staining identified HepG2 cells with (steatosis group) or without (normal group) FFA-induced steatosis (200 ×); B: The steatosis group exhibited significant up-regulation of TG content; C: circRNA_0046366 quantification revealed inhibition of its expression after hepatocellular steatosis; D: circRNA_0046366 expression was inversely correlated with hepatocellular TG level in the steatosis group. Results are expressed as mean ± SD. bP < 0.01. FFA: Free fatty acid; TG: Triglyceride.
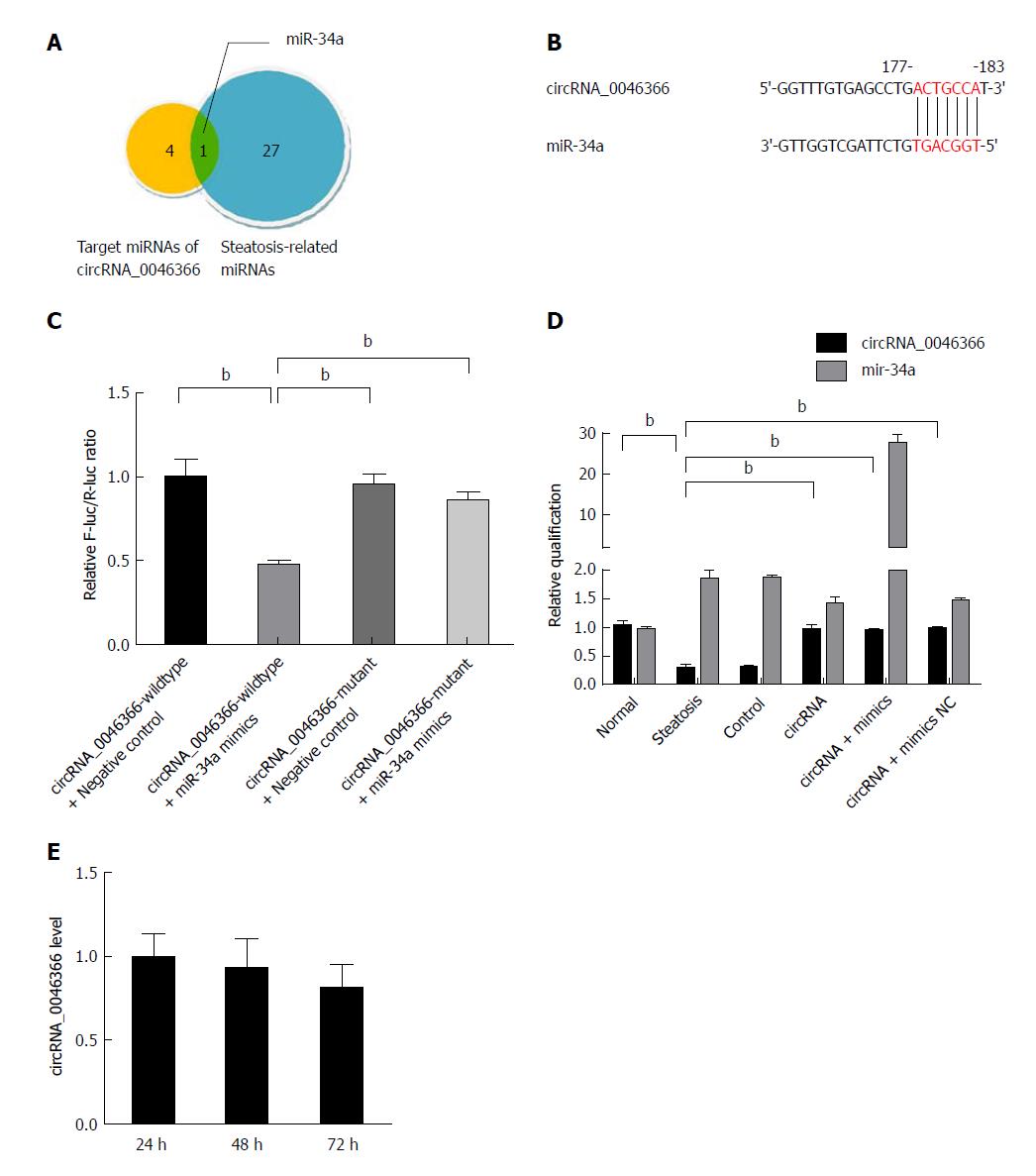
Figure 2 circRNA_0046366 functions as the antagonist of miR-34a.
A: Set intersection filters circRNA_0046366-targeting miRNAs associated with hepatocellular steatosis; B: circRNA-miRNA interaction recognized by the base complementation between MRE of circRNA_0046366 and seed sequence of miR-34a; C: Dual-luciferase reporter assay verified the antagonistic effect of circRNA_0046366 on miR-34a; D: Treatment of circRNA-carrying vector normalized expression level of circRNA_0046366 with counteracting impact on miR-34a; E: Results are expressed as mean ± SD. bP < 0.01.
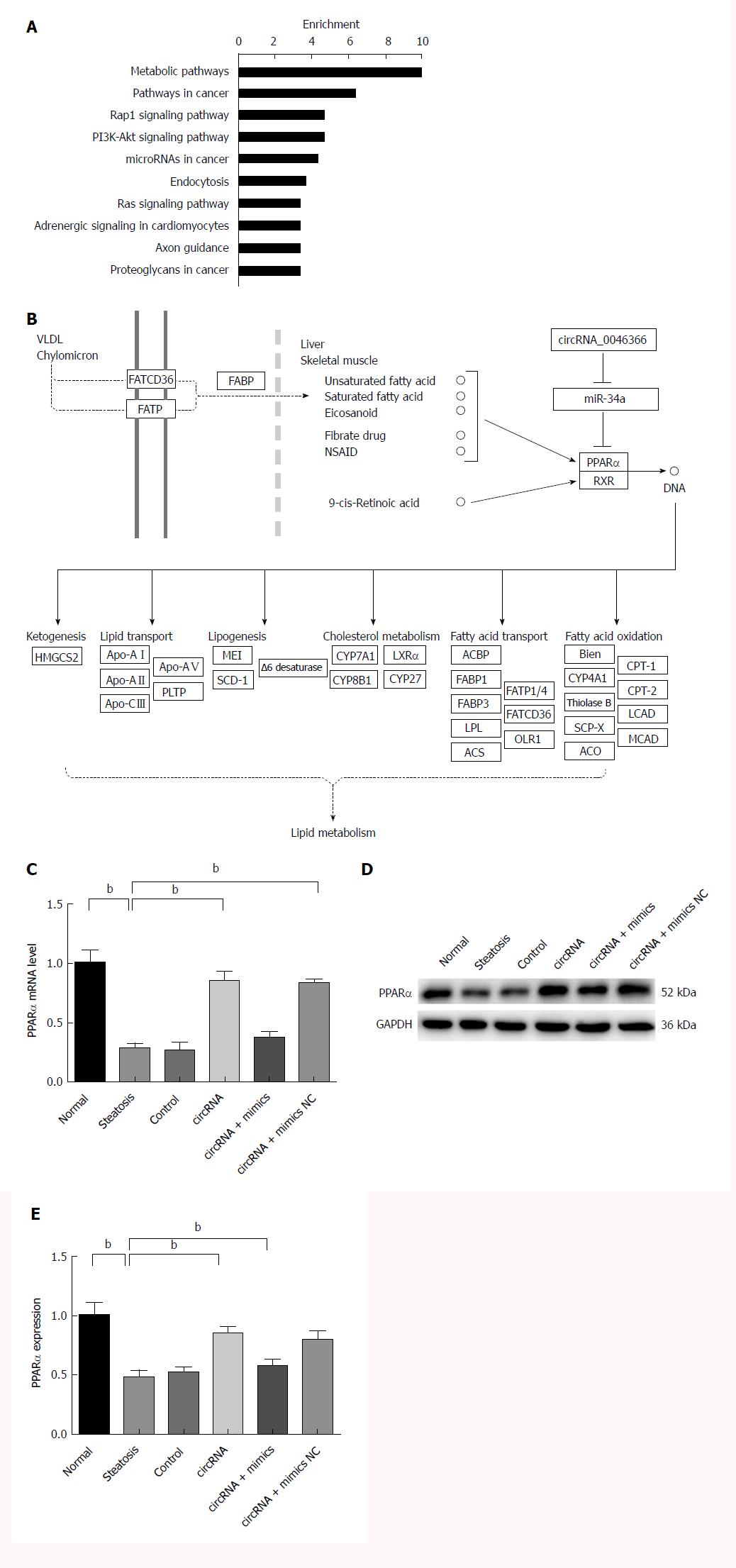
Figure 3 circRNA_0046366 restoration rescued PPARα expression by miR-34a inactivation.
A: Metabolic pathway (hsa01100) was the top-enriched pathway that was affected by circRNA_0046366-induced miR-34a antagonism; B: PPARα mediated the regulatory role of miR-34a in lipid metabolism signaling by controlling expression of multiple lipometabolic genes (adapted from the KEGG database). miR-34a inactivation leads to the up-regulated expression of PPARα at transcriptional (C) and translational (D) levels. Results are expressed as mean ± SD. bP < 0.01. KEGG: Kyoto Encyclopedia of Genes and Genomes; PPAR: Peroxisome proliferator-activated receptor.
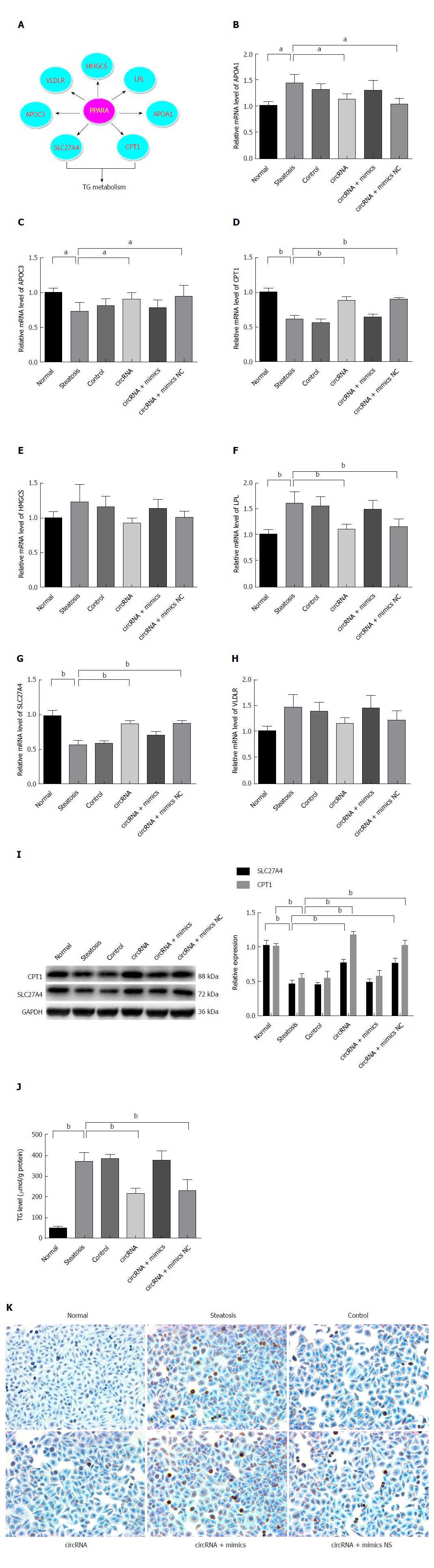
Figure 4 PPARα normalization improved hepatocellular steatosis by transcriptional promotion of lipometabolic genes.
A: PPARα restoration by circRNA-0046366 promoted expression of CPT1A and SLC27A at the transcriptional level; B: Transcriptional activation of CPT1A and SLC27A resulted in their significant up-regulation. Improved expression of lipometabolic genes reduced the TG level (C) and attenuated hepatocellular steatosis (D) (200 ×). Results are expressed as mean ± SD. aP < 0.05, bP < 0.01. PPAR: Peroxisome proliferator-activated receptor; TG: Triglyceride.
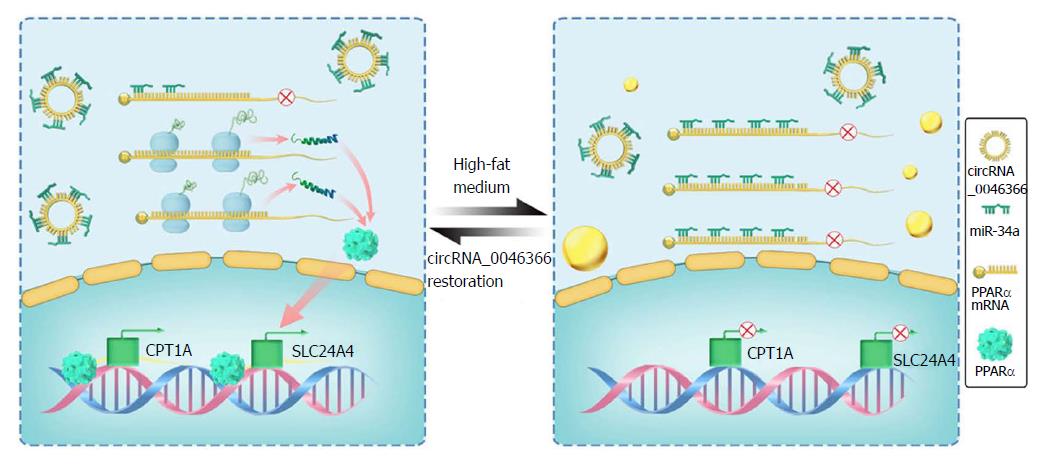
Figure 5 Schematic representation exhibits the circRNA_0046366/miR-34a/PPARα axis underlying occurrence and resolution of hepatocellular steatosis.
In contrast to the hepatic steatogenesis that occurs upon its deficiency, circRNA_0046366 restoration abrogates the inhibitory effect of miR-34a on PPARα by antagonizing the miRNA-mRNA interaction. PPARα normalization transcriptionally activates the lipometabolic genes, which further ameliorates hepatic steatosis by regaining lipid homeostasis. mi: Micro; PPAR: Peroxisome proliferator-activated receptor.
- Citation: Guo XY, Sun F, Chen JN, Wang YQ, Pan Q, Fan JG. circRNA_0046366 inhibits hepatocellular steatosis by normalization of PPAR signaling. World J Gastroenterol 2018; 24(3): 323-337
- URL: https://www.wjgnet.com/1007-9327/full/v24/i3/323.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i3.323