Published online Jun 21, 2008. doi: 10.3748/wjg.14.3693
Revised: April 1, 2008
Accepted: April 8, 2008
Published online: June 21, 2008
AIM: To evaluate the protective effect of 2'-p-hydroxybenzoylmussaenosidic acid [negundoside (NG), against carbon tetrachloride (CCl4)-induced toxicity in HuH-7 cells.
METHODS: CCl4 is a well characterized hepatotoxin, and inducer of cytochrome P450 2E1 (CYP2E1)-mediated oxidative stress. In addition, lipid peroxidation and accumulation of intracellular calcium are important steps in the pathway involved in CCl4 toxicity. Liver cells (HuH-7) were treated with CCl4, and the mechanism of the cytoprotective effect of NG was assessed. Silymarin, a known hepatoprotective drug, was used as control.
RESULTS: NG protected HuH-7 cells against CCl4 toxicity and loss of viability without modulating CYP2E1 activity. Prevention of CCl4 toxicity was associated with a reduction in oxidative damage as reflected by decreased generation of reactive oxygen species (ROS), a decrease in lipid peroxidation and accumulation of intracellular Ca2+ levels and maintenance of intracellular glutathione homeostasis. Decreased mitochondrial membrane potential (MMP), induction of caspases mediated DNA fragmentation and cell cycle arrest, as a result of CCl4 treatment, were also blocked by NG. The protection afforded by NG seemed to be mediated by activation of cyclic adenosine monophosphate (cAMP) synthesis and inhibition of phospholipases (cPLA2).
CONCLUSION: NG exerts a protective effect on CYP2E1-dependent CCl4 toxicity via inhibition of lipid peroxidation, followed by an improved intracellular calcium homeostasis and inhibition of Ca2+-dependent proteases.
-
Citation: Tasduq SA, Kaiser PJ, Gupta BD, Gupta VK, Johri RK. Negundoside, an iridiod glycoside from leaves of
Vitex negundo , protects human liver cells against calcium-mediated toxicity induced by carbon tetrachloride. World J Gastroenterol 2008; 14(23): 3693-3709 - URL: https://www.wjgnet.com/1007-9327/full/v14/i23/3693.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3693
Natural products from plant sources have extensive past and present use in treatment of diverse diseases and serve as compounds of interest both in their natural form and as templates for synthetic modification. The importance of natural products in modern medicine has been well recognized. More than 20 new drugs, launched world over between 2000 and 2005, originate from natural products. Scrutiny of medical indications by source of compounds has demonstrated that natural products and related drugs are used to treat 87% of all categorized human diseases (infectious and non-infectious)[1].
Vitex negundo (verbenaceae) is an important source of such natural drugs. It is a reputed medicinal herb and its parts have been employed as a traditional cure in Asian systems of medicine (Indian, Chinese, Malaysian) for a variety of disease conditions. A number of pharmacological activities have been attributed to V. negundo, such as: analgesic and anti-inflammatory activity[2], enzymes inhibition[3], nitric oxide scavenging activity[4], snake venom neutralization activity[5], antifeeding activity[6], antiradical and antilipoperoxidative[7], CNS activity[8], hepatoprotective activity[9], anti-bacterial activity[10], anti-fungal[11], larvicidal activity [12], antiandrogenic effects[13], mosquito repellent activity[14].
In the recent past, some of our work and work as reported by others on botanical products from V. negundo have shown a promising hepatoprotective activity[915]. This activity has been evaluated against various hepatotoxic agents including carbon tetrachloride (CCl4). CCl4 is a well established and widely used hepatotoxin and the principle cause of CCl4-induced liver injury is proposed to be lipid peroxidation by free radical derivatives of CCl4. CCl4 is activated by NADH-CYP 450 2E1 system of the liver endoplasmic reticulum and converted into trimethyl CCl3 radicals (via reductive dehalogenation) and, under aerobic conditions, in the more reactive trichloromethyl peroxy radical CCl3OO*. Formation of the radicals CCl3* and CCl3OO* causes oxidative stress. The CYP 2E1-mediated metabolism results in generation of reactive oxygen species, which further contributes to the development of cellular injury[16]. Also, considerable evidence suggests that CCl4 modifies the expression levels of several pro-apoptotic and anti-apoptotic growth factors and receptors[17] especially during chronic administration. CCl4 has been shown to be a carcinogen and has been classified as a group 2B carcinogen by inducing gene conversion, homozygosity and intra-chromosomal recombinations[18].
It was, therefore, our interest to investigate, in-depth, the mechanism of modulation of CCl4-induced toxic manifestations with 2'-p-hydroxybenzoylmussaenosidic acid [negundoside (NG)] (a purified irridoid glycoside from leaves of Vitex negundo), particularly inhibition of downstream CYP 2E1 cascade of pro-apoptotic events with reference to the following: (1) role of CYP 450 2E1 activation on calcium-mediated oxidative stress; (2) involvement of calcium in phospholipase A2 (PLA2) and cyclic adenosine monophosphate (cAMP) regulation; (3) effect of activated cPLA2 on mitochondrial depolarization, inducing cytochrome C release resulting in caspase mediated apoptosis.
DMEM F12 medium, Fetal calf serum, trypsin-EDTA solution, 2′, 7′-dichlorofluoresceine diacetate (DCF-DA), rhodamine-123 (Rh-123), propidium iodide (PI), DNase-free RNase, proteinase K, 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Hoechst 33258, cyclosporine A, penicillin, streptomycin, l-glutamine, pyruvic acid, camptothecin, malondialdehyde (MDA) and other biochemicals were purchased from Sigma Chemicals Co. (St. Louis, Mo). Caspase-3 (ApoAlert caspases assay kits) were from B.D. Clontech, USA. Phospholipase A2 (PLA2) and cyclic adenosine triphosphatase (cAMP) were measured by commercially available kits from Cyman company, USA, and R&D systems, USA respectively. Protein concentration was measured using the BCA Protein Assay Kit from Pierce (Rockford, IL, USA).
Aerial parts of the plant Vitex negundo Linn were collected locally during August to October. Plant material was identified and authenticated by examination of the morphological characteristics by taxonomist of the Institute. A voucher specimen has been deposited in Indian Institute of Integrative Medicine (I.I.I.M.) Jammu Herbarium under collection No. 17814.
The shade dried and powdered leaves (1 kg) of V. negundo were soaked in ethanol (5 L) and kept overnight. The percolate was filtered and concentrated under reduced pressure at below 50°C. The extraction procedure was repeated three times more using 3 L of ethanol each time. The combined ethanol extract was stirred with water (300 mL) for 1 h and filtered through Celite. The aqueous extract was concentrated at 50°C and finally dried in vacuum desiccators.
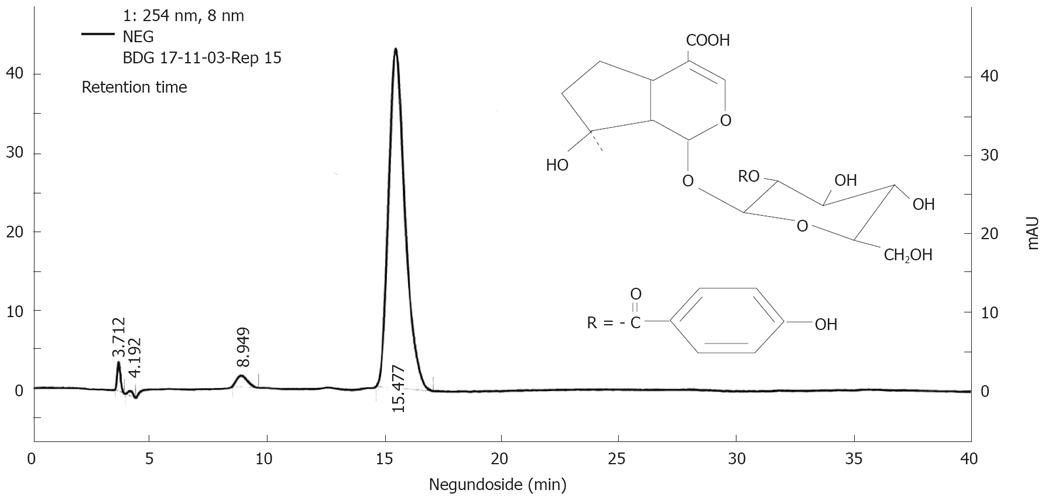
The ethanol extract (50 g) of V. negungo was adsorbed over silica gel (100 g) to make slurry which was packed over a column of silica gel (1 kg) packed in chloroform. Elution was done with chloroform followed by mixture of chloroform and methanol. Elution with 10% methanol in chloroform gave agnuside followed by mixture of agnuside and negundoside and then negundoside. The compounds were characterized on the basis of 1HNMR, 13CNMR mass spectral data (data not shown) and standardized by HPLC (Figure 1).
The study was carried out using as a model a human hepatoma HuH-7 cells line (ATCC-USA), a generous gift from Dr. Vijai Kumar, International Centre for Genetic Engineering and Biotechnology (ICGEB), New Delhi, India. Cells were grown in Dulbecco's Modified Eagle's Medium (DMEM) containing 10% fetal calf serum (FCS), supplemented with 100 Units/mL penicillin, 100 mg/L streptomycin in a humidified atmosphere in 5% CO2 at 37°C, and were sub-cultured at 1:5 ratio once a week.
FCS was reduced to 3% for the experiments. Cells were plated at a density of 3 × 104 cells/cm2 and maintained in culture medium for 12 h. Stock solutions of CCl4, NG and silymarin were prepared fresh to avoid oxidation. For CCl4 toxicity experiments, test substances, were added to the cell cultures an hour prior to CCl4 treatment. Cells or supernatant were then collected for determination of various parameters.
Cells were seeded onto 24-well plates, and after the corresponding treatment, the medium was removed and cell viability was evaluated by assaying for the ability of functional mitochondria to catalyze the reduction of MTT to form formazan salt by mitochondrial dehydrogenases, as described[19] and determined by ELISA reader at 565 nm (Multiskan Spectrum; Thermo Electron Corporation, USA).
The cellular and nuclear morphology was observed under the light microscope (Nikon Eclipse TE2000U), at 40 × magnification, or under fluorescent microscopy, using Hoechst 33 258 staining method as described[20] with certain modifications. Briefly, untreated and treated HuH-7 cells in 6 well plates were harvested by trypsinization, centrifuged at 100 ×g for 5 min and washed twice with PBS. Cells were gently suspended in 100 &mgr;L PBS and fixed in 400 &mgr;L cold acetic acid: methanol (v/v = 1:3) overnight at 4°C. Cells were washed again in 1 mL of fixing solution, suspended in the residual volume of about 50 &mgr;L, spread on a clean slide and dried overnight at room temperature. One milliliter of staining solution (Hoechst 33 258, 10 mg/L in 0.01 mol/L citric acid and 0.45 mol/L disodium phosphate containing 0.05% Tween 20) was poured on each slide and stained for 30 min under subdued light at room temperature. Slides were washed under gentle flow of tap water, rinsed in distilled water followed by in PBS. While wet, 50 &mgr;L of mounting fluid (PBS:glycerol, 1:1) was poured over the center of slide and covered with glass cover slip. The slides were sealed with nail polish and observed for any nuclear morphological alterations and apoptotic bodies under inverted fluorescence microscope (Nikon Eclipse TE2000U, magnification 40X) using UV excitation.
Anti-hemolytic activity of NG and silymarin was studied as described[21]. Briefly, blood was collected from healthy volunteers and centrifuged (3000 r/min) with an equal volume of sterilized Alsver solution (2% dextrose, 0.8% sodium citrate, 0.05% citric acid, and 0.42% sodium chloride in water) to obtain the packed cells. The cells were washed with isosaline (0.85%, pH 7.4) and diluted with phosphate buffer (0.15 mol/L, pH 7.4). RBCs (108 cells/mL) were incubated with triton × 100 (1 g/L) to induce 100% cell lysis, in absence and presence of test materials at 37°C for 1 h. The A of the supernatant was determined at 540 nm.
CYP2E1 activity was determined by assaying P-nitrophenol hydroxylation in rat liver microsomes prepared by calcium precipitation method as described earlier[22]. In brief, 1 mg microsomes in presence and absence of test material caused hydroxylation of 100 mmol/L aniline hydrochloride in presence of 30 mmol/L cumene hydroperoxide in 0.1 mol/L Tris buffer, pH 7.5. Liberated p-aminophenol was treated with 1 mL of 1 mol/L Na2CO3 and 1 mL of 2% phenol solution in 0.5 mol/L NaOH. The samples were allowed to stand at room temperature for 30 min and read at 630 nm.
Cells were plated onto 60 mm Petri dishes, and at the end of the treatment they were washed twice in cold PBS and harvested using rubber policeman in 1 mL PBS. To the resulting cell suspension, we added 2 mL of TCA-TBA reagent (15% TCA + 0.375% TBA in 5 mol/L HCl) in glass tubes. Tubes were kept in a boiling water bath (100°C) for 1 h, and then cooled to room temp. The contents were centrifuged at 1500 ×g for 10 min. Absorbance of the supernatants was read at 535 nm against blank. Malonyldialdehyde (MDA) was used to draw the standard curve. The results were expressed as nmoles MDA/mg protein.
In other set of experiments, 1 g/L of rat liver microsomes in 0.15 mol/L NaCl were incubated with 0.1 mmol/L FeSO4 and 35 mmol/L H2O2 in the presence and absence of test materials to stimulate lipid peroxidation. Generation of MDA was determined by assaying for thiobarbituric acid-reactive substances (TBARS) as described[22].
Cells were seeded onto 60 mm Petri dishes and collected after the corresponding treatments. The total GSH content (reduced form) of samples was assayed as described[23]. Briefly, after treatments, cells were washed with ice cold PBS containing 10 mmol/L EDTA and centrifuged at 1500 ×g. The cell pellets were resuspended in PBS/EDTA solution with 35% perchloric acid. The cell suspensions were kept on ice for 10 min and vortexed in between 5 times. Cell suspension was centrifuged at 13 000 r/min for 10 min at 4°C. The supernatant was transferred to fresh tubes and the pH adjusted to 7 with triethanolamine, 1 mol/L, K2CO3 1.65 mol/L and EDTA 30 mmol/L. The contents were centrifuged at 13 000 r/min for 10 min. To the 50 &mgr;L of the supernatant, we added 1850 &mgr;L of PBS/EDTA solution and 100 &mgr;L of o-phthaldehyde (1 g/L in methanol). The samples were incubated in the dark for 20 min and read at 350 nm (excitation) and 420 nm (emission) with a fluorescent spectrofluorometer (Perkin Elmer; LS-55).
Cytochrome C was determined as described[24]. Briefly, the reaction medium (2.5 mL) containing mitochondria (1 mg protein/mL) was 0.2 mol/L sucrose, 1 mmol/L KH2PO4, 5 mmol/L succinate, 2 &mgr;mol/L rotenone, 10 mmol/L Tris-MOPS, pH 7.3 at 25°C. Mitochondria were preincubated with test materials [NG, silymarin and cyclosporin A (CsA; 0.2 &mgr;mol/L)] for 10 min, and then in presence of CCl4 (2 mmol/L) for 30 min. After incubation, aliquots of mitochondrial suspensions were centrifuged at 11 000 ×g for 8 min to obtain the supernatant. Finally, the supernatant (50 &mgr;L) was introduced into an HPLC system equipped with a reverse-phase C4 Cosmosil column (150 mm × 4.6 mm, 5-&mgr;m particle size, equipped with a UV-visible detector (393 nm). The column (Spelco; Supercosil LC-304; 24 &mgr;m × 4.6 &mgr;m × 5 &mgr;m) was eluted with a linear gradient of acetonitrile-water (solvents modified with 0.1 mL/L trifluoroacetic acid); the gradient started at 20% acetonitrile and changed to 60% during 12 min and the flow rate was 1.0 mL/min. The column was then washed with the 60% acetonitrile for 5 min followed by reequilibration for 5 min in the 20% acetonitrile.
Mitochondria from livers of rats (Wistar) were prepared as described[25]. In brief, rat livers were washed once with physiological saline, dissected and washed twice in cold isolation solution (200 mmol/L D-mannitol, 70 mmol/L sucrose, 2 mmol/L HEPES, 0.5 g/L BSA). Dissected livers were minced with two volumes of isolation solution and homogenized (IKA homogenizer-WERK, Ultra Turrax, T 25 B). The homogenate was diluted with 7 volumes of isolation buffer and centrifuged for 10 min at 560 ×g. The supernatant collected was again centrifuged for 15 min at 7000 ×g. The mitochondrial pellet was collected and resuspended in 2.5 volumes of isolation solution and stored at -80°C until use.
Intracellular calcium levels were determined with the fluorescent calcium indicator fura2-AM by ratiometric fluorimetry as described[26]. HuH-7 cells were detached from the plate using HBSS buffer (118 mmol/L NaCl, 4.6 mmol/L KCl, 10 mmol/L glucose, 20 mmol/L Hepes, pH 7.2) containing 0.02% EDTA, resuspended in HBSS with 1 mmol/L CaCl2, and incubated with 1 &mgr;mol/L fura2-AM in the dark for 1 h at 37°C. Cells were washed with HBSS/CaCl2, and resuspended in HBSS/CaCl2 at a density of 1 × 106 cells/mL. EGTA (10 mmol/L) was added at the beginning of the experiment, followed by a 60 s-equilibration period[27]. Intracellular free calcium measurements were performed at 37°C using a ratiometric fluorescence spectrophotometer (Perkin-Elmer LS 50B). Intracellular Ca2+ concentration was estimated as described[28] based on the equation: [Ca2+]i = Kd[(R-Rmin)/(Rmax-R)]Fmin(380)/Fmax(380), where R is F340/F380 ratio, Rmin and Rmax are the ratios with 50 mmol/L digitonin, and 50 mmol/L digitonin + 11 mmol/L CaCl2, respectively. Kd represents the apparent dissociation constant of Fura-2 (224 nmol/L) and Fmin(380)/Fmax(380) are the fluorescence values of digitonized cells without or with 11 mmol/L CaCl2, respectively.
Changes in the mitochondrial membrane potential (δψm) were examined by monitoring the cell fluorescence after staining with rhodamine 123 (Rh123) as described[29]. Rh123 is a membrane permeable fluorescent cationic dye that is selectively taken up by mitochondria directly proportional to the MMP[30]. The intensities from Rh123 and PI were determined using a BD-LSR flow cytometer equipped with electronic doublet discriminating capability.
The production of ROS was monitored with DCF-DA as the probe[31]. DCF-DA diffuses through the cell membrane and is enzymatically hydrolyzed by intracellular esterases to nonfluorescent DCF-H, which is then rapidly oxidized to the highly fluorescent DCF in presence of ROS. Treated and non-treated HuH-7 cells were incubated for 1 h with DCF-DA (2 &mgr;mol/L) and, after the washing of cells, the production of free radicals were assessed in situ as the enhancement of fluorescence at excitation wavelength of 500 nm and emission wavelength of 520 nm and was measured in a fluorescent plate reader (Perkin Elmer LS 55, USA).
Intracellular H2O2 levels were analyzed using 123 dihydrorhodamine (123-DHR) as specific fluorescent dye probe as described by Katiyar et al with modifications for HuH-7 cells[32]. Briefly, non-treated and treated HuH-7 were washed twice with PBS and incubated in the culture medium without FCS and loaded with 123-DHR (5 &mgr;mol/L). Cells were further incubated for 45 min to irreversibly oxidize and convert DHR to fluorescent compound rhodamine 123 and fluorescence was read with aid of spectrofluorometer (Perkin Elmer LS 55, USA) with excitation wavelength 485 nm and emission wavelength of 530 nm. The cells for fluorescent-based assays were grown onto sterile black fluorescent plates (96 well format; Nunc, Denmark).
Cell cycle was analysed as described by Yang et al[33]. Briefly, non-treated and treated HuH-7 cells were harvested by trypsinization, centrifuged at 1500 ×g for 5 min, washed with PBS, and fixed in 70% ethanol at 4°C overnight. Fixed cells were washed twice with PBS and incubated in PBS containing 1.5 mg/L RNase A for 1 h at 37°C, followed by staining with 5 &mgr;L PI (1 mmol/L stock) for 20 min on ice. The cells were analyzed for DNA content using BD-LSR flow cytometer equipped with electronic doublet discrimination capability using blue (488 nm) excitation from argon laser. Data were collected in list mode on 10 000 events for FL2-A vs FL2-W.
DNA fragmentation was analysed as described by Yang et al[33]. Briefly, HuH-7 cells treated or untreated were harvested and centrifuged at 1500 ×g for 5 min. After washing twice with PBS/ethylenediamine-N, N, N', N'-tetraacetic acid (EDTA). Cells were incubated in a lysis buffer [0.5 mL/L Triton X-100, 10 mmol/L EDTA, 0.4 g/L proteinase K, and 10 mmol/L Tris-HCl, pH 7.4] at 56°C for 1 h. Cell lysate was treated with 0.4 g/L RNase at 37°C for 30 min. The genomic DNAs were purified by phenol/chloroform extraction and ethanol precipitation, and resuspended in a Tris-EDTA buffer, DNA fragments were stained with ethidium bromide and visualized by 2.5% agarose electrophoresis.
Caspase activation was measured using a caspase 3 fluorometric assay kit (BD Apoalert caspase 3 fluorescent assay kit). HuH-7 cells, treated or untreated were harvested, and centrifuged (approximately 1 mg protein) at 400 ×g for 5 min. The cell pellets were re-suspended in 50 &mgr;L of chilled cell lysis buffer and incubated on ice for 10 min, and the lysates were centrifuged at 15 000 ×g for 10 min at 4°C to precipitate cellular debris. A total of 50 &mgr;L of cell lysates was incubated with 50 &mgr;L of reaction buffer/DTT mix. DEVD-CHO was used as an inhibitor of caspase 3 in an induced sample. Five &mgr;L of 1 mmol/L caspase-3 substrate (DEVD-AFC at a final concentration of 50 &mgr;mol/L) was added to each sample. The samples were incubated at 37°C for 1 h and read on a spectrofluorometer (Perkin Elmer LS 55) with excitation wavelength 400 nm and emission wavelength of 505 nm.
cPLA2 activity was measured in cell lysates, using cPLA2 assay kit (Cayman Chemical Company cPLA2 assay kit) as per the instructions of the supplier. The kit involves the principle that cPLA2 exhibits specificity towards arachidonic acid. Arachiconyl thio-PC is used as a synthetic substrate to detect the phospholipase activity. Hydrolysis of the arachiconoyl thioester bond at the sn-2 position by cPLA2 releases free thiol which are detected by DTNB (5, 5'-dithiobis-2-dinitrobenzoic acid).
cAMP activity was measured in the cell lysates, using cAMP assay kit (R&D Systems, Inc, cAMP assay kit) as per the instructions of the supplier. The kit is based on the competitive binding technique in which cAMP present in a sample competes with a fixed amount of horseradish peroxidase (HRP)-labeled cAMP for sites on a mouse monoclonal antibody. During the incubation, the monoclonal antibody becomes bound to the goat anti-mouse antibody coated onto the microplate. Following a wash to remove excess conjugate and unbound sample, a substrate solution is added to the wells to determine the bound activity. The colour developed was stopped and the absorbance read at 450 nm. The intensity of the colour is inversely proportional to the concentration of cAMP in the sample.
The assay was performed using antioxidant assay kit from Cayman Chemical Company, as per the instructions by the manufacturer. The assay was used to access the ability of the test materials (NG, Silymarin etc) to inhibit the oxidation of ABTS to ABTS.+ by metmyoglobin. The amount of ABTS.+ produced in absence and presence of test material was monitored by reading absorbance at 750 nm. Suppression of the absorbance at 750 nm in presence of test material is proportional to their antioxidant activity. The capacity of the antioxidant activity (inhibition of ABTS.+ formation) in the test sample was compared with that of Trolox, a water-soluble tocopherol analogue.
This assay was performed as described by Gonzalez et al[34]. In brief, for the assay, various concentrations of test compound were added to 3 mL of DPPH solution (20 mg/L methanol) and incubated for 5 min at 25°C. The absorbance was measured al 517 nm. A 100% decoloration was established using methanol-water 2:1 and the percentage of DPPH decoloration was calculated.
In vitro effect of NG on superoxide anion radical generation was studied as described previously[35]. Two reaction systems used were: (a) enzymic ; (b) non-enzymic. System (a) comprised of 100 &mgr;mol/L xanthine, 600 &mgr;mol/L nitroblue tetrazolium (NBT) and 0.07 U/mL xanthine oxidase in 50 mmol/L sodium carbonate pH 9.2, incubated for 10 min in presence and absence of test materials and A read at 560 nm. System (b) comprised of 10 &mgr;mol/L phenazine methosulphate, 78 &mgr;mol/L NADH, 25 &mgr;mol/L NBT, incubated for 2 min in presence and absence of test materials and A read at 560 nm.
Protein concentration was measured with BCA Protein Assay Kit from Pierce (Rockford, IL, USA) as per the instructions of the manufacturer.
Results are expressed as mean ± SD. Comparisons were made between control and treated groups unless otherwise indicated using unpaired Student's t-test and P values < 0.05 were considered statistically significant.
For better scientific and clinical acceptability and proper global positioning of plant based products, it has become implicit to determine their chemical profile data on the basis of purity of compound. In this respect we have developed the HPLC protocol for NG (Figure 1). HPLC profile confirmed that NG was isolated as < 95% pure.
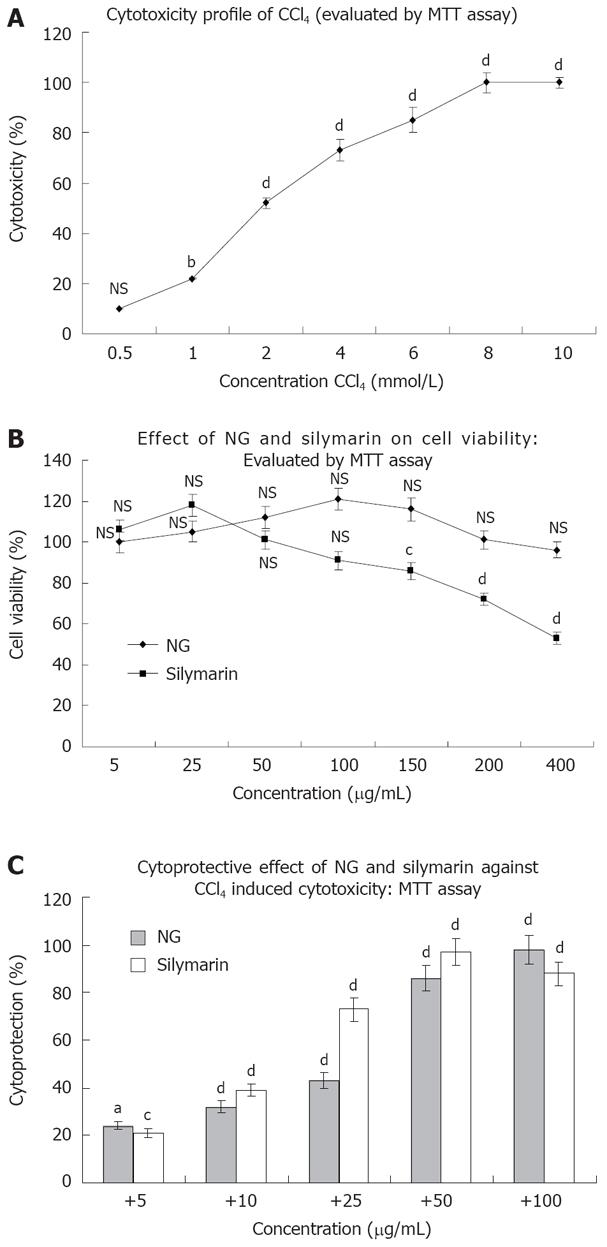
CCl4 produced a concentration dependent loss of viability in HuH-7 cells as evaluated by MTT assay. At 24 h of incubation, the IC50 value of CCl4 was found to be 2 mmol/L approximately (1.958 mmol/L; Figure 2A). This concentration was used to generate oxidative stress to study the cytoprotective effect of NG and silymarin in further experimentations. NG alone was not toxic under the assay conditions up to a concentration of 400 mg/L. However, silymarin at concentrations above 50 mg/L produced loss of cell viability, with an estimated IC50 value of 413.38 mg/L (Figure 2B).
To characterize the protective effect of NG and silymarin on CCl4 induced cytotoxicity in HuH-7 cells, dose-response experiments were conducted using various concentrations of NG and silymarin. NG showed a significant dose dependent protective effect against CCl4 induced loss of cell viability. The PC50 (50% protective concentration) for NG was estimated to be 24.46 mg/L and 15.30 mg/L for silymarin (Figure 2C).
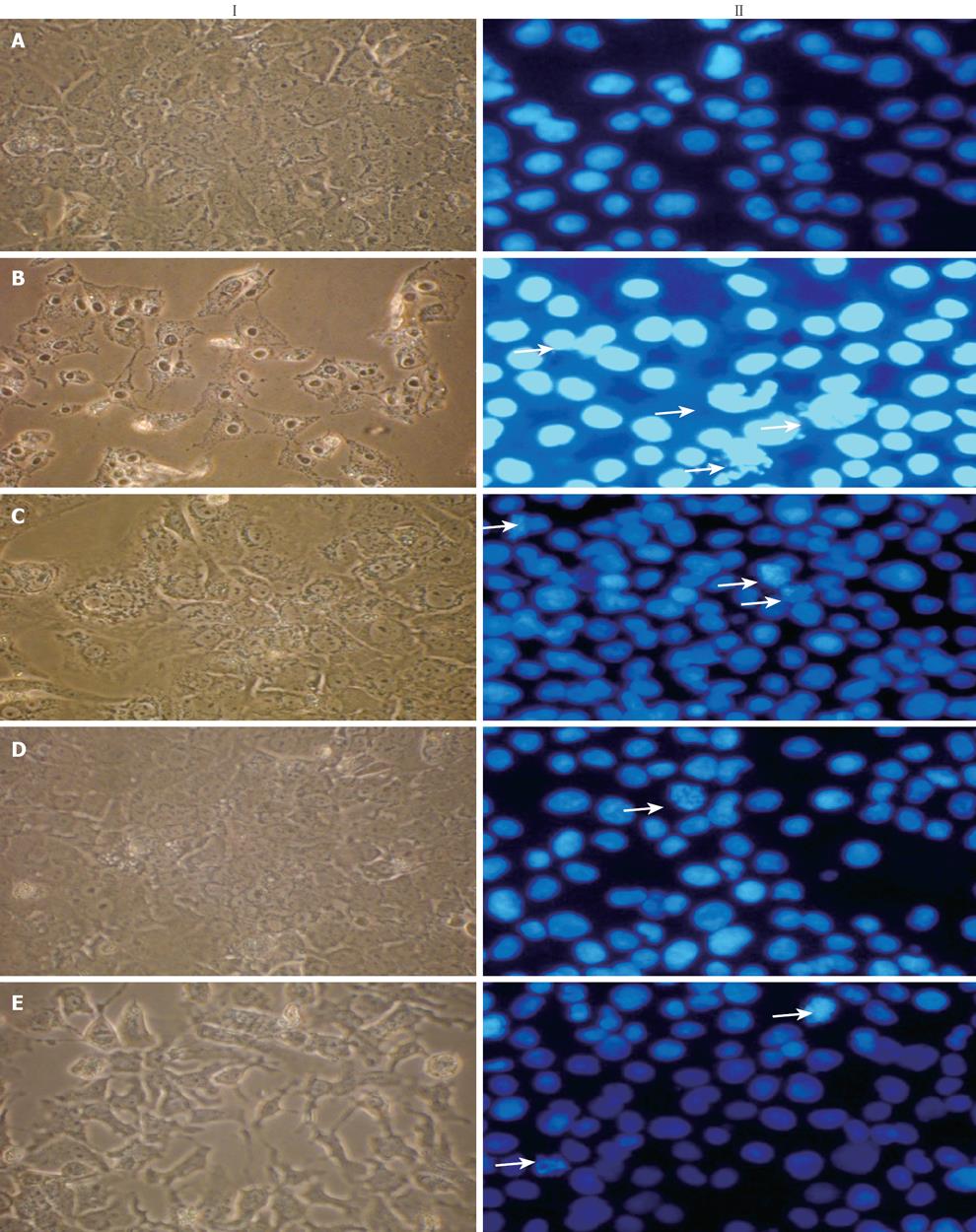
Results obtained from MTT assay were in total correlation with the extent of cell death as confirmed by morphological changes observed under light microscope and Hoechst 33 258 staining under fluorescence microscopy (Figure 3). Treatment with CCl4 caused HuH-7 cells to lose their normal structure with signs of cell swelling, most of the cells were detached and monolayer was disturbed (Figure 3IA compared with Figure 3IB). These structural changes were prevented to a large extent by 30 and 100 &mgr;g/mL of NG and were comparable with the protection offered by silymarin at 50 mg/L (Figure 3IC-F).
Nuclei of untreated HuH-7 cells appeared prominently round in shape (Figure 3 IIA). After exposure with CCl4, cells showed morphological alterations and condensation of nuclei (Figure 3IIB). The prominent changes were accompanied by an increase in apoptotic bodies and increase in cellular debris. All these alterations were prevented by co-exposure with NG and silymarin in a dose dependent manner (Figure 3IIC-E).
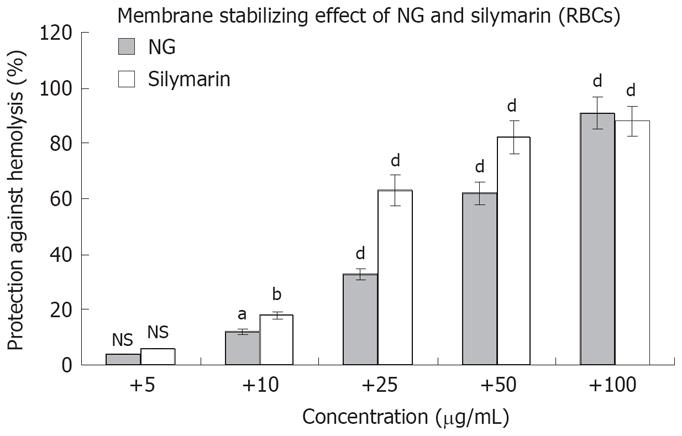
The above mentioned cytoprotective results were further correlated with the membrane stabilizing effect of NG and silymarin against Triton X 100 (1 g/L)-induced membrane disruption in human RBCs. NG showed an effect in the range of 4% to 91% in a concentration dependent manner (5 mg/L to 100 mg/L) against a protective effect of 6% to 88% at the same concentration, shown by silymarin (Figure 4).
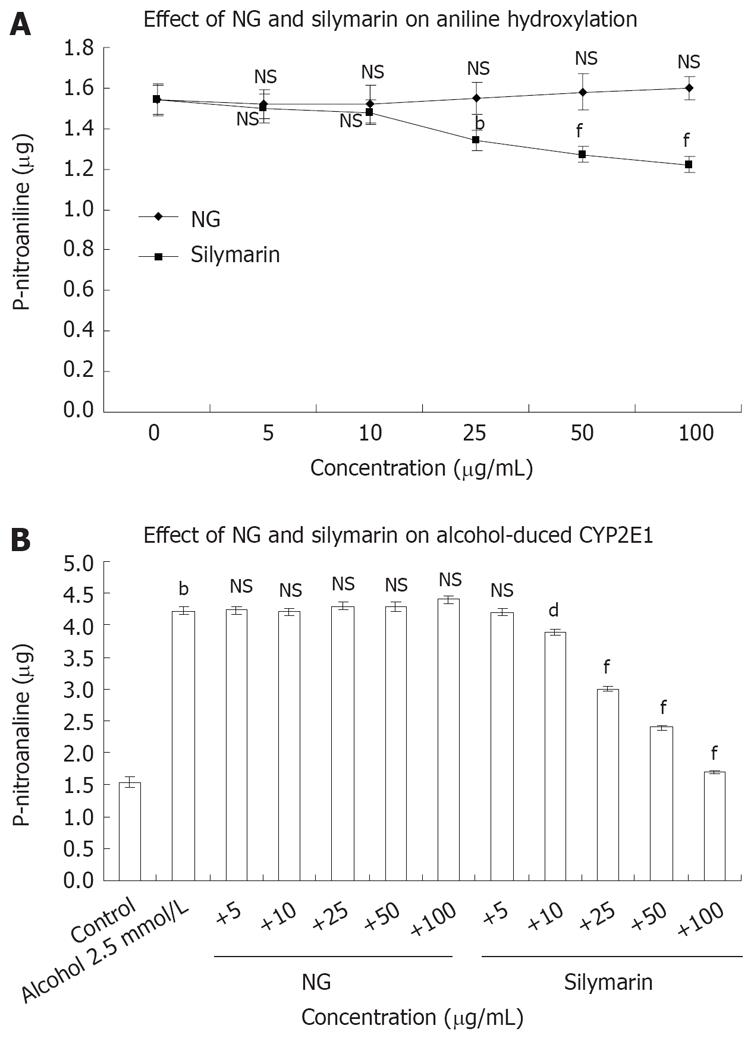
To study the mechanism by which NG was preventing CCl4-induced toxicity in HuH-7 cells, the possible interference of NG and silymarin on CYP2E1 activity (aniline hydroxylation) was studied. Hepatic microsomes from Wistar rats were used as a source to assay in vitro effect of NG and silymarin on CYP2E1 levels (Figure 5A). In another set of experiments, the CYP2E1 protein levels were analyzed to assay the protective effect of NG and silymarin against oxidation of 2.5 mmol/L ethanol (Figure 5B).
NG alone showed no significant effect on aniline hydroxylation levels at any concentrations used (5 to 100 mg/L), whereas silymarin produced an inhibitory effect in the range of 2.6% to 26.2% at the same concentrations (Figure 5A). Isoniazid (used as a positive control for inhibition of CYP2E1) showed a dose dependent inhibition, with IC50 at 500 &mgr;mol/L (data not shown).
Treatment of microsomes with 2.5 mmol/L ethanol caused an increase of 63% in aniline hydroxylation levels. Co-treatment of NG with ethanol showed no inhibitory effect on aniline hydroxylation levels induced by ethanol. However, silymarin caused inhibitions of 8.4%, 41%, 76% and 149% at 10 mg/L, 25 mg/L, 50 mg/L and 100 mg/L, respectively in aniline hydroxylation levels compared to microsomes treated with ethanol alone (Figure 5B).
The results (Figure 5A and B) show that NG did not inhibit CYP2E21 activity, suggesting that NG is most probably acting as an antioxidant, and not as a CYP2E1 inhibitor and silymarin acts both as an antioxidant, and an inhibitor of CYP2E1.
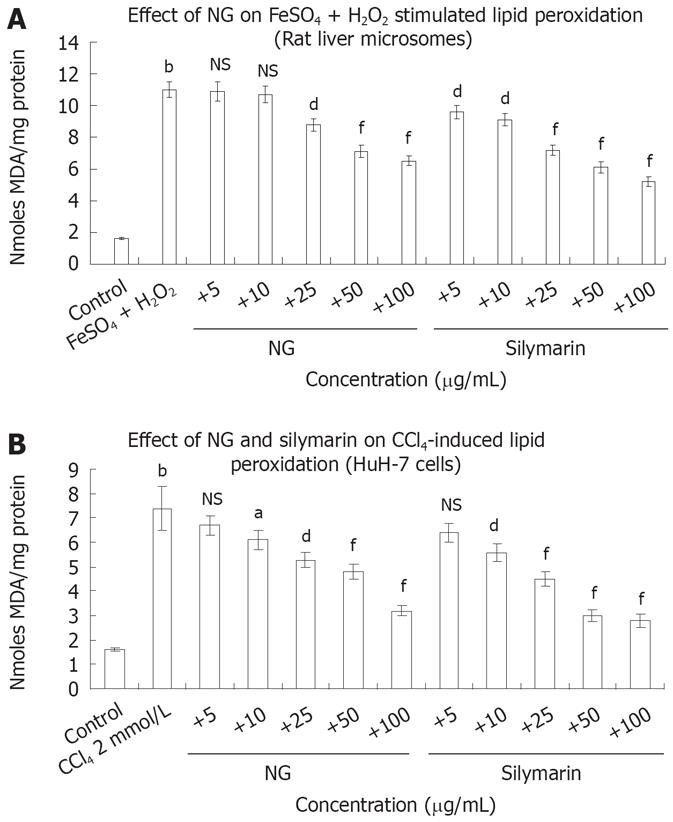
FeSO4 + H2O2 increased LPO in rat liver microsomes by 6.8-fold. Incubation with NG (10 to 100 mg/L) decreased in a range of 3% to 69%, this increase in LPO levels (Figure 6A). Silymarin at the equivalent concentrations showed an enhanced inhibitory effect of 21% to 111%. Treatment of HuH-7 cells with CCl4, increased LPO levels up to 4.6-fold. NG offered a protective effect against this increase by 10.4%, 21%, 39%, 54% and 131% at 5 mg/L, 10 mg/L, 25 mg/L, 50 mg/L and 100 mg/L, respectively. At similar concentrations, silymarin produced respective inhibitory effects of 17%, 32%, 64%, 146% and 164% (Figure 6B).
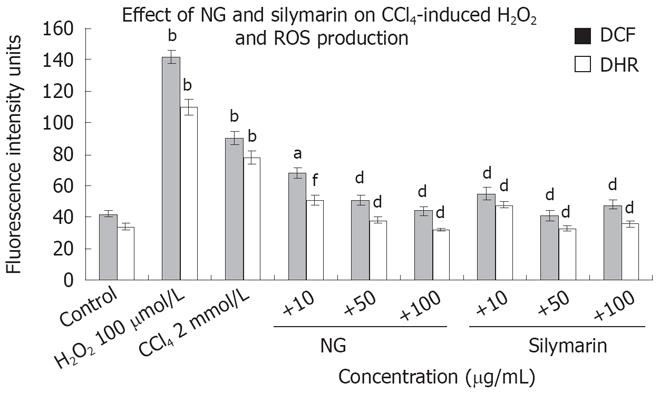
Oxidative stress was studied by fluorescence spectro-photometrical analysis of the levels of ROS, using DCF-DA and DHR as the probes. Figure 7 shows the mean values of DCF and DHR fluorescence for cell populations with various treatments. Treatment with CCl4, increased by 2.1 (DCF) and 2.2-fold (DHR) the production of ROS in HuH-7 cells in comparison with no addition control. NG and silymarin (10 to 100 mg/L) reduced the increase in the ROS levels produced by CCl4 in a significant and dose dependent manner. NG at 10 mg/L decreased DCF fluorescence intensity by 32% and DHR by 52%. At 50 mg/L, DCF and DHR intensities were decreased by 76% and 105%, respectively. At 100 mg/L, both the fluorescence intensities were down by 104% and 143%. H2O2 (500 &mgr;mol/L) was used as positive control for ROS generation.
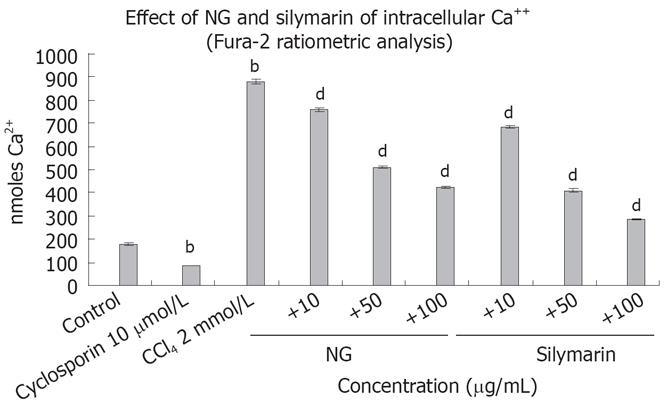
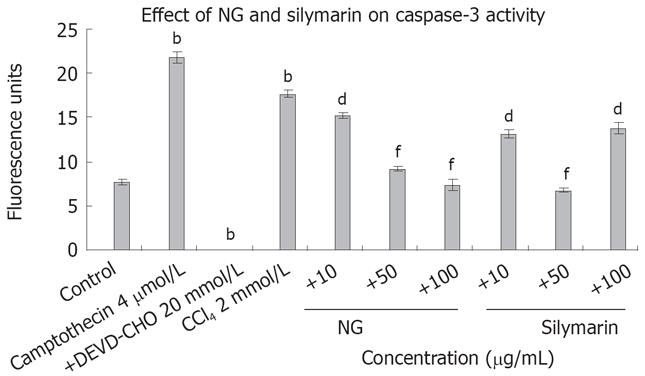
Intracellular Ca2+ and caspase 3 levels were increased significantly by CCl4 treatment, which reflects the requirement of Ca2+ and caspase 3 in the overall toxicity pathway of CCl4 (Figures 8 and 9). CCl4 caused an increase of 4.8-fold in Ca2+ and 2.3-fold in caspase 3 levels in HuH-7 cells. This abnormal rise in Ca2+ levels were decreased by NG by 15%, 72% and 106% and caspase 3 levels were decreased by 16%, 92% and 139% at 10 mg/L, 50 mg/L and 100 mg/L, respectively. Silymarin also showed a dose dependent inhibitory effect on Ca2+ and caspase 3 levels increased by CCl4. The effect was 28%, 114% and 207% in Ca2+ levels at 10 mg/L, 50 mg/L and 100 mg/L, and 34% and 160% in caspase 3 levels at 10 mg/L and 50 mg/L, respectively. However at 100 mg/L, silymarin showed a less significant effect (28% decrease). Thus, silymarin showed a higher Ca2+ inhibitory effect compared to NG, but less caspase 3 inhibition at concentrations above 50 mg/L. Cyclosporine (10 &mgr;mol/L) was used as positive control for Ca2+ inhibition and camptothecin (inducer, 4 &mgr;mol/L) and DEVD-CHO (inhibitor, 20 &mgr;mol/L) were used as positive controls.
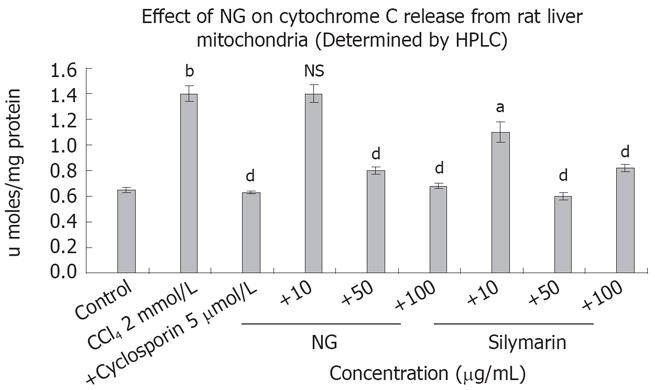
Isolated rat liver mitochondria were used to study the effect of NG and silymarin on cytochrome C release induced by CCl4 (Figure 10). CCl4 caused an increase of 2.1-fold in cytochrome C levels, which was inhibited by 75% and 105% at 50 mg/L and 100 mg/L of NG treatment, respectively. Silymarin showed an effect in the range of 27% at 10 mg/L, 135% at 50 mg/L. However at a higher concentration (100 mg/L), silymarin showed a biphasic effect, with a slight increase in cytochrome C levels compared to 50 mg/L (36% increase). Cyclospo-rine 5 &mgr;mol/L was used as a positive control for cytochrome C inhibition.
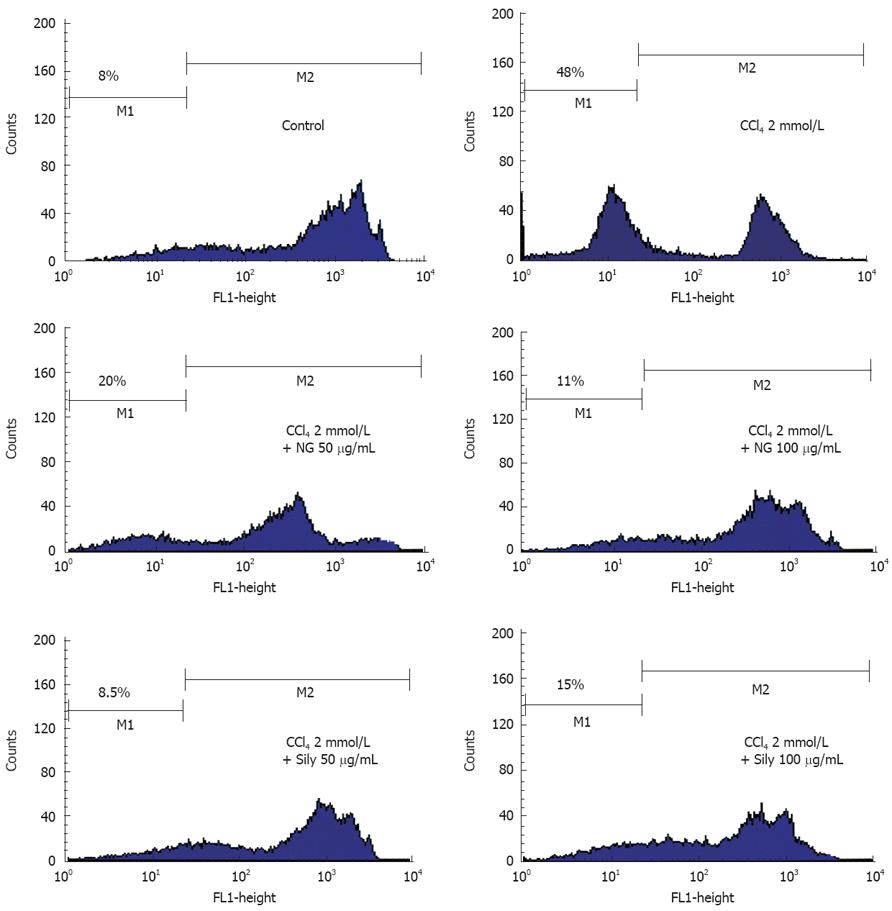
Oxidative damage to mitochondria and the onset of MMP transition seems to play an important role in CCl4-induced toxicity in HuH-7 cells. MMP transitions were analyzed by flow cytometry after staining with Rh123 (Figure 11). Untreated HuH-7 cells were strong in Rh123 fluorescence intensity, suggestive of intact viable cells. A very small percentage of cells (8%) were showing low Rh123 fluorescence, reflective of damaged cells. CCl4 caused a 6-fold increase in percentage of cells with low Rh123 fluorescence. Incubation in the presence of 50 mg/L and 100 mg/L of NG and silymarin, respectively significantly protected HuH-7 cells from decline in MMP produced by CCl4.
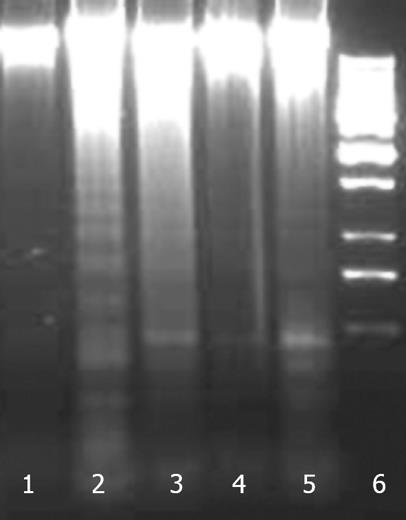
Apoptosis was studied by internucleosomal DNA fragmentation analysis and cell cycle analysis by flow cytometry. A DNA ladder formation was found with cells treated with CCl4 (Figure 12). Treatment with NG and silymarin protected HuH-7 cells against CCl4 induced DNA fragmentation.
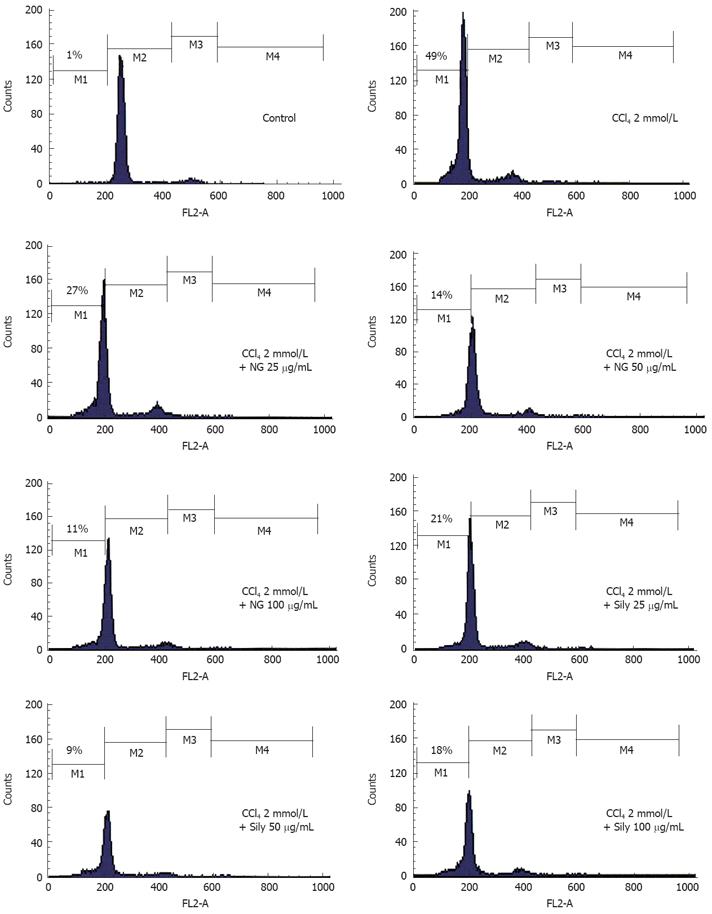
In cell cycle analysis, treatment with CCl4 markedly increased proportion of apoptotic cells significantly (49%). NG and silymarin had an obvious anti-apoptosis effect. As shown in Figure 13, the co-treatment with 25 mg/L, 50 mg/L and 100 mg/L of NG markedly reduced the percentage of the apoptotic cells to 27%, 14% and 11%, respectively. Silymarin showed a reduction in apoptotic cells by 21% and 9% at 25 mg/L and 50 mg/L, respectively. At 100 mg/L, however, silymarin had an effect of 18% (Figure 13).
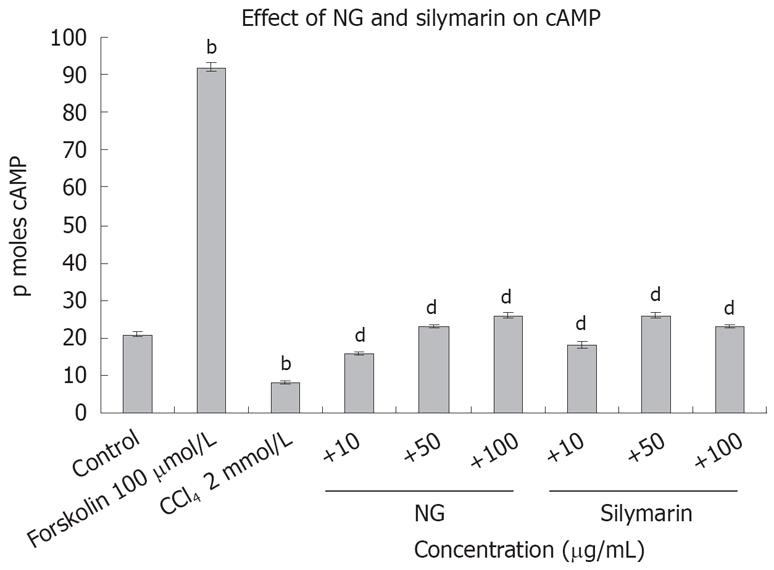
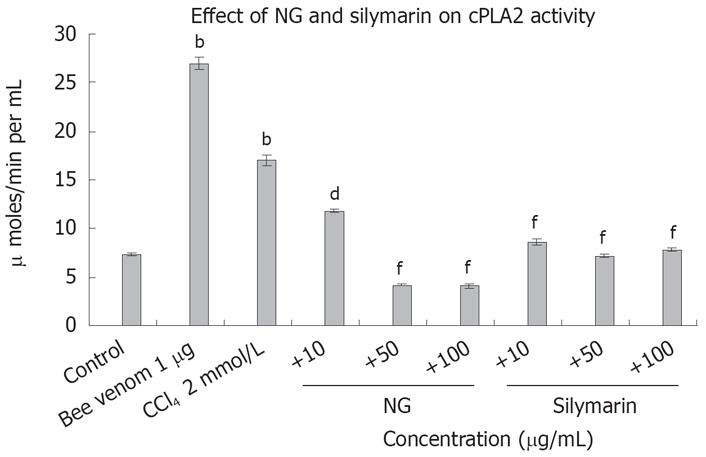
To test the effect of CCl4-induced oxidative stress on cAMP levels in HuH-7 cells, we evaluated cAMP with and without CCl4 and then in the presence of CCl4 with NG and silymarin. As hypothesized, cAMP levels were significantly reduced by CCl4. Concomitant treatment of HuH-7 cells with NG significantly increased the levels of cAMP. Similar results were evident with treatment with silymarin. Forskolin (100 &mgr;mol/L) was used as a positive inducer of cAMP (Figure 14). On the contrary, phospholipase A2 levels were significantly increased with the CCl4 treatment (2.3-fold). NG effectively reduced this increase by 44% at 10 mg/L and 304% at 50 mg/L. Silymarin reduced these levels by 97% at 10 mg/L and 136% at 50 mg/L. Bee venom (1 mg/L) was used as positive control to induce cPLA2 levels (Figure 15).
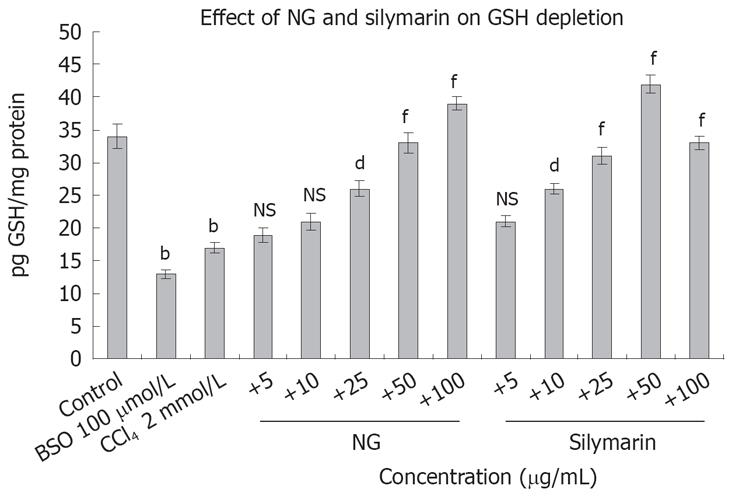
Figure 16 depicts the effect of CCl4 on GSH levels and restorative effect of NG and silymarin in a dose-response manner. Treatment of HuH-7 cells with CCl4-depleted the GSH content by 2 folds. Co-exposure with NG and silymarin effectively restored the depleted levels of GSH in a dose response manner. Restorative effect of NG was in the range of 17% to 147% at 5 mg/L to 100 mg/L, respectively. Silymarin showed an effect in the range of 23% to 152% at a concentration of 5 to 50 mg/L. However at higher concentration (100 mg/L), there was a slight decrease in this effect. BSO (100 &mgr;mol/L) was used as a positive inhibitor of GSH.
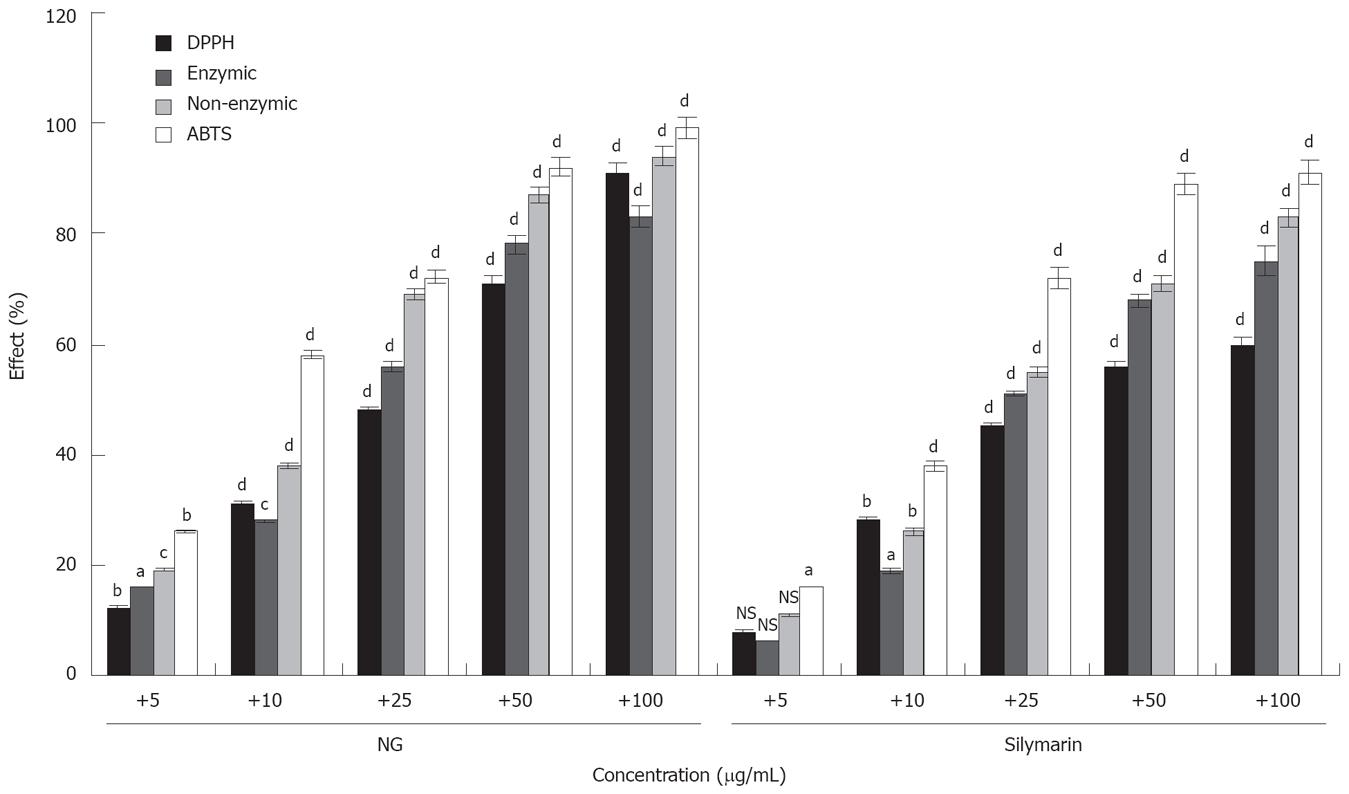
Figure 17 is representative of anti-oxidant activity of NG and silymarin. NG showed a strong activity, with I.C. 50 values of 17.31 mg/L for DPPH, 22.75 mg/L for enzymatic reaction, 13.49 mg/L for non-enzymatic reaction and 8.71 mg/L for ABTS assay. The IC 50 values of silymarin for the same assays were 34.07, 24.35, 21.10 and 12.36 mg/L respectively.
Liver cells exposed to various chemicals/drugs (pro-oxidants) appear to be a useful in vitro model to characterize the biochemical and toxicological properties of such entities, and the possible protection provided by added agents[36]. The main goal of this work was to investigate the influence of an irridoid glycoside compound negundoside (NG) on CYP2E1-mediated toxicity in HuH-7 cells induced by CCl4. Overall, the results of the present study indicate that NG is effective in protecting against the toxicity and the loss of viability induced by CCl4.
The main mechanism by which CCl4 is known to mediate its toxic effects is through oxidative stress and oxidative damage due to an increased production of ROS[37]. Induction of CYP2E1 by CCl4 is one of the main pathways by which CCl4 increases ROS production and generates a state of oxidative stress in the liver[3839]. Since CYP2E1 is a key contributor to injury produced by CCl4, one possible mechanism involved in the prevention of this toxicity by NG could have been an inhibition of CYP2E1 catalytic activity. Results in this study indicate that NG does not affect p-nitrophenol metabolism by CYP2E1 in liver microsomes under the experimental conditions (Figure 5A and B); therefore, the mechanism by which NG affords its protection is not by inhibition of CYP2E1 activity. This is in confirmation to earlier reports in which many plant derived products like, Scutellariae radix[40], Humulus lupulus[41], green tea compounds[42] have also been shown to be hepatoprotective in other systems without any effect on CYP2E1 catalytic activity.
Bio-metals, such as iron are powerful catalysts of free radical formation and lipid peroxidation processes, and polyunsaturated fatty acids in cellular membranes (microsomes) provide basic substrates for these reactions[43]. Scavenging or preventing formation of lipid radicals may prevent damage when cellular antioxidant defense mechanism is strengthened or iron overload is sequestered by exogenous treatment with cytoprotective drugs as NG. As lipid peroxidation (LPO) has been shown to play an important role in the ensuing toxicities in CYP2E1-induced conditions[4445], in this respect, NG strongly inhibited lipid peroxidation promoted by H2O2+Fe in microsomes and CCl4 in HuH-7 cells (Figure 6A and B). It has been reported that glycosides, such as NG, are potent cytoprotective agents against oxidative stress induced cytotoxicity[46]. Therefore, one major mechanism underlying the effectiveness of NG in protecting against the CCl4-induced LPO in HuH-7 cells may involve its capability to prevent lipid peroxidation chain reactions as a consequence of scavenging free radicals or chelating iron.
Intracellular calcium has been suggested to play a critical role in the oxidative damage of liver cells. Earlier, it has been reported that treatment of liver cells with CCl4 increases calcium levels and produce cellular toxicity through calcium dependent pathways. Elevated levels of calcium initiates a cascade of signaling events leading to activation of calcium dependent degredative enzymes as phospholipases A2, endonucleases, or proteases[47]. Our results are in corroboration with this and showed that CCl4-induced cell death in HuH-7 cells was mediated by release of intracellular calcium with subsequent activation of caspase 3 and cPLA2 (Figures 9 and 15) and simultaneous inhibition of cAMP levels (Figure 14). Increased intracellular calcium, activation of PLA2 and inhibition of cAMP were almost parallel to toxicity. Oxidative stress-mediated LPO is suggested to be the initiator of intracellular calcium release[25], which later influences down stream apoptotic signaling processes. cAMP levels are known to be regulated by catalytic activity of adenylate cyclase and phosphodiesterase. Increasing concentration of intracellular cAMP has been directly associated with inhibition of phosphodiesterase, reduced release of ROS and inhibition of chemotaxis, degranulation and cell death[48]. NG restored the calcium and cAMP to normal levels, inhibited lipid peroxidation, activated cPLA2 levels were inhibited and cytotoxicity was reversed without altering CYP2E1 levels. Therefore we hypothesize that NG inhibits CCl4-induced oxidative stress and, hence LPO, which increases intracellular calcium and PLA2 activation and converge on mitochondria, inducing mitochondrial damage. All these downstream events of CYP2E1 mediated toxicity were effectively inhibited by NG, thus demonstrating its strong anti-oxidant capacity. We also suggest that inhibition of intracellular calcium release mediated cPLA2 activation, increase in cAMP levels, and restoration of MMP are the key factors in cytoprotection afforded by NG. Recently, it has been suggested that calcium levels do not play a direct role in toxicity[26], but that activation of PLA2, promotion of the mitochondrial permeability transition and loss of mitochondrial function, which are secondary manifestations of increased calcium levels, form a general pathway involved in the toxicity: all these events were restored to normal by NG.
As the main antioxidant inside mammalian cells, GSH plays a pivotal role in preventing oxidative stress and mitochondrial damage caused by numerous toxins[49]. Therefore, the effect of CCl4 in the absence or presence of NG on GSH content was evaluated. CCl4 treatment drastically depleted intracellular GSH in HuH-7 cells, an effect prevented in the presence of NG (Figure 16). Accordingly, the maintenance of intracellular GSH levels by NG may help in protecting against the oxidative toxicity induced by CCl4 in HuH-7 cells and avoid cell degeneration and death. Previously as well, depletion of GSH has been shown to enhance CYP2E1 resulting in CYP2E1-derived ROS leading to toxicity[50].
Decreased MMP has been proposed to be a key mechanism by which CYP2E1-dependent LPO causes loss in cell viability. Mitochondria are a main source for generating ROS and, hence, a target for damage by oxidative stress[51]. In this respect, CCl4 treatment caused a decrease in the MMP in HuH-7 cells, and this effect was prevented by NG as well as by silymarin (Figure 11). These results suggest that NG and silymarin may protect the cells by preventing oxidant-induced MMP transition leading to pathogenesis of necrotic or apoptotic cell death[52]. It has been proposed earlier that mitochondrial injury derived from oxidative damage can lead not only to necrosis by depleting ATP, but also to apoptotic cell death by inducing the release of mitochondrial factors such as cytochrome C, which activates the caspase cascade[5354].
Regardless of its precise mechanism of action, numerous studies in various animal models and in humans describe protective effects of NG against oxidative stress-related disease states[2–15]. This enhances its potential usefulness as a preventive agent toward oxidative damage involved in the development of liver injury caused by oxidative stress. Since NG acts as a very potent membrane stabilizer, it is also suggested that NG, may be acting as amphipathic substance, localizing near the membrane surface, trapping any radicals generated in the lipid environment of the membranes as well as in the cytosol. Such localization is suggested from the fact that CYP2E1 is found in the microsomes, and mitochondria appear to be a target for the CYP2E1-mediated damage in the presence of hepatotoxins such as CCl4, which was effectively inhibited by NG. Moreover, NG is well tolerated without adverse health effects by humans even after oral administration at high doses as evident from its use in Asian traditional medicine practices for various ailments.
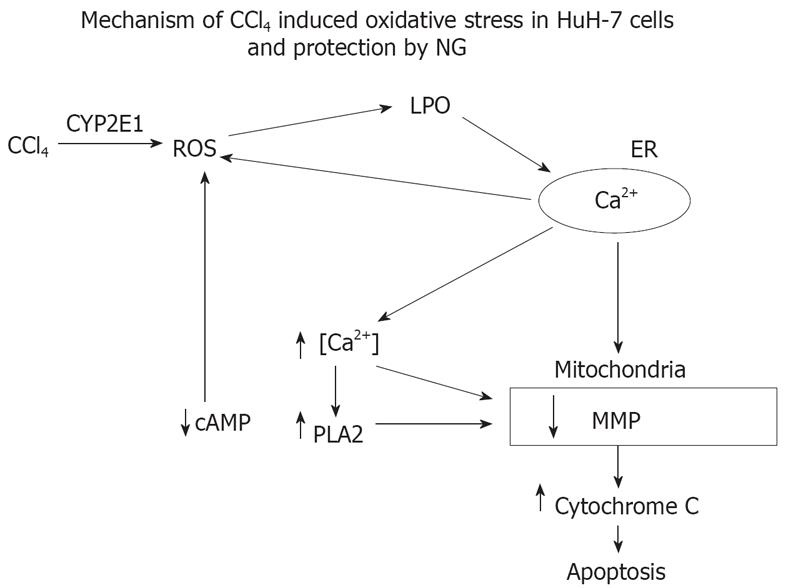
In conclusion, this report shows that NG can protect against CCl4-induced toxicity and oxidative stress. The mechanism of protection involves decreased production of ROS and lipid peroxidation when the CYP2E1 mediated oxidative stress was produced in HuH-7 cells with pro-oxidant as CCl4. The main mechanism involved in the cytoprotection of NG seems to be its ability to protect the mitochondria against depletion in its membrane potential, an event that is very critical in the loss of cell viability as a consequence of oxidative stress. This mechanism has been postulated in the Figure 18. NG has been shown to prevent CCl4-induced liver injury, which may be, in part, due to the protection against CYP2E1-dependent oxidative stress as demonstrated in this study. NG supplementation could also prove to be protective against numerous toxicants that involve induction of oxidative stress through increased generation of ROS.
Vitex negundo is a reputed medicinal herb of Indian sub-continent. All plant parts are considered important in Ayurvedic system of medicine for various indications.
Several pharmacological studies validate the medicinal claims of Vitex negundo. Diverse chemical constituents have been reported from various parts of this plant which are considered responsible for its varied pharmacological activities. The negundoside seems to be a potential constituent that exerts a protective effect on CYP2E1-dependent toxicity caused by carbon tetrachloride (CCl4) via inhibition of lipid peroxidation, followed by an improved intracellular calcium homeostasis and inhibition of Ca2+-dependent proteases.
The present investigation shows that negundoside is a potent phytopharmaceutical that acts in a novel way in inhibiting liver toxicity by interfering in the key events that are the main causative factors leading to liver dysfunction.
Negundoside exerts a protective action on CYP2E1-dependent oxidative stress and toxicity that may contribute to preventing chemically-induced liver injury, and may be useful in preventing toxicity by various other hepatotoxins as well.
The authors investigated the anti-apoptotic effect of negundoside (NG), being extracted from leaves of Vitex negundo, on cultured human hepatoma cell line, HuH-7G2. The authors performed a large amount of experiments, and found that NG inhibited ROS formation, lipid peroxidation, intracellular calcium elevation, GSH depletion, elevated anti-oxidant activity, declined MMP, cytochrome C release, and finally carbon tetrachloride-medicated apoptosis. The manuscript addressed the authors' hypothesis with sufficient data.
| 1. | Chin YW, Balunas MJ, Chai HB, Kinghorn AD. Drug discovery from natural sources. AAPS J. 2006;8:E239-E253. [Cited in This Article: ] |
| 2. | Dharmasiri MG, Jayakody JR, Galhena G, Liyanage SS, Ratnasooriya WD. Anti-inflammatory and analgesic activities of mature fresh leaves of Vitex negundo. J Ethnopharmacol. 2003;87:199-206. [Cited in This Article: ] |
| 3. | Azhar-Ul-Haq , Malik A, Khan MT, Anwar-Ul-Haq , Khan SB, Ahmad A, Choudhary MI. Tyrosinase inhibitory lignans from the methanol extract of the roots of Vitex negundo Linn. and their structure-activity relationship. Phytomedicine. 2006;13:255-260. [Cited in This Article: ] |
| 4. | Jagetia GC, Baliga MS. The evaluation of nitric oxide scavenging activity of certain Indian medicinal plants in vitro: a preliminary study. J Med Food. 2004;7:343-348. [Cited in This Article: ] |
| 5. | Alam MI, Gomes A. Snake venom neutralization by Indian medicinal plants (Vitex negundo and Emblica officinalis) root extracts. J Ethnopharmacol. 2003;86:75-80. [Cited in This Article: ] |
| 6. | Chandramu C, Manohar RD, Krupadanam DG, Dashavantha RV. Isolation, characterization and biological activity of betulinic acid and ursolic acid from Vitex negundo L. Phytother Res. 2003;17:129-134. [Cited in This Article: ] |
| 7. | J Munasinghe TC, Seneviratne CK, Thabrew MI, Abeysekera AM. Antiradical and antilipoperoxidative effects of some plant extracts used by Sri Lankan traditional medical practitioners for cardioprotection. Phytother Res. 2001;15:519-523. [Cited in This Article: ] |
| 8. | Gupta M, Mazumder UK, Bhawal SR. CNS activity of Vitex negundo Linn. in mice. Indian J Exp Biol. 1999;37:143-146. [Cited in This Article: ] |
| 9. | Avadhoot Y, Rana AC. Hepatoprotective effect of Vitex negundo against carbon tetrachloride-induced liver damage. Arch Pharm Res. 1991;14:96-98. [Cited in This Article: ] |
| 10. | Perumal Samy R, Ignacimuthu S, Sen A. Screening of 34 Indian medicinal plants for antibacterial properties. J Ethnopharmacol. 1998;62:173-1782. [Cited in This Article: ] |
| 11. | Damayanti M, Susheela K, Sharma GJ. Effect of plant extracts and systemic fungicide on the pineapple fruit-rotting fungus, Ceratocystis paradoxa. Cytobios. 1996;86:155-165. [Cited in This Article: ] |
| 12. | Pushpalatha E, Muthukrishnan J. Larvicidal activity of a few plant extracts against Culex quinquefasciatus and Anopheles stephensi. Indian J Malariol. 1995;32:14-23. [Cited in This Article: ] |
| 13. | Bhargava SK. Antiandrogenic effects of a flavonoid-rich fraction of Vitex negundo seeds: a histological and biochemical study in dogs. J Ethnopharmacol. 1989;27:327-339. [Cited in This Article: ] |
| 14. | Hebbalkar DS, Hebbalkar GD, Sharma RN, Joshi VS, Bhat VS. Mosquito repellent activity of oils from Vitex negundo Linn. leaves. Indian J Med Res. 1992;95:200-203. [Cited in This Article: ] |
| 15. | Prabhakar A, Gupta BD, Suri KA, Satti NK, Malhotra S, Gupta KK, Sharma VK, Johri RK, Jaggi BS, Chandan BK; Hepatoprotective activity of 2’-p-hydroxybenzoylmussaenodidic acid. United States patent, US Patent 7, 259, 148. 2007;259:148. [Cited in This Article: ] |
| 16. | Dey A, Caro AA, Cederbaum AI. S-adenosyl methionine protects ob/ob mice from CYP2E1-mediated liver injury. Am J Physiol Gastrointest Liver Physiol. 2007;293:G91-G103. [Cited in This Article: ] |
| 17. | Kume Y, Ikeda H, Inoue M, Tejima K, Tomiya T, Nishikawa T, Watanabe N, Ichikawa T, Kaneko M, Okubo S. Hepatic stellate cell damage may lead to decreased plasma ADAMTS13 activity in rats. FEBS Lett. 2007;581:1631-1634. [Cited in This Article: ] |
| 18. | Beddowes EJ, Faux SP, Chipman JK. Chloroform, carbon tetrachloride and glutathione depletion induce secondary genotoxicity in liver cells via oxidative stress. Toxicology. 2003;187:101-115. [Cited in This Article: ] |
| 19. | Jimenez-Lopez JM, Cederbaum AI. Green tea polyphenol epigallocatechin-3-gallate protects HepG2 cells against CYP2E1-dependent toxicity. Free Radic Biol Med. 2004;36:359-370. [Cited in This Article: ] |
| 20. | Lee CS, Kim YJ, Han ES. Glycyrrhizin protection against 3-morpholinosydnonime-induced mitochondrial dysfunction and cell death in lung epithelial cells. Life Sci. 2007;80:1759-1767. [Cited in This Article: ] |
| 21. | Gandhidasan R, Thamaraichelvan , Baburaj S. Anti inflammatory action of Lannea coromandelica by HRBC membrane stabilization. Fitoterapia. 1991;62:81-83. [Cited in This Article: ] |
| 22. | Tasduq SA, Kaiser P, Sharma SC, Johri RK. Potentiation of isoniazid-induced liver toxicity by rifampicin in a combinational therapy of antitubercular drugs (rifampicin, isoniazid and pyrazinamide) in Wistar rats: A toxicity profile study. Hepatol Res. 2007;37:845-853. [Cited in This Article: ] |
| 23. | Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502-522. [Cited in This Article: ] |
| 24. | Nakagawa Y, Suzuki T, Kamimura H, Nagai F. Role of mitochondrial membrane permeability transition in N-nitrosofenfluramine-induced cell injury in rat hepatocytes. Eur J Pharmacol. 2006;529:33-39. [Cited in This Article: ] |
| 25. | Kawamura-Sato K, Hirama Y, Agata N, Ito H, Torii K, Takeno A, Hasegawa T, Shimomura Y, Ohta M. Quantitative analysis of cereulide, an emetic toxin of Bacillus cereus, by using rat liver mitochondria. Microbiol Immunol. 2005;49:25-30. [Cited in This Article: ] |
| 26. | Caro AA, Cederbaum AI. Role of intracellular calcium and phospholipase A2 in arachidonic acid-induced toxicity in liver cells overexpressing CYP2E1. Arch Biochem Biophys. 2007;457:252-263. [Cited in This Article: ] |
| 27. | Rameh LE, Rhee SG, Spokes K, Kazlauskas A, Cantley LC, Cantley LG. Phosphoinositide 3-kinase regulates phospholipase Cgamma-mediated calcium signaling. J Biol Chem. 1998;273:23750-23757. [Cited in This Article: ] |
| 28. | Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440-3450. [Cited in This Article: ] |
| 29. | Caro AA, Cederbaum AI. Ca2+-dependent and independent mitochondrial damage in HepG2 cells that overexpress CYP2E1. Arch Biochem Biophys. 2002;408:162-170. [Cited in This Article: ] |
| 30. | Emaus RK, Grunwald R, Lemasters JJ. Rhodamine 123 as a probe of transmembrane potential in isolated rat-liver mitochondria: spectral and metabolic properties. Biochim Biophys Acta. 1986;850:436-448. [Cited in This Article: ] |
| 31. | Rothe G, Valet G. Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2',7'-dichlorofluorescin. J Leukoc Biol. 1990;47:440-448. [Cited in This Article: ] |
| 32. | Katiyar SK, Afaq F, Azizuddin K, Mukhtar H. Inhibition of UVB-induced oxidative stress-mediated phosphorylation of mitogen-activated protein kinase signaling pathways in cultured human epidermal keratinocytes by green tea polyphenol (-)-epigallocatechin-3-gallate. Toxicol Appl Pharmacol. 2001;176:110-117. [Cited in This Article: ] |
| 33. | Yang D, Yaguchi T, Yamamoto H, Nishizaki T. Intracellularly transported adenosine induces apoptosis in HuH-7 human hepatoma cells by downregulating c-FLIP expression causing caspase-3/-8 activation. Biochem Pharmacol. 2007;73:1665-1675. [Cited in This Article: ] |
| 34. | Gonzalez-Avila M, Arriaga-Alba M, de la Garza M, del Carmen HernandezPretelin M, Dominguez-Ortiz MA, Fattel-Fazenda S, Villa-Trevino S. Antigenotoxic, antimutagenic and ROS scavenging activities of a Rhoeo discolor ethanolic crude extract. Toxicol In Vitro. 2003;17:77-83. [Cited in This Article: ] |
| 35. | Tasduq SA, Kaisar P, Gupta DK, Kapahi BK, Maheshwari HS, Jyotsna S, Johri RK. Protective effect of a 50% hydroalcoholic fruit extract of Emblica officinalis against anti-tuberculosis drugs induced liver toxicity. Phytother Res. 2005;19:193-197. [Cited in This Article: ] |
| 36. | Cao J, Jiang LP, Liu Y, Yang G, Yao XF, Zhong LF. Curcumin-induced genotoxicity and antigenotoxicity in HepG2 cells. Toxicon. 2007;49:1219-1222. [Cited in This Article: ] |
| 37. | Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 2003;124:778-790. [Cited in This Article: ] |
| 38. | Morimoto M, Hagbjork AL, Nanji AA, Ingelman-Sundberg M, Lindros KO, Fu PC, Albano E, French SW. Role of cytochrome P4502E1 in alcoholic liver disease pathogenesis. Alcohol. 1993;10:459-464. [Cited in This Article: ] |
| 39. | Tsukamoto H. Cyp2e1 and ALD. Hepatology. 2000;32:154-156. [Cited in This Article: ] |
| 40. | Kim JY, Lee S, Kim DH, Kim BR, Park R, Lee BM. Effects of flavonoids isolated from Scutellariae radix on cytochrome P-450 activities in human liver microsomes. J Toxicol Environ Health A. 2002;65:373-381. [Cited in This Article: ] |
| 41. | Henderson MC, Miranda CL, Stevens JF, Deinzer ML, Buhler DR. In vitro inhibition of human P450 enzymes by prenylated flavonoids from hops, Humulus lupulus. Xenobiotica. 2000;30:235-251. [Cited in This Article: ] |
| 42. | Obermeier MT, White RE, Yang CS. Effects of bioflavonoids on hepatic P450 activities. Xenobiotica. 1995;25:575-584. [Cited in This Article: ] |
| 43. | Wilhelm J. Metabolic aspects of membrane lipid peroxidation. Acta Univ Carol Med Monogr. 1990;137:1-53. [Cited in This Article: ] |
| 44. | Caro AA, Cederbaum AI. Synergistic toxicity of iron and arachidonic acid in HepG2 cells overexpressing CYP2E1. Mol Pharmacol. 2001;60:742-752. [Cited in This Article: ] |
| 45. | Chen Q, Galleano M, Cederbaum AI. Cytotoxicity and apoptosis produced by arachidonic acid in Hep G2 cells overexpressing human cytochrome P4502E1. J Biol Chem. 1997;272:14532-14541. [Cited in This Article: ] |
| 46. | Moon MK, Choi BM, Oh GS, Pae HO, Kim JD, Oh H, Oh CS, Kim DH, Rho YD, Shin MK. Catalposide protects Neuro 2A cells from hydrogen peroxide-induced cytotoxicity via the expression of heme oxygenase-1. Toxicol Lett. 2003;145:46-54. [Cited in This Article: ] |
| 47. | Manibusan MK, Odin M, Eastmond DA. Postulated carbon tetrachloride mode of action: a review. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2007;25:185-209. [Cited in This Article: ] |
| 48. | Matsuhashi T, Otaka M, Odashima M, Jin M, Komatsu K, Konishi N, Wada I, Sato T, Horikawa Y, Ohba R. Specific type IV phosphodiesterase inhibitor ameliorates thioacetamide-induced liver injury in rats. J Gastroenterol Hepatol. 2005;20:135-140. [Cited in This Article: ] |
| 49. | Balasubramaniyan V, Shukla R, Murugaiyan G, Bhonde RR, Nalini N. Mouse recombinant leptin protects human hepatoma HepG2 against apoptosis, TNF-alpha response and oxidative stress induced by the hepatotoxin-ethanol. Biochim Biophys Acta. 2007;1770:1136-1144. [Cited in This Article: ] |
| 50. | Zhuge J, Cederbaum AI. Depletion of S-adenosyl-l-methionine with cycloleucine potentiates cytochrome P450 2E1 toxicity in primary rat hepatocytes. Arch Biochem Biophys. 2007;466:177-185. [Cited in This Article: ] |
| 51. | Wu D, Cederbaum AI. Cyclosporine A protects against arachidonic acid toxicity in rat hepatocytes: role of CYP2E1 and mitochondria. Hepatology. 2002;35:1420-1430. [Cited in This Article: ] |
| 52. | Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta. 1998;1366:177-196. [Cited in This Article: ] |
| 53. | Lemasters JJ, Qian T, Bradham CA, Brenner DA, Cascio WE, Trost LC, Nishimura Y, Nieminen AL, Herman B. Mitochondrial dysfunction in the pathogenesis of necrotic and apoptotic cell death. J Bioenerg Biomembr. 1999;31:305-319. [Cited in This Article: ] |
| 54. | Hoek JB, Cahill A, Pastorino JG. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology. 2002;122:2049-2063. [Cited in This Article: ] |