Published online Dec 26, 2021. doi: 10.12998/wjcc.v9.i36.11495
Peer-review started: August 19, 2021
First decision: September 5, 2021
Revised: September 25, 2021
Accepted: November 14, 2021
Article in press: November 14, 2021
Published online: December 26, 2021
Processing time: 126 Days and 3.6 Hours
Hepatocellular carcinoma (HCC) accompanied by a tumor thrombus is very common. However, the treatment strategy is controversial and varies by the location of the thrombus.
We report herein a case of HCC with a tumor thrombus in the suprahepatic inferior vena cava (IVC), which was successfully treated by hepatectomy combined with thrombectomy following sorafenib chemotherapy. A 47-year-old woman with chronic hepatitis was diagnosed with HCC. Computed tomography and magnetic resonance imaging showed that the tumor lesion was located in the right half of the liver, and a tumor thrombus was detected in the suprahepatic IVC near the right atrium. After multi-departmental discussion and patient informed consent, right major hepatectomy and total removal of the tumor thrombus were successfully performed under cardiopulmonary bypass. There were no serious complications after surgery. Following sorafenib treatment, no recurrence has been detected so far (11 mo later).
Surgical treatment followed by adjuvant sorafenib therapy might be an acceptable choice for HCC patients with tumor thrombosis in the IVC.
Core Tip: Hepatocellular carcinoma (HCC) is the most common type of liver cancer with a high mortality rate worldwide. For HCC patients with tumor thrombosis in the inferior vena cava (IVC), in addition to tumor progression, acute pulmonary embolism induced by tumor thrombosis is also a vital factor decreasing patient survival. Once a pulmonary embolism occurs, there is no effective therapy, and the patients usually die. Therefore, in Asia-Pacific regions such as China, Japan and South Korea, surgical treatment is recommended in highly selected patients, which might provide better survival outcomes than other treatments. Here, we report a case of a resectable HCC patient with tumor thrombosis in the IVC who was treated successfully by liver resection, tumor thrombosis removal and systemic treatment.
- Citation: Zhang ZY, Zhang EL, Zhang BX, Zhang W. Surgery for hepatocellular carcinoma with tumor thrombosis in inferior vena cava: A case report. World J Clin Cases 2021; 9(36): 11495-11503
- URL: https://www.wjgnet.com/2307-8960/full/v9/i36/11495.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i36.11495
Hepatocellular carcinoma (HCC) is the most common type of liver cancer with a high mortality rate worldwide. The percentage of HCC patients with vascular invasion at the time of initial diagnosis is 10%-40%[1-3]. The median survival time (MST) has been reported to be only 3-10.1 mo without any treatment[4]. According to the American Association for the Study of Liver Disease (AASLD)/Barcelona Clinic for Liver Cancer (BCLC) staging system and treatment guidelines, HCC associated with vascular invasion or bile duct invasion is regarded as an advanced stage of disease[1]. The only suggested treatment for such patients is systemic treatment, such as with sorafenib or lenvatinib.
However, the efficacy of these treatments is not satisfactory. Especially for HCC patients with tumor thrombosis in the inferior vena cava (IVC), in addition to tumor progression, acute pulmonary embolism induced by tumor thrombosis is also a vital factor decreasing patient survival. Once a pulmonary embolism occurs, there is no effective therapy, and most patients die. Therefore, in Asia-Pacific regions such as China, Japan and South Korea, surgical treatment is recommended in highly selected patients, which might provide better survival outcomes than other treatments[5,6].
Here, we report a case of a resectable HCC patient with tumor thrombosis in the IVC who was treated successfully by liver resection, tumor thrombosis removal and sorafenib treatment.
A 47-year-old woman with hepatitis B virus-associated chronic hepatitis without obvious complaining was diagnosed with HCC at the clinic.
This patient did not have any obvious complaint. She came to our clinic and ask for regular examination.
This patient had hepatitis B infection for a long time.
No special personal and family history.
Physical examination showed no positive results.
The patient’s alpha fetoprotein (AFP) was 105.5 ng/mL. The protein induced by vitamin K absence/agonist-II (PIVKA-II) was 77505 mAU/mL. The indocyanine green retention rate at 15 min (ICGR15) was 5.4%. The Child-Pugh Score was A with 5 points.
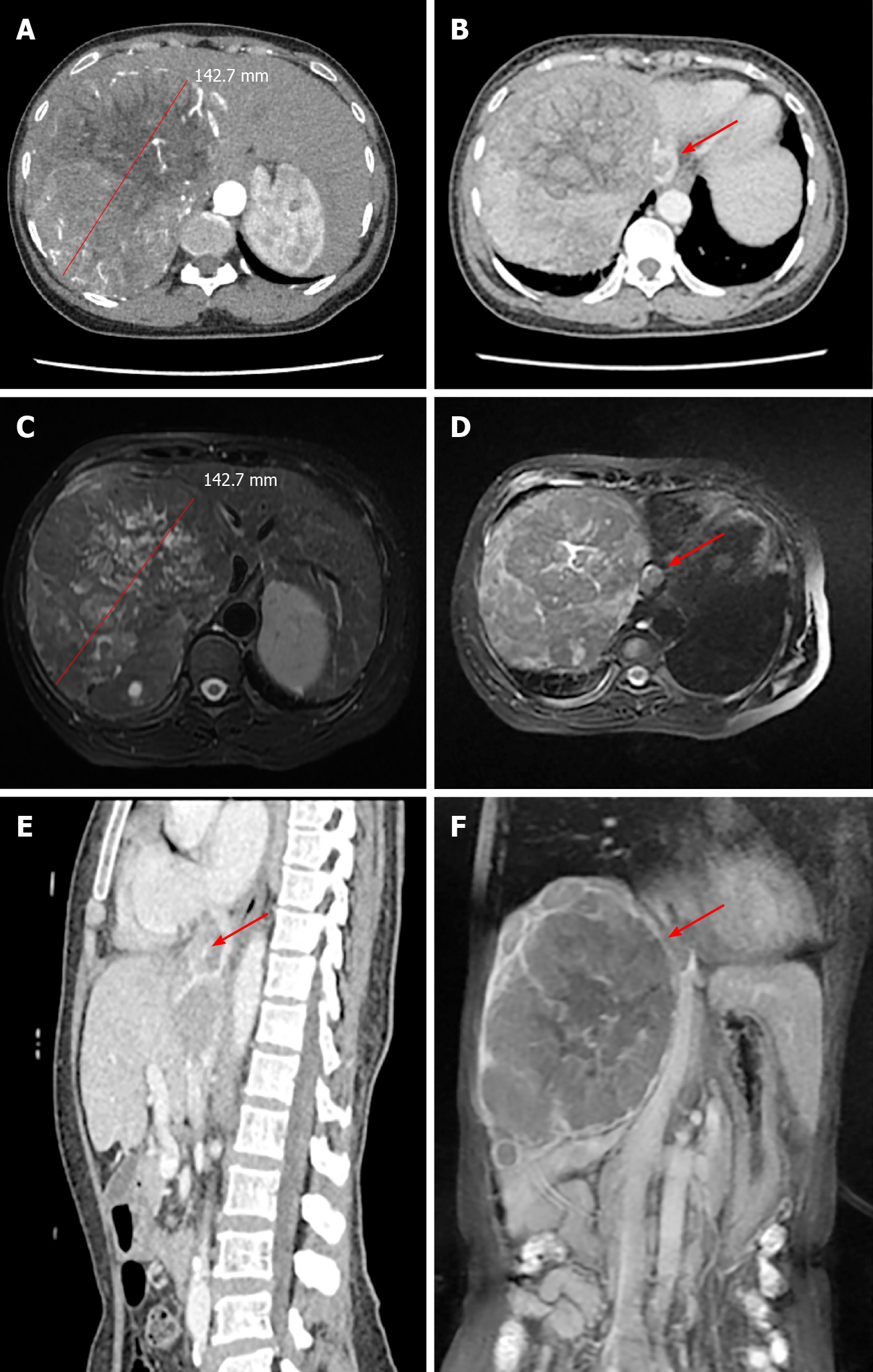
Computed tomography (CT) and magnetic resonance imaging (MRI) scans showed a solitary tumor 14 cm × 12 cm in diameter located in the right half of the liver (Figure 1A and C). In addition, tumor thrombosis was detected in the IVC flattened to the right atrium, nearly 2 cm in diameter (Figure 1B and D-F). The upper side of the tumor thrombosis extended beyond the diaphragm but did not extend into the right atrium. Distant metastasis and intrahepatic metastasis was not observed.
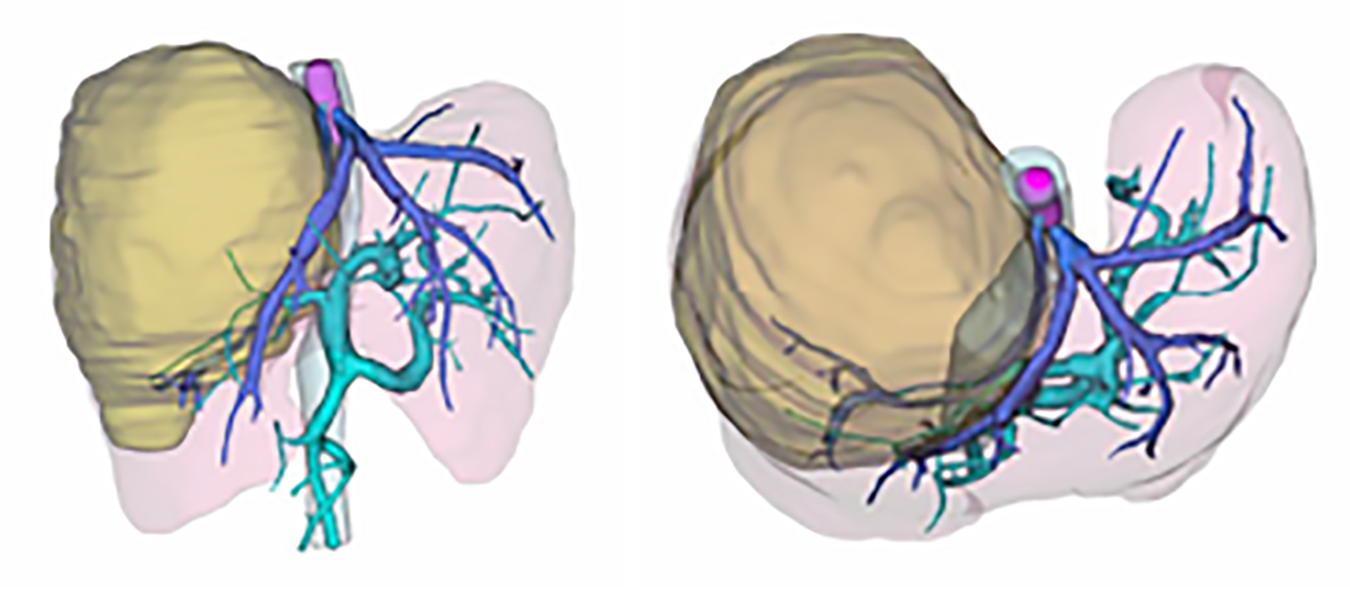
After three-dimensional reconstruction using CT scanning (Figure 2), the left remnant liver volume (RLV) was determined to be 781.16 mL. The standard liver volume (SLV), which was calculated in accordance with the patient’s body surface area, was 1034 mL according to Urata’s formula[7]. Thus, her RLV was over SLV × 50%. According to Western guidelines, such as the AASLD/BCLC, hepatectomy is not recommended in cases with vascular invasion[8]. However, according to the 2019 Chinese clinical guidelines for the management of HCC, resection might be a possible choice because the tumor and thrombosis were resectable and her RLV was sufficient[5].
Based on the AFP and PIVKA-II tests and CT/MRI scanning results, the patient was diagnosed with HCC and tumor thrombosis in the IVC.
After multidisciplinary discussion, two treatment schemes were proposed. One of the treatment options was to completely remove the tumor and thrombus together followed by sorafenib or lenvatinib molecular targeted therapy. The other was transarterial chemoembolization (TACE) combined with radiotherapy and molecular targeted therapy. We discussed in detail the advantages and disadvantages of the two treatments with the patient. In the end, this patient and her family members selected the first treatment strategy.
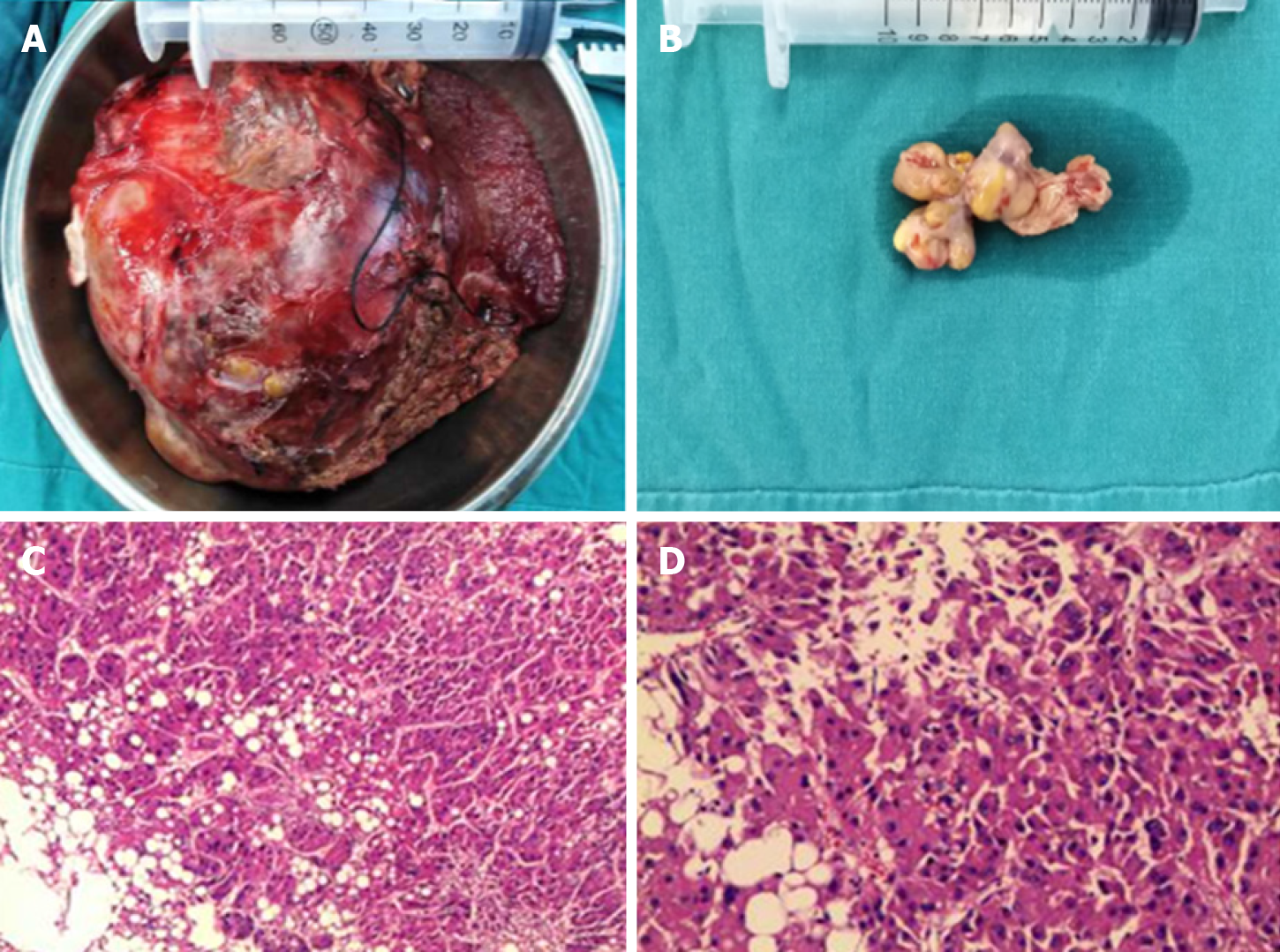
Accordingly, a right hemihepatectomy and total removal of the tumor thrombus from the IVC were performed. Surgery was performed through a subcostal incision with xiphoid extension and median sternotomy. There was no ascites or metastasis observed. Because the thrombus was flattened to the right atrium, total hepatic vascular exclusion (THVE) and cardiopulmonary bypass (CPB) were performed before the removal of the tumor and tumor thrombus. Normothermic CPB was chosen to minimize the ischemic damage to the heart caused by intraoperative hypotension. CPB was established with cannulation of the femoral artery, suprahepatic vena cava and infrahepatic vena cava for drainage and the administration of heparin (25000 units).
Then, THVE was performed. First, the right hepatic pedicle was transected to stop the inflow to the right liver. Next, the right atrium, infrahepatic vena cava, and hepatoduodenal ligament were clamped. The time for clamping of the hepato
The postoperative recovery was uneventful. The patient was discharged on posto
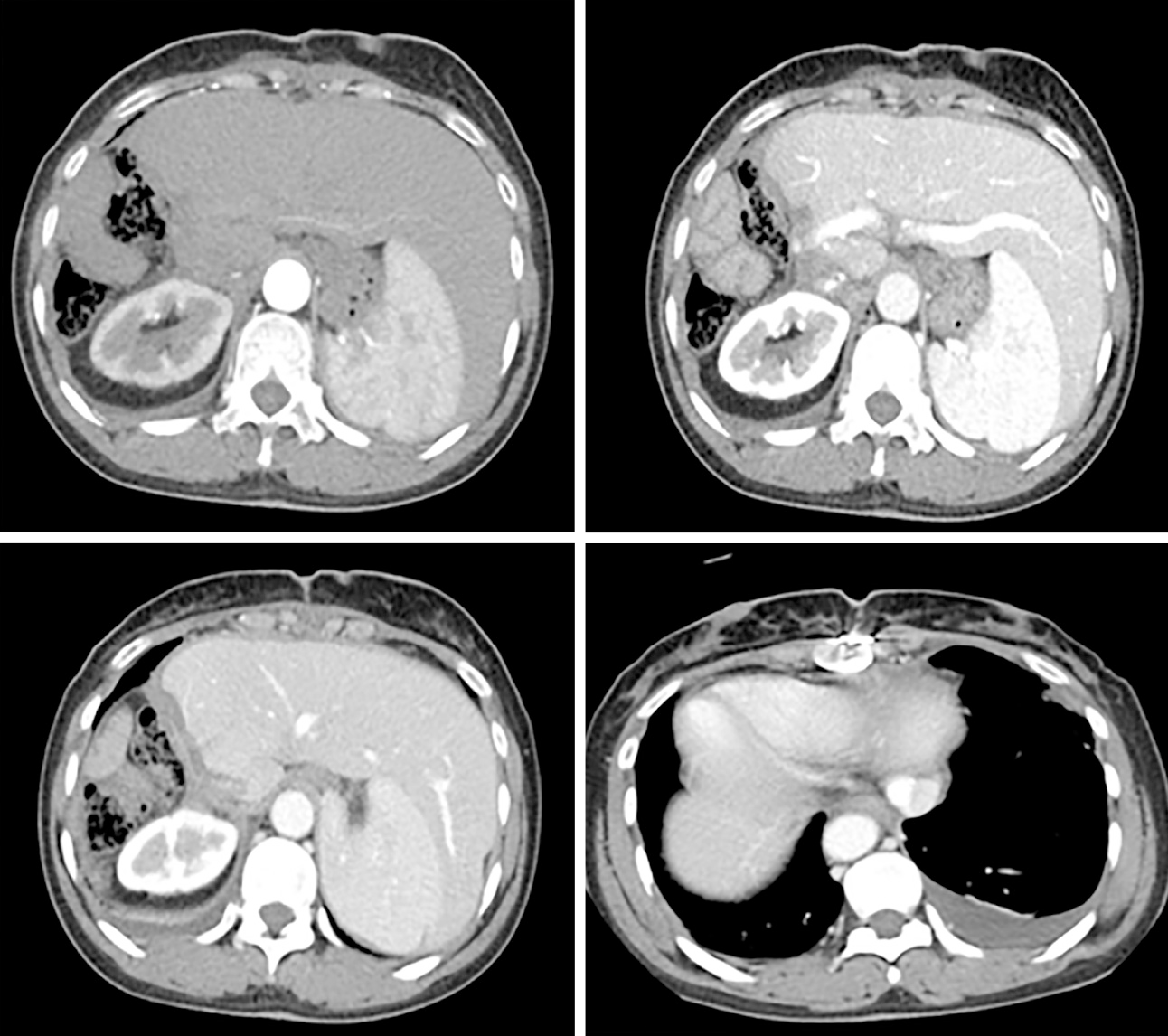
One month later, this patient underwent digital subtraction angiography (DSA) to check her liver. No recurrence was found. Every two months, she returned to our clinic to recheck her tumor biomarkers (AFP, PIVKA-II) and to undergo radiology tomography (ultrasonography, MRI or CT) (Figure 4). So far, she has survived for 11 mo after the surgery, and no recurrence has been detected.
The percentage of HCC cases with vascular invasion at the initial time of diagnosis is high. However, the incidence of HCC with a tumor thrombus in the IVC is relatively lower, from 1% to 4%[9-12]. The treatment strategy for HCC patients associated with vascular invasion remains controversial. According to the existing guidelines, such as AASLD/BCLC, tumor thrombosis in the vessels is a contraindication for liver resection. Conservative treatment or molecular targeted therapy is recommended. However, according to some treatment guidelines[5,13], liver resection might be an acceptable choice for selected patients. Furthermore, most of the research has focused on the treatment of portal vein tumor thrombosis (PVTT). Once the tumor thrombus has extended to the main portal vein or superior mesenteric vein, hepatectomy would no longer be suggested[4,13].
Recently, a study from Japan showed that liver resection for selected patients with tumor thrombus in hepatic vein could provide a longer median overall survival time (2.87 years vs 1.10 years) compared with nonresection therapies, including TACE, radiotherapy, sorafenib, or conservative treatment[14]. As an curative treatment option or bridging therapy, transaterial radioemblization (TARE) has been used in HCC patients with BCLC stage A to C patients[15]. And good outcomes without significant adverse events have also been reported when compared conservative TACE. In BCLC stage C patients, TARE showed median overall survivals ranging from 6-10 mo which might be very similar to patients who received sorafenib treatment[16]. Due to the lack of significant macroembolic effect causing liver decompensation, PVTT is no longer contraindication for TARE treatment[17]. As we have discussed before, the prognosis for HCC with tumor thrombus in vessel varied based on the extension and location in the vessel[18]. The prognosis of HCC patients with tumor thrombus in portal vein system was different with patients with tumor thrombus in hepatic vein system. Actually, the prognosis of patients with main PVTT (OS ranging from 4-7 mo) was worse when compared with patients with segmentary or lobar PVTT (OS ranging from 7-13 mo)[4]. So the prognosis for HCC patients followed TARE treatment in BCLC C stage should be evaluated based on the location and extension status. However, to date, well designed clinical trial focused on the comparison of the prognosis in HCC patients with tumor thrombus in hepatic vein system after different treatment including TACE, TARE, surgery or conservative treament was rare. So which treatment should be selected according to the extent of tumor thrombus in the hepatic vein system is still uncertain.
In China, local ablation therapies, such as RFA, TACE, surgical therapy, or systemic therapy, are all recommended for HCC patients with vascular invasion. Compared with tumor thrombosis in the portal vein, tumor thrombosis in the IVC or right atrium is accompanied by a high risk of sudden death because of pulmonary embolism or heart failure[19,20]. Surgical resection combined with postoperative molecular targeted therapy might benefit resectable patients with tumor thrombi in the IVC. Therefore, according to our experience, resection of the tumor and tumor thrombosis might be an acceptable choice, although the risk of intraoperative and postoperative complications is high.
One of the problems associated with the surgical treatment of HCC patients with tumor thrombi in the IVC or right atrium is the high operative risk. In our case, procedures including THVE, CPB, and hypothermic cardiocirculatory arrest were used to minimize surgical stress. CPB and hypothermic cardiocirculation could preserve the intraoperative circulation and minimize the damage caused by ischemia-reperfusion[11]. In our case, no functional damage to the heart or liver was detected during the recovery term after the operation.
THVE is one of the most commonly used techniques in hepatectomy and can minimize bleeding during surgery[21]. However, in our case, the volume of blood lost during the surgery was very high. The coagulation dysfunction caused by CPB might be one of the potential reasons. Moreover, cirrhosis might be another possible reason. Nearly 80% of HCC patients in China have cirrhosis[22]. The cirrhotic state could damage coagulation function and change the anatomical structure in the liver. Then, it could eventually increase the difficulty of surgery and bleeding and decrease the functional reserve[23].
ICGR15 is one technique used before surgery to evaluate hepatic functional reserve. It can partially evaluate the cirrhosis state. Major resection should not be performed if the ICGR15 exceeds 20%[13]. In this case, the ICGR15 was 5.4%, which meets the standard for major hepatectomy. However, coagulation dysfunction still emerged, especially after the administration of heparin. The volume of diffused blood oozing from the surgical surface exceeded our expectations.
The other problem associated with the surgical treatment of HCC patients with tumor thrombi in the IVC is the high rate of recurrence. According to some retrospective studies, patients with tumor thrombi in only the hepatic vein had a better prognosis than those with tumor thrombi in the IVC. The MST after surgery in patients with only hepatic vein tumor thrombus was 3.95-5.27 years. The median time to recurrence (TTR) was 0.4-1.06 years[24,25]. Once the tumor thrombus extends into the IVC, the MST and TTR could be shortened to only 1.39-1.6 years and 0.25 years, respectively[25]. In some reports, no patients with tumor thrombi invading the IVC survived for more than 2 years after surgery[24]. The major factor shortening their survival time is early distant organ metastasis, especially lung metastasis accompanied by or without metastasis to other organs. Therefore, based on our experience, the control of intrahepatic recurrence and distant metastasis should be prioritized. In this case, one month after the surgery, the patient returned to our center and underwent DSA, and no recurrence sites were detected in the liver or lungs. Her serum tumor markers were found to be reduced to within the normal range. To control recurrences, sorafenib treatment was recommended for this patient.
Sorafenib is an oral multikinase inhibitor that blocks the activity of protein kinases associated with angiogenesis and metastasis. Sorafenib has become a standard treatment for HCC patients with vascular invasion[5,26]. According to the results of the STORM study (http://clinicaltrials.gov/ct2/show/NCT00692770), sorafenib as an adjuvant treatment after resection or ablation showed no benefits for survival or recurrence[27]. However, that study did not include patients with vascular invasion or distant metastasis. According to the SHARP trial, the median survival in the subgroup with MVI disease was 8.1 mo with sorafenib and 4.9 mo with placebo[26]. Several recent retrospective studies from China demonstrated that sorafenib could reduce recurrence and prolong the survival rate in patients with a high risk of recurrence after surgery[28,29]. Therefore, sorafenib might be useful in decreasing the risk of recurrence in patients with tumor thrombi after surgery. In this case, we recommended sorafenib treatment after recovery from surgery. At the time of writing, no sign of recurrence had been detected in this patient.
In conclusion, we report a case of advanced HCC that was treated with hepatectomy and thrombectomy. During the surgery, CPB, THVE and hypothermic cardiocirculation were used. After the surgery, sorafenib was administered as an adjuvant treatment. This treatment strategy was selected after discussion by a multidisciplinary department. After the surgery, this patient showed good outcomes. Therefore, hepatectomy and thrombectomy with adjuvant sorafenib treatment might be an acceptable choice for selected HCC patients with tumor thrombosis in the IVC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Broering DC S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3596] [Article Influence: 276.6] [Reference Citation Analysis (4)] |
| 2. | Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, Wu MC, Cheng SQ. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2010;17:2073-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 3. | Chen XP, Qiu FZ, Wu ZD, Zhang ZW, Huang ZY, Chen YF, Zhang BX, He SQ, Zhang WG. Effects of location and extension of portal vein tumor thrombus on long-term outcomes of surgical treatment for hepatocellular carcinoma. Ann Surg Oncol. 2006;13:940-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Shuqun C, Mengchao W, Han C, Feng S, Jiahe Y, Guanghui D, Wenming C, Peijun W, Yuxiang Z. Tumor thrombus types influence the prognosis of hepatocellular carcinoma with the tumor thrombi in the portal vein. Hepatogastroenterology. 2007;54:499-502. [PubMed] [DOI] [Full Text] |
| 5. | Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9:452-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 332] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 6. | Ikeda M, Mitsunaga S, Shimizu S, Ohno I, Takahashi H, Okuyama H, Kuwahara A, Okusaka T. Current status of hepatocellular carcinoma in Japan. Chin Clin Oncol. 2013;2:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 7. | Urata K, Kawasaki S, Matsunami H, Hashikura Y, Ikegami T, Ishizone S, Momose Y, Komiyama A, Makuuchi M. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 704] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 8. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4108] [Article Influence: 586.9] [Reference Citation Analysis (6)] |
| 9. | Pandya H, Shah C, Lakhani J, Patel M. Intra-atrial tumour thrombus secondary to hepatocellular carcinoma. Australas Med J. 2013;6:321-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Mukai K, Shinkai T, Tominaga K, Shimosato Y. The incidence of secondary tumors of the heart and pericardium: a 10-year study. Jpn J Clin Oncol. 1988;18:195-201. [PubMed] |
| 11. | Ohta M, Nakanishi C, Kawagishi N, Hara Y, Maida K, Kashiwadate T, Miyazawa K, Yoshida S, Miyagi S, Hayatsu Y, Kawamoto S, Matsuda Y, Okada Y, Saiki Y, Ohuchi N. Surgical resection of recurrent extrahepatic hepatocellular carcinoma with tumor thrombus extending into the right atrium under cardiopulmonary bypass: a case report and review of the literature. Surg Case Rep. 2016;2:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, Kudo M, Kubo S, Sakamoto M, Nakashima O, Kumada T, Kokudo N; Liver Cancer Study Group of Japan. Liver resection for hepatocellular carcinoma associated with hepatic vein invasion: A Japanese nationwide survey. Hepatology. 2017;66:510-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 13. | Kudo M, Kitano M, Sakurai T, Nishida N. General Rules for the Clinical and Pathological Study of Primary Liver Cancer, Nationwide Follow-Up Survey and Clinical Practice Guidelines: The Outstanding Achievements of the Liver Cancer Study Group of Japan. Dig Dis. 2015;33:765-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 235] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 14. | Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, Kudo M, Ku Y, Sakamoto M, Nakashima O, Kaneko S, Kokudo N; Liver Cancer Study Group of Japan. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 2016;65:938-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 365] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 15. | Nam JY, Lee YB, Lee JH, Yu SJ, Kim HC, Chung JW, Yoon JH, Kim YJ. A Prognostic Prediction Model of Transarterial Radioembolization in Hepatocellular Carcinoma: SNAP-HCC. Dig Dis Sci. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, Baker T, Gates V, Miller FH, Sato KT, Wang E, Gupta R, Benson AB, Newman SB, Omary RA, Abecassis M, Kulik L. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 781] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 17. | Mosconi C, Cappelli A, Pettinato C, Golfieri R. Radioembolization with Yttrium-90 microspheres in hepatocellular carcinoma: Role and perspectives. World J Hepatol. 2015;7:738-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Zhang ZY, Zhang EL, Zhang BX, Chen XP, Zhang W. Treatment for hepatocellular carcinoma with tumor thrombosis in the hepatic vein or inferior vena cava: A comprehensive review. World J Gastrointest Surg. 2021;13:796-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Ulus T, Birdane A, Dündar E, Tünerir B. Asymptomatic course of a metastatic mass completely filling the right atrium in a patient with hepatocellular carcinoma. Turk Kardiyol Dern Ars. 2012;40:52-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Dedeilias P, Nenekidis I, Koukis I, Anagnostakou V, Paparizou N, Zompolos S, Apostolakis E. Acute heart failure caused by a giant hepatocellular metastatic tumor of the right atrium. J Cardiothorac Surg. 2011;6:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Chen XP, Zhang ZW, Zhang BX, Chen YF, Huang ZY, Zhang WG, He SQ, Qiu FZ. Modified technique of hepatic vascular exclusion: effect on blood loss during complex mesohepatectomy in hepatocellular carcinoma patients with cirrhosis. Langenbecks Arch Surg. 2006;391:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Chen XP, Wu ZD, Huang ZY, Qiu FZ. Use of hepatectomy and splenectomy to treat hepatocellular carcinoma with cirrhotic hypersplenism. Br J Surg. 2005;92:334-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Huang ZY, Chen G, Hao XY, Cai RY, Zhao YF, Chen XP. Outcomes of non-anatomic liver resection for hepatocellular carcinoma in the patients with liver cirrhosis and analysis of prognostic factors. Langenbecks Arch Surg. 2011;396:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Ikai I, Yamamoto Y, Yamamoto N, Terajima H, Hatano E, Shimahara Y, Yamaoka Y. Results of hepatic resection for hepatocellular carcinoma invading major portal and/or hepatic veins. Surg Oncol Clin N Am. 2003;12:65-75, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Kokudo T, Hasegawa K, Yamamoto S, Shindoh J, Takemura N, Aoki T, Sakamoto Y, Makuuchi M, Sugawara Y, Kokudo N. Surgical treatment of hepatocellular carcinoma associated with hepatic vein tumor thrombosis. J Hepatol. 2014;61:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 26. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10270] [Article Influence: 604.1] [Reference Citation Analysis (2)] |
| 27. | Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY, Lee HC, Song T, Roayaie S, Bolondi L, Lee KS, Makuuchi M, Souza F, Berre MA, Meinhardt G, Llovet JM; STORM investigators. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 788] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 28. | Huang Y, Zhang Z, Zhou Y, Yang J, Hu K, Wang Z. Should we apply sorafenib in hepatocellular carcinoma patients with microvascular invasion after curative hepatectomy? Onco Targets Ther. 2019;12:541-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Li J, Hou Y, Cai XB, Liu B. Sorafenib after resection improves the outcome of BCLC stage C hepatocellular carcinoma. World J Gastroenterol. 2016;22:4034-4040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |