Published online Jun 26, 2021. doi: 10.12998/wjcc.v9.i18.4542
Peer-review started: January 14, 2021
First decision: March 15, 2021
Revised: March 27, 2021
Accepted: April 22, 2021
Article in press: April 22, 2021
Published online: June 26, 2021
Processing time: 148 Days and 1.3 Hours
Colon cancer is one of the most common malignancies worldwide, and chemotherapy is a widely used strategy in colon cancer clinical therapy. However, chemotherapy resistance is a major cause of disease recurrence and progression in colon cancer, and thus novel drugs for treatment are urgently needed. Tetramethylpyrazine (TMP), a component of the traditional Chinese medicine Chuanxiong Hort, has been proven to exhibit a beneficial effect in tumors.
To investigate the potential anticancer activity of TMP in colon cancer and its underlying mechanisms.
Colon cancer cells were incubated with different concentrations of TMP. Cell viability was evaluated by crystal violet staining assay and cell counting kit-8 assay, and cell apoptosis and cell cycle were assessed by flow cytometry.
TMP significantly inhibited the proliferation of colon cancer cells in a dose- and time-dependent manner. In addition, flow cytometry revealed that TMP induced cell cycle arrest at the G0/G1 phase. TMP treatment caused early stage apoptosis in SW480 cells, whereas it caused late stage apoptosis in HCT116 cells.
Our studies demonstrated that TMP inhibits the proliferation of colon cancer cells in a dose- and time-dependent manner by inducing apoptosis and arresting the cell cycle at the G0/G1 phase. Our findings suggest that TMP might serve as a potential novel therapeutic drug in the treatment of human colon cancer.
Core Tip: Colon cancer is one of the most common malignancies worldwide, and chemotherapy is a widely used strategy in colon cancer clinical therapy. However, many patients eventually relapse and develop drug resistance and chemotherapy resistance. This is one of the main causes of chemotherapeutic failure. Tetramethylpyrazine (TMP) has been proven to exhibit a beneficial effect in many types of malignant tumors. Here, we find out that TMP can induce apoptosis and inhibits proliferation of colon cancer cells, suggesting that TMP might serve as a potential novel therapeutic drug in the treatment of human colon cancer.
- Citation: Li H, Hou YX, Yang Y, He QQ, Gao TH, Zhao XF, Huo ZB, Chen SB, Liu DX. Tetramethylpyrazine inhibits proliferation of colon cancer cells in vitro. World J Clin Cases 2021; 9(18): 4542-4552
- URL: https://www.wjgnet.com/2307-8960/full/v9/i18/4542.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i18.4542
Colon cancer is one of the most commonly diagnosed cancers and its incidence is growing in many countries. The incidence of colon cancer is high in developed countries, reflecting a prevalence of risk factors, including an unhealthy diet and obesity[1]. Although patients with early stage colon cancer can be treated by surgical resection, due to a lack of characteristic clinical manifestations and colon cancer screening, many patients are at an advanced stage at the time of diagnosis[2]. Chemotherapy is a commonly used strategy for colon cancer treatment, as many patients have regional or distant spread at the time of diagnosis. However, many patients eventually relapse and develop drug resistance and chemotherapy resistance. This is one of the main causes of chemotherapeutic failure. Therefore, clinical treatment of colon cancer is still a great challenge, and searching for novel drugs for colon cancer treatment with high selectivity and low toxicity has become a recent focus of attention.
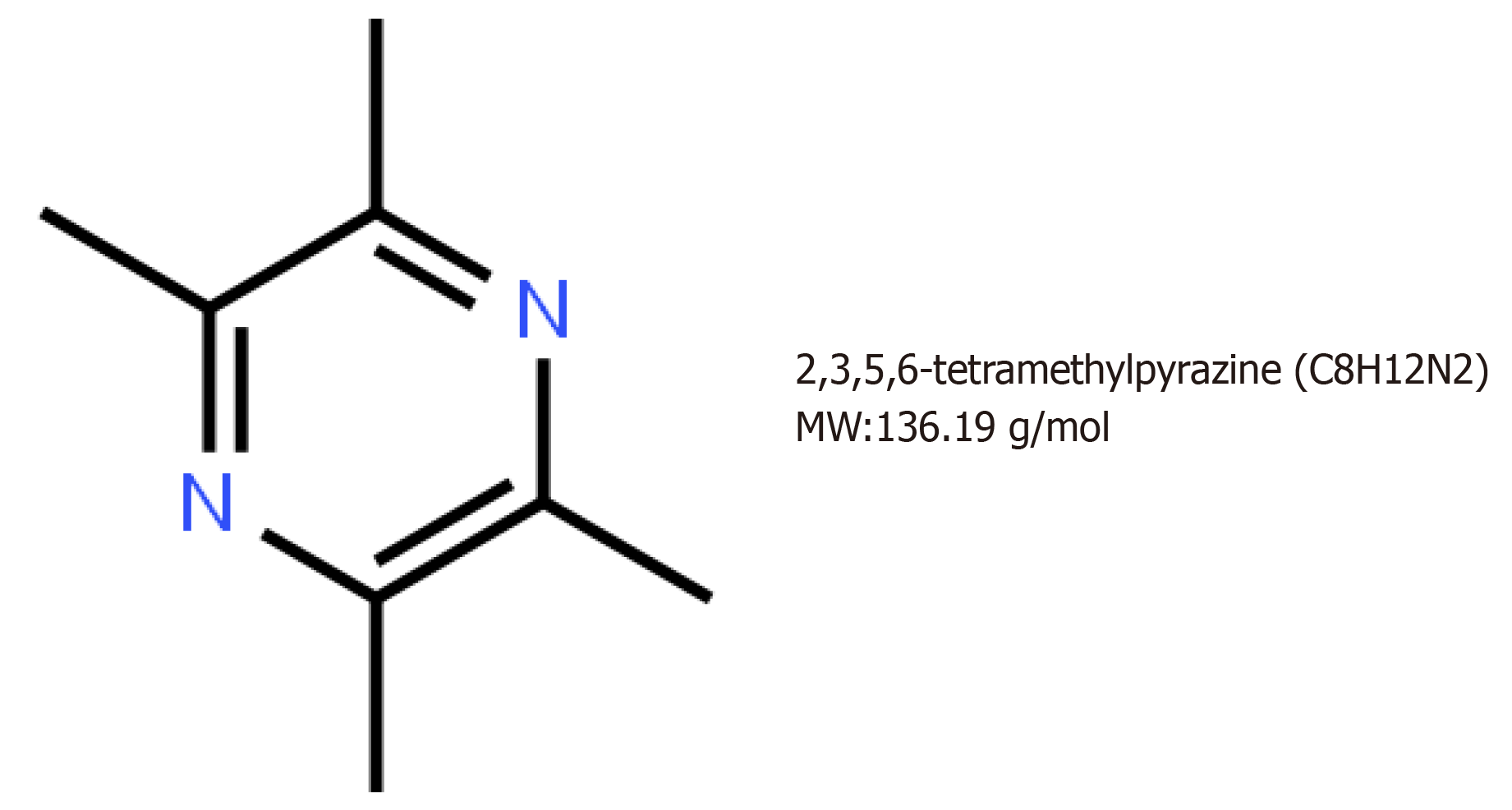
Ligusticum chuanxiong, a Chinese herb, has a long history in traditional Chinese medicine[3]. Tetramethylpyrazine (2,3,5,6-tetramethylpyrazine, TMP) is an alkaloid monomer that exists in the roots or stems of Ligusticum chuanxiong[4]. TMP has been widely used for the treatment of neurovascular and cardiovascular disorders, such as acute ischemic stroke, angina pectoris, and atherosclerosis[5-7]. The underlying mechanisms involve inhibition of platelet aggregation, suppression of apoptosis, and scavenging peroxyl radicals, superoxide, and hydroxyl radicals[8]. Recent studies have demonstrated that TMP has potent inhibitory effects on a number of types of tumors, such as lung, breast, ovarian carcinoma, gastric carcinoma, osteosarcoma, leukemia, and hepatocellular carcinoma, through affecting the proliferation and apoptosis of tumor cells[9-13]. However, the effects of TMP on colon cancer have not been investigated. In the present study, we evaluated the effects of TMP on the viability, cell cycle distribution, and apoptosis of colon cancer cell lines.
Six human colon cancer cell lines, DLD1, HCT116, LOVO, LS1747, SW480, and SW620, were purchased from the Cell Resource Center of the Institute of Basic Medicine, Chinese Academy of Medical Sciences. All cell lines were cultured in RPMI 1640 medium (Gibco, United States) with 10% fetal bovine serum (FBS, HyClone, United States) in a cell incubator with an atmosphere of 50 mL/L CO2 at 37 °C.
TMP was purchased from Sigma-Aldrich (United States) and dissolved in DMSO (Sigma-Aldrich, United States). Cell counting kit-8 (CCK-8) was purchased from Med Chem Express (United States). The Annexin V-FITC/ propidium iodide (PI) kit and PI were purchased from BD (United States) and Sigma, respectively.
Cells were seeded in 6-well plates (1 × 105 cells/well) and incubated in RPMI 1640 supplemented with 10% FBS for 24 h. Cells were treated with TMP at a final concentration of 0 µg/mL, 300 µg/mL, 600 µg/mL, 900 µg/mL, 1200 µg/mL, or 1500 µg/mL for 24 h, 48 h, or 72 h. The morphology of the cells was examined under an inverted light microscope.
Cell viability was evaluated by crystal violet staining assay. Cells were seeded in 6-well plates (5 × 104 cells/well) and incubated in RPMI 1640 supplemented with 10% FBS for 24 h. The cells were then incubated with TMP at various concentrations for 48 h; cells incubated with 0 µg/mL TMP served as a control. The treated cells were washed with PBS and then mixed with crystal violet solution and incubated within 10 min. The morphology of the cells was examined under an inverted light microscope. The experiment was performed in triplicate.
CCK-8 assay was used to detect cell proliferation following the manufacturer's instructions. SW480 and HCT116 cells were seeded in 96-well microtiter plates at a density of 5 × 103 cells/well. After 24 h, the medium was replaced with RPMI 1640 containing different concentrations of TMP (0 µg/mL, 300 µg/mL, 600 µg/mL, 900 µg/mL, 1200 µg/mL, or 1500 µg/mL) and cells were cultured for 24 h, 48 h, or 72 h. At the indicated time points, 10 µL of CCK-8 kit solution was added to the cells, and cells were incubated for an additional 2 h. Absorbance was measured at 450 nm. Six duplicate wells were run for each treatment. All experiments were performed at least three times. The cell viability index was calculated according to the formula: Experimental OD value/control OD value × 100%.
SW480 and HCT116 cells were treated with TMP at a final concentration of 0 µg/mL, 300 µg/mL, 600 µg/mL, 900 µg/mL, or 1200 µg/mL for 24 h. The cells were centrifuged, fixed in 70% ethanol for at least 24 h, washed with PBS, and treated with RNase A. Cells were then incubated with 300 µl of PI (50 µg/mL) at room temperature for 15 min. The cell cycle distribution was examined by flow cytometry. The experiment was conducted in triplicate.
For apoptosis assays, SW480 and HCT116 cells were treated with TMP at a final concentration of 0 µg/mL, 300 µg/mL, 600 µg/mL, 900 µg/mL, or 1200 µg/mL for 24 h. Cells were then stained using the reagents in the Annexin V-FITC/PI kit for 30 min at 0 °C in the dark. The percentage of apoptotic cells was determined by flow cytometry. The experiment was conducted in triplicate.
All the experiments were repeated three times independently and each sample was analyzed in triplicate. Data from all experiments are presented as the mean ± SD. One-way ANOVA with the least-significant difference tests was used to determine the significance of difference. Student’s t-test was used for comparing two treatments. Graph Pad Prism 6 software (Graph Pad Software, United States) was used to make statistical charts. FlowJo 7.0 (BD, United States) was used to process and analyze the flow cytometer data, and Modfit (Verity Software House, United States) software was used to analyze the cell cycle distribution. P < 0.05 was considered statistically significant.
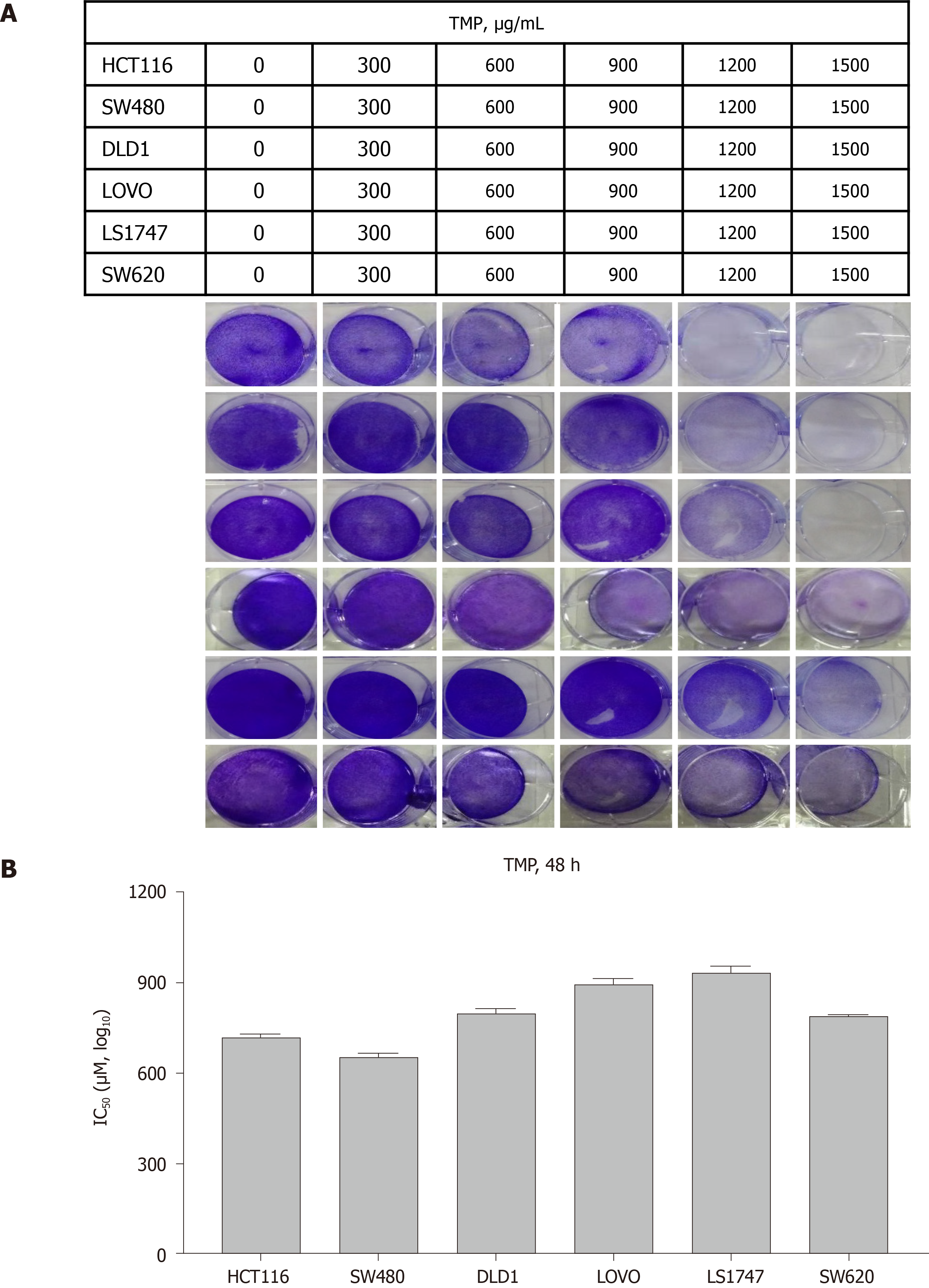
The chemical structure of TMP is presented in Figure 1. To investigate the effect of TMP on the proliferation of colon cancer cells, six colon cancer cell lines, DLD1, HCT116, LOVO, LS1747, SW480, and SW620, were used for experiments. The colon cancer cell lines were exposed to different concentrations of TMP for 48 h. As shown in Figure 2A, the results showed that TMP suppressed the cell viability in a dose-dependent manner, especially in SW480 and HCT116 cells. Logistic regression analysis was used to calculate the concentration of TMP that resulted in 50% inhibition (IC50) of cell proliferation through CCK-8 assay. The results showed that the IC50 values in SW480 and HCT116 cells were the lowest among all cell lines (Figure 2B). Therefore, the SW480 and HCT116 colon cancer cell lines were selected for further investigation.
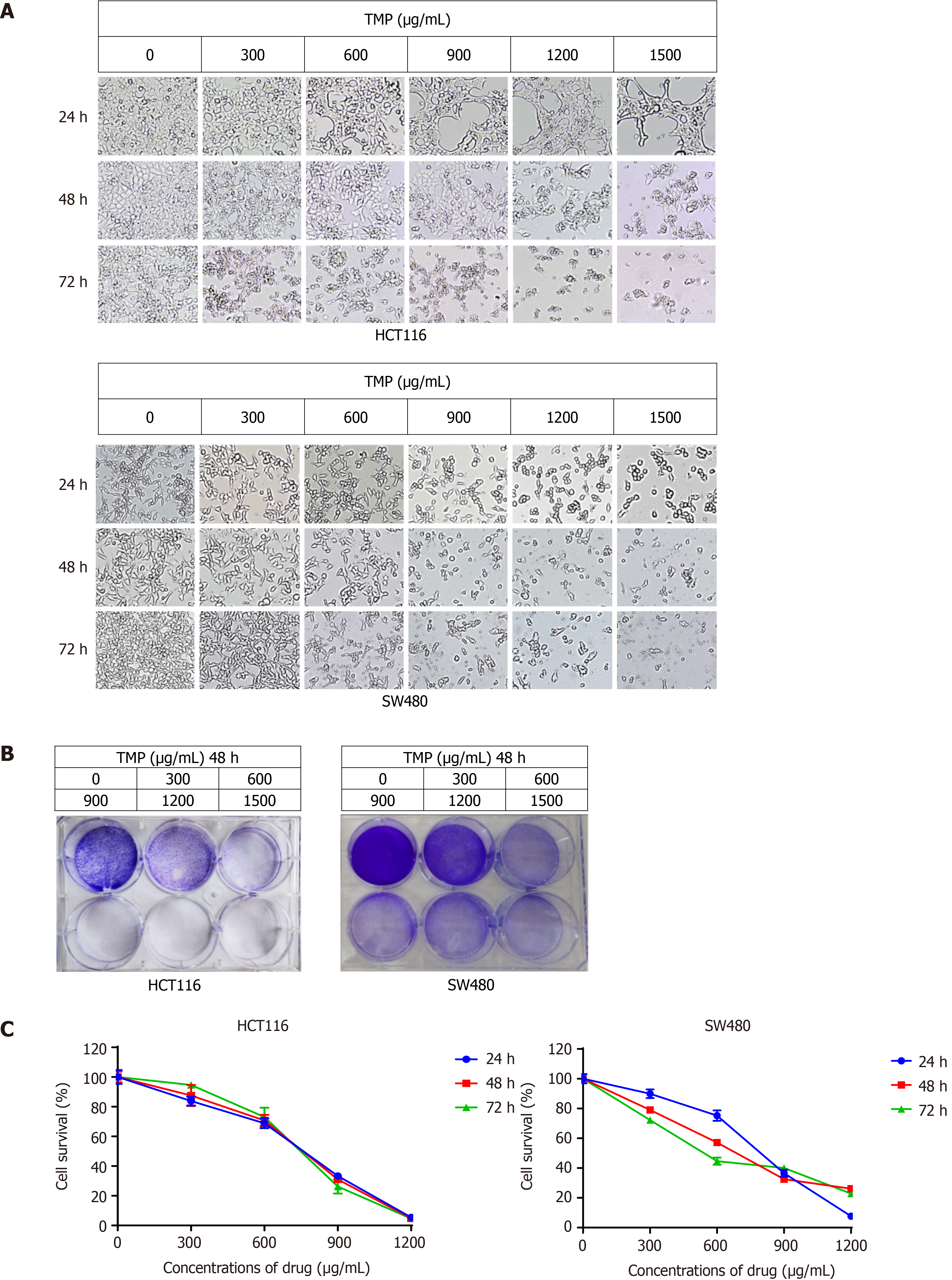
To identify the optimum drug level of TMP in colon cancer cells, we treated colon cancer cell lines SW480 and HCT116 with various concentrations of TMP for 24 h, 48 h, and 72 h. The morphological changes of the SW480 and HCT116 cells were examined under an inverted light microscope. SW480 and HCT116 cell morphology became distorted, round, and fragmented after TMP treatment, especially when the concentration of TMP reached 600 µg/mL (Figure 3A). The results of crystal violet staining of SW480 and HCT116 cells treated with TMP for 48 h were consistent with the microphotograph results (Figure 3B). CCK-8 assays showed that the proliferation and viability of SW480 and HCT116 cells gradually decreased with increasing TMP concentration and prolonged exposure time (Figure 3C). Together, these results show that TMP significantly inhibits colon cancer cell proliferation in a dose- and time-dependent manner.
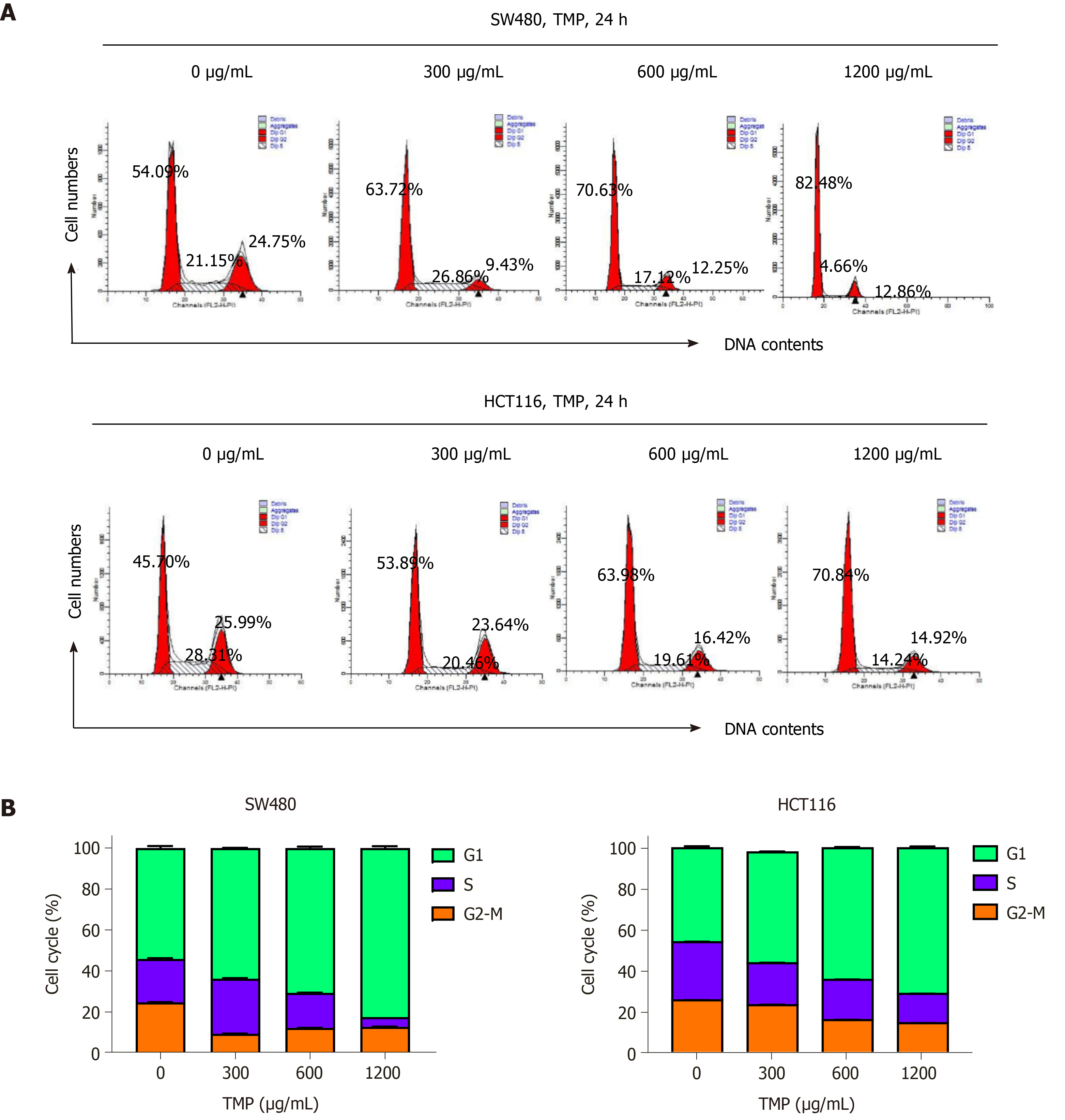
Cell cycle arrest is always accompanied with inhibition of cell proliferation. Based on the proliferation inhibition of SW480 and HCT116 cells treated with TMP, we next tested the cell cycle distribution. Flow cytometry analysis showed that TMP treatment for 24 h increased the G0/G1 phase cell proportion, whereas it decreased the proportion of cells in S phase (Figure 4A). Moreover, a significant increase in the percentage of G1 phase was detected in cells treated with TMP at 600 µg/mL for 24 h (Figure 4B). These data suggest that TMP treatment inhibits the proliferation of colon cancer cells by inducing a G0/G1 phase arrest.
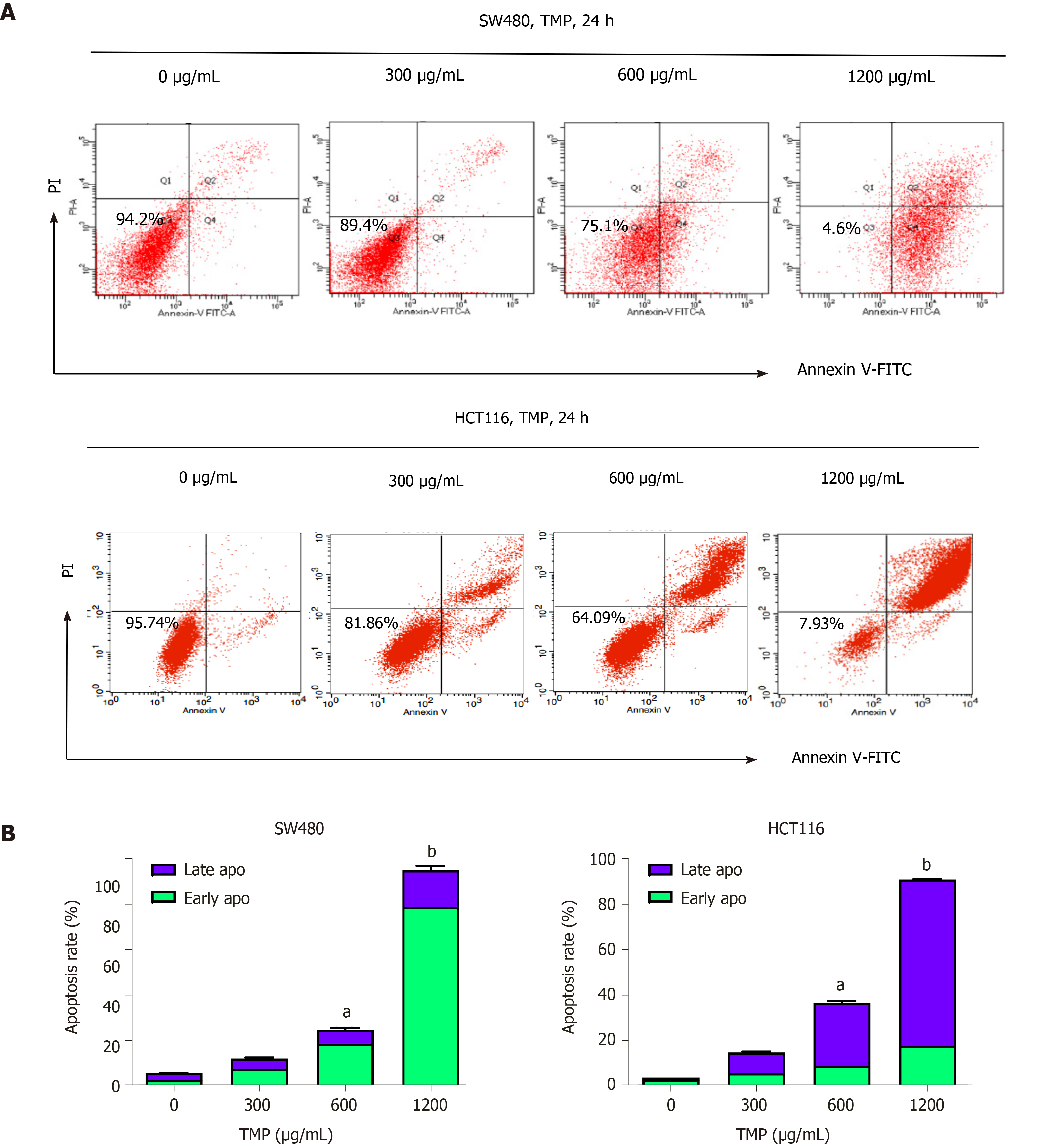
TMP has been reported to induce apoptosis in some cancer cells[14-17]. To determine whether TMP similarly induces colon cancer cell apoptosis, we performed Annexin-V/PI double staining and analyzed cell apoptosis. We treated SW480 and HCT116 cells with different concentrations of TMP for 24 h, and flow cytometry analysis was performed to measure the apoptosis rates. As shown in Figure 5A, SW480 and HCT116 cells treated with TMP showed a marked increase in apoptosis rate, especially when the concentration of TMP reached 600 µg/mL. TMP significantly induced apoptosis of colon cancer cells in a dose-dependent manner. As shown in Figure 5B, we found further evidence that SW480 cells treated with TMP showed increased cell numbers in early apoptosis (Annexin + / PI -, lower right quadrant in Figure 5A), whereas the HCT116 cells showed late apoptosis (Annexin + / PI +, upper right quadrant in Figure 5A).
Colon cancer is one of the most commonly diagnosed cancers worldwide and one of the most fatal malignancies in terms of estimated new cases and estimated deaths[18]. Although surgical resection is the main curative therapy for early stage colon cancer, chemotherapy still has an important role, as many patients have regional or distant spread at the time of diagnosis[19]. However, many patients have died from chemotherapy resistance, disease progression, and recurrence. Progress in improving overall survival has been relatively slow[20]. Hence, searching for effective chemothe
Over the last few decades, plants have been shown to contain a variety of anti-tumor components, and many plant compounds, such as vincristine, paclitaxel, topside, and topotecan, have been used in the clinic to treat cancer[21]. TMP is an alkaloid monomer that exists in the roots or stems of a Chinese herbal medicine plant and is considered to be safe due to its long history of use in Chinese traditional medicine. Recent studies confirmed its anti-tumor effects in lung, breast, ovarian carcinoma, osteosarcoma, gastric carcinoma, leukemia, and hepatocellular carcinoma[22,23]. However, the anti-tumor activity of TMP against colon cancer cells has not been investigated.
In the present study, we examined the potential effects of TMP on six colon cancer cell lines, DLD1, HCT116, LOVO, LS1747, SW480, and SW620. We found that TMP significantly suppressed colon cancer cell viability in a dose-dependent manner, especially in SW480 and HCT116 cells. The IC50 values in SW480 and HCT116 cells were the lowest, and therefore, the SW480 and HCT116 colon cancer cell lines were selected for further investigation.
To examine the effect of TMP in regulating colon cancer cell proliferation, we initially examined the dose-response effect of TMP on cell viability. After treatment with a concentration gradient of TMP, the morphology of SW480 and HCT116 cells became distorted, round, and fragmented, especially when the concentration of TMP reached 600 µg/mL. CCK-8 assays demonstrated that TMP markedly inhibited colon cancer cell proliferation in a dose- and time-dependent manner. Our results are consistent with those of previous reports on the effect of TMP on the proliferation of cancer cells[24] and demonstrate that TMP significantly inhibits the proliferation of colon cancer cells in a dose- and time-dependent manner.
Cell cycle arrest in cancer cells is often accompanied with inhibition of cell proliferation[25]. Uncontrolled cell proliferation is an important biological characteristic that distinguishes tumor cells from ordinary somatic cells[26]. The loss of cell cycle control plays a critical role in tumor development and proliferation[27]. Ji et al[28] showed that TMP inhibited the proliferation of human gastric cancer cell line SGC-7901 and caused G1 phase cell cycle arrest. We performed flow cytometry to examine the effect of TMP on the cell cycle and found that TMP induced a significant cell cycle arrest in the G0/G1 phase, with a decrease in S phase cells. Moreover, there was a significant increase in the percentage of the G0/G1 phase in cells treated with TMP at 600 µg/mL for 24 h. These data suggest that TMP treatment could inhibited the proliferation of colon cancer cells by inducing a G0/G1 cell cycle arrest.
Apoptosis and proliferation are always closely related. Several studies have shown that TMP can induce tumor cell apoptosis[29-31]. We used flow cytometry to examine the effect of TMP on apoptosis and found that HCT116 and SW480 cells treated with TMP showed a marked increase in the proportion of apoptotic cells, especially with TMP at 600 µg/mL. These data suggest that TMP significantly induced apoptosis of colon cancer cells in a dose-dependent manner. Further analysis revealed that TMP treatment caused early stage apoptosis in SW480 cells, whereas it caused late stage apoptosis in HCT116 cells.
In conclusion, our study demonstrated that TMP inhibited the proliferation of colon cancer cells in a dose- and time-dependent manner by inducing apoptosis and arresting the cell cycle at the G0/G1 phase. The results of the present study suggest that TMP may be a novel drug candidate for the treatment of colon cancer patients. Further studies of the mechanisms of TMP in human colon cancer are needed.
Colon cancer is one of the most common malignancies worldwide, and chemotherapy is a widely used strategy in clinical therapy for colon cancer. However, chemotherapy resistance is a major cause of recurrence and disease progression in colon cancer, and thus novel drugs for treatment are urgently needed. Tetramethylpyrazine (TMP), a component of the traditional Chinese medicine Chuanxiong Hort, has been proven to exhibit a beneficial effect in a number of types of malignant tumors.
To investigate the potential anticancer activity of TMP in colon cancer and its underlying mechanisms.
To investigate the potential anticancer activity of TMP in colon cancer and its underlying mechanisms.
Colon cancer cells were incubated with different concentrations of TMP. Cell viability was evaluated by crystal violet staining assay and Cell Counting Kit-8 assay, and cell apoptosis and cell cycle were assessed by flow cytometry.
TMP significantly inhibited the proliferation of colon cancer cells in a dose- and time-dependent manner. In addition, flow cytometry revealed that TMP induced cell cycle arrest at the G0/G1 phase.
Our study demonstrated that TMP inhibits the proliferation of colon cancer cells in a dose- and time-dependent manner by inducing apoptosis and arresting the cell cycle at the G0/G1 phase.
Further studies of the mechanisms of TMP in human colon cancer are needed.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Reiff T S-Editor: Liu M L-Editor: Wang TQ P-Editor: Li JH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55852] [Article Influence: 7978.9] [Reference Citation Analysis (132)] |
| 2. | Wang S, Xia B, Qiao Z, Duan L, Wang G, Meng W, Liu Z, Wang Y, Zhang M. Tetramethylpyrazine attenuated bupivacaine-induced neurotoxicity in SH-SY5Y cells through regulating apoptosis, autophagy and oxidative damage. Drug Des Devel Ther. 2019;13:1187-1196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Pan J, Shang JF, Jiang GQ, Yang ZX. Ligustrazine induces apoptosis of breast cancer cells in vitro and in vivo. J Cancer Res Ther. 2015;11:454-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Yang Q, Huang DD, Li DG, Chen B, Zhang LM, Yuan CL, Huang HH. Tetramethylpyrazine exerts a protective effect against injury from acute myocardial ischemia by regulating the PI3K/Akt/GSK-3β signaling pathway. Cell Mol Biol Lett. 2019;24:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Wu X, Zhang F, Xiong X, Lu C, Lian N, Lu Y, Zheng S. Tetramethylpyrazine reduces inflammation in liver fibrosis and inhibits inflammatory cytokine expression in hepatic stellate cells by modulating NLRP3 inflammasome pathway. IUBMB Life. 2015;67:312-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Zhang C, Teng F, Tu J, Zhang D. Ultrasound-enhanced protective effect of tetramethylpyrazine against cerebral ischemia/reperfusion injury. PLoS One. 2014;9:e113673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Kim M, Kim SO, Lee M, Lee JH, Jung WS, Moon SK, Kim YS, Cho KH, Ko CN, Lee EH. Tetramethylpyrazine, a natural alkaloid, attenuates pro-inflammatory mediators induced by amyloid β and interferon-γ in rat brain microglia. Eur J Pharmacol. 2014;740:504-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Xu D, Chi G, Zhao C, Li D. Ligustrazine Inhibits Growth, Migration and Invasion of Medulloblastoma Daoy Cells by Up-Regulation of miR-211. Cell Physiol Biochem. 2018;49:2012-2021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Huang HH, Liu FB, Ruan Z, Zheng J, Su YJ, Wang J. Tetramethylpyrazine (TMPZ) triggers S-phase arrest and mitochondria-dependent apoptosis in lung cancer cells. Neoplasma. 2018;65:367-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Zhou Y, Ji Z, Yan W, Zhou Z, Li H, Xiao Y. Tetramethylpyrazine inhibits prostate cancer progression by downregulation of forkhead box M1. Oncol Rep. 2017;38:837-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Han J, Song J, Li X, Zhu M, Guo W, Xing W, Zhao R, He X, Liu X, Wang S, Li Y, Huang H, Xu X. Ligustrazine Suppresses the Growth of HRPC Cells through the Inhibition of Cap- Dependent Translation Via Both the mTOR and the MEK/ERK Pathways. Anticancer Agents Med Chem. 2015;15:764-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Yi B, Liu D, He M, Li Q, Liu T, Shao J. Role of the ROS/AMPK signaling pathway in tetramethylpyrazine-induced apoptosis in gastric cancer cells. Oncol Lett. 2013;6:583-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Zheng J, Ma LT, Ren QY, Hu Y, Bai Y, Bian H, Zhang Y, Zhou YC, Yang MH. Anti-fibrotic effects of Salvia miltiorrhiza and Ligustrazine Injection on LX-2 cells involved with increased N-myc downstream-regulated gene 2 expression. Chin J Integr Med. 2017;23:923-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Wang K, Zhu X, Zhang K, Zhou F, Zhu L. Neuroprotective effect of tetramethylpyrazine against all-trans-retinal toxicity in the differentiated Y-79 cells via upregulation of IRBP expression. Exp Cell Res. 2017;359:120-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Yin J, Yu C, Yang Z, He JL, Chen WJ, Liu HZ, Li WM, Liu HT, Wang YX. Tetramethylpyrazine inhibits migration of SKOV3 human ovarian carcinoma cells and decreases the expression of interleukin-8 via the ERK1/2, p38 and AP-1 signaling pathways. Oncol Rep. 2011;26:671-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Wang Y, Fu Q, Zhao W. Tetramethylpyrazine inhibits osteosarcoma cell proliferation via downregulation of NF-κB in vitro and in vivo. Mol Med Rep. 2013;8:984-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11573] [Cited by in RCA: 13170] [Article Influence: 1881.4] [Reference Citation Analysis (4)] |
| 18. | Gelibter AJ, Caponnetto S, Urbano F, Emiliani A, Scagnoli S, Sirgiovanni G, Napoli VM, Cortesi E. Adjuvant chemotherapy in resected colon cancer: When, how and how long? Surg Oncol. 2019;30:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Chakrabarti S, Peterson CY, Sriram D, Mahipal A. Early stage colon cancer: Current treatment standards, evolving paradigms, and future directions. World J Gastrointest Oncol. 2020;12:808-832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 91] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 20. | Zhao CP, Xu ZJ, Guo Q, Li YX, Gao XZ, Peng YY. Overexpression of suppressor of IKBKE 1 is associated with vincristine resistance in colon cancer cells. Biomed Rep. 2016;5:585-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Szychowski J, Truchon JF, Bennani YL. Natural products in medicine: transformational outcome of synthetic chemistry. J Med Chem. 2014;57:9292-9308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Shen J, Zeng L, Pan L, Yuan S, Wu M, Kong X. Tetramethylpyrazine regulates breast cancer cell viability, migration, invasion and apoptosis by affecting the activity of Akt and caspase-3. Oncol Lett. 2018;15:4557-4563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Zheng CY, Xiao W, Zhu MX, Pan XJ, Yang ZH, Zhou SY. Inhibition of cyclooxygenase-2 by tetramethylpyrazine and its effects on A549 cell invasion and metastasis. Int J Oncol. 2012;40:2029-2037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Wang XJ, Xu YH, Yang GC, Chen HX, Zhang P. Tetramethylpyrazine inhibits the proliferation of acute lymphocytic leukemia cell lines via decrease in GSK-3β. Oncol Rep. 2015;33:2368-2374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17:93-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1413] [Cited by in RCA: 1377] [Article Influence: 172.1] [Reference Citation Analysis (0)] |
| 26. | McLaughlin F, Finn P, La Thangue NB. The cell cycle, chromatin and cancer: mechanism-based therapeutics come of age. Drug Discov Today. 2003;8:793-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Liu Y, Cao Y, Zhang W, Bergmeier S, Qian Y, Akbar H, Colvin R, Ding J, Tong L, Wu S, Hines J, Chen X. A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther. 2012;11:1672-1682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 434] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 28. | Ji AJ, Liu SL, Ju WZ, Huang XE. Anti-proliferation effects and molecular mechanisms of action of tetramethypyrazine on human SGC-7901 gastric carcinoma cells. Asian Pac J Cancer Prev. 2014;15:3581-3586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Bi L, Yan X, Chen W, Gao J, Qian L, Qiu S. Antihepatocellular Carcinoma Potential of Tetramethylpyrazine Induces Cell Cycle Modulation and Mitochondrial-Dependent Apoptosis: Regulation of p53 Signaling Pathway in HepG2 Cells In Vitro. Integr Cancer Ther. 2016;15:226-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Sun X, Liao W, Wang J, Wang P, Gao H, Wang M, Xu C, Zhong Y, Ding Y. CSTMP induces apoptosis and mitochondrial dysfunction in human myeloma RPMI8226 cells via CHOP-dependent endoplasmic reticulum stress. Biomed Pharmacother. 2016;83:776-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Cao J, Miao Q, Zhang J, Miao S, Bi L, Zhang S, Yang Q, Zhou X, Zhang M, Xie Y, Wang S. Inhibitory effect of tetramethylpyrazine on hepatocellular carcinoma: possible role of apoptosis and cell cycle arrest. J Biol Regul Homeost Agents. 2015;29:297-306. [PubMed] |