Published online Jun 16, 2021. doi: 10.12998/wjcc.v9.i17.4423
Peer-review started: February 9, 2021
First decision: February 28, 2021
Revised: March 9, 2021
Accepted: March 25, 2021
Article in press: March 25, 2021
Published online: June 16, 2021
Processing time: 105 Days and 17.9 Hours
Paraneoplastic cerebellar degeneration (PCD), which is rare in clinical practice, is closely related to autoimmunity. Cases positive for anti-Yo antibodies (anti-Purkinje cytoplasmic antibody 1) are the main subtype of PCD. PCD is subacute cerebellar degeneration, and while it progresses over weeks to months, its resultant deficits last much longer. Cancer patients with anti-Yo antibody-positive PCD are very rare. Most of them are breast cancer or ovarian cancer patients but also occasionally lung cancer patients.
A 61-year-old woman presented with sudden vertigo, nausea, and vomiting for approximately 10 d. The patient's neurological examination showed torsion with downbeat nystagmus and ataxia of the right limb and trunk. Laboratory examination found that the patient's cerebrospinal fluid and serum were anti-Yo antibody-positive, positron emission tomography computed tomography showed an increased metabolic rate in the retroperitoneal lymph nodes, and the pathology of lymph node punctures in the retroperitoneum and neck suggested adenocarcinoma of the pancreaticobiliary duct, which strengthens the hypothesis of paraneoplastic origin. Intravenous immunoglobulin (IVIg) 0.4 g/kg/d for 5 d and methylprednisolone 160 mg for 3 d were initiated, which was reduced to 80 mg for 3 d and then to 40 mg for 7 d. After treatment with IVIg and a steroid, the patient's vertigo and ataxia alleviated.
The patient's vertigo and ataxia alleviated after treatment, suggesting that early immunotherapeutic intervention may have certain value in stopping neurological loss.
Core Tip: We report for the first time a female patient with anti-Yo antibody-positive paraneoplastic cerebellar degeneration (PCD), who was later diagnosed with possible cholangiocarcinoma. She presented with sudden vertigo, nausea, and vomiting for approximately 10 d. The brain magnetic resonance imaging examination showed no obvious abnormalities. Finally, the pathology of lymph node punctures in the retroperitoneum and neck suggested adenocarcinoma of the pancreaticobiliary duct. The patient's symptoms alleviated with intravenous immunoglobulin and steroid treatment. This case highlighted that early immunotherapeutic intervention may stop and reverse neurological loss in PCD.
- Citation: Lou Y, Xu SH, Zhang SR, Shu QF, Liu XL. Anti-Yo antibody-positive paraneoplastic cerebellar degeneration in a patient with possible cholangiocarcinoma: A case report and review of the literature. World J Clin Cases 2021; 9(17): 4423-4432
- URL: https://www.wjgnet.com/2307-8960/full/v9/i17/4423.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i17.4423
Paraneoplastic neurological syndrome (PNS) refers to a syndrome of "distant" nervous system damage caused by tumors[1], the incidence of PNS was 1/100000 person-years, and the prevalence was 4/100000 or 1 PNS in every 334 cancers[2]. The current academic consensus is to classify paraneoplastic cerebellar degeneration (PCD) as a "classic" PNS. PCD[3] is rare in the clinic and closely related to autoimmunity. At present, nearly 30 different autoantibodies have been reported to be related to PCD, including anti-Yo antibodies, anti-Tr antibodies, anti-Hu antibodies, and anti-Ma antibodies. Anti-Yo-positive cases are the main subtype of PCD, accounting for nearly 50% of all cases[4]. At present, more than 90% of patients with cerebellar ataxia and anti-Yo antibodies have been diagnosed with cancer. Most of these patients are breast cancer or ovarian cancer patients, with occasional lung cancer or Hodgkin's disease patients[5]. However, cholangiocarcinoma with anti-Yo antibody-positive PCD is rare. To our knowledge, we are the first to report cholangiocarcinoma with anti-Yo anti
A 61-year-old woman was referred to Affiliated Zhejiang Hospital, Zhejiang University School of Medicine due to sudden vertigo with nausea and vomiting for 10 d.
Ten day prior to referral to our hospital, the patient suffered from vertigo. The vertigo was persistent, preventing her from opening her eyes. She felt dizzy when she opened her eyes, which was accompanied by nausea and vomiting. The vertigo became worse when her posture changed, and her symptoms were relieved when lying on her left side, while the symptoms were worse when she turned her head to the right. The patient had no palpitations, tinnitus, hearing loss, headache, limb weakness, numbness, limb convulsions, vague speech, or consciousness loss. She went to a nearby hospital and underwent a computed tomography (CT) scan of the brain, which showed no obvious abnormalities. She was treated with Betahistine, but her vertigo did not relieve.
She had a history of hypertension. She underwent "pancreatic tumor resection" more than 20 years ago and recovered well after surgery.
There was no history of dizziness, headache, or tumor in the family.
The patient's neurological examination showed spontaneous downbeat nystagmus, gaze in all directions showed torsion with downbeat nystagmus, and nystagmus was stronger when she gazed to the right. The patient had clear speech and normal hearing. Her right finger-to-nose and heel-to-shin tests were unstable, her limb muscle strength was grade 5, her limb tendon reflex was (+), Romberg's sign could not be completed, and the bedside head impulse test was negative.
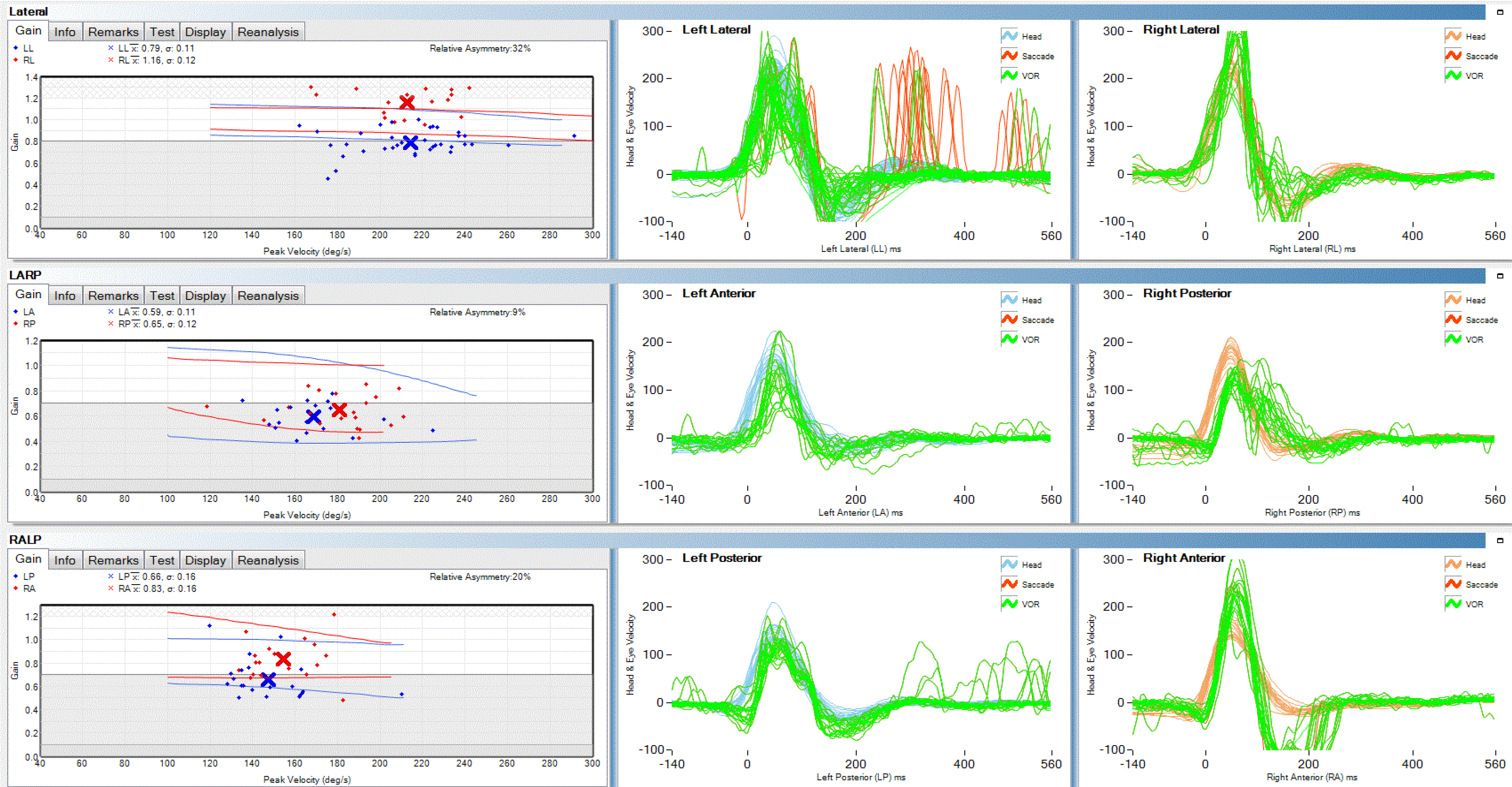
Laboratory investigation revealed hemoglobin 147 g/L, white blood cell count (WBC) 9.3 × 109/L (N 70.8, L 21.8, M 7%), platelet count 119 × 109/L, sodium 139.79 mmol/L, potassium 3.72 mmol/L, chloride 103.1 mmol/L, glucose 4.3 mmol/L, TSH 1.487 μU/mL, FT 41.41 ng/dL, FT3 2.79 pg/mL, anti-thyroglobulin < 15, anti-thyroid peroxidase < 28 IU/mL, C3 1.25 and C4 3.8 g/L, folic acid 12.39 nmol/L, and vitamin B12 284 pmol/L. However, the level of the tumor marker carbohydrate antigen 125 (CA125) was significantly increased; her CA125 level was 332.50 U/mL (the normal level of CA125 is < 35 U/mL); and the level of the tumor marker carbohydrate antigen 199 (CA199) was 21.63 U/mL (the normal level of CA199 is < 39 U/mL), which was normal. Next, we performed a lumbar puncture on this patient, and cerebrospinal fluid (CSF) analysis revealed a colourless fluid with CSF pressure 100 mmH2O, WBC 0 cells/mm3, protein 0.31 g/L, and glucose 5.0 mmol/L. Serum and CSF analysis of paratumor antibodies and autoimmune cerebellitis antibodies showed that the patient was positive for anti-Yo antibody, through the immunospot assay. The video head pulse test (vHIT) results suggested that the left semicircular canal gain, which was used to evaluate vestibulo-ocular reflex function, was slightly lower than the right gain (Figure 1).
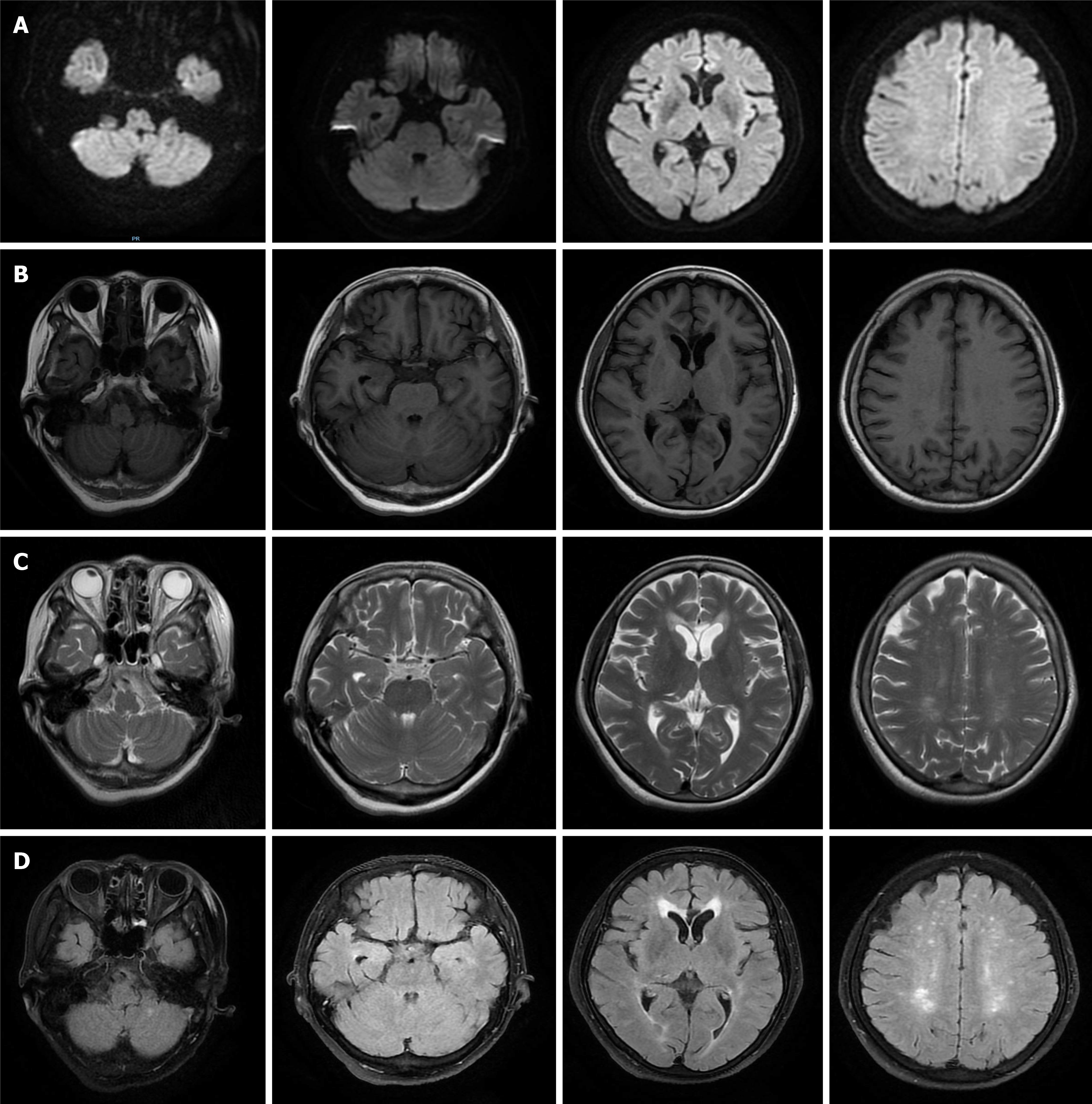
Because the patient had a history of hypertension, which is a risk factor for cerebrovascular disease, and signs of ataxia, although there was no obvious abnormality on her skull CT, central vertigo was still considered, and cerebral infarction was considered first. Therefore, the patient underwent a brain magnetic resonance imaging (MRI) examination that showed no obvious abnormalities (Figure 2). Then she underwent a brainstem magnetic resonance scan that also showed no obvious abnormalities.
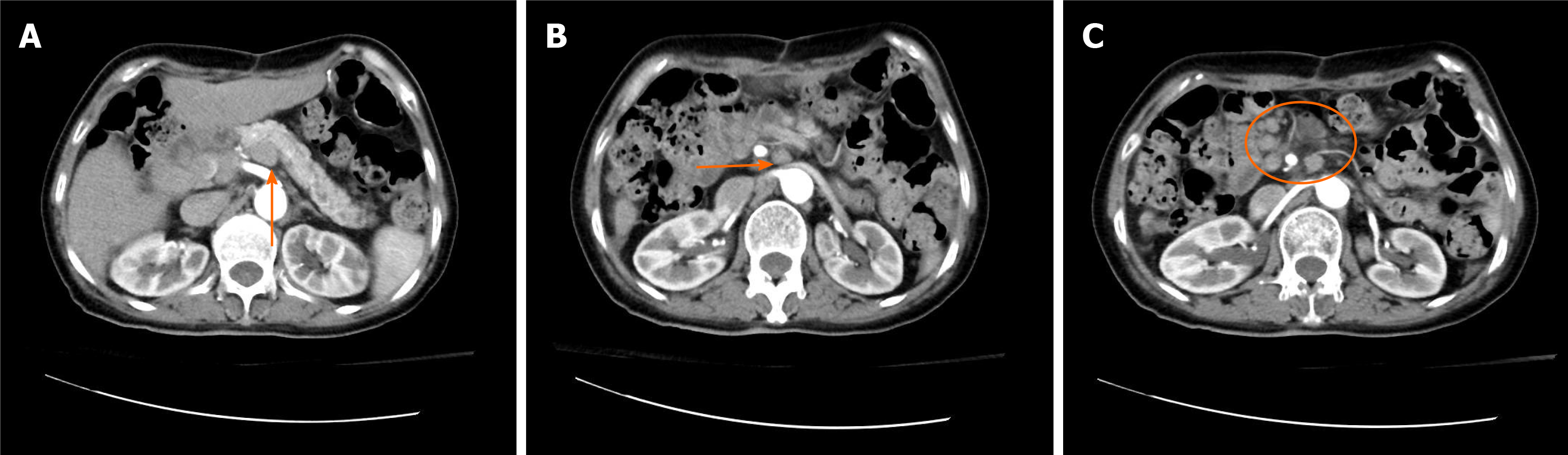
Based on the results of the serum and CSF paratumor antibodies tests, we looked for the primary tumor. Abdominal para-aortic lymph node B-ultrasound indicated multiple sites of peritoneal lymphadenopathy. Subsequently, positron emission tomography-CT examination was performed, which revealed that the patient had increased fluorodeoxyglucose metabolism in the porta hepatis region, mesenteric roots, and back of the pancreas and multiple retroperitoneal soft tissue nodules and enlarged lymph nodes that were considered malignant. Contrast-enhanced abdominal CT examination revealed multiple enlarged lymph nodes in the retroperitoneum and porta hepatis region, which might be a metastatic tumor (Figure 3). Because of severe nausea and vomiting, the patient and her family members worried that the patient could not tolerate endoscopic retrograde cholangiopancreatography (ERCP) examination, then refused to take ERCP and magnetic resonance cholangiopancreatography (MRCP) examinations. Therefore, the patient did not receive endoscopic ultrasound, MRCP, and ERCP examinations.
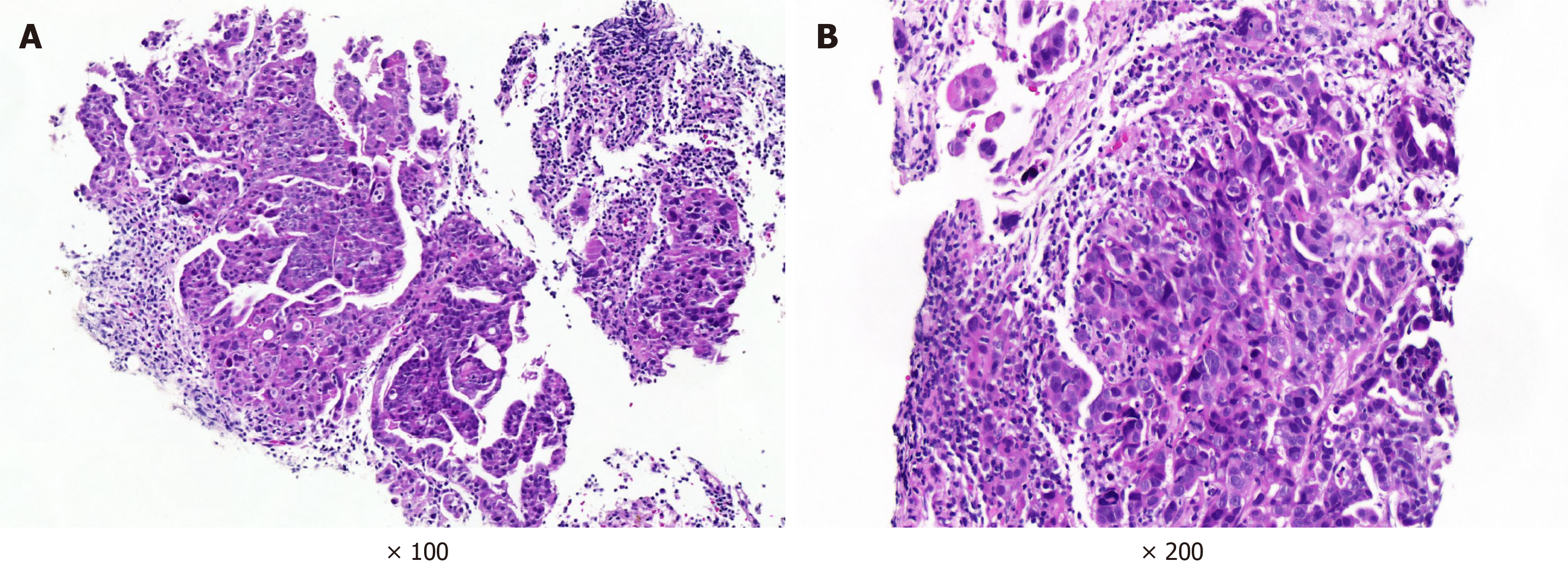
Then, we performed an ultrasound-guided lymph node puncture on this patient and biopsied the posterior peritoneal and cervical lymph nodes. Pathological results suggested metastatic cancer, and pancreaticobiliary duct origin was considered. The immunohistochemical findings were as follows: Cytokeratin (CK) 7 (+), CK19 (+), CK20 (-), caudal-related homeobox transcription factor 2 (CDX2) (-), Ki67 (+; 40%), and carcinoembryonic antigen (CEA) (-) (left cervical lymph node pathology); and CK7 (+), CK19 (+), CK20 (-), CDX2 (-), Ki67 (+; 40%), and CEA (partial +) (retroperitoneal lymph node pathology) (Figure 4).We reviewed the pathology of the "pancreatic tumor" resected from the patient more than 20 years ago. The pathological examination result was a grade II duodenal ampulla bile duct adenocarcinoma.
The sudden vertigo that presented in this patient belonged to the category of acute vestibular syndrome. The vHIT showed that the left horizontal semicircular canal, anterior semicircular canal, and posterior semicircular canal gains were lower than the right gains, and the left horizontal semicircular canal was accompanied by saccade. The patient had an acute onset, and the semicircular canal gain of the vHIT test was low, so her diagnosis was somewhat confusing, as the symptoms could also indicate peripheral vestibular system diseases such as vestibular neuritis. However, the patient had ataxia of the right limb and trunk at the same time, and the symptoms continued to progress for more than 10 d, which did not match the manifestations of vestibular neuronitis. Therefore, we believed that the cause was positioned in the central vestibular system. Because the onset of this patient was acute, and there was a history of hypertension, which is a risk factor for cerebrovascular disease, we first considered cerebrovascular disease, but cerebellar hemorrhage, infarction, and metastasis were excluded by skull CT/MRI examinations, and the thin-layer MRI scan of the brainstem that she received later also did not reveal any infarcts. Due to the central compensation mechanism, patients with cerebral infarction will improve after the acute phase (about 7 d). However, the patient's symptoms continued to develop after 10 d, which did not match the manifestations of cerebrovascular disease.
Subsequently, we found a significant increase in CA125 and positive anti-YO antibodies in serum and cerebrospinal fluid, then the patient was diagnosed with anti-YO positive PCD. Finally, through lymph node puncture, the patient’s pathology suggested adenocarcinoma of pancreaticobiliary duct origin, therefore, this patient was diagnosed as possible anti-Yo antibody-positive PCD with cholangiocarcinoma.
For treatment, we administered 0.4 g/kg/d IVIg intravenously for 5 d and simultaneously administered 160 mg methylprednisolone intravenously for 3 d, which was reduced to 80 mg administered intravenously for 3 d and then to 40 mg administered intravenously for 7 d. Based on her pathological results, the patient received anti-tumor treatment. Because of severe nausea and vomiting, the patient could not tolerate to gemcitabine based chemotherapy, and was treated with anlotinib hydrochloride (a targeted drug).
Beginning on the third day of treatment with IVIg, the patient's symptoms of vertigo and vomiting eased, she was able to stand and walk with help, the modified rankin scale (MRS) score changed from 5 to 4. Her symptoms relieved (MRS score 4) for about 2 mo, and she was alive now at 16 mo after the onset of the disease, but was bedrid
Anti-Yo antibody-positive PCD usually presents as symptoms of subacute cerebellar degeneration. The main clinical manifestation is cerebellar ataxia of the trunk and limbs, which lasts for weeks to months[4]. If the patient has dysarthria, nystagmus, diplopia, and other symptoms that indicate brainstem involvement, without any intervention, symptom development will peak within 6 mo[6]. PCD with acute onset is relatively rare[7,8], Vogrig et al[7]reported two cases of acute stroke-like onset of PCD, similar to the onset of our patient. However, currently accurate explanations for the variability of the onset and progression of PCD are lacking. Recent studies have found that large-scale cerebellar inflammatory cytokines are produced in the PCD mouse model[9], and this inflammatory response can induce Purkinje cell death at an early stage[10].
In the early stages of PCD with anti-Yo antibody positivity, magnetic resonance examinations of the brain are usually normal, and cerebellar atrophy usually appears in the late stages of disease[4].The majority of patients with anti-Yo antibody-positive cerebellar ataxia are eventually diagnosed with cancer. The most common tumors are breast and ovarian cancers, followed by occasional lung cancer and Hodgkin's disease[5]. A retrospective survey by Monstad et al[11] and others found that the rates of anti-Yo antibody positivity in 557 ovarian cancer patients and 253 breast cancer patients were 2.3% and 1.6%, respectively, but only 12% of the anti-Yo antibody-positive patients had PCD. However, there have been relatively few reports of PCD with cholangiocarcinoma. At present, only Bruhnding et al[12] reported the case of a male PCD patient with cholangiocarcinoma; the patient received IVIg treatment, but the ataxia did not improve, and given this poor overall prognosis, the patient declined further treatment. We reported for the first time that a female PCD patient who was probably caused by cholangiocarcinoma.
Our patient's CA125 was significantly higher. Elevated CA125 is most common in ovarian cancer, and there is also a certain positive rate in other non-ovarian malignancies[13]. It has been shown that serum CA125 levels are elevated in patients with cholangiocarcinoma, and the specificity of elevated CA125 in diagnosing cholangiocarcinoma is 79.2%, and CA125 is barely elevated in patients with benign biliary tract diseases[14].
Subsequently, we performed a lumbar puncture on the patient and found specific onconeural anti-Yo antibody positivity through serum and cerebrospinal fluid tests. Finally, biopsy of the lymph node was consistent with a pancreaticobiliary duct tumor. However, as mentioned above, PCD often occurs in patients with breast or ovarian cancer. In this case, there is no evidence of breast cancer or ovarian cancer. Follow-up still needs to monitor whether there are related cancers.
PCD mainly involves the vermis and midline structures of the cerebellum in the early stage, and under physiological conditions, the midline structure of the cerebellum mainly participates in the integration of otolith and semicircular canal signals[15], controls the otolith-ocular reflex, and participates in the regulation of the semicircular canal-ocular reflex. When the midline structure of the cerebellum is dysfunctional, the integration of otolith and semicircular canal signals becomes dysfunctional. The imbalance in the integration of otolith and semicircular canal signals may lead to differences in the regulation of semicircular canal function on both sides[16], which may be the reason for the higher asymmetry of semicircular canal gains in this patient seen by the vHIT.
The exact mechanism of Purkinje cell death in anti-Yo antibody-positive PCD is unknown and may be related to the activation of CD8+ T cells. Early pathological changes in anti-Yo antibody-positive PCD include infiltration of lymphocytes around the blood vessels, activation of microglia, and infiltration of CD8+ T lymphocytes into the cerebellar Purkinje cell layer[17,18]. With disease progression, the pathological manifestation of PCD is mainly large and rapid loss of noninflammatory Purkinje cells[19,20]. An autopsy study on two Yo antibody-positive PCD patients found that both patients had extensive loss of Purkinje cells, activated microglia, and CD8+ T cells infiltration in all parts of the cerebellum, and it was found that despite the long disease duration, there were still surviving Purkinje cells[21].
In terms of treatment, as PCD cases are rare and it is difficult to design randomized controlled trials, the currently reported treatments for PCD also lack a foundation in evidence-based medicine. In addition, currently, research on anti-Yo antibody-positive PCD has not found promising and consistent treatments. Immunotherapy is currently controversial. Vernino[22] reported no clinical benefit in 23 patients receiving immunosuppressive therapy, such as corticosteroids, plasma exchange (PLEX), and IVIg[22]. However, there are reports that immunotherapy at the beginning of symptoms can control the development of the disease[23]. Rojas et al[24] reported that one of the 22 patients treated with PLEX showed a moderate clinical benefit. One of the 17 patients treated with high doses of steroids experienced a slight, transient improvement, while Shams'ili et al[25] reported improvement in 2 out of 6 patients receiving immunosuppressive therapy. In our patient, the symptoms of vertigo, vomiting, and ataxia of the trunk were moderately relieved after IVIg and methylprednisolone treatment, and she was able to stand and walk with help. Immunotherapy had a moderate benefit in her, and clinical benefit may be due to the removal of pathological antibodies that had not been found to date through immunotherapy[6]. In addition, guidelines from the Agency for Healthcare Research and Quality recommend early antitumor therapy, in addition to immunotherapy, as the approach that offers the greatest chance for PNS stabilization[6]. According to the research of Shams'ili et al[25], antitumor therapy combined with immunotherapy improved and sustained the condition of 2 out of 6 patients with PCD[25], but other studies have shown that antitumor treatment has no clear effects[24,26]. According to our pathological results, our patient, who received a targeted drug (anlotinib hydrochlo
However, unlike other paraneoplastic syndromes, anti-Yo antibody-associated PCD is unlikely to respond significantly to the removal of the occult tumor. Disability progression of anti-Yo antibody-positive PCD results in < 10% of patients being able to walk unassisted for a long period of time, and most patients end up being bedridden. Anti-Yo antibody-positive patients have a median survival time of 13 mo, which is higher than that for anti-Hu syndrome patients[6,25]. In addition, some studies have shown that the prognosis of breast cancer combined with anti-Yo antibody-positive PCD is better than that of ovarian cancer. The former has a survival time of 100 mo, while the latter has a survival time of only 22 mo[24,29]. Early treatment is important to improve the prognosis of disease. Widdess-Walsh et al[30] reviewed 15 cases of anti-Yo antibody-positive PCD patients treated with IVIg and found that the best prognosis was achieved when treatment was administered within 1 mo of the onset of symptoms. In this case, the patient was given IVIg and methylprednisolone half a month after symptom onset. After treatment, she was able to stand and walk with help, and she was alive at 16 mo after the onset of the disease, but was bedridden. The patient's improvement may be due to immunotherapy before extensive Purkinje cell loss; as a result of intervention, cerebellar Purkinje cells are preserved, and neurolo
To our knowledge, this is the first report on female cholangiocarcinoma with anti-Yo antibody-positive PCD. The patient in this report had a moderate therapeutic response to IVIg, a steroid, and a tumor-targeted drug, and the patient’s duration of improvement (able to standing) was 2 mo. Afterwards, due to economic reasons, she did not continue IVIg therapy, but was treated with a tumor-targeted drug. Currently, she is still alive 16 mo after the onset, but is bedridden. The moderate therapeutic response suggested that early immunotherapy is of great value in stopping and reversing neurological loss.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huguet JM, Sempokuya T S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Li JH
| 1. | Rahman SU, Sana MK, Tahir Z, Ali A, Shah PA. Paraneoplastic syndromes in cholangiocarcinoma. World J Hepatol. 2020;12:897-907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Vogrig A, Gigli GL, Segatti S, Corazza E, Marini A, Bernardini A, Valent F, Fabris M, Curcio F, Brigo F, Iacono D, Passadore P, Rana M, Honnorat J, Valente M. Epidemiology of paraneoplastic neurological syndromes: a population-based study. J Neurol. 2020;267:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 3. | Höftberger R, Lassmann H. Immune-mediated disorders. Handb Clin Neurol. 2017;145:285-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Vatankulu B, Yilmaz Aksoy S, Asa S, Sager S, Sayman HB, Halac M, Sonmezoglu K. Accuracy of FDG-PET/CT and paraneoplastic antibodies in diagnosing cancer in paraneoplastic neurological syndromes. Rev Esp Med Nucl Imagen Mol. 2016;35:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B, Grisold W, Honnorat J, Smitt PS, Vedeler Ch, Verschuuren JJ, Vincent A, Voltz R. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75:1135-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1122] [Cited by in RCA: 1109] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 6. | Venkatraman A, Opal P. Paraneoplastic cerebellar degeneration with anti-Yo antibodies - a review. Ann Clin Transl Neurol. 2016;3:655-663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Vogrig A, Bernardini A, Gigli GL, Corazza E, Marini A, Segatti S, Fabris M, Honnorat J, Valente M. Stroke-Like Presentation of Paraneoplastic Cerebellar Degeneration: a Single-Center Experience and Review of the Literature. Cerebellum. 2019;18:976-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Mitoma H, Adhikari K, Aeschlimann D, Chattopadhyay P, Hadjivassiliou M, Hampe CS, Honnorat J, Joubert B, Kakei S, Lee J, Manto M, Matsunaga A, Mizusawa H, Nanri K, Shanmugarajah P, Yoneda M, Yuki N. Consensus Paper: Neuroimmune Mechanisms of Cerebellar Ataxias. Cerebellum. 2016;15:213-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 9. | Yshii LM, Gebauer CM, Pignolet B, Mauré E, Quériault C, Pierau M, Saito H, Suzuki N, Brunner-Weinzierl M, Bauer J, Liblau R. CTLA4 blockade elicits paraneoplastic neurological disease in a mouse model. Brain. 2016;139:2923-2934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 10. | Panja D, Vedeler CA, Schubert M. Paraneoplastic cerebellar degeneration: Yo antibody alters mitochondrial calcium buffering capacity. Neuropathol Appl Neurobiol. 2019;45:141-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Monstad SE, Storstein A, Dørum A, Knudsen A, Lønning PE, Salvesen HB, Aarseth JH, Vedeler CA. Yo antibodies in ovarian and breast cancer patients detected by a sensitive immunoprecipitation technique. Clin Exp Immunol. 2006;144:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Bruhnding A, Notch D, Beard A. Anti-Yo positive paraneoplastic cerebellar degeneration in the setting of cholangiocarcinoma. J Clin Neurosci. 2017;36:71-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Funston G, Hamilton W, Abel G, Crosbie EJ, Rous B, Walter FM. The diagnostic performance of CA125 for the detection of ovarian and non-ovarian cancer in primary care: A population-based cohort study. PLoS Med. 2020;17:e1003295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 14. | Qiu Y, He J, Chen X, Huang P, Hu K, Yan H. The diagnostic value of five serum tumor markers for patients with cholangiocarcinoma. Clin Chim Acta. 2018;480:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Laurens J, Angelaki DE. Simple spike dynamics of Purkinje cells in the macaque vestibulo-cerebellum during passive whole-body self-motion. Proc Natl Acad Sci USA. 2020;117:3232-3238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Shaikh AG, Ghasia FF, Dickman JD, Angelaki DE. Properties of cerebellar fastigial neurons during translation, rotation, and eye movements. J Neurophysiol. 2005;93:853-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Small M, Treilleux I, Couillault C, Pissaloux D, Picard G, Paindavoine S, Attignon V, Wang Q, Rogemond V, Lay S, Ray-Coquard I, Pfisterer J, Joly F, Du Bois A, Psimaras D, Bendriss-Vermare N, Caux C, Dubois B, Honnorat J, Desestret V. Genetic alterations and tumor immune attack in Yo paraneoplastic cerebellar degeneration. Acta Neuropathol. 2018;135:569-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 18. | Carpenter EL, Vance BA, Klein RS, Voloschin A, Dalmau J, Vonderheide RH. Functional analysis of CD8+ T cell responses to the onconeural self protein cdr2 in patients with paraneoplastic cerebellar degeneration. J Neuroimmunol. 2008;193:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | van Coevorden-Hameete MH, de Graaff E, Titulaer MJ, Hulsenboom E, Sabater L, Hoogenraad CC, Sillevis Smitt PA. Plasticity-related gene 5: A novel surface autoantigen in paraneoplastic cerebellar degeneration. Neurol Neuroimmunol Neuroinflamm. 2015;2:e156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Klein JP, Stover DG, Oxnard GR, Levy BD, Loscalzo J. Restoring Balance: Paraneoplastic Cerebellar Degeneration. Am J Med. 2017;130:e85-e87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Storstein A, Krossnes BK, Vedeler CA. Morphological and immunohistochemical characterization of paraneoplastic cerebellar degeneration associated with Yo antibodies. Acta Neurol Scand. 2009;120:64-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Vernino S. Paraneoplastic cerebellar degeneration. Handb Clin Neurol. 2012;103:215-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Tsuboguchi S, Yajima R, Higuchi Y, Ishikawa M, Kawachi I, Koyama Y, Nishizawa M. A case of slowly progressive anti-Yo-associated paraneoplastic cerebellar degeneration successfully treated with antitumor and immunotherapy. Rinsho Shinkeigaku. 2016;56:477-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Rojas I, Graus F, Keime-Guibert F, Reñé R, Delattre JY, Ramón JM, Dalmau J, Posner JB. Long-term clinical outcome of paraneoplastic cerebellar degeneration and anti-Yo antibodies. Neurology. 2000;55:713-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 199] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Shams'ili S, Grefkens J, de Leeuw B, van den Bent M, Hooijkaas H, van der Holt B, Vecht C, Sillevis Smitt P. Paraneoplastic cerebellar degeneration associated with antineuronal antibodies: analysis of 50 patients. Brain. 2003;126:1409-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 345] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 26. | Bradley WH, Dottino PR, Rahaman J. Paraneoplastic cerebellar degeneration in ovarian carcinoma: case report with review of immune modulation. Int J Gynecol Cancer. 2008;18:1364-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Raibagkar P, Ho D, Gunturu KS, Srinivasan J. Worsening of anti-Hu paraneoplastic neurological syndrome related to anti-PD-1 treatment: Case report and review of literature. J Neuroimmunol. 2020;341:577184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 28. | Manson G, Maria ATJ, Poizeau F, Danlos FX, Kostine M, Brosseau S, Aspeslagh S, Du Rusquec P, Roger M, Pallix-Guyot M, Ruivard M, Dousset L, Grignou L, Psimaras D, Pluvy J, Quéré G, Grados F, Duval F, Bourdain F, Maigne G, Perrin J, Godbert B, Taifas BI, Forestier A, Voisin AL, Martin-Romano P, Baldini C, Marabelle A, Massard C, Honnorat J, Lambotte O, Michot JM. Worsening and newly diagnosed paraneoplastic syndromes following anti-PD-1 or anti-PD-L1 immunotherapies, a descriptive study. J Immunother Cancer. 2019;7:337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 29. | Vedeler CA, Antoine JC, Giometto B, Graus F, Grisold W, Hart IK, Honnorat J, Sillevis Smitt PA, Verschuuren JJ, Voltz R; Paraneoplastic Neurological Syndrome Euronetwork. Management of paraneoplastic neurological syndromes: report of an EFNS Task Force. Eur J Neurol. 2006;13:682-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 197] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 30. | Widdess-Walsh P, Tavee JO, Schuele S, Stevens GH. Response to intravenous immunoglobulin in anti-Yo associated paraneoplastic cerebellar degeneration: case report and review of the literature. J Neurooncol. 2003;63:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Gomez GG, Read SB, Gerschenson LE, Santoli D, Zweifach A, Kruse CA. Interactions of the allogeneic effector leukemic T cell line, TALL-104, with human malignant brain tumors. Neuro Oncol. 2004;6:83-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |