Published online Oct 26, 2019. doi: 10.12998/wjcc.v7.i20.3226
Peer-review started: September 5, 2019
First decision: September 23, 2019
Revised: October 13, 2019
Accepted: October 15, 2019
Article in press: October 15, 2019
Published online: October 26, 2019
Processing time: 51 Days and 0.3 Hours
Higher intraocular pressure (IOP) is a major risk factor for developing glaucoma, and the leading cause of irreversible blindness worldwide. High altitude (HA) may be involved in IOP, but the reported results were conflicting. Ascent to HA directly by plane from low altitude regions is an acute, effortless exposure. However, the effects of such exposure to different altitudes on IOP have rarely been reported.
To investigate changes in IOP after rapid effortless exposure to HA in stages and compare it with systemic parameters.
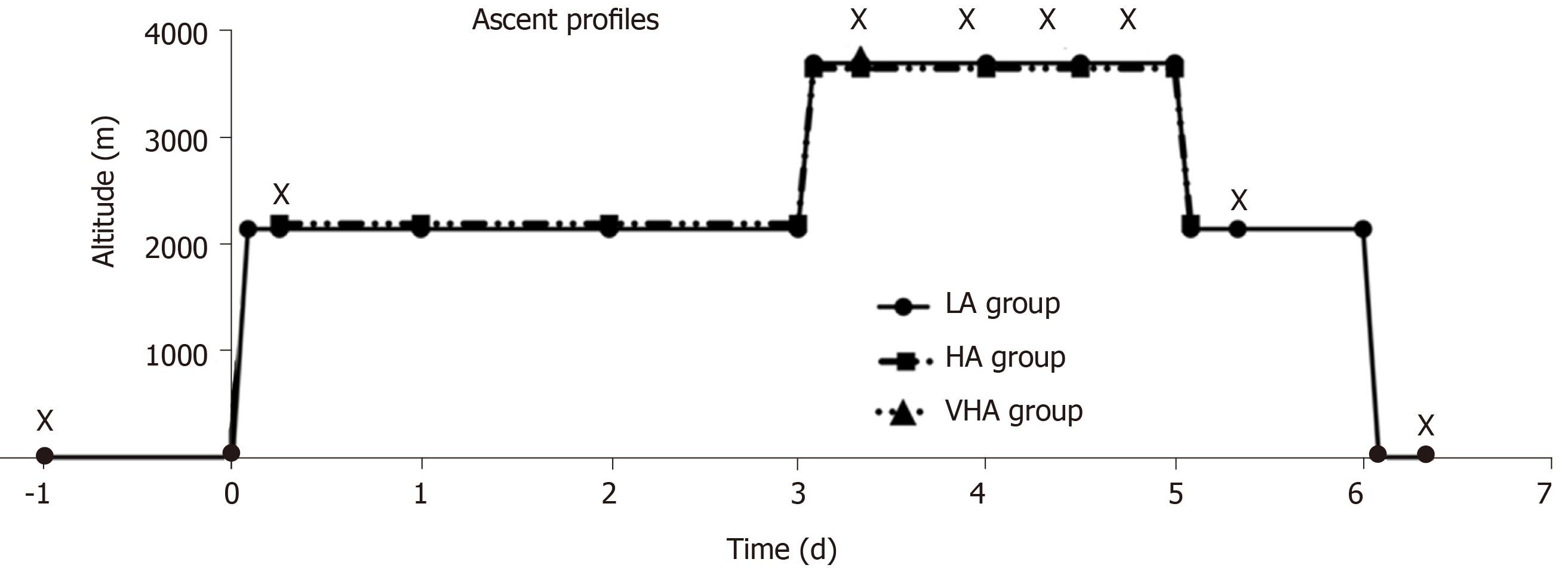
Fifty-eight healthy subjects (116 eyes) were divided into three groups: 17 low-altitude (LA) residents [44 m above sea level (ASL)], 22 HA residents (2261 m ASL) and 19 very HA (VHA) residents (3750 m ASL). The LA group flew to HA first. Three days later, they flew with the HA group to VHA where both groups stayed for 2 d. Then, the LA group flew back to HA and stayed for 1 d before flying back to 44 m. IOP, oxygen saturation (SpO2) and pulse rate were measured. The linear mixed model was used to compare repeated measurements.
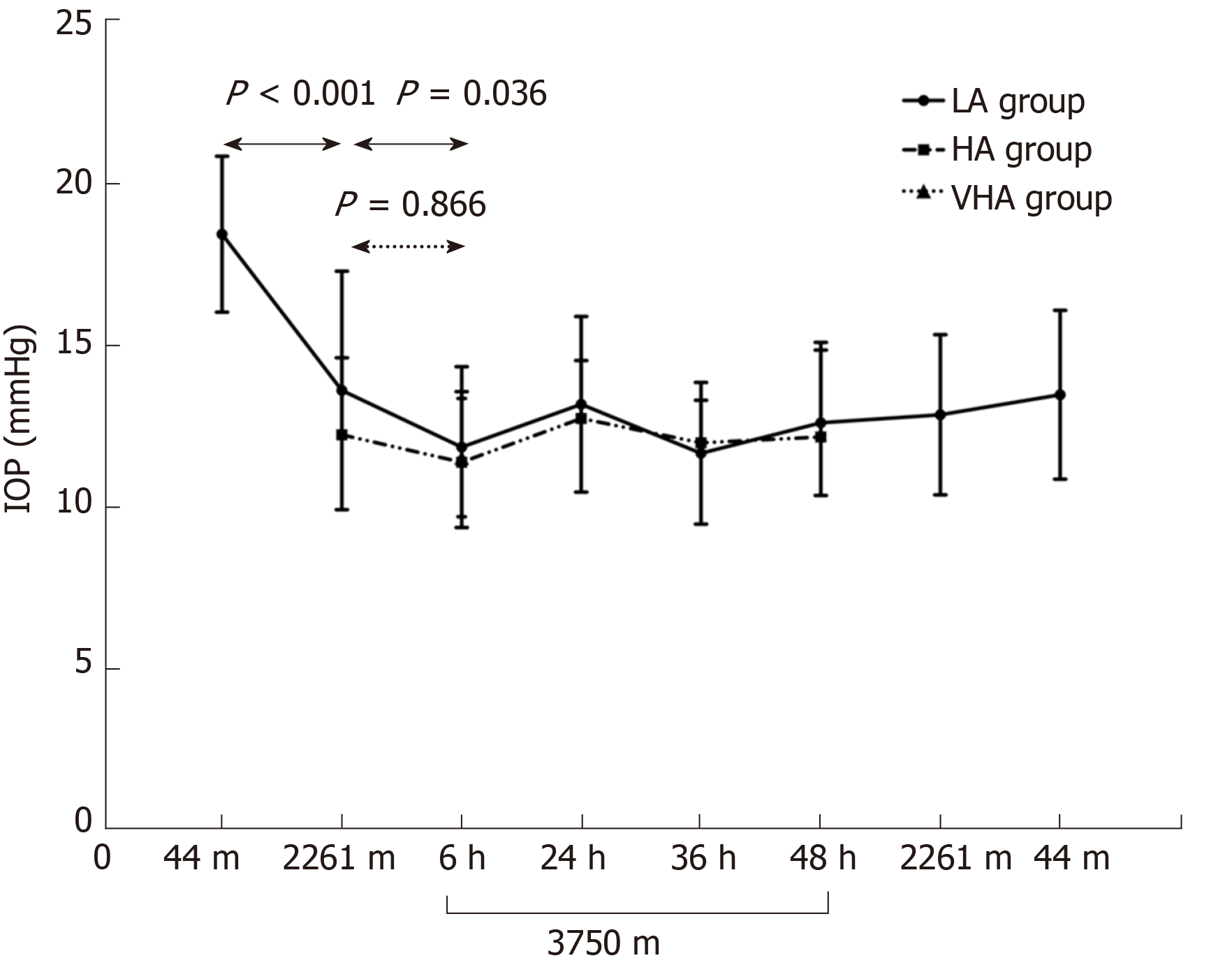
IOP in the LA group significantly decreased from 18.41 ± 2.40 mmHg at 44 m to 13.60 ± 3.68 mmHg at 2261 m ASL (P < 0.001), and then to 11.85 ± 2.48 mmHg at 3750 m ASL (P = 0.036 compared to IOP at 2261 m ASL) and partially recovered to 13.47 ± 2.57 mmHg upon return to 44 m. IOP in the LA group at HA and VHA was comparable to that in the local residents (12.2 ± 2.4 mmHg for HA,11.5 ± 1.8 mmHg for VHA). IOP was positively associated with SpO2 while negatively associated with pulse rate.
IOP in the LA group gradually reduced as altitude elevated in stages and became comparable to IOP in local residents. Hypoxia may be associated with IOP, which deserves further study.
Core tip: Intraocular pressure (IOP) in the lowlanders gradually reduced as altitude elevated in stages. Higher baseline IOP correlated with greater IOP changes in the lowlanders. Lower systemic oxygen saturation was associated with lower IOP as altitude increases. IOP may not be a useful screening method for incipient and potentially harmful altitude-dependent diseases. The findings may provide a potential environmental factor that can lower IOP, which deserves further study.
- Citation: Xie Y, Sun YX, Han Y, Yang DY, Yang YQ, Cao K, Li SN, Li X, Lu XX, Wu SZ, Wang NL. Longitudinal observation of intraocular pressure variations with acute altitude changes. World J Clin Cases 2019; 7(20): 3226-3236
- URL: https://www.wjgnet.com/2307-8960/full/v7/i20/3226.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i20.3226
Glaucoma is the leading cause worldwide of irreversible blindness[1], and intraocular pressure (IOP) is a major risk factor for developing glaucoma. Accurate measurement of IOP is crucial for diagnosis and management of glaucoma.
The International Society for Mountain Medicine defines 1500–3500 m above sea level (ASL) as high altitude (HA), 3500–5500 m ASL as very high altitude (VHA) and > 5500 m ASL as extremely high altitude. Around 140 million people live permanently at altitudes > 2500 m[2]. Large numbers of people travel to HA for recreational or research purposes. IOP was previously proposed as an indicator for acute mountain sickness and HA cerebral edema[3,4]. Thus, change in IOP at HA has been a topic of interest for many years. Nevertheless, the results have been inconsistent. Findings range from a decrease[4-9], to no change[10], to an increase[11-15] in IOP after exposure to moderate and VHA. The conflict in IOP variation with altitude may be due to duration and rate of ascent, altitude reached, limitations of measuring instruments, and individual susceptibility. Many lowlanders now ascend to high altitude directly by plane, which is a relatively acute, effortless exposure. However, the effects of such exposure to different altitudes on IOP have rarely been reported.
Different from previous studies, this is the first study to explore IOP change from sea level to HA and then up to VHA and back to sea level for the same subjects. The IOP changes in highlanders exposure to higher elevation were also investigated. The changes in IOP may provide additional information on how altitude alters IOP readings. Secondly, in this study, subjects flew from one altitude to another. The effects of acute effortless exposure to different altitudes have rarely been reported. Thirdly, we compared the IOP in ascending groups with local highlanders at HA, which has not been explored before. Finally, we correlated the IOP results with concurrently measured systemic changes such as oxygen saturation, blood pressure, pulse rate, erythrocyte, and hemoglobin concentration.
This prospective study was performed on a medical team who participated in a medical mission at HA from August 9, 2015 to August 15, 2015. We included 116 eyes in 58 healthy doctors who were separated into three groups according to the altitude where they permanently resided. The first group was the low altitude (LA) group, composed of 17 healthy lowlanders who were all native inhabitants of Beijing (44 m ASL) and permanently resided there. The second group was the HA group, composed of 22 healthy HA dwellers who were all native inhabitants of Xining (2261 m ASL). The third group was the VHA group, composed of 19 healthy highlanders who were all native inhabitants of Yushu (3750 m ASL). All subjects were originated in China. Exclusion criteria for participants were any type of cardiac, respiratory or nervous system disease; a history of HA pulmonary edema or HA cerebral edema; a history of ophthalmic disease including refractive errors ≥ ± 6 diopters, glaucoma, retinal disease, and narrow-anterior chamber angle; a history of ophthalmic surgery; a history of contact lens wear; and intake of any drugs affecting IOP such as acetazolamide, corticosteroids, or beta-blockers.
This study was approved by the Ethics Committee of Beijing Tongren Hospital, and adhered to the principles of the Declaration of Helsinki. All subjects gave written, informed consent after having been informed of the nature of the research expedition. The study was registered at http://www.chictr.org.cn (Study NO. ChiCTR18000 15513).
The LA group was flown from Beijing, China (44 m ASL) to Xining, China (2261 m ASL), where they acclimatized for 3 d before being flown with the HA group for 2 h to Yushu, China (3750 m ASL). They stayed at Yushu for 2 d. The LA and HA groups flew back to Xining. After 1 d of rest at Xining, the LA group flew back to Beijing (Figure 1). At Xining and Yushu, subjects were advised to avoid any strenuous activity and were not allowed to descend below 2000 m and 4000 m ASL, respectively. The medical mission was carried out for 3 d in Xining and 2 d in Yushu.
All participants initially underwent general and ophthalmic baseline examinations at their local hospitals: The LA group at Beijing Tongren Hospital, the HA group at the Qinghai Provincial People’s Hospital, and the VHA group at Yushu Hospital. The LA group was examined 6 h after flying to 2261 m ASL. The same measurements were simultaneously performed on the LA and HA groups at 6, 24, 36 and 48 h after flying to 3750 m ASL (Figure 2). IOP measurements in lowlanders were also performed 6 h after flying back to Xining and Beijing.
IOP was measured with a handheld tonometer (24–3000, 12K1031; Accutome, Malvern, PA, United States) by placing the tip of the device onto the central cornea under local anesthesia with 0.5% proparacaine hydrochloride (Alcon-Couvreur, Puurs, Belgium). Three complete measurements were taken, and the average was calculated and recorded. The tonometer was calibrated according to the manufacturer’s guidelines before each examination session. Concurrently with IOP measurement, blood pressure and pulse oximetry were measured with an electronic sphygmomanometer (HEM-7133; Omron Corporation, Kyoto, Japan) and a finger pulse oximeter (PC-60NW; Health Force, Shanghai, China), respectively. Subjects were resting in a sitting position at this time. Stable values after at least 3 min rest were recorded. Mean blood pressure (MBP) was calculated as 1/3 × systolic blood pressure + 2/3 × diastolic blood pressure. Ocular perfusion pressure was calculated as 2/3 × MBP – IOP. All the systemic values were measured at least 6 h after exposure to a new altitude and also at similar times of the day to minimize the effect of fatigue due to flying and diurnal variation[16]. Venous blood was drawn while subjects were oriented in the sitting position and the blood was placed in separate tubes. Erythrocyte and hemoglobin concentrations were examined and recorded for further analysis. To avoid hemolysis, specimens were observed by a senior biochemist. All equipment used was calibrated to ensure accuracy before each testing session.
Statistical analysis was performed with commercially available statistical software packages (SPSS for Mac, version 24.0; IBM-SPSS, Chicago, IL, United States). The Shapiro–Wilk W test was used to check for normal distribution of data. When variables were not normally distributed, the Kruskal–Wallis test with post hoc Dunn multiple comparisons was used to compare them among the three altitude groups. When variables were normally distributed, one-way analysis of variance with post hoc least significant difference analysis was performed among the groups. Levene’s test was used to examine the homogeneity of the variance. The linear mixed model was used to compare repeated measurements at each time point for the LA and HA groups as altitude elevated, and to adjust for correlation between two eyes within each subject. Bonferroni correction was applied for multiple comparisons. Spearman correlation was used to examine for associations between IOP and other variables. A linear regression was utilized to explore the association of IOP changes in the LA group before and after ascending. A two-sided error P < 0.05 was considered as statistically significant.
The three groups were comparable in gender, age, height, weight, body mass index, and waist and hip measurements (Table 1). As expected, compared to the LA group, the HA and VHA groups had lower pulse oxygen saturation (SpO2), and higher erythrocyte and hemoglobin levels (all P < 0.001) (Table 2). The VHA group had the lowest SpO2 (P < 0.001) and highest erythrocyte and hemoglobin levels (P = 0.03 and P = 0.004, respectively). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) in the altitude groups were higher, but not significantly, than in the LA group (P = 0.138 and P = 0.336, respectively). In terms of IOP, the LA group had the highest value while the HA and VHA groups had comparable values (P < 0.001) (Table 2).
| LA group | HA group | VHA group | P value | |
| n | 17 | 22 | 19 | |
| Gender (male/ female)1 | 6/11 | 11/11 | 10/9 | 0.548 |
| Age (yr)(range)2 | 28.1 ± 8.1(22-56) | 29.6 ± 4.3 (20-37) | 28.6 ± 6.0 (22-42) | 0.716 |
| Height (m)2 | 1.7 ± 5.7 | 1.7 ± 8.8 | 1.7 ± 6.3 | 0.19 |
| Weight (kg)2 | 62.2 ± 8.7 | 64.9 ± 16.0 | 64.0 ± 10.6 | 0.898 |
| BMI (kg/m2)2 | 21.8 ± 2.1 | 22.5 ± 3.9 | 23.6 ± 3.1 | 0.253 |
| Waistline (cm)2 | 77.0 ± 8.4 | 80.7 ± 13.1 | 80.7 ± 9.7 | 0.492 |
| Hipline (cm)2 | 93. 7± 5.3 | 90.3 ± 7.9 | 90.6 ± 7.1 | 0.279 |
| Parameters | LA group | HA group | VHA group | P value | Post hoc analysis |
| n | 17 (34 eyes) | 22 (44 eyes) | 19 (38 eyes) | ||
| SpO2 (%)1 | 97.06 ± 0.43 | 94.55 ± 1.18 | 90.05 ± 2.09 | < 0.001 | A > B > C |
| IOP (mmHg)2 | 18.4 ± 2.4 | 12.2 ± 2.4 | 11.5 ± 1.8 | < 0.001 | A > B, C |
| OPP (mmHg)2 | 36.5 ± 6.3 | 47.9 ± 7.3 | 49.2 ± 9.2 | < 0.001 | A > B, C |
| SBP (mmHg)3 | 109.7 ± 11.5 | 118.6 ± 14.9 | 118.3 ± 18.3 | 0.149 | NS |
| DBP (mmHg)3 | 68.7 ± 10.3 | 76.1 ± 10.3 | 77.5 ± 14.5 | 0.069 | NS |
| MBP (mmHg)3 | 82.4 ± 10.2 | 90.2 ± 11.2 | 91.1 ± 14.9 | 0.051 | NS |
| Pulse rate (bpm)3 | 78.6 ± 9.1 | 79.6 ± 10.6 | 87.6 ± 8.5 | 0.009 | A, B < C |
| Erythrocyte (1012/L)1 | 4.58 ± 0.6 | 5.24 ± 0.63 | 5.67 ± 0.62 | < 0.001 | A < B, C |
| Hemoglobin (g/L)1 | 137.63 ± 21.14 | 157.09 ± 20.26 | 180.84 ± 23.37 | < 0.001 | A < B < C |
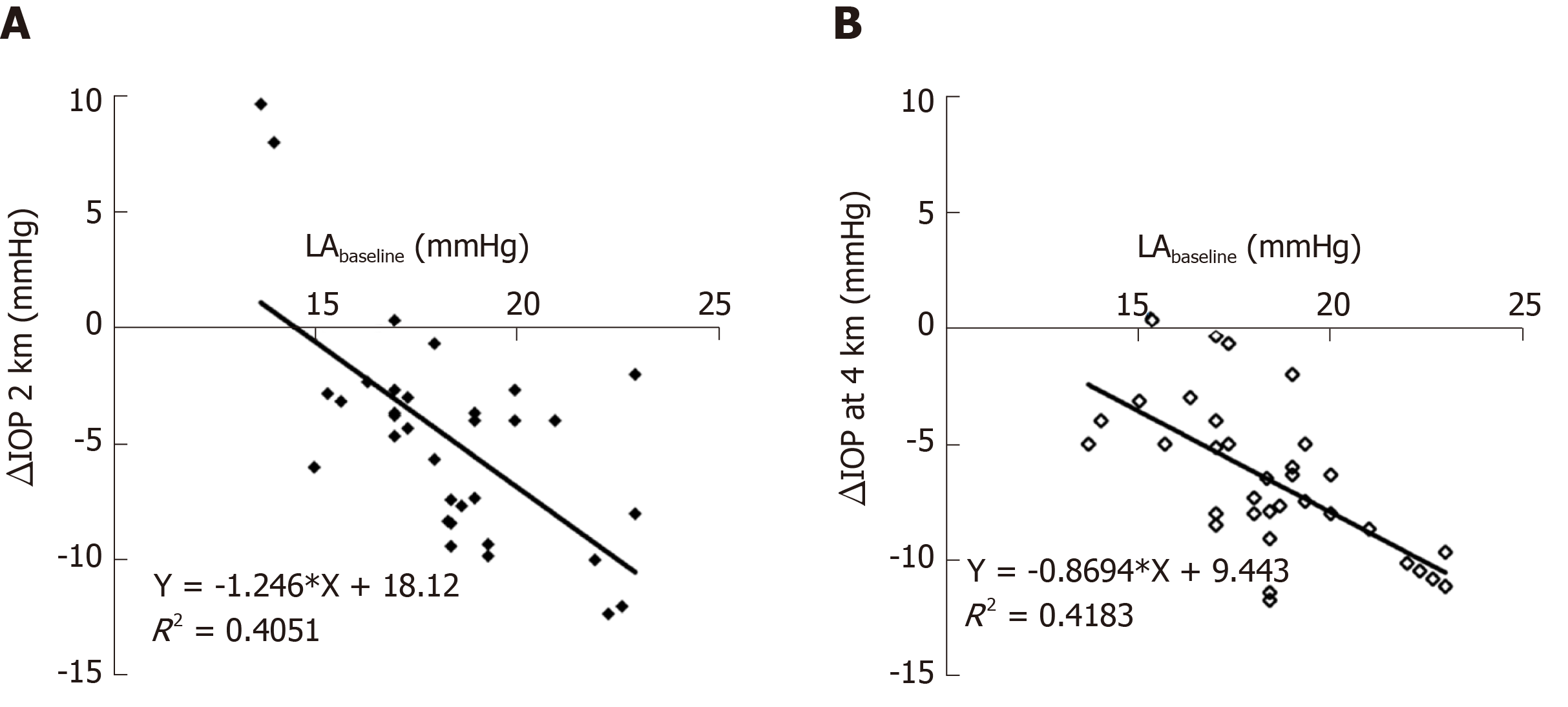
All subjects in the LA and HA groups finished the ascent procedure without discomfort such as headache, nausea and dehydration, and without medication. Longitudinal observation of IOP in the LA group elucidated a gradual and significant decrease from 18.41 ± 2.40 mmHg at sea level (LA baseline) to 13.60 ± 3.68 mmHg at 2261 m ASL (P < 0.001), and then to 11.85 ± 2.48 mmHg at 3750m ASL (P = 0.036 compared to IOP at 2261 m ASL). This IOP at 3750 m ASL was maintained for 2 d (all P > 0.05). When the LA group flew back to 2261 m ASL, the IOP did not change (12.84 ± 2.34 mmHg). When the LA group flew back to 44 m, the IOP increased to 13.47 ± 2.57 mmHg but was still lower than LA baseline (P < 0.001). The IOP in the LA group at 2261 and 3750 m was comparable to that in the native residents: 12.23 ± 2.38 mmHg in HA residents (P = 0.178) and 11.53 ± 1.83 mmHg in VHA residents (P = 0.463). Figure 3 shows IOP changes in the LA groups were negatively associated with LA baseline (LA at 2261 m ASL, r = –0.546, P < 0.001; LA at 3750 m ASL, r = –0.636, P < 0.001).
No significant changes were found in the IOP of the HA group throughout the observation time at 3750 m ASL (11.39 ± 2.18 mmHg at 6 h, 12.73 ± 1.79 mmHg at 24 h, 11.98 ± 1.32 mmHg at 36 h, and 12.16 ± 2.92 mmHg at 48 h, all P > 0.05) compared to that at 2261 m ASL (12.23 ± 2.38 mmHg, HA baseline).
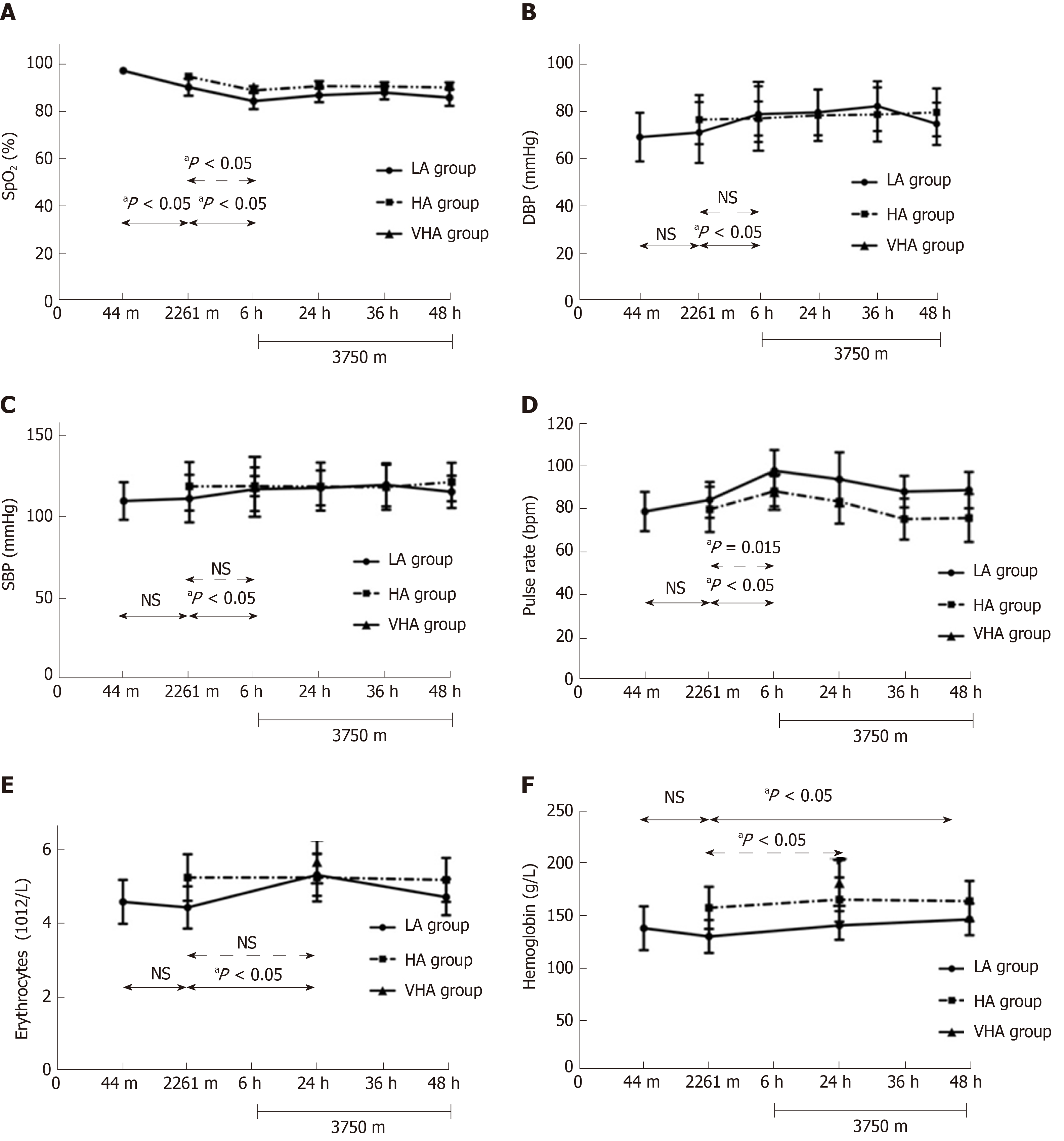
Figure 4 shows vital and hematological parameters variations in the two ascending groups as altitude elevated. SpO2 of the LA group gradually decreased from 97.06 ± 0.43% at 44 m ASL to 90.06 ± 3.60% at 2261 m ASL (P < 0.001), and then to 84.18 ± 3.41% at 3750 m ASL (P < 0.001). All other systemic parameters in the LA group did not change after exposure to 2261 m ASL. However, there were increases in pulse rate (97.41 ± 9.6 bpm, P < 0.001), DBP (78.41 ± 11.77 mmHg, P < 0.001), SBP (116.88 ± 13.36 mmHg, P = 0.006), erythrocyte level (5.31 ± 0.57 1012/L, P < 0.001), and hemoglobin level (146.18 ± 15.26 g/L, P = 0.027) after exposure to 3750 m ASL when compared to that at 2261 m ASL.
After 6 h exposure to 3750 m ASL, SpO2 of HA group significantly dropped from 94.55 ± 1.18% to 88.67 ± 1.83% (P < 0.001) and remained stable during the observation time. Pulse rate significantly increased from 79.59 ± 10.58 to 87.92 ± 7.02 bpm (P = 0.015) and recovered at 24 h exposure to 3750 m ASL (83.14 ± 10.19 bpm, P = 1.000). There was an increase in hemoglobin level (157.09 ± 20.26 vs 165 ± 21.32 g/L, P < 0.001). No significant changes were found in erythrocyte level and BP (all P = 1.000).
Table 3 shows the association between IOP and systemic parameters. IOP was positively correlated with SpO2 in the LA and HA groups. There was a positive correlation between IOP and hemoglobin level in the LA group. IOP was not associated with SBP, DBP or erythrocyte level.
| LA group | HA group | |||
| r | P value | r | P value | |
| SpO2 (%) | 0.388 | < 0.001 | 0.117 | 0.223 |
| Pulse rate (bpm) | –0.186 | 0.062 | –0.176 | 0.065 |
| Systolic blood pressure (mmHg) | –0.008 | 0.938 | 0.076 | 0.432 |
| Diastolic blood pressure (mmHg) | –0.110 | 0.270 | 0.127 | 0.187 |
| Erythrocyte (1012/L) | 0.069 | 0.578 | 0.001 | 0.994 |
| Hemoglobin (g/L) | 0.099 | 0.427 | –0.041 | 0.746 |
We prospectively studied the IOP changes at a series of altitude elevations and found that LA group demonstrated a consistent trend of decrease in IOP with acute, effortless altitude elevation from sea level; and at the highest altitude, the decreased IOP was comparable to the IOP of native residents. However, IOP in the HA group was sustained after exposure to VHA. The change in IOP of the LA group was correlated with systemic parameters.
The decrease in IOP in the LA group after acute effortless exposure was consistent with previous studies performed at similar altitude[4,5,7,9]. However, these findings contradict those of Somner et al[10], who found an increase in both raw IOP and IOP corrected by central corneal thickness (CCT) after exposure to 5200 m ASL. The subjects in that study were flown to 3700 m ASL, where they acclimatized for 4 d before being driven for 2 h to 5200 m ASL. The authors compared IOP at 5200 m ASL and upon return to sea level. The baseline value measured after exposure to HA might be influenced by altitude exposure and thus yielded different results[10]. Our findings were also inconsistent with a study that reported increased IOP in lowlanders travelled to 2000 m ASL by bus and trekked to 2800 m ASL[11]. The effect of acute altitudinal change on intraocular pressure was different from the effect of chronic exposure and thus yielded different results. A previous study has also reported a reduction in IOP from 14.2 ± 2.7 mmHg at ground level to 12.3 ± 2.5 mmHg in the second hour of flight and 12.0 ± 1.7 mmHg at 3.5 h after landing. The cabin pressure was maintained around 8000 feet, equivalent to 2440 m ASL[17]. This pressure was similar to that at the first altitude our subjects reached. It is possible that air travel might also have contributed to some extent to the IOP changes found at HA.
In the current study, IOP in lowlanders did not return to baseline upon return to sea level. Nebbioso et al[8] reported a further decrease in IOP after immediate return to sea level from 5486 m ASL in a simulation chamber. Bosch et al[18] demonstrated partially recovered IOP compared with that at 5200 m ASL. Willmann et al[9] reported a significant reduction in IOP after correction by CCT and recovered to baseline level 14 d after return to 374 m. Thus, previous studies, although they rarely showed complete IOP recordings from pre-ascent to HA then back to sea level, combined with our results indicate that IOP should spend some time recovering to sea level after exposure to altitude. Whether IOP changes after exposure to higher elevations in highlanders has never been reported. Our results showed comparable IOP in highlanders throughout the observation time at VHA (3750 m ASL), indicating that adaptation in highlanders protected them from hypoxia at higher altitude.
Our data show that HA and VHA permanent residents had lower IOP than LA residents had. Epidemiological studies at different altitudes found that IOP in healthy Han Chinese subjects in Shunyi (35 m)[19] and Yongnian (65 m)[20] were 16.11 ± 3.39 and 15.0 ± 2.8 mmHg, respectively, while the IOP of Bai Chinese healthy subjects in Dali (2179 m)[21] and of Tibetans in Qushui (3593 m)[22] were 14.4 ± 3.3 and 12.9 ± 2.7 mmHg, respectively. The IOP levels at the three altitudes in the present study were higher than those reported in previous studies, which could be due to the smaller samples and younger age, but the tendency for IOP decrease is in agreement with these pooled results. To obtain more powerful results, larger study samples are needed to investigate the normal range of IOP in different-altitude native residents. Because different altitudes are shown to be associated with different IOP ranges, the effects of altitude changes should be considered when diagnosing subgroups of glaucoma, especially for normal-tension glaucoma, and when developing target IOPs for glaucomatous patients.
The fact that the IOP dropped to a plateau and then remained stable suggests that the hypoxic and hypobaric environment at HA may affect the balance between aqueous production and outflow. Although the exact mechanisms are unclear, our data showing a positive correlation between SpO2 and IOP may support the hypothesis that reduced oxygen saturation experienced at HA may decrease aqueous production and thus IOP[23]. It is possible that aqueous humor production may be impaired during systemic hypoxia and, after a certain hypoxia threshold has been reached, IOP no longer changes. Moreover, hypoxia provokes hyperventilation. This can decrease IOP through decreased PCO2 and bicarbonate effects on alteration of intraocular blood volume and altered formation of aqueous humor[24]. Two outliers on the left graph in Figure 3 stemmed from paired eyes of one person and indicate an increase in IOP after exposure to 2261 m ASL. The reason for this is not clear. This subject showed more stable heart rate and BP and this may partly explain these two singular values. Despite the parameters above being associated with IOP changes at HA, a post-hoc study should be performed to examine further the underlying mechanisms of IOP changes at HA. This may provide a potential environmental factor that can lower IOP. Although IOP decreased on ascent to 2261 m ASL, other systemic parameters did not change besides SpO2. This suggests that IOP may be not a useful screening method for incipient and potentially harmful altitude-dependent diseases.
The present study had some limitations. Firstly, CCT was not measured. However, Aptel et al[25] reported the pooled effects of HA exposure on CCT, which showed a mean increase of 19.3 μm after 3–5 d of exposure. If we compensate IOP with the change in CCT based on the range of values suggested in the literature[26-28], the true IOP in our subjects might have been 0.2–0.4 mmHg lower than that measured at HA or VHA. Adjusting for this would yield an even more significant decrease in IOP from LA to 2261 m and 3750 m ASL. Secondly, all participants in the present study were young adults and it is unclear how well the results obtained from our study population can be applied to older subjects. Thirdly, IOP was measured by Accupen applanation tonometer. The tonometer effect on IOP readings should be considered in future studies. Lastly, the present study did not follow the IOP changes over a long duration of exposure to HA.
In summary, this study showed that, for young healthy individuals, IOP gradually reduced when altitude acutely elevated in stages and became comparable to IOP in native residents. Systemic SpO2 reduction might be involved in the mechanisms of decrease in IOP as altitude increases. This might provide a potential environmental factor to lower IOP. Measuring IOP might not be a useful screening method for incipient and potentially harmful altitude-dependent diseases.
High intraocular pressure (IOP) is a major risk factor for glaucoma. Previous studies suggested that high altitude affected intraocular pressure, but the results were inconsistent. Now many lowlanders ascend to high altitude by plane. The effects of such an acute, effortless exposure to different altitudes have rarely been reported.
This study is a longitudinal observation of intraocular pressure variation with acute, effortless altitude changes. Our findings may provide additional information on how altitude changes affect IOP.
To investigate changes in IOP after acute effortless exposure to high altitude in stages and compare it with systemic parameters.
This prospective study included three groups according to the place of residence: Low-altitude (LA) group [44 m above sea level (ASL)], high altitude (HA) group (2261 m ASL), and very high altitude (VHA)group (3750 m ASL). The LA group flew to HA first. Three days later, LA group flew with the HA group to VHA where both groups stayed for 2 d. Then, the LA group flew back to 2261 m ASL and stayed for 1 d before flying back to 44 m. IOP, vital values and hematological values were measured and compared before, during and after exposure to 2261 m and 3750 m ASL. The mixed linear model was used to compare repeated measurements and Bonferroni correction was applied for multiple comparisons. Spearman correlation was used to analyze the associations between IOP and systemic parameters. Different from previous studies, this is the first study to observe IOP changes in the same subjects from sea level to high altitude and then up to very high altitude, and then back to sea level. In addition, subjects flew from one altitude to another altitude. We also compared the IOP in ascending groups from low altitude with that in local highlanders.
The LA group had the highest IOP (18.41 ± 2.40 mmHg) compared with the HA (12.23 ± 2.38 mmHg) and VHA (11.53 ± 1.83 mmHg) groups. IOP in the LA group significantly decreased from 18.41 ± 2.40 mmHg at 44 m to 13.60 ± 3.68 mmHg at 2261 m ASL, and then to 11.85 ± 2.48 mmHg at 3750 m ASL and partially recovered to 13.47 ± 2.57 mmHg upon return to 44 m. IOP in the LA group at HA and VHA decreased to that in the native residents. IOP was positively associated with pulse oxygen saturation. Higher baseline IOP correlated with greater IOP changes in the LA group. The findings indicated that acute, effortless exposure to high altitude reduced IOP. It would be interesting to investigate the effects of long-term altitude exposure on IOP in the future. Furthermore, the mechanisms of IOP changes at high altitude deserve further study.
IOP in the lowlanders reduced as altitude elevated in stages and became comparable to IOP in native residents. The lower IOP was associated with hypoxia at high altitude. The higher the baseline IOP, the greater the decrease in IOP. The findings may provide a potential environmental factor to lower IOP, that deserves further study.
The results suggested that high and very high altitude residents have lower IOP than lowlanders had. The IOP in ascending groups from low altitude dropped to the level of local highlanders after acute, effortless exposure to higher altitude. Lower IOP is associated with hypoxia at high altitude. Larger sample studies are needed to investigate the normal range of IOP in native residents at high altitude. Furthermore, the mechanisms of IOP variation and IOP changes after long-term exposure to high altitude deserves further study.
The authors thank Dale Norton Bongbong for his contributions to the manuscript revision.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Orbell JH, Sogabe I S-Editor: Wang JL L-Editor: Ma JY E-Editor: Ma YJ
| 1. | Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4855] [Cited by in RCA: 4941] [Article Influence: 260.1] [Reference Citation Analysis (0)] |
| 2. | Basnyat B, Murdoch DR. High-altitude illness. Lancet. 2003;361:1967-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 356] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 3. | Cushing T, Paterson R, Haukoos J, Harris NS. Intraocular pressure is not associated with acute mountain sickness. High Alt Med Biol. 2013;14:342-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Cymerman A, Rock PB, Muza SR, Lyons TP, Fulco CS, Mazzeo RS, Butterfield G, Moore LG. Intraocular pressure and acclimatization to 4300 M altitude. Aviat Space Environ Med. 2000;71:1045-1050. [PubMed] |
| 5. | Pavlidis M, Stupp T, Georgalas I, Georgiadou E, Moschos M, Thanos S. Intraocular pressure changes during high-altitude acclimatization. Graefes Arch Clin Exp Ophthalmol. 2006;244:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Bosch MM, Barthelmes D, Merz TM, Truffer F, Knecht PB, Petrig B, Bloch KE, Hefti U, Schubiger G, Landau K. Intraocular pressure during a very high altitude climb. Invest Ophthalmol Vis Sci. 2010;51:1609-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Nazari H, Nilforushan N, Sedaghat A, Soudi R, Irani A, Gordiz A, Hatamkhani S. Intraocular pressure after exposure to moderate altitude. Graefes Arch Clin Exp Ophthalmol. 2013;251:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Nebbioso M, Fazio S, Di Blasio D, Pescosolido N. Hypobaric hypoxia: effects on intraocular pressure and corneal thickness. ScientificWorldJournal. 2014;2014:585218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Willmann G, Schommer K, Schultheiss M, Fischer MD, Bartz-Schmidt KU, Gekeler F, Schatz A. Effect of High Altitude Exposure on Intraocular Pressure Using Goldmann Applanation Tonometry. High Alt Med Biol. 2017;18:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Somner JE, Morris DS, Scott KM, MacCormick IJ, Aspinall P, Dhillon B. What happens to intraocular pressure at high altitude? Invest Ophthalmol Vis Sci. 2007;48:1622-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Karakucuk S, Mujdeci M, Baskol G, Arda H, Gumus K, Oner A. Changes in central corneal thickness, intraocular pressure, and oxidation/antioxidation parameters at high altitude. Aviat Space Environ Med. 2012;83:1044-1048. [PubMed] |
| 12. | Ersanli D, Yildiz S, Sonmez M, Akin A, Sen A, Uzun G. Intraocular pressure at a simulated altitude of 9000 m with and without 100% oxygen. Aviat Space Environ Med. 2006;77:704-706. [PubMed] |
| 13. | Karadag R, Sen A, Golemez H, Basmak H, Yildirim N, Karadurmus N, Koseoglu E, Akin A. The effect of short-term hypobaric hypoxic exposure on intraocular pressure. Curr Eye Res. 2008;33:864-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Foulsham W, Tatham AJ. High Altitude-associated Changes in Intraocular Pressure Abrogated by Trabeculectomy. J Glaucoma. 2017;26:957-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Karadag R, Sen A, Yildirim N, Basmak H, Golemez H, Cakir E, Akin A. The relation between intraocular pressure change and plasma natriuretic peptide under simulated hypobaric conditions. Indian J Ophthalmol. 2010;58:195-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Wilensky JT. Diurnal variations in intraocular pressure. Trans Am Ophthalmol Soc. 1991;89:757-790. [PubMed] |
| 17. | Mehdizadeh M. An assessment of intraocular pressure change in healthy subjects during air flight. Curr Eye Res. 2008;33:810-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Bosch MM, Merz TM, Barthelmes D, Petrig BL, Truffer F, Bloch KE, Turk A, Maggiorini M, Hess T, Schoch OD, Hefti U, Sutter FK, Pichler J, Huber A, Landau K. New insights into ocular blood flow at very high altitudes. J Appl Physiol (1985). 2009;106:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Xu L, Li J, Zheng Y, Cui T, Zhu J, Ma K, Yang H, Ma B, Jonas JB. Intraocular pressure in Northern China in an urban and rural population: the Beijing eye study. Am J Ophthalmol. 2005;140:913-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Zhou Q, Liang YB, Wong TY, Yang XH, Lian L, Zhu D, Sun LP, Wang NL, Friedman DS. Intraocular pressure and its relationship to ocular and systemic factors in a healthy Chinese rural population: the Handan Eye Study. Ophthalmic Epidemiol. 2012;19:278-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Zhong H, Li J, Li C, Wei T, Cha X, Cai N, Luo T, Yu M, Yuan Y. The prevalence of glaucoma in adult rural Chinese populations of the Bai nationality in Dali: the Yunnan Minority Eye Study. Invest Ophthalmol Vis Sci. 2012;53:3221-3225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Wang G Q, Bai ZX, Luo S, Shi J, Cao LQ, Chang HF, Sai XY. Characteristic of intraocular pressure distribution in population of 1115 Tibetan aged 40 years old or more. International Eye Science. 2014;1181-1185. [DOI] [Full Text] |
| 23. | Kiel JW, Hollingsworth M, Rao R, Chen M, Reitsamer HA. Ciliary blood flow and aqueous humor production. Prog Retin Eye Res. 2011;30:1-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Smith RB, Aass AA, Nemoto EM. Intraocular and intracranial pressure during respiratory alkalosis and acidosis. Br J Anaesth. 1981;53:967-972. [PubMed] |
| 25. | Aptel F, Weinreb RN, Chiquet C, Mansouri K. 24-h monitoring devices and nyctohemeral rhythms of intraocular pressure. Prog Retin Eye Res. 2016;55:108-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Bhan A, Browning AC, Shah S, Hamilton R, Dave D, Dua HS. Effect of corneal thickness on intraocular pressure measurements with the pneumotonometer, Goldmann applanation tonometer, and Tono-Pen. Invest Ophthalmol Vis Sci. 2002;43:1389-1392. [PubMed] |
| 27. | Wolfs RC, Klaver CC, Vingerling JR, Grobbee DE, Hofman A, de Jong PT. Distribution of central corneal thickness and its association with intraocular pressure: The Rotterdam Study. Am J Ophthalmol. 1997;123:767-772. [PubMed] |
| 28. | Tonnu PA, Ho T, Newson T, El Sheikh A, Sharma K, White E, Bunce C, Garway-Heath D. The influence of central corneal thickness and age on intraocular pressure measured by pneumotonometry, non-contact tonometry, the Tono-Pen XL, and Goldmann applanation tonometry. Br J Ophthalmol. 2005;89:851-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 229] [Article Influence: 11.5] [Reference Citation Analysis (0)] |