Published online Oct 6, 2019. doi: 10.12998/wjcc.v7.i19.3039
Peer-review started: July 17, 2019
First decision: August 2, 2019
Revised: August 7, 2019
Accepted: September 13, 2019
Article in press: September 13, 2019
Published online: October 6, 2019
Processing time: 82 Days and 2.5 Hours
Bronchobiliary fistula (BBF) is a rare disease characterized by an abnormal connection between the biliary system and bronchi. Traditional causes of BBF include trauma and infections, and more recent causes include malignancies and certain cancer treatments. Ramucirumab is an antivascular endothelial growth factor receptor 2 monoclonal antibody, currently used as a second-line treatment for gastric cancer.
A 43-year-old man visited our hospital with the complaint of jaundice. He was diagnosed with inoperable advanced gastric cancer owing to invasion of the hepatic hilum by the tumor. After percutaneous transhepatic biliary drainage (PTBD) and stent placement, capecitabine and oxaliplatin were administered as first-line palliative chemotherapy. The tumor progressed, and paclitaxel and ramucirumab were administered as second-line chemotherapy. However, on the first day of the second cycle, the patient suddenly developed dyspnea and pneumonia. BBF was diagnosed on the basis of the presence of bilious sputum and the results of computed tomography, and PTBD was repeated.
This is the first report of BBF after administration of the new antiangiogenic agent ramucirumab.
Core tip: This report is significant and will be of interest to readers because it describes an extremely rare case of bronchobiliary fistula (BBF) developing after chemotherapy for advanced gastric cancer. Moreover, it is the first report on BBF occurring after the administration of the new antiangiogenic agent ramucirumab.
- Citation: Kim HB, Na YS, Lee HJ, Park SG. Bronchobiliary fistula after ramucirumab treatment for advanced gastric cancer: A case report. World J Clin Cases 2019; 7(19): 3039-3046
- URL: https://www.wjgnet.com/2307-8960/full/v7/i19/3039.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i19.3039
Bronchobiliary fistula (BBF) is a rare disease with a poor prognosis that presents with respiratory symptoms due to an abnormal connection between the bile ducts and the bronchial tree. First reported in 1850, its best-known cause is an infection-derived liver abscess; other causes include cholestasis, trauma, postoperative complications, and invasion by a malignant tumor[1-3]. Owing to advanced procedures that better detect primary and metastatic malignant liver tumors (e.g., stereotactic radiosurgery, transcatheter arterial chemoembolization, and radiofrequency ablation), the rate at which tumor-associated BBF is reported has increased[4-8]. Here, to the best of our knowledge, we present the first report of BBF after ramucirumab administration for advanced gastric cancer.
Jaundice and itching.
A 43-year-old man visited our hospital complaining of jaundice and itching two weeks earlier.
He had undergone gastrointestinal (GI) resection owing to a road-traffic accident 20 years earlier.
His medical history and that of his family were otherwise unremarkable.
His abdomen was smooth and soft without tenderness and palpable mass.
The complete blood count results were as follows, with normal ranges in parentheses: White blood cells (WBCs), 8.60 × 103/μL (4.0–10.0 103/μL); hemoglobin, 7.7 g/dL (12-16 g/dL); platelets, 694 × 103/μL (150-400 103/μL). Blood biochemistry results were as follows: T-otal bilirubin, 11.4 mg/dL (0.2–1.1 mg/dL); aspartate aminotransferase, 48 U/L (5-40 U/L); alanine aminotransferase, 59 U/L (5-40 U/L); alkaline phosphatase, 413 U/L (42-128 U/L); gamma-glutamyl transferase, 242 U/L (16-73 U/L). Based on these findings, obstructive jaundice was most strongly suspected. C-reactive protein (CRP) was at 2.19 mg/dL (0-0.5 mg/dL). Among the tumor markers, carbohydrate antigen 19-9 was slightly elevated, at 71.1 U/mL (0-37 U/mL), but carcinoembryonic antigen was normal, at 2.84 ng/mL (0-5.0 ng/mL).
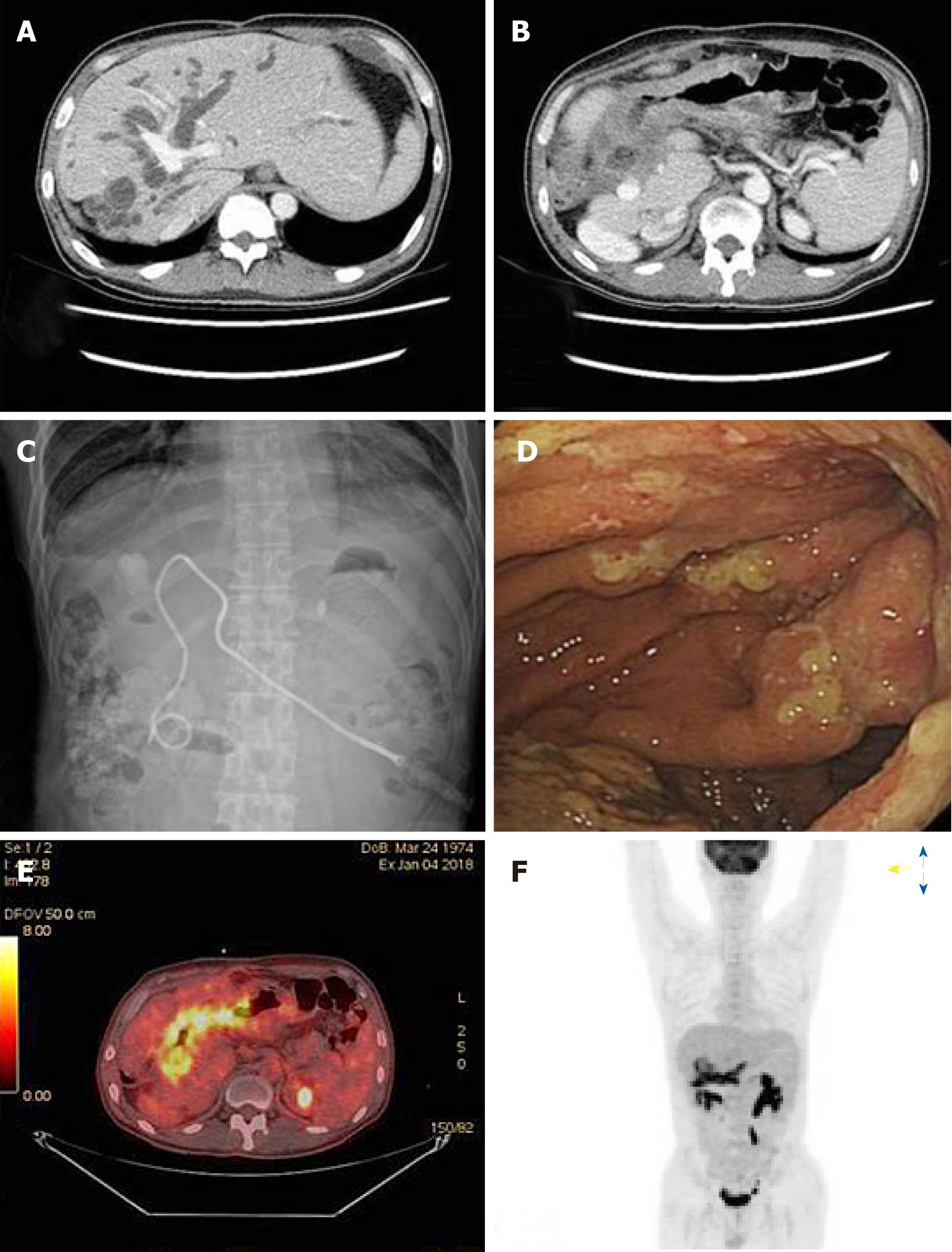
Abdominopelvic computed tomography (CT) revealed a gastric mass with significant wall thickening that directly infiltrated the hilar area of the liver and severe bile duct dilatation (Figure 1A and B). Under ultrasonograpy guidance, we decided the percutaneous transhepatic biliary drainage (PTBD) via left anterolateral approach. Left segment 3 intrahepatic duct was punctured and pigtail catheter was inserted into the deuodenum portion, and tubogram was done. Obstructive jaundice was confirmed, and there was no leakage or complication after procedure (Figure 1C). Direct liver invasion by an advanced gastric cancer was suspected. Consistent with Bormann type 4 gastric cancer, gastroenteroscopy showed a diffuse lesion accompanied by luminal narrowing and mucosal ulceration (Figure 1D). On positron emission (PET)-CT, the mass appeared hypermetabolic, but there were no distant metastases (Figure 1E and F).
Histological examination confirmed human epidermal growth factor 2-negative, poorly differentiated adenocarcinoma. Based on the combined CT, gastroenteroscopy, and PET-CT findings, the patient was diagnosed with locally advanced gastric cancer directly invading the hilar area of the liver.
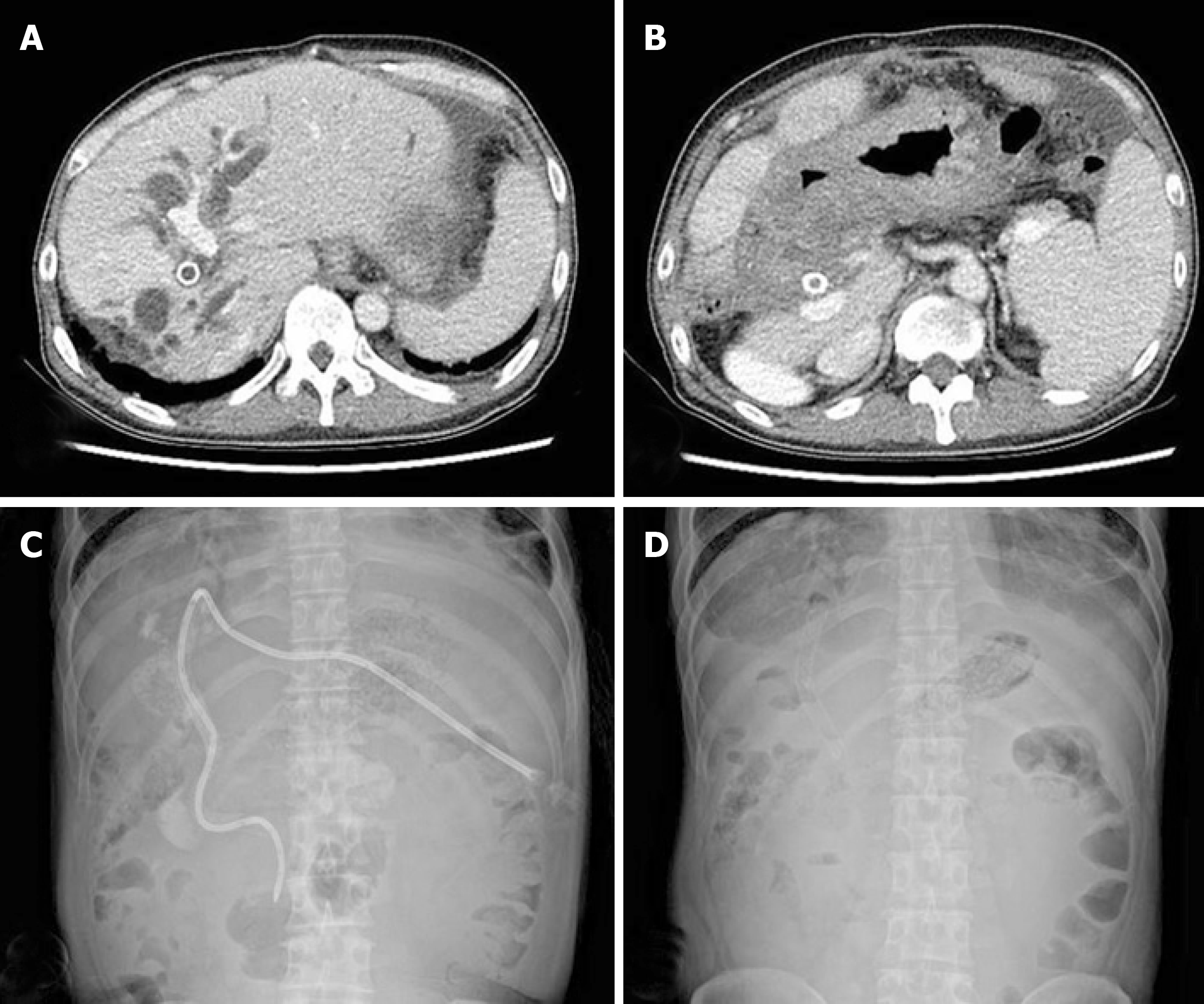
Total bilirubin levels returned to normal after PTBD, and a metallic stent was placed in the bile duct. The patient was considered inoperable owing to locally advanced gastric cancer; hence, palliative chemotherapy was administered. The patient completed 3 cycles of XELOX combination chemotherapy (capecitabine oxaliplatin, 2000 and 139 mg/body surface area, respectively) without any specific adverse effects, and the treatment response was stable disease. However, after 6 cycles, CT showed signs of disease progression, namely, increased mass size and dilation of the bile duct (Figure 2A and 2B). Because total bilirubin was elevated to 6.1 mg/dL, PTBD and stent placement were repeated again (Figure 2C and 2D).
As second-line chemotherapy, ramucirumab (8 mg/kg; days 1 and 15) and paclitaxel (80 mg/body surface area; days 1, 8, and 15; Q28DAYS) were administered. Ramucirumab is a monoclonal antibody to vascular endothelial growth factor receptor 2 (VEGFR-2). After completing the first cycle, the patient showed no specific discomfort or adverse effects.
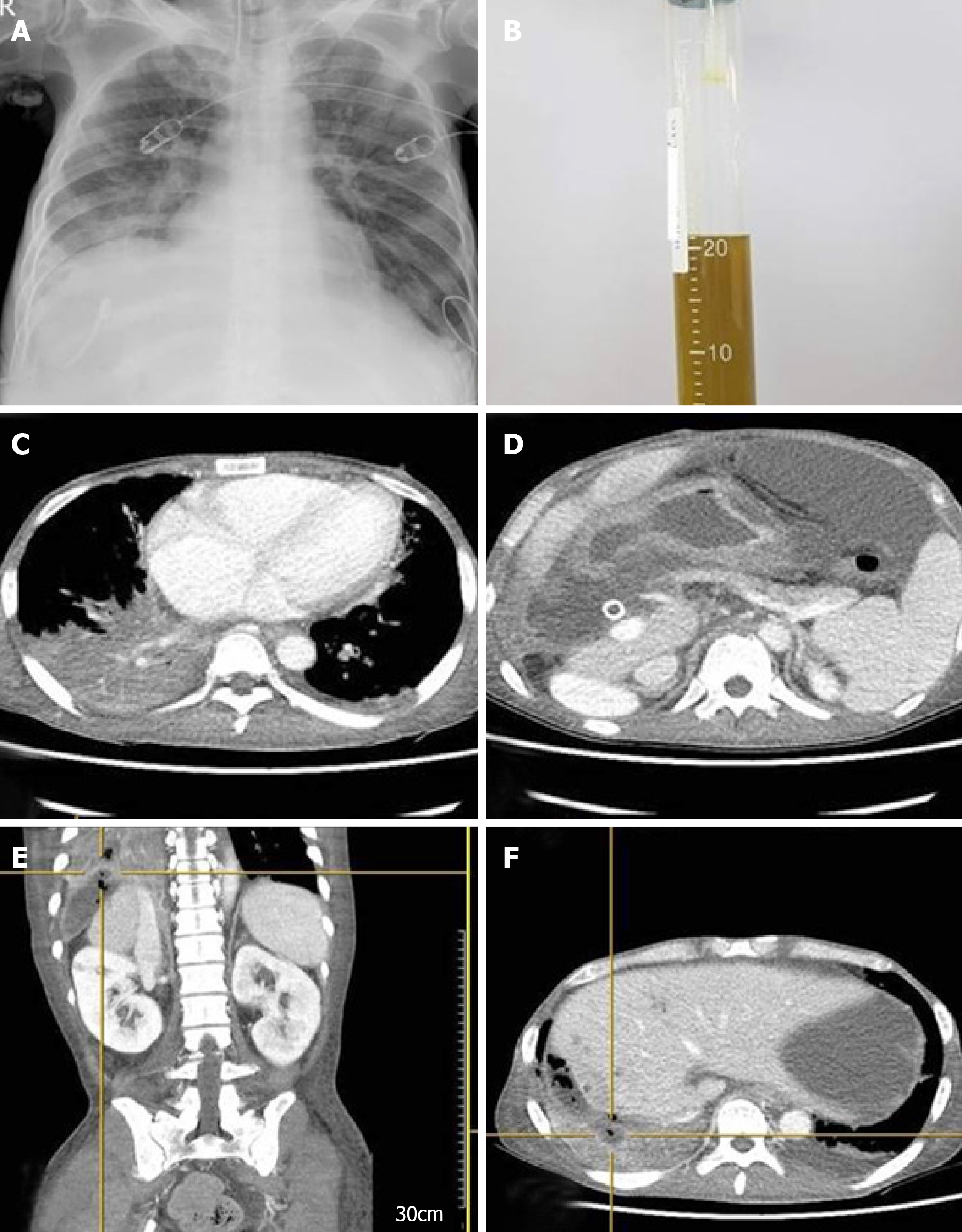
However, on the morning of the first day of the second cycle, he suddenly developed a severe cough and dyspnea and was admitted to the emergency room. Upon admission, arterial blood gas tests showed severe hypoxia: pH, 7.267 (7.35–7.45); PCO2, 52.1 mmHg (35-45 mmHg); PO2, 40.6 mmHg (80–100 mmHg); HCO3, 23.2 mmol/L (21–27 mmol/L); O2 saturation, 68.1% (92.0-100%). Simple chest X-ray imaging showed pneumonic infiltration in the right lower lobe (Figure 3A), and the patient was immediately intubated and placed on a mechanical ventilator.
The complete blood count results were as follows: WBCs, 33.31 × 103/μL; hemoglobin, 8.9 g/dL; platelets, 418× 103/mL; neutrophils, 89% (29.86 × 103/μL). Owing to highly elevated CRP levels (34.5 mg/dL) and the patient’s history of vomiting, aspiration pneumonia was strongly suspected. The biochemistry results were as follows: Total bilirubin, 1.57 mg/dL; aspartate aminotransferase, 40.8 U/L; alanine aminotransferase, 11.0 U/L. The patient was immediately started on broad-spectrum antibiotics.
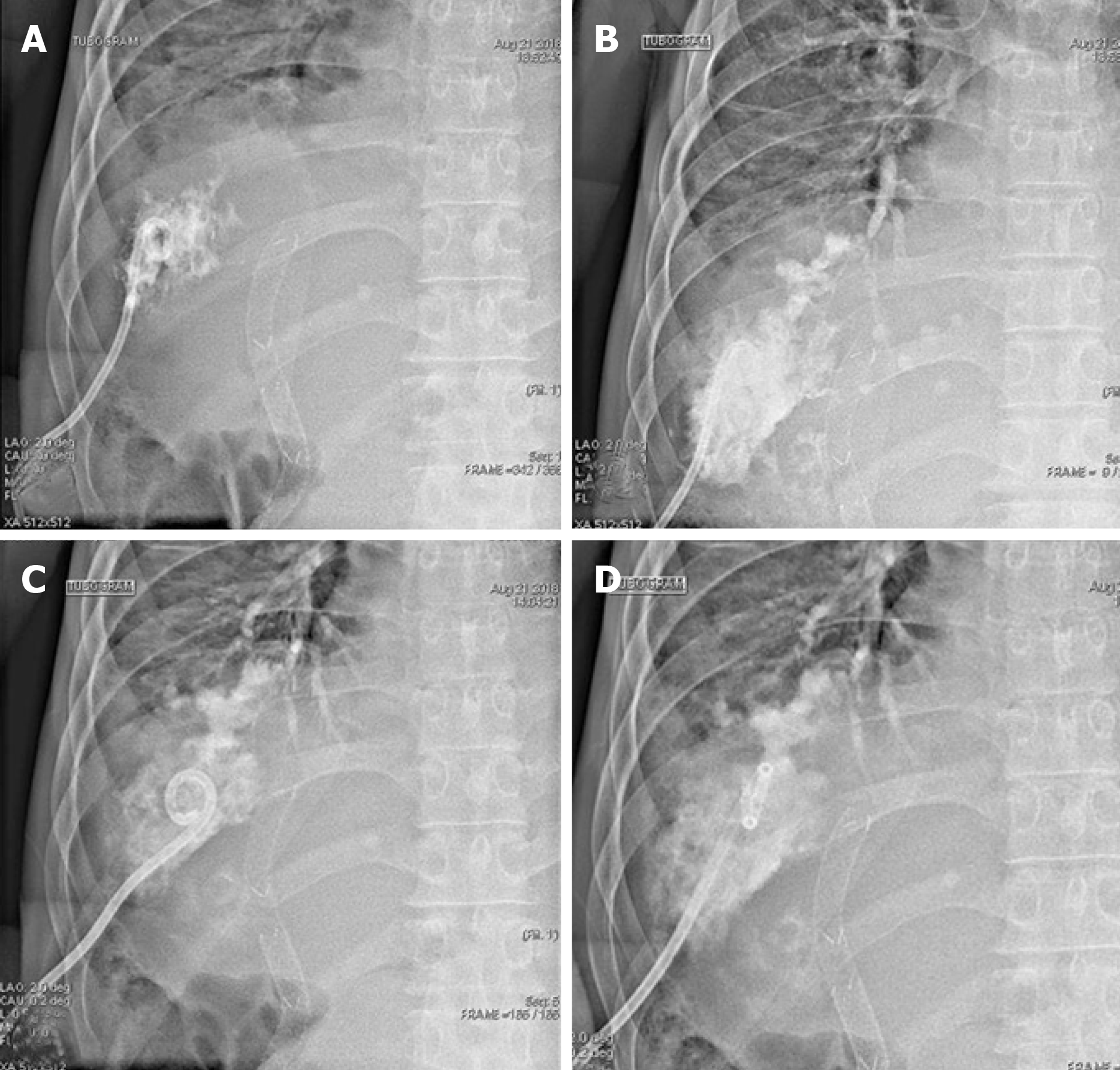
After intubation, a large amount of yellowish sputum was observed; the sputum gradually became greenish in color as time passed (Figure 4B). Thorax and abdomen CT showed widespread pneumonic infiltration in the lower lobe of the right lung and free air in the perihepatic area, as well as fluid collection and perforation of the diaphragm (Figure 4C and 4D). Based on the CT findings and the greenish sputum, BBF was strongly suspected. Multidisciplinary consultation was performed to determine the treatment plan. Surgical treatment was initially considered; however, since the patient had terminal cancer, surgery was not performed. Because it was necessary to reduce the amount of accumulated fluid, which was thought to be bile irritating the lung parenchyma, percutaneous drainage and a tubogram were performed in the perihepatic area. The tubogram showed bile juice leaking from the biliary tract and passing through the diaphragm, forming a direct fistula with the bronchus; bile drainage was observed directly, confirming the diagnosis of BBF (Figure 4A-D). The patient continued to receive broad spectrum antibiotics and mechanical ventilation with bile drainage, but the pneumonia continually worsened without improvement until he eventually died.
Bronchobiliary fistula after ramucirumab treatment for advanced gastric cancer
Bile is a very strong irritant that causes a severe inflammatory reaction when it leaks out of the bile duct. In BBF, the leaked bile directly irritates and perforates the diaphragm, causing a severe inflammatory reaction at the lung pleura, ultimately forming a connection between the bile duct and the bronchial tree. BBF has a very poor prognosis: The bile aggravates severe pneumonia, potentially leading to death due to sepsis or acute respiratory distress syndrome (ARDS)[1-8]. Bile leakage in BBF has multiple causes.
In patients with a history of hepatobiliary tract disease, especially those who recently underwent a liver procedure, signs of BBF include respiratory symptoms (e.g., dyspnea or cough), severe pneumonia in the right lower lobe, and a sudden elevation in the levels of liver and inflammatory markers[9,10]. However, owing to its rarity, BFF is difficult to diagnose, with a confirmed diagnosis requiring the presence of bilious sputum and the identification of diaphragmatic perforation on CT or bile connecting to the bronchus on endoscopic retrograde cholangiopancreatography (ERCP) or PTBD[1-4,11,12].
The first aim of BBF treatment is to reduce the amount of bile that drains into the bronchial system. The most effective treatment is surgery to directly suture the fistula after early diagnosis. For BBFs caused by bile duct obstructions of benign origin or liver abscesses, procedures such as ERCP or PTBD can reduce the amount of bile juice entering the bronchus and detect and suture the fistula directly[11-13]. However, once bilious sputum is observed (i.e., BFF is diagnosed), the disease has already progressed to pneumonia in most patients owing to bile-induced inflammation, with dyspnea, ARDS, and sepsis soon following. Moreover, most BBF patients have underlying disorders (e.g., an abscess or tumor) that considerably weaken their systems, making treatment difficult and prognosis extremely poor[1-4,14-18].
Ramucirumab is a monoclonal antibody for VEGFR-2 that is currently used in combination with paclitaxel as an antiangiogenic agent in second-line chemotherapy for gastric cancer[19,20]. Ramucirumab is in the same class as bevacizumab, which is widely used in colorectal cancer. One of the severe effects of bevacizumab is GI perforation, which occurs in 1%-3% of cases[21-23]. The mechanisms for GI perforation are diverse. One mechanism involves the inhibition of angiogenesis and neovascularization by antiangiogenic agents; these processes are required for an adequate blood supply for tissue regeneration after chemotherapy-induced tumor necrosis. Insufficient healing of severe inflammation around the cancer can also result in perforation, as can bowel ischemia due to a tumor thrombus[23-25]. Like bevacizumab, ramucirumab causes GI perforation, although the incidence is lower (around 1.5%). The mechanisms whereby ramucirumab induces GI perforation are thought to be similar to those of bevacizumab, but more investigations are needed[26]. The precise incidence of GI perforation in patients taking ramucirumab also remains to be determined.
In this case study, the main symptom at initial presentation was jaundice due to the direct invasion of the hepatic hilum by the advanced gastric cancer. After PTBD and stent placement, the patient received first-line chemotherapy consisting of capecitabine and oxaliplatin, but the cancer still progressed. Paclitaxel and ramucirumab were administered as second-line chemotherapy. Although the patient’s condition appeared to be stable after the first cycle, he suddenly developed dyspnea and pneumonia. BBF was suspected owing to the presence bilious sputum and was confirmed via CT and PTBD. The following mechanism may be possible: During ramucirumab chemotherapy, we observed perforation of the diaphragm and biloma at the site of the stent in the bile duct. The bile may have irritated the diaphragm and formed a fistula that eventually connected to the bronchus, resulting in BBF.
This is a valuable report as it presents an extremely rare case of BBF after chemotherapy in a gastric cancer patient. Moreover, it is the first reported case of BBF development after administration of the new antiangiogenic agent ramucirumab.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Merrett ND S-Editor: Gong ZM L-Editor: A E-Editor: Liu MY
| 1. | Gugenheim J, Ciardullo M, Traynor O, Bismuth H. Bronchobiliary fistulas in adults. Ann Surg. 1988;207:90-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Mandal A, Sen S, Baig SJ. Bronchobiliary fistula. J Minim Access Surg. 2008;4:111-113. [PubMed] |
| 3. | Liao GQ, Wang H, Zhu GY, Zhu KB, Lv FX, Tai S. Management of acquired bronchobiliary fistula: A systematic literature review of 68 cases published in 30 years. World J Gastroenterol. 2011;17:3842-3849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 119] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 5. | Moreira VF, Arocena C, Cruz F, Alvarez M, San Roman AL. Bronchobiliary fistula secondary to biliary lithiasis. Treatment by endoscopic sphincterotomy. Dig Dis Sci. 1994;39:1994-1999. [PubMed] |
| 6. | Baudet JS, Medina A, Moreno A, Navazo L, Avilés J, Soriano A. Bronchobiliary fistula secondary to ruptured hepatocellular carcinoma into the bile duct. J Hepatol. 2004;41:1066-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Kim HY, Kwon SH, Oh JH, Shin JS, Dong SH, Park MJ, Park SJ. Percutaneous Transhepatic Embolization of a Bronchobiliary Fistula Developing Secondary to a Biloma After Conventional Transarterial Chemoembolization in a Patient with Hepatocellular Carcinoma. Cardiovasc Intervent Radiol. 2016;39:628-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, De Wever I, Michel L. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 496] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 9. | Poullis M, Poullis A. Biliptysis caused by a bronchobiliary fistula. J Thorac Cardiovasc Surg. 1999;118:971-972. [PubMed] |
| 10. | Matsumoto T, Otsuka K, Kaihara S, Tomii K. Biliary Pneumonia due to the Presence of a Bronchobiliary Fistula. Intern Med. 2017;56:1451-1452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Brem H, Gibbons GD, Cobb G, Edgin RA, Ellison EC, Carey LC. The use of endoscopy to treat bronchobiliary fistula caused by choledocholithiasis. Gastroenterology. 1990;98:490-492. [PubMed] |
| 12. | Kim JH, Kim MD, Lee YK, Hwang SG, Lee JH, Kim EK, Jeong HC. Bronchobiliary fistula treated with histoacryl embolization under bronchoscopic guidance: a case report. Respir Med CME. 2008;1:164-168. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Katsinelos P, Paroutoglou G, Chatzimavroudis G, Beltsis A, Mimidis K, Katsinelos T, Pilpilidis I, Papaziogas B. Successful treatment of intractable bronchobiliary fistula using long-term biliary stenting. Surg Laparosc Endosc Percutan Tech. 2007;17:206-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Rose DM, Rose AT, Chapman WC, Wright JK, Lopez RR, Pinson CW. Management of bronchobiliary fistula as a late complication of hepatic resection. Am Surg. 1998;64:873-876. [PubMed] |
| 15. | Crnjac A, Pivec V, Ivanecz A. Thoracobiliary fistulas: literature review and a case report of fistula closure with omentum majus. Radiol Oncol. 2013;47:77-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Kim DH, Choi DW, Choi SH, Heo JS, Jeong J, Rhu J. Surgical treatment of bronchobiliary fistula due to radiofrequency ablation for recurrent hepatocellular carcinoma. Korean J Hepatobiliary Pancreat Surg. 2013;17:135-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Chua HK, Allen MS, Deschamps C, Miller DL, Pairolero PC. Bronchobiliary fistula: principles of management. Ann Thorac Surg. 2000;70:1392-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Chong CF, Chong VH, Jalihal A, Mathews L. Bronchobiliary fistula successfully treated surgically. Singapore Med J. 2008;49:e208-e211. [PubMed] |
| 19. | Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Zalcberg JR, Chau I, Campbell W, Sivanandan C, Pikiel J, Koshiji M, Hsu Y, Liepa AM, Gao L, Schwartz JD, Tabernero J; REGARD Trial Investigators. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1541] [Cited by in RCA: 1576] [Article Influence: 143.3] [Reference Citation Analysis (0)] |
| 20. | Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A; RAINBOW Study Group. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1613] [Cited by in RCA: 1770] [Article Influence: 160.9] [Reference Citation Analysis (0)] |
| 21. | Tanyi JL, McCann G, Hagemann AR, Coukos G, Rubin SC, Liao JB, Chu CS. Clinical predictors of bevacizumab-associated gastrointestinal perforation. Gynecol Oncol. 2011;120:464-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Sliesoraitis S, Tawfik B. Bevacizumab-induced bowel perforation. J Am Osteopath Assoc. 2011;111:437-441. [PubMed] |
| 23. | Badgwell BD, Camp ER, Feig B, Wolff RA, Eng C, Ellis LM, Cormier JN. Management of bevacizumab-associated bowel perforation: a case series and review of the literature. Ann Oncol. 2008;19:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Kim HS, Kim SS, Park SG. Bowel perforation associated sunitinib therapy for recurred gastric gastrointestinal stromal tumor. Ann Surg Treat Res. 2014;86:220-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Park SG, Chung CH, Park CY. Colon perforation during sorafenib therapy for advanced hepatocelluar carcinoma. A case report. Tumori. 2011;97:794-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Sashegyi A, Zhang Y, Lin Y, Binder P, Ferry D. Comment on: Risk of gastrointestinal perforation in cancer patients receiving ramucirumab: a meta-analysis of randomized controlled trials. J Chemother. 2017;29:62-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |